Abstract
Background
Nonspecific induction of local innate immune responses by mucosally administered immunotherapy is a new approach to protection from upper respiratory tract infections. Therefore, a new liposome‐toll‐like receptor complex (LTC) immune stimulant was developed and investigated for its ability to activate innate immune responses in cats, both in vitro and in vivo, as part of an initial evaluation of LTC for use as an immunotherapeutic agent in cats.
Objectives
We hypothesized that LTC could activate innate immune responses in cats after topical application to nasal and oropharyngeal mucosal surfaces.
Animals
Mucosal immune responses to topical administration of LTC were assessed in 7 healthy, purpose‐bred cats, and in vitro responses were assessed using blood samples from healthy cats.
Methods
Cytokine and cellular immune responses to LTC were evaluated in blood samples, nasal lavage specimens, and pharyngeal swabs from cats, using reverse transcriptase polymerase chain reaction assays, ELISA assays, and flow cytometry.
Results
Liposome‐TLR complexes rapidly activated leukocytes in vitro, including upregulation of costimulatory molecule expression and cytokine production. Topical administration of LTC in healthy cats triggered rapid recruitment of monocytes to the nasal and oropharyngeal mucosa.
Conclusions and Clinical Importance
Liposome‐TLR complexes were found to effectively activate innate immune responses in cats after mucosal administration. These findings suggest that LTC have potential for use as a new mucosally administered immunotherapy for nonspecific protection from viral and bacterial respiratory tract infections.
Keywords: cytokines, mucosal immunity, T cells, upper respiratory tract
Abbreviations
- FBS
fetal bovine serum
- IL
interleukin
- INF
interferon
- LTC
liposome‐TLR complexes
- NALT
nasopharyngeal‐associated lymphoid tissue
- NK
natural killer
- OX40
tumor necrosis factor receptor superfamily, member 4
- PBMC
peripheral blood mononuclear cells
- PBS
phosphate‐buffered saline
- RT‐PCR
reverse transcriptase polymerase chain reaction
- TLR
toll‐like receptor
- TNF
tumor necrosis factor
1. INTRODUCTION
Upper respiratory infection (URI) is a common and serious problem in cats in boarding facilities, animal hospitals, and shelters. Unfortunately, few quickly effective options are available for preventing these infections in newly exposed cats. Immunoprophylaxis may be most effective if focused at the likely portal of pathogen entry. The upper respiratory tract is the site of entry for several important respiratory pathogens of cats, consisting of a diverse array of viral and bacterial organisms, including feline herpesvirus 1 (FHV‐1), calicivirus, Mycoplasma sp., Bordetella bronchiseptica, and Chlamydia felis.1, 2, 3, 4, 5, 6 Activation of host innate immune defenses, including airway epithelial cells and immune cells in oropharyngeal lymphoid tissues, results in production of antiviral and antibacterial cytokines, including type I (INF‐α) and II (INF‐γ) interferons, tumor necrosis factor alpha (TNF‐α), and interleukin 12 (IL‐12) and chemokines such as monocyte chemoattractant protein 1 and IL‐8 that recruit monocytes, T cells, and neutrophils. Local activation of innate immune responses may create an environment that is less susceptible to viral entry and replication or to bacterial invasion and colonization.7 Clinically, such an immune protective effect could be manifested as either partial or complete protection from infection or as a decrease in the severity and duration of clinical signs of illness, along with decreased viral or bacterial shedding.
One potential solution to the problem of complex upper respiratory tract infections in cats would be an immunotherapeutic agent capable of inducing nonspecific immune activation at mucosal surfaces in the oropharynx, such as liposome‐toll‐like receptor complexes (LTC). These complexes have been shown previously to induce strong nonspecific protection from lethal bacterial and viral respiratory tract infections after intranasal administration in several different mouse infection models.8, 9, 10, 11, 12 In addition, we demonstrated induction of therapeutic immune responses in cats with chronic rhinitis after parenteral administration of LTC.13 Therefore, we reasoned that immune stimulatory complexes could be further optimized for mucosal delivery and generation of local mucosal immune responses in the upper respiratory tract of cats. Therefore, the original LTC formulation was modified to improve its innate immune stimulatory potency and to improve adhesion and contact time with mucosal surfaces.
In the present study, the immune activating properties of modified LTC were evaluated in cats.12, 14 The modified LTC consisted of cationic liposomes formulated with 2 TLR agonists: polyinosinic‐polycytidylic acid (poly I:C), as a TLR3, retinoic acid‐inducible gene I, melanoma differentiation‐associated protein 5 agonist, and noncoding plasmid DNA (pDNA), as a TLR9 agonist rich in cytosine‐guanine. In addition, the modified LTC contained a mucosal adhesive agent (carboxymethylcellulose) to increase adhesion to mucosal cell surfaces. We also assessed cytokine and cell activation responses by feline leukocytes in vitro and also assessed the local mucosal immune responses to topical administration of LTC in healthy cats.
2. MATERIALS AND METHODS
2.1. Preparation of liposome‐TLR agonist complexes for mucosal immune stimulation
Liposomes were prepared by dissolving the cationic lipid, 1,2‐dioleoyl‐3‐trimentylammonium‐propane (DOTAP) and cholesterol (Avanti Polar Lipids, Alabaster, AL) in chloroform, and the solution was dried overnight in a vacuum desiccator to a thin film. The lipids were rehydrated in 5% sterile dextrose in water at 50°C as described previously.12, 14 Poly I:C (InVivoGen, San Diego, California) and pDNA (noncoding commercial plasmid PCR2.1) Thermo Fisher Scientific, Waltham, MA were added to form liposome complexes, as reported previously.12, 14 The endotoxin content of the plasmid was between 0.04 and 0.25 EU/μg. Carboxymethylcellulose (Sigma‐Aldrich, St. Louis, Missouri) was added to the LTCs to increase mucosal adhesion. In studies in which fluorescent LTCs were delivered, the liposomes were formulated to contain 10% (vol/vol) Topfluor‐labeled cholesterol (Avanti Polar Lipids).
2.2. Animal studies
Animal protocols were approved by the Institutional Animal Care and Use Committee of an independent contract research facility in Fort Collins, Colorado. Purpose‐bred cats for the studies were purchased from Liberty Research (Waverly, New York). All animals used in the studies were adopted to owners in the Fort Collins area after completion of the studies, and no animals were euthanized as part of these studies.
For collection of nasal lavage samples, cats were restrained manually in a head‐down position, and 0.5 mL of prewarmed sterile phosphate‐buffered saline (PBS) solution was administered into each nostril, and the resulting fluid backflow was collected from the nostrils using a 15 mL conical tube. The procedure was repeated once for each nostril, and all of the fluid collected was pooled for later analysis and stored on ice. For oropharyngeal samples, alginate swabs were gently rubbed and rolled against the mucosa of the caudal oropharyngeal region and placed in tubes with 1 mL PBS and stored on ice before analysis. Nasal and oropharyngeal samples were collected 24 hours before LTC administration, 6 hours after LTC administration, and again 20 and 72 hours after LTC administration.
Cats were treated with LTC administered on day 0. Control cats were treated with an equivalent volume of sterile PBS. Each cat was given 1 mL of LTC (0.2 mL intranasally in each nostril and 0.6 mL PO) while being gently restrained. In a separate experiment designed to track LTC uptake by mucosal cells in the nostril and oropharynx of cats, 1 mL of LTC labeled with TopFluor fluorescent dye was administered by instilling 0.2 mL intranasally in each nostril and 0.6 mL (equivalent to 60 μg of Poly I:C and PCR2.1) PO. Average weight of the cats was approximately 3 kg; thus, the intranasal dose of each TLR ligand was approximately 20 μg per kilogram body weight. No adverse effects, either acute or longer term, were observed after intranasal LTC administration (data not shown).
2.3. Preparation of peripheral blood mononuclear cells
Whole blood was obtained by jugular venipuncture from a separate group healthy young adult cats and collected into EDTA tubes. For separation of peripheral blood mononuclear cell (PBMC), blood was diluted 1:2 with sterile PBS, then layered over a Ficoll (GE Healthcare, Uppsala, Sweden) gradient and centrifuged. The PBMC were collected and washed twice in PBS and then resuspended in complete tissue culture medium before flow cytometric analysis.
2.4. Cell culture and PBMC activation with LTC
Peripheral blood mononuclear cells were cultured in complete medium, which consisted of Dulbecco's modified eagle medium (Thermo Fisher Scientific, Watham, Massachusetts) containing 15% fetal bovine serum (FBS; Avanti Polar Lipids), essential and nonessential amino acids, penicillin, and streptomycin (Gibco‐Thermo Fisher Scientific, Pittsburgh, Pennsylvania). Cells were plated in 96‐well flat bottom plates (Celltreat, Pepperell, Massachusetts) at a density of 1 × 106 cells/well in 200 μL medium. For assays involving LTC activation of PBMC, LTC were added at 3 different dilutions (1 μL per well, 0.3 μL per well, and 0.1 μL per well) in triplicate wells of PBMC in 100 μL complete medium, with careful mixing, and then the cells were incubated for an additional 48 hours. Conditioned medium was collected for IFN‐γ assay, and cells were collected for flow cytometric analysis of activation markers (see below) and for analysis of cytokine gene expression by quantitative reverse transcriptase polymerase chain reaction (qRT‐PCR) (see below). Assays were repeated at least twice, using PBMC from different donor animals.
2.5. Quantitative RT‐PCR analysis of cytokine gene expression in vitro
Ribonucleic acid was extracted from PBMC incubated for 48 hours with LTC, as noted above, and expression of IL‐1, IL‐12, IFN‐α, IFN‐γ, and TNF‐α genes was determined using quantitative RT‐PCR. Briefly, RNA was isolated and reverse transcribed using a commercial kit (Qiagen, Germantown, Maryland), and the complementary DNA then was amplified using Cyber green primers (Bio‐Rad, Hercules, California) using a qPCR MX3000p system instrument (Agilent, Santa Clara, California). Cytokine transcript copy numbers were determined by quantitative RT‐PCR, as noted previously.13
2.6. IFN‐γ ELISA
Cell culture supernatants from PBMC cultures were analyzed for IFN‐γ concentrations, using a feline IFN‐γ specific ELISA (DuoSet Feline IFN‐γ kit; R&D systems, Minneapolis, Minnesota) according to the manufacturer's instructions.
2.7. Flow cytometry
Cells were harvested after 48 hours of in vitro stimulation and immunostained with the following antibodies conjugated with fluorochromes: CD5‐PE (clone f43; Southern Biotech, Birmingham, Alabama), CD21‐APC (clone CA2.1Dg; Bio‐Rad), and OX40‐FITC (clone 7D6; Bio‐Rad), or CD14‐APC (clone TÜK4; Bio‐Rad) and MHCII‐FITC (clone TÜ39; BD Biosciences, San Jose, California). Cells were immunostained with primary antibodies for 20 minutes at 4°C in FACs buffer (PBS with 2% FBS and 0.05% sodium azide) after a 5‐minute incubation with normal cat serum (Jackson ImmunoResearch) to block nonspecific binding.
Cells obtained by nasal lavage and oropharyngeal swabs were washed once with PBS, then immunostained in 96‐well plates with the directly conjugated antibodies noted above. Isotype‐matched antibodies were used as control for each primary antibody. Cells from in vitro activated PBMC cultures were collected 48 hours after stimulation and measured using the same panel of antibodies, with appropriate controls. Cells were analyzed using a Beckman Coulter Gallios flow cytometer (Beckman Coulter, Indianapolis, Indiana), and data were analyzed using FlowJo software (Tree Star, Ashland, Oregon).
2.8. Preparation and analysis of nasal and pharyngeal samples
Swabs were collected from the distal oropharynx and immediately placed in 2 mL of complete tissue culture medium in 15 mL polypropylene tubes on ice. The samples were lightly vortexed to free cells from the swabs. Fluid was transferred to a new tube, and 2 mL of PBS was added to the empty tube containing the swab, vortexed again, and pooled with the first sample. The sample then was filtered using a 70 μm cell strainer (Celltreat, Pepperell, Massachusetts) to remove debris and mucus. Samples were centrifuged, the cell pellets were resuspended in 0.5 mL of PBS, and the cells were counted.
Nasal lavage samples were filtered, washed, centrifuged, resuspended, and counted in the same fashion as for oropharyngeal swab samples. Cells were stained in the same manner as described for PBMC, and equal numbers were analyzed by flow cytometry to determine the cell type and activation.
2.9. Confocal microscopy
Monocyte‐derived macrophages were generated from cat PBMC by incubation of plastic adherent monocytes with 10 ng/mL human recombinant macrophage colony stimulating factor for 5 days. The macrophages that were generated were then incubated with 0.1, 1, or 2 μL per well of TopFluor‐labeled LTC in complete medium at 37°C for 4 hours, then washed gently 3 times, fixed with 2% paraformaldehyde for 30 minutes, washed again with distilled water, cover‐slipped, and analyzed by confocal microscopy. Confocal images of feline monocyte‐derived macrophages (untreated control cells or cells incubated with labeled LTC) were obtained using an Olympus IX3 confocal microscope (Waltham, Massachusetts). Images were processed and analyzed using Olympus cellSens software.
2.10. Statistical methods
Statistical comparisons between data sets with 2 treatment groups were performed using the nonparametric Mann‐Whitney test. Comparisons among 3 or more groups were performed using analysis of variance, followed by the Tukey multiple means post‐test. Analyses were performed using Prism6 software (GraphPad, La Jolla, California). For all analyses, statistical significance was set at P < .05.
3. RESULTS
3.1. B cells, T cells, and monocytes from feline PBMC activated by LTC in vitro
Feline lymphoid tissues express TLRs such as TLR3 and TLR9, and feline immune effector cells (T cells and B cells) express TNF receptor superfamily, member 4 (OX40). When bound to its ligand OX40L, OX40 augments immune responses by prolonging survival of T cells, generation of immune memory, preventing emergence of T‐cell tolerance, and mediating decreased immunosuppressive activity of regulatory T cells. Thus, we chose to examine the effect of in vitro treatment of PBMC with LTC on OX40 expression in these cells.13, 15, 16, 17 Unfractionated PBMC were used because LTC may have both a direct effect on leukocytes as well as an indirect effect through crosstalk among various cell types in the mixture. B lymphocytes that were CD21+ were found to exhibit significant upregulation of expression of the costimulatory molecule OX40, in a dose‐dependent fashion with respect to the amount of added LTC (Figure 1, panel A). For T‐cell responses to LTC stimulation, the percentage of CD5+ T cells expressing OX40 also increased significantly as a function of LTC concentration (Figure 1, panel B). Finally, a significant increase in expression of MHCII by CD14+ monocytes was observed in cultures of PBMC treated with LTC (Figure 1, panel C). Similar results were observed in 3 separate studies.
Figure 1.
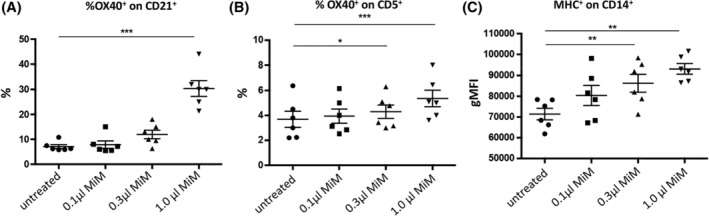
Treatment of PBMC from 6 healthy cats with LTC. A, OX40 expression by CD21+ B cells. B, OX40 expression by CD5+ T cells. C, MHCII expression by gated CD14+ monocytes. *, P < .05, **, P < .01 and ***, P < .005. LTC, liposome‐toll‐like receptor complexes; OX40, tumor necrosis factor receptor superfamily, member 4; PBMC, peripheral blood mononuclear cells
3.2. LTC treatment activates cytokine gene expression by PBMC
Quantitative RT‐PCR analysis was used to assess induction of cytokine gene expression by PBMC treated with LTC (Figure 2). Significant upregulation of expression of mRNA for INF‐α as well as upregulation of IL‐1β, IL‐12, and TNF‐α expression after LTC stimulation was identified in most cats.
Figure 2.
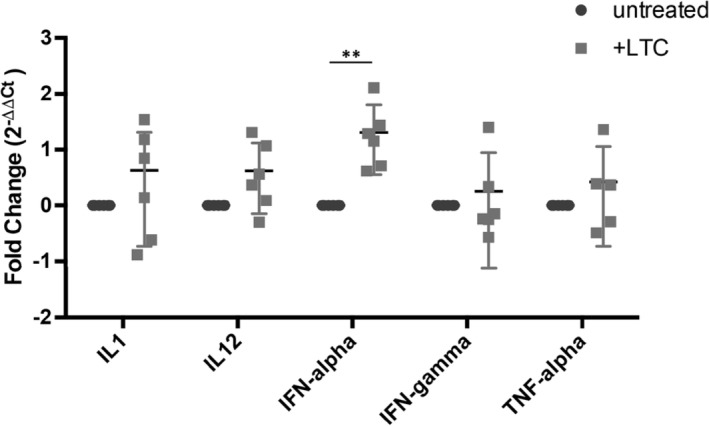
Activation of cytokine gene expression in PBMC after treatment with LTC. Mean gene expression levels (with SEM) for triplicate samples (1 × 106 cells/well) of unstimulated PBMC and PBMC stimulated with LTC (1 μL LTC per 200 μL; incubation period 8 hours). **, P < .01. LTC, liposome‐toll‐like receptor complexes; PBMC, peripheral blood mononuclear cells
3.3. LTC treatment triggers IFN‐γ secretion by PBMC
Supernatants were collected from PBMC after 48 hours stimulation with LTC, for analysis of IFN‐γ production (Figure 3). Liposome‐TLR complexes treatment stimulated a dose‐dependent, significant secretion of IFN‐γ secretion, as determined by ELISA analysis of supernatants.
Figure 3.
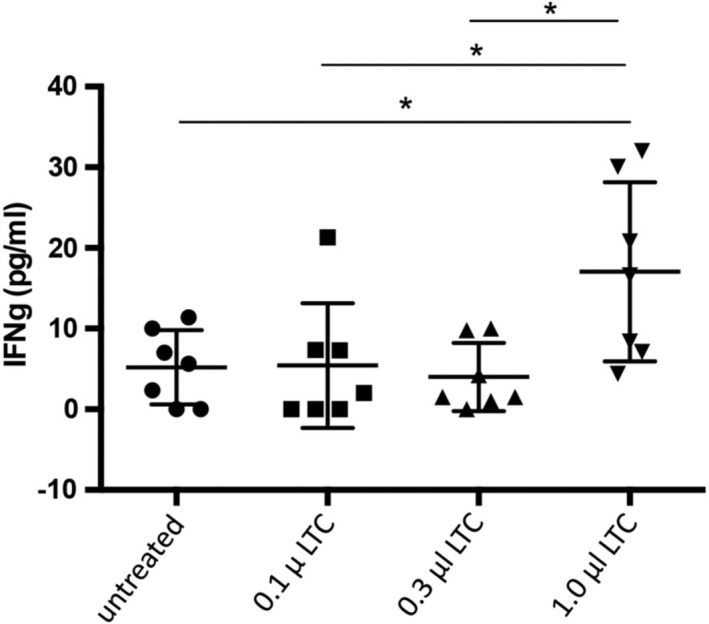
Secretion of interferon gamma (IFN‐γ) by PBMC after stimulation in vitro with LTC. Mean IFN‐γ concentrations and SEM for the LTC and untreated groups. *, P < 0.05. LTC, liposome‐toll‐like receptor complexes; PBMC, peripheral blood mononuclear cells
3.4. Uptake of labeled LTC by macrophages in vitro
Monocyte‐derived macrophages were generated from PBMC of cats by plastic adherence, followed by culture for 4 days in complete medium, after which macrophage uptake of LTC was assessed. Extensive uptake of LTC complexes by macrophages was observed (Figure 4), consistent with the known targeting of liposome complexes to monocytes and macrophages and other phagocytic immune cells.
Figure 4.
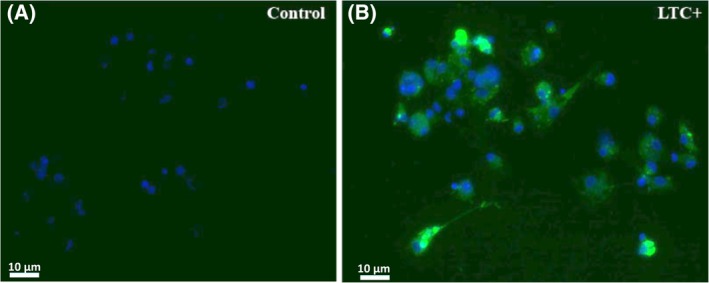
Uptake of labeled LTC by macrophages. Macrophages from PBMC incubated with labeled LTC for 4 hours with uptake assessed by confocal microscopy. Cell nuclei were delineated with DAPI staining (blue), whereas labeled LTC were visualized as bright green staining. A, macrophages alone. B, Macrophages incubated with labeled LTC. LTC, liposome‐toll‐like receptor complexes; PBMC, peripheral blood mononuclear cells
3.5. Retention of labeled LTC by cells in the oropharynx and nasal cavity
Seven healthy adult cats were given 0.1 mL of labeled LTC bilaterally into the nostrils and 0.6 mL PO. Nasal lavage and throat swab samples were obtained before administration, and 6, 20, and 72 hours after LTC administration; cell populations were analyzed by flow cytometry, as described in the Materials and Methods section. Compared to pretreatment samples, samples obtained from the nose and oropharynx at 6 and 20 hours after LTC administration contained a readily identifiable population of cells containing fluorescently‐labeled LTC (Figure 5). The LTC‐containing cells were detected in both nasal lavage cells (Figure 5A) and in throat swab cells (oropharynx, Figure 5B). Peak uptake of LTC‐labeled cells in the nasal lavage samples occurred 6 hours after LTC administration, whereas in the oropharynx peak of LTC uptake occurred 20 hours after administration.
Figure 5.
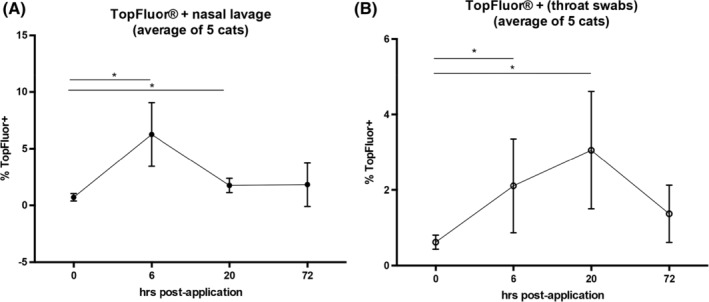
Binding of LTC to mucosal cells in the nasal cavity and oropharynx of healthy cats. Labeled LTC administered intranasally and PO to 5 healthy adult cats. Mean percentages of LTC‐positive cells are plotted versus time post‐treatment. *, P < 0.05. LTC, liposome‐toll‐like receptor complexes
3.6. Intranasal and PO administration of LTC triggers influx of inflammatory leukocytes to mucosal surfaces
Cells from the nasal cavity or oropharynx were obtained by nasal lavage or swabbing the back of the throat, respectively, as described above. Cells were immunostained with fluorescently conjugated antibodies against specific cell markers for T cells (CD5+) and monocytes (CD14+) and analyzed by flow cytometry. Neutrophils were identified by their characteristic forward versus side scatter profiles. Pretreatment samples from the nasal cavity of 7 healthy cats contained an average of 7% CD5+ T cells, whereas the average percentage of CD5+ T cells in the oropharynx of cats before treatment was 73%. The percentage of CD14+ monocytes in the nasal cavity before treatment was 2.5%, similar to the 3% of CD14+ monocytes found in the oropharynx (Figure 6). Percentages of neutrophils found in the nasal cavity were 10%, and 2% in the oropharynx of untreated animals.
Figure 6.
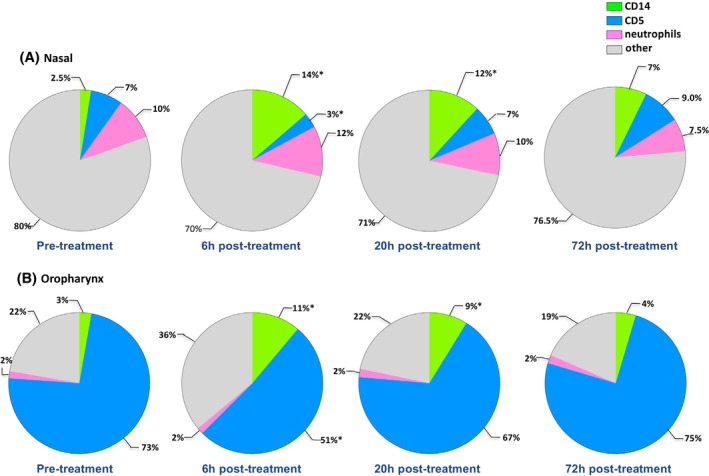
Inflammatory cell changes in mucosal surfaces as a response to topical treatment with LTC. Average results from 7 healthy adult cats; an average 2.4 × 105 (which ranged between 4.5 × 104 and 1.7 × 106) cells were obtained from nasal or oropharyngeal samples. A, Nasal passages. B, The oropharynx. Color key: green, monocytes; blue, T cells; rose, neutrophils; and gray, all other nonleukocytes, consisting primarily of mucosal epithelial cells. Significant differences are indicated by asterisks (*). LTC, liposome‐toll‐like receptor complexes
Six hours after topical administration of LTC, a marked increase in the percentage of CD14+ monocytes was observed in both the nasal mucosa (Figure 6A, increasing from an average of 2.5% to an average of 14%) and in the oropharynx (Figure 6B, increasing from an average of 3% to an average of 11%). The percentage of CD14+ monocytes from both nasal and oropharynx samples had decreased by 20 and 72 hours compared to the 6 hours samples but still remained higher than in pretreatment samples. Interestingly, the percentages of CD5+ T cells from the nasal mucosa decreased significantly 6 hours post‐LTC treatment (decreasing from 7% to 3%) and from 73% to 51% in the oropharynx. Nasal CD5+ T cells returned to pretreatment values by 72 hours after treatment. In the oropharynx, the decrease in CD5+ cells observed at 6 hours was also transient, with percentages of CD5+ T cells returning to nearly baseline values (67%) at 20 hours post‐LTC treatment.
The neutrophil population was essentially unchanged throughout the study time course in both the nose and the oropharynx after LTC administration (Figure 6).
4. DISCUSSION
An effective, broad‐spectrum immunotherapeutic agent capable of activating protective mucosal immune responses would provide a valuable means of inducing rapid protection from a variety of upper respiratory tract pathogens in cats. Therefore, we investigated innate immune responses to LTC, a new immune therapeutic agent designed for mucosal application. We found that LTC induced activation of feline leukocytes, including upregulation of costimulatory molecule (OX40 and MHCII) expression. In addition, LTC induced production of cytokines associated with innate immune responses, including INF‐α, INF‐γ, TNF‐α, and IL‐12. in vivo studies in healthy cats demonstrated uptake of LTC by immune cells and epithelial cells in the upper airways, as well as recruitment of monocytes to the upper airways. Our studies indicated that cats were capable of responding to mucosal immune activation by LTC. Significant upregulation of IFN‐γ along with increases in lymphocyte expression of OX40 and monocyte expression of MHCII all would be associated with innate immune system activation for better viral and bacterial pathogen clearance.7, 18, 19 These findings are consistent with LTC activation of mucosal innate immune responses in cats and induction of partial protection against inhalational challenge with FHV‐1.19
Nasopharyngeal‐associated lymphoid tissue (NALT) is a network of immune cells located in the oropharyngeal region that is primed for immune recognition of viral, bacterial, and fungal pathogens.20, 21, 22, 23 When activated by infections, innate immune effector cells in the NALT (including monocytes, neutrophils, innate lymphoid cells, and natural killer [NK] cells) respond by producing cytokines and chemokines, and by activating other immune effector cells including tissue macrophages, epithelial cells, and T and B cells. In our study, several key antiviral and antibacterial cytokines were produced after LTC treatment, including significant increases in secretion of IFN‐γ protein, and increases in INF‐α and IL‐12 transcription, as assessed by qRT‐PCR. These results together suggest broad activation of innate immune responses in nasal and oropharyngeal tissues after administration of LTC in cats.
Distinct cellular trafficking responses to LTC treatment were noted when monocyte and T‐cell responses were compared in the nose and oropharynx. For example, LTC administration triggered strong recruitment of CD14+ monocytes to both the nasal cavity and the oropharynx, while at the same time triggering significant, transient disappearance of T cells from these mucosal sites (see Figure 6). Interestingly, minimal neutrophil responses to LTC administration were observed at any time point evaluated, in either the nose or oropharynx. The recruitment of monocytes by LTC could be attributed to production of the chemokine CCL2, which has been noted previously after treatment with LTC.11 In contrast, the transient disappearance of T cells from mucosal sites after LTC administration most likely could be attributed to transient redistribution of T cells after adherence to capillary blood vessels or migration into tissues. A similar effect has been observed previously in dogs treated systemically with LTC.24 In support of transient redistribution, we observed that CD5+ T lymphocytes were rapidly restored in mucosal sites within 72 hours after LTC administration.
We also observed distinct differences in resident immune cell populations in the nasal cavity and oropharynx, especially with respect to the density of mucosal T cells. For example, the oropharynx was found to be highly enriched in mucosal CD5+ T cells, whereas T cells were much less numerous in the nasal cavity (see Figure 6). These differences are considered real (and not simply a reflection of differences in sampling techniques), because the percentages of unmarked cells (assumed to represent primarily mucosal epithelial cells, see Figure 6) were similar in samples from the oropharynx, compared to nasal lavage samples. Thus, the oropharynx in cats appears to be enriched in superficially located T cells compared to the nasal cavity and thus well suited to induction of mucosal immune responses after PO administration of nonspecific immune stimuli.
The ability of LTC to rapidly induce mucosal immune responses in the upper airway and oropharynx of cats suggests potential use as a nonspecific immune therapeutic agent for prevention of viral and bacterial infections in cats. Administration of LTC significantly decreased signs of FHV‐1 infection and viral shedding in experimentally inoculated cats.19 In addition, we have found that topical application of LTC in dogs can induce mucosal activation and cellular recruitment, similar to the responses seen in cats (Dow S, unpublished data). Thus, topical treatment in the upper airways with LTC may have utility for preventing or mitigating infections with common respiratory tract pathogens in both dogs and cats.
The present study had several limitations. For example, the lack of available reagents for full phenotyping of feline leukocytes precluded a more complete assessment of cellular responses to topical LTC administration. In addition, the relatively small numbers of cells collected by nasal lavage or oral swabs precluded cytokine analysis after LTC administration. The in vitro studies using PBMC did not determine the cellular source of specific cytokine production after LTC activation. However, previous studies of LTC in mice indicated that dendritic cells were the primary cellular source of IL‐12 production after LTC administration, and that NK cells were the primary cellular source of IFN‐γ.25, 26
In summary, our studies describe the innate immune activation properties of a novel mucosally active immune stimulant in cats. Innate immune activation by LTC was associated with production of key antiviral and antibacterial cytokines, and with upregulation of important costimulatory molecules on immune effector cells. These findings suggest further evaluation of the LTC mucosal immune stimulant for prevention of URI in cats and for treatment of established URI refractory to treatment with antibiotics or other medications such as lysine.
CONFLICT OF INTEREST DECLARATION
Drs. Lappin and Dow both hold stock options and corporate positions in Poudre Canyon Therapeutics, a Fort Collins company developing the LTC immunotherapy platform technology.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL AND CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The cats were managed by a contract research facility in Fort Collins under an approved IACUC protocol.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENTS
The authors acknowledge the assistance of Ms. Jade Kurihara with preparation of LTC used in the study, and Ms. Jennifer Hawley for performing RT‐PCR assays. Portions of this research were presented at the 2017 American College of Veterinary Internal Medicine Forum, National Harbor, Maryland. This study was supported by a grant from the state of Colorado Office of Economic Development and International Trade.
Wheat W, Chow L, Coy J, Contreras E, Lappin M, Dow S. Activation of upper respiratory tract mucosal innate immune responses in cats by liposomal toll‐like receptor ligand complexes delivered topically. J Vet Intern Med. 2019;33:838–845. 10.1111/jvim.15426
Funding information State of Colorado Office of Economic Development and International Trade; Colorado Office of Economic Development and International Trade
REFERENCES
- 1. Gaskell RM, Radford AD, Dawson S. Feline infectious respiratory disease Feline Medicine and Therapeutics. New York, NY: Wiley; 2004. [Google Scholar]
- 2. Lara VM, Benassi JC, Bisetto SP, et al. Molecular detection of infectious pathogens of the upper respiratory tract in captive nondomestic felids. J Zoo Wildl Med. 2017;48:529‐531. [DOI] [PubMed] [Google Scholar]
- 3. Radford AD, Coyne KP, Dawson S, Porter CJ, Gaskell RM. Feline calicivirus. Vet Res. 2007;38:319‐335. [DOI] [PubMed] [Google Scholar]
- 4. Lee‐Fowler T. Feline respiratory disease: what is the role of Mycoplasma species? J Feline Med Surg. 2014;16:563‐571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bradley A, Kinyon J, Frana T, Bolte D, Hyatt DR, Lappin MR. Efficacy of intranasal administration of a modified live feline herpesvirus 1 and feline calicivirus vaccine against disease caused by Bordetella bronchiseptica after experimental challenge. J Vet Intern Med. 2012;26:1121‐1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patel JR, Heldens JG. Review of companion animal viral diseases and immunoprophylaxis. Vaccine. 2009;27:491‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Netea MG, Joosten LA, Latz E, et al. Trained immunity: A program of innate immune memory in health and disease. Science. 2016;352:aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Logue CH, Phillips AT, Mossel EC, et al. Treatment with cationic liposome‐DNA complexes (CLDCs) protects mice from lethal Western equine encephalitis virus (WEEV) challenge. Antiviral Res. 2010;87:195‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Henderson A, Propst K, Kedl R, Dow S. Mucosal immunization with liposome‐nucleic acid adjuvants generates effective humoral and cellular immunity. Vaccine. 2011;29:5304‐5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Troyer RM, Propst KL, Fairman J, Bosio CM, Dow SW. Mucosal immunotherapy for protection from pneumonic infection with Francisella tularensis. Vaccine. 2009;27:4424‐4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goodyear A, Kellihan L, Bielefeldt‐Ohmann H, Troyer R, Propst K, Dow S. Protection from pneumonic infection with burkholderia species by inhalational immunotherapy. Infect Immun. 2009;77:1579‐1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dow S. Liposome‐nucleic acid immunotherapeutics. Expert Opin Drug Deliv. 2008;5:11‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Veir JK, Lappin MR, Dow SW. Evaluation of a novel immunotherapy for treatment of chronic rhinitis in cats. J Feline Med Surg. 2006;8:400‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zaks K, Jordan M, Guth A, et al. Efficient immunization and cross‐priming by vaccine adjuvants containing TLR3 or TLR9 agonists complexed to cationic liposomes. J Immunol. 2006;176:7335‐7345. [DOI] [PubMed] [Google Scholar]
- 15. Gao J, Bernatchez C, Sharma P, Radvanyi LG, Hwu P. Advances in the development of cancer immunotherapies. Trends Immunol. 2013;34:90‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shimojima M, Miyazawa T, Ikeda Y, et al. Use of CD134 as a primary receptor by the feline immunodeficiency virus. Science. 2004;303:1192‐1195. [DOI] [PubMed] [Google Scholar]
- 17. Ignacio G, Nordone S, Howard KE, Dean GA. Toll‐like receptor expression in feline lymphoid tissues. Vet Immunol Immunopathol. 2005;106:229‐237. [DOI] [PubMed] [Google Scholar]
- 18. Quintin J, Cheng SC, van der Meer JW, et al. Innate immune memory: towards a better understanding of host defense mechanisms. Curr Opin Immunol. 2014;29:1‐7. [DOI] [PubMed] [Google Scholar]
- 19. Contreras ET, Olea‐Popelka F, Wheat, et al. Evaluation of liposome toll‐like receptor ligand complexes for non‐specific mucosal immunoprotection from feline herpesvirus‐1 infection. J Vet Int Med. 2019;1‐7. 10.1111/jvim.15427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Asanuma H, Thompson AH, Iwasaki T, et al. Isolation and characterization of mouse nasal‐associated lymphoid tissue. J Immunol Methods. 1997;202:123‐131. [DOI] [PubMed] [Google Scholar]
- 21. Kiyono H, Fukuyama S. NALT‐ versus Peyer's‐patch‐mediated mucosal immunity. Nat Rev Immunol. 2004;4:699‐710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6:148‐158. [DOI] [PubMed] [Google Scholar]
- 23. Pabst R. Mucosal vaccination by the intranasal route. Nose‐associated lymphoid tissue (NALT)‐Structure, function and species differences. Vaccine. 2015;33:4406‐4413. [DOI] [PubMed] [Google Scholar]
- 24. Dow S, Elmslie R, Kurzman I, Macewen G, Pericle F, Liggitt D. Phase I study of liposome‐DNA complexes encoding the interleukin‐2 gene in dogs with osteosarcoma lung metastases. Hum Gene Ther. 2005;16:937‐946. [DOI] [PubMed] [Google Scholar]
- 25. Dow SW, Fradkin LG, Liggitt DH, et al. Lipid‐DNA complexes induce potent activation of innate immune responses and antitumor activity when administered intravenously. J Immunol. 1999;163:1552‐1561. [PubMed] [Google Scholar]
- 26. U'Ren L, Kedl R, Dow S. Vaccination with liposome—DNA complexes elicits enhanced antitumor immunity. Cancer Gene Ther. 2006;13:1033‐1044. [DOI] [PubMed] [Google Scholar]


