Abstract
The neurophysiological underpinnings involved in susceptibility to and maintenance of anxiety are not entirely known. However, two stress-responsive systems, the hypothalamic-pituitary-adrenal axis and the endocannabinoid system, may interact in anxiety. Here, we examine the relationship between FAAH genotype, CRFR1 genotype, baseline cortisol, and state anxiety in a rural adult population using data from Project FRONTIER. We predicted that FAAH A (AA and AC vs CC; rs324420) and three CRFR1 SNP minor alleles (rs7209436 C→ T [minor allele]; rs110402, G → A [minor]; and rs242924 G→ T [minor]), would interact to predict low baseline cortisol and low state anxiety scores. We found partial support for our prediction. In CRFR1 minor carriers, the FAAH AA or AC (vs. CC) genotype was associated with higher cortisol and with lower anxiety. In CRFR1 non-minors, those with FAAH AA or AC (vs. CC) showed decreased cortisol and higher anxiety. These results suggest that FAAH CC genotype only conveys risk for anxiety in individuals who are also carriers of the CRFR1 minor combination. FAAH genotype was significantly associated with baseline cortisol but was not independently associated with anxiety. Contrary to our predictions, baseline cortisol was negatively associated with anxiety. Lastly, we did not find any independent relationships between any of our SNPs and baseline cortisol or anxiety. These data suggest FAAH and cortisol interact to predict state anxiety, but that the relationship depends on CRFR1 genotype. The Project FRONTIER dataset is supported by Texas Tech University Health Sciences Center Garrison Institute on Aging.
Keywords: FAAH, CRFR1, Anxiety, Cortisol, Project FRONTIER
1. Introduction
According to National Institute of Mental Health (NIMH), over 40 million adults in the US, or roughly 18% of the population, suffer from anxiety, and nearly 30% of adults will experience an anxiety disorder during their lifetime (National Institute of Mental Health, 2017; Anxiety and Depression Association of America). Despite its prevalence, the neurobiological underpinnings of anxiety onset and maintenance are not fully understood. Using the NIMH Research Domain Criteria (RDoC) approach (Cuthbert and Insel, 2013), recent studies using humans and animal models have determined various biomarkers specific to anxiety. The RDoC aims to incorporate many dimensions including behavior, genetics, neurobiology, environment, and experiential effects into diagnosis and categorization of mental disorders, with the goal that doing so will aid in understanding disease etiology and will enhance treatment options (Insel, 2014) also see [Holmes and Patrick, 2018]). Due to the reliance on fear and threat in the diagnosis of anxiety disorders, studies have focused on the physiological systems associated with response to threats (stressors), specifically the hypothalamic-pituitary-adrenal (HPA) axis and the endocannabinoid system (Clinchy et al., 2011; Harris and Carr, 2016; Lang et al., 2016; McEwen et al., 2015; Perusini and Fanselow, 2015). Data suggest these two systems play both independent and interacting roles in the onset and maintenance of anxiety.
Dysregulation of the HPA axis, resulting in alteration to both baseline and stress-induced glucocorticoid levels (e.g., cortisol and corticosterone), has been implicated in several diseases and psychopathologies, including anxiety (McEwen et al., 2015, 2016; Shin and Liberzon, 2010). For example, baseline levels of cortisol were positively associated with anxiety scores in young (Takahashi et al., 2005), middle aged (Van Eck et al., 1996) and aged (Lenze et al., 2011) adults, and awakening cortisol levels were higher in individuals with anxiety disorders compared to those without (Vreeburg et al., 2010). Moreover, acute and chronic stress can both lead to increased HPA axis activity, resulting in release of glucocorticoid hormones, and an increase in anxiety (Pulopulos et al., 2015; Shin and Liberzon, 2010). These data suggest that elevated baseline and post-stress glucocorticoids are positively related to anxiety.
Additionally, anxiety is thought to be at least partially mediated by corticotropin-releasing factor (CRF) binding to CRFR1 in the limbic system, mainly the amygdala (Arborelius et al., 1999; Binder and Nemeroff, 2010; Reul and Holsboer, 2002; Rosen and Schulkin, 1998; Schulkin et al., 2005; Shin and Liberzon, 2010; Zorrilla et al., 2002). CRF primarily binds to one of two receptors, CRFR1 or CRFR2, but has higher affinity for CRFR1 and this receptor predominates in the hypothalamus, amygdala, cerebellum, pituitary and cerebral cortex (Sanders and Nemeroff, 2016). Hypothalamic CRF is important for initiation of the hypothalamic-pituitary-adrenal (HPA) axis, whereas CRF in the limbic system is important for the autonomic (sympathetic nervous system) and behavioral responses to stress, and is involved in fear and anxiety (Arborelius et al., 1999; Heinrichs and Koob, 2004; McEwen et al., 2015; Müller et al., 2003; Perusini and Fanselow, 2015). Hypothalamic and limbic CRF are independently regulated, but HPA axis output can potentiate the effect of limbic CRF as glucocorticoid binding in the amygdala increases CRF concentrations in that area (Binder and Nemeroff, 2010; Rosen and Schulkin, 1998), providing a possible link between elevated circulating glucocorticoids, CRF, and anxiety.
Although various manipulation studies in rodents (e.g., knockout of CRFR1, injection of CRF, injection of CRF antagonists) have shown that increased limbic CRF is anxiogenic, and that this response is driven by binding to CRFR1, the correlational data from humans is mixed (Arborelius et al., 1999; Sanders and Nemeroff, 2016). But, recent evidence suggests that CRF-related single nucleotide polymorphisms (SNPs) might be partially responsible for these discrepancies in the human literature and may help to explain risk and resilience (Binder and Nemeroff, 2010; Feder et al., 2009; Gillespie et al., 2009; Rogers et al., 2013; Weber et al., 2016). For example, three SNPs in the CRFR1 gene (rs7209436 C→ T [minor allele]; rs110402, G → A [minor]; and rs242924 G→ T [minor]) have been linked to HPA axis responsiveness (Mahon et al., 2013; Polanczyk et al., 2009; Tyrka et al., 2009), impact of childhood trauma on adult affect (depression; Bradley et al., 2008), and anxiety (Binder and Nemeroff, 2010; Feder et al., 2009). It seems when combined, the 3 minor CRFR1 SNP alleles are associated with decreased HPA axis responsiveness in adults and may be protective from (stress-related) affective disorders.
The endocannabinoid (eCB) system is a neuromodulatory network involved in the regulation of several stress-responsive neural circuits and can impact HPA axis activity (Gorzalka et al., 2008; Hill and McEwen, 2010; Hill and Tasker, 2012; McEwen et al., 2015). The two major signaling molecules of the eCB system are N-arachidonoylethanolamine (anandamide; AEA) and 2-arachidonoylglycerol (2-AG), which are synthesized post-synaptically (Hillard et al., 2011). Endocannabinoids bind to CB1 and CB2 receptors. CB1 receptors are the most abundant G-protein-coupled receptors in the central nervous system and are found on GABAergic, glutamatergic, serotonergic, noradrenergic, and dopaminergic terminals, but the predominant effects of eCB signaling result from modifying GABA and glutamate release (see Morena et al., 2016). Regulation of eCBs is done primarily via degrading enzymes, namely fatty acid amide hydrolase (FAAH) which metabolizes AEA, and monoacylglyceride (MAG) lipase, which metabolizes 2-AG. Genetic variability in the gene encoding FAAH may impact anxiety. Specifically, a substitution of a proline at amino-acid 129 with a threonine (C385A, rs324420), alters the regulation of FAAH, but not its enzymatic activity, making it more vulnerable to proteolytic degradation (Chiang et al., 2004; Sipe et al., 2002). The AA or AC genotype is associated with decreased FAAH, increased AEA levels, and reduced amygdala activity (increased inhibitory tone) comparison to the CC genotype (Gunduz-Cinar et al., 2013). Thus, CC individuals seem to be at higher risk for anxiety disorders and anxious behavior as compared to A carriers, and this effect has been shown in humans and in a knock-in mouse model (Dincheva et al., 2015; Hariri et al., 2009). Given these findings, FAAH has recently been tagged as a possible therapeutic target for anxiety disorders (Hill et al., 2013a; Patel et al., 2017).
When taken together, data suggest CRF plays a central role in linking the HPA axis, the eCB system, and the amygdala with anxiety (Hill et al., 2009, 2010a; Hillard et al., 2011). In elaborate studies in mice, Gray and colleagues (Gray et al., 2015) showed stress-associated increase in CRF concentration in the amygdala resulted in CRFR1 activation, an increase in FAAH activity, and thus decrease AEA levels. Overall, this cascade results in decreased AEA binding to CB1 receptors resulting in increased glutamate release, and increased activity of the basolateral amygdala resulting in increased expression of anxiety-like behaviors (Gray et al., 2015); this effect appeared to be independent of initial stress-related glucocorticoid increase. These data suggest one mechanism by which stress increases anxiety is via CRFR1 impacts on FAAH activity and AEA binding in the basolateral amygdala. Another recent study using a FAAH inhibitor in rats corroborates these findings (Natividad et al., 2017).
Given the relationships among the HPA axis, CRFR1, FAAH, and anxiety, we hypothesized that baseline cortisol and SNPs in CRFR1 and FAAH genes interact to influence state anxiety in humans (see Fig. 1). Here, we determined if baseline cortisol, FAAH, and CRFR1 SNPs are related to state anxiety levels, measured via the Beck Anxiety Inventory (BAI), in an aged, majority Hispanic, rural, community dwelling human population. We proposed a moderated mediation in that baseline cortisol mediates the relationship between FAAH SNP (rs324420) and state anxiety, and that effect is moderated by CRFR1 genotype. Specifically, we predict that FAAH A (AA and AC vs CC) and CRFR1 SNP minor alleles (AA, TT, TT) will interact to predict low baseline cortisol and those individuals will have the lowest state anxiety scores. Additionally, for comparison with published data on other sample populations, we determined if baseline cortisol and any of the four SNPs are individually associated with anxiety scores, and if any of the four SNPs are related to baseline cortisol. To date, only one study, to our knowledge, has investigated the interactive effects of CRFR1 (rs110402; AA vs. GG and GA) and FAAH (rs324420; CC vs. AC and AA) genotype on anxiety-related markers in humans (Demers et al., 2016). Contrary to our predictions based on previous data (see above), using fMRI data and a moderated mediation analysis, Demers and colleagues found that individuals high in AEA signaling (FAAH AA or AC) with CRFR1 protective allele (AA vs. AG and GG) had blunted basolateral amygdala habituation and increased risk of anxiety disorders (via the DSM-IV criteria); they did not assess cortisol. Thus, it is possible that we could find results predicted by the literature outlined above, or, we may corroborate findings of Demers and colleagues and find the opposite relationship.
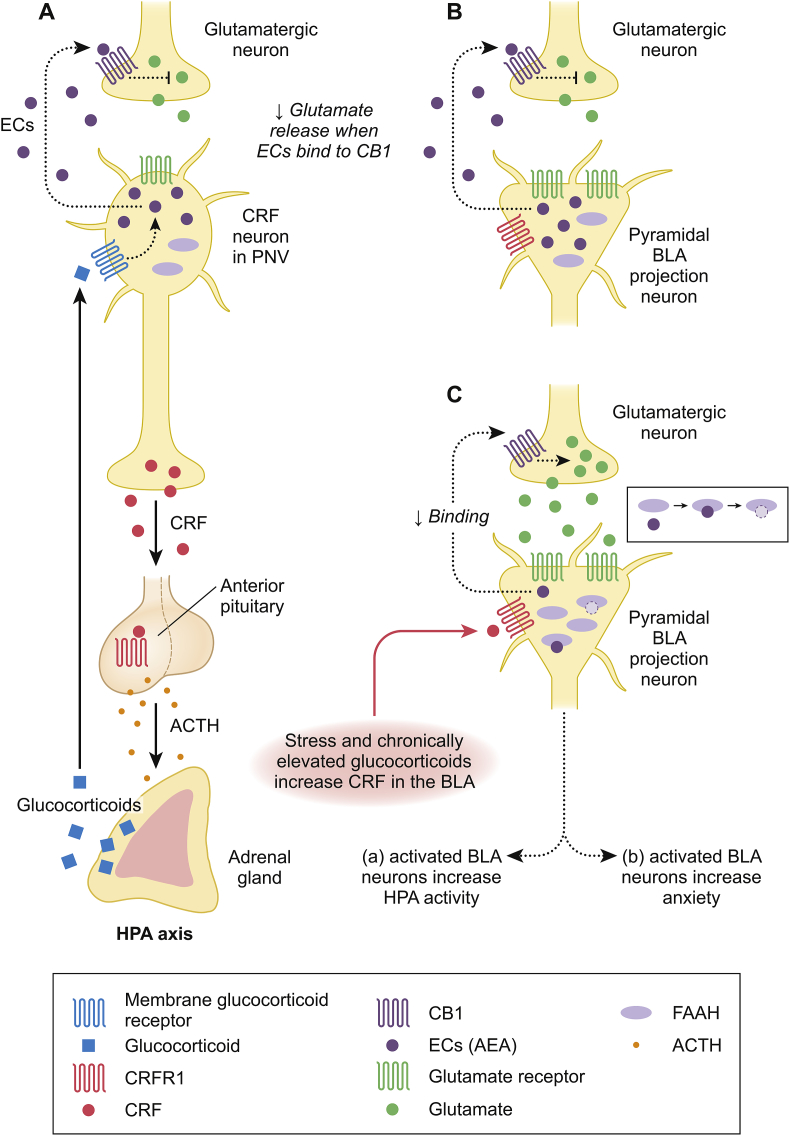
Fig. 1.
Schematic representation of the hypothesized relationship among FAAH, CRF, and cortisol in the paraventricular nucleus (PVN) of the hypothalamus and the basolateral amygdala (BLA). Based on the above information, we predict that individuals with the CC FAAH genotype (increased FAAH activity) will have increased BLA output and thus increased baseline cortisol and increased anxiety. Given that the CRFR1 minor alleles are associated with decreased response to stress in other studies, we predict minor allele carriers will be less stress responsive and therefore will show decreased cortisol and decreased anxiety. We predict having both protective alleles will be associated with the lowest cortisol levels and lowest anxiety scores. A) The HPA axis is active under basal conditions with constitutive release of CRF from the PVN; CRF binds to CRFR1 in the anterior pituitary resulting in increased ACTH, ACTH stimulates release of glucocorticoids from the adrenal cortex. This axis is under negative feedback inhibition with glucocorticoids inhibiting the axis at the PVN, the prefrontal cortex, and the hippocampus. In the PVN, glucocorticoids bind to a membrane bound glucocorticoid receptors on the CRF neurons, resulting in an increase in endocannabinoids (ECs) which bind to CB1 receptors on glutamatergic neurons and decrease release of glutamate and thus decreased activity of the HPA axis. The endocannabinoids seem to play a role in both baseline (mainly via BLA) and post-stress (via prefrontal cortex and PVN regulation) constraint of the axis. B) Within the BLA, tonic release of ECs (AEA) from pyramidal projection neurons keeps glutamate levels low via CB1 binding in the glutamatergic neuron. C) Acute stress initially results in increased CRF in the PVN as well as in the BLA and these seem to be independent of one another. Increased CRF in the BLA binds to CRFR1 on the pyramidal neurons which results in an increase in FAAH. FAAH then metabolizes AEA resulting in decreased binding to CB1 and therefore increased output of glutamate. This glutamate activates the BLA neurons which a) increases HPA axis output and b) increases anxiety. Chronically increased glucocorticoids lead to decreased CRF in the PVN but increased CRF in the amygdala, specifically the central amygdala and the basolateral amygdala. Figure is based on rodent and human data: (Gorzalka et al., 2008; Gray et al., 2015; Heinrichs and Koob, 2004; Hill et al., 2010a; Hill and Tasker, 2012; Müller et al., 2003; Roozendaal et al., 2008, Norris and Carr, 2013; Zajkowska et al., 2014; Morena et al., 2016).
2. Methods
2.1. Participants
Data were obtained from the Texas Tech University Health Sciences Project FRONTIER (Facing Rural Obstacles to healthcare Now through Intervention, Education, and Research) database (https://www.ttuhsc.edu/ruralhealth/researchgroup/frontier.aspx). Project FRONTIER is funded by Texas Tech University Health Sciences Center Garrison Institute on Aging and is currently ongoing. Study coordinators set-up appointments at study participant's rural county hospital every 3 years for testing and data collection. All data are collected by Project FRONTIER personnel and archived on a secure computer or in biobank freezers (biological specimens). Whole blood is collected by a trained phlebotomist at each visit. All data are collected with patient consent and TTUHSC Institutional Review Board approval was obtained by Project FRONTIER coordinators. Authors of this study did not directly interact with any of the participants. Authors obtained the following de-identified samples or variable data for 193 individuals: frozen whole blood, frozen serum, Beck Anxiety Inventory scores, last grade level completed in school, sex, age, race (white/Caucasian, black, or other), and ethnicity (Hispanic or not Hispanic). Participants from this study were community dwelling at the time of data collection and as long as they were over 40, were not excluded from the study for any reason. The average age of entry into the study was 58.27 yrs (range 40–87). Data obtained for this study were from the second appointment (year 3 of study).
Demographic information for the 193 participants is as follows: 92.2% Caucasian, 50.5% Hispanic, and 72.5% female. Self-reported highest grade level completed averaged 10.1 (range: 0–19). The average age at interview and data collection for samples obtained for our analysis was 61.09 yrs (std dev. 11.66, range: 42–90). All procedures performed by the authors were approved by the Texas Tech University IRB.
2.2. Project FRONTIER Beck Anxiety Inventory (BAI)
The BAI is a commonly used 21-item, self-report questionnaire which asks participants to rank anxiety symptoms on their severity over the past week using a 0–3 point Likert scale (Beck et al., 1988; Beck and Steer, 1993). The test has high internal reliability and discriminant validity (Fydrich et al., 1992; Kabacoff et al., 1997). Scores for each item are added and possible total score ranges from 0 to 63 (max score of 3 on each of 21 items). Recommended clinical cut-offs are: 0–7 minimal anxiety, 8–15 mild anxiety,16–25 moderate anxiety, over 26, severe anxiety (Carney et al., 2011); clinically significant anxiety according to Beck and Steer (1993) is 16. For all participants, BAI scores averaged 4.94 (range: 0–34; n = 193) and were thus in the minimally anxious range. Only 11 participants scored over 16 with 5 of those being higher than 26. Thus, only a handful of participants involved were likely clinically anxious, allowing us to correlate genotypes with measures of state anxiety in a majority non-clinical, aged adult, rural population.
2.3. DNA extraction and SNP genotyping
Whole blood was used for DNA extraction. Extraction was done using a commercially available kit as per the manufacturer's instructions (FlexiGene kit # 51206, Qiagen), except for the following modification: extracted DNA was suspended in molecular grade water instead of the storage buffer provided with the Qiagen kit. Following extraction, concentration of DNA in each sample was determined by Nanodrop spectrophotometry (average: 222 ng/ul) and the 260/280 ratio was inspected for purity. Extracted DNA was diluted with molecular grade water to a concentration of 10 ng/ul, aliquoted, and frozen for future use.
Genotyping analysis was performed on an Applied Biosystems 7300 quantitative PCR machine using commercially available TaqMan allelic discrimination SNP genotyping assays (Applied Biosystems, ThermoFisher Scientific). Participant genotype for FAAH (rs324420 [A or C]; kit C1897306), and three CRFR1 loci (rs110402 [A or G], kit C2544843; rs7209436 [C or T], kit C1570087; rs242924 [T or G], kit C2257689) as per the kit manufacturer's instructions. Genotype data for 176–186 individuals were available for analysis (FAAH: 186; CRFR1: 176 [rs110402], 185 [rs7209436], and 181 [rs242924]).
2.4. Serum cortisol assay
Project FRONTIER personnel obtained serum samples from blood collected at each visit. Of the 193 participant datasets requested, 191 had serum samples available. Serum was collected from blood and frozen in aliquots in the biobank. All blood samples were collected between 7:30 a.m. and 2:45 p.m. (only two samples were collected after 12 noon), and 90% of samples were collected between 7:00 and 9:30 a.m., thus we used time of day as a covariate in analyses. Concentration of serum cortisol was determined using a commercially available radioimmunoassay kit (ImmuChem, 07–221102; MP Biomedicals, Solon, OH) according to manufacturer's instructions.
2.5. Statistical analysis
All analyses were completed using SPSS v. 22 and alpha was set at 0.05. Moderated mediation analyses (Preacher, 2007, Preacher and Hayes, 2008) were conducted to examine the conditional indirect effect of baseline cortisol on the interaction of FAAH_A (AA and AC vs CC) and CRF1_minor (minor at all 3 SNPs: AA, TT, TT) on anxiety (for the benefits of this approach see [Antonakis et al., 2010; Hayes, 2009]), controlling for time of day the blood was drawn. The sexes were analyzed together. For this analysis we used model 7 in the process macro for SPSS (Hayes, 2018) with 50,000 bootstrapped samples (for the benefits of bootstrapping see [Hayes and Preacher, 2010; MacKinnon et al., 2000],). Note, for the CRFR1 minor grouping, the total n is 185 as we were able to include all participants with known CRFR1 genotype because even if they were missing data for one SNP, the other two did not meet the CRFR1 minor criteria.
Additional analyses for association between SNP and anxiety or cortisol concentration were completed via one-way ANOVA; effect sizes are reported as partial eta squared. Anxiety and cortisol association was assessed using Pearson's r.
3. Results
3.1. Moderated mediation
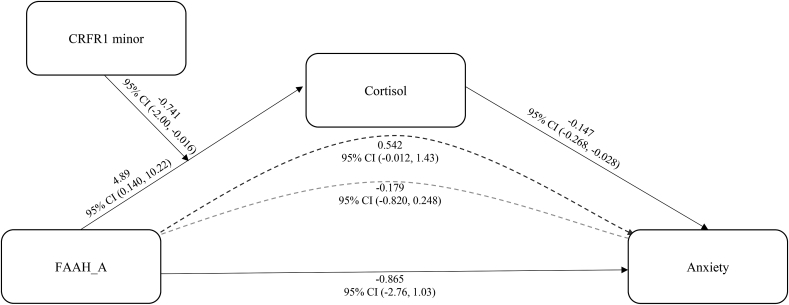
The total effect of the interaction between FAAH_A and CRF1_minor on anxiety was not statistically significant (b = −0.890, SE = 0.941, 95% CI bias corrected [-2.75, 0.967]). Results from the moderated mediation analysis indicated that the total indirect effect (interaction between FAAH_A and CRF1_minor to cortisol to anxiety) was significant – b = −0.721, SE = 0.505, 95% CI bias corrected [-2.00, −0.016]. Examining this moderated mediation, the conditional indirect effect of cortisol on anxiety was positive for CRF1_minor carriers, b = 0.542, SE = 0.380, 95% CI bias corrected [-0.012, 1.43], meaning that in CRF1_minor carriers, having the FAAH CC (compared to AA, AC) genotype is associated with lower cortisol and here lower cortisol is associated with increased anxiety. The conditional indirect effect was negative for CRF1_non-minor carriers, b = −0.180, SE = 0.254, 95% CI bias corrected [-0.820, 0.248], meaning that in CRF1_non-minor carriers, having the FAAH CC (compared to AA, AC) genotype is associated with increased cortisol and here increased cortisol is associated with lowered anxiety. Overall, the moderated mediation results suggest that the mediation of cortisol between FAAH_A and anxiety goes in one direction for CRF1_minor carriers and goes in the opposite direction for CRF1_non-minors. A summary of predictions and results are presented in Table 1 and results from the model are presented in Fig. 2.
Table 1.
Summary of predictions and results. Results that correspond with predictions are shown in bold, results that did not are italicized. We predicted that FAAH A (AA and AC vs CC) and CRFR1 SNP minor alleles (AA, TT, TT) would interact to predict low baseline cortisol and the lowest state anxiety scores. We predicted the CC FAAH genotype (increased FAAH activity) would have high baseline cortisol and high anxiety scores. Additionally, we predicted CRFR1 minor allele carriers would show low cortisol and low anxiety.
| CRFR1 Minor SNP carriers (“protective”, decreased HPA activity) | CRFR1 non-minor SNP carriers (“more risky”, increased HPA activity) | ||
| FAAH AA/AC (High AEA) | FAAH CC (Low AEA) | FAAH AA/AC (High AEA) | FAAH CC (Low AEA) |
|
Higher cortisol Lower anxiety |
Lower cortisol Higher anxiety |
Lower cortisol Higher anxiety |
Higher cortisol Lower anxiety |
Fig. 2.
Cortisol mediated the interaction between FAAH genotype and CRFR1 minor on anxiety. Pathway coefficients represent unstandardized b values. The 95% confidence interval (95% CI) is presented below the b value, if the interval does not include zero then it is significant at p < 0.05. The dark dotted line is the mediation of cortisol on FAAH and anxiety for CRFR1 minor allele carriers and the gray dotted line is the mediation of cortisol on FAAH and anxiety for CRFR1 non-minor allele carriers.
3.2. Genotypes and associations between SNPs, cortisol, and anxiety
Genotype data are shown in Table 2. Individual genotype did not impact baseline cortisol (Table 3) or scores on the Beck Anxiety Inventory (Table 4). Baseline cortisol and state anxiety were negatively correlated in our total sample: as cortisol decreased, anxiety increased (r = −0.175, p = 0.015, n = 191).
Table 2.
Genotype frequencies.
| Gene | Restriction site | Genotype (n) | Total n | ||
|---|---|---|---|---|---|
| FAAH | rs324420 | AA (20) | AC (74) | CC (92) | 186 |
| CRFR1 | rs110402 | AA (64) | AG (74) | GG (38) | 176 |
| CRFR1 | rs7209436 | TT (63) | CT (80) | CC (42) | 185 |
| CRFR1 | rs242924 | TT (65) | TG (75) | GG (41) | 181 |
| CRFR1 minor | rs110402; rs7209436; rs242924 | AA/TT/TT (60) | All others (125) | 185 | |
Table 3.
Relationship between genotype and baseline cortisol (ug/dl) for all participants (sexes combined). Cortisol data presented are means and standard error, statistical results are from ANOVA, effects size is partial eta squared.
| Gene | Restriction site | Cortisol (genotype) | Main effect of genotype | Effect size | ||
|---|---|---|---|---|---|---|
| FAAH | rs324420 | 18.90 ± 0.82 (AA/AC) | 18.38 ± 0.83 (CC) | F 1,179 = 0.19, P = 0.660 | 0.001 | |
| CRFR1 | rs110402 | 19.28 ± 1.00 (AA) | 18.77 ± 0.94 (AG) | 16.63 ± 1.31 (GG) | F 2,171 = 1.38, P = 0.256 | 0.016 |
| CRFR1 | rs7209436 | 19.40 ± 1.00 (TT) | 18.65 ± 0.89 (CT) | 17.49 ± 1.23 (CC) | F 2,180 = 0.73, P = 0.484 | 0.008 |
| CRFR1 | rs242924 | 19.24 ± 0.98 (TT) | 19.45 ± 0.91 (TG) | 17.10 ± 1.23 (GG) | F 2,176 = 1.31, P = 0.272 | 0.015 |
| CRFR1 minor | rs110402 rs7209436 rs242924 | 19.78 ± 1.02 (AA/TT/TT) | 18.02 ± 0.71 (All others) | F 1,182, = 1.99, P = 0.159 | 0.011 | |
| CRFR1 | rs110402 | 19.28 ± 1.00 (AA) | 18.04 ± 0.76 (AG/GG) | F 1,172 = 0.97, P = 0.326 | 0.006 | |
| CRFR1 | rs7209436 | 19.40 ± .99 (TT) | 18.25 ± 0.72 (CT/CC) | F 1,181 = 0.88, P = 0.349 | 0.005 | |
| CRFR1 | rs242924 | 19.24 ± 0.97 (TT) | 18.61 ± 0.73 (TG/GG) | F 1,177 = 0.26, P = 0.608 | 0.002 | |
Table 4.
Relationship between genotype and Beck Anxiety Inventory (BAI) score. BAI data presented are means and standard error, statistical results are from ANOVA, effects size is partial eta squared.
| Gene | Restriction site | BAI (genotype) | Main effect of genotype | Effect size | ||
|---|---|---|---|---|---|---|
| FAAH | rs324420 | 5.15 ± 1.45 (AA) | 5.55 ± .75 (AC) | 4.47 ± 0.67 (CC) | F 2,183 = 0.59, P = 0.558 | 0.006 |
| CRFR1 | rs110402 | 4.72 ± 0.81 (AA) | 4.78 ± .75 (AG) | 5.18 ± 1.05 (GG) | F 2,173 = 0.07, P = 0.935 | 0.001 |
| CRFR1 | rs7209436 | 4.37 ± .81 (TT) | 5.02 ± .72 (CT) | 5.40 ± 0.99 (CC) | F 2,182 = 0.37, P = 0.694 | 0.004 |
| CRFR1 | rs242924 | 4.57 ± .80 (TT) | 5.00 ± .75 (TG) | 5.24 ± 1.01 (GG) | F 2,178 = 0.15, P = 0.859 | 0.002 |
| CRFR1 minor | rs110402 rs7209436 rs242924 | 4.40 ± 0.823 (AA/TT/TT) | 5.08 ± 0.568 (All others) | F 1,184 = 0.46, P = 0.498 | 0.003 | |
| CRFR1 | rs110402 | 4.72 ± 0.81 (AA) | 4.92 ± 0.61 (AG/GG) | F 1,174 = 0.04, P = 0.843 | <0.001 | |
| CRFR1 | rs7209436 | 4.37 ± 0.80 (TT) | 5.16 ± 0.58 (CT/CC) | F 1,183 = 0.64, P = 0.426 | 0.003 | |
| CRFR1 | rs242924 | 4.57 ± 0.80 (TT) | 5.09 ± 0.60 (TG/GG) | F 1,179 = 0.27, P = 0.605 | 0.001 | |
4. Discussion
We found partial support for our prediction that FAAH A (AA and AC vs CC) and CRFR1 SNP minor alleles (AA, TT, TT), would interact to predict low baseline cortisol and low state anxiety scores. In CRFR1 minor carriers, the FAAH AA or AC (vs. CC) genotype was associated with higher cortisol and with lower anxiety. Additionally, in CRFR1 non-minors, those with FAAH AA or AC (vs. CC) showed decreased cortisol and higher anxiety. Based on data from this population, it seems the minor alleles of CRFR1 and the AA or AC genotype of FAAH are related to reduced anxiety, but, surprisingly, related to higher, and not lower, baseline cortisol. Additionally, among individuals with the protective CRFR1 minor combination, those with the FAAH CC genotype that confers risk had higher anxiety, but, those with the FAAH CC genotype and without the protective CRFR1 minor combination had lower anxiety. Thus, it seems the FAAH CC genotype only confers risk for anxiety in individuals who are also carriers of the CRFR1 minor combination. When dissecting our model overall, FAAH genotype was significantly associated with baseline cortisol, in that CC individuals had higher cortisol than did AA and AC individuals, which is in line with our initial predictions. But, FAAH genotype was not independently associated with anxiety, and baseline cortisol was negatively associated with anxiety score; as cortisol decreased, anxiety increased, which is contrary to our prediction. Lastly, contrary to other studies we did not find any independent relationships between any SNPs and baseline cortisol or anxiety score.
Although unexpected based on our predictions, low baseline and post-stress cortisol being associated with high anxiety has been previously documented in both clinical and healthy populations of humans (Beaton et al., 2006; Jezova et al., 2004; Shirotsuki et al., 2009; Vingerhoets et al., 1996), and in rodents (Cohen et al., 2006). But, none of the above studies assess genotype. The reason for the association between decreased baseline and post-stress cortisol and anxiety is not clear. However, recent studies exploring the interaction between the HPA axis and the endocannabinoid system may provide insight (Balsevich et al., 2017). For example, it is well documented (in rodents) that acute stress (likely via increased CRF) decreases the amount of AEA in the BLA resulting in increased anxiety and in HPA axis activation (Gray et al., 2015), but glucocorticoids can have different effects from stress. Exposure to elevated glucocorticoids without stress results in increased AEA in the amygdala of rats within 10 min (Hill et al., 2010b). Additionally, the site of sampling, for example brain vs. periphery, matters. Whereas in rodents, acute stress decreases amygdala AEA, in humans, acute psychosocial stress increased circulating AEA (Dlugos et al., 2012), and circulating cortisol and AEA have bene shown to be positively correlated (Hill et al., 2013b). This AEA-enhancing effect of stress and glucocorticoids is thought to help reign in the initial response to stress (Morena et al., 2016), suggesting that individuals with lower baseline and post-acute-stress cortisol may have decreased AEA and thus more activity in the BLA. Moreover, circulating AEA is negatively correlated with anxiety in healthy and psychiatric populations – lower AEA is related to higher anxiety (Dlugos et al., 2012; Hill et al., 2008), suggesting that when taking this evidence into account lower cortisol could be associated with higher anxiety. But, individuals with lower baseline AEA tended to have higher post-stress cortisol, and baseline cortisol is not always correlated with baseline AEA (Dlugos et al., 2012), suggesting more information is needed. Glucocorticoids are necessary for sculpting the functional connectivity between the amygdala and prefrontal cortex and for normal fear extinction (Hill et al., 2018), and reduced cortisol, along with elevated CRF, is found in individuals with PTSD, a disorder characterized, at least in part, by overactivity of the amygdala and impaired fear processing (Hill et al., 2018). Taken together, these data suggest low cortisol can be associated with endocannabinoid signaling and anxiety.
It is important to note, however, that the bidirectional interactions between the endocannabinoid system and the HPA axis are complex (Balsevich et al., 2017; Morena et al., 2016) and involve not just the BLA and PVN, but several other brain regions (e.g., hippocampus, prefrontal cortex, ventral tegmental area, bed nucleus of the stria terminalis) and mediators (e.g., 2-AG, arginine vasopressin, GABA, 5-HT). Additionally, the time course of hormone elevation, time point of sample collection, location of sample collection, level of hormone (baseline vs. post-stress), duration of stress (acute vs. chronic), past life experiences (e.g., childhood or adult trauma), and species (e.g. rodents vs humans) can all impact outcomes (Balsevich et al., 2017; Hill et al., 2018; Morena et al., 2016). Less is known about the baseline interactions of these systems as more work has investigated impacts of acute or chronic stress and/or artificial glucocorticoid elevation, and, due to ability to control variables and manipulate and analyze brains, rodents have been the primary study species. Future studies to illuminate more precisely how durations (acute vs. chronic) and species-specific levels (e.g., circadian peak or post-stress) of glucocorticoid increase alter amygdalar AEA and FAAH, and anxiety-like behavior are needed to determine the interacting role these systems play in anxiety. For our study, it is important to point out that no specific stressor was applied prior to collection of blood samples used for assay of cortisol, but it is possible that hormone levels were not true representations of baseline as some participants may have found anticipation of a medical exam and venipuncture stressful. We did use time of day of blood sample as a covariate to control for circadian variation in cortisol. However, even if our samples are not true baseline values, our data are still supported by other studies showing baseline and post-stress cortisol are lower in those with increased anxiety. Additionally, we were not able to measure AEA in our study, but future studies should measure levels of circulating glucocorticoids and endocannabinoids in the same participants.
We found the FAAH CC genotype only confers risk for anxiety if the CRFR1 minor allele combination is also present. Additionally, the FAAH AA/AC genotype was riskier than the CC if the three CRFR1 minor alleles were not present. This result is somewhat contrary to our prediction as we hypothesized the FAAH AA/AC and the CRFR1 minor combination would buffer individuals from anxiety both in combination and on their own. While it is unknown why we found this flipped effect of FAAH SNP depending on CRFR1 SNP status, it may be that in response to CRF signaling (which may vary by genotype and/or stress), individuals with higher baseline AEA (those with AA/AC) experience a greater relative drop in AEA compared to the CC individuals that started with less AEA. If the greater drop corresponds to proportionally greater release of inhibition (by decreased binding at more CB1 receptors) it may therefore relate to more glutamatergic output and thus higher BLA activity and increased anxiety. To our knowledge, information on the specific AEA binding dynamics and numbers of CB1 receptors in the BLA among individuals who differ in FAAH genotype is not available. However, data from rodents suggest that the level of CB1 binding is important for signaling - the higher the CB1 agonist concentration, the lower the firing rate of BLA neurons (Pistis et al., 2004), suggesting that a more pronounced decrease in AEA could possible result in greater BLA output. Additionally, recent work highlights the importance of CB1 in understanding the interactions among the EC system, stress, and anxiety. For example, chronic stress, sustained glucocorticoids, and early life stress can decrease CB1 receptor density in the brain; blocking CB1 can increase anxiety in baseline and post-stress scenarios and can increase HPA axis activity (Morena et al., 2016; Balsevich et al., 2017; Ibarra-Lecue et al., 2018; Hill et al., 2019). Moreover, another recent study found FAAH A carriers had higher anxiety (and depression) than CC individuals, but only if they were exposed to childhood adversity (Lazary et al., 2016); CRFR1 genotype, CB1, and cortisol were not assessed. These data suggest that the role of CB1 is likely important and may help to explain how gene by environment interactions impact anxiety. Future studies should determine if CRFR1 minor alleles play a role in the relationship between FAAH genotype, CB1 dynamics, and anxiety.
It is important to note that despite the abundance of data on the role of CRFR1 SNPS in various stress and affective state outcomes, it is unclear what the functional significance of these SNPS are at the molecular/cellular level (Binder and Nemeroff, 2010). The gene encoding CRFR1 is located on chromosome 17 and contains 13 exons spanning 51 kB (Binder and Nemeroff, 2010). The three CRFR1 SNPS included here are located near the 5’ end and are in the first and second intron region (Chen et al., 1993; Vita et al., 1993). But it is unclear what these SNPs do to CRFR1 functionality; future studies addressing this would be beneficial for interpreting data. Additionally, several previous reports of CRFR1 minor alleles being protective are in situations that involved early life trauma (Tyrka et al., 2009; Bradley et al., 2008; Polanczyk et al., 2009). Specifically, in white, non-Hispanic individuals (n = 129), those with the common homozygous genotypes of GG (vs. AG or AA; rs110402) or GG (vs. GT or TT; rs242924) who experienced childhood trauma showed increased cortisol response to the Dex/CRF challenge test; but, there was no impact of genotype on cortisol in individuals without childhood trauma (Tyrka et al., 2009). Moreover, the presence of two copies of the minor allele for each of the three SNPs was found to be protective against adult depression in African American, non-Hispanic individuals (n = 422) who experienced severe early life stress/trauma (Bradley et al., 2008). Additionally, in an additive model, individuals (n = 368, Caucasians) homozygous for the minor allele at each SNP (TT, AA, or TT) showed decreased cortisol response to an acute stressor (Trier Social Stress Test; Mahon et al., 2013). And, higher baseline cortisol was associated with increased trait anxiety (Spielberger STAI-T) in individuals homozygous for the common allele of rs7209436 (CC) and rs110402 (GG); authors did not report main effects of genotype on anxiety measures (Mahon et al., 2013). Thus, it is therefore possible that a gene by environment interaction is important for interpreting the role of CRFR1 and FAAH genotype (see Lazary et al., 2016) in stress physiology and affect (Heim and Binder, 2012). In line with this, recent studies have shown that early life trauma can lead to differential DNA methylation (Roberts et al., 2018) and that this impact is directly related to stress-responsive genes (FKBP5) and affect, mainly depression (Binder, 2009; Binder et al., 2008; A. Zannas and Binder, 2014; Zannas et al., 2016). We do not have information on exposure to early life trauma in our study population, but this would be an interesting area for future study.
Overall, we partially confirmed our predictions, and, when compared to the other existing dataset exploring the interaction among CRFR1, FAAH, and anxiety, our data are somewhat confirmatory. Demers and colleagues (Demers et al., 2016) found one (protective) CRFR1 minor allele (rs110402) and the (less risky) FAAH AA or AC alleles were associated with increased anxiety disorder diagnosis; they did not measure baseline cortisol. However, if the individuals in the Demers study were non-minor at CRFR1 rs7209436 and rs242924, then their participants would match our results of FAAH AA or AC being riskier for anxiety in individuals who were not carriers of all three minor alleles. But, the sample populations in the two studies were not identical. In our sample, it is important to note that individuals were not clinically anxious, and our anxiety scores represent natural variation in state (current) anxiety in a population; whereas the individuals in the Demers et al. study met DSM-5 criteria for anxiety disorder. However, data suggest that state, trait, and clinical anxiety are related and therefore biomarkers related to one of these measures may be relevant for all of them. For example, state anxiety increases after stress (Ackerl et al., 2002) and this is true for individuals high and low for trait anxiety (Jezova et al., 2004; Roelofs et al., 2007), thus is it plausible that baseline cortisol at the time of state anxiety measure and genotypes related to anxiety and stress responsiveness would be associated with both state and trait anxiety. Moreover, state and trait anxiety are highly correlated (Spielberger, 1983) and individuals with higher levels of anxiety sensitivity are more at-risk for clinical anxiety disorders (Naragon-Gainey, 2010). Nonetheless, it is possible that biomarkers of state anxiety and those for trait or pathological anxiety differ.
In conclusion, in our study population, the minor alleles of CRFR1 and the AA or AC genotype of FAAH are related to reduced anxiety, but, surprisingly, related to higher, and not lower, baseline cortisol. Additionally, the FAAH CC genotype is only confers risk for anxiety in individuals who are also carriers of the CRFR1 minor combination. But, FAAH genotype was not independently associated with anxiety, and baseline cortisol was negatively associated with anxiety score. These results may help to interpret how genetic differences in the HPA axis and EC system impact stress-related disorders.
Acknowledgements
Project FRONTIER is currently funded by TTUHSC Garrison Institute on Aging; the TTUHSC F. Marie Hall Institute for Rural and Community Health participated in previous management of Project FRONTIER. This research was partially funded by the National Science Foundation (PRISM; NSF Grant No. 1035096) and by the TTU Center for Active Learning and Undergraduate Engagement (CALUE; now TRUE). We thank the PRISM PIs (Drs. G. Brock Williams, Sophia Jang, Nancy McIntyre, Jaclyn Canas-Carrell, and Jerry Dwyer), Dr. James Carr, Dr. Andrew Littlefield, Jessica Spott, Jerylme Robins, Dr. P. Hemachandra Reddy, and the Garrison Institute on Aging for their support. We extend a special thank you to Dr. Linda Yin for her support and wisdom, and for her exceptional work on, and dedication to, the Project FRONTIER biobank. Lastly, we would like to thank two anonymous reviewers for their helpful comments and critiques of an earlier version of this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2019.100154.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Ackerl K., Atzmueller M., Grammer K. The scent of fear. Neuroendocrinol. Lett. 2002;23(2):79–84. [PubMed] [Google Scholar]
- Antonakis J., Bendahan S., Jacquart P., Lalive R. On making causal claims: a review and recommendations. Leader. Q. 2010;21:1086–1120. [Google Scholar]
- Anxiety and Depression Association of America Facts & Statistics. Retrieved April 4, 2018. https://adaa.org/about-adaa/press-room/facts-statistics
- Arborelius L., Owens M.J., Plotsky P.M., Nemeroff C.B. The role of corticotropin-releasing factor in depression and anxiety disorders. J. Endocrinol. 1999;160(1):1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- Balsevich G., Petrie G.N., Hill M.N. Endocannabinoids: effectors of glucocorticoid signaling. Front. Neuroendocrinol. 2017;47:86–108. doi: 10.1016/j.yfrne.2017.07.005. [DOI] [PubMed] [Google Scholar]
- Beaton E.A., Schmidt L.A., Ashbaugh A.R., Santesso D.L., Antony M.M., McCabe R.E., Segalowitz S.J., Schulkin J. Low salivary cortisol levels among socially anxious young adults: preliminary evidence from a selected and a non-selected sample. Pers. Indiv. Differ. 2006;41(7):1217–1228. [Google Scholar]
- Beck A.T., Steer R.A. Psychological Corporation; San Antonio, TX: 1993. Beck Anxiety Inventory Manual. [Google Scholar]
- Beck A.T., Epstein N., Brown G., Steer R.A. An inventory for measuring clinical anxiety: psychometric properties. J. Consult. Clin. Psychol. 1988;56(6):893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Binder E.B. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34(Suppl. 1):S186–S195. doi: 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Binder E.B., Nemeroff C.B. The CRF system, stress, depression and anxiety – insights from human genetic studies. Mol. Psychiatr. 2010;15:574–588. doi: 10.1038/mp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder E.B., Bradley R.G., Liu W., Epstein M.P., Deveau T.C., Mercer K.B. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. J. Am. Med. Assoc. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley R.G., Binder E.B., Epstein M.P., Tang Y., Nair H.P., Liu W., Gillespie C.F., Berg T., Evces M., Newport D.J., Stowe Z.N. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch. Gen. Psychiatr. 2008;65(2):190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney C.E., Moss T.G., Harris A.L., Edinger J.D., Krystal A.D. Should we be anxious when assessing anxiety using the Beck Anxiety Inventory in clinical insomnia patients? J. Psychiatr. Res. 2011;45(9):1243–1249. doi: 10.1016/j.jpsychires.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Lewis K.A., Perrin M.H., Vale W.W. Expression cloning of a human corticotropin-releasing-factor receptor. Proc. Natl. Acad. Sci. U. S. A. 1993;90:8967–8971. doi: 10.1073/pnas.90.19.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang K.P., Gerber A.L., Sipe J.C., Cravatt B.F. Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: evidence for a link between defects in the endocannabinoid system and problem drug use. Hum. Mol. Genet. 2004;13:2113–2119. doi: 10.1093/hmg/ddh216. [DOI] [PubMed] [Google Scholar]
- Clinchy M., Schulkin J., Zanette L.Y., Sheriff M.J., McGowan P.O., Boonstra R. The neurological ecology of fear: insights neuroscientists and ecologists have to offer one another. Front. Behav. Neurosci. 2011;5:21. doi: 10.3389/fnbeh.2011.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H., Zohar J., Gidron Y., Matar M.A., Belkind D., Loewenthal U., Kozlovsky N., Kaplan Z. Blunted HPA axis response to stress influences susceptibility to posttraumatic stress response in rats. Biol. Psychiatry. 2006;59(12):1208–1218. doi: 10.1016/j.biopsych.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Cuthbert B.N., Insel T.R. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11(1):126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demers C.H., Conley E.D., Bogdan R., Hariri A.R. Interactions between anandamide and corticotropin-releasing factor signaling modulate human amygdala function and risk for anxiety disorders: an imaging genetics strategy for modeling molecular interactions. Biol. Psychiatry. 2016;80(5):356–362. doi: 10.1016/j.biopsych.2015.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dincheva I., Drysdale A.T., Hartley C.A., Johnson D.C., Jing D., King E.C., Ra S., Gray J.M., Yang R., DeGruccio A.M., Huang C. FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nat. Commun. 2015;6:6395. doi: 10.1038/ncomms7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlugos A., Childs E., Stuhr K.L., Hillard C.J., De Wit H. Acute stress increases circulating anandamide and other N-acylethanolamines in healthy humans. Neuropsychopharmacology. 2012;37(11):2416–2427. doi: 10.1038/npp.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder A., Nestler E.J., Charney D.S. Psychobiology and molecular genetics of resilience. Nat. Rev. Neurosci. 2009;10(6):446. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fydrich T., Dowdall D., Chambless D.L. Reliability and validity of the Beck anxiety inventory. J. Anxiety Disord. 1992;6(1):55–61. [Google Scholar]
- Gillespie C.F., Phifer J., Bradley B., Ressler K.J. Risk and resilience: genetic and environmental influences on development of the stress response. Depress. Anxiety. 2009;26(11):984–992. doi: 10.1002/da.20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorzalka B.B., Hill M.N., Hillard C.J. Regulation of endocannabinoid signaling by stress: implications for stress-related affective disorders. Neurosci. Biobehav. Rev. 2008;32(6):1152–1160. doi: 10.1016/j.neubiorev.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Gray J.M., Vecchiarelli H.A., Morena M., Lee T.T., Hermanson D.J., Kim A.B., McLaughlin R.J., Hassan K.I., Kühne C., Wotjak C.T., Deussing J.M. Corticotropin-releasing hormone drives anandamide hydrolysis in the amygdala to promote anxiety. J. Neurosci. 2015;35(9):3879–3892. doi: 10.1523/JNEUROSCI.2737-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz-Cinar O., Hill M.N., McEwen B.S., Holmes A. Amygdala FAAH and anandamide: mediating protection and recovery from stress. Trends Pharmacol. Sci. 2013;34(11):637–644. doi: 10.1016/j.tips.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri A.R., Gorka A., Hyde L.W., Kimak M., Halder I., Ducci F., Ferrell R.E., Goldman D., Manuck S.B. Divergent effects of genetic variation in endocannabinoid signaling on human threat-and reward-related brain function. Biol. Psychiatry. 2009;66(1):9–16. doi: 10.1016/j.biopsych.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris B.N., Carr J.A. The role of the hypothalamus-pituitary-adrenal/interrenal axis in mediating predator-avoidance trade-offs. Gen. Comp. Endocrinol. 2016;230:110–142. doi: 10.1016/j.ygcen.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Hayes A.F. Beyond Barron and Kenny: statistical mediation analysis in the new millennium. Commun. Monogr. 2009;76:408–420. [Google Scholar]
- Hayes A.F. second ed. Guildford Press; New York, New York: 2018. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. [Google Scholar]
- Hayes A.F., Preacher K.J. Quantifying and testing indirect effects in simple mediation models when the constituent paths are nonlinear. Multivariate Behav. Res. 2010;45:627–660. doi: 10.1080/00273171.2010.498290. [DOI] [PubMed] [Google Scholar]
- Heim C., Binder E.B. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene–environment interactions, and epigenetics. Exp. Neurol. 2012;233(1):102–111. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Heinrichs S.C., Koob G.F. Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. J. Pharmacol. Exp. Ther. 2004;311(2):427–440. doi: 10.1124/jpet.103.052092. [DOI] [PubMed] [Google Scholar]
- Hill M.N., McEwen B.S. Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2010;34(5):791–797. doi: 10.1016/j.pnpbp.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.N., Tasker J.G. Endocannabinoid signaling, glucocorticoid-mediated negative feedback, and regulation of the hypothalamic-pituitary-adrenal axis. Neuroscience. 2012;204:5–16. doi: 10.1016/j.neuroscience.2011.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.N., Miller G.E., Ho W.S.V., Gorzalka B.B., Hillard C.J. Serum endocannabinoid content is altered in females with depressive disorders: a preliminary report. Pharmacopsychiatry. 2008;41(2):48–53. doi: 10.1055/s-2007-993211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.N., McLaughlin R.J., Morrish A.C., Viau V., Floresco S.B., Hillard C.J., Gorzalka B.B. Suppression of amygdalar endocannabinoid signaling by stress contributes to activation of the hypothalamic–pituitary–adrenal axis. Neuropsychopharmacology. 2009;34(13):2733–2745. doi: 10.1038/npp.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.N., Patel S., Campolongo P., Tasker J.G., Wotjak C.T., Bains J.S. Functional interactions between stress and the endocannabinoid system: from synaptic signaling to behavioral output. J. Neurosci. 2010;30(45):14980–14986. doi: 10.1523/JNEUROSCI.4283-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.N., Karatsoreos I.N., Hillard C.J., McEwen B.S. Rapid elevations in limbic endocannabinoid content by glucocorticoid hormones in vivo. Psychoneuroendocrinology. 2010;35(9):1333–1338. doi: 10.1016/j.psyneuen.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.N., Kumar S.A., Filipski S.B., Iverson M., Stuhr K.L., Keith J.M., Cravatt B.F., Hillard C.J., Chattarji S., McEwen B.S. Disruption of fatty acid amide hydrolase activity prevents the effects of chronic stress on anxiety and amygdalar microstructure. Mol. Psychiatr. 2013;18(10):1125–1135. doi: 10.1038/mp.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.N., Bierer L.M., Makotkine I., Golier J.A., Galea S., McEwen B.S., Hillard C.J., Yehuda R. Reductions in circulating endocannabinoid levels in individuals with post-traumatic stress disorder following exposure to the World Trade Center attacks. Psychoneuroendocrinology. 2013;38(12):2952–2961. doi: 10.1016/j.psyneuen.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.N., Campolongo P., Yehuda R., Patel S. Integrating endocannabinoid signaling and cannabinoids into the biology and treatment of posttraumatic stress disorder. Neuropsychopharmacology. 2018;43(1):80–102. doi: 10.1038/npp.2017.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.N., Eiland L., Lee T.T.Y., Hillard C.J., McEwen B.S. Early life stress alters the developmental trajectory of corticolimbic endocannabinoid signaling in male rats. Neuropharmacology. 2019;146:154–162. doi: 10.1016/j.neuropharm.2018.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard C.J., Beatka M., Sarvaideo J. Endocannabinoid signaling and the hypothalamic‐pituitary‐adrenal axis. Compr. Physiol. 2011;7(1):1–15. doi: 10.1002/cphy.c160005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A.J., Patrick L.M. The myth of optimality in clinical neuroscience. Trends Cognit. Sci. 2018;22:241–257. doi: 10.1016/j.tics.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarra-Lecue I., Pilar-Cuéllar F., Muguruza C., Florensa-Zanuy E., Díaz Á., Urigüen L., Castro E., Pazos A., Callado L.F. The endocannabinoid system in mental disorders: evidence from human brain studies. Biochem. Pharmacol. 2018;157:97–107. doi: 10.1016/j.bcp.2018.07.009. [DOI] [PubMed] [Google Scholar]
- Insel T.R. The NIMH research domain criteria (RDoC) project: precision medicine for psychiatry. Am. J. Psychiatry. 2014;171(4):395–397. doi: 10.1176/appi.ajp.2014.14020138. [DOI] [PubMed] [Google Scholar]
- Jezova D., Makatsori A., Duncko R., Moncek F., Jakubek M. High trait anxiety in healthy subjects is associated with low neuroendocrine activity during psychosocial stress. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2004;28(8):1331–1336. doi: 10.1016/j.pnpbp.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Kabacoff R.I., Segal D.L., Hersen M., Van Hasselt V.B. Psychometric properties and diagnostic utility of the Beck Anxiety Inventory and the State-Trait Anxiety Inventory with older adult psychiatric outpatients. J. Anxiety Disord. 1997;11:33–47. doi: 10.1016/s0887-6185(96)00033-3. [DOI] [PubMed] [Google Scholar]
- Lang P.J., McTeague L.M., Bradley M.M. RDoC, DSM, and the reflex physiology of fear: a biodimensional analysis of the anxiety disorders spectrum. Psychophysiology. 2016;53(3):336–347. doi: 10.1111/psyp.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazary J., Eszlari N., Juhasz G., Bagdy G. Genetically reduced FAAH activity may be a risk for the development of anxiety and depression in persons with repetitive childhood trauma. Eur. Neuropsychopharmacol. 2016;26(6):1020–1028. doi: 10.1016/j.euroneuro.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Lenze E.J., Mantella R.C., Shi P., Goate A.M., Nowotny P., Butters M.A., Andreescu C., Thompson P.A., Rollman B.L. Elevated cortisol in older adults with generalized anxiety disorder is reduced by treatment: a placebo-controlled evaluation of escitalopram. Am. J. Geriatr. Psychiatry. 2011;19(5):482–490. doi: 10.1097/JGP.0b013e3181ec806c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon D.P., Krull J.L., Lockwood C.M. Equivalence of the mediation, confounding, and suppression effect. Prev. Sci. 2000;1:173–181. doi: 10.1023/a:1026595011371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon P.B., Zandi P.P., Potash J.B., Nestadt G., Wand G.S. Genetic association of FKBP5 and CRHR1 with cortisol response to acute psychosocial stress in healthy adults. Psychopharmacology. 2013;227(2):231–241. doi: 10.1007/s00213-012-2956-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., Gray J.D., Nasca C. Recognizing resilience: Learning from the effects of stress on the brain. Neurobiol. Stress. 2015;1:1–11. doi: 10.1016/j.ynstr.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., Nasca C., Gray J.D. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2016;41(1):3–23. doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morena M., Patel S., Bains J.S., Hill M.N. Neurobiological interactions between stress and the endocannabinoid system. Neuropsychopharmacology. 2016;41(1):80. doi: 10.1038/npp.2015.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M.B., Zimmermann S., Sillaber I., Hagemeyer T.P., Deussing J.M., Timpl P., Kormann M.S., Droste S.K., Kuhn R., Reul J.M., Holsboer F. Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat. Neurosci. 2003;6(10):1100. doi: 10.1038/nn1123. [DOI] [PubMed] [Google Scholar]
- Naragon-Gainey K. Meta-analysis of the relations of anxiety sensitivity to the depressive and anxiety disorders. Psychol. Bull. 2010;136(1):128. doi: 10.1037/a0018055. [DOI] [PubMed] [Google Scholar]
- National Institute of Mental Health . 2017. Any Anxiety Disorder.https://www.nimh.nih.gov/health/statistics/any-anxiety-disorder.shtml Retrieved May 13, 2018, from. [Google Scholar]
- Natividad L.A., Buczynski M.W., Herman M.A., Kirson D., Oleata C.S., Irimia C., Polis I., Ciccocioppo R., Roberto M., Parsons L.H. Constitutive increases in amygdalar corticotropin-releasing factor and fatty acid amide hydrolase drive an anxious phenotype. Biol. Psychiatry. 2017;82(7):500–510. doi: 10.1016/j.biopsych.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris D.O., Carr J.A. Academic Press; 2013. Vertebrate endocrinology. [Google Scholar]
- Patel S., Hill M.N., Cheer J.F., Wotjak C.T., Holmes A. The endocannabinoid system as a target for novel anxiolytic drugs. Neurosci. Biobehav. Rev. 2017;76:56–66. doi: 10.1016/j.neubiorev.2016.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perusini J.N., Fanselow M.S. Neurobehavioral perspectives on the distinction between fear and anxiety. Learn. Mem. 2015;22(9):417–425. doi: 10.1101/lm.039180.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistis M., Perra S., Pillolla G., Melis M., Gessa G.L., Muntoni A.L. Cannabinoids modulate neuronal firing in the rat basolateral amygdala: evidence for CB1-and non-CB1-mediated actions. Neuropharmacology. 2004;46(1):115–125. doi: 10.1016/j.neuropharm.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Polanczyk G., Caspi A., Williams B., Price T.S., Danese A., Sugden K., Uher R., Poulton R., Moffitt T.E. Protective effect of CRHR1 gene variants on the development of adult depression following childhood maltreatment: replication and extension. Arch. Gen. Psychiatr. 2009;66(9):978–985. doi: 10.1001/archgenpsychiatry.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher K.J., Rucker D.D., Hayes A.F. Assessing moderated mediation hypotheses: theory, methods, and prescriptions. Multivariate Behavioral Research. 2007;42:185–227. doi: 10.1080/00273170701341316. [DOI] [PubMed] [Google Scholar]
- Preacher K.J., Hayes A.F. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Pulopulos M.M., Hidalgo V., Almela M., Puig-Perez S., Villada C., Salvador A. Acute stress and working memory in older people. Stress. 2015;18(2):178–187. doi: 10.3109/10253890.2015.1004538. [DOI] [PubMed] [Google Scholar]
- Reul J.M., Holsboer F. Corticotropin-releasing factor receptors 1 and 2 in anxiety and depression. Curr. Opin. Pharmacol. 2002;2(1):23–33. doi: 10.1016/s1471-4892(01)00117-5. [DOI] [PubMed] [Google Scholar]
- Roberts A.L., Gladish N., Gatev E., Jones M.J., Chen Y., MacIsaac J.L., Tworoger S.S., Austin S.B., Tanrikut C., Chavarro J.E., Baccarelli A.A., Kobor M.S. Exposure to childhood abuse is associated with human sperm DNA methylation. Transl. Psychiatry. 2018;8:194. doi: 10.1038/s41398-018-0252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs K., Bakvis P., Hermans E.J., van Pelt J., van Honk J. The effects of social stress and cortisol responses on the preconscious selective attention to social threat. Biol. Psychol. 2007;75(1):1–7. doi: 10.1016/j.biopsycho.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Rogers J., Raveendran M., Fawcett G.L., Fox A.S., Shelton S.E., Oler J.A., Cheverud J., Muzny D.M., Gibbs R.A., Davidson R.J., Kalin N.H. CRHR1 genotypes, neural circuits and the diathesis for anxiety and depression. Mol. Psychiatr. 2013;18(6):700–707. doi: 10.1038/mp.2012.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B., Schelling G., McGaugh J.L. Corticotropin-releasing factor in the basolateral amygdala enhances memory consolidation via an interaction with the β-adrenoceptor–cAMP pathway: dependence on glucocorticoid receptor activation. Journal of Neuroscience. 2008;28(26):6642–6651. doi: 10.1523/JNEUROSCI.1336-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen J.B., Schulkin J. From normal fear to pathological anxiety. Psychol. Rev. 1998;105(2):325–350. doi: 10.1037/0033-295x.105.2.325. [DOI] [PubMed] [Google Scholar]
- Sanders J., Nemeroff C. The CRF system as a therapeutic target for neuropsychiatric disorders. Trends Pharmacol. Sci. 2016;37(12):1045–1054. doi: 10.1016/j.tips.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulkin J., Morgan M.A., Rosen J.B. A neuroendocrine mechanism for sustaining fear. Trends Neurosci. 2005;28(12):629–635. doi: 10.1016/j.tins.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Shin L.M., Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35(1):169. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirotsuki K., Izawa S., Sugaya N., Yamada K.C., Ogawa N., Ouchi Y., Nagano Y., Nomura S. Salivary cortisol and DHEA reactivity to psychosocial stress in socially anxious males. Int. J. Psychophysiol. 2009;72(2):198–203. doi: 10.1016/j.ijpsycho.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Sipe J.C., Chiang K., Gerber A.L., Beutler E., Cravatt B.F. A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proc. Natl. Acad. Sci. Unit. States Am. 2002;99(12):8394–8399. doi: 10.1073/pnas.082235799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C.D. Consulting Psychologists Press; Palo Alto, CA: 1983. Manual for the State-Trait Anxiety Inventory (Form Y) [Google Scholar]
- Takahashi T., Ikeda K., Ishikawa M., Kitamura N., Tsukasaki T., Nakama D., Kameda T. Anxiety, reactivity, and social stress-induced cortisol elevation in humans. Neuroendocrinol. Lett. 2005;26(4):351–354. [PubMed] [Google Scholar]
- Tyrka A.R., Price L.H., Gelernter J., Schepker C., Anderson G.M., Carpenter L.L. Interaction of childhood maltreatment with the corticotropin-releasing hormone receptor gene: effects on hypothalamic-pituitary-adrenal axis reactivity. Biol. Psychiatry. 2009;66(7):681–685. doi: 10.1016/j.biopsych.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eck M., Berkhof H., Nicolson N., Sulon J. The effects of perceived stress, traits, mood states, and stressful daily events on salivary cortisol. Psychosom. Med. 1996;58(5):447–458. doi: 10.1097/00006842-199609000-00007. [DOI] [PubMed] [Google Scholar]
- Vingerhoets A.D.J.J.M., Ratliff-Crain J., Jabaaij L., Tilders F.J., Moleman P., Menges L.J. Self-reported stressors, symptom complaints and psychobiological functioning II: psychoneuroendocrine variables. J. Psychosom. Res. 1996;40(2):191–203. doi: 10.1016/0022-3999(95)00528-5. [DOI] [PubMed] [Google Scholar]
- Vita N., Laurent P., Lefort S., Chalon P., Lelias J.M., Kaghad M., Le Fur G., Caput D., Ferrara P. Primary structure and functional expression of mouse pituitary and human brain corticotrophin releasing factor receptors. FEBS Lett. 1993;335(1):1–5. doi: 10.1016/0014-5793(93)80427-v. [DOI] [PubMed] [Google Scholar]
- Vreeburg S.A., Zitman F.G., van Pelt J., DeRijk R.H., Verhagen J.C., van Dyck R., Hoogendijk W.J.G., Smit J.H., Penninx B.W. Salivary cortisol levels in persons with and without different anxiety disorders. Psychosom. Med. 2010;72(4):340–347. doi: 10.1097/PSY.0b013e3181d2f0c8. [DOI] [PubMed] [Google Scholar]
- Weber H., Richter J., Straube B., Lueken U., Domschke K., Schartner C., Klauke B., Baumann C., Pané-Farré C., Jacob C.P., Scholz C.J. Allelic variation in CRHR1 predisposes to panic disorder: evidence for biased fear processing. Mol. Psychiatr. 2016;21(6):813–822. doi: 10.1038/mp.2015.125. [DOI] [PubMed] [Google Scholar]
- Zajkowska Z.E., Englund A., Zunszain P.A. Towards a personalized treatment in depression: endocannabinoids, inflammation and stress response. Pharmacogenomics. 2014;15(5):687–698. doi: 10.2217/pgs.14.40. [DOI] [PubMed] [Google Scholar]
- Zannas A.S., Binder E.B. Gene–environment interactions at the FKBP5 locus: sensitive periods, mechanisms and pleiotropism. Genes Brain Behav. 2014;13(1):25–37. doi: 10.1111/gbb.12104. [DOI] [PubMed] [Google Scholar]
- Zannas A.S., Wiechmann T., Gassen N.C., Binder E.B. Gene–stress–epigenetic regulation of FKBP5: clinical and translational implications. Neuropsychopharmacology. 2016;41(1):261. doi: 10.1038/npp.2015.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla E.P., Valdez G.R., Nozulak J., Koob G.F., Markou A. Effects of antalarmin, a CRF type 1 receptor antagonist, on anxiety-like behavior and motor activation in the rat. Brain Res. 2002;952(2):188–199. doi: 10.1016/s0006-8993(02)03189-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.