Abstract
Disseminated breast cancer cells employ adaptive molecular responses following cytotoxic therapeutic insult which promotes their survival and subsequent outgrowth. Here we demonstrate that expression of the pro-metastatic lncRNA BORG (BMP/OP-Responsive Gene) is greatly induced within triple-negative breast cancer (TNBC) cells subjected to environmental and chemotherapeutic stresses commonly faced by TNBC cells throughout the metastatic cascade. This stress-mediated induction of BORG expression fosters the survival of TNBC cells and renders them resistant to the cytotoxic effects of doxorubicin both in vitro and in vivo. The chemoresistant traits of BORG depend upon its robust activation of the NF-κB signaling axis via a novel BORG-mediated feed-forward signaling loop, and via its ability to bind and activate RPA1. Indeed, genetic and pharmacologic inhibition of NF-κB signaling or the DNA-binding activity of RPA1 abrogates the pro-survival features of BORG and renders BORG-expressing TNBCs sensitive to doxorubicin-induced cytotoxicity. These findings suggest that therapeutic targeting of BORG or its downstream molecular effectors may provide a novel means to alleviate TNBC recurrence.
Introduction
Breast cancer persists as the most commonly diagnosed malignancy, as well as the leading cause of cancer-related deaths among females worldwide [1]. The treatment of breast cancer is complicated by the fact that breast cancers are highly heterogeneous in nature and comprised of at least five genetically distinct subtypes, including normal-like, Luminal A and B, HER2 over-expressing, and basal breast cancers [2–4]. Along these lines, >75% of basal breast cancers are characterized as triple-negative breast cancers (TNBCs) [5], a highly lethal class of breast cancers that are particularly prone to metastasize to distant tissues [6]. Indeed, metastasis remains the principal cause for >90% of breast cancer-associated deaths [7]. Despite the clinical burden attributed to metastasis, the molecular underpinnings of this process remain incompletely understood. Current dogma posits that the competency of disseminated tumor cells to successfully navigate the metastatic cascade largely depends upon their ability to transcend the “metastatic bottleneck,” a process whereby circulating tumor cells survive transit through the vasculature and remain viable in foreign tissue microenvironments upon vascular extravasation [8]. Indeed, the metabolic, hypoxic, and physical stress placed upon carcinoma cells as they traverse the metastatic cascade makes metastasis a supremely inefficient event, wherein only ~0.01% of circulating tumor cells are capable of initiating some form of metastatic outgrowth [9, 10]. As such, the malignant cells proficient in ultimately forming clinically relevant metastases rely heavily upon pro-survival responses in order to maintain cellular viability that permits their outgrowth [11].
Moreover, these same pro-survival traits that emerge in response to the selective pressures associated with metastasis are frequently exploited by disseminated cancer cells to circumvent the genotoxic and metabolic insults induced by chemotherapeutic treatment [12–14]. The pathways associated with the development of chemoresistance are multifold and frequently depend upon (i) the inactivation of apoptotic pathways, and (ii) the adaptive activation of pro-survival signals [15]. A potent signaling axis shown to be critical for the acquisition of chemoresistant phenotypes in breast cancers is the NF-κB pathway. Indeed, signaling through NF-κB functions as an evolutionarily conserved method for coordinating inflammatory-, immune-, and stress-associated responses. Importantly, these signals and pathways are routinely hijacked by malignant cells, including TNBCs, in order to promote their survival and growth [16, 17], especially in response to cytotoxic drugs [18, 19]. Thus, deciphering the upstream and downstream molecular mediators of NF-κB signaling during the acquisition of chemoresistance may provide targetable insights into sensitizing disseminated breast cancer cells to chemotherapeutic agents.
Long non-coding RNAs (lncRNAs) are a class of heterogeneous RNA molecules that carry out an extensive array of cellular functions despite lacking the capacity to code for proteins. Amongst the functions attributed to lncRNAs are their ability to govern a variety of oncogenic processes operant during primary tumor formation and metastatic progression [20, 21]. Notably, lncRNAs have been shown to promote the formation of metastases by exerting pro-survival and anti-apoptotic activities on malignant cells [20, 22]. Similarly, breast cancers have been shown to heavily rely upon the activities of lncRNAs during the development of chemoresistance [23]. Along these lines, we recently identified BORG (BMP/OP-Responsive Gene) as a pro-metastatic lncRNA whose expression correlates with poor long-term outcomes in breast cancer patients; its expression also drove the reactivation of proliferative programs in indolent breast cancer lesions, leading to their recurrence [24]. Interestingly, we also determined that aberrant BORG expression prevented TNBCs from succumbing to anoikis [24], and from apoptosis elicited by mechanically rigid cellular microenvironments [24–26].
In light of these potential interactions between BORG and survival signals, we speculated that the pro-metastatic activities of BORG are critically dependent upon its activation of pro-survival signals in disseminated breast cancer cells, particularly during (i) metastatic seeding, colonization, and eventual outgrowth; and (ii) adaptation to stress elicited by administration of cancer chemotherapies. Accordingly, we show herein that BORG expression is highly responsive to cytotoxic drug treatment, especially doxorubicin, and orchestrates a transcriptional response that promotes the survival and chemoresistance of TNBC cells. Mechanistically, the chemoresistant activities of BORG reflect its strong induction and activation of the NF-κB pathway, which in turn further activates BORG expression in a doxorubicin-mediated feed-forward loop. Pharmacologic and genetic inactivation of NF-κB, as well as that of BORG and its downstream effector RPA1, readily promoted apoptosis in TNBCs and restored their sensitivity to chemotherapeutic agents. Collectively, these findings establish BORG as a novel and essential driver of disseminated TNBC cell survival and chemoresistance; they also imply that therapeutic targeting of either BORG or its effectors, NF-κB and RPA1, may provide novel inroads to alleviate the metastatic outgrowth and disease recurrence of disseminated TNBCs.
Results
Chemotherapeutic and metabolic insults induce BORG expression in TNBC cells
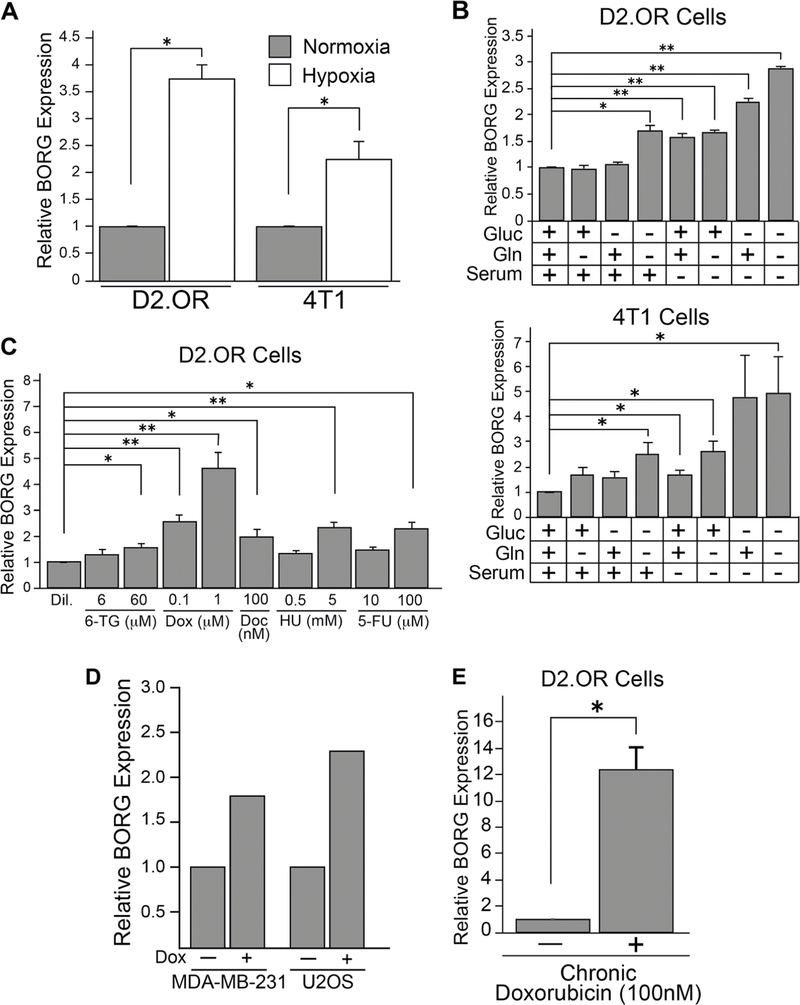
The expression of lncRNAs is robustly regulated by conditions that challenge the viability of breast cancer cells undergoing the process of metastasis (e.g., hypoxia and metabolic stress; [27]). Furthermore, we [24, 28] and others [29] demonstrated that BORG expression is robustly induced in cells exposed to stress-associated cytokines. These findings, together with our recent study identifying BORG as a novel driver of metastatic progression [24], led us to surmise that upregulated BORG expression may function as a master regulator of cell survival signaling activated in response to cellular stressors experienced by breast cancer cells as they traverse the metastatic cascade. As an initial test of this hypothesis, we subjected multiple TNBC cell lines (i.e., 4T1 and D2.OR cells) to growth under hypoxic conditions. Figure 1a shows that BORG expression was induced significantly in both 4T1 and D2. OR cells in response to hypoxia. We also monitored the impact of progressive nutrient deprivation on BORG expression and found that withdrawal of serum, glucose, and glutamine, both singly and in multiple combinations, significantly induced the quantity of BORG transcripts in these same cells (Fig. 1b). Lastly, we monitored alterations in BORG expression in D2.OR cells treated with a wide panel of chemotherapeutic drugs, including doxorubicin, hydroxyurea, docetaxel, 5-fluorouracil, and 6-thioguanine. As shown in Fig. 1c, administering these cytotoxic agents to D2.OR cells resulted in their significant upregulation of BORG. Moreover, targeted pharmacological therapies (e.g., trastuzumab and lapatinib) led to the augmentation of BORG levels in human BT474 and SKBR3 cells (Supplementary Fig. 1a), implying that drug-mediated upregulation of BORG transpires independently of drug class and mechanism of cytotoxicity. Notably, BORG expression was highly sensitive to treatment with the anthracycline doxorubicin both in D2.OR cells (Fig. 1c), as well as in human TNBC and osteosarcoma cells (i.e., MDA-MB-231 and U2OS cells [30], respectively; Fig. 1d). To further explore doxorubicin-dependent regulation of BORG expression, we chronically treated D2.OR cells with sub-lethal doses of doxorubicin (e.g., 100 nM), thereby generating a population of D2.OR cells that were resistant to doxorubicin. Interestingly, the doxorubicin-resistant D2.OR cells that emerged after this treatment scheme showed a striking elevation of BORG expression (Fig. 1e). Collectively, these findings imply that the regulation of BORG expression in TNBC cell lines is highly responsive to environmental stressors, particularly those associated with chemotherapeutic insult.
Fig. 1.
BORG expression is enhanced by chemotherapeutic treatment and metabolic stress in TNBC cells. a BORG expression is significantly increased in D2.OR and 4T1 cells grown under hypoxic conditions (2% oxygen tension; 24 h). Data represent mean (±SEM) of three independent qRT-PCR trials performed in triplicate (*P < 0.05). b BORG expression was significantly elevated in D2.OR or 4T1 cells subjected to 24 h growth in media proficient (+) or deficient (−) in various nutrients or 6 h growth in Krebs–Henseleit Buffer (i.e., deplete of glc, gln, and serum). Data represent mean (±SEM) of three independent qRT-PCR trials performed in triplicate (*P < 0.05; **P < 0.005); (glc: glucose; gln: glutamine). c BORG expression was significantly increased in D2.OR cells treated with various cytotoxic drugs at denoted concentrations for 24 h. Data represent mean (±SEM) of three independent qRT-PCR trials performed in triplicate (*P < 0.05; **P < 0.005) (dil. diluent; 6-TG: 6-thioguanine; Dox: doxorubicin; HU: hydroxyurea; 5-FU: 5-fluorouracil). d RNA-seq analyses indicate that BORG expression is elevated in human MDA-MB-231 and U2OS cells treated with doxorubicin. e Doxorubicin-resistant D2. OR clones that emerged after 4 weeks of chronic doxorubicin treatment (100 nM) harbored significantly higher levels of BORG as quantified by qRT-PCR. Data represent mean (±SEM) of three independent trials performed in triplicate (*P < 0.05)
BORG drives the development of chemoresistant properties in TNBC cells
Based on the observation that doxorubicin treatment selected for a population of chemoresistant, BORG-enriched D2.OR cells (Fig. 1e), we hypothesized that BORG may be capable of affording chemoresistant traits to TNBC cells. Along these lines, Fig. 2a shows that BORG-expressing D2.OR cells were significantly less likely than their parental (i.e., empty vector) counterparts to undergo apoptosis when exposed to high doses of doxorubicin. Similarly, BORG-expressing D2.OR cells (Supplementary Fig. 1b) more readily formed chemoresistant colonies when exposed to sub-lethal concentrations of doxorubicin as compared to their parental counterparts (Supplementary Fig. 1c). Importantly, shRNA-mediated knockdown of BORG in doxorubicin-resistant D2.OR cells (Fig. 2b) significantly resensitized these cells to the cytotoxic effects of doxorubicin (Fig. 2c). Similarly, depletion of BORG in parental D2.OR cells (Supplementary Fig. 1d) delayed the development of doxorubicin-resistant colonies subsequent to chronic treatment with 100 nM doxorubicin (Supplementary Fig. 1e). Additionally, shRNA-mediated depletion of BORG in 4T1 cells (Supplementary Fig. 1f), a TNBC cell line shown to harbor elevated expression of BORG [24], sensitizes these cells to the cytotoxic effects of doxorubicin (Supplementary Fig. 1g). Thus, these results indicate that BORG protects against doxorubicin-induced cell death in multiple TNBC cell lines.
Fig. 2.
BORG confers resistance to cyto- and genotoxic effects of doxorubicin. a BORG significantly mitigates doxorubicin-induced apoptosis in D2.OR cells treated with 4 μM doxorubicin for 24 h. Data represent mean (±SEM) of three independent trials performed in triplicate (*P < 0.05). b shRNA-mediated depletion of BORG in D2.OR cells rendered resistant to doxorubicin via chronic treatment with 100 nM doxorubicin. c Depletion of BORG restores the cytotoxic effects of doxorubicin in these doxorubicin-resistant D2.OR cells. d BORG abrogates doxorubicin-mediated G2/M arrest in D2.OR cells. Left: representative cell-cycle distribution of diluent or doxorubicin-treated D2.OR cells; right: quantification of cell-cycle distribution of diluent or doxorubicin-treated D2.OR cells. Data represent mean (±SEM) of three independent trials performed in triplicate (*P < 0.05; **P < 0.005). e BORG inhibits doxorubicin-induced DNA damage in D2.OR cells, as evidenced by neutral Comet assays. Left: representative photos of D2.OR cells analyzed via Comet assays following diluent or doxorubicin treatment; right: quantification of percent tail DNA (i.e., proportion of total DNA that resides in the comet tail of each cell) or tail length in the resulting D2.OR cells. 75 cells were analyzed in each condition from three independent slides (*P < 0.05; **P < 0.005)
To further investigate the chemoresistant properties of BORG, we performed cell-cycle analyses on D2.OR derivatives treated with varying concentrations of doxorubicin. While parental D2.OR cells underwent profound G2/M arrest following doxorubicin treatment, their BORG-expressing counterparts exhibited a distinct cell-cycle distribution, wherein a preserved fraction of cells persisted in G0/G1 phase (Fig. 2d). Doxorubicin-induced G2/M arrest has been shown to be associated not only with a susceptibility to post-treatment apoptosis in various cancer cells, but also with the accumulation of substantial DNA damage and an enhanced sensitivity to the genotoxic effects of doxorubicin [31–34]. To determine whether BORG did indeed protect against the genotoxicity of doxorubicin, we carried out Comet assays using our D2.OR derivatives to quantify the extent of their DNA damage upon doxorubicin exposure. Figure 2e shows that parental and BORG-expressing D2.OR cells both succumbed to doxorubicin-induced double-stranded DNA breaks in a dose-dependent manner; however, heterologous expression of BORG significantly mitigated the extent of DNA damage at all doxorubicin concentrations tested, as evidenced by (i) shorter DNA tail lengths, and (ii) reduced quantities of tail DNA in the resulting comets. Collectively, these findings suggest that BORG promotes chemoresistance in part by reducing the extent of DNA damage engendered by cyto-toxic agents.
BORG confers pro-survival properties to TNBC cells
Due to our recent findings demonstrating that exogenous expression of BORG in D2.OR cells orchestrated a transcriptional signature associated with numerous pro-survival pathways [24], we speculated that BORG exerts pro-survival effects beyond those seen in response to cytotoxic treatment. In testing this hypothesis, we subjected parental and BORG-expressing D2.OR cells to multiple forms of apoptotic stress, and subsequently monitored their ability to survive and grow in an anchorage-independent manner. As shown in Fig. 3a, heterologous expression of BORG in D2. OR cells readily promoted their growth and survival in soft agar; it also enhanced their colony forming ability in extreme dilution clonogenic assays (Fig. 3b). Additionally, BORG protected D2.OR cells against apoptosis induced by both hypoxia (Fig. 3c) and extreme nutrient deprivation (Fig. 3d). Taken together, these findings indicate that BORG promotes the survival and growth of D2.OR cells following their exposure to metastasis-associated cellular stress insults.
Fig. 3.
BORG confers pro-survival traits to D2.OR cells subjected to environmental stresses. a BORG enhanced the anchorage-independent growth of D2.OR cells plated into soft agar, as evidenced by colony formation efficiency and average size of colony. Representative photos of the resulting wells are depicted; inset depicts enlarged view of colonies. Data represent mean (±SEM) of soft agar assays plated in triplicate (*P < 0.05). b BORG promotes colony formation capacity of D2.OR cells subjected to clonogenic assays. Colony formation was quantified via ImageJ-mediated calculation of percent area or via a parameter that incorporates both area and density of colonies formed (intensity percent). Representative photos of resulting wells from each condition are depicted. Data represent mean (±SEM) of clonogenic assays plated in triplicate (*P < 0.05). c Parental D2.OR cells were more susceptible to hypoxia-induced cell death relative to BORG-expressing D2.OR cells. Cells were grown at 2% O2 tension for 24 h and apoptosis was quantified by Caspase-Glo 3/7 assays. Data represent mean (±SEM) of three independent trials performed in triplicate (*P < 0.05). d BORG protected D2.OR cells against apoptosis induced by growth under extreme nutrient deprivation as determined by Caspase-Glo 3/7 assays. Data represent mean (±SEM) of three independent trials performed in triplicate (*P < 0.05); (CM: complete media; KHB: Krebs–Henseleit Buffer; -gluc: glucose deprivation)
BORG alters the transcriptional landscape of D2.OR cells and stimulates NF-κB signaling
To garner additional mechanistic insights into how BORG promotes chemoresistant and pro-survival activities in TNBCs, we performed RNA-seq on parental and BORG-expressing D2.OR organoids propagated in 3D-cultures [24]. As shown in Fig. 4a, enforced expression of BORG led to profound transcriptomic alterations in D2.OR cells, resulting in 1156 genes being significantly upregulated and another 1313 genes being significantly downregulated (±2-fold cutoff; FDR < 0.05). In performing Gene Set Enrichment Analyses, we observed BORG-regulated transcripts to be significantly associated with aggressive breast cancers (e.g., basal-like; Supplementary Fig. 2a), a pattern we demonstrated previously [24]. Interestingly, multiple transcriptional profiles associated with cellular stress and survival programs were amongst the top enriched pathways in BORG-expressing D2.OR cells. Most notably, BORG enriched for gene signatures that were critical for the development of chemoresistance towards doxorubicin (Supplementary Fig. 2b). Similarly, BORG enriched for the expression of a core set of genes upregulated in response to hypoxia, radiotherapy, and serum-deprivation (Supplementary Fig. 2c-e). Collectively, these transcriptional signatures lend support to the notion that upregulated BORG expression confers a protective and pro-survival response in cells exposed to a variety of chemotherapeutic and metabolic insults.
Fig. 4.
BORG stimulates flux through the NF-κB signaling axis in D2. OR cells. a Volcano plot depicting RNA-seq data identifying differentially expressed transcripts in BORG-expressing vs. parental D2.OR cells grown in 3D-culture. Blue points represent significantly altered transcripts (i.e., fold change ≥±2; FDR < 0.05). b BORG expression in D2.OR cells enriches for a transcriptional signature that corresponds to the maintenance of NF-κB signaling in carcinoma cells. c BORG significantly enhances NF-κB signaling in D2.OR cells, as evidenced by NF-κB luciferase reporter assays. Data represent mean (±SEM) of three independent trials performed in triplicate (*P < 0.05). d Western blot depicting increased quantities of activated p65 in BORG-expressing D2.OR cells. Whole cell lysates were incubated with biotinylated NF-κB consensus sequence oligonucleotides prior to streptavidin-mediated pulldown of active p65 complexes. e Western blot depicting expression of phosphorylated or unphosphorylated IκBα in parental or BORG-expressing D2.OR cells grown in 3D-culture. f ChIP assays demonstrate significantly enhanced localization of p65 to the promoters of multiple NF-κB-responsive genes in BORG-expressing D2.OR cells. Data represent mean (±SEM) of three independent trials performed in triplicate (*P< 0.05). g Scatter plot depicting quantification and correlation of internally normalized expression of BORG and hallmark NF-κB-associated transcripts in 18 RNA-seq datasets (16 derived from normal or malignant mammary tissues, 1 derived from airway epithelial cells, 1 derived from osteosarcoma cells)
In addition to the aforementioned survival-associated transcriptional patterns, BORG-expressing D2.OR cells also exhibited significant enrichment of a hallmark gene signature that correlated with the induction of NF-κB activity (Fig. 4b). Accordingly, our RNA-seq analyses revealed a similar enrichment of NF-κB-associated gene signatures (Supplementary Fig. 2f), including significant amplification of numerous transcripts associated with the NF-κB pathway (Supplementary Fig. 2g), as well as those that house NF-κB consensus DNA-binding sequences within their promoters (Supplementary Fig. 2h). This transcriptional signature led us to hypothesize BORG as a novel activator of the NF-κB signaling axis. As such, we performed NF-κB luciferase reporter assays in D2.OR derivatives and established that BORG significantly induced the expression of luciferase driven by the NF-κB promoter (Fig. 4c). Along these lines, BORG stimulated the phosphorylation and DNA-binding activity of the NF-κB effector, p65/RelA, as determined by NF-κB oligonucleotide capture assays [35, 36] (Fig. 4d). This augmented activation of p65 correlated with the ability of BORG to induce the phosphorylation of IκBα and its subsequent partial degradation in D2.OR cells grown in 3D-culture (Fig. 4e). Accordingly, chromatin immunoprecipitation (ChIP) assays demonstrated that the localization of p65 to its consensus binding sequence within the promoters of multiple NF-κB-responsive genes (e.g., Tnfaip3, Tlr2, and Nfkbia [37]) was significantly enhanced in D2.OR cells engineered to overexpress BORG (Fig. 4f). Collectively, these findings establish BORG as a novel regulator and activator of NF-κB signaling in D2.OR cells.
Although BORG led to the activation of NF-κB in D2. OR cells, we further sought to determine whether BORG expression governs NF-κB signaling in a more ubiquitous manner (i.e., in a variety of malignant cells and tissues, including human TNBCs). In doing so, we utilized 18 RNA-seq datasets derived from malignant and normal human mammary epithelial cells to quantify the expression of BORG, as well as the enrichment of hallmark NF-κB-associated transcripts. Figure 4g illustrates that BORG expression significantly correlates with the enrichment (i.e., activation) of NF-κB signaling in these human tissues (Spearman’s rho = 0.55, p = 0.021; Pearson’ r = 0.72, p = 0.008, R2 = 0.39). Such a correlation was particularly notable in highly metastatic TNBCs [38, 39], as they harbored markedly elevated levels of BORG and substantial enrichment of NF-κB-associated transcripts (Supplementary Fig. 2I). These results therefore signify that BORG expression correlates with the activation of NF-κB signaling pathways in a wide array of malignant human breast tissues.
BORG-mediated chemoresistance requires augmented NF-κB signaling
The aforementioned findings, as well as those linking NF-κB to pro-survival signaling and chemoresistance in breast cancers [40–44], led us to examine the impact of BORG-mediated NF-κB activation on D2.OR sensitivity to chemotherapy. In doing so, we stably expressed an inactive form of IκBα (i.e., super-repressor, or SR [45]) in parental (SR-D2.OR) and BORG-expressing D2.OR (SR-D2.OR-BORG) cells as a means to inhibit the activation and nuclear translocation of p65 [46]. When stably expressed in D2.OR cells, the IκBα super-repressor clearly prevented BORG from stimulating the phosphorylation (Fig. 5a) and transcriptional activity of NF-κB (Fig. 5b). Notably, SR-D2. OR-BORG cells were significantly more sensitive to treatment with doxorubicin both in acute (Fig. 5c) and chronic (Fig. 5d) settings. Additionally, expression of the IκBα super-repressor in D2.OR-BORG cells markedly reduced their ability to survive under hypoxic conditions (Fig. 5e), as well as to grow and survive in soft agar assays (Fig. 5f). Similarly, SR-D2.OR-BORG cells were significantly more sensitive to anoikis (Supplementary Fig. 3a) and exhibited elevated levels of cleaved caspase 8 (Supplementary Fig. 3b) relative to their BORG-expressing counterparts that possessed an intact NF-κB signaling system. Finally, expression of the IκBα super-repressor diminished the capacity of D2.OR cells to thrive in clonogenic assays (Supplementary Fig. 3c) and restored the susceptibility of BORG-expressing D2.OR cells to apoptosis induced by nutrient deprivation (Supplementary Fig. 3d). Thus, these findings suggest that the broad chemoresistant and prosurvival activities mediated by BORG transpire in an NF-κB-dependent manner.
Fig. 5.
BORG-mediated stimulation of NF-κB activity is necessary to confer chemoresistant and pro-survival traits to TNBCs. Stable expression of a dominant-negative (DN) form of IκBα (i.e., ‘super-repressor’; SR) inhibits BORG-mediated induction of NF-κB activity, as evidenced by a Western blot analysis depicting decreased phosphorylation of p65 in BORG-expressing D2.OR cells and b NF-κB luciferase assays (pBABE: empty vector). c BORG inhibits doxorubicin-induced apoptosis in D2.OR cells across a wide range of acute concentrations, an effect that is abrogated by inhibition of NF-κB signaling. Apoptosis measured by Caspase-Glo 3/7 assays. d BORG-expressing D2.OR cells require intact NF-κB signaling to confer resistance to chronic, sub-lethal doses of doxorubicin. Photos depict representative cell quantity and phenotype following 2 weeks of doxorubicin treatment at 100 nM. e Inhibition of NF-κB signaling significantly increases hypoxia-induced apoptosis in BORG-expressing D2.OR cells, as measured by Caspase-Glo 3/7 assays. f Stable expression of DN-IκBα significantly decreases BORG-mediated anchorage-independent growth in D2.OR cells, as evidenced by colony formation efficiency. Representative photos of the resulting wells are depicted; inset depicts enlarged view of colonies. All data represent mean (±SEM) of three independent trials performed in triplicate (*P < 0.05)
The IκBα super-repressor inhibits NF-κB signaling by preventing p50/p65 NF-κB heterodimers to undergo nuclear translocation [46], thus offering few insights into the upstream regulatory events operant during BORG-mediated activation of NF-κB. To address this question, we inter-rogated our RNA-seq data and observed a striking transcriptional upregulation of NEMO/IKKγ (Supplementary Fig. 2g), a regulatory subunit of the IκB kinase (IKK) complex [47]. Upregulated expression of NEMO/IKKγ transcripts (Fig. 6a) and protein levels (Fig. 6b) was independently verified in BORG-expressing D2.OR cells pro-pagated in 3D-culture. As anticipated, CRISPR/Cas9-mediated knockout of NEMO/IKKγ in BORG-expressing D2.OR cells (BORG/sgNEMO; Fig. 6b) prevented BORG from activating NF-κB in D2.OR cells (Fig. 6c). More importantly, BORG/sgNEMO-D2.OR cells became susceptible to anoikis (Fig. 6d) and doxorubicin treatment (Fig. 6e) as compared to their NEMO-expressing counterparts. Finally, administration of the IKKβ inhibitor, IKK-2 Inhibitor VI [35], prevented BORG from activating NF-κB (Fig. 6f) and correspondingly mitigated BORG-mediated inhibition of anoikis in D2.OR cells [24] (Fig. 6g). Collectively, these findings indicate that BORG relies upon the activation of NF-κB, particularly through an intact IKK complex, to confer pro-survival effects to D2.OR cells.
Fig. 6.
Inhibition of the IKK complex abrogates BORG-dependent chemoresistance. a BORG-expressing D2.OR cells harbor significantly higher quantities of NEMO/IKKγ transcripts. Data represent mean (±SEM) of three independent qRT-PCR trials performed in triplicate in 3D-culture (*P < 0.05). b Western blot analysis depicts enhanced protein expression of NEMO in BORG-expressing D2.OR cells as compared to parental D2.OR cells. CRISPR/Cas9-mediated knockout of NEMO was performed in these D2.OR derivatives using two distinct sgRNA designs (sgNT: non-targeting sgRNA). c CRISPR/Cas9-mediated knockout of NEMO prevents BORG-dependent stimulation of NF-κB signaling, as evidenced by NF-κB luciferase assays. Data represent mean (±SEM) of three independent trials performed in triplicate (*P < 0.05). d NEMO knockout sensitizes BORG-expressing D2.OR cells to anoikis, as measured by Caspase-Glo 3/7 assays. Data represent mean (±SEM) of three independent trials performed in triplicate (*P < 0.05). e NEMO knockout sensitizes BORG-expressing D2.OR cells to apoptosis subsequent to 4 μM treatment with doxorubicin for 48 h. f Treatment of BORG-expressing D2.OR cells with 1 μM IKK-2 Inhibitor VI (i.e., inhibitor of IKK-β) prevents BORG-mediated activation of NF-κB signaling, as measured by NF-κB luciferase assays. Data represent mean (±SEM) of three independent trials performed in triplicate (*P < 0.05). g BORG-expressing D2. OR cells are sensitized to anoikis when simultaneously treated with 1 μM IKK-2 Inhibitor VI, as quantified by Caspase-Glo 3/7 assays. Data represent mean (±SEM) of three independent trials performed in triplicate (*P < 0.05)
Doxorubicin-mediated activation of NF-κB drives BORG expression and initiates a feed-forward NF-κB signaling loop in TNBC cells
We recently established the importance of BORG in promoting TNBC metastasis, and in driving latent disseminated TNBCs to emerge from dormancy and recur; [24] however, the precise mechanism(s) that govern the induction of BORG expression in TNBCs remain to be fully elucidated. Although BORG expression is responsive to cytokine administration and microenvironmental composition [24, 28, 29], no unifying regulatory pathway capable of reliably controlling the transcriptional regulation of BORG has emerged. Curiously, the promoter region of BORG harbors multiple motifs containing high sequence homology for the p50/p65 NF-κB consensus DNA-binding sequence (Supplementary Fig. 4a), thereby implicating NF-κB as a potential inducer of BORG expression. Indeed, ChIP assays coupled to quantitative PCR (ChIP-qPCR) demonstrated that p65 is capable of binding to various regions within the BORG promoter that are associated with these consensus binding sites (Supplementary Fig. 4b). Accordingly, Fig. 7a shows that exposing D2.OR cells to either doxorubicin or hypoxia, two BORG-inducing stimuli, triggered their activation of NF-κB and enhanced the binding of p65 to the BORG promoter (Supplementary Fig. 4b). Similarly, treatment of human MDA-MB-231 and U2OS cells with doxorubicin not only resulted in elevated BORG expression (Fig. 1d), but also led to the enrichment of a transcriptional profile associated with hallmark NF-κB signaling (Supplementary Fig. 4b). Importantly, the doxorubicin-mediated activation of NF-κB in D2.OR cells was significantly diminished upon reduction of BORG expression in D2.OR cells exposed to acute, high-dose or chronic, sub-lethal levels of doxorubicin (Fig. 7b). These findings thereby signify the requisite nature of BORG in promoting NF-κB signaling subsequent to doxorubicin treatment.
Fig. 7.
Inhibition of NF-κB signaling prevents environmental-and chemotherapeutic-induced upregulation of BORG as part of a feed-forward loop. a Activation of the NF-κB signaling pathway is significantly enhanced in parental D2.OR cells following doxorubicin treatment (4 μM, 48 h; left) or growth under hypoxic conditions (2% O2, 24 h; right). Data represent mean (±SEM) of three independent NF-κB reporter assays performed in triplicate (*P < 0.05). b shRNA-mediated depletion of BORG significantly prevents the activation of NF-κB signaling following treatment of D2.OR cells with 4 μM (24 h; left) or 100 nM (3 weeks; right) doxorubicin. Data represent mean ( ± SEM) of three independent qRT-PCR trials performed in triplicate (*P < 0.05). c Stable expression of a dominant-negative (DN) form of IκBα (SR) in D2.OR cells prevents the induction of BORG in response to doxorubicin treatment. Data represent mean (±SEM) of three independent qRT-PCR trials performed in triplicate (*P < 0.05). d Stable expression of DN-IκBα in 4T1 and D2.OR cells prevents the induction of BORG in response to hypoxia (2% O2, 24 h). Data represent mean (±SEM) of three independent qRT-PCR trials performed in triplicate (*P < 0.05). e D2.OR cells stably expressing DN-IκBα (SR) harbor significantly reduced quantities of BORG compared to empty vector expressing D2.OR cells (pBABE) when grown in basal growth conditions. Data represent mean (±SEM) of three independent qRT-PCR trials performed in triplicate (*P < 0.05). f Expression of DN-IκBα in D2.OR cells prevents upregulation of BORG in response to growth on rigid (R) 3D-cultures supplemented with 3 mg/ml collagen. (C: compliant; i.e., void of collagen). Data represent mean (±SEM) of three independent qRT-PCR trials performed in triplicate (*P < 0.05). g Expression of DN-IκBα in the 4T1 progression series prevents upregulation of BORG in response to growth on rigid 3D-cultures, as well as the basal expression of BORG in 4T1 cells grown in compliant cultures. Data represent mean (±SEM) of three independent qRT-PCR trials performed in triplicate (*P < 0.05)
Because doxorubicin and hypoxia both elicited BORG expression in D2.OR cells (Fig. 1), we sought to determine whether stress-mediated induction of BORG expression was dependent upon NF-κB. Remarkably, doxorubicin failed to induce BORG expression in D2.OR cells that (i) expressed the IκBα super-repressor (Fig. 7c), or (ii) lacked expression of NEMO (Supplementary Fig. 4c). Similarly, stable expression of the IκBα super-repressor wholly prevented the upregulation of BORG in response to hypoxia (Fig. 7d) and nutrient deprivation (Supplementary Fig. 4d) in both D2.OR and 4T1 cells. Interestingly, treatment of D2.OR cells with cobalt, an element known to mimic hypoxia by stabilizing and activating HIF1-α signaling [48], had no effect on the expression of BORG (Supplementary Fig. 4e), implying that hypoxia-induced upregulation of BORG is dependent primarily upon the activation of NF-κB. Collectively, these findings suggest that chemotherapy-and stress-induced upregulation of BORG requires intact NF-κB signaling in multiple TNBC cell types.
It is interesting to note that D2.OR cells engineered to stably express the IκBα super-repressor exhibited a moderate, but significant decrease in BORG expression under basal growth conditions (Fig. 7e); they also failed to upregulate BORG expression when propagated in 3D-cultures supplemented with collagen (Fig. 7f), a condition shown to promote the expression of BORG [24]. Likewise, IκBα super-repressor expression in 4T1 and 4T07 cells markedly reduced their activation of BORG expression when cultured on rigid, collagen-containing 3D-cultures, and in 4T1 cells cultured on compliant 3D-cultures (Fig. 7g). Taken together, these results identify NF-κB as a major regulatory node coupled to BORG expression in normal and malignant cells; they also demonstrate the presence of a novel feed-forward signaling loop involving NF-κB-mediated induction of BORG expression, which in turn further amplifies NF-κB activation.
In vivo chemoresistance driven by BORG depends upon NF-κB activation
We next sought to determine whether BORG is capable of conferring pro-survival and chemoresistant phenotypes to orthotopic D2.OR tumors produced in syngeneic BALB/c mice. As expected, BORG-expressing D2.OR cells formed primary tumors more rapidly than their parental D2.OR counterparts (Fig. 8a), a finding that aligns with our previous observations that BORG provides proliferative cues for dormant disseminated D2.OR cells in the lungs of mice [24]. More importantly, Fig. 8b shows that BORG-expressing D2.OR cells were resistant to the cytotoxic effects of doxorubicin treatment (5 mg/kg; once weekly), whereas the growth of tumors formed by parental and SR-D2.OR-BORG cells was significantly impeded by doxorubicin treatment, as determined by monitoring differences in tumor volume caliper measurements (Fig. 8b), tumor mass (Supplementary Fig. 5a), and tumor bioluminescent output (Supplementary Fig. 5b, c). Furthermore, following the ex vivo culture of primary D2.OR tumor cells, we observed significantly greater quantities of BORG in parental D2.OR cells isolated from animals treated with doxorubicin as compared to D2.OR cells isolated from vehicle-treated animals (Fig. 8c). Moreover, the ability of doxorubicin to induce BORG expression in D2.OR tumors was abrogated by expression of the IκBα super-repressor in parental D2.OR cells (Fig. 8c). Collectively, these findings establish two important features related to the development of acquired doxorubicin resistance: (i) BORG is dependent upon NF-κB to confer chemoresistant properties to TNBC cells in vivo, and (ii) doxorubicin administration selects for TNBC cells that contain robust expression of BORG, an event that requires intact NF-κB signaling.
Fig. 8.
BORG relies upon the activation of NF-κB to confer chemoresistance to doxorubicin in vivo. a BORG-expressing D2.OR cells form larger primary mammary fat pad as compared to parental D2.OR cells. Tumor volume derived from caliper measurements of tumor dimensions. (n = 5 mice; *P < 0.05). b Mammary fat pad tumors derived from BORG-expressing D2.OR cells are resistant to the growth-inhibitory effects of doxorubicin (5 mg/kg, weekly i.p. administration), an effect that is abolished by expression of a dominant-negative form of IκBα (SR). Tumor volume was derived from caliper measurements of tumor dimensions and plotted relative to tumor volume at time of doxorubicin treatment. Resulting tumors following 4 weeks of doxorubicin treatment are depicted (n = 5 mice per treatment arm; *P < 0.05). c Cell lines were derived from dissociated vehicle- and doxorubicin-treated mammary fat pad tumors. Parental D2.OR tumors (pBABE) exposed to doxorubicin exhibited significantly enhanced expression of BORG compared to their vehicle-treated counterparts, whereas doxorubicin had no effect on BORG expression in tumors derived from D2.OR cells expressing DN-IκBα. d Treatment with varying concentrations of bortezomib (Bzb) led to the inhibition of NF-κB signaling in BORG-expressing D2.OR and 4T1 cells, as measured by NF-κB luciferase assays. Data represent mean (±SEM) of three independent trials performed in triplicate (*P < 0.05). e Treatment of BORG-expressing D2.OR cells with 0.5 nM bortezomib sensitized these cells to the cytotoxic effects of 4 μM doxorubicin treatment, as determined by Caspase-Glo 3/7 assays. Data represent mean (±SEM) of three independent trials performed in triplicate (*P < 0.05)
Historically, the targeted inactivation of NF-κB in preclinical and clinical therapy settings has presented numerous efficacy-and toxicity-related challenges [49]. Nevertheless, the proteasome inhibitor bortezomib has demonstrated anti-neoplastic activity via its capacity to inhibit NF-κB activity both in vitro and in vivo [50, 51]. As such, we monitored the impact of bortezomib on NF-κB activity in various TNBC cell lines. Figure 8d demonstrates that bortezomib dose-dependently inhibited the NF-κB signaling axis in BORG-expressing D2.OR cells, and in 4T1 cells that endogenously harbor constitutive-activation of NF-κB [35]. Importantly, treating BORG-expressing D2. OR cells with bortezomib (0.5 nM) restored their sensitivity to the cytotoxic actions of doxorubicin (Fig. 8e). Collectively, these findings imply that combining cytotoxic agents with inhibitors of NF-κB may provide new inroads to inactivate the chemoresistant and pro-survival activities of BORG in metastatic breast cancers.
Pharmacological targeting and inactivation of BORG-induced chemoresistance
Although the ability of bortezomib to inactivate NF-κB in BORG-expressing TNBCs may offer some feasibility to alleviate BORG-dependent metastases, the clinical efficacy of bortezomib is unfortunately limited by its wide toxicity profile and the propensity for malignant cells to acquire resistance against its cytotoxic effects [50, 52]. Informed treatment strategies using therapeutic agents that specifically target molecular mediators of malignancy often mitigate toxic effects and deter the acquisition of chemoresistant traits [53]. As such, we sought to identify the precise BORG-dependent molecular mediators that confer chemoresistance to TNBCs, doing so by performing a mass-spectrometry based screen that captured biotinylated antisense BORG transcripts as a means to identify BORG-interacting proteins [24]. Through this strategy, we identified the protein RPA1 as a strong binding partner of BORG in D2.OR cells (Fig. 9a; S. Valadkhan, data not shown). Interestingly, quantifying RPA1 expression in breast cancer specimens housed within The Cancer Genome Atlas demonstrated that RPA1 levels were significantly elevated in basal breast cancer subtypes, which are highly aggressive and typically resistant to chemotherapies, as compared to their less aggressive luminal A or normal subtypes (Fig. 9b). Because RPA1 is a single-strand DNA-binding protein that is essential for the repair of damaged DNA [54, 55], we speculated that BORG may confer resistance to the genotoxic effects of doxorubicin by enhancing the activities of RPA1. Accordingly, CRISPR/Cas9-mediated depletion of RPA1 expression (Supplementary Fig. 6a) re-sensitized BORG-expressing D2.OR cells to both the genotoxic and apoptotic effects of doxorubicin (Fig. 9c–e; Supplementary Fig. 6b). Importantly, RPA1-deficiency in parental D2.OR cells did not amplify the cytotoxic effects of doxorubicin (Fig. 9e). These findings imply that RPA1-deficiency specifically enhanced the cytotoxic activity of doxorubicin in BORG-dependent cells, as opposed to simply enhancing the general sensitivity of breast cancer cells to doxorubicin. Finally, we monitored the impact of the small molecule RPA1 inhibitor, TDRL-505 [56], in altering the sensitivity of D2.OR derivatives to doxorubicin. In doing so, we observed BORG-expressing D2.OR cells treated with TDRL-505 to exhibit significantly greater doxorubicin-induced apoptosis than those treated with DMSO (Fig. 9f). Moreover, D2.OR cells subjected to 48 h treatment of TDRL-505 showed no significant change in cell viability at concentrations known to inhibit RPA1 activity (Supplementary Fig. 6c). Taken together, these results establish RPA1 as an essential BORG-interacting molecule that participates in the acquisition of chemoresistant phenotypes by BORG.
Fig. 9.
BORG elicits its chemoresistant effects through its interaction with RPA1, a pharmacologically targetable complex. a BORG is enriched in RPA1 immunocomplexes, as demonstrated by RNA-immunoprecipitation analyses of parental and BORG-expressing D2. OR cells. Identical IP of RelA/p65 serves as negative control. Data represent mean (±SEM) of three independent trials performed in triplicate (*P < 0.05). b RPA1 transcript abundance is significantly higher in aggressive basal breast cancer lesions as compared to luminal A and normal-like lesions. Data were acquired from TCGA BRCA dataset (*P <1× 10−4; **P <1 × 10−12). c, d Comet assays reveal that RPA1-deficiency significantly increases the magnitude of DNA damage acquired by BORG-expressing D2.OR cells during 48 h treatment with varying concentrations of doxorubicin. c Representative photos of D2.OR derivatives analyzed via Comet assays following diluent or doxorubicin treatment. d 75 cells were analyzed in each condition from three independent slides (**P < 0.005). e BORG-expressing D2.OR cells depleted of RPA1 are sensitized to the cytotoxic effects of doxorubicin treatment, as quantified by Caspase-Glo 3/7 assays. Data represent mean (±SEM) of three independent trials performed in triplicate (*P < 0.05). f Pharmacological inhibition of RPA1 via 48 h treatment with 30 μM TDRL-505 significantly increases doxorubicin-induced apoptosis in BORG-expressing D2.OR cells, as evidenced by Caspase-Glo 3/7 assays. Data represent mean (±SEM) of three independent trials performed in triplicate (*P < 0.05)
Discussion
Numerous studies have cemented lncRNAs as potent modulators of nearly every conventional hallmark of cancer, thereby allowing non-coding RNAs to effectively govern the metastatic competence of disseminated cells [20]. Despite the frequency at which pro-metastatic lncRNAs are being discovered, our previous and current studies establish BORG as a uniquely robust lncRNA that influences multiple facets of the metastatic cascade, namely (i) the colonization, outgrowth, and recurrence of dormant disseminated breast cancer cells [24], and (ii) the acquisition of chemoresistant and pro-survival phenotypes as shown herein. Interestingly, these two BORG-dependent features of metastatic progression are contingent upon disparate molecular mediators and interacting protein partners, thereby pointing to a bifurcation in the oncogenic signaling system governed by BORG. For instance, the pro-proliferative features of BORG are dependent upon its binding to TRIM28, which represses the expression of various tumor suppressors [24], while the chemoresistant activities of BORG are contingent upon its binding to RPA1, as well as its concomitant stimulation of NF-κB signaling. Interestingly, the activation of RPA1 and NF-κB by BORG are most strongly associated with circumventing the cytotoxic activities of DNA-damaging agents, as opposed to imposing direct effects on primary tumor development and progression. For instance, while inhibition of NF-κB clearly abrogated BORG-dependent doxorubicin resistance in vivo (Fig. 8b), this same cellular condition failed to significantly impact the growth of tumors derived from D2.OR cells (Supplementary Fig. 5b, d) or the growth of organoids derived from BORG-expressing D2.OR cells (data not shown). Such divergence of phenotype-associated pathways speaks to the multifunctional nature of BORG, and to the necessity of strategically tailoring therapies aimed at inactivating essential mediators and signaling nodes activated by BORG during the metastatic progression of breast cancers.
Recent evidence suggests that the expression of numerous lncRNAs in a variety pathophysiological conditions, including cancer, is tightly regulated by a multitude of cellular stresses, including genotoxicity, nutrient deprivation, heat and oxidative stress, and hypoxia [27, 57]. As such, it is unsurprising to learn that lncRNAs are exploited during the maintenance of cellular homeostasis in response to stress, as these non-coding RNAs possess multiple characteristics that make them uniquely poised to respond to noxious stimuli and conditions. For example, the synthesis of lncRNAs is extremely rapid and energetically favorable as compared to protein synthesis. As such, BORG and other lncRNAs represent ideal homeostatic tools that can be readily deployed when cells are strained by metabolic or nutrient stressors [27]. Similarly, lncRNAs are capable of rapid evolution due to the frequent presence of excess, non-essential bases in many lncRNAs that act as a ‘drawing board’ through which lncRNAs mutate and acquire positively-selected modules and structural alterations [57]. Indeed, it is tempting to speculate that BORG evolved in a manner that permits its expression and fosters a pro-survival response in cells and tissues exposed to various cellular stressors, a phenotype likely exploited by disseminated TNBCs as they encounter the environmental and therapeutic stresses routinely associated with metastasis [11, 58–63]. A clear manifestation of these pro-survival effects is the ability of BORG to confer in vivo resistance to the potent, commonly used chemotherapeutic doxorubicin (Fig. 8). These chemoresistant properties are even more note-worthy given the fact that BORG induces the proliferation of non-metastatic cells (i.e., D2.OR cells) in vivo and that doxorubicin is classically known to be more efficacious against rapidly dividing cells [64, 65]. Quantifying BORG expression in primary TNBC lesions may therefore represent a novel strategy that predicts for the clinical response to doxorubicin, much the same way that BORG expression in primary breast lesions correlates with the likelihood of metastatic relapse in TNBC patients [24].
BORG promotes NF-κB-dependent signaling and transcriptional patterns to foster survival in response to environmental and therapeutic stresses. Importantly, BORG appears to be uniquely equipped to activate the NF-κB signaling pathway, an event that is reinforced by the ability of BORG to initiate a novel feed-forward NF-κB signaling loop whereby the activation of NF-κB amplifies BORG expression, which further enhances NF-κB activation (Fig. 7). Although the precise molecular mediators linking BORG to enhanced NF-κB signaling remain uncertain, our findings point to an important role of NEMO/IKKγ in bridging this knowledge gap. Indeed, we observed BORG to induce the expression of NEMO transcripts and protein (Fig. 6a, b), an event that was critical for BORG-dependent activation of NF-κB. Additionally, the transcriptional and phenotypic outcomes activated downstream of p65 (e.g., that seen in BORG-expressing D2.OR cells; Fig. 4d) greatly depend upon the machinery coupled to chromatin remodeling at relevant genomic loci [66–68]. Moreover, we observe large alterations in histone methylation patterns (e.g., H3K4me1) upon manipulating BORG levels in varying cell types (data not shown), leading to the hypothesis that BORG induces widespread changes in the epigenetic landscape of TNBC cells. Indeed, such BORG-mediated alterations of the chromatin state could explain the role of BORG in propagating an NF-κB feed-forward loop, as BORG-induced chromatin remodeling could dictate the genomic binding patterns of p65, including its binding to the BORG promoter (Supplementary Fig. 4b).
Numerous lncRNAs have recently been shown to alter signaling through the NF-κB axis [69]. The ability of NF-κB to specifically target lncRNAs (i.e., BORG) represents an exciting avenue through which to inhibit NF-κB-dependent malignancies, owing to the fact that broad, non-specific inhibitors of NF-κB signaling can lead to immense off-target side effects that limit therapeutic utility [69]. Conversely, targeting lncRNAs or their direct effectors offers the ability to precisely inhibit the arms and molecular mediators of the NF-κB pathway that malignancies are most dependent upon, while sparing those essential for physiologic cell maintenance. Indeed, we show that pharmacologic inactivation of specific BORG binding proteins selectively sensitizes chemoresistant cells to the cytotoxic effects of doxorubicin (Fig. 9). Coupled with emerging technologies that show preclinical efficacy in altering the expression and activity of lncRNAs [70], BORG represents a realistic target whose inhibition could mitigate pro-survival pathways in disseminated breast cancer cells, thereby preventing their metastatic outgrowth both pre-and post-therapeutic insult.
Materials and methods
Cell culture
D2.OR cells and the 4T1 progression series (67NR, 4T07, and 4T1) were obtained from Fred Miller (Wayne State University, Detroit, MI) and grown in DMEM (Sigma-Aldrich, St. Louis, MO) supplemented with 10% FBS and 1% Pen/Strep. Growth under hypoxic conditions was accomplished using a variable oxygen control CO2 incubator (Thermo Fisher Scientific, Waltham, MA) set at 2% O2 tension. Cells were grown in DMEM supplemented with 1% FBS for 24 h at 2% O2. Nutrient deprivation was conducted via growth of D2.OR cells in DMEM, no glucose (Gibco, Grand Island, NY), or MEM, NEAA, no glutamine (Gibco), or Krebs–Henseleit Buffer (118 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 1.25 mM CaCl2, 1.2 mM KH2PO4, 25 mM NaHCO3). Small molecule inhibitors or chemotherapeutic agents used during the course of these studies are listed in Supplementary Table 1. Cell lines were frequently confirmed to be negative for mycoplasma contamination using the MycoAlert Mycoplasma Detection Kit (Lonza, Basel, Switzerland).
In long-term doxorubicin treatment assays, D2.OR derivatives were plated into 6-well plates (250,000 cells/well) containing DMEM/10% FBS with or without 100 nM doxorubicin. Media was replaced every other day, and at 7 days post plating, the cells were collected and replated into identical 6-well plates containing 100 nM doxorubicin (i.e., 1:1 split). Photomicrographs of resulting cells were taken 14 days following initial plating; cells were collected 28 days following initial plating for RNA extraction.
When denoted, cells were grown atop solidified cushions of Cultrex reconstituted basement extract (Trevigen, Gaithersburg, MD), containing 3 mg/ml collagen type I (BD Biosciences, Franklin Lakes, NJ) when specified as rigid as described [24].
Generation of stable cell lines
D2.OR cells were engineered to stably express firefly luciferase by transfection with pNifty-CMV-luciferase, followed by zeocin selection (500 μg/ml; Invitrogen, Carlsbad, CA) as described [71]. The full-length BORG transcript and mutant BORG deletions were created as described [28] and used to transfect D2.OR cells, followed by selection with G418 (500 μg/ml) to generate stable cell lines. Expression of DN-IκBα was performed via VSVG lentiviral transduction of D2.OR, 4T1, 4T07, or 67NR cells with pBabe-Puro-IKBα-mut (super repressor [45]; William Hahn, Addgene plasmid #15291), followed by selection with puromycin (5 μg/ml). CRISPR/Cas9-mediated knockout of NEMO and RPA1 was performed by transducing D2.OR derivatives with lentiviral particles produced from vector containing expression cassettes for both SpCas9 and the chimeric single guide RNA scaffold (pLentiCRISPRv2 [72]), followed by selection with puromycin (5 μg/ml). sgRNA design was carried out using CHOPCHOP design tool [73] to target various exons of NEMO and RPA1, while minimizing off-target binding of sgRNAs. Primers used for generation of CRISPR/Cas9 sgRNAs are listed in Supplementary Table 2 (bold bases correlated with genomic sequence).
Soft agar assays
Soft agar assays were performed in 6-well plates containing a bottom layer of 2 ml 0.6% noble agar, topped with 3 ml of a cell suspension containing 75,000 cells in 0.54% noble agar in DMEM with 10% FBS. After solidification, agar was topped with 2 ml DMEM/10% FBS and was changed every other day. After two weeks, media was aspirated and colonies were stained with NBT (200 μl; 1 mg/ml; Sigma-Aldrich) at 37 °C overnight. Photos of colonies were acquired using the Chemidoc Imaging System (Bio-Rad, Hercules, CA) and analysis was automated using ImageJ software to determine the number and average size of colonies.
Clonogenic assays
D2.OR derivatives were plated into 6-well plates (200 cells/well) and grown for 10 days in complete media, which was replaced every other day. After 10 days, cells were washed 2× with PBS and fixed in acetic acid/methanol (1:7 [vol/vol]) at room temperature for 5 min, followed by staining with 0.5% crystal violet solution at room temperature for 2 h. Plates were rinsed with ddH2O for 5 min and dried overnight at room temperature. Photographs of wells were acquired using the Chemidoc Imaging System (Bio-Rad) and automated quantification of colony formation was performed using the ColonyArea ImageJ Plugin [74] to determine percentage area of the well covered by colonies, as well as the density of the colonies.
Anoikis and caspase-Glo assays
Anoikis assays were performed as previously described [24] and Caspase-Glo Assays (Promega, Madison, WI) were performed in 96-well plates under conditions denoted by the manufacturer.
Cell-cycle analysis
D2.OR derivatives were plated into standard 6-well tissue culture dishes and synchronized by serum starvation prior to doxorubicin or vehicle treatment for 48 h, at which point cells were trypsinized and subjected to PI staining and subsequent cell-cycle analysis using FlowJo software, as described [24].
Comet assays
D2.OR derivatives were plated into 6-well plates and treated with vehicle or varying concentrations of doxorubicin for 48 h prior to subjecting cells to neutral Comet assays as described [75, 76]. Briefly, 13,000 cells were resuspended in 0.7% low-melting point agarose in PBS and plated onto pre-chilled (4 °C) Superfrost Plus microscope slides (Fisher Scientific, Hampton, NH) that were coated with 0.8% agarose in PBS. After solidification, a subsequent layer of 0.7% LMP agarose was added, followed by lysis in Comet Lysis Buffer (2.5 M NaCl, 0.1 M EDTA, 10 mM Tris base, 1% N-laurylsarcosine, 0.5% Triton X-100, 10% DMSO) for 1 h at 4 °C. Cells were washed 3 × (5 min; 4 °C) in Comet electrophoresis buffer (300 mM sodium acetate, 100 mM Tris–HCl, pH 8.3) then subjected to electrophoresis in Comet electrophoresis buffer for 1 h at 18 V. Slides were then washed 2× in PBS and 2× in ethanol, prior to drying and staining of DNA with 2 μg/ml ethidium bromide. Photos of slides were taken on a Leica DM6000 upright microscope and tail length and percent tail DNA were quantified with assistance from an ImageJ Comet Assay macro (https://www.med.unc.edu/microscopy/resources/imagej-plugins-and-macros/comet-assay).
RNA extraction and qRT-PCR analysis
When denoted, cells were isolated from 3D-culture or standard tissue culture, and subjected to RNA extraction using TRIzol reagent as described [24]. One μg of total RNA was subsequently reverse-transcribed with iScript cDNA Synthesis Kit (Bio-Rad) and subjected to semi-quantitative real-time PCR using iQ SYBR Green Supermix (Bio-Rad) as previously described [24]. The gene primers used in these studies are listed in Supplementary Table 2.
RNA-seq analysis
BORG-expressing and vector-transfected D2.OR derivatives were grown in 3D-culture for 7 days prior to isolation from 3D-matrix and subsequent extraction of total cellular RNA as described [24]. One μg of total cellular RNA was used for preparation of sequencing libraries using the Tru-Seq Stranded Total RNA library prep kit (Illumina), followed by RNA sequencing on the HiSeq 2500 Platform (Illumina) using rapid run mode. Two replicate RNA-seq experiments were performed for each condition (total of 4 samples) with an average of ~66 million paired end, 100 nucleotide long reads obtained for each sample. Generated sequencing reads were assessed for quality using FastQC. The reads were then trimmed for adapter sequences and low-quality nucleotides at the ends of the reads using Trim Galore (Babraham Bioinformatics). Reads that passed quality control were then aligned to the mouse genome (Mus musculus, GRCm38/mm10) using TopHat [77]. The alignment of the reads was guided using the ENCODE reference annotation for GRCm38/mm10. The TopHat output was then analyzed for differential expression using the Cufflinks suite of tools. Differentially expressed genes were identified using a significance cutoff of FDR < 0.05 [78].
A parallel analysis was performed using HISAT2 as the alignment tool, followed by counting the reads mapped to each gene with HTSeq-count using the GRCm38/mm10 and Gencode M10 release of the mouse transcriptome as the reference annotation. The data were fit to a negative binomial generalized log-linear model with quasi-likelihood tests using EdgeR followed by genewise statistical tests to identify differentially expressed genes. The differential expression results of the two pipelines were used to identify positive-and negatively enriched pathways and genesets using the C2 module of the MSigDB database of gene sets and GSEA tool [79].
To define the relationship between the expression of BORG and activation of the NF-κB pathway, additional TNBC-related datasets were selected from public RNA-seq repositories and analyzed as described above. The level of BORG and NF-κB pathway enrichment levels were calculated for every sample within each dataset. To assess the impact of BORG on NF-κB activation between different datasets, the levels of BORG and NF-κB pathway enrichment for the samples within each dataset were normalized to the sample with the lowest level of BORG expression. The normalized values were used to calculate Pearson’s correlation coefficient using the statistics module of SciPy. Datasets utilized in these correlation experiments include PRJNA278881, PRJNA278887, PRJNA256302, PRJNA292572, PRJNA251383, PRJNA193090, PRJNA335388, PRJNA278888, PRJNA284949, PRJNA238231, PRJNA260526, and PRJNA227137.
Immunoblotting
Cells were isolated from 3D-culture as described [24] and homogenized on ice in RIPA buffer (50 mM Tris, 150 mM NaCl, 6 mM sodium deoxycholate, 1.0% NP-40, 0.1% SDS, pH 7.4) supplemented with protease inhibitor cocktail (Sigma-Aldrich) and phosphatase inhibitors (10 mM sodium orthovanadate, 40 mM β-glycerophosphate, 20 mM NaF). Lysates were cleared by centrifugation and subjected to immunoblot analysis as described [24] with antibodies denoted in Supplementary Table 1. ImageJ software was used to quantify band density of protein of interest and respective actin loading control. Alternatively, 500 μg of whole cell D2.OR lysate was incubated overnight at 4 °C with 1 μg of biotinylated double-stranded DNA oligonucleotides containing the NF-κB consensus DNA-binding sequence. Active p65:oligonucleotide complexes were captured by streptavidin-agarose beads (Thermo Fisher Scientific), washed, and resolved by SDS-PAGE gels, followed by immobilization on nitrocellulose membranes and probing with anti-p65 antibodies [35]. Biotinylated oligonucleotide sequences are listed in Supplementary Table 1.
NF-κB luciferase assays
D2.OR derivatives were set into 24 well plates (40,000 cells/well) and transfected using TransIT-LT1 (Mirus Bio, Madison, WI), 100 ng pCMV-βGal (gift from Dr. Gerard Blobe), and 300 ng pGL4.76-Rluc (Promega) containing an NF-κB-driven promoter derived from an NF-κB luciferase reporter that was a gift from Dr. John Routes [36]. Twenty-four hour post-transfection, media was removed, washed with PBS, and replaced with media containing 1% FBS supplemented with doxorubicin or bortezomib when denoted. Forty-eight hour post-transfection, cells were washed with PBS and lysed in 1× Renilla Luciferase Assay Lysis Buffer (Promega). Renilla luciferase activity (i.e., NF-κB activity) in lysates was quantified using Renilla Luciferase Assay Reagent (Promega) and read on GloMax-Multi detection system (Promega). Normalization of Renilla Luciferase output in each well was performed via chemiluminescent quantification of β-galactosidase activity (Takara Bio) per the manufacturer’s protocol.
In vivo mouse studies
Firefly-expressing D2.OR derivatives were resuspended in PBS and injected orthotopically into the mammary fat pads of 8 week old female Balb/C mice (Jackson Laboratory, Bar Harbor, ME; 2.5 × 106 cells/mouse). Each treatment arm was comprised of 5 mice (i.e., 40 mice randomly and blindly divided into 8 groups), a sample size based on previous experience with preclinical breast cancer models, as well as power analyses using G*Power software [80] to estimate the sufficient number of animals necessary to achieve statistical significance. Primary tumors were measured using digital calipers (Fisher Scientific) and tumor volume was calculated using the formula: volume = (width2)*(length/2). Additionally, bioluminescent output of fat pad tumors was quantified at stated times post-injection as described [24]. Mice were treated intraperitoneally in an unblinded manner with doxorubicin (5 mg/kg) or vehicle (PBS) upon tumor volume reaching 100 mm3. Weekly i.p. injections were continued prior to sacrifice of mice 4 weeks following initial doxorubicin treatment. Upon sacrifice, tumors were excised, weighed, and either processed for histopathological analysis by the Case Comprehensive Cancer Center’s Tissue Resources Core or dissociated to generate an ex vivo cell line. Tissue dissociation was performed manually, using sterile surgical tools followed by incubation in 0.25% trypsin at 37 °C for 1 h. Tumor homogenate was passed through a cell strainer and plated into 10 cm dishes containing complete media and 500 μg/ml zeocin (Invitrogen).
RNA-immunoprecipitation assays
RIP was performed as previously described [24] using antibodies against RPA1 (Santa Cruz Biotechnology, Dallas, TX; sc-28304; 5 μg) or RelA (Cell Signaling Technology, Danvers, MA; #8242; 5 μg), as listed in Supplementary Table 1.
BORG promoter scanning and chromatin Immunoprecipitation
Identification of homologous NF-κB consensus DNA-binding sequences within the BORG promoter was carried out using the publicly available PROMO and MALGEN software [81, 82] with a dissimilarity sequence less than 15%. Overall, 5700 base pairs upstream of the transcriptional start site was used as the BORG promoter sequence. ChIP assays were performed as previously described [24]. Parental D2.OR cells were treated with either doxorubicin or vehicle for 24 h prior to fixation. p65 antibody and qPCR primers are annotated in Supplementary Table 1 or Supplementary Table 2, respectively.
Cell viability assays
D2.OR cells were cultured in 96-well plates (10,000 cells/well) and treated with DMSO or varying concentrations of TDRL-505 for 48 h, at which point the cells were washed with PBS and assessed for cell viability using CellTiter-Glo Luminescent Cell Viability Assay per manufacturer’s protocol (Promega). Luminescent output was quantified using GloMax-Multi detection system (Promega).
Statistics
Unless otherwise stated, all experiments were performed in biological triplicate where significance was determined by unpaired two-tailed Student’s t-tests. p-values ≤ 0.05 were considered to be statistically significant. GSEA analyses were performed using a geneset-based permutation test to yield FDR and nominal p-values that indicated the significance of enrichment of the corresponding collection of genes. The mRNA expression values from the TCGA breast cancer dataset (TCGA BRCA) were downloaded from the dUCSC Xena Cancer Genome Browser [83]. As denoted in figure legends, all results were conveyed as mean ± SEM unless otherwise stated.
Study approval
All animal studies were performed in accordance with the Institutional Animal Care and Use Committees for Case Western Reserve University.
Supplementary Material
Acknowledgements
Members of the Schiemann Laboratory are thanked for critical comments and reading of the manuscript. We thank Dr. Ricky Chan for assistance in our analyses of RNA-seq data. We also acknowledge the expertise provided by members of the Case Comprehensive Cancer Center’s Core Facilities, including the Gene Expression & Genotyping Core, the Imaging Research Core, Tissue Resources Core, and the Genomics Core. Research support was provided in part by the National Institutes of Health to W.P.S. (CA129359, CA177069, and CA194518) and A.J.G (T32GM007250 and F30CA203233). Additional support was graciously provided by the METAvivor Foundation (W.P.S.), and by pilot funding from the Case Comprehensive Cancer Center’s Research Innovation Fund, which is supported by the Case Council and Friends of the Case Comprehensive Cancer Center (W.P.S. and S.V.).
Footnotes
Data availability
All sequencing data provided herein have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus (GEO) and can be accessed through the GEO Series accession number GSE116656. All additional data are available from the corresponding authors upon request.
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Torre LA, Islami F, Siegel RL, Ward EM, Jemal A. Global cancer in women: burden and trends. Cancer Epidemiol Biomark Prev 2017;26:444–57. [DOI] [PubMed] [Google Scholar]
- 2.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 2001;98:10869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA 2003;100:8418–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature 2000;406:747–52. [DOI] [PubMed] [Google Scholar]
- 5.Alluri P, Newman LA. Basal-like and triple-negative breast cancers: searching for positives among many negatives. Surg Oncol Clin N Am 2014;23:567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 2007;13:4429–34. [DOI] [PubMed] [Google Scholar]
- 7.Chiang AC, Massague J. Molecular basis of metastasis. N Engl J Med 2008;359:2814–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vanharanta S, Massague J. Origins of metastatic traits. Cancer Cell 2013;24:410–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luzzi KJ, MacDonald IC, Schmidt EE, Kerkvliet N, Morris VL, Chambers AF, et al. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol 1998;153:865–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein CA. Framework models of tumor dormancy from patient-derived observations. Curr Opin Genet Dev 2011;21:42–49. [DOI] [PubMed] [Google Scholar]
- 11.Senft D, Ronai ZE. Adaptive stress responses during tumor metastasis and dormancy. Trends Cancer 2016;2:429–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell 2017;168:670–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tiligada E Chemotherapy: induction of stress responses. Endocr Relat Cancer 2006;13(Suppl 1):S115–124. [DOI] [PubMed] [Google Scholar]
- 14.Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell 2012;150:165–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer 2013;13:714–26. [DOI] [PubMed] [Google Scholar]
- 16.Hoesel B, Schmid JA. The complexity of NF-kappaB signaling in inflammation and cancer. Mol Cancer 2013;12:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayden MS, Ghosh S. NF-kappaB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev 2012;26:203–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Godwin P, Baird AM, Heavey S, Barr MP, O’Byrne KJ, Gately K. Targeting nuclear factor-kappa B to overcome resistance to chemotherapy. Front Oncol 2013;3:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montagut C, Tusquets I, Ferrer B, Corominas JM, Bellosillo B, Campas C, et al. Activation of nuclear factor-kappa B is linked to resistance to neoadjuvant chemotherapy in breast cancer patients. Endocr Relat Cancer 2006;13:607–16. [DOI] [PubMed] [Google Scholar]
- 20.Schmitt AM, Chang HY. Long noncoding RNAs in cancer pathways. Cancer Cell 2016;29:452–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huarte M The emerging role of lncRNAs in cancer. Nat Med 2015;21:1253–61. [DOI] [PubMed] [Google Scholar]
- 22.Di Gesualdo F, Capaccioli S, Lulli M. A pathophysiological view of the long non-coding RNA world. Oncotarget 2014;5:10976–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malhotra A, Jain M, Prakash H, Vasquez KM, Jain A. The regulatory roles of long non-coding RNAs in the development of chemoresistance in breast cancer. Oncotarget 2017;8:110671–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gooding AJ, Zhang B, Jahanbani FK, Gilmore HL, Chang JC, Valadkhan S, et al. The lncRNA BORG drives breast cancer metastasis and disease recurrence. Sci Rep 2017;7:12698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yap WT, Salvay DM, Silliman MA, Zhang X, Bannon ZG, Kaufman DB, et al. Collagen IV-modified scaffolds improve islet survival and function and reduce time to euglycemia. Tissue Eng Part A 2013;19:2361–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Somaiah C, Kumar A, Mawrie D, Sharma A, Patil SD, Bhattacharyya J, et al. Collagen promotes higher adhesion, survival and proliferation of mesenchymal stem cells. PLoS ONE 2015;10: e0145068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valadkhan S, Valencia-Hipolito A. lncRNAs in stress response. Curr Top Microbiol Immunol 2016;394:203–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang B, Gunawardane L, Niazi F, Jahanbani FK, Chen X, Valadkhan S. A novel RNA motif mediates the strict nuclear localization of a long noncoding RNA. Mol Cell Biol 2014;34:2318–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeda K, Ichijo H, Fujii M, Mochida Y, Saitoh M, Nishitoh H, et al. Identification of a novel bone morphogenetic protein-responsive gene that may function as a noncoding RNA. J Biol Chem 1998;273:17079–85. [DOI] [PubMed] [Google Scholar]
- 30.Zanotto-Filho A, Masamsetti VP, Loranc E, Tonapi SS, Gorthi A, Bernard X, et al. Alkylating agent-induced NRF2 blocks endoplasmic reticulum stress-mediated apoptosis via control of glutathione pools and protein thiol homeostasis. Mol Cancer Ther 2016;15:3000–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lupertz R, Watjen W, Kahl R, Chovolou Y. Dose-and time-dependent effects of doxorubicin on cytotoxicity, cell cycle and apoptotic cell death in human colon cancer cells. Toxicology 2010;271:115–21. [DOI] [PubMed] [Google Scholar]
- 32.Tyagi AK, Singh RP, Agarwal C, Chan DC, Agarwal R. Silibinin strongly synergizes human prostate carcinoma DU145 cells to doxorubicin-induced growth Inhibition, G2-M arrest, and apoptosis. Clin Cancer Res 2002;8:3512–9. [PubMed] [Google Scholar]
- 33.DiPaola RS. To arrest or not to G(2)-M Cell-cycle arrest: commentary re: A. K. Tyagi et al. , Silibinin strongly synergizes human prostate carcinoma DU145 cells to doxorubicin-induced growth inhibition, G(2)-M arrest, and apoptosis. Clin. cancer res, 8: 3512–3519, 2002. Clin Cancer Res. 2002;8:3311–4. [PubMed] [Google Scholar]
- 34.Ling YH, el-Naggar AK, Priebe W, Perez-Soler R. Cell cycle-dependent cytotoxicity, G2/M phase arrest, and disruption of p34cdc2/cyclin B1 activity induced by doxorubicin in synchronized P388 cells. Mol Pharmacol 1996;49:832–41. [PubMed] [Google Scholar]
- 35.Neil JR, Schiemann WP. Altered TAB1:I kappaB kinase interaction promotes transforming growth factor beta-mediated nuclear factor-kappaB activation during breast cancer progression. Cancer Res 2008;68:1462–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neil JR, Tian M, Schiemann WP. X-linked inhibitor of apoptosis protein and its E3 ligase activity promote transforming growth factor-beta-mediated nuclear factor-kappaB activation during breast cancer progression. J Biol Chem 2009;284:21209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tong AJ, Liu X, Thomas BJ, Lissner MM, Baker MR, Senagolage MD, et al. A stringent systems approach uncovers gene-specific mechanisms regulating inflammation. Cell 2016;165:165–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furlow PW, Zhang S, Soong TD, Halberg N, Goodarzi H, Mangrum C, et al. Mechanosensitive pannexin-1 channels mediate microvascular metastatic cell survival. Nat Cell Biol 2015;17:943–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gomis RR, Alarcon C, Nadal C, Van Poznak C, Massague J. C/EBPbeta at the core of the TGFbeta cytostatic response and its evasion in metastatic breast cancer cells. Cancer Cell 2006;10:203–14. [DOI] [PubMed] [Google Scholar]
- 40.Piva R, Belardo G, Santoro MG. NF-kappaB: a stress-regulated switch for cell survival. Antioxid Redox Signal 2006;8:478–86. [DOI] [PubMed] [Google Scholar]
- 41.Tornatore L, Sandomenico A, Raimondo D, Low C, Rocci A, Tralau-Stewart C, et al. Cancer-selective targeting of the NF-kappaB survival pathway with GADD45beta/MKK7 inhibitors. Cancer Cell 2014;26:495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wullaert A, Bonnet MC, Pasparakis M. NF-kappaB in the regulation of epithelial homeostasis and inflammation. Cell Res 2011;21:146–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahmed KM, Cao N, Li JJ. HER-2 and NF-kappaB as the targets for therapy-resistant breast cancer. Anticancer Res 2006;26:4235–43. [PMC free article] [PubMed] [Google Scholar]
- 44.Khan S, Lopez-Dee Z, Kumar R, Ling J. Activation of NFkB is a novel mechanism of pro-survival activity of glucocorticoids in breast cancer cells. Cancer Lett 2013;337:90–95. [DOI] [PubMed] [Google Scholar]
- 45.Boehm JS, Zhao JJ, Yao J, Kim SY, Firestein R, Dunn IF, et al. Integrative genomic approaches identify IKBKE as a breast cancer oncogene. Cell 2007;129:1065–79. [DOI] [PubMed] [Google Scholar]
- 46.Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation. Science 1995;267:1485–8. [DOI] [PubMed] [Google Scholar]
- 47.Israel A The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harb Perspect Biol 2010;2:a000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yuan Y, Hilliard G, Ferguson T, Millhorn DE. Cobalt inhibits the interaction between hypoxia-inducible factor-alpha and von Hippel-Lindau protein by direct binding to hypoxia-inducible factor-alpha. J Biol Chem 2003;278:15911–6. [DOI] [PubMed] [Google Scholar]
- 49.Gupta SC, Sundaram C, Reuter S, Aggarwal BB. Inhibiting NF-kappaB activation by small molecules as a therapeutic strategy. Biochim Biophys Acta 2010;1799:775–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kouroukis TC, Baldassarre FG, Haynes AE, Imrie K, Reece DE, Cheung MC. Bortezomib in multiple myeloma: systematic review and clinical considerations. Curr Oncol 2014;21:e573–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paramore A, Frantz S. Bortezomib. Nat Rev Drug Discov 2003;2:611–2. [DOI] [PubMed] [Google Scholar]
- 52.Lu S, Wang J. The resistance mechanisms of proteasome inhibitor bortezomib. Biomark Res 2013;1:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lopez JS, Banerji U. Combine and conquer: challenges for targeted therapy combinations in early phase trials. Nat Rev Clin Oncol 2017;14:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao M, Geng R, Guo X, Yuan R, Zhou X, Zhong Y, et al. PCAF/GCN5-mediated acetylation of RPA1 promotes nucleotide excision repair. Cell Rep 2017;20:1997–2009. [DOI] [PubMed] [Google Scholar]
- 55.Haring SJ, Mason AC, Binz SK, Wold MS. Cellular functions of human RPA1. Multiple roles of domains in replication, repair, and checkpoints. J Biol Chem 2008;283:19095–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shuck SC, Turchi JJ. Targeted inhibition of replication protein A reveals cytotoxic activity, synergy with chemotherapeutic DNA-damaging agents, and insight into cellular function. Cancer Res 2010;70:3189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lakhotia SC. Long non-coding RNAs coordinate cellular responses to stress. Wiley Interdiscip Rev Rna 2012;3:779–96. [DOI] [PubMed] [Google Scholar]
- 58.Jezierska-Drutel A, Rosenzweig SA, Neumann CA. Role of oxi-dative stress and the microenvironment in breast cancer development and progression. Adv Cancer Res 2013;119:107–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.White E, Mehnert JM, Chan CS. Autophagy, metabolism, and cancer. Clin Cancer Res 2015;21:5037–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mahadevan NR, Zanetti M. Tumor stress inside out: cell-extrinsic effects of the unfolded protein response in tumor cells modulate the immunological landscape of the tumor microenvironment. J Immunol 2011;187:4403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Catalano V, Turdo A, Di Franco S, Dieli F, Todaro M, Stassi G. Tumor and its microenvironment: a synergistic interplay. Semin Cancer Biol 2013;23:522–32. [DOI] [PubMed] [Google Scholar]
- 62.Victorino VJ, Pizzatti L, Michelletti P, Panis C. Oxidative stress, redox signaling and cancer chemoresistance: putting together the pieces of the puzzle. Curr Med Chem 2014;21:3211–26. [DOI] [PubMed] [Google Scholar]
- 63.Huang Z, Zhou L, Chen Z, Nice EC, Huang C. Stress management by autophagy: Implications for chemoresistance. Int J Cancer 2016;139:23–32. [DOI] [PubMed] [Google Scholar]
- 64.Baral E, Auer G. In vitro effect of doxorubicin on non-proliferating and proliferating epithelial cells. Int J Radiat Oncol Biol Phys 1990;19:963–5. [DOI] [PubMed] [Google Scholar]
- 65.Li S, Kennedy M, Payne S, Kennedy K, Seewaldt VL, Pizzo SV, et al. Model of tumor dormancy/recurrence after short-term chemotherapy. PLoS One 2014;9:e98021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wan F, Lenardo MJ. Specification of DNA binding activity of NF-kappaB proteins. Cold Spring Harb Perspect Biol 2009;1: a000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhong H, May MJ, Jimi E, Ghosh S. The phosphorylation status of nuclear NF-kappa B determines its association with CBP/p300 or HDAC-1. Mol Cell 2002;9:625–36. [DOI] [PubMed] [Google Scholar]
- 68.Huang B, Yang XD, Zhou MM, Ozato K, Chen LF. Brd4 coactivates transcriptional activation of NF-kappaB via specific binding to acetylated RelA. Mol Cell Biol 2009;29:1375–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mao X, Su Z, Mookhtiar AK. Long non-coding RNA: a versatile regulator of the nuclear factor-kappaB signalling circuit. Immunology 2017;150:379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li CH, Chen Y. Targeting long non-coding RNAs in cancers: progress and prospects. Int J Biochem Cell Biol 2013;45:1895–910. [DOI] [PubMed] [Google Scholar]
- 71.Wendt MK, Taylor MA, Schiemann BJ, Schiemann WP. Down-regulation of epithelial cadherin is required to initiate metastatic outgrowth of breast cancer. Mol Biol Cell 2011;22:2423–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelson T, et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 2014;343:84–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Labun K, Montague TG, Gagnon JA, Thyme SB, Valen E. CHOPCHOPv2: a web tool for the next generation of CRISPR genome engineering. Nucleic Acids Res 2016;44:W272–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guzman C, Bagga M, Kaur A, Westermarck J, Abankwa D. ColonyArea: an ImageJ plugin to automatically quantify colony formation in clonogenic assays. PLoS One 2014;9:e92444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Courilleau C, Chailleux C, Jauneau A, Grimal F, Briois S, Boutet-Robinet E, et al. The chromatin remodeler p400 ATPase facilitates Rad51-mediated repair of DNA double-strand breaks. J Cell Biol 2012;199:1067–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boutet-Robinet E, Trouche D, Canitrot Y. Neutral comet assay. Bio Protoc 2013;3:12–17. [Google Scholar]
- 77.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 2009;25:1105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 2010;28:511–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 2005;102:15545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 2009;41:1149–60. [DOI] [PubMed] [Google Scholar]
- 81.Farre D, Roset R, Huerta M, Adsuara JE, Rosello L, Alba MM, et al. Identification of patterns in biological sequences at the ALGGEN server: PROMO and MALGEN. Nucleic Acids Res 2003;31:3651–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Messeguer X, Escudero R, Farre D, Nunez O, Martinez J, Alba MM. PROMO: detection of known transcription regulatory elements using species-tailored searches. Bioinformatics 2002;18:333–4. [DOI] [PubMed] [Google Scholar]
- 83.Goldman M, Craft B, Brooks A, Zhu J, Haussler D. The UCSC Xena Platform for cancer genomics data visualization and interpretation. BioRxiv 2018. 10.1101/326470. [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.