Abstract
Purpose:
To determine the feasibility, safety, and tolerability of concomitant chemoradiotherapy administered at standard doses in HIV-infected women with locally-advanced cervical cancer (LACC) receiving antiretroviral therapy (ART).
Patients and Methods:
Eligible participants had HIV infection and untreated, histologically-confirmed, invasive carcinoma of the uterine cervix, FIGO stages IB2, IIA (if tumor greater than 4 cm), IIB, IIIA, IIIB, or IVA and met standard eligibility criteria. Subjects were prescribed 41.4-45 Gy external beam radiation therapy followed by high dose rate brachytherapy concomitant with up to six weekly doses of cisplatin 40 mg/m2 and were followed for 12 months.
Results:
Sixty-four women were screened at two sites in sub-Saharan Africa, of whom 40 eligible participants were enrolled, for a screening ratio of 1.60. Of the 38 eligible participants who initiated study treatment, 31 (82%) completed treatment. By the 12-month follow-up visit, 7 women had died of disease and 29 of 31 (94%) returned for follow-up. One-year progression-free survival was 76.3% (95% CI, 59.4-86.9%), and did not significantly differ according to stage at entry (p=0.581). Participant-reported adherence to ART was high; by 12 months, 93% of participants had an undetectable viral load. The most common grade 3 or 4 adverse event was decreased lymphocyte count that affected all treated participants. Non-hematologic serious adverse events were similar to those observed in women with LACC without HIV infection.
Conclusions:
The majority of HIV-infected women with LACC can complete concomitant chemoradiotherapy with the same cisplatin dose used in HIV-uninfected women with comparable tolerability and high ART adherence while on treatment.
Background
Cervical cancer is the second most commonly diagnosed cancer and third leading cause of cancer death among women in less-developed countries. Nearly 90% of cervical cancer deaths occur in the developing parts of the world. The incidence rates of cervical cancer are highest in sub-Saharan Africa where a high percentage of patients with cervical cancer have not participated in screening programs, present with more advanced local disease and have substantially lower cure rates[1-4].
Cervical cancer is also more common in women infected with the human immunodeficiency virus (HIV) than HIV-uninfected women[5]. Among women with HIV infection, locally-advanced cervical cancer (LACC) causes significant morbidity often at the prime of their reproductive lives[6, 7]. In a large cohort of HIV-positive women with LACC in Botswana, nearly all (96.9%) participants presented with symptomatic cancer and 80.8% were stage IIB or greater at the time of diagnosis[8].
Concomitant chemoradiotherapy using cisplatin as the chemotherapeutic radiosensitizing agent is the standard of care for the treatment of LACC. Multiple randomized, controlled trials (RCT) in HIV-uninfected women with LACC showed improved efficacy when cisplatin was added to radiation therapy, even in women who had histologic evidence of metastatic disease to pelvic and/or para-aortic lymph nodes [9]. Data from 5 RCTs (GOG 85, RTOG 9001, GOG 120, SWOG 8797, GOG 123) prompted an NCI alert in February 1999 that recommended consideration of concomitant chemoradiotherapy for all women with cervical cancer[2, 10]. A Cochrane database review of 19 RCTs confirmed that concomitant chemoradiotherapy improved overall survival (OS) and progression-free survival (PFS[11]) in LACC compared to radiotherapy alone.
The effects of anti-retroviral therapy (ART) on standard treatments for cervical cancer are unknown largely because HIV-infected women with LACC have typically been excluded from large-scale clinical trials[12]. Many antiretroviral agents are known to cause drug-drug interactions, so co-administration of standard therapies with ART could influence the efficacy and increase the toxicity of these treatments. Additionally, HIV-infected women with cervical cancer often have a poor nutritional status with limited marrow reserve while they are undergoing high-dose pelvic radiation therapy concomitant with chemotherapy, further accentuating potential toxicities. [5]. Due to these factors, some clinicians initiate therapy with lower than standard weekly radiosensitizing doses of cisplatin[13], despite a paucity of data suggesting that reduced cisplatin doses result in reduced toxicity. Here we report the data from a prospective phase II open-label study, AMC-081 (NCT01590017), to evaluate the feasibility and safety of concomitant chemoradiotherapy with ART administered at standard doses in HIV-infected women with LACC at two sites in sub-Saharan Africa that were part of the AIDS Malignancy Consortium (AMC), a National Cancer Institute-supported clinical trials group.
Study Design
Objectives
The primary objectives of this study were to evaluate the feasibility, safety, and tolerability of concomitant chemoradiotherapy with cisplatin in HIV-infected women with LACC who were receiving concomitant ART. Feasibility was assessed based on 1) the proportion of participants who completed the concomitant chemoradiotherapy regimen, 2) the screening ratio defined as the number of potential participants screened per enrolled participant, and 3) availability proportion defined as the completion proportion at 6 and 12 months. Exploratory objectives also included determination of 1) the 1-year PFS and OS, 2) the effects of treatment on HIV control, 3) the recurrence patterns for HIV-infected women with LACC, and 4) self-reported adherence to ART during concomitant chemoradiotherapy.
Eligibility Criteria
Eligible participants were at least 18 years old with an ECOG performance status of ≤ 2 with untreated, histologically-confirmed, documented invasive squamous cell carcinoma, adenocarcinoma, or adenosquamous carcinoma of the uterine cervix, FIGO stages IB2, IIA (if tumor greater than 4 cm), IIB, IIIA, IIIB, or IVA (2009 standard). All participants were required to have HIV-1 infection documented by a primary rapid test or E/CIA test kit and confirmed by Western blot or secondary antibody test and the following laboratory criteria: hemoglobin ≥10 g/dL (6.2 mmol/L), platelet count ≥ 100,000/mm3 (100 × 109/L), ANC ≥ 1500/mm3 (1.5 × 109/L), creatinine clearance ≥ 60 mL/min (1.00 mL/s) calculated by the Cockcroft-Gault equation for women, AST and ALT ≤3 times the upper limit of normal (ULN), total bilirubin ≤2 times the ULN unless related to antiretroviral use (e.g., atazanavir and indinavir), in which case the direct bilirubin had to be ≤2 times the ULN. Participants receiving transfusion, erythropoietin, or myeloid growth factor support were eligible for this study. All participants were to be prescribed combination ART with the goal of virological suppression using an acceptable regimen that adhered to national guidelines for treatment of HIV infection. ART treatment-experienced participants, defined as women with a HIV viral load > 400 copies/mL who have been on ART for more than 4 months could be enrolled if an alternative ART regimen was available that included at least two ART drugs that, in the opinion of the site investigator, were expected to have activity based on genotypic testing and treatment history.
Women were excluded if they had a hysterectomy, prior malignancy within the past 24 months (Kaposi sarcoma that did not require systemic therapy was allowed), were pregnant or breast feeding, had an acute active uncontrolled infection such as tuberculosis or malaria or had a history of significant cardiovascular disease. Women were excluded if imaging showed enlarged para-aortic lymph nodes that were suspicious for metastasis.
Recruitment procedures
Participants were enrolled at two AIDS Malignancy Consortium sites located in Johannesburg, South Africa and Harare, Zimbabwe. Quality assurance for radiation equipment and review of treatment planning at both sites was provided by the NCI-supported Imaging and Radiation Oncology Core (IROC, MD Anderson Houston, Texas, USA). Sites had the protocol approved by their Institutional Review Board (IRB)/Independent Ethics Committee (IEC) and any relevant national and local authorities.
The local pathology review was done prior to enrollment to confirm participant eligibility. A central pathology review (K.W.) was performed for all study participants, which confirmed the local diagnosis and histology in all cases. One participant was enrolled with histology that did not meet eligibility criteria (adenoid basal carcinoma). She was treated as per protocol and her toxicity data was included in this report, but efficacy and other data were excluded.
Study modalities
Radiation therapy procedures
After clinical staging and study-mandated CT of the abdomen and pelvis to document the absence of extra-pelvic disease, all participants received cisplatin concomitant with external beam radiation therapy and brachytherapy (see Figure 1 for study schema). Radiation therapy (RT) began within 7 days of enrollment. All participants underwent CT treatment planning and IMRT was not allowed in this study. All participants received 41.4-45 Gy external beam RT delivered to the pelvis in 23-25 fractions of 1.8 Gy. Treatments were delivered daily, five fractions per week and completed within 5 weeks ± 3 days. Bladder distention and bowel exclusion devices were allowed. Attempts were made to exclude all small bowel from treatment field after 50.4 Gy.
Figure 1:
Study schema
Following completion of whole pelvic RT each participant received high dose rate (HDR) intracavity brachytherapy using vaginal applicators and a standard medical source listed on the American Association of Physicists in Medicine (AAPM) source registry [14]. Dose specifications were calculated by accepted global standards. All participants received 35-43.6Gy equivalent dose (EQD2) to Point A by brachytherapy using one of the following regiments: two fractions of 9Gy, three fractions of 7-8Gy, or four fractions of 6-7Gy. Thus the combination of whole pelvic RT and brachytherapy resulted in a total equivalent dose of 80-85Gy to the target.
Six participants received a parametrial boost at the discretion of the treating radiation oncologist. The radiation oncologist chose the dose to be delivered to the involved parametrium based on bulk of parametrial disease at presentation. The parametrial boost was 5.4-9.0 Gy in 3-5 fractions of 1.8 Gy per fraction given using opposed fields – AP/PA (anterior-posterior/posterior-anterior), daily to midplane if unilateral or bilateral parametrial boost was used.
Every effort was made to encourage the participant to comply with the radiation treatment prescribed and complete therapy within 56 days. Quality control for all of the treatment plans were performed by IROC and final review was performed by one of the radiation oncology investigators (M.G.).
Chemotherapy and Antiretroviral therapy
Cisplatin 40 mg/m2 was administered intravenously over 30-60 minutes weekly on days 1, 8, 15, 22, 29 and 36 for up to a total of 6 weekly cycles. Three participants with CD4+ T-cell counts under 200cells/mm3 were started at 25 mg/m2 as per protocol. All participants were prescribed combination ART. Participants already on an effective ART regimen remained on that regimen during study treatment. Participants could begin ART on the day of study enrollment to meet eligibility criteria. Although specific drugs were not prohibited, investigators were advised to avoid didanosine, stavudine, zidovudine and tenofovir because of potentially overlapping neurological, hematologic, and renal toxicities with cisplatin. If co-administration was unavoidable and graded toxicities occurred that required modification of the cisplatin dose, the use of alternative antiretroviral drugs without overlapping toxicities were considered before modifying the cisplatin dose.
All toxicities were graded according to the Common Toxicity Criteria (CTCAE v4.0). Cisplatin dose modifications were prespecified for grade 2 or higher hematologic and non-hematologic toxicities. The following levels were applied sequentially for cisplatin dose reductions: dose level −1=30 mg/m2, dose level −2=25 mg/m2. If graded adverse events recurred on dose level −2, cisplatin was discontinued. Dose reductions based on hematologic parameters are described in Table 1. Adverse event reporting was performed according to NCI Cancer Therapy Evaluation Program (CTEP, http://ctep.cancer.gov) guidelines.
Table 1:
Dose reduction for granulocyte, absolute neutrophil count and/or platelet counts*
| ANC or granulocytes/μL |
Platelets/μL | Cisplatin | |
|---|---|---|---|
| ≥ 1,500 | and | ≥ 100,000 | No dose reduction |
| 1,499-1,000 | or | 99,999-75,000 | Reduce by one dose level* |
| < 1,000 | or | < 75,000 | Hold* |
If the maximum number of prior dose reductions of cisplatin have been made, treatment was held for up to two weeks for blood count recovery
Monitoring
All participants were monitored weekly during chemoradiotherapy with complete blood count and differential, serum creatinine, electrolytes, review of the interim medical history including concomitant medications and adverse events, and assessment of ART adherence, and these assessments were repeated at completion of chemoradiotherapy. Physical examination was repeated at day 22 and at the completion of chemoradiotherapy. After completing protocol therapy, participants were followed every 3 months for one year. Physical examination and review of the interim medical history, concomitant medications and review of adverse events was performed at each follow-up visit, and CD4 T-cell count, HIV viral load, and CT scan of the abdomen and pelvis were performed at 6-month intervals.
Statistical considerations
The sample size was based on testing the null hypothesis that the treatment completion rate is 0.50 against the alternative that it is ≥ 0.70 at the one-sided 0.10 significance level with power of 0.90 using a one-sample Chi-square test. This yielded a sample size of 38 study participants. The primary outcome measure of treatment completion rate was estimated as the proportion of all women initiating treatment who completed cisplatin treatment with 95% confidence interval. Successful completion of concomitant chemoradiotherapy is defined as receiving 5 or more cycles of cisplatin and completion of the equivalent doses per fraction of >78Gy to Point A. The screening ratio was estimated as the number of women screened divided by the number of enrolled subjects and its 95% confidence interval was based on the Poisson distribution. The Kaplan-Meier method was used to describe PFS and OS.
Results
Sixty-four women were screened at two sites, one in Zimbabwe (Parirenyatwa Hospital) and one in South Africa (Charlotte Maxeke Johannesburg Academic Hospital), of whom 40 eligible participants were enrolled, for a screening ratio of 1.60 (95% CI, 1.25 to 2.04). 41 were screened at the Zimbabwe site of whom 26 were enrolled. 23 were screened at the Johannesburg site of whom 14 were enrolled. Of the 24 women who were screened and ineligible, the most common reasons were metastatic disease identified on screening CT scan (n=10, 43%) and abnormal laboratory results (n=10, 43%). Additionally, one had an active pelvic thrombus, one was reclassified as stage IB1, and one had an ectopic kidney. One participant was inadvertently enrolled with a diagnosis of adenoid basal carcinoma. This participant was considered evaluable for toxicity but was excluded from all other analyses.
Eligible participants were an average (SD) of 44.0 (5.5) years of age, all having acquired HIV through heterosexual contact with a median (range) CD4 count of 424 cells/mm3 (139-1204). ART for participants is shown in Table 2. Most participants had invasive squamous cell carcinoma (90%); the remaining eligible participants had adenocarcinoma or adenosquamous carcinoma. Of the 40 enrolled patients, 70% had stage IIB disease and 60% had bilateral parametrial involvement. Sites of involvement included vagina (60%), nodes (28%), bladder (5%), rectum (3%) and other pelvic sites (43%) (Table 3).
Table 2:
ART regimens in AMC-081
| ART regimen | N (%) |
|---|---|
| NNRTI-based regimens | 38 |
| Efavirenz-based regimen | 30 (79) |
| Nevirapine-based regimen | 8 (21) |
| Protease inhibitor-based regimen | 2 |
| Atazanavir | 1(2) |
| Lopinavir/ritonavir | 1(2) |
Table 3:
Cervical cancer characteristics at enrollment
| Disease Characteristics | N(%) |
|---|---|
| Histologic Diagnosis | |
| Squamous cell carcinoma | 36 (90) |
| Adenocarcinoma | 3 (8) |
| Adenosquamous carcinoma | 1 (3) |
| Grade of Disease | |
| Well-differentiated | 1 (3) |
| Moderately-differentiated | 25 (63) |
| Poorly-differentiated | 14 (35) |
| FIGO Stage at Entry (Clinical Staging) | |
| Stage IIA | 1 (3) |
| Stage IIB | 28 (70) |
| Stage IIIA | 1 (3) |
| Stage IIIB | 10 (25) |
Of the 40 enrolled participants, two withdrew prior to receiving treatment. Of the 38 treated participants, 31 (82%) completed treatment (90% one-sided confidence interval: 69% to 100%). The null hypothesis that the treatment completion rate is 50% was rejected (p<0.001). Three participants were treated at a lower protocol-specified dose of cisplatin (25mg/m2) due to low CD4 counts (<200mm3/ml). Of 38 treated participants, 50% received 5-6 cisplatin cycles with radiation therapy at the full prescribed dose without delays or modifications, and 100% of the treated participants completed 5-6 cycles of cisplatin with radiation therapy. In the safety population, which included the one ineligible treated participant, 20 participants experienced 44 adverse events that resulted in treatment delay or discontinuation. Three participants developed decreased white count/neutropenia resulting in treatment discontinuation (all decreases in white count/neutropenia), two of whom also had treatment delays.
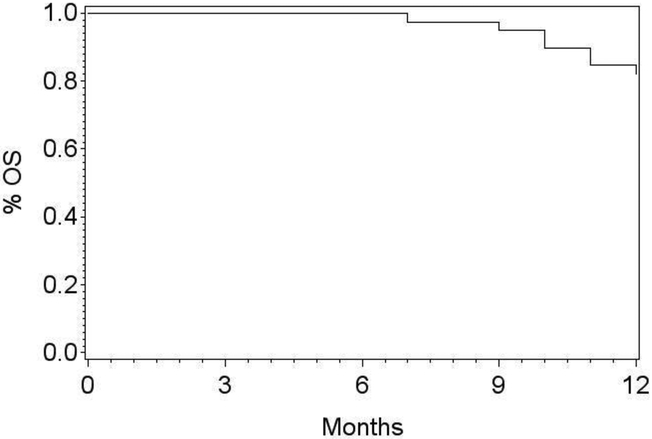
Of the 38 eligible participants who initiated study treatment, 33 (87%) returned at the 6-month follow-up. By the time of the 12-month follow-up, 7 women had died of progressive cervical cancer. Of the remaining 31 subjects, 29 (94%) returned at the 12-month follow-up. One-year overall survival was 81.6% (95% CI, 65.2 to 90.8%) (Figure 2). The one-year PFS was 76.3% (95% CI, 59.4-86.9%), and did not significantly differ according to stage at entry (p=0.581). The estimate of one-year PFS was 75.9% (95% CI, 55.9 to 87.7%) for stage IIA/B and 77.8% (95% CI, 36.5 to 93.9%) for stage IIIA/B. Among the 31 women who completed treatment, the one-year PFS was 77.4 (95% CI, 58.4 to 88.5%). By 12 months, 11 treated participants had shown tumor recurrence, three in the pelvis only, seven in both the pelvis and one or more new extra-pelvic sites and one at a new extra-pelvic site only. Sites of extra-pelvic recurrence included paraaortic nodes (n=5), lungs (n=3) and anterior abdominal wall (n=1). Participant-reported adherence to ART was high; 89% reported full adherence and 11% reported missing one dose. HIV viral load was undetectable at screening in 29 (73%) participants. By 6 months post-treatment, this improved to 94% and by 12 months, 93% of participants who remained alive had an undetectable viral load. As expected, there was an overall reduction in CD4 counts after initiation of therapy with partial recovery (data not shown). By 12 months following concomitant chemoradiotherapy the median CD4 count was 296 (range 108-766). Changes in HIV viral load and CD4 count did not contribute to significant dose delays or reductions.
Figure 2:
The one-year overall survival in AMC-081 was 81.6% (95% CI: 65.2-90.8%).
Adverse events
Adverse events at the event level are shown in Table 4. The most common adverse event at grade 3 or 4 was decreased lymphocyte count which affected all treated participants and decreased white blood cell count (all participants), followed by decreased neutrophil count. Non-hematologic serious adverse events are similar to those observed in women with LACC without HIV infection.
Table 4:
Grade 3-4 adverse events in treated participants (n=39*)
| No. Participants (%) |
|
|---|---|
| Gastrointestinal | |
| Diarrhea | 1 (3) |
| Vomiting | 2 (5) |
| Renal and Urinary | |
| Chronic Kidney Disease | 1 (3) |
| Blood/Bone Marrow | |
| Lymphocyte Count Decreased | 39 (100) |
| Other hematological** | 16 (41) |
| Nervous System | |
| Syncope | 2 (5) |
| Metabolism | |
| Hypermagnesemia | 1 (3) |
| Hypokalemia | 3 (8) |
| Hypomagnesemia | 1 (3) |
| Vascular | |
| Hypertension | 1 (3) |
Safety population includes one participant who was enrolled in error and treated.
Anemia or low platelet or neutrophil counts.
Discussion
AMC-081 is the first clinical trial in sub-Saharan Africa designed to prospectively collect detailed treatment, adverse events and outcomes in HIV-infected women with LACC on ART who met pre-determined criteria for receiving concomitant chemoradiotherapy. Our data adds to, and contrasts with, the existing literature on the treatment of LACC in HIV-positive women in sub-Saharan Africa, which has described the single-institution experience in South Africa and Botswana using local standard-of-care treatment guidelines. [15] compared treatment tolerability among HIV-infected and HIV-uninfected women with LACC treated between 2009 and 2011 with concomitant chemoradiotherapy in South Africa. Of the 22 HIV-positive women who were prescribed this treatment, 15 (69%) completed 4 or more cycles of chemotherapy and received adequate RT compared with 129 (94.2%) of the 137 HIV-uninfected patients undergoing concomitant chemoradiotherapy[15]. ART treatment, ART adherence and HIV viral loads were not monitored. In a more recently-published study of a much larger number of women with LACC, [16] reported on 5-year OS among 71 HIV-positive and 421 HIV-negative women treated with radiation therapy at the same institution between 2007 and 2011. Only 53 of 71 HIV-positive women were prescribed concomitant platinum-based chemotherapy, of whom only 31 (58.5%) received four or more chemotherapy cycles. Both the 2-year and 5-year OS of all HIV-positive women were significantly lower than that of their HIV-negative counterparts treated at the same medical center. As in their previous report, ART treatment, ART adherence and HIV viral loads were not monitored in HIV-positive women, nor were causes of death. In another study conducted in Botswana that compared treatment and outcomes of HIV-infected and HIV-uninfected women with cervical cancer who were treated with curative intent between 2012 and 2015, 85 (48.0%) participants with HIV infection and 40 (48.0%) without HIV infection completed the recommended radiation therapy (79 Gy [EQD2]). No information was given about either the number of cycles or total dose of cisplatin administered in the Botswana study, nor is there any information about hematologic adverse events[8] or adherence to ART reported in that study. Our study showed that the majority of selected participants who met pre-defined entry criteria could complete therapy with the same cisplatin dose used in HIV-uninfected women with comparable tolerability while maintaining high ART adherence while on treatment. We found that dose reductions and delays were similar to those in HIV-uninfected women with LACC treated with concomitant chemoradiotherapy. As anticipated in sub-Saharan Africa, there were many women with stage IIIB tumors, rendering comparison with other reported prospective LACC trials that had few stage IIIB participants difficult. However, while we did not conduct long term follow-up in this study, one-year overall survival rates observed among women with high risk advanced tumors were similar to those reported studies of women with generally smaller tumors and without HIV infection.
This was the first AMC-sponsored trial to test combined modality treatment for LACC in HIV-infected women. We were able to generate high-quality feasibility and safety data that will be applied to the design of future trials in a population with historically poor outcomes. Prior to this trial, it was common practice to prescribe lower and potentially less effective doses of cisplatin for women with HIV and LACC based on poorly documented concerns about potential drug interactions and additive toxicities. Our findings suggest that standard doses of concomitant chemoradiotherapy should be considered the standard of care for treatment of appropriately selected HIV-positive women with LACC.
Highlights.
HIV-infected women with advanced cervical cancer on ART tolerate concomitant chemoradiotherapy
Most HIV+ women with cervical cancer on ART complete all prescribed cycles of radiosensitizing chemotherapy at standard dose
1 year PFS of HIV+ women with advanced cervical cancer on ART is similar to their HIV-negative counterparts
Hematologic toxicities from this standard dosing in this patient population is mostly self-limiting and manageable
Acknowledgments
Funding Source: National Cancer Institute AIDS Malignancy Consortium UM1CA121947
The investigators would like to acknowledge the efforts of the group chair, Dr. Ron Mitsuyasu as well as insights from Cancer Therapy Evaluation (CTEP) Program members Dr. Rich Little and the NCI office of HIV and AIDS Malignancy (OHAM) members Dr. Mostafa Nokta and Dr. Rebecca Huppi. Administrative support was provided by members of the Emmes Corporation (Rockville, MD)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures/Conflicts of Interest
Dr. Mark Einstein has advised or participated in educational speaking activities but does not receive an honorarium from any companies. In specific cases, his employers have received payment for his time spent for these activities from Papivax, Cynvec, Altum Pharma, Photocure, Becton Dickenson, and PDS Biotechnologies. If travel required for meetings with industry, the company pays for Dr. Einstein’s travel expenses. Rutgers has received grant funding for research-related costs of clinical trials that Dr. Einstein has been the overall or local PI within the past 12 months from J&J, Pfizer, AstraZeneca, Advaxis, and Inovio. Dr. Garg reports other from Varian medical systems, outside the submitted work. Dr. Palefsky reports grants and non-financial support from Merck and Co., grants and other from Vir Biotechnologies, personal fees from Vaccitech, other from Ubiome, grants, personal fees and non-financial support from Antiva, personal fees from Janssen Pharmaceuticals, personal fees from Novan, other from Virion, outside the submitted work. Ms. Lensing reports grants from NIH/NCI, during the conduct of the study. Dr. Krown reports grants from NIH/NCI, during the conduct of the study; non-financial support from Celgene, non-financial support from Merck, non-financial support from Gilead, personal fees from Pfizer, outside the submitted work. The other contributing authors declare no competing financial interests.
Contributor Information
Mark H. Einstein, Department of Obstetrics, Gynecology, & Women’s Health, Rutgers New Jersey Medical School, Newark, NJ, mark.einstein@rutgers.edu.
Ntokozo Ndlovu, College of Health Sciences, University of Zimbabwe Harare, Zimbabwe, ntokozosqo@gmail.com.
Jeannette Lee, Department of Biostatistics, University of Arkansas for Medical Sciences, Little Rock, AR JYLee@uams.edu.
Elizabeth A. Stier, Department of Obstetrics and Gynecology, Boston University School of Medicine, Boston, MA elstier@bu.edu.
Jeffrey Kotzen, Department of Radiation Oncology, University of the Witwatersrand, Johannesburg, South Africa Jeffrey.Kotzen@wits.ac.za.
Madhur Garg, Department of Radiation Oncology, Montefiore Medical Center, Albert Einstein College of Medicine, Yeshiva University, Bronx, NY MGARG@montefiore.org.
Kathleen Whitney, Department of Pathology, Montefiore Medical Center, Albert Einstein College of Medicine, Yeshiva University, Bronx, NY kwhitney@montefiore.org.
Shelly Y. Lensing, Department of Biostatistics University of Arkansas for Medical Sciences, Little Rock, AR SYLensing@uams.edu.
Mariza Tunmer, Radiation Oncology Wits Donald Gordon Medical Centre, Johannesburg, South Africa mariza.tunmer@wits.ac.za.
Webster Kadzatsa, Department Radiotherapy and Oncology, College of Health Science University of Zimbabwe, Harare, Zimbabwe wkadzatsa@gmail.com.
Joel Palefsky, Department of Medicine, University of California, San Francisco, San Francisco, CA Joel.Palefsky@ucsf.edu.
Susan E. Krown, AIDS Malignancy Consortium, New York, NY krowns@mskcc.org.
References
- 1.Spayne J, et al. , Invasive cervical cancer: a failure of screening. Eur J Public Health, 2008. 18(2): p. 162–5. [DOI] [PubMed] [Google Scholar]
- 2.Denny L, Cervical cancer: prevention and treatment. Discov Med, 2012. 14(75): p. 125–31. [PubMed] [Google Scholar]
- 3.Pfaendler KS and Tewari KS, Changing paradigms in the systemic treatment of advanced cervical cancer. Am J Obstet Gynecol, 2016. 214(1): p. 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ndlovu N, K.R., Factors associated with tumour stage at presentation in invasive cervical cancer. Cent Afr J Med., 2003. ;49(9-10): p. 107–111. [PubMed] [Google Scholar]
- 5.Einstein MH and Phaeton R, Issues in cervical cancer incidence and treatment in HIV. Curr Opin Oncol, 2010. 22(5): p. 449–55. [DOI] [PubMed] [Google Scholar]
- 6.Bateman AC, et al. , The burden of cervical pre-cancer and cancer in HIV positive women in Zambia: a modeling study. BMC Cancer, 2015. 15: p. 541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denslow SA, et al. , Incidence and progression of cervical lesions in women with HIV: a systematic global review. Int J STD AIDS, 2014. 25(3): p. 163–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dryden-Peterson S, et al. , HIV Infection and Survival Among Women With Cervical Cancer. J Clin Oncol, 2016. 34(31): p. 3749–3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morris M, et al. , Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med, 1999. 340(15): p. 1137–43. [DOI] [PubMed] [Google Scholar]
- 10.Cervical Cancer Treatment (PDQ®)-Health Professional Version. Available from: https://www.cancer.gov/types/cervical/hp/cervical-treatment-pdq#link/_605.
- 11.Green J, et al. , Concomitant chemotherapy and radiation therapy for cancer of the uterine cervix. Cochrane Database Syst Rev, 2005(3): p. CD002225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deeken JF, Pantanowitz L, and Dezube BJ, Targeted therapies to treat non-AIDS-defining cancers in patients with HIV on HAART therapy: treatment considerations and research outlook. Curr Opin Oncol, 2009. 21(5): p. 445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nyongesa C, et al. , A phase I study of concurrent cisplatin chemotherapy in patients with carcinoma of the cervix receiving pelvic radiotherapy. Int J Gynecol Cancer, 2006. 16(4): p. 1614–9. [DOI] [PubMed] [Google Scholar]
- 14.Imaging and Radiation Oncology Core - MDAnderson - Houston Quality Assuance Center. May/21/2018]; Available from: http://rpc.mdanderson.org/RPC/home.htm.
- 15.Simonds HM, Neugut AI, and Jacobson JS, HIV Status and Acute Hematologic Toxicity Among Patients With Cervix Cancer Undergoing Radical Chemoradiation. Int J Gynecol Cancer, 2015. 25(5): p. 884–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simonds HM, et al. , Five-year overall survival following chemoradiation among HIV-positive and HIV-negative patients with locally advanced cervical carcinoma in a South African cohort. Gynecol Oncol, 2018. 151(2): p. 215–220. [DOI] [PubMed] [Google Scholar]