Abstract
Tissue engineering has gained considerable attention in the development of small diameter tissue engineered vascular grafts (TEVGs) for treating coronary heart disease. A properly designed acellular and biodegradable TEVG must encourage the infiltration and growth of vascular smooth muscle cells (SMCs). Our group has previously shown that increasing levels of TGFβ2 can differentially modulate SMC migration and proliferation. In this study, tubular electrospun scaffolds loaded with TGFβ2 were fabricated using various ratios of gelatin/polycaprolactone (PCL), resulting in scaffolds with porous nano-woven architecture suitable for tissue ingrowth. Scaffold morphology, degradation rate, TGβ2 release kinetics, and bioactivity were assessed. TGFβ2 was successfully integrated into the electrospun biomaterial that resulted in a differential release profile depending on the gelatin/PCL ratio over the course of 42 days. Higher TGFβ2 elution was obtained in scaffolds with higher gelatin content, which may be related to the biodegradation of gelatin in culture media. The biological activity of the released TGFβ2 was evaluated by its ability to affect SMC proliferation as a function of its concentration. SMCs seeded on TGFβ2-loaded scaffolds also showed higher densities and infiltration after 5 days in culture as compared to scaffolds without TGFβ2. Our results demonstrate that the ratio of synthetic and natural polymers in electrospun blends can be used to tune the release of TGFβ2. This method can be used to intelligently modulate the SMC response in gelatin/PCL scaffolds making the TGFβ2-loaded conduits attractive for cardiovascular tissue engineering applications.
Keywords: TGFβ2 release, vascular tissue engineering, gelatin, polycaprolactone electrospinning, smooth muscle cells
Introduction
The development of a functional tissue engineering vascular graft (TEVG) is one of the urgent necessities in the effort to improve the treatment of coronary heart disease [1, 2]. An acellular and biodegradable TEVG must encourage healthy host cell infiltration while also providing a suitable environment for matrix production and proper long term function [3, 4]. SMCs play an essential role in the medial layer where they are responsible for modulating vascular tone and maintaining a homeostatic environment via tightly regulating extracellular matrix (ECM) remodeling [5]. Therefore, control of SMCs recruitment, infiltration, and proliferation is an important aspect of TEVG development and design. TEVG scaffolds can also provide additional biofunctionality by locally delivering biomolecules to regulate SMCs response [6, 7], TGFβ2 is a pleiotropic cytokine that regulates different cellular processes including the cell cycle, cell differentiation, cell growth, cell death, and ECM deposition and organization [8-10]. This growth factor has received particular interest in the study of cardiovascular development and disease as its signaling pathway is implicated in ECM remodeling of vascular and cardiac tissue in health and disease [11-14]. It has also been found to play a critical role in vascular remodeling during embryogenesis [15]. More specifically, TGFβ2 can have a differential effect on SMCs proliferation, migration, and matrix deposition as was demonstrated in our previous work [10]. We showed that the proliferation and migration of SMCs seeded in gelatin/fibrinogen scaffolds was increased at low concentrations of exogenous TGFβ2 (≤ 2ng/ml in culture media) whereas the proliferation and migration of SMCs decreased at high concentrations of exogenous TGFβ2 (≥ 5ng/ml) [10]. These results suggested that it may be possible to modulate SMC behavior in a TEVG using TGFβ2.
Electrospinning is a widely used robust and cost-efficient technique to fabricate fibrous scaffolds for tissue engineering applications [16, 17]. This technique permits the fine-tuning of microstructure, mechanical properties, biodegradability, and biocompatibility of TEVGs [16, 17]. Electrospun scaffolds encourage cells to attach, infiltrate, and proliferate due to their ECM-like interconnected pores that allow for the efficient exchange of nutrients and wastes [18]. These porous matrices are also characterized by their high surface-to-volume ratio improving their mass transfer properties and drug loading capacity [18]. Additionally, electrospinning has been shown to be an effective method for sustained integrated delivery of growth factors [18, 19].
Gelatin is a cost effective natural polymer obtained by the denaturation of collagen triplehelix with numerous cell binding sites [20, 21]. For biomedical applications, gelatin structure needs to be stabilized before putting it in contact with aqueous solutions such as culture media in the case of in vitro research studies or blood in the case of in vivo implementation [22, 23]. The most common process for gelatin stabilization is crosslinking, which avoids fast dissolution and loss of the three dimensional structure of the scaffold, giving it mechanical strength [22-25]. Various methods have been studied for crosslinking of gelatin electrospun scaffolds including the treatment with liquid phase genipin [30]. Genipin is a natural agent extracted from the gardenia fruit that spontaneously crosslinks bioproteins including gelatin [23, 26]. Several studies have noted the low cytotoxicity and genotoxicity of genipin, and there are no reports of problems of genipin induced calcification [27-29]. Although the crosslinking of gelatin significantly improves its mechanical strength, scaffolds fabricated exclusively by gelatin possess low suture retention and lose their mechanical integrity over time [30, 31]. The use of synthetic biodegradable polymers such as PCL in a blend with natural polymers have been used to improve the mechanical properties and patency of scaffolds, and delay their degradation allowing for new tissue ingrowth [32-34].
The goal of this work was to create hybrid gelatin:polycaprolactone (G:PCL) tubular scaffolds capable of releasing bioactive TGFβ2 to encourage SMC attachment, migration, and proliferation. Electrospinning was used to prepare tubular scaffolds with increasing ratios of a G:PCL blend and TGFβ2 was loaded into the polymeric blends during the electrospinning process. Genipin was then used to crosslink the gelatin contained in the polymeric blends. The resulting scaffolds were evaluated for their porosity, fiber thickness, degradation rates, TGFβ2 release kinetics, bioactivity of the released growth factor, and their suitability for in vitro SMC culture.
Materials and Methods
Preparation of TGFβ2 loaded polymeric scaffolds
Gelatin from porcine skin (Sigma-Aldrich, USA) and PCL (80,000 MW, Sigma-Aldrich, USA) were mixed at three different percentages: 80% gelatin 20% PCL (80G:20PCL), 50% gelatin 50% PCL (50G:50PCL), and 20% gelatin 80% PCL (20G:80PCL). The polymeric blends were dissolved in 1,1,1,3,3,3-Hexafluoro-2-propanol (HFP) (Sigma-Aldrich, USA) to create a 10% (w/v) solution under continuous stirring. The polymeric solutions were loaded into a 5 ml BD syringe and lyophilized recombinant human TGFβ2 (R&D systems) was reconstituted with 4mM HCL containing 1mg/ml bovine serum albumin (BSA) (Sigma-Aldrich, USA). The TGFβ2 solution was mixed with the polymeric blends directly in the syringe immediately prior to electrospinning to create a final concentration of 1.3 μg/ml for a low TGFβ2 releasing scaffold and 10.3 μg/ml for high TGFβ2 releasing scaffolds. A 23-gauge stainless steel dispensing blunt tip needle (CML supply, USA) was attached to the syringe which was then loaded onto a NE-1000 single syringe pump (New era pump systems Inc., USA) set to a pumping rate of 30 μL/min and a total dispensing volume of 300 μL. The distance from the needle tip to the target was 10 cm. The polymeric solutions containing TGFβ2 were electrospun at a voltage of 15 kV onto a grounded 1.4-mm metallic rod mounted onto a rotating and translating mandrel set to a rotation rate of 300 rpm and a translational speed of 300 mm/sec. Each scaffold had an approximate length of 8 cm and dry weight of 10 mg, for a total mass loss of 66.6% during the electrospinning process. The loss of scaffold material during electrospinning is probably due to the inherent static forces of the electrospinning hood that can attract charged polymeric fibers throughout the fabrication process. We assumed that the amount of TGFβ2 loss was proportional to the total material loss during electrospinning. Then, the remaining amount of TGFβ2 after electrospinning was calculated as the 33.4% of the initial amount loaded, for a total of 133.3 ng/scaffold for the low TGFβ2 releasing scaffolds and 1035 ng/scaffold for the high TGFβ2 releasing scaffold. Therefore, the determined TGFβ2 density were 13.3 ng TGFβ2/ mg scaffold (13ng TGFβ2/ mg scaffold hereafter), and 100.35 ng TGFβ2/ mg scaffold (100 ng TGFβ2/ mg scaffold hereafter). After electrospinning, scaffolds were air dried for 1 h to remove any trace of HFP. The resulting tubular scaffolds were crosslinked in a 0.5% (m/v) genipin solution (Wako Chemicals, USA) in 200 proof ethanol for 24 hours at 37°C under constant stirring. The tubes were rinsed with ethanol to remove any remaining genipin residues.
Scaffold characterization
Scaffold characterization was performed using scanning electron microscopy (SEM) on scaffolds with and without 13 ng of TGFβ2/ mg scaffold. Briefly, samples were mounted onto aluminum stubs, grounded with silver paint, and sputter coated with 6 nm gold/palladium (Cressington Sputter Coater Auto 108, Cressington, Watford, UK). Cross-sectional and en face views of the samples were imaged in a JEOL JSM-6335F scanning electron microscope (Peabody, MA) at 3 kV at a magnification of 2000x. The en face SEM images (n=3 for each group) were binarized and the porosity was calculated by dividing the total number of fiber pixels by the total number of pixels in the image. The fiber diameter was calculated by manually measuring the diameter of 40 randomly selected fibers per scaffold (n=3 for each group) via freehand lines superimposed over the SEM images in ImageJ.
Scaffold degradation rate
The degradation rate of the electrospun scaffolds containing 13 ng of TGFβ2/ mg scaffold was assessed in terms of dry weight change. For each group, 8-cm long dry crosslinked scaffolds were weighed and placed into 15 ml conical tubes containing 10 ml of 1X phosphate buffered saline (PBS) (Gibco, Life technologies) and incubated at 37°C. Lee et al. 2017, demonstrated that the weight loss of crosslinked gelatin electrospun scaffolds started to stabilize at 28 days of incubation in PBS [35]. In order to observe any difference in mass loss after 28 days, the samples were taken from the PBS at 7, 14, 21, 28, and 42 days, rinsed 2 times with deionized water, dried in a convection oven for 1 day at 42 °C and weighed. The samples were then put back in fresh 1X PBS and incubated at 37°C to continue the degradation study. The degradation of each scaffold was calculated as a percentage of dry weight remaining compared to their initial dry weight (n=4 in each group).
In vitro TGFβ2 release kinetics
TGFβ2 loaded scaffolds containing 13 ng of TGFβ2/ mg scaffold (8-cm long, 10 mg total dry weight approximately) were put in 15 mL conical tubes and incubated in 10 mL of Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS), 100U/mL of penicillin, 100 μg/mL of streptomycin, 5 μg/mL of amphotericin B and 25 mM HEPES (Gibco, Life technologies, USA) at 37°C and 5% CO2. The release media was collected at 1, 4, 8, 15, 21, 28, and 42 days and frozen at −80°C until assaying. The release medium was replenished with fresh medium to continue the release study. TGFβ2 concentrations were quantified using the Quantiquine human TGFβ2 immunoassay ELISA kit form R&D systems (n=4 in each group).
TGFβ2 bioactivity assay
The bioactivity of the released TGFβ2 was measured by its ability to modulate SMC proliferation. Scaffold groups of 80G:20PCL, 50G:50PCL, and 20G:80PCL containing 13 ng TGFβ2/mg scaffold and 100 ng TGFβ2/mg scaffold were fabricated by electrospinning as previously described. Porcine aortic SMCs from passage 4 were seeded in wells of 96-well plates at a density of 7 × 103 cells/well. Cells were cultured in the above described culture media for 24 h at 37°C and 5% CO2 in a humidified atmosphere. Culture media was then changed in each cell seeded well and the scaffolds containing either 0ng, 13 ng, or 100 ng TGFβ2/mg of scaffold were cut into 7 mm pieces and submerged into the culture media without touching the cells. For this purpose, an HTS Transwell®-96 well permeable support (Corning, USA) was used, which allowed cells to interact with the released TGFβ2 without directly contacting the scaffold. The system was incubated for 5 days at 37°C and 5% CO2 in a humidified atmosphere. After the incubation period, the scaffolds and the permeable support were removed, and the cells were detached from the wells using 0.25% trypsin (Gibco, Life technologies, USA). Cell number was determined using the Countess II-automated cell counter from Invitrogen using a cell sample pre-stained with trypan blue (n=5 in each group). To calibrate the effect of TGFβ2 on porcine vascular SMC growth, cells were grown in culture media containing different concentrations of TGFβ2 ranging from 1 pg/ml to 10 ng/ml. Porcine aortic SMCs (PAOSMCs) were seeded in the wells of 96-well plates at a density of 7 × 103 cells/well in culture media for 24 hours. The media was then replaced with culture media with different concentrations of TGFβ2. The SMCs were incubated for additional 5 days and cell proliferation was evaluated by cell count (n=4 in each group).
In vitro SMC growth in the TGFβ2 loaded scaffolds
To evaluate the ability of the TGFβ2 releasing scaffolds to support growth, PAOSMCs were seeded onto the scaffolds with or without TGFβ2. Cylindrical 80G:20PCL scaffolds containing 13 ng TGFβ2/mg of scaffold, were cut into 3 mm pieces. Each piece was placed upright in a well of a 96-well plate, and PAOSMCs from passage 4 were pipette-seeded into the lumen at a density of 1 × 104 cells/scaffold, followed by a 15 min incubation period. A second seeding was performed by submerging the samples in 100 μl of a cell solution containing 1 × 104 cells, follow by a 15 min incubation period to let the cells attach to the outer surface. The samples were then rinsed with 1X PBS and transferred to new wells of a 96-well plate containing fresh culture media prepared as described above. The seeded scaffolds were cultured for 5 days in a humidified atmosphere at 37°C and 5% CO2. After the incubation, the samples were immediately fixed in 10% formalin for 24 hours at 4°C. Cell nuclei were stained with VECTSHIELD® mounting media containing 4',6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, USA). The Pitt Advanced Intravital Microscope (AIM) for multiphoton imaging at the University of Pittsburgh’s Soft Tissue Biomechanics Laboratory was used to observe the total cell distribution along the scaffold thickness. The Pitt AIM is an Olympus BX51 upright laser-scanning microscope coupled to a Coherent 120-fs tunable pulsed Titanium-Sapphire laser (Santa Clara, CA). For this study a 4X air objective was used. Incident light was focused on the sample and the backscattered signal was collected over a 1500 ×1500 μm field of view at 5 μm steps, imaging through the scaffold depth. Cross-sections and en face images of each scaffold were captured. The laser was centered at λ=750 nm to excite DAPI, and 2-photon excited fluorescence (2PEF) was split with a 505 nm dichroic mirror and collected through a 460/80 bandpass filter. The fibers were imaged colocalized using the autofluorescence signal from genipin collecting the light with a 620/50 bandpass filter. The optics were chosen to maximize discrimination between the polymeric fibers and DAPI fluorescence. The colocalized image stacks from nuclei and fibers were merged to visualize the 3D cell location in the scaffolds. Maximum intensity projections from the cross-sectional images were binarized and used to calculate the area covered by the cells in 3 different regions: inner (luminal), middle (medial), and outer (abluminal) third through the thickness (n=4 in each group).
Statistical methods
All values are presented as the mean ± standard deviation unless otherwise specified. Individual Students t-tests were performed for the porosity and fiber thickness values. For all statistical tests, a critical p-value of 0.05 was used to define significance. The statistical analysis for the effect of different concentrations of TGFβ2 on SMCs proliferation was performed using a one-way ANOVA with the Dunnett’s comparison procedure. For the bioactivity assay and nuclei area coverage in the cell seeded scaffolds the analysis was completed using a two-way ANOVA with a post hoc analysis using a Bonferroni correction to account for family-wise error.
Results
Scaffold porosity and fiber thickness
The tubular electrospun scaffolds with and without TGFβ2 were found to have porosities above 35% and fiber thickness below 1μm (Table 1).
Table 1.
Scaffold porosity and fiber thickness (n=3 in each)
| Without TGFβ2 | With TGFβ2 | |||||
|---|---|---|---|---|---|---|
| Scaffold | 80G:20PCL | 50G:50 PCL | 20G:80PCL | 80G:20PCL | 50G:50 PCL | 20G:80PCL |
| Porosity (%) | 43.6 ± 3.1 | 37.5 ± 4.6 | 41.1 ±1.7 | 45.6 ± 1.6 | 38.9 ± 3.7 | 41.24 ±3.7 |
| Fiber thickness (μm) | 0.69 ± 0.59 | 0.63 ± 0.51 | 0.44 ± 0.35 | 0.60 ± 0.46 | 0.57 ± 0.48 | 0.44 ± 0.36 |
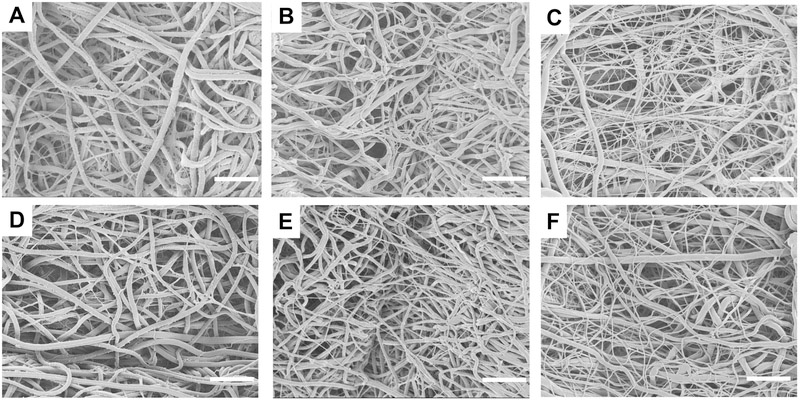
The addition of TGFβ2 did not significantly change the porosity or fiber thickness in scaffolds with the same gelatin:PCL (referred to as G:PCL) composition. Fiber thickness was significantly lower (p<0.05) in the 20G:80PCL compared to the 80G:20PCL in scaffolds with and without TGFβ2, and to the 50G:50PCL with TGFβ2. The en face SEM images of the electrospun scaffolds are shown in Figure 1, where inter-connected pores are observed in all the samples. It is also noticeable that thick and thin fibers were formed regardless of the scaffold composition. However, as the content of gelatin decreases, and PCL increases the difference in thickness between the thick and thin fibers is lower which contributed to the reduction in the fiber size variance. This behavior was observed in scaffolds with and without TGFβ2. Histograms showing the change in fiber size distribution are presented in the supplementary material (Figure S1)
Figure 1.
Representative en face SEM images of tubular scaffolds excluding (A-C) and including (D-F) 13 ng of TGFβ2/ mg scaffold. A and D are composed of 80G:20PCL; B and E are composed of 50G:50PCL; C and F are composed of 20G:80PCL. Scale bar is 10μm.
Degradation rate
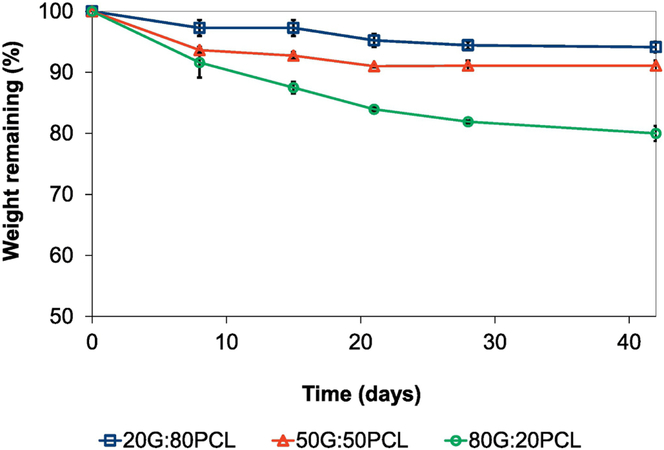
Figure 2 shows the scaffolds’ weight percentage remaining as a function of time after incubation in PBS.
Figure 2.
Degradation rate of TGFβ2 releasing scaffolds over the course of 42 days. Error bars shown are standard deviation (n=4). Scaffolds containing 13 ng of TGFβ2/ mg scaffold were used.
These results demonstrate that the degradation rate of the blended G:PCL scaffolds increased as gelatin content increased, with the lowest weight percentage remaining of 80% ± 1.2% in the 80G:20PCL scaffolds after 42 days of incubation in an aqueous solution. The weight remaining after 42 days was 91±0.81% and 94.1±0.88% for the 50G:50PCL and 20G:80PCL grafts, respectively.
We measured the degradation of the 80G:20PCL, 50G:50PCL, and 20G:80PCL scaffolds without TGFβ2 after 1 week of incubation in PBS at 37°C. No significant difference was found indicating that the presence of TGFβ2 does not influence the degradation rate of the scaffold (supplementary material, Figure S2).
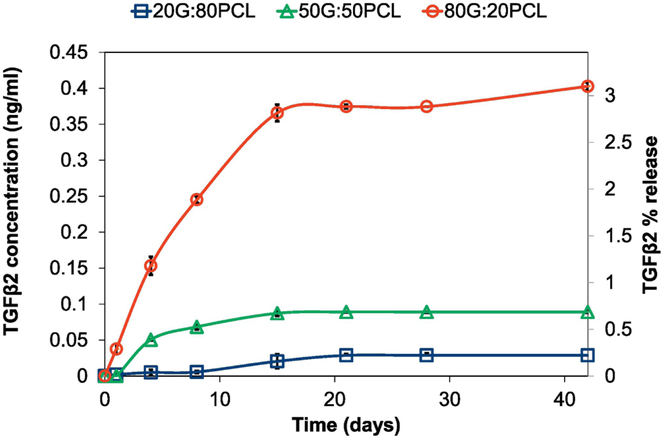
Release of TGFβ2 from crosslinked gelatin:PCL electrospun scaffolds
TGFβ2 release from crosslinked G:PCL scaffolds was evaluated using an ELISA kit. Figure 3 shows the release profile of TGFβ2 from the 80G:20PCL, 50G:50PCL, and 20G:80PCL constructs. All samples showed a slow and sustained release over the course of approximately 15 days prior to reaching a plateau. The scaffolds with higher percentage of gelatin exhibited higher amounts of eluted TGFβ2. 80G:20PCL constructs had the maximum TGFβ2 percentage release of 3.1% over the course of 42 days. The maximum 42-day percentage release for the 50G:50PCL and 20G:80PCL constructs was 0.22% and 0.07%, respectively.
Figure 3.
Cumulative release of TGFβ2 from electrospun scaffolds with varying G:PCL compositions. Error bars shown are standard deviation (n=4). Scaffolds containing 13 ng of TGFβ2/ mg scaffold were used.
Bioactivity of the released TGFβ2
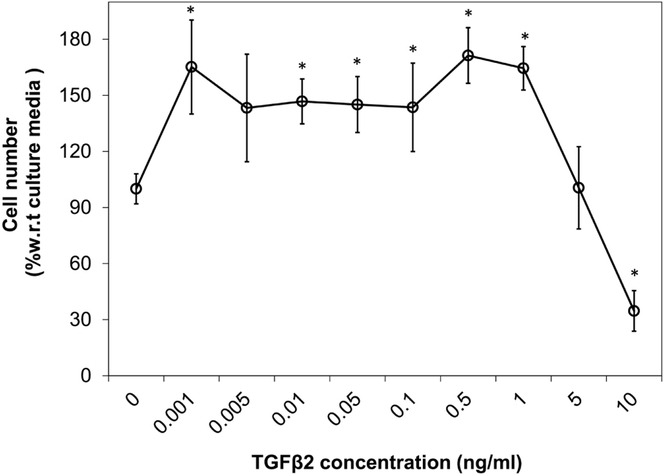
A calibration curve of the cell number following 5 days of culture with a range of TGFβ2 concentrations (Figure 4) shows a significant increase in cell number when growing in media with added TGFβ2 at 0.001 ng/ml (p=0.002), 0.01 ng/ml (p=0.036), 0.05ng/ml (p=0.074), 0.1ng/ml (0.057), 0.5 ng/ml (p=0.001), and 1ng/ml (p=0.002) compared to culture media without TGFβ2. A significant decrease in cell number was obtained when cells were growing in media with 10 ng/ml of exogenous TGFβ2 compared to culture media alone (p=0.004).
Figure 4.
Effect of exogenous TGFβ2 dose on proliferation of SMCs growing in monolayer for 5 days. Cell number was normalized with the value obtained for culture media without TGFβ2. Error bars shown are standard deviation (*p < 0.05 compared to culture media; n=4).
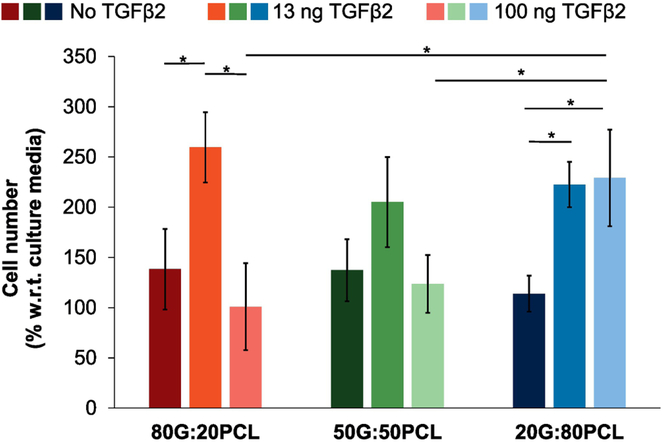
Scaffolds loaded with 13 ng TGFβ2/mg scaffold had a positive effect on cell proliferation as shown in Figure 5. 80G:20PCL constructs with 13 ng TGFβ2/mg exhibited a significantly increased cell number relative to both scaffolds without TGFβ2 (p=0.0106) and those containing 100 ng TGFβ2/mg of scaffold (p=0.001). Scaffolds composed of 20G:80PCL with 13 ng TGFβ2/mg and 100 ng TGFβ2/mg had a significant positive effect on cell proliferation compared to scaffolds without TGFβ2 (p=0.0003, and p=0.032 respectively). The 80G:20PCL and 50G:50PCL constructs loaded with 100 ng TGFβ2/mg of scaffold had an inhibitory effect on cell proliferation, while the 20G:80PCL scaffolds with this high TGFβ2 content showed an increase in cell number (p=0.016, and p=0.035 respectively).
Figure 5.
Bioactivity of released TGFβ2 from electrospun scaffolds on cells growing in monolayers. Scaffolds loaded with 13 ng and 100 ng of TGFβ2/mg of scaffold were submerged in the culture media without having direct contact with the cells. A permeable support was used to elevate the scaffolds above the cell level. Cell count was assessed after 5 days in culture. Error bars shown are standard deviation (*p < 0.05; n=5).
The relationship between the results from the cumulative release of TGFβ2 (Figure 3), the effect of exogenous TGFβ2 dose on proliferation of SMCs (Figure 4), and bioactivity of released TGFβ2 from electrospun scaffolds (Figure 5) is provided in Table 2. It is possible to observe that the obtained cell number in Figure 5 is higher than the expected cell number in Figure 4, where TGFβ2 was added directly to the cultures by pipetting. In Figure 5 TGFβ2 was released from the scaffolds submerged in culture media. In Table 2 it is also possible to observe that the obtained cell number increases or decreases depending on the concentration of TGFβ2 in the media.
Table 2:
Expected TGFβ2 concentration in media from the electrospun scaffolds used in the bioactivity assay and the corresponding expected and obtained cell number. Average % release was obtained from the cumulative release curves presented in Figure 3. Expected TGFβ2 concentration was calculated using the scaffold weight (7 mm piece, 87.5 mg), volume of culture media (150μl), and percentage of release from each scaffold at 5 days. Expected cell number was obtained from the curve presented in Figure 4. The % TGFβ2 release for the 100 ng/mg scaffolds is assumed to be comparable to that from the 13 ng/mg scaffolds.
| TGFβ2 Loaded (ng/mg scaffold) |
Scaffold | Average % release at 5 days |
Expected TGFβ2 concentration (ng/ml) |
Expected cell number |
Average obtained cell number |
|---|---|---|---|---|---|
| 80G:20PCL | 1.42 | 1.07 | 164.5 | 259.57 | |
| 13 | 50G:50PCL | 0.45 | 0.34 | 155.8 | 204.88 |
| 20G:80 PCL | 0.038 | 0.03 | 145 | 222.78 | |
| 80G:20PCL | 1.42 | 8.28 | 60 | 100.92 | |
| 100 | 50G:50PCL | 0.45 | 2.63 | 130 | 123.62 |
| 20G:80 PCL | 0.038 | 0.22 | 143.6 | 229.19 |
This effect is in accordance with the calibration curve in Figure 4. In Figure 5 it can be seen that all scaffolds loaded with 13 ng of TGFβ2/mg scaffold have a positive effect on cell growth. This is related to the expected concentration of TGFβ2 ranging from 0.03 ng/ml to 1.07 ng/ml which will increase cell proliferation (Figure 4). It is very interesting to observe that 80G:20 PCL and 50G:50PCL scaffolds loaded with 100 ng TGFβ2/mg scaffold have a negative effect on cell growth as their expected TGFβ2 released concentration at 5 days is 8.28 ng/ml and 2.62 ng/ml, respectively. However, the 20G:80PCL scaffolds have a released TGFβ2 concentration of 0.22 ng/ml which have been demonstrated to increase cell proliferation (Figure 4).
In vitro cell growth of SMCs in the TGFβ2-releasing scaffolds.
In order to evaluate the in vitro growth of SMCs in the TGFβ2 releasing scaffolds, we used scaffolds containing 13 ng TGFβ2/mg as we observed the highest effect on cell number of SMCs growing in monolayer (Figure 5). We also have considered cost of fabrication in selecting the 80G:20PCL constructs. Scaffolds with 13 ng TGFβ2 /mg scaffold have a cost of fabrication almost 10X lower than the scaffolds with 100 ng TGFβ2 /mg. Moreover, our research group have biomechanically characterized scaffolds with different gelatin and PCL content and compared mechanical properties to the ones of a native rat aorta. The 80G:20PCL (0.0008 ± 0.00021 mmHg-1) have a closer compliance value to the native tissue (0.00056 ± 0.000318 mmHg-1) compared to the 20G:80PCL (0.00019 ± 0.0008 mmHg-1) which can be considered hypocompliant.
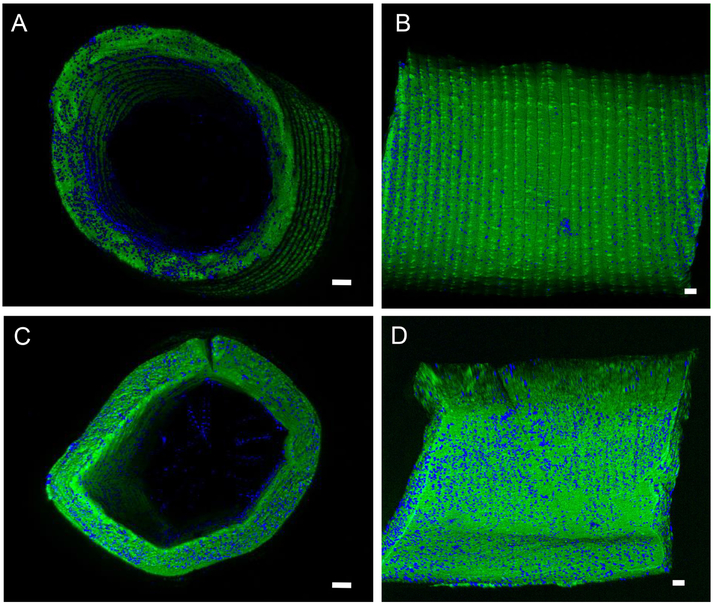
A representative image from the 2-photon 3D reconstructions of the seeded 80G:20PCL scaffolds with and without 13 ng TGFβ2/mg is shown in Figure 6. The TGFβ2 eluting scaffolds had a more even cell distribution in both the radial direction and along the circumference. These scaffolds also exhibited higher number of nuclei along the scaffold length.
Figure 6.
Representative multiphoton images of the 80G:20PCL electrospun scaffolds without (A and B) and with (C and D) 13 ng of TGFβ2/mg scaffold cultured with SMCs for 5 days. (A) and (B) are cross-sectional and en face images of the scaffold without TGFβ2, respectively. (C) and (D) are cross-sectional and en face images of the TGFβ2 releasing scaffolds. Green - electrospun fibers; Blue – nuclei; Scale bar is 100μm
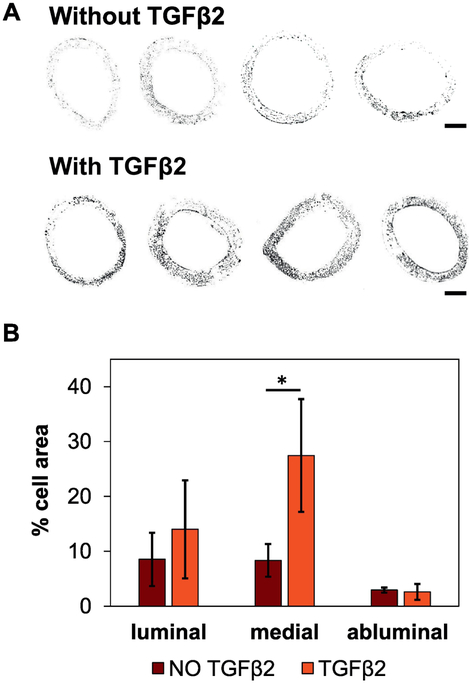
Binary images (Figure 7A) were used to calculate the percentage of the cross-sectional area occupied by cell nuclei. These results showed that scaffolds releasing TGFβ2 have significantly higher cell numbers in the middle section of the construct relative to scaffolds without TGFβ2 (p=0.037, Figure 7B).
Figure 7.
A) Binary multiphoton images of the 80G:20PCL electrospun scaffolds without (top row) and with (bottom row) 13 ng TGFβ2/mg scaffold cultured with SMCs for 5 days. The four cross-sections in each group correspond to four different replicates. Scale bar is 200 μm. B) Quantification of the cross-sectional area occupied by cell nuclei. The thickness of the scaffold was divided in 3 different regions, each one corresponding to one third of the thickness: inner (luminal), middle (medial), and outer (abluminal). Error bars shown are standard deviation (*p < 0.05; n = 4)
Discussion
We have demonstrated here that electrospun scaffolds composed of a blend of natural and synthetic polymers have the ability to elute bioactive and functional TGFβ2. These grafts also present porosities and fiber diameters that promote SMC attachment, migration, and proliferation. Our results also show that TGFβ2 elution increases with increasing gelatin content, with all the studied grafts exhibiting a slow and sustained release over 15 days and a lack of burst release. This eluted TGFβ2 is bioactive and has a differential effect on SMC migration and proliferation both in monolayer and 3D culture.
Rnjak-Kovacina et al. in 2014 [35] used image analysis to calculate the porosity of their electrospun scaffolds. The authors demonstrated that tubular scaffolds fabricated from synthetic human elastin with porosities higher than 35% promoted early attachment, spreading and proliferation of primary dermal fibroblasts, as well as migration and infiltration into the construct. In our work we also used image analysis to determine the porosity of the scaffolds obtaining values ranging from 35% to 47%. This combined with the fact that the cells seeded in the scaffolds were able to migrate through the construct indicates that the studied porous scaffolds are suitable for cell infiltration. Heydarkhan-Hagvall et al. [36] created three-dimensional gelatin:PCL and collagen:elastin:PCL electrospun scaffolds with fiber sizes ranging from 590-880 nm, which favored the attachment of adipose derived stem cells. Similarly, Fu et al., [37] fabricated tubular electrospun gelatin/PCL and collagen/PCL constructs. These researchers obtained scaffolds with fiber diameters ranging from 300 nm to 400 nm, favoring the adhesion and proliferation of human umbilical cord SMCs in vitro and in vivo. Here, the electrospun scaffolds are composed of fibers with thicknesses ranging from 440 nm - 700 nm on average which are similar to that obtained by Heydarkhan-Hagvall et al. and Fu et al. Looking closer into the scaffold fiber structure, the blends of gelatin and PCL resulted in electrospun matrices with both thick and thin fibers and thickness distributions becoming more uniform as gelatin content decreased. These results are comparable with that of Gautam, et al., 2013 [38] who also showed a relationship between increasing PCL content and fiber size distribution.
Our results suggest that the hydrolysis of hybrid biomaterials and the elution of growth factors strongly depend on gelatin content. These findings are consistent with the study of Munj et al. 2017 [39] who studied the release of rhodamine B from gelatin:PCL scaffolds with varying compositions of the two polymers. Their study concluded that the degradation of the polymeric matrix is faster as gelatin content increases in the scaffold. The authors also found that increasing the PCL content decreased the elution of the dye which also prevented its burst release. Our results show that degradation of 80G:20PCL constructs in PBS is faster compared to that of 50G:50PCL, and 20G:80PCL; obtaining the slowest degradation rate for the 20G:80PCL constructs. The release profiles obtained in our work also suggest that TGFβ2 elution rate from the scaffolds depends directly on the gelatin amount in the polymeric blend, where the highest and lowest percentage released was obtained for the 80G:20PCL and 20G:80PCL constructs, respectively. All of our materials lacked an observable burst release. This type of profile may be due to the addition of PCL to the scaffolds and also to the crosslinking of gelatin, which prevents the fast hydrolysis of gelatin. Here, the release profiles are also similar to the ones obtained in the work of Nada et al. 2016 [40] where the elution of chloramphenicol from electrospun gelatin crosslinked with poly aldehyde β- cyclodextrin (PA-β-CD) was studied. They show that crosslinked gelatin fibers have a sustained release of the drug with a lack of rapid release, as opposed to the profile obtained using uncrosslinked fibers where more than 90% of the chloramphenicol was eluted in the first 30 min of the study. Nada et al. concluded that the addition of a crosslinker is necessary to maintain the integrity of the gelatin containing electrospun fibers avoiding rapid elution of the drug.
Different authors have studied the release of growth factors from polymeric matrices for a number of tissue engineering applications. The obtained percentage release values depend on the materials used for the scaffold fabrication. For instance, Sohier et al. 2006 [41] studied the release of TGFβ1 from Poly(ethylene glycol)-terephthalate/poly(butylene terephthalate)(PEGT/PBT) and poly(ethylene glycol)-succinate)/ poly(butylene succinate) (PEGS/PBS) in view of cartilage regeneration. TGFβ1 was loaded into the PEG/PBS using an emulsion-coating method which used chloroform and high vacuum to force the emulsion solution through a premade porous scaffold. The release of the TGFβ1 depended on the PEG hydrolysis in media which was incomplete after 50 days obtaining a maximum percentage release of 13% of the total amount loaded. Another example is the work of Wang et al. 2016 [42] who created electrospun PCL:hyluronan (HA) constructs loaded with epidermal growth factor (EGF) for wound healing. EGF was directly mixed with the polymers prior to electrospinning. The authors found that the addition of HA increased the hydrophilicity of the scaffold and increased the release speed of the EGF to a maximum of 30% elution after 33 days. In that study the researchers did not use a crosslinker to stabilize the HA, however they obtained an incomplete release of EGF that can be attributed to the presence of PCL which may have prevented the short-term degradation of the scaffold. These studies have release systems that either only contained synthetic polymers that don’t need to be crosslinked or are aimed at applications that don’t require the natural polymer to display mechanical strength. In our study the maximum TGFβ2 release from the 80G:20PCL scaffolds was 3.1% of the total amount loaded. These results are comparable to those obtained by Wang et al. 2015 [43] who fabricated electrospun and EDC crosslinked gelatin:PCL scaffolds functionalized with heparin and loaded with VEGF. The growth factor was bound to the scaffold through its affinity to heparin. The authors observed a sustained release of the VEGF that increases by increasing the amount of gelatin in the blend. The percentage release never exceeded 2.7%. However, the released VGEF was able to promote the in vitro proliferation of endothelial cells and the in vivo vascularization of a subcutaneous implant. We believe that the addition of PCL, which slows the degradation of the construct, as well as crosslinking method, may have been responsible for our low growth factor recovery. In this study, we added a relatively high amount of TGFβ2 to the scaffolds due to our a priori knowledge of the inhibiting effect of crosslinking on gelatin hydrolysis. This high degree of crosslinking may have significantly decreased the degradation of gelatin which would have influenced the release of TGFβ2. Additionally, since the crosslinking method used here consisted in submerging the scaffolds in a liquid solution of genipin in ethanol, it is possible that surface adsorbed TGFβ2 was released in this crosslinking fluid resulting in a low content of TGFβ2 in the scaffold. Moreover, since genipin is a protein crosslinking agent [44, 45], it is possible that it may have reacted with some of the available amine groups from TGFβ2, rending the growth factor unrecognizable by the antibodies in the ELISA kit. However, gelatin is a very linear molecule that has all its amine groups exposed and available for crosslinking, increasing the likelihood that the genipin reacts with the gelatin over the amine groups of TGFβ2, which exhibit a non-linear three dimensional structure and post-translational glycosylation [46]. This hypothesis is also supported by the bioactivity results in Figure 5, where it is observed that the released TGFβ2 either promoted or prevented SMCs proliferation depending on its concentration in the media. This demonstrates that the eluted TGFβ2 maintained its structure as evidendenced by its ability to influence SMC growth.
Overall, the behavior of SMCs exposed to eluted TGFβ2 are consistent with the behavior of SMCs exposed to exogenous TGFβ2 in media (Figure 4). Additionally, our previous work demonstrates similar SMC behavior, in which increasing concentrations of exogenous TGFβ2 were added to PAOSMCs growing on our electrospun materials, where cell migration, proliferation, and collagen production were differentially modulated by TGFβ2 [10]. Furthermore, the in vitro experiments of SMCs growing on 80G:20PCL scaffolds with and without TGFβ2 showed that it is possible to obtain higher cell numbers and improved migration from TGFβ2-eluting constructs loaded with 13 ng TGFβ2/mg of scaffold. After 5 days of culture, the cells growing on the TGFβ2-eluting scaffolds were more evenly distributed throughout the construct. These results confirmed both qualitatively and quantitatively the bioactivity of the TGFβ2 released from our graft material.
The exposure of TGFβ2 to a liquid ethanol solution during the crosslinking process is a potential limitation of our approach. Not only would this result in a potentially low yield of TGFβ2, it may also be creating a gradient of TGFβ2 in the radial direction with the highest concentration of the growth factor in the middle region of the scaffold decreasing towards the inner and outer surfaces (luminal and abluminal regions, respectively). We believe this may be the reason for the increased number of cells in the middle region of the TGFβ2 containing scaffolds in Figure 7. To overcome the low recovery of TGFβ2, and possible TGFβ2 gradient, further experiments with different drug-loading methods will be necessary to further explore this hypothesis. One possibility could be to incorporate TGFβ2 by adsorption after the crosslinking of the scaffolds, which would limit TGFβ2 exposure to the fluid and to the crosslinker. However, this may result in an initial burst release from the fiber surface [39]. Another alternative would be to covalently immobilize TGFβ2 to already crosslinked fibers which could reduce the initial burst release. However, this may cause the loss of bioactivity, since the immobilization can change the conformation of the growth factor. Another alternative would be to encapsulate TGFβ2 into biodegradable microspheres that would be further incorporated into the polymeric blend before electrospinning. With this method the TGFβ2 would be protected from the crosslinker, and the release would mainly depend on the degradation of the microspheres and not the degradation of the scaffold. Therefore, it may be important to choose encapsulating materials with similar degradation rates to the currently studied electrospun fibers. It is important to note that any inclusion of particles to electrospun fibers could have implications on the electrospinning process and consequently influence the mechanical properties of the scaffold. In our laboratory, we have developed techniques to fully control and optimize the mechanical properties of our grafts allowing target vessel compliance matching [25].
Another limitation of the study is that we assumed that release profile from the scaffolds with 100 ng of TGFβ2/mg scaffold is comparable to the obtained for the ones with 13 ng of TGFβ2/mg scaffold. In this work we created scaffolds with high content TGFβ2 to better demonstrate that the released TGFβ2 is bioactive and that it has a concentration dependent effect on SMC growth. As we demonstrated in our previous work and here in Figure 4, high concentrations of TGFβ2 have a negative effect on cell proliferation. In Figure 5 shows that scaffolds releasing high amounts of TGFβ2 decreased cell proliferation. However, to make further conclusions about effect of the TGFβ2 content in the release kinetics, future experiments will recreate the release kinetic profiles using scaffolds loaded with different amounts of TGFβ2.
In this study we show that it is possible to modulate the release of TGFβ2 from tubular electrospun scaffolds by varying the ratio of gelatin and PCL in polymeric solution. We also demonstrated that the TGFβ2 released from our scaffolds is bioactive and influences SMC growth. However, to make further conclusions about the suitability of the TGFβ2 releasing scaffolds for vascular tissue engineering applications, it will be necessary to study the influence of the growth factor on the biomechanical properties of the scaffolds. The absence of this comparative data is therefore a limitation of this study. While we have demonstrated the ability fully control the mechanical properties of our graft materials in the absence of TGFβ2 [25, 47], ongoing studies in our laboratory are focusing in the full mechanical characterization of scaffolds containing TGFβ2.
Another limitation of this work is that we have only evaluated TGFβ2 release in vitro. We expect a faster degradation of the studied constructs in vivo due to the presence and effect of macrophages and other immune cells. This may contribute to an accelerated TGFβ2 release from the implanted construct. However, we are also expecting a decrease in the local concentration of the growth factor due to intraluminal and interstitial flow increasing washout from the scaffold. Further in vivo experiments will be required using TGFβ2 eluting-scaffolds to evaluate the effect of the released TGFβ2 on scaffold cell infiltration, growth, and remodeling.
Our future work will focus on continuing to design a TGFβ2-eluting electrospun scaffold that can release low amounts of TGFβ2 initially to increase SMC proliferation and migration through the scaffold. The scaffolds will also be designed to subsequently provide a late release of higher amounts of TGFβ2, thus reducing SMC proliferation and migration while also promoting collagen production as the TEVG degrades. In this study we have evaluated the effect of TGFβ2 on SMC migration and proliferation from TGFβ2-loaded constructs. However, we recognize that it is important to gain knowledge on how SMC phenotype is altered using our TGFβ2 stimulus. Further in vitro studies will investigate the expression levels of proliferative and contractile markers of SMC stimulated with different concentrations of TGFβ2.
Conclusions
To the best of our knowledge this is the first study investigating a release system to modulate SMC growth and infiltration in TEVGs using TGFβ2. SMCs are essential for the formation of a vascular media that can modulate vascular tone and ECM remodeling, making the control of SMC recruitment, infiltration, and proliferation an important aspect of a TEVG development. Here, we demonstrated the fabrication of gelatin/PCL hybrid biodegradable scaffolds with the ability of releasing bioactive TGFβ2. This release can be tuned using the degradation properties of natural and synthetic polymers in a blend allowing control of SMC growth and infiltration of the TGFβ2-releasing conduits, thus making these scaffolds attractive for vascular tissue engineering applications.
Supplementary Material
HIGHLIGHTS.
Electrospun scaffolds composed of a blend of gelatin and PCL can elute TGFβ2
TGFβ2 presented a differential release profile depending on the gelatin/PCL ratio.
Higher TGFβ2 elution was obtained in scaffolds with higher gelatin content.
Released TGFβ2 is bioactive and has a differential effect on SMCs growth.
Acknowledgements
This research was funded by the NIH [1R56HL136517-01, PI: J.P. Vande Geest]. D. C. Ardila was also funded by the Cellular Approaches to Tissue Engineering and Regeneration training grant (CATER) [5T32EB001026] at the University of Pittsburgh.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benjamin EJ, et al. , Heart disease and stroke statistics—2018 update: a report from the American Heart Association. 2018. 137(12): p. e67–e492. [DOI] [PubMed] [Google Scholar]
- 2.Nieponice A, et al. , In vivo assessment of a tissue-engineered vascular graft combining a biodegradable elastomeric scaffold and muscle-derived stem cells in a rat model. 2010. 16(4): p. 1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukunishi T, et al. , Preclinical study of patient-specific cell-free nanofiber tissue-engineered vascular grafts using 3-dimensional printing in a sheep model. 2017. 153(4): p. 924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ong CS, et al. , Tissue engineered vascular grafts: current state of the field. Expert review of medical devices, 2017. 14(5): p. 383–392. [DOI] [PubMed] [Google Scholar]
- 5.Wang G, et al. , Origin and differentiation of vascular smooth muscle cells. The Journal of physiology, 2015. 593(14): p. 3013–3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rychter M, Baranowska-Korczyc A, and Lulek J, Progress and perspectives in bioactive agent delivery via electrospun vascular grafts. RSC Advances, 2017. 7(51): p. 32164–32184. [Google Scholar]
- 7.Han J and Lelkes PI, Drug-Eluting Vascular Grafts, in Focal Controlled Drug Delivery. 2014, Springer; p. 405–427. [Google Scholar]
- 8.Boileau C, et al. , TGFB2 mutations cause familial thoracic aortic aneurysms and dissections associated with mild systemic features of Marfan syndrome. Nature genetics, 2012. 44(8): p. 916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramnath N, et al. , Fibulin-4 deficiency increases TGF-β signalling in aortic smooth muscle cells due to elevated TGF-β2 levels. Scientific reports, 2015. 5: p. 16872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ardila DC, et al. , TGFβ2 differentially modulates smooth muscle cell proliferation and migration in electrospun gelatin-fibrinogen constructs. Biomaterials, 2015. 37: p. 164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Artman M, et al. , Cardiovascular Development and Congenital Malformations: Molecular & Genetic Mechanisms. 2008: John Wiley & Sons. [Google Scholar]
- 12.Azhar M, et al. , Transforming growth factor Beta2 is required for valve remodeling during heart development. Developmental Dynamics, 2011. 240(9): p. 2127–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doetschman T, et al. , Transforming growth factor beta signaling in adult cardiovascular diseases and repair. Cell and tissue research, 2012. 347(1): p. 203–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braverman AC, et al. , Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 7: aortic diseases, including marfan syndrome: a scientific statement from the American Heart Association and American College of Cardiology. Journal of the American College of Cardiology, 2015. 66(21): p. 2398–2405. [DOI] [PubMed] [Google Scholar]
- 15.Molin DG, et al. , TGFb2 does not affect neural crest cell migration but is a key player in vascular remodeling during embryogenesis. Cardiovascular Development and Congenital Malformations, 2008: p. 148. [Google Scholar]
- 16.Agarwal S, Wendorff JH, and Greiner A, Progress in the field of electrospinning for tissue engineering applications. Advanced Materials, 2009. 21(32-33): p. 3343–3351. [DOI] [PubMed] [Google Scholar]
- 17.Hasan A, et al. , Electrospun scaffolds for tissue engineering of vascular grafts. Acta biomaterialia, 2014. 10(1): p. 11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weng L and Xie J, Smart electrospun nanofibers for controlled drug release: recent advances and new perspectives. Current pharmaceutical design, 2015. 21(15): p. 1944–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ji W, et al. , Bioactive electrospun scaffolds delivering growth factors and genes for tissue engineering applications. Pharmaceutical research, 2011. 28(6): p. 1259–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davidenko N, et al. , Evaluation of cell binding to collagen and gelatin: a study of the effect of 2D and 3D architecture and surface chemistry. Journal of Materials Science: Materials in Medicine, 2016. 27(10): p. 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo L, et al. , Fabrication of gelatin-micropatterned surface and its effect on osteogenic differentiation of hMSCs. Journal of Materials Chemistry B, 2018. 6(7): p. 1018–1025. [DOI] [PubMed] [Google Scholar]
- 22.Zhan J, et al. , In vitro evaluation of electrospun gelatin–glutaraldehyde nanofibers. Frontiers of Materials Science, 2016. 10(1): p. 90–100. [Google Scholar]
- 23.Zhang Y, et al. , Preparation, characterization, and evaluation of genipin crosslinked chitosan/gelatin three-dimensional scaffolds for liver tissue engineering applications. Journal of Biomedical Materials Research Part A, 2016. 104(8): p. 1863–1870. [DOI] [PubMed] [Google Scholar]
- 24.Wen C, Lu L, and Li X, Mechanically Robust Gelatin–A lginate IPN Hydrogels by a Combination of Enzymatic and Ionic Crosslinking Approaches. Macromolecular Materials and Engineering, 2014. 299(4): p. 504–513. [Google Scholar]
- 25.Tamimi E, et al. , Biomechanical comparison of glutaraldehyde-crosslinked gelatin fibrinogen electrospun scaffolds to porcine coronary arteries. Journal of biomechanical engineering, 2016. 138(1): p. 011001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panzavolta S, et al. , Electrospun gelatin nanofibers: optimization of genipin cross-linking to preserve fiber morphology after exposure to water. Acta biomaterialia, 2011. 7(4): p. 1702–1709. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, et al. , Biocompatibility and anti-calcification of a biological artery immobilized with naturally-occurring phytic acid as the crosslinking agent. Journal of Materials Chemistry B, 2017. 5(40): p. 8115–8124. [DOI] [PubMed] [Google Scholar]
- 28.Tsai CC, et al. , In vitro evaluation of the genotoxicity of a naturally occurring crosslinking agent (genipin) for biologic tissue fixation. Journal of biomedical materials research, 2000. 52(1): p. 58–65. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, et al. , Genipin crosslinking reduced the immunogenicity of xenogeneic decellularized porcine whole-liver matrices through regulation of immune cell proliferation and polarization. Scientific reports, 2016. 6: p. 24779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, et al. , Electrospinning of gelatin fibers and gelatin/PCL composite fibrous scaffolds. Journal of Biomedical Materials Research Part B: Applied Biomaterials: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials, 2005. 72(1): p. 156–165. [DOI] [PubMed] [Google Scholar]
- 31.Rajzer I, et al. , Electrospun gelatin/poly (ε-caprolactone) fibrous scaffold modified with calcium phosphate for bone tissue engineering. Materials Science and Engineering: C, 2014. 44: p. 183–190. [DOI] [PubMed] [Google Scholar]
- 32.Goyal R, et al. , Development of hybrid scaffolds with natural extracellular matrix deposited within synthetic polymeric fibers. Journal of Biomedical Materials Research Part A, 2017. 105(8): p. 2162–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norouzi M, et al. , PLGA/gelatin hybrid nanofibrous scaffolds encapsulating EGF for skin regeneration. Journal of Biomedical Materials Research Part A, 2015. 103(7): p. 2225–2235. [DOI] [PubMed] [Google Scholar]
- 34.Fukunishi T, et al. , Tissue-engineered small diameter arterial vascular grafts from cell-free nanofiber PCL/chitosan scaffolds in a sheep model. PLoS One, 2016. 11(7): p. e0158555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rnjak-Kovacina J, et al. , Tailoring the porosity and pore size of electrospun synthetic human elastin scaffolds for dermal tissue engineering. Biomaterials, 2011. 32(28): p. 6729–6736. [DOI] [PubMed] [Google Scholar]
- 36.Heydarkhan-Hagvall S, et al. , Three-dimensional electrospun ECM-based hybrid scaffolds for cardiovascular tissue engineering. Biomaterials, 2008. 29(19): p. 2907–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fu W, et al. , Electrospun gelatin/PCL and collagen/PLCL scaffolds for vascular tissue engineering. International journal of nanomedicine, 2014. 9: p. 2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gautam S, Dinda AK, and Mishra NC, Fabrication and characterization of PCL/gelatin composite nanofibrous scaffold for tissue engineering applications by electrospinning method. Materials Science and Engineering: C, 2013. 33(3): p. 1228–1235. [DOI] [PubMed] [Google Scholar]
- 39.Munj HR, Lannutti JJ, and Tomasko DL, Understanding drug release from PCL/gelatin electrospun blends. Journal of Biomaterials Applications, 2017. 31(6): p. 933–949. [DOI] [PubMed] [Google Scholar]
- 40.Nada AA, et al. , Eco-friendly gelatin-based electrospun fibers to control the release of chloramphenicol. Fibers and Polymers, 2016. 17(12): p. 1985–1994. [Google Scholar]
- 41.Sohier J, et al. , Tailored release of TGF-β1 from porous scaffolds for cartilage tissue engineering. International journal of pharmaceutics, 2007. 332(1-2): p. 80–89. [DOI] [PubMed] [Google Scholar]
- 42.Wang Z, et al. , Evaluation of emulsion electrospun polycaprolactone/hyaluronan/epidermal growth factor nanofibrous scaffolds for wound healing. Journal of biomaterials applications, 2016. 30(6): p. 686–698. [DOI] [PubMed] [Google Scholar]
- 43.Wang K, et al. , Enhanced vascularization in hybrid PCL/gelatin fibrous scaffolds with sustained release of VEGF. BioMed research international, 2015 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rose JB, et al. , Gelatin-based materials in ocular tissue engineering. Materials, 2014. 7(4): p. 3106–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Butler MF, Ng YF, and Pudney PD, Mechanism and kinetics of the crosslinking reaction between biopolymers containing primary amine groups and genipin. Journal of Polymer Science Part A: Polymer Chemistry, 2003. 41(24): p. 3941–3953. [Google Scholar]
- 46.Lin HY, et al. , The Soluble Exoplasmic Domain of the Type II Transforming Growth Factor (TGF)-ß Receptor A HETEROGENEOUSLY GLYCOSYLATED PROTEIN WITH HIGH AFFINITY AND SELECTIVITY FOR TGF-ß LIGANDS. Journal of Biological Chemistry, 1995. 270(6): p. 2747–2754. [DOI] [PubMed] [Google Scholar]
- 47.Harrison S, et al. , Computationally optimizing the compliance of a biopolymer based tissue engineered vascular graft. 2016. 138(1): p. 014505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.