Abstract
Objective:
Roux-en-Y gastric bypass (RYGB) surgery and Vertical Sleeve Gastrectomy (VSG) are most commonly performed bariatric procedures. While studies report new onset alcohol misuse following RYGB, the impact of VSG on alcohol intake is less clear. We evaluated hedonic feeding, alcohol drinking and hypothalamic obesity-related gene expression following VSG.
Methods:
Male Long Evans rats underwent VSG or sham surgery. To evaluate hedonic feeding, rats received a high-fat diet following behavioral satiation on chow. Alcohol (5-10% v/v) drinking was assessed in a two-bottle choice paradigm. Finally, PCR array evaluated gene expression.
Results:
VSG induced a moderate but significant weight loss. Sham rats significantly escalated high-fat diet intake following behavioral satiation, an effect significantly reduced in VSG rats. A moderate decrease in alcohol intake was observed in VSG rats at low (5%) alcohol concentration. However, overall no significant between group differences were evident. Key hypothalamic orexigenic transcripts linked to stimulation of food and alcohol intake were significantly decreased in VSG rats.
Conclusions:
VSG attenuated hedonic feeding without impacting alcohol drinking, an effect potentially mediated by alterations in genetic information flow within the hypothalamus. Importantly, these data highlight VSG as an effective bariatric procedure with potentially reduced risk of developing alcohol use disorder.
Keywords: Sleeve Gastrectomy, alcoholism, hedonic eating
INTRODUCTION
Bariatric surgery is a well-documented treatment option for obesity. In this context, vertical sleeve gastrectomy (VSG), a procedure where the fundus is surgically reduced to create a tubular gastric sleeve, has emerged as a prevalent surgical manipulation (1). In addition to a significant reduction in appetite and body weight, VSG patients experience improved metabolic profile. Specifically, decreased consumption of palatable/energy dense foods, weight loss, decreased hepatic glucose production and improvement in glucose homeostasis and dyslipidemia have been reported following VSG (2–4). One possibility explaining these observations is that VSG reduces appetite by mitigating hedonic hunger to restore metabolic homeostasis.
In the central nervous system, the hypothalamus is a brain region that integrates metabolic signals with internal need to direct behaviors that maintain homeostasis (5). The hypothalamus contains both orexigenic neuropeptides and anorectic neuropeptides whose release is coordinated to control energy balance and feeding behavior (6). In this regard, genetic events control the quality of a feeding event whereas physiologic mechanisms initiate or terminate a particular meal. Notably, genetic expression changes within the hypothalamus are sensitive to fluctuations in metabolic status (7) and feeding behavior (8). Currently, the behavioral and genetic mechanisms that contribute to reduced appetite and body weight loss after VSG are unresolved.
The current study tested corollaries of the central hypothesis that adaptations in feeding behavior and hypothalamic gene expression contribute to the positive benefits of VSG on body weight loss. Separate from feeding behavior, other bariatric techniques, namely Roux-en-Y Gastric Bypass (RYGB) surgery, stimulates new onset alcohol intake (9–12). We recently discovered that RYGB rats increase alcohol intake at the expense of palatable food intake, suggesting that surgery-induced changes in appetite and alcohol intake may be casual (11). Thus, a separate goal of the current work was to determine if VSG surgery impacted alcohol intake in rats that are otherwise non-preferring prior to surgery. To address these issues, we utilized a rodent model of VSG where male Long Evans rats characterized for body weight loss underwent a battery of behavioral tests designed to assess feeding in the absence of caloric need and new onset alcohol intake. Following behavioral characterization, PCR array analysis was conducted to elucidate alterations in the obesity-related gene expression within the hypothalamus that contributed to VSG-induced behavioral changes.
METHODS
Animals
Male Long Evans rats (Harlan, IN) housed in an environmentally controlled vivarium on a reverse light cycle (lights off at 7 a.m.) were used with food and water available ad libitum, except when indicated. All procedures were approved by Institutional Animal Care and Use Committee guidelines at Washington State University. Rats (age= ~14 wks) were initially exposed to the high fat diet (HFD; Research Diets, New Brunswick, NJ, 4.41 kcal/g, 1.71 kcal/g from fat) for ~ 8 weeks. Subsequently, rats (n=10/group) received sham or VSG surgery. Out of this, 9 sham and 8 VSG rats completed the study. Following surgery, all rats were maintained on liquid osmolyte and water for the first 5-8 days and were then slowly returned to and maintained on standard rodent chow (Teklad, 3.41 kcal/g, 0.51 kcal/g from fat) throughout the remainder of the study.
Surgery
All rats were fasted at-least 24hr before surgery as described previously (11). On the day of surgery, VSG group of rats were anesthetized and received an incision in the abdominal wall. The stomach was gently removed from the abdominal cavity and lateral stomach (70-80% of total stomach volume) was excised using a stapler (Ethicon, Ithaca, New York), creating a tubular gastric piece connecting esophagus and duodenum. The newly created gastric sleeve was gently placed back, and abdominal cavity was closed. Rats in the sham surgery group were anesthetized, received an incision in the abdominal wall, stomach was gently removed from the cavity and placed back before closing abdominal cavity. All rats received appropriate post-operative care and were allowed to recover until body weight was stable. Subsequently, a subset of rats was tested in hedonic feeding or alcohol drinking paradigms.
Hedonic Food Intake
To assess hedonic food intake, rats were food deprived overnight as described previously (11, 13). Following 21 hrs, all rats received pre-weighed rodent chow. Food was weighed each hour for 2hrs. Following the second hour of chow access, a preweighed HFD was presented to both Sham and VSG group of rats and food was weighed one hr later. The HFD intake after satisfying homeostatic caloric needs constitutes the hedonic portion of this test.
Alcohol Intake
Unsweetened alcohol (5, 8, or 10% v/v) bottles were presented on alternating days in a counterbalanced fashion in the rat home cages using a two-bottle choice paradigm (one bottle water and another alcohol). Alcohol was introduced 4 hrs into the rat’s subjective dark cycle and alcohol and water intake was evaluated 24 hrs later. All animals had ad libitum access to water and food and no water/food deprivation occurred during these testing. The position of alcohol/water bottles were switched at each testing session. Bottles were weighed, gently placed in the cages and re-weighed manually following each session to evaluate alcohol intake (g/kg).
PCR Array
Following behavioral assessment, all rats were euthanized, and brain tissues were snap frozen and stored at −80°C until further analysis. Hypothalamus from sham and VSG (n=3/group) was microdissected and placed in RNAlater (Ambion). Tissue Ruptor (QIAGEN, Germantown, MD, USA), QIAshredder (QIAGEN cat# 79654) and RNeasy Plus mini kit (QIAGEN cat#74134) were used for total RNA extraction and isolation as described in the manufacturer’s protocol. The concentration and purity of RNA samples were determined by Nanodrop spectrophotometer. The degradation and integrity were assessed by Experion Automated Electrophoresis (BioRad, Hercules, CA, USA). All RNA samples were high quality and passed all necessary requirements. cDNA was synthesized from 350ng of total RNA for each sample using RT2 First Strand kit (QIAGEN cat# 330401) following manufacturer’s protocol. PCR amplification was conducted using MyiQ Real Time quantitative PCR system (Bio Rad). Baseline threshold was manually set to 100 RFU in primary data analysis for all arrays. The Rat Obesity RT2 Profiler PCR arrays (QIAGEN cat# PARN-017Z) were used to profile expression of a total of 84 genes (Table 1), which included orexigenic genes, anorectic genes and genes involved in energy expenditure. All array passed quality control tests (PCR array reproducibility, RT efficiency and genomic DNA contamination). Web based data analysis tool (QIAGEN) was used to calculate fold change and p-values. Two housekeeping genes (Hprt1 and Rplp1) were used for qPCR data normalization. The CT cut-off was set to 35 and any gene with measurements > 35 were excluded from further analysis. Fold-Change (2^ (- Delta Delta CT)) is the normalized gene expression (2^ (- Delta Delta CT)) in the VSG samples divided by the normalized gene expression (2^ (- Delta CT)) in the sham samples. Fold-regulation represents fold-change results in a biologically meaningful way. Fold-change values greater than one indicates a positive-or an up-regulation, and the fold-regulation is equal to the fold-change. Fold-change values less than one indicate a negative or down-regulation, and the fold-regulation is the negative inverse of the fold-change.
Table 1.
A Panel of Gene Examined Using a Rat RT2 Profiler PCR Array
| OREXIGENIC GENES |
| Neuropeptides & Receptors: Adra2b, Agrp, Cnr1, Gal, Galr1, Mchr1, Hcrt, Hcrtr1, Npy, Npy1r, Nr3c1 (Grl), Oprk1, Oprm1, Sigmar1 (Oprs1). |
| Gut Hormones & Receptors: Ghrl (Ghrelin, Obestatin), Ghsr. |
| ANORECTIC GENES |
| Neuropeptides & Receptors: Atrn, Bdnf, Brs3, Calca, Calcr, Cartpt, Cntf, Cntfr, Crh, Crhr1, Drd1, Drd2, Gh1, Ghr, Prlhr (Gpr10), Grp, Grpr, Hrh1, Htr2c, Il1a, Il1b, Il1r1, Il6, Il6r, Mc3r, Nmb, Nmbr, Nmu, Nmur1 (Gpr66), Ntrk1, Nts, Ntsr1, Pomc, Sort1, Trh, Trhr, Ucn. |
| Gut Hormones & Receptors: Apoa4, Cck, Cckar, Glp1r, Pyy. |
| Adipocyte-Derived Peptides & Receptors: Lep (Leptin), Lepr, Tnf. |
| Pancreas Derived Peptides & Receptors: Calcr, Clps, Gcg, Gcgr, Glp1r, Iapp, Ins1, Ins2, Insr, Ramp3, Sst, Sstr1. |
| ENERGY EXPENDITURE |
| Adipocyte-Derived Peptides & Receptors: Adipoq (Acrp30), Adipor1, Adipor2, Adrb1, C3, Ppara, Pparg, Ppargc1a (Ppargc1), Ptpn1 (Ptp), Ucp1. |
| CNS-Derived Peptides & Receptors: Adcyap1, Adcyap1r1, Thrb. |
Statistical Analysis
Body weight, food intake and alcohol intake data over a period of time were analyzed by a mixed-model two-way ANOVA, with post-hoc tests to compare within group effects. The within-subject variable was time interval (time or conditions of measurements) and the between-groups variable was surgical procedure (sham or VSG surgery). HFD intake data were analyzed using unpaired t-test. PCR array data were analyzed using unpaired t-test only as per the manufacturer recommended web-based RT2 Profiler PCR Array Data Analysis software and others using this method to evaluate gene expression. All statistical comparisons were conducted at 0.05α level.
RESULTS
VSG Surgery: Body Weight
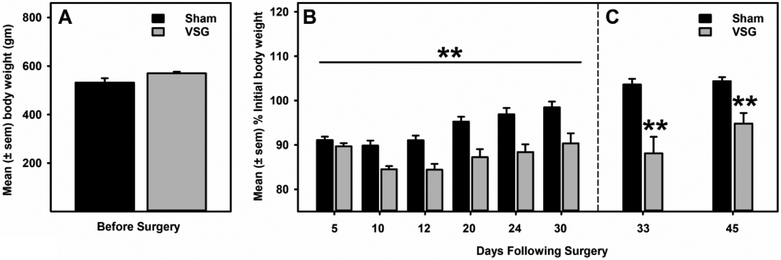
No statistically significant between group (p>0.05) differences existed before surgery (Fig 1A). Sham rats lost an average of 10 gm (~ 2.0 %) of their initial body weight, whereas VSG rats lost an average of 55.0 gm (~10%) of their initial body weight in the first 30 days following surgery (Fig 1B). A mixed- model ANOVA identified a main effect of time (F2.5, 37.5 = 52.255, p= 0.000), significant time and treatment interaction (F2.5, 37.5 = 7.970, p= 0.001) and a significant between group differences (F1.0, 15.0 = 15.420, p= 0.001) in body weight. In addition, these significant between group differences persisted across time during hedonic feeding (p= 0.0065) and alcohol drinking (p=0.0047) testing (Fig 1C).
Figure 1. VSG surgery promoted moderate but sustained decreases in body weight.
A) There were no significant differences in the baseline pre-surgery body weight between sham and VSG rats. B) VSG surgery led to a moderate but significant (p= 0.001) decrease in body weight relative to sham controls. **p<0.01 main effect of VSG Surgery. C) Significant (p<0.01) body weight differences existed between sham and VSG groups at each behavioral testing interval after surgery.
VSG Surgery: Hedonic Feeding
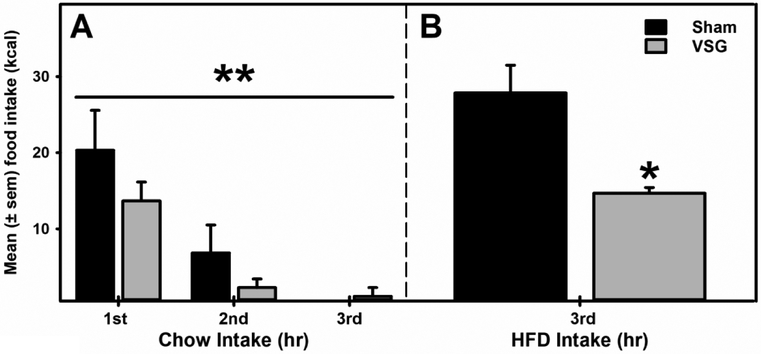
A mixed model ANOVA identified a significant effect of time (F2.0, 10.0 = 10.21, p= 0.004), but no significant (p>0.05) interaction or between groups differences were evident, suggesting that chow intake in both groups decreased over the duration but chow intake was not significantly (p>0.05) different between sham and VSG group of rats following overnight fasting (a homeostatic driven re-feeding; Fig 2A). However, a palatable food (HFD) intake following a chow pre-load was significantly reduced in the VSG rats compared to the Sham controls (Fig 2B).
Figure 2. VSG Surgery Selectively Attenuated Hedonic Feeding.
A) No significant between group differences in chow intake were observed when rat received chow following an overnight deprivation. **p<0.01 main effect of time. B) In contrast, when HFD was presented during 3rd hr, after re-feeding had ceased, VSG rats displayed a significant reduction in HFD intake (p= 0.029) compared to the sham controls. *p<0.05 compared to sham controls.
VSG Surgery: Alcohol Drinking
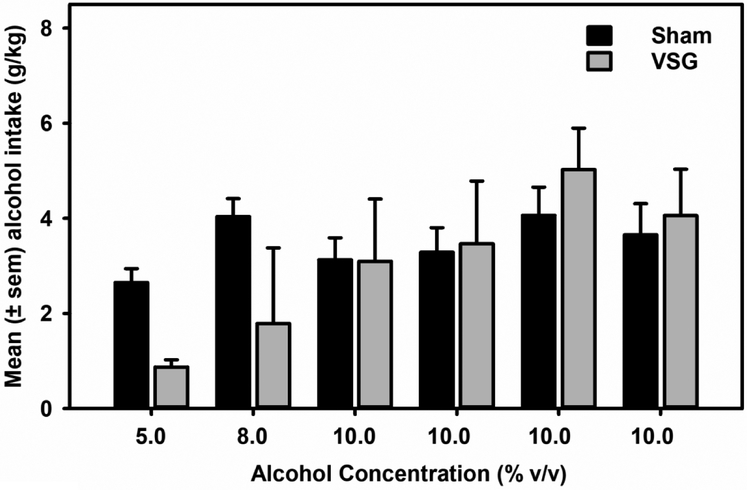
Alcohol drinking at lower alcohol (5%) but not higher concentrations (8-10%) appeared to be reduced in the VSG group of rats compared to the Sham controls. However, mixed-model ANOVA did not identify any statistically significant with-in or between-group differences (Fig 3). Water intake was not significantly different between sham and VSG groups.
Figure 3. VSG surgery did not impact alcohol drinking.
Data represent mean (±sem) alcohol (5.0-10.0% v/v) consumption (g/kg). When tested around 45 days following VSG surgery, alcohol drinking levels did not differ between groups.
VSG Surgery: Obesity-related Gene Expression in the Hypothalamus
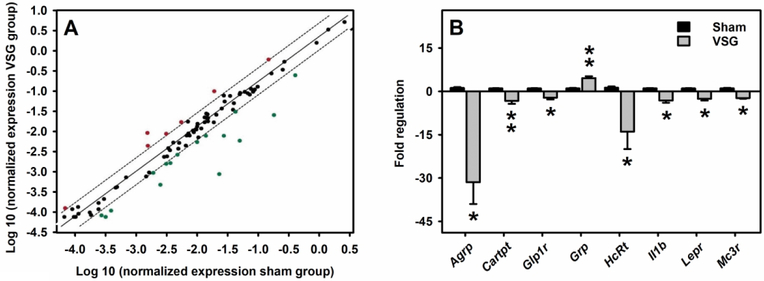
A Rat Obesity RT2 Profiler PCR array examined expression of 84 obesity-related genes (i.e., orexigenic, anorectic and energy expenditure) in the hypothalamus following Sham and VSG surgery. Several of these genes were significantly impacted following VSG surgery as shown in the scatter plot (Fig 4A). A total of 71 genes were expressed in detectable amount, whereas 13 genes (Adipoq, Adra2b, Apoa4, Clps, Gcgr, Gh1, Iapp, Ins1, Lep, Nmur1, Ntrk1, Pparg, Ucp1) were deemed undetectable (based on CT values). Table 2 summarizes the list of these 71 genes and their corresponding PCR array data. Of these 71 genes, 8 genes (Adipor1, Agrp, Cartpt, Glp1r, HcRt, llb, Lepr, Mc3r) were significantly (p<0.05) downregulated and one gene (Grp) was significantly (p<0.05) upregulated in the hypothalamus of VSG rats compared to sham controls. Furthermore, ≥ 2-fold statistical increase and decrease were evident in Agrp, Cartpt, Glp1r, HcRt, llb, Lepr, Mc3r and Grp, respectively (Fig 4B).
Figure 4. Alterations in the hypothalamic obesity-related gene expression following VSG surgery in rats.
A) Scatter plot analysis of differential expression of obesity-related genes in the hypothalamus following VSG compared to the sham controls, using the Rat Obesity RT2 Profiler PCR Array. Each dot represents one gene and the top and bottom genes outside the dotted lines represent 2-fold increase and decrease, respectively. B) Fold regulation (≥ 2-fold) is plotted for only statistically significant (p<0.05) increased or decreased gene expression following VSG compared to the sham controls. **p<0.01 and *p<0.05 compared to sham controls.
TABLE 2.
Impact of Vertical Sleeve Gastrectomy on Obesity-Related Gene Expression in Hypothalamus Using the Rat Obesity RT2 Profiler PCR Array.
| # | Unigene | Refseq | Symbol | Description | Fold Regulation |
p value |
|---|---|---|---|---|---|---|
| 1 | Rn.202559 | NM_016989 | Adcyap1 | Adenylate cyclase activating polypeptide 1 | −2.0 | 0.192 |
| 2 | Rn.234543 | NM_133511 | Adcyap1r1 | Adenylate cyclase activating polypeptide 1 receptor 1 | −1.4 | 0.174 |
| 3 | Rn.104556 | NM_207587 | Adipor1 | Adiponectin receptor 1 | −1.3 | 0.021* |
| 4 | Rn.101984 | NM_001037979 | Adipor2 | Adiponectin receptor 2 | −1.2 | 0.122 |
| 5 | Rn.87064 | NM_012701 | Adrb1 | Adrenergic, beta-1-, receptor | −1.2 | 0.715 |
| 6 | Rn.137597 | NM_033650 | Agrp | Agouti related protein homolog (mouse) | −27.1 | 0.024* |
| 7 | Rn.53846 | NM_031351 | Atrn | Attractin | −1.4 | 0.226 |
| 8 | Rn.11266 | NM_012513 | Bdnf | Brain-derived neurotrophic factor | −1.9 | 0.230 |
| 9 | Rn.86415 | NM_152845 | Brs3 | Bombesin-like receptor 3 | −2.1 | 0.127 |
| 10 | Rn.11378 | NM_016994 | C3 | Complement component 3 | −4.5 | 0.300 |
| 11 | Rn.90085 | NM_017338 | Calca | Calcitonin-related polypeptide alpha | −1.6 | 0.188 |
| 12 | Rn.10062 | NM_053816 | Calcr | Calcitonin receptor | −1.0 | 0.928 |
| 13 | Rn.89164 | NM_017110 | Cartpt | CART prepropeptide | −3.0 | 0.004** |
| 14 | Rn.9781 | NM_012829 | Cck | Cholecystokinin | −1.7 | 0.547 |
| 15 | Rn.10184 | NM_012688 | Cckar | Cholecystokinin A receptor | −1.3 | 0.145 |
| 16 | Rn.89774 | NM_012784 | Cnr1 | Cannabinoid receptor 1 (brain) | 1.4 | 0.408 |
| 17 | Rn.6067 | NM_013166 | Cntf | Ciliary neurotrophic factor | −1.1 | 0.603 |
| 18 | Rn.55036 | NM_001003929 | Cntfr | Ciliary neurotrophic factor receptor | −1.1 | 0.595 |
| 19 | Rn.10349 | NM_031019 | Crh | Corticotropin releasing hormone | 2.2 | 0.100 |
| 20 | Rn.10499 | NM_030999 | Crhr1 | Corticotropin releasing hormone receptor 1 | −1.0 | 0.905 |
| 21 | Rn.24039 | NM_012546 | Drd1 | Dopamine receptor D1A | 2.2 | 0.286 |
| 22 | Rn.87299 | NM_012547 | Drd2 | Dopamine receptor D2 | 1.1 | 0.826 |
| 23 | Rn.8929 | NM_033237 | Gal | Galanin prepropeptide | −1.4 | 0.234 |
| 24 | Rn.10213 | NM_012958 | Galr1 | Galanin receptor 1 | 1.0 | 0.955 |
| 25 | Rn.54383 | NM_012707 | Gcg | Glucagon | −1.2 | 0.490 |
| 26 | Rn.2178 | NM_017094 | Ghr | Growth hormone receptor | −1.2 | 0.628 |
| 27 | Rn.42103 | NM_021669 | Ghrl | Ghrelin/obestatin prepropeptide | −1.2 | 0.544 |
| 28 | Rn.74241 | NM_032075 | Ghsr | Growth hormone secretagogue receptor | −2.3 | 0.054 |
| 29 | Rn.11408 | NM_012728 | Glp1r | Glucagon-like peptide 1 receptor | −2.1 | 0.023* |
| 30 | Rn.10930 | NM_133570 | Grp | Gastrin releasing peptide | 4.6 | 0.006** |
| 31 | Rn.10316 | NM_012706 | Grpr | Gastrin releasing peptide receptor | −1.0 | 0.860 |
| 32 | Rn.7628 | NM_013179 | HcRt | Hypocretin | −10.5 | 0.039* |
| 33 | Rn.88262 | NM_013064 | Hcrtr1 | Hypocretin (orexin) receptor 1 | −1.5 | 0.191 |
| 34 | Rn.81032 | NM_017018 | Hrh1 | Histamine receptor H 1 | −1.8 | 0.149 |
| 35 | Rn.9935 | NM_012765 | Htr2c | 5-hydroxytryptamine (serotonin) receptor 2C | 1.0 | 0.800 |
| 36 | Rn.12300 | NM_017019 | Il1a | Interleukin 1 alpha | 1.1 | 0.512 |
| 37 | Rn.9869 | NM_031512 | Il1b | Interleukin 1 beta | −2.9 | 0.016* |
| 38 | Rn.9758 | NM_013123 | Il1r1 | Interleukin 1 receptor, type I | −1.2 | 0.341 |
| 39 | Rn.9873 | NM_012589 | Il6 | Interleukin 6 | −1.1 | 0.457 |
| 40 | Rn.1716 | NM_017020 | Il6r | Interleukin 6 receptor | −1.2 | 0.435 |
| 41 | Rn.989 | NM_019130 | Ins2 | Insulin 2 | −1.7 | 0.193 |
| 42 | Rn.9876 | NM_017071 | Insr | Insulin receptor | −1.1 | 0.436 |
| 43 | Rn.9891 | NM_012596 | Lepr | Leptin receptor | −2.4 | 0.014* |
| 44 | Rn.215838 | NM_001025270 | Mc3r | Melanocortin 3 receptor | −2.3 | 0.023* |
| 45 | Rn.10822 | NM_031758 | Mchr1 | Melanin-concentrating hormone receptor 1 | 1.1 | 0.120 |
| 46 | Rn.18763 | NM_001109149 | Nmb | Neuromedin B | 1.1 | 0.715 |
| 47 | Rn.89709 | NM_012799 | Nmbr | Neuromedin B receptor | 2.4 | 0.064 |
| 48 | Rn.47720 | NM_022239 | Nmu | Neuromedin U | −5.0 | 0.076 |
| 49 | Rn.9714 | NM_012614 | Npy | Neuropeptide Y | −1.4 | 0.088 |
| 50 | Rn.11642 | NM_001113357 | Npy1r | Neuropeptide Y receptor Y1 | −1.0 | 0.984 |
| 51 | Rn.90070 | NM_012576 | Nr3c1 | Nuclear receptor subfamily 3, group C, member 1 | −1.3 | 0.082 |
| 52 | Rn.60814 | NM_001102381 | Nts | Neurotensin | 3.2 | 0.089 |
| 53 | Rn.200149 | NM_001108967 | Ntsr1 | Neurotensin receptor 1 | −1.1 | 0.708 |
| 54 | Rn.89571 | NM_017167 | Oprk1 | Opioid receptor, kappa 1 | 1.2 | 0.206 |
| 55 | Rn.10118 | NM_013071 | Oprm1 | Opioid receptor, mu 1 | 1.3 | 0.255 |
| 56 | Rn.108195 | NM_139326 | Pomc | Proopiomelanocortin | −10.3 | 0.327 |
| 57 | Rn.9753 | NM_013196 | Ppara | Peroxisome proliferator activated receptor alpha | −1.1 | 0.584 |
| 58 | Rn.19172 | NM_031347 | Ppargc1a | Peroxisome proliferator-activated receptor gamma, coactivator 1 alpha | 1.2 | 0.396 |
| 59 | Rn.138127 | NM_139193 | Prlhr | Prolactin releasing hormone receptor | 1.8 | 0.137 |
| 60 | Rn.11317 | NM_012637 | Ptpn1 | Protein tyrosine phosphatase, nonreceptor type 1 | −1.1 | 0.834 |
| 61 | Rn.13173 | NM_001034080 | Pyy | Peptide YY (mapped) | −1.9 | 0.143 |
| 62 | Rn.48672 | NM_020100 | Ramp3 | Receptor (G protein-coupled) activity modifying protein 3 | −1.4 | 0.147 |
| 63 | Rn.1129 | NM_030996 | Sigmar1 | Sigma non-opioid intracellular receptor 1 | 1.1 | 0.455 |
| 64 | Rn.11286 | NM_031767 | Sort1 | Sortilin 1 | −1.1 | 0.543 |
| 65 | Rn.34418 | NM_012659 | Sst | Somatostatin | 2.1 | 0.138 |
| 66 | Rn.42915 | NM_012719 | Sstr1 | Somatostatin receptor 1 | −1.0 | 0.975 |
| 67 | Rn.34019 | NM_012672 | Thrb | Thyroid hormone receptor beta | −1.6 | 0.101 |
| 68 | Rn.2275 | NM_012675 | Tnf | Tumor necrosis factor (TNF superfamily, member 2) | −1.1 | 0.442 |
| 69 | Rn.22 | NM_013046 | Trh | Thyrotropin releasing hormone | 1.7 | 0.241 |
| 70 | Rn.9962 | NM_013047 | Trhr | Thyrotropin releasing hormone receptor | 1.2 | 0.368 |
| 71 | Rn.10190 | NM_019150 | Ucn | Urocortin | −2.1 | 0.156 |
Bold text highlights statistically significant changes.
p<0.01 and
p<0.05 compared to sham controls.
DISCUSSION
The present study was designed to test corollaries of the central hypothesis that VSG surgery selectively attenuates palatable food intake through modulation of genetic information flow within the hypothalamus. From these efforts, we discovered that VSG surgery in male rodents led to anticipated reductions in body weight that was sustained throughout the study period. In addition, VSG surgery attenuated palatable food intake following a caloric preload, but spared deprivation-induced re-feeding behavior. This observation supports the contention that VSG is not a restrictive surgery but rather limits excess intake in the absence of a caloric need. Although, alcohol drinking was moderately impacted at lower alcohol concentration, this effect diminished as the concentration of alcohol was escalated. Behaviorally characterized VSG rats displayed decreased expression of key orexigenic hypothalamic transcripts linked to stimulation of both food and alcohol intake. Collectively, these results suggest that weight loss after surgical reconstruction of the stomach in VSG is accompanied by behavioral and neurobiological events that signify reduced drive for palatable food.
Roux-en-Y Gastric Bypass (RYGB) surgery and Vertical Sleeve Gastrectomy (VSG) are the most widely performed surgical procedures to induce sustained weight loss for the treatment of obesity and related metabolic complications (1). VSG has emerged as a popular and frequently performed surgical procedure for obesity treatment given its efficiency (several metabolic benefits even at ~ 20% mean reduction in the body weight) and allowance of revisional procedures (1). Furthermore, a significant reduction in the fat and fat-free mass has been reported following VSG (14). However, large variability exists in body weight reduction following VSG (15) and both preclinical and clinical studies report body weight regain following bariatric procedures, including VSG (16–18). It is important to note that marked variability in the maximal weight loss following bariatric surgeries could be attributed to several factors, including age at which the surgical procedure is performed, pre-operative BMI, % early weight loss following surgery, poor-diet quality, incompliance with post-operative dietary recommendations, emotional eating, increase food craving and/or pre-operative food preferences (17–22).
In this context, hedonic feeding (consumption of a palatable food in the absence of a caloric need) could be a major contributor to the individual difference in weight regain after VSG. Notably, preclinical evidence indicates that preoperative food preference for palatable food is maintained after VSG (18, 23), highlighting perseverance for unhealthy food as a means to stimulate body weight regain. However, it is unknown if palatable food intake is impacted in the absence of caloric needs following VSG. The data we present here indicate for the first time that VSG rats selectively reduced intake of palatable food, when offered a high fat diet after consuming a caloric pre-load. Supporting this finding, preclinical studies indicate that VSG rats decrease preference for high fat foods despite displaying persistence of food-motivated behavior (24). Importantly re-feeding on low fat food after deprivation did not differ between VSG rats and sham controls, suggesting that VSG is not a restrictive surgical procedure. Instead, relative to controls, VSG rats selectively reduced intake when offered a palatable high fat diet after consuming a caloric pre-load. Consistent with this, clinical evidence indicates that VSG reduces preference and liking for high caloric foods (rich in fat and sugar) (3, 25). Taken together, these results suggest that VSG selectively abrogates hedonic feeding in the absence of a caloric need.
Previous work from our group and others indicate that RYGB surgery increases alcohol intake in rodents. Specifically, RYGB surgery increases sensitivity, consumption and motivation to obtain alcohol (9–11) in rats that are non-alcohol preferring at baseline. Moreover, we recently discovered that this phenomenon occurs at the expense of reduced hedonic feeding (11). That is, RYGB rats that displayed increased alcohol intake also selectively reduced hedonic intake of palatable food after a caloric pre-load. To determine if surgical effects on alcohol extend to the VSG procedure, we examined alcohol intake over a range of alcohol concentrations. Our data indicate that VSG reduced intake of alcohol at low concentration and that this phenomenon is diminished as the concentration of alcohol is escalated. These data are in agreement with a recent preclinical study that found reduced alcohol intake after VSG surgery (26). However, in that study, VSG rats and mice consumed significantly less alcohol at all concentrations tested. It is important to note that VSG rats were directly compared to non-surgical controls in that study. Whereas in the current study, sham rats that served as controls experienced the same degree of perioperative stress but did not undergo surgical reduction of the fundus. Thus, these differences in alcohol consumption among control groups may have contributed to the disparity in results between the two studies. In either case, we conclude that if anything, VSG reduces alcohol intake as opposed to RYGB, which stimulates new onset alcohol intake in both patients (12) and rodents (9, 10).
It is important to note that anatomical and resultant physiological alterations following surgeries that re-structure the gastrointestinal tract can impact alcohol pharmacokinetics. For example, rapid absorption, higher blood alcohol concentration and delayed alcohol elimination have been reported following RYGB, which may contribute to the development of AUD in these patients, nicely reviewed elsewhere (27). On the other hand, fewer studies have examined alcohol pharmacokinetics and alcohol intoxication following VSG and have generated conflicting results. For example, a prospective study (n=10) compared alcohol metabolism, peak blood alcohol concentration, alcohol elimination and intoxication before and after (3 and 12 months) VSG and found no significant changes (28). Similar results were obtained by an additional study that examined the impact of VSG (n=7) and gastric banding (n=9) on alcohol pharmacokinetics and intoxication following 3- and 6-months of surgery (29). These data are consistent with our preclinical finding demonstrating no change in alcohol drinking following VSG. In contrast, Maluenda et al. (2010) reported increased blood alcohol levels and delayed alcohol clearance 2 months following VSG in 12 patients (30). Therefore, additional well-controlled clinical studies are needed to assess alcohol pharmacokinetics and the risk of development of AUD following VSG.
Interestingly, males and females exhibit similar rates of obesity. Although, the number of male patients seeking bariatric procedures continues to grow, females constitute the majority of patients undergoing these surgeries. Since the current study examined the impact of VSG on hedonic feeding and alcohol drinking in male rats only, future studies are needed to determine if similar effects persist in females. In this context, a clinical study reported no significant changes in the alcohol pharmacokinetics (examined using Breathalyzer) and intoxication levels 3- and 12-months following VSG, primarily in females (28). However, a recent study argued that the Breathalyzer method may be unreliable and that more robust analytical methods (e.g., gas chromatography) are needed to assess alcohol pharmacokinetics following bariatric surgery (31). For example, Acevedo et al found faster absorption, higher peak blood alcohol levels and heightened intoxications in 11 women ~2.0 years following VSG using this method (31). Interestingly, a very recent, large, multi-institutional study based on self-reported alcohol consumption found similar pre- and post-operative (1 and 2 yr) AUD prevalence following RYGB (n=1006, 78.4% female) and VSG (n=4718, 78.4% female). In this study, AUD was detected 2-yrs following RYGB or VSG. Notably income, education, baseline alcohol consumption or alcohol misuse were predictive for AUD after surgery (32). Collectively, these data highlight the importance of method, time following surgery and history of alcohol misuse as critical factors that should be considered when evaluating the impact of VSG on new onset of AUD.
The hypothalamus integrates metabolic information with internal need to adapt behavioral responses. Specifically gastrointestinal (GI) derived peptides target the hypothalamus to regulate energy balance through modulation of feeding behavior (5). To examine potential mechanisms for the observed reduced hedonic feeding, we measured mRNA changes in the hypothalamus of VSG rats that were behaviorally characterized for reduced hedonic feeding and low concentration alcohol intake. Our results indicate that VSG dramatically reduced expression of key orexigenic peptides known to stimulate food intake. For example, Agouti related peptide (AgRP) is a neuropeptide with well-established effects on appetite stimulation (33). Activation of AgRP neurons drives feeding in untrained rodents (34), demonstrating that these neurons are necessary and sufficient to initiate feeding. We find that AgRP mRNA is significantly decreased after VSG relative to sham control rats. Pharmacologic AgRP selectively drives preferences for fat and stimulates mesocortical dopamine release (35). Thus, reduced hedonic feeding in VSG may derive from reduced AgRP expression. We also detected decreased HcRt or orexin mRNA in the hypothalamus of VSG rats. Notably, orexin signaling is required for hedonic feeding behavior in rodents (36). In addition, orexin promotes alcohol intake in rodents (37). Thus, decreased orexin expression may also contribute to the reduction in hedonic feeding and/or low concentration alcohol intake observed after VSG surgery. We also detected decreased expression (a strong trend p=0.05) of ghrelin receptor-1a (GHSR), the central target of the appetite hormone ghrelin (38). In addition to promoting appetite, ghrelin also stimulates alcohol intake (39). Notably, VSG reduces circulating ghrelin in humans (40) and rodents (41) indicating that hypothalamic reduction in GHSR mRNA expression may derive from reduced circulating ghrelin. In support of this contention, we recently discovered that RYGB rats with reduced circulating ghrelin have diminished GHSR control of dopamine neuronal firing (11), suggesting that reduced plasma ghrelin may lead to reductions in central GHSR function. A significant decrease in leptin receptor mRNA expression was also observed in the hypothalamus of VSG rats. Notably, studies have reported decreased circulating leptin hormone following VSG (42). It is important to note that leptin concentration and sensitivity closely correlate with the body weight as obesity increases whereas weight loss decreases plasma leptin levels (43). Therefore, a decreased leptin receptor expression in the present study could be a consequence of reduced body weight following VSG surgery. Interestingly, leptin positively regulates hypothalamic CART (cocaine- and amphetamine-regulated transcript) mRNA levels (44). We observed a significant decrease in the CART prepropeptide mRNA expression in the hypothalamus of VSG rats, which is in agreement with the decreased leptin functional activity following VSG. Notably, we also discovered that VSG led to up-regulation of gastrin releasing peptide (GRP) a peptide produced in the gastrointestinal tract and hypothalamus that inhibits food intake (45). Collectively, these results indicate that VSG surgery exerts dramatic alterations in the genetic expression in the brain’s endogenous appetite center that potentially contributes to reduced drive to feed. It is important to note whether similar changes could be attributed to the weight loss itself, independent of surgery, is unclear which requires further investigation.
To summarize, the current data indicate that VSG surgery may exert positive benefits on body weight loss through reductions in hedonic intake of palatable food, an effect that occurs without risk of new onset alcohol misuse and potentially explained by alterations in genetic information flow within the hypothalamus. These data can inform development of new therapies designed to reduce body weight with reduced risk of developing alcohol use disorder.
Roux-en-Y gastric bypass (RYGB) surgery and Vertical Sleeve Gastrectomy (VSG) are the most effective and commonly performed bariatric procedures for sustained body weight loss.
Both preclinical and clinical studies report new onset alcohol misuse following RYGB surgery.
The impact of VSG on alcohol intake is less clear.
VSG induced a moderate but significant and sustained weight loss in rats.
VSG attenuated hedonic feeding without impacting alcohol drinking, an effect potentially mediated by alterations in genetic information flow within the hypothalamus.
Importantly, these data highlight VSG as an effective bariatric procedure with potentially reduced risk of developing alcohol use disorder.
ACKNOWLEDGEMENTS
We thank Arriel Van Cleef and RCMI/LCRC/LBRN Cell and Molecular Biology Core services, College of Pharmacy, Xavier University of Louisiana, New Orleans, LA for providing technical assistance.
Funding: JFD: This project was supported, in part, by the Alcohol and Drug Abuse Program (ADARP) at Washington State University grant # 2550-1324. SS: This publication was also made possible by funding, in part, from the NIMHD-RCMI grant number 5G12MD007595-10 from the National Institute on Minority Health and Health Disparities and the NIGMS-BUILD grant number 5UL1GM118967 and 5TL4GM118968-04.
Footnotes
Authors declare no conflict of interest.
BIBLIOGRAPHY:
- 1.Angrisani L, Vitiello A, Santonicola A, Hasani A, De Luca M, lovino P. Roux-en-Y Gastric Bypass Versus Sleeve Gastrectomy as Revisional Procedures after Adjustable Gastric Band: 5-Year Outcomes. Obes Surg 2017;27:1430–1437. [DOI] [PubMed] [Google Scholar]
- 2.Coluzzi I, Raparelli L, Guarnacci L, et al. Food Intake and Changes in Eating Behavior After Laparoscopic Sleeve Gastrectomy. Obes Surg 2016;26:2059–2067. [DOI] [PubMed] [Google Scholar]
- 3.Ammon BS, Bellanger DE, Geiselman PJ, Primeaux SD, Yu Y, Greenway FL. Short-Term Pilot Study of the Effect of Sleeve Gastrectomy on Food Preference. Obes Surg 2015;25:1094–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bužga M, Holéczy P, Švagera Z, Švorc P, Zavadilová V. Effects of sleeve gastrectomy on parameters of lipid and glucose metabolism in obese women – 6 months after operation. Wideochir Inne Tech Maloinwazyjne 2013;8:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blouet C, Schwartz GJ. Hypothalamic nutrient sensing in the control of energy homeostasis. Behav Brain Res 2010;209:1–12. [DOI] [PubMed] [Google Scholar]
- 6.Leibowitz SF, Wortley KE. Hypothalamic control of energy balance: different peptides, different functions. Peptides 2004;25:473–504. [DOI] [PubMed] [Google Scholar]
- 7.Schaffhauser AO, Madiehe AM, Braymer HD, Bray GA, York DA. Effects of a high-fat diet and strain on hypothalamic gene expression in rats. Obes Res 2002;10:1188–1196. [DOI] [PubMed] [Google Scholar]
- 8.Sirohi S, Van Cleef A, Davis JF. Binge-like intake of HFD attenuates alcohol intake in rats. Physiol Behav 2017;178:187–195. [DOI] [PubMed] [Google Scholar]
- 9.Davis JF, Tracy AL, Schurdak JD, et al. Roux en Y gastric bypass increases ethanol intake in the rat. Obes Surg 2013;23:920–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hajnal A, Zharikov A, Polston JE, et al. Alcohol reward is increased after Roux-en-Y gastric bypass in dietary obese rats with differential effects following ghrelin antagonism. PLoS OnE 2012;7:e49121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sirohi S, Richardson BD, Lugo JM, Rossi DJ, Davis JF. Impact of Roux-en-Y gastric bypass surgery on appetite, alcohol intake behaviors, and midbrain ghrelin signaling in the rat. Obesity (Silver Spring) 2017;25:1228–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King WC, Chen J-Y, Mitchell JE, et al. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA 2012;307:2516–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sirohi S, Schurdak JD, Seeley RJ, Benoit SC, Davis JF. Central & peripheral glucagon-like peptide-1 receptor signaling differentially regulate addictive behaviors. Physiol Behav 2016;161:140–144. [DOI] [PubMed] [Google Scholar]
- 14.Golzarand M, Toolabi K, Djafarian K. Changes in Body Composition, Dietary Intake, and Substrate Oxidation in Patients Underwent Laparoscopic Roux-en-Y Gastric Bypass and Laparoscopic Sleeve Gastrectomy: a Comparative Prospective Study. Obes Surg 2018. [DOI] [PubMed] [Google Scholar]
- 15.Manning S, Pucci A, Carter NC, et al. Early postoperative weight loss predicts maximal weight loss after sleeve gastrectomy and Roux-en-Y gastric bypass. Surg Endosc 2015;29:1484–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braghetto I, Csendes A, Lanzarini E, Papapietro K, Cárcamo C, Molina JC. Is laparoscopic sleeve gastrectomy an acceptable primary bariatric procedure in obese patients? Early and 5-year postoperative results. Surg Laparosc Endosc Percutan Tech 2012;22:479–486. [DOI] [PubMed] [Google Scholar]
- 17.Sarwer DB, Wadden TA, Moore RH, et al. Preoperative eating behavior, postoperative dietary adherence, and weight loss after gastric bypass surgery. Surg Obes Relat Dis 2008;4:640–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hao Z, Townsend RL, Mumphrey MB, Morrison CD, Münzberg H, Berthoud H-R. RYGB Produces more Sustained Body Weight Loss and Improvement of Glycemic Control Compared with VSG in the Diet-Induced Obese Mouse Model. Obes Surg 2017;27:2424–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freire RH, Borges MC, Alvarez-Leite JI, Toulson Davisson Correia MI. Food quality, physical activity, and nutritional follow-up as determinant of weight regain after Roux-en-Y gastric bypass. Nutrition 2012;28:53–58. [DOI] [PubMed] [Google Scholar]
- 20.Janse Van Vuuren MA, Strodl E, White KM, Lockie PD. Emotional food cravings predicts poor short-term weight loss following laparoscopic sleeve gastrectomy. Br J Health Psychol 2018. [DOI] [PubMed] [Google Scholar]
- 21.Peacock JC, Schmidt CE, Barry K. A Qualitative Analysis of Post-operative Nutritional Barriers and Useful Dietary Services Reported by Bariatric Surgical Patients. Obes Surg 2016;26:2331–2339. [DOI] [PubMed] [Google Scholar]
- 22.Rossell J, González M, Mestres N, et al. Diet Change After Sleeve Gastrectomy Is More Effective for Weight Loss Than Surgery Only. Obes Surg 2017;27:2566–2574. [DOI] [PubMed] [Google Scholar]
- 23.Saeidi N, Nestoridi E, Kucharczyk J, Uygun M, Yarmush M, Stylopoulos N. Sleeve gastrectomy and Roux-en-Y gastric bypass exhibit differential effects on food preferences, nutrient absorption and energy expenditure in obese rats. Int J Obes (Lond) 2012;36:1396–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson-Pérez HE, Chambers AP, Sandoval DA, et al. The effect of vertical sleeve gastrectomy on food choice in rats. Int J Obes (Lond) 2013;37:288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Primeaux SD, Tzeng TH, Allerton TD, et al. Differences in short-term food preferences following vertical sleeve gastrectomy and Roux-en-Y gastric bypass surgery. Obes Res Clin Pract 2015;9:628–632. [DOI] [PubMed] [Google Scholar]
- 26.Orellana ER, Jamis C, Horvath N, Hajnal A. Effect of vertical sleeve gastrectomy on alcohol consumption and preferences in dietary obese rats and mice: A plausible role for altered ghrelin signaling. Brain Res Bull 2018;138:26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blackburn AN, Hajnal A, Leggio L. The gut in the brain: the effects of bariatric surgery on alcohol consumption. Addict Biol 2017;22:1540–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gallo AS, Berducci MA, Nijhawan S, et al. Alcohol metabolism is not affected by sleeve gastrectomy. Surg Endosc 2015;29:1088–1093. [DOI] [PubMed] [Google Scholar]
- 29.Changchien EM, Woodard GA, Hernandez-Boussard T, Morton JM. Normal alcohol metabolism after gastric banding and sleeve gastrectomy: a case-cross-over trial. J Am Coll Surg 2012;215:475–479. [DOI] [PubMed] [Google Scholar]
- 30.Maluenda F, Csendes A, De Aretxabala X, et al. Alcohol absorption modification after a laparoscopic sleeve gastrectomy due to obesity. Obes Surg 2010;20:744–748. [DOI] [PubMed] [Google Scholar]
- 31.Acevedo MB, Eagon JC, Bartholow BD, Klein S, Bucholz KK, Pepino MY. Sleeve gastrectomy surgery: when 2 alcoholic drinks are converted to 4. Surg Obes Relat Dis 2018;14:277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ibrahim N, Alameddine M, Brennan J, Sessine M, Holliday C, Ghaferi AA. New onset alcohol use disorder following bariatric surgery. Surg Endosc 2018. [DOI] [PubMed] [Google Scholar]
- 33.Ilnytska O, Argyropoulos G. The role of the Agouti-Related Protein in energy balance regulation. Cell Mol Life Sci 2008;65:2721–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci 2011;14:351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis JF, Choi DL, Shurdak JD, et al. Central melanocortins modulate mesocorticolimbic activity and food seeking behavior in the rat. Physiol Behav 2011;102:491–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi DL, Davis JF, Fitzgerald ME, Benoit SC. The role of orexin-A in food motivation, reward-based feeding behavior and food-induced neuronal activation in rats. Neuroscience 2010;167:11–20. [DOI] [PubMed] [Google Scholar]
- 37.Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. BrJPharmacol 2006;148:752–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zigman JM, Nakano Y, Coppari R, et al. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest 2005;115:3564–3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jerlhag E, Egecioglu E, Landgren S, et al. Requirement of central ghrelin signaling for alcohol reward. Proc Natl Acad Sci USA 2009;106:11318–11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langer FB, Reza Hoda MA, Bohdjalian A, et al. Sleeve gastrectomy and gastric banding: effects on plasma ghrelin levels. Obes Surg 2005;15:1024–1029. [DOI] [PubMed] [Google Scholar]
- 41.CHAMBERS AP, KIRCHNER H, WILSON-PEREZ HE, et al. The Effects of Vertical Sleeve Gastrectomy in Rodents Are Ghrelin Independent. Gastroenterology 2013;144:50–52.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bužga M, Zavadilová V, Holéczy P, et al. Dietary intake and ghrelin and leptin changes after sleeve gastrectomy. Wideochir Inne Tech Maloinwazyjne 2014;9:554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Friedman JM. The function of leptin in nutrition, weight, and physiology. Nutr Rev 2002;60:S1–14; discussion S68-84, 85-87. [DOI] [PubMed] [Google Scholar]
- 44.Kristensen P, Judge ME, Thim L, et al. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature 1998;393:72–76. [DOI] [PubMed] [Google Scholar]
- 45.Gutzwiller JP, Drewe J, Hildebrand P, Rossi L, Lauper JZ, Beglinger C. Effect of intravenous human gastrin-releasing peptide on food intake in humans. Gastroenterology 1994;106:1168–1173. [DOI] [PubMed] [Google Scholar]