Abstract
The ultrasmall nanoparticle AGuIX is a versatile platform that tolerates a range of chemical diversity for theranostic applications. Our previous work showed that AGuIX clears rapidly from normal tissues, while durably accumulating within the tumor microenvironment. On this basis, AGuIX was used to detect tumor tissue with Gd3+ enhanced MRI, and can sensitize tumors to radiation therapy. As we begin the translation of AGuIX, we appreciated that coupling AGuIX to a long-lived radioisotope would help to more completely measure the magnitude and duration of its retention within the tumor microenvironment. Therefore, we developed 89Zr-DFO-AGuIX. AGuIX was coupled to DFO and then to 89Zr in ~99% radiochemical yield. Stability studies showed that 89Zr-DFO-AGuIX did not dissociate after 72 hours. In animals bearing U87MG xenografts, it was detectable at levels above background for 72 hours. Lastly, 89Zr-DFO-AGuIX did not accumulate in inflammatory abscesses in vivo, highlighting its specificity for well vascularized tumors.
Keywords: AGuIX, nanoparticle, Positron emission tomography, cancer
Introduction:
Owing to their chemical versatility and encouraging properties in vivo, nanotechnologies have been aggressively developed and refined over the past two decades for biomedical applications1, 2. We have previously developed AGuIX, an ultrasmall nanoparticle (NP) for theranostic applications in cancer2–5. AGuIX is ~10 kDa NP with a hydrodynamic diameter of ~3 nm. The core of the NP is a polysiloxane matrix, onto which chemically diverse chelators can be grafted. For instance, about 10 DOTAGA chelators have been engineered per NP to bind Gd3+ for contrast enhanced MRI in vivo6, 7. Moreover, alternative chelators can be attached onto AGuIX for nuclear imaging, and we recently engineered one additional NODAGA chelator to bind 68Ga3+ to AGuIX for PET in vivo8. Lastly, near-infrared fluorescent molecules can be conjugated to the NP to track its tissue localization with microscopy or to follow them by optical imaging9, 10.
Applying these constructs in vivo to study the biodistribution of AGuIX longitudinally with MRI and PET has shown that the construct does not accumulate substantially in normal tissues, and rapidly clears from rodents via the kidneys. After 24 hours, ~90% of the NPs are cleared in the urine, with ~10% of the injected NPs bound within the kidneys and ~0.2% distributed in all other normal tissues. Microscopy with fluorescently tagged AGuIX showed retention within the proximal tubules of the kidneys, with subsequent excretion of the native or partially degraded NPs.11. Lastly, sufficient accumulation of the NP has been observed in the microenvironment of preclinical tumor models to amplify the therapeutic effects of external beam radiation therapy11, 12,. Collectively, these observations strongly justify first in man studies with AGuIX.
Long lived radioisotopes (e.g. zirconium-89, iodine-124) are essential to fully study the pharmacokinetics and biodistribution of molecules with higher order structure in vivo13. Because AGuIX can be readily engineered with chemically discrete chelators, we reasoned that 89Zr could be incorporated onto chelates grafted at the surface of the polysiloxane core for nuclear imaging. Indeed, we and others have shown that 89Zr is an excellent radionuclide to characterize monoclonal antibodies in preclinical models of cancer, and that 89Zr can be stably coupled to large biomolecules with a desferrioxamine (DFO) chelator13, 14. These considerations motivated us to design a synthetic route to DFO-conjugated AGuIX, and to evaluate the properties of 89Zr-DFO-AGuIX in vitro and in vivo.
Experimental Section:
Chemicals:
Sodium hydroxide (NaOH, 99.99%), hydrochloric acid (HCl, 36.5−38%) and dimethylsulfoxide (DMSO, >99.5%) were purchased from Aldrich Chemical (France), acetonitrile (CH3CN, >99.9%) was purchased from Carlo Erba (France), trifluoroacetic acid (TFA, >99%) was purchased from Alfa Aesar (United Kingdom), copper sulfate pentahydrate (CuSO4.5H2O, 98%) was purchased from Merck (Germany). AGuIX particles were purchased from Nano-H (Saint-Quentin Fallavier, France). The desferrioxamine chelate, p-NCS-Bz-DFO (N1-hydroxy-N1-(5-(4-(hydroxy(5-(3-(4-isothiocyanatophenyl)thioureido)pentyl)amino)-4-oxobutanamido)pentyl)-N4-(5-(N-hydroxyacetamido)pentyl)succinamide) was purchased from ChemaTech (France). All products were used without further purification. Only Mili-Q water (ρ>18MΩ.cm) was used for the aqueous solution preparation.
Synthesis of DFO-AGuIX:
AGuIX nanoparticles (500 μmol based on [Gd3+], 1 eq.) were dispersed in 6.67 mL of water for 1 hour at a pH of 7.4. 37.6 mg of p-NCS-Bz-DFO (50 μmol, 1 eq.) were dissolved in 4.80 mL of DMSO. The DMSO solution was then gradually added over 8 hours to the AGuIX solution under stirring at 50°C. The addition was performed gradually to prevent precipitation of the ligands and of the functionalized product. After addition was complete, the pH was decreased to 5.0 with 1M HCl solution and stirred at room temperature overnight to precipitate free unreacted DFO. Unreacted DFO was firstly removed by centrifugation; all other other unreacted products were removed by tangential filtration trough Vivaspin® membranes (molecular weight threshold = 5 kDa, Sartorius Stedim Biotech). The DFO-AGuIX solution was concentrated to approximately [Gd3+] = 100 mM after many cycles of tangential filtration. The solution was sterile filtered through 0.2 μm syringe filter in order to remove the largest impurities. It was then freeze-dried for storage, using a Christ Alpha 1–2 lyophilizer. The Gd3+ yield for the synthesis of AGuIX prior to conjugating to DFO was 48%, which was determined with relaxometry and inductively coupled plasma mass spectrometry as reported previously8. After conjugation of DFO to AGuIX, the yield of the final molecule was determined to be 68% using ICP-MS.
Radiochemistry:
To a solution of [89Zr]Zr-oxalic acid (1.2mCi; 12 μl) and 2 M Na2CO3 (12 μl) was added 0.30 ml of 0.5 M HEPES (pH 7.1–7.3), 0.50 ml of DFO-AGuIX (pH = 7, 22.5 μmoles Gd) or 0.50 of DFO (Deferoxamine mesylate salt, pH = 7, 15 mg), and 0.70 ml 0.5 M HEPES (pH 7.1–7.3) into the reaction vial were added. After incubation (120 minutes) at 37° C, the radiolabeling yield was determined by iTLC. The final product was filtered through a 0.2 micron filter into a sterile vial. Radiolabeling efficiency (typically > 98.5%) was determined by ITLC using chromatography strips and 20 mM citric acid (pH 4.9–5.1) as the mobile phase. In vitro stability of 89Zr-AGuIX was assessed in serum for up to 3 days. For the serum stability studies, 50 μL 89Zr-AGuIX at 50 mM in Gd3+ were added to 450 μL serum and the mixture was incubated at 37 °C. Samples were taken at 1h, 4h, 24h, and 75h and assessed by ITLC. 89Zr-transferrin was prepared as previously described15.
Serum stability determination:
Blood samples were immediately subjected to centrifugation at 2300g for 15 min at 4 °C, and the serum supernatants were collected. The supernatant was immediately aliquoted and studied. In vitro, 50 μCi 89Zr-DFO-AGuIX were incubated with 100% fetal bovine serum and aliquots were reserved for analysis at 1 hr, 4 hr, 8 hr, 24 hr and 72 hr. Healthy nu/nu mice (8–10 weeks old, n = 3) were injected with 50 ± 3 uCi of 89Zr-DFO-AGuIX preparation in 100 μL HEPES solution (pH=7.4). Blood was sampled from the submandibular vein at 1 hr, 4 hr, 8 hr, 24 hr and 72 hr post injection. Activity within the blood samples was resolved by ITLC and each experiment was carried out in triplicate.
Animal studies:
All animal protocols were approved by the UCSF Administrative on Laboratory Animal Care. For tumor imaging studies, 8-wk-old male nude (nu/nu) mice (Charles River Laboratories) were injected subcutaneously with 1 × 106 cells containing 50% Matrigel (v/v) in the flank while anesthetized with 2% isoflurane. Animals were used for imaging studies with MRI and PET once the tumors became palpable. For MRI studies, AGuIX (20 μmol) and DOTAREM (20 μmol) were administered via tail vein injection. Animals were imaged with MRI 20 minutes post injection. For biodistribution studies with inflammatory abscesses, ~50 μL of turpentine oil (Sigma Aldrich) was injected subcutaneously in the right hind limb per a previously established methodology16. Fifteen minutes was allowed to pass to generate the acute phase response, whereupon the mice were injected with 89Zr-transferrin or 89Zr-DFO-AGuIX. Animals were euthanized 30 min post injection with CO2 (g) asphyxiation.
Magnetic resonance imaging:
All MRI acquisitions were performed on a 7T Small Animal MRI facility at UCSF, using a 7T 300 MHz Horizontal Bore Varian MR System. Pre and Post-treatment MR imaging was performed. A multislice spin-echo sequence was used to generate spin-density weighted images, with a TR of 3000 ms and a TE of 18 ms. Sample scans had an acquisition matrix of 128×128 points, with the field of view measuring 19.2×19.2 mm. Prior to injection in mice, a concentrated colloid (AGuIX in water, [Gd3+] = 100 mM) was diluted by saline solution in order to obtain an intravenous use solution ([Gd3+] = 50 mM). The pH was adjusted to 7.4. Before use, this solution was filtered onto syringe filter with nylon membrane (pore diameter 0.22 μm). The chelate used was DOTAREM® (laboratories Guerbet, Aulnay sous Bois France, 0.5 mM/mL) as available in MRI units. The aqueous AGuIX was manually injected in the tail vein at 200 μL volume. The gadolinium chelates were injected via the tail vein at the same concentration (50mM) at 200 μL volume.
Results:
AGuIX NPs were synthesized according to our previously established, top-down protocol. Briefly, gadolinium oxide cores were first synthesized in diethylene glycol. The oxide core was coated with a polysiloxane shell using hydrolysis–condensation of aminopropyl triethoxysilane (APTES) and tetraethyl orthosilicate (TEOS). DOTAGA groups (1,4,7,10-tetra-azacyclododecane-1-glutaric anhydride-4,7,10-triacetic acid) were then covalently ligated onto the nanoparticles via a primary amine from APTES. To induce core dissolution, the NPs were transferred from DEG to water. The resulting polysiloxane hollow cores were collapsed and fragmented into small and rigid platforms of polysiloxane. The resulting NPs bear on their surface DOTAGA molecules that are chelated to Gd3+ cations.7
To prepare DFO-AGuIX, 1-(4-isothiocyanatophenyl)-3- [6,17-dihydroxy-7,10,18,21-tetraoxo-27-(N-acetylhydroxylamino)-6,11,17,22-tetraazaheptaeicosine] thiourea (p-NCS-Bz-DFO) was incubated with AGuIX for one hour at room temperature. DFO was covalently ligated to the solvent exposed primary amines contained within APTES, and the resulting functional group was a thiourea. DFO-AGuIX was subjected to tangential filtration over a 5 kDa membrane to remove any unconjugated p-NCS-Bz-DFO at pH 5. DFO-AGuIX NPs were then freeze−dried for storage.
We expected that adding DFO to AGuIX would change its retention on reverse phase HPLC. On this basis, we subjected DFO-AGuIX to RP-HPLC to determine its purity. Naked AGuIX, DFO-AGuIX, and free DFO were loaded into the injection loop separately. The corresponding chromatogram from the DFO-AGuIX showed peaks at 2.5 min corresponding to degradation fragments of DFO-AGuIX due to the hydrolysis of silyl ether bonds in diluted medium17. The major peak at 13–14 min corresponded to DFO-AGuIX (Figure 1A). Moreover, DFO-AGuIX eluted later than AGuIX and no free DFO was detected in the DFO-AGuIX formulation. The final colloidal solution was characterized by dynamic light scattering and DFO-AGuIX was determined to have a hydrodynamic diameter of 4.4 ± 1 nm (slightly larger to the hydrodynamic diameter of naked AGuIX that is 3.6 ± 0.8 nm, Figure 1B), which is suitable for renal excretion.
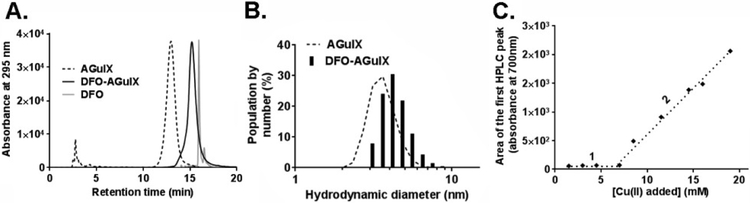
Figure 1.
Synthesis and characterization of DFO-AGuIX. A. An overlay of reverse phase HPLC traces showing the resolution of DFO-AGuIX at ~15 min compared to naked AGuIX (~13 min) and free DFO (~16 min). These data also underscore the purity of DFO-AGuIX after filtration. The early eluting peaks on the AGuIX HPLC trace are minor degradation products. They do not appear on the DFO-AGuIX trace because they are removed with reaction purification. B. Dynamic light scattering data showing that the hydrodynamic diameter of DFO-AGuIX is larger than naked AGuIX, as expected. These data were acquired using the purified DFO-AGuIX material. C. Determination of the number of DFO chelates per AGuIX nanoparticle using Cu2+ titration and UV/Vis spectroscopy. The area under the peak corresponding to free Cu2+ was calculated after addition of Cu2+ on DFO-AGuIX and injection in HPLC (see also Supplemental Figure 1). Under sub-saturating conditions (1), no peak was detected, while increasing the concentration of Cu2+, free Cu2+ ions were detected as the concentration exceeded ~7 mM (2). This assay was conducted with 38.4 mM AGuIX (in [Gd3+]).
To quantify the number of DFO molecules per AGuIX, we titrated Cu2+ into a preparation of DFO-AGuIX ([Gd3+] = 38.4 mM) and resolved the free and AGuIX bound Cu2+ with HPLC. We chose to utilize Cu2+ for several reasons. First, the constant of complexation between Cu2+ and DFO has been calculated18, and is only two fold less than Fe3+ (log β = 23.98 for [CuLH]0, log β = 41.01 for [FeLH]+). Moreover, Cu(II) sulfate is easily detected at 700 nm. Lastly, we have previously developed, validated and published this HPLC based approach using Cu(II) sulfate to determine the number of NODA chelators on the AGuIX scaffold8.
On titration, a free fraction of Cu(II) sulfate was initially detected at ~7 mM of Cu2+, and this point of inflection allowed us to estimate between 1–2 DFO chelators per DFO-AGuIX (Figure 1C and Supplemental Figure 1). A detectable but negligible amount of unknown Cu(II) conjugated degradation products were also observed (Supplemental Figure 2). These early eluting peaks did not interfere with the integration of the Cu(II) sulfate peak on the HPLC trace. ICP-MS performed on DFO-AGuIX also resulted in a relative composition consistent with 1–2 DFO molecules per AGuIX: Gd10APTES*27.8TEOS*26.6DOTAGA*10.2DFO*1.4. Lastly, a separate set of experiments with Cu2+ and naked AGuIX lacking DFO showed almost no evidence of binding (Supplemental Figure 3). This indicates that the DOTAGA chelators within AGuIX are saturated with Gd3+ (free chelate < 2%), and the Cu2+ binding observed with DFO-AGuIX is due to an interaction between Cu2+ and DFO.
DFO-AGuIX was then metallated with 89Zr-oxalate in one hour to a radiochemical yield of 99%. Unreacted 89Zr-oxalate was removed by centrifugal membrane filtration per a previous protocol19. The radiochemical purity was determined to be 100% by iTLC using 20 mM citric acid as the mobile phase (Figure 2A and Supplemental Figure 4).
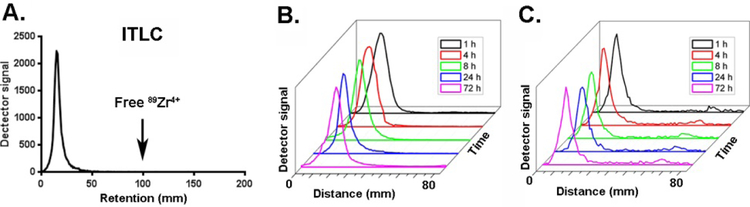
Figure 2.
Synthesis and characterization of 89Zr-DFO-AGuIX. A. A representative ITLC showing the near complete metallation of DFO-AGuIX with 89Zr-oxalate. The large peak corresponds to activity at the baseline, interpreted to be 89Zr-AGuIX, and an arrow indicates the expected Rf for 89Zr-oxalate (see also Supplemental Figure 5). B. Representative ITLC traces showing the stability of 89Zr-DFO-AGuIX over time in neat fetal bovine serum. No peaks were resolved from baseline, suggesting that 89Zr-DFO-AGuIX is not metabolized to smaller radioactive byproducts. C. Representative iTLC traces showing the stability of 89Zr-DFO-AGuIX over time in in mouse serum. Mice were injected with ~50 μCi of 89Zr-DFO-AGuIX, and blood was harvested at the indicated time point. The isolated serum was spotted and resolved by iTLC.
We next evaluated the stability of 89Zr-DFO-AGuIX in vitro and in vivo. No detectable degradation of 89Zr-DFO-AGuIX was observed after 72 hours of incubation at 37° C in bovine serum (Figure 2B). Moreover, no evidence of degradation was observed in the serum harvested from mice injected intravenously with 89Zr-DFO-AGuIX (Figure 2C and Supplemental Figure 5). Because 89Zr-DFO-AGuIX was very stable, we determined the biological half-life of the construct in normal mice. Serial measurements of total activity in tumor naïve nu/nu mice injected with 89Zr-AGuIX intravenously showed that the biological half-life is ~ 67 hours (Supplemental Figure 6).
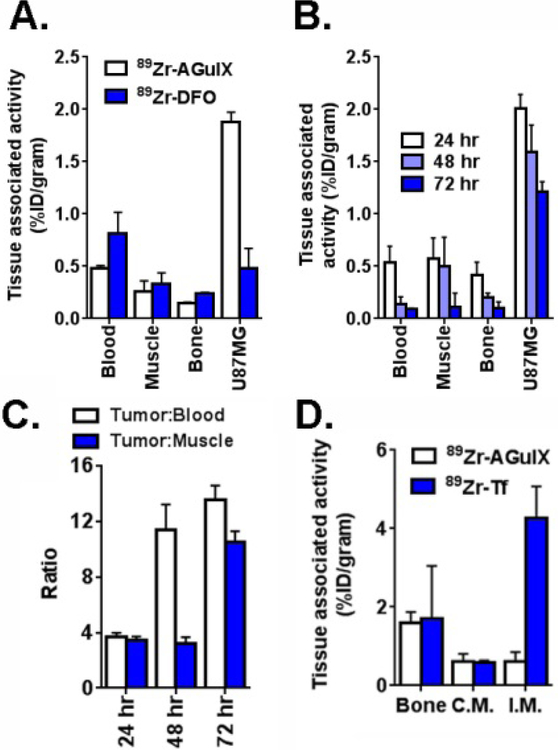
We next asked if 89Zr-DFO-AGuIX accumulates within a subcutaneous tumor model in mice. Male nu/nu mice inoculated with subcutaneous U87MG tumors were injected with 89Zr-DFO-AGuIX intravenously, and the biodistribution was monitored over time. After observing evidence of accumulation in the tumor with MRI 20 min post injection due to the positive contrast agent properties of DFO-AGuIX (r1 = 16.7 mmol−1.s−1 and r2/r1 = 1.5 at 37°C and 1.4 T) (Supplemental Figure 7), we investigated the tumor associated activity from 24 – 72 hours with biodistribution studies. Durable retention of the NPs (~2% ID/g) was observed in human glioma U87MG tumors, and was statistically greater than the accumulation of 89Zr-DFO (~0.5% ID/g at 24 hours post injection, see Figure 3A and 3B, and Supplemental Figures 8 and 9 for biodistribution from a larger panel of normal tissues.). The tumor to blood and tumor to muscle ratio for 89Zr-AGuIX incrementally increased over time to values greater than 10 at 72 hours post injection (Figure 3C). The uptake in other normal tissues was low, as expected, with the notable exception of the kidneys (Supplemental Figure 9). Autoradiography of the tumor slices ex vivo also showed that the highest degree of activity within the tumor slices co-localized with areas harboring visually obvious pericellular compartments (Supplemental Figure 10). Lastly, we tested whether 89Zr-DFO-AGuIX accumulated in inflammatory abscesses in vivo. After inducing an acute phase response with an intramuscular injection of turpentine, normal nu/nu mice were treated with 89Zr-DFO-AGuIX or 89Zr-transferrin, a molecule we previously showed to localize to sites of inflammation15. After 30 min, a biodistribution study was conducted to determine the amount of activity in the inflamed muscle and normal tissues. The uptake of 89Zr-Tf was higher in the inflamed muscle compared to the untreated contralateral muscle, as expected. However, no difference in activity was observed between the inflamed and untreated muscles of mice treated with 89Zr-AGuIX (Figure 3D and Supplemental Figure 11). Moreover, the level of tissue associated activity in the inflamed muscle was ~0.5% ID/g, or roughly equal to what was observed for 89Zr-DFO in U87MG tumors.
Figure 3.
89Zr-DFO-AGuIX accumulates in the tumor microenvironment, but not in inflammatory abscesses. A. Biodistribution data at 24 hours post injection of 89Zr-DFO-AGuIX or 89Zr-DFO shows significantly higher uptake of the NP in the microenvironment of subcutaneous U87MG tumors compared to 89Zr-DFO. No substantial differences were observed in muscle or bone, two normal reference tissues, from the cohorts receiving either radionuclide. B. Biodistribution data showing that 89Zr-DFO-AGuIX persists in the tumor microenvironment for several days post injection. At 72 hours, the tumor associated activity was ~1.0% ID/g, which is above background. C. A graphical representation of the mean tumor to muscle and tumor to blood ratios over time for mice treated with 89Zr-DFO-AGuIX. D. Biodistribution data showing no uptake of 89Zr-DFO-AGuIX in the inflamed muscles within the hindlimbs of a mouse cohort. By comparison, 89Zr-transferrin showed robust uptake in the inflamed muscle, presumably owing to the abundant expression of the transferrin receptor on peripheral mononuclear blood cells. I.M. = inflamed muscle, C.M. = contralateral unmanipulated muscle.
Discussion:
In this report, we describe the synthesis and characterization of AGuIX functionalized with DFO, and conjugated to 89Zr. The construct was synthesized from AGuIX in two steps to >98% radiochemical yield. Consistent with our expectations, the radiolabeled construct is stable out to 72 hours in vitro and in vivo, and durably accumulates into subcutaneous tumors during this time period. Finally, 89Zr-DFO-AGuIX did not accumulate in inflammatory abscesses in vivo, as expected. Collectively, these data support the use of 89Zr-DFO-AGuIX as a tool to measure the biodistribution and pharmacokinetics of AGuIX in a first-in-man study.
To our knowledge, this is the first example of a 89Zr-labeled ultrasmall nanoparticle, though others have conjugated 89Zr to larger nanoparticles with longer circulation times20–24. As we demonstrate, the use of 89Zr is justified, as AGuIX can persist for days in the tumor microenvironment due to the enhanced permeability and retention effect. Consistent with previous MR and microscopy data, accumulation within the kidney also persists for days, extending the biological half-life of AGuIX25. Moreover, we felt a radiometal-based approach (as opposed to a halogen) was appropriate, as we have previously shown that chemically discrete chelators can be added to the polysiloxane core after functionalization with DOTAGA and saturation with Gd3+. Lastly, 89Zr was an attractive radionuclide to work with, owing to its long half-life (~78 hours), and the recently clinical data showing it is well tolerated in patients when attached to monoclonal antibodies26, 27.
Incorporating a long lived radioisotope for PET onto AGuIX presents a more realistic approach to quantify the amount of NPs in cancer at later time points post injection. While our preclinical experience with AGuIX shows that its concentration in tumors can be calculated with MRI within minutes to hours post injection, contrast becomes more difficult to detect in tumors >24 hours post injection4. By comparison, a ~1 hour PET acquisition should be sufficient to acquire the ~10 million coincident events needed for tumor contrast based on the magnitude of radioactivity detected in the biodistribution study. PET also broadens the dynamic range of concentrations that can be measured by lowering the limit of NP detection compared to contrast enhanced MRI. All of these considerations are significant, as the pharmacokinetics of the NP will dictate the design of a pending clinical trial to determine its value as a radiosensitizer.
Using our current synthetic scheme, approximately 1 molecule of DFO was incorporated per AGuIX NP. In the future, we anticipate more DFO chelators can be added to the unreacted primary amines on the polysiloxane core, which in turn could favorably raise the specific activity of the construct. Along these lines, our data also argues strongly for the ligation of 89Zr-AGuIX onto large biomolecules (e.g. IgG, Fab) for immunoPET applications14. We are currently exploring these frontiers.
Supplementary Material
Acknowledgements:
The authors acknowledge Sergio Wong, and Drs Youngho Seo and Sergey Magnitsky for assistance with the biodistribution studies and MRI experiments, and Frederic Boschetti from CheMatech SAS for the furnishing of p-NCS-Bz-DFO. M.J.E., C.T., and L.T.H. were supported by the 2013 David H. Koch Young Investigator Award from the Prostate Cancer Foundation, by the National Institutes of Health (R00CA172695, 1R01CA17661-01), and the Department of Defense Prostate Cancer Research Program (PC140107, PC151060). E.T., F.L. and O.T. were supported by the French National Research Agency (ANR6126RPIB-0010 Multimage).
References:
- 1.Hainfeld JF; Smilowitz HM; O’Connor MJ; Dilmanian FA; Slatkin DN Gold nanoparticle imaging and radiotherapy of brain tumors in mice. Nanomedicine 2013, 8, (10), 1601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lux F; Mignot A; Mowat P; Louis C; Dufort S; Bernhard C; Denat F; Boschetti F; Brunet C; Antoine R; Dugourd P; Laurent S; Vander Elst L; Muller R; Sancey L; Josserand V; Coll JL; Stupar V; Barbier E; Remy C; Broisat A; Ghezzi C; Le Duc G; Roux S; Perriat P; Tillement O Ultrasmall rigid particles as multimodal probes for medical applications. Angewandte Chemie 2011, 50, (51), 12299–303. [DOI] [PubMed] [Google Scholar]
- 3.Sancey L; Lux F; Kotb S; Roux S; Dufort S; Bianchi A; Cremillieux Y; Fries P; Coll JL; Rodriguez-Lafrasse C; Janier M; Dutreix M; Barberi-Heyob M; Boschetti F; Denat F; Louis C; Porcel E; Lacombe S; Le Duc G; Deutsch E; Perfettini JL; Detappe A; Verry C; Berbeco R; Butterworth KT; McMahon SJ; Prise KM; Perriat P; Tillement O The use of theranostic gadolinium-based nanoprobes to improve radiotherapy efficacy. The British journal of radiology 2014, 87, (1041), 20140134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Duc G; Roux S; Paruta-Tuarez A; Dufort S; Brauer E; Marais A; Truillet C; Sancey L; Perriat P; Lux F; Tillement O Advantages of gadolinium based ultrasmall nanoparticles vs molecular gadolinium chelates for radiotherapy guided by MRI for glioma treatment. Cancer nanotechnology 2014, 5, (1), 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Detappe A; Kunjachan S; Rottmann J; Robar J; Tsiamas P; Korideck H; Tillement O; Berbeco R AGuIX nanoparticles as a promising platform for image-guided radiation therapy. Cancer nanotechnology 2015, 6, (1), 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bianchi A; Dufort S; Fortin PY; Lux F; Raffard G; Tassali N; Tillement O; Coll JL; Cremillieux Y In vivo MRI for effective non-invasive detection and follow-up of an orthotopic mouse model of lung cancer. NMR in biomedicine 2014, 27, (8), 971–9. [DOI] [PubMed] [Google Scholar]
- 7.Mignot A; Truillet C; Lux F; Sancey L; Louis C; Denat F; Boschetti F; Bocher L; Gloter A; Stephan O; Antoine R; Dugourd P; Luneau D; Novitchi G; Figueiredo LC; de Morais PC; Bonneviot L; Albela B; Ribot F; Van Lokeren L; Dechamps-Olivier I; Chuburu F; Lemercier G; Villiers C; Marche PN; Le Duc G; Roux S; Tillement O; Perriat P A top-down synthesis route to ultrasmall multifunctional Gd-based silica nanoparticles for theranostic applications. Chemistry 2013, 19, (19), 6122–36. [DOI] [PubMed] [Google Scholar]
- 8.Truillet C; Bouziotis P; Tsoukalas C; Brugiere J; Martini M; Sancey L; Brichart T; Denat F; Boschetti F; Darbost U; Bonnamour I; Stellas D; Anagnostopoulos CD; Koutoulidis V; Moulopoulos LA; Perriat P; Lux F; Tillement O Ultrasmall particles for Gd-MRI and (68) Ga-PET dual imaging. Contrast media & molecular imaging 2015, 10, (4), 309–19. [DOI] [PubMed] [Google Scholar]
- 9.Morlieras J; Dufort S; Sancey L; Truillet C; Mignot A; Rossetti F; Dentamaro M; Laurent S; Vander Elst L; Muller RN; Antoine R; Dugourd P; Roux S; Perriat P; Lux F; Coll JL; Tillement O Functionalization of small rigid platforms with cyclic RGD peptides for targeting tumors overexpressing alphavbeta3-integrins. Bioconjugate chemistry 2013, 24, (9), 1584–97. [DOI] [PubMed] [Google Scholar]
- 10.Morlieras J; Chezal JM; Miot-Noirault E; Roux A; Heinrich-Balard L; Cohen R; Tarrit S; Truillet C; Mignot A; Hachani R; Kryza D; Antoine R; Dugourd P; Perriat P; Janier M; Sancey L; Lux F; Tillement O Development of gadolinium based nanoparticles having an affinity towards melanin. Nanoscale 2013, 5, (4), 1603–15. [DOI] [PubMed] [Google Scholar]
- 11.Lux F; Sancey L; Bianchi A; Cremillieux Y; Roux S; Tillement O Gadolinium-based nanoparticles for theranostic MRI-radiosensitization. Nanomedicine 2015, 10, (11), 1801–15. [DOI] [PubMed] [Google Scholar]
- 12.Le Duc G; Miladi I; Alric C; Mowat P; Brauer-Krisch E; Bouchet A; Khalil E; Billotey C; Janier M; Lux F; Epicier T; Perriat P; Roux S; Tillement O Toward an image-guided microbeam radiation therapy using gadolinium-based nanoparticles. ACS nano 2011, 5, (12), 9566–74. [DOI] [PubMed] [Google Scholar]
- 13.Zeglis BM; Houghton JL; Evans MJ; Viola-Villegas N; Lewis JS Underscoring the influence of inorganic chemistry on nuclear imaging with radiometals. Inorganic chemistry 2014, 53, (4), 1880–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans MJ Measuring oncogenic signaling pathways in cancer with PET: an emerging paradigm from studies in castration-resistant prostate cancer. Cancer discovery 2012, 2, (11), 985–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holland JP; Evans MJ; Rice SL; Wongvipat J; Sawyers CL; Lewis JS Annotating MYC status with 89Zr-transferrin imaging. Nature medicine 2012, 18, (10), 1586–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jennings G; Elia M The acute-phase response to turpentine-induced abscesses in malnourished rats at different environmental temperatures. Metabolism: clinical and experimental 1992, 41, (2), 141–7. [DOI] [PubMed] [Google Scholar]
- 17.Truillet C; Lux F; Tillement O; Dugourd P; Antoine R Coupling of HPLC with electrospray ionization mass spectrometry for studying the aging of ultrasmall multifunctional gadolinium-based silica nanoparticles. Analytical chemistry 2013, 85, (21), 10440–7. [DOI] [PubMed] [Google Scholar]
- 18.Crisponi G; Nurchi VM; Crespo-Alonso M; Sanna G; Zoroddu MA; Alberti G; Biesuz R A Speciation Study on the Perturbing Effects of Iron Chelators on the Homeostasis of Essential Metal Ions. PloS one 2015, 10, (7), e0133050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vosjan MJ; Perk LR; Visser GW; Budde M; Jurek P; Kiefer GE; van Dongen GA Conjugation and radiolabeling of monoclonal antibodies with zirconium-89 for PET imaging using the bifunctional chelate p-isothiocyanatobenzyl-desferrioxamine. Nature protocols 2010, 5, (4), 739–43. [DOI] [PubMed] [Google Scholar]
- 20.Keliher EJ; Yoo J; Nahrendorf M; Lewis JS; Marinelli B; Newton A; Pittet MJ; Weissleder R 89Zr-labeled dextran nanoparticles allow in vivo macrophage imaging. Bioconjugate chemistry 2011, 22, (12), 2383–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen F; Goel S; Valdovinos HF; Luo H; Hernandez R; Barnhart TE; Cai W In Vivo Integrity and Biological Fate of Chelator-Free Zirconium-89-Labeled Mesoporous Silica Nanoparticles. ACS nano 2015, 9, (8), 7950–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller L; Winter G; Baur B; Witulla B; Solbach C; Reske S; Linden M Synthesis, characterization, and biodistribution of multiple 89Zr-labeled pore-expanded mesoporous silica nanoparticles for PET. Nanoscale 2014, 6, (9), 4928–35. [DOI] [PubMed] [Google Scholar]
- 23.Perez-Medina C; Abdel-Atti D; Zhang Y; Longo VA; Irwin CP; Binderup T; Ruiz-Cabello J; Fayad ZA; Lewis JS; Mulder WJ; Reiner T A modular labeling strategy for in vivo PET and near-infrared fluorescence imaging of nanoparticle tumor targeting. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 2014, 55, (10), 1706–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perez-Medina C; Tang J; Abdel-Atti D; Hogstad B; Merad M; Fisher EA; Fayad ZA; Lewis JS; Mulder WJ; Reiner T PET Imaging of Tumor-Associated Macrophages with 89Zr-Labeled High-Density Lipoprotein Nanoparticles. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 2015, 56, (8), 1272–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sancey L; Kotb S; Truillet C; Appaix F; Marais A; Thomas E; van der Sanden B; Klein JP; Laurent B; Cottier M; Antoine R; Dugourd P; Panczer G; Lux F; Perriat P; Motto-Ros V; Tillement O Long-term in vivo clearance of gadolinium-based AGuIX nanoparticles and their biocompatibility after systemic injection. ACS nano 2015, 9, (3), 2477–88. [DOI] [PubMed] [Google Scholar]
- 26.Pandit-Taskar N; O’Donoghue JA; Durack JC; Lyashchenko SK; Cheal SM; Beylergil V; Lefkowitz RA; Carrasquillo JA; Martinez DF; Fung AM; Solomon SB; Gonen M; Heller G; Loda M; Nanus DM; Tagawa ST; Feldman JL; Osborne JR; Lewis JS; Reuter VE; Weber WA; Bander NH; Scher HI; Larson SM; Morris MJ A Phase I/II Study for Analytic Validation of 89Zr-J591 ImmunoPET as a Molecular Imaging Agent for Metastatic Prostate Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 2015, 21, (23), 5277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dijkers EC; Oude Munnink TH; Kosterink JG; Brouwers AH; Jager PL; de Jong JR; van Dongen GA; Schroder CP; Lub-de Hooge MN; de Vries EG Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clinical pharmacology and therapeutics 2010, 87, (5), 586–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.