Supplemental Digital Content is Available in the Text.
This experimental highly controlled trial in 20 patients with fibromyalgia shows that the cannabinoid THC, but not CBD, is effective in the treatment of fibromyalgia pain.
Keywords: Cannabis, Chronic pain, Fibromyalgia, Pharmacokinetics, THC, CBD, Placebo cannabis, Pain models
Abstract
In this experimental randomized placebo-controlled 4-way crossover trial, we explored the analgesic effects of inhaled pharmaceutical-grade cannabis in 20 chronic pain patients with fibromyalgia. We tested 4 different cannabis varieties with exact knowledge on their ∆9-tetrahydrocannabinol (THC) and cannabidiol (CBD) content: Bedrocan (22.4-mg THC, <1-mg CBD; Bedrocan International BV, Veendam, the Netherlands), Bediol (13.4-mg THC, 17.8-mg CBD; Bedrocan International BV, Veendam, the Netherlands), Bedrolite (18.4-mg CBD, <1-mg THC; Bedrocan International BV, Veendam, the Netherlands), and a placebo variety without any THC or CBD. After a single vapor inhalation, THC and CBD plasma concentrations, pressure and electrical pain thresholds, spontaneous pain scores, and drug high were measured for 3 hours. None of the treatments had an effect greater than placebo on spontaneous or electrical pain responses, although more subjects receiving Bediol displayed a 30% decrease in pain scores compared to placebo (90% vs 55% of patients, P = 0.01), with spontaneous pain scores correlating with the magnitude of drug high (ρ = −0.5, P < 0.001). Cannabis varieties containing THC caused a significant increase in pressure pain threshold relative to placebo (P < 0.01). Cannabidiol inhalation increased THC plasma concentrations but diminished THC-induced analgesic effects, indicative of synergistic pharmacokinetic but antagonistic pharmacodynamic interactions of THC and CBD. This experimental trial shows the complex behavior of inhaled cannabinoids in chronic pain patients with just small analgesic responses after a single inhalation. Further studies are needed to determine long-term treatment effects on spontaneous pain scores, THC–CBD interactions, and the role of psychotropic symptoms on pain relief.
1. Introduction
In the current opioid epidemic, there is the need for pharmaceutical alternatives to opioid treatment in patients with chronic pain. An alternative may be found in the chemicals of the cannabis plant (Cannabis sativa L.), which contains over 500 chemical components, with more than 100 of them being cannabinoids.8 Cannabinoids, or more specifically phytocannabinoids, are the main active chemical components of the cannabis plant. They exhibit most of their pharmacological effects via cannabinoid type 1 (CB1) and type 2 (CB2) G-protein-coupled receptors. CB1 receptors are located mainly in the central nervous system, whereas CB2 receptors are mostly found on immune cells.21 These receptors form part of the endocannabinoid system, a modulatory biological system that influences the activity of different neurotransmitters with their own ligands, the endocannabinoids, such as anandamide and 2-arachidonoylglycerol.22 As for cannabis, its major cannabinoid is ∆9-tetrahydrocannabinol (THC), a partial CB1-receptor agonist, that produces a variety of effects including altered cognition and motor function, analgesia, and psychotropic effects (eg, drug high).3 Another key component of cannabis is cannabidiol (CBD) that, while nonintoxicating, does affect mood and cognition.16 It is a CB2 receptor antagonist and additionally has agonist activity at the 5HT-receptor and stimulates the vanilloid receptor type 1 with similar efficacy as capsaicin.2,7,11,29
In this experimental trial, we explored the effect of pharmaceutical-grade cannabis in patients with chronic pain caused by the fibromyalgia (FM) syndrome. Fibromyalgia is characterized by chronic widespread pain, often accompanied by secondary symptoms including sleep disturbance, tiredness, and cognitive symptoms such as memory deficits.10 This condition predominantly affects women, with a worldwide prevalence of 2% to 8% and conventional pharmacologic treatment is considered only mildly effective.5,8,17
We explored the analgesic effects of inhaled pharmaceutical-grade cannabis using the cannabis plant with all its natural components. We tested 4 different varieties with exact knowledge on their THC and CBD content. The varieties used were Bedrocan with a high THC/low CBD content, Bedrolite with a high CBD/low THC content, Bediol with a combined high THC/high CBD content, and a placebo variety without any THC or CBD content. This approach enabled exploration of cannabis effects on pain relief relative to placebo cannabis that was similar in smell, appearance, and handling compared with the other varieties. We assessed relief of experimental pressure pain, electrical pain, and spontaneous pain (primary endpoints), as well as the subjective and psychotropic effects. We hypothesized that compared with placebo treatment, all THC-containing treatments would cause greater analgesic responses for both spontaneous pain and evoked pain models.
2. Methods
2.1. Ethics and trial registration
This single-center, double-blind, placebo-controlled, 4-way crossover study, with acronym Spirocan, was performed at the Anesthesia and Pain Research Unit of the Department of Anesthesiology at LUMC. The protocol was approved by the local institutional review board and the Central Committee on Research Involving Human Subjects in The Hague. The study was registered at trialregister.nl under identifier NTR6091 and in the European Union Drug Regulating Authorities Clinical Trials (EUDRACT) database under identifier 2015‐003811‐39. Before enrollment, all patients gave written informed consent.
2.2. Patients: inclusion and exclusion criteria
Female patients diagnosed with FM were approached to participate in the study through announcements in local newspapers and the web site of the association of patients with FM. When patients indicated interest in the study and were diagnosed with FM by a rheumatologist, they were queried for inclusion and exclusion criteria. Inclusion criteria were: a pain score ≥5 for most of the day (on a verbal pain scale from 0 = no pain to 10 = most pain imaginable) and positive diagnostic criteria of the 2010 American College of Rheumatology.28 These criteria include a widespread pain index (WPI) ≥7 (on a scale from 0 to 19) and a symptom severity (SyS) score ≥5 (on a scale from 0 to 12) or a WPI of 3 to 6 and a SyS score ≥9. The WPI defines the number of body areas in which a patient experienced pain during the past week; the SyS score indicates the level of other main symptoms of FM such as fatigue, nonrefreshing sleep, and cognitive symptoms. The presence of autonomic complaints such as diarrhea or obstipation, dizziness, dry mouth/eyes, etc. was not a reason for exclusion, as we consider these symptoms consistent with the FM syndrome. Exclusion criteria included age <18 years, any medical, neurological, or psychiatric illness, use of strong opioids or other painkillers except paracetamol and/or ibuprofen, benzodiazepine use, any known allergies to study medication, illicit drug or alcohol use, recent use of cannabis, pregnancy, breast feeding, and the presence of pain syndromes other than FM. On the day of screening and on the morning of each of the 4 study days, the urine of the patient was tested for illicit drug use using a dipstick (Alere Toxicology Plc, Oxfordshire, United Kingdom; the stick tests for cocaine, amphetamine, cannabinoids, phencyclidine, methadone, benzodiazepines, tricyclic antidepressants, and barbiturates). In case of a positive test, the subject was excluded from the study. Subjects were instructed not to eat for at least 6 hours and drink for at least 2 hours before the study visit. Any foods or beverages containing caffeine such as coffee, tea, or chocolate were not allowed for 24 hours before the study visit.
2.3. Study design: drugs, inhalation, and blood sampling
Patients visited the research unit on 5 occasions. On their first visit, the patients were screened (medical history, physical examination, and urinalysis) and familiarized with the experimental setup (they were, for example, trained in the inhalation process). On each of their next visits, the patients received 1 of 4 possible cannabis treatments (in random order) with at least 2 weeks between visits.
The active cannabis substances were composed of the dried, milled, and homogenized flowers of the plant Cannabis sativa L., which were cultivated under standardized conditions in line with the requirements of good manufacturing practices (GMP). We used 4 distinct pharmaceutical-grade cannabis varieties, all obtained from Bedrocan International BV (Veendam, the Netherlands) and all prepared by Proxy Laboratories BV (Leiden, the Netherlands) under GMP conditions:
(1) Bedrocan: The Bedrocan cannabis variety contains 22% THC (220 mg per gram) and less than 1% CBD. It was developed in the Netherlands out of a requirement by the Dutch Health Ministry to have a “high THC” variety available to patients. We used 100 mg that contained 22.4-mg THC and less than 1-mg CBD.
(2) Bediol: The Bediol cannabis variety is characterized by the combination of 6.3% THC (63 mg per gram) and 8% CBD (80 mg per gram). We used 200 mg that contained 13.4-mg THC and 17.8-mg CBD.
(3) Bedrolite: This variety is composed of 9% CBD (90 mg per gram) and less than 1% THC. We used 200 mg that contained 18.4-mg CBD and less than 1-mg THC.
(4) Placebo: The placebo was derived from the Bedrocan cannabis variety after selective removal of the cannabinoids by solvent extraction by Proxy Laboratories BV under GMP conditions. After removal of the cannabinoids, the specific terpene profile (responsible for smell and taste) was restored in a subsequent manufacturing step. Consequently, the placebo had a moisture content and terpenoid profile matching the active drug (Bedrocan).
Study medication was analyzed for cannabinoid content, terpene profile, and water content by an independent quality control laboratory. In addition, tests were performed to ensure that unwanted elements were absent such as adulterants, microbes, heavy metals, and pesticides. The pharmacy and ethics committee reviewed and approved the products' quality certificates before dispensing the cannabis to the research team. During the study, all varieties were refrigerated at 2 to 8 °C in triple-layer laminated foil pouches.
Patients were dosed with cannabis vapor. All cannabinoids are mostly present in the plant in their acid form. Application of heat is needed for decarboxylation of the cannabinoid acids into their active forms (eg, THC acid into THC).9 All 4 cannabis varieties were vaporized using the Volcano Medic vaporizer (Storz & Bickel GmbH & Co, Tuttlingen, Germany)—a safe and reliable method of intrapulmonary administration of cannabinoids.13,30 The Volcano heated the homogenized plant material to 210 °C to allow for conversion of the THC acid and CBD acid into THC and CBD vapor for inhalation. The vapor was collected in an 8-L plastic balloon that, after inflation, was detached from the vaporizer and subsequently equipped with a mouthpiece for inhalation. For the purpose of blinding, the balloon was covered with an opaque plastic bag so that no variation in density of the vapor was visible between visits. The evaporation process was performed by a member of the research team not involved in the study proceedings. Before and after each evaporation, the device was cleaned with alcohol. The complete content of the balloon was inhaled through the mouth within 3 to 7 minutes, and each breath was held for 5 seconds after each inhalation.
On each occasion, an arterial line was placed in the left or right radial artery for blood sampling. Five milliliter of blood was obtained at t = 0 (control sample, before inhalation), 5, 10, 20, 30, 40, 50, 60, 90, 120, and 180 minutes after the start of inhalation. Blood was collected in EDTA tubes (covered with an aluminum foil), centrifuged at 2000g at 4°C; separated plasma was stored at −80°C until analysis. The samples were analyzed by Analytical Biochemical Laboratory BV, Assen, the Netherlands. All handling of the samples was done in a darkened room to prevent the cannabis molecules from disintegrating. Determination of the CBD, THC, and its active metabolite 11‐hydroxy‐THC (11-OH-THC) plasma concentrations was performed using liquid chromatography with tandem mass spectrometer detection (LC‐MS/MS). In Supplemental Materials 1 to 3, the analysis specifications including chromatograms of the 3 cannabis varieties are given for 2 (low and high) concentrations (available at http://links.lww.com/PAIN/A705).
2.4. Study design: pain tests, questionnaires, and safety
All subjects rated their FM pain on an 11-point visual analogue scale (from 0 = no pain to 10 = most severe pain imaginable) at baseline (before cannabis inhalation) and at 1, 2, and 3 hours after inhalation.
Two experimental pain tests were performed:
(1) Pressure pain test18: A pressure algometer (FDN 100; Wagner Instruments Inc, Greenwich, CT) was used to deliver pressure pain on a skin area of 1 cm2 between the thumb and index finger; the affected area overlays the adductor pollicis muscle. The algometer has a force capacity (±accuracy) of 100 ± 2 N (10 ± 0.2 kgf) and graduation of 1 N (100 gf), respectively. A gradually increasing pressure was manually applied, and the subjects were asked to indicate when the procedure became painful (pressure pain threshold). All measurements were obtained in triplicate at t = 0 (baseline), 12, 22, 32, 42, 62, 92, 122, 152, and 182 minutes after the start of inhalation. The 3 measurements were averaged for further analysis.
(2) Electrical pain test20: Electrical pain was induced using a locally designed computer interfaced electrical currents stimulator (CICS, Leiden University Medical Center, Leiden, the Netherlands). The stimulator was connected to 2 electrodes (surface area 0.8 cm2) placed on the tibial surface of the right leg, approximately 10 cm above the medial malleolus. The stimulator produced a stimulus train (stimulus duration 0.2 ms at 10 Hz) that increased from 0 mA at 0.5 mA/second (cutoff 128 mA). The subjects were instructed to press a control button when pain was first felt (pain threshold) and when the pain became unbearable (pain tolerance; this ended the stimulus train). Measurements were obtained at t = 0, 10, 20, 30, 40, 60, 90, 120, 150, and 180 minutes after the start of cannabis inhalation.
Two questionnaires were taken to assess the effect of drug treatment on mental and psychoactive cannabis effects:
(1) Bowdle questionnaire4,30: This questionnaire evaluates 3 psychedelic effects (drug high, alterations in internal perception, and alterations in external perception) from 13 questions scored on a 100-mm visual analogue scale (from 0, no effect, to 100, maximum effect). Internal perception reflects inner feelings that do not correspond with the reality and is derived from questions regarding the hearing of unrealistic voices or sounds and having unrealistic thoughts and paranoid or anxious feelings. The external perception indicates a misperception of an external stimulus or change in the awareness of the subject's surroundings and is derived from questions regarding the perceptual change of body parts, the change of surroundings, the altered passing of time, the difficulty of controlling thoughts, and the change in color and sound intensity.
(2) Bond and Lader questionnaire3,30: The Bond and Lader scales are calculated from sixteen 100-mm visual analogue scales. The endpoints are set at antonymous word pairs such as “alert–drowsy,” “well coordinated–clumsy,” “mentally slow–quick witted,” and “incompetent–proficient.” The study participant's task is to make a mark on each scale at the point that best describes how they currently feel considering that the 2 anchors reflect the greatest extent they experience each state. Responses from these 16 scales are then scored to yield 3 main factors of alertness (alert, strong, clear‐headed, coordinated, energetic, quick‐witted, attentive, proficient, and interested), contentment (contented, happy, amicable, gregarious, and tranquil), and calmness (calm and relaxed). A high score indicates impairment.
The subjects were queried before drug inhalation and at 30-min intervals after the start of inhalation. Adverse events and serious adverse events were collected in the case record form. In case of a serious adverse event, the event was treated, and no further measurements were obtained. In case of an adverse event (eg, nausea, vomiting, headache, and dizziness), no further action was taken apart from supportive care.
2.5. Randomization, allocation, and blinding
Randomization was performed by the pharmacy using a computer-generated randomization list. A distinct randomization sequence was created for each subject; randomization sequence was controlled with just 2 subjects with an identical treatment sequence. On the day before the experiment, the subject was allocated to treatment by the pharmacy after receiving a fax message from the investigators with the participant's identifier code and study visit number. Treatment was prepared on the day of the study and collected by a technician from the pharmacy in a closed opaque canister labeled with the patient's identifier code and study visit number; the contents of the canister were emptied in the vaporizer. The study team was next presented with the filled opaque balloon just before the actual cannabis inhalation. The investigators (and patients) remained blinded until data analysis was complete (June 2018). The study was independently monitored ensuring that all good clinical practice requirements were met.
2.6. Statistical analysis: sample size and assessment of treatment effects
Considering the data from Wallace et al.,25 we calculated the need for 20 subjects to allow for a significant separation between treatments with a power >0.9 and alpha = 0.05. In case of dropout after one visit, the data were discarded, and a new subject was recruited. Before the data analyses, all variables were screened for missing data, homoscedasticity, distribution abnormalities, and outliers. For both primary and secondary endpoints, the effect of active treatment (Bedrocan, Bedrolite, or Bediol) on the change in effect was compared between treatments using a mixed model. Treatment was set as a fixed effect, a random effect for the subject was added to account for repeated measurements over time, and treatment order was added as a covariate. For spontaneous pain, the responder rate was determined for each treatment and compared with placebo responder rates using a χ2 test. A responder was defined as having a reduction in spontaneous pain score of at least 30% or 50% at one or more measurements. In addition, the change in spontaneous pain score relative to baseline was related to the drug high score by Spearman's ρ. The number of adverse events between the 3 active treatments and placebo was analyzed using a χ2 test. SPSS (IBM Corp Released 2017; IBM SPSS Statistics for Windows, Version 25.0, Armonk, NY: IBM Corp) was used for all analyses with P values <0.05 considered significant. All data are reported as mean ± SD, unless otherwise stated.
3. Results
Twenty-five patients were recruited for participation. Five patients ended their participation after their first study visit for unknown reasons (n = 1), side effects such as dizziness and nausea (n = 3), and fear of needles (n = 1) (Fig. 1). All were replaced by another patient according to the protocol. The 20 patients who completed the trial were on average 39 ± 13 years with an average weight of 82 ± 20 kg and height of 169 ± 7 cm (body mass index 29 ± 7 kg/m2). At screening, patients reported an average verbal pain score of 7.20 ± 1.24 units and were all diagnosed with FM with a WPI of 13.9 ± 2.6, SyS of 9.2 ± 1.3, and 14.9 ± 2.9 of positive tender points.
Figure 1.
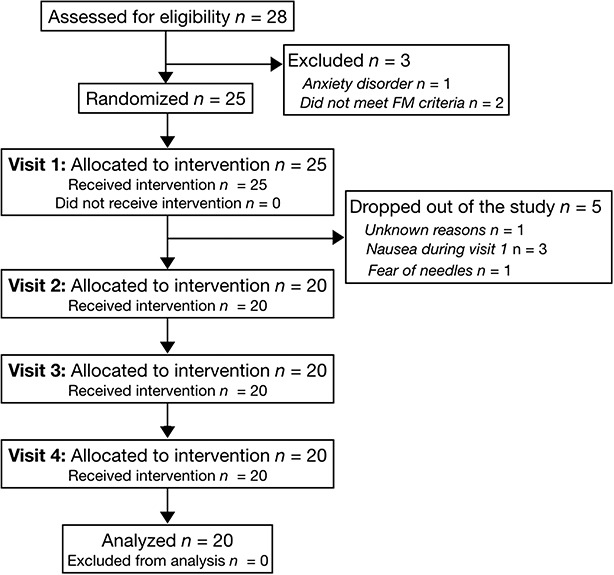
Consort flow diagram. FM, fibromyalgia.
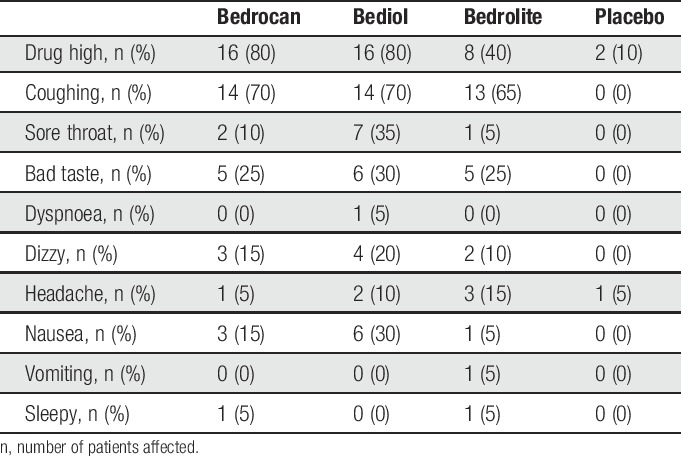
Cannabis inhalation was achieved in (minutes:seconds) 5:03 ± 2:54 (21 ± 11 inhalations; Bedrocan), 6:57 ± 4:05 (23 ± 11 inhalations; Bediol), 5:30 ± 2:37 (22 ± 10 inhalations; Bedrolite), and 2:48 ± 1:40 (14 ± 6 inhalations; Placebo). The complete content of the balloon was inhaled by all subjects. All 3 active treatments, but not placebo, were associated with several adverse effects (Table 1), with frequent effects related to the inhalation of cannabis (coughing during inhalation in 65%-70%, sore throat and bad taste during inhalation in 25%-35% of participants). Most adverse effects unrelated to the inhalation process were drug high in 40% to 80%, dizziness in 15% to 20%, and nausea in 5% to 30% of participants. Two patients reported feelings of drug high after placebo treatment. There were no differences in frequency of adverse effects between active treatments (P > 0.05). No serious adverse events occurred.
Table 1.
Incidence of adverse events.
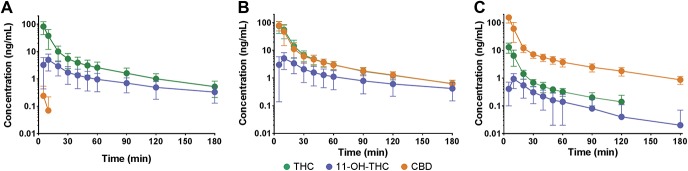
After inhalation of all 3 active treatments, THC, its metabolite 11-OH-THC, and CBD, were detectable with the following CMAX and TMAX values (Fig. 2). Bedrocan: THC 82 ± 20 ng/mL at t = 5 minutes, 11-OH-THC 5 ± 3 ng/mL at 10 minutes, and CBD 0.2 ± 0.3 ng/mL at 5 minutes; Bediol: THC 76 ± 35 ng/mL at t = 5 minutes, 11-OH-THC 5 ± 3 ng/mL at 10 minutes, and CBD 80 ± 029 ng/mL at 5 minutes; and Bedrolite: THC 13 ± 5 ng/mL at t = 5 minutes, 11-OH-THC 0.9 ± 0.5 ng/mL at 10 minutes, and CBD 155 ± 57 ng/mL at 5 minutes. No cannabinoids were detectable after placebo inhalations.
Figure 2.
Plasma concentrations of ∆9-tetrahydrocannabinol (THC), its metabolite 11-hydroxy-THC (11-OH-THC), and cannabidiol (CBD) after inhalation of 3 cannabis varieties, Bedrocan (A), Bediol (B), and Bedrolite (C). Data are mean ± 95% confidence interval.
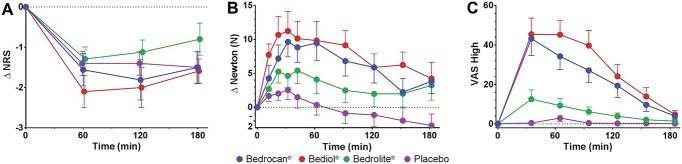
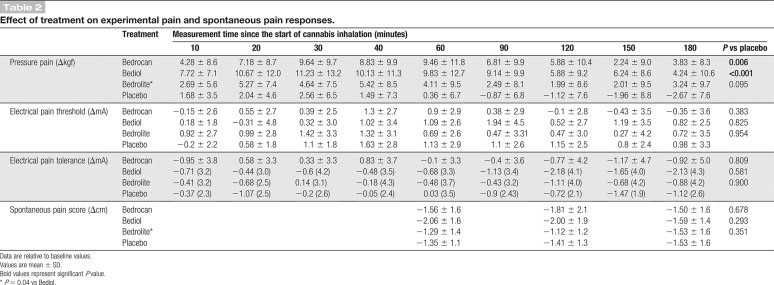
None of the treatments had an effect greater than placebo on spontaneous pain scores or electrical pain responses (Fig. 3 and Table 2). By contrast, both Bedrocan and Bediol caused a significant increase in tolerance to the pressure applied to the skin over the adductor pollicis muscle for the duration of the study. The largest effect was observed for the cannabis variety that contained high doses of both THC and CBD (Bediol) with an increase in tolerated pressure of 9 to 11 kgf from t = 20 to 90 minutes (P < 0.001 vs placebo; t = 0 minutes is the start of cannabis inhalation). Over this same time range, Bedrocan increased the tolerated pressure by 7 to 9 kgf (P = 0.006 vs placebo). With respect to spontaneous pain scores and tolerance to pressure pain, Bediol had significantly greater effects than Bedrolite (P = 0.04 for both endpoints, Table 2 and Fig. 3).
Figure 3.
Effect of cannabis varieties Bedrocan, Bediol, Bedrolite, and placebo cannabis on spontaneous pain scores (A), pressure pain threshold (B), and drug high (C). Data are mean ± SEM and are relative to baseline. NRS, numerical rating score; VAS, visual analogue scale.
Table 2.
Effect of treatment on experimental pain and spontaneous pain responses.
After placebo treatment, 11 and 6 patients had 30% and 50% reduction in pain scores on at least one measurement period, respectively. Comparing these responder rates to active treatment, significantly more patients responded to Bediol with a decrease in spontaneous pain by 30% (n = 18, P = 0.01; Fig. 4) but not with a decrease by 50% (n = 9, P = 0.052). At both responder rates, all other treatments had response profiles not different from placebo (Fig. 4). Spontaneous pain scores were strongly correlated with the magnitude of drug high for Bedrocan (ρ = −0.5, P < 0.001) and for Bediol (ρ = −0.5, P < 0.001).
Figure 4.
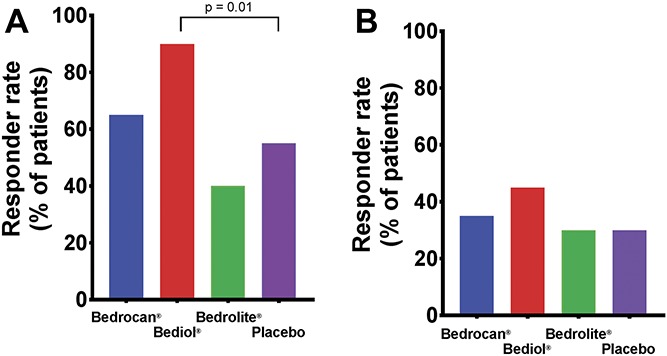
Cannabis responder rates: (A) Percentage responders with a decrease of at least 30% in spontaneous pain scores on at least one measurement. (B) Percentage responders with a decrease of at least 50% in spontaneous pain scores on at least one measurement.
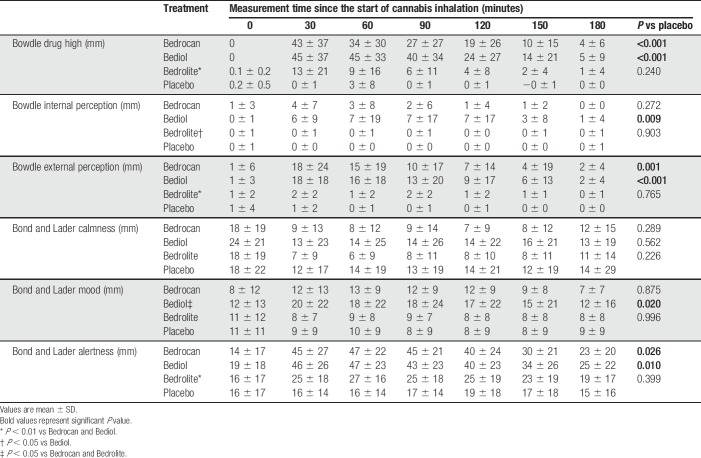
Psychoactive effects of treatment, as measured by the Bowdle questionnaire, are given in Table 3. Bedrocan and Bediol caused moderate drug high responses, on average just below 50% of the maximum possible response (Fig. 3B), but significantly greater than placebo (P < 0.001). Bedrolite had less intense drug high responses compared with either Bedrocan (P = 0.003) or Bediol (P < 0.001). Small effects were seen for changes in internal perception (Bediol vs placebo, max. mean difference with placebo 7 mm, P = 0.009, Table 3) and external perception (Bedrocan and Bediol vs placebo, max. mean difference with placebo 17 mm, P < 0.001), indicative of limited psychosis-like effects after Bedrocan and Bediol treatment. Bedrolite caused smaller changes in internal perception than Bediol (P = 0.04) and smaller changes in external perception than both Bediol (P = 0.004) and Bedrocan (P = 0.01). The responses to the Bond and Lader questionnaire indicate mild deterioration in mood observed during Bediol treatment (max. mean difference with placebo 11 mm, P = 0.02, Table 3) and mild deterioration in alertness during Bedrocan (max. mean difference with placebo 21 mm, P = 0.02). Some small differences in mood and alertness were observed among the 3 active treatments (Table 3).
Table 3.
Effect of treatment on subjective feelings derived from the Bowdle questionnaire and Bond and Lader questionnaire.
To assess whether blinding of active vs placebo treatment was successful, we calculated Bang's blinding index (Bang's BI),1,26 which translates correct vs random guessing into a single number. Bang's BI ranges between −1 and 1 with 0 a perfect blinding and values >0.5 or <−0.5 indicative of failure of blinding above random guessing in the majority of subjects. Bang's BI values were between 0.3 and 0.4 just after inhalation for the 3 active treatments (40% of patients correctly guessed that they received active treatment, whereas 50% of patients were unable to determine what treatment they received). At the end of the experiment, more subjects correctly guessed that they received active treatment after Bedrocan (Bang's BI 0.85) or Bediol (Bang's BI 0.90) inhalation. After placebo treatment, Bang's BI was −0.05 just after inhalation and 0.45 at the end of the study. Assessment of a possible order effect on the measured pain-related endpoints did not show a significant effect (P > 0.05), indicating that starting with placebo or with active treatment had no significant effect on outcome.
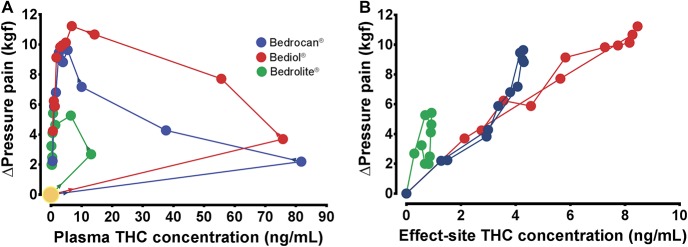
In Figure 5A, the plasma THC concentration vs Δpressure pain for the 3 active cannabis varieties is plotted showing loops with counterclockwise direction. Using a nonparametric collapsing approach, we closed the loops to give the relationship between the estimated THC effect-site (or steady-state) concentration and Δpressure pain (Fig. 5B)19 (Using ke0obj, written and kindly provided by Dr. S.L. Shafer [Stanford University, Palo Alto, CA]). The effect of Bedrocan (blue dots) is derived from just THC (reference drug). The effect of Bediol (red dots) is lower than expected from its steady-state THC concentration range, indicative of an antagonist effect of CBD (when combined with THC) on the pressure pain response. By contrast, when CBD is administered without relevant THC content (Bedrolite, green dots), a small THC-independent analgesic effect is apparent.
Figure 5.
(A) Plasma THC concentration (CP) vs the change in pressure pain threshold after treatment with Bedrocan (blue dots), Bediol (red dots), and Bedrolite (green dots). The arrows indicate the direction of effect, starting at the large yellow circle. (B) Estimated steady-state or effect-site (CE) concentration vs the change in pressure pain threshold for the 3 active cannabis varieties. THC, tetrahydrocannabinol.
4. Discussion
The main findings of this experimental study in chronic pain patients with FM are that:
(1) none of the treatments had an effect greater than placebo on spontaneous pain scores; (2) compared to placebo responder rates, significantly more patients responded to Bediol (containing high doses of THC and CBD) with a decrease in spontaneous pain by 30%; the 2 other active treatments had response profiles not different from placebo; (3) the reduction in spontaneous pain scores was correlated with the magnitude of drug high; (4) pressure pain threshold increased significantly in patients treated with Bedrocan and Bediol, 2 cannabis varieties with a high THC content; (5) Bedrolite, a cannabis variety with a high CBD content was devoid of analgesic activity in any of the spontaneous or evoked pain models; and (6) CBD increased plasma concentrations of THC but had an antagonistic effect on analgesia when combined with THC.
Major strengths of our study are the measurement of plasma concentrations of the inhaled cannabinoids enabling the correlation of plasma concentration rather than dose to effect, the use of a placebo cannabis variety exempt from THC and CBD but with the original terpene profile of the Bedrocan variety, and the testing of well-defined cannabis varieties in a group of patients with a well-defined chronic pain condition. Limitations of the study are the short treatment period and lack of validation of the experimental measures in FM.
Over the past years, cannabis has become increasingly popular for medical use. Currently, an increasing number of countries legalized or are planning to legalize cannabis for medicinal purposes. For instance, in the Netherlands, standardized cannabis has been available in pharmacies on prescription since 2003. However, cannabinoids typically have modest effects with small effect sizes and numbers needed to treat >20.24 In addition, the effect of cannabinoids in relieving chronic pain seems to diminish over time.24 Still, many patients report using cannabis for the treatment of chronic pain with promising results.23 We performed a small experimental study to explore the acute analgesic effects on experimental measures of 3 cannabis varieties that ranged in THC and CBD content after a single inhalation.
Our experimental study was not designed to provide direct evidence for the clinical use of cannabis in FM but may be used to design future clinical trials. In addition, our approach allows to link the observed effect with THC and CBD plasma concentrations and to detect possible pharmacokinetic and/or pharmacodynamic interactions. Here, we discuss the performance and outcome of the study with focus on the use of placebo cannabis, pharmacokinetics, potential analgesic efficacy of THC and CBD, and adverse effects.
4.1. Placebo cannabis
We used a placebo cannabis variety (ie, a cannabis plant devoid of THC or CBD but with the full terpene profile) as a comparator to ensure blinding of treatment. Cannabis placebo varieties without cannabinoids have been used before.27 Our placebo plant material had a similar smell and appearance as the other cannabis varieties. The importance of successful blinding in clinical trials on cannabis analgesia has recently been highlighted.26 Although our approach theoretically allows for blinding of treatment during inhalation, we cannot exclude that lack of psychoactive symptoms from placebo inhalation during the course of the study had some influence on the outcome in some of the pain models. This is especially relevant given the study crossover design. Indeed, at the end of the study, 13/20 (65%) patients guessed correctly that they had received placebo treatment. On the other hand, the terpenes present in the placebo plant may have exerted some effects. Terpenes are assumed to interact with cannabinoids (entourage effect), improving their pharmacodynamic effects (eg, by increasing pulmonary uptake and change binding of cannabinoids to their receptors), but also have effects of their own, including anti-inflammatory, antidepressant, and analgesic effects.12,22 This then suggests that the placebo cannabis variety used in our study is best considered an active placebo. Hence, the observation of an appreciable placebo effect in the relief of spontaneous pain is not surprising.
4.2. Pharmacokinetics
The pharmacokinetic analysis showed that peak THC concentrations were similar after Bedrocan and Bediol inhalation, whereas the peak THC concentration after Bedrolite inhalation was about one-sixth of that of the other 2 varieties (Fig. 2). These are important observations and indicate that magnitude of THC plasma concentrations was partly dependent on the presence of CBD in the inhalant. In Bedrocan, 24-mg inhaled THC (and <1-mg CBD) produced a mean THC peak plasma concentration of 82 ng/mL. In the other 2 cannabis varieties with CBD contents of about 18 mg, THC plasma concentrations were at least 50% higher than expected from the Bedrocan pharmacokinetic data. The THC–CBD pharmacokinetic interaction may be explained by (1) a possible CBD-induced increase in pulmonary THC uptake, for example, due to an increase in pulmonary blood flow. We are unaware of any data that support this mechanism; (2) CBD-induced inhibition of THC metabolism. Although CBD potently inhibits THC metabolism in the rat,14 our data do not support any inhibition of THC conversion to 11-OH-THC (Fig. 2); and (3) cyclizing of CBD into THC. Because both compounds are chemically related, CBD can convert into THC; this has been observed after subcutaneous administration of CBD in the rat.14 To further improve our understanding of the pharmacokinetic behavior of THC under different CBD conditions, we plan a compartmental pharmacokinetic analysis of our data.
4.3. Outcome of the acute experimental pain tests
Two cannabis varieties, Bedrocan and Bediol, were analgesic in the pressure pain model but had no effect in the electrical pain model or on relief of spontaneous pain. The pressure pain test seems especially suited for exploring treatment effects in FM pain, as it elicits mechanical muscle stimulation through Aδ- and C-fiber activation and better reflects the symptoms of patients with FM than electrical pain, which produces direct sensory nerve stimulation.19 We previously used electrical noxious stimulation as a model of acute pain and showed high sensitivity of opioids in alleviating transcutaneous electrical pain.20 The current data suggest that cannabis may have limited use in acute pain treatment.
Interestingly, when CBD and THC were combined (in Bediol), CBD had antagonistic pharmacodynamic effects (Fig. 5B), possibly because of an antagonistic or negative modulatory action at the CB1 receptor.6 Despite this pharmacodynamic antagonism, the analgesic responses exceeded those of Bedrocan, possibly because of the CBD-induced increase in THC concentrations. The opposed direction of the pharmacokinetic and pharmacodynamic CBD–THC interactions is an indication of the complex pharmacological behavior of cannabinoids in humans. When CBD is given without relevant THC content (ie, Bedrolite, containing predominantly CBD), just small analgesic effects not different from placebo became apparent. This is somewhat surprising, as it is our experience and that of others that patients with chronic pain report beneficial effects from CBD treatment.23 Possibly, such effects are related to improvement of insomnia, anxiety, cognition, and/or mood. In addition, it may well be that a single CBD administration may be insufficient to elicit analgesic responses, or that the dose was too low.
4.4. Side effects
Some side effects of active treatment were observed. One-third of patients reported sore throat and bad taste, whereas two-thirds coughed during the 5- to 7-minute inhalation of the active treatments. In the course of the study, one-third of patients experienced nausea without vomiting. All symptoms were rated as mild. An important observation was that most patients disliked the feeling of drug high after inhalation, although the intensity was rated as moderate (Fig. 3C). Because this is a general observation in chronic pain patients treated with psychedelic medication, we recently studied the ability to temper the feeling of drug high induced by racemic ketamine. We observed that drug high intensity was reduced by 30% during administration of the nitric oxide donor sodium nitroprusside.15 Because cannabis and ketamine produce their psychotropic effects through separate pathways (N-methyl-d-aspartate receptor antagonism vs CB1-receptor agonism), further studies are needed to discover viable options to reduce THC-related drug high without reducing analgesia. Still, it may be that this may have a negative effect on analgesic efficacy because we observed that relief of spontaneous pain was correlated with drug high scores. This suggests that some level of intoxication is required for an analgesic cannabis effect, or that the lack of complete blinding due to the occurrence of psychotropic side effects (or symptoms during inhalation) influenced pain scoring to some extent.
In conclusion, in this experimental and highly controlled study, we explored the pharmacokinetics and pharmacodynamics of 3 active cannabis varieties in chronic pain patients with FM. The most important observation is that when simultaneously inhaled, THC and CBD interact in complex fashions with synergistic pharmacokinetic but antagonistic pharmacodynamic interactions. The analgesic efficacy of active treatment was limited to varieties that contained THC and was observed exclusively in the evoked pressure pain model. None of the active treatments were effective in reducing spontaneous pain scores more than placebo. Further studies are needed to assess efficacy and safety (including addictive behavior) in clinical trials with prolonged treatment periods and explore the role of psychotropic effects in the development of analgesia.
Conflict of interest statement
This investigator-initiated trial was performed in collaboration with Bedrocan International BV (Veendam, the Netherlands). Bedrocan International BV was responsible for the production and delivery of the cannabis products and the Volcano device for cannabis inhalation. M.A. Kowal is an employee of Bedrocan International BV, the Netherlands. He commented on the protocol and final version of the paper. The other authors have no conflict of interest to declare.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/A705.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
References
- [1].Bang H, Ni L, Davis C. Assessment of blinding in clinical trials. Control Clin Trials 2004;25:143–56. [DOI] [PubMed] [Google Scholar]
- [2].Bisogno T, Hanuš L, De Petrocellis L, Tchilibon S, Ponde D, Brandi I, Moriello AS, Davis JB, Mechoulam R, Di Marzo V. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol 2001;134:845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bond A, Lader M. The use of visual analogue scales in rating of subjective feelings. Br J Med Psychol 1974;47:211–8. [Google Scholar]
- [4].Bowdle TA, Radant AD, Cowley DS, Kharasch ED, Strassman RJ, Roy-Byrne PP. Psychedelic effects of ketamine in healthy volunteers. Anesthesiology 1998;88:82–8. [DOI] [PubMed] [Google Scholar]
- [5].Calandre EP, Rico-Villadermoros F, Slim M. An update on pharmacotherapy for the treatment of fibromyalgia. Expert Opin Pharmacother 2015;16:1347–68. [DOI] [PubMed] [Google Scholar]
- [6].Campos AC, Fogaça MV, Scarante FF, Joca SRL, Sales AJ, Gomes FP, Sonego AB, Rodrigues NS, Galve-Roperh I, Guimarães FS. Plastic and neuroprotective mechanisms involved in the therapeutic effects of cannabidiol in psychiatric disorders. Front Pharmacol 2017;8:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Campos A, Guimarães F. Involvement of 5HT1A receptors in the anxiolytic-like effects of cannabidiol injected into the dorsolateral periaqueductal gray of rats. Psychopharmacology (Berl) 2008;199:223–30. [DOI] [PubMed] [Google Scholar]
- [8].Clauw DJ. Fibromyalgia: a clinical review. JAMA 2014;311:1547–55. [DOI] [PubMed] [Google Scholar]
- [9].ElSohly M, Gul W. Constituents of Cannabis sativa. In: Pertwee R, editor. Handbook of Cannabis. Oxford: Oxford University Press, 2014. [Google Scholar]
- [10].Fiz J, Durán M, Capellà D, Carbonell J, Farré M. Cannabis use in patients with fibromyalgia: effect on symptoms relief and health-related quality of life. PLoS One 2011;6:e18440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gomes F, Resstel L, Guimarães F. The anxiolytic-like effects of cannabidiol injected into the bed nucleus of the stria terminalis are mediated by 5-HT1A receptors. Psychopharmacology (Berl) 2011;213:465–73. [DOI] [PubMed] [Google Scholar]
- [12].Guimarães AG, Serafini MR, Quintans-júnior LJ. Terpenes and derivatives as a new perspective for pain treatment: a patent review. Expert Opin Ther Pat 2014;24:243–65. [DOI] [PubMed] [Google Scholar]
- [13].Hazekamp A, Ruhaak R, Zuurman L, van Gerven J, Verpoorte R. Evaluation of a vaporizing device (Volcano) for the pulmonary administration of tetrahydrocannabinol. J Pharm Sci 2006;95:1308–17. [DOI] [PubMed] [Google Scholar]
- [14].Hlozek T, Uttl L, Kadarabek L, Balikova M, Lhotkova E, Horsley RR, Novakova P, Sichova K, Stefkova K, Tyls F, Kuchar M, Palenicek T. Pharmacokinetic and behavioral profile of THC, CBD, and THC + CBD combination after pulmonary, oral, and subcutaneous administration in rats and conformation of conversion in vivo of CBD to THC. Eur Neuropsychopharmacol 2017;27:1223–37. [DOI] [PubMed] [Google Scholar]
- [15].Jonkman K, van der Schrier R, van Velzen M, Aarts L, Olofsen E, Sarton E, Niesters M, Dahan A. Differential role of nitric oxide in the psychedelic symptoms induced by racemic ketamine and esketamine in human volunteers. Br J Anaesth 2018;120:1009–18. [DOI] [PubMed] [Google Scholar]
- [16].Kowal M, Hazekamp A, Colzato L, van Steenbergen H, Hommel B. Modulation of cognitive and emotional processing by cannabidiol: the role of the anterior cingulate cortex. Front Hum Neurosc 2013;7:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Krasselt M, Baerwald C. Fibromyalgie-Syndrom: aktuelle empfehlungen zu diagnsostik und Therapie. Dtsch Med Wochenschr 2018;143:1103–8. [DOI] [PubMed] [Google Scholar]
- [18].Martini CH, Proto P, Olofsen E, van Velzen M, Aarts L, Dahan A, Niesters M. A randomized controlled trial and novel mathematical analysis of the analgesic effect of oxycodone versus paracetamol orodispersible tablets. Eur J Pain 2015;19:295–304. [DOI] [PubMed] [Google Scholar]
- [19].Olesen AE, Andresen T, Staahl C, Drewes AD. Human experimental pain models for assessing the therapeutic efficacy of analgesic drugs. Pharmacol Rev 2012;64:772–9. [DOI] [PubMed] [Google Scholar]
- [20].Olofsen E, Romberg R, Bijl H, Mooren R, Engbers F, Kest B, Dahan A. Alfentanil and placebo analgesia: absence of sex differences. Anesthesiology 2005;103:130–9. [DOI] [PubMed] [Google Scholar]
- [21].Pertwee R, Cascio M. Known pharmacological actions of delta-9-tetrahydrocannabinol and of four other chemical constituents of cannabis that activate cannabinoid receptors. In: Pertwee R, editor. Handbook of Cannabis. Oxford: Oxford University Press, 2014. [Google Scholar]
- [22].Ruso EB. Taming THC. Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Phramacol 2011;163:1344–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sexton M, Cuttler C, Finnell J, Mischley L. A cross-sectional survey of medical cannabis users: patterns of use and perceived efficacy. Cannabis Cannabinoid Res 2016;1:131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Stockings E, Campbell G, Hall WD, Nielsen S, Zagic D, Rahman R, Murnion B, Farrell M, Weier M, Degenhardt L. Cannabis and cannabinoids for the treatment of chronic non-cancer pain conditions: a systematic review and meta-analysis of controlled and observational studies. PAIN 2018;159:1932–44. [DOI] [PubMed] [Google Scholar]
- [25].Wallace MS, Marcotte TD, Umlauf A, Gouaux B, Atkinson JH. Efficacy of inhaled cannabis on painful diabetic neuropathy. J Pain 2015;16:616–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wilsey B, Deutsch R, Marcotte TD. Maintenance of blinding in clinical trials and the implications for studying analgesia using cannabinoids. Cannabis Cannabinoid Res 2016;1:139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wilsey B, Marcotte TD, Deutsch R, Zhao H, Prasad H, Phan A. An exploratory human laboratory experiment evaluating vaporized cannabis is in the treatment of neuropathic pain from spinal cord injury and disease. J Pain 2016;17:982–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg GL, Katx RS, Mease P, Russell AS, Russell IJ, Winfield JB, Yunus MB. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62:600–10. [DOI] [PubMed] [Google Scholar]
- [29].Zanelati T, Biojone C, Moreira F, Guimarães F, Joca S. Antidepressant-like effects of cannabidiol in mice: possible involvement of 5-HT1A receptors. Br J Pharmacol 2010;159:122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zuurman L, Roy C, Schoemaker R, Hazekamp A, den Hartigh J, Bender J, Verpoorte R, Pinquier JL, Cohen AF, van Gerven JM. Effect of intrapulmonary tetrahydrocannabinol administration in humans. J Psychopharmacol 2008;22:707–16. [DOI] [PubMed] [Google Scholar]