Abstract
Rates of alcohol use disorder (AUD) have increased in women by 84% over the past ten years relative to a 35% increase in men. This substantive increase in female drinking is alarming given that women experience greater alcohol-related health consequences compared to men. Stress is strongly associated with all phases of alcohol addiction, including drinking initiation, maintenance, and relapse for both women and men, but plays an especially critical role for women. The purpose of the present narrative review is to highlight what is known about sex differences in the relationship between stress and drinking. The critical role stress reactivity and negative affect play in initiating and maintaining alcohol use in women is addressed, and the available evidence for sex differences in drinking for negative reinforcement as it relates to brain stress systems is presented. This review discusses the critical structures and neurotransmitters that may underlie sex differences in stress-related alcohol use (e.g., prefrontal cortex, amygdala, norepinephrine, corticotropin releasing factor, and dynorphin), the involvement of sex and stress in alcohol-induced neurodegeneration, and the role of ovarian hormones in stress-related drinking. Finally, the potential avenues for the development of sex-appropriate pharmacological and behavioral treatments for AUD are identified. Overall, women are generally more likely to drink to regulate negative affect and stress reactivity. Sex differences in the onset and maintenance of alcohol use begin to develop during adolescence, coinciding with exposure to early life stress. These factors continue to affect alcohol use into adulthood, when reduced responsivity to stress, increased affect-related psychiatric comorbidities and alcohol-induced neurodegeneration contribute to chronic and problematic alcohol use, particularly for women. However, current research is limited regarding the examination of sex in the initiation and maintenance of alcohol use. Probing brain stress systems and associated brain regions is an important future direction for developing sex-appropriate treatments to address the role of stress in AUD.
Keywords: Alcohol use disorder, Stress, Sex differences, Female, Brain stress systems
Highlights
-
•
Rates of AUD have increased in women by 84% in the past 10 years.
-
•
The consideration of sex in addiction research has historically been inadequate.
-
•
Stress plays a critical role in initiating and maintaining alcohol use in women.
-
•
Alterations in stress pathophysiology may drive continued alcohol use in women.
-
•
Treatments targeted at sex-dependent factors which maintain drinking is critical.
1. Introduction
Currently in the U.S. population, 13% or 30 million adults have an alcohol use disorder (AUD) (Grant et al., 2017), which is primarily characterized by a loss of control over drinking, preoccupation with drinking, continued use despite negative consequences (e.g., health, job function, interpersonal relationships), tolerance, and withdrawal. Alcohol consumption is the third leading cause of preventable morbidity and mortality in the U.S. (Mokdad et al., 2004), and losses to the economy exceed $249 billion per year (Sacks et al., 2015). Alcohol causes the most harm to self and others when considered in the context of all other substances (including tobacco and opioids) (Nutt et al., 2010) and the U.S. exceeds global per capita alcohol consumption by 50%, potentially impacting the prevalence of AUD in the U.S. (Shield et al., 2013; Manthey et al., 2017).
Historically, rates of AUD have been greater in men when compared to women, however, this gap is closing (White et al., 2015). Over the past ten years, rates of AUDs have increased in women by 84%, relative to a 35% increase in men (Grant et al., 2017), such that currently the weighted rate of AUDs among adults in the United States is 9.0% in women and 16.7% in men (Grant et al., 2017). Similarly, there have been substantial increases in the prevalence of alcohol use and binge drinking in women, but not men, assessed over the past 16 years in several national data sources (Grucza et al., 2018). Factors associated with increasing rates of AUD in women include drinking to regulate negative affect, the experience of negative emotions or being in a negative emotional state, and stress, as well as greater sensitivity to alcohol-induced neurodegeneration (Agabio et al., 2016a; Nolen-Hoeksema and Hilt, 2006; Koob and White, 2017; Sharrett-Field et al., 2013). While stress and negative affect are strongly associated with all phases of alcohol addiction, including drinking initiation, maintenance, and relapse, for both women and men, they play an especially critical role for women (Koob and White, 2017; Koob, 2009; Verplaetse et al., 2018).
This substantive increase in female alcohol consumption is especially alarming as women have exacerbated alcohol-related health consequences when compared to men. While drinking is strongly associated with significant health risks in both sexes, women with AUDs have a higher risk of developing alcohol-related liver injury, including liver cirrhosis, and hepatitis compared to men (National Institute of Alcohol Abuse and Alcoholism, 2017; Agabio et al., 2017; Szabo, 2018), even though women may consume less alcohol and have a shorter duration of use (Rehm et al., 2010). Alcohol use increases the risk of cancers of the mouth, esophagus, pharynx, larynx, liver, and breast, and women are at greater risk of alcohol-related cancer than men (National Institute of Alcohol Abuse and Alcoholism, 2017; National Cancer Institute, 2013). Additionally, women are more vulnerable to alcohol-related cardiovascular conditions than men (Agabio et al., 2016a; National Institute of Alcohol Abuse and Alcoholism, 2017). While there is some evidence that low to moderate alcohol consumption protects against ischemic stroke, among women consuming three or more drinks per day there is an increased risk of stroke relative to men (Patra et al., 2010).
In addition to experiencing greater relative risk than men for the most common and serious alcohol-related diseases, women also face other sex-dependent health consequences from drinking. Excessive alcohol consumption is associated with menstrual irregularity and altered hormone levels during menstrual cycle phases (Martel et al., 2017; Mary Ann Emmanuel and Nicholas, 2003), including increases in endogenous estradiol (Erol et al., 2017) and temporary increases in testosterone levels (Mary Ann Emmanuel and Nicholas, 2003; Erol et al., 2017). These hormonal increases have been associated with incidence of female-related health problems, including cancers (e.g., breast) and poor reproductive health. There is a clear linear relationship between increased alcohol consumption and incidence of breast cancer in women (Chen et al., 2011), with a 22% excess risk that rises about 10% for each 10 g of alcohol consumed per day (Key et al., 2006). Pregnancy- and perinatal-related consequences of drinking include infertility and spontaneous abortion (Andersen et al., 2012), as well as significant cognitive, psychological, and behavioral problems in offspring, including fetal alcohol syndrome (National Institute of Alcohol Abuse and Alcoholism, 2017). Alcohol use is also associated with increased risk of physical and sexual assaults among women, with approximately one half of cases involving alcohol consumption by either the victim, perpetrator, or both (Abbey et al., 2001). Despite the significant increase in alcohol consumption among women and the associated health risks, few attempts have been made to develop treatments to address sex-dependent factors associated with problematic alcohol consumption in women versus men. This highlights the urgent need to include sex as a biological variable (SABV) in future alcohol-related research.
Sex differences exist at all levels of biological organization including brain anatomy, neurochemistry, connectivity, and function (Cosgrove et al., 2007a; Lind et al., 2017; Choleris et al., 2018; Miller et al., 2017), with relevance for understanding sex-based differences in addiction (Sharrett-Field et al., 2013). However, the consideration of sex in preclinical and human research has historically been inadequate. For example, an examination of sex bias in preclinical biomedical research found male bias in eight fields, with neuroscience demonstrating the largest bias (Beery and Zucker, 2011). Regarding addiction, male participants are included at higher rates than females, and many studies that include both sexes do not analyze results by sex. In an effort to combat male bias, the National Institutes of Health (NIH) required the inclusion of women in clinical research (1993) (Clayton and Collins, 2014) and, more recently, SABV in basic and preclinical research (Guizzetti et al., 2016). The inclusion of SABV is a key element to increase scientific rigor and reproducible findings, in order to guide clinical studies and practice. As advances are made in the biomedical field to include SABV in preclinical and clinical research, it is critical to consider the role of sex in mechanisms underlying disease, including AUD (Guizzetti et al., 2016), and translate these findings towards effective treatment for both women and men.
The purpose of the present narrative review is to highlight what is known about sex differences in the relationship between stress (see Table 1 for a list of stress paradigms in preclinical and clinical research included in this review) and drinking. An exhaustive search was conducted to identify relevant manuscripts related to sex, stress, and alcohol use in the preclinical and human literature (see supplementary materials for search details). Specifically, the critical role stress reactivity and negative affect play in initiating and maintaining alcohol use in women will be addressed, including how sex differences emerge in neuroadaptations of stress pathophysiology with continued alcohol use. Furthermore, this review aims to highlight the state of the field, identify gaps in our knowledge, and suggest ways to translate this knowledge to effectively target sex-dependent mechanisms to improve AUD treatment.
Table 1.
Stress paradigms in preclinical and clinical research included in the present review.
| Paradigm | Indication | Reference in manuscript |
|---|---|---|
| Preclinical | ||
| Learned helplessness | Depression | Vengeliene et al., 2005 |
| SDPSa | Depression | Riga et al., 2014 |
| Predator odor | Psychological stress/PTSDb | Cozzoli et al., 2014 |
| Tail suspension | Psychological stress/acute stress | Cozzoli et al., 2014 |
| Restraint | Physical stress/acute stress | Cozzoli et al., 2014 |
| Foot shock | Physical stress/acute stress | Cozzoli et al., 2014; Le et al., 2005; Zorrilla et al., 2013 |
| Tail pinch | Physical stress/acute stress | Cozzoli et al., 2014 |
| Noise stress | Mild stress exposure/acute stress | Arnsten and Goldman-Rakic, 1998 |
| Yohimbinee |
Pharmacologic stress/acute stress |
Bertholomey et al., 2016; Le et al., 2005; Zorrilla et al., 2013 |
| Clinical | ||
| CRFc/cortisol administration | Increase pituitary-adrenal axis response | Heim et al., 2001 |
| Citalopram stimulation test | Assess neuroendocrine reaction | Anthenelli et al., 2018 |
| Combined dexamethasone/CRFc test | Assess glucocorticoid feedback loop | Schwandt et al., 2016; Anthenelli et al., 2018 |
| Cosyntropin | Assess adrenocortical response | Adinoff et al., 2010 |
| Combined Cosyntropin/dexamethasone | Assess neuroendocrine feedback | Adinoff et al., 2010 |
| Trier Social Stress Test | Psychological stress | Kwako et al., 2015 |
| Imagery Scripts | Psychological stress | Chaplin et al., 2008; McKee et al., 2011; Seo et al., 2011; Kwako et al., 2015 |
| Emotionally arousing films | Arouse negative emotions | Cahill et al., 2001 |
| IAPSd | Induce highly charged emotions | Garavan et al., 2001 |
| Public speaking challenge | Psychological stress | Bernardy, 1995; Lovallo et al., 2000; Sorocco et al., 2006 |
| Mental arithmetic task | Psychological stress | Bernardy et al., 1996; Bernardy, 1995; Errico et al., 2002; Sorocco et al., 2006 |
SDPS = social defeat-induced persistent stress.
PTSD = post-traumatic stress disorder.
CRF = corticotropin-releasing factor.
IAPS = International Affective Picture System.
= also used in clinical paradigms; self-report questionnaires/measures are not described above.
2. Do stress and negative affect drive initiation and maintenance of alcohol use for women?
2.1. Drinking for negative affect regulation in adolescence
Negative affect is strongly associated with the onset of drinking and subsequent dependence among adolescents in both preclinical models of anxiety-like behavior and clinical depressive and anxiety symptomatology (Johannessen et al., 2017; Hammerslag and Gulley, 2016). For example, a clinical study of older adolescents (ages 15–18 years old) demonstrated that the severity of depressive symptoms, as measured by Hopkins Symptom Checklist-25, was associated with earlier onset of alcohol use, frequent alcohol consumption, and intoxication in both boys and girls (Johannessen et al., 2017). However, the association between early drinking onset and anxiety/depressive symptoms was stronger in girls (Johannessen et al., 2017). Initial depressive symptoms during early adolescence in girls were also associated with increased problematic alcohol use later in adolescence (Edwards et al., 2014). This is consistent with findings in the preclinical literature showing that female adolescent rodents with higher social anxiety-like behavior exhibit increased alcohol drinking compared to male adolescent rodents (Varlinskaya and Spear, 2015). During mid-adolescence, there is a decline in sensation-seeking as well as more control of impulsivity among girls when compared to adolescent boys (Shulman et al., 2015). Overall, given the association between negative affect and onset of alcohol use, these differences in developmental trajectories between boys and girls may contribute to adolescent girls being more apt to drink to cope with negative affect.
2.2. Role of early life stress and childhood maltreatment
Both preclinical and clinical research have demonstrated that exposure to early life stress has long-term effects on stress reactivity and alcohol use (Bunea et al., 2017; Stephens and Wand, 2012; Heim and Nemeroff, 2001). Repeated exposure to early life stressors, prior to 17 years of age, is associated with an increased risk for subsequent problematic alcohol use among young adults (18–25 years of age) (Shin et al., 2018). Recent findings also suggest that women endorsing a history of maltreatment before age 18 may display more vulnerability to negative alcohol outcomes, including earlier drinking onset and increased rates of AUDs (Evans et al., 2018; Fenton et al., 2013). Specifically, sexual abuse, emotional neglect, and emotional abuse were associated with increased rates of AUDs in women compared to men (Fenton et al., 2013; Sartor et al., 2018).
Of note, data from a national survey of cigarette smokers suggest that approximately 41% of women and 49% of men endorse physical or emotional maltreatment prior to age 18, while approximately 30% of women and 18% of men endorse serious psychological distress as children (Smith et al., 2015). Additional epidemiological data demonstrated that 10.8% of women and 6.6% of men who endorse maltreatment during childhood later met criteria for a lifetime alcohol dependence diagnosis (Oberleitner et al., 2015). This suggests that while base rates should be considered when investigating the underlying effects of childhood maltreatment on drinking, women have unique mechanisms contributing to higher rates of subsequent alcohol dependence. Preclinical research also supports the association between early life stress and subsequent alcohol use; corticosterone exposure during adolescence (aged 27–28 days) increased alcohol drinking and seeking in female rodents to a greater degree than males (Bertholomey et al., 2016), suggesting an effect of early stress on alcohol-motivated behavior.
2.3. Sex differences in brain morphology in adolescence
Early life stress is associated with long-lasting alterations in the mesolimbic dopamine (DA) pathway, changes in neurotransmitter systems implicated in the stress response (e.g., norepinephrine [NE], corticotropin-releasing factor [CRF]), and changes in brain morphometry, including decreased volume in the corpus callosum, anterior cingulate cortex and amygdala, as well as reduction in volume and synaptic density in the hippocampus (for review see (Enoch, 2011; Gonzalez, 2013)). It has been postulated that the strong relationship between negative affect and the initiation of alcohol use in adolescent girls (Edwards et al., 2014) may be related to the effect of early life stress on corticolimbic function, which is less responsive to alcohol in women, and thus increases vulnerability for alcohol use and misuse (Koob and White, 2017).
There is emerging evidence that the brains of women and men further differentiate in adolescence. In youth, girls tend to display more interhemispheric communication than boys, possibly facilitating connectivity between subcortical areas related to analytical and intuitive processing, whereas boys display more intrahemispheric communication than girls (Koob and White, 2017; Ingalhalikar et al., 2014). While the impact of these differences on behavior is not yet clear, it is likely that these differences in corticolimbic function during adolescence contribute to the observation that girls are more likely to drink to regulate negative affect. These differences in brain morphology also continue to emerge in adulthood, such that adult women have more gray matter in the medial prefrontal cortex (PFC), an area important for regulating executive function, while males have more gray matter in the anterior cingulate cortex, an area involved in hedonic and impulsive activity (Koob and White, 2017; Ruigrok et al., 2014). It is likely that these sex differences in human brain structure alter brain function leading to sex differences in the cycle of AUD, including maintenance and relapse (Koob and Volkow, 2016).
2.4. Relationship between drinking and negative affect into adulthood
The relationship between stress, negative affect and alcohol use continues into adulthood, with particularly salient consequences for women (Brady and Sonne, 1999). For example, women endorsing two or more past year stressful life events were 4 times more likely to have a new onset AUD, whereas men with two or more past year stressful life events were 2.5 times more likely to have a new onset AUD (Verplaetse et al., 2018). Additionally, women are more likely to relapse in response to stress (Becker and Koob, 2016), whereas acute stress has been generally shown to have minimal impact on subjective responses to alcohol among men (Childs et al., 2011). Furthermore, women have sensitized responses to stress during periods of alcohol withdrawal (Sharrett-Field et al., 2013; Becker and Koob, 2016). These findings are consistent with the preclinical literature demonstrating that female rodents consume more alcohol in an animal model of depression (Vengeliene et al., 2005) and are more reactive to stress-induced relapse (Bertholomey et al., 2016).
Psychiatric comorbidities such as depression, anxiety disorders, and posttraumatic stress disorder (PTSD) are strongly associated with problematic drinking, and to a higher degree in women compared to men. There is a stronger relationship between negative affect and alcohol-related measures, including alcohol consumption, craving, binge drinking, problematic use, and dependence in women than men (Boykoff et al., 2010; Kendler et al., 2015; Choi and DiNitto, 2011; Anker et al., 2017, 2018). For example, data from NESARC-III suggests there are higher rates of co-morbidity between AUD and major depression in women than in men (24% vs. 13%; unpublished data). Social drinking women report higher levels of sadness and anxiety following stress-induction as compared to men, and women with alcohol dependence report higher levels of depression and anxiety than their male counterparts, indicating that women are particularly vulnerable to emotional stress (Chaplin et al., 2008; King et al., 2003). This has been shown to affect alcohol consumption, as women diagnosed with alcohol dependence report a higher frequency of heavy alcohol consumption during negative emotional states (Abulseoud et al., 2013). Additionally, rumination is a prospective predictor of alcohol-related problems among women but not men (Nolen-Hoeksema and Harrell, 2002). When compared to men, women report drinking more in response to unpleasant emotions and interpersonal conflict, and this relationship is mediated by depressive symptom severity (Lau-Barraco et al., 2009).
Unsurprisingly, the relationship between stress, negative affect, and alcohol use is also observed when exploring PTSD symptomatology and comorbid alcohol use (PTSD-AUD). Women are more likely than their male counterparts to be diagnosed with PTSD-AUD (14% vs. 6%) and women are also more likely to endorse that PTSD preceded AUD (Sonne Susan et al., 2010). Women also endorse greater intensity and frequency of avoiding trauma-related cognitions/feelings and social impairment compared to males, and thus may use alcohol and drugs to alleviate PTSD-related negative affect (Sonne Susan et al., 2010; Hien et al., 2009). Overall, both women and men with PTSD-AUD report drinking to cope; however, men display greater drinking for enhancement of positive emotions, while alcohol consumption is related to drinking to cope among women (Lehavot et al., 2014). Preclinical research supports such conclusions as female rodents are more likely to increase alcohol intake in an animal model of PTSD compared to males (Cozzoli et al., 2014).
As reviewed in the prior section, sex differences regarding alcohol onset and maintenance begin to emerge in early adolescence, with exposure to early life stress, developmental changes and emerging differences in brain morphology underlying the role of stress and negative affect in alcohol use, particularly for women. The relationship between stress, negative affect, and alcohol use continues into adulthood with increased vulnerability to alcohol use related to negative emotional states and past year stress and increased prevalence of psychiatric comorbidities related to negative affect observed in women.
3. Are there sex differences in neuroadaptations related to stress pathophysiology as a consequence of continued drinking?
3.1. Overview of stress-induced pathophysiology and the effects of alcohol
The interactions between pathophysiology related to stress and neuroadaptations induced as a result of AUD are complex and involve several brain and neurotransmitter systems. Stress-related changes in reward circuitry (e.g., mesocorticolimbic dopaminergic system) likely sensitize individuals to the reinforcing effects of substances of abuse (McKee et al., 2011). Stress co-activates this reward circuitry simultaneously with brain stress systems, such as the hypothalamic-pituitary-adrenal (HPA) axis, and induces signaling through many neurotransmitters, including NE, CRF and dynorphin. Activation of these pathways likely enhances the effects of drugs of abuse on mood, craving and physiological reactivity, thus contributing to stress-related substance use (McKee et al., 2011).
While a detailed review of stress pathophysiology and the effects of substance use on these systems is outside the scope of this review, the following sections focus on particular brain structures and neurotransmitters that may underlie the interactions between sex, stress, and alcohol. Specifically, the PFC, amygdala, and hippocampus as well as NE, CRF, dynorphin, and gamma-Aminobutyric acid (GABA) are identified as important structures and neurotransmitters implicated in stress-related alcohol use (see Table 2 for a summary of findings on brain structures and neuromodulators underlying sex differences in stress-related alcohol use). For a comprehensive review of the neurobiology underlying the relationship between stress pathophysiology and addiction, see (Koob and Volkow, 2010, 2016; Kwako and Koob, 2017).
Table 2.
Summary of the literature on brain structures and neuromodulators hypothesized to underlie sex differences in stress-related alcohol use.
Legend: ? = unknown, not been studied; ✓ = studied with positive findings; mixed = mixed findings.
PFC = prefrontal cortex.
CRF = corticotropin-releasing factor.
HPA = hypothalamic-pituitary-adrenal.
GABA = gamma-Aminobutyric acid.
Correspondence of findings between preclinical and clinical.
3.2. Brain structures
3.2.1. PFC
The PFC-amygdala axis is critical for the processing of reward, inhibitory control, stress reactivity, and emotion regulation. Neuroimaging studies demonstrate that alcohol and stress induce alterations in PFC activity in both men and women (Seo et al., 2011). For example, alcohol-related stimuli activated the medial PFC in abstinent individuals with AUD compared to healthy controls, and PFC activation was enhanced in those who subsequently relapsed to alcohol use (Grüsser et al., 2004). Alcohol cues can also activate the dorsolateral PFC (dlPFC) in non-treatment-seeking individuals with AUD compared to social drinkers (George et al., 2001). Regarding stress, HPA axis and autonomic responses to stress are enhanced following lesions to the ventromedial PFC (Sinha, 2008). The release of catecholamines, such as NE and DA, following stress significantly impairs PFC-dependent cognitive function (Arnsten and Goldman-Rakic, 1998), whereas high levels of NE and DA in the amygdala and NAc strengthen habitual responding (Arnsten, 2000).
Regarding sex differences, female rodents are more reactive to stress-induced PFC dysfunction than males, an effect mediated by high levels of estrogen (Shansky et al., 2004); thus, may account for the higher vulnerability to anxiety disorders in women. However, research on region-specific sex differences indicates that healthy social-drinking men show greater stress-related neural activation in the medial PFC compared to women (Seo et al., 2011; Goldstein et al., 2010). To our knowledge, there has not been any work examining sex differences in stress-induced PFC dysfunction as it relates to AUD. However, work by our group suggests that women smokers with smaller or blunted amphetamine-induced DA changes in the dlPFC were more likely to relapse to smoking following stress but not male smokers (Zakiniaeiz et al., 2017). This finding suggests that stress may compromise the ability of the PFC to regulate inhibitory control, which in turn may be related to stress-induced drug use, particularly in women. As such, this work should be extended to examine sex differences in the PFC in individuals with AUD, especially considering recent evidence demonstrating sex differences in striatal cue-reactivity in problem drinkers; men showed stronger activation in response to alcohol cues than women in the striatum (Kaag et al., 2018).
3.2.2. Amygdala
The amygdala is a critical structure related to stress, arousal, and negative reinforcement. The bed nucleus of the stria terminalis (BNST; also referred to as the extended amygdala) is thought to comprise the brain stress systems, including the NE and CRF systems, that generate the negative affective states leading to compulsive drug-seeking (e.g., negative reinforcement) (Koob, 2009). Negative affective stimuli increase amygdala activity partially through release of NE, leading to heightened arousal and attention, fear memory formation, and facilitated motor responses. NE release in response to stressors subsequently stimulates CRF release in the amygdala. Data from our group has demonstrated that reductions in NE signaling through alpha2-adrenergic receptors in the amygdala increases resilience to social stress, while decreasing anxiety- and depressive-like behaviors in mice (Mineur et al., 2018). CRF1 receptors are upregulated in the extended amygdala following a history of alcohol dependence (Kwako et al., 2015), and CRF1 antagonists administered directly into the amygdala reverse alcohol withdrawal-induced anxiety-like behavior in rodents (Koob, 2009). Alcohol exposure enhances transmission of the inhibitory neurotransmitter GABA in the central amygdala of alcohol-dependent rodents, while increasing anxiety-like behavior and withdrawal-induced alcohol self-administration (Clemens and Vendruscolo, 2008); although, no data on sex differences in GABA signaling have been reported. Overall, increased weekly alcohol consumption has been associated with decreased right amygdala-orbital frontal cortex connectivity, as well as sensation-seeking; however no sex differences in amygdala functioning were explored (Crane et al., 2018).
Regarding sex differences in the amygdala, imaging studies have demonstrated that men have larger amygdala volume (Cosgrove et al., 2007b), men have larger amygdala activation in response to stress compared to women (Seo et al., 2011), and there are sex-related differences in left and right amygdala activation in men and women recalling memories of negative emotional stimuli (Cahill et al., 2001). Thus, it has been hypothesized that women may drink more to achieve a desired reduction in negative affect or anxiety (Koob and White, 2017). However, it should be noted that other studies suggest that men and women both show similar activation of the amygdala following negative stimuli (Garavan et al., 2001). Furthermore, excitatory signaling in the amygdala may be suppressed by alcohol to a greater degree in male rodents compared to females (Logrip et al., 2017), warranting additional research of sex differences regarding stress in the amygdala.
3.2.3. Hippocampus
The hippocampus is a key brain structure in the limbic system, which plays a key role in the formation of memories. Women may experience exacerbated cognitive deficits associated with drinking compared to men. Hippocampal damage after a binge alcohol paradigm is associated with spatial learning deficits in female but not male rodents (Maynard et al., 2018), and clinical studies demonstrate that women either with AUD or following alcohol administration have poorer performance on measures of working memory than men (Flannery et al., 2007; Liu et al., 2010). Women who are heavy drinkers have greater impaired inhibition (Weafer et al., 2015; Smith et al., 2016) and consistently perform more poorly on tasks involving spatial planning, problem solving and cognitive flexibility (Flannery et al., 2007; Liu et al., 2010) when compared to men. Neuroimaging studies identify effects of AUD on activation in regions associated with spatial working memory in both men and women, but women with AUD differed from controls to a greater degree than men (Nixon et al., 2014). However, it is important to note that sex-dependent findings on the effects of AUD on cognitive function remain mixed (Hoffman et al., 2015; Van den Berg et al., 2017) and warrant further investigation, especially because cognitive impairments have been linked to poorer treatment outcomes across most substance using-populations (Sofuoglu et al., 2013).
3.3. Neurotransmitters & peptides
3.3.1. Norepinephrine
Alcohol activates the noradrenergic system in both animals and humans (Koob, 2009; McDougle et al., 1995; Weinshenker and Schroeder, 2007), and the noradrenergic system, in turn, plays a role in alcohol-related arousal, reinforcement, withdrawal, and stress reactivity (Koob, 2009). Elevated plasma levels of epinephrine and NE have been documented in recently abstinent alcoholics (Patkar et al., 2004), and noradrenergic agents have been used to treat sympathetic hyperactivity during alcohol withdrawal (Muzyk et al., 2011). Alcohol administration in rodents upregulates c-fos signaling, a marker of neuronal activity, in the locus coeruleus (LC), the nucleus containing noradrenergic cell bodies that projects to forebrain structures, including the amygdala (Weinshenker and Schroeder, 2007). Reductions in NE release occur by stimulation of inhibitory presynaptic alpha2 noradrenergic receptors or by blocking alpha1 or beta noradrenergic receptors.
Preclinical evidence suggests that increased NE signaling enhances stress-induced reinstatement to alcohol following extinction, whereas reduced noradrenergic activity, by blocking alpha1, alpha2a, or beta receptors, attenuates alcohol self-administration and stress-induced reinstatement to alcohol-seeking (Haass-Koffler et al., 2018). For example, the noradrenergic alpha-2 agonist guanfacine has been shown to reverse relapse vulnerability to alcohol-seeking in rats after social defeat-induced persistent stress (SDPS) (Riga et al., 2014), an animal model of depression. Further, yohimbine, an alpha2-antagonist and pharmacologic stressor that increases noradrenergic activity, can reinstate alcohol-seeking in rodents (Le et al., 2005), and female rodents may be more sensitive to yohimbine-induced reinstatement to alcohol-seeking than males (Bertholomey et al., 2016). Indeed, electrophysiological studies confirm that female rodents have greater LC activation following hypotensive stress (Curtis et al., 2006), an effect that may be mediated by CRF. Probing the noradrenergic system with agents that perturb or attenuate noradrenergic activity to evaluate sex differences may help elucidate mechanisms underlying stress-precipitated drinking, but generally sex differences in the effect of NE on stress-related drinking have not been thoroughly examined.
In humans, noradrenergic agents have demonstrated efficacy in reducing drinking days per week, drinks per week, and stress- or cue-induced alcohol craving in individuals with AUD (Haass-Koffler et al., 2018); although, clinical trials of prazosin, an alpha1-adrenergic antagonist, for AUD with comorbid PTSD have produced mixed results (Haass-Koffler et al., 2018). To our knowledge, no studies have yet examined sex differences in treatment response for medications targeting the noradrenergic system for AUD in humans. Only two studies have evaluated sex differences in the effect of guanfacine on smoking and cocaine use outcomes. In daily cigarette smokers, our group has found that guanfacine preferentially reduced smoking lapse, cigarettes smoked, and tobacco craving following stress in women but not men (McKee, 2013). Similarly, guanfacine decreased cocaine craving, alcohol craving, anxiety, and negative emotion following stress and drug imagery in cocaine-dependent women but not men (Fox et al., 2014). The effect of guanfacine on stress-related drug self-administration and craving may be related to its ability to improve PFC connectivity during periods of stress (Arnsten and Jin, 2012). Further, the sex-dependent differences in treatment response to guanfacine may be related to the ability of estrogen to regulate alpha2 receptors (Millan, 2003) and facilitate the synthesis of NE in the LC in female vs. male rodents (Thanky et al., 2002). Overall, research is lacking in the examination of sex differences in noradrenergic mechanisms underlying stress-related alcohol use.
3.3.2. CRF
CRF regulates HPA axis reactivity, is a primary mediator of the stress response, and is hypothesized to play a key role in stress-related drug use, drug withdrawal, and relapse to drug-taking (for review see Becker (2012)). CRF is a 41-amino acid polypeptide, and CRF-containing neurons are located throughout the brain, and particularly in regions important to stress pathophysiology (e.g., amygdala). CRF signals through two receptor subtypes, CRF1 and CRF2; however, the CRF1 receptor has been more extensively studied in addiction (Koob, 2010). It is known that chronic alcohol exposure dysregulates CRF neurotransmission (Becker, 2012). CRF administration reinstates alcohol-seeking in rodents (Le et al., 2002) and CRF1 receptor knockout mice exhibit reduced alcohol drinking (Kaur et al., 2012). CRF1 antagonists administered systemically or directly into the ventricles can reduce alcohol self-administration, attenuate yohimbine- and footshock-induced reinstatement of alcohol-seeking, and block withdrawal-induced anxiety-like behavior in alcohol-dependent animals (Zorrilla et al., 2013). However, the translation of these findings to humans is equivocal (Kwako et al., 2015), perhaps because sex differences have not been taken into account fully.
There are significant gaps in the literature regarding sex differences in CRF-mediated regulation of alcohol use in response to stress. Preclinical research has demonstrated that CRF-containing neurons are less able to adapt to high levels of CRF and LC sensitivity to CRF is greater in female vs. male rodents, suggesting that CRF-related dysfunction may contribute to the greater vulnerability to stress-related psychiatric disorders in women (Bangasser et al., 2010). Indeed, alcohol withdrawal-induced CRF reactivity may be greater in female vs. male rodents (Silva and Madeira, 2012), but sex-dependent mechanisms underlying this difference are not well understood. Findings in anxious, alcohol-dependent women suggest that the CRF1 antagonist verucerfont reduced HPA axis reactivity to stress (Kwako et al., 2015; Schwandt et al., 2016). Interestingly, estrogen is thought to regulate CRF gene expression and the CRF response to stress (Vamvakopoulos and Chrousos, 1993; Handa and Weiser, 2014; Novais et al., 2017; Li et al., 2006). For example, estrogen replacement therapy may moderate the CRF response to emotional stress in postmenopausal women (Lindheim et al., 1992). However, work in this area remains limited.
Sex-dependent effects of stress and alcohol on CRF regulation of the HPA axis have also been understudied, but a picture is emerging. Preclinically, corticosterone exposure during adolescence may sensitize animals to stress-induced responding for alcohol later in life and corticosterone-treated adolescent female rodents were more sensitive to yohimbine-induced reinstatement of alcohol-seeking than males in adulthood (Bertholomey et al., 2016). Similarly, in human subjects exposure to early life stress, including childhood trauma or maltreatment, is related to heightened stress reactivity, increased depressive symptomology (Heim and Nemeroff, 2001), and higher adrenocorticotropic hormone (ACTH) levels in response to CRF administration as adults (Heim et al., 2001).
Acute alcohol consumption can also increase plasma corticosterone and cortisol levels in both rodents and humans, respectively, in a sex-dependent manner. For example, while heavy drinkers have demonstrated increases in plasma cortisol following acute alcohol intake (Stephens and Wand, 2012), one study found that regardless of alcohol dependence history, women demonstrated a more robust response of ACTH and cortisol following the neuroendocrine stimulation test (peripherally-acting dexamethasone/CRF) than men (Anthenelli et al., 2018). Other studies have demonstrated that the cortisol awakening response was higher in heavy drinking women than moderate drinking women following chronic consumption (Adinoff et al., 2010). In contrast, separate studies of recently abstinent (up to 4 weeks) alcohol-dependent men (Bernardy et al., 1996; Lovallo et al., 2000; Errico et al., 2002) and women (Bernardy, 1995) or social drinkers with a family history of alcoholism (Sorocco et al., 2006) indicate a blunted cortisol response to stress (e.g., public speaking, mental arithmetic) compared to healthy controls; although, direct comparison of sex differences in stress reactivity was not conducted in these studies due to small sample size.
In preclinical work, female rodents exhibited greater ACTH and corticosterone responses following alcohol and stress exposure compared to males (Bertholomey et al., 2016; Cozzoli et al., 2014). Female mice deficient in the endogenous opioid peptide β-endorphin, a regulator of the HPA axis, also demonstrated increases in voluntary binge-like alcohol intake compared to wild-type controls, an effect not present in males (Nentwig et al., 2018). In the same study, females deficient in β-endorphin exhibited increased baseline anxiety, corticosterone, and CRF mRNA in the extended amygdala, all of which normalized following alcohol consumption (Nentwig et al., 2018). However, other studies indicate that male rodents exhibited greater withdrawal-induced elevations in plasma corticosterone relative to females (Alele and Devaud, 2007). While the literature is mixed both among and between preclinical and clinical findings, it is likely that chronic alcohol consumption produces profound alterations in HPA axis reactivity. The discrepancy in findings related to sex differences may be associated with study timeframe (e.g., early HPA dysregulation in acute alcohol use vs. the withdrawal period of rodents and recently abstinent individuals with AUD).
Overall, the data suggest that females may be more sensitive to alterations in CRF and HPA axis reactivity induced by stress and continued alcohol use compared to males. Females may also be more sensitive to withdrawal-induced changes in CRF activity, whereas males may exhibit a heightened corticosterone response following withdrawal. Research is needed to extend and reproduce these preliminary findings as there is promise in using these interconnected systems as probes to understand mechanisms underlying drinking to regulate negative affect and stress reactivity.
3.3.3. Dynorphin/kappa
The dynorphin/kappa opioid receptor (KOR) system has also been implicated in the stress response and addiction (Anderson and Becker, 2017) but studies examining sex differences in the role of the dynorphin/KOR system on alcohol intake have shown mixed results. Dynorphin, an endogenous opioid peptide, binds exclusively to the KOR and is released in response to stress. KOR activation by dynorphin is associated with stress-related behaviors in rodents and humans (McLaughlin et al., 2003), and may contribute to negative affective states associated with drug withdrawal (Racz et al., 2013). Indeed, KOR antagonists have demonstrated efficacy in reducing anxiety- and depressive-like behavior in studies using only male rodents (McLaughlin et al., 2003). In rodents, KORs may also be dysregulated following chronic alcohol exposure (e.g., 4-week intermittent vapor exposure [14 h on/10 h off]), consequently leading to an increase in alcohol self-administration (Walker and Koob, 2008). The KOR antagonists JDTic and nor-binaltorphimine (nor-BNI) have been shown to attenuate alcohol self-administration, cue-induced reinstatement of alcohol-seeking, and alcohol-induced conditioned place preference (CPP) in rodents (Anderson and Becker, 2017; Racz et al., 2013; Walker and Koob, 2008), although this effect is dependent on rodent strain. It should be noted that nor-BNI may only be effective in alcohol-dependent vs. non-dependent animals (Walker and Koob, 2008), and most of the aforementioned studies were conducted in males only.
Sex differences in the dynorphin/KOR system have been studied for pain, mood dysregulation, and responses to drugs of abuse (Rasakham and Liu-Chen, 2011). For example, in non-human primates, females are less sensitive to the analgesic effect of KOR agonists (Negus et al., 2002). Female rats are less sensitive to the depressive-like effect of a KOR agonist than males (Russell et al., 2014), possibly indicative of an enhanced role for KORs in anxiety- and depressive-like states in male rodents. Regarding alcohol, pharmacologic studies suggest that nor-BNI reduces alcohol intake in female rodents, but increases alcohol intake in male rats following social isolation stress (Morales et al., 2014). However, other studies have found that nor-BNI decreased drinking in male rodents, with no effect in females (Zhou et al., 2015).
Sex dependent effects on alcohol-motivated behavior in mice with genetic manipulations of KOR have also been variable. In one study, female prodynorphin knockout mice exhibited reduced alcohol preference (Blednov et al., 2006), but others have shown that female prodynorphin knockouts exhibit increased alcohol intake (Racz et al., 2013), likely because different mouse strains were used in these studies. Both female and male KOR knockout mice also exhibit reduced alcohol consumption, however (Kovacs et al., 2005). Overall, results are mixed with regard to sex-related differences in the role of the dynorphin/KOR system on alcohol-motivated behavior. While changes in science (e.g., NIH) policy (Clayton and Collins, 2014) ensure that female rodents are included in studies probing the dynorphin/kappa system for stress-related drinking behavior, current research in this area is lacking.
3.3.4. GABA
One of the primary molecular targets for alcohol is the family of GABAA receptors, which are required for its acute sedative effects (Davies, 2003). Imaging studies show that cortical GABA levels are higher in women compared to men (Sanacora et al., 1999). Our group has also shown that women have greater GABAA-benzodiazepine receptor availability than men (Esterlis et al., 2013). Similar observations have been made in animal models (Stefanova, 1998). GABA acts tonically in the amygdala at GABAA receptors to blunt anxiety-like responses, as does acute alcohol (Sanders and Shekhar, 1995), and chronic alcohol exposure, by way of chronic intermittent ethanol (CIE), induces GABAA receptor plasticity in the amygdala (Lindemeyer et al., 2014). Female mice also have more high affinity GABA binding sites than males, and these sites are more sensitive to stress-induced dysregulation in females than in males (Skilbeck et al., 2008). Together, these results suggest that decreasing GABA transmission could have more profound effects in women than in men.
As discussed above, another effect of chronic alcohol use is NE hyperexcitability (Fitzgerald, 2013), leading to greater stress reactivity and anxiety. This hyperarousal could be enhanced if GABA neurons are inhibited. NE can also activate GABAergic neurons in the amygdala, which could counteract the stimulatory effects of NE on principal neurons. However, chronically elevated NE signaling induced by long-term alcohol use can desensitize β-adrenergic receptors on GABA neurons of the amygdala (for review see (Patkar et al., 2016)). This allostatic imbalance, a disruption in the adaptive processes that sustain homeostasis of the stress response (McEwen, 2005), is likely to promote stress and anxiety-like behaviors. Thus, chronic alcohol use may increase the susceptibility to stress-induced behaviors and promote relapse to alcohol drinking by inducing NE hyperstimulation and decreasing amygdala GABA signaling. The increased GABA receptor number in the female brain may also contribute to the more pronounced effects in female mice (Skilbeck et al., 2008).
3.4. Alcohol-induced neurodegeneration and relationship to stress and drinking
While markers of peripheral inflammation have long been associated with a variety of psychiatric disorders, including AUD (Szabo and Mandrekar, 2009; Gonzalez-Quintela et al., 2008), the role that neuroinflammation plays has only recently been examined (Hillmer et al., 2017; Kalk et al., 2017). Microglia, the resident macrophages of the brain, are involved in a variety of physiologic and pathologic processes, most notably in the initiation and maintenance of neuroinflammation. Resting microglia are tightly regulated by interactions with neurons, and microglia normally protect neurons (Ponomarev et al., 2011). For example, when provided with signals that indicate the presence of tissue damage or pathogens, microglia become activated and carry out repair functions. However, excessive activation leads to the release of substances that cause neuronal dysfunction and death, such as inflammatory cytokines, chemokines, reactive oxygen species, nitric oxide, and glutamate (Yakovleva et al., 2011; van Gool et al., 2010; McNally, 2007; Block et al., 2007), and through these pathways may contribute to alcohol-induced neurodegeneration. It is now generally accepted that alcohol initially activates microglia (McClain et al., 2011), which plays a role in alcohol-induced adaptations in the brain; but more recent neuroimaging studies suggest that with chronic alcohol consumption (e.g., alcohol-dependent subjects imaged 1–4 days after their last drink but before the occurrence of major brain changes following abstinence (Hillmer et al., 2017) or recently detoxified alcohol-dependent subjects within 1 month of medically assisted withdrawal (Kalk et al., 2017)) the neuroimmune and peripheral immune systems become suppressed (Hillmer et al., 2017; Kalk et al., 2017).
Women are more susceptible to alcohol-related impaired peripheral immunity than men, including alcohol-induced liver disease (Rehm et al., 2010), cardiac myopathy (Mogos et al., 2017), and hypertension (Briasoulis et al., 2012). In the brain, it has long been thought that women with AUD have more brain atrophy, including gray and white matter volume loss, than do men with AUD (Pfefferbaum et al., 2009; Hommer et al., 2001; Sohrabji, 2002), although some findings are conflicting (Pfefferbaum et al., 2001, 2006). Markers of neurodegeneration, including neuroimmune function, have been linked to decrements in executive function (Flannery et al., 2007; Liu et al., 2010) and poor AUD treatment response (Goldstein and Volkow, 2011; Durazzo et al., 2011; Noël et al., 2002). Taken together, these observations suggest that women may be more susceptible to the neuroimmune consequences of alcohol than men, which has critical behavioral and cognitive implications.
In addition to neuroimmune function, synaptic density may be a marker of neurodegeneration. Preclinical studies have shown that synaptic density was lower in rodents chronically exposed to alcohol vs. control animals in cerebellar Purkinje cells (Dlugos and Pentney, 1997) and cerebellar cortex (Dlugos and Pentney, 2002). In a subset of chronically alcohol-treated animals that recovered for 20 weeks, there was an apparent restoration of synaptic density to control levels (Dlugos and Pentney, 1997). Additionally, lower synaptic density may contribute to neuronal instability (Feng et al., 2009), particularly a glutamate-GABA imbalance, which is known to develop with chronic alcohol consumption (Volkow et al., 2011).
There is also evidence to suggest that neurodegeneration alters the stress response, and possibly the effect of stress on alcohol consumption. A critical role of microglia is to support and protect neurons, to prune dendritic spines, and to promote synapse formation. Stress perturbates this homeostatic balance. For example, preclinical studies demonstrate that stress induces changes in the shape and molecular makeup of microglia (Rohan Walker et al., 2013) and causes synaptic loss on neurons in the PFC (Ota et al., 2014) and hippocampus (Milior et al., 2016), which were associated with depressive behaviors. More recently, stress-induced activation of microglia was shown to contribute directly to neuronal “remodeling” and synaptic deficits in the PFC, which underlie symptoms of anxiety and depression (Wohleb et al., 2018). Finally, sensitization of stress-induced microglial activation has been observed following alcohol exposure (Walter and Crews, 2017; Walter et al., 2017). However, to our knowledge, sex differences in the role of stress on alcohol-induced neurodegeneration have not been well-characterized. Because women may be more susceptible to the neuroimmune consequences of alcohol than men, probing systems that may rescue dendritic spine loss and protect against alcohol and stress-induced changes in synaptic density, including the noradrenergic and GABA systems, may be a critical step in translational research for identifying markers of neurodegeneration and sex-appropriate treatment development.
4. How are sex hormones related to stress and drinking?
Alterations in sex steroid levels, associated with the hypothalamic pituitary gonadal (HPG) axis, have effects on various neurotransmitter systems, including brain stress systems. For instance, progesterone and its metabolites allopregnanolone and pregnanolone regulate neuronal function, particularly through non-genomic action of GABAA receptors (Guennoun et al., 2015; Lynch and Sofuoglu, 2010). The relationship between sex hormones and GABAA receptors likely contribute to the anxiolytic effects of alcohol; namely because the anxiolytic effects of alcohol have been attributed to GABAA receptor function (Lobo and Harris, 2008). Progesterone and its metabolites also interact with other neurotransmitter systems, including the glutamate and nicotinic acetylcholine receptor systems (α4β2, α5; for review see (Lynch and Sofuoglu, 2010; Turkmen et al., 2011)), which likely modulate the effect of alcohol on the brain. Additionally, serotonin may mediate various physiological actions of estrogen, as estradiol alters the concentration of serotonin (Rybaczyk et al., 2005). Estrogens, including estradiol, have been implicated as mediators of DA efflux in women but not men, likely affecting motivation for rewards such as drugs of abuse (Yoest et al., 2014). Finally, there is initial evidence that estrogen may up-regulate α2A-adrenergic receptors, which would likely impact stress reactivity and substance use (Pedersen et al., 2004) due to perturbations in NE signaling.
Increasing evidence suggests that ovarian hormones may account for sex differences in alcohol self-administration, alcohol withdrawal, and reinstatement to alcohol-seeking in rodents and humans (Carroll and Anker, 2010; Anker and Carroll, 2010). Women with consistently high estradiol levels also have higher alcohol intake than those characterized by lower estradiol levels (Muti et al., 1998). Similarly, chronic estradiol replacement can increase alcohol drinking in female rodents, whereas chronic testosterone can decrease drinking in male rodents (Bertholomey and Torregrossa, 2017). Increases in progesterone and allopregnanolone have been associated with decreased alcohol withdrawal severity, with female rodents exhibiting more sensitivity to the anxiolytic-like effect of allopregnanolone during withdrawal (Becker and Koob, 2016; Carroll and Anker, 2010).
These results are consistent with evidence that drinking behavior, including intake, fluctuates over the course of the menstrual or estrus cycles, when sex steroids are also in flux (Becker and Koob, 2016). Phases of the estrus and menstrual cycles are characterized by higher levels of estradiol associated with higher risk of drinking and binge drinking (Martel et al., 2017; Becker and Koob, 2016). Among women in the early follicular menstrual phase (days 1–5; characterized by low levels of ovarian hormones), drinking to cope with negative affect predicted increased alcohol consumption (Joyce Kayla et al., 2017). These results should be viewed with caution as there are methodological variations associated with dichotomizing the cycles (see review (Allen et al., 2016)), making it difficult to determine the influence of ovarian hormones on alcohol consumption in these studies.
5. Reducing stress and negative affect as a treatment target for AUD
5.1. Pharmacological
Abstinence from alcohol or reduction of high-risk drinking can prevent and reduce many of the harmful health consequences of drinking. Successful treatment of AUD is associated with lower blood pressure, improved liver function, and stabilization of many conditions, including, cardiomyopathy, gastritis, ascites, and edema (Substance Abuse and Mental Health Services Adminstration & National Institute on Alcohol Abuse and Alcoholism, 2015). There are three FDA-approved medications for AUD; disulfiram, naltrexone (oral and depot formulations), and acamprosate, each with limitations regarding their efficacy and use. Research on disulfiram has been primarily conducted in males, and there is insufficient data to determine if there are sex differences in treatment response (Agabio et al., 2016b). Post-hoc evaluation of sex differences in medication response to naltrexone and acamprosate suggests that they may be equally effective for women (Agabio et al., 2016b; Yoon et al., 2016; Greenfield et al., 2010). However, in a large U.S.-based study, effect sizes for % days abstinent for acamprosate (effect size = 0.04) and naltrexone (effect size = 0.22) were small to moderate (Anton et al., 2006), and lack of sex differences may simply represent a floor effect.
As reviewed above, NE, CRF, dynorphin/KOR, and GABA all play a role in stress pathophysiology and associated alcohol consumption. Medications targeted to each system have been successful in reducing alcohol-motivated behavior, including alcohol craving, alcohol-seeking and consumption, and stress-induced alcohol relapse. While research on sex differences in each of these systems has been limited, there is some indication that targeting brain stress systems for sex-appropriate pharmacotherapeutic treatment options holds promise. Preliminary work suggests that noradrenergic agents may preferentially reduce stress-related drug outcomes in women compared to men. For example, in daily smokers, guanfacine attenuated cigarettes smoked and tobacco craving following stress in women (McKee, 2013). Similarly, guanfacine reduced alcohol craving, cocaine craving, anxiety, and negative emotion following stress in cocaine-dependent women but not men (Fox et al., 2014). However, sex difference research in humans with AUD is limited.
Research with CRF antagonists is mixed but some data suggest that they are efficacious in reducing HPA axis reactivity following stress in women with AUD (Schwandt et al., 2016). KOR antagonists also show promise, as preclinical data demonstrate that these agents preferentially reduce alcohol self-administration in female rodents compared to males (Morales et al., 2014). Because GABA binding sites are more sensitive to stress-induced dysregulation in female rodents (Skilbeck et al., 2008), GABAergic agents may also be a promising target for problematic alcohol use, particularly in women drinking for negative reinforcement. Medications targeting neuroinflammation and neurodegeneration are a growing interest in addiction (Ray et al., 2014). Women appear to be more susceptible to the neuroimmune consequences of alcohol than men, with stress and stress-related drinking likely playing a critical role in this relationship. Examining sex-dependent treatment response to medications which target neuroinflammation or neurodegeneration is a promising line of inquiry.
Overall, sex difference research in treatment efficacy for agents targeting each of these stress systems is limited in both rodent models of high alcohol drinking and in humans with AUD. The considerable body of data suggesting that women are more likely to drink to regulate negative affect and stress, while men are more likely to drink for the reinforcing properties of alcohol, indicates an important direction in the development of sex-appropriate treatments for AUD. Given the involvement of the NE, CRF, dynorphin/KOR, GABA, and neuroinflammation systems in stress pathophysiology and addiction, targeting these systems to attenuate sex-dependent mechanisms that maintain drinking is critical.
5.2. Behavioral measures related to AUD
5.2.1. Heart rate variability (HRV)
HRV, changes in time between beat-to-beat intervals, is thought to reflect vagal reactivity and autonomic nervous system flexibility (Berntson et al., 1997). Emerging evidence suggests that HRV may be a potential mechanism underlying the relationship between stress and substance use. A recent meta-analysis found that reductions in tonic HRV are associated with psychiatric disorders, including anxiety and depression, whereas elevations in tonic HRV are reflective of healthier states (Berntson et al., 2008; Chalmers et al., 2014). For example, individuals with higher tonic HRV were better able to adapt to stress (Park et al., 2014). Women have greater vagal mediation of heart rate, autonomic balance at rest, and vagal withdrawal in response to stress compared to men (Liao et al., 1995; Li et al., 2009). Thus, HRV biofeedback may have clinical benefits for women seeking non-pharmacological treatment for AUD. HRV has been shown to predict alcohol craving (Quintana et al., 2013) and is reduced by heavy alcohol consumption. Women experience dysregulated HRV in response to alcohol, less suppression of HRV in alcohol challenges and have greater increases in HRV in response to visual cues, including alcohol cues (Bates et al., 2011; Udo et al., 2009). Consistent with these findings, HRV biofeedback has been shown to improve alcohol abstinence outcomes at 1-year follow-up (Penzlin et al., 2017) and has recently gained support as an adjunctive therapy for depression (Fonoberova et al., 2014). While support for HRV as a mechanism underlying negative affect and substance use is relatively new, HRV may be dysregulated during alcohol exposure and HRV-based interventions may improve AUD treatment outcomes, especially among women drinkers.
5.2.2. Psychosocial interventions
To date, several psychosocial interventions, including Cognitive Behavioral Therapy (CBT), 12-Step Facilitation/Alcoholics Anonymous (TSF/AA), Contingency Management (CM), and Motivational Interviewing (MI)-Based Interventions have proven to be effective treatments for AUD (Project MATCH Research Group, 1997; Project MATCH Research Group, 1998; Petry et al., 2000). CBT has also been shown to effectively treat comorbid AUD and depressive/anxiety symptomatology (Olthuis et al., 2015). However, limited research has focused on sex differences in behavioral treatments as they relate to reductions in alcohol consumption and stress reactivity.
Two recent studies used female-specific CBT, including modules to target social support, interpersonal functioning, and coping with negative affect/anxiety, to treat women with AUD (Epstein et al., 2018a, 2018b); although, when compared to sex-neutral treatment, both treatments showed similar positive outcomes (Epstein et al., 2018b). Another study has demonstrated that women were more likely to participate in AA, have better alcohol-related outcomes, and show decreases in depressive symptoms and drinking to cope/reduce tension (Moos et al., 2006). It should be noted that Project MATCH results demonstrated equivalent, modest response rates to CBT, TSF/AA, and MI-based interventions (Project MATCH Research Group, 1997; Project MATCH Research Group, 1998) across sexes; however, research regarding the overall effectiveness of these interventions for women remains understudied and requires more sophisticated methodologies (Greenfield et al., 2011), with Project Match including 70–80% men in their treatment populations (Project MATCH Research Group, 1997; Project MATCH Research Group, 1998). Additionally, research has demonstrated that CM is equally beneficial for both sexes, although its use is often limited due to financial resources (Rash and Petry, 2015). Thus, additional research is needed to improve treatment outcomes for women receiving psychosocial interventions. Specifically, it is imperative that future studies include more advanced methodologies, as well as enough women in sample sizes to adequately explore sex-related factors that may mediate or moderate treatment outcomes and thus elucidate underlying mechanisms between men and women with AUD or problematic drinking (Greenfield et al., 2011). Based upon the current evidence, it appears that concurrently targeting stress reactivity, as well as alcohol use, would serve to improve treatment outcomes; however more targeted research on sex differences is needed.
5.2.3. Mindfulness-based interventions (MBIs)
There is growing evidence that MBIs are associated with reductions in negative affect and anxiety (Hofmann et al., 2010), as well as support for its use to treat a variety of substance use disorders (SUDs Zgierska et al., 2009). Mindfulness-based relapse prevention (MBRP) has shown particular promise in the treatment of SUDs, including AUD. For instance, participants receiving MBRP- or CBT-based relapse prevention (RP), both showed a lower risk of relapse to heavy drinking at 6-month follow-up; however, MBRP demonstrated significant decreases in heavy drinking compared to CBT-based RP or treatment as usual at 12-month follow-up (Bowen et al., 2014). When tailored to women substance users with a history of trauma, those attending more sessions of MBRP showed greater reductions in alcohol addiction severity and perceived stress, although it should be noted that these results were preliminary (Amaro et al., 2014). Overall, there appears to be promise in the utility of MBIs among women to decrease alcohol consumption and perceived stress.
6. Conclusions
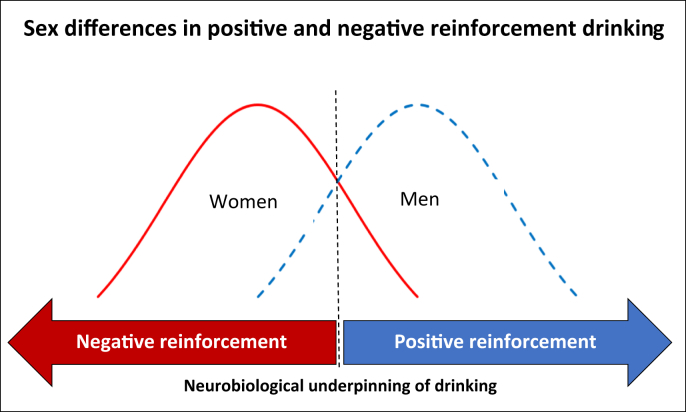
The present evidence suggests that sex difference research (SABV) and treatment development is needed to address the growing rates of alcohol use and AUD as well as the exacerbated alcohol-related health consequences among women. Current FDA-approved medications for AUD have not yet been adequately studied across sexes and likely do not directly address the factors contributing to the onset and maintenance of alcohol use in women. Overall, women are generally more likely to drink to regulate negative affect and stress reactivity (i.e., negative reinforcement) while men may be more likely to drink for positive reinforcement (see Fig. 1). Drinking motivated by negative or positive reinforcement differs in its neurobiological underpinnings, and probing stress pathophysiology may be an important direction to develop tailored treatments for women. Sex differences in onset and maintenance of alcohol use begin to develop during adolescence, coinciding with exposure to early life stress and emerging developmental changes, including differences in brain morphology. These factors continue to affect alcohol use into adulthood, when reduced responsivity to stress, increased affect-related psychiatric comorbidities and alcohol-induced neurodegeneration contribute to chronic and problematic alcohol use, particularly for women.
Fig. 1.
Schematic summarizing findings from the present review that the general population of women is more likely to drink for negative reinforcement (e.g., stress and negative affect), and the general population of men is more likely to drink for positive reinforcement (e.g., stimulation), which reflects sex differences in the neurobiological underpinnings of drinking behavior. However, as also indicated in Fig. 1, these populations overlap, and some women may drink primarily for positive reinforcement and some men may drink primarily for negative reinforcement. This line of evidence suggests that probing stress pathophysiology may be an important direction to develop tailored treatments for women.
Sex-dependent interventions targeting stress pathophysiology are likely an important direction in AUD treatment development for women, as these systems and structures underlie and maintain alcohol consumption in women. Probing the brain stress systems, such as the noradrenergic system, and its associated brain regions may be relevant for examining sex differences in stress responses and chronic alcohol use. For example, noradrenergic agents, such as yohimbine, may be utilized to probe stress-induced PFC dysfunction in preclinical samples, and decreasing NE with noradrenergic targets may attenuate stress reactivity and associated effects on PFC function among individuals with AUD. In fact, preliminary work highlighted in this review suggests that noradrenergic agents may preferentially reduce smoking self-administration and craving following stress in women; however, surprisingly little work has extended these findings to AUD. It should be noted that the impact of comorbid drug use (e.g., cigarette smoking, cocaine use) and AUD on stress pathophysiology and brain neuroadaptations was not discussed in this review and adds to the complex findings documented across human studies. Future research should build on the findings presented on sex differences in stress-related alcohol use and examine how relationships between sex and stress may be impacted by polysubstance use. There is growing evidence across other substances of abuse that women use drug and non-drug reinforcers (e.g., food) for stress and affect regulation (McKee, 2013; Verplaetse et al., 2015; Tomiyama et al., 2011; Laitinen et al., 2002).
Additional evidence demonstrates that targeting other brain stress systems may also be promising targets for sex-appropriate pharmacological treatment development for AUD. Preliminary data suggests that CRF antagonists may reduce HPA-axis reactivity following stress among women with AUD and KOR antagonists have been shown to reduce alcohol self-administration in female rodents, illustrating the promise of pharmacological developments targeting these systems. Similarly, GABAergic agents may be a promising avenue for treatment development for women with AUD, as women have higher cortical GABA levels; thus, these agents may reduce problematic alcohol use among those drinking for negative reinforcement. Because evidence suggests that women may be more susceptible to the neuroimmune consequences of alcohol than men, future research with agents such as glial modulators to target neuroinflammation or probing systems that protect against alcohol or stress-induced alterations in synaptic density may be an important next step in SABV and alcohol research. Finally, emerging psychosocial and behavioral interventions, including HRV and MBIs, may be beneficial in the treatment of AUD in women by targeting relevant stress pathophysiology.
Overall, further research is warranted to elucidate sex differences in brain systems and regions that underlie mechanisms associated with alcohol consumption and relapse among women, with particular emphasis on modulating stress reactivity in women (e.g., PFC-amygdala axis; brain stress systems); Table 2 highlights that much of this work has yet to be completed. This line of investigation will result in the development of treatments to target these sex-dependent systems to attenuate mechanisms that maintain drinking in women.
Declarations of interest
Authors have no conflicts of interest as related to the present review.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2019.100149.
Funding
This work was supported by National Institutes of Health grants: P50DA033945 (SAM), P01AA027473 (SAM), R01AA022285 (SAM), K01AA025670 (TLV), T32DA007238 (ILP), MH077681 (MRP), and DA14241 (MRP). Funding sources had no involvement in the interpretation of data, writing of the review or decision to submit this article for publication.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abbey A., Zawacki T., Buck P.O. Alcohol and sexual assault. Alcohol Res. Health. 2001;25(1):43–51. [PMC free article] [PubMed] [Google Scholar]
- Abulseoud O.A., Karpyak V.M., Schneekloth T. A retrospective study of gender differences in depressive symptoms and risk of relapse in patients with alcohol dependence. Am. J. Addict. 2013;22(5):437–442. doi: 10.1111/j.1521-0391.2013.12021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff B., Best S.E., Ye W., Williams M.J., Iranmenesh A. Adrenocortical and pituitary glucocorticoid feedback in abstinent alcohol‐dependent women. Alcohol Clin. Exp. Res. 2010;34(5):915–924. doi: 10.1111/j.1530-0277.2010.01164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agabio R., Campesi I., Pisanu C., Gessa G.L., Franconi F. Sex differences in substance use disorders: focus on side effects. Addict. Biol. 2016;21(5):1030–1042. doi: 10.1111/adb.12395. [DOI] [PubMed] [Google Scholar]
- Agabio R., Pani P.P., Preti A., Gessa G.L., Franconi F. Efficacy of medications approved for the treatment of alcohol dependence and alcohol withdrawal syndrome in female patients: a descriptive review. Eur. Addict. Res. 2016;22(1):1–16. doi: 10.1159/000433579. [DOI] [PubMed] [Google Scholar]
- Agabio R., Pisanu C., Luigi Gessa G., Franconi F. Sex differences in alcohol use disorder. Curr. Med. Chem. 2017;24(24):2661–2670. doi: 10.2174/0929867323666161202092908. [DOI] [PubMed] [Google Scholar]
- Alele P., Devaud L. Sex differences in steroid modulation of ethanol withdrawal in male and female rats. J. Pharmacol. Exp. Therapeut. 2007;320(1):427–436. doi: 10.1124/jpet.106.107896. [DOI] [PubMed] [Google Scholar]
- Allen A.M., McRae-Clark A.L., Carlson S. Determining menstrual phase in human biobehavioral research: a review with recommendations. Exp. Clin. Psychopharmacol. 2016;24(1):1–11. doi: 10.1037/pha0000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaro H., Spear S., Vallejo Z., Conron K., Black D.S. Feasibility, acceptability, and preliminary outcomes of a mindfulness-based relapse prevention intervention for culturally-diverse, low-income women in substance use disorder treatment. Subst. Use Misuse. 2014;49(5):547–559. doi: 10.3109/10826084.2013.852587. [DOI] [PubMed] [Google Scholar]
- Andersen A.-M.N., Andersen P.K., Olsen J., Grønbæk M., Strandberg-Larsen K. Moderate alcohol intake during pregnancy and risk of fetal death. Int. J. Epidemiol. 2012;41(2):405–413. doi: 10.1093/ije/dyr189. [DOI] [PubMed] [Google Scholar]
- Anderson R.I., Becker H.C. Role of the dynorphin/kappa opioid receptor system in the motivational effects of ethanol. Alcohol Clin. Exp. Res. 2017;41(8):1402–1418. doi: 10.1111/acer.13406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker J.J., Carroll M.E. The role of progestins in the behavioral effects of cocaine and other drugs of abuse: human and animal research. Neurosci. Biobehav. Rev. 2010;35(2):315–333. doi: 10.1016/j.neubiorev.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker J.J., Forbes M.K., Almquist Z.W. A network approach to modeling comorbid internalizing and alcohol use disorders. J. Abnorm. Psychol. 2017;126(3):325–339. doi: 10.1037/abn0000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker J.J., Kummerfeld E., Rix A., Burwell S.J., Kushner M.G. Causal network modeling of the determinants of drinking behavior in comorbid alcohol use and anxiety disorder. Alcohol Clin. Exp. Res. 2018;0(ja) doi: 10.1111/acer.13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthenelli R.M., Heffner J.L., Blom T.J., Daniel B.E., McKenna B.S., Wand G.S. Sex differences in the ACTH and cortisol response to pharmacological probes are stressor-specific and occur regardless of alcohol dependence history. Psychoneuroendocrinology. 2018;94:72–82. doi: 10.1016/j.psyneuen.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton R.F., O'Malley S.S., Ciraulo D.A. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. Jama. 2006;295(17):2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Arnsten A.F. vol. 126. Elsevier; 2000. pp. 183–192. (Stress Impairs Prefrontal Cortical Function in Rats and Monkeys: Role of Dopamine D1 and Norepinephrine α-1 Receptor Mechanisms. Progress in Brain Research). [DOI] [PubMed] [Google Scholar]
- Arnsten A.F., Goldman-Rakic P.S. Noise stress impairs prefrontal cortical cognitive function in monkeys: evidence for a hyperdopaminergic mechanism. Arch. Gen. Psychiatr. 1998;55(4):362–368. doi: 10.1001/archpsyc.55.4.362. [DOI] [PubMed] [Google Scholar]
- Arnsten A.F., Jin L.E. Guanfacine for the treatment of cognitive disorders: a century of discoveries at Yale. Yale J. Biol. Med. 2012;85(1):45. [PMC free article] [PubMed] [Google Scholar]
- Bangasser D.A., Curtis A., Reyes B.A. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol. Psychiatr. 2010;15(9):896. doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates M.E., Buckman J.F., Vaschillo E.G. The redistribution of power: neurocardiac signaling, alcohol and gender. PLoS One. 2011;6(12) doi: 10.1371/journal.pone.0028281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker H.C. Effects of alcohol dependence and withdrawal on stress responsiveness and alcohol consumption. Alcohol Res. Curr. Rev. 2012;34(4):448. doi: 10.35946/arcr.v34.4.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J.B., Koob G.F. Sex differences in animal models: focus on addiction. Pharmacol. Rev. 2016;68(2):242–263. doi: 10.1124/pr.115.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery A.K., Zucker I. Sex bias in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 2011;35(3):565–572. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardy N.C. University of Oklahoma; 1995. Cardiovascular and Adrenocortical Effects of Chronic Alcohol Abuse in Sober Female Inpatients. Unpublished doctoral dissertation. [Google Scholar]
- Bernardy N.C., King A.C., Parsons O.A., Lovallo W.R. Altered cortisol response in sober alcoholics: an examination of contributing factors. Alcohol. 1996;13(5):493–498. doi: 10.1016/0741-8329(96)00043-2. [DOI] [PubMed] [Google Scholar]
- Berntson G.G., Bigger J.T., Jr., Eckberg D.L. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Berntson G.G., Norman G.J., Hawkley L.C., Cacioppo J.T. Cardiac autonomic balance versus cardiac regulatory capacity. Psychophysiology. 2008;45(4):643–652. doi: 10.1111/j.1469-8986.2008.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholomey M.L., Torregrossa M.M. Gonadal hormones affect alcohol drinking, but not cue+ yohimbine-induced alcohol seeking, in male and female rats. Physiol. Behav. 2017 doi: 10.1016/j.physbeh.2017.10.025. pii:S0031-9384(17) 30376–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholomey M., Nagarajan V., Torregrossa M.M. Sex differences in reinstatement of alcohol seeking in response to cues and yohimbine in rats with and without a history of adolescent corticosterone exposure. Psychopharmacology. 2016;233(12):2277–2287. doi: 10.1007/s00213-016-4278-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov Y.A., Walker D., Martinez M., Harris R.A. Reduced alcohol consumption in mice lacking preprodynorphin. Alcohol. 2006;40(2):73–86. doi: 10.1016/j.alcohol.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block M.L., Zecca L., Hong J.-S. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007;8:57. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Bowen S., Witkiewitz K., Clifasefi S.L. Relative efficacy of mindfulness-based relapse prevention, standard relapse prevention, and treatment as usual for substance use disorders: a randomized clinical trial. JAMA Psychiatry. 2014;71(5):547–556. doi: 10.1001/jamapsychiatry.2013.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boykoff N., Schneekloth T.D., Hall-Flavin D. Gender differences in the relationship between depressive symptoms and cravings in alcoholism. Am. J. Addict./American Academy of Psychiatrists in Alcoholism and Addictions. 2010;19(4):352–356. doi: 10.1111/j.1521-0391.2010.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady K.T., Sonne S.C. The role of stress in alcohol use, alcoholism treatment, and relapse. Alcohol Res. Health. 1999;23(4) 263-263. [PMC free article] [PubMed] [Google Scholar]
- Briasoulis A., Agarwal V., Messerli F.H. Alcohol consumption and the risk of hypertension in men and women: a systematic review and meta‐analysis. J. Clin. Hypertens. 2012;14(11):792–798. doi: 10.1111/jch.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunea I.M., Szentágotai-Tătar A., Miu A.C. Early-life adversity and cortisol response to social stress: a meta-analysis. Transl. Psychiatry. 2017;7(12):1274. doi: 10.1038/s41398-017-0032-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L., Haier R.J., White N.S. Sex-related difference in amygdala activity during emotionally influenced memory storage. Neurobiol. Learn. Mem. 2001;75(1):1–9. doi: 10.1006/nlme.2000.3999. [DOI] [PubMed] [Google Scholar]
- Carroll M.E., Anker J.J. Sex differences and ovarian hormones in animal models of drug dependence. Horm. Behav. 2010;58(1):44–56. doi: 10.1016/j.yhbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Chalmers J.A., Quintana D.S., Abbott M.J.A., Kemp A.H. Anxiety disorders are associated with reduced heart rate variability: a meta-analysis. Front. Psychiatry. 2014;5:80. doi: 10.3389/fpsyt.2014.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin T.M., Hong K., Bergquist K., Sinha R. Gender differences in response to emotional stress: an assessment across subjective, behavioral, and physiological domains and relations to alcohol craving. Alcohol Clin. Exp. Res. 2008;32(7):1242–1250. doi: 10.1111/j.1530-0277.2008.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.Y., Rosner B., Hankinson S.E., Colditz G.A., Willett W.C. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. Jama. 2011;306(17):1884–1890. doi: 10.1001/jama.2011.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs E., O'Connor S., de Wit H. Bidirectional interactions between acute psychosocial stress and acute intravenous alcohol in healthy men. Alcohol Clin. Exp. Res. 2011;35(10):1794–1803. doi: 10.1111/j.1530-0277.2011.01522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi N.G., DiNitto D.M. Psychological distress, binge/heavy drinking, and gender differences among older adults. Am. J. Addict. 2011;20(5):420–428. doi: 10.1111/j.1521-0391.2011.00149.x. [DOI] [PubMed] [Google Scholar]
- Choleris E., Galea L.A.M., Sohrabji F., Frick K.M. Sex differences in the brain: implications for behavioral and biomedical research. Neurosci. Biobehav. Rev. 2018;85:126–145. doi: 10.1016/j.neubiorev.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton J.A., Collins F.S. Policy: NIH to balance sex in cell and animal studies. Nature News. 2014;509(7500):282. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens K.J., Vendruscolo L.F. Anxious to drink: gabapentin normalizes GABAergic transmission in the central amygdala and reduces symptoms of ethanol dependence. J. Neurosci. 2008;28(37):9087–9089. doi: 10.1523/JNEUROSCI.2928-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove K.P., Mazure C.M., Staley J.K. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol. Psychiatry. 2007;62(8):847–855. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove K.P., Mazure C.M., Staley J.K. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol. Psychiatry. 2007;62(8):847–855. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli D.K., Tanchuck-Nipper M.A., Kaufman M.N., Horowitz C.B., Finn D.A. Environmental stressors influence limited-access ethanol consumption by C57BL/6J mice in a sex-dependent manner. Alcohol. 2014;48(8):741–754. doi: 10.1016/j.alcohol.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane N.A., Gorka S.M., Phan K.L., Childs E. Amygdala-orbitofrontal functional connectivity mediates the relationship between sensation seeking and alcohol use among binge-drinking adults. Drug Alcohol Depend. 2018;192:208–214. doi: 10.1016/j.drugalcdep.2018.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis A.L., Bethea T., Valentino R.J. Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology. 2006;31(3):544. doi: 10.1038/sj.npp.1300875. [DOI] [PubMed] [Google Scholar]
- Davies M. The role of GABAA receptors in mediating the effects of alcohol in the central nervous system. J. Psychiatr. Neurosci. 2003;28(4):263–274. [PMC free article] [PubMed] [Google Scholar]
- Dlugos C.A., Pentney R.J. Morphometric evidence that the total number of synapses on Purkinje neurons of old F344 rats is reduced after long-term ethanol treatment and restored to control levels after recovery. Alcohol Alcohol. 1997;32(2):161–172. doi: 10.1093/oxfordjournals.alcalc.a008250. [DOI] [PubMed] [Google Scholar]
- Dlugos C.A., Pentney R.J. Quantitative immunocytochemistry of GABA and synaptophysin in the cerebellar cortex of old ethanol‐fed rats. Alcohol Clin. Exp. Res. 2002;26(11):1728–1733. doi: 10.1097/01.ALC.0000037139.60009.2D. [DOI] [PubMed] [Google Scholar]
- Durazzo T.C., Tosun D., Buckley S. Cortical thickness, surface area and volume of the brain reward system in alcohol dependence: relationships to relapse and extended abstinence. Alcohol Clin. Exp. Res. 2011;35(6):1187–1200. doi: 10.1111/j.1530-0277.2011.01452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A.C., Joinson C., Dick D.M. The association between depressive symptoms from early to late adolescence and later use and harmful use of alcohol. Eur. Child Adolesc. Psychiatry. 2014;23(12):1219–1230. doi: 10.1007/s00787-014-0600-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch M.-A. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology. 2011;214(1):17–31. doi: 10.1007/s00213-010-1916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E.E., McCrady B.S., Hallgren K.A. Individual versus group female-specific cognitive behavior therapy for alcohol use disorder. J. Subst. Abuse Treat. 2018;88:27–43. doi: 10.1016/j.jsat.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E.E., McCrady B.S., Hallgren K.A., Cook S., Jensen N.K., Hildebrandt T. A randomized trial of female-specific cognitive behavior therapy for alcohol dependent women. Psychol. Addict. Behav. 2018;32(1):1. doi: 10.1037/adb0000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erol A., Ho A.M.C., Winham S.J., Karpyak V.M. Sex hormones in alcohol consumption: a systematic review of evidence. Addict. Biol. 2017 doi: 10.1111/adb.12589. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errico A.L., King A.C., Lovallo W.R., Parsons O.A. Cortisol dysregulation and cognitive impairment in abstinent male alcoholics. Alcohol Clin. Exp. Res. 2002;26(8):1198–1204. doi: 10.1097/01.ALC.0000025885.23192.FF. [DOI] [PubMed] [Google Scholar]
- Esterlis I., McKee S.A., Kirk K. Sex‐specific differences in GABAA‐benzodiazepine receptor availability: relationship with sensitivity to pain and tobacco smoking craving. Addict. Biol. 2013;18(2):370–378. doi: 10.1111/j.1369-1600.2011.00403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E.A., Upchurch D.M., Simpson T., Hamilton A.B., Hoggatt K.J. Differences by Veteran/civilian status and gender in associations between childhood adversity and alcohol and drug use disorders. Soc. Psychiatr. Psychiatr. Epidemiol. 2018;53(4):421–435. doi: 10.1007/s00127-017-1463-0. [DOI] [PubMed] [Google Scholar]
- Feng G., Xiao F., Lu Y. Down-regulation synaptic vesicle protein 2A in the anterior temporal neocortex of patients with intractable epilepsy. J. Mol. Neurosci. 2009;39(3):354–359. doi: 10.1007/s12031-009-9288-2. [DOI] [PubMed] [Google Scholar]
- Fenton M.C., Geier T., Keyes K., Skodol A.E., Grant B.F., Hasin D.S. Combined role of childhood maltreatment, family history, and gender in the risk for alcohol dependence. Psychol. Med. 2013;43(5):1045–1057. doi: 10.1017/S0033291712001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald P.J. vol. 7. SART; 2013. Elevated Norepinephrine May Be a Unifying Etiological Factor in the Abuse of a Broad Range of Substances: Alcohol, Nicotine, Marijuana, Heroin, Cocaine, and Caffeine. Substance Abuse: Research and Treatment; p. S13019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery B., Fishbein D., Krupitsky E. Gender differences in neurocognitive functioning among alcohol‐dependent Russian patients. Alcohol Clin. Exp. Res. 2007;31(5):745–754. doi: 10.1111/j.1530-0277.2007.00372.x. [DOI] [PubMed] [Google Scholar]
- Fonoberova M., Mezić I., Buckman J.F. A computational physiology approach to personalized treatment models: the beneficial effects of slow breathing on the human cardiovascular system. Am. J. Physiol. Heart Circ. Physiol. 2014;307(7):H1073–H1091. doi: 10.1152/ajpheart.01011.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox H.C., Morgan P.T., Sinha R. Sex differences in guanfacine effects on drug craving and stress arousal in cocaine-dependent individuals. Neuropsychopharmacology. 2014;39(6):1527. doi: 10.1038/npp.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H., Pendergrass J.C., Ross T.J., Stein E.A., Risinger R.C. Amygdala response to both positively and negatively valenced stimuli. Neuroreport. 2001;12(12):2779–2783. doi: 10.1097/00001756-200108280-00036. [DOI] [PubMed] [Google Scholar]
- George M.S., Anton R.F., Bloomer C. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Arch. Gen. Psychiatr. 2001;58(4):345–352. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- Goldstein R.Z., Volkow N.D. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat. Rev. Neurosci. 2011;12(11):652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J.M., Jerram M., Abbs B., Whitfield-Gabrieli S., Makris N. Sex differences in stress response circuitry activation dependent on female hormonal cycle. J. Neurosci. 2010;30(2):431–438. doi: 10.1523/JNEUROSCI.3021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A. The impact of childhood maltreatment on biological systems: implications for clinical interventions. Paediatr. Child Health. 2013;18(8):415–418. [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Quintela A., Campos J., Loidi L., Quinteiro C., Perez L.-F., Gude F. Serum TNF-α levels in relation to alcohol consumption and common TNF gene polymorphisms. Alcohol. 2008;42(6):513–518. doi: 10.1016/j.alcohol.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Grant B.F., Chou S.P., Saha T.D. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA psychiatry. 2017;74(9):911–923. doi: 10.1001/jamapsychiatry.2017.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield S.F., Pettinati H.M., O'Malley S., Randall P.K., Randall C.L. Gender differences in alcohol treatment: an analysis of outcome from the COMBINE study. Alcohol Clin. Exp. Res. 2010;34(10):1803–1812. doi: 10.1111/j.1530-0277.2010.01267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield S.F., Rosa C., Putnins S.I. Gender research in the national Institute on drug abuse national treatment clinical trials network: a summary of findings. Am. J. Drug Alcohol Abuse. 2011;37(5):301–312. doi: 10.3109/00952990.2011.596875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Project MATCH Research Group Matching alcoholism treatments to client heterogeneity: project MATCH posttreatment drinking outcomes. J. Stud. Alcohol. 1997;58(1):7–29. [PubMed] [Google Scholar]
- Project MATCH Research Group Matching alcoholism treatments to client heterogeneity: project MATCH three‐year drinking outcomes. Alcohol Clin. Exp. Res. 1998;22(6):1300–1311. doi: 10.1111/j.1530-0277.1998.tb03912.x. [DOI] [PubMed] [Google Scholar]
- Grucza R.A., Sher J.K., Kerr W.C. Trends in adult alcohol use and binge drinking in the early 21st-century United States: a meta-analysis of 6 national survey series. Alcohol Clin. Exp. Res. 2018;42(10):1939–1950. doi: 10.1111/acer.13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grüsser S.M., Wrase J., Klein S. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology. 2004;175(3):296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Guennoun R., Labombarda F., Gonzalez Deniselle M.C., Liere P., De Nicola A.F., Schumacher M. Progesterone and allopregnanolone in the central nervous system: response to injury and implication for neuroprotection. J. Steroid Biochem. Mol. Biol. 2015;146:48–61. doi: 10.1016/j.jsbmb.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Guizzetti M., Davies D.L., Egli M. Sex and the lab: an alcohol-focused commentary on the NIH initiative to balance sex in cell and animal studies. Alcohol Clin. Exp. Res. 2016;40(6):1182–1191. doi: 10.1111/acer.13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass-Koffler C.L., Swift R.M., Leggio L. Noradrenergic targets for the treatment of alcohol use disorder. Psychopharmacology. 2018:1–10. doi: 10.1007/s00213-018-4843-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerslag L.R., Gulley J.M. Sex differences in behavior and neural development and their role in adolescent vulnerability to substance use. Behav. Brain Res. 2016;298(0 0):15–26. doi: 10.1016/j.bbr.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa R.J., Weiser M.J. Gonadal steroid hormones and the hypothalamo–pituitary–adrenal axis. Front. Neuroendocrinol. 2014;35(2):197–220. doi: 10.1016/j.yfrne.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C., Nemeroff C.B. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol. Psychiatry. 2001;49(12):1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Heim C., Newport D.J., Bonsall R., Miller A.H., Nemeroff C.B. Altered pituitary-adrenal Axis responses to provocative challenge tests in adult survivors of childhood abuse. Am. J. Psychiatry. 2001;158(4):575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- Hien D. In: Trauma, Posttraumtic Stress Disorder, and Addiction Among Women. Brady Kathleen T., Back Sudie E., Greenfield Shelly F., editors. Guilfrod Publications; New York, NY: 2009. (Women & Addiction) [Google Scholar]
- Hillmer A., Sandiego C., Hannestad J. In vivo imaging of translocator protein, a marker of activated microglia, in alcohol dependence. Mol. Psychiatr. 2017;22(12):1759. doi: 10.1038/mp.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman L.A., Sklar A.L., Nixon S.J. The effects of acute alcohol on psychomotor, set-shifting, and working memory performance in older men and women. Alcohol. 2015;49(3):185–191. doi: 10.1016/j.alcohol.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann S.G., Sawyer A.T., Witt A.A., Oh D. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. J. Consult. Clin. Psychol. 2010;78(2):169–183. doi: 10.1037/a0018555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommer D.W., Momenan R., Kaiser E., Rawlings R.R. Evidence for a gender-related effect of alcoholism on brain volumes. Am. J. Psychiatry. 2001;158(2):198–204. doi: 10.1176/appi.ajp.158.2.198. [DOI] [PubMed] [Google Scholar]
- Ingalhalikar M., Smith A., Parker D. Sex differences in the structural connectome of the human brain. Proc. Natl. Acad. Sci. Unit. States Am. 2014;111(2):823–828. doi: 10.1073/pnas.1316909110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen E.L., Andersson H.W., Bjørngaard J.H., Pape K. Anxiety and depression symptoms and alcohol use among adolescents-a cross sectional study of Norwegian secondary school students. BMC Public Health. 2017;17(1):494. doi: 10.1186/s12889-017-4389-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce K.M., Hudson A., O'Connor R. Changes in coping and social motives for drinking and alcohol consumption across the menstrual cycle. Depress. Anxiety. 2017;35(4):313–320. doi: 10.1002/da.22699. [DOI] [PubMed] [Google Scholar]
- Kaag A.M., Wiers R.W., de Vries T., Pattij T., Goudriaan A.E. Striatal alcohol cue‐reactivity is stronger in male than female problem drinkers. Eur. J. Neurosci. 2018 doi: 10.1111/ejn.13991. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalk N., Guo Q., Owen D. Decreased hippocampal translocator protein (18 kDa) expression in alcohol dependence: a [11 C] PBR28 PET study. Transl. Psychiatry. 2017;7(1):e996. doi: 10.1038/tp.2016.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S., Li J., Stenzel‐Poore M.P., Ryabinin A.E. Corticotropin‐Releasing factor acting on Corticotropin‐Releasing factor receptor type 1 is critical for binge alcohol drinking in mice. Alcohol Clin. Exp. Res. 2012;36(2):369–376. doi: 10.1111/j.1530-0277.2011.01610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler K.S., Edwards A.C., Gardner C.O. Sex differences in the pathways to symptoms of alcohol use disorder: a study of opposite‐sex twin pairs. Alcohol Clin. Exp. Res. 2015;39(6):998–1007. doi: 10.1111/acer.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key J., Hodgson S., Omar R.Z. Meta-analysis of studies of alcohol and breast cancer with consideration of the methodological issues. Cancer Causes Control. 2006;17(6):759–770. doi: 10.1007/s10552-006-0011-0. [DOI] [PubMed] [Google Scholar]
- King A.C., Bernardy N.C., Hauner K. Stressful events, personality, and mood disturbance:: gender differences in alcoholics and problem drinkers. Addict. Behav. 2003;28(1):171–187. doi: 10.1016/s0306-4603(01)00264-7. [DOI] [PubMed] [Google Scholar]
- Koob G.F. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61–75. doi: 10.1016/j.brainres.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 2010;1314:3–14. doi: 10.1016/j.brainres.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F., Volkow N.D. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F., Volkow N.D. Neurobiology of addiction: a neurocircuitry analysis. The Lancet Psychiatry. 2016;3(8):760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F., White A. Paper Presented at: 2017 National Conference on Alcohol and Opioid Use in Women & Girls: Advances in Prevention, Treatment, and Recovery Research. 2017. Alcohol and the female brain. (Washington D.C) [Google Scholar]
- Kovacs K.M., Szakall I., O’brien D. Decreased oral self‐administration of alcohol in κ‐opioid receptor knock‐out mice. Alcohol Clin. Exp. Res. 2005;29(5):730–738. doi: 10.1097/01.alc.0000164361.62346.d6. [DOI] [PubMed] [Google Scholar]
- Kwako L.E., Koob G.F. Neuroclinical framework for the role of stress in addiction. Chronic Stress. 2017;1 doi: 10.1177/2470547017698140. 2470547017698140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwako L.E., Spagnolo P.A., Schwandt M.L. The corticotropin releasing hormone-1 (CRH1) receptor antagonist pexacerfont in alcohol dependence: a randomized controlled experimental medicine study. Neuropsychopharmacology. 2015;40(5):1053. doi: 10.1038/npp.2014.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitinen J., Ek E., Sovio U. Stress-related eating and drinking behavior and body mass index and predictors of this behavior. Prev. Med. 2002;34(1):29–39. doi: 10.1006/pmed.2001.0948. [DOI] [PubMed] [Google Scholar]
- Lau-Barraco C., Skewes M.C., Stasiewicz P.R. Gender differences in high-risk situations for drinking: are they mediated by depressive symptoms? Addict. Behav. 2009;34(1):68–74. doi: 10.1016/j.addbeh.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le A., Harding S., Juzytsch W., Fletcher P., Shaham Y. The role of corticotropin-releasing factor in the median raphe nucleus in relapse to alcohol. J. Neurosci. 2002;22(18):7844–7849. doi: 10.1523/JNEUROSCI.22-18-07844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le A., Harding S., Juzytsch W., Funk D., Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology. 2005;179(2):366–373. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- Lehavot K., Stappenbeck C.A., Luterek J.A., Kaysen D., Simpson T.L. Gender differences in relationships among PTSD severity, drinking motives, and alcohol use in a comorbid alcohol dependence and PTSD sample. Psychol. Addict. Behav. : journal of the Society of Psychologists in Addictive Behaviors. 2014;28(1):42–52. doi: 10.1037/a0032266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Bowe J., Kinsey‐Jones J., Brain S., Lightman S., O'byrne K. Differential role of corticotrophin‐releasing factor receptor types 1 and 2 in stress‐induced suppression of pulsatile luteinising hormone secretion in the female rat. J. Neuroendocrinol. 2006;18(8):602–610. doi: 10.1111/j.1365-2826.2006.01450.x. [DOI] [PubMed] [Google Scholar]
- Li Z., Snieder H., Su S. A longitudinal study in youth of heart rate variability at rest and in response to stress. Int. J. Psychophysiol. 2009;73(3):212–217. doi: 10.1016/j.ijpsycho.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D., Barnes R.W., Chambless L.E. Age, race, and sex differences in autonomic cardiac function measured by spectral analysis of heart rate variability—the ARIC study. Am. J. Cardiol. 1995;76(12):906–912. doi: 10.1016/s0002-9149(99)80260-4. [DOI] [PubMed] [Google Scholar]
- Lind K.E., Gutierrez E.J., Yamamoto D.J., Regner M.F., McKee S.A., Tanabe J. Sex disparities in substance abuse research: evaluating 23 years of structural neuroimaging studies. Drug Alcohol Depend. 2017;173:92–98. doi: 10.1016/j.drugalcdep.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemeyer A.K., Liang J., Marty V.N. Ethanol-induced plasticity of GABA A receptors in the basolateral amygdala. Neurochem. Res. 2014;39(6):1162–1170. doi: 10.1007/s11064-014-1297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindheim S.R., Legro R.S., Bernstein L. Behavioral stress responses in premenopausal and postmenopausal women and the effects of estrogen. Am. J. Obstet. Gynecol. 1992;167(6):1831–1836. doi: 10.1016/0002-9378(92)91783-7. [DOI] [PubMed] [Google Scholar]
- Liu I.-C., Chiu C.-H., Yang T.-T. The effects of gender and a co-occurring depressive disorder on neurocognitive functioning in patients with alcohol dependence. Alcohol Alcohol. 2010;45(3):231–236. doi: 10.1093/alcalc/agq016. [DOI] [PubMed] [Google Scholar]
- Lobo I.A., Harris R.A. GABA(A) receptors and alcohol. Pharmacol., Biochem. Behav. 2008;90(1):90–94. doi: 10.1016/j.pbb.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip M.L., Oleata C., Roberto M. Sex differences in responses of the basolateral-central amygdala circuit to alcohol, corticosterone and their interaction. Neuropharmacology. 2017;114:123–134. doi: 10.1016/j.neuropharm.2016.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo W.R., Dickensheets S.L., Myers D.A., Thomas T.L., Nixon S.J. Blunted stress cortisol response in abstinent alcoholic and polysubstance‐abusing men. Alcohol Clin. Exp. Res. 2000;24(5):651–658. [PubMed] [Google Scholar]
- Lynch W.J., Sofuoglu M. Role of progesterone in nicotine addiction: evidence from initiation to relapse. Exp. Clin. Psychopharmacol. 2010;18(6):451–461. doi: 10.1037/a0021265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthey J., Imtiaz S., Neufeld M., Rylett M., Rehm J. Quantifying the global contribution of alcohol consumption to cardiomyopathy. Popul. Health Metrics. 2017;15:20. doi: 10.1186/s12963-017-0137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel M.M., Eisenlohr-Moul T., Roberts B. Interactive effects of ovarian steroid hormones on alcohol use and binge drinking across the menstrual cycle. J. Abnorm. Psychol. 2017;126(8):1104–1113. doi: 10.1037/abn0000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mary Ann Emmanuel F.W., Nicholas V. 2003. Emanuele. Alcohol's Effects on Female Reproductive Function. [Google Scholar]
- Maynard M.E., Barton E.A., Robinson C.R., Wooden J.I., Leasure J.L. Sex differences in hippocampal damage, cognitive impairment, and trophic factor expression in an animal model of an alcohol use disorder. Brain Struct. Funct. 2018;223(1):195–210. doi: 10.1007/s00429-017-1482-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain J.A., Morris S.A., Deeny M.A. Adolescent binge alcohol exposure induces long-lasting partial activation of microglia. Brain Behav. Immun. 2011;25:S120–S128. doi: 10.1016/j.bbi.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougle C.J., Price L.H., Heninger G.R., Krystal J.H., Charney D.S. Noradrenergic response to acute ethanol administration in heathly subjects: comparison with intravenous yohimbine. Psychopharmacology. 1995;118(2):127–135. doi: 10.1007/BF02245830. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Stressed or stressed out: what is the difference? J. Psychiatr. Neurosci. 2005;30(5):315. [PMC free article] [PubMed] [Google Scholar]
- McKee S. American Psychological Association Annual Convention; Honolulu, HI: 2013. Why Is it More Difficult for Women to Quit Smoking? Translating Knowledge into Practice. [Google Scholar]
- McKee S.A., Sinha R., Weinberger A.H. Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. J. Psychopharmacol. 2011;25(4):490–502. doi: 10.1177/0269881110376694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J.P., Marton-Popovici M., Chavkin C. κ opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J. Neurosci. 2003;23(13):5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally R.J. Mechanisms of exposure therapy: how neuroscience can improve psychological treatments for anxiety disorders. Clin. Psychol. Rev. 2007;27(6):750–759. doi: 10.1016/j.cpr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Milior G., Lecours C., Samson L. Fractalkine receptor deficiency impairs microglial and neuronal responsiveness to chronic stress. Brain Behav. Immun. 2016;55:114–125. doi: 10.1016/j.bbi.2015.07.024. [DOI] [PubMed] [Google Scholar]
- Millan M.J. The neurobiology and control of anxious states. Prog. Neurobiol. 2003;70(2):83–244. doi: 10.1016/s0301-0082(03)00087-x. [DOI] [PubMed] [Google Scholar]
- Miller L.R., Marks C., Becker J.B. Considering sex as a biological variable in preclinical research. FASEB J. : official publication of the Federation of American Societies for Experimental Biology. 2017;31(1):29–34. doi: 10.1096/fj.201600781R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur Y.S., Cahuzac E.L., Mose T.N. Interaction between noradrenergic and cholinergic signaling in amygdala regulates anxiety- and depression-related behaviors in mice. Neuropsychopharmacology. 2018;43(10):2118–2125. doi: 10.1038/s41386-018-0024-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogos M.F., Salemi J.L., Russell K.D., Piano M.R. Contemporary appraisal of sex differences in prevalence, correlates, and outcomes of alcoholic cardiomyopathy in the United States. Am Heart Assoc. 2017;136(suppl 1) doi: 10.1093/alcalc/agz050. A14554. [DOI] [PubMed] [Google Scholar]
- Mokdad A.H., Marks J.S., Stroup D.F., Gerberding J.L. Actual causes of death in the United States, 2000. J. Am. Med. Assoc. 2004;291(10):1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Moos R.H., Moos B.S., Timko C. Gender, treatment and self-help in remission from alcohol use disorders. Clin. Med. Res. 2006;4(3):163–174. doi: 10.3121/cmr.4.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M., Anderson R.I., Spear L.P., Varlinskaya E.I. Effects of the kappa opioid receptor antagonist, nor‐binaltorphimine, on ethanol intake: impact of age and sex. Dev. Psychobiol. 2014;56(4):700–712. doi: 10.1002/dev.21137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muti P., Trevisan M., Micheli A. Alcohol consumption and total estradiol in premenopausal women. Cancer Epidemiology and Prevention Biomarkers. 1998;7(3):189–193. [PubMed] [Google Scholar]
- Muzyk A.J., Fowler J.A., Norwood D.K., Chilipko A. Role of α2-agonists in the treatment of acute alcohol withdrawal. Ann. Pharmacother. 2011;45(5):649–657. doi: 10.1345/aph.1P575. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute Alcohol and cancer risk. 2013. https://www.cancer.gov/about-cancer/causes-prevention/risk/alcohol/alcohol-fact-sheet
- National Institute of Alcohol Abuse and Alcoholism . 2017. Women and Alcohol. [Google Scholar]
- Negus S.S., Zuzga D.S., Mello N.K. Sex differences in opioid antinociception in rhesus monkeys: antagonism of fentanyl and U50, 488 by quadazocine. J. Pain. 2002;3(3):218–226. doi: 10.1054/jpai.2002.124734. [DOI] [PubMed] [Google Scholar]
- Nentwig T.B., Wilson D.E., Rhinehart E.M., Grisel J.E. Sex differences in binge‐like EtOH drinking, corticotropin‐releasing hormone and corticosterone: effects of β‐endorphin. Addict. Biol. 2018 doi: 10.1111/adb.12610. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon S.J., Prather R., Lewis B. vol. 125. Elsevier; 2014. pp. 253–272. (Sex Differences in Alcohol-Related Neurobehavioral Consequences. Handbook of Clinical Neurology). [DOI] [PubMed] [Google Scholar]
- Noël X., Sferrazza R., Van der Linden M. Contribution of frontal cerebral blood flow measured by 99mTc-Bicisate SPECT and executive function deficits to predicting treatment outcome in alcohol-dependent patients. Alcohol Alcohol. 2002;37(4):347–354. doi: 10.1093/alcalc/37.4.347. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S., Harrell Z.A. Rumination, depression, and alcohol use: tests of gender differences. J. Cognit. Psychother. 2002;16(4):391–404. [Google Scholar]
- Nolen-Hoeksema S., Hilt L. Possible contributors to the gender differences in alcohol use and problems. J. Gen. Psychol. 2006;133(4):357–374. doi: 10.3200/GENP.133.4.357-374. [DOI] [PubMed] [Google Scholar]
- Novais A., Monteiro S., Roque S., Correia-Neves M., Sousa N. How age, sex and genotype shape the stress response. Neurobiology of Stress. 2017;6:44–56. doi: 10.1016/j.ynstr.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt D.J., King L.A., Phillips L.D. Drug harms in the UK: a multicriteria decision analysis. Lancet. 2010;376(9752):1558–1565. doi: 10.1016/S0140-6736(10)61462-6. [DOI] [PubMed] [Google Scholar]
- Oberleitner L.M., Smith P.H., Weinberger A.H., Mazure C.M., McKee S.A. Impact of exposure to childhood maltreatment on transitions to alcohol dependence in women and men. Child. Maltreat. 2015;20(4):301–308. doi: 10.1177/1077559515591270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olthuis J.V., Watt M.C., Mackinnon S.P., Stewart S.H. CBT for high anxiety sensitivity: alcohol outcomes. Addict. Behav. 2015;46:19–24. doi: 10.1016/j.addbeh.2015.02.018. [DOI] [PubMed] [Google Scholar]
- Ota K.T., Liu R.-J., Voleti B. REDD1 is essential for stress-induced synaptic loss and depressive behavior. Nat. Med. 2014;20(5):531. doi: 10.1038/nm.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park G., Vasey M.W., Van Bavel J.J., Thayer J.F. When tonic cardiac vagal tone predicts changes in phasic vagal tone: the role of fear and perceptual load. Psychophysiology. 2014;51(5):419–426. doi: 10.1111/psyp.12186. [DOI] [PubMed] [Google Scholar]
- Patkar A.A., Marsden C.A., Naik P.C. Differences in peripheral noradrenergic function among actively drinking and abstinent alcohol‐dependent individuals. Am. J. Addict. 2004;13(3):225–235. doi: 10.1080/10550490490459898. [DOI] [PubMed] [Google Scholar]
- Patkar O.L., Belmer A., Bartlett S.E. InTech; 2016. Contribution of Noradrenaline, Serotonin, and the Basolateral Amygdala to Alcohol Addiction: Implications for Novel Pharmacotherapies for AUDs. Recent Advances in Drug Addiction Research and Clinical Applications. [Google Scholar]
- Patra J., Taylor B., Irving H. Alcohol consumption and the risk of morbidity and mortality for different stroke types-a systematic review and meta-analysis. BMC Public Health. 2010;10(1):258. doi: 10.1186/1471-2458-10-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen S.B., Kristensen K., Hermann P.A., Katzenellenbogen J.A., Richelsen B. Estrogen controls lipolysis by up-regulating α2a-adrenergic receptors directly in human adipose tissue through the estrogen receptor α. Implications for the female fat distribution. J. Clin. Endocrinol. Metab. 2004;89(4):1869–1878. doi: 10.1210/jc.2003-031327. [DOI] [PubMed] [Google Scholar]
- Penzlin A.I., Barlinn K., Illigens B.M.-W., Weidner K., Siepmann M., Siepmann T. Effect of short-term heart rate variability biofeedback on long-term abstinence in alcohol dependent patients – a one-year follow-up. BMC Psychiatry. 2017;17(1):325. doi: 10.1186/s12888-017-1480-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry N.M., Martin B., Cooney J.L., Kranzler H.R. vol. 68. American Psychological Association; US: 2000. pp. 250–257. (Give Them Prizes and They Will Come: Contingency Management for Treatment of Alcohol Dependence). [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A., Rosenbloom M., Deshmukh A., Sullivan E.V. Sex differences in the effects of alcohol on brain structure. Am. J. Psychiatry. 2001;158(2):188–197. doi: 10.1176/appi.ajp.158.2.188. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A., Adalsteinsson E., Sullivan E.V. Dysmorphology and microstructural degradation of the corpus callosum: interaction of age and alcoholism. Neurobiol. Aging. 2006;27(7):994–1009. doi: 10.1016/j.neurobiolaging.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A., Rosenbloom M., Rohlfing T., Sullivan E.V. Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biol. Psychiatry. 2009;65(8):680–690. doi: 10.1016/j.biopsych.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev E.D., Veremeyko T., Barteneva N., Krichevsky A.M., Weiner H.L. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-α–PU. 1 pathway. Nat. Med. 2011;17(1):64. doi: 10.1038/nm.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana D.S., Guastella A.J., McGregor I.S., Hickie I.B., Kemp A.H. Heart rate variability predicts alcohol craving in alcohol dependent outpatients: further evidence for HRV as a psychophysiological marker of self-regulation. Drug Alcohol Depend. 2013;132(1):395–398. doi: 10.1016/j.drugalcdep.2013.02.025. [DOI] [PubMed] [Google Scholar]
- Racz I., Markert A., Mauer D., Stoffel-Wagner B., Zimmer A. Long-term ethanol effects on acute stress responses: modulation by dynorphin. Addict. Biol. 2013;18(4):678–688. doi: 10.1111/j.1369-1600.2012.00494.x. [DOI] [PubMed] [Google Scholar]
- Rasakham K., Liu-Chen L.-Y. Sex differences in kappa opioid pharmacology. Life Sci. 2011;88(1–2):2–16. doi: 10.1016/j.lfs.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash C.J., Petry N.M. Contingency management treatments are equally efficacious for both sexes in intensive outpatient settings. Exp. Clin. Psychopharmacol. 2015;23(5):369. doi: 10.1037/pha0000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray L.A., Roche D.J., Heinzerling K., Shoptaw S. Opportunities for the development of neuroimmune therapies in addiction. Int. Rev. Neurobiol. 2014;118:381–401. doi: 10.1016/B978-0-12-801284-0.00012-9. [DOI] [PubMed] [Google Scholar]
- Rehm J., Taylor B., Mohapatra S. Alcohol as a risk factor for liver cirrhosis: a systematic review and meta‐analysis. Drug Alcohol Rev. 2010;29(4):437–445. doi: 10.1111/j.1465-3362.2009.00153.x. [DOI] [PubMed] [Google Scholar]
- Riga D., Schmitz L.J., Van Der Harst J.E. A sustained depressive state promotes a guanfacine reversible susceptibility to alcohol seeking in rats. Neuropsychopharmacology. 2014;39(5):1115. doi: 10.1038/npp.2013.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohan Walker F., Nilsson M., Jones K. Acute and chronic stress-induced disturbances of microglial plasticity, phenotype and function. Curr. Drug Targets. 2013;14(11):1262–1276. doi: 10.2174/13894501113149990208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruigrok A.N., Salimi-Khorshidi G., Lai M.-C. A meta-analysis of sex differences in human brain structure. Neurosci. Biobehav. Rev. 2014;39:34–50. doi: 10.1016/j.neubiorev.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell S.E., Rachlin A.B., Smith K.L. Sex differences in sensitivity to the depressive-like effects of the kappa opioid receptor agonist U-50488 in rats. Biol. Psychiatry. 2014;76(3):213–222. doi: 10.1016/j.biopsych.2013.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybaczyk L.A., Bashaw M.J., Pathak D.R., Moody S.M., Gilders R.M., Holzschu D.L. An overlooked connection: serotonergic mediation of estrogen-related physiology and pathology. BMC Women's Health. 2005;5 doi: 10.1186/1472-6874-5-12. 12-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks J.J., Gonzales K.R., Bouchery E.E., Tomedi L.E., Brewer R.D. 2010 national and state costs of excessive alcohol consumption. Am. J. Prev. Med. 2015;49(5):e73–e79. doi: 10.1016/j.amepre.2015.05.031. [DOI] [PubMed] [Google Scholar]
- Sanacora G., Mason G.F., Rothman D.L. Reduced cortical γ-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch. Gen. Psychiatr. 1999;56(11):1043–1047. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- Sanders S., Shekhar A. Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacol. Biochem. Behav. 1995;52(4):701–706. doi: 10.1016/0091-3057(95)00153-n. [DOI] [PubMed] [Google Scholar]
- Sartor C.E., Bachrach R.L., Stepp S.D., Werner K.B., Hipwell A.E., Chung T. The relationship between childhood trauma and alcohol use initiation in Black and White adolescent girls: considering socioeconomic status and neighborhood factors. Soc. Psychiatr. Psychiatr. Epidemiol. 2018;53(1):21–30. doi: 10.1007/s00127-017-1461-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwandt M.L., Cortes C.R., Kwako L.E. The CRF1 antagonist verucerfont in anxious alcohol-dependent women: translation of neuroendocrine, but not of anti-craving effects. Neuropsychopharmacology. 2016;41(12):2818. doi: 10.1038/npp.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D., Jia Z., Lacadie C.M., Tsou K.A., Bergquist K., Sinha R. Sex differences in neural responses to stress and alcohol context cues. Hum. Brain Mapp. 2011;32(11):1998–2013. doi: 10.1002/hbm.21165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky R., Glavis-Bloom C., Lerman D. Estrogen mediates sex differences in stress-induced prefrontal cortex dysfunction. Mol. Psychiatr. 2004;9(5):531. doi: 10.1038/sj.mp.4001435. [DOI] [PubMed] [Google Scholar]
- Sharrett-Field L., Butler T.R., Reynolds A.R., Berry J.N., Prendergast M.A. Sex differences in neuroadaptation to alcohol and withdrawal neurotoxicity. Pflueg. Arch. Eur. J. Physiol. 2013;465(5):643–654. doi: 10.1007/s00424-013-1266-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shield K.D., Rylett M., Gmel G., Gmel G., Kehoe‐Chan T.A., Rehm J. Global alcohol exposure estimates by country, territory and region for 2005—a contribution to the comparative risk assessment for the 2010 global burden of disease study. Addiction. 2013;108(5):912–922. doi: 10.1111/add.12112. [DOI] [PubMed] [Google Scholar]
- Shin S.H., McDonald S.E., Conley D. Patterns of adverse childhood experiences and substance use among young adults: a latent class analysis. Addict. Behav. 2018;78:187–192. doi: 10.1016/j.addbeh.2017.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman E.P., Harden K.P., Chein J.M., Steinberg L. Sex differences in the developmental trajectories of impulse control and sensation-seeking from early adolescence to early adulthood. J. Youth Adolesc. 2015;44(1):1–17. doi: 10.1007/s10964-014-0116-9. [DOI] [PubMed] [Google Scholar]
- Silva S.M., Madeira M.D. Effects of chronic alcohol consumption and withdrawal on the response of the male and female hypothalamic–pituitary–adrenal axis to acute immune stress. Brain Res. 2012;1444:27–37. doi: 10.1016/j.brainres.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann. N. Y. Acad. Sci. 2008;1141(1):105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skilbeck K., Hinton T., Johnston G. Sex-differences and stress: effects on regional high and low affinity [3H] GABA binding. Neurochem. Int. 2008;52(6):1212–1219. doi: 10.1016/j.neuint.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Smith P.H., Saddleson M.L., Homish G.G., McKee S.A., Kozlowski L.T., Giovino G.A. The relationship between childhood physical and emotional abuse and smoking cessation among US women and men. Psychol. Addict. Behav. 2015;29(2):338. doi: 10.1037/adb0000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.L., Iredale J.M., Mattick R.P. Sex differences in the relationship between heavy alcohol use, inhibition and performance monitoring: disconnect between behavioural and brain functional measures. Psychiatr. Res. Neuroimaging. 2016;254:103–111. doi: 10.1016/j.pscychresns.2016.06.012. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M., DeVito E.E., Waters A.J., Carroll K.M. Cognitive enhancement as a treatment for drug addictions. Neuropharmacology. 2013;64(1):452–463. doi: 10.1016/j.neuropharm.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F. Neurodegeneration in women. Alcohol Res. Health. 2002;26(4):316–318. [PMC free article] [PubMed] [Google Scholar]
- Sonne Susan C., Back Sudie E., Zuniga Claudia D., Randall Carrie L., Brady Kathleen T. Gender differences in individuals with comorbid alcohol dependence and post‐traumatic stress disorder. Am. J. Addict. 2010;12(5):412–423. [PubMed] [Google Scholar]
- Sorocco K.H., Lovallo W.R., Vincent A.S., Collins F.L. Blunted hypothalamic–pituitary–adrenocortical axis responsivity to stress in persons with a family history of alcoholism. Int. J. Psychophysiol. 2006;59(3):210–217. doi: 10.1016/j.ijpsycho.2005.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova N. γ-Aminobutyric acid-immunoreactive neurons in the amygdala of the rat–sex differences and effect of early postnatal castration. Neurosci. Lett. 1998;255(3):175–177. doi: 10.1016/s0304-3940(98)00735-6. [DOI] [PubMed] [Google Scholar]
- Stephens M.A.C., Wand G. Stress and the HPA Axis: role of glucocorticoids in alcohol dependence. Alcohol Res. Curr. Rev. 2012;34(4):468–483. doi: 10.35946/arcr.v34.4.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Adminstration & National Institute on Alcohol Abuse and Alcoholism . 2015. Medication for the Treatment of Alcohol Use Disorder: A Brief Guide. [Google Scholar]
- Szabo G. Women and alcoholic liver disease — warning of a silent danger. Nature Reviews Gastroenterology & Hepatology. 2018;15:253. doi: 10.1038/nrgastro.2018.8. [DOI] [PubMed] [Google Scholar]
- Szabo G., Mandrekar P. A recent perspective on alcohol, immunity, and host defense. Alcohol Clin. Exp. Res. 2009;33(2):220–232. doi: 10.1111/j.1530-0277.2008.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanky N.R., Son J.H., Herbison A.E. Sex differences in the regulation of tyrosine hydroxylase gene transcription by estrogen in the locus coeruleus of TH9-LacZ transgenic mice. Mol. Brain Res. 2002;104(2):220–226. doi: 10.1016/s0169-328x(02)00383-2. [DOI] [PubMed] [Google Scholar]
- Tomiyama A.J., Dallman M.F., Epel E.S. Comfort food is comforting to those most stressed: evidence of the chronic stress response network in high stress women. Psychoneuroendocrinology. 2011;36(10):1513–1519. doi: 10.1016/j.psyneuen.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkmen S., Backstrom T., Wahlstrom G., Andreen L., Johansson I.-M. Tolerance to allopregnanolone with focus on the GABA-A receptor. Br. J. Pharmacol. 2011;162(2):311–327. doi: 10.1111/j.1476-5381.2010.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udo T., Bates M.E., Mun E.Y. Gender differences in acute alcohol effects on self-regulation of arousal in response to emotional and alcohol-related picture cues. Psychol. Addict. Behav. 2009;23(2):196. doi: 10.1037/a0015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vamvakopoulos N.C., Chrousos G.P. Evidence of direct estrogenic regulation of human corticotropin-releasing hormone gene expression. Potential implications for the sexual dimophism of the stress response and immune/inflammatory reaction. J. Clin. Invest. 1993;92(4):1896–1902. doi: 10.1172/JCI116782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg J.F., Dogge B., Kist N., Kok R.M., Van der Hiele K. Gender differences in cognitive functioning in older alcohol-dependent patients. Subst. Use Misuse. 2017;52(5):574–580. doi: 10.1080/10826084.2016.1245341. [DOI] [PubMed] [Google Scholar]
- van Gool W.A., van de Beek D., Eikelenboom P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet. 2010;375(9716):773–775. doi: 10.1016/S0140-6736(09)61158-2. [DOI] [PubMed] [Google Scholar]
- Varlinskaya E.I., Spear L.P. Social consequences of ethanol: impact of age, stress, and prior history of ethanol exposure. Physiol. Behav. 2015;148:145–150. doi: 10.1016/j.physbeh.2014.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengeliene V., Vollmayr B., Henn F.A., Spanagel R. Voluntary alcohol intake in two rat lines selectively bred for learned helpless and non-helpless behavior. Psychopharmacology. 2005;178(2–3):125–132. doi: 10.1007/s00213-004-2013-5. [DOI] [PubMed] [Google Scholar]
- Verplaetse T.L., Weinberger A.H., Smith P.H. Targeting the noradrenergic system for gender-sensitive medication development for tobacco dependence. Nicotine Tob. Res. 2015;17(4):486–495. doi: 10.1093/ntr/ntu280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verplaetse T.L., Moore K.E., Pittman B.P. vol. 2. 2018. (Intersection of Stress and Gender in Association with Transitions in Past Year DSM-5 Substance Use Disorder Diagnoses in the United States. Chronic Stress). Thousand Oaks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Wang G.-J., Fowler J.S., Tomasi D., Telang F. Addiction: beyond dopamine reward circuitry. Proc. Natl. Acad. Sci. Unit. States Am. 2011;108(37):15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker B.M., Koob G.F. Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology. 2008;33(3):643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter T.J., Crews F.T. Microglial depletion alters the brain neuroimmune response to acute binge ethanol withdrawal. J. Neuroinflammation. 2017;14(1):86. doi: 10.1186/s12974-017-0856-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter T.J., Vetreno R.P., Crews F.T. Alcohol and stress activation of microglia and neurons: brain regional effects. Alcohol Clin. Exp. Res. 2017;41(12):2066–2081. doi: 10.1111/acer.13511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J., De Arcangelis J., de Wit H. Sex differences in behavioral impulsivity in at-risk and non-risk drinkers. Front. Psychiatry. 2015;6:72. doi: 10.3389/fpsyt.2015.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker D., Schroeder J.P. There and back again: a tale of norepinephrine and drug addiction. Neuropsychopharmacology. 2007;32(7):1433. doi: 10.1038/sj.npp.1301263. [DOI] [PubMed] [Google Scholar]
- White A., Castle I‐Jen P., Chen Chiung M., Shirley M., Roach D., Hingson R. Converging patterns of alcohol use and related outcomes among females and males in the United States, 2002 to 2012. Alcohol Clin. Exp. Res. 2015;39(9):1712–1726. doi: 10.1111/acer.12815. [DOI] [PubMed] [Google Scholar]
- Wohleb E.S., Terwilliger R., Duman C.H., Duman R.S. Stress-induced neuronal colony stimulating factor 1 provokes microglia-mediated neuronal remodeling and depressive-like behavior. Biol. Psychiatry. 2018;83(1):38–49. doi: 10.1016/j.biopsych.2017.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovleva T., Bazov I., Watanabe H., Hauser K.F., Bakalkin G. Transcriptional control of maladaptive and protective responses in alcoholics: a role of the NF-κB system. Brain Behav. Immun. 2011;25:S29–S38. doi: 10.1016/j.bbi.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoest K.E., Cummings J.A., Becker J.B. Estradiol, dopamine and motivation. Cent. Nerv. Syst. Agents Med. Chem. 2014;14(2):83–89. doi: 10.2174/1871524914666141226103135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon G., Kim S.W., Petrakis I.L., Westermeyer J. High-dose naltrexone treatment and gender in alcohol dependence. Clin. Neuropharmacol. 2016;39(4):165–168. doi: 10.1097/WNF.0000000000000152. [DOI] [PubMed] [Google Scholar]
- Zakiniaeiz Y., Hillmer A., Goetz L., Morris E. College on Problems of Drug Dependence Annual Meeting. 2017. Sex differences in dorsolateral prefrontal cortex dopamine release and the relationship to tobacco smoking treatment outcomes. (Montreal, Canada) [Google Scholar]
- Zgierska A., Rabago D., Chawla N., Kushner K., Koehler R., Marlatt A. Mindfulness meditation for substance use disorders: a systematic review. Subst. Abuse : official publication of the Association for Medical Education and Research in Substance Abuse. 2009;30(4):266–294. doi: 10.1080/08897070903250019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Stahl E.L., Lovell K.M. Characterization of kappa opioid receptor mediated, dynorphin-stimulated [35S] GTPγS binding in mouse striatum for the evaluation of selective KOR ligands in an endogenous setting. Neuropharmacology. 2015;99:131–141. doi: 10.1016/j.neuropharm.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla E.P., Heilig M., de Wit H., Shaham Y. Behavioral, biological, and chemical perspectives on targeting CRF1 receptor antagonists to treat alcoholism. Drug Alcohol Depend. 2013;128(3):175–186. doi: 10.1016/j.drugalcdep.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.