Abstract
The stress response differs between women using hormonal contraception and naturally cycling women. Yet, despite ample evidence showing that the stress response differs across the menstrual cycle in naturally cycling women, limited work has investigated whether the stress response differs across the hormonal contraceptive cycle, during which synthetic hormones are taken most of the month but not all of it. To induce a stress response, women using hormonal contraception completed the cold pressor test during either the active phase, when hormones are present, or during the inactive phase, when hormones are not present. Saliva was collected and assayed for free cortisol and progesterone levels prior to stress onset, immediately after stress termination, and 15-min post stress onset. Free cortisol and progesterone increased to a similar degree across both hormonal contraceptive phases in response to the cold pressor test. Post-hoc investigation indicates that the progestin “generation” (classification of synthetic progestins based on the compounds they are derived from) can differentially affect the free steroid response to cold pressor test stress, with the largest effects observed in women using formulations containing second-generation progestins. These findings indicate that progestin generation, particularly second-generation progestins, may have a more impactful influence on the stress response than hormonal contraceptive cycle phase. Potential mechanisms driving this effect and need for additional research are discussed.
Keywords: Hormonal contraception, Stress, Cortisol, Progesterone, Progestins, Progestin generation
1. Introduction
Women using hormonal contraception (HC) exhibit smaller free cortisol responses than naturally cycling (NC) women to psychosocial stressors, physical stressors, and even athletic competitions (Crewther et al., 2015; Kirschbaum et al., 1999; Nielsen et al., 2013, 2014), despite HC and NC women showing similar diurnal cortisol rhythms (Vibarel-Rebot et al., 2015). Methodologically, however, although menstrual cycle phase is controlled and reported for NC women, studies examining differences between HC and NC women rarely report or control for position in the HC cycle. Lack of reporting or controlling whether HC women are in the active, hormone-containing phase, or inactive, no-hormone phase, of the HC cycle makes it difficult to interpret how HC is leading to reported differences. For instance, various forms of HC have been shown to increase corticosteroid binding globulin (Wiegratz et al., 2003), which could explain the reduced salivary cortisol response to stress observed with HC but would likely only be seen during the active HC phase. Yet, this is only one possibility. The synthetic estradiol, ethinyl estradiol, and synthetic progestins contained in HC affect a multitude of systems beyond the reproductive system. Further complicating matters are findings showing that the different progestins can affect these systems differently, which makes understanding the mechanisms at play substantially more difficult. One way to begin uncovering which mechanism(s) contribute to the effect of HC on the stress response is to investigate patterns across the active and inactive HC phases.
A less intensively studied portion of the hypothalamo-pituitary-adrenal (HPA) axis is the adrenal progesterone response to stress. The adrenal release of progesterone in response to stress is well documented in animal models and men (Breier and Buchanan, 1992; Brown et al., 1976; Cooper et al., 1995; Deis et al., 1989; Duncan et al., 1998; Elman and Breier, 1997; Fajer et al., 1971; Romeo et al., 2004, 2006), with more limited work investigating the response in women (Childs et al., 2010; Gaffey and Wirth, 2014; Herrera et al., 2016; Schoofs and Wolf, 2011; Wirth et al., 2011). Even more limited is work examining this portion of the HPA response in women using HC (Wirth et al., 2007), which also did not report HC cycle phase.
In this preliminary report on the effects of HC phase on free cortisol and progesterone response to stress, women were exposed to cold pressor stress during either the active or inactive HC phase. We hypothesized that both cortisol and progesterone responses would differ between the active and inactive HC phases, such that responses would be larger during the inactive phase when synthetic hormones are no longer available to impact systems which might limit adrenal steroid response to stress. In a post-hoc analysis we also tested whether the class of progestins in the HC formulations was related to the magnitude of the free cortisol and progesterone responses to stress. The classes of progestins contained in HC are referred to as generations and are typically characterized by what the progestin was derived from (Davtyan, 2012): first-generation progestins are created from estranes derived from testosterone or from pregnanes derived from 17-OH progesterone, second-generation progestins are created from gonanes derived from testosterone, third-generation progestins are created from second-generation gonane derivatives, and fourth-generation progestins are created from non-ethylated estranes or from pregnanes.
2. Materials and methods
Eighty-five participants were recruited from the University of Southern California psychology subject pool and provided written informed consent approved by the University of Southern California Institutional Review Board. Eligibility criteria included using monophasic oral HC containing 4–7 inactive days, or vaginal rings, for a minimum of 2 months. Additional eligibility criteria included being a non-smoker, having no chronic illness contraindicated for exposure to the cold pressor task, no pregnancy or nursing within the last 12 months, and no use of medications that might affect the stress response (e.g., corticosteroids, beta-blockers, and anxiolytics). See Table 1 for demographic information.
Table 1.
Demographic and mood information for all participants.
| Age (years) | |
| Mean | 19.8 |
| Range | 18–23 |
| Education (years) | |
| Mean | 13.9 |
| Range | 12–16 |
| Ethnicity (n) | |
| Non-Hispanic | 61 |
| Hispanic | 7 |
| Decline to State | 2 |
| Race (n) | |
| American Indian/Alaska Native | 0 |
| Asian | 14 |
| Pacific Islander/Native Hawaiian | 0 |
| Black or African American | 0 |
| White | 37 |
| More than one race | 14 |
| Unknown or not reported | 5 |
| Trait Anxiety | |
| Active | 41.8 |
| Inactive | 39 |
| Depression | |
| Active | 16.8 |
| Inactive | 15.3 |
| Time of year tested (n) | |
| September | 11 |
| October | 15 |
| November | 21 |
| December | 4 |
| January | 3 |
| February | 12 |
| March | 7 |
| April | 12 |
Women did not differ on scores of depression (CES-D; Radloff, 1977) or trait anxiety (STAI-Y2; Spielberger et al., 1983) between the HC phases. Women were tested during the fall and spring academic semesters.
Women were tested once during either the active or inactive phase of their HC regimen. During the sessions, women provided 3 saliva samples and completed the cold pressor test (Lovallo, 1975). The cold pressor test (CPT) involved holding their dominant hand in ice-cold water (0–3 °C) for up to 3 min and has been shown to effectively increase salivary cortisol levels over the tested time course in our (Herrera et al., 2016, 2017; Lighthall et al., 2009, 2011, 2013) and other (Buchanan et al., 2006; Cahill et al., 2003; Nielsen et al., 2013, 2014; Schoofs et al., 2009) labs. Prior to beginning the CPT, participants were instructed to keep their hand immersed up to the wrist for as long as possible up to 3 min, at which time the researcher would terminate the task. Throughout the duration of hand immersion, a researcher watched the participant while holding a stopwatch the participant was unable to see.
Salivary samples are a reliable source for determining biologically available, unbound, levels of hormones (Duplessis et al., 2010; Gozansky et al., 2005; Tunn et al., 1992; Vining et al., 1983). Participants passively drooled ∼1 mL of saliva into a collection tube for each sample. To ensure stable baseline hormone levels, sessions were conducted in the afternoons between 1200 h and 1800h, and participants were asked to refrain from food/drink within 1 h, sleep within 3 h, and caffeine, alcohol, and exercise within 24 h of their session start time. Women arrived for sessions an average of 23 min prior to CPT exposure, during which time they drank 8oz of water to clean the mouth for saliva samples (completed at least 10 min before the first saliva sample was collected) and completed questionnaires (i.e., demographics and mood measures). Saliva samples were collected prior to CPT (baseline), immediately after the hand was removed from the water (0 m post offset), and 15 min after the hand was placed in the water (15 m post onset). The 15 m-post-onset time point was selected for consistency with our previous work (Herrera et al., 2016, 2017), which aimed to have cognitive tasks performed during the peak times for salivary cortisol reactivity, between 21 and 40 min after stressor onset (Dickerson and Kemeny, 2004). All samples were processed for cortisol and progesterone using Salimetrics, LLC (State College, PA) ELISA kits and measured optically using Molecular Devices, LLC SpectraMax M3 Multi-mode Microplate Reader (Sunnyvale, CA). The inter- and intra-assay variations for cortisol (6.5%; 11.6%) and progesterone (10.4%; 16.5%) were within the expected ranges from our lab.
Fifteen women were excluded; four for using multiphasic HC, two for unknown HC day, one for not yet taking the first pill of her current pack, seven for not completing the stress task, and one for insufficient sleep the night prior (<2 h). Two baseline cortisol outliers, both in the active phase, were excluded from the cortisol analyses, and three progesterone baseline outliers, one in the inactive phase and two in the active phase, were excluded from the progesterone analyses. Due to their low enrollment (n = 4 per HC phase), women using fourth-generation formulations were also excluded from analyses. With these exclusions, cortisol analyses included 32 active HC phase women and 28 inactive HC phase women, while progesterone analyses included 30 active HC phase women and 27 inactive HC phase women (see Table 2 for HC information).
Table 2.
Breakdown of the different hormonal contraceptive formulations used by all participants.
| Progestin |
Active N |
Inactive N |
Progestin Generation | Ethinyl Estradiol Dose (mg) | Progestin Dose (mg) | Number of Inactive Pills |
|---|---|---|---|---|---|---|
| HC Formulation | 38 | 32 | ||||
| Ethynodiol Diacetate | 1 | 0 | ||||
| Zovia | 1 | 0 | 1 | 0.035 | 1.00 | 7 |
| Norethindrone Acetate | 4 | 6 | ||||
| Gildess | 0 | 2 | 1 | 0.020 | 1.00 | 7 |
| Menastrin | 0 | 1 | 1 | 0.020 | 1.00 | 4 |
| Minastrin | 1 | 0 | 1 | 0.020 | 1.00 | 4 |
| Gildess Fe 1/20 | 1 | 1 | 1 | 0.020 | 1.00 | 7 |
| Junel Fe | 0 | 2 | 1 | 0.020 | 1.00 | 7 |
| Junel Fe 1/20 | 1 | 0 | 1 | 0.020 | 1.00 | 7 |
| Microgestin Fe | 1 | 0 | 1 | 0.020 | 1.00 | 7 |
| Norethindrone | 4 | 3 | ||||
| GeneressFe | 1 | 0 | 1 | 0.025 | 0.80 | 4 |
| Microgestin | 1 | 1 | 1 | 0.030 | 1.50 | 7 |
| Nortrel | 0 | 1 | 1 | 0.035 | 0.50 | 7 |
| Microgestin Fe | 0 | 1 | 1 | 0.030 | 1.50 | 7 |
| Ortho Novum | 1 | 0 | 1 | 0.035 | 1.00 | 7 |
| Wymzya Fe | 1 | 0 | 1 | 0.035 | 0.40 | 4 |
| Levonorgestrel | 14 | 10 | ||||
| Aubra | 1 | 3 | 2 | 0.020 | 0.10 | 7 |
| Aviane | 1 | 0 | 2 | 0.020 | 0.10 | 7 |
| Chateal | 1 | 1 | 2 | 0.030 | 0.15 | 7 |
| Levora | 2 | 1 | 2 | 0.030 | 0.15 | 7 |
| Lutera | 7 | 4 | 2 | 0.020 | 0.10 | 7 |
| Portia | 1 | 0 | 2 | 0.030 | 0.15 | 7 |
| Sronyx | 0 | 1 | 2 | 0.020 | 0.10 | 7 |
| Amethia Lo | 1 | 0 | 2 | 0.020 | 0.10 | 7 |
| Norgestrel | 1 | 1 | ||||
| Elinest | 0 | 1 | 2 | 0.030 | 0.30 | 7 |
| Lo'Ovral | 1 | 0 | 2 | 0.030 | 0.30 | 7 |
| Etonorgestrel | 5 | 3 | ||||
| NuvaRing | 5 | 3 | 3 | 0.015 | 0.12 | 7 |
| Norgestimate | 5 | 5 | ||||
| Estarylla | 0 | 1 | 3 | 0.035 | 0.25 | 7 |
| Mononessa | 1 | 1 | 3 | 0.035 | 0.25 | 7 |
| Previfem | 1 | 1 | 3 | 0.035 | 0.22 | 7 |
| Sprintec | 2 | 1 | 3 | 0.035 | 0.25 | 7 |
| Ortho Cyclen | 1 | 1 | 3 | 0.035 | 0.25 | 7 |
| Drospirenone | 4 | 4 | ||||
| Gianvi | 0 | 1 | 4 | 0.020 | 3.00 | 4 |
| Loryna | 1 | 0 | 4 | 0.020 | 3.00 | 7 |
| Ocella | 1 | 0 | 4 | 0.030 | 3.00 | 7 |
| Safyral | 0 | 1 | 4 | 0.030 | 3.00 | 7 |
| Vestura | 1 | 0 | 4 | 0.020 | 3.00 | 4 |
| Yaz | 1 | 0 | 4 | 0.020 | 3.00 | 4 |
| Zarah | 0 | 1 | 4 | 0.030 | 3.00 | 7 |
| Generic Drospirenone | 0 | 1 | 4 | 0.030 | 3.00 | 7 |
N is reported for each progestin and corresponding HC formulations for the active and inactive groups. Ethinyl estradiol and progestin dosages and number of inactive pills or days are also reported.
Statistical analyses were conducted using SPSS. The baseline cortisol and progesterone outliers were identified as values more extreme than 1.5x the interquartile range using boxplots. 3 (progestin generation) x 2 (HC phase) x 3 (time) mixed-model ANOVAs were conducted to test changes in cortisol and progesterone responses between women seen during the active and inactive HC phases using different generation progestins. Bonferroni Corrected pairwise comparisons for time and progestin generation were modeled into the ANOVAs. Follow-up analyses were conducted where appropriate.
3. Results
3.1. Influence of progestin generation on cortisol response to CPT between the HC phases
Over half the women held their hand in the ice water for the full 3 min, with only eight women removing their hand before completing 1 min. There was no relationship between immersion time and salivary cortisol reactivity 15 m post onset (r = −0.086, p = .480, n = 70).
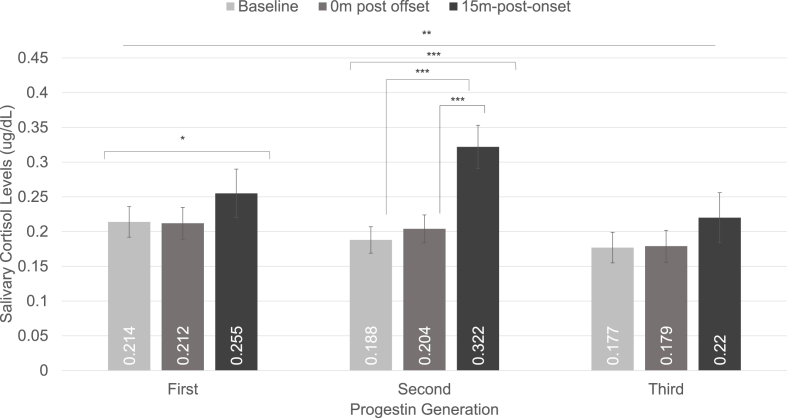
CPT increased free cortisol levels over time across generations and HC phase, F(2,108) = 20.929, p < .001, ηp2 = 0.279. There was a significant generation × time interaction, F(4,108) = 3.836, p = .006, ηp2 = 0.124, suggesting that cortisol response to CPT differed between generations 1, 2, and 3 (see Fig. 1). Follow-up analyses revealed that women using generation 1 progestins experienced significant increases in free cortisol over time, F(2,34) = 4.722, p = .016, ηp2 = 0.217, although pairwise comparisons revealed this was only driven by marginally higher free cortisol levels 15 m post onset compared with 0 m post offset, p = .072, 95% CI = −0.003, 0.088. Women using generation 2 progestins also showed significant increases in free cortisol over time, F(2,46) = 17.255, p <. 001, ηp2 = 0.429, driven by significantly higher free cortisol levels at 15 m post onset than at baseline, p = .001, 95% CI = 0.053, 0.210, or at 0 m post offset, p = .001, 95% CI = 0.042, 0.187. Women using generation 3 progestins only exhibited marginally significant changes across time, F(2,34) = 2.735, p = .079, ηp2 = 0.139, with no differences in pairwise comparisons between baseline, 0 m post offset, and 15 m post onset (see Fig. 1).
Fig. 1.
Salivary cortisol response to cold pressor stress within different progestin generations. The two-way time × generation interaction was significant. Women using formulations containing first-generation progestins showed a main effect of time, but pairwise comparisons revealed no differences between specific timepoints for the first-generation progestins. Women using formulations containing second-generation progestins showed significant increases in free cortisol levels at the 15 m-post-onset time point compared to the baseline and 0 m-post-offset time points. Women using formulations containing third-generation progestins showed no change in cortisol. *p < .05, **p ≤ .01, ***p ≤ .001.
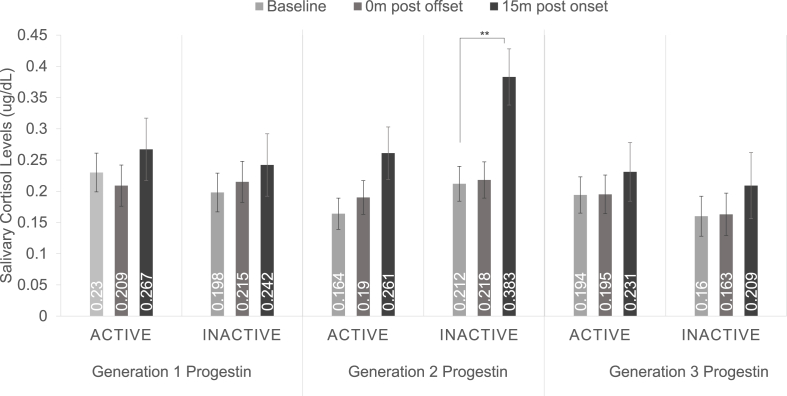
Pairwise comparisons indicate the larger free cortisol response in women using second-generation progestins was driven by the free cortisol response during the inactive HC phase. Women using second-generation progestins and seen during the inactive phase showed a significant increase in free cortisol levels 15 m post onset compared with baseline, p = .003, 95% CI = 0.056, 0.286, while women seen during the active phase did not, p = .079, 95% CI = −0.009, 0.203 (see Fig. 2). The three-way generation x HC phase × time interaction was not significant, F(4,108) = 1.223, p = .305, ηp2 = 0.043.
Fig. 2.
Salivary cortisol response to cold pressor stress during the active and inactive hormonal contraceptive phases within different progestin generations. Pairwise comparisons indicate women using second-generation progestins showed a significant increase in free cortisol levels at the 15 m-post-onset time point compared with baseline during the inactive phase, while women seen during the active phase did not. The three-way phase x time × generation interaction was not significant. **p ≤ .01.
3.2. Influence of progestin generation on progesterone response to CPT between the HC phases
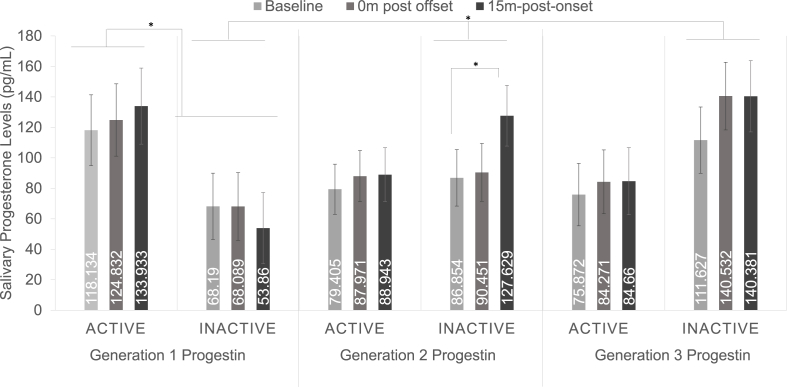
CPT increased free progesterone levels over time across generations and HC phase, F(2,102) = 3.847, p = .025, ηp2 = 0.070. There was a significant HC phase × generation interaction, F(2,51) = 3.706, p = .031, ηp2 = 0.127, driven by higher progesterone levels during the active phase in women using generation 1 progestins, p = .047, 95% CI = 0.779, 123.728 (see Fig. 3). The three-way generation x phase × time interaction was not quite significant, F(4,102) = 2.102, p = .086, ηp2 = 0.076. Follow-up analyses of the active group revealed no effect of time, generation, or interactions with progesterone response. In contrast, in the inactive phase group there was a significant time × generation interaction, F(4,48) = 3.300, p = .018, ηp2 = 0.216, suggesting that progestin generation differentially affected progesterone responses to CPT during the inactive phase only. Additional follow-up analyses of the inactive HC phase group revealed that women using either generation 1 progestins, F(2,14) = 0.902, p = .428, ηp2 = 0.114, or generation 3 progestins, F(2,14) = 2.997, p = .083, ηp2 = 0.300, did not experience significant increases in progesterone over time, whereas women using generation 2 progestins did show significant increases in progesterone, F(2,20) = 5.435, p = .013, ηp2 = 0.352, driven by significantly higher progesterone levels 15 m post onset compared with baseline, p = .036, 95% CI = 2.614, 78.908 (see Fig. 3).
Fig. 3.
Salivary progesterone response to cold pressor stress during the active and inactive hormonal contraceptive phases within different progestin generations. The three-way phase x time × generation interaction was not significant. However, there was a two-way phase × generation interaction. Women using formulations containing first-generation progestins showed significantly lower overall progesterone levels during the inactive phase. There also was significant time × generation interaction when looking only at the inactive phase where only women using formulations containing second-generation progestins showed a significant progesterone response to stress. This was not observed in women using formulations containing first- or third-generation progestins. *p < .05.
4. Discussion
We examined whether free cortisol and free progesterone responses to cold pressor stress differed in women during the active and inactive HC phases. We found that although free cortisol response to stress did not systematically differ between phases, there was an effect of progestin generation. Women whose HC formulation contained second-generation progestins showed more robust free cortisol responses 15 m post-stress onset than women using formulations containing first- or third-generation progestins. Pairwise comparisons suggest the larger free cortisol response in women using second-generation progestins are driven by larger responses during the inactive HC phase than the active HC phase. Unlike the free cortisol response, we did find a significant effect of HC phase on CPT-induced progesterone response, with women experiencing a progesterone response during the inactive HC phase only. Interestingly, this effect was also limited to women using HC formulations containing second-generation progestins.
These progesterone response findings extend previous work suggesting that progesterone is released from the adrenal gland in addition to the ovaries in women (Wirth et al., 2007) and findings that CPT increases progesterone in naturally cycling women (Herrera et al., 2016). Although progesterone is a known portion of the HPA axis response (Cooper et al., 1995; Elman and Breier, 1997; Gaffey and Wirth, 2014; Romeo et al., 2004; Wirth et al., 2011), it is more difficult to ascertain whether stress-induced progesterone increases are of an adrenal or ovarian source in naturally cycling women (Wirth et al., 2007). Finding a progesterone response during the active HC phase would be a clear indicator of adrenal progesterone release since HC inhibits ovarian production of progesterone by preventing ovulation (Lobo and Stanczyk, 1994). However, our current results indicate that the progesterone response is observed during the inactive HC phase only, and only within users of second-generation progestins. The overall pattern suggests that the same or similar mechanisms of HC use which limit the free cortisol response to stress also limit stress-induced progesterone release.
Why free cortisol and progesterone responses differ as a function of progestin generation is difficult to elucidate as the synthetic hormones contained in HC affect so many systems, further complicated by progestins within a generation often times exerting different effects across systems (for reviews see, Benagiano et al., 2004; Sitruk-Ware and Nath, 2010, 2011, 2013). Since the effects of progestin generation on cortisol and progesterone response appear limited to women using second-generation progestins, it is likely that the mechanisms driving the effects are similar. These effects include, but are not limited to, the upregulation of the corticosteroid binding globulin (Wiegratz et al., 2003), sex hormone binding globulin (Gaspard et al., 1983; Rad et al., 2006; Van der Vange et al., 1990), and altering both high-density and low-density lipoprotein cholesterol levels (Bergink et al., 1982; Gaspard et al., 1985; Godsland et al., 1990; Knopp et al., 2001; van Rooijen et al., 2002; Wynn and Niththyananthan, 1982).
4.1. Corticosteroid binding globulin levels
A likely candidate mechanism for the smaller stress-induced free cortisol responses observed in prior research when comparing HC women to NC women (Crewther et al., 2015; Kirschbaum et al., 1999; Nielsen et al., 2013, 2014) is the upregulating effect of the estradiol component of HC on corticosteroid binding globulin levels (Wiegratz et al., 2003). Although to different degrees, the progestins are anti-estrogenic (for review see, Sitruk-Ware, 2004), leading to the possibility that second-generation progestins exert a greater anti-estrogenic effect on corticosteroid binding globulin upregulation and blunt this effect relative to first- and third-generation progestins. Lesser upregulation of corticosteroid binding globulin relative to the other progestin generations would result in a greater level of free, unbound, cortisol. However, levonorgestrel, the most used second-generation progestin in our sample, does not appear to block or lessen the estrogen-induced increase in corticosteroid binding globulin, with some even reporting greater corticosteroid binding globulin upregulation in formulations using levonorgestrel (Ågren et al., 2011; Endrikat et al., 2002; Hammond et al., 1984; Kivelä et al., 2001; Ruokonen and Käär, 1985; Spona et al., 1996). As such this does not appear to be a differentiating mechanism between progestin generations.
4.2. Competitive binding to corticosteroid binding globulin
Progesterone binds to corticosteroid binding globulin with some affinity (Dunn et al., 1981). Coupled with evidence that progestins contained in HC promiscuously bind to other receptor complexes, such as glucocorticoid receptors (for reviews see, Schindler et al., 2003; Sitruk-Ware, 2004, 2006; Sitruk-Ware and Nath, 2010, 2011, 2013), perhaps progestins contained in HC also have some binding affinity to corticosteroid binding globulin. This could account for the observed pattern of results without affecting the estrogen-related upregulation of corticosteroid binding globulin. This would require that second-generation progestins display a relatively greater affinity for corticosteroid binding globulin than first- and third-generation progestins, comparatively displacing the amount of cortisol able to bind to the plasma protein, leading to higher levels of free cortisol. However, studies have shown that despite the varying affinity to other receptor complexes such as androgen, glucocorticoid, and mineralocorticoid receptors, synthetic progestins preferentially bind to sex hormone binding globulin and do not bind to corticosteroid binding globulin is any significant manner (Endrikat et al., 2002; Hammond et al., 1984; Kuhnz et al., 1994; Schindler et al., 2003).
4.3. Androgens and androgenicity
Androgen levels are negatively correlated with glucocorticoid responses to stress. In rodent models, orchiectomy, or removal of the testes, is associated with larger corticosterone responses to multiple stressors, an effect often reversed with androgen replacement (for reviews see, Goel et al., 2014; Handa and Weiser, 2014). Yet, despite HC-induced decreases in free androgen levels (Vibarel-Rebot et al., 2015), studies consistently find that HC users show smaller free cortisol responses than non-users (Crewther et al., 2015; Kirschbaum et al., 1999; Nielsen et al., 2013, 2014), suggesting the reductions in androgen levels are not a primary contributor to the HC-related effect on cortisol response.
Although HC inhibits the endogenous production and release of androgens, the synthetic progestins contained in HC differentially bind to and activate androgen receptors (for reviews see, Schindler et al., 2003; Sitruk-Ware, 2004, 2006; Sitruk-Ware and Nath, 2010, 2011, 2013). The varying androgenicity of the progestins may contribute to the differences in free cortisol response to stressors across progestin generation, with more androgenic progestins leading to blunted cortisol responses to stress. However, levonorgestrel, the most prevalently used second-generation progestin in this study, is highly androgenic (Kaplan, 1995; Lemus et al., 1992; McGuire et al., 1990; Schindler et al., 2003; Sitruk-Ware, 2004; Sitruk-Ware and Nath, 2011; Van der Vange et al., 1990). Thus, despite the negative relationship between androgens and the steroid response to stress, the androgenicity of progestins does not appear to impart a similar effect on the stress steroid response.
4.4. Androgen metabolites
The presumed mechanism of action for androgen-related blunting of the steroid response to stress (for reviews see, Goel et al., 2014; Handa and Weiser, 2014), would be direct action of androgens on androgen receptor complexes. Thus, it would follow that progestins that bind to and activate the androgen receptor would also be associated with relatively smaller cortisol responses. However, research suggests it is not activation of the androgen receptor complex that directly inhibits the stress response. For instance, flutamide, a classic androgen receptor antagonist (Nguyen et al., 2007), failed to fully block the dihydrotestosterone (DHT)-related dampening of HPA axis reactivity (Lund et al., 2004, 2006). DHT is a potent androgen that is converted from testosterone by the 5-alpha reductase enzyme. Thus, finding that an androgen receptor antagonist does not block the ability of DHT to dampen the HPA response suggests that classic androgen receptor is not involved in quieting the stress response. Rather, evidence suggests the primary mechanism for androgen-related quiescence of the HPA response is through androgen metabolites, particularly the DHT metabolite 5α-androstan-3β,17β-diol (3β-diol), via action on the estrogen receptor β (Lund et al., 2006).
Reports that androgen metabolites and/or androgen action via estrogen receptor β suggests that the androgenicity of a progestin would not contribute to the relative magnitude of the stress steroid response. Importantly, if the mechanism of action for androgen-related quiescence of HPA reactivity is related to androgen metabolites, then the affinity of the progestin for the androgen receptor is irrelevant as these synthetic hormones will not be converted to androgen metabolites.
4.5. 17β-estradiol and estrogenicity
HC use results in very low estradiol levels (for review see, Rivera et al., 1999). Based on work showing post-menopausal women randomly assigned to 17β-estradiol showed blunted cortisol responses to CPT stress compared with women receiving placebo (Herrera et al., 2017), HC-related blunting of estradiol levels should be associated with greater free cortisol response to stressors. Yet, that is not the case (Crewther et al., 2015; Kirschbaum et al., 1999; Nielsen et al., 2013, 2014).
According to the above-reviewed work suggesting that the androgen-related blunting of the HPA axis is a function of androgen metabolite activation of the estrogen receptor β (Lund et al., 2006), it may be that the relative estrogenicity of HC is affecting the stress steroid response. If some progestins exert their anti-estrogenic effects (for review see, Sitruk-Ware, 2004) by having antagonistic effects on the estrogen receptor β, this may contribute to the larger relative free cortisol responses to stress in second-generation progestins such as levonorgestrel, which some have reported to be highly anti-estrogenic (Gaspard et al., 1983; Schindler et al., 2003). However, this theoretical assertion can only address the interaction between time and generation on free cortisol response, not the findings from follow-up analyses showing the cortisol response was larger during the inactive phase only.
4.6. Cholesterol levels
Cholesterol is the precursor to sex and stress steroids. Levels of high-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol are reportedly affected by HC, although the pattern of effects are mixed (Bergink et al., 1982; Bradley et al., 1978; Gaspard et al., 1985; Godsland et al., 1990; Kaupplnen-Makelln et al., 1992; Knopp et al., 2001; Tikkanen et al., 1982; van Rooijen et al., 2002; Wahl et al., 1983; Wynn et al., 1969; Wynn and Niththyananthan, 1982). Importantly, LDL and HDL cholesterol can lead to increased cortisol levels in a dose-dependent manner in vitro and in vivo (Hammami et al., 1986; Liu et al., 2000; Terao et al., 2000; Yaguchi et al., 1998). Additionally, smaller cortisol responses are reportedly related to human patient populations with fewer LDL receptors (Illingworth et al., 1982, 1983).
This may be one potential mechanism for greater cortisol response in users of second-generation progestins, although results are mixed. Some reports indicate that levonorgestrel increases LDL levels (Kiley and Hammond, 2007; Knopp et al., 2001; Sitruk-Ware and Nath, 2011, 2013) and decreases HDL (Bergink et al., 1982; Gaspard et al., 1985; Godsland et al., 1990; Kaupplnen-Makelln et al., 1992; Kiley and Hammond, 2007; McGuire et al., 1990; Ruokonen and Käär, 1985; Sitruk-Ware and Nath, 2013; Tikkanen et al., 1982; Wynn and Niththyananthan, 1982), while other reports indicate deceases in LDL (Gaspard et al., 1985; Shaaban et al., 1984), or no effect on HDL, LDL, or both (Godsland et al., 1990; Kaupplnen-Makelln et al., 1992; Knopp et al., 2001; Shaaban et al., 1984; van Rooijen et al., 2002; Wynn and Niththyananthan, 1982). These mixed effects appear to be, in part, a result of progestin dosage, whether administered alone or in combination with ethinyl estradiol, or duration of use. Further complicating this matter are additional mixed results regarding whether LDL or HDL cholesterol levels and/or receptors are the primary contributor to corticosteroidogenesis (Bochem et al., 2013; for review see, Gwynne and Strauss III, 1982; Hammami et al., 1986; Illingworth et al., 1982; Illingworth et al., 1983; Liu et al., 2000).
4.7. Conclusions
Discussed herein are six possible mechanisms for our observed pattern, none of which offers a straightforward account for why second-generation containing HC formulations are associated with a relatively larger free cortisol response to cold pressor stress. Other possible mechanisms may also drive this effect including, but not limited to, the imbalance between endogenous hormone production and levels and receptor activation; progestins exerting differential effects higher up in the HPA axis, such as the hypothalamus (i.e., corticotropin releasing factor) or the pituitary (i.e., adrenocorticotropin); differential effects on the endocannabinoid system, which is also involved in suppressing corticotropin releasing hormone neurons and terminating the HPA response (Evanson et al., 2010; for review see, Hill and Tasker, 2012) and interacts with sex steroids and the hypothalamo-pituitary-gonadal axis (for review see, Gorzalka and Dang, 2012); and differential binding to mineralocorticoid receptors, which exhibit a high affinity for glucocorticoids and are theorized to regulate both the basal and stress-induced levels of glucocorticoids (Rozeboom et al., 2007; van Haarst et al., 1997).
Our study was a preliminary investigation into how the adrenal response to stressors varies across the hormonal contraceptive cycle. Although our hypotheses did not require inclusion of these factors, the study would have been strengthened by including a naturally cycling group and a no-stress condition, as well as including additional extended time points for salivary cortisol testing. An important next step is to replicate the observed progestin generation pattern while potentially also including for comparison a naturally cycling group and/or a no-stress control condition. Inclusion of additional time points for salivary cortisol analysis would also be useful to see if the time course of cortisol reactivity differs across the HC phase. For instance, women might return to baseline levels faster during one phase over the other, or have different delays in reaching peak cortisol levels. Though the 15 m-post-onset time point is commonly used in studies assessing reactivity to CPT (Buchanan et al., 2006; Herrera et al., 2016, 2017; Nielsen et al., 2013, 2014), and cortisol does not increase significantly from 15 to 30 min (Buchanan et al., 2006) nor from 15 to 42 min post onset (Herrera et al., 2016), finding differences with additional later time points is plausible given work showing not only a delay between plasma and salivary cortisol levels but also peak levels occurring approximately 40 min after stress exposure (Duplessis et al., 2010; Gozansky et al., 2005). We also did not record what time of day participants typically took their oral contraception. Given that different progestins are metabolized at different rates and can reach peak serum levels at different times (for review see, Benagiano et al., 2004), this information would have been useful in further investigating these generational effects. Another aim for future research on this topic will be a priori inclusion of progestin generation, which will help address issues related to power that the post-hoc nature of our analyses cannot address. Despite these limitations, this preliminary work highlights the need to better understand how hormonal contraception is affecting women beyond the reproductive, cardiovascular, and lipid outcomes typically measured. Furthermore, as evident in our discussion of possible mechanisms, this approach will need to be multifaceted and translational, with the use of animal models to help determine the physiological and behavioral phenotypes observed in women.
Declarations of interest
None.
Funding
National Institute on Aging, United States (grant numbers R01AG038043 and R01AG025340).
Acknowledgements
This work was supported by the National Institute on Aging, United States (grant numbers R01AG038043 and R01AG025340).
References
- Ågren U.M., Anttila M., Mäenpää-Liukko K., Rantala M.-L., Rautiainen H., Sommer W.F., Mommers E. Effects of a monophasic combined oral contraceptive containing nomegestrol acetate and 17β-oestradiol compared with one containing levonorgestrel and ethinylestradiol on haemostasis, lipids and carbohydrate metabolism. Eur. J. Contracept. Reprod. Health Care. 2011;16:444–457. doi: 10.3109/13625187.2011.604450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benagiano G., Primiero F., Farris M. Clinical profile of contraceptive progestins. Eur. J. Contracept. Reprod. Health Care. 2004;9:182–193. doi: 10.1080/13625180400007736. [DOI] [PubMed] [Google Scholar]
- Bergink E., Borglin N., Klottrup P., Liukko P. Effects of desogestrel and levonorgestrel in low-dose oestrogen oral contraceptives on serum lipoproteins. Contraception. 1982;25:477–485. doi: 10.1016/0010-7824(82)90037-3. [DOI] [PubMed] [Google Scholar]
- Bochem A.E., Holleboom A.G., Romijn J.A., Hoekstra M., Dallinga-Thie G.M., Motazacker M.M., Hovingh G.K., Kuivenhoven J.A., Stroes E.S. High-density lipoprotein as a source of cholesterol for adrenal steroidogenesis; a study in individuals with low plasma HDL-C. J. Lipid Res. 2013;54:1698–1704. doi: 10.1194/jlr.P033449. jlr. P033449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D.D., Wingerd J., Petitti D.B., Krauss R.M., Ramcharan S. Serum high-density-lipoprotein cholesterol in women using oral contraceptives, estrogens and progestins. N. Engl. J. Med. 1978;299:17–20. doi: 10.1056/NEJM197807062990104. [DOI] [PubMed] [Google Scholar]
- Breier A., Buchanan R.W. The effects of metabolic stress on plasma progesterone in healthy volunteers and schizophrenic patients. Life Sci. 1992;51:1527–1534. doi: 10.1016/0024-3205(92)90563-5. [DOI] [PubMed] [Google Scholar]
- Brown G., Courtney G., Marotta S. Progesterone secretion by adrenal glands of hamsters and comparison of ACTH influence in rats and hamsters. Steroids. 1976;28:275–282. doi: 10.1016/0039-128x(76)90115-x. [DOI] [PubMed] [Google Scholar]
- Buchanan T.W., Tranel D., Adolphs R. Impaired memory retrieval correlates with individual differences in cortisol response but not autonomic response. Learn. Mem. 2006;13:382–387. doi: 10.1101/lm.206306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L., Gorski L., Le K. Enhanced human memory consolidation with post-learning stress: interaction with the degree of arousal at encoding. Learn. Mem. 2003;10:270–274. doi: 10.1101/lm.62403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs E., Dlugos A., De Wit H. Cardiovascular, hormonal, and emotional responses to the TSST in relation to sex and menstrual cycle phase. Psychophysiology. 2010;47:550–559. doi: 10.1111/j.1469-8986.2009.00961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper C., Evans A., Cook S., Rawlings N. Cortisol, progesterone and β-endorphin response to stress in calves. Can. J. Anim. Sci. 1995;75:197–201. [Google Scholar]
- Crewther B.T., Hamilton D., Casto K., Kilduff L.P., Cook C.J. Effects of oral contraceptive use on the salivary testosterone and cortisol responses to training sessions and competitions in elite women athletes. Physiol. Behav. 2015;147:84–90. doi: 10.1016/j.physbeh.2015.04.017. [DOI] [PubMed] [Google Scholar]
- Davtyan C. Four generations of progestins in oral contraceptives. Proc. Ucla Healthcare. 2012;16:1–3. [Google Scholar]
- Deis R., Leguizamon E., Jahn G. Feedback regulation by progesterone of stress-induced prolactin release in rats. J. Endocrinol. 1989;120:37–43. doi: 10.1677/joe.0.1200037. [DOI] [PubMed] [Google Scholar]
- Dickerson S.S., Kemeny M.E. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol. Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Duncan G.E., Knapp D.J., Carson S.W., Breese G.R. Differential effects of chronic antidepressant treatment on swim stress-and fluoxetine-induced secretion of corticosterone and progesterone. J. Pharmacol. Exp. Ther. 1998;285:579–587. [PMC free article] [PubMed] [Google Scholar]
- Dunn J.F., Nisula B.C., Rodbard D. Transport of steroid hormones: binding of 21 endogenous steroids to both testosterone-binding globulin and corticosteroid-binding globulin in human plasma. J. Clin. Endocrinol. Metab. 1981;53:58–68. doi: 10.1210/jcem-53-1-58. [DOI] [PubMed] [Google Scholar]
- Duplessis C., Rascona D., Cullum M., Yeung E. Salivary and free serum cortisol evaluation. Mil. Med. 2010;175:340–346. doi: 10.7205/milmed-d-09-00166. [DOI] [PubMed] [Google Scholar]
- Elman I., Breier A. Effects of acute metabolic stress on plasma progesterone and testosterone in male subjects: relationship to pituitary-adrenocortical axis activation. Life Sci. 1997;61:1705–1712. doi: 10.1016/s0024-3205(97)00776-5. [DOI] [PubMed] [Google Scholar]
- Endrikat J., Blode H., Gerlinger C., Rosenbaum P., Kuhnz W. A pharmacokinetic study with a low-dose oral contraceptive containing 20 μg ethinylestradiol plus 100 μg levonorgestrel. Eur. J. Contracept. Reprod. Health Care. 2002;7:79–90. [PubMed] [Google Scholar]
- Evanson N.K., Tasker J.G., Hill M.N., Hillard C.J., Herman J.P. Fast feedback inhibition of the HPA axis by glucocorticoids is mediated by endocannabinoid signaling. Endocrinology. 2010;151:4811–4819. doi: 10.1210/en.2010-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajer A., Holzbauer M., Newport H.M. The contribution of the adrenal gland to the total amount of progesterone produced in the female rat. J. Physiol. 1971;214:115–126. doi: 10.1113/jphysiol.1971.sp009422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffey A.E., Wirth M.M. Stress, rejection, and hormones: cortisol and progesterone reactivity to laboratory speech and rejection tasks in women and men. F1000 Research. 2014;3 doi: 10.12688/f1000research.5142.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspard U., Romus M., Gillain D., Duvivier J., Demey-Ponsart E., Franchimont P. Plasma hormone levels in women receiving new oral contraceptives containing ethinyl estradiol plus levonorgestrel or desogestrel. Contraception. 1983;27:577–590. doi: 10.1016/0010-7824(83)90023-9. [DOI] [PubMed] [Google Scholar]
- Gaspard U., Buret J., Gillain D., Romus M., Lambotte R. Serum lipid and lipoprotein changes induced by new oral contraceptives containing ethinylestradiol plus levonorgestrel or desogestrel. Contraception. 1985;31:395–408. doi: 10.1016/0010-7824(85)90006-x. [DOI] [PubMed] [Google Scholar]
- Godsland I.F., Crook D., Simpson R., Proudler T., Felton C., Lees B., Anyaoku V., Devenport M., Wynn V. The effects of different formulations of oral contraceptive agents on lipid and carbohydrate metabolism. N. Engl. J. Med. 1990;323:1375–1381. doi: 10.1056/NEJM199011153232003. [DOI] [PubMed] [Google Scholar]
- Goel N., Workman J.L., Lee T.T., Innala L., Viau V. Sex differences in the HPA Axis. In: Terjung R., editor. Comprehensive Physiology. 2014. pp. 1121–1155. [DOI] [PubMed] [Google Scholar]
- Gorzalka B.B., Dang S.S. Minireview: endocannabinoids and gonadal hormones: bidirectional interactions in physiology and behavior. Endocrinology. 2012;153:1016–1024. doi: 10.1210/en.2011-1643. [DOI] [PubMed] [Google Scholar]
- Gozansky W., Lynn J., Laudenslager M., Kohrt W. Salivary cortisol determined by enzyme immunoassay is preferable to serum total cortisol for assessment of dynamic hypothalamic–pituitary–adrenal axis activity. Clin. Endocrinol. 2005;63:336–341. doi: 10.1111/j.1365-2265.2005.02349.x. [DOI] [PubMed] [Google Scholar]
- Gwynne J.T., Strauss III J.F. The role of lipoproteins in steroidogenesis and cholesterol metabolism in steroidogenic glands. Endocr. Rev. 1982;3:299–329. doi: 10.1210/edrv-3-3-299. [DOI] [PubMed] [Google Scholar]
- Hammami M., Legendre C., Maume B.F. Induction of corticosteroid biosynthetic pathway by ACTH and high-density lipoprotein in newborn rat adrenocortical cells cultured in serum-free medium. Biochim. Biophys. Acta Mol. Cell Res. 1986;886:457–467. doi: 10.1016/0167-4889(86)90182-5. [DOI] [PubMed] [Google Scholar]
- Hammond G.L., Langley M.S., Robinson P.A., Nummi S., Lund L. Serum steroid binding protein concentrations, distribution of progestogens, and bioavailability of testosterone during treatment with contraceptives containing desogestrel or levonorgestrel. Fertil. Steril. 1984;42:44–51. doi: 10.1016/s0015-0282(16)47956-2. [DOI] [PubMed] [Google Scholar]
- Handa R.J., Weiser M.J. Gonadal steroid hormones and the hypothalamo–pituitary–adrenal axis. Front. Neuroendocrinol. 2014;35:197–220. doi: 10.1016/j.yfrne.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera A.Y., Nielsen S.E., Mather M. Stress-induced increases in progesterone and cortisol in naturally cycling women. Neurobiol. Stress. 2016;3:96–104. doi: 10.1016/j.ynstr.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera A.Y., Hodis H.N., Mack W.J., Mather M. Estradiol therapy after menopause mitigates effects of stress on cortisol and working memory. J. Clin. Endocrinol. Metab. 2017;102:4457–4466. doi: 10.1210/jc.2017-00825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M., Tasker J. Endocannabinoid signaling, glucocorticoid-mediated negative feedback, and regulation of the hypothalamic-pituitary-adrenal axis. Neuroscience. 2012;204:5–16. doi: 10.1016/j.neuroscience.2011.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illingworth D.R., Kenny T.A., Connor W.E., Orwoll E.S. Corticosteroid production in abetalipoproteinemia: evidence for an impaired response to ACTH. J. Lab. Clin. Med. 1982;100:115–126. [PubMed] [Google Scholar]
- Illingworth D.R., Lees A.M., Lees R.S. Adrenal cortical function in homozygous familial hypercholesterolemia. Metab. Clin. Exp. 1983;32:1045–1052. doi: 10.1016/0026-0495(83)90075-6. [DOI] [PubMed] [Google Scholar]
- Kaplan B. Desogestrel, norgestimate, and gestodene: the newer progestins. Ann. Pharmacother. 1995;29:736–742. doi: 10.1177/106002809502907-817. [DOI] [PubMed] [Google Scholar]
- Kaupplnen-Makelln R., Kuusl T., Ylikorkala O., Tlkkanen M.J. Contraceptives containing desogestrel or levonorgestrel have different effects on serum lipoproteins and post‐heparin plasma lipase activities. Clin. Endocrinol. 1992;36:203–209. doi: 10.1111/j.1365-2265.1992.tb00959.x. [DOI] [PubMed] [Google Scholar]
- Kiley J., Hammond C. Combined oral contraceptives: a comprehensive review. Clin. Obstet. Gynecol. 2007;50:868–877. doi: 10.1097/GRF.0b013e318159c06a. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C., Kudielka B., Gaab J., Schommer N.C., Hellhammer D.H. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom. Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kivelä A., Ruuskanen M., Ågren U., Dieben T. The effects of two progestogen-only pills containing either desogestrel (75 μg/day) or levonorgestrel (30 μg/day) on carbohydrate metabolism and adrenal and thyroid function. Eur. J. Contracept. Reprod. Health Care. 2001;6:71–77. [PubMed] [Google Scholar]
- Knopp R.H., Broyles F.E., Cheung M., Moore K., Marcovina S., Chandler W.L. Comparison of the lipoprotein, carbohydrate, and hemostatic effects of phasic oral contraceptives containing desogestrel or levonorgestrel. Contraception. 2001;63:1–11. doi: 10.1016/s0010-7824(00)00196-7. [DOI] [PubMed] [Google Scholar]
- Kuhnz W., Staks T., Jütting G. Pharmacokinetics of levonorgestrel and ethinylestradiol in 14 women during three months of treatment with a tri-step combination oral contraceptive: serum protein binding of levonorgestrel and influence of treatment on free and total testosterone levels in the serum. Contraception. 1994;50:563–579. doi: 10.1016/0010-7824(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Lemus A.E., Vilchis F., Damsky R., Chávez B.A., García G.A., Grillasca I., Pérez-Palacios G. Mechanism of action of levonorgestrel: in vitro metabolism and specific interactions with steroid receptors in target organs. J. Steroid Biochem. Mol. Biol. 1992;41:881–890. doi: 10.1016/0960-0760(92)90442-l. [DOI] [PubMed] [Google Scholar]
- Lighthall N.R., Mather M., Gorlick M.A. Acute stress increases sex differences in risk seeking in the balloon analogue risk task. PLoS One. 2009;4 doi: 10.1371/journal.pone.0006002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighthall N.R., Sakaki M., Vasunilashorn S., Nga L., Somayajula S., Chen E.Y., Samii N., Mather M. Gender differences in reward-related decision processing under stress. Soc. Cognit. Affect Neurosci. 2011;7:476–484. doi: 10.1093/scan/nsr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighthall N.R., Gorlick M.A., Schoeke A., Frank M.J., Mather M. Stress modulates reinforcement learning in younger and older adults. Psychol. Aging. 2013;28:35. doi: 10.1037/a0029823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Heikkila P., Meng Q.-H., Kahri A.I., Tikkanen M.J., Voutilainen R. Expression of low and high density lipoprotein receptor genes in human adrenals. Eur. J. Endocrinol. 2000;142:677–682. doi: 10.1530/eje.0.1420677. [DOI] [PubMed] [Google Scholar]
- Lobo R.A., Stanczyk F.Z. New knowledge in the physiology of hormonal contraceptives. Am. J. Obstet. Gynecol. 1994;170:1499–1507. doi: 10.1016/s0002-9378(94)05011-8. [DOI] [PubMed] [Google Scholar]
- Lovallo W. The cold pressor test and autonomic function: a review and integration. Psychophysiology. 1975;12:268–282. doi: 10.1111/j.1469-8986.1975.tb01289.x. [DOI] [PubMed] [Google Scholar]
- Lund T.D., Munson D.J., Haldy M.E., Handa R.J. Dihydrotestosterone may inhibit hypothalamo–pituitary–adrenal activity by acting through estrogen receptor in the male mouse. Neurosci. Lett. 2004;365:43–47. doi: 10.1016/j.neulet.2004.04.035. [DOI] [PubMed] [Google Scholar]
- Lund T.D., Hinds L.R., Handa R.J. The androgen 5α-dihydrotestosterone and its metabolite 5α-androstan-3β, 17β-diol inhibit the hypothalamo–pituitary–adrenal response to stress by acting through estrogen receptor β-expressing neurons in the hypothalamus. J. Neurosci. 2006;26:1448–1456. doi: 10.1523/JNEUROSCI.3777-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire J.L., Phillips A., Tolman E.L., Flor S., Kafrissen M.E. Pharmacologic and pharmacokinetic characteristics of norgestimate and its metabolites. Am. J. Obstet. Gynecol. 1990;163:2127–2131. doi: 10.1016/0002-9378(90)90552-i. [DOI] [PubMed] [Google Scholar]
- Nguyen T.-V.V., Yao M., Pike C.J. Flutamide and cyproterone acetate exert agonist effects: induction of androgen receptor-dependent neuroprotection. Endocrinology. 2007;148:2936–2943. doi: 10.1210/en.2006-1469. [DOI] [PubMed] [Google Scholar]
- Nielsen S.E., Segal S.K., Worden I.V., Yim I.S., Cahill L. Hormonal contraception use alters stress responses and emotional memory. Biol. Psychol. 2013;92:257–266. doi: 10.1016/j.biopsycho.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S.E., Ahmed I., Cahill L. Postlearning stress differentially affects memory for emotional gist and detail in naturally cycling women and women on hormonal contraceptives. Behav. Neurosci. 2014;128:482–493. doi: 10.1037/a0036687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rad M., Kluft C., Ménard J., Burggraaf J., de Kam M.L., Meijer P., Sivin I., Sitruk-Ware R.L. Comparative effects of a contraceptive vaginal ring delivering a nonandrogenic progestin and continuous ethinyl estradiol and a combined oral contraceptive containing levonorgestrel on hemostasis variables. Am. J. Obstet. Gynecol. 2006;195:72–77. doi: 10.1016/j.ajog.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Radloff L.S. The CES-D scale: a self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977;1:385–401. [Google Scholar]
- Rivera R., Yacobson I., Grimes D. The mechanism of action of hormonal contraceptives and intrauterine contraceptive devices. Am. J. Obstet. Gynecol. 1999;181:1263–1269. doi: 10.1016/s0002-9378(99)70120-1. [DOI] [PubMed] [Google Scholar]
- Romeo R.D., Lee S.J., McEwen B.S. Differential stress reactivity in intact and ovariectomized prepubertal and adult female rats. Neuroendocrinology. 2004;80:387–393. doi: 10.1159/000084203. [DOI] [PubMed] [Google Scholar]
- Romeo R.D., Karatsoreos I.N., McEwen B.S. Pubertal maturation and time of day differentially affect behavioral and neuroendocrine responses following an acute stressor. Horm. Behav. 2006;50:463–468. doi: 10.1016/j.yhbeh.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Rozeboom A.M., Akil H., Seasholtz A.F. Mineralocorticoid receptor overexpression in forebrain decreases anxiety-like behavior and alters the stress response in mice. Proc. Natl. Acad. Sci. 2007;104:4688–4693. doi: 10.1073/pnas.0606067104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruokonen A., Käär K. Effects of desogestrel, levonorgestrel and lynestrenol on serum sex hormone binding globulin, cortisol binding globulin, ceruloplasmin and HDL-cholesterol. Eur. J. Obstet. Gynecol. 1985;20:13–18. doi: 10.1016/0028-2243(85)90078-4. [DOI] [PubMed] [Google Scholar]
- Schindler A.E., Campagnoli C., Druckmann R., Huber J., Pasqualini J.R., Schweppe K.W., Thijssen J.H.H. Classification and pharmacology of progestins. Maturitas. 2003;46:7–16. doi: 10.1016/j.maturitas.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Schoofs D., Wolf O.T. Are salivary gonadal steroid concentrations influenced by acute psychosocial stress? A study using the Trier Social Stress Test (TSST) Int. J. Psychophysiol. 2011;80:36–43. doi: 10.1016/j.ijpsycho.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Schoofs D., Wolf O.T., Smeets T. Cold pressor stress impairs performance on working memory tasks requiring executive functions in healthy young men. Behav. Neurosci. 2009;123:1066–1075. doi: 10.1037/a0016980. [DOI] [PubMed] [Google Scholar]
- Shaaban M., Elwan S.I., Abdalla S., Darwish H. Effect of subdermal levonorgestrel contraceptive implants, Norplant®, on serum lipids. Contraception. 1984;30:413–419. doi: 10.1016/0010-7824(84)90033-7. [DOI] [PubMed] [Google Scholar]
- Sitruk-Ware R. Pharmacological profile of progestins. Maturitas. 2004;47:277–283. doi: 10.1016/j.maturitas.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Sitruk-Ware R. New progestagens for contraceptive use. Hum. Reprod. Update. 2006;12:169–178. doi: 10.1093/humupd/dmi046. [DOI] [PubMed] [Google Scholar]
- Sitruk-Ware R., Nath A. The use of newer progestins for contraception. Contraception. 2010;82:410–417. doi: 10.1016/j.contraception.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Sitruk-Ware R., Nath A. Metabolic effects of contraceptive steroids. Rev. Endocr. Metab. Disord. 2011;12:63. doi: 10.1007/s11154-011-9182-4. [DOI] [PubMed] [Google Scholar]
- Sitruk-Ware R., Nath A. Characteristics and metabolic effects of estrogen and progestins contained in oral contraceptive pills. Best Pract. Res. Clin. Endocrinol. Metabol. 2013;27:13–24. doi: 10.1016/j.beem.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene R., Vagg P., Jacobs G. Consulting Psychologists Press; Palo Alto, CA: 1983. 1983. Manual for the State-Trait Anxiety Inventory. [Google Scholar]
- Spona J., Feichtinger W., Kindermann C., Wünsch C., Brill K. Inhibition of ovulation by an oral contraceptive containing 100 μg levonorgestrel in combination with 20 μg ethinylestradiol. Contraception. 1996;54:299–304. doi: 10.1016/s0010-7824(96)00183-7. [DOI] [PubMed] [Google Scholar]
- Terao T., Nakamura J., Yoshimura R., Ohmori O., Takahashi N., Kojima H., Soeda S., Shinkai T., Nakano H., Okuno T. Relationship between serum cholesterol levels and meta-chlorophenylpiperazine-induced cortisol responses in healthy men and women. Psychiatr. Res. 2000;96:167–173. doi: 10.1016/s0165-1781(00)00197-9. [DOI] [PubMed] [Google Scholar]
- Tikkanen M., Nikkilä E., Kuusi T., Sipinen S. Effects of oestradiol and levonorgestrel on lipoprotein lipids and postheparin plasma lipase activities in normolipoproteinaemic women. Acta Endocrinol. 1982;99:630–635. doi: 10.1530/acta.0.0990630. [DOI] [PubMed] [Google Scholar]
- Tunn S., Mollmann H., Barth J., Derendorf H., Krieg M. Simultaneous measurement of cortisol in serum and saliva after different forms of cortisol administration. Clin. Chem. 1992;38:1491–1494. [PubMed] [Google Scholar]
- Van der Vange N., Blankenstein M., Kloosterboer H., Haspels A., Thijssen J. Effects of seven low-dose combined oral contraceptives on sex hormone binding globulin, corticosteroid binding globulin, total and free testosterone. Contraception. 1990;41:345–352. doi: 10.1016/0010-7824(90)90034-s. [DOI] [PubMed] [Google Scholar]
- van Haarst A.D., Oitzl M.S., De Kloet E.R. Facilitation of feedback inhibition through blockade of glucocorticoid receptors in the hippocampus. Neurochem. Res. 1997;22:1323–1328. doi: 10.1023/a:1022010904600. [DOI] [PubMed] [Google Scholar]
- van Rooijen M., von Schoultz B., Silveira A., Hamsten A., Bremme K. Different effects of oral contraceptives containing levonorgestrel or desogestrel on plasma lipoproteins and coagulation factor VII. Am. J. Obstet. Gynecol. 2002;186:44–48. doi: 10.1067/mob.2002.119179. [DOI] [PubMed] [Google Scholar]
- Vibarel-Rebot N., Rieth N., Lasne F., Jaffré C., Collomp K. Oral contraceptive use and saliva diurnal pattern of metabolic steroid hormones in young healthy women. Contraception. 2015;91:245–247. doi: 10.1016/j.contraception.2014.11.010. [DOI] [PubMed] [Google Scholar]
- Vining R.F., McGinley R.A., Symons R.G. Hormones in saliva: mode of entry and consequent implications for clinical interpretation. Clin. Chem. 1983;29:1752–1756. [PubMed] [Google Scholar]
- Wahl P., Walden C., Knopp R., Hoover J., Wallace R., Heiss G., Rifkind B. Effect of estrogen/progestin potency on lipid/lipoprotein cholesterol. N. Engl. J. Med. 1983;308:862–867. doi: 10.1056/NEJM198304143081502. [DOI] [PubMed] [Google Scholar]
- Wiegratz I., Kutschera E., Lee J.H., Moore C., Mellinger U., Winkler U.H., Kuhl H. Effect of four different oral contraceptives on various sex hormones and serum-binding globulins. Contraception. 2003;67:25–32. doi: 10.1016/s0010-7824(02)00436-5. [DOI] [PubMed] [Google Scholar]
- Wirth M.M., Meier E.A., Fredrickson B.L., Schultheiss O.C. Relationship between salivary cortisol and progesterone levels in humans. Biol. Psychol. 2007;74:104–107. doi: 10.1016/j.biopsycho.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Wirth M.M., Scherer S.M., Hoks R.M., Abercrombie H.C. The effect of cortisol on emotional responses depends on order of cortisol and placebo administration in a within-subject design. Psychoneuroendocrinology. 2011;36:945–954. doi: 10.1016/j.psyneuen.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn V., Niththyananthan R. The effect of progestins in combined oral contraceptives on serum lipids with special reference to high-density lipoproteins. Am. J. Obstet. Gynecol. 1982;142:766–772. doi: 10.1016/s0002-9378(16)32486-3. [DOI] [PubMed] [Google Scholar]
- Wynn V., Doar J., Mills G., Stokes T. Fasting serum triglyceride, cholesterol, and lipoprotein levels during oral-contraceptive therapy. Lancet. 1969;294:756–760. doi: 10.1016/s0140-6736(69)90476-0. [DOI] [PubMed] [Google Scholar]
- Yaguchi H., Tsutsumi K., Shimono K., Omura M., Sasano H., Nishikawa T. Involvement of high density lipoprotein as substrate cholesterol for steroidogenesis by bovine adrenal fasciculo-reticularis cells. Life Sci. 1998;62:1387–1395. doi: 10.1016/s0024-3205(98)00077-0. [DOI] [PubMed] [Google Scholar]