Introduction
Cheyne-Stokes respiration (CSR) and periodic breathing (PB) have been associated with heart failure, 1–8 acute coronary syndrome, 9–10 arrhythmias, 11–12 cardioverter-defibrillator therapy in response to ventricular tachycardia, 11 stroke, 13–15 increased stroke severity, 16 and death. 1–2,4–6,8,17 Both CSR and PB are forms of central sleep apnea associated with change ventilation and loss of ventilatory drive. Homeostasis is usually maintained when carbon dioxide and oxygen levels in the blood are balanced through the process of ventilation. However, in heart failure patients, CSR and PB are observed when there is increased chemosensitivity due to high heart and lung pressures, low carbon dioxide and oxygen stores in the body, and delayed circulatory time. 18–19 CSR and PB are regular cycles of breathing with a crescendo-decrescendo pattern. CSR is characterized by cycles of hyperpnea, hypopnea and apnea,18 while PB is characterized by cycles of hyperpnea and hypopnea.19
CSR and PB may result in frequent arousal from sleep and surges in sympathetic nervous system activity, which when coupled with hypoxia may lead to arrhythmias. 19 However, few studies have examined CSR and PB in the intensive care unit (ICU). Richards et al, found a 34% prevalence of CSR and PB in patients admitted to the ICU with acute cardiovascular illness, 12 while Van et al, showed an 82% prevalence in patients admitted with heart failure.9 Given the limited studies examining CSR and PB in the ICU, which have been linked to adverse outcomes in outpatient populations with chronic disease, warrants the study of CSR and PB in the ICU and whether these breathing patterns are associated with adverse outcomes.
Patients in the ICU are at highest risk for deterioration and death, making recognition of physiological changes of paramount importance. In 2007, the National Patient Safety Agency reported that 11% of in-hospital deaths were the result of unrecognized patient deterioration. 20 Furthermore, in 2014, after reviewing 73 sentinel events, The Joint Commission Office of Quality and Safety found that inadequate patient assessment contributed to 48 (66%) of these patient deaths.21 While the importance of monitoring and recognizing critical illness have been described,22 a solution has not been achieved, with ICU mortality rates at 5.6% and hospital mortality at 8.7%.23
Although clinicians utilize bedside physiologic monitors to detect and quantify dynamic vital sign and electrocardiographic (ECG) changes, ICU patients are still at high risk for adverse respiratory events. Importantly, these events may go unrecognized since detailed respiratory assessment (e.g., rate, depth) is typically assessed visually and represents only a “snapshot” of time.24 Hence, respiratory patterns associated with CSR and PB, which require a longer and more focused observation and assessment, could be missed altogether. Recognizing CSR and PB might help clinicians characterize and gauge chronic illness. Given current limitations, clinicians need a better way to monitor respiratory changes associated with CSR and PB, particularly given that these breathing patterns are associated with arrhythmias and adverse events.
The current standard of care for patients in the ICU includes continuous ECG monitoring, typically using 7 ECG leads, for detection of arrhythmias, myocardial ischemia, and QT-interval lengthening. 25 Although, research using continuous ECG data to measure respiration is longstanding, 26 monitoring and documentation of respirations via ECG continues to be deficient 27 due to lack of consensus in the measurement technique and poor availability of electronic equipment capable of measuring its presence. 28 Our research group has been exploring the use of continuous ECG data to measure respiration (Figure 1) and CSRPB (Figure 2) in both community-based healthy participants and hospitalized patients with suspected acute coronary syndrome (ACS). 10 In our prior work, using 12-lead ECG Holter data, we found that hospitalized telemetry unit patients with confirmed ACS had CSR episodes 6 times more frequently and PB episodes 1.3 times more frequently than patients with a non-ACS cardiac diagnosis (i.e. valvular heart disease, heart failure, pericarditis, aortic dissection). 10 In the current study, we expand our research on this topic to the ICU setting.
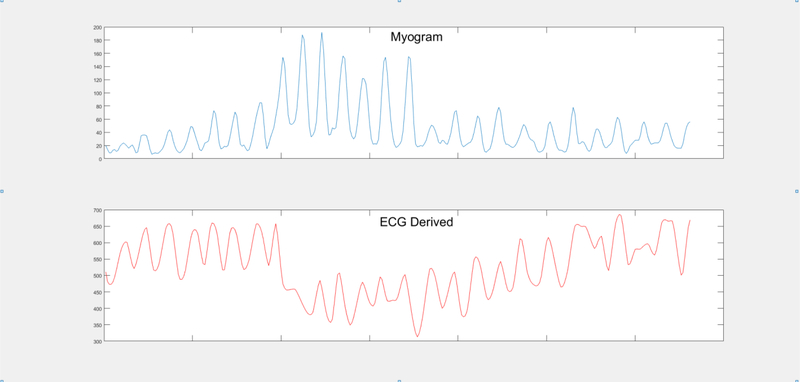
Figure 1. ECG derived respiration from mean QRS variation.
Respiration over 1 min. Top figure: Myogram derived respiration. Bottom figure: ECG derived respiration from mean QRS amplitude variation.
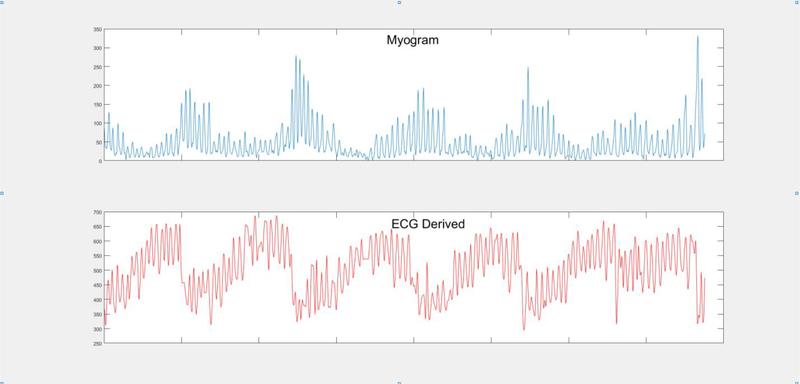
Figure 2. Cheyne–Stokes Respiration Periodic Breathing derived from myogram and ECG.
Cheyne Stokes Respiration Periodic Breathing over 5 min. Top figure: Myogram derived Cheyne Stokes Respiration Periodic Breathing. Bottom figure: ECG derived Cheyne Stokes Respiration Periodic Breathing from mean QRS amplitude variation.
This study was designed to address two research questions: 1) Do ICU patients have a higher mean number of CSRPB episodes when compared to healthy community-based participants?; 2) Are ICU patients with higher rates of CSRPB more likely to have adverse outcomes (i.e., cardiorespiratory arrest, emergent intubation, or in-hospital mortality) when compared to patients with low rates of CSRPB?
Methods
Only a few studies have used the gold standard, polysomnography (PSG), to investigate CSR or PB in hospitalized, critically ill patients. 7,9,12 This is not surprising since PSG is expensive, not readily available, and uncomfortable for patients to wear because this test requires numerous electrodes and wires for electrocardiography, electroencephalography, electromyography, and electrooculography. 29 While there are a number of commercially available devices capable of measuring the Apnea Hypopnea Index, 30–34 which is often used to report CSR and PB, these devices are costly and would require a critically ill patient to wear yet another piece of equipment. In contrast, the algorithm that we are testing in this study uses already available continuous ECG data from the bedside monitors to derive CSRPB.
Study design.
This is an observational study using ECG data from two studies, one including a group of 461 ICU patients (Alarm Study General Electric Study number 123.042012-GES-0003) 35 and, the second including a group of 100 healthy community-based participants (T32 NR007088, F31 NR015196). 10 Both studies received Institutional Review Board approval, and the methods for these have been described previously. 10,35 A waiver of consent was granted for the ICU group, which allowed inclusion of all consecutive patients admitted to the ICU. A research nurse obtained written informed consent from participants in the healthy community-based group.
Data Collection
ICU Group.
This group included 461 consecutive patients ≥18 years old who were treated in one of five adult ICUs (cardiac, neurological/neuro-surgical, medical-surgical) during March, 2013. Patients were monitored with a Solar 8000i physiologic monitor as part of their routine ICU care (version 5.4 software, GE Healthcare, Milwaukee, WI) using a five skin electrode lead configuration, which recorded 7 ECG leads (leads I, II, III, aVR, aVL, aVF and V1). A sophisticated research infrastructure was used to capture continuous ECG data for offline analysis. 35 Briefly, continuous ECG data were captured, and then securely transferred using a specially developed research system (CARESCAPE Gateway system, GE Healthcare, Milwaukee, WI) to an external server for off-line analysis (BedMaster, Excel Medical Electronics, Inc, Jupiter, FL). The ECG data stored in the BedMaster system is initially in flat file format. These files are then converted into Extensible Markup Language (XML) format using BedMaster software, and then converted into binary files (AD Instruments, Dunedin, New Zealand) for final analysis. Patient demographic and clinical data were obtained from electronic medical records (Epic software, Madison, WI).
Of the 461 ICU patients, 13 (2.8%) patients were excluded because they had less than 4 hours of continuous ECG data; thus limiting our ability to identify CSRPB episodes. In addition, 107 (23.2%) patients were excluded because they were not breathing spontaneously (i.e., ventilator, bilevel positive airway pressure, continuous positive airway pressure). The final ICU group included 341 (74%) patients from the parent study.
Healthy Group.
This group included 100 community-based participants ≥18 years old enrolled between March 2013 and June 2013. Participants were carefully screened to ensure only “healthy” subjects were included. Excluded were individuals with flu symptoms, chronic illnesses (e.g., coronary heart disease, heart failure, heart transplantation, hypertension requiring medication, abnormal heart rhythm, stroke, diabetes mellitus, chronic obstructive pulmonary disease, asthma requiring inhaler use, sleep apnea, cancer with treatment in the past 12 months, end-stage renal failure), or patients taking crtain medications (nitroglycerin, coumadin, pradaxa, beta blockers). Additionally, individuals responding affirmatively to two or more of the Geisinger Health Tool criteria questions for sleep apnea were excluded. 36 Once consented, participants wore a H12+ Holter recorder (Mortara Instrument Inc, Milwaukee, WI) for 24 hours, with skin electrodes placed in the Mason-Likar electrode configuration. 37 While all 12-ECG leads were recorded in the healthy group, only the seven leads that matched the hospital ECG leads (I, II, III, aVR, aVL, aVF and V1) were used to derive CSRPB so that both the ICU and healthy group’s ECG leads matched.
ECG Derived Cheyne-Stokes and Periodic Breathing Analysis
To ensure ECG waveforms were of high quality, each patient’s file was carefully examined visually for excessive artifact or missing data. For the ICU group we examined the ECG data using Lab Chart software (version 7.2.1, AD Instruments), and H-Scribe software was used for the healthy group (version 4.34, Mortara Instrument Inc, Milwaukee, WI).
Specially designed research software was used to derive CSRPB (ECG Algorithm). A prior version of this software algorithm has been used by our group and others. 10,38 More recently, this algorithm was compared by Grasso and colleagues to Polysomnography in a prospective cohort of patients being screened for obstructive sleep apnea. 39
The algorithm derives CSRPB episodes from continuously acquired ECG data by examining beat to beat changes in the QRS complex that occur when the heart shifts in the thorax during inhalation and are associated with an increase in tidal volume. The ECG algorithm computes mean square QRS amplitude variations in 15 second intervals (area divided by width in ¼ microvolts) and derives a mean QRS amplitude waveform at a rate of one sample per second, which is then used to measure changes in tidal volume and calculate CSRPB. Figure 2 illustrates how the algorithm identifies CSRPB, defined as three consecutive cycles of a crescendo-decrescendo breathing pattern lasting an average of 180 seconds. Because continuous ECG recording length varied between 4 to 24 hours and started at varying hours of the day for each patient, we divided the total number of CSRPB episodes by the total number of hours of recorded ECG data; thus, creating an event rate per patient of CSRPB per hour of recording, which was used in the analysis.
Sleep data from progress notes was not collected. To evaluate if there were differences in CSRPB experience during the day versus the night, we analyzed the average number of CSRPB per hour in patients who were continuously monitored for at least 4 hours between 12:00 am and 4:00 am, and in those who were not continuously monitored between 12:00 am and 4:00 am. Additionally, to measure if supplemental oxygen or opioid intake had an effect in CSRPB per hour we analyzed these variables comparing ICU patients who received supplemental oxygen or opioids during the period of recording to ICU patients who did not receive supplemental oxygen or opioids. To determine if body mass index (BMI) and a history of obstructive sleep apnea had effect on CSRPB, we analyzed the association between BMI and history of sleep apnea and CSRPB in both the healthy and ICU patients. Lastly, we examined CSRPB per hour in all ICU patients and then by discharge diagnosis (cardiovascular, neurological/neuro-surgical, medical surgical).
ICU Outcomes
Adverse outcomes were identified through a detailed review of the electronic medical record and included: cardiorespiratory arrest; emergent intubation; and in-hospital mortality. A researcher reviewed procedure notes, flowsheets, medication administration records, clinician“s progress notes and clinician”s discharge notes for every patient. Cardiorespiratory arrest was defined as patients requiring cardiopulmonary resuscitation, defibrillation and/or advanced cardiac life support medications. Emergent intubation was defined as patients requiring endotracheal intubation followed by mechanical ventilation. In hospital mortality was defined as all-cause mortality during a patient’s hospital stay. For patients who suffered multiple adverse outcomes, the first adverse outcome was selected for analysis. A composite variable was created for each patient with an adverse outcome (cardiorespiratory arrest, emergent intubation, inhospital mortality), patients who suffered multiple adverse outcomes were only counted once in the analysis. ICU length of stay was determined from the electronic medical record by subtracting the patient’s discharge date and time from the patient’s admission date and time.
Statistical Analysis
Statistical analyses were conducted using Stata Release 14 (StataCorp, TX, USA). Continuous data are reported as means ± standard deviations (SD) and categorical data as proportions. To compare continuous demographic and clinical data between the ICU versus the healthy group, Student’s t-tests were used for normally distributed data, and the non-parametric Wilcoxon rank-sum test for non-normally distributed data. Pearson chi-square tests were used to compare categorical variables (i.e., demographics, clinical data, and adverse outcomes) between ICU versus healthy participants. Because the CSRPB data were not normally distributed among the ICU patient group, comparisons were examined by quartile. It was hypothesized that the frequency of CSRPB in the healthy group would not be different from ICU patients in the lower quartile; hence, a chi-square test was used for this comparison (healthy versus lower quartile ICU). A chi-square test was also used to test for a difference in adverse outcomes between ICU patients in the lower versus upper quartile. To assess for external validity, the Kruskal Wallis non-parametric test was used to compare CSRPB per hour by ICU setting (cardiac, neurological/neuro-surgical, medical-surgical). A p-value <0.05 was considered statistically significant.
Results
A total of 341 patients were in the ICU group, and 100 individuals made up the healthy group. The ICU patient group had more males (52% versus 35%, <0.01), were older (59 years versus 34 years, p<0.001), and had a higher BMI (28 versus 25, p<0.001) when compared to the healthy group. The groups were different when compared by race, which was attributed to a large number of ICU patients with an „unknown race’ designation (ICU = 13%, Healthy = 0%, p = 0.001).
Of the 341 ICU patients, 286 (84%) had at least one CSRPB episode, and 97 (97%) of the 100 healthy participants had at least one CSRPB episode. The mean hourly rate of CSRPB was not different when comparing ICU patients to healthy participants (0.39 versus 0.18, p = 0.85).
However, the range of CSRPB was broad, 0 to 6 for the ICU group versus 0 to 0.51 for healthy participants.
Because the distribution of CSRPB in the ICU patient group was so varied, the group was divided into four quartiles for further analysis. Quartile 1 included 86 patients with a mean hourly CSRPB rate of 0.02; quartile 2 included 85 patients with a mean hourly CSRPB rate of 0.09; quartile 3 included 85 patients with a mean hourly CSRBP rate of 0.23; and quartile 4 included 85 patients with a mean hourly CSRPB of 1.19.
A comparison between the lower quartile (quartile 1) ICU group and the healthy group is shown in Table 1. ICU patients in quartile 1 were older (54 years versus 34 years, p<0.001), had a higher body mass index (28 versus 25, p=0.02), and a lower mean hourly CSRPB count than the healthy group (0.02 versus 0.18, p<0.001).
Table 1.
Demographic and clinical characteristics for healthy, Q1 and Q4 patients with a p-value comparison shown in the last two columns as labeled.
| Variable | Healthy | Q1 | Q4 | Healthy vs Q1 p-values | Q1 vs Q4 p-values |
|---|---|---|---|---|---|
| Number | 100 | 86 | 85 | ||
| Age, mean (±SD) | 34 (10) | 55 (19) | 63 (16) | <0.001 | 0.001a |
| BMI, kg/m2, mean (± SD) | 25 (3.6) | 28 (7.9) | 28 (6.5) | 0.02 | 0.42a |
| CSRPB/hr, mean (± SD) | 0.18 (0.09) | 0.02 (0.02) | 1.19 (1.12) | <0.001 | <0.001a |
| CSRPB/hr, range | 0–0.51 | 0–0.06 | 0.34–6 | NA | NA |
| Supplemental Oxygen | NA | 59 (69) | 58 (68) | NA | 0.96b |
| Opioid Intake | NA | 61 (71) | 60 (71) | NA | 0.55b |
| H Obstructive Sleep Apnea, n (%) | NA | 10 (12) | 7 (8) | NA | 0.46b |
| H Heart Failure, n (%) | NA | 6 (7) | 12 (14) | NA | 0.13b |
| H Myocardial Infarction, n (%) | NA | 2 (2) | 3 (4) | NA | 0.64b,c |
| H Atrial Fibrillation, n (%) | NA | 7 (8) | 8 (9) | NA | 0.77b |
| H Hypertension, n (%) | NA | 34 (40) | 52 (61) | NA | <0.01b |
| H Coronary Artery Disease, n (%) | NA | 8 (9) | 15 (18) | NA | 0.11b |
| H Pulmonary Hypertension, n (%) | NA | 2 (2) | 3 (4) | NA | 0.64b,c |
| H Stroke, n (%) | NA | 11 (13) | 15 (18) | NA | 0.38b |
| Cardiovascular discharge diagnosis, n (%) | NA | 9 (11) | 21 (25) | NA | 0.014b |
| Neurological/Neurosurgical, discharge diagnosis, n (%) | NA | 26 (30) | 28 (33) | NA | NSb |
| Medical-Surgical, discharge diagnosis, n (%) | NA | 51 (59) | 36 (42) | NA | 0.024b |
= Wilcoxon rank-sum test,
= Chi-square test,
= Fisher exact test.
BMI = body mass index;
CSRPB = Cheyne-Stokes Respiration Periodic Breathing; H = History; hr = hour; ICU = Intensive Care Unit; Q = Quartile; n = Number; SD = Standard Deviation.
The proportion of ICU patients receiving supplemental oxygen, opioid treatment, or a history of obstructive sleep apnea or heart failure was not significantly different between quartile 1 and quartile 4 (Table 2). Body Mass Index was not associated to CSRPB per hour in the ICU group (rs = 0.08, p = 0.18). Based on these results, we did not control for supplemental oxygen therapy, opioid administration, history of sleep apnea, history of heart failure or BMI in subsequent statistical analyses.
Table 2.
Adverse events and length of stay for ICU patients by CSRPB quartile. The last column shows the comparison between ICU quartile 1 versus ICU quartile 4.
| Adverse Event in ICU | Q1 | Q2 | Q3 | Q4 | Q1 vs Q4 p-value |
|---|---|---|---|---|---|
| n | 86 | 85 | 85 | 85 | |
| Emergent Intubation, n (%) | 4 (5) | 3 (4) | 7 (8) | 8 (9) | 0.22a,b |
| Cardiorespiratory Arrest, n (%) | 0 (0) | 2 (2) | 0 (0) | 4 (5) | 0.042a,b |
| In Hospital Mortality, n (%) | 6 (7) | 4 (5) | 0 (0) | 8 (9) | 0.56a |
| Composite All, n (%) | 7 (8) | 6 (7) | 7 (8) | 16 (19) | 0.041a |
| ICU Length of Stay, in hours, mean (±SD) | 90 (135) | 111 (245) | 92 (101) | 128 (220) | 0.031c |
= Chi-square Test,
= Fisher exact test;
= Wilcoxon Rank.
CSRPB = Cheyne-Stokes Respiration Periodic Breathing; ICU = Intensive Care Unit; Q = Quartile; n = Number.
We also examined our data by time of day. Time of recording, day versus night, did not have a significant effect on CSRPB per hour. And 282 (83%) patients who were monitored between 12:00 am and 4:00 am had 0.41 CSRPB/hr while 59 (17%) patients who were not monitored between 12:00 am and 4:00 am had 0.26 CSRPB/hr (p=0.17).
To examine whether CSRPB were associated with adverse outcomes we compared ICU patients in quartile 4 to ICU patients in quartile 1. Patients in quartile 4 were older (63 years versus 54 years, p = 0.001) and had a higher mean hourly rate of CSRPB (1.19 versus 0.02, p<0.001) as compared to ICU patients in quartile 1 (Table 1). Patients in quartile 4 were also more likely to have a history of hypertension (61% versus 40%, p <0.01), or a cardiovascular discharge diagnosis (25% versus 11%, p = 0.014) than patients in quartile 1.
Table 2 displays adverse outcomes among the ICU group by quartiles. ICU patients in quartile 4 had a higher proportion of cardiorespiratory arrests (5% versus 0%, p = 0.042), and more adverse events over all (composite any adverse event 19% versus 8%, p = 0.041) as compared to patients in quartile 1.
Examining CSRPB per hour by patient’s discharge diagnosis showed that the 55 (16%) patients with a cardiovascular discharge diagnosis had the highest average CSRPB per hour (0.61±0.91), followed by the 112 (33%) patients with a neurological/neurosurgical discharge diagnosis (0.40±0.71), followed by the 174 (51%) patients with a medical-surgical diagnosis (0.31 ±0.67; p = 0.015). Dunn’s post-hoc analysis showed significance when comparing CSRPB per hour in patients with a cardiovascular discharge diagnosis versus patients with a medical surgical-diagnosis (p=0.002); but not when comparing a cardiovascular diagnosis versus neurological/neurosurgical diagnosis (p = 0.026), which was just above the significance level for the Dunn’s post-hoc test a p-value of 0.025 (alpha/2).
We then decided to look more closely at the average CSRPB for patients in quartile 4 based on cardiovascular, neurological/neurosurgical, and medical-surgical discharge diagnosis. Although not statistically significant, we found the same trend, with the 21(25%) patients with cardiovascular discharge diagnosis having the highest number of CSRPB per hour (1.36 ± 1.1), followed by the 28 (33%) patients with a neurological/neurosurgical discharge diagnosis (1.24 ± 1.02), and then the 36 (42%) patients with a medical-surgical discharge diagnosis (1.05 ± 1.2) (p=0.15). Additionally, patients in quartile 4 had an even distribution of overall adverse events by discharge diagnosis: 4/21 (19%) patients with cardiovascular discharge diagnosis, 5/28 (18%) patients with neurological/neurosurgical discharge diagnosis, and 7/36 (19%) patients with a medical-surgical discharge diagnosis (p = 0.99).
Discussion
The results of this study revealed that 84% of ICU patients had at least one episode of CSRPB, which is in line with the 82% prevalence reported by Van, who also used a rate of ≥1 CSRPB episode to measure prevalence. 9 In contrast, the prevalence of CSRPB was higher than Richards et al, who found that 34% of patients spent a minimum of 0.1 minutes in CSR. 12 These two very different prevalence rates are likely explained by the different measurement units used (i.e., CSRPB episodes/hours versus minutes spent in CSR) and the fact that the algorithm used in our study combines both Cheyne-Stokes and Periodic Breathing.
Remarkably, healthy participants experienced more CSRPB than ICU patients in quartile 1. It is possible that ICU patients in quartile 1 experienced fewer CSRPB than the healthy participants because they were receiving oxygen, which has been found to reduce CSR; 40 or because they were receiving opioids, which has been found to lower the drive to breathe. 41
To our knowledge, this appears to be the first study to report an association between CSRPB, measured from bedside monitoring ECG data, and inpatient adverse outcomes among ICU patients. Specifically, ICU patients who experience higher rates of CSRPB (quartile 4) experienced a higher rate of cardiorespiratory arrests and a composite variable of any adverse outcome when compared to ICU patients with low rates of CSRPB (quartile 1). While not statistically different, there was a trend for a higher rate of mortality among patients in the fourth CSRPB quartile group. Additionally, ICU patients in the fourth CSRPB quartile had longer length of ICU admission as compared to ICU patients in quartile 1.
Not surprisingly, when analyzing the entire sample of 341 ICU patients we found a trend showing that patients with a cardiovascular discharge diagnosis had a higher rate of CSRPB. In these patients, a high CSRPB rate may be a consequence of poor ventricular function. Altogether, heart and lung congestion, increased peripheral baroreceptor chemosensitivity, decreased carbon dioxide and oxygen stores, and delayed circulatory time, may perpetuate cycles of over-ventilation (hyperpnea) in response to low levels of carbon dioxide in the blood and under-ventilation (hypopnea) in response to low levels of carbon dioxide in the blood. 19 Patients with a high CSRPB rate experience more surges of sympathetic nervous system activity during hyperpnea and arousal. The combination of increased sympathetic nervous system activity, hypoxia and acute illness may potentiate arrhythmias and adverse outcomes. 19
Our outcome results are in congruence with work from other research teams who found sleep apnea to be associated with myocardial infarction, 42–43 unstable angina, 42 arrhythmias, 12 acute decompensated heart failure, 7 and more recently with a discharge diagnosis of acute coronary syndrome. 9–10
There was also a trend showing that patients with a neurological/neurosurgical discharge diagnosis had more CSRPB per hour than patients with a medical-surgical discharge diagnosis; which may be the result of injury to the pons 44 or cerebellum 45–46 and impact the breathing center. Consequently, this study supports findings that abnormal breathing patterns, namely CSRPB, are found in ICU patients with a neurological discharge diagnosis. 44–49
Lastly, overall adverse outcomes were evenly distributed among patients with cardiovascular, neurological/neurosurgical and medical-surgical diagnosis, suggesting that the higher rate of CSRPB is associated with adverse events, regardless of discharge diagnosis disposition.
Limitations
Though efforts were made to reduce error and bias, certain limitations still exist. A major limitation to this study is that although this research software has been used previously to derive CSRPB, 10,38 it has not been validated with the gold standard, polysomnography. Additionally, the study included a small sample size of adverse outcomes. The variable of interest, CSRPB, was not normally distributed among the ICU group; hence, the data were distributed into quartiles and analyzed as such. Furthermore, because we excluded patients on mechanical ventilation, the results cannot be generalized to critically ill patients who are mechanically ventilated. We did not measure sleep nor did we perform visual assessment to confirm the presence of CSRPB, rather we used an ECG derived method. CSRPB may occur during wake or sleep, which may influence outcomes, but cannot be examined from our study. Short and fragmented sleep may also contribute to negative outcomes; hence, whether this factor contributed to adverse outcomes cannot be determined from this study. A history of obstructive sleep apnea was determined only by medical record review. Because obstructive sleep apnea is often undiagnosed it is possible some of the patients had this disorder. It is not uncommon for CSRPB and obstructive sleep apnea to co-occur; however, we were not able to differentiate these variables in this study. Finally the ICU data were obtained in an academic hospital setting, in patients with high acuity, and thus, it may not be representative of ICU units not matching this setting.
Conclusion
In summary, the measurement of CSRPB derived from already available continuous ECG waveform data in ICU patients is feasible. There is an association between the average rate of CSRPB, cardiopulmonary arrest, and the composite of any adverse event. Patients with higher average rates of CSRPB also have longer ICU length of stay.
Additional research using a larger sample size is needed to better understand the predictive value of CSRPB in the ICU setting. The value of using CSRPB for early detection of patient deterioration also warrants further examination, ideally with a prospective randomized clinical trial to determine whether monitoring this new parameter would lead to early identification of impending cardiopulmonary arrest, which may lead to improved patient outcomes.
Supplementary Material
Acknowledgments
Funding:
Dr. Tinoco’s institution received funding from the National Institutes of Nursing Research (T32 NR007088 and F31 NR015196). Supported by a grant from General Electric (GE) Healthcare and entitled “Analysis of Patient Monitor Alarms in Adult Intensive Care Units”. Study number 123.04–2012-GES-0003. GE had a minimal role in study design and no role in data collection and analysis, decision to publish, or preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Findley LJ, Zwillich CW, Ancoli-Israel S, Kripke D, Tissi G, Moser KM. Cheyne-Stokes breathing during sleep in patients with left ventricular heart failure. Southern Medical Journal. 1985;78(1):11–15. [DOI] [PubMed] [Google Scholar]
- 2.Andreas S, Hagenah G, Möller C, Werner GS, Kreuzer H. Cheyne-Stokes respiration and prognosis in congestive heart failure. The American Journal of Cardiology. 1996;78(11):1260–1264. [DOI] [PubMed] [Google Scholar]
- 3.Lanfranchi PA, Braghiroli A, Bosimini E, Mazzuero G, Colombo R, Donner CF, & Giannuzzi P (1999). Prognostic Value of Nocturnal Cheyne-Stokes Respiration in Chronic Heart Failure. Circulation, 99, 1435–1440. [DOI] [PubMed] [Google Scholar]
- 4.Sin DD, Logan AG, Fitzgerald FS, Liu PP, Bradley TD. Effects of continuous positive airway pressure on cardiovascular outcomes in heart failure patients with and without Cheyne-Stokes Respiration. Circulation. 2000;102(1):61–66. [DOI] [PubMed] [Google Scholar]
- 5.Leite JJ, Mansur AJ, de Freitas HFG, Chizola PR, Bocchi EA, Terra-Filho M, … Lorenzi-Filho G. (2003). Periodic breathing during incremental exercise predicts mortality in patients with chronic heart failure evaluated for cardiac transplantation. Journal of the American College of Cardiology, 41(12), 2175–2181. 10.1016/S0735-1097(03)00460-1 [DOI] [PubMed] [Google Scholar]
- 6.Brack T, Thüer I, Clarenbach CF, et al. Daytime Cheyne-Stokes respiration in ambulatory patients with severe congestive heart failure is associated with increased mortality. Chest. 2007;132(5):1463–1471. [DOI] [PubMed] [Google Scholar]
- 7.Padeletti M, Green P, Mooney AM, Basner RC, Mancini DM. Sleep disordered breathing in patients with acutely decompensated heart failure. Sleep Medicine. 2009;10(3):353–360. [DOI] [PubMed] [Google Scholar]
- 8.Amir O, Reisfeld D, Sberro H, Paz H, Mintz S, Lewis BS. Implications of Cheyne-Stokes breathing in advanced systolic heart failure. Clinical Cardiology. 2010;33(3):E8–E12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van den Broecke S, Jobard O, Montalescot G, et al. Very early screening for sleep-disordered breathing in acute coronary syndrome in patients without acute heart failure. Sleep Medicine. 2014;15(12):1539–1546. [DOI] [PubMed] [Google Scholar]
- 10.Tinoco A, Drew BJ, Hu X, Mortara D, Cooper BA, Pelter MM. ECG-Derived Cheyne-Stokes Respiration and Periodic Breathing in Healthy and Hospitalized Populations. Annals of Noninvasive Electrocardiology. 2017;22(6):e12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bitter T, Westerheide N, Prinz C, et al. Cheyne-Stokes respiration and obstructive sleep apnoea are independent risk factors for malignant ventricular arrhythmias requiring appropriate cardioverter-defibrillator therapies in patients with congestive heart failure. European Heart Journal. 2011;32(1):61–74. [DOI] [PubMed] [Google Scholar]
- 12.Richards KC, Anderson WM, Chesson AL Jr, Nagel CL. Sleep-related breathing disorders in patients who are critically ill. Journal of Cardiovascular Nursing. 2002;17(1):42–55. [DOI] [PubMed] [Google Scholar]
- 13.Bassetti C, Aldrich MS, Chervin RD, & Quint D (1996). Sleep Apnea in Patients with Transient Ischemic Attack and Stroke: A Prospective Study of 59 Patients. American Academy of Neurology, 47(5), 1167–1173. [DOI] [PubMed] [Google Scholar]
- 14.Nachtmann A, Siebler M, Rose G, Sitzer M, & Steinmetz H (1995). Cheyne Stokes Respiration in Stroke. Neurology, 45, 820–821. [DOI] [PubMed] [Google Scholar]
- 15.Nopmaneejumruslers C, Kaneko Y, Hajek V, Zivanovic V, & Bradley TD (2005). Cheyne-Stokes Respiration in Stroke: Relationship to Hypocapnia and Occult Cardiac Dysfunction. American Journal of Respiratory and Critical Care Medicine, 171(9), 1048–1052. 10.1164/rccm.200411-1591OC [DOI] [PubMed] [Google Scholar]
- 16.Siccoli MM, Valko PO, Hermann DM, & Bassetti CL (2008). Central periodic breathing during sleep in 74 patients with acute ischemic stroke – Neurogenic and cardiogenic factors. Journal of Neurology, 255(11), 1687–1692. 10.1007/s00415-008-0981-9 [DOI] [PubMed] [Google Scholar]
- 17.Hanly PJ, Zuberi Khokhar NS. Increased mortality associated with Cheyne-Stokes respiration in patients with congestive heart failure. American Journal of Respiratory and Critical Care Medicine. 1996;153:272–276. [DOI] [PubMed] [Google Scholar]
- 18.Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, … Young T. (2008). Sleep Apnea and Cardiovascular Disease: An American Heart Association/American College of Cardiology Foundation Scientific Statement From the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing In Collaboration With the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation, 118(10), 1080–1111. 10.1161/CIRCULATIONAHA.107.189420 [DOI] [PubMed] [Google Scholar]
- 19.Leung RST, Comondore VR, Ryan CM, Stevens D. Mechanisms of sleep-disordered breathing: causes and consequences. Pflügers Archiv - European Journal of Physiology. 2012;463(1):213–230. [DOI] [PubMed] [Google Scholar]
- 20.Recognizing and responding appropriately to early signs of deterioration in hospitalized patients. The National Patient Safety Agency website. http://www.nrls.npsa.nhs.uk/resources/?entryid45=59834.Issued2007. Accessed October 15, 2017.
- 21.Joint Commission. Preventing Delays in Treatment. Quick Safety. 2015;(9):1–2. [Google Scholar]
- 22.Smith GB. In-hospital cardiac arrest: Is it time for an in-hospital “chain of prevention”? Resuscitation. 2010;81(9):1209–1211. [DOI] [PubMed] [Google Scholar]
- 23.Lilly CM, Swami S, Liu X, Riker RR, Badawi O. Five-Year Trends of Critical Care Practice and Outcomes. Chest. 2017;152(4):723–735. [DOI] [PubMed] [Google Scholar]
- 24.Moss TJ, Lake DE, Calland JF, et al. Signatures of Subacute Potentially Catastrophic Illness in the ICU: Model Development and Validation. Critical Care Medicine. 2016;44(9):1639–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandau KE, Funk M, Auerbach A, et al. Update to Practice Standards for Electrocardiographic Monitoring in Hospital Settings: A Scientific Statement From the American Heart Association. Circulation. 2017;136(19):e273–e344. [DOI] [PubMed] [Google Scholar]
- 26.Moody GB, Mark RG, Bump MA, et al. Clinical Validation of the ECG-Derived Respiration (EDR) Technique. Computers in Cardiology. 1986;(13):507–510. [Google Scholar]
- 27.Cretikos MA, Bellomo R, Hillman K, Chen J, Finfer S, Flabouris A. Respiratory rate: the neglected vital sign. Medical Journal of Australia. 2008;188(11):657–659. [DOI] [PubMed] [Google Scholar]
- 28.Hogan J Why don’t nurses monitor the respiratory rates of patients? British Journal of Nursing. 2006;15(9):489–492. [DOI] [PubMed] [Google Scholar]
- 29.Chokroverty S, Thomas RJ, & Bhatt M (2013). Atlas of sleep medicine. Elsevier Health Sciences. [Google Scholar]
- 30.Erman MK, Stewart D, Einhom D, Gordon N, & Casal E (2007). Validation of the ApneaLink™ for the Screening of Sleep Apnea: a Novel and Simple Single-Channel Recording Device. Journal of Clinical Sleep Medicine, 3(4), 387–392. [PMC free article] [PubMed] [Google Scholar]
- 31.Ayappa I, Norman RG, Seelall V, & Rapoport DM (2008). Validation of Self-Applied Unattended Monitor for sleep Disordered Breathing. Journal of Clinical Sleep Medicine, 4(1), 26–37. [PMC free article] [PubMed] [Google Scholar]
- 32.Ng SSS, Chan T-O, To K-W, Ngai J, Tung A, Ko FWS, & Hui DSC (2010). Validation of Embletta portable diagnostic system for identifying patients with suspected obstructive sleep apnoea syndrome (OSAS). Respirology, 15(2), 336–342. 10.1111/j.1440-1843.2009.01697.x [DOI] [PubMed] [Google Scholar]
- 33.Driver HS, Pereira E, Bjerring K, Toop F, Stewart SC, Munt PW, & Fitzpatrick MF (2011). Validation of the MediByte® type 3 portable monitor compared with polysomnography for screening of obstructive sleep apnea. Canadian Respiratory Journal, 18(3), 137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuceege M, Firat H, Demir A, & Ardic S (2013). Reliability of the Watch-PAT 200 in Detecting Sleep Apnea in Highway Bus Drivers. Journal of Clinical Sleep Medicine. 10.5664/jcsm.2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drew BJ, Harris P, Zègre-Hemsey JK, et al. Insights into the Problem of Alarm Fatigue with Physiologic Monitor Devices: A Comprehensive Observational Study of Consecutive Intensive Care Unit Patients. PLoS ONE. 2014;9(10):e110274. doi.org/ 10.1371/journal.pone.0110274.g002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: A tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108(5):812–821. [DOI] [PubMed] [Google Scholar]
- 37.Mason RE, Likar I. A new system of multiple-lead exercise electrocardiography. American Heart Journal. 1966;71(2):196–205. [DOI] [PubMed] [Google Scholar]
- 38.Haigney M, Zareba W, La Rovere MT, Grasso I, Mortara D. Assessing the interaction of respiration and heart rate in heart failure and controls using ambulatory Holter recordings. Journal of Electrocardiology. 2014;47(6):831–835. [DOI] [PubMed] [Google Scholar]
- 39.Grasso I, Haigney M, Mortara D, Collen JF, Hostler J, Moores A, Kelly W (2018). Detection of sleep-disordered breathing with ambulatory Holter monitoring. Sleep and Breathing. 10.1007/s11325-018-1623-9 [DOI] [PubMed] [Google Scholar]
- 40.Sasayama S, Izumi T, Seino Y, Ueshima K, Asanoi H, Group ChHS. Effects of nocturnal oxygen therapy on outcome measures in patients with chronic heart failure and Cheyne-Stokes respiration. Circulation Journal. 2006;70(1):1–7. [DOI] [PubMed] [Google Scholar]
- 41.Pattinson KTS (2008). Opioids and the control of respiration. British Journal of Anaesthesia, 100(6), 747–758. 10.1093/bja/aen094 [DOI] [PubMed] [Google Scholar]
- 42.Saito T, Yoshikawa T, Sakamoto Y, Tanaka K, Inoue T, Ogawa R. Sleep Apnea in Patients with Acute Myocardial Infarction. Critical Care Medicine. 1991;19:938–941. [DOI] [PubMed] [Google Scholar]
- 43.Moruzzi P, Sarzi-Braga S, Rossi M, Contini M. Sleep apnoea in ischaemic heart disease: differences between acute and chronic coronary syndromes. Heart. 1999;82(3):343–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee MC, Klassen AC, Heaney LM, & Resh JM (1976). Respiratory Rate and Pattern Disturbances in Acute Brain Stem Infarction. Stroke, 7(4), 382–385. 10.1161/01.STR.7.4.382 [DOI] [PubMed] [Google Scholar]
- 45.North B, & Jennett S (1974). Abnormal Breathing Patterns Associated With Acute Brain Damage. Arch Neurol, 31(5), 338–344. [DOI] [PubMed] [Google Scholar]
- 46.Lee A, Chen ML, Abeshaus S, Poliakov A, & Ojemann JG (2013). Posterior fossa tumors and their impact on sleep and ventilatory control: A clinical perspective. Respiratory Physiology & Neurobiology, 189(2), 261–271. 10.1016/j.resp.2013.05.027 [DOI] [PubMed] [Google Scholar]
- 47.Vapalahti M, & Troupp H (1971). Prognosis for Patients with Severe Brain Injuries. Brithish Medical Journal, 14(3), 404–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Henriques-Filho PS, & Pratessi R (2008). SLEEP APNEA AND REM SLEEP BEHAVIOR DISORDER IN PATIENTS WITH CHIARI MALFORMATIONS. Arquivos de Neuro-Psichiatria, 66(2B), 344–349. [DOI] [PubMed] [Google Scholar]
- 49.Shibazaki K, Kimura K, Aoki J, Uemura J, Fujii S, & Sakai K (2014). Dysarthria plus dysphagia is associated with severe sleep-disordered breathing in patients with acute intracerebral hemorrhage. European Journal of Neurology, 21(2), 344–348. 10.1111/ene.12323 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.