Abstract
Background:
Nonspecific ST-T repolarization (NST) abnormalities alter the ST-segment for reasons often unrelated to acute myocardial ischemia, which could contribute to misdiagnosis or inappropriate treatment. We sought to define the prevalence of NST patterns in patients with chest pain and evaluate how such patterns correlate with the eventual etiology of chest pain and course of hospitalization.
Methods:
This was a prospective observational study that included consecutive prehospital chest pain patients from three tertiary care hospitals in the U.S. Two independent reviewers blinded from clinical data audited the prehospital 12-lead ECG for the presence or absence of NST patterns (i.e., right or left bundle branch block, left ventricular hypertrophy with strain pattern, ventricular pacing, ventricular rhythm, or coarse atrial fibrillation). The primary outcome was 30-day major adverse cardiac events (MACE) defined as cardiac arrest, acute heart failure, post-discharge infarction, or all-cause death.
Results:
The final sample included 750 patients (age 59±17, 58% males). A total of 40 patients (5.3%) experienced 30-MACE and 131 (17.5%) had NST patterns. The presence of NST patterns was an independent multivariate predictor of 30-day MACE (9.9% vs. 4.4%, OR = 2.2 [95% CI = 1.1–4.5]. Patients with NST patterns had increased median length of stay (1.0 [IQR 0.5–3] vs. 2.0 [IQR 1–4] days, p<0.05) independent of the etiology of chest pain.
Conclusions:
One in six prehospital ECGs of patients with chest pain has NST patterns. This pattern is associated with increased length of stay and adverse cardiac outcomes, suggesting the need of preventive measures and close follow up in such patients.
Keywords: chest pain, electrocardiogram, nonspecific ST-T changes
INTRODUCTION
Chest pain is the second leading cause of emergency department (ED) visits in the United States; accounting for nearly seven million ED visits every year.1 Chest pain can be caused by a wide spectrum of etiologies, ranging from benign musculoskeletal pain to life-threatening cardiopulmonary emergencies. The initial ED evaluation of chest pain is focused on ruling out life-threatening conditions, primarily acute coronary syndrome (ACS). Such initial ED evaluation commonly involves serial ECG analysis for evaluating the presence or absence of acute myocardial ischemia.2,3 Significant ST-segment elevation or depression constitutes the universal standard for stratifying patients with suspected ACS.4
There are many morphological ECG patterns that preclude the proper interpretation of the ST-segment on the ECG.5 These confounders lead to secondary nonspecific ST-segment (NST) changes that may not be related to ischemia or the underlying cause of chest pain. These NST changes, therefore, create a diagnostic dilemma in patients with chest pain.6 There are two sources of such ST confounders: (1) abnormalities that prolong the QRS duration leading to repolarization discordance (i.e., pacing, bundle branch block, hypertrophy, ventricular rhythm),5 or (2) abnormalities that interfere with proper measurement of the ST-segment amplitude (i.e., atrial flutter or coarse atrial fibrillation). In either case, the resulting ST deviation would likely be due to reasons other than ischemia. Although these ECG confounders have been acknowledged by the most recent update to practice standards for ECG monitoring, ST-segment monitoring was not recommended in patients with such NST changes.7 These patients are not excluded from ST monitoring because they are at minimal risk for ischemia, but rather because computer measurements of ST amplitude are invalid. Unfortunately, these very same patients with NST changes who are excluded from ST monitoring are potentially at higher risk for cardiovascular complications and coronary death.8 Accordingly, we sought to define the prevalence of NST changes on the initial ECG in patients evaluated at the ED for chest pain and evaluate how these confounders correlate with the eventual etiology of chest pain and with the course of hospitalization.
Methods
Sample and Setting
This was a secondary analysis of Electrocardiographic Methods for Prompt Identification of Coronary Events (EMPIRE)9. EMPIRE is an ongoing, prospective, observational cohort study that enrolls consecutive patients with chest pain admitted to the ED through Emergency Medical Services. Inclusion criteria were age 18 or older, chief complaint of non-traumatic chest pain or equivalent (e.g., shortness of breath, palpitation, etc.), and a prehospital 12-lead ECG obtained in the field by onsite paramedics. Patients with traumatic chest pain were not enrolled. There were no interventions or modification to routine care. The parent study collected key data elements recommended by the American College of Cardiology for measuring the management and outcomes of patients with ACS including demographics, past medical history, medications, clinical presentation and course of hospitalization, laboratory tests, imaging studies, cardiac catheterization, treatments, and in-hospital complications.10 The study was approved by the University of Pittsburgh Institutional Review Board (IRB).
Defining Clinical Variables
ACS (myocardial infarction or unstable angina) ACS was defined as the presence of symptoms of ischemia with one of the following two criteria: (1) elevation of cardiac troponin (i.e., > 99th percentile), and/or (2) the presence of focal myocardial ischemia on cardiac imaging (e.g., echocardiogram, nuclear imaging, or angiographic evidence).11,12 In addition, we further audited the etiology of chest pain and categorized each patient into one of these four categories (1) ACS, (2) other cardiovascular conditions (e.g., stable coronary disease, pulmonary embolism, pericarditis, heart failure, etc.), (3) non-cardiac causes (e.g., GI-related, substance-related, etc.), and (4) undifferentiated chest pain (e.g., musculoskeletal pain, unknown, etc.).
The primary study outcome was 30-day Major Adverse Cardiac Events (MACE), defined as the occurrence of one of the followings within 30 days of initial presentation: (1) resuscitated or un-resuscitated sudden cardiac arrest, (2) development of acute heart failure with pulmonary edema, (3) post-discharge infarction requiring cardiac revascularization, or (4) all-cause death. Using UPMC billing data, an independent reviewer audited each case to ascertain the 30-day outcomes. The reviewer was provided full access to patient index and discharge records, serial ECGs, results of cardiac diagnostic tests (e.g., imaging scans and catheterization laboratory reports), and other information pertinent to the course of hospitalization (e.g., interventions, procedures, and prescribed medications).
ECG Analysis
Two independent reviewers blinded from study outcomes examined the prehospital 12-lead ECG for each patient. The initial analysis was performed using a computer-assisted algorithm, then the presence or absence of NST changes were manually determined by the two independent reviewers. All disagreements were resolved by a third reviewer. We followed the most recent American Heart Association (AHA) / Heart Rhythm Society (HRS) standardized recommendations for interpreting the electrocardiogram.5 NST repolarization pattern was defined as the presence of at least one of the following: (1) complete right or left bundle branch block, (2) wide-QRS complex ventricular rhythm, (3) ventricular pacing, (4) left ventricular hypertrophy with strain pattern (Sokolow-Lyon voltage criteria), or (5) atrial flutter or coarse atrial fibrillation.
Statistical Analysis
All analyses were performed using SPSS v. 22 with significance level of alpha < 0.05 two tailed for hypothesis testing. All variables were reviewed for normal distribution, and descriptive analyses were reported as mean ± standard deviation or median (inter-quartile range) for continuous variables and n (%) for categorical variables. Groups were compared using chi-square for discrete variables (or Mann-Whitney U test with non-normal data), and using ANOVA for continuous variables. Interactions between variables were examined using generalized linear equation models. Finally, logistic regression was used to identify the significant bivariate predictors of the primary study outcome. Predictors significant at p<0.10 in the bivariate analysis were entered in an age and sex-adjusted multivariate logistic regression model with backward selection. Odds ratios (95% CI) were computed for significant predictors.
Results
Baseline Characteristics
This study included 750 patients with a mean age of 59±17; 58% were males and 40% were black. As expected, ACS risk factors were very prevalent in this sample, including hypertension (70%), overweight or obesity (59%), current or prior smoking (59%), hyperlipidemia (35%), known coronary artery disease (33%), old myocardial infarction (28%), diabetes (26%), prior coronary revascularization (24%), and diagnosed heart failure (18%).
ECG Findings
On the prehospital ECG, most patients (86%) were in normal sinus rhythm and 9.5% were in atrial fibrillation. Overall, a total of 131 patients (17.5%) had NST changes on their presenting ECG, including left (n=19) or right (n=31) bundle branch block, left ventricular hypertrophy with strain pattern (n=37), ventricular pacing (n=26), atrial flutter or coarse atrial fibrillation (n=18), and wide-complex ventricular arrhythmia (n=12) (groups are not mutually exclusive). Table 1 compares the baseline characteristics between patients with or without NST changes. As expected, those with NST changes were older and were more likely to have a significant cardiac history, including hypertension, known coronary artery disease, and prior coronary revascularization.
Table 1:
Demographic and Clinical Characteristics of Study Sample
| Variables | All Patients (n=750) |
NST Changes | |
|---|---|---|---|
| Present (n=131) |
Absent (n=619) |
||
| Demographics | |||
| Age (years) | 59 ± 17 | 65 ± 17*** | 58 ± 16 |
| Sex (Male) | 433 (58%) | 85 (65%) | 348 (56%) |
| Race (Black) | 301 (40%) | 58 (44%) | 243 (39%) |
| ACS Risk Factors | |||
| Obesity (BMI > 30) | 285 (38%) | 41 (31%) | 244 (40%) |
| Ever Smoked | 436 (58%) | 70 (53%) | 366 (59%) |
| Hypertension | 519(70%) | 103(79%)** | 416(68%) |
| Diabetes Mellitus | 196(26%) | 41 (32%) | 155(25%) |
| Hyperlipidemia | 259 (35%) | 53(41%) | 206 (33%) |
| CAD | 248 (33%) | 65 (50%) *** | 183(30%) |
| History of Ml | 205 (28%) | 42 (32%) | 163(27%) |
| Diagnosed HF | 130(18%) | 42 (32%) *** | 88 (14%) |
| Prior PCI | 177(24%) | 41 (32%) * | 136(22%) |
| Prior CABG | 73(10%) | 30 (23%) *** | 43 (7%) |
| Clinical Presentation | |||
| Chest Pain | 645 (86%) | 110(85%) | 535 (87%) |
| Shortness of Breath | 215(29%) | 43(31%) | 172(28%) |
| Positive Initial Troponin | 95(13%) | 25(19%)* | 70(11%) |
| ACS event | 115(15%) | 24(18%) | 91 (15%) |
| PCI Done | 65 (9%) | 11 (9%) | 54 (9%) |
| CABG Done | 9(1.2%) | 2 (1.5%) | 7(1.1%) |
| LOS (median [IQR]) | 1.0(0.5–3.0) | 2.0(1.0–4.0)* | 1.0(0.5–3.0) |
| 30-Day Follow Up | |||
| 30-day readmission | 146(20%) | 29 (22%) | 117(19%) |
| 30-day MACE | 40 (5.3%) | 13(9.9%)** | 27 (4.4%) |
p < 0.05;
p < 0.01;
p < 0.001
CAD: coronary artery disease; MI: myocardial infarction; HF: heart failure; PCI: percutaneous coronary intervention; CABG: coronary artery bypass graft; ACS: acute coronary syndrome; LOS: length of stay; MACE: major adverse cardiac events.
Clinical Outcomes
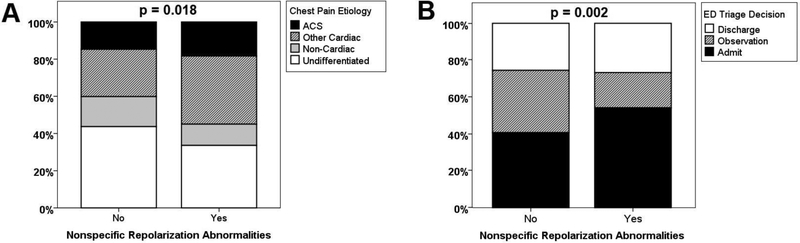
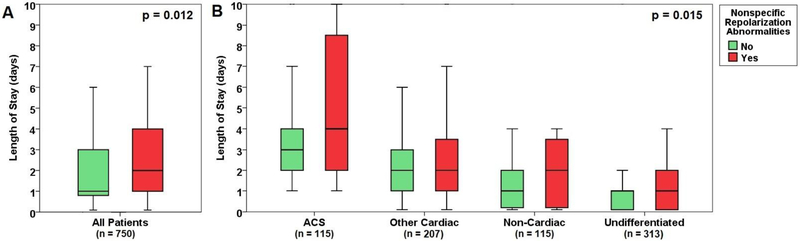
A total of 115 patients (15%) had a final diagnosis of ACS. Although patients with NST changes were more likely to have an initially positive troponin level (19% vs. 11%, p=0.015), there was no difference in the incidence of ACS events in those with or without such NST pattern (18% vs 15%, p=NS, Table 1). However, patients with NST patterns were more likely to have a cardiac cause of chest pain and less likely to have an undifferentiated chest pain (Figure 1A). As a result, patients with NST changes were more likely to be admitted to the hospital during initial ED triage (54% vs. 41%, p = 0.002, Figure 1B). Specifically, those with NST patterns had on average 1-day increased length of stay compared to those without such pattern (2.0 vs. 1.0, p = 0.012, Figure 2A). This increased length of stay remained significant after accounting for etiology of chest pain (Figure 2B).
Figure 1: Chest pain etiology and initial triage decisions in patients with or without nonspecific repolarization abnormalities.
(A) Comparison of the distribution of chest pain etiology in patients with (n=131) or without (n=619) nonspecific ST-T repolarization abnormalities. P value is based on chi-square.
(B) Comparison of the initial triage decision in the emergency department (ED) of patients with (n=131) or without (n=619) nonspecific ST-T repolarization abnormalities. P value is based on chi-square.
Figure 2: The length of stay in patients with or without nonspecific repolarization abnormalities.
Comparison of length of stay in based on the presence or absence of nonspecific ST-T repolarization abnormalities in the entire sample (A) and in each subgroup of chest pain etiologies (B). The dark bold horizontal lines indicate the median, the box sides indicate the 25% and 75% interquartile range, and the whiskers indicate the 95% CI of the standard error. The p values are based on Mann-Whitney U test.
A total of 40 patients (5.3%) experienced 30-day MACE. The rate of MACE events in those with or without NST patterns was 9.9% vs. 4.4% (p=0.011). Table 2 shows the bivariate and multivariate predictors of 30-day MACE. Non-black race, history of heart failure, a final diagnosis of ACS, and the presence of NST pattern were significant and independent predictors or 30-day MACE. Patients with NST patterns were at two-fold increased risk of 30-day MACE, independent of other baseline patient characteristics and etiology of chest pain.
Table 2:
Bivariate and Multivariate Predictors of Primary Study Outcome
| Variables | Bivariate | Multivariatea | |||
|---|---|---|---|---|---|
| p value | OR [95% CI] | p value | β | OR [95% CI] | |
| Demographics | |||||
| Age (per 10-year increment) | 0.150 | - | 0.732 | - | - |
| Male Sex | 0.719 | - | 0.351 | - | - |
| Non-Black Race | 0.010 | 2.8 [1.3–6.2] | 0.048 | 0.828 | 2.3 [1.0–5.2] |
| ACS Risk Factors | |||||
| Obesity (BMI > 30) | 0.431 | - | X | ||
| Ever Smoked | 0.791 | - | X | ||
| Hypertension | 0.983 | - | X | ||
| Diabetes Mellitus | 0.100 | 1.7 [0.9–3.4] | 0.467 | - | - |
| Hyperlipidemia | 0.508 | - | X | ||
| CAD | 0.642 | - | X | ||
| History of Ml | 0.476 | - | X | ||
| Diagnosed HF | 0.036 | 2.1 [1.1–4.3] | 0.040 | 0.791 | 2.2 [1.0–4.7] |
| Prior PCI | 0.840 | - | X | ||
| Prior CABG | 0.750 | - | X | ||
| Clinical Presentation | |||||
| ACS event | <0.001 | 6.5 [3.4–12.5] | <0.001 | 1.783 | 5.9 [3.0–11.7] |
| Presenting 12-lead ECG | |||||
| NST pattern | 0.013 | 2.4 [1.2–4.8] | 0.039 | 0.775 | 2.2 [1.1–4.5] |
CAD: coronary artery disease; MI: myocardial infarction; HF: heart failure; PCI: percutaneous coronary intervention; CABG: coronary artery bypass graft; ACS: acute coronary syndrome; NST: non-specific STT pattern on the presenting ECG; NS = not significant.
Bold indicates statistical significance
Age and sex adjusted multivariate logistic regression model with backward selection method. All variables significant at p<0.10 at the bivariate analysis were entered in the multivariate model. “x” indicates the variable was not entered in the multivariate model. Hosmer and Lemshow goodness-of-fit Chi-square =2.39, p = 0.664.
Finally, a total of 146 (20%) patients were re-hospitalized within 30 days of initial presentation. There was no difference in readmission rates between those with or without NST patterns (22% vs. 19%, p=NS, Table 1).
Discussion
To our knowledge, this is the first study to examine the prevalence and outcomes of NST patterns on the prehospital ECG in high-risk chest pain patients evaluated in the ED. In this analysis, we found that at least one in six patients with chest pain manifested NST changes on the prehospital ECG during first medical contact. Patients with such ECG patterns were more likely to be admitted to the hospital and more likely to have a cardiac-related etiology of chest pain. Additionally, they had on average 1-day increased length of stay and two times higher risk of 30-day adverse cardiac events, independent of demographic and clinical characteristics.
It is well established that NST changes are associated with adverse patient outcomes in various clinical populations. In 2014, Al-Zaiti and colleagues performed a meta-analysis on more than 250,000 patients from 31 studies that examined the prognostic value of NST changes in different disease populations, including hypertension, diabetes mellitus, post MI, post-menopausal women, the elderly, as well as the general population.13 This meta-analysis showed that NST patterns are associated with increased risk of sudden death (hazard ratio 2.6 [95% CI 1.7–3.9]) and cardiovascular death (hazard ratio 2.2 [95% CI 1.9–2.5]). Only a few studies looked specifically at patients with chest pain. Kesek and colleagues examined the presenting 12-lead ECG of 800 consecutive chest pain patients admitted to critical care at a single hospital in Sweden.14 At 6-month follow up, the authors found that the rate of all-cause death in patients with NST changes was 11% compared to <1% in those with normal QRS-T pattern. Similarly, Ho and colleagues examined the baseline 12-lead ECG of 2255 consecutive chest pain patients referred for exercise testing at a single hospital in the U.S.15 The authors found that the rate of cardiac death or nonfatal MI in patients with NST changes was 11% compared to 7% in those with normal ECG findings (mean follow up period 6.9 ± 1.5 years). Despite the lower rate of cardiac events in our data, which is probably due to the significantly shorter duration of follow-up, our results are congruent with these earlier reports. The presence of NST changes significantly increases the risk of death or nonfatal MI in high-risk patients with prehospital chest pain.
The association between NST changes and subsequent adverse cardiac events follows logical clinical reasoning. First, NST abnormalities are predictive of abnormal left ventricular function. In chest pain populations, it has been shown that 15%–28% of patients with NST have diminished left ventricular ejection fraction (i.e., <0.35), compared to only 5% in patients with normal ECG findings.16 Second, NST abnormalities have been previously linked to compromised coronary circulation in apparently healthy adults. In the general population for instance, it has been shown that 4.3%–15.1% of patients with NST developed fatal or nonfatal MI during long-term follow up, compared to only 3.8% in patients with normal ECG findings.17 Such associations between NST patterns and cardiac function / coronary circulation provide a plausible explanation for the association observed in our data between this ECG pattern and other chronic comorbidities (i.e., hypertension, heart failure, and coronary artery disease).
Our findings have several important clinical implications. During the initial ED triage of chest pain, the priority is to distinguish those in acute cardiac distress from those who are not. Whereas patients with diagnostic ECG changes (e.g., STEMI) are usually referred for urgent catheterization, all other chest pain patients, including those with normal or borderline ECG abnormalities, are routinely triaged using established clinical decision rules (e.g., risk scores). Although there is no consensus on how to triage those with NST patterns, the recent HEART score gives partial points to these patients.18 However, the fact that many of the patients with NST changes had cardiac risk factors suggest that applying clinical decision rules on these patients would duplicate the points already given to risk factors. Having a better understanding of the outcomes of these patients with NST changes from the ED perspective is necessary. As such, our data suggest that patients with NST patterns are more likely to have a cardiac-related etiology versus non-cardiac or undifferentiated chest pain (Figure 1A). Specifically, the absence of NST pattern on the presenting ECG has a moderate accuracy in ruling out the possibility of a cardiac-related etiology of chest pain (specificity = 86%, negative predictive value = 63%).
Another important clinical implication is that the presence of NST changes is associated with increased length of stay and subsequent 30-day death or non-fatal MI, after controlling for the etiology of chest pain. This suggests that the presence of NST patterns on the prehospital ECG may warrant vigorous preventive management and close follow up. More importantly, around 25% of chest pain patients admitted for telemetry have ST-segment monitoring confounders (Figure 1B). Cardiac telemetry monitoring in these patients would fail to accurately detect subsequent or transient episodes of acute myocardial ischemia.7 Lack of valid tools for monitoring ischemia in patients with NST patterns is a double-edged sword given that these patients have higher rate of adverse cardiac events. If patients with NST abnormalities are improperly placed on telemetry monitoring, ST alarms would be likely invalid, contributing to alarm fatigue, and potentially leading to unnecessary testing or treatment.19 This diagnostic dilemma might explain the increased length of stay we observed in this subgroup, which was independent from the etiology of chest pain. Healthcare providers need to be aware of the limitations of ECG monitoring in this prevalent subgroup of patients (i.e., one in four telemetry patients), and explore alternative methods of monitoring patients with ST confounders (e.g., more frequent vital signs, more cardiac biomarkers assays, etc.). As such, further research is needed to enhance existing computerized ST algorithms to account for baseline ST confounders.20
Limitations
There are a few limitations of this study. First, the sample size was relatively small and the rate of events for the primary outcome was low. A sample size of 750 with x and y response variables prevalent at 20% and 5% can detect a significant OR of 2.4 at 80% power. As such, it is possible that we were underpowered to detect significant changes in some of the predictors presented in Table 2 (type II error). Second, the EMPIRE dataset included chest pain patients who were transported to the hospital by Emergency Medical Services and may not be representative of all ED patients. Our patients may be at higher acuity level and at higher risk for adverse events compared to typical chest pain populations seen at a given ED. Third, another limitation is the possibility of loss to follow up due to the observational nature of our study. We used inpatient and outpatient electronic medical records of a large hospital network to assure complete ascertainment. Using this approach, we found that 4.4% of patients in our dataset experienced 30-day MACE, which is comparable to the rate reported in chest pain populations in the United States using Medicare billing data (i.e., 3.2–4.3%).21 Finally, time to event data were not available thereby precluding proportional hazards analysis.
CONCLUSION
Nonspecific repolarization abnormalities in the ST-T segment are prevalent in patients with prehospital chest pain. Patients with such ECG patterns are more likely to be admitted to the hospital, are more likely to have a cardiac-related etiology of chest pain, have on average 1-day increased length of stay, and have two-fold increased risk of 30-day MACE. These observations suggest an important area for further investigation so we can appropriately risk stratify and treat patients with NST patterns, both from the prehospital and ED perspective. Patients with such ECG abnormalities may require additional preventive management and close follow up. Healthcare providers need to be aware of the limitations of ECG monitoring in this high-risk subgroup of patients and should explore alternative methods for monitoring these patients during their hospital stay.
Supplementary Material
Acknowledgments
Funding:
NIH / NHLBI (R01 HL 137761)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
None
References
- 1.Rui P, K. K. National Hospital Ambulatory Medical Care Survey: Emergency Department Summary Tables. US Department of Health and Human Services, National Center for Health Statistics. 2014;Available from: http://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2014_ed_web_tables.pdf.
- 2.Fox KAA, McLean S. Nice guidance on the investigation of chest pain. Heart. 2010;96(12):903–906. [DOI] [PubMed] [Google Scholar]
- 3.Ringstrom E, Freedman J. Approach to undifferentiated chest pain in the emergency department: a review of recent medical literature and published practice guidelines. Mount Sinai Journal of Medicine: A Journal of Translational and Personalized Medicine. 2006;73(2):499–505. [PubMed] [Google Scholar]
- 4.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. European heart journal. 2012;33(20):2551–2567. [DOI] [PubMed] [Google Scholar]
- 5.Rautaharju PM, Surawicz B, Gettes LS. AHA/ACCF/HRS Recommendations for the Standardization and Interpretation of the Electrocardiogram: Part IV: The ST Segment, T and U Waves, and the QT Interval. J Am Coll Cardiol. 2009;53(11):982–991. [DOI] [PubMed] [Google Scholar]
- 6.Hayden GE, Brady WJ, Perron AD, Somers MP, Mattu A. Electrocardiographic T-wave inversion: Differential diagnosis in the chest pain patient. The American Journal of Emergency Medicine. 2002;20(3):252–262. [DOI] [PubMed] [Google Scholar]
- 7.Sandau KE, Funk M, Auerbach A, et al. Update to Practice Standards for Electrocardiographic Monitoring in Hospital Settings: A Scientific Statement From the American Heart Association. Circulation. 2017;136(19):e273–e344. [DOI] [PubMed] [Google Scholar]
- 8.Eriksson P, Wilhelmsen L, Rosengren A. Bundle-branch block in middle-aged men: risk of complications and death over 28 years The Primary Prevention Study in Göteborg, Sweden. European Heart Journal. 2005;26(21):2300–2306. [DOI] [PubMed] [Google Scholar]
- 9.Al-Zaiti SS, Martin-Gill C, Sejdic E, Alrawashdeh M, Callaway C. Rationale, development, and implementation of the Electrocardiographic Methods for the Prehospital Identification of Non-ST Elevation Myocardial Infarction Events (EMPIRE). J Electrocardiol. 2015;48(6):921–926. [DOI] [PubMed] [Google Scholar]
- 10.Cannon CP, Battler A, Brindis RG, et al. American College of Cardiology key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes: A report of the American College of Cardiology Task Force on Clinical Data Standards (Acute Coronary Syndromes Writing Committee) Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation, American College of Emergency Physicians, American Heart Association, Cardiac Society of Australia & New Zealand, National Heart Foundation of Australia, Society for Cardiac Angiography and Interventions, and the Taiwan Society of Cardiology. Journal of the American College of Cardiology. 2001;38(7):2114–2130. [DOI] [PubMed] [Google Scholar]
- 11.Kushner FG, Hand M, Smith SC, et al. 2009 Focused Updates: ACC/AHA Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction (Updating the 2004 Guideline and 2007 Focused Update) and ACC/AHA/SCAI Guidelines on Percutaneous Coronary Intervention (Updating the 2005 Guideline and 2007 Focused Update). Catheterization and Cardiovascular Interventions. 2009;74(7). [DOI] [PubMed] [Google Scholar]
- 12.Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction: Statement from ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. European Heart Journal. 2007;28(20):2525–2538. [DOI] [PubMed] [Google Scholar]
- 13.Al-Zaiti SS, Fallavollita JA, Wu YB, Tomita MR, Carey MG. Electrocardiogram-Based Predictors of Clinical Outcomes: A Meta-Analysis of the Prognostic Value of ventricular repolarization. Heart & Lung. 2014;43(6):516–526. [DOI] [PubMed] [Google Scholar]
- 14.Kesek M, Jernberg T, Lindahl B, Xue J, Englund A. Principal component analysis of the T wave in patients with chest pain and conduction disturbances. Pacing & Clinical Electrophysiology. 2004;27(10):1378–1387. [DOI] [PubMed] [Google Scholar]
- 15.Ho K-T, Miller TD, Hodge DO, Bailey KR, Gibbons RJ. Use of a Simple Clinical Score to Predict Prognosis of Patients With Normal or Mildly Abnormal Resting Electrocardiographic Findings Undergoing Evaluation for Coronary Artery Disease. Mayo Clinic proceedings Mayo Clinic. 2002;77(6):515–521. [DOI] [PubMed] [Google Scholar]
- 16.O’Keefe JH Jr., Zinsmeister AR, Gibbons RJ. Value of normal electrocardiographic findings in predicting resting left ventricular function in patients with chest pain and suspected coronary artery disease. Am J Med. 1989;86(6 Pt 1):658–662. [DOI] [PubMed] [Google Scholar]
- 17.Larsen CT, Dahlin J, Blackburn H, et al. Prevalence and prognosis of electrocardiographic left ventricular hypertrophy, ST segment depression and negative T-wave. The Copenhagen City Heart Study. European Heart Journal. 2002;23(4):315–324. [DOI] [PubMed] [Google Scholar]
- 18.Mahler SA, Riley RF, Hiestand BC, et al. The HEART Pathway Randomized Trial: Identifying Emergency Department Patients With Acute Chest Pain for Early Discharge. Circulation: Cardiovascular Quality and Outcomes. 2015;8(2):195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai Q, Mehta N, Sgarbossa EB, et al. The left bundle-branch block puzzle in the 2013 ST-elevation myocardial infarction guideline: from falsely declaring emergency to denying reperfusion in a high-risk population. Are the Sgarbossa Criteria ready for prime time? American heart journal. 2013;166(3):409–413. [DOI] [PubMed] [Google Scholar]
- 20.Xu M, Wei S, Qin X, Zhang Y, Liu C. Rule-based method for morphological classification of ST segment in ECG signals. Journal of Medical and Biological Engineering. 2015;35(6):816–823. [Google Scholar]
- 21.Cotterill PG, Deb P, Shrank WH, Pines JM. Variation in chest pain emergency department admission rates and acute myocardial infarction and death within 30 days in the Medicare population. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 2015;22(8):955–964. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.