Abstract
BACKGROUND:
Neoadjuvant chemoradiation (CRT) impairs bowel function in patients with rectal cancer treated with total mesorectal excision (TME). The impact of other forms of neoadjuvant therapy such as neoadjuvant chemotherapy alone (NC) and induction chemotherapy followed by CRT (total neoadjuvant therapy or TNT) on postoperative bowel function has not been investigated.
METHODS:
We conducted a retrospective review of 176 rectal cancer patients treated between November 1, 2011, and August 31, 2017. All patients completed the MSKCC Bowel Function Instrument (BFI), a validated bowel function questionnaire, at least 6 months after TME and/or ileostomy reversal. Differences in BFI scores were compared across 4 groups (surgery alone, CRT, NC, and TNT) and also according to exposure to neoadjuvant RT and neoadjuvant chemotherapy. A multivariable linear regression model was used to evaluate the independent relationship between exposure to neoadjuvant RT or chemotherapy and BFI.
RESULTS:
BFI total scores were significantly different between the 4 groups (p = 0.008). Exposure to RT correlated with worse BFI total scores (p = 0.002), and no differences were found in BFI total score after exposure to neoadjuvant chemotherapy (p = 0.92). In a linear regression model, only exposure to RT (β = −5.1; 95% CI −8.9 to −1.3; p = 0.008) and tumor distance from the anal verge (β = 1.23; 95% CI 0.48 to 1.97; p = 0.001) were significantly correlated with BFI total score.
CONCLUSION:
NC, whether administered alone or added to CRT, does not seem to impair bowel function. These data should be used to counsel rectal cancer patients when discussing neoadjuvant therapy options.
Keywords: Neoadjuvant therapy, Patient-reported outcomes, Rectal neoplasm, Total Neoadjuvant Therapy, Induction chemotherapy
Introduction
In the past two decades, advances in the management of rectal cancer have resulted in lower rates of recurrence and longer survival.[1–4] With the introduction of new surgical techniques and neoadjuvant therapies (NAT), more patients are undergoing sphincter-preserving surgery and fewer patients require a permanent stoma.[5, 6] Nevertheless, a significant proportion of patients who have undergone sphincter-preserving total mesorectal excision (TME) report significant bowel dysfunction.[7, 8]
Bowel dysfunction after sphincter-preserving surgery encompasses a broad spectrum of symptoms including, among others, fecal incontinence, urgency and difficulty with evacuation. This group of heterogeneous symptoms, referred to as low anterior resection syndrome (LARS)[9], arise after surgery, with a considerable proportion of patients suffering a lifelong major impact in their quality of life[10].
Since chemoradiotherapy (CRT) is one of the most important risk factors for bowel dysfunction after TME,[7, 11, 12] novel NAT strategies have been devised with the goal of striking a balance between oncological and functional outcomes. One approach uses neoadjuvant chemotherapy (NC) alone, which according to preliminary findings does not seem to compromise oncological outcomes for selected patients with clinical stage II or III disease.[13] Alternatively, total neoadjuvant therapy (TNT), comprising a full dose of induction chemotherapy followed by CRT, has been increasingly adopted and is associated with comparatively higher rates of clinical and pathologic complete responses and a possible greater likelihood of organ preservation.[14] However, the effects of these novel NATs relative to patients’ postoperative bowel function have not been thoroughly evaluated. Our study was therefore aimed at comparing postoperative bowel function between patients who received NC, TNT, CRT, or surgery alone (SA).
Materials and Methods
Patients
The study was approved by the institutional review board of Memorial Sloan Kettering Cancer Center (MSK), and a waiver of informed consent was obtained. We retrospectively identified patients who underwent sphincter-preserving TME at MSK between November 1, 2011, and August 31, 2017, for primary stage I, II, or III rectal adenocarcinoma located within 10 cm from the anal verge. Patients were excluded if they had a previous history of bowel dysfunction or inflammatory bowel disease, underwent extended rectal resection, or had incomplete data on postoperative bowel function. Standard demographic, clinical, surgical and pathological data were collected.
Treatment
The choice of NAT was based on patient-specific recommendations of the multidisciplinary disease management team. SA was considered as the initial approach only for T1-T2 tumors with low-risk features, and NAT was reserved mainly for stage II/III disease. During the initial period of this study, patients were more likely to receive CRT alone as the standard of care. Based on the results of a pilot institutional trial [13], NC alone was considered for selected stage II/III rectal cancer patients with a good tumor response as assessed by clinical, endoscopic, and imaging evaluation after completion of the full dose of upfront chemotherapy. TNT has been increasingly used at our institution as the standard treatment for locally advanced rectal cancer and for selected patients with distal stage I tumors who are interested in avoiding a stoma. This shift toward TNT was justified by the potential improved compliance with the delivery of planned systemic therapy, increased rates of complete response (clinical and pathological), earlier administration of systemic chemotherapy for possible micrometastases, and quicker reversal of diverting loop ileostomies [14].
Four groups of patients were defined based on the type of NAT received: SA, CRT, NC, and TNT. The CRT group received a total of 50 or 50.4 Gy in 25 or 28 radiotherapy fractions, respectively, with concurrent infusional fluorouracil or oral capecitabine twice daily for 5 to 6 weeks. The NC group received either eight cycles of mFOLFOX6 (leucovorin, fluorouracil, and oxaliplatin), five cycles of CAPOX (capecitabine and oxaliplatin),[15–17] or FLOX (weekly fluorouracil-leucovorin and biweekly oxaliplatin).[18] The TNT group received induction chemotherapy as per the NC group, followed by CRT 2 to 3 weeks later.
TME was performed using a standard open or minimally invasive approach (depending on the surgeon’s preference), usually 8 to 12 weeks after completion of NAT in the CRT and TNT groups or 4 weeks after completion of NAT in the NC group. A colorectal or coloanal anastomosis was performed using either the hand-sewing or double-stapling technique depending on tumor location. A temporary diverting loop ileostomy was created depending on tumor location, patient characteristics, and the surgeon’s judgment.
Postoperative Bowel Function
Postoperative bowel function was assessed as part of the routine clinical care using the MSK Bowel Function Instrument (MSK BFI) at least 6 months after TME or ileostomy closure. The MSK BFI questionnaire was specifically designed to assess bowel function after sphincter-preserving surgery for rectal cancer[19] and is the most comprehensive of the questionnaires currently in use[20]. It consists of 18 questions recalling a 4-week time frame. Fourteen questions are grouped in 3 subscales, each one of them evaluating an important dimension of bowel function (diet, urgency/soilage and frequency), with 4 individual questions. The BFI total score is obtained using a linear scale and an equal-weighting scoring system in which each question has five possible answers ranging from “never” to “always,” except for one question asking about the number of bowel movements per 24 h. The BFI total score ranges from 18 to 90, with accounting for the possibility of missing data and with a score of 90 indicating the best possible bowel function measured with this questionnaire (Appendix 1). The main strength of this questionnaire is in the meticulous design and comprehensive evaluation of bowel dysfunction after TME as well correlation with clinically significant variables and quality-of-life instruments.
As an initial approach, BFI total scores were compared among the four groups of patients. Then, the scores were compared between patients who were exposed to radiotherapy (CRT and TNT groups) and patients who were not (SA and NC groups) and also between patients who received neoadjuvant chemotherapy (NC and TNT groups) and those who did not (SA and CRT groups). In an exploratory analysis, differences between the BFI subscale scores of the four groups were evaluated.
Statistical Analysis
Statistical analysis was performed using SAS v9.4 ® (SAS Institute Inc., NC, USA). Frequencies and percentages were calculated for categorical variables, and medians and ranges were calculated for continuous variables. The chi-square test for categorical variables and the Wilcoxon rank-sum test or Kruskal-Wallis test for continuous variables were used to compare across the treatment groups. To evaluate whether treatment group was an independent correlate of BFI, a multivariable normal regression analysis was fit with BFI as the dependent variable. This model included known clinically relevant variables as well as variables found to be significantly associated with BFI scores in univariate analysis. Variables that did not reach statistical significance were excluded. Patients with missing data were excluded from analysis and all patients were analyzed in the treatment group initially determined. The manuscript was prepared in accordance with STROBE guidelines.[21]
Results
A total of 176 patients were included in the analysis: 32 (18.2%) in the SA group, 12 (6.8%) in the NC group, 34 (19.3%) in the CRT group and 98 (55.7%) in the TNT group (Table 1). On average, patients in the CRT group had tumors located closer to the anal verge, were more likely to have a hand-sewn anastomosis, had a diverting ileostomy for a longer time, and completed the BFI after a longer interval following restorative colonic surgery than patients in the other groups (Table 1).
Table 1.
Patient demographics, tumor characteristics and treatment variables in rectal cancer patients treated with TME and no adjuvant therapy (SA), neoadjuvant chemoradiation (CRT), neoadjuvant chemotherapy, or total neoadjuvant therapy (TNT).
| Characteristic | SA (n = 32) |
CRT (n = 34) |
NC (n = 12) |
TNT (n = 98) |
p valuea |
|---|---|---|---|---|---|
| Age, median (range) | 55.5 (30–75) | 54.0 (34–78) | 47.5 (25–77) | 53.0 (26–72) | 0.30 |
| Female, n (%) | 17 (53.1) | 13 (38.2) | 6 (50) | 46 (46.9) | 0.67 |
| BMI, median kg/m2 (range) | 27.3 (18.2–39.5) | 26.85 (18.9–41.5) | 26.45 (19.9–38.6) | 27.1 (18.3–43.6) | 0.96 |
| Tumor distance,b median cm (range) | 8.0 (4.0–10.0) | 6.75 (1.0–10.0) | 8 (4.0–10.0) | 8 (1.0–10.0) | 0.04 |
| Surgical approach, n (%) | 0.46 | ||||
| Robotic | 23 (71.9) | 21 (61.8) | 11 (91.7) | 72 (73.5) | |
| Open | 6 (18.8) | 10 (29.4) | 1 (8.3) | 22 (22.4) | |
| Lapar oscopic | 3 (9.4) | 3 (8.8) | 0 | 4 (4.1) | |
| Hand-sewn anastomosis, n (%) | 3 (9.4) | 11 (32.4) | 1 (8.3) | 19 (19.4) | 0.08 |
| Intersphincteric resection, n (%) | 2 (6.3) | 3 (8.8) | 0 | 6 (6.1) | 0.53 |
| Diverting loop ileostomy, n (%) | 23 (71.9) | 31 (94.0) | 10 (83.3) | 85 (86.7) | 0.14 |
| Months to ileostomy closure, median (range) | 4 (2.0–9.0) | 7.0 (3.0–23.0) | 4.0 (2.0–6.0) | 4.0 (1.0–20.0) | 0.001 |
| Pathological AJCC stage, n (%) | |||||
| 0 | 4 (12.5) | 12 (35.3) | 3 (25.0) | 21 (21.4) | 0.38 |
| I | 16 (50.0) | 9 (26.5) | 5 (41.7) | 32 (32.7) | |
| II | 2 (6.3) | 6 (17.6) | 3 (25.0) | 19 (19.4) | |
| III | 10 (31.3) | 7 (20.6) | 1 (8.3) | 26 (26.5) | |
| Postoperative complication, n (%) | 12 (37.5) | 13 (38.2) | 4 (33.3) | 22 (22.4) | 0.20 |
| Months to survey, median (range) | 18.0 (6.0–43.0) | 22.0 (6.0–38.0) | 13.0 (6.0–30.0) | 13.0 (6.0–39.0) | 0.03 |
Kruskal-Wallis test for comparison of all four groups.
From the anal verge.
In univariate analysis, there was a statistically significant difference in total BFI scores between the four groups (p = 0.008) (Figure 1). Patients exposed to neoadjuvant RT (CRT and TNT groups) had significantly lower (p = 0.002) total scores than patients who did not receive RT (SA and NC groups) (Figure 2A). We found no differences (p = 0.92) in total scores between patients who received neoadjuvant chemotherapy (NC and TNT groups) and patients who did not (SA and CRT groups) (Figure 2B).
Figure 1.

MSK BFI total score for rectal cancer patients treated with TME and surgery alone (SA, n=32), neoadjuvant chemotherapy (NC, n=12), neoadjuvant chemoradiation (CRT, n=34) or total neoadjuvant therapy (TNT, n=98). Total scores are presented as boxplot with whiskers (median, interquartile and full range). The Y-axis does not start at zero.
Figure 2.
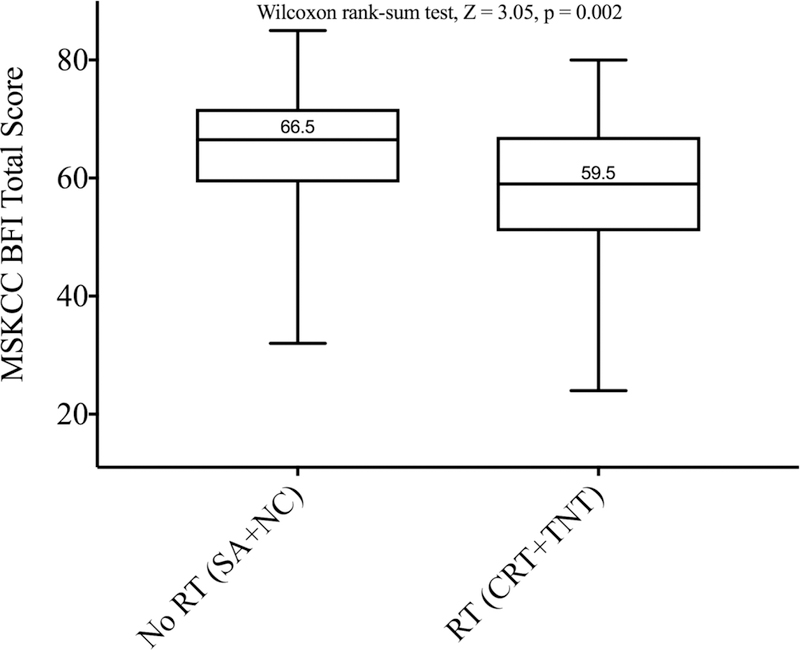
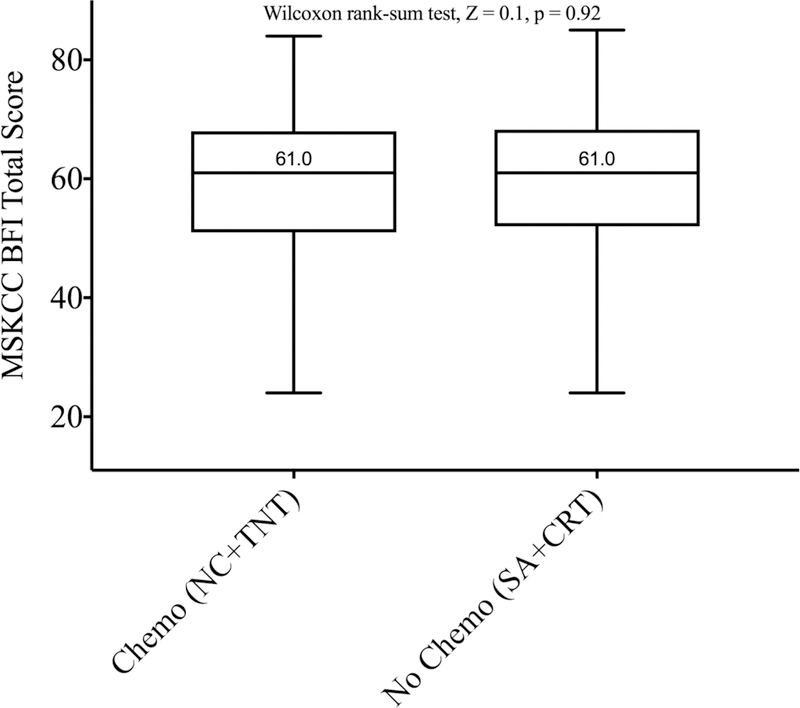
MSK BFI total score according to whether (A) the patients received preoperative radiation (RT, n=44) or not (no RT, n=132) or (B) they received preoperative chemotherapy (Chemo, n=110) or not (No Chemo, n=66). Total scores are presented as boxplot with whiskers. The Y-axis does not start at zero.
Total BFI scores were significantly correlated with exposure to RT (β = −5.1; 95% CI −8.9 to −1.3; p = 0.008) and tumor distance from the anal verge (β = 1.23; 95% CI 0.48 to 1.97; p = 0.001) in a multivariable regression model that included exposure to RT (CRT and TNT groups), time from surgery to survey, and tumor distance. For each centimeter of tumor distance from the anal verge, the BFI total score increased by 1.23 (corresponding to better bowel function), and exposure to RT decreased the total score by 5.31 points (corresponding to worse bowel function), when all the other variables remained constant in the model.
An exploratory univariate analysis of the different BFI subscales between groups is shown in Table 2. We did not observe any differences in the number of bowel movements and values on the diet subscale between groups. However, differences in the frequency and urgency/soilage subscales (p = 0.02 and p = 0.01, respectively) were observed.
Table 2.
Postoperative bowel function in rectal cancer patients treated with TME and no adjuvant therapy (SA), neoadjuvant chemoradiation (CRT), neoadjuvant chemotherapy, or total neoadjuvant therapy (TNT). Data is presented as medians with ranges in parentheses.
| Variable | SA (n = 32) |
CRT (n = 34) |
NC (n = 12) |
TNT (n = 98) |
p valuea |
|---|---|---|---|---|---|
| Bowel movements/day | 5 (1–15) | 6 (0–20) | 4 (2–18) | 5 (0–15) | 0.26 |
| BFI subscales | |||||
| Diet | 14.0 (4–20) | 14.0 (5–18) | 14.5 (8–20) | 12.0 (4–20) | 0.30 |
| Frequency | 23.0 (14–30) | 21.0 (8–29) | 24.0 (17–30) | 21.0 (6–30) | 0.02 |
| Urgency/soilage | 16.0 (8–20) | 14.0 (4–20) | 18.0 (6–20) | 13.5 (4–20) | 0.01 |
Kruskal Wallis test for comparison of all four groups.
Discussion
Our study indicates that the addition of neoadjuvant chemotherapy, independent of whether the patients received CRT or not, does not seem to be associated with a negative impact on postoperative bowel function in rectal cancer patients treated with TME. Conversely, the inclusion of RT is associated with worse postoperative bowel function.
Our results show that bowel function did not vary significantly after the addition of neoadjuvant or induction chemotherapy. Peripheral neuropathy is one of the most common side effects of oxaliplatin-based chemotherapy regimens[22], but the possible effects of this agent on bowel function has not being properly addressed. A small retrospective study tried to evaluate the effects of adjuvant oxaliplatin on anal continence, showing no differences between incontinence rate in both groups using the Wexner Fecal Incontinence Score[23]. Additionally, a recent cross-sectional study evaluating possible predictors of bowel dysfunction between patients who received NC compared with CRT[24], showed that CRT alone was associated with a significantly higher risk of developing major LARS, assessed using the LARS score, a previously validated screening tool for bowel dysfunction[25]. The ongoing PROSPECT trial[26], which is investigating selective use of radiotherapy after six cycles of induction FOLFOX6, will probably help to answer this question.
Bowel dysfunction is a well-known adverse effect of neoadjuvant or adjuvant CRT.[27] Our results are in agreement with those of a large population-based study[11] that showed that CRT (either short or long term) and complete TME were the strongest independent risk factors for major bowel dysfunction. However, in our study, the addition of induction chemotherapy to CRT did not have a negative impact on postoperative bowel function. TNT has been progressively adopted, mainly because of higher rates of completion of planned systemic chemotherapy, earlier closure of temporary ileostomy, and higher rates of complete response (pathologic and sustained clinical).[14] Another potential benefit of TNT could be the conservation of normal rectal function by increasing the rate of organ preservation due to higher rates of clinical complete response after NAT. However, a recent cross-match study showed that about a third of the patients who underwent watch-and-wait (WW) management after a clinical complete response had major LARS[28]. These findings must be interpreted with caution, as the prevalence of major LARS may be as high as 19% in healthy women between the ages 50 and 75 [29]. Conversely, our own preliminary unpublished data show that patients with a clinical complete response who underwent a WW strategy after NAT had better bowel function on all MSKCC BFI subscales and higher total scores after matching with patients who underwent sphincter-preserving surgery and TME. Future results of the OPRA trial[30], which is investigating TNT and nonoperative management, will provide additional information on the role of TNT relative to bowel function in patients with locally advanced rectal cancer.
Our study’s limitations include its retrospective design and the relatively small sample size, which limits the generalizability of the findings. In addition, the following considerations should be taken into account.
Tumor distance from the anal verge may not be an appropriate surrogate for anastomosis height in some cases. This potential discrepancy is mitigated somewhat by the study’s inclusion only of patients whose tumor was within 10 cm from the anal verge and by inclusion of tumor distance in the multivariate analysis.
The higher rate of hand-sewn anastomoses in the CRT group may have contributed to poorer bowel function, but only three patients in the CRT group underwent an intersphincteric resection, with no significant difference in the proportion of intersphincteric resections among the four groups. Exclusion of patients with a hand-sewn anastomosis did not change the direction of the results, and adjustment for anastomosis type in multivariate analysis did not reveal any statistical difference.
Another concern is the potential effect of disuse proctitis on bowel function, given that the CRT group had a longer median interval from TME to ileostomy closure secondary to higher rates of planned adjuvant chemotherapy. After multivariable adjustment, interval to ileostomy closure was omitted from the final model due to lack of statistical significance.
Finally, because BFI was administered as part of routine clinical care, the interval from surgery to survey was highly variable between groups. In a preliminary analysis, we had grouped patients on the basis of whether they completed the BFI <1 or ≥1 year after surgery, and the scores showed similar directionality.
Notwithstanding the above limitations, our study is to our knowledge the first to compare different strategies of NATs for rectal cancer in relation to postoperative bowel function. An important strength of the study is the use of the BFI, which is the most comprehensive tool available for evaluating postoperative bowel function.
Conclusion
Neoadjuvant chemotherapy, alone or in combination with CRT, does not seem to impair postoperative bowel function after TME. Selective use of radiotherapy is likely to benefit postoperative bowel function in rectal cancer patients. These data should be taken into consideration when counseling patients about different strategies of NAT and bowel dysfunction.
Acknowledgments
Funding: NCI grant P30 CA008748.
Appendix 1. MSK Bowel Function Instrument
Please answer the following questions based on your experience over the last 4 weeks.
| 1.How many bowel movements did you generally have in 24 hours? | ––––––––bowel movements/ 24 hours. | ||||
| Always | Most of the time | Sometimes | Rarely | Never | |
| 2. Do certain solid foods increase the number of bowel movements in a day? | □ | □ | □ | □ | □ |
| 3. Do certain liquids that you drink increase the number of bowel movements in a day? | □ | □ | □ | □ | □ |
| 4. Do you feel like you have totally emptied your bowels after a bowel movement? | □ | □ | □ | □ | □ |
| 5. Do you get to the toilet on time? | □ | □ | □ | □ | □ |
| 6. Do you have another bowel movement within 15 minutes of your last bowel movement? | □ | □ | □ | □ | □ |
| 7. Do you know the difference between having to pass gas (air) and needing to have a bowel movement? | □ | □ | □ | □ | □ |
| 8. Have you used medicines to decrease the number of bowel movements (drugs like Imodium®, Lomotil®)? | □ | □ | □ | □ | □ |
| 9. Have you had diarrhea (no form, watery stools)? | □ | □ | □ | □ | □ |
| 10. Have you had loose stool (slight form, but mushy)? | □ | □ | □ | □ | □ |
| 11. Have you been able to wait 15 minutes to get to the toilet when you feel like you are going to have a bowel movement? | □ | □ | □ | □ | □ |
| 12. Have you been able to control the passage of gas (air)? | □ | □ | □ | □ | □ |
| 13. Have you limited the types of solid food you eat to control your bowel movements? | □ | □ | □ | □ | □ |
| 14. Have you limited the types of liquids you drink to control your bowel movements? | □ | □ | □ | □ | □ |
| 15. Have you had soilage (leakage of stool) of your undergarments during the day? | □ | □ | □ | □ | □ |
| 16. Have you used a tissue, napkin, and/or pad in your undergarments during the day in case of stool leakage? | □ | □ | □ | □ | □ |
| 17. Have you had soilage (leakage of stool) of your undergarments when you go to bed? | □ | □ | □ | □ | □ |
| 18. Have you had to alter your activities because of your bowel function? | □ | □ | □ | □ | □ |
REFERENCES
- 1.Heald RJ, Moran BJ, Ryall RD, Sexton R, MacFarlane JK. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978–1997. Arch Surg 1998;133:894–899 [DOI] [PubMed] [Google Scholar]
- 2.Kapiteijn E, Putter H, van de Velde CJH, Cooperative investigators of the Dutch ColoRectal Cancer Group. Impact of the introduction and training of total mesorectal excision on recurrence and survival in rectal cancer in The Netherlands. Br J Surg 1998;89:1142–1149. doi: 10.1046/j.1365-2168.2002.02196.x [DOI] [PubMed] [Google Scholar]
- 3.van Gijn W, Marijnen CAM, Nagtegaal ID, Kranenbarg EM-K, Putter H, Wiggers T, Rutten HJT, Påhlman L, Glimelius B, van de Velde CJH, Dutch Colorectal Cancer Group. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol 2011;12:575–582. doi: 10.1016/S1470-2045(11)70097-3 [DOI] [PubMed] [Google Scholar]
- 4.Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, Becker H, Raab H-R, Villanueva M-T, Witzigmann H, Wittekind C, Beissbarth T, Rödel C. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012;30:1926–1933. doi: 10.1200/JCO.2011.40.1836 [DOI] [PubMed] [Google Scholar]
- 5.Engel AF, Oomen JLT, Eijsbouts Q a. J, Cuesta MA, van de Velde CJH Nationwide decline in annual numbers of abdomino-perineal resections: effect of a successful national trial? Colorectal Dis 2003;5:180–184. doi: 10.1046/j.1463-1318.2003.00454.x [DOI] [PubMed] [Google Scholar]
- 6.Tilney HS, Heriot AG, Purkayastha S, Antoniou A, Aylin P, Darzi AW, Tekkis PP. A national perspective on the decline of abdominoperineal resection for rectal cancer. Ann Surg 2008;247:77–84. doi: 10.1097/SLA.0b013e31816076c3 [DOI] [PubMed] [Google Scholar]
- 7.Loos M, Quentmeier P, Schuster T, Nitsche U, Gertler R, Keerl A, Kocher T, Friess H, Rosenberg R. Effect of preoperative radio(chemo)therapy on long-term functional outcome in rectal cancer patients: a systematic review and meta-analysis. Ann Surg Oncol 2013;20:1816–1828. doi: 10.1245/s10434-012-2827-z [DOI] [PubMed] [Google Scholar]
- 8.Scheer AS, Boushey RP, Liang S, Doucette S, O’Connor AM, Moher D. The long-term gastrointestinal functional outcomes following curative anterior resection in adults with rectal cancer: a systematic review and meta-analysis. Dis Colon Rectum 2011;54(12):1589–1597. doi: 10.1097/DCR.0b013e3182214f11 [DOI] [PubMed] [Google Scholar]
- 9.Bryant CLC, Lunniss PJ, Knowles CH, Thaha MA, Chan CLH. Anterior resection syndrome. Lancet Oncol 2012;13(9):e403–408. doi: 10.1016/S1470-2045(12)70236-X [DOI] [PubMed] [Google Scholar]
- 10.Emmertsen KJ, Laurberg S, Rectal Cancer Function Study Group. Impact of bowel dysfunction on quality of life after sphincter-preserving resection for rectal cancer. Br J Surg 2013;100:1377–1387. doi: 10.1002/bjs.9223 [DOI] [PubMed] [Google Scholar]
- 11.Bregendahl S, Emmertsen KJ, Lous J, Laurberg S. Bowel dysfunction after low anterior resection with and without neoadjuvant therapy for rectal cancer: a population-based cross-sectional study. Colorectal Dis 2013;15:1130–9. doi: 10.1111/codi.12244 [DOI] [PubMed] [Google Scholar]
- 12.Peeters KCMJ, van de Velde CJH, Leer JWH, Martijn H, Junggeburt JMC, Kranenbarg EK, Steup WH, Wiggers T, Rutten HJ, Marijnen CAM Late Side Effects of Short-Course Preoperative Radiotherapy Combined With Total Mesorectal Excision for Rectal Cancer: Increased Bowel Dysfunction in Irradiated Patients—A Dutch Colorectal Cancer Group Study. J Clin Oncol 2005;23:6199–6206 . doi: 10.1200/JCO.2005.14.779 [DOI] [PubMed] [Google Scholar]
- 13.Schrag D, Weiser MR, Goodman KA, Gonen M, Hollywood E, Cercek A, Reidy-Lagunes DL, Gollub MJ, Shia J, Guillem JG, Temple LKF, Paty PB, Saltz LB. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol 2014;32:513–518. doi: 10.1200/JCO.2013.51.7904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cercek A, Roxburgh CSD, Strombom P, Smith JJ, Temple LKF, Nash GM, Guillem JG, Paty PB, Yaeger R, Stadler ZK, Seier K, Gonen M, Segal NH, Reidy DL, Varghese A, Shia J, Vakiani E, Wu AJ, Crane CH, Gollub MJ, Garcia-Aguilar J, Saltz LB, Weiser MR. Adoption of Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer. JAMA Oncol 2018, in press. doi: 10.1001/jamaoncol.2018.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haller DG, Tabernero J, Maroun J, de Braud F, Price T, Van Cutsem E, Hill M, Gilberg F, Rittweger K, Schmoll H-J. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 2011;29:1465–1471. doi: 10.1200/JCO.2010.33.6297 [DOI] [PubMed] [Google Scholar]
- 16.Schmoll H-J, Twelves C, Sun W, O’Connell MJ, Cartwright T, McKenna E, Saif M, Lee S, Yothers G, Haller D. Effect of adjuvant capecitabine or fluorouracil, with or without oxaliplatin, on survival outcomes in stage III colon cancer and the effect of oxaliplatin on post-relapse survival: a pooled analysis of individual patient data from four randomised controlled trials. Lancet Oncol 2014;15:1481–1492. doi: 10.1016/S1470-2045(14)70486-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmoll H-J, Tabernero J, Maroun J, de Braud F, Price T, Van Cutsem E, Hill M, Hoersch S, Rittweger K, Haller DG. Capecitabine Plus Oxaliplatin Compared With Fluorouracil/Folinic Acid As Adjuvant Therapy for Stage III Colon Cancer: Final Results of the NO16968 Randomized Controlled Phase III Trial. J Clin Oncol 2015; 33:3733–3740. doi: 10.1200/JCO.2015.60.9107 [DOI] [PubMed] [Google Scholar]
- 18.Yothers G, O’Connell MJ, Allegra CJ, Kuebler JP, Colangelo LH, Petrelli NJ, Wolmark N. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol 2011;29:3768–3774. doi: 10.1200/JCO.2011.36.4539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Temple LK, Bacik J, Savatta SG, Gottesman L, Paty PB, Weiser MR, Guillem JG, Minsky BD, Kalman M, Thaler HT, Schrag D, Wong DW. The Development of a Validated Instrument to Evaluate Bowel Function After Sphincter-Preserving Surgery for Rectal Cancer. Dis Colon Rectum 2005;48:1353–1365 . doi: 10.1007/s10350-004-0942-z [DOI] [PubMed] [Google Scholar]
- 20.Chen TY-T, Emmertsen KJ, Laurberg S. What Are the Best Questionnaires To Capture Anorectal Function After Surgery in Rectal Cancer? Curr Colorectal Cancer Rep 2015;11:37–43 . doi: 10.1007/s11888-014-0217-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elm E von, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–7 . doi: 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 22.Kanat O, Ertas H, Caner B. Platinum-induced neurotoxicity: A review of possible mechanisms. World J Clin Oncol 2017;8:329–335 . doi: 10.5306/wjco.v8.i4.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fern, Arias O, Viudez A, Eito C, Ibanez-Beroiz B, Asin G, Hern I, ez, Cambra K, Errasti M, Barrado M, Campo M, Visus I, Flamarique S, Ciga MA Effects of Adjuvant Oxaliplatin on Anal Function in Locally Advanced Rectal Cancer Treated with Preoperative Chemo-Radiotherapy and Low Anterior Resection. Colorectal Cancer 2017;3:1.doi: 10.21767/2471-9943.100033 [DOI] [Google Scholar]
- 24.Qin Q, Huang B, Cao W, Zhou J, Ma T, Zhou Z, Wang J, Wang L. Bowel Dysfunction After Low Anterior Resection With Neoadjuvant Chemoradiotherapy or Chemotherapy Alone for Rectal Cancer: A Cross-Sectional Study from China. Dis Colon Rectum 2017;60:697–705. doi: 10.1097/DCR.0000000000000801 [DOI] [PubMed] [Google Scholar]
- 25.Emmertsen KJ, Laurberg S. Low Anterior Resection Syndrome Score: Development and Validation of a Symptom-Based Scoring System for Bowel Dysfunction After Low Anterior Resection for Rectal Cancer. Ann Surg 2012;255:922–928 . doi: 10.1097/SLA.0b013e31824f1c21 [DOI] [PubMed] [Google Scholar]
- 26.PROSPECT: Chemotherapy Alone or Chemotherapy Plus Radiation Therapy in Treating Patients With Locally Advanced Rectal Cancer Undergoing Surgery In: https://clinicaltrials.gov/ct2/show/NCT01515787. Accessed 12 May 2018
- 27.Emmertsen KJ, Laurberg S. Bowel dysfunction after treatment for rectal cancer. Acta Oncol 2008;47:994–1003. doi: 10.1080/02841860802195251 [DOI] [PubMed] [Google Scholar]
- 28.Hupkens BJP, Martens MH, Stoot JH, Berbee M, Melenhorst J, Beets-Tan RG, Beets GL, Breukink SO. Quality of Life in Rectal Cancer Patients After Chemoradiation: Watch-and-Wait Policy Versus Standard Resection - A Matched-Controlled Study. Dis Colon Rectum 2017;60:1032–1040 . doi: 10.1097/DCR.0000000000000862 [DOI] [PubMed] [Google Scholar]
- 29.Juul T, Elfeki H, Christensen P, Laurberg S, Emmertsen KJ, Bager P. Normative Data for the Low Anterior Resection Syndrome Score (LARS Score). Ann Surg 2018, in press. doi: 10.1097/SLA.0000000000002750 [DOI] [PubMed] [Google Scholar]
- 30. Smith JJ, Chow OS, Gollub MJ, Nash GM, Temple LK, Weiser MR, Guillem JG, Paty PB, Avila K, Garcia-Aguilar J, Rectal Cancer Consortium. Organ Preservation in Rectal Adenocarcinoma: a phase II randomized controlled trial evaluating 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total mesorectal excision or nonoperative management. BMC Cancer 2015;15:767. doi: 10.1186/s12885-015-1632-z [DOI] [PMC free article] [PubMed] [Google Scholar]


