Abstract
Recently, monolayer molybdenum disulphide (MoS2) has emerged as a promising and non–precious electrocatalyst for hydrogen evolution reaction. However, its performance is largely limited by the low density and poor reactivity of active sites within its basal plane. Here, we report that domain boundaries in the basal plane of monolayer MoS2 can greatly enhance its hydrogen evolution reaction performance by serving as active sites. Two types of effective domain boundaries, the 2H-2H domain boundaries and the 2H-1T phase boundaries, were investigated. Superior hydrogen evolution reaction catalytic activity, long-term stability and universality in both acidic and alkaline conditions were achieved based on a multi-hierarchy design of these two types of domain boundaries. We further demonstrate that such superior catalysts are feasible at a large scale by applying this multi-hierarchy design of domain boundaries to wafer-scale monolayer MoS2 films.
While water-splitting electrocatalysts enable energy storage in carbon-neutral fuels, a recent challenge has been the discovery and understanding of catalyst active sites. Here, authors find domain boundaries in MoS2 materials to present high-activity, stable, and scalable sites for H2 evolution.
Introduction
Hydrogen evolution reaction (HER) process is crucial to the production of hydrogen, the most efficient and environmental-friendly fuel. Platinum (Pt) and Pt-based materials are known as the best electrocatalysts for HER so far, but they are also very scarce and expensive1. Recently, molybdenum disulfide (MoS2) has emerged as an active, earth-abundant, and inexpensive alternative to Pt and Pt-based electrocatalysts2–7. It is generally believed that the catalytic activity of MoS2 originates from its edges while its basal plane is rather inert, which limits the practical application of this material for HER8–11. In order to overcome the limited catalytic activity of the MoS2 basal plane, various techniques have been developed, such as phase engineering12–15, interface electronic coupling16, introducing active unsaturated defects14 and strain17. These techniques could improve the restricted factors (poor conductivity and limited active sites) for the potential of MoS2 in HER18–20. Recently, a pioneer strategy has been proposed by introducing S vacancies into the basal plane17,21–25, where gap states around the Fermi level allow hydrogen to bind directly to exposed Mo atoms. Considering the presence of dangling bonds in vacancy defects, these vacancy defects in MoS2 are easy to be poisoned and would lead to the surface instability from a HER point of view. To fully exploit MoS2 materials in realistic application, searching for alter
Herein, we report a facile route toward the activation of the monolayer MoS2 basal plane for HER by introducing domain boundaries, including both 2H–2H domain boundaries and 2H–1T-phase boundaries. We found that the domain boundaries can provide ultrahigh-density active sites, while still maintain the surface stability. Utilizing a multi-hierarchy design of these two types of boundaries, we are able to achieve a high basal-plane electrocatalytic performance with an exchange current density of 0.57 × 10−4 A cm−2, a Tafel slope of 73 mV dec−1, and a remarkable long-term operation stability over 200 h. We also demonstrate that such catalysts are scalable, e.g., over 4-inch wafer scale, pushing a crucial technological step toward practical applications.
Results
2H–2H domain boundaries and 2H–1T-phase boundaries for HER
In this study, we investigated both single-crystalline and polycrystalline 2H-phase monolayer MoS2 samples grown by chemical vapor deposition (CVD) for HER. The former samples (type-I) were grown on sapphire substrates with individual domain size of a few hundred micrometers (refer to ref. 25 for our previous work)26. The latter samples were continuous film samples grown either on sapphire substrates (type-II) with highly orientated domains of a few micrometers (refer to ref. 26 for our previous work)27 or on SiO2/Si substrates (type-III) with randomly orientated domains of a few hundred nanometers (refer to ref. 27 for our previous work)28. Figure 1a–c show typical optical and false-color transmission electron microscope (TEM) images of these different monolayer MoS2 samples to illustrate their domain sizes and the 2H–2H domain boundaries. Hence, we have three types of MoS2 samples for comparable investigation of the HER performances: type-I free of 2H–2H domain boundaries, type-II with low density of 2H–2H domain boundaries, and type-III with high density of 2H–2H domain boundaries (further details in Supplementary Fig. 1). Note that the 2H–2H domain boundaries in our MoS2 samples are not perfectly straight. These boundaries usually consist of various configurations, typically including arrays of 4–6 rings (4|6), 6–8 rings (6|8), 5–7 rings (5|7), and 4–4 rings (4|4) as shown in Supplementary Fig. 2. The high-resolution TEM and X-ray photoelectron spectroscopy (XPS) were used to confirm that all samples are of high quality with low-defect density and very clean surfaces even after transfer processes (Supplementary Fig. 3). Hence, the influence of defects in different types of samples can be excluded.
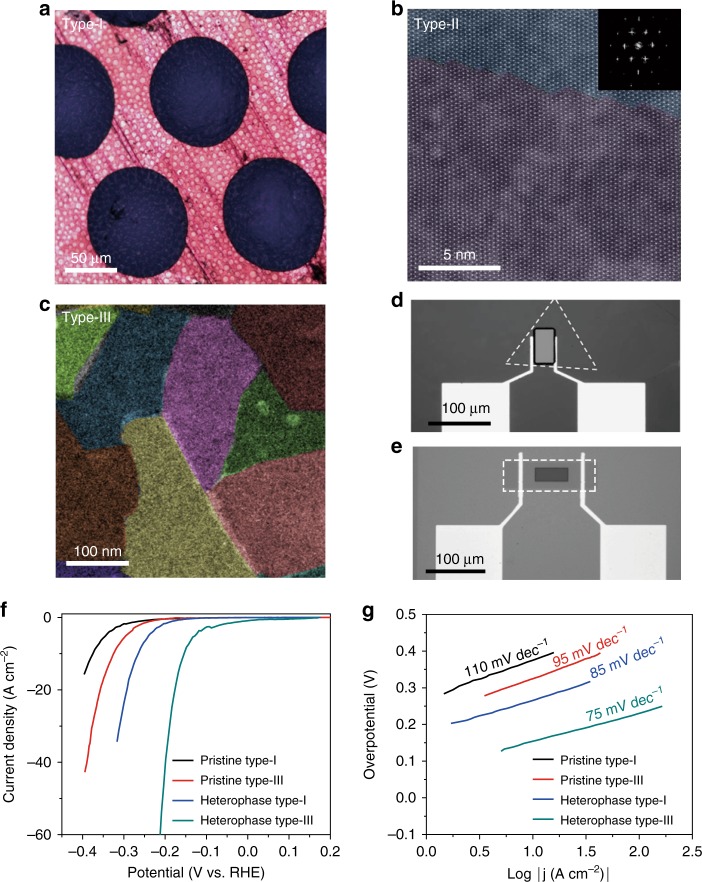
Fig. 1.
Activity of 2H–2H domain boundaries and 2H–1T-phase boundaries for HER. a Optical image of the type-I MoS2 with individual domain size of few hundreds microns transferred onto a TEM grid. b HRTEM images of as-grown type-II MoS2 film with highly orientated domains of few microns for the domain sizes. c False-color dark-field TEM image of type-III MoS2 with high-density 2H–2H domain boundaries. d, e Optical microscope image of a individual MoS2 single-crystal domain without 2H–2H domain boundaries (d) and polycrystalline ML–MoS2 with domain boundaries (e). Dashed regions indicate the HER window opened on the basal plane. f Polarization curves of the pristine type-I MoS2 (without any domain boundaries), pristine type-III MoS2 (with 2H–2H domain boundaries), heterophase type-I MoS2 (with 2H–1T-phase domain boundaries), and heterophase type-III MoS2 (with both 2H–2H and 2H–1T domain boundaries), respectively. g Tafel plots of the corresponding curves in f
In order to investigate whether the 2H–2H domain boundaries could serve as active sites in HER, local probe characterizations were first performed (further details in Supplementary Note 1 and Supplementary Fig. 4). Figure 1d and e show two typical HER devices fabricated from type-I and type-III samples, respectively. Note that these devices were protected by polymethyl methacrylate (PMMA) masks with only small windows exposed on the MoS2 samples in the center to avoid the contribution from the MoS2 edges during the HER characterizations. The corresponding HER polarization curves and Tafel plots of both devices are shown in Fig. 1f, g, respectively. It can be clearly seen that type-III samples have better catalytic performances than type-I samples as evidenced by the drop of the overpotential from ~ 375 mV to ~ 325 mV at current density of 10 A cm−2 and the Tafel slope from ~ 110 to ~ 95 mV dec−1. Since the qualities of the two samples are very similar, except for the density of domain boundaries, we thus can draw a conclusion that the enhanced HER activity in type-III samples comes from the 2H–2H domain boundaries.
Then we explored the possibility of further introducing 2H–1T-phase boundaries as active sites in HER. In order to produce such boundaries, we performed post low-energy Ar–plasma bombardments on pristine type-I and type-III samples to induce the 2H-to-1T-phase transition (refer to ref. 28 for our previous work)29. Note that this phase transition is quite local and the resulted samples consist of a mosaic texture of nearly half 2H- and half 1T-phases, exhibiting an average domain size of a few nanometers and high-density phase boundaries (Supplementary Fig. 5). HER performance of two typical heterophase devices similar to those described above is shown in Fig. 1f, g. For type-I sample after phase transition, a drop of the overpotential from ~ 370 to ~ 260 mV at current density of 10 A cm−2 and the Tafel slope from ~ 110 to ~ 85 mV dec−1 can be clearly seen, suggesting that 2H–1T-phase boundaries are more efficient for HER as active reaction sites than 2H–2H boundaries. Besides, the metallic 1 T phase offers better charge transport capability than 2 H phase, which is consistent with previous report16. Notably, the heterophase type-III sample exhibits the lowest overpotential of 200 mV and Tafel slope of 75 mV dec−1. In order to confirm that no other defects in the heterophase structure contributed prominently to the enhanced HER activity, we performed atomic force microscopy (AFM) and STM characterizations. Only few S vacancies (~1.9%) having little effect on HER can be found without any other defects in the heterophase sample (details in Supplementary Figs. 6–7 and Supplementary Note 2)17,25,30. Particularly, we also found that these S vacancies would not introduce gap states that allow favorable hydrogen adsorption (Supplementary Fig. 7). We have also performed thermal annealing for our phase-changed samples to investigate the effect of phase boundaries. Thermal annealing was carried out in vacuum at 600 °C for 1 h. This process can recover from all 1 T phases back to 2 H phases in our sample (as confirmed in Supplementary Fig. 8a). Meanwhile, after annealing treatment, the HER performance degrades to the same level of pristine MoS2, as shown in Supplementary Fig. 8b. On the basis of these results, we concluded that the phase boundaries are dominant active sites in our heterophase MoS2 samples while those S vacancies contribute less to enhance the HER performance. Thus the composite structure containing both high-density domain and phase boundaries is the most promising candidate for HER.
Mechanism of the activation of the MoS2 basal plane by boundaries
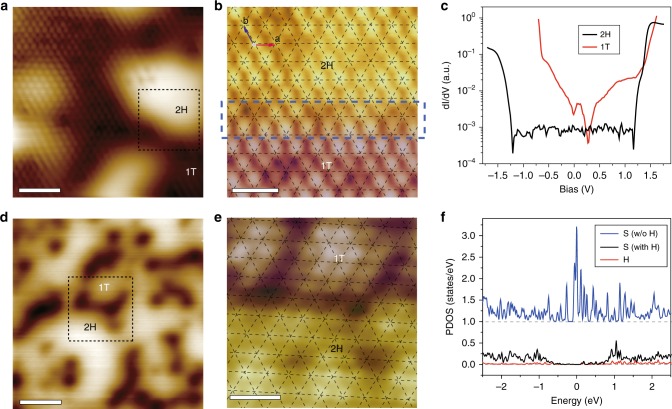
In order to confirm the role of 2H–1T-phase boundaries in the basal plane of MoS2 in HER, we performed scanning tunneling microscopy (STM) and scanning tunneling spectroscopy (STS) characterizations. Since STM/STS measurements require conductive substrates, MoS2 samples were epitaxially grown on graphite by CVD and treated by Ar–plasma to induce phase transitions subsequently. Figure 2a shows a typical topographic STM image of the monolayer heterophase MoS2, where bright and dark regions correspond to the 2 H and 1 T phases, respectively. A zoom-in image at one-phase boundary is shown in Fig. 2b with the top sulfur (S) atoms clearly resolved. The sliding of S atoms in 1 T phase with respect to the 2 H phase further confirmed coexistence of the two phases (details in Supplementary Fig. 9). As shown in Fig. 2c, 2H-phase domains have bandgaps as usual, e.g., ~2.5 eV; in contrast, 1T-phase domains are metallic without bandgaps.
Fig. 2.
Hydrogen adsorption at 2H–1T-phase boundaries. a STM topography of as-treated MoS2 showing mixed 2 H (bright) and 1 T (dark) domains. b Zoom-in image of a domain boundary as denoted by a dashed square in a, where 2H and 1T phases are rendered with yellow and purple colors, respectively. The lattice grid of 2H phase is superimposed on the image to highlight the lateral sliding of S atoms in 1T phase. The black arrows indicate the sliding direction [120]. The two in-plane primitive vectors are shown in the upper left of b. c dI/dV spectra taken on the 2H and 1T phases, suggesting the semiconducting 2H phase with a 2.5-eV bandgap and metallic 1T phase. d STM image of the MoS2 after hydrogenation, showing prominent depression features. The sample bias was chosen such that the apparent heights of 2 H and 1 T phases become similar, to highlight the depression features. e Zoom-in image of the depression features as denoted by a dashed square in d, indicating that the depression features are located exactly at the boundary of 2H and 1T phases. Set points of the STM images: a: 1.5 V, 50pA; b: 1 V, 10 pA; d: 2 V, 10 pA; e: 0.5 V, 50pA. Set point of dI/dV spectra:1.5 V, 50 pA c. f Projected density of states (PDOS) of S and H atoms at the phase boundary before and after hydrogenation. Blue curve: PDOS of S atoms before hydrogenation, black curve: PDOS of S atoms with H bonded, red curve: PDOS of absorbed H atoms. Scale bar: 2 nm a, 0.7 nm b, 1.5 nm d, 0.7 nm e
In HER process, stable hydrogen adsorption at active sites is a crucial step. Therefore, we simulated this step via hydrogenating the heterophase sample surface by the atomic hydrogen (see Supporting Information for more details). After hydrogenation, additional depression features with atomic-size width appear exactly at the phase boundaries (Fig. 2d, e), suggesting that the atomic hydrogen prefers to adsorb at the S sites of phase boundary. Density functional theory (DFT) calculations were also carried out to confirm the STM observations. Simulated results indicate that the formation of S–H covalent bonds can lead to a significant reduction of the density of states (DOS) of S atoms around the Fermi level (Fig. 2f), which is consistent with the apparent depression observed in the STM images due to the adsorption of H atoms on the S atoms.
Next, we performed further DFT calculations on the 2H–2H grain boundaries and the 2H–1T-phase boundaries to investigate their catalytic activities (see Supporting Information for computational details). Figure 3a, b show the structural model of 2H–1T-phase boundaries and four typical kinds of 2H–2H boundary configurations (4|8, 6|8, 4|4, 5|7)24. Figure 3c shows the calculated Gibbs free energy of the adsorbed atomic hydrogen (ΔGH*). Note that ΔGH* is a widely accepted indicator for the catalytic activity and the optimal value is ΔGH* = 0 eV, where hydrogen is bounded neither too strongly nor too weakly31. For comparison, we also performed calculations on Pt (111) surface, basal plane of 2H–MoS2 and 1T–MoS2 with the same sized supercells as for the phase boundaries, yielding ΔGH* = −0.18 eV, 1.87 eV, and −6.97 eV, respectively (Fig. 3b). These numbers are consistent with previous calculations10,32–36. Due to the high instability of the pristine 1 T phase, the initial binding of hydrogen on a pure 1T-phase basal plane is quite strong, which results in heavy relaxation of the adsorption area. This releases most of the 1 T energy, making the structure rather inert for the further adsorption of hydrogen atoms14,37,38. Moreover, for the 1 T phase confined within 2 H phase by interfaces, the strong relaxations at the interfaces have the similar effect as the initial H adsorption, which prevents the confined 1 T phase to adsorb more H atoms favorably (further details in Supplementary Fig. 10). This can explain the absence of H adsorption at the nanometer-size 1 T phase as shown in the STM image (Fig. 2d, e). The Gibbs free energy of all the H adsorption sites in 2H–2H domain boundaries are much smaller than that of the perfect MoS2 basal plane (1.87 eV), indicating that 2H–2H boundaries can indeed break the inertia of the basal plane and enhance the interaction between the H atom and the adsorption sites. Impressively, the 2H–1T-phase boundaries exhibit ΔGH* = −0.13 eV (Fig. 3b), very close to that of the Pt (111) surface and Mo-edge of 2H–MoS2. Thus, phase boundaries in the basal plane of monolayer MoS2 could serve as effective sites to tune hydrogen reaction barriers and optimize the overall kinetics of H2 evolution39,40.
Fig. 3.
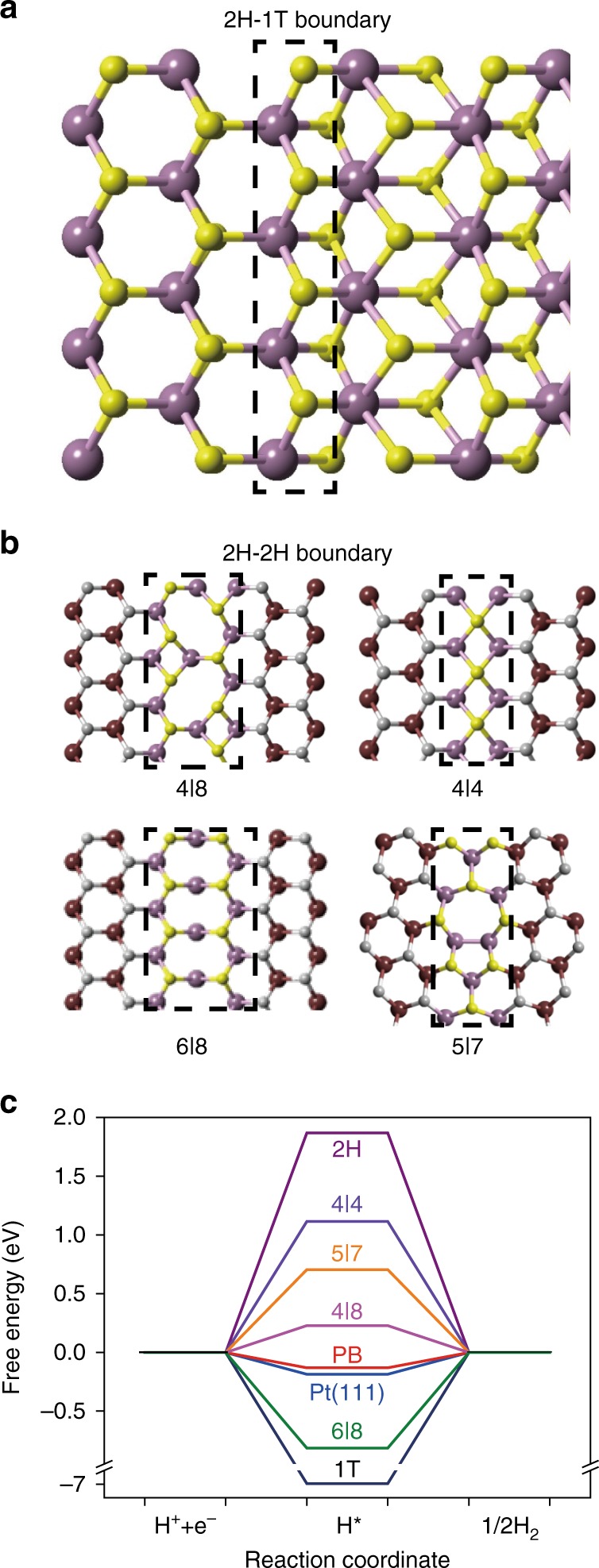
Theoretical simulations. a, b The top views of the atomic model of the zigzag type 2H–1T-phase boundaries and four kinds of 2H–2H boundaries (4|8, 6|8, 4|4, 5|7). c A comparison of the Gibbs free energies of the adsorbed H on 2H-phase of MoS2, 1T-phase of MoS2, Pt(111) surface, 2H–1T-phase boundaries (PBs) and four kinds of 2H–2H boundaries in the context of HER
Multi-hierarchy monolayer MoS2 catalysts for HER
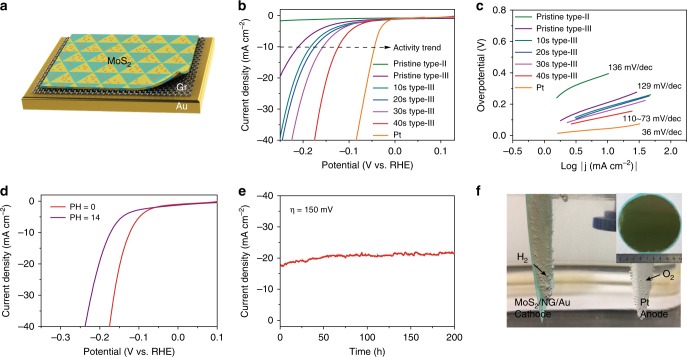
Based on the above experimental and theoretical results, we can conclude that both domain boundaries and phase boundaries can serve as active sites in HER. More boundaries, in principle, should offer better HER performance. We thus investigated the effect of boundary density in a systematic way. MoS2 electrodes for electrocatalytic HER testing were fabricated from pristine type-II samples (with low density of 2H–2H domain boundaries), pristine type-III samples (with high density of 2H–2H domain boundaries), and a series of heterophase type-III samples with varying density of 2H–1T-phase boundaries. As mentioned above, the heterophase MoS2 can be produced by Ar–plasma bombardments; while the percentage of 1T-phase in 2H-phase matrix, thus the density of phase boundaries, can be actually tuned by the treatment durations. The illustrated multi-hierarchy catalysts for HER characterizations is shown in Fig. 4a. ML–MoS2 catalysts were supported on graphene (Gr)/Au films (structure and fabrication details discussed in Supplementary Note 1 and Supplementary Figs. 11–13). Note that the underlying graphene layers, as an internal electron transport channels, can decrease the resistive loss and accelerate electron transport from the MoS2 film to electrodes11. Polarization curves (Fig. 4b) and Tafel plots (Fig. 4c) of these samples were measured in 0.5 M sulfuric acid electrolyte using a standard three-electrodes configuration (see the Methods section for more details). Note that, the overpotential at 10 mA/cm2 decreased linearly with increasing the phase-boundary density of MoS2 tuned by the treatment durations, which confirmed the effect of phase boundaries in HER (further details see Supplementary Figs. 14, 15).
Fig. 4.
Multi-hierarchy monolayer MoS2 catalysts for HER. a Schematic structure of the multi-hierarchy MoS2 catalysts with both high density of domain and phase boundaries. b Polarization curves for pristine type-II samples, pristine type-III samples, a series of heterophase type-III samples with different phase boundary densities and Pt. c Tafel plots of the corresponding curves in b. d HER performance of a multi-hierarchy MoS2 catalyst in 0.5-M H2SO4 (red curve) and 1-M KOH (purple curve). e Time-dependent current density curve for a multi-hierarchy MoS2 catalyst under static overpotential of 150 mV for 200 h. f Demonstration of the catalytic HER activity in 0.5 M H2SO4 from a multi-hierarchy MoS2 catalyst with a size of 4 inches in diameter. Inset: photograph of pristine as-grown wafer-scale MoS2 on sapphire substrate
The control sample is a commercial Pt foil, which exhibits a nearly zero overpotential. From these data shown in Fig. 4b, we can clearly see that samples with either more domain boundaries or more phase boundaries can yield better HER performance in terms of reduced overpotentials and Tafel slopes. The best structure is a composite with both high-density domain and phase boundaries, as shown in the illustrated drawing of Fig. 4a. Notably, the best sample presents a very low onset potential of ~100 mV, a low overpotential of ~136 mV, a Tafel slope of ~73 mV dec−1, an extremely large cathodic current density of ~78 mA cm−2 at η = 200 mV, and an exchange current density of 57 μA cm−2, all of which are better than or comparable to sulfur vacancies (details see Supplementary Fig. 16) and previous results from the edge-dominated MoS2 catalysts33,41,42.
A remarkable feature of these composite boundary catalysts is that they can work in both acidic and alkaline conditions with similar performance, considering that only a few candidates could do this job43. Polarization curves (Fig. 4d) measured in 1 M KOH (pH = 14) present an overpotential of ~176 mV at 10 mA cm−2, which is slightly larger than that in acidic conditions. We also carried out stability testing, which is another important aspect in practical applications, for the multi-hierarchy MoS2 catalysts in Fig. 4e. Surprisingly, it can work for over 200 h without any noticeable degradation of current density. We also investigated these two type of boundaries, respectively, and both of them show excellent durability (see Supplementary Fig. 17). Thus we attribute this excellent durability to the stable structures of monolayer MoS2 with 2H–2H and 2H–1T boundaries. Such structures will not be degraded during the HER process, although they are just monolayers. Finally, the catalysts with composite boundary can also be easily scaled up. As a proof-of-concept demonstration, a 4-inch wafer-scale catalysts, exhibited in Fig. 4f, was prepared for HER. The wafer-scale catalysts sample still exhibit good HER properties (see Supplementary Fig. 18 and Supplementary Movie 1 for more details).
Discussion
In conclusion, we have both experimentally and theoretically verified that 2H–2H and 2H–1T domain boundaries in basal plane of ML–MoS2 could act as new highly active and tunable catalytic sites for HER. Based on the observed phenomena, we then achieved multi-hierarchy ML–MoS2 electrocatalysts containing both types of domain boundaries for HER. These electrocatalysts not only show remarkable electrocatalytic performances with a small overpotential of ~0.1 V and large cathodic currents, but have long-term stability and universality in both acidic and alkaline conditions. Moreover, the ML–MoS2 electrocatalysts with composite boundaries can be easily scaled up. Our results provide a comprehensive understanding of the HER mechanism for the MoS2 basal plane, as well as a facile route to design high-performance electrocatalysts.
Methods
Growth of the monolayer 2H–MoS2 using CVD method
A three zone furnace was used for CVD growth of MoS2. SiO2 (300 nm)/P + + Si and highly oriented pyrolytic graphite (HOPG) served as substrates. Sulfur (S) (Alfa Aesar 99.9%) and molybdenum trioxide (MoO3) (Alfa Aesar 99.999%) were used as precursors and loaded in zone I and II, respectively. The distance between the two sources was 22 cm. The substrates were put in the third zone. The temperatures of MoO3, S, and substrates were 560, 120, and 780 °C, respectively. Each temperature zone was kept stable for 20 min before the growth. During the growth, argon was used as carrying gas at a flow rate of 130 sccm, and the vacuum pressure was kept at 0.67 Torr.
Formation of heterophase MoS2
Phase transition of MoS2 was also performed in the home-made, remote plasma system reported in our previous work29. An inductively coupled plasma was generated by dispersing a 20 W RF power at the entrance of a 4-inch quartz-tube furnace. The pressure in the tube furnace was fixed at ≈0.69 Torr for phase transition by flowing argon at 100 sccm and vacuum pumping. The process was carried out for 10, 20, 30, 40 s at room temperature, respectively.
Characterization details
The as-grown layer monolayer MoS2 samples were characterized by optical microscopy, AFM (Bruker Icon microscope, tapping mode), Raman spectroscopy (532 nm laser, Horiba Jobin Yvon LabRAM HR-Evolution Raman), X-ray photoelectron spectroscopy (Kratos Analytical Axis Ultra).
Electrochemical characterizations
All of the electrochemical measurements were performed in a typical three-electrode system on electrochemical workstation (Autolab PGSTAT 302 N). A Pt foil or a glassy carbon electrode (for long time test) were used as counter electrodes, and saturated Ag/AgCl electrode serve as the reference electrode. The linear sweep voltammetry (LSV) with the scan rate of 5 mV s−1 was carried out. All the applied potentials were converted to reversible hydrogen electrode (RHE) potentials scaled using the equation E (vs. RHE) = E (vs. Ag/AgCl) + 0.204 V + 0.0591 VPH, after IR correction. The stability tests for the heterophase MoS2 was performed using chronoamperometry at a constant applied overpotential.
Computational details
First-principles calculations based on DFT were carried out by using the Vienna Ab initio Simulation Package (VASP)7,10. The interactions between valence electrons and ions were treated with the projector-augmented wave (PAW) method8. The exchange-correlation interactions were described by generalized gradient approximation (GGA) with the Perdew−Burke−Ernzerhof (PBE) functional11. The electron wave functions were expanded in a plane-wave basis set with cutoff energy of 520 eV. The convergence criterion for residual force on each atom during structure relaxation was set to 0.02 eV/Å, and the geometries were relaxed to minimize the total energy of the system until a precision of 10–4 eV was reached.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
G.Z. thanks the supports from NCSF under the grant No. 11834017 and 61888102, the Strategic Priority Research Program of CAS under the grant No. XDB30000000, the Key Research Program of Frontier Science of CAS under the grant No. QYZDB-SSW-SLH004, and the National Key R&D program under grant No. 2016YFA0300904. Y.J. acknowledges support by the National Key R&D program under Grant No. 2016YFA0300901 and 2017YFA0205003, the National Natural Science Foundation of China under Grant No. 11634001, 11888101 and 21725302, Beijing Municipal Science & Technology Commision (grant No. Z181100004218006) and the Strategic Priority Research Program of Chinese Academy of Sciences under Grant no. XDB28000000. L.Z. is supported by the National Science Foundation of China with Grant No. 11574157. R.Y. acknowledges support by the National Science Foundation of China (NSFC, Grant No. 11574361) and the Youth Innovation Promotion Association CAS (No. 2018013).
Author contributions
G.Z. and R.Y designed and supervised the research. J.Z., Q.W., Z.W., N.L. and L.D. prepared the samples. J.Z., Q.W. and R.Y. performed AFM, Raman, XPS, and electrochemical characterizations. J.Z., H.Y., J.Z. and W.C. performed TEM characterizations. Z.C.W. and Y.J. performed STM and STS characterizations. H.D. and L.Z. performed calculations. W.W. provided the electrochemical workstation. Data were collected and analyzed by J.Z., Z.C.W., H.D., L.Z., Y.J., D.S. and G.Z. Figures were prepared by J.Z. and M.L. The paper was written by J.Z., L.Z., Y.J. and G.Z. All authors discussed the results and commented on the paper.
Data availability
All data are available from the authors upon reasonable request. All source data underlying Figs. 1a, f, 2c, f, 3c, 4b–e and Supplementary Figs. 1g, h, 3d, e, 5a–c, 7c, 8a, 12a, d, 13a–c, 16, 17 and 18d are provided as a Source Data file.
Competing interests
The authors declare no competing interests.
Footnotes
Journal Peer Review Information: Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jianqi Zhu, Zhi-Chang Wang, Huijia Dai.
Contributor Information
Rong Yang, Email: ryang@iphy.ac.cn.
Lixin Zhang, Email: lxzhang@nankai.edu.cn.
Ying Jiang, Email: yjiang@pku.edu.cn.
Guangyu Zhang, Email: gyzhang@iphy.ac.cn.
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-09269-9.
References
- 1.Conway B, Tilak B. Interfacial processes involving electrocatalytic evolution and oxidation of H2, and the role of chemisorbed H. Electrochim. Acta. 2002;47:3571–3594. doi: 10.1016/S0013-4686(02)00329-8. [DOI] [Google Scholar]
- 2.Hinnemann B, et al. Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J. Am. Chem. Soc. 2005;127:5308–5309. doi: 10.1021/ja0504690. [DOI] [PubMed] [Google Scholar]
- 3.Greeley J, Jaramillo TF, Bonde J, Chorkendorff I, Nørskov JK. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nat. Mater. 2006;5:909. doi: 10.1038/nmat1752. [DOI] [PubMed] [Google Scholar]
- 4.Chhowalla M, et al. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013;5:263–275. doi: 10.1038/nchem.1589. [DOI] [PubMed] [Google Scholar]
- 5.Cao X, Tan C, Zhang X, Zhao W, Zhang H. Solution-processed two-dimensional metal dichalcogenide-based nanomaterials for energy storage and conversion. Adv. Mater. 2016;28:6167. doi: 10.1002/adma.201504833. [DOI] [PubMed] [Google Scholar]
- 6.Jaramillo TF, et al. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science. 2007;317:100–102. doi: 10.1126/science.1141483. [DOI] [PubMed] [Google Scholar]
- 7.Bonde J, Moses PG, Jaramillo TF, Nørskov JK, Chorkendorff I. Hydrogen evolution on nano-particulate transition metal sulfides. Faraday Discuss. 2009;140:219–231. doi: 10.1039/B803857K. [DOI] [PubMed] [Google Scholar]
- 8.Merki D, Vrubel H, Rovelli L, Fierro S, Hu X. Fe, Co, and Ni ions promote the catalytic activity of amorphous molybdenum sulfide films for hydrogen evolution. Chem. Sci. 2012;3:2515–2525. doi: 10.1039/c2sc20539d. [DOI] [Google Scholar]
- 9.Chang K, et al. MoS2/graphene cocatalyst for efficient photocatalytic H2 evolution under visible light irradiation. ACS nano. 2014;8:7078–7087. doi: 10.1021/nn5019945. [DOI] [PubMed] [Google Scholar]
- 10.Xie J, et al. Controllable disorder engineering in oxygen-incorporated MoS2 ultrathin nanosheets for efficient hydrogen evolution. J. Am. Chem. Soc. 2013;135:17881–17888. doi: 10.1021/ja408329q. [DOI] [PubMed] [Google Scholar]
- 11.Bentley CL, et al. Electrochemical maps and movies of the hydrogen evolution reaction on natural crystals of molybdenite (MoS2): basal vs. edge plane activity. Chem. Sci. 2017;8:6583–6593. doi: 10.1039/C7SC02545A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lukowski MA, et al. Enhanced hydrogen evolution catalysis from chemically exfoliated metallic MoS2 nanosheets. J. Am. Chem. Soc. 2013;135:10274–10277. doi: 10.1021/ja404523s. [DOI] [PubMed] [Google Scholar]
- 13.Voiry D, et al. Conducting MoS2 nanosheets as catalysts for hydrogen evolution reaction. Nano. Lett. 2013;13:6222–6227. doi: 10.1021/nl403661s. [DOI] [PubMed] [Google Scholar]
- 14.Yin Y, et al. Contributions of phase, sulfur vacancies, and edges to the hydrogen evolution reaction catalytic activity of porous molybdenum disulfide nanosheets. J. Am. Chem. Soc. 2016;138:7965. doi: 10.1021/jacs.6b03714. [DOI] [PubMed] [Google Scholar]
- 15.Yu Y, et al. High phase-purity 1T′-MoS2-and 1T′-MoSe2-layered crystals. Nat. Chem. 2018;10:638–643. doi: 10.1038/s41557-018-0035-6. [DOI] [PubMed] [Google Scholar]
- 16.Voiry D, et al. The role of electronic coupling between substrate and 2D MoS2 nanosheets in electrocatalytic production of hydrogen. Nat. Mater. 2016;15:1003. doi: 10.1038/nmat4660. [DOI] [PubMed] [Google Scholar]
- 17.Li H, et al. Activating and optimizing MoS2 basal planes for hydrogen evolution through the formation of strained sulphur vacancies. Nat. Mater. 2016;15:48–53. doi: 10.1038/nmat4465. [DOI] [PubMed] [Google Scholar]
- 18.Ding Q, Song B, Xu P, Jin S. Efficient electrocatalytic and photoelectrochemical hydrogen generation using MoS2 and related compounds. Chem. 2016;1:699–726. doi: 10.1016/j.chempr.2016.10.007. [DOI] [Google Scholar]
- 19.Zhang J, et al. Unveiling active sites for the hydrogen evolution reaction on monolayer MoS2. Adv. Mater. 2017;29:1701955–1701962. doi: 10.1002/adma.201701955. [DOI] [PubMed] [Google Scholar]
- 20.Yin Y, et al. Synergistic phase and disorder engineering in 1T-MoSe2 nanosheets for enhanced hydrogen-evolution reaction. Adv. Mater. 2017;29:1700311–1700318. doi: 10.1002/adma.201700311. [DOI] [PubMed] [Google Scholar]
- 21.Lin SH, Kuo JL. Activating and tuning basal planes of MoO2, MoS2, and MoSe2 for hydrogen evolution reaction. Phys. Chem. Chem. Phys. 2015;17:29305–29310. doi: 10.1039/C5CP04760A. [DOI] [PubMed] [Google Scholar]
- 22.Lu AY, et al. High-sulfur-vacancy amorphous molybdenum sulfide as a high current electrocatalyst in hydrogen evolution. Small. 2016;12:5530–5537. doi: 10.1002/smll.201602107. [DOI] [PubMed] [Google Scholar]
- 23.Li H, et al. Kinetic study of hydrogen evolution reaction over strained MoS2 with sulfur vacancies using scanning electrochemical microscopy. J. Am. Chem. Soc. 2016;138:5123–5129. doi: 10.1021/jacs.6b01377. [DOI] [PubMed] [Google Scholar]
- 24.Ouyang Y, et al. Activating inert basal planes of MoS2 for hydrogen evolution reaction through the formation of different intrinsic defects. Chem. Mater. 2016;28:4390–4396. doi: 10.1021/acs.chemmater.6b01395. [DOI] [Google Scholar]
- 25.Tsai C, et al. Electrochemical generation of sulfur vacancies in the basal plane of MoS2 for hydrogen evolution. Nat. Commun. 2017;8:15113–15120. doi: 10.1038/ncomms15113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen W, et al. Oxygen-assisted chemical vapor deposition growth of large single-crystal and high-quality monolayer MoS2. J. Am. Chem. Soc. 2015;137:15632–15635. doi: 10.1021/jacs.5b10519. [DOI] [PubMed] [Google Scholar]
- 27.Yu H, et al. Wafer-scale growth and transfer of highly-oriented monolayer MoS2 Continuous films. ACS nano. 2017;11:12001–12007. doi: 10.1021/acsnano.7b03819. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, et al. Scalable growth of high-quality polycrystalline MoS2 monolayers on SiO2 with tunable grain sizes. ACS nano. 2014;8:6024–6030. doi: 10.1021/nn5020819. [DOI] [PubMed] [Google Scholar]
- 29.Zhu J, et al. Argon plasma induced phase transition in monolayer MoS2. J. Am. Chem. Soc. 2017;139:10216–10219. doi: 10.1021/jacs.7b05765. [DOI] [PubMed] [Google Scholar]
- 30.Cho S, et al. Phase patterning for ohmic homojunction contact in MoTe2. Science. 2015;349:625–628. doi: 10.1126/science.aab3175. [DOI] [PubMed] [Google Scholar]
- 31.Jiao Y, Zheng Y, Jaroniec M, Qiao SZ. Design of electrocatalysts for oxygen-and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 2015;44:2060–2086. doi: 10.1039/C4CS00470A. [DOI] [PubMed] [Google Scholar]
- 32.Tauster S, Pecoraro T, Chianelli R. Structure and properties of molybdenum sulfide: Correlation of O2 chemisorption with hydrodesulfurization activity. J. Catal. 1980;63:515–519. doi: 10.1016/0021-9517(80)90109-8. [DOI] [Google Scholar]
- 33.Salmeron M, Somorjai G, Wold A, Chianelli R, Liang K. The adsorption and binding of thiophene, butene and H2S on the basal plane of MoS2 single crystals. Chem. Phys. Lett. 1982;90:105–107. doi: 10.1016/0009-2614(82)83620-8. [DOI] [Google Scholar]
- 34.Byskov LS, Nørskov JK, Clausen BS, Topsøe H. DFT calculations of unpromoted and promoted MoS2-based hydrodesulfurization catalysts. J. Catal. 1999;187:109–122. doi: 10.1006/jcat.1999.2598. [DOI] [Google Scholar]
- 35.Wang H, et al. Transition-metal doped edge sites in vertically aligned MoS2 catalysts for enhanced hydrogen evolution. Nano. Research. 2015;8:566–575. [Google Scholar]
- 36.Tsai C, Chan K, Nørskov JK, Abild-Pedersen F. Rational design of MoS2 catalysts: tuning the structure and activity via transition metal doping. Catalysis Science &. Technology. 2015;5:246–253. [Google Scholar]
- 37.Hu T, Li R, Dong J. A new (2 × 1) dimerized structure of monolayer 1T-molybdenum disulfide, studied from first principles calculations. J. Chem. Phys. 2013;139:174702–174708. doi: 10.1063/1.4827082. [DOI] [PubMed] [Google Scholar]
- 38.Tang Q, Jiang D. Mechanism of hydrogen evolution reaction on 1T-MoS2 from first principles. Acs. Catalysis. 2016;6:4953–4961. [Google Scholar]
- 39.Kibsgaard J, Chen Z, Reinecke BN, Jaramillo TF. Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis. Nat. Mater. 2012;11:963–969. doi: 10.1038/nmat3439. [DOI] [PubMed] [Google Scholar]
- 40.Kong D, et al. Synthesis of MoS2 and MoSe2 films with vertically aligned layers. Nano. Lett. 2013;13:1341–1347. doi: 10.1021/nl400258t. [DOI] [PubMed] [Google Scholar]
- 41.Shi J, et al. Controllable growth and transfer of monolayer MoS2 on Au foils and its potential application in hydrogen evolution reaction. ACS nano. 2014;8:10196–10204. doi: 10.1021/nn503211t. [DOI] [PubMed] [Google Scholar]
- 42.Ye G, et al. Defects engineered monolayer MoS2 for improved hydrogen evolution reaction. Nano. Lett. 2016;16:1097–1103. doi: 10.1021/acs.nanolett.5b04331. [DOI] [PubMed] [Google Scholar]
- 43.Yin J, et al. Ni–C–N nanosheets as catalyst for hydrogen evolution reaction. J. Am. Chem. Soc. 2016;138:14546–14549. doi: 10.1021/jacs.6b09351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All data are available from the authors upon reasonable request. All source data underlying Figs. 1a, f, 2c, f, 3c, 4b–e and Supplementary Figs. 1g, h, 3d, e, 5a–c, 7c, 8a, 12a, d, 13a–c, 16, 17 and 18d are provided as a Source Data file.