Supplemental Digital Content is available in the text.
Keywords: anticoagulants, atrial fibrillation, brain infarction, cerebral hemorrhage
Abstract
Background and Purpose—
Recurrent bleeding associated with oral anticoagulants (OACs) causes a dilemma in patients with atrial fibrillation (AF) sustaining an intracerebral hemorrhage. Treatment recommendations guiding clinical practice on optimal OAC agent selection in this population are lacking. This study aimed to investigate the comparative effectiveness and safety of non–vitamin K antagonist OAC (NOAC) versus warfarin in patients with AF sustaining an intracerebral hemorrhage.
Methods—
We conducted a nationwide observational cohort study including patients with AF sustaining an intracerebral hemorrhage and who subsequently claimed an OAC prescription. Contrasts of 1-year risks for ischemic stroke and intracerebral hemorrhage risks were obtained and evaluated by inverse probability treatment weighted absolute risk reduction and risk ratios.
Results—
Among 622 AF patients with intracerebral hemorrhage, 274 claimed a warfarin prescription and 348 a NOAC prescription. Mean age was 76 years (39% females); 72% had an index nonsevere event and 28% moderate to severe index event according to the Scandinavian Stroke Severity scale. The 1-year ischemic stroke risk was 7.85% for warfarin and 4.01% for NOACs, with a weighted absolute risk reduction of 3.78% (95% CI, −0.15% to 7.71%); the weighted risk ratio was 0.52 (0.27–1.00). For recurrent intracerebral hemorrhage, the risk was 7.00% for warfarin and 5.07% for NOACs. The absolute risk reduction was 1.93% (−2.02% to 5.87%), with an a weighted risk ratio of 0.72 (0.38–1.38).
Conclusions—
NOACs were associated with a nonsignificant lower risk of ischemic stroke and recurrent intracerebral hemorrhage compared with warfarin. The results add to current recommendations of selecting a NOAC agent for stroke prophylaxis treatment in patients with AF, including those with sustaining an intracerebral hemorrhage.
Patients presented with atrial fibrillation (AF) are at increased risk of stroke, and prophylactic treatment with oral anticoagulants (OAC) is central to the management of this common arrhythmia.1 The increase in AF prevalence and change in guideline recommendations have increased the proportion of patients with AF at risk of bleeding related to OAC treatment.2,3 The most feared bleeding complication related to OAC is intracranial bleeding (including intracerebral hemorrhage), which is associated with a poor prognosis of functionality and a 30-day mortality up to 40%.4,5 OAC treatment will usually be discontinued immediately in patients with OAC-related intracranial bleeding. If OAC is to be reintroduced in these clinically complicated patients with AF, the risk of ischemic stroke should be balanced against the risk of recurrent intracranial bleeding.6
Previous observational studies have indicated a clinical benefit of resuming OAC treatment in patients with AF sustaining an intracerebral hemorrhage or a trauma-induced intracranial bleeding.6–8 Common for these investigations was that the antithrombotic treatment options included vitamin K antagonists or antiplatelet therapy, and all showed lower risk of ischemic stroke if warfarin treatment was resumed.9
The landmark non–vitamin K antagonist OAC (NOAC) trials showed a clear benefit over warfarin in terms of lower risk for intracerebral hemorrhage.10 Nevertheless, a universal exclusion criteria for all 4 trials were patients with prior intracranial hemorrhage (including both trauma-induced bleeding events and intracerebral hemorrhages).11–14 Thus, no evidence exists for whether the efficacy and safety for NOAC versus warfarin are maintained in patients with AF sustaining an intracerebral hemorrhage. To guide clinical practice, we investigated the comparative effectiveness and safety of NOACs versus warfarin in patients with AF sustaining an intracerebral hemorrhage.
Methods
We conducted an observational cohort study using data from the nationwide Danish registries including patients with AF sustaining an intracerebral hemorrhage with subsequent OAC treatment initiation. Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to The Danish Health Data Agency at forskerservice@sundhedsdata.dk.
Sources of Data
Study data were obtained from 4 Danish nationwide databases using a unique identification number allowing linkage on individual level between databases. (1) We included data from the Danish Stroke Registry holding quality data on stroke patients since 2003 including data on type of stroke, stroke severity, smoking status, and alcohol consumption15; (2) the Danish National Prescription Registry holding information on purchase date, Anatomical Therapeutic Chemical classification code, and package size for every prescription claim since 199416; (3) the Danish Civil Registration System holding information on sex, date of birth, vital, and emigration status17; and (4) the Danish National Patient Register, which includes admission/discharge date, and discharge International Classification of Diseases (ICD) diagnoses for hospital admissions since 1977.18
Study Population
Patients with an incident discharge diagnosis for primary spontaneous intracerebral hemorrhage registered in the Danish Stroke Registry were considered for inclusion. Because we focused on the comparative effectiveness and safety of 2 different OAC treatments, patients were eligible for study inclusion when they claimed a prescription of warfarin or a NOAC agent (dabigatran, rivaroxaban, or apixaban) in the period from January 2003 to April 2017 (end of inclusion) after the index event. Edoxaban treatment was not considered because of late entry in the study period. The following Anatomical Therapeutic Chemical codes were used to define OAC treatment exposure status: B01AE07, BF01AF01, and BF01AF02 for NOACs and B01AA03 for warfarin. We restricted the study to patients with a prior hospital diagnosis of AF at the time of OAC treatment initiation (index date) recorded in the Danish National Patient Registry or in the Danish Stroke Registry. This was done to ensure a strong indication for OAC treatment recommendation. Patients with hospital diagnoses indicating valvular AF, defined as mitral stenosis (ICD: I05) or mechanical heart valves (ICD: Z952, Z953, and Z954) at the time of an OAC prescription claim were excluded. Patients were followed from the date of an OAC prescription claim until June 30, 2017, in the Danish National Patient Registry. The 10th revision of ICD codes was used to identify outcomes and clinical characteristics. The Danish Health Data Authority provided the data material. No ethical approval was obtained, as this is not mandated for registry studies in Denmark.
Outcomes and Comorbidities
Information on outcomes was obtained from the Danish National Patient Registry. The primary effectiveness outcome was a hospital diagnosis of ischemic stroke (ICD: I63 and I64.9), and the primary safety outcome was a hospital diagnosis of recurrent intracerebral hemorrhage (ICD: I61). For study outcomes, we only included primary diagnosis codes and restricted to patients who were admitted to the hospital to increase the validity of the coded diagnosis. The coding validity of these outcomes has previously been validated in the Danish National Patient Registry with a positive predictive values of ≥80%.19,20
Index intracerebral hemorrhage severity was evaluated according to the Scandinavian Stroke Scale, which (among others) include assessment of the patient’s level of consciousness, eye movements, coordination ability, and ability to speak.21 The total score of maximum 58 (lower scores indicate more severe intracerebral hemorrhage events) was stratified as nonsevere 39 to 58, moderately severe 20 to 38, and severe 0 to 19. Thromboembolic risk was quantified based on comorbidity information included in the CHA2DS2-VASc score.22 Concomitant cardiovascular medication and comorbidities were also collected from the Danish Nationwide Patient Registry and the Danish Nationwide Prescription Registry (Table I in the online-only Data Supplement).
Statistics
Time-to-event data were used to analyze risk of outcomes associated with treatment exposure groups. Time at risk was measured from the date of the initial OAC prescription until the outcome of interest, emigration, death, or end of study period, whichever came first. Outcomes were examined at 1 year of follow-up and 3 years follow-up period; the latter was selected to restrict follow-up time for warfarin users, which could not be achieved among NOAC users because of more recent market entry (August 2011).
To depict risk development over time in treatment groups, we calculated the cumulative incidence of outcomes based on the Aalen-Johansen estimator, to account for the competing risk of death.22 The pseudovalue approach was used to estimate the cumulative incidence at fixed time points with death considered as a competing event. The risks obtained from the pseudovalues were analyzed in a generalized linear model to estimate the relative risk in terms of risk ratios as well as absolute risk reduction.23,24 The latter measure was included to reflect the clinical impact of selecting either treatment approaches.
The analyses were conducted in agreement with the causal inference framework of potential outcomes. To allow for balanced comparison between treatment groups, propensity score methodology was applied to control for baseline confounding. We used an inverse probability of treatment weight and applied stabilized weights to balance covariate differences between exposure groups (see Methods in the online-only Data Supplement for details). The underlying propensity score was calculated as the probability for receiving either a NOAC or a warfarin treatment.25 The underlying logistic regression model to predict probability of treatment included the following covariates: sex, age (restricted cubic spline), stroke severity category (measured according to the Scandinavian Stroke Scale), days since hospital discharge (restricted cubic spline), length of hospital stay for the index intracerebral hemorrhage event (restricted cubic spline), reduced renal function, alcohol consumption, smoking status, CHA2DS2-VASc score (categorical), and aspirin treatment.
To allow for an evaluation of treatment effectiveness and safety in different clinical scenarios, we conducted a stratified analysis based on the severity of the index event grouped as nonsevere and moderately to severe intracerebral hemorrhages.
Sensitivity Analyses
We conducted 3 sensitivity analyses to assess the robustness of our methodological approach, and to evaluate the internal validity of the observed associations.
First, the study population was restricted to patients who claimed an OAC prescription within the first year after hospital discharge following the index intracerebral hemorrhage event. Second, to investigate the presence of residual confounding, we conducted analyses using falsification outcomes, that is, outcomes that were unlikely to be causally associated to the treatment exposure.26 For this analysis, we used hospital diagnosis of pneumonia (ICD: J12 J13 J14 J15 J16 J17 J18 A481 A709) as falsification outcome); and urinary tract infections ascertained by a prescription claim for trimethoprim (J01EA01), pivmecillinam (J01CA08), sulfamethizole (J01EB02), or nitrofurantoin (J01XE01), which are used specifically for urinary tract treatment in Denmark.27 Last, we restricted the patient inclusion period by exclusion of patients enrolled before year 2011 (first year of NOAC market availability in Denmark).
Stata/MP, version 15 (StataCorp) and R version 3.1.1 (The R Foundation) were used for the statistical analysis. A P value <0.05 was considered statistically significant.
Results
From a population of 9846 patients with incident intracerebral hemorrhage between January 2003 and April 2017, we identified 1864 AF patients discharged from the hospital with an intracerebral hemorrhage. A total of 274 patients initiated warfarin treatment, whereas 348 initiated NOAC treatment, which comprised the study population (Figure 1). The mean age was 77.4 years for NOAC-treated patients (43% females) and 74.6 years for warfarin-treated patients (34% females). The mean Scandinavian Stroke Scale severity score was 42.2 and similar between treatment groups. The proportion of patients with severe intracerebral hemorrhage was marginally higher among NOAC users (12.4% versus 8.4%), whereas the proportion of nonsevere was higher among warfarin users (69.5% among NOAC users versus 74.8% among warfarin users; Table 1). The overall proportion of patients with prior thromboembolism was 52%, and 62% of the patients were hypertensive. The mean CHA2DS2-VASc score was 4.4 (SD=1.7) and similar in both treatment groups.
Figure 1.
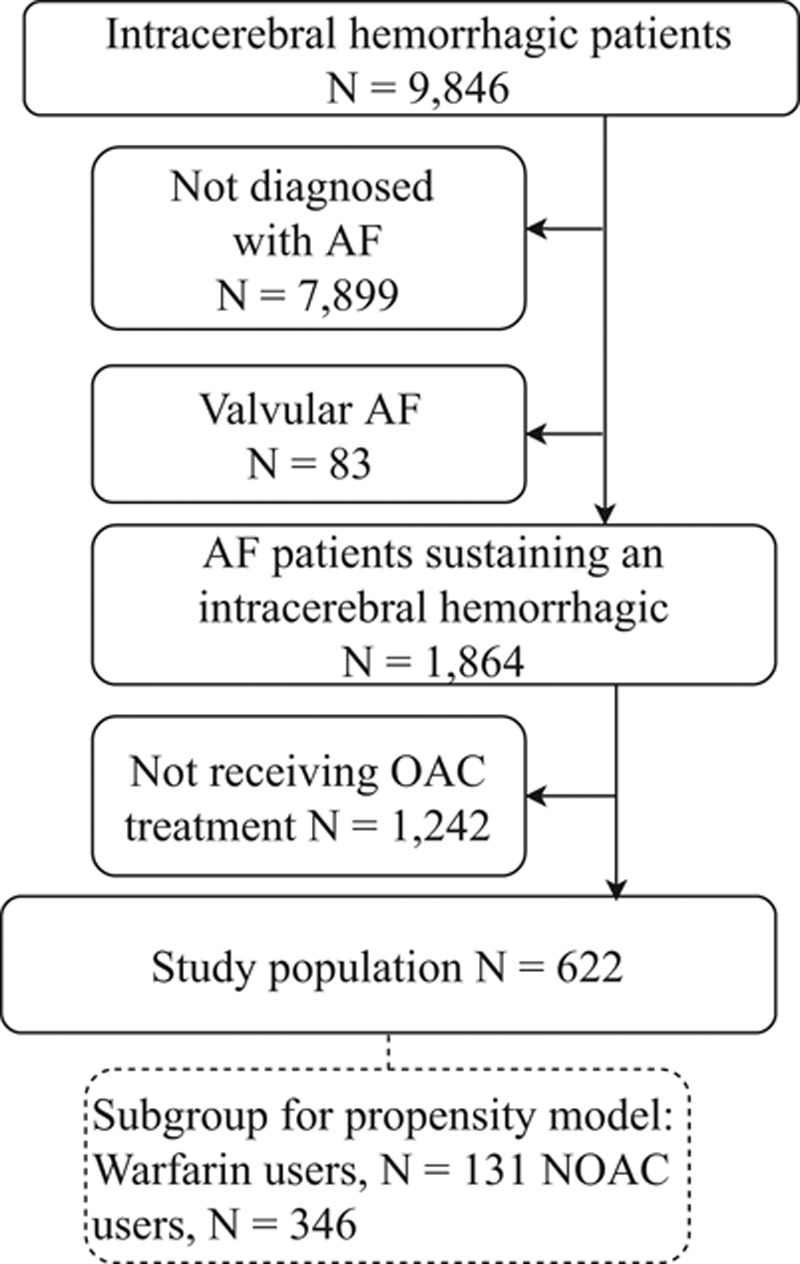
Flowchart of the study population. The subgroup of 477 patients was used to obtain the model for the propensity score (inclusion from August 2011 through April 2017). AF indicates atrial fibrillation; NOAC, non–vitamin K antagonist OAC; and OAC, oral anticoagulant.
Table 1.
Patient Characteristics
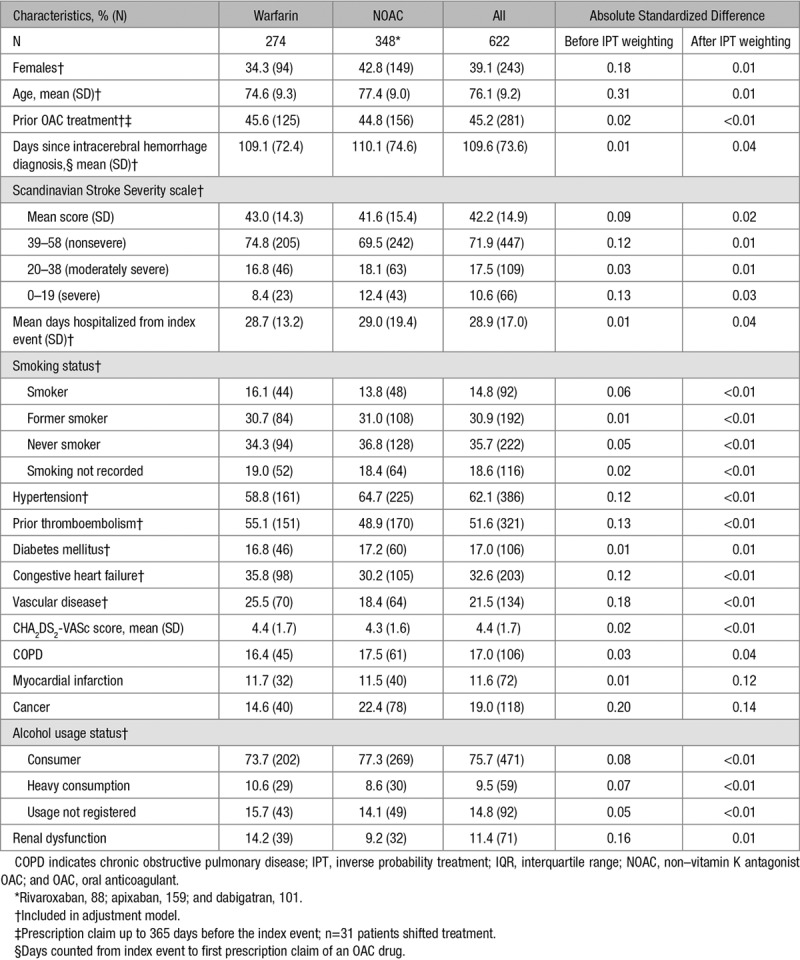
The population for creating a model for the propensity score was based on 477 patients (Figure 1). Graphical inspection of the propensity score distribution for NOAC users and warfarin users revealed sufficient overlap between treatment groups; no extreme values were observed (Figure I in the online-only Data Supplement).
Risks for Ischemic Stroke
During 1 year of follow-up, a total of 36 ischemic stroke events were observed (Table 2), 21 for warfarin-treated patients and 15 for NOAC-treated patients. Inverse probability weighted cumulative incidence curves indicated a difference in risk over time with warfarin being associated with a higher risk in comparison with NOAC-treated patients (Figure 2). The 1-year ischemic stroke risk for warfarin was 7.85% versus 4.01% for NOAC-treated patients, with an absolute weighted risk reduction of 3.78% (95% CI, −0.15% to 7.71%). The corresponding risk ratio indicated nonsignificantly lower risk among NOAC users: 0.52 (95% CI, 0.27–1.00; Figure 3). The absolute risk reduction and risk ratio were slightly attenuated when using 3-year follow-up time, but the direction of the results was maintained (Figure II in the online-only Data Supplement).
Table 2.
Event Count and Inverse Probability Treatment Weighted Absolute Risks for Investigated Outcomes According to Treatment Status at 1 Year and 3 Years of Follow-Up
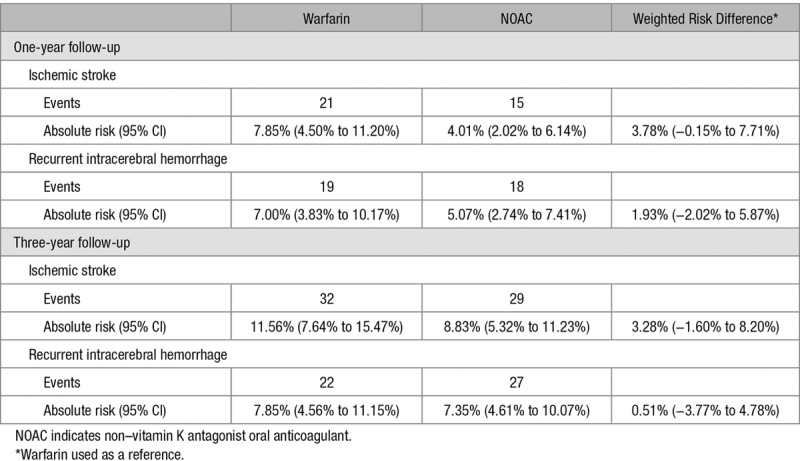
Figure 2.
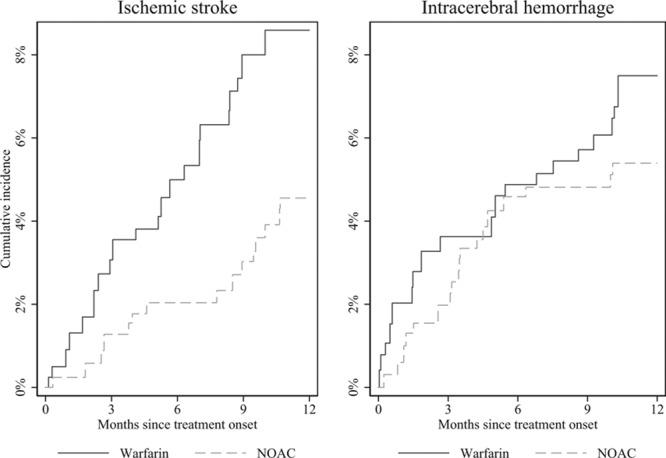
Inverse probability treatment weighted cumulative incidence of ischemic stroke and intracerebral hemorrhage for warfarin and non–vitamin K antagonist oral anticoagulant (NOAC) treatments.
Figure 3.
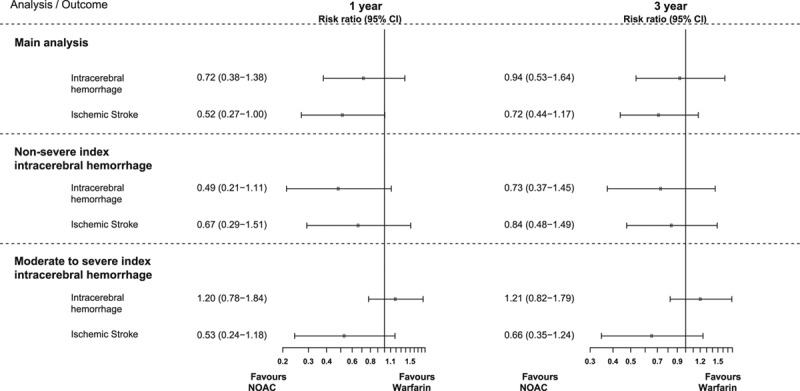
Forest plot of inverse probability treatment weighted risk ratios contrasting non–vitamin K antagonist oral anticoagulants (NOACs) vs warfarin (reference) and associated outcomes under different analytic approaches.
Restricting the analysis to patients who received OAC treatment within 1 year after hospital discharge reduced the study population to 179 warfarin-treated patients and 205 NOAC-treated patients. The 1-year risk reduction was 3.89% (95% CI, −1.34% to 9.13%), and the corresponding risk ratio was 0.54 (95% CI, 0.12–2.43); both results were in accordance with the main analysis but with wider CIs because of a lower sample size.
Risks for Recurrent Intracerebral Hemorrhage
Nineteen intracerebral hemorrhages were observed among warfarin users and 18 among NOAC users during 1 year of follow-up. Risk development during this period indicated a marginally higher risk associated with warfarin treatment compared with NOAC treatment during the first 3 months (Figure 2). The inverse probability weighted 1-year intracerebral hemorrhage risk was 7.00% for warfarin, and 5.07% for NOAC; the absolute weighted risk reduction was 1.93% (95% CI, −2.02% to 5.87%; Table 2). The corresponding risk ratio was statistically nonsignificantly different 0.72 (95% CI, 0.38–1.38; Figure 3). Using 3-year follow-up time attenuated the difference in risk of recurrence (Figure 2): the risk reduction was 0.51% (95% CI, −3.77% to 4.78%), and the risk ratio was 0.94 (95% CI, 0.53–1.64).
When restricting the study population to patients who received OAC treatment within 1 year after hospital discharge (sensitivity analysis), the 1-year risk reduction of recurrence was 0.97% (95% CI, −4.01% to 5.95%), and the adjusted risk ratio was 1.07 (95% CI, 0.39–2.95).
Intracerebral Hemorrhage Severity
A total of 447 patients were classified as having a nonsevere intracerebral hemorrhage and 175 patients survived a moderate to severe (Scandinavian Stroke Scale ≤38) intracerebral hemorrhage. In the former group, we observed 23 ischemic stroke events and 24 recurrent intracerebral hemorrhages, whereas in patients with moderate to severe index event, we observed 13 ischemic strokes and 16 intracerebral hemorrhages —using 1 year of follow-up data.
The risk reduction for ischemic stroke among nonsevere patients was 2.08% (95% CI, −2.03% to 6.18%), and the risk ratio was 0.67 (95% CI, 0.29–1.51; Figure 3). For intracerebral hemorrhage, the risk reduction and risk ratio were 4.31% (95% CI, −0.03% to 8.95%) and 0.49 (95% CI, 0.21–1.11), respectively.
Among patients classified with moderate to severe intracerebral hemorrhage, the absolute risk reduction for ischemic stroke was 7.77% (95% CI, −1.23% to 16.77%), and the risk ratio was 0.53 (95% CI, 0.24–1.18; Figure 3). The risk reduction for recurrent intracerebral hemorrhage was −3.86% (95% CI, −11.18% to 4.00%) indicating lower risk among warfarin users compared with NOAC users. The corresponding risk ratio was 1.20 (95% CI, 0.78–1.84). Caution is warranted in interpretation of the subgroup results of moderate to severe index event, as the propensity score distribution did not indicate sufficient overlap between the 2 treatment options (Figure III in the online-only Data Supplement).
Sensitivity Analyses
Examining the treatment-associated falsification outcomes revealed nonsignificant adjusted risk ratios of 1.07 (95% CI, 0.67–1.72) for pneumonia and 1.13 (95% CI, 0.87–1.48) for urinary tract infection treatment when comparing NOAC treatment versus warfarin. The results from the falsification analyses may indicate low risk of residual confounding in the main analysis.
Restricting the patient population by only including patients diagnosed after August 11, 2011, restricted the patient population to 497 patients (see Table II in the online-only Data Supplement for patient characteristics before and after the NOAC marked availability). The 1-year risk ratio for ischemic stroke was 0.74 (95% CI, 0.30–1.84), for intracerebral hemorrhage the risk ratio was 0.52 (95% CI, 0.26–1.05).
Discussion
This is the first nationwide cohort study to compare the effectiveness and safety of NOAC versus warfarin among patients with AF sustaining an intracerebral hemorrhage. Our principal finding was that NOAC treatment was associated with a lower risk of ischemic stroke in comparison with warfarin, although the adjusted risk ratio did not reach statistical significance. Second, we observed a nonsignificant different risk ratio of recurrent intracerebral hemorrhage among NOAC-treated patients in comparison with warfarin. The risk differences indicated that NOAC treatment was associated with >3 percentage point lower 1-year risks of both ischemic stroke and intracerebral hemorrhage compared with warfarin.
Patients with AF and concomitant intracerebral hemorrhage poses a clinical conundrum, and guideline recommendations have been lacking to guide treating physicians. The patient will often be considered at high stroke risk (from comorbidities associated with stroke in AF), whereas the bleeding event may be the primary concern for both the patient and the healthcare providers. Thus, the latent ischemic stroke risk might be of less concern, albeit this should still be among the primary concerns in the management of AF. Previous research in this field has focused on resuming treatment with vitamin K antagonists and have indicated that warfarin treatment may be a safe option.6,7,9,28 The landmark NOAC trials excluded all patients with previous intracranial hemorrhage, but reported a class effect of the drugs in reducing the risk of intracranial hemorrhage in comparison with warfarin. Study-level meta-analysis showed a relative risk reduction in intracerebral hemorrhage of 0.49 (95% CI, 0.38–64).10 Although this result is generalizable to a broad AF population, it has not been established whether this risk reduction would be achieved in an AF population with intracerebral hemorrhage. In this observational study including data reflecting clinical practice, we obtained a similar relative risk estimate for intracerebral hemorrhage when contrasting NOACs versus warfarin. Moreover, our results also suggest a clear trend of an appealing effectiveness profile for NOACs in comparison with warfarin in this population. Although this could be connected to differences in drug effects, perhaps a more plausible explanation would be because of dose management of the selected drugs. With the NOAC agents, the treating physician does not have dose titration options, because these are provided in fixed dosages (standard dose or reduced dose, administrated mainly dependent on renal function).
The mechanism for lower risk of intracerebral hemorrhage with NOAC treatment compared to warfarin is not fully understood. One possible explanation relates to how warfarin affects the coagulation system, which among others includes inhibition of activated tissue factor (factor VIIa). The cerebral blood vessels contain high concentrations of factor VII and promote coagulation in the event of endothelia disruption with bleeding into the brain wall.29 Although this may explain improved prognosis for NOAC patients experiencing an intracranial hemorrhage in contrast to warfarin patients,30 it remains unsure if this mechanism may also in part explain our observations of a better safety profile in terms of recurrent intracerebral hemorrhage. Currently, randomized controlled trials and prospective cohort studies are either in preparation or ongoing to investigate the efficacy of specific NOAC agents versus warfarin, antiplatelet therapy, or no treatment.31–33
Selecting the optimal timing for resuming (or initiating) OAC treatment post an intracerebral hemorrhage event in AF is debatable. Contemporary guidelines suggest reinitiating of OAC after 4 to 8 weeks (Class IIb) provided that the bleeding cause and risk factors are controlled.3 In our study, observation time commenced at the first OAC prescription claim post the index event of intracerebral hemorrhage. As we included prevalent OAC users, we could not assess the exact date of OAC treatment initiation. To guide optimal timing of OAC initiation after an intracerebral hemorrhage would require carefully conducted randomized controlled trials, and observational data may have a high risk of bias by indication.34
Limitations
The nature of this study was observational, and the data applied was collected for administrative purposes—both must be considered in interpretation of the results. Residual or unmeasured confounding is likely to persist and may partly explain the observed associations. Additionally, treatment strategies have evolved during the applied study period, including statin use and blood pressure control, which we could not investigate because of the size of the study population. We lacked imaging information and could not assess the volume and location of the hematoma. Hence, we cannot rule out the influence of the underlying cerebral small vessel diseases that could cause the hemorrhage itself, therefore also the possible risk of recurrence. Indeed, lobar versus nonlobar bleeding have been shown to carry high prognostic value in terms of bleeding recurrence. We also lacked information on intensity and quality of warfarin treatment. Indeed, the higher risk of ischemic stroke among warfarin users could indicate dose titration to a lower threshold than usually suggests (international normalized ratio between 2 and 3).
The falsification end point analysis did not indicate that confounding was the primary explanation of the results. Indication bias might also be present and may have influenced the selection of treatment strategy and choice of agent. However, the distributions of the propensity for receiving either NOAC or warfarin were very similar, indicating that our modeling strategy sufficiently controlled for baseline confounding (including indication bias assuming correct model specifications) to allow for causal inference of the obtained associations. We could not assess risk differences within the NOAC agents due, and low sample size, in general, preclude firm conclusions based on these data.
We did not restrict our main analysis to patients who commenced OAC treatment within a certain time after the index intracerebral hemorrhage event, but investigated this in a sensitivity analysis. Although this information was included in the underlying propensity model, time since stroke is likely a strong predictor of time to recurrence and warrants further investigations.35,36
Our results may potentially not be generalizable to the general AF population who sustain an intracerebral hemorrhage as we focused on NOACs versus warfarin among those who were selected to resume OAC treatment. Among those patients where OAC treatment has been deemed suitable, a NOAC agent seems preferable over warfarin.
Conclusions
In this Danish nationwide observational cohort study of patients with AF sustaining an intracerebral hemorrhage, NOAC treatment was associated with a lower risk of ischemic stroke and intracerebral hemorrhage in comparison with warfarin treatment, but low sample size prevented from statistically significant conclusions. Our results add to the current recommendation for selecting a NOAC agent as the preferred stroke prophylaxis in this subgroup of patients with AF when suitable.
Acknowledgments
Dr Nielsen had full access to all of the data in this study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to the design, analysis, interpretation of data, drafting the article, or revising it critically for important intellectual content and approved the final version to be published.
Sources of Funding
This study was supported by “The Bristol-Myers Squibb/Pfizer European Thrombosis Investigator Initiated Research Program 2016”. The sponsor had no role in the design; conduct of study; collection, management, analysis, and interpretation data; preparation, review, or approval of the article; and decision to submit the article for publication.
Disclosures
All authors have completed the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Lip has served as a consultant for Bayer/Janssen, Bristol-Myers Squibb/Pfizer, Medtronic, Boehringer Ingelheim, Novartis, Verseon, and Daiichi-Sankyo; and Speaker for Bayer, Bristol-Myers Squibb/Pfizer, Medtronic, Boehringer Ingelheim, and Daiichi-Sankyo. No fees were directly received personally. Dr Larsen has served as an investigator for Janssen Scientific Affairs, LLC, and Boehringer Ingelheim and received speaking fees from Bayer, Bristol-Myers Squibb/Pfizer, Boehringer Ingelheim, MSD, and AstraZeneca. Dr Nielsen has received speaking fees from Boehringer Ingelheim, consulting fees from Bayer and Daiichi-Sankyo, and grant support from Bristol-Myers Squibb/Pfizer. Dr Skjøth has received consulting fees from Bayer. The other authors report no conflicts.
Supplementary Material
Footnotes
Drs Lip and Larsen are joint senior authors.
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.118.023797.
References
- 1.Lip GYH, Freedman B, De Caterina R, Potpara TS. Stroke prevention in atrial fibrillation: past, present and future. Thromb Haemost. 2017;117:1230–1239. doi: 10.1160/TH16-11-0876. doi: 10.1160/TH16-11-0876. [DOI] [PubMed] [Google Scholar]
- 2.Gaist D, García Rodríguez LA, Hellfritzsch M, Poulsen FR, Halle B, Hallas J, et al. Association of antithrombotic drug use with subdural hematoma risk. JAMA. 2017;317:836–846. doi: 10.1001/jama.2017.0639. doi: 10.1001/jama.2017.0639. [DOI] [PubMed] [Google Scholar]
- 3.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. ESC Scientific Document Group. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 4.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–176. doi: 10.1016/S1474-4422(09)70340-0. doi: 10.1016/S1474-4422(09)70340-0. [DOI] [PubMed] [Google Scholar]
- 5.Fang MC, Go AS, Chang Y, Hylek EM, Henault LE, Jensvold NG, et al. Death and disability from warfarin-associated intracranial and extracranial hemorrhages. Am J Med. 2007;120:700–705. doi: 10.1016/j.amjmed.2006.07.034. doi: 10.1016/j.amjmed.2006.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nielsen PB, Larsen TB, Skjøth F, Gorst-Rasmussen A, Rasmussen LH, Lip GY. Restarting anticoagulant treatment after intracranial hemorrhage in patients with atrial fibrillation and the impact on recurrent stroke, mortality, and bleeding: a Nationwide Cohort Study. Circulation. 2015;132:517–525. doi: 10.1161/CIRCULATIONAHA.115.015735. doi: 10.1161/CIRCULATIONAHA.115.015735. [DOI] [PubMed] [Google Scholar]
- 7.Chao TF, Liu CJ, Liao JN, Wang KL, Lin YJ, Chang SL, et al. Use of oral anticoagulants for stroke prevention in patients with atrial fibrillation who have a history of intracranial hemorrhage. Circulation. 2016;133:1540–1547. doi: 10.1161/CIRCULATIONAHA.115.019794. doi: 10.1161/CIRCULATIONAHA.115.019794. [DOI] [PubMed] [Google Scholar]
- 8.Pennlert J, Asplund K, Carlberg B, Wiklund PG, Wisten A, Åsberg S, et al. Antithrombotic treatment following intracerebral hemorrhage in patients with and without atrial fibrillation. Stroke. 2015;46:2094–2099. doi: 10.1161/STROKEAHA.115.009087. doi: 10.1161/STROKEAHA.115.009087. [DOI] [PubMed] [Google Scholar]
- 9.Korompoki E, Filippidis FT, Nielsen PB, Del Giudice A, Lip GYH, Kuramatsu JB, et al. Long-term antithrombotic treatment in intracranial hemorrhage survivors with atrial fibrillation. Neurology. 2017;89:687–696. doi: 10.1212/WNL.0000000000004235. doi: 10.1212/WNL.0000000000004235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–962. doi: 10.1016/S0140-6736(13)62343-0. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 11.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 12.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 13.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 14.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 15.Johnsen SP, Ingeman A, Hundborg HH, Schaarup SZ, Gyllenborg J. The Danish Stroke Registry. Clin Epidemiol. 2016;8:697–702. doi: 10.2147/CLEP.S103662. doi: 10.2147/CLEP.S103662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kildemoes HW, Sørensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39(7 suppl):38–41. doi: 10.1177/1403494810394717. doi: 10.1177/1403494810394717. [DOI] [PubMed] [Google Scholar]
- 17.Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39(7 suppl):22–25. doi: 10.1177/1403494810387965. doi: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- 18.Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39(7 suppl):30–33. doi: 10.1177/1403494811401482. doi: 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wildenschild C, Mehnert F, Thomsen RW, Iversen HK, Vestergaard K, Ingeman A, et al. Registration of acute stroke: validity in the Danish Stroke Registry and the Danish National Registry of Patients. Clin Epidemiol. 2014;6:27–36. doi: 10.2147/CLEP.S50449. doi: 10.2147/CLEP.S50449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindenstrøm E, Boysen G, Waage Christiansen L, à Rogvi Hansen B, Würtzen Nielsen P. Reliability of Scandinavian neurological stroke scale. Cerebrovasc. Dis. 1991;1:103–107. [Google Scholar]
- 22.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 23.Klein JP, Logan B, Harhoff M, Andersen PK. Analyzing survival curves at a fixed point in time. Stat Med. 2007;26:4505–4519. doi: 10.1002/sim.2864. doi: 10.1002/sim.2864. [DOI] [PubMed] [Google Scholar]
- 24.Parner ET, Andersen PK. Regression analysis of censored data using pseudo-observations. Stata J. 2010;10:408–422. [Google Scholar]
- 25.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prasad V, Jena AB. Prespecified falsification end points: can they validate true observational associations? JAMA. 2013;309:241–242. doi: 10.1001/jama.2012.96867. doi: 10.1001/jama.2012.96867. [DOI] [PubMed] [Google Scholar]
- 27.Guldberg R, Kesmodel US, Brostrøm S, Kærlev L, Hansen JK, Hallas J, et al. Use of antibiotics for urinary tract infection in women undergoing surgery for urinary incontinence: a cohort study. BMJ Open. 2014;4:e004051. doi: 10.1136/bmjopen-2013-004051. doi: 10.1136/bmjopen-2013-004051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pennlert J, Overholser R, Asplund K, Carlberg B, Van Rompaye B, Wiklund PG, et al. Optimal timing of anticoagulant treatment after intracerebral hemorrhage in patients with atrial fibrillation. Stroke. 2017;48:314–320. doi: 10.1161/STROKEAHA.116.014643. doi: 10.1161/STROKEAHA.116.014643. [DOI] [PubMed] [Google Scholar]
- 29.Mackman N. The role of tissue factor and factor VIIa in hemostasis. Anesth Analg. 2009;108:1447–1452. doi: 10.1213/ane.0b013e31819bceb1. doi: 10.1213/ane.0b013e31819bceb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson D, Charidimou A, Shakeshaft C, Ambler G, White M, Cohen H, et al. CROMIS-2 collaborators. Volume and functional outcome of intracerebral hemorrhage according to oral anticoagulant type. Neurology. 2016;86:360–366. doi: 10.1212/WNL.0000000000002310. doi: 10.1212/WNL.0000000000002310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Nieuwenhuizen KM, van der Worp HB, Algra A, Kappelle LJ, Rinkel GJ, van Gelder IC, et al. APACHE-AF Investigators. Apixaban versus Antiplatelet drugs or no antithrombotic drugs after anticoagulation-associated intraCerebral HaEmorrhage in patients with Atrial Fibrillation (APACHE-AF): study protocol for a randomised controlled trial. Trials. 2015;16:393. doi: 10.1186/s13063-015-0898-4. doi: 10.1186/s13063-015-0898-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charidimou A, Wilson D, Shakeshaft C, Ambler G, White M, Cohen H, et al. The Clinical Relevance of Microbleeds in Stroke study (CROMIS-2): rationale, design, and methods. Int J Stroke. 2015;10(suppl A100):155–161. doi: 10.1111/ijs.12569. doi: 10.1111/ijs.12569. [DOI] [PubMed] [Google Scholar]
- 33.Shoamanesh A. NASPAF-ICH: NOACs for Stroke Prevention in Patients With Atrial Fibrillation and Previous ICH [Internet]. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02998905. Accessed February 7, 2019.
- 34.Nielsen PB, Johnsen SP. Letter by Nielsen and Johnsen regarding article, “Optimal timing of anticoagulant treatment after intracerebral hemorrhage in patients with atrial fibrillation”. Stroke. 2017;48:e115. doi: 10.1161/STROKEAHA.117.016747. doi: 10.1161/STROKEAHA.117.016747. [DOI] [PubMed] [Google Scholar]
- 35.Glynn RJ, Schneeweiss S, Stürmer T. Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol. 2006;98:253–259. doi: 10.1111/j.1742-7843.2006.pto_293.x. doi: 10.1111/j.1742-7843.2006.pto_293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneeweiss S, Gagne JJ, Glynn RJ, Ruhl M, Rassen JA. Assessing the comparative effectiveness of newly marketed medications: methodological challenges and implications for drug development. Clin Pharmacol Ther. 2011;90:777–790. doi: 10.1038/clpt.2011.235. doi: 10.1038/clpt.2011.235. [DOI] [PubMed] [Google Scholar]


