Abstract
Transcranial direct current stimulation (tDCS) is a neuromodulation technique with potential to treat eating disorders and obesity. As for any potential treatment, it is important to assess the degree to which expectation effects contribute to its reported efficacy. This study assessed the effect of tDCS on amount of food craving and eating while tightly controlling treatment expectation. N=74 adults with overweight or obesity were informed of the known effects of tDCS to suppress craving and eating. Once electrodes were on the head, half of the participants were told they were receiving real, and the other half sham tDCS. Within these groups, approximately half actually received real and the other half sham tDCS. Stimulation parameters used were those previously found to reduce craving and eating, including in our lab: 2mA, anode right/cathode left targeting the dorsolateral prefrontal cortex for 20 minutes (real), or only for the first and last minute (sham). Analyses controlled for demographics, hunger, trait impulsiveness, eating motives, dieting, binge eating, suggestibility, and baseline craving and eating. Participants told they were receiving real tDCS craved and ate less than participants told they were receiving sham tDCS (both p<0.01), regardless of tDCS condition administered. There was no main effect of real vs. sham tDCS on craving or eating or an interaction between tDCS condition and expectation. The scientific validation of tDCS as a treatment for eating-related conditions hinges on controlling for the powerful effects of expectation. This can include the type of information provided on consent forms and participants’ ability to guess real from sham conditions.
Keywords: neuromodulation, placebo, dorsolateral prefrontal cortex, suggestibility, psychological, cognition
1. Introduction
Transcranial direct current stimulation (tDCS) is an inexpensive, non-invasive neuromodulatory tool shown to alter neural activity and behavior in humans (Das et al., 2016). As a result, tDCS has been tested as a potential treatment for a plethora of diseases and conditions including learning, language, memory, motor, pain, sensory, and emotional disorders (Kekic et al., 2016;Nitsche et al., 2008;Shin, Foersterand Nitsche, 2015;Tremblay et al., 2014). It has also shown promise to treat eating-related conditions including eating disorders (Burgess et al., 2016;Kekic et al., 2017;Val-Laillet et al., 2015), obesity (Alonso-Alonso, 2013;Ray et al., 2017), and frequent food cravings (Fregni et al., 2008). However, there are inconsistent findings despite the use of very similar tDCS parameters. Specifically, most studies aimed at reducing food craving and eating have targeted the dorsolateral prefrontal cortex (DLPFC) with 2 mA current using a right anode/left cathode montage in a single-session. Some studies using these parameters obtained a reduction of caloric consumption and food craving despite testing populations with different body weight status and eating patterns (i.e. healthy vs. disordered eating) (Burgess et al., 2016;Fregni et al., 2008;Lapenta et al., 2014;Ray et al., 2017). However, other studies using these parameters either obtained reductions in craving but not eating (Goldman et al., 2011;Kekic et al., 2014), or no reduction in craving (Kekic et al., 2017). Stimulation with 1 mA instead of 2 mA also did not affect craving or eating (Georgii et al., 2017). Studies that employed a left anode/right cathode or reference montage over the DLPFC also found equivocal craving and eating outcomes (Fregni et al., 2008;Montenegro et al., 2012).
A potential explanation for the inconsistency is expectation of treatment outcomes. It is well documented that patients’ beliefs about the actions of a treatment can influence whether or not they respond to that treatment and to what degree (Evers et al., 2018). For example, when patients expect a treatment to have beneficial or rewarding effects, the expectation alone can produce positive outcomes that are unrelated to the physiological actions of the actual treatment, (i.e., a placebo effect) even when they know the treatment is a placebo (Fontaine et al., 2016). This is a particular issue with tDCS studies because the standard shamming procedure used as a control is not always effective at masking active from sham tDCS conditions (Horvath, Carter and Forte, 2014). This is especially true when delivering 2mA of current (O’Connell et al., 2012), the most common level of current used among all tDCS studies (Bikson et al., 2016). Active stimulation induces transient physical sensations such as itching and tingling throughout the session (Bikson et al., 2016) while the standard sham condition delivers current only for the first and last minute of the session. Hence participants are more likely to guess that the active condition is the real one (Horvath et al. 2014;O’Connell et al., 2012), logically increasing the likelihood of treatment expectations. In fact, there is evidence that correct guessing of conditions can affect tDCS outcomes. Brunoni et al. found that 83% of participants with major depression correctly guessed when they received real tDCS and 37% correctly guessed when they received sham tDCS. Importantly, upon further exploration, they found that participants who correctly guessed their tDCS allocation had greater clinical improvement in depression symptoms (Brunoni et al., 2014).
In addition to correct guessing, simply having tDCS electrodes placed on the head can result in expectation effects. Aslaksen et al. found that participants who received a standard sham procedure incurred a significant reduction in pain intensity compared to a group that had no electrodes placed on the head. Further, participants in this sham tDCS group responded similarly to individuals in the real tDCS group (Aslaksenand Flaten, 2008). Other sources of expectation effects not unique to tDCS but likely affecting its assessment include personal beliefs about treatments in general, and written or verbal information about the treatment (Arnold, Finnissand Kerridge, 2014). To the latter, Rabipour et al. recently reported that individuals administered tDCS who were verbally primed to expect greater efficacy from tDCS on a working memory and executive function task performed better than individual who were stimulated but primed to expect lower efficacy from tDCS. Rabipour and colleagues published these findings while the present study was in peer-review. Both studies used similar methods to systematically evaluate the influence of expectation on tDCS outcomes but this is the first study to do so on appetite outcomes. Given the popular opinion that tDCS has promise to treat obesity and eating disorders, understanding the effects of expectation in the putative action of tDCS to suppress food craving and eating is critical as it could be masking the true efficacy of tDCS. This, in turn, affects the rigorous scientific validation of tDCS as a treatment for these highly prevalent medical conditions.
Therefore, the purpose of this study was to systematically control expectation to assess if, and to what degree, it influenced the effect of tDCS on food craving and eating. We hypothesized that expectation and tDCS would independently reduce food craving and amount of food consumed. We further hypothesized that expectation and tDCS would work synergistically, exerting a greater suppression of craving and eating than each alone.
2. Methods
2.1. Participants
The University of Alabama at Birmingham (UAB) Institutional Review Board for Human Subjects approved this study. Participants were N=74 consenting UAB students and employees. Participation was compensated with either a $50 check or research credits if enrolled in an Introduction to Psychology course. The sample was ethnically diverse with 28% African American, 47% White, 16% Asian/Indian, and 9% “Other” and was made up of 30 men and 44 women. Inclusion criteria were: age 18–55 (sample mean was 19.9, SD 3.4, range 18 – 41), and a measured body mass index (BMI) ≥ 25 (sample mean was 31.8, SD 5.5, range 24.8 – 46.5). The BMI criteria allowed a sample representative of a population likely to seek a novel weight-loss treatment like tDCS. Exclusion criteria were: pregnancy, breastfeeding, current enrollment in a commercial weight-loss program, uncontrolled diabetes or hypertension, allergy to any of the test food ingredients, use of prescription medications that influenced appetite, intention of starting or stopping a medication during the research period, recreational drug use, anorexia nervosa, bulimia nervosa, schizophrenia, bipolar disorder, current suicidal ideation, past or impending brain surgery, brain trauma, loss of consciousness, and implanted metal or biomedical devices.
2.2. Experimental variables
2.2.1. Groups
Participants were randomly assigned, based on scheduling order and blocked for sex, to one of four experimental groups: (1) Told Fake/Got Fake, (2) Told Fake/Got Real, (3) Told Real/Got Fake, and (4) Told Real/Got Real (see Table 1). The word, “fake” was used instead of “sham” with the participants, as described below, because it had a more common meaning.
2.2.2.. Manipulation of expectation
Research assistants (RAs) verbally informed participants two times of the known effects of tDCS to suppress craving and eating. Once electrodes were placed on the head, and immediately before tDCS was administered, the RA said, “You were randomly assigned to the group that will receive real (or fake) tDCS. Research has shown that real tDCS makes people crave less and eat less junk food.” For the sham condition, the script concluded with, “but you are receiving fake tDCS.” Prior to this point, the RA was blind to the actual stimulation condition the participant would receive. Immediately after reading the above script, the RA sat behind the participant and opened an envelope divulging which tDCS condition (real or sham) to administer. The second time the RAs gave verbal information was after the tDCS session and immediately before the eating task. A different RA, blind to the actual stimulation condition, stated, “As I mentioned earlier, real tDCS stimulation is known to decrease craving and eating of junk food. People tend to crave less and eat less junk food after real tDCS.” For the sham condition, the script concluded with, “but you received fake tDCS.” All RAs received training and practiced delivering the scripts in a uniform fashion. The study title on the consent form also conveyed that tDCS was to be tested for its effect to suppress craving and eating. This was a standard IRB procedure and, while RAs instructed the participants to read the protocol, there was no final check to ascertain that all attended to it carefully, as there was for the verbal manipulation of expectation. We have no reason to believe that the number of participants who read it more carefully than others differed by randomized assignment.
2.2.3. Manipulation of tDCS
Participants received a single session of real or sham tDCS. Real tDCS was 2mA of current for 20 minutes; sham tDCS was 2mA of current only during the first and the last minute of the 20-minute session. A TCT Research Limited (Hong Kong, China) device was used to deliver current via 4×6 cm electrodes (current density = 0.083mA/cm2) soaked with 0.9% saline solution. The anode was placed over the right dorsolateral prefrontal cortex (DLPFC) and the cathode was placed over the left DLPFC. These correspond to F4 and F3 positions of the EEG 10–20 system, respectively. The right anode/left cathode DLPFC montage, stimulation duration, and stimulation frequency, is the most common tDCS design that aimed to reduce food craving and eating in humans (Burgess, 2016 #2863;Ray, 2017 #2876;Fregni, 2008 #2696; Lapenta, 2014 #2664; Goldman, 2011 #2733; Kekic, 2014 #2680:Kekic, 2017 #2920}.
2.3. Primary measures
2.3.1. Food craving task
An electronic food photo-rating task measured amount of food craving. Participants viewed 24 images of highly palatable foods of four types (sweet, fatty protein, carbohydrate, and mixed macronutrient) on a computer screen. For each food, they were asked to rate, how much they liked each food on a scale of 1 (“hate this food”) to 5 (“love this food”), and if they would want the food if it were available to them right now on a scale of 1 (“definitely not”) to 5 (“definitely”). Any food rated a 1 or 2 for liking was removed from the want ratings before analyses to avoid floor effects since unliked foods are not likely to be craved.
2.3.2. Eating task
To measure amount of food eaten, each participant was offered a generous pre-measured amount of Double-Stuff Oreo® cookies, Lay’s® potato chips, Skittles® candy, and a small bottle of water placed on a table. We left participants alone in the room for 20 minutes after they were told to consume as much of the foods as they wished. Total kilocalories (kcals) consumed of all three foods was used in analyses. Also obtained was a baseline measure of craving and eating, described below under Procedures.
2.4. Secondary measures
2.4.1. Hunger assessment
Prior to the day of their lab visit, RAs instructed participants to arrive at the lab feeling not overly hungry or overly full. Upon arrival, RAs asked the participants if they were overly hungry or full and were rescheduled if they were. The hunger assessment also asked them to rate their current level of hunger on a scale from 1 (“I am not hungry at all”) to 10 (“I have never been hungrier”).
2.4.2. Electronic baseline surveys
A demographics survey obtained participants’ sex, age, and ethnicity. They then completed a battery of standardized psychological trait surveys which included: the Barratt Impulsiveness Scale (BIS) to measure attention, motor, and planning impulsiveness (Stanford et al., 2009), the Palatable Eating Motives Scale (PEMS) to assess frequency of eating tasty foods for reasons other than hunger (Burgess et al., 2014), the Dutch Eating Behavior Questionnaire-Restraint (DEBQ-R) to measure dieting attitudes and successful dieting (Van Strien et al., 1986), the Binge-Eating Scale (BES) to assess severity of binge-eating and binge-eating behavior risk (Gormally et al., 1982), and the Short Suggestibility Scale (SSS) to evaluate tendency to internalize and accept messages (Kotov, Bellmanand Watson, 2004).
2.5. Procedures
Each participant made two lab visits separated by at least 48 hours and scheduled as close to the same time of day as possible. During the first visit, height and weight were taken for a BMI (kg/m2), hunger was assessed, and baseline surveys were completed. Participants were then administered a baseline eating task as described above except without electrodes on the head. This provided a measure of as normal of an eating episode as possible in a laboratory setting. During the second lab visit, another BMI was obtained and they were administered the food photo craving task described above. RAs then placed the tDCS electrodes on the head, recited the expectation script, and administered stimulation. After stimulation, the participants completed the same food-craving task and then the RA escorted them to sit at the eating table. Once at the table with food in front of them, the RA read the second expectation script and left the participant to eat. An RA then debriefed the participants and asked them to confirm what tDCS condition they were told they had received. The true purpose of the study was not revealed until the end of the study with a mass email. This was to ensure the participants did not divulge the true purpose of the study to other potential participants.
2.6. Statistical analysis
SPSS was used to conduct all statistical analyses. Separate 2 × 2 univariate ANOVAs using expectation (Told Real tDCS vs. Told Fake tDCS) and tDCS (Got Real tDCS vs. Got Fake tDCS) as fixed factors were conducted to determine if there were any main effects of expectation, of tDCS, and expectation × tDCS interactions on food craving and eating. Pre-tDCS food craving ratings and kcals consumed during the first-visit eating task were covariates for the respective ANCOVAs to control for individual differences in baseline craving and eating. After the initial analyses, demographics, BMI, hunger ratings, time of day (morning vs. afternoon), and the psychological trait survey scores were entered as separate covariates/fixed factors. Lastly, partial correlations assessed the relationship between craving and eating, while controlling for pre-tDCS craving scores and baseline kcals consumed. Eta-squared determine effect sizes with 0.01, 0.06, and 0.14 as cutoffs for the small, medium, and large effect sizes, respectively (Cohen, 1988). A more conservative p value of 0.01 for significance controlled for multiple comparisons when analyzing the craving subgroups (sweet, fatty, carbohydrate, mixed, total).
3. Results
3.1. Effect of expectation and tDCS on food craving
There was a significant main effect of expectation on craving for the sweet [F (1, 69) = 9.914, p = 0.002, η2 = 0.08], carbohydrate [F (1, 69) = 9.893, p = 0.002, η2 = 0.08], and all-foods [F (1, 69) = 10.012, p = 0.002, η2 = 0.09] categories. As shown in Figure 2A, participants told they were receiving real tDCS craved less of all the food types than participants who were told they were receiving fake tDCS. In contrast, and as shown in Figure 2B, there was no main effect of tDCS on craving [F (1, 69) = 0.08, p = 0.776, η2 = 0.00].
Fig. 2.
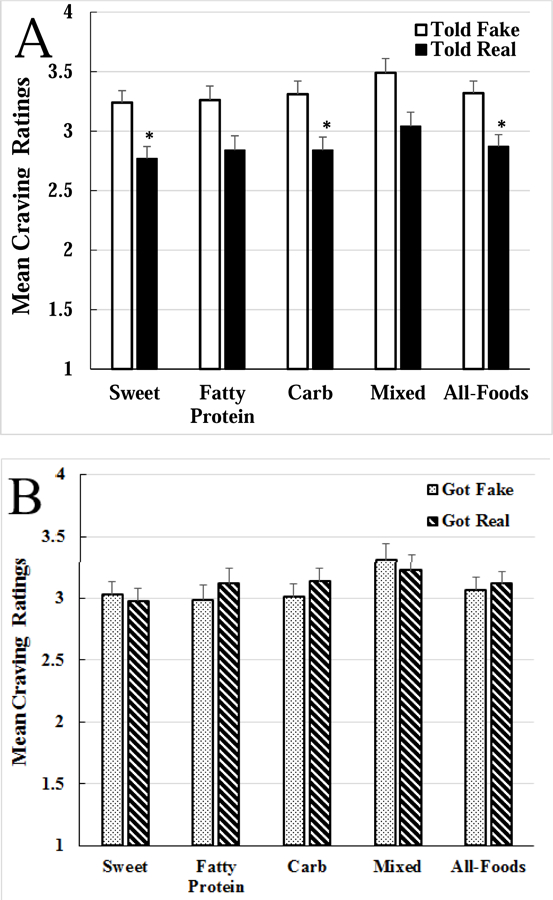
Food craving results. A. The main effect of expectation on craving. Participants who were told they were receiving real transcranial direct current stimulation (tDCS; “Told Real,” black bars) craved significantly less of the sweet, carbohydrate, and all-foods (average of the 4 food types) categories compared to participants told they were receiving sham tDCS (“Told Fake,” white bars); *p<0.01. B. No main effect of transcranial direct current stimulation (tDCS) on craving. Those who received real tDCS (“Got Real,” striped bars) did not differ from those who received sham tDCS (“Got Fake,” dotted bars) on any of the craving categories.
That is, craving ratings did not differ between those who received real tDCS and fake tDCS. There was also no expectation × tDCS interaction such that individuals told they were getting real tDCS craved less than those told they were getting fake tDCS, regardless of what condition they actually received [F (1, 69) = 0.555, p = 0.459, η2 = 0.00]. Controlling for demographics, BMI, time of day, hunger ratings, or the trait survey scores did not change the main effect of expectation over tDCS to reduce craving. Supplementary Table 1 provides the ANCOVA summary for craving of the all-foods category.
3.2. Effect of expectation and tDCS on eating
There was a significant main effect of expectation for eating [F (1, 69) = 8.425, p = 0.005, η2 = 0.09]. As shown in Figure 3A, participants told they were receiving real tDCS ate significantly less kcals (37.4%) than those told they were receiving fake tDCS. In contrast, as shown in Figure 3B, there was no main effect of tDCS on eating [F (1, 69) = 0.006, p = 0.936, η2 = 0.00]. That is, there was no difference in kcals consumed between those who received real tDCS and fake tDCS. There was also no significant expectation × tDCS interaction such that individuals told they were getting real tDCS ate less than those told they were getting fake tDCS, regardless of what condition they actually received [F (1, 69) = 2.513, p = 0.118, η2 = 0.035]. Controlling for demographics, BMI, time of day, hunger ratings, or the trait survey scores did not change the main effect of expectation over tDCS to reduce eating. See Supplementary Table 1 for the ANCOVA summary on eating.
Fig. 3.
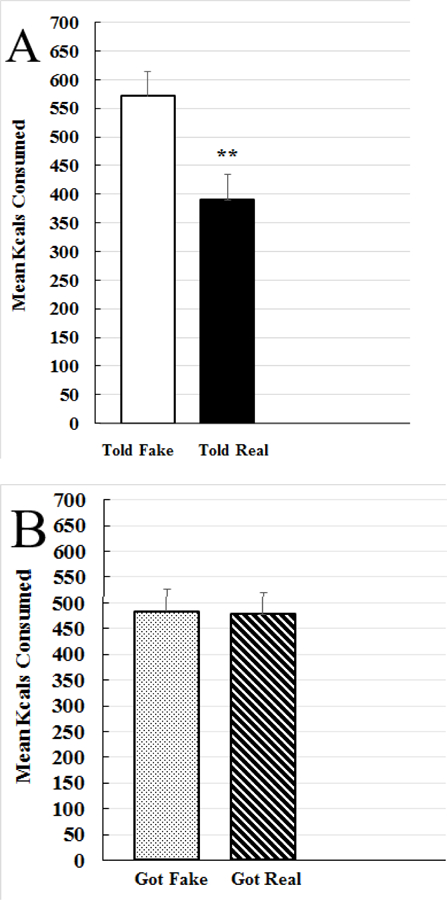
Food consumption results. A. The main effect of expectation on amount of food consumed. Participants who were told they were receiving real transcranial direct current stimulation (tDCS; “Told Real,” black bars) consumed significantly less than participants who were told they were receiving fake tDCS (“Told Fake,” white bars); *p<0.05, **p<0.01. B. No main effect of transcranial direct current stimulation (tDCS) on eating. There was no difference in kcals consumed between participants who received real tDCS (“Got Real,” striped bars) and sham tDCS (“Got Fake,” dotted bars).
3.3. Correlations between change in craving and eating
Amount of food craved was positively correlated with amount of food eaten following the tDCS session, r = 0.49, p=0.000. Controlling for baseline craving and intake strengthened the correlation to r = 0.56, p=0.000.
4. Discussion
The main purpose of this study was to determine the effect of treatment expectation on tDCS to suppress food craving and eating. Investigations seeking to validate the efficacy of a new pharmaceutical or other treatment must consider the high probability that positive expectations contribute to any ameliorative effects. Expectations are also likely to influence tDCS outcomes, but prior to this study, were not systematically investigated in food craving or eating. In this study, we controlled expectations by telling participants that tDCS had been found to reduce craving and eating and what tDCS condition they were going to receive. Therefore, all participants could be assumed to have the same beliefs about the actions of the tDCS condition they were receiving. There were three major findings: 1) expectation reduced food craving and eating, 2) tDCS did not reduce food craving or eating, and 3) expectation × tDCS did not work synergistically to reduce food craving or eating.
Expectation alone yielded a 37.4% reduction in kcals consumed, an amount much higher than that attributed to tDCS in previously published studies. Particularly surprising was the fact that participants were told only once that tDCS was expected to reduce craving of junk food and, that they learned what tDCS condition they were going to receive only seconds before the onset of current. The participants were also told only once more of the known effects of tDCS on eating, this just before the eating task, which followed stimulation. It clearly takes little time for expectations to influence behavior. These results are consistent with the greater performance of mental tasks with tDCS in healthy individuals when they are primed to expect greater vs. lower efficacy from tDCS on an executive function task (Rabipour et al., 2018).
Unlike expectation, tDCS had no independent or synergistic effect on food craving or eating. This is in contrast to some studies that reported significant reductions in craving and /or eating (Fregni et al., 2008; Goldman et al., 2011; Kekic et al., 2014; Lapenta et al., 2014), including two from our own lab. We found tDCS-suppression of craving and eating in individuals with binge-eating disorder (Burgess et al., 2016) and with frank obesity, the latter only when controlling for trait variables such as impulsiveness (Ray et al., 2017). To our knowledge, these studies did not control for potential sources of expectation outside of using a sham control, which unfortunately is a weak control method (Horvath et al., 2014). Therefore, we cannot rule out that uncontrolled expectation effects contributed to the positive tDCS outcomes in these studies. In the present study, controlled expectation may have cancelled any tDCS effects on craving and eating. In a critical review, Tremblay et al. pointed out that the inconsistent findings across tDCS studies is especially true of studies targeting frontal regions of the brain, known to affect cognitive-related functions (Tremblay et al., 2014). They propose that when it comes to studying cognitive-based behaviors, there is already a pre-existing, highly interconnected network of neurons (one need only consider all the factors that influence thinking!) such that any current from tDCS cannot compete with factors activating the pre-existing network. We propose that expectation of what a treatment can do is a potent shaper of this pre-existing neural network, possibly too potent for tDCS to alter enough to exert a behavioral change.
The effect of expectation, the lack of an effect of tDCS, and the equivocal findings across previous tDCS studies, begs the question, “Does tDCS have any effect on behavior, independent of expectation effects?” Animals cannot formulate expectation effects when initially treated with tDCS yet have been found to respond to stimulation (Das et al., 2016). However, in animals, the electrodes are also placed directly on the brain or skull so there is considerably less shunting of current compared to the amount of current shunting that occurs in humans (Bestmannand Walsh, 2017). Using human cadavers, Vöröslakos et al. found that the skull and scalp shunted approximately 75% of delivered current (Vöröslakos et al., 2018). Therefore, the effects observed in animal tDCS studies may not be translatable to humans. There was one study conducted in humans who were incapable of formulating treatment expectations that could help answer this question. Bai et al found that real tDCS targeting the DLPFC compared to sham tDCS increased global brain excitability, as measured by EEG, in individuals with minimal consciousness or in a vegetative state (Bai et al., 2017). However, they obviously could not measure changes in behavior. Therefore, there is limited evidence to explain the behavioral effects of tDCS in the absence of expectation effects in humans.
In addition, and contrary to our hypothesis, there was no interaction between expectation and tDCS. Hence, contrary to our prediction, tDCS did not augment the suppression of craving and eating produced by expectation compared to sham. We based this prediction on a study which found that tDCS augmented placebo effects (Egorova et al., 2015) and on reasoning that the actions of tDCS depend on the current state of already activated neural networks involved in cognitive processes (Tremblay et al., 2014). However, we observed no additive or synergistic effect between tDCS and expectation.
We acknowledge that the study is not without limitations. First, participants received a single session of tDCS. Repeated sessions, higher current intensity (Vöröslakos et al., 2018), and/or longer stimulation duration may be required for tDCS to affect eating and craving beyond the influence of expectation effects. However, it is worth pointing out that 1) the strength of the expectation manipulation – telling participants what to expect from tDCS, and what tDCS condition they were getting- may not be that much more powerful than expectation effects arising from reading about the effects of tDCS on a consent form and correctly guessing real from sham conditions. Both are sources of expectation that can occur in traditional tDCS studies; and 2) even positive outcomes from using these stronger ‘doses’ of tDCS conditions may be biased by expectation effects (Brunoni et al., 2014). Second, we did not ask participants if they thought they actually received the tDCS condition that they were told they received. We chose not to ask for this information for fear that they would share the deception with other potential participants, which would undermine the integrity of the study. The risk of this happening was high given that most of the participants were students in same-semester Introduction to Psychology classes. We also did not assess the participants’ degree of knowledge or familiarity with tDCS coming into the experiment so we cannot rule out influence of foreknowledge to the controlled expectation procedures. Lastly, the results may not generalize to older adults. There is evidence that they are less suggestible than younger adults (Huff and Umanath, 2018) so they may not be as influenced by the expectation manipulation used. Lastly, we must parsimoniously conclude that the effect of expectation was only tested on food craving and eating in the current study. Further studies are needed to assess the effects of tDCS and controlled expectations for other behaviors.
Despite the limitations, the study has notable strengths. This was the first study to systematically manipulate and assess the effect of expectation on appetite-related outcomes. Second, we confirmed that participants had in mind the stimulation condition they were told they were getting by having them report the condition they were told at the end of the study. Only those with correct recall were included in the study, which strengthened the integrity of the expectation manipulation. Third, although the present study used a between-subjects design, we obtained baseline measures of craving and eating and used them in the analyses to allow for statistical control of within-subject differences. Fourth, the method used for the baseline-eating task was novel. Participants ate with no electrodes on the head. Hence, despite that eating took place in the lab, this method approximated natural eating more closely than with sham tDCS providing a better control for individual differences in amount of eating. The in-lab eating test was in itself a strength compared to assessing only craving. Fifth, while many tDCS studies on appetite have used healthy controls, we recruited only individuals with overweight and obesity since ultimately this is a group that will be targeted to treat with tDCS.
It is important to note that in this study, we observed a significant correlation between craving ratings and amount of food eaten, which was not observed in our previous studies that used the same craving and eating-task methods. However, our past studies did not systematically control expectation effects (Burgess et al., 2016; Ray et al., 2017). The positive association between reduced eating and craving found here might have been due to the powerful effect of the expectation manipulation on both of these outcomes. The fact that craving does not always predict eating in tDCS studies may hint at a limitation of the efficacy of tDCS as a treatment or, at minimum, is a reason to conduct both craving and eating tests in the same study.
In conclusion, the results of the study have important implications for improving studies designed to estimate the effects of tDCS. The strong effects of communicated expectation highlight the importance of keeping verbal information about tDCS tightly controlled and uniform from participant to participant, between RAs, and between research groups. Consent forms should also use ambiguous language when describing the possible effects of tDCS. Even titles of studies on the consent form should be carefully inspected for information that may set up expectation effects. Although the outcome variables in this study were appetite related, it is very likely that the influence of expectation extends to studies using tDCS for other conditions, especially those targeting frontal regions of the brain, but further studies are needed. Expectation effects are a cognitive phenomenon; hence, conditions most susceptible to expectation confounds are likely to be those that involve cognitive function (Tremblay et al., 2014). TDCS is being heralded as a promising treatment and, while it may prove to have efficacy, its scientific validation as a treatment, and the precise understanding of its actions in the brain, is contingent on controlling expectation effects.
Supplementary Material
Fig. 1.
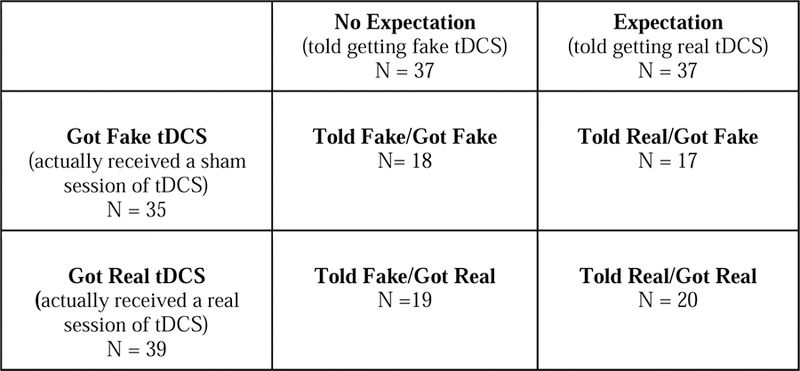
Conditions making up the four experimental groups. Participants were told they were receiving either fake (i.e., sham) or real transcranial direct current stimulation (tDCS). Within these groups, roughly half actually received fake tDCS and half received real tDCS.
Acknowledgements:
We thank Dr. Ed Cook for helping with recruitment. We also thank Ashley Hazlitt, Lauren Osborn, and Brooke Walker for their help in collecting the data.
Funding: This work was supported by the NIH National Institute of Diabetes and Digestive and Kidney Disease (P30DK0563360); National Heart, Lung, and Blood Institute through the UAB Nutrition Obesity Research Center predoctoral training program (T32HL105349) and UAB Psychology Merit Funds. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDDK, NHLBI, or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest: The authors declare no conflicts of interest.
References
- Alonso-Alonso M (2013). Translating tDCS into the field of obesity: mechanism-driven approaches. Frontiers in Human Neuroscience 7, 512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold MH, Finniss DGand Kerridge I (2014). Medicine’s inconvenient truth: the placebo and nocebo effect. Internal Medicine 44, 398–405. 10.1111/imj.12380. [DOI] [PubMed] [Google Scholar]
- Aslaksen PMand Flaten MA (2008). The roles of physiological and subjective stress in the effectiveness of a placebo on experimentally induced pain. Psychosocial Medicine 70, 811–818. 10.1097/PSY.0b013e31818105ed [DOI] [PubMed] [Google Scholar]
- Bai Y, Xiab X, Kangd J, Yang Y, Heb Jand Xiaoli L (2017). TDCS modulates cortical excitability in patients with disorders of consciousness. NeuroImage: Clinical 15, 702–709. 10.1016/j.nicl.2017.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F, Mayberg HS, Wager TD, Stohler CSand Zubieta J (2005). Neurobiological mechanisms of the placebo effect. The Journal of Neuroscience 25, 10390–10402. 10.1523/JNEUROSCI.3458-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann Sand Walsh V (2017). Transcranial electrical stimulation. Current Biology 27, R1258–R1262. 10.1016/j.cub.2017.11.001 [DOI] [PubMed] [Google Scholar]
- Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, Mourdoukoutas AP, Kronberg G, Truong D, Boggio P, Brunoni AR, Charvet L, Fregni F, Fritsch B, Gillick B, Hamilton RH, Hampstead BM, Jankord RK,A, Knotkova H, Liebetanz D, Liu A, Loo C, Nitsche MA, Reis J, Richardson JD, Rotenberg A, Turkeltaub PE and Woods AJ (2016). Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimulation 9, 641–661. 10.1016/j.brs.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoni AR, Schestatsky P, Lotufo PA, Benseñor IMand Fregni F (2014). Comparison of blinding effectiveness between sham tDCS and placebo sertraline in a 6-week major depression randomized clinical trial. Clinical Neurophysiology 125, 298–305. 10.1016/j.clinph.2013.07.020 [DOI] [PubMed] [Google Scholar]
- Burgess EE, Sylvester MD, Morse KE, Amthor FR, Mrug S, Lokken KL, Osborn MK, Soleymani Tand Boggiano MM (2016). Effects of transcranial direct current stimulation (tDCS) on binge-eating disorder. International Journal of Eating Disorders 49, 930–936. 10.1002/eat.22554 [DOI] [PubMed] [Google Scholar]
- Burgess EE, Turan B, Lokken KL, Morse Aand Boggiano MM (2014). Profiling motives behind hedonic eating. Preliminary validation of the Palatable Eating Motives Scale. Appetite 72, 66–72. 10.1016/j.appet.2013.09.016 [DOI] [PubMed] [Google Scholar]
- Cohen JE (1988). Statistical Power Analysis for the Behavioral Sciences Hillsdale, NJ, Lawrence Erlbaum Associates, Inc. [Google Scholar]
- Das S, Holland P, Frens MAand Donchin O (2016). Impact of transcranial direct current stimulation (tDCS) on neuronal functions. Frontiers in Neuroscience 10, 550 10.3389/fnins.2016.00550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorova N, Yu R, Kaur N, Vangel M, Gollub RL, Dougherty DD, Kong Jand Camprodon JA (2015). Neuromodulation of conditioned placebo/nocebo in heat pain: anodal vs cathodal transcranial direct current stimulation to the right dorsolateral prefrontal cortex. Pain 156, 1342–1347. 10.1097/j.pain.0000000000000163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers AWM, Colloca L, Blease C, Annoni M, Atlas LY, Benedetti F, Bingel U, Büchel C, Carvalho C, Colagiuri B, Crum AJ, Enck P, Gaab J, Geers AL, Howick J, Jensen K, Kirsch I, Meissner K, Napadow V, Peerdeman KJ, Raz A, Rief W, Vase L, Wager TD, Wampold BE, Weimer K, Wiech K, Kaptchuk TJ, Klinger Rand Kelley JM (2018). Implications of Placebo and Nocebo Effects for Clinical Practice: Expert Consensus. Psychother Psychosom 87, 204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine KR, Williams MS, Hoenemeyer TW, Kaptchuk TJand Dutton GR (2016). Placebo effects in obesity research. Obesity (Silver Spring) 24, 769–771. 10.1002/oby.21456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Orsati F, Pedrosa W, Fecteau S, Tome FA, Nitsche MA, Mecca T, Macedo EC, Pascual-Leone Aand Boggio PS (2008). Transcranial direct current stimulation of the prefrontal cortex modulates the desire for specific foods. Appetite 51, 34–41. 10.1016/j.appet.2007.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgii C, Goldhofer P, Meule A, Richard Aand Blechert J (2017). Food craving, food choice and consumption: The role of impulsivity and sham-controlled tDCS stimulation of the right dlPFC. Physiology and Behavior 177, 20–26. 10.1016/j.physbeh.2017.04.004 [DOI] [PubMed] [Google Scholar]
- Goldman RL, Borckardt JJ, Frohman HA, O’Neil PM, Madan A, Campbell LK, Budak Aand George MS (2011). Prefrontal cortex transcranial direct current stimulation (tDCS) temporarily reduces food cravings and increases the self-reported ability to resist food in adults with frequent food craving. Appetite 56, 741–746. 10.1016/j.appet.2011.02.013 [DOI] [PubMed] [Google Scholar]
- Gormally J, Black S, Daston Sand Rardin D (1982). The assessment of binge eating severity among obese persons. Addictive Behaviors 7, 47–55. [DOI] [PubMed] [Google Scholar]
- Horvath JC, Carter Oand Forte JD (2014). Transcranial direct current stimulation: five important issues we aren’t discussing (but probably should be). Frontiers in Systems Neuroscience 8, 2 10.3389/fnsys.2014.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff Mand Umanath S (2018). Evaluating suggestibility to additive and contradictory misinformation following explicit error detection in younger and older adults. Journal of Experimental Psychology: Applied 24, 180–195. 10.1037/xap0000138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekic M, Boysen E, Campbell Iand Schmidt U (2016). A systematic review of the clinical efficacy of transcranial direct current stimulation (tDCS) in psychiatric disorders. Journal of Psychiatric Research 74, 70–86. 10.1016/j.jpsychires.2015.12.018 [DOI] [PubMed] [Google Scholar]
- Kekic M, McClelland J, Bartholdy S, Boysen E, Musiat P, Dalton B, Tiza M, David AS, Campbell ICand Schmidt U (2017). Single-session transcranial direct current stimulation temporarily improves symptoms, mood, and self-regulatory control in bulimia nervosa: a randomised controlled trial. PLoS One 12, e0167606 10.1371/journal.pone.0167606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekic M, McClelland J, Campbell I, Nestler S, Rubia K, David ASand Schmidt U (2014). The effects of prefrontal cortex transcranial direct current stimulation (tDCS) on food craving and temporal discounting in women with frequent food cravings. Appetite 78, 55–62. 10.1016/j.appet.2014.03.010 [DOI] [PubMed] [Google Scholar]
- Kotov RI, Bellman SBand Watson DB (2004). Multidimensional Iowa Suggestibility Scale (MISS) brief manual https://medicine.stonybrookmedicine.edu/system/files/MISSBriefManual.pdf.
- Lapenta OM, Sierve KD, de Macedo EC, Fregni Fand Boggio PS (2014). Transcranial direct current stimulation modulates ERP-indexed inhibitory control and reduces food consumption. Appetite 83, 42–48. 10.1016/j.appet.2014.08.005 [DOI] [PubMed] [Google Scholar]
- Montenegro RA, Okano AH, Cunha FA, Gurgel JL, Fontes EBand Farinatti PT (2012). Prefrontal cortex transcranial direct current stimulation associated with aerobic exercise change aspects of appetite sensation in overweight adults. Appetite 58, 333–338. 10.1016/j.appet.2011.11.008 [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, Paulus W, Hummel F, Boggio PS, Fregni Fand Pascual-Leone A (2008). Transcranial direct current stimulation: State of the art 2008. Brain Stimulation 1, 206–223. 10.1016/j.brs.2008.06.004 [DOI] [PubMed] [Google Scholar]
- O’Connell NE, Cossar J, Marston L, Wand BM, Bunce D, Moseley GLand De Souza LH (2012). Rethinking clinical trials of transcranial direct current stimulation: participant and assessor blinding is inadequate at intensities of 2mA. PLoS One 7, e47514 10.1371/journal.pone.0047514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabipour S, Wu AD, Davidson PSand Iacoboni M (2018). Expectations may influence the effects of transcranial direct current stimulation. Neuropsychologia 119, 524–534. 10.1016/j.neuropsychologia.2018.09.005. [DOI] [PubMed] [Google Scholar]
- Ray MK, Sylvester MD, Osborn L, Helms J, Turan B, Burgess EEand Boggiano MM (2017). The critical role of cognitive-based trait differences in transcranial direct current stimulation (tDCS) suppression of food craving and eating in frank obesity. Appetite 116, 568–574. 10.1016/j.appet.2017.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin YI, Foerster Áand Nitsche MA (2015). Transcranial direct current stimulation (tDCS) - application in neuropsychology. Neuropsychologia 69, 154–175. 10.1016/j.neuropsychologia.2015.02.002 [DOI] [PubMed] [Google Scholar]
- Stanford MS, Mathias CW, Dougherty DM, Lake SL, Anderson NEand Patton JH (2009). Fifty years of the Barratt Impulsiveness Scale: An update and review. Personality and Individual Differences 47, 385–395. 10.1016/j.paid.2009.04.008 [DOI] [Google Scholar]
- Tremblay S, Lepage J, Latulipe-Loiselle A, Fregni F, Pascual-Leone Aand Théoret H (2014). The uncertain outcome of prefrontal tDCS. Brain Stimulation 7, 773–783. 10.1016/j.brs.2014.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Val-Laillet D, Aarts E, Weber B, Ferrari M, Quaresima V, Stoeckel L, Alonso-Alonso M, Audette M, Malbert Cand Stice E (2015). Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. Neuroimage: Clinical 8, 1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Strien T, Fritjers JER, Bergers GPAand Defares PB (1986). Dutch Eating Behaviour Questionnaire for assessment of restrained, emotional and external eating behaviour. International Journal of Eating Disorders 20, 295–315. [Google Scholar]
- Vöröslakos M, Takeuchi Y, Brinyiczki K, Zombori T, Oliva A, Fernández-Ruiz A, Kozák G, Kincses ZT, Iványi B, Buzsáki Gand Berényi A (2018). Direct effects of transcranial electric stimulation on brain circuits in rats and humans. Nature Communications 9, 483 10.1038/s41467-018-02928-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


