Abstract
Emerging evidence from the preclinical and human research suggests sex differences in response to different types of stress exposure, and that developmental timing, reproductive status, and biological sex are important factors influencing the degree of HPA activation/function. Here we review data regarding: i) sex differences in behavioral and neural responses to uncontrollable and controllable stressors; ii) distinct trajectories of behavioral development and HPA-axis function in male and female rats following adolescent stress exposure; iii) normative changes in behavior and dopamine function in early postpartum rats; iv) aberrant HPA-axis function and its link to abnormal behaviors in two independent, preclinical mouse models of postpartum depression; and, v) data indicating that gender, in addition to sex, is an important determinant of stress reactivity in humans. Based on these findings, we conclude it will be important for future studies to investigate the short and long-term effects of a wide variety of stressors, how these effects may differ according to developmental timing and in relation to gonadal function, the relationship between aberrant HPA-axis activity during the postpartum and mood disorders, and influences of both sex and gender on stress reactivity in humans.
Keywords: Sex differences, Stress, Animal models, HPA-Axis, Development, Postpartum
The hypothalamic-pituitary-adrenal (HPA) axis mediates the neuroendocrine stress response and is mediated by glucocorticoids (Smith and Vale, 2006; Herman et al., 2016). Stress triggers the activation of the HPA-axis, which culminates in the production of glucocorticoids by the adrenals (Lupien et al., 2009). Briefly, corticotrophin-releasing hormone (CRH) is released from the paraventricular nucleus in the hypothalamus (PVN) in response to stressors and stimulates the release of adrenocorticotropic hormone (ACTH) from the pituitary gland (Herman et al., 2016). ACTH then stimulates the adrenal cortex to secrete cortisol (in humans) or corticosterone (i.e. CORT, in rodents) into the bloodstream (Lupien et al., 2009; Herman et al., 2016). Cortisol/CORT feeds back via glucocorticoid and mineralocorticoid receptors in the pituitary, hypothalamus and multiple other sites in brain regions and periphery to regulate its own secretion as well as the magnitude and duration of HPA-axis activation (Keller-Wood and Dallman, 1984). These receptors are expressed throughout the brain and have widespread actions beyond HPA-axis regulation (de Kloet et al., 1993; Herman, 1993). Activation of the HPA-axis and the subsequent stress response is necessary to promote adaptation and enable a response to threats and other homeostatic challenges (i.e. allostasis) (McEwen, 1998; Smith and Vale, 2006). However, chronic or repeated stress drives constant activation of the HPA-axis and related circuitry (i.e. high allostatic load), leads to HPA-axis dysregulation and increases risk for developing psychiatric disorders that differentially affect men and women, such as mood and anxiety disorders (McEwen and Stellar, 1993; McEwen, 2003; Fernandez-Guasti et al., 2012; Altemus et al., 2014; Oyola and Handa, 2017).
Clinical and preclinical research have demonstrated significant sex differences in HPA-axis function and regulation at baseline and in response to stress (Kudielka and Kirschbaum, 2005; Goel et al., 2014; Oyola and Handa, 2017). Female rodents exhibit greater basal concentrations of CORT (Kitay, 1961) and secrete higher concentrations of CORT in response to physical and psychological stressors (Goel et al., 2014; Oyola and Handa, 2017) (see Table 1 for summary). In addition, clinical studies have shown enhanced stress sensitivity and greater susceptibility towards affective dysfunction in women, which may be related to sexual dimorphisms in the HPA-axis response to stress (Altemus, 2006; Solomon and Herman, 2009). This is significant given that increased stress responsiveness is implicated in the etiology of mood and anxiety disorders, which have a higher incidence in women (Altemus, 2006; Parker and Brotchie, 2010), and heightened stress sensitivity in women has been proposed as a key factor in the development of these disorders (Becker et al., 2007). Collectively, these data indicate that sex is an important determinant of disease susceptibility. For these reasons, here we synthesize novel research highlighting: i) sex differences in neurobehavioral responding to stressors; ii) sex differences in the developmental trajectories and adult outcomes following adolescent stress exposure; iii) sex-specific changes in HPA-axis function during the postpartum period and HPA-axis dysregulation in animal models of postpartum mood disorders; and, iv) sex and gender interactions in responding to stress in humans. Finally, although here we focus on the developmental period spanning from adolescence through the reproductive years, it is important to note that there is evidence for a role of stress in aging (Sapolsky, 1999; Goosens and Sapolsky, 2007), as well as sex differences in the stress response in aged populations of both humans and rodents (Bowman et al., 2006; Luine et al., 2007; Lupien et al., 2009; Bale and Epperson, 2015). These differences are thought to be mediated by the normative hormonal changes required for the transition to reproductive senescence, which lead to alterations in stress responsivity within and between the sexes, as well as increase risk for psychiatric disorders (Bale and Epperson, 2015).
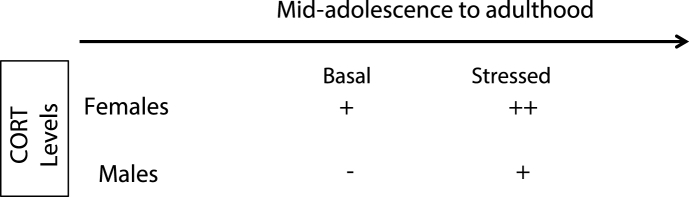
Table 1.
Summary of sex differences in corticosterone (CORT) secretion from adolescence to adulthood. Basal levels of CORT are higher in female rodents than in male rodents. Stress-induced increases in CORT levels are greater in female rodents and remain elevated longer than in male rodents.
1. Sex differences in response to uncontrollable and controllable stressors
Stress-related disorders such as depression, anxiety and post-traumatic stress disorder (PTSD) have a higher incidence in women than in men (Solomon and Herman, 2009; Bangasser and Valentino, 2014; Shansky, 2015). The direct mechanisms that drive these sex differences are unclear, but may emerge, at least partially, from different coping styles in response to adversity. Thus, there is a need for validated animal models that provide insights into the neural mechanisms associated with the selection and implementation of relevant stress coping behavior in both sexes. Below, we focus on recent findings obtained using uncontrollable and controllable stress paradigms covered in the session.
1.1. Uncontrollable stressors
The forced swim test (FST), although initially developed as a screen for antidepressant efficacy (Porsolt et al., 1977), constitutes an acute inescapable stressor that can be used to distinguish between active (i.e. swimming, climbing) and passive (i.e. floating) coping responses (de Kloet and Molendijk, 2016). FST exposure induces a robust activation of the HPA-axis and alterations within stress-responsive brain circuits, and these effects differ between the sexes (Dalla et al., 2008, 2011). For example, swim stress increases CORT levels in male and female rats, but females exhibit elevated CORT levels post-FST compared with males (Drossopoulou et al., 2004), suggesting heightened stress reactivity. Female Sprague Dawley rats also display greater immobility (i.e. passive) behavior during the FST compared with males (Rincon-Cortes and Grace, 2017), which is consistent with results previously observed in female Wistar rats (Drossopoulou et al., 2004; Dalla et al., 2008). Recently, it has been shown that forced swim stress has a sex-specific effect on the mesolimbic dopamine (DA) system: it reduces the number of spontaneously active DA neurons (i.e. population activity) within the ventral tegmental area (VTA) in females, but not in males (Rincon-Cortes and Grace, 2017). Importantly, this attenuation of VTA DA activity is comparable to that observed in male and female rats following chronic mild stress (CMS) (Chang and Grace, 2014; Rincon-Cortes and Grace, 2017). These data suggest sex differences in behavioral responses to forced swim stress, in which females adopt a more passive response, as well as sex-dependent effect of swim stress on VTA population activity in which females are more susceptible to stress-induced DA downregulation.
Using a modified and extended version of the FST consisting of a pre-test (Day 1) and a test (Day 2) 4 weeks later, Shansky and colleagues have identified effects of sex and strain (Long Evans, Sprague Dawley) on the selection of behavioral responses and neural activity patterns within the medial prefrontal cortex (mPFC) in response to the FST on Day 2 (Colom-Lapetina et al., 2017). Sprague Dawley females exhibited lower overall immobility than Sprague Dawley males or Long Evans females on Day 1; whereas Sprague Dawley males exhibited more overall immobility than Sprague Dawley females or Long Evans males on Day 2 (Colom-Lapetina et al., 2017). Sprague Dawley males had lower neural activity in the mPFC, as indexed by reduced counts of c-fos + cells within the prelimbic and infralimbic cortices, which corresponded to the increased immobility during test day (Day 2). These findings show that sexually-divergent coping responses to long-term forced swim are strain dependent and that the mPFC may contribute to sexually dimorphic behavior in long-term forced swim.
1.2. Controllable stressors
A key feature of coping is the perceived or actual behavioral control over some aspect of the adverse event (Maier, 2015). The degree of behavioral control that an organism has over a stressor is a potent modulator of the stressor's impact. In male rats, uncontrollable stressors (i.e. inescapable shock, IS) produce numerous outcomes that do not occur if the stressor is controllable (i.e. escapable shock, ES) (Maier, 2015). Controllable stressors can also exert protective effects against future uncontrollable stressors, a process known as behavioral immunization that is mediated by the ventral mPFC (Amat et al., 2006; Maier et al., 2006). But what about females? Do protective neurobehavioral effects of stressor controllability extend to female rats? To address this, Baratta and colleagues employed the stressor controllability paradigm, in which animals can terminate tail shocks by performing a wheel-turn escape response. Surprisingly, control over the stressor had no impact on shock-induced behavioral outcomes in females: female ES rats exhibited potentiated freezing, poor escape behavior and reduced social exploration, similar to that observed in female IS rats (Baratta et al., 2018).
The lack of benefit afforded by behavioral control in females is in sharp contrast to what has been reported in males and suggests that the neural processing of control differs between the sexes. In males, IS induces potent activation of serotonin (5-HT) in the dorsal raphe nucleus (DRN) (Maswood et al., 1998; Grahn et al., 1999), which is critical for producing the behavioral sequelae following IS (e.g. impaired shuttle box escape, exaggerated freezing, decreased social exploration). These outcomes are prevented in male ES subjects because the experience of control engages prefrontal top-down inhibition over DRN 5-HT activity (Amat et al., 2005), with a specific projection from the prelimbic (PL) cortex to the DRN mediating these protective effects. Females respond to behavioral control differently. Consistent with behavioral results, controllable stress in females does not blunt stress-induced activation of DRN 5-HT: ES and IS females showed equally elevated Fos expression in 5-HT labelled neurons (Baratta et al., 2018). Moreover, unlike in prior studies with males, behavioral control (i.e. ES) did not engage the PL-DRN pathway in females, suggesting that the absence of a modulating effect of control on DRN 5-HT is due to a lack of top-down inhibition provided by the PL cortex.
Given that the perception of control during an adverse event (i.e. tail shock) can promote resilience against the effects of future stressors in males, a separate study was conducted to determine whether the lack of ES effects on females extend to behavioral immunization to a future stressor. In females, stressor controllability did not protect against the behavioral effects induced by IS: ES females that were exposed to IS exhibited attenuated social exploration and exaggerated freezing responses (Barrata et al. under review). This finding suggests that the behavioral immunization induced by ES is sex-specific. In addition, the ES- and IS-induced structural plasticity within the PL-DRN also differed within the sexes. In males, IS elicited broad, non-specific alterations in PL spine size, while ES elicited PL-DRN circuit-specific spine changes. In contrast, females exhibited broad, non-specific spine enlargement after ES but only minor structural changes after IS (Barrata et al. under review). Together, these data provide evidence for a circuit specific mechanism of structural plasticity that could underlie sexual divergence in the protective effects of stress controllability.
2. Short and long-term effects of adolescent stress exposure in male and female rats
The onset of stress-related psychopathologies, including anxiety and depression, often occurs in late adolescence and is frequently precipitated by exposure to chronic stress (McCormick and Green, 2013). Adolescence is an important developmental window in brain maturation and a period of active neuroplasticity within brain areas critically involved in stress regulation and HPA-axis function, such as the hippocampus, prefrontal cortex, and amygdala (Andersen and Teicher, 2008; Eiland and Romeo, 2013; McEwen et al., 2016). Hence, there are major changes in HPA-axis function and stress responsiveness during adolescence in both humans and rodents (Lupien et al., 2009; Klein and Romeo, 2013). For example, adolescent male and female rats exhibit greater and prolonged HPA-axis stress responses to a wide variety of stressors relative to adults (Romeo et al., 2016), suggesting a link between immaturity of stress-responsive brain circuits and enhanced HPA-axis drive. This developmental change in stress reactivity is thought to be a contributing factor to the increased vulnerabilities during adolescence conferring risk to later life psychopathology (Spear, 2009). Nonetheless, stress susceptibility in adolescents, particularly in females, is poorly understood because the bulk of stress studies are focused on the study of stress in prenatal, early postnatal, and adult life in male animals.
To this end, Dr. Herman's group employed a 14-day chronic variable stress (CVS) paradigm during adolescence and examined the short and long-term effects of chronic stress on neuroendocrine function and depressive-like behavior in rats of both sexes. In male rats, exposure to CVS during late adolescence (PND50-64) resulted in reduced body weight gain, decreased body fat, adrenal hypertrophy, increased basal CORT secretion and increased HPA-axis response to a novel stressor (Jankord et al., 2011). Females rats exposed to CVS during late adolescence (PND 45–58) exhibited reduced body weight gain, increased basal CORT secretion and blunted HPA-axis response to a novel stressor (Wulsin et al., 2016). Unlike males, female rats did not show the CVS-induced adrenal hypertrophy and CORT hyper-secretion in response to a novel stressor, suggesting that adolescent females may have some degree of resistance to the effects of CVS on neuroendocrine stress responses and helplessness related behaviors when tested shortly after stress cessation. The immediate effects of adolescent CVS exposure differed from those observed in adulthood. Exposure to chronic stress during late adolescence produced long-lasting neuroendocrine (i.e. hyposecretion of CORT and ACTH) and behavioral effects (i.e. increased FST immobility) in adult female rats that are thought to reflect blunted central and hormonal stress reactivity as well as increased depression-like behavior (Wulsin et al., 2016). This is consistent with clinical data suggesting that HPA-axis dysregulation is a key factor in the development of mood and anxiety disorders, which have a higher incidence in women (Altemus, 2006; Parker and Brotchie, 2010). Adult males exposed to adolescent CVS exposure exhibited increased depressive-like behavior in the FST, but no changes in HPA-axis reactivity to an acute stressor, suggesting a resilient phenotype (Cotella et al., 2018). Collectively, these data suggest that adolescent stress exposure reprograms subsequent HPA-axis baseline activity (i.e. alters adult HPA function), stress reactivity, and behavioral responses in a sex-dependent way, in which males exhibit greater resilience to the enduring influence of stress on behavioral coping strategies adult HPA function.
Although recent rodent studies demonstrate adolescent chronic stress exposure results in greater and longer-lasting changes in behavior and HPA-axis function in females than in males (Bourke and Neigh, 2011; Romeo et al., 2016; Wulsin et al., 2016; Cotella et al., 2018), there is also evidence that adolescent females may benefit more from environmental interventions aimed at ameliorating the enduring effects of stress. For instance, a recent study showed that exposure to an enriched environment (EE) prior and during adolescent chronic stress exposure (PND33-60) can buffer against the long-term effects of stress in a sex-specific manner. Adolescent EE prevented increased adult depression-like behavior in CVS females and blocked the effects of stress on adrenal responsivity, while having little to no effect on males or control (i.e. unstressed) females (Smith et al., 2017). Since adolescent EE confers protection from the delayed behavioral and hormonal effects of CVS in females, this suggests that adolescence is a sensitive period for mitigating the impact of stress in females and underscores the importance of early intervention.
3. Stress and the postpartum period
The HPA-axis undergoes dramatic changes during pregnancy and lactation (Russell et al., 2001; Brunton et al., 2008). During this time, the metabolic demands placed on the mother are increased, and specific adaptations of both basal and stress-induced HPA-axis activity occur to facilitate the transition into this new role and fulfill the needs of the offspring (Lightman et al., 2001; Brunton et al., 2008; Windle et al., 2013). Pregnancy and the early postpartum are associated with elevated basal levels of circulating glucocorticoids in both humans and rodents, which is a similar hormonal profile to that observed in depressed patients and animal models relevant to depression (Bonnin, 1992; Parker et al., 2003; Glynn et al., 2013; Brummelte and Galea, 2016). In humans, these normative changes in HPA-axis function have been associated with the postpartum blues (O'Keane et al., 2011), which refers to the transient changes in mood and affect during the first couple of weeks postpartum (Pitt, 1973; Henshaw, 2003). Yet, the neural substrates associated with increased affective dysregulation during the early postpartum remain poorly understood. Recently, time-dependent changes in affect-related behavior have been identified in rodents, with postpartum females exhibiting greater negative affect (i.e. low social motivation, increased FST immobility) that is associated with attenuation of VTA DA neuron activity and limited to the first 3 days postpartum (Rincón-Cortés and Grace, under review). Thus, changes in maternal affect with the onset of motherhood may be due to alterations in HPA-axis functioning that transiently alter mesolimbic DA system activity, though this remains to be determined in humans.
In addition to changes in basal HPA activity (i.e. baseline CORT hypersecretion), stress-induced HPA activity is markedly attenuated in postpartum female rodents (Stern et al., 1973; Slattery and Neumann, 2008). Thus, the increase of HPA activity normally seen in response to either physical or psychological stressors in the non-reproductive state become severely attenuated or absent in lactating animals (Lightman et al., 2001; Slattery and Neumann, 2008). Dysregulation of the HPA-axis, particularly in the postpartum period, may increase vulnerability to postpartum depression (PPD) (Stowe and Nemeroff, 1995; Dickens and Pawluski, 2018). For instance, inability to suppress the stress-induced activation of the HPA-axis during pregnancy and the postpartum period has been proposed to play a role in PPD, which is supported by evidence of altered levels of cortisol, ACTH and CRH in patients suffering from PPD (Bloch et al., 2003; de Rezende et al., 2016). Dr. Maguire's research program utilizes preclinical mouse models of postpartum depression. The first model characterized lacks neurosteroid-sensitive GABAA receptors (GABAARs), which exhibit depression and anxiety-like behaviors that are restricted to the postpartum period as well as abnormal maternal care behaviors (Maguire and Mody, 2008). Specifically, Gabrd−/− mice exhibit reduced latency to immobility as well as increased time spent immobile in the FST, decreased sucrose consumption, and reduced aggression towards an intruder, while failing to build a proper nest and dispersing pups which leads to decreased pup survival rate (Maguire and Mody, 2008). Notably, abnormal postpartum behaviors in Gabrd−/− mice are associated with altered stress reactivity and involve hyperexcitability of the HPA-axis during the postpartum period (Maguire and Mody, 2016).
To investigate the contribution of HPA-axis hyperexcitability during the peripartum period in postpartum depression-like behaviors, the Maguire lab generated another preclinical mouse model which lacks the K+/Cl-co-transporter, KCC2 on CRH neurons (KCC2/Crh mice). Prior reports have demonstrated a critical role for KCC2 in the PVN in the regulation of stress-induced activation of the HPA-axis, which involves dephosphorylation of KCC2 at residue Ser940 and downregulation of KCC2 (Sarkar et al., 2011). Through a series of elegant experiments, Maguire and colleagues have demonstrated that suppression of HPA-axis during pregnancy and the postpartum period involves maintenance of KCC2 expression in the PVN (Melon et al., 2018). Mice lacking functional KCC2 specifically in CRH neurons (KCC2/Crh) exhibited inability to suppress the HPA axis in response to acute restraint stress during pregnancy and the postpartum period (Melon et al., 2018). In addition, KCC2/Crh dams also exhibited increased depressive-like behaviors (i.e. FST) and anxiety-like behaviors (i.e. elevated plus maze, light/dark box) during the postpartum period as well as deficits in maternal approach test. Consistent with the role for hypothalamic CRH activity as a contributing factor in abnormal postpartum stress reactivity and postpartum behaviors, chemogenetic activation of CRH neurons in the PVN induced an abnormal postpartum phenotype in wildtype (CRH-Cre) dams. Furthermore, inhibition of CRH neurons in the PVN reversed the abnormal postpartum phenotype observed in KCC2/Crh dams. Taken together, these findings demonstrate that HPA-axis dysregulation is sufficient to induce abnormal postpartum behaviors and deficits in maternal behaviors in preclinical models, and support a novel role for KCC2 in the PVN in peripartum stress hyporeactivity necessary for adaptive maternal behaviors.
4. Influence of sex and gender on stress reactivity in humans
Gendered sexuality comprises a complex set of sexual behaviors, identities and orientations with important health implications (Mayer et al., 2008; O'Hanlan et al., 2018). For example, lesbian, gay and bisexual (LGB) populations are at greater risk for psychiatric disorders compared with heterosexual individuals and these disparities are hypothesized to represent forms of minority stress, referring to the cumulative stress individuals from stigmatized minority groups experience (Cochran et al., 2003; Meyer, 2003). But, do LGB individuals differ in stress reactive cortisol compared with heterosexual individuals? After a trier social stress test (TSST), lesbian/bisexual women manifested peak cortisol concentrations late during recovery from the TSST compared with heterosexual women (Juster et al., 2015). Specifically, peak levels were attained 40 min after the TSST rather than the typical peak at 10–20 min. In contrast, gay/bisexual men displayed lower overall cortisol concentrations throughout testing as well as specifically 10 and 20 min after stress exposure when compared with heterosexual men, which may suggest HPA-axis downregulation (Juster et al., 2015). These findings were significant even while adjusting for sex hormones (estradiol to progesterone ratio in women and testosterone in men), age, self-esteem, and disclosure status. These data provide evidence for gender-based modulation of cortisol stress reactivity based on sexual orientation, which can help differentiate between sex-based versus gender-based factors that modulate endocrine stress reactivity and underline the importance of assessing sexual orientation during tests of endocrine stress reactivity in humans.
To add complexity to the study of stress reactivity in humans, there is also evidence that sex hormones vary according to sexual orientation in women, but also according to stress indices (Juster et al., 2016). Lesbian/bisexual women showed higher overall testosterone and progesterone concentrations as a group in comparison to heterosexual women, while no differences were found among gay/bisexual men when compared to heterosexual men. Lesbian, bisexual women and heterosexual men showed positive associations between mean estradiol concentrations and allostatic load, while gay/bisexual men and heterosexual women showed positive associations between mean testosterone and cortisol systemic output. In sum, these data suggest that sex hormone variations appear to differ according to sexual orientation among women, but also as a function of cortisol systemic output allostatic load and perceived stress for both sexes. However, it is unclear whether these differences are due to biological factors and/or arise from cultural and/or environmental influences.
5. Conclusions
Stress-induced activation of the HPA-axis varies between the sexes. Recent preclinical research indicates sex-differences in response to both uncontrollable and controllable stressors, in which females exhibit adverse effects that are not modulated by stressor controllability as well as distinct patterns of stress-induced neuronal activity and morphology. Since males and female rats exhibit differential effects of stress exposure on cortical (PFC) and midbrain structures (VTA, DRN) implicated in the etiology of mood and anxiety disorders in humans, sex-specific alterations induced by stress within these areas may increase risk for psychiatric disorders that are more prevalent in women (i.e. depression, anxiety, PTSD). Thus, the sex-dependent effects of stress in the structure and function of cortical and midbrain systems may not only reflect enhanced stress sensitivity in females but also serve as underlying mechanism leading to sex-biased stress-related disorders. Furthermore, the developmental context in which stressor exposure occurs has important implications for programming later life behavior and brain function. Adolescent chronic stress results in short and long-term changes in the selection of behavioral and HPA responses to stress that vary between the sexes. Importantly, environmental enrichment can confer resilience to the adult neurobiological sequelae induced by adolescent stress, although this effect is sex-specific and seems to be limited to females. This is significant given that: a) mood and anxiety disorders frequently emerge during adolescence and are more common in females; and, b) environmental manipulations are highly beneficial to humans because they provide a safe therapeutic alternative to pharmacological interventions. In females, the postpartum represents a period of marked adaptation of the HPA-axis and is characterized by basal hypersecretion and stress-induced hyposecretion of CORT. In rodents, these normative hormonal changes are associated with time-limited changes in affect-related behaviors as well as within the mesolimbic DA system. In a mouse model of PPD, KCC2 loss within PVN CRH neurons overrides this dampened stress reactivity and results in inappropriate HPA-axis responses to stress and abnormal maternal behaviors, suggesting a mechanism by which HPA-axis dysfunction during the postpartum confers susceptibility to affective dysregulation and aberrant maternal behavior. Finally, biological sex differences and sociocultural gender diversity influence endocrine stress reactivity in humans; and, interactions between the HPA-axis and sex hormones (e.g., testosterone, estrogen) can influence biological sex differences in stress response patterns.
Funding and disclosures
M.R.C was supported by F32-MH110128. J.P.H. is supported by MH101729 and MH049698. J.M. was supported by NS073574 and NS102937.
References
- Altemus M. Sex differences in depression and anxiety disorders: potential biological determinants. Horm. Behav. 2006;50:534–538. doi: 10.1016/j.yhbeh.2006.06.031. [DOI] [PubMed] [Google Scholar]
- Altemus M., Sarvaiya N., Neill Epperson C. Sex differences in anxiety and depression clinical perspectives. Front. Neuroendocrinol. 2014;35:320–330. doi: 10.1016/j.yfrne.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J., Paul E., Zarza C., Watkins L.R., Maier S.F. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J. Neurosci. 2006;26:13264–13272. doi: 10.1523/JNEUROSCI.3630-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J., Baratta M.V., Paul E., Bland S.T., Watkins L.R., Maier S.F. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat. Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Andersen S.L., Teicher M.H. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Bale T.L., Epperson C.N. Sex differences and stress across the lifespan. Nat. Neurosci. 2015;18:1413–1420. doi: 10.1038/nn.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D.A., Valentino R.J. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front. Neuroendocrinol. 2014;35:303–319. doi: 10.1016/j.yfrne.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratta M.V., Leslie N.R., Fallon I.P., Dolzani S.D., Chun L.E., Tamalunas A.M. Behavioural and neural sequelae of stressor exposure are not modulated by controllability in females. Eur. J. Neurosci. 2018;47:959–967. doi: 10.1111/ejn.13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J.B., Monteggia L.M., Perrot-Sinal T.S., Romeo R.D., Taylor J.R., Yehuda R. Stress and disease: is being female a predisposing factor? J. Neurosci. 2007;27:11851–11855. doi: 10.1523/JNEUROSCI.3565-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch M., Daly R.C., Rubinow D.R. Endocrine factors in the etiology of postpartum depression. Compr. Psychiatr. 2003;44:234–246. doi: 10.1016/S0010-440X(03)00034-8. [DOI] [PubMed] [Google Scholar]
- Bonnin F. Cortisol levels in saliva and mood changes in early puerperium. J. Affect. Disord. 1992;26:231–239. doi: 10.1016/0165-0327(92)90100-k. [DOI] [PubMed] [Google Scholar]
- Bourke C.H., Neigh G.N. Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Horm. Behav. 2011;60:112–120. doi: 10.1016/j.yhbeh.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman R.E., Maclusky N.J., Diaz S.E., Zrull M.C., Luine V.N. Aged rats: sex differences and responses to chronic stress. Brain Res. 2006;1126:156–166. doi: 10.1016/j.brainres.2006.07.047. [DOI] [PubMed] [Google Scholar]
- Brummelte S., Galea L.A. Postpartum depression: etiology, treatment and consequences for maternal care. Horm. Behav. 2016;77:153–166. doi: 10.1016/j.yhbeh.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Brunton P.J., Russell J.A., Douglas A.J. Adaptive responses of the maternal hypothalamic-pituitary-adrenal axis during pregnancy and lactation. J. Neuroendocrinol. 2008;20:764–776. doi: 10.1111/j.1365-2826.2008.01735.x. [DOI] [PubMed] [Google Scholar]
- Chang C.H., Grace A.A. Amygdala-ventral pallidum pathway decreases dopamine activity after chronic mild stress in rats. Biol. Psychiatry. 2014;76:223–230. doi: 10.1016/j.biopsych.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran S.D., Mays V.M., Sullivan J.G. Prevalence of mental disorders, psychological distress, and mental health services use among lesbian, gay, and bisexual adults in the United States. J. Consult. Clin. Psychol. 2003;71:53–61. doi: 10.1037//0022-006x.71.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colom-Lapetina J., Begley S.L., Johnson M.E., Bean K.J., Kuwamoto W.N., Shansky R.M. Strain-dependent sex differences in a long-term forced swim paradigm. Behav. Neurosci. 2017;131:428–436. doi: 10.1037/bne0000215. [DOI] [PubMed] [Google Scholar]
- Cotella E.M., Gomez A.S., Lemen P., Chen C., Fernandez G., Hansen C., Herman Long-term impact of chronic variable stress in adolescence versus adulthood. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2018;88:303–310. doi: 10.1016/j.pnpbp.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla C., Pitychoutis P.M., Kokras N., Papadopoulou-Daifoti Z. Sex differences in response to stress and expression of depressive-like behaviours in the rat. Curr Top Behav Neurosci. 2011;8:97–118. doi: 10.1007/7854_2010_94. [DOI] [PubMed] [Google Scholar]
- Dalla C., Antoniou K., Kokras N., Drossopoulou G., Papathanasiou G., Bekris S. Sex differences in the effects of two stress paradigms on dopaminergic neurotransmission. Physiol. Behav. 2008;93:595–605. doi: 10.1016/j.physbeh.2007.10.020. [DOI] [PubMed] [Google Scholar]
- de Kloet E.R., Molendijk M.L. Coping with the forced swim stressor: towards understanding an adaptive mechanism. Neural Plast. 2016;2016:6503162. doi: 10.1155/2016/6503162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet E.R., Oitzl M.S., Joels M. Functional implications of brain corticosteroid receptor diversity. Cell. Mol. Neurobiol. 1993;13:433–455. doi: 10.1007/BF00711582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rezende M.G., Garcia-Leal C., de Figueiredo F.P., Cavalli Rde C., Spanghero M.S. Altered functioning of the HPA axis in depressed postpartum women. J. Affect. Disord. 2016;193:249–256. doi: 10.1016/j.jad.2015.12.065. [DOI] [PubMed] [Google Scholar]
- Dickens M.J., Pawluski J.L. The HPA Axis during the perinatal period: implications for perinatal depression. Endocrinology. 2018;159:3737–3746. doi: 10.1210/en.2018-00677. [DOI] [PubMed] [Google Scholar]
- Drossopoulou G., Antoniou K., Kitraki E., Papathanasiou G., Papalexi E., Dalla C. Sex differences in behavioral, neurochemical and neuroendocrine effects induced by the forced swim test in rats. Neuroscience. 2004;126:849–857. doi: 10.1016/j.neuroscience.2004.04.044. [DOI] [PubMed] [Google Scholar]
- Eiland L., Romeo R.D. Stress and the developing adolescent brain. Neuroscience. 2013;249:162–171. doi: 10.1016/j.neuroscience.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Guasti A., Fiedler J.L., Herrera L., Handa R.J. Sex, stress, and mood disorders: at the intersection of adrenal and gonadal hormones. Horm. Metab. Res. 2012;44:607–618. doi: 10.1055/s-0032-1312592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn L.M., Davis E.P., Sandman C.A. New insights into the role of perinatal HPA-axis dysregulation in postpartum depression. Neuropeptides. 2013;47:363–370. doi: 10.1016/j.npep.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Goel N., Workman J.L., Lee T.T., Innala L., Viau V. Sex differences in the HPA axis. Comp. Physiol. 2014;4:1121–1155. doi: 10.1002/cphy.c130054. [DOI] [PubMed] [Google Scholar]
- Goosens K.A., Sapolsky R.M. Stress and glucocorticoid contributions to normal and pathological aging. In: Riddle D.R., editor. Brain Aging: Models, Methods, and Mechanisms. 2007. Boca Raton (FL) [PubMed] [Google Scholar]
- Grahn R.E., Will M.J., Hammack S.E., Maswood S., McQueen M.B., Watkins L.R. Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Res. 1999;826:35–43. doi: 10.1016/s0006-8993(99)01208-1. [DOI] [PubMed] [Google Scholar]
- Henshaw C. Mood disturbance in the early puerperium: a review. Arch Womens Ment Health. 2003;6(Suppl. 2):S33–S42. doi: 10.1007/s00737-003-0004-x. [DOI] [PubMed] [Google Scholar]
- Herman J.P. Regulation of adrenocorticosteroid receptor mRNA expression in the central nervous system. Cell. Mol. Neurobiol. 1993;13:349–372. doi: 10.1007/BF00711577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J.P., McKlveen J.M., Ghosal S., Kopp B., Wulsin A., Makinson R. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Comp. Physiol. 2016;6:603–621. doi: 10.1002/cphy.c150015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankord R., Solomon M.B., Albertz J., Flak J.N., Zhang R., Herman J.P. Stress vulnerability during adolescent development in rats. Endocrinology. 2011;152:629–638. doi: 10.1210/en.2010-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster R.P., Almeida D., Cardoso C., Raymond C., Johnson P.J., Pfaus J.G. Gonads and strife: sex hormones vary according to sexual orientation for women and stress indices for both sexes. Psychoneuroendocrinology. 2016;72:119–130. doi: 10.1016/j.psyneuen.2016.06.011. [DOI] [PubMed] [Google Scholar]
- Juster R.P., Hatzenbuehler M.L., Mendrek A., Pfaus J.G., Smith N.G., Johnson P.J. Sexual orientation modulates endocrine stress reactivity. Biol. Psychiatry. 2015;77:668–676. doi: 10.1016/j.biopsych.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller-Wood M.E., Dallman M.F. Corticosteroid inhibition of ACTH secretion. Endocr. Rev. 1984;5:1–24. doi: 10.1210/edrv-5-1-1. [DOI] [PubMed] [Google Scholar]
- Kitay J.I. Sex differences in adrenal cortical secretion in the rat. Endocrinology. 1961;68:818–824. doi: 10.1210/endo-68-5-818. [DOI] [PubMed] [Google Scholar]
- Klein Z.A., Romeo R.D. Changes in hypothalamic-pituitary-adrenal stress responsiveness before and after puberty in rats. Horm. Behav. 2013;64:357–363. doi: 10.1016/j.yhbeh.2013.01.012. [DOI] [PubMed] [Google Scholar]
- Kudielka B.M., Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol. Psychol. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Lightman S.L., Windle R.J., Wood S.A., Kershaw Y.M., Shanks N., Ingram C.D. Peripartum plasticity within the hypothalamo-pituitary-adrenal axis. Prog. Brain Res. 2001;133:111–129. doi: 10.1016/s0079-6123(01)33009-1. [DOI] [PubMed] [Google Scholar]
- Luine V.N., Beck K.D., Bowman R.E., Frankfurt M., Maclusky N.J. Chronic stress and neural function: accounting for sex and age. J. Neuroendocrinol. 2007;19:743–751. doi: 10.1111/j.1365-2826.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- Lupien S.J., McEwen B.S., Gunnar M.R., Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Maguire J., Mody I. GABA(A)R plasticity during pregnancy: relevance to postpartum depression. Neuron. 2008;59:207–213. doi: 10.1016/j.neuron.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J., Mody I. Behavioral deficits in juveniles mediated by maternal stress hormones in mice. Neural Plast. 2016;2016:2762518. doi: 10.1155/2016/2762518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier S.F. Behavioral control blunts reactions to contemporaneous and future adverse events: medial prefrontal cortex plasticity and a corticostriatal network. Neurobiol Stress. 2015;1:12–22. doi: 10.1016/j.ynstr.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier S.F., Amat J., Baratta M.V., Paul E., Watkins L.R. Behavioral control, the medial prefrontal cortex, and resilience. Dialogues Clin. Neurosci. 2006;8:397–406. doi: 10.31887/DCNS.2006.8.4/smaier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maswood S., Barter J.E., Watkins L.R., Maier S.F. Exposure to inescapable but not escapable shock increases extracellular levels of 5-HT in the dorsal raphe nucleus of the rat. Brain Res. 1998;783:115–120. doi: 10.1016/s0006-8993(97)01313-9. [DOI] [PubMed] [Google Scholar]
- Mayer K.H., Bradford J.B., Makadon H.J., Stall R., Goldhammer H., Landers S. Sexual and gender minority health: what we know and what needs to be done. Am. J. Public Health. 2008;98:989–995. doi: 10.2105/AJPH.2007.127811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick C.M., Green M.R. From the stressed adolescent to the anxious and depressed adult: investigations in rodent models. Neuroscience. 2013;249:242–257. doi: 10.1016/j.neuroscience.2012.08.063. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Stress, adaptation, and disease. Allostasis and allostatic load. Ann. N. Y. Acad. Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Mood disorders and allostatic load. Biol. Psychiatry. 2003;54:200–207. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Stellar E. Stress and the individual. Mechanisms leading to disease. Arch. Intern. Med. 1993;153:2093–2101. [PubMed] [Google Scholar]
- McEwen B.S., Nasca C., Gray J.D. Stress effects on neuronal structure: Hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2016;41:3–23. doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melon L.C., Hooper A., Yang X., Moss S.J., Maguire J. Inability to suppress the stress-induced activation of the HPA axis during the peripartum period engenders deficits in postpartum behaviors in mice. Psychoneuroendocrinology. 2018;90:182–193. doi: 10.1016/j.psyneuen.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer I.H. Prejudice, social stress, and mental health in lesbian, gay, and bisexual populations: conceptual issues and research evidence. Psychol. Bull. 2003;129:674–697. doi: 10.1037/0033-2909.129.5.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hanlan K.A., Gordon J.C., Sullivan M.W. Biological origins of sexual orientation and gender identity: impact on health. Gynecol. Oncol. 2018;149:33–42. doi: 10.1016/j.ygyno.2017.11.014. [DOI] [PubMed] [Google Scholar]
- O'Keane V., Lightman S., Patrick K., Marsh M., Papadopoulos A.S., Pawlby S. Changes in the maternal hypothalamic-pituitary-adrenal axis during the early puerperium may be related to the postpartum 'blues'. J. Neuroendocrinol. 2011;23:1149–1155. doi: 10.1111/j.1365-2826.2011.02139.x. [DOI] [PubMed] [Google Scholar]
- Oyola M.G., Handa R.J. Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: sex differences in regulation of stress responsivity. Stress. 2017;20:476–494. doi: 10.1080/10253890.2017.1369523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G., Brotchie H. Gender differences in depression. Int. Rev. Psychiatry. 2010;22:429–436. doi: 10.3109/09540261.2010.492391. [DOI] [PubMed] [Google Scholar]
- Parker K.J., Schatzberg A.F., Lyons D.M. Neuroendocrine aspects of hypercortisolism in major depression. Horm. Behav. 2003;43:60–66. doi: 10.1016/s0018-506x(02)00016-8. [DOI] [PubMed] [Google Scholar]
- Pitt B. Maternity blues. Br. J. Psychiatry. 1973;122:431–433. doi: 10.1192/bjp.122.4.431. [DOI] [PubMed] [Google Scholar]
- Porsolt R.D., Le Pichon M., Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Rincon-Cortes M., Grace A.A. Sex-dependent effects of stress on immobility behavior and VTA dopamine neuron activity: modulation by ketamine. Int. J. Neuropsychopharmacol. 2017;20:823–832. doi: 10.1093/ijnp/pyx048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo R.D., Patel R., Pham L., So V.M. Adolescence and the ontogeny of the hormonal stress response in male and female rats and mice. Neurosci. Biobehav. Rev. 2016;70:206–216. doi: 10.1016/j.neubiorev.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J.A., Douglas A.J., Ingram C.D. Brain preparations for maternity-adaptive changes in behavioral and neuroendocrine systems during pregnancy and lactation. An overview. Prog. Brain Res. 2001;133:1–38. doi: 10.1016/s0079-6123(01)33002-9. [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M. Glucocorticoids, stress, and their adverse neurological effects: relevance to aging. Exp. Gerontol. 1999;34:721–732. doi: 10.1016/s0531-5565(99)00047-9. [DOI] [PubMed] [Google Scholar]
- Sarkar J., Wakefield S., MacKenzie G., Moss S.J., Maguire J. Neurosteroidogenesis is required for the physiological response to stress: role of neurosteroid-sensitive GABAA receptors. J. Neurosci. 2011;31:18198–18210. doi: 10.1523/JNEUROSCI.2560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky R.M. Sex differences in PTSD resilience and susceptibility: challenges for animal models of fear learning. Neurobiol Stress. 2015;1:60–65. doi: 10.1016/j.ynstr.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery D.A., Neumann I.D. No stress please! Mechanisms of stress hyporesponsiveness of the maternal brain. J. Physiol. 2008;586:377–385. doi: 10.1113/jphysiol.2007.145896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B.L., Morano R.L., Ulrich-Lai Y.M., Myers B., Solomon M.B., Herman J.P. Adolescent environmental enrichment prevents behavioral and physiological sequelae of adolescent chronic stress in female (but not male) rats. Stress. 2017:1–10. doi: 10.1080/10253890.2017.1402883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Vale W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006;8:383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M.B., Herman J.P. Sex differences in psychopathology: of gonads, adrenals and mental illness. Physiol. Behav. 2009;97:250–258. doi: 10.1016/j.physbeh.2009.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear L.P. Heightened stress responsivity and emotional reactivity during pubertal maturation: implications for psychopathology. Dev. Psychopathol. 2009;21:87–97. doi: 10.1017/S0954579409000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern J.M., Goldman L., Levine S. Pituitary-adrenal responsiveness during lactation in rats. Neuroendocrinology. 1973;12:179–191. doi: 10.1159/000122167. [DOI] [PubMed] [Google Scholar]
- Stowe Z.N., Nemeroff C.B. Women at risk for postpartum-onset major depression. Am. J. Obstet. Gynecol. 1995;173:639–645. doi: 10.1016/0002-9378(95)90296-1. [DOI] [PubMed] [Google Scholar]
- Windle R.J., Wood S.A., Kershaw Y.M., Lightman S.L., Ingram C.D. Adaptive changes in basal and stress-induced HPA activity in lactating and post-lactating female rats. Endocrinology. 2013;154:749–761. doi: 10.1210/en.2012-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulsin A.C., Wick-Carlson D., Packard B.A., Morano R., Herman J.P. Adolescent chronic stress causes hypothalamo-pituitary-adrenocortical hypo-responsiveness and depression-like behavior in adult female rats. Psychoneuroendocrinology. 2016;65:109–117. doi: 10.1016/j.psyneuen.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]