Summary
Since Marx reported on osteonecrosis of the jaw caused by the injectable bisphosphonates (BPs) pamidronate and zoledronate, there have been numerous reports not only in Western countries, but in Japan as well, of bisphosphonate-related osteonecrosis of the jaw (BRONJ) as a pathology similar to radiation-related osteonecrosis/osteomyelitis of the jaw, which is accompanied by exposure of the bone. Osteonecrosis of the jaw similar to that occurring with BPs is also produced with the anti-receptor activator of nuclear factor kappa-Β ligand (RANKL) antibody denosumab, a bone resorption inhibitor that has a different mode of action from BPs, and there is also a report of osteonecrosis of the jaw related to bevacizumab, an angiogenic inhibitor. Because of this, in its newest position paper (2014), the AAOMS changed the nomenclature from BRONJ to “medication-related osteonecrosis of the jaw” (MRONJ). This article presents an overview of the position paper on medication-related osteonecrosis of the jaw and the current status of the MRONJ in Japan.
Keyword: Bisphosphonates, Anti-RANKL antibody, Bisphosphonate-related osteonecrosis of the jaw, Medication-related osteonecrosis of the jaw, Osteoporosis, Position paper
1. Introduction
Radiation-related osteonecrosis/osteomyelitis of the jaw is a delayed-onset disorder of radiation therapy that has long been known in the field of oral and maxillofacial surgery, and tooth extraction or implant therapy within the irradiation field is generally considered to be contraindicated. Since Marx reported on osteonecrosis of the jaw caused by the injectable bisphosphonates (BPs) pamidronate and zoledronate [1], there have been numerous reports not only in Western countries, but in Japan as well, of bisphosphonate-related osteonecrosis of the jaw (BRONJ) as a pathology similar to radiation-related osteonecrosis/osteomyelitis of the jaw, which is accompanied by exposure of the bone.
In response to the increase in BRONJ patients, the American Association of Oral and Maxillofacial Surgeons (AAOMS) published a paper setting out the position of the Association on the subject in 2006 (AAOMS 2006), and this was subsequently revised in 2009 and 2014. In Japan as well, a joint investigative commission into BRONJ comprising five academic societies (the Japanese Society for Bone and Mineral Research, the Japan Osteoporosis Society, the Japanese Society for Oral and Maxillofacial Radiology, the Japanese Society of Periodontology, and the Japanese Society of Oral and Maxillofacial Surgeons) released its position paper in 2010, with an abridged version of the Japanese text (partially revised edition) published in 2012 and a fully revised edition published in 2017. This has come to be one of the clinical guidelines for ARONJ (anti-resorptive agents-related osteonecrosis of the jaw) in Japan [2].
Osteonecrosis of the jaw similar to that occurring with BPs is also produced with the anti-receptor activator of nuclear factor kappa-Β ligand (RANKL) antibody denosumab, a bone resorption inhibitor that has a different mode of action from BPs [3], and there is also a report of osteonecrosis of the jaw related to bevacizumab, an angiogenic inhibitor [4]. Because of this, in its newest position paper (2014), the AAOMS changed the nomenclature from BRONJ to “medication-related osteonecrosis of the jaw” (MRONJ).
In the present position paper, the following changes are made with respect to the AAOMS 2009 diagnostic criteria: (1) the criteria are expanded to include “bone resorption inhibitors or angiogenic inhibitors,” rather than only BPs; (2) “bone that can be probed through an intra- or extra-oral fistula” is also included, rather than only bone exposure; and, of course, (3) it is specified that “there is no metastasis to the jawbone.”
2. MRONJ is a side effect of medication
MRONJ is listed on the package inserts of BPs and denosumab as a “major side effect,” and it states that “the patient should be carefully observed and appropriate measures such as discontinuation of administration should be taken in the case of any abnormality.” Thus, discontinuing the injections is one option when MRONJ occurs as a side effect.
MRONJ due to angiogenic inhibitors is viewed as a problem in AAOMS 2014, but there have as yet been few reports in Japan.
MRONJ is on the increase in Japan. The system of reporting drug side effects not only by pharmaceutical companies, but also by medical institutions has been legislated in this country. However, this does not mean that institutions diagnosing MRONJ have reported every case to the Ministry of Health, Labour and Welfare, and it is not easy to gain an accurate picture of the number of MRONJ patients at present.
The Research Planning Committee of the Japanese Society of Oral and Maxillofacial Surgeons published a national survey in 2008 (survey period: April 2006–June 2008) [5], in which there were 568 recorded cases. After exclusion of those in which the presence of bone exposure and the name or administration period of the BP agents was not known, 263 cases remained and were regarded as BRONJ patients. As noted above, the position paper in Japan was published in 2010, and since prevention measures were put forward in this paper, increases or decreases in the number of new patients are a matter of concern. However, in a national survey in 2015 (https://www.jsoms.or.jp/medical/work/study/bronj/, in Japanese), there were 4797 cases (1,599 cases in 1 year), which shows a worrying increase of 19.3 times the 2008 figure and 17.3 times the 2006 figure (Table 1). These results indicate the rapidity of the increase in the number of MRONJ patients and the inadequacy of the response in recent years [6].
Table 1.
BRONJ trends according to a survey in Japan.
| Survey year | 2006 | 2008 | 2015 |
|---|---|---|---|
| Period of survey | ∼2006 | 2006–2008 | 2011–2013 |
| No. of institutions surveyed | 239 | 248 | 501 |
| Response rate, % | 64.0 | 75.8 | 70.3 |
| Patients with BRONJ | 28 | 263 | 4,797 |
| Age at onset of BRONJ, mean years | 68.1 | 68.1 | 74.6 |
| Sex, male/female | 3/25 | 44/219 | 1,404/3,393 |
| Site of onset, % maxilla/mandible/both | 14.3/82.1/3.6 | 30.8/60.8/8.4 | 28.1/64.7/7.2 |
| BRONJ stage, % 1/2/3/unknown | 7.1/71.4/14.4/7.1 | 16.0/71.1/12.1/0.8 | 20.7/61.4/16.8/1.1 |
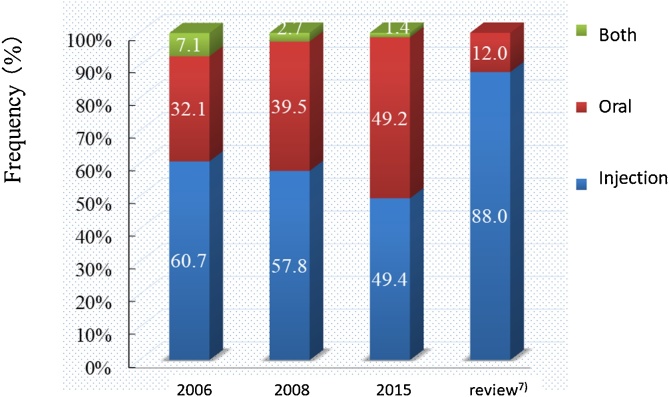
| Route of BP administration, %, parenteral/oral/both | 60.7/32.2/7.1 | 57.8/39.5/2.7 | 49.4/49.2/1.4 |
It was initially believed that MRONJ did not readily occur in osteoporosis patients with oral BPs. In fact, a study based on 2400 BRONJ cases listed in PubMed from 2003 to 2009 showed that injectable BPs were given in approximately 90% of cases [7]. However, a national survey in Japan in 2008 showed that injectable BPs were given in 62% of cases and oral BPs were given in 38%, so that Japan was characterized by a different pattern of use than other countries [6]. The results of the 2015 national survey showed that the proportion of oral BP drugs had risen to 49.2%. This indicates that the importance of oral BPs should not be underestimated (Fig. 1).
Fig. 1.
Route of BP administration.
The number of patients currently using oral BPs is estimated to be over 1.3 million, and the number of patients looks set to rise with the expected increase in the elderly population. This surely cannot be ignored by private clinicians working in general dental practice. While there appears to have been a steady increase in the number of MRONJ cases due to oral BPs, a surprisingly high increase of just over 10% has been found in serious stage 3 cases (cutaneous fistula or pathological fracture of mandible, etc.).
3. MRONJ countermeasures through coordination between doctors and dentists
The drugs that are suspected of being related to osteonecrosis/osteomyelitis of the jaw are prescribed by doctors, and it is mainly dentists that are responsible for MRONJ prevention, diagnosis, and treatment. With the patients as the focus, a great many professionals are involved, including not only doctors and dentists, but also pharmacists, nurses, dental hygienists, etc. However, a shared understanding of MRONJ among these professionals is essential to ensure smooth cooperation.
There are many areas of MRONJ that are yet to be clarified, but the expertise from basic and clinical research to date was set out in AAOMS 2014. Realistically, application of this expertise in clinical settings is most likely to be on a case-by-case basis through cooperation mainly between doctors and dentists. Below is an exploration of matters that can easily produce misunderstanding and should be discussed between medical doctors and dentists.
Prospective studies of the intravenous bone-modifying agents (zoledronate and denosumab) used for bone lesions in patients with malignant tumors have shown that MRONJ occurs in 1–2% of patients with either drug [3]. At the same time, the prevalence of MRONJ due to oral BPs is generally reported to be lower in osteoporosis patients with osteoporosis than in patients with malignant tumors [8]. Bone exposure (osteonecrosis) does occur even in patients not using bone resorption inhibitors, although this is extremely rare (<0.001%). Some people therefore consider that, if the prevalence of MRONJ is extremely low, it cannot be attributed to bone resorption inhibitors.
MRONJ due to oral BPs is more common in Japan than in other countries, although the reasons for this are not clear (a higher population aging rate than other countries and a large number of patients with teeth with apical periodontitis due to inadequate dental treatment are possible causes). The prevalence is estimated at 0.01–0.02% in the position paper, and this probably reflects the number of cases in the national survey.
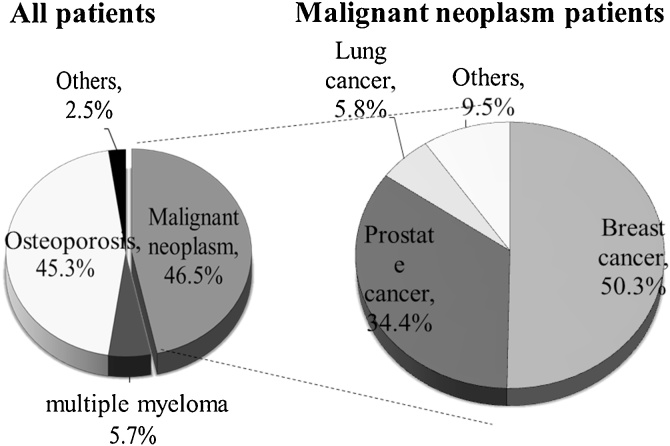
The risk with using injectable BPs in patients with malignant tumors is unmistakably greater than with using oral BPs in patients with osteoporosis, but an intravenous BP for osteoporosis has gone on sale in Japan. The risk with an injectable BP for osteoporosis is conjectured to be about the same as with an oral BP (an injectable BP does not have the same problem as oral BPs of the patient forgetting to take it, and this is estimated to make up for the slightly higher prevalence), and the anti-RANKL antibody denosumab is also an injectable drug. Therefore, rather than classifying in terms of oral or injection administration route, surely it is more appropriate to classify in terms of the primary disease, whether the drug is for osteoporosis or for a malignant tumor (Fig. 2).
Fig. 2.
Primary indications for BP therapy.
With the exception of etidronate, which does not contain nitrogen and is considered to have an extremely low risk of MRONJ, there is currently no evidence to indicate any differences between drugs such as, for example, whether alendronate or risedronate has the higher risk of MRONJ (it was predicted that risedronate, which is considered to have stronger avidity for bone, would have a higher risk, but there are currently no studies of this).
With regard to angiogenic inhibitors, concomitant use with a bone resorption inhibitor is believed to present the same or higher risk of MRONJ as use of other antitumor agents, but the risk with use in monotherapy is probably lower than the risk with bone resorption inhibitors.
Incidentally, in the case of non-hospital prescription of an oral drug, the name of the drug can easily be checked in the drug diary. However, injectable drugs are not recorded in the drug diary and thus need to be checked with the prescribing doctor.
4. MRONJ risk factors
The overall dose (roughly approximating the administration period) of the drug is the biggest problem. In particular, this is important for the amount of BP that accumulates in the bone, and the AAOMS 2014 makes 4 years a cut-off point for oral BPs [9]. While there is no cut-off period shown for injectable BPs, the prevalence increases with time. It is particularly interesting that denosumab appears to reach a peak at 2–3 years.
Further, zoledronate is used for both malignant tumors and osteoporosis, but the dosage is 10 or more times higher for malignant tumors. A simple comparison of the prevalence of MRONJ (the ratio of MRONJ patients to patients using the drug) between malignant tumor and osteoporosis is not possible because of the differences in patient background characteristics (age, ability to defend against infection, drugs used, etc.), but it may be conjectured that differences in the dosage are reflected in the difference in prevalence.
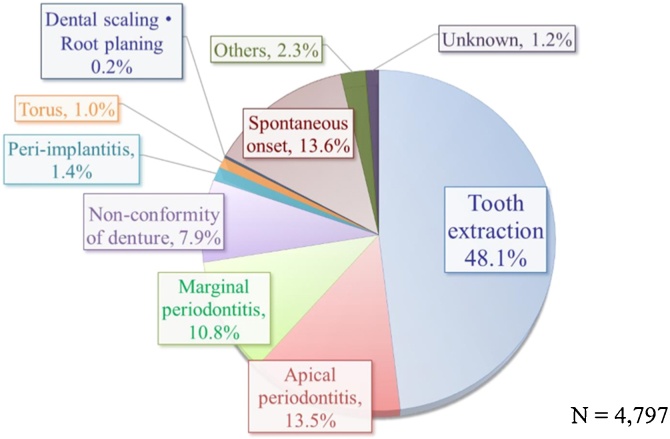
Local factors can be seen as closely related to the question of why drug-related osteonecrosis occurs specifically in the jaw and not in other bones. Among such factors, tooth extraction and periodontitis (apical or marginal) are important. With tooth extraction, the bone is temporarily exposed, and reduced resistance to infection is a risk (there have been a number of studies trying primary closure of the wound or platelet-rich plasma (PRP) injection for prevention of infection, but the effectiveness of these measures is unclear). At the same time, periodontitis is naturally a risk factor because it is a cause of osteomyelitis of the jaw, but also BPs readily accumulate at the inflamed site, and considering the dose-dependency mentioned above, this accumulation will also increase the risk of MRONJ (Fig. 3).
Fig. 3.
Trigger for onset BRONJ.
The majority of extractions are carried out as treatment of periodontitis. Therefore, rather than the invasive extraction procedure, it may perhaps be the underlying periodontitis that is important as a risk factor. This is supported by the fact that MRONJ does not frequently occur following surgical procedures to place implants in healthy jaws.
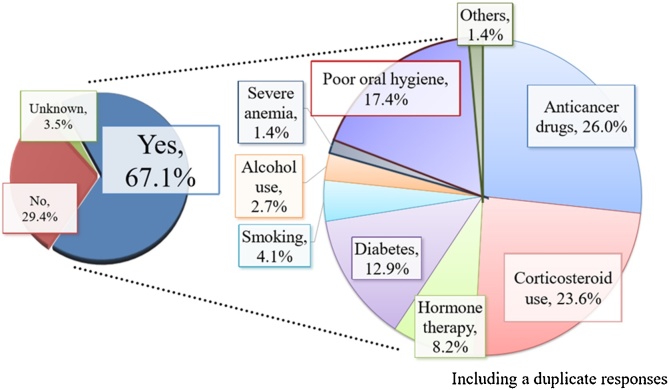
Diabetes, smoking, and steroid administration are important risk factors. While studies may be found that contradict the relationship with smoking, diabetes and smoking are both factors that exacerbate periodontal disease, and as with patients with osteoporosis, care should be taken if the risk of MRONJ increases over the long term.
Steroids have long been known to be a risk factor for (aseptic) osteonecrosis, they decrease bony tissue and reduce resistance to infection, and long-term use is believed to increase the risk of MRONJ (Fig. 4).
Fig. 4.
Risk factor for onset of BRONJ.
Use of the angiogenic inhibitor bevacizumab, as well as anticancer drugs and immunosuppressive agents, can readily aggravate dental infections, and from this perspective, they present a risk for MRONJ.
5. Can MRONJ be prevented by avoiding extraction?
The idea that MRONJ developed as a result of extraction is a common one, and there is consequently a strong tendency to avoid invasive dental treatment. However, a considerable number of cases have been seen recently in which excessive avoidance of invasive dental treatment has led to neglect of the source of infection, resulting in the frequent appearance of MRONJ. Invasive dental treatments that are judged to be necessary from a dental medical point of view should not be avoided due to excessive concern.
With implant therapy, which is one of the invasive dental treatments, it has been reported that the success rate is no different when BPs are administered to patients, and that MRONJ did not occur with invasive treatments carried out on areas with no initial osteomyelitis (implying that MRONJ onset comes not from the treatment, but from the underlying infection).
There are cases in which MRONJ pathology has become established as a result of dental infection, but diagnosis may be difficult if X-ray examination does not show the characteristic sequestrum formation and bone exposure. There is probably a common pattern whereby the presence of MRONJ is not noticed, and inflammatory symptoms such as pain are diagnosed as the result of marginal or apical periodontitis. If the tooth close to the MRONJ pathology is then extracted, the socket in which osteonecrosis is produced naturally does not heal, and the MRONJ diagnosis is finally made due to the persisting bone exposure. Not only in the case of extraction, but also if marginal or apical periodontitis is diagnosed and periodontal surgery or apicectomy is performed, the wound will not heal if osteonecrosis has already occurred.
A similar situation may be seen with gingival cancer, since there are many cases that unfortunately are only detected because of incomplete healing following extraction or periodontal treatment. Naturally, the gingival cancer did not occur as a result of extraction or periodontal treatment. The tooth was extracted because a diagnosis was made of periodontitis or apical periodontitis, which was effectively a misdiagnosis. It is not the case at all that gingival cancer or BRONJ would not have occurred if the tooth had not been extracted. In the case where the condition progresses with none of the characteristic pain of the so-called “dry socket” even though the bone was exposed following extraction, it would be natural to suppose that latent MRONJ, which is hard to diagnose, was already present, and the diagnosis was established as a result of incomplete healing following extraction. If extraction is avoided and efforts are made to conserve the tooth when BRONJ is already present, diagnosis and the start of treatment will be delayed, thus simply prolonging the period during which the patient suffers the condition.
6. Can MRONJ be improved only through conservative treatment?
Regardless of whether BPs have been used, no improvement in necrotic bone (sequestra) can be expected through conservative therapy, unless of course the necrotic bone is removed. There are cases in which surgical treatment is difficult because it is a systemic condition, but, nonetheless, AAOMS 2014 [9] states that regardless of the stage of the disease, “sequestra should be removed to facilitate soft tissue healing. The extraction of symptomatic teeth within exposed, necrotic bone should be considered, since it is unlikely that the extraction will exacerbate the established necrotic process.” Surgical treatment should be considered in line with the position set out in AAOMS 2014 [9].
It should be borne in mind that bone resorption inhibitors are prescribed because they are needed. Basically, medical prescriptions should be given priority, and in many cases, cessation of bone resorption inhibitors will therefore not be possible. However, there are pathological cases where switching to a different medication may be a possibility, so it is always worth consulting with the doctor.
First, the dentist should appreciate the disadvantages of drug cessation, which are the risk of bone fracture or SRE onset. There is a report that the risk of bone fracture increases by 20% with drug cessation in patients with osteoporosis who have taken bone resorption inhibitors for 3 years or less. It has also been reported that when patients switched to the selective estrogen receptor modulator raloxifene, not only was the fracture preventive effect reduced, but the risk of venous thrombosis and stroke increased. In addition, there is scant evidence for the effectiveness of short-term (2–3 months) drug cessation, and taking into account the disadvantages of drug cessation, it should be considered that there are many cases in which drug cessation for the purpose of MRONJ onset prevention would be problematic.
On the other hand, MRONJ is a side effect (adverse event), and the package inserts state that if this occurs, the patient should be carefully observed and appropriate measures such as discontinuation of administration should be taken in the case of any abnormality. Drug cessation is therefore one possible option.
There have been reports stating that there should be a temporary cessation of oral BPs about every 5 years or so, and that with the injectable BP zoledronate, there is no difference in effect with monthly administration or with 3-monthly administration. The evidence is far from complete, and the methods of administration used to date may well be revised in the future. Consequently, there may perhaps be a greater range of cases in which drug cessation is a possibility.
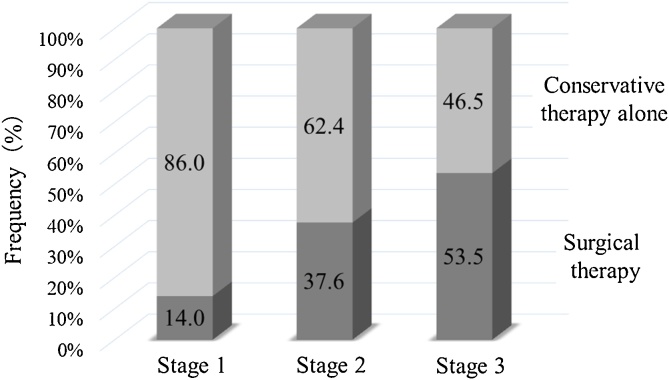
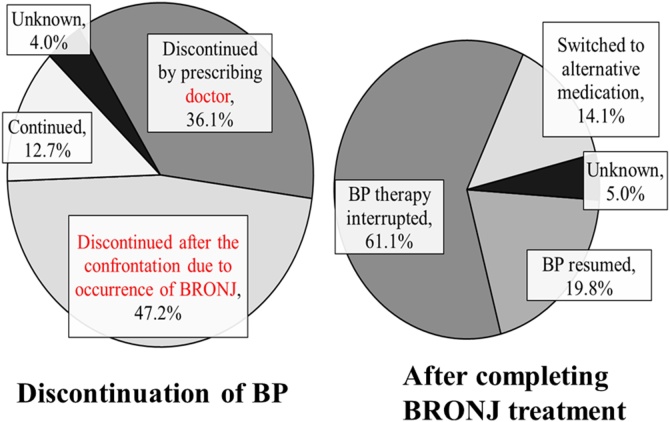
Collaboration between doctors and dentists is desirable, so that when no side effects (MRONJ) occur, the dentist does not hinder treatment by the doctor and gives the doctor all possible support; while the frequency of occurrence is low, if there should be any side effects, the doctor asks the dentist to take measures to address the side effects and at the same time stops administration of the responsible drug (Fig. 5, Fig. 6).
Fig. 5.
Therapy of BRONJ according to stage.
Fig. 6.
Discontinuation and resumption of BP therapy.
7. Can MRONJ be cured?
There are limits to the response by conservative treatment alone, and even at stage 2, proactive surgery is indicated, with recovery now becoming a possibility.
When surgical treatment is judged to be necessary, the possibility or otherwise of therapeutic cessation of the responsible drug should be examined, regardless of the primary disease. This is because sequestrum formation and separation can be facilitated by cessation of bone resorption inhibitors following MRONJ onset. If the sequestra detach, they are expelled naturally or may be treated by surgical extirpation.
In patients with osteoporosis, the parathyroid hormone (PTH) preparation teriparatide is useful for ensuring safer drug cessation [10]. The administration period for teriparatide can be extended to a maximum of 2 years. Therapeutic drug cessation may not always be possible in patients with bone metastatic malignant tumors because of the primary disease, but if drug cessation were possible for a certain period through switching to a different anticancer drug, sequestrum separation would be facilitated, and treatment could be carried out.
If there is no recovery despite these measures, resection of the lesion site of the jaw (jawbone margin resection, jawbone segmental resection) is required, but there is a risk of recurrence if the resection is insufficient [9]. In addition, the procedure carries the risk of exposure of the reconstruction plate if the anti-inflammatory effect is insufficient or if the segmental resection is extensive.
The establishment of therapeutic methods, including prevention, to address MRONJ is now a matter of urgency. There is also a need for education and awareness activities to publicize the rapid increase in MRONJ. Treatment of MRONJ when it occurs can be entrusted to the dentist, but diagnosis and prevention of MRONJ need to be carried out through collaboration between doctors and dentists. Prescribing doctors should encourage dental examinations in advance for the prevention of MRONJ, and dentists, who are the ones actually treating MRONJ, should strive for complete oral management (Fig. 7).
Fig. 7.
Invasive dental treatment during the administration of BP.
It would be very desirable for doctors to appreciate the reality of dental infections and to be aware of the need for oral care. Dentists should have an accurate understanding of the symptoms indicating MRONJ and of the risks of onset in order to give patients the appropriate dental treatment. For this reason, close coordination among medicine, dentistry, and pharmacy, including prescribing doctors and pharmacists, is absolutely essential.
Conflict of interest
There are no conflicts of interest to declare.
References
- 1.Marx R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115–1117. doi: 10.1016/s0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 2.Yoneda T., Hagino H., Sugimoto T., Ohta H., Takahashi S., Soen S. Erratum to: antiresorptive agent-related osteonecrosis of the jaw: position paper 2017 of the Japanese allied committee on osteonecrosis of the jaw. J Bone Miner Metab. 2017;35(January (20)) doi: 10.1007/s00774-017-0816-9. [DOI] [PubMed] [Google Scholar]
- 3.Saad F., Brown J.E., Van Poznak C., Ibrahim T., Stemmer S.M., Stopeck A.T. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol. 2013;23:1341–1347. doi: 10.1093/annonc/mdr435. [DOI] [PubMed] [Google Scholar]
- 4.Ramirez L., Lopez-Pintor R.M., Casanas E., Arriba Ld Hernandez G. New non-bisphosphonate drugs that produce osteonecrosis of the jaws. Oral Health Prev Dent. 2015;13:385–393. doi: 10.3290/j.ohpd.a34055. [DOI] [PubMed] [Google Scholar]
- 5.Urade M., Tanaka N., Furusawa K., Shimada J., Shibata T., Kirita T. Nationwide survey for bisphosphonate-related osteonecrosis of the jaws in Japan. J Oral Maxillofac Surg. 2011;69:e364–e371. doi: 10.1016/j.joms.2011.03.051. [DOI] [PubMed] [Google Scholar]
- 6.Shibahara T., Morikawa T., Yago K., Kishimoto H., Imai Y., Kurita K. National survey on bisphosphonate-related osteonecrosis of the jaws in Japan. J Oral Maxillofac Surg. 2018;(April) doi: 10.1016/j.joms.2018.04.009. pii: S0278-2391(18)30312-4. [DOI] [PubMed] [Google Scholar]
- 7.Filleul O., Crompot E., Saussez S. Bisphosphonate-induced osteonecrosis of the jaw: a review of 2400 patient cases. J Cancer Res Clin Oncol. 2010;136:1117–1124. doi: 10.1007/s00432-010-0907-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan A.A., Morrison A., Hanley D.A., Felsenberg D., McCauley L.K., O’Ryan F. International task force on osteonecrosis of the Jaw. Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus. J Bone Miner Res. 2015;30:3–23. doi: 10.1002/jbmr.2405. [DOI] [PubMed] [Google Scholar]
- 9.Ruggiero S.L., Dodson T.B., Fantasia J., Goodday R., Aghaloo T., Mehrotra B. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw-2014 update. J Oral Maxillofac Surg. 2014;72:1938–1956. doi: 10.1016/j.joms.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 10.Chan H.L., McCauley L.K. Parathyroid hormone applications in the craniofacial skeleton. J Dent Res. 2013;92:18–25. doi: 10.1177/0022034512464779. [DOI] [PMC free article] [PubMed] [Google Scholar]