Short Summary
Cardiac regeneration strategies and de novo generation of cardiomyocytes have long been significant areas of research interest in cardiovascular medicine. In this review, we outline a variety of common cell sources and methods used to regenerate cardiomyocytes, and highlight the important role that key Circulation Research papers have played in this flourishing field.
Keywords: Cardiac regeneration, Embryonic stem cells, Induced pluripotent stem cells, Differentiation, Cardiomyocytes
Introduction
Heart disease, whether inherited or acquired, is the leading cause of mortality in both men and women worldwide, accounting for 17.3 million deaths per year.1 The urgent need to improve existing therapies has driven researchers to seek a better understanding of the diverse but inter-related mechanistic origins of heart development and failure, with the ultimate goals of identifying novel pharmacological treatments and/or cell-based engineering approaches to replace damaged heart tissue. Animal models are widely used as surrogates for studying human disease, both in order to recapitulate the complex clinical course of human heart failure and to generate in vitro tools for studying specific aspects of tissue dysfunction.2 Although useful insights have been gained, experimental findings from animal models have not always extrapolated to human disease presentation due to considerable species variation3. Here we describe prominent routes taken towards the goal of cardiac regeneration by focusing on key contributing papers published by Circulation Research in the 60 years since its establishment.
Multipotent adult stem cells
Multipotent adult stem cells have been the focus of most preclinical and clinical studies carried out to date in the field of cardiac regeneration. They represent an attractive source of stem cells since they are relatively abundant, accessible and autologous, and their mechanisms of action for any observed improvement in cardiac function can be potentially delineated. In 1998, Anversa et al. published a field-changing paper challenging the notion that the myocardium is a non-regenerating tissue, by describing the presence of multipotent cardiac stem cells (CSCs) in the adult myocardium that are positive for the hematopoietic progenitor marker c-kit.4 Methods for isolating functionally competent CSCs and mechanisms proving that their activation can reverse cardiac dysfunction were later published by the same group.5, 6 It was this pioneering work and the ability to adequately expand CSCs ex vivo that formed the basis for the first randomized clinical trial of CSC implant in ischemic heart disease patients (SCIPIO trial).7 Phase I of the trial demonstrated a sound safety profile and potential for efficacy in improving ventricular function. In 2004, Messina et al. were able to isolate and expand c-kit+ CSCs from adult murine hearts as self-adherent clusters of progenitor cells, termed cardiospheres.8 This isolation technique later became feasible for human hearts and was used to test the therapeutic efficacy of cardiosphere-derived cells (CDCs) in the CADUCEUS trial.9 The Phase I trial demonstrated a good safety profile and potential for reducing in scar size and regional function compared to controls. More recently, Dey et al. performed detailed characterization of multiple stem cell populations and concluded that c-kit+ CSCs represent the most primitive population of multipotent cardiac progenitors when compared to bone marrow-derived c-kit+ populations, and that CDCs are more closely related to bone marrow stem cells in terms of their gene and protein expression profiles.10 The exact mechanistic and functional outcome implications of such differences are not yet known, but may aid ongoing clinical trials in understanding the biology of these promising cell populations.
Bone marrow-derived mononuclear cells (MNCs) have also garnered considerable interest in regenerative cell therapy as they are easily accessible and autologous, and require minimal expansion. Significantly, evidence of MNC mobilization after myocardial infarction (MI) in mice have supported that bone marrow cells play a role in myocardial healing following injury.11, 12 Randomized human clinical studies of injected MNCs demonstrated a modest increase in left ventricular ejection fraction (LVEF) and a decrease in the New York Heart Association (NYHA) functional classification system.13 Ischemic cardiomyopathy patients receiving MNCs also demonstrated a significant reduction in natriuretic peptide levels.14 Notably, infusion of MNCs with higher colony-forming capacity was associated with lower mortality, raising awareness to the notion that cell viability and quality have a significant impact on therapeutic effect. Mechanistic investigations have suggested that beneficial effects of MNC therapy were a result of neovascularization and paracrine effects rather than cardiomyocyte differentiation.15
Studies of bone marrow-derived mesenchymal stem cells (MSCs) revealed yet another adult stem cell source thought to be suitable for cardiac regeneration. MSCs were reported to readily express phenotypic characteristics of CMs and, when introduced into infarcted animal hearts by intravenous injections, to localize at sites of myocardial injury, prevent tissue remodeling, and improve cardiac recovery.16, 17 Intracoronary infusion of allogeneic mesenchymal precursors (Stro-3+ subpopulation) was also shown to decrease infarct size, improve systolic function, and increase neovascularization in animal MI models.18 These observations led to a pilot human clinical study which confirmed the safety and tolerability of MSCs in humans, and subsequently to a Phase I/II randomized trial.19, 20 More recently, additional evidence has questioned the ability of MSCs to transdifferentiate into cardiomyocytes, instead attributing the mechanism of their therapeutic properties to paracrine effects, neovascularization, and activation of endogenous CSCs.19, 21
Another class of multipotent adult stem cells of particular interest in cardiac cell therapy are CD34+ angiogenic precursors. This interest stems from the relatively impaired angiogenesis seen in ischemic heart disease patients as well as from findings that patients with coronary artery disease have reduced number and migratory activity of angiogenic precursors.22 It has also been observed that CD34+ cell injection ameliorates cardiac recovery in human MI patients by improving perfusion and/or by paracrine effects rather than cardiomyocyte differentiation.23 In one of the largest cell therapy trials to date, Losordo et al. demonstrated that patients with refractory angina who received intramyocardial injections of CD34+ cells experienced significant improvements in angina frequency and exercise tolerance.24 In a subsequent publication, the group identified that CD34+ cells secrete exosomes that might account for some of the improved phenotypes.25 The benefit of CD34+ cells was also shown for non-ischemic cardiomyopathy, when intracoronary injections resulted in a small, but significant improvement in ventricular function and survival.26 More importantly, this study demonstrated that higher intramyocardial homing was associated with better cell therapy response, providing support to prior observations with MNCs that cell delivery method and quality play a significant role in their therapeutic efficacy.
Finally, adipose-derived stem cells (ADSCs) abundantly available from liposuction surgeries have been considered as potential sources of CMs. In 2004, Planart-Blenard et al. reported potential derivation of CMs from human ADSCs by treatment with transferrin, IL-3, IL-6, and VEGF, although at very low event rate (Figure 1).27 Ongoing trials are evaluating the efficacy of this cell population in regeneration of ischemic myocardium, and although complete results have yet to be published, preliminary data are encouraging (Trial identifier: NCT00426868).
Figure 1 – Historic landmarks in the field of cardiac stem cell biology.
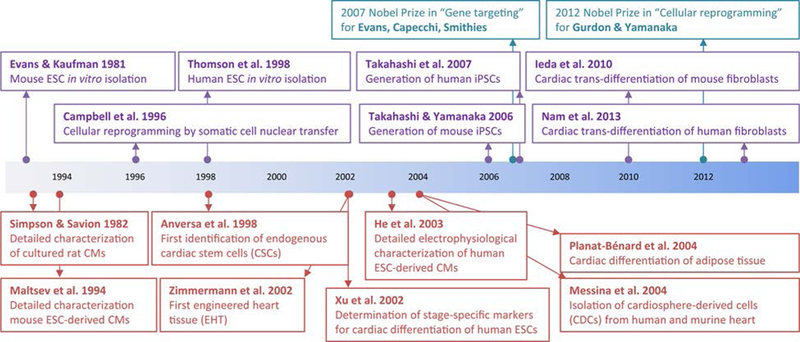
Timeline of important discoveries contributing to the field of stem cell cardiac differentiation and characterization (purple and green boxes, above timeline), including the key Top 100 Circulation Research papers discussed in this review (red boxes, below timeline). ESC, embryonic stem cell; iPSC, induced pluripotency stem cell; CMs, cardiomyocytes.
Trans-differentiation of committed cells
Early attempts at inducing cardiac regeneration involved transplant of skeletal myoblasts or fetal CMs to infarcted canine or rat hearts. Unfortunately, these studies ultimately disappointed the field as myoblasts remained firmly committed to form mature skeletal muscle in the heart28, while extensive cell death coupled with limited proliferation after transplant prevented fetal cardiomyocytes from repairing injury.29 Transplantation of non-contractile committed cells such as fibroblasts and smooth muscle cells into infarcted rat hearts was then briefly thought to enhance heart function, possibly due to aforementioned paracrine effects.30 More recently, several studies have demonstrated in vitro31 and in vivo32 transdifferentiation of mouse fibroblasts into seemingly functional CMs by over-expressing combinations of the cardiac transcription factors Gata4, Mef2c, Tbx5, Hand2, and Nkx2.5. Mouse CMs generated by direct transdifferentiation are positive for CM-specific sarcomeric markers, exhibit electrophysiological and gene expression profiles similar to those of fetal CMs, although this was disputed by other investigators.33 In vitro transdifferentiation towards CM-like cells was also reported for human fibroblasts, albeit by more time consuming and less efficient protocols that generated mostly partially reprogrammed CMs.34 Current efforts in this research area focus on optimizing transdifferentiation efficiency and CM maturation, further characterizing derived CMs, and validating that in vitro and in vivo transdifferentiation occur in the absence of experimental artifacts, which can include incomplete silencing of transgene expression from Cre-lox systems, cell fusion events, as well as the possibility of retrovirus transfecting not only dividing fibroblasts but also non-dividing cardiomyocytes in vivo. For this technology to be fully applied in the clinic, a greater understanding of issues that have plagued the field must be reached: (1) the potential consequences of depleting endogenous cardiac fibroblasts to replenish cardiomyocytes; (2) the ability to transfect bystander cells such as smooth muscle and endothelial cells with cardiac transcription factors; and (3) the challenge of triggering immune response against the host cells transfected with viral versus non-viral vectors.
Pluripotent stem cells
Embryonic stem cells
The isolation by Evans and Kaufman of mouse embryonic stem cells (mESCs) in 198135 and the generation of human embryonic stem cells (hESCs) by Thomson in 199836 opened new horizons for in vitro generation of CMs. Many protocols have been developed over the years to maximize the yield and efficiency of pluripotent ESC differentiation to CMs.37 One of the most utilized methods has been the formation of 3D aggregates named “embryoid bodies” within which cardiac differentiation occurs. In 2002, Xu et al. were amongst the first to optimize cardiac differentiation protocols for hESCs by using DNA demethylating agent 5-azacytidine and enrichment with Percoll separation gradients to obtain up to 70% pure cardiomyocyte populations (Figure 1).38 Later on, rigorous protocol standardization and the use of key signaling factors such as BMP4 and Activin A enabled conversion of hESCs to CMs with over 90% efficiency.39 Consequently, the formation of 3D aggregates, a labor intensive process, has now been largely replaced by differentiation in monolayer cultures, which are more amenable to scale-up and automation.40
Induced pluripotent stem cells
The discovery of induced pluripotent stem cell (iPSC) technology41, based partly on principles highlighted by early somatic cell nuclear transfer experiments42, has meant that mature somatic cells such as skin fibroblasts and peripheral blood mononuclear cells (PBMCs) can be reprogrammed with relative ease to acquire an ESC-like phenotype. iPSCs retain the same capacity for high efficiency cardiac differentiation as ESCs, with the added advantages of avoiding ethical debates related to use of human embryos and enabling autologous transplantation of CMs without the need for immunosuppression. These characteristics make iPSCs ideal cellular models to provide a renewable source of CMs for basic research, pharmacological testing, and cell therapy (Figure 2).43
Figure 2 – Uses of induced pluripotent stem cells.
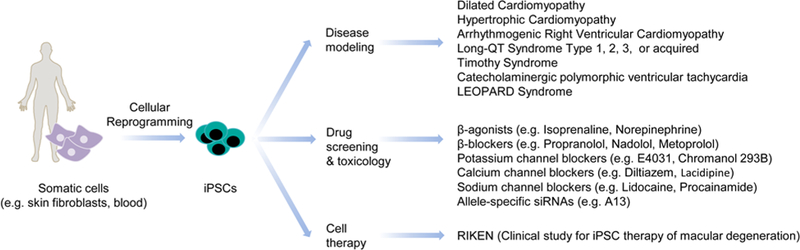
iPSCs are ideal cellular models to provide a renewable source of cardiomyocytes for in vitro disease modeling, pharmacological testing, and therapeutic applications in regenerative medicine.
Characterization of pluripotent stem cell-derived cardiomyocytes
The use of pluripotent stem cell-derived cardiomyocytes (PSC-CMs), which include both hESC-CMs and iPSC-CMs, for downstream applications requires that their properties be physiologically analogous to human cardiomyocytes in vivo. Assays for CM characterization, such as assessment for cross striations, ultrastructure, and chronotropic drug response, were established decades ago for primary rodent myocytes and published in a highly cited Circulation Research paper by Simpson and Savion in 1982.44 In 1994, Maltsev et al. were able to apply the same assays for extensive characterization of mESC-CMs.45 In addition, rigorous experimental optimization enabled them to identify internal and external solutions for patch-clamp electrophysiological analysis to confirm that CM populations comprised of ventricular, atrial, and nodal sub-types, and exhibited most basic cardiac-specific ionic currents (L-type, ICa, INa, Ito, IK, IK1, IK, ATP, IK, Ach, and If). In 2003, He et al. were among the first to perform similar characterizations of hESC-CMs.46
Disease modelling, drug screening, and cell therapy with PSC-CMs
In vitro derived PSC-CMs have been assessed as potential screening platforms for drug discovery and toxicology studies. Despite their immature fetal phenotype, extensive pharmacological validation confirms their potential utility in drug evaluation.47 Clinically relevant drugs (e.g., adrenergic receptor agonists, anti-arrhythmic agents) have been shown to exert chronotropic and inotropic effects on PSC-CMs. In addition, experimental drugs have been used for in vitro amelioration of diseased phenotypes in human iPSC models of cardiovascular diseases48 and prediction of cytotoxic drug-induced side-effects.49, 50 Accumulated evidence suggests that PSC-CMs can offer the pharmaceutical industry a valuable physiologically relevant tool for validation of novel drug candidates and identification of potential cardiotoxic effects in early drug development stages, thereby easing the huge associated economic and patient care burdens.51, 52
The most successful and widely acknowledged use of PSCs-CMs has so far been in disease modeling. The development of disease models by genome editing of mESCs, a technology that led to award of the Nobel Prize in 2007 for Sir Martin Evans, Mario Capecchi, and Oliver Smithies (Figure 1), has offered new tools for in vivo mechanistic investigation into cardiac illnesses. The discovery of induced pluripotency technologies, which likewise led to the Nobel Prize in 2012 for Sir John Gurdon and Shinya Yamanaka, allowed the generation of patient-specific iPSC-CMs for studying human disease models of familial hypertrophic cardiomyopathy53, familial dilated cardiomyopathy54, long QT syndrome55, Timothy syndrome56, arrhythmogenic right ventricular dysplasia57, and others44 (Figure 2). Beyond the potential ability of these models to reveal insights into pathological disease mechanisms, they also offer unique opportunities to explore promising new genetic therapies58 and to identify loci or pathways related to predisposition towards cardiac disorders, thus enabling refinement of phenotype-to-genotype correlations to improve risk stratification and disease management.
The use of PSC-CMs has also expanded to in vivo applications, with transplantation shown to improve cardiac function in rat and guinea pig models of acute myocardial infarct (MI).59, 60 Effective strategies to deplete potential tumorigenic cells61, 62, induce immunotolerance63, 64, and enhance cell survival65 are being sought. Novel tissue engineering approaches to create engineered heart tissues (EHTs) for aiding cell delivery, survival, alignment and functionality of transplanted PSC-CMs are being developed in parallel.66 Notably, these technologies were pioneered by Thomas Eschenhagen’s group, who published one of the very first EHT papers in Circulation Research in 2002.67
Conclusions and future outlook
Extensive progress has been made in the field of cardiac stem cell biology to promote heart tissue repair by introduction of exogenous stem cells, such as MSCs, MNCs, ADSCs, CD34+ cells, c-kit+ CSCs, and CDCs, as evidenced by recent early phase clinical trials shown to reduce infarct size in patients (Table 1). New clinical trials are underway to validate the efficacy of these therapies. Investigation into identifying ideal patient populations, cell delivery timing, and efficacy endpoints will certainly be needed to optimize their full potential. At the same time, hESCs and iPSCs are progressively being used to reliably generate de novo CMs. A major hurdle, however, is their closer resemblance to fetal rather than adult CMs.68 Combination of increasingly efficient CM generation protocols40 and next generation sequencing technology69, as well as other high-throughput screening assays, can lead to identification of molecular markers to further enhance CM maturation. Taken together, these advances in adult and pluripotent stem cell biology over the past decades may herald a new area of cardiovascular regenerative and personalized medicine, in upcoming years.
Table 1 – Cell sources for cardiac repair.
Advantages and disadvantages of the various cell sources used for cardiac regeneration studies, with examples of clinical trials in which cells were used for cardiac regeneration or, in brackets, other conditions. ADSCs, adipose-derived stem cells; MSCs, mesenchymal stem cells; MNCs, mononuclear cells; CSCs, cardiac stem cells; CDCs, cardiosphere-derived cells; DFs, dermal fibroblasts; CFs, cardiac fibroblasts; ESCs, embryonic stem cells; iPSCs, induced pluripotent stem cells; NA, not available.
| Category | Cell type | Advantages | Disadvantages | Published clinical trials |
Radiographic improvement | Symptomatic improvement | Unpublished/ongoing clinical trials |
|---|---|---|---|---|---|---|---|
| Adult Stem Cells | ADSCs | Relatively abundant Accessible by minimally invasive procedures Autologous cell population |
Limited proliferation potential Inefficient in vitro or in vivo cardiac differentiation Modest improvements in cardiac function observed to date |
None to date | (ACELLDream) | ||
| CD34+ | ACT34-CMI NOGA-DCM |
NA + |
+ + |
NOGA-DCM, RENEW PreSERVE-AMI |
|||
| MSCs | POSEIDON C-CURE TAC-HFT |
+/− + + |
+ + + |
PROMETHEUS POSEIDON-DCM NCT00644410 CHART-1, Ixcell-DCM |
|||
| MNCs | FOCUS-HF, FOCUS Swiss-AMI LateTIME, TIME TOPCARE-CHD ASTAMI BOOST |
+/− - - - - + - - |
+ - NA - NA + +/− NA |
||||
| CSCs | Adequately expanded ex vivo Autologous cell population | Procured by relatively invasive procedures | SCIPIO | + | + | SCIPIO | |
| CDCs | CADUCEUS | + | + | ALLSTAR | |||
| Committed Cells | DFs CFs |
Potential for in vivo direct transdifferentiation in humans Bypass need for stem cell progenitors |
In vitro cardiac transdifferentiation extremely inefficient, mostly generating partially reprogrammed cardiomyocytes | None to date | None to date | ||
| Pluripotent Stem Cells | ESCs | Indefinite self-renewal Efficient in vitro cardiac differentiation |
Ethically problematic Allogeneic transplant requires immunosuppression Immature fetal-like differentiated cells |
None to date | (GERON, ACT) | ||
| iPSCs | Additional potential for autologous transplant compared to ESCs | Immature fetal-like differentiated cells | None to date | (RIKEN) |
Acknowledgements
We gratefully acknowledge Joseph D. Gold and Blake Wu for critical reading and funding support from Leducq Foundation, American Heart Association 13EIA14420025, National Institutes of Health (NIH) R01 HL113006, NIH U01 HL099776, California Institute for Regenerative Medicine (CIRM) TR3–05556, and CIRM DR2–05394 (JCW).
Non-standard Abbreviations and Acronyms
- ADSC
Adipose-derived stem cell
- CM
Cardiomyocyte
- CDC
Cardiosphere-derived cell
- CSC
Cardiac stem cell
- EHT
Engineered heart tissue
- ESC
Embryonic stem cell
- iPSC
Induced pluripotent stem cell
- LVEF
Left ventricular ejection fraction
- MI
Myocardial infarction
- MNC
Mononuclear Cell
- MSC
Mesenchymal stem cell
- NYHA
New York Heart Association
- PBMC
Peripheral blood mononuclear cells
- PSC
Pluripotent stem cell
Footnotes
Disclosures
JCW is a consultant for Merck and Novartis and is a co-founder of Stem Cell Theranostics.
References
- 1.Laslett LJ, Alagona P, Clark BA, Drozda JP, Saldivar F, Wilson SR, Poe C, Hart M. The worldwide environment of cardiovascular disease: Prevalence, diagnosis, therapy, and policy issuesa report from the american college of cardiology. Journal of the American College of Cardiology. 2012;60:S1–S49 [DOI] [PubMed] [Google Scholar]
- 2.Milan DJ, MacRae CA. Animal models for arrhythmias. Cardiovasc Res. 2005;67:426–437 [DOI] [PubMed] [Google Scholar]
- 3.Rajamohan D, Matsa E, Kalra S, Crutchley J, Patel A, George V, Denning C. Current status of drug screening and disease modelling in human pluripotent stem cells. Bioessays. 2013;35:281–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anversa P, Kajstura J. Ventricular myocytes are not terminally differentiated in the adult mammalian heart. Circ Res. 1998;83:1–14 [DOI] [PubMed] [Google Scholar]
- 5.D’Amario D, Fiorini C, Campbell PM, Goichberg P, Sanada F, Zheng H, Hosoda T, Rota M, Connell JM, Gallegos RP, Welt FG, Givertz MM, Mitchell RN, Leri A, Kajstura J, Pfeffer MA, Anversa P. Functionally competent cardiac stem cells can be isolated from endomyocardial biopsies of patients with advanced cardiomyopathies. Circ Res. 2011;108:857–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rota M, Padin-Iruegas ME, Misao Y, De Angelis A, Maestroni S, Ferreira-Martins J, Fiumana E, Rastaldo R, Arcarese ML, Mitchell TS, Boni A, Bolli R, Urbanek K, Hosoda T, Anversa P, Leri A, Kajstura J. Local activation or implantation of cardiac progenitor cells rescues scarred infarcted myocardium improving cardiac function. Circ Res. 2008;103:107–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolli R, Chugh AR, D’Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (scipio): Initial results of a randomised phase 1 trial. The Lancet. 2011;378:1847–1857 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MV, Coletta M, Vivarelli E, Frati L, Cossu G, Giacomello A. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–921 [DOI] [PubMed] [Google Scholar]
- 9.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LEJ, Berman D, Czer LSC, Marbán L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marbán E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (caduceus): A prospective, randomised phase 1 trial. The Lancet. 2012;379:895–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dey D, Han L, Bauer M, Sanada F, Oikonomopoulos A, Hosoda T, Unno K, De Almeida P, Leri A, Wu JC. Dissecting the molecular relationship among various cardiogenic progenitor cells. Circ Res. 2013;112:1253–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kucia M, Dawn B, Hunt G, Guo Y, Wysoczynski M, Majka M, Ratajczak J, Rezzoug F, Ildstad ST, Bolli R, Ratajczak MZ. Cells expressing early cardiac markers reside in the bone marrow and are mobilized into the peripheral blood after myocardial infarction. Circ Res. 2004;95:1191–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheikh AY, Lin S-A, Cao F, Cao Y, van der Bogt KEA, Chu P, Chang C- P, Contag CH, Robbins RC, Wu JC. Molecular imaging of bone marrow mononuclear cell homing and engraftment in ischemic myocardium. STEM CELLS. 2007;25:2677–2684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanganalmath SK, Bolli R. Cell therapy for heart failure: A comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res. 2013;113:810–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Assmus B, Fischer-Rasokat U, Honold J, Seeger FH, Fichtlscherer S, Tonn T, Seifried E, Schächinger V, Dimmeler S, Zeiher AM. Transcoronary transplantation of functionally competent bmcs is associated with a decrease in natriuretic peptide serum levels and improved survival of patients with chronic postinfarction heart failure: Results of the topcare-chd registry. Circ Res. 2007;100:1234–1241 [DOI] [PubMed] [Google Scholar]
- 15.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9–20 [DOI] [PubMed] [Google Scholar]
- 17.Suzuki G, Iyer V, Lee T-C, Canty JM. Autologous mesenchymal stem cells mobilize ckit+ and cd133+ bone marrow progenitor cells and improve regional function in hibernating myocardium. Circ Res. 2011;109:1044–1054 [DOI] [PubMed] [Google Scholar]
- 18.Houtgraaf JH, de Jong R, Kazemi K, de Groot D, van der Spoel TIG, Arslan F, Hoefer I, Pasterkamp G, Itescu S, Zijlstra F, Geleijnse ML, Serruys PW, Duckers HJ. Intracoronary infusion of allogeneic mesenchymal precursor cells directly after experimental acute myocardial infarction reduces infarct size, abrogates adverse remodeling, and improves cardiac function. Circ Res. 2013;113:153–166 [DOI] [PubMed] [Google Scholar]
- 19.Williams AR, Trachtenberg B, Velazquez DL, McNiece I, Altman P, Rouy D, Mendizabal AM, Pattany PM, Lopera GA, Fishman J, Zambrano JP, Heldman AW, Hare JM. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: Functional recovery and reverse remodeling. Circ Res. 2011;108:792–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The poseidon randomized trial. JAMA. 2012;308:2369–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reinecke H, Minami E, Zhu W-Z, Laflamme MA. Cardiogenic differentiation and transdifferentiation of progenitor cells. Circ Res. 2008;103:1058–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vasa M, Fichtlscherer S, Adler K, Aicher A, Martin H, Zeiher AM, Dimmeler S. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001;103:2885–2890 [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Zhang S, Rabinovich B, Bidaut L, Soghomonyan S, Alauddin MM, Bankson JA, Shpall E, Willerson JT, Gelovani JG, Yeh ETH. Human cd34+ cells in experimental myocardial infarction: Long-term survival, sustained functional improvement, and mechanism of action. Circ Res. 2010;106:1904–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Losordo DW, Henry TD, Davidson C, Sup Lee J, Costa MA, Bass T, Mendelsohn F, Fortuin FD, Pepine CJ, Traverse JH, Amrani D, Ewenstein BM, Riedel N, Story K, Barker K, Povsic TJ, Harrington RA, Schatz RA, Investigators tA-C. Intramyocardial, autologous cd34+ cell therapy for refractory angina. Circ Res. 2011;109:428–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sahoo S, Klychko E, Thorne T, Misener S, Schultz KM, Millay M, Ito A, Liu T, Kamide C, Agrawal H, Perlman H, Qin G, Kishore R, Losordo DW. Exosomes from human cd34+ stem cells mediate their proangiogenic paracrine activity. Circ Res. 2011;109:724–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vrtovec B, Poglajen G, Lezaic L, Sever M, Domanovic D, Cernelc P, Socan A, Schrepfer S, Torre-Amione G, Haddad F, Wu JC. Effects of intracoronary cd34+ stem cell transplantation in non-ischemic dilated cardiomyopathy patients: 5-year follow up. Circ Res. 2012 [DOI] [PubMed] [Google Scholar]
- 27.Planat-Benard V, Menard C, Andre M, Puceat M, Perez A, Garcia-Verdugo JM, Penicaud L, Casteilla L. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ Res. 2004;94:223–229 [DOI] [PubMed] [Google Scholar]
- 28.Reinecke H, Poppa V, Murry CE. Skeletal muscle stem cells do not transdifferentiate into cardiomyocytes after cardiac grafting. J Mol Cell Cardiol. 2002;34:241–249 [DOI] [PubMed] [Google Scholar]
- 29.Muller-Ehmsen J, Whittaker P, Kloner RA, Dow JS, Sakoda T, Long TI, Laird PW, Kedes L. Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J Mol Cell Cardiol. 2002;34:107–116 [DOI] [PubMed] [Google Scholar]
- 30.Fujii T, Yau TM, Weisel RD, Ohno N, Mickle DA, Shiono N, Ozawa T, Matsubayashi K, Li RK. Cell transplantation to prevent heart failure: A comparison of cell types. Ann Thorac Surg. 2003;76:2062–2070; discussion 2070 [DOI] [PubMed] [Google Scholar]
- 31.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu JD, Srivastava D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen JX, Krane M, Deutsch MA, Wang L, Rav-Acha M, Gregoire S, Engels MC, Rajarajan K, Karra R, Abel ED, Wu JC, Milan D, Wu SM. Inefficient reprogramming of fibroblasts into cardiomyocytes using gata4, mef2c, and tbx5. Circ Res. 2012;111:50–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu J-D, Stone Nicole R, Liu L, Spencer CI Qian L, Hayashi Y, Delgado-Olguin P, Ding S, Bruneau Benoit G, Srivastava D. Direct reprogramming of human fibroblasts toward a cardiomyocyte-like state. Stem Cell Reports. 2013;1:235–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156 [DOI] [PubMed] [Google Scholar]
- 36.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147 [DOI] [PubMed] [Google Scholar]
- 37.Burridge PW, Keller G, Gold JD, Wu JC. Production of de novo cardiomyocytes: Human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell. 2012;10:16–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu C, Police S, Rao N, Carpenter MK. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ Res. 2002;91:501–508 [DOI] [PubMed] [Google Scholar]
- 39.Burridge PW, Thompson S, Millrod MA, Weinberg S, Yuan X, Peters A, Mahairaki V, Koliatsos VE, Tung L, Zambidis ET. A universal system for highly efficient cardiac differentiation of human induced pluripotent stem cells that eliminates interline variability. PLoS One. 2011;6:e18293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang J, Klos M, Wilson GF, Herman AM, Lian X, Raval KK, Barron MR, Hou L, Soerens AG, Yu J, Palecek SP, Lyons GE, Thomson JA, Herron TJ, Jalife J, Kamp TJ. Extracellular matrix promotes highly efficient cardiac differentiation of human pluripotent stem cells: The matrix sandwich method. Circ Res. 2012;111:1125–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872 [DOI] [PubMed] [Google Scholar]
- 42.Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–813 [DOI] [PubMed] [Google Scholar]
- 43.Matsa E, Denning C. In vitro uses of human pluripotent stem cell-derived cardiomyocytes. J Cardiovasc Transl Res. 2012;5:581–592 [DOI] [PubMed] [Google Scholar]
- 44.Simpson P, Savion S. Differentiation of rat myocytes in single cell cultures with and without proliferating nonmyocardial cells. Cross-striations, ultrastructure, and chronotropic response to isoproterenol. Circ Res. 1982;50:101–116 [DOI] [PubMed] [Google Scholar]
- 45.Maltsev VA, Wobus AM, Rohwedel J, Bader M, Hescheler J. Cardiomyocytes differentiated in vitro from embryonic stem cells developmentally express cardiac-specific genes and ionic currents. Circ Res. 1994;75:233–244 [DOI] [PubMed] [Google Scholar]
- 46.He JQ, Ma Y, Lee Y, Thomson JA, Kamp TJ. Human embryonic stem cells develop into multiple types of cardiac myocytes: Action potential characterization. Circ Res. 2003;93:32–39 [DOI] [PubMed] [Google Scholar]
- 47.Narsinh K, Narsinh KH, Wu JC. Derivation of human induced pluripotent stem cells for cardiovascular disease modeling. Circ Res. 2011;108:1146–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsa E, Rajamohan D, Dick E, Young L, Mellor I, Staniforth A, Denning C. Drug evaluation in cardiomyocytes derived from human induced pluripotent stem cells carrying a long qt syndrome type 2 mutation. Eur Heart J. 2011;32:952–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liang P, Lan F, Lee AS, Gong T, Sanchez-Freire V, Wang Y, Diecke S, Sallam K, Knowles JW, Nguyen PK, Wang PJ, Bers DM, Robbins RC, Wu JC. Drug screening using a library of human induced pluripotent stem cell-derived cardiomyocytes reveals disease specific patterns of cardiotoxicity. Circulation. 2013;127:1677–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Navarrete EG, Liang P, Lan F, Sanchez-Freire V, Simmons C, Gong T, Sharma A, Burridge P, Patlolla B, Lee A, Wu H, Beygui R, Wu S, Robbins RC, Bers D, Wu JC. Screening adverse drug-induced arrhythmia events using human induced pluripotent stem cell-derived cardiomyocytes and low-impedance microelectrode arrays Circulation. 2013;In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Engle SJ, Puppala D. Integrating human pluripotent stem cells into drug development. Cell Stem Cell. 2013;12:669–677 [DOI] [PubMed] [Google Scholar]
- 52.Mordwinkin NM, Lee AS, Wu JC. Patient-specific stem cells and cardiovascular drug discovery. JAMA. 2013;310:2039–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lan F, Lee Andrew S, Liang P, Sanchez-Freire V, Nguyen Patricia K, Wang L, Han L, Yen M, Wang Y, Sun N, Abilez Oscar J, Hu S, Ebert Antje D, Navarrete Enrique G, Simmons Chelsey S, Wheeler M, Pruitt B, Lewis R, Yamaguchi Y, Ashley Euan A, Bers Donald M, Robbins Robert C, Longaker Michael T, Wu Joseph C. Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient-specific induced pluripotent stem cells. Cell Stem Cell. 2013;12:101–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun N, Yazawa M, Liu J, Han L, Sanchez-Freire V, Abilez OJ, Navarrete EG, Hu S, Wang L, Lee A, Pavlovic A, Lin S, Chen R, Hajjar RJ, Snyder MP, Dolmetsch RE, Butte MJ, Ashley EA, Longaker MT, Robbins RC, Wu JC. Patient-specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Sci Transl Med. 2012;4:130ra147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moretti A, Bellin M, Welling A, Jung CB, Lam JT, Bott-Flugel L, Dorn T, Goedel A, Hohnke C, Hofmann F, Seyfarth M, Sinnecker D, Schomig A, Laugwitz KL. Patient-specific induced pluripotent stem-cell models for long-qt syndrome. N Engl J Med. 2010;363:1397–1409 [DOI] [PubMed] [Google Scholar]
- 56.Carvajal-Vergara X, Sevilla A, D’Souza SL, Ang YS, Schaniel C, Lee DF, Yang L, Kaplan AD, Adler ED, Rozov R, Ge Y, Cohen N, Edelmann LJ, Chang B, Waghray A, Su J, Pardo S, Lichtenbelt KD, Tartaglia M, Gelb BD, Lemischka IR. Patient-specific induced pluripotent stem-cell-derived models of leopard syndrome. Nature. 2010;465:808–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim C, Wong J, Wen J, Wang S, Wang C, Spiering S, Kan NG, Forcales S, Puri PL, Leone TC, Marine JE, Calkins H, Kelly DP, Judge DP, Chen HS. Studying arrhythmogenic right ventricular dysplasia with patient-specific ipscs. Nature. 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matsa E, Dixon JE, Medway C, Georgiou O, Patel MJ, Morgan K, Kemp PJ, Staniforth A, Mellor I, Denning C. Allele-specific rna interference rescues the long-qt syndrome phenotype in human-induced pluripotency stem cell cardiomyocytes. European Heart Journal. 2013;doi: 10.1093/eurheartj/eht067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Caspi O, Huber I, Kehat I, Habib M, Arbel G, Gepstein A, Yankelson L, Aronson D, Beyar R, Gepstein L. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. Journal of the American College of Cardiology. 2007;50:1884–1893 [DOI] [PubMed] [Google Scholar]
- 60.Shiba Y, Fernandes S, Zhu WZ, Filice D, Muskheli V, Kim J, Palpant NJ, Gantz J, Moyes KW, Reinecke H, Van Biber B, Dardas T, Mignone JL, Izawa A, Hanna R, Viswanathan M, Gold JD, Kotlikoff MI, Sarvazyan N, Kay MW, Murry CE, Laflamme MA. Human es-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012;489:322–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee AS, Tang C, Rao MS, Weissman IL, Wu JC. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat Med. 2013;19:998–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tang C, Lee AS, Volkmer J-P, Sahoo D, Nag D, Mosley AR, Inlay MA, Ardehali R, Chavez SL, Pera RR, Behr B, Wu JC, Weissman IL, Drukker M. An antibody against ssea-5 glycan on human pluripotent stem cells enables removal of teratoma-forming cells. Nat Biotech. 2011;29:829–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Almeida PE, Ransohoff JD, Nahid A, Wu JC. Immunogenicity of pluripotent stem cells and their derivatives. Circ Res. 2013;112:549–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pearl JI, Lee AS, Leveson-Gower DB, Sun N, Ghosh Z, Lan F, Ransohoff J, Negrin RS, Davis MM, Wu JC. Short-term immunosuppression promotes engraftment of embryonic and induced pluripotent stem cells. Cell Stem Cell. 2011;8:309–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu S, Huang M, Nguyen PK, Gong Y, Li Z, Jia F, Lan F, Liu J, Nag D, Robbins RC, Wu JC. Novel microrna prosurvival cocktail for improving engraftment and function of cardiac progenitor cell transplantation. Circulation. 2011;124:S27–S34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tulloch NL, Muskheli V, Razumova MV, Korte FS, Regnier M, Hauch KD, Pabon L, Reinecke H, Murry CE. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ Res. 2011;109:47–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zimmermann WH, Schneiderbanger K, Schubert P, Didie M, Munzel F, Heubach JF, Kostin S, Neuhuber WL, Eschenhagen T. Tissue engineering of a differentiated cardiac muscle construct. Circ Res. 2002;90:223–230 [DOI] [PubMed] [Google Scholar]
- 68.Cao F, Wagner RA, Wilson KD, Xie X, Fu JD, Drukker M, Lee A, Li RA, Gambhir SS, Weissman IL, Robbins RC, Wu JC. Transcriptional and functional profiling of human embryonic stem cell-derived cardiomyocytes. PLoS One. 2008;3:e3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Churko JM, Mantalas GL, Snyder MP, Wu JC. Overview of high throughput sequencing technologies to elucidate molecular pathways in cardiovascular diseases. Circ Res. 2013;112:1613–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]


