Abstract
Stress can disrupt memory and contribute to cognitive impairments in psychiatric disorders, including schizophrenia and attention deficit hyperactivity disorder. These diseases are more common in men than in women, with men showing greater cognitive impairments. Mnemonic deficits induced by stress are mediated, in part, by corticotropin releasing factor (CRF). However, where CRF is acting to regulate memory, and sex differences therein, is understudied. Here we assessed whether CRF in the medial septum (MS), which projects to the hippocampus, affected memory formation in male and female rats. CRF in the MS did not alter hippocampal-independent object recognition memory, but impaired hippocampal-dependent object location memory in both sexes. Interestingly, males were more sensitive than females to the disruptive effect of a low dose of CRF in the MS. Female resistance was not due to circulating ovarian hormones. However, compared to males, females had higher MS expression of CRF binding protein, which reduces CRF bioavailability and thus may mitigate the effect of the low dose of CRF in females. In contrast, there was no sex difference in CRF1 expression in the MS. Consistent with this finding, CRF1 antagonism blocked the memory impairment caused by the high dose of CRF in the MS in both sexes. Collectively, these results suggest that males are more vulnerable than females to the memory impairments caused by CRF in the MS. In both sexes, CRF1 antagonists prevented MS-mediated memory deficits caused by high levels of CRF, and such levels can result from very stressful events. Thus, CRF1 antagonists may be a viable option for treating cognitive deficits in stressed individuals with psychiatric disorders.
Keywords: Corticotropin releasing hormone, Medial septum, Memory, Sex differences, Ovarian hormones, Stress
1. Introduction
Stress is an environmental factor that can exacerbate symptoms of psychiatric disorders characterized by cognitive deficits, including schizophrenia and attention deficit hyperactivity disorder (ADHD) (American Psychiatric Association, 2013; Hirvikoski et al., 2009; Walker and Diforio, 1997). Another shared feature of these disorders is that there are sex differences, such that men are diagnosed more frequently and present with more cognitive disruptions than women (Gershon and Gershon, 2002; Mendrek and Mancini-Marïe, 2016). These epidemiological data have led to the idea that sex differences in response to stress contribute to sex biases in certain psychiatric disorders (Bangasser and Wicks, 2017).
Corticotropin releasing factor (CRF) is a key neuropeptide that initiates and orchestrates the stress response by activating the hypothalamic pituitary adrenal axis (Bale and Vale, 2004; Owens and Nemeroff, 1991). It also acts centrally as a neuromodulator to regulate cognitive changes due to stress (reviewed in, Bangasser and Kawasumi, 2015). For example, spatial memory is disrupted in transgenic mice with central CRF overexpression (Heinrichs et al., 1996), and attention is impaired by a central infusion of CRF in rats (Cole et al., 2016; Van't Veer et al., 2012). However, the target of CRF's actions within the brain to regulate cognition is still being explored. One brain region that is critical for cognitive function and in a position to be affected by CRF regulation is the medial septum (MS). The MS contains GABAergic and cholinergic neurons that project to the hippocampus to regulate aspects of learning and memory (Amaral and Kurz, 1985; Cai et al., 2012; Pang et al., 2011). The MS also has a high density of CRF1 receptors (CRF1) and contains CRF axon terminals, although CRF is not produced within this region (Merchenthaler et al., 1982; Sauvage and Steckler, 2001; Van Pett et al., 2000). Yet, whether CRF in the MS modulates memory had not been investigated previously.
The present study was designed to assess whether CRF in the MS disrupts memory in male and female rats. Given emerging research demonstrating a range of sex differences in CRF function, from its expression to its receptor signaling (Bangasser et al., 2010; Iwasaki-Sekino et al., 2009), we assessed the effects of CRF in the MS in both sexes. Based on prior reports of the modulatory effect of ovarian hormones on CRF's regulation of other cognitive processes (Cole et al., 2016), we also evaluated whether ovarian hormones influenced sensitivity of the MS to CRF. Additionally, sex differences in CRF1 or CRF binding protein (CRF-BP), which limits CRF's bioavailability, were also measured, because these differences could impact the efficacy of CRF in this region.
2. Material and methods
2.1. Subjects and cytology
Adult (>60 days old) male and female Long Evans rats (Charles River Laboratories, Wilmington MA, USA) were housed in same sex pairs with food (LabDiet 5001) and water available ad libitum. Rats were maintained under a 12-h reverse light/dark cycle (dark onset at 9:00am). Rats were allowed 7 days of acclimation to the animal facility and were handled for 5 min/day for five days prior to behavioral testing, which was conducted between 2:00–5:00 p.m. Female rats were lavaged daily in order to evaluate estrous cycle phase as previously described (Bangasser and Shors, 2008). All studies were conducted in accordance with the Temple University Institutional Animal Use and Care Committee and the Institutional Animal Care and Use Committee.
2.2. Stereotaxic and ovariectomy surgeries
Stereotaxic surgery was conducted under aseptic conditions as previously described (Bangasser et al., 2013). Briefly, a 22 gauge guide cannula (Plastics One, Roanoke, VA) was implanted into the MS (+0.4 mm A/P from Bregma, +1.0 mm M/L from Bregma, −4.9 mm D/V from cortical surface, 10° drive depth) (Paxinos and Watson, 2007). Rats undergoing ovariectomy (OVX) or sham surgery received these manipulations immediately prior to the stereotaxic surgery on the same day. Following a dorsal incision, the ovary and the oviduct were exteriorized through the muscle wall. For the OVX surgical procedure only, the ovaries were removed. Rats in all surgical conditions received analgesic, Flunixin (Bimeda) 2.5 mg/kg, s.c., prior to and 24 h after surgery. Rats were singly housed following surgery and allowed at least 1 week to recover, unless they were in the OVX and sham surgery study, in which case they were given at least 2 weeks to recover to ensure adequate time for the uterus to regress (Kleinstreuer et al., 2016; Owens and Ashby, 2002).
2.3. Drugs and microinfusions
All drugs were reconstituted in water, aliquoted, lyophilized, and frozen until the day of use, when they were reconstituted with artificial cerebrospinal fluid (aCSF). Studies examining both male and female rats have found that there are no CRF2 in the MS, yet there is a high density of CRF1 (Van Pett et al., 2000). Thus, we chose to use ovine CRF (American Peptides, Vista, CA, USA), which preferentially binds to CRF1, the receptor subtype present in the MS. The doses (3 ng, 30 ng, and 100 ng) were chosen based on their efficacy in other brain regions and their ability to alter physiological endpoints to a similar degree as stressor exposure (Curtis et al., 2006; Snyder et al., 2015). The CRF1 antagonist NBI 35965 (Tocris, Bristol, United Kingdom) was used at 0.44 ng as previously detailed (Howerton et al., 2014). All drugs were infused at a flow rate of 0.1 μl/min for 5 min by an infusion pump (Harvard Apparatus) connected to a 10 μL syringe (Hamilton) attached to polyethylene tubing. After each drug infusion, the infusion needle was left in place for 1 min before removal. Testing began 10 min after each CRF infusion. For studies where the antagonist was used in combination with CRF dosing, the antagonist (or control infusion) was delivered 1 h prior to the administration of CRF.
2.4. Behavioral testing
Given the projections from the MS to the hippocampus (Amaral and Kurz, 1985), we predicted that CRF in the MS would disrupt hippocampal-dependent memory. While there are many ways to test this, we chose to use the object location task because it does not use aversive stimuli that would activate the endogenous CRF system (e.g., shock in contextual fear conditioning, cold water in the Morris water maze). Also, the object location task is very similar to the object recognition task, which can be run in such a way that it is hippocampal-independent thereby serving as a useful control procedure. To ensure that our version of the object recognition task did not require the hippocampus, we used a 5 min training phase with a 5 min delay between the training and testing phases, because rats with hippocampal lesions can successfully complete this task using these parameters (Mumby et al., 2002). Additionally, we employed a habituation procedure that involved repeated exposure to the training context, a method that renders the object recognition task independent of the hippocampus (Oliveira et al., 2010).
All testing occurred under red light and was recorded (Noldus EthoVision XT, Version 10) by a camera suspended above the test arena. For the object location task, rats were habituated to a black plexiglass square arena (76.2 cm × 76.2 cm × 43.18 cm, open top) containing bedding (1 cm deep), and a black and white vertical striped cue on the inside wall, for 5 mins/day for three consecutive days. On test day, following infusion(s), rats were exposed to a 5 min training phase to two identical metal objects (builder's hardware framing connector - vertical orientation, 5.5 cm × 15.3 cm) at one end of the arena placed 18 cm from arena walls. Following training, rats were returned to their home cage for a 5 min delay during which one of the objects was displaced to the opposite side of the arena (locations of objects were counterbalanced). Rats were then placed back in the chamber for a 5 min test of time spent exploring each object.
A subset of rats was tested for novel object recognition following the object location task. The day after completing the object location task, rats were habituated to the arena for an additional 5 min/day for five consecutive days. The test day was similar to the object location task in that it consisted of 5 min training, delay, and testing phases. However, in the object recognition task, rats were exposed to two plastic objects (PVC pipe with cover, 18 cm × 2.75 cm) during the training phase, and then one plastic object was replaced with a glass object (125 mL flask, 10.5 cm × 6.5 cm), located in the same position, prior to the test phase. Objects and their replacement location were counterbalanced. Drug administration between the object location task and object recognition task was counterbalanced.
Behavior was hand-scored with the assistance of Kinoscope software (Kokras et al., 2017). Object exploration was defined as being within 2 cm of the object and actively sniffing the object. Animals that climbed to the top of an object or exhibited rearing behavior were not considered to be exploring the object. Animals that failed to explore both objects were dropped from analysis. For both the object location task and the object recognition task, preference scores were calculated by determining the ratio of time spent with the displaced or novel object, respectively, divided by the time spent with the familiar object. All videos were scored by a rater blind to the experimental condition.
2.5. Tissue collection and processing
Following experimental procedures, rats were deeply anesthetized with an intraperitoneal injection of Euthasol and transcardially perfused with 4% paraformaldehyde. Brain tissue was coronal sectioned at 30 μm on a cryostat (Microm HM 500 M) and MS cannula placement slices were assessed. For qPCR experiments, animals were sacrificed by decapitation. Brains were flash frozen and stored in −80 °C until punches of the MS were taken during cryostat sectioning. RNA was extracted using the RNeasy kit (Qiagen) according to manufacturer's instructions. RNA concentration and purity were quantified by NanoDrop spectrophotometry (Thermo Fisher Scientific). Generation of cDNA was carried out using the RETROscript kit (Ambion) with 500 ng of RNA as template. Reactions were prepared in 96-well optical reaction plates (ABI) with optical adhesive covers (ABI) using Fast SYBR Green Master Mix (ABI). Three technical replicates were used for each animal. Reactions were carried out in the qTower (Analytik Jena) with an initial incubation at 95 °C for 10 min, and 40 subsequent cycles of 95 °C for 15 s, 60 °C for 30 s. The primers used were as follows: Crhr1 F: TTGGCAAACGTCCTGGGGTAT; R: GCGGACAATGTTGAAGAGAAAG; Crhbp primers were purchased from BioRad (assay ID qRnoCID0004271). Housekeepers primers were as follows Tuba4a F: GCTGGAAAACATGTGCCTCG; R: GCTGCATCTTCCTTCCCAGT; Gapdh F: GTTTGTGATGGGTGTGAACC; R: TGTTGTGAGTGGCAGTGATG; Hprt F: TCCCAGCGTCGTGATTAGTG; R: ATGGCCTCCCATCTCCTTCA. ΔCt values were corrected using housekeeping gene expression levels for each sample and fold change was calculated as 2exp(-ΔΔCt). The data presented is the calculated mean for the biological replicates with n being equal to the number of biological replicates (i.e. the number of rats examined).
2.6. Statistical analysis, cannula placement assessment, and other exclusion criteria
The behavioral experiments were analyzed with two-way ANOVAs and all designs were between subjects. For studies investigating dose-response effects, sex and dose served as factors. Studies investigating an estrous cycle effect or the effect of ovariectomy were analyzed with 2 × 2 ANOVAs with hormone condition and dose as factors with two levels. The CRF1 antagonist study was analyzed with a 2-way ANOVA with the factors of sex and drug treatment (with NBI 35965 + Vehicle, Vehicle + 100 ng CRF, NBI 35965 + 100 ng CRF as the different levels). Significant interactions and simple main effects were followed up with Tukey's post-hoc tests. The gene expression data were analyzed with independent samples t-tests. Values that exceeded 2 SDs above or below their respective group mean for the dependent variables assessed (e.g., ratio of time spent with the displaced/familiar object during the testing phase) were considered outliers and dropped. Results were considered statistically significant at p < .05.
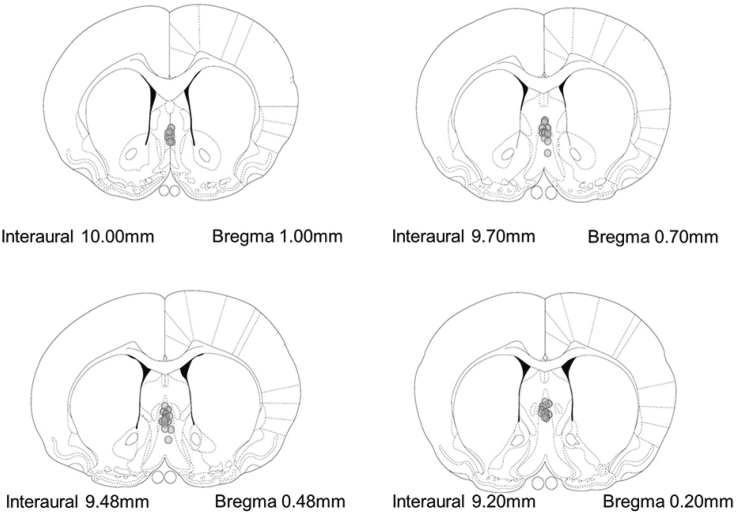
Nine rats with incorrect MS cannula placements were dropped from the statistical analysis. An observer blind to treatment conditions verified all incorrect cannula placement exclusions. Cannula placements located within the MS for rats included in the study are depicted in Fig. 1. Additionally, outliers (more than ±2 standard deviations) were dropped from the following groups: object location male/aCSF (n = 1), male/CRF 3 ng (n = 1), male/CRF 30 ng (n = 1); object recognition male/aCSF (n = 1), male/CRF 30 ng, female/CRF 30 ng (n = 1); female/CRF 100 ng (n = 1); gonadectomy study sham/aCSF (n = 1), OVX/aCSF (n = 1); antagonist study female/NBI 35965 + CRF (n = 1).
Fig. 1.
Location of cannula tips for all rats included in the studies. Because of extensive overlap cannula tip locations, not all individual injector locations are indicated. Coronal brain section images were adapted from (Paxinos and Watson, 2007).
The n's reported reflect only the subjects included in the analysis. For the object location task: controls (male, n = 11; female, n = 9), 3 ng CRF (male, n = 8; female, n = 9), 30 ng CRF (male, n = 7; female, n = 10), and 100 ng CRF (male, n = 9; female, n = 9). For the object recognition task: controls (male, n = 9; female, n = 8), 30 ng CRF (male, n = 8; female, n = 9), and 100 ng CRF (male, n = 9; female, n = 8). For the object location cycle data study: diestrus (I and II) (aCSF, n = 5; CRF 3 ng, n = 2), proestrus and estrus (aCSF, n = 4; CRF 3 ng, n = 7). For the OVX study: sham controls (aCSF, n = 8; CRF 3 ng, n = 11) and OVX (aCSF, n = 8; CRF 3 ng, n = 9). For the CRF antagonist study: NBI 35965 followed by aCSF (male, n = 10; female, n = 12), vehicle followed by 100 ng CRF (male, n = 10; female, n = 7), NBI 35965 followed by 100 ng CRF (male, n = 9; female, n = 9). For qPCR studies: male n = 8; female n = 8.
3. Results
3.1. CRF in the MS impaired performance on the object location task and males were more sensitive to this effect
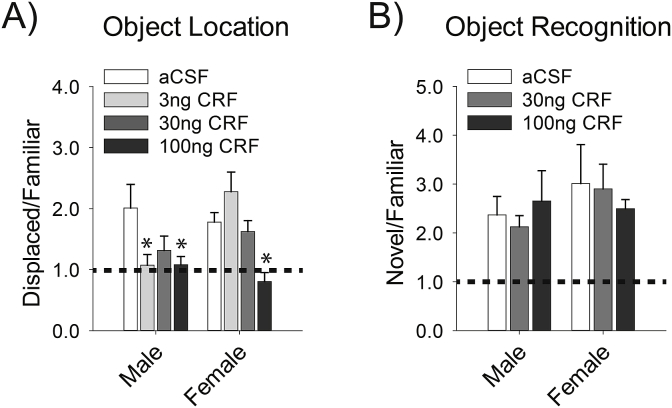
We found that CRF in the MS reduced relative time spent exploring the displaced vs. familiar object [F(3,64) = 5.52, p = .002] (Fig. 2A). This result indicates that CRF impairs object location memory. There was no main effect of sex [F(1,64) = 2.02, p = .160], but there was a significant sex by CRF treatment interaction [F(3,64) = 3.75, p = .015] (Fig. 2A). A post-hoc test revealed that, in females, only the highest dose of CRF (100 ng) reduced the relative time spent with the displaced object compared to vehicle (p = .035). In males, the 30 ng dose reduced the relative time spent with the displaced object in males, but this effect was not significant (p = .226). However, the 3 ng and 100 ng CRF doses in the MS of males significantly reduced the relative time spent with the displaced object (p = .043, and p = .036, respectively). Notably, the 3 ng dose was only effective in males, which suggests that males are more sensitive to CRF in the MS than females.
Fig. 2.
CRF in the MS disrupted spatial memory and males were more sensitive to this effect, but recognition memory was unaffected. A) In the object location task, the 100 ng dose of CRF in the MS reduced the ratio of time spent with the displaced compared to the familiar object in both sexes, but the 3 ng dose only reduced this ratio in males when compared to aCSF-treated controls. B) In the object recognition task, CRF in the MS did not alter the ratio of time spent with the novel, relative to the familiar object. The dashed line marks a ratio of 1, when an equal amount of time is spent with both objects, indicative of no memory. Data are represented as mean ± SEM, asterisks indicate (p < .05) from same sex aCSF-treated controls.
3.2. CRF in the MS does not impair performance on the object recognition task
We also tested memory in a version of the object recognition task that does not require the hippocampus (Mumby et al., 2002; Oliveira et al., 2010). This design allowed us to determine whether CRF in the MS specifically impaired spatial memory, or disrupted memory more generally. We found no effect of CRF treatment on preference scores for the novel object relative to the familiar object [F(2, 45)<1], suggesting that CRF in the MS does not impair performance in this task. In addition, there was no effect of sex [F(1, 45 = 1.37, p = .248], nor an interaction between sex and CRF treatment [F(2, 45)<1] (Fig. 2B). This result suggests that CRF in the MS selectively impairs spatial memory.
3.3. Assessment of factors that could contribute to decreased female sensitivity to CRF in the MS
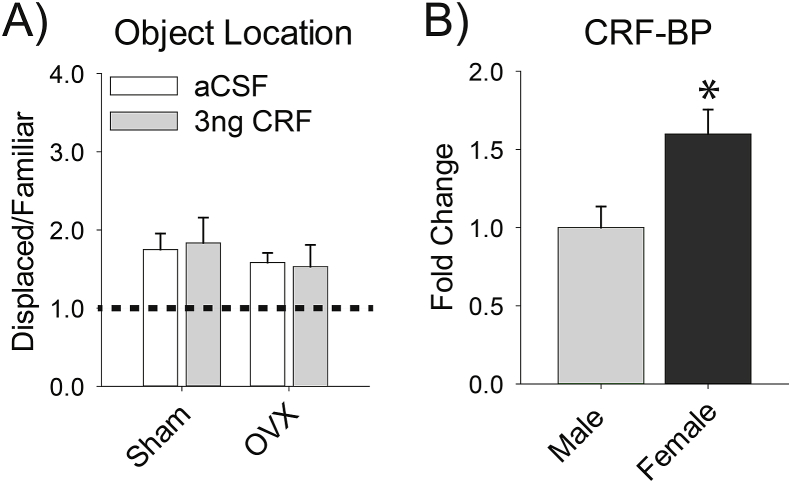
Unlike male rats, females were protected against the adverse effect of 3 ng of CRF in the MS on the object location task. To determine whether circulating ovarian hormones contributed to this protection, we reanalyzed the data for the aCSF infusion and the 3 ng infusion separating females by estrous cycle stage. We compared females in diestrus (I and II), when ovarian hormones are lower, to females in proestrus and estrus, when ovarian hormones are higher. There was no main effect of dose [F(1, 14) = 1.59, p = .229], cycle stage [F(1, 14)< 1], or interaction [F(1, 14)< 1] (data not depicted). Although these results indicated that estrous cycle stage did not modulate the effect of CRF in the MS on spatial memory, this analysis was limited because it was underpowered, as the initial study was not designed specifically to assess estrous cycle effects. To determine more thoroughly whether circulating ovarian hormones protect females from the negative effect of 3 ng of CRF in the MS, an additional study compared the effects of aCSF and 3 ng of CRF in the MS in OVX to sham controls. There was no main effect of hormonal status [F(1,32)<1], CRF treatment [F(1,32)<1], nor interaction [F(1,32)<1] for time spent with the displaced relative to familiar object (Fig. 3A). Thus, OVX females do not show the male-like sensitivity to the disruptive effect of the 3 ng of CRF in the MS on spatial memory. This finding indicates that circulating ovarian hormones do not account for female resistance to low doses of CRF in the MS.
Fig. 3.
Female resistance to low levels of CRF in the MS is not due to circulating ovarian hormones, but is linked to a sex difference in CRF-BP. A) OVX females responded similarly to the 3 ng dose of CRF as their sham-operated counterparts. The dashed line marks a ratio of 1, when an equal amount of time is spent with both objects, indicative of no memory. B) In the MS, females have more CRF-BP expression than males. Data are represented as mean ± SEM, asterisks indicate (p < .05).
We also investigated another factor that could contribute to reduced female sensitivity to low levels of CRF in the MS, CRF-BP, which sequesters CRF thereby limiting its bioavailability. Females had ∼1.5 × the amount of CRF-BP in the MS than males [t(14) = 2.33, p = .035] (Fig. 3B). This increase in CRF-BP in the female MS could help reduce the effect of CRF in this region. There were no significant differences in the housekeeping genes [for Gapdh, t(14) = 0.24, p = .816; for Hprt t(14) = 0.45, p = .662, for Tuba4a t(14) = 0.27, p = .795].
3.4. There are no sex differences in CRF1 expression in the MS or in its ability to mediate the impairing effect of CRF in the MS on spatial memory
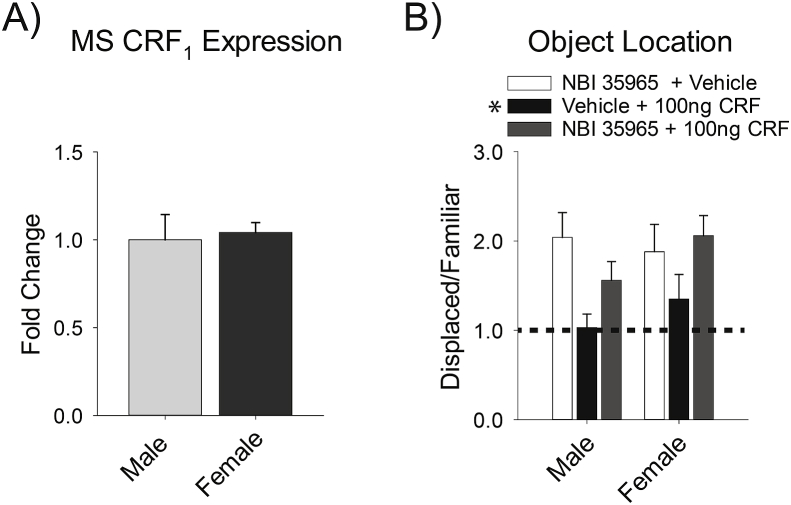
Previous studies have found that the receptor subtype in the MS is CRF1 (Radulovic et al., 1998; Van Pett et al., 2000). However, these initial studies either used only male animals or did not compare the sexes. It was therefore possible that male sensitivity to CRF was also due to greater CRF1 expression in the MS. Yet, qPCR studies revealed no sex difference in CRF1 expression [t(14) = 0.016, p = .987] (Fig. 4A). There were no significant differences in the housekeeping genes [for Gapdh t(14) = 0.02, p = .989; for Hprt t(14) = 0.03, p = .976; for Tuba4a t(14) = 0.07, p = .944].
Fig. 4.
CRF1 expression and its ability to mediate the effect of CRF in the MS is similar in both sexes. A) Male and female rats have similar CRF1 expression in the MS. B) Pretreatment with the CRF1 antagonist, NBI 35965, blocked the effect of 100 ng of CRF in the MS in both sexes. The dashed line marks a ratio of 1, when an equal amount of time is spent with both objects, indicative of no memory. Data are represented as mean ± SEM, asterisk in legend indicates (p < .05) compared to other treatment groups.
This result suggested that CRF1 should mediate the spatial memory deficit that results from high levels of CRF in the MS in both sexes. To confirm this idea, the CRF1 antagonist, NBI 35965, was infused into the MS prior to local administration of the 100 ng dose of CRF, which was effective in both sexes. This manipulation was compared to rats given aCSF followed by 100 ng of CRF, and NBI 35965 followed by aCSF. There was no main effect of sex [F(1,51) = 1.11, p = .296] nor significant interaction [F(2,51) < 1] (Fig. 4B). However, there was a main effect of treatment [F(2,51) = 4.83, p = .012]. Post-hoc analysis on the simple main effect revealed that pretreatment with the CRF1 antagonist blocked the negative effect of the high dose of CRF in the MS on spatial memory (p = .048) (Fig. 4B). These results reveal that CRF in the MS impairs spatial memory via CRF1 in both sexes. Additionally, performance of vehicle infused rats pretreated with the CRF1 antagonist did not exceed performance of CRF infused rats pretreated with the CRF1 antagonist (p = .828). This result indicates that blocking CRF1 in the MS does not improve spatial memory and suggests that there is no endogenous effect of CRF in the MS on spatial memory.
4. Discussion
The high density of CRF receptors in the MS was first documented decades ago (Potter et al., 1994), yet the effect of CRF in the MS had not been previously evaluated. Here we addressed this gap by demonstrating that CRF administered to the MS disrupted object location, but not object recognition memory. Our findings suggest that CRF treatment in the MS selectively impaired hippocampal-dependent memory, which is consistent with the known projections from the MS to the hippocampus (Amaral and Kurz, 1985). Surprisingly, males were more sensitive than females to the deleterious impact of increased CRF signaling in the MS on object location memory. Indeed, male performance was impaired by a low dose of CRF, which spared female performance on the object location task. This finding is in contrast to the locus coeruleus (LC), where female LC neurons are more sensitive than male LC neurons to the effects of CRF, suggesting that sex differences in CRF-sensitivity are region specific (Bangasser et al, 2010, 2018; Curtis et al., 2006).
Estradiol can be neuroprotective, and high levels of ovarian hormones protect against the negative effect of CRF on another cognitive process mediated by a different basal forebrain cholinergic structure (Cole et al., 2016; Garcia-Segura et al., 2001). However, the sex difference in spatial deficits induced by CRF in the MS was not attributable to circulating adult ovarian hormones, because their removal did not increase MS sensitivity to CRF in females. Instead, increased CRF-BP in the MS of females compared to males can explain female resistance to the low dose of CRF in the MS on spatial memory. CRF-BP is a negative regulator of CRF activity, so the high levels of CRF-BP in the MS of females may be sufficient to protect females from low levels of CRF in this region.
Unlike the low dose of CRF in the MS, which only disrupted spatial memory in males, the high dose of CRF impaired this type of memory in both males and females. This effect was mediated by CRF1 in the MS in both sexes. Consistent with this finding, there were no sex differences in CRF1 expression in this region.
4.1. CRF in the MS impaired spatial memory
As noted, the high dose of CRF in the MS impaired spatial memory in the object location task in both sexes. In contrast, recognition memory was unaffected by this manipulation. The sparing of recognition memory reveals that CRF in the MS neither disrupts mnemonic processes generally nor causes performance deficits that prevent successful completion of memory tasks involving objects. Given that CRF was administered into the MS prior to the start of the training phase in the object location task, it is difficult to determine the exact mnemonic processes disrupted (i.e., acquisition, consolidation, or recall). However, long-term consolidation can be ruled out, because the training and testing phases were only 5 min apart. Future studies that administer CRF in the MS prior to testing will be needed to dissociate effects on acquisition of spatial information from those on other mnemonic processes.
The MS projects to the hippocampus, which is required for spatial memory. Therefore, our results indicate that CRF in the MS causes a downstream alteration in hippocampal function that disrupts spatial memory. Hippocampal-dependent memory is regulated by both MS cholinergic and GABAergic efferents, and CRF1 are found on cholinergic neurons and non-cholinergic cells in the MS (Cai et al., 2012; Pang et al., 2011; Sauvage and Steckler, 2001). The present studies do not distinguish the MS cell type mediating the CRF-induced spatial memory deficit. However, it is likely that these effects are attributable to changes in hippocampal acetylcholine levels. Central administration of CRF alters hippocampal acetylcholine and this effect is likely mediated via the MS, as it is a major source of acetylcholine for the hippocampus (Amaral and Kurz, 1985; Day et al., 1998). Moreover, behavioral studies found that selective lesions to MS cholinergic neurons disrupt performance on the object location task (Cai et al., 2012). In contrast, selective MS GABAergic lesions fail to disrupt spatial reference memory, the type of mnemonic processes assessed with the object location task, but MS GABAergic lesions do impair spatial working memory (Pang et al., 2011). Collectively, these findings indicate that CRF in the MS alters hippocampal acetylcholine to disrupt spatial reference memory, but confirmation of this likely mechanism is needed.
4.2. Sex differences in CRF sensitivity in the MS
An interesting outcome of the present study was the discovery that the low dose of CRF in the MS disrupted spatial memory in male, but not female rats. This finding is different than our previous research on the LC-arousal system, which determined that females are more sensitive than males to the effect of CRF in the LC (Bangasser et al., 2010; Curtis et al., 2006). This combination of findings reveals that sex differences in CRF sensitivity are region specific. Importantly, these sex differences could bias males and females towards different responses to stress: low levels of CRF would be expected to impair certain types of memory in males but result in high levels of arousal in females.
Our work raised questions about the mechanisms underlying sex differences in MS CRF signaling and its impact on hippocampal memory function. Circulating ovarian hormones seemed like a logical candidate because we previously found that these hormones modulate female sensitivity to the impairing effect of CRF on sustained attention, a processes mediated by another cholinergic region in the basal forebrain (Cole et al., 2016). Specifically, female rats in the estrous cycle phases with higher ovarian hormones, but not phases with lower ovarian hormones, were protected from the negative effect of CRF on sustained attention (Cole et al., 2016). Therefore, we predicted that ovarian hormones would similarly protect against the impairing effect of low levels of CRF in the MS on spatial memory. To our surprise, this was not the case. Moreover, ovariectomizing females did not increase their MS sensitivity to CRF. These studies demonstrate that circulating ovarian hormones do not contribute to decreased female sensitivity to the mnemonic deficit caused by CRF administration to the MS.
Instead, we found a sex difference in CRF-BP that may help explain female resistance to low doses of CRF in the MS. Given that CRF-BP limits the bioavailability of CRF, the higher levels of CRF-BP found in the MS of females could prevent the low dose of CRF from affecting their MS function. In contrast, the high dose of CRF in the MS may overwhelm the capacity of CRF-BP to protect females, leading to a spatial memory deficit. One consideration is that ovine CRF was used in this study and CRF-BP binds ovine CRF with a lower affinity than human/rat CRF (Eckart et al., 2001). Thus, the increase in CRF-BP in the female rat MS would offer even more protection against endogenous CRF released by stress. It is also possible that the sex difference in CRF-BP does not fully explain the decreased female CRF sensitivity and that other sex differences in CRF function also contribute. In the LC, we have identified sex differences in CRF1 signaling that confer female sensitivity to CRF (Bangasser et al., 2010). Perhaps a similar sex difference in CRF1 signaling in the MS confers male sensitivity to CRF. Future studies will assess this possibility.
The sex difference in MS CRF-BP is consistent with other reports of CRF-BP being higher in the female than male pituitary (Speert et al., 2002; Stinnett et al., 2015). In the pituitary, estradiol increases the expression of CRF-BP through estrogen responsive element half-sites on the CRF-BP promoter (van de Stolpe et al., 2004). Here we have determined that circulating ovarian hormones do not contribute to the sex difference in CRF sensitivity in the MS. Perhaps instead it is the surge of estradiol during puberty that causes a lasting increase in CRF-BP in the MS of females. Alternatively, androgens may suppress CRF-BP in the MS via the activation of androgen receptors found in this region (Simerly et al., 1990). Future studies are needed to determine how the sex difference in CRF-BP in the MS is established.
4.3. CRF1 in the MS and its potential as a target for improving cognition during stress
Prior studies found CRF1, but not CRF2, in the MS of male and female rats (Radulovic et al., 1998; Van Pett et al., 2000). However, data from males and females were combined, such that these studies did not compare expression between the sexes. It therefore remained to be determined whether there were sex differences in CRF1 expression in the MS that could explain male sensitivity to CRF in this region. We tested this possibility here and found that MS CRF1 expression was similar in male and female rats. This result indicated that blocking CRF1 in the MS would prevent the effect of CRF on spatial memory in both sexes. Yet, in other brain regions, sex differences in antagonist efficacy have been reported even when receptor levels are comparable between males and females. Specifically, there are no sex differences in CRF1 expression in the mouse dorsal raphe, but males are more sensitive than females to the anxiolytic effect of a CRF1 antagonists in this region, likely due to sex differences in CRF1 distribution on neuronal subpopulations (Howerton et al., 2014). Thus, we tested whether blocking CRF1 in the MS would prevent the impairing effect of CRF in the MS on spatial memory. Indeed, this antagonism was effective in both sexes. When considered along with the report of the sex difference in the effect of the CRF1 antagonist in the dorsal raphe (Howerton et al., 2014), these results reveal that sex differences in CRF1 antagonist efficacy are brain region specific.
The antagonist results have clinical implications. The basic research studies that characterized the anxiolytic and antidepressant-like effects of CRF1 antagonists used male rodents (Chaki et al., 2004; Deak et al., 1999; Mansbach et al., 1997; Schulz et al., 1996; Zorrilla et al., 2002). In contrast, clinical trials tested the efficacy of these drugs for treating disorders, such as depression, generalized anxiety disorder, and post-traumatic stress disorder, only in females or in both sexes with male and female data combined for analysis (Binneman et al., 2008; Coric et al., 2010; Dunlop et al., 2017; Howerton et al., 2014). Unfortunately, these clinical trials have failed (Murrough and Charney, 2017). One likely contributing factor is the faulty assumption that the effects of CRF1 antagonists in males are the same as in females. Although there is evidence that CRF1 antagonists may better treat anxiety in male rodents (Howerton et al., 2014), our results suggest that CRF1 antagonists, at least in the MS, would ameliorate certain stress-induced cognitive deficits effectively in both sexes. More research is needed, but perhaps these compounds, which may be inadequate for treating affective disorders in women, are instead a viable option for effectively treating cognitive deficits in both men and women with disorders, such as schizophrenia and ADHD, in which stress exacerbates symptoms.
Conflicts of interest
None of the authors have any conflicts of interest to declare.
Acknowledgements
We would like to thank Hanna Lefebo, Victoria Cantoral, Natalie Newcamp, and Shivam Bhakta for their technical assistance. This work was supported by the National Science Foundation [NSF CAREER IOS-1552416 to D.A.B] and the National Institutes of Health [K01 DA039308 and DP1 DA046537].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2019.100150.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Amaral D.G., Kurz J. An analysis of the origins of the cholinergic and noncholinergic septal projections to the hippocampal formation of the rat. J. Comp. Neurol. 1985;240(1):37–59. doi: 10.1002/cne.902400104. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . 5 edn. vol. 5. American Psychiatric Publishing; Washington, D.C: 2013. (Diagnostic and Statistical Manual of Mental Disorders: DSM-5). [Google Scholar]
- Bale T.L., Vale W.W. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu. Rev. Pharmacol. Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Bangasser D.A., Curtis A., Reyes B.A., Bethea T.T., Parastatidis I., Ischiropoulos H. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol. Psychiatr. 2010;15(9):896–904. doi: 10.1038/mp.2010.66. 877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D.A., Eck S.R., Telenson A.M., Salvatore M. Sex differences in stress regulation of arousal and cognition. Physiol. Behav. 2018;187:42–50. doi: 10.1016/j.physbeh.2017.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D.A., Kawasumi Y. Cognitive disruptions in stress-related psychiatric disorders: a role for corticotropin releasing factor (CRF) Horm. Behav. 2015;76:125–135. doi: 10.1016/j.yhbeh.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D.A., Lee C.S., Cook P.A., Gee J.C., Bhatnagar S., Valentino R.J. Manganese-enhanced magnetic resonance imaging (MEMRI) reveals brain circuitry involved in responding to an acute novel stress in rats with a history of repeated social stress. Physiol. Behav. 2013;122:228–236. doi: 10.1016/j.physbeh.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D.A., Shors T.J. The bed nucleus of the stria terminalis modulates learning after stress in masculinized but not cycling females. J. Neurosci. 2008;28(25):6383–6387. doi: 10.1523/JNEUROSCI.0831-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D.A., Wicks B. Sex-specific mechanisms for responding to stress. J. Neurosci. Res. 2017;95(1–2):75–82. doi: 10.1002/jnr.23812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binneman B., Feltner D., Kolluri S., Shi Y., Qiu R., Stiger T. A 6-week randomized, placebo-controlled trial of CP-316,311 (a selective CRH 1 antagonist) in the treatment of major depression. Am. J. Psychiatry. 2008;165(5):617–620. doi: 10.1176/appi.ajp.2008.07071199. [DOI] [PubMed] [Google Scholar]
- Cai L., Gibbs R.B., Johnson D.A. Recognition of novel objects and their location in rats with selective cholinergic lesion of the medial septum. Neurosci. Lett. 2012;506(2):261–265. doi: 10.1016/j.neulet.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaki S., Nakazato A., Kennis L., Nakamura M., Mackie C., Sugiura M. Anxiolytic- and antidepressant-like profile of a new CRF1 receptor antagonist, R278995/CRA0450. Eur. J. Pharmacol. 2004;485(1):145–158. doi: 10.1016/j.ejphar.2003.11.032. [DOI] [PubMed] [Google Scholar]
- Cole R.D., Kawasumi Y., Parikh V., Bangasser D.A. Corticotropin releasing factor impairs sustained attention in male and female rats. Behav. Brain Res. 2016;296:30–34. doi: 10.1016/j.bbr.2015.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coric V., Feldman H.H., Oren D.A., Shekhar A., Pultz J., Dockens R.C. Multicenter, randomized, double-blind, active comparator and placebo-controlled trial of a corticotropin-releasing factor receptor-1 antagonist in generalized anxiety disorder. Depress. Anxiety. 2010;27(5):417–425. doi: 10.1002/da.20695. [DOI] [PubMed] [Google Scholar]
- Curtis A.L., Bethea T., Valentino R.J. Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology. 2006;31(3):544–554. doi: 10.1038/sj.npp.1300875. [DOI] [PubMed] [Google Scholar]
- Day J.C., Koehl M., Le Moal M., Maccari S. Corticotropin-releasing factor Administered centrally, but not peripherally, stimulates hippocampal acetylcholine release. J. Neurochem. 1998;71(2):622–629. doi: 10.1046/j.1471-4159.1998.71020622.x. [DOI] [PubMed] [Google Scholar]
- Deak T., Nguyen K.T., Ehrlich A.L., Watkins L.R., Spencer R.L., Maier S.F. The impact of the nonpeptide corticotropin-releasing hormone antagonist antalarmin on behavioral and endocrine responses to Stress**This research was supported by NIMH grant MH-50479 and the undergraduate research opportunities program at the university of Colorado at boulder. Endocrinology. 1999;140(1):79–86. doi: 10.1210/endo.140.1.6415. [DOI] [PubMed] [Google Scholar]
- Dunlop B.W., Binder E.B., Iosifescu D., Mathew S.J., Neylan T.C., Pape J.C. Corticotropin-releasing factor receptor 1 antagonism is ineffective for women with posttraumatic stress disorder. Biol. Psychiatry. 2017;82(12):866–874. doi: 10.1016/j.biopsych.2017.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckart K., Jahn O., Radulovic J., Tezval H., van Werven L., Spiess J. A single amino acid serves as an affinity switch between the receptor and the binding protein of corticotropin-releasing factor: implications for the design of agonists and antagonists. Proc. Natl. Acad. Sci. Unit. States Am. 2001;98(20):11142. doi: 10.1073/pnas.211424998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Segura L.M., Azcoitia I., DonCarlos L.L. Neuroprotection by estradiol. Prog. Neurobiol. 2001;63(1):29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- Gershon J., Gershon J. A meta-analytic review of gender differences in ADHD. J. Atten. Disord. 2002;5(3):143–154. doi: 10.1177/108705470200500302. [DOI] [PubMed] [Google Scholar]
- Heinrichs S.C., Stenzel-Poore M.P., Gold L.H., Battenberg E., Bloom F.E., Koob G.F. Learning impairment in transgenic mice with central overexpression of corticotropin-releasing factor. Neuroscience. 1996;74(2):303–311. doi: 10.1016/0306-4522(96)00140-6. [DOI] [PubMed] [Google Scholar]
- Hirvikoski T., Lindholm T., Nordenström A., Nordström A.-L., Lajic S. High self-perceived stress and many stressors, but normal diurnal cortisol rhythm, in adults with ADHD (attention-deficit/hyperactivity disorder) Horm. Behav. 2009;55(3):418–424. doi: 10.1016/j.yhbeh.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Howerton A.R., Roland A.V., Fluharty J.M., Marshall A., Chen A., Daniels D. Sex differences in corticotropin-releasing factor receptor-1 action within the dorsal raphe nucleus in stress responsivity. Biol. Psychiatry. 2014;75(11):873–883. doi: 10.1016/j.biopsych.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki-Sekino A., Mano-Otagiri A., Ohata H., Yamauchi N., Shibasaki T. Gender differences in corticotropin and corticosterone secretion and corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychological stress in rats. Psychoneuroendocrinology. 2009;34(2):226–237. doi: 10.1016/j.psyneuen.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Kleinstreuer N.C., Ceger P.C., Allen D.G., Strickland J., Chang X., Hamm J.T. A curated database of rodent uterotrophic bioactivity. Environ. Health Perspect. 2015;124(5):556–562. doi: 10.1289/ehp.1510183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokras N., Baltas D., Theocharis F., Dalla C. Kinoscope: an open-Source computer program for behavioral pharmacologists. Front. Behav. Neurosci. 2017;11:88. doi: 10.3389/fnbeh.2017.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansbach R.S., Brooks E.N., Chen Y.L. Antidepressant-like effects of CP-154,526, a selective CRF1 receptor antagonist. Eur. J. Pharmacol. 1997;323(1):21–26. doi: 10.1016/s0014-2999(97)00025-3. [DOI] [PubMed] [Google Scholar]
- Mendrek A., Mancini-Marïe A. Sex/gender differences in the brain and cognition in schizophrenia. Neurosci. Biobehav. Rev. 2016;67:57–78. doi: 10.1016/j.neubiorev.2015.10.013. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I., Vigh S., Petrusz P., Schally A.V. Immunocytochemical localization of corticotropin-releasing factor (CRF) in the rat brain. Am. J. Anat. 1982;165(4):385–396. doi: 10.1002/aja.1001650404. [DOI] [PubMed] [Google Scholar]
- Mumby D.G., Gaskin S., Glenn M.J., Schramek T.E., Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn. Mem. 2002;9(2):49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough J.W., Charney D.S. Corticotropin-releasing factor type 1 receptor antagonists for stress-related disorders: time to call it quits? Biol. Psychiatry. 2017;82(12):858–860. doi: 10.1016/j.biopsych.2017.10.012. [DOI] [PubMed] [Google Scholar]
- Oliveira A.M.M., Hawk J.D., Abel T., Havekes R. Post-training reversible inactivation of the hippocampus enhances novel object recognition memory. Learn. Mem. 2010;17(3):155–160. doi: 10.1101/lm.1625310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens J.W., Ashby J. Critical review and evaluation of the uterotrophic bioassay for the identification of possible estrogen agonists and antagonists: in support of the validation of the OECD uterotrophic protocols for the laboratory rodent. Crit. Rev. Toxicol. 2002;32(6):445–520. doi: 10.1080/20024091064291. [DOI] [PubMed] [Google Scholar]
- Owens M.J., Nemeroff C.B. Physiology and pharmacology of corticotropin-releasing factor. Pharmacol. Rev. 1991;43(4):425–473. [PubMed] [Google Scholar]
- Pang K.C.H., Jiao X., Sinha S., Beck K.D., Servatius R.J. Damage of GABAergic neurons in the medial septum impairs spatial working memory and extinction of active avoidance: effects on proactive interference. Hippocampus. 2011;21(8):835–846. doi: 10.1002/hipo.20799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G., Watson C. sixth ed. Academic Press/Elsevier; Amsterdam ; Boston: 2007. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- Potter E., Sutton S., Donaldson C., Chen R., Perrin M., Lewis K. Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proc. Natl. Acad. Sci. U. S. A. 1994;91(19):8777–8781. doi: 10.1073/pnas.91.19.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic J., Sydow S., Spiess J. Characterization of native corticotropin-releasing factor receptor type 1 (cRFR1) in the rat and mouse central nervous system. J. Neurosci. Res. 1998;54(4):507–521. doi: 10.1002/(SICI)1097-4547(19981115)54:4<507::AID-JNR8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Sauvage M., Steckler T. Detection of corticotropin-releasing hormone receptor 1 immunoreactivity in cholinergic, dopaminergic and noradrenergic neurons of the murine basal forebrain and brainstem nuclei--potential implication for arousal and attention. Neuroscience. 2001;104(3):643–652. doi: 10.1016/s0306-4522(01)00137-3. [DOI] [PubMed] [Google Scholar]
- Schulz D.W., Mansbach R.S., Sprouse J., Braselton J.P., Collins J., Corman M. CP-154,526: a potent and selective nonpeptide antagonist of corticotropin releasing factor receptors. Proc. Natl. Acad. Sci. U. S. A. 1996;93(19):10477–10482. doi: 10.1073/pnas.93.19.10477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly R.B., Chang C., Muramatsu M., Swanson L.W. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J. Comp. Neurol. 1990;294(1):76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Snyder K.P., Hill-Smith T.E., Lucki I., Valentino R.J. Corticotropin-releasing factor in the rat dorsal raphe nucleus promotes different forms of behavioral flexibility depending on social stress history. Neuropsychopharmacology. 2015;40:2517. doi: 10.1038/npp.2015.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speert D.B., McClennen S.J., Seasholtz A.F. Sexually dimorphic expression of corticotropin-releasing hormone-binding protein in the mouse pituitary. Endocrinology. 2002;143(12):4730–4741. doi: 10.1210/en.2002-220556. [DOI] [PubMed] [Google Scholar]
- Stinnett G.S., Westphal N.J., Seasholtz A.F. Pituitary CRH-binding protein and stress in female mice. Physiol. Behav. 2015;150:16–23. doi: 10.1016/j.physbeh.2015.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van't Veer A., Yano J.M., Carroll F.I., Cohen B.M., Carlezon W.A., Jr. Corticotropin-releasing factor (CRF)-induced disruption of attention in rats is blocked by the kappa-opioid receptor antagonist JDTic. Neuropsychopharmacology. 2012;37(13):2809–2816. doi: 10.1038/npp.2012.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Stolpe A., Slycke A.J., Reinders M.O., Zomer A.W., Goodenough S., Behl C. Estrogen receptor (ER)-mediated transcriptional regulation of the human corticotropin-releasing hormone-binding protein promoter: differential effects of ERalpha and ERbeta. Mol. Endocrinol. 2004;18(12):2908–2923. doi: 10.1210/me.2003-0446. [DOI] [PubMed] [Google Scholar]
- Van Pett K., Viau V., Bittencourt J.C., Chan R.K., Li H.Y., Arias C. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J. Comp. Neurol. 2000;428(2):191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Walker E.F., Diforio D. Schizophrenia: a neural diathesis-stress model. Psychol. Rev. 1997;104(4):667. doi: 10.1037/0033-295x.104.4.667. [DOI] [PubMed] [Google Scholar]
- Zorrilla E.P., Valdez G.R., Nozulak J., Koob G.F., Markou A. Effects of antalarmin, a CRF type 1 receptor antagonist, on anxiety-like behavior and motor activation in the rat. Brain Res. 2002;952(2):188–199. doi: 10.1016/s0006-8993(02)03189-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.