ABSTRACT
Purpose
Previous studies have suggested that extreme endurance exercise may induce cardiac microdamage that could lead to subsequent myocardial fibrosis. Soluble suppression of tumorigenicity 2 (sST2) is a cardiac biomarker for assessment of myocardial fibrosis, inflammation, and strain. We evaluated baseline and exercise-induced sST2 concentrations in a heterogeneous cohort of marathon runners to identify predictors for sST2 concentrations.
Methods
Ninety-two runners supplied demographic data, health status, physical activity levels, and marathon experience. Before (baseline) and immediately after (finish) the marathon, blood was collected for analysis of sST2 and cardiac troponin I (cTnI).
Results
Eighty-two participants (45 ± 8 yr, 79% male) finished the race in 227 ± 28 min at 92% (88%–94%) of their predicted maximum heart rate (exercise intensity). sST2 concentrations increased in all runners, from 34 (25–46) ng·mL−1 to 70 (53–87) ng·mL−1 (P < 0.001), and cTnI increased from 9 (5–21) ng·L−1 to 60 (34–102) ng·L−1 (P < 0.001). sST2 concentrations were higher in the fastest marathon runners. Sex and marathon personal best time were associated with baseline sST2 (R2 = 0.27); baseline sST2, weight loss, and exercise intensity during marathon were associated with finish sST2 (R2 = 0.54); baseline sST2, height, sex, and weekly training hours were associated with the exercise-induced increase in sST2 (R2 = 0.47). We observed no association between sST2 and cTnI concentrations.
Conclusion
An exercise-induced increase in sST2 was observed in all marathon runners with sST2 concentrations exceeding cutoff values both at baseline (48%) and finish (94%). Faster runners had higher sST2 concentrations. Our data suggest complex variables determine sST2 concentrations in marathon runners.
Key Words: EXERCISE, CARDIAC BIOMARKERS, SST2, MYOCARDIAL FIBROSIS
Vigorous-intensity exercise increases myocardial workload as evidenced by a higher heart rate, stroke volume, and systolic blood pressure during exercise. Previous studies have demonstrated increases in cardiac biomarkers such as cardiac troponin I (cTnI) (1) and B-type natriuretic peptide (2) after prolonged exercise. The normalization of such elevated biomarker concentrations is typically observed within 24–72 h (3,4). The clinical relevance of exercise-induced changes in cTnI and B-type natriuretic peptide is unknown.
A recent systematic review found an increased prevalence of myocardial fibrosis in endurance athletes (5). This finding is unexpected and may suggest that the accumulation of acute prolonged, high-intensity exercise induces detrimental adaptations to cardiac tissue possibly reflected in acute change in cardiac biomarkers. Suppression of tumorigenicity 2 (ST2) is a member of the interleukin-1 (IL-1) receptor family and functions as the receptor of IL-33. ST2 has two main isoforms: a cell surface receptor (ST2L) and a soluble (sST2) form (6). IL-33 is a cytokine that is secreted by cardiac fibroblasts in response to cell damage and exerts its function by binding with ST2L on cardiomyocytes. The IL-33/ST2 system is upregulated in the heart after mechanical overload or injury and reduces myocardial fibrosis and hypertrophy (7). The beneficial effects of IL-33, however, only occur when IL-33 binds with ST2L. sST2 acts as a decoy receptor, and IL-33/sST2 binding consequently eliminates the beneficial cardiac effects (6). Both sST2 and ST2L are increased in response to myocardial strain and infarction (8), and sST2 is associated with hemodynamic stress (9). Moreover, clinical studies have shown an association between sST2 and (cardiovascular) outcomes (10,11). As such, sST2 is used as a novel cardiac biomarker for myocardial fibrosis, inflammation, and strain in heart failure patients and has added value as an independent prognostic biomarker, both using single and serial measurements (10,11). Exploring exercise-induced changes in sST2 may inform clinicians of potential effects of exercise when evaluating this biomarker.
The purpose of this study was to assess baseline and exercise-induced changes in sST2 in a heterogeneous cohort of amateur athletes, before and after a marathon run. Furthermore, we aimed to identify predictors of baseline and postexercise sST2 concentrations.
METHODS
Study population
A total of 92 moderately to highly trained runners (26 to 71 yr old) competing in the 2010 Eindhoven Marathon (The Netherlands) were recruited after an online advertisement. Before participation, all participants provided written informed consent. The Medical Ethical Committee of the Radboud University Nijmegen Medical Center approved the study (NL33270.091.10), which was conducted in line with the Declaration of Helsinki.
Study procedures
All participants completed an online questionnaire about personal characteristics, including daily physical activity, marathon experience (e.g., previous completed marathons and personal record), and health status (e.g., medical history and medication use). On the day of the marathon, further demographic data were obtained, weight was measured, and a venous blood sample was taken. Heart rate was monitored continuously during the race by using a two-channel ECG chest band system (Polar Electro Oy, Kempele, Finland). Mean heart rate (HRmean) was determined as the average heart rate between the start and the finish of the marathon. HRmax (208 – 0.7 × age) and exercise intensity (exercise intensity = 100 × HRmean/HRmax) were calculated (12). Immediately after the marathon (<5 min), again a venous blood sample was taken and weight was measured.
At each sample point, 10 mL of blood was drawn from an antecubital vein. Whole venous blood was collected in serum-gel vacutainer tubes and allowed to clot for ∼45 min. After centrifugation, serum was aliquoted, frozen, and stored at −80°C for later analysis. Analysis was performed on a single day using the same calibration and setup to minimize variation. sST2 was analyzed using the Presage ST2 Assay (Critical Diagnostics, San Diego, CA). The assay imprecision has an average intra-assay CV of 5.1%, with a detection limit of 1.8 ng·mL−1. sST2 was stable for 2 d at room temperature, 10 d at 4°C, and 30 d at −20°C (13). An sST2 value of 35 ng·mL−1 is used as a standard analysis cut point for assessing risk of adverse events in patients with heart failure (14). This cut point is based on a receiver operating characteristics curve analysis for the prediction of adverse outcomes in chronic heart failure patients (15). In reference range analysis for sST2, healthy males showed slightly higher 95% nonparametric reference interval values (8.6–49.3 ng·mL−1) compared with healthy females (7.2–33.5 ng·mL−1). Median concentration and reference range values of the two healthy reference cohorts were comparable (males: median 24.2 and 25.7 ng·mL−1; females: 17.5 and 16.3 ng·mL−1) (13). cTnI was analyzed using a high-sensitive cTnI assay (Centaur TnI-Ultra; Siemens Healthcare Diagnostics, Breda, The Netherlands). The assay imprecision was 5.3% at 80 ng·L−1 and 3.0% at 27,200 ng·L−1, with a detection limit of 6 ng·L−1. A cTnI value of 40 ng·L−1 was used as the clinical cutoff value for an acute myocardial infarction (16).
An additional 2 mL of blood was drawn in a subset of participants (n = 53) at baseline and finish to determine plasma levels of hematocrit and hemoglobin (RapidLab®1265; Siemens Healthcare Diagnostics Inc., Tarrytown, NY) to determine plasma volume changes using the Dill and Costill formula (17).
Finish time (exercise duration) was obtained using the ChampionChip time registration (ChampionChip®, MYLAPS, The Netherlands), out of which mean running speed was calculated (speed = 42.195/exercise duration in hours).
Baseline and postexercise body mass were assessed (Seca 888 Scale; Seca, Hamburg, Germany) to provide a surrogate of change in hydration status. The relative change (%) in body mass between the measurements was calculated, and body mass losses ≥2% were indicated as dehydration.
Data analysis
Statistical analyses were performed using SPSS Statistics 21 (SPSS Inc., Chicago, IL). All parameters were visually inspected for normality, checked for kurtosis and skewness, and tested using Shapiro–Wilk normality tests. Continuous variables were reported as mean ± SD when normally distributed, or as median (interquartile range) when not normally distributed, and categorical variables were presented as proportions. Paired t-tests were used to compare continuous variables between baseline and finish. Mann–Whitney U-tests were used when continuous variables where not normally distributed. Mann–Whitney U-tests were used to compare sST2 concentrations between individuals above and below median marathon personal best time (using personal best time as a marker of lifelong training status). Pearson chi-square tests were used to compare categorical variables. Spearman’s correlations were used to assess correlations between baseline, finish, and ΔsST2 and cTnI concentrations. Statistical significance was assumed at P < 0.05. sST2 concentrations at baseline and finish were transformed with the natural logarithm before regression analysis. To assess predictors of sST2, linear regression analysis was used to identify predictors of baseline, finish, and ΔsST2 (Ln Finish sST2 − Ln Baseline sST2). First, univariate linear regression analysis of variables was used to select potential predictors for multivariate analysis. Variables that were significantly (P ≤ 0.10) associated with sST2 concentrations using univariate analysis were then included in a multivariate analysis. We performed a backward multivariate linear regression analysis to gain insight into which variables could independently predict baseline, finish, and ΔsST2. Because of missing values in exercise intensity data due to limited availability of heart rate monitors (70 of 82 finishers had a heart rate monitor), we imputed exercise intensity in the data set for regression analysis. Missing data were imputed with multivariable imputation by chained equations with predictive mean matching. We checked patterns of missing data and followed the “missing at random” assumption. All available variables were used to predict missing values in 15 imputed data sets with 50 burn-in iterations. Healthy convergence, imputed distribution, and plausibility were verified (results not shown).
RESULTS
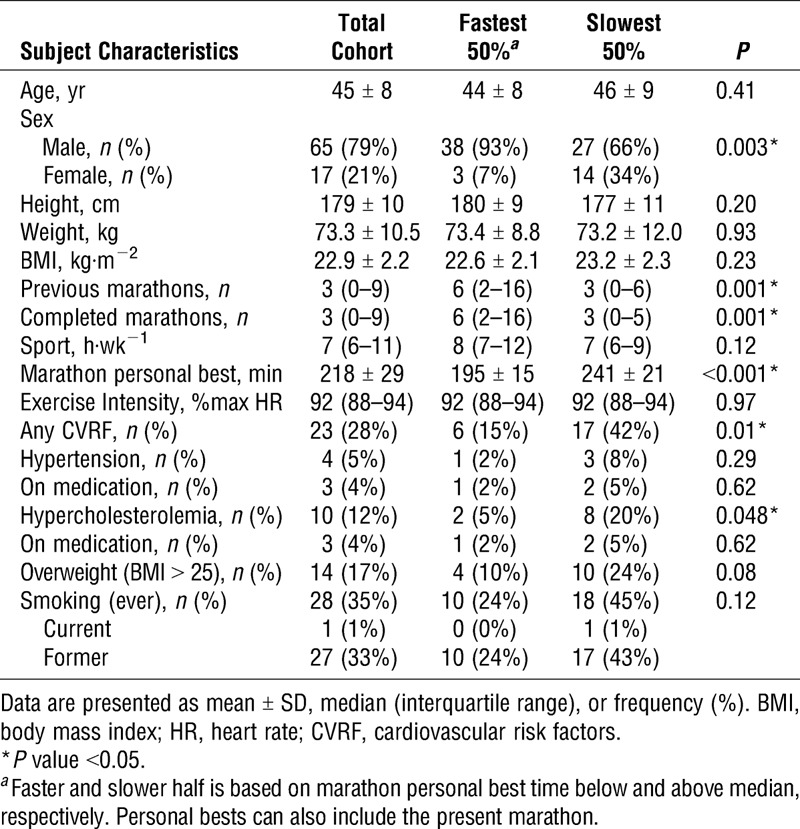
During the race, mean wet bulb globe temperature was 18.8°C (min, 17.0; max, 20.5), with a relative humidity of 52%. Of the 92 participants who started the race, 9 participants did not finish the marathon because of exhaustion (n = 2), acute knee problems (n = 1), heat illness (n = 1), dyspnea (n = 1), hip problems (n = 1), headache (n = 1), or other sport-related injuries (n = 2) and were therefore not included in the analysis. One participant was excluded afterward because of missing multiple data points. Therefore, we included 82 participants in the data analysis (mean age, 48 ± 5 yr; 79% men) although postrace blood withdrawal failed in two participants. Demographic characteristics and the medical history of the full cohort and both the faster and the slower half of the cohort are presented in Table 1. On average, participants performed exercise training for 7 (6–11) h·wk−1 and had completed 3 (0–9) marathons in the past. Marathon personal best time, including the finish time of the present marathon, was 218 ± 29 min.
TABLE 1.
Subject characteristics and health status of the 82 athletes that finished the Eindhoven marathon, shown as total cohort and split by marathon personal best time.
Marathon performance
The average finish time of the marathon (42.2 km) was 227 ± 28 min, with a mean speed of 11.3 ± 1.4 km·h−1. Mean heart rate was 161 ± 9 bpm, which equates to 92% (88%–94%) of the maximal predicted heart rate. There was a significant decrease in body mass (−2.4% ± 1.2%) after finishing the marathon (P < 0.001), and 63% of our cohort was dehydrated. Median plasma volume change was 0% (−5% to 4%). The faster half of the cohort was more often male, had run more marathons, and less often had cardiovascular risk factors (Table 1).
Exercise-induced changes in sST2 and cTnI
Baseline sST2 concentrations were 34 (25–46) ng·mL−1, with 39 (48%) marathon runners exceeding the cutoff value of ≥35 ng·mL−1. Baseline cTnI concentrations were 9 (5–21) ng·L−1, with 5 (6%) marathon runners exceeding the cutoff value of ≥40 ng·L−1. sST2 concentrations increased in every athlete from baseline to finish (34 [25–46] vs 70 [53–87] ng·mL−1, P < 0.001; Fig. 1), with 75 (94%) marathon runners exceeding the sST2 cutoff value at the finish. cTnI concentrations increased in 77 marathon runners (96%) from baseline to finish (9 [5–21] ng·L−1 to 60 [34–102] ng·L−1; P < 0.001), with 55 (69%) marathon runners exceeding the cTnI cutoff value at the finish. There was no association between baseline (P = 0.24), finish (P = 0.42), and Δ (P = 0.69) sST2 and cTnI concentrations.
FIGURE 1.
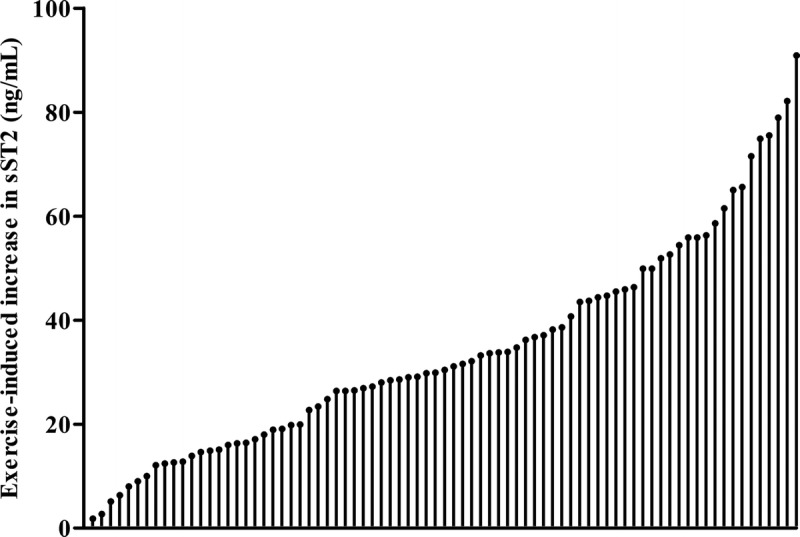
Frequency distribution of ΔsST2. Every runner shows an increase in sST2 concentration.
Baseline (41 [30–50] vs 34 [23–42], P = 0.01), finish (81 [61–98] vs 63 [48–72], P = 0.001), and Δ (36 [27–52] vs 30 [16–40] ng·mL−1, P = 0.03) sST2 concentrations were higher in the fastest marathon runners (Fig. 2).
FIGURE 2.
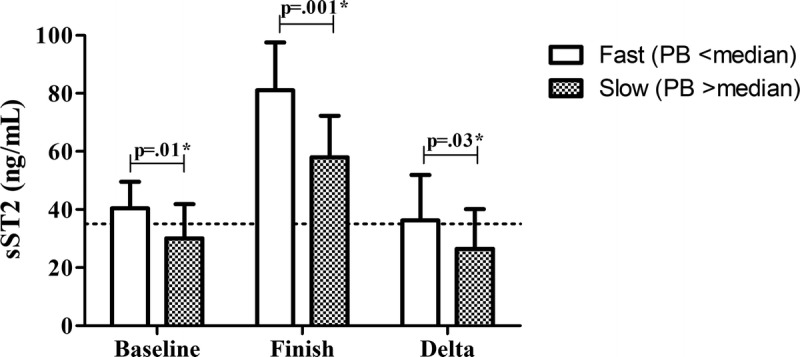
sST2 concentrations in the fastest 50% vs slowest 50% of the cohort based on marathon personal best time. Dotted line shows the cutoff value for sST2 (35 ng·mL−1). Data are shown as median with interquartile range.
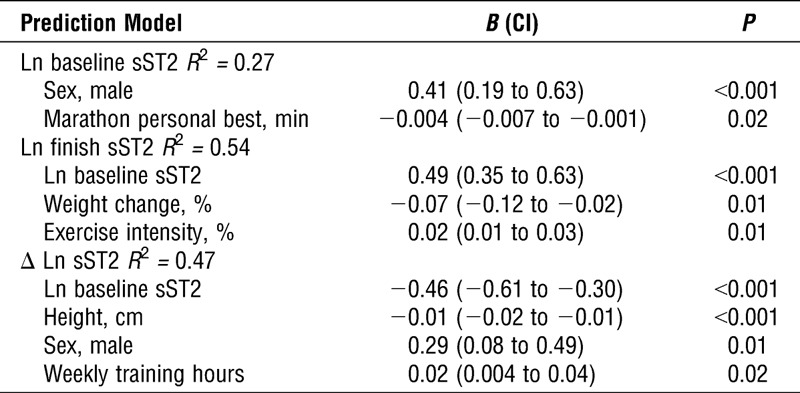
Predictors of sST2
Univariate analysis revealed that sex, height, weight, running speed, number of previous marathons, and marathon personal best time were associated with baseline sST2 concentrations (Table 2); sex, body mass index, weight change, exercise intensity and running speed during marathon, number of previous marathons, marathon personal best, and baseline sST2 were associated with sST2 at finish (Table 2); sex, height, weight, training volume, and baseline sST2 were associated with ΔsST2 (Table 2). Multivariate analysis revealed that marathon personal best time inversely predicted baseline sST2, whereas male sex was associated with higher sST2 concentrations (R2 = 0.27; Table 3). Baseline sST2 and exercise intensity during marathon were directly associated with finish sST2, whereas greater weight loss (negative weight change) during marathon was associated with higher finish sST2 (R2 = 0.54; Table 3). Baseline sST2 and height were inversely associated, whereas male sex and weekly training hours were directly associated with ΔsST2 (R2 = 0.47; Table 3).
TABLE 2.
Univariate linear regression analysis of cohort characteristics.
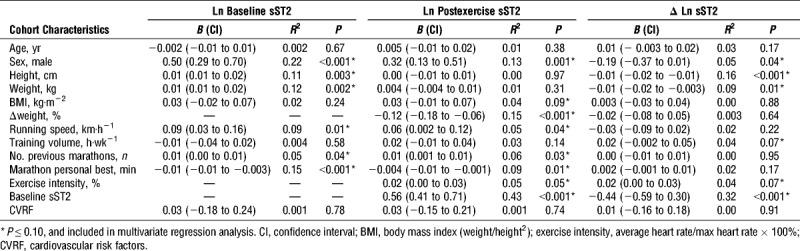
TABLE 3.
Multivariate linear regression analysis.
DISCUSSION
Our results indicate that a marathon run causes a significant increase in sST2 concentrations in all participants and sST2 concentrations were higher in the faster runners. Moreover, a large proportion of marathon runners had sST2 concentrations above cutoff values both at baseline (48%) and finish (94%). sST2 and cTnI concentrations were not correlated at any time point. We observed that male sex, baseline sST2 (with finish sST2), weekly training hours, exercise intensity, and greater weight loss (negative weight change) during marathon were directly associated, whereas marathon personal best time, height, and baseline sST2 (with ΔsST2) were inversely associated with (changes in) sST2 concentrations. These findings suggest that both personal and exercise characteristics influence sST2 concentrations.
Effects of exercise
We observed an exercise-induced increase in sST2 concentrations in all runners, whereas sST2 concentrations above the reference cutoff value were found in ∼50% of the runners at baseline and in nearly all runners postmarathon. sST2 is established as a predictor of mortality in heart failure patients and sST2 values vary between 17.8 and 38.1 ng·mL−1 in chronic heart failure patients (11), whereas acute heart failure patients demonstrate a median sST2 value of 71 ng·mL−1 (10). Although produced in abundance in the myocardium, the site of production of circulating ST2 is thought to be largely extramyocardial and related to diastolic loading on the pulmonary vasculature (18). Indeed, in animal models of heart failure, abundant production and secretion of sST2 from pneumocytes and pulmonary vascular endothelium is evident (unpublished material; J. L. Januzzi, personal communication). The IL-33/ST2 system may also play a role in the exercise-induced immune and inflammatory pathways, indicating that elevated sST2 concentrations may present beneficial anti-inflammatory properties (19).
We found that runners demonstrate similar sST2 concentrations as chronic heart failure patients and acute heart failure patients at baseline and postmarathon, respectively. It is unlikely that apparently healthy marathon runners express equal myocardial inflammation and fibrosis as acute heart failure patients. Thus, the high sST2 concentrations of our population may be explained by regular exposure to increased hemodynamic load of cardiac and pulmonary tissue during exercise, as this may trigger sST2 elevations (7–9). Chronic exercise training may therefore elevate baseline values of sST2 in some individuals, whereas marathon running may further increase sST2 concentrations because of acute increases in cardiac load (20). On the other hand, acute increases in sST2 after exercise may represent beneficial anti-inflammatory properties. It is unknown whether high sST2 concentrations may limit the beneficial cardiac effects of a physically active lifestyle and future studies that explore the clinical relevance of this phenomenon are warranted.
Predictors of sST2
The magnitude of sST2 elevations in marathon runners was heterogeneous (Fig. 1), but baseline, finish, and ΔsST2 concentrations were higher in the fastest runners (Fig. 2). Emerging evidence suggests that the accumulation of multiple acute bouts of high-intensity exercise during prolonged training and competitive periods may induce detrimental cardiac adaptations such as myocardial fibrosis and pathological right ventricular remodeling (21). Indeed, a systematic review confirmed that the prevalence of myocardial fibrosis was strongly associated with the cumulative lifelong exercise dose (5). Although only studying a single acute bout of exercise, we found that a faster marathon personal best and a higher exercise intensity were significantly associated with higher sST2 concentrations. Whether these elevated sST2 concentrations in specific individuals may predict the development of myocardial fibrosis over a longer training exposure is unclear but worthy of follow-up. sST2 elevations have been linked to right ventricular pressure overload and dysfunction (22). Cardiac wall stress of the right ventricle disproportionally increases during exercise compared with left ventricle (23), and elevated sST2 concentrations after exercise may reflect a potential and selective detrimental effect of long-term high-intensity exercise on the right ventricle. It would be interesting to assess whether myocardial fibrosis is more prevalent in runners with the highest sST2 concentrations as a recent pilot study found coronary artery disease in cyclists with the highest postexercise cardiac troponin concentrations (24).
Clinical relevance
We observed that weight loss during exercise and exercise intensity were directly associated with finish sST2 concentrations, which may reflect hemodynamic stress during exercise. When evaluating sST2 concentrations after an exercise bout, this may overestimate the “normal” sST2 values. Therefore, the amount of physical activity/exercise an individual performs (both acute effect of exercise and chronic exercise training) may affect the interpretation of sST2 concentrations.
Myocardial fibrosis is an important risk factor for adverse cardiovascular outcomes in clinical populations (5), and sST2 concentrations have prognostic value in chronic heart failure patients (11). The short- and long-term effects of myocardial fibrosis and sST2 elevations in athletes are unclear. Future studies are required to evaluate whether sST2 can be an early indicator of adverse outcomes in athletes.
Limitations
In the present study, we only measured sST2 and cTnI concentrations before and directly after the marathon. Measuring sST2 concentrations at multiple time points would have provided more information on the kinetics of sST2 in combination with exercise and would allow to observe how quickly concentrations increase after initiation of exercise and with increasing duration of exercise, and subsequently how quickly concentrations return to baseline values after exercise cessation. Correlating sST2 with cTnI on the basis of a single time point simplifies the potential relationship and may therefore miss a potential association. Moreover, this was a cross-sectional study and therefore does not allow for the determination of the prognostic value of exercise-induced sST2 elevations, for which a longitudinal study is required. Finally, we did not perform cardiac MRI scans during this study, which would have been helpful to image acute myocardial (micro)damage and/or (diffuse) myocardial fibrosis and relate this to baseline and exercise-induced elevations of sST2 concentrations.
CONCLUSION
Marathon running causes a significant increase in sST2 concentrations in amateur athletes with sST2 concentrations exceeding cutoff values both at baseline (48%) and finish (94%). Faster runners had higher sST2 concentrations. There was no correlation between sST2 and cTnI at any time point. Our data suggest complex variables determine sST2 concentrations in marathon runners. Furthermore, the long-term prognostic meaning of elevated sST2 concentrations in athletes and whether the substantial rise in sST2 after a marathon is related to cardiopulmonary stress or anti-inflammatory properties deserves further exploration.
Acknowledgments
V. L. A. is financially supported by a grant from the Radboud Institute for Health Sciences. J. L. J. is partially supported by the Hutter Family Professorship. T. M. H. E. is supported by a Horizon 2020 grant from the European Commission (Marie Sklodowska-Curie Fellowship 655502). The authors thank Critical Diagnostics for the analyses of the sST2 samples. Furthermore, they thank Siemens Healthcare Diagnostics for provision of the troponin assays.
J. V. S. is Chief Scientific Officer and president of Critical Diagnostics. The results of the present study do not constitute endorsement by the American College of Sports Medicine. The authors declare that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
REFERENCES
- 1.Eijsvogels TM, Hoogerwerf MD, Maessen MF, et al. Predictors of cardiac troponin release after a marathon. J Sci Med Sport. 2015;18(1):88–92. [DOI] [PubMed] [Google Scholar]
- 2.Aengevaeren VL, Hopman MT, Thijssen DH, van Kimmenade RR, de Boer MJ, Eijsvogels TM. Endurance exercise-induced changes in BNP concentrations in cardiovascular patients versus healthy controls. Int J Cardiol. 2017;227:430–5. [DOI] [PubMed] [Google Scholar]
- 3.Scharhag J, George K, Shave R, Urhausen A, Kindermann W. Exercise-associated increases in cardiac biomarkers. Med Sci Sports Exerc. 2008;40(8):1408–15. [DOI] [PubMed] [Google Scholar]
- 4.Scherr J, Braun S, Schuster T, et al. 72-h kinetics of high-sensitive troponin T and inflammatory markers after marathon. Med Sci Sports Exerc. 2011;43(10):1819–27. [DOI] [PubMed] [Google Scholar]
- 5.van de Schoor FR, Aengevaeren VL, Hopman MT, et al. Myocardial fibrosis in athletes. Mayo Clin Proc. 2016;91(11):1617–31. [DOI] [PubMed] [Google Scholar]
- 6.Pascual-Figal DA, Januzzi JL. The biology of ST2: the International ST2 Consensus Panel. Am J Cardiol. 2015;115(7 Suppl):3B–7. [DOI] [PubMed] [Google Scholar]
- 7.Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007;117(6):1538–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinberg EO, Shimpo M, De Keulenaer GW, et al. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. 2002;106(23):2961–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broch K, Andreassen AK, Ueland T, et al. Soluble ST2 reflects hemodynamic stress in non-ischemic heart failure. Int J Cardiol. 2015;179:378–84. [DOI] [PubMed] [Google Scholar]
- 10.van Vark LC, Lesman-Leegte I, Baart SJ, et al. Prognostic value of serial ST2 measurements in patients with acute heart failure. J Am Coll Cardiol. 2017;70(19):2378–88. [DOI] [PubMed] [Google Scholar]
- 11.Aimo A, Vergaro G, Passino C, et al. Prognostic value of soluble suppression of tumorigenicity-2 in chronic heart failure: a meta-analysis. JACC Heart Fail. 2017;5(4):280–6. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37(1):153–6. [DOI] [PubMed] [Google Scholar]
- 13.Lu J, Snider JV, Grenache DG. Establishment of reference intervals for soluble ST2 from a United States population. Clin Chim Acta. 2010;411(21–22):1825–6. [DOI] [PubMed] [Google Scholar]
- 14.Felker GM, Fiuzat M, Thompson V, et al. Soluble ST2 in ambulatory patients with heart failure: association with functional capacity and long-term outcomes. Circ Heart Fail. 2013;6(6):1172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ky B, French B, McCloskey K, et al. High-sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ Heart Fail. 2011;4(2):180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Apple FS, Smith SW, Pearce LA, Ler R, Murakami MM. Use of the Centaur TnI-Ultra assay for detection of myocardial infarction and adverse events in patients presenting with symptoms suggestive of acute coronary syndrome. Clin Chem. 2008;54(4):723–8. [DOI] [PubMed] [Google Scholar]
- 17.Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol. 1974;37(2):247–8. [DOI] [PubMed] [Google Scholar]
- 18.Bartunek J, Delrue L, Van Durme F, et al. Nonmyocardial production of ST2 protein in human hypertrophy and failure is related to diastolic load. J Am Coll Cardiol. 2008;52(25):2166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De la Fuente M, MacDonald TT, Hermoso MA. The IL-33/ST2 axis: role in health and disease. Cytokine Growth Factor Rev. 2015;26(6):615–23. [DOI] [PubMed] [Google Scholar]
- 20.Billat VL, Petot H, Landrain M, Meilland R, Koralsztein JP, Mille-Hamard L. Cardiac output and performance during a marathon race in middle-aged recreational runners. ScientificWorldJournal. 2012;2012:810859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eijsvogels TM, Fernandez AB, Thompson PD. Are there deleterious cardiac effects of acute and chronic endurance exercise? Physiol Rev. 2016;96(1):99–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.AbouEzzeddine OF, McKie PM, Dunlay SM, et al. Suppression of tumorigenicity 2 in heart failure with preserved ejection fraction. J Am Heart Assoc. 2017;6(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.La Gerche A, Heidbüchel H, Burns AT, et al. Disproportionate exercise load and remodeling of the athlete’s right ventricle. Med Sci Sports Exerc. 2011;43(6):974–81. [DOI] [PubMed] [Google Scholar]
- 24.Skadberg O, Kleiven O, Bjorkavoll-Bergseth M, et al. Highly increased troponin I levels following high-intensity endurance cycling may detect subclinical coronary artery disease in presumably healthy leisure sport cyclists: the North Sea Race Endurance Exercise Study (NEEDED) 2013. Eur J Prev Cardiol. 2017;24(8):885–94. [DOI] [PubMed] [Google Scholar]


