Abstract
Background
Although antimicrobial resistance is increasingly common in equine medicine, molecular and epidemiological data remains scarce.
Objectives
We estimated the prevalence of, and risk factors for, shedding of multidrug resistant (MDR), extended spectrum β‐lactamase (ESBL)‐producing, and AmpC β‐lactamase‐producing, or some combination of these in Escherichia coli in horses in France. We characterized ESBL/AmpC isolates for antimicrobial susceptibility and the presence of virulence and ESBL/AmpC‐associated resistance genes.
Animals
Fecal samples from healthy adult horses at 41 premises were collected. A questionnaire was completed by each premises manager. A subset of these samples was tested to build 2 bacterial collections.
Methods
Indicator (without enrichment) and specific (enrichment with ceftriaxone) E. coli tested for antimicrobial susceptibility. Prevalence of isolates nonsusceptible to antimicrobials was estimated at the horse and the premises level. The ESBL/AmpC and virulence genes were identified by PCR. Multivariable logistic regression was used to investigate risk factors for MDR and ESBL/AmpC isolates at premises.
Results
Approximately 44% of horses shed MDR E. coli. Resistance most commonly was observed to ampicillin, streptomycin, and amoxicillin/clavulanic acid. Twenty‐nine percent of premises housed horses shedding ESBL/AmpC‐producing isolates. The ESBL/AmpC gene most commonly identified was bla CTX‐M‐1. Virulence gene iutA was identified in 1 ESBL/AmpC‐producing isolate. Medical treatment, staff numbers, and activity were identified as risk factors for housing horses shedding ESBL/AmpC‐producing E. coli isolates.
Conclusions and Clinical Importance
Prevalence of healthy horses harboring ESBL/AmpC genes and MDR isolates in their intestinal microbiota is substantial. Risk factors could be used to elaborate guidelines to prevent their dissemination.
Keywords: antimicrobial resistance, cephalosporinase, equine, microbiota
Abbreviations
- AMC
amoxicillin‐clavulanic acid
- AMK
amikacin
- AMP
ampicillin
- AmpC
AmpC β‐lactamase
- ATM
aztreonam
- BHI
brain heart infusion
- bla
β‐lactamase
- CAZ
cefoperazone
- CHL
chloramphenicol
- CI
confidence interval
- CIP
ciprofloxacin
- CIPARS
Canadian Integrated Program of Antimicrobial Resistance Surveillance
- CN
cefalexin
- CRO
ceftriaxone
- Ecl
Reference Laboratory for Escherichia coli
- ENR
enrofloxacin
- ESBL
extended spectrum β‐lactamase
- ExPEC
extra‐intestinal pathogenic E. coli
- GEN
gentamicin
- IFCE
Institut Français du Cheval et de l'Equitation (French Institute of the Horse and Equitation)
- KAN
kanamycin
- MDR
multidrug resistant
- NAL
nalidixic acid
- PIP
piperacillin
- SSS
sulfisoxazole
- TET
tetracycline
- TIC
ticarcillin
- XNL
ceftiofur
1. INTRODUCTION
Antimicrobial resistance was declared by the World Health Organization (WHO) in 2014 as the greatest threat for human and veterinary medicine.1 Equine medicine also is affected, as demonstrated by the recent publication of several case reports.2, 3 Nevertheless, molecular and epidemiological data in this species remains scarce.4 Since their domestication, horses mainly have been considered working animals or livestock, although more recently some also may be considered companion animals. Therefore, they represent a potential source of contamination by direct contact with their owners or through the food chain. Horses have been overlooked in the global approach to antimicrobial resistance.5 Nevertheless, they received more antimicrobials per kilogram than did cattle in France in 2013.6
Escherichia coli is present in the intestinal microbiota of mammals, being mostly commensal although some strains can be pathogenic. Because of its ubiquity, frequent exposure to systemic antimicrobial treatment, and its great genomic plasticity, this bacterium is considered by the European Center of Disease Control as an excellent indicator for antimicrobial surveillance.7
An important mechanism of resistance that can be found in E. coli is the production of extended spectrum β‐lactamases (ESBL), AmpC cephalosporinases (AmpC),5 or both, resulting in the enzymatic inactivation of β‐lactams. The global prevalence of β‐lactamase genes (eg, bla) has increased considerably over the last 30 years throughout the world, both in humans and in animals. This increase might be due to spatial dispersal of mobile genetic elements or high‐risk clones or could arise from antimicrobial pressure. In horses, phenotypic resistance to ceftiofur (XNL) has been documented in foals8 and in adults.9 The ESBL gene variant bla CTX‐M‐1 is most often identified.10 However, bla CTX‐M‐2, bla CTX‐M‐9,11 bla CTX‐M‐15,12 and several variants of bla CMY also have been detected.10 All these variants also have been found in other animal10 species and in humans.13 The gene bla CTX‐M‐15 is the most prevalent variant in humans because it disseminates through epidemic plasmids and high‐risk E. coli clones,14 such as the sequence‐type ST131. The β‐lactamase genes mostly are carried by plasmids, which also may convey resistance to other antimicrobial classes, thus conferring multidrug resistance (MDR).15 Moreover, owning a horse is a risk factor for carriage of ESBL in humans,16 highlighting potential concern for public health. The use of third‐ and fourth‐generation cephalosporins marketed for veterinary medicine is authorized in horses in France, possibly enhancing the dissemination of ESBL/AmpC genes.
Antimicrobial resistance genes may coexist with virulence genes on the same plasmids in pathogenic bacteria.17 Although E. coli is not considered an enteric pathogen in adult horses, extra‐intestinal pathogenic E. coli (ExPEC) sporadically have been recognized as potential pathogenic agents in horses,18 and the presence of virulence factors has been reported in E. coli isolated from horses.19 The ExPEC are of public health concern because some isolates may be zoonotic. The presence of highly virulent and antimicrobial‐resistant strains in the intestinal microbiota of horses could represent a risk for horse handlers because of the possibility of transmission through close contact.20
No data is available on the presence of MDR or ESBL/AmpC‐producing isolates and ExPEC in the healthy equine population in France. Our objective was to determine the prevalence of, and risk factors for, shedding MDR or ESBL/AmpC‐producing E. coli isolates in horses. We characterized potential ESBL/AmpC isolates for antimicrobial susceptibility and the presence of virulence and ESBL/AmpC‐associated resistance genes.
2. MATERIALS AND METHODS
2.1. Sampling
This work was part of a larger study on resistance to anthelmintics and antimicrobials in horses in France. Sampling details were described elsewhere.21 Briefly, premises housing more than 40 horses, as mentioned in the French Horse and Riding Institute (IFCE) database, were contacted by telephone and selected on a voluntary basis. For each breeding premises, a riding school within a radius of 50 km was recruited when available. At time of the study, we estimated the number of riding schools in the source population as 1600 and the number of breeding premises as 249 in the study area (only riding schools located in French departments including selected breeding premises were considered). To be included in the study, horses had to be >2 years old and considered healthy by the premises manager. They also had to be present on the premises for >4 weeks before sampling and must not have received anthelmintic treatment within the last 2 months. On the premises, a convenience sample of horses was assembled based on accessibility.
At each premises, between 8 and 36 horses were sampled. Two grams of feces were obtained, either from the rectum or within 5 minutes of defecation and stored at 4°C up to 6 hours. On the collection day, 18 mL of 30% glycerol were added to each sample, and the samples were stored at −20°C before analysis.
A questionnaire was developed based on published risk factors in the horse.22 It was administered on the farm during an interview with the manager. Questions were directed to the general manager of the premises and were always at the premises level. The questionnaire was written in French and is available on request.
2.2. Indicator E. coli collection: Nonenriched culture, antimicrobial susceptibility testing, ESBL/AmpC gene identification, and descriptive statistics
Because of financial and logistic restrictions, we were not able to test all samples. At first, 3 individual fecal samples per premises were randomly selected, by mixing them up by premises and picking them blinded to horse identification. Because of loss of viability of E. coli during transportation observed for the first set of samples (see results), the number of selected samples per premises was increased to 6 on remaining premises where we did not initially obtain samples with viable E. coli. One milliliter of the sample suspension in 30% glycerol was inoculated in 4 mL of peptone water. After ≤15 minutes, 100 μL of this suspension was inoculated onto a tryptic soy agar slope tube which was sent to the Reference Laboratory for Escherichia coli (Ecl). On receipt, tubes were incubated for 24 hours at 37°C, scraped and bacterial growth passaged onto MacConkey agar and incubated at 37°C overnight. All colonies, up to a maximum of 3, were selected for each fecal sample. Lactose‐positive colonies were selected preferentially, but when not present, lactose‐negative colonies were selected and retained for further analysis if indole‐positive and citrate‐negative.23 Isolates were confirmed as E. coli by PCR detection of the uidA gene.24
Isolates were tested for susceptibility to the 14 antimicrobial agents examined in the Canadian Integrated Program for Antimicrobial Surveillance (CIPARS 2008) using the disk‐diffusion assay as previously described.25 The following antimicrobial disks were used: azithromycin (15 μg), streptomycin (STR; 10 μg), gentamicin (GEN; 10 μg), trimethoprim‐sulfamethoxazole (23.75 μg), sulfisoxazole (SSS; 250 μg), tetracycline (TET; 30 μg), nalidixic acid (NAL; 30 μg), ciprofloxacin (CIP; 5 μg), chloramphenicol (CHL; 30 μg), ampicillin (AMP; 10 μg), amoxicillin/clavulanic acid (AMC; 30 μg), cefoxitin (FOX; 30 μg), ceftriaxone (CRO; 30 μg), and XNL (30 μg). The E. coli strain ATCC 25922 was used as a susceptible quality control. Veterinary CLSI 2015 clinical breakpoints were used when available; CLSI 2015 clinical breakpoints were used otherwise.
When isolates were nonsusceptible (intermediate or resistant) to third‐generation cephalosporins, we looked for 5 β‐lactamase resistance genes (bla SHV, bla TEM, bla CMY‐2, bla OXA, bla CTX‐M) by PCR multiplex. We used the primer sequences described previoulsy.26 The ESBL/AmpC resistance genes were detected by PCR performed on DNA extracted by heat lysis, as previously described.27 The protocol was provided by the National Microbiology Laboratory of the Public Health Agency of Canada28 with control strains ECL3482, PMON38, and CTX‐M15. When bla CTX‐M was detected, the CTX‐M group was determined by PCR.29 The amplicon was purified using the QIAquick PCR Purification Kit, according to manufacturer instructions. The variant was identified by Sanger sequencing and compared using the Comprehensive Antibiotic Resistance Database (CARD) (https://card.mcmaster.ca/).
We estimated the prevalence of horses shedding resistant or nonsusceptible (resistant and intermediate) isolates for each antimicrobial and of premises housing these horses. We then estimated the global prevalence and 95% confidence intervals (CI) of horses shedding isolates nonsusceptible to >1, 3, 5, 7, or 9 classes of antimicrobials and of premises housing these horses. An isolate was considered MDR if nonsusceptible to ≥1 agent of ≥3 antimicrobial classes.30 Positive MDR status for a horse was defined as detection of ≥1 MDR isolate for that horse. A premise was attributed positive MDR status if it housed ≥1 MDR‐positive horse.
At the horse level, prevalence estimates were adjusted for sampling weights to consider the different sampling probabilities of premises (riding school versus breeding) and horses within each premises (ie, the proportion of horses sampled was not constant across all premises). For the estimation of CIs, variances were adjusted for stratification by type of premises (riding school versus breeding) and for clustering to consider potential nonindependence of horses sampled within the same premises. At the premises level, prevalence estimates also were adjusted for sampling weights (at the premises level only) and stratification. The Surveyfreq procedure of SAS 9.4 was used for estimation, based on the Taylor series method.
2.3. Potential ESBL/AmpC producing E. coli collection: Culture, antimicrobial susceptibility testing, ESBL/AmpC and virulence gene identification, and descriptive statistics
Bacteria producing ESBL/AmpC may be shed in small quantities in healthy animals.31 To improve detection sensitivity and permit more accurate estimation of the proportion of positive premises, 2 different approaches were used for enrichment with CRO. In the first approach, samples used to produce the indicator collection were processed. After receipt at the Ecl and first incubation, plates were scraped and inoculated in 10 mL of MacConkey broth containing 1 mg/L of CRO and incubated overnight at 37°C. When bacterial growth was positive, 100 μL of MacConkey broth was inoculated on MacConkey agar containing 1 mg/L of CRO and incubated at 37°C overnight. Between 1 and 3 lactose‐positive isolates per sample were selected.
In the second approach, 8 individual fecal samples per premises were examined individually. When available, these fecal samples were different from those used to produce the indicator collection. In addition, pools of samples from 6 to 10 horses were examined for each premises, and these pooled samples also came from different horses. One milliliter of the initial glycerol/feces mixture was added to 9 mL of Brain Heart Infusion (BHI) broth and incubated 4 hours at 37°C. One hundred microliters of the BHI broth were inoculated on MacConkey agar with CRO, 1 mg/L. When bacterial growth was observed, 1‐3 lactose‐positive isolates were selected.
All isolates of this collection were confirmed as E. coli by the detection of the uidA gene using PCR24 and were tested for susceptibility against 30 antimicrobials by the disk diffusion method. The following disks were used: STR (10 μg), GEN (10 μg), amikacin (AMK; 30 μg), kanamycin (KAN; 30 μg), spectinomycin (100 μg), trimethoprim‐sulfonamide (5 μg), SSS (200 μg), TET (30 μg), NAL (30 μg), CIP (5 μg), flumequin (30 μg), enrofloxacin (ENR; 5 μg), CHL (30 μg), florfenicol (30 μg), AMP (10 μg), AMC (30/10 μg), piperacillin (PIP; 100 μg), piperacillin‐tazobactam (100/10 μg), ticarcillin (TIC; 75 μg), TIC‐clavulanic acid (75/10 μg), cefalexin (CN; 30 μg), FOX (30 μg), cefuroxim (30 μg), CRO (30 μg), ceftazidim (30 μg), XNL (30 μg), cefoperazone (CAZ; 75 μg), cefepime (30 μg), aztreonam (ATM; 30 μg), and imipenem (10 μg). The E. coli strain ATCC 25922 was used as a control. Veterinary CLSI 2015 clinical breakpoints were used when available, CLSI 2015 clinical breakpoints otherwise were used when available, and EUCAST clinical breakpoints were used if criteria could not be found in the 2 first sources.
For all isolates in this collection, we looked for 5 β‐lactamase resistance genes (bla SHV, bla TEM, bla CMY‐2, bla OXA, bla CTX‐M) by PCR multiplex (PCR and gene identification protocols described above).
The potential pathogenicity of ESBL/AmpC‐producing isolates was determined based on the presence of virulence genes which define E. coli pathotypes in animals (Enterotoxigenic E. coli [eltB, estA, estB], Enteropathogenic E. coli [eae], Shiga‐toxin producing E. coli [stxA and stx2A], and extra‐intestinal E. coli [ExPEC] [iutA]). The PCR procedures were performed following the protocol of the Ecl available at http://www.apzec.ca/en/APZEC/Protocols/APZEC_PCR_e-n.aspx.
Descriptive statistics were used to present susceptibility profiles per antimicrobial. We also estimated the prevalence and 95% CIs of premises housing horses shedding isolates nonsusceptible to >1, 3, 5, 7, or 9 classes of antimicrobials in this collection, adjusted for stratification and sampling weights as previously described. The ESBL/AmpC status for premises was attributed in the same manner as MDR status.
2.4. Risk factors
Two outcome variables were defined: MDR and ESBL/AmpC status of premises. Putative risk factors from the questionnaire with P < .20 (likelihood ratio test) in univariable logistic regression analysis were selected for inclusion in a multivariable model for each outcome. Pairwise associations between these selected variables were assessed by χ2 test. In the presence of significant association (P < .05), only 1 of 2 correlated variables was kept based on the biological relevance with the outcome. For the ESBL/AmpC model, the number of horses tested per premises was categorized (≤10, 11‐20, and ≥21) and included as a covariate in the full model to consider potential impact on the sensitivity of ESBL/AmpC detection. The final multivariable model then was refined by sequentially omitting variables with P > .05. However, if the removal of a variable caused a >20% change in the odds ratio of another variable, it was kept in the model as a potential confounder. No interaction was tested. The fit of the final model was evaluated using the Hosmer and Lemeshow goodness‐of‐fit test. The Genmod procedure of SAS 9.4 software was used.
3. RESULTS
3.1. Indicator collection
Overall, 1061 fecal samples from individual horses were collected from 41 premises, distributed as shown in Figure 1. Because of financial and logistic restrictions, a subset of 195 samples, from 41 premises, was sent to the Ecl. We detected viable E. coli in 132 of these 195 samples (68%). A total of 348 E. coli isolates were selected from these 132 horses, originating from 38 premises. Prevalence estimates were calculated based on the E. coli‐positive samples only. Prevalence estimates of horses and premises shedding resistant and nonsusceptible isolates per antimicrobial are shown in Figure 2. Over 40% of the horses and 80% of the premises showed nonsusceptibility to AMP, STR, and AMC.
Figure 1.
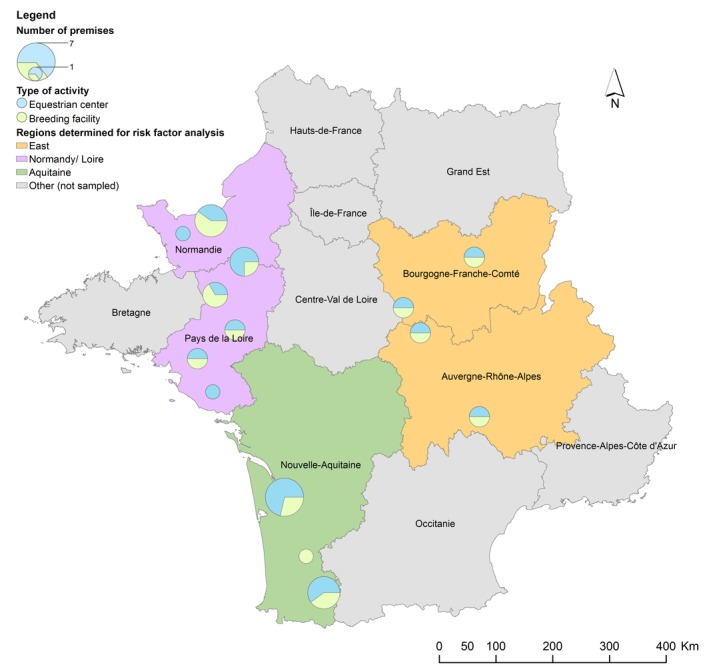
Geographical distribution of sampled premises over the French territory in a cross‐sectional study of 132 healthy adult horses, in 41 premises, performed in 2015. Colored regions were defined for the risk factor calculation. The size of the circle is proportional to the number of premises sampled
Figure 2.
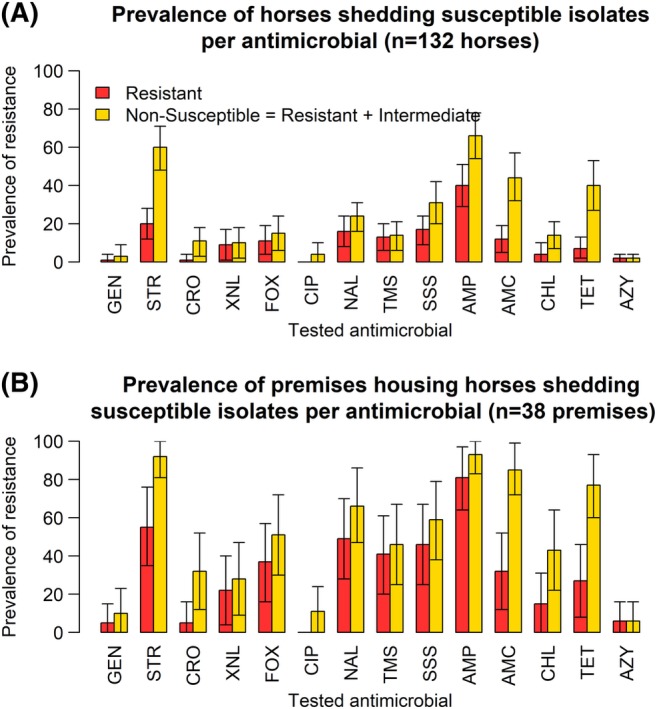
Prevalence estimates of resistance per antimicrobial, at the horse level (A), and at the premises level (B), in a cross‐sectional study of 132 healthy adult horses, in 38 premises, performed in 2015, in France. AMC, amoxicillin‐Clavulanic acid; AMP, ampicillin; AZY, azithromycin; CHL, chloramphenicol; CIP, ciprofloxacin; CRO, ceftriaxone; FOX, Cefoxitin; GEN, gentamicin; NA, Nalidixic acid; SSS, Sulfisoxazole; STR, streptomycin; TET, tetracycline; TMS, trimethoprim‐sulfamethoxazole; XNL, Ceftiofur. Bars represent 95% confidence intervals. We tested 348 isolates in total
Nonsusceptibility to third‐generation cephalosporins was observed in isolates from 10.6% of horses and 31.7% of premises. None of these isolates demonstrated the presence of bla genes by PCR.
Prevalence estimates of nonsusceptibility to 1st‐generation quinolone (NAL) was substantial (23.8% of horses and 66.7% of premises) even though nonsusceptibility to fluoroquinolones was lower (3.8% of horses and 10.6% of premises).
Prevalence estimates of horses shedding isolates nonsusceptible to >1, 3, 5, 7, or 9 classes of antimicrobials, and premises housing these horses, are summarized in Table 1. The prevalence of horses shedding, isolates nonsusceptible to ≥1 antimicrobial and MDR isolates was high (84.4% and 44.4%, respectively). A total of 79.7% of premises housed horses shedding MDR isolates. In addition, 7.6% of horses shed isolates nonsusceptible to 7 classes of antimicrobials.
Table 1.
Prevalence estimates of healthy adult horses
| Number of resistant antimicrobial classes | Bacterial collection | |||||
|---|---|---|---|---|---|---|
| Indicator | ESBL/AmpC | |||||
| Horse level (n = 132) | Premise level (n = 38) | Premise level (n = 41) | ||||
| Prev (%) | CI (95%) | Prev (%) | CI (95%) | Prev (%) | CI (95%) | |
| ≥1 | 84.4 | 77.4‐91.4 | 99.2 | 97.5‐100 | 29.0 | 11.5‐46.5 |
| ≥3 (MDR) | 44.4 | 33.1‐55.6 | 79.7 | 63.5‐96.0 | 29.0 | 11.5‐46.5 |
| ≥5 | 21.9 | 14.1‐29.6 | 60.2 | 39.9‐80.5 | 29.0 | 11.5‐46.5 |
| ≥7 | 7.6 | 1.5‐13.7 | 26.1 | 7.0‐45.2 | 28.3 | 10.8‐45.8 |
| ≥9 | 0 | NA | 0 | NA | 2.1 | 0.0‐4.4 |
Left of the table: Prevalence estimates of healthy adult horses shedding E. coli isolates nonsusceptible to more than 1, 3, 5, 7, or 9 classes of antimicrobials and premises housing these horses based on the indicator collection results in a cross‐sectional study of 132 horses, in 38 premises, in France in 2015. Right of the table: Prevalence estimates of premises housing healthy adult horses shedding isolates nonsusceptible to more than 1, 3, 5, 7, or 9 classes of antimicrobial in the ESBL/AmpC collection, based on the ESBL/AmpC collection results in a cross‐sectional study on healthy horses, in 38 premises, in France in 2015.
Abbreviations: AmpC, AmpC β‐lactamase; CI, confidence interval; ESBL, extended spectrum β‐lactamase; MDR, multidrug resistant; Prev, prevalence.
3.2. ESBL/AmpC collection
Twenty‐nine percent (95% CI, 11.5‐46.5) of premises housed horses that shed isolates nonsusceptible to CRO and thus belonging to the ESBL/AmpC collection. In the first approach, 195 samples from individual horses from 41 premises were tested, and we detected positive samples for 5 premises. In the second approach, 744 samples from the 41 premises were tested either in pools or individually. We detected positive samples for16 premises (including the 5 premises identified using the first approach).
Nonsusceptibility was observed for a wide range of antimicrobial classes among the 50 isolates of this collection originating from the 16 positive premises (Figure 3). Indeed, all isolates were nonsusceptible to AMP, PIP, CN, and CRO, although only 1 isolate was nonsusceptible to AMC and FOX (2nd‐generation cephalosporin classified as a cephamycin). In addition, > 60% of these isolates were nonsusceptible to aminoglycosides (GEN, KAN, STR), TET, and folate inhibitors (trimethoprim‐sulfonamides, sulfizoxasole). The proportion of isolates nonsusceptible to quinolones was lower in this collection than in the indicator collection. Nevertheless, we found 1 isolate that was resistant to fluoroquinolones in this collection. All isolates were susceptible to carbapenems and AMK. For all premises that housed horses shedding ESBL/AmpC isolates (29.0% of total premises), at least isolate was nonsusceptible to 5 classes of antimicrobials, and 2.1% of all premises housed horses that shed isolates nonsusceptible to up to 9 classes of antimicrobials (Table 1).
Figure 3.
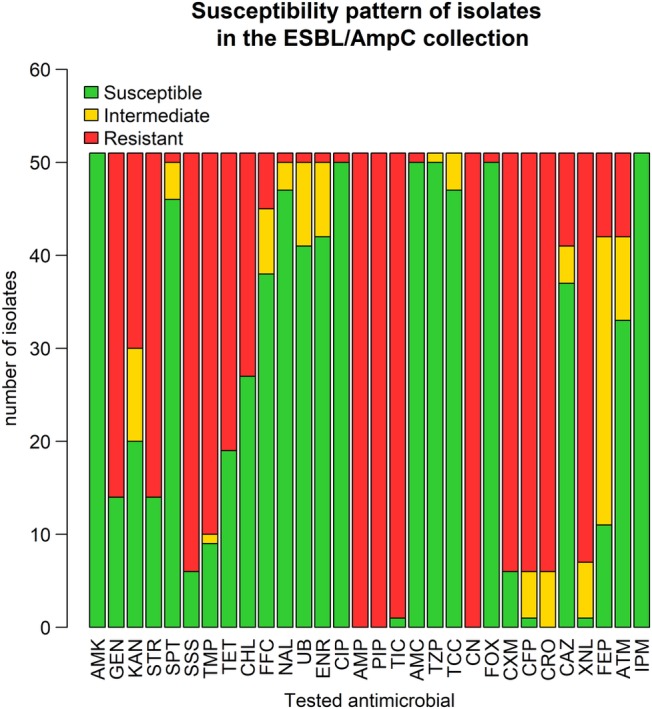
Characterization of susceptibility profiles of E. coli isolates in the ESBL/AmpC collection, in a cross‐sectional study performed on healthy adult horses, in France, in 2015 (n = 50 isolates). AMC, amoxicillin‐Clavulanic acid; AMK, Amikacin; AMP, ampicillin; ATM, Aztreonam; CAZ, Cefoperazon; CFP, Ceftazidime; CHL, chloramphenicol; CIP, ciprofloxacin; CN, Cefalexin; CRO, ceftriaxone; CXM, Cefuroxim; ENR, Enrofloxacin; FEP, Cefepime; FFC, Florfenicol; FOX, Cefoxitin; GEN, gentamicin; IPM, Imipenem; KAN, kanamycin; NAL, Nalidixic acid; PIP, Piperacillin; SPT, Spectinomycin; SSS, Sulfisoxazole; STR, streptomycin; TCC, Ticarcillin‐Clavulanic acid; TET, tetracycline; TIC, Ticarcillin; TMP, trimethoprim; TZP, Piperacillin‐Tazobactam; UB, Flumequine; XNL, Ceftiofur
The main ESBL genes identified were bla CTX‐M‐1 (33/50 tested isolates) and bla CTX‐M‐2 (8/50), but we also identified isolates that carried the ESBL genes bla CTX‐M‐14 (2/50) and bla SHV‐12 (6/50). Only 1 isolate carried the AmpC gene bla CMY‐2. No isolate with ≥2 ESBL/AmpC genes was observed.
A high diversity of non‐β‐lactam resistance patterns was observed among isolates that shared the same bla gene, but the 1 isolate carrying bla CMY‐2 was resistant only to FOX and clavulanic acid. The presence of bla SHV‐12 most often was associated with intermediate resistance to CRO and XNL, but with resistance to CAZ (third‐generation cephalosporin) and ATM (monobactam). No virulence genes associated with enteric pathogenic E. coli were observed. Only 1 isolate carried iutA.
3.3. Risk factors
Risk factors were calculated for 38 premises, because 3 questionnaires were not filled out completely. Sixteen potential risk factors were identified from the questionnaire (Table 2). All were dichotomous or categorical.
Table 2.
Potential risk factors for ESBL/AmpC and multidrug resistant (MDR) status at the premises level, in a cross‐sectional study performed on healthy adult horses, in France, in 2015
| Risk factor (MDR) | ESBL/AmpC status | MDR status | ||||
|---|---|---|---|---|---|---|
| No of stables per category | % of positive stables per category | P (univariate analysis) | No of stables per category | % of positive stables per category | P (univariate analysis) | |
| Number of horses tested | .82 | NA | ||||
| 10 and less | 1 | 0.0 | ||||
| Between 11 and 20 | 36 | 41.7 | ||||
| 21 and more | 4 | 25.0 | ||||
| Region | .26 | .56 | ||||
| Normandy | 19 | 42.1 | 18 | 66.7 | ||
| Aquitaine | 14 | 50.0 | 12 | 75.0 | ||
| East | 8 | 12.5 | 8 | 87.5 | ||
| Transportation | .06 | .39 | ||||
| Twice per month or more | 27 | 44.4 | 24 | 75.0 | ||
| Less than twice per month | 11 | 9.1 | 10 | 60.0 | ||
| Total number of horses in the stable | .09 | .76 | ||||
| Less than 50 | 13 | 15.4 | 9 | 66.7 | ||
| 50 and more | 25 | 44.0 | 25 | 72.0 | ||
| Staff taking care of horses (daily) | .007 | .03 | ||||
| 5 persons and less | 21 | 14.3 | 17 | 52.9 | ||
| More than 5 persons | 17 | 58.9 | 17 | 88.2 | ||
| Decision to administrate antimicrobials without medical advice | .77 | .84 | ||||
| Yes | 33 | 33.3 | 30 | 70.0 | ||
| No | 5 | 40.0 | 4 | 75.0 | ||
| Contact with wild life | .20 | .85 | ||||
| Yes | 27 | 40.7 | 23 | 69.6 | ||
| No | 11 | 18.2 | 11 | 72.7 | ||
| Presence of other animals on the farm | .58 | .20 | ||||
| Farm animals | 4 | 25.0 | 4 | 75.0 | ||
| Pets | 17 | 35.3 | 14 | 85.7 | ||
| Farm animals and pets | 12 | 25.0 | 11 | 45.5 | ||
| No | 5 | 60.0 | 5 | 80.0 | ||
| Fertilizer spread on the pasture | .65 | .76 | ||||
| Yes | 10 | 40.0 | 9 | 66.7 | ||
| No | 28 | 32.1 | 25 | 72.0 | ||
| Stall cleansing frequency | .44 | .90 | ||||
| Less than once a week | 8 | 50.0 | 7 | 71.4 | ||
| Once a week and more | 26 | 34.5 | 23 | 73.9 | ||
| Activity | .05 | .28 | ||||
| Breeding facility | 20 | 55.0 | 17 | 82.4 | ||
| Riding school | 21 | 23.8 | 21 | 66.7 | ||
| One person of the staff in contact with human or veterinarian medical environment | .23 | .58 | ||||
| Yes | 28 | 28.6 | 25 | 68.0 | ||
| No | 10 | 50.0 | 9 | 77.8 | ||
| Vet is specialized in equine medicine | .97 | .97 | ||||
| Yes | 36 | 36.1 | 32 | 68.8 | ||
| No | 2 | 0.0 | 2 | 100 | ||
| One horse has been hospitalized in the last 3 months | .49 | .84 | ||||
| Yes | 4 | 50.0 | 4 | 75.0 | ||
| No | 34 | 32.4 | 30 | 70.0 | ||
| One horse has undergone surgery in the last 3 months | .49 | .35 | ||||
| Yes | 4 | 50.0 | 4 | 50.0 | ||
| No | 34 | 32.4 | 30 | 73.3 | ||
| One horse has been medically treated in the last 3 months (all treatments considered) | .04 | .60 | ||||
| Yes | 20 | 50.0 | 18 | 66.7 | ||
| No | 18 | 16.7 | 16 | 75.0 | ||
A positive MDR status for a horse was defined as the detection of a least 1 MDR isolate for that horse. A premises was attributed a positive MDR status if it housed at least 1 positive MDR horse. P‐values are derived from the likelihood ratio test in univariate analysis.
Five risk factors were considered for multivariate modeling (all P < .20 in univariable analysis) for the ESBL/AmpC outcome, but “transportation” and “contact with wild life” were excluded because they were associated with “the number of persons taking care of the horses” and “activity,” respectively. According to the multivariate model, the odds of being an ESBL/AmpC premises were 14.6 times higher (P = .03) among the riding schools compared to breeding premises, 9.6 times higher (P = .03) if the premises housed a horse that had been medically treated within the last 3 months and 35.7 times higher (P = .006) in premises where the staff consisted of >5 persons (Table 3). The Hosmer‐Lemeshow goodness‐of‐fit analysis indicated that our model fitted the data (P = .6). Transportation was marginally but not statistically significant (P = .06) in the univariate analysis but was excluded from multivariable analysis.
Table 3.
Parameter estimates and odds ratio from a multivariable logistic regression modeling ESBL/AmpC‐positive status at the premises level, based on the results of a cross‐sectional study performed on 38 premises housing healthy adult horses, sampled in France in 2015
| Risk factor | Odds ratios | ||
|---|---|---|---|
| Estimate | 95% CI | P | |
| Riding school versus breeding facility | 14.6 | 1.3‐164.6 | .03 |
| One horse has been medically treated in the last 3 months (all treatments considered) versus no horse treated | 9.6 | 1.2‐76.9 | .03 |
| More than 5 persons taking care of horses daily versus 5 persons or less | 35.7 | 2.9‐500.0 | .006 |
Abbreviations: AmpC, AmpC β‐lactamase; CI, confidence interval; ESBL, extended spectrum β‐lactamase.
The odds of being an MDR premises were 6.7 (95% CI 1.2‐38.4) times higher (P = .03) in premises where the staff consisted of >5 persons. This was the only statistically significant variable for this outcome. No regional trends were observed from these analyses.
4. DISCUSSION
We demonstrated that the fecal microbiota of healthy horses harbors MDR and ESBL/AmpC E. coli isolates. The prevalence of premises housing horses shedding ESBL/AmpC E. coli isolates (29.0%) is comparable to that found in pig farms in other European countries.32 This finding is both surprising and worrisome because horses can be considered companion animals.10 Until now, the focus concerning antimicrobial resistance in animals has been on food‐producing animals.7 These results suggest that companion animals, including horses, also are important in the persistence of antimicrobial resistance genes.
Ceftiofur is a wide‐spectrum third‐generation cephalosporin approved for use in veterinary medicine that is very well tolerated by horses. Thus, its common use by horse practitioners33 may promote ESBL/AmpC gene persistence in this species. Nevertheless, the occurrence of ESBL/AmpC genes in other animal species and in the environment is a global problem affecting both animal and public health34 and also may contribute to the prevalence of ESBL/AmpC genes in horses. Although most ESBL/AmpC‐positive isolates in our study were commensals, ESBL/AmpC genes may be transmissible to potential pathogenic or zoonotic strains.
The predominant family of ESBL genes found in the equine microbiota was ESBL bla CTX‐M. This ESBL family has been of public health concern since the 2000s. In Europe, a dramatic increase in the incidence and diversity of this family has occurred, and over the last 2 decades, a shift from other ESBL enzymes in Enterobacteriaceae such as bla TEM and bla SHV to bla CTX‐M variants has been observed. The bla CTX‐M encoded ESBL family is characterized by the ability to hydrolyze oxy‐imino‐cephalosporins (third and fourth generation) and monobactams but not cephamycins and carbapenems. These bacteria generally are susceptible to β‐lactam inhibitors. The predominance of the bla CTX‐M‐1 suggests global dissemination of this gene in the equine population. Moreover, it also has been found in all other food‐producing animal species in Europe,13 suggesting dissemination among species.
In addition to CTX‐M ESBLs, the AmpC resistance gene bla CMY‐2 is frequently found in food production animals in North America,35 but it also has been found in Europe.36 To the best of our knowledge, this gene has not previously been identified in healthy horses, and this finding reinforces the idea that AmpC genes may spread through the healthy animal population.
The ESBL/AmpC genes often are carried by MDR plasmids, which play a key role in their dissemination.15 The high prevalence of ESBL/AmpC and MDR E. coli isolates observed in our study may be explained by the combination of the presence of such plasmids and antimicrobial pressure. Nevertheless, their mobile genetic elements, such as transposons and conjugative plasmids, and their ability to disseminate, should be investigated further.13
Our approach of examining an indicator E. coli collection identified extensive drug resistance in E. coli in the intestinal microbiome of healthy horses in France, as previously observed in the United Kingdom.4 The large amount of nonsusceptibility to AMP in the indicator collection could be explained by the frequent use of penicillin in equine medicine.
Acquired resistance to quinolones and fluoroquinolones is a result of the appearance of chromosomal mutations. Generally, the mutations appear in sequence and involve the genes gyrA (coding for the gyrase) and parC (coding for a subunit of the topoisomerase). The first mutation confers resistance to quinolones and the combination of the 2 mutations confers resistance to fluoroquinolones. Hence, nonsusceptibility to NAL generally is considered a predictor for fluoroquinolone treatment failure.37 The finding in the indicator collection that >55% of premises featured this type of resistance suggests that ENR should be used with caution in horses.
Although we detected nonsusceptibility to third‐generation cephalosporins in the indicator collection, none of the isolates carried tested ESBL/AmpC genes, suggesting that other ESBL/AmpC genes may be present in the population, (eg, bla OXA , bla PER , bla GES‐1, and bla VER‐1) or other mechanisms of resistant to cephalosporins may be present (eg, an efflux pump). These other mechanisms would less likely be transmissible by mobile elements but also could account for treatment failure when using cephalosporins and therefore could still have an impact on the health of horses.
In the indicator collection, we detected viable E. coli in only 68% of the fecal samples. This viability rate is low compared to other published studies in healthy horses,4 and compared to internal data available in our laboratory. Some E. coli isolates may have been lost during shipping. This issue questions the validity of the indicator collection results, but if it had any effect on the results it would have underestimated resistance. Indeed, antimicrobial resistance has a fitness cost,38 and resistant isolates should have died first. Nevertheless, the level of resistance we found in the indicator collection, based only on E. coli‐positive samples, is comparable to that of other studies.4 Therefore, we do not believe shipping had considerable influence on the results of the indicator collection.
Our results showed that medication represented a risk factor for ESBL/AmpC‐positive premises. Antimicrobial treatment already has been identified as a risk factor at the individual level in other studies.22 Although our results pertain to the premises level, we could hypothesize that medically treated horses are more likely to shed ESBL/AmpC isolates. Based on this information, we could suggest isolating horses that are medically treated or at least implementing appropriate biosecurity measures. As an example, limiting contact between treated and healthy horses and handling healthy horses before treated horses might be beneficial to limit antimicrobial gene dissemination. More longitudinal studies are needed to establish the duration of shedding in nonhospitalized horses and to develop more accurate recommendations. Nevertheless, based on other studies,39, 40 the isolation of the horses should be for at least 2 weeks.
The finding that the number of persons taking care of horses influences the presence of ESBL/AmpC genes or the presence of MDR isolates has not been documented previously, to our knowledge. Although more studies are needed to confirm such an association, considering that this information is easy to obtain, it could be helpful for elaborating guidelines to improve the health of horses. For example, it could help horse practitioners to define “at‐risk” populations and justify the performance of antimicrobial susceptibility testing more frequently.
Riding schools also seem to be more at risk, as compared to breeding premises, for the presence of ESBL/AmpC genes. This risk factor is relevant because riding schools are an interface with the general population. This finding could provide the impetus to set up prevention measures to limit dissemination of ESBL/AmpC genes and MDR isolates. Appropriate recommendations could include washing hands after touching horses or limiting contact between horses and infants at this type of premises, although further studies are needed to quantify the risk of transmission from horses to humans, and reciprocally.
Our study has some limitations. First, horses within a premises were not selected randomly. However, we have no reason to believe that this selection process would have biased our results, considering that the people in charge of field sampling were blinded to the outcome status and drug use history of the horses. In addition, CIs for odd ratios were wide because of the small sample size of premises. Based on these results, it is not possible to compare the strength of association among the identified risk factors, and this low precision should be considered in interpreting the impact of these risk factors in the horse population. Small sample size also limited the statistical power of our study, and thus true associations with risk factors might have been missed. Finally, because many risk factors were tested, some risk factors may have been statistically significant based on chance alone, and thus more studies should be conducted to confirm these associations.
In conclusion, we found a high prevalence of ESBL/AmpC genes and MDR isolates in the microbiota of healthy horses. Surveillance of ESBL/AmpC gene dissemination and quantification of MDR isolates would be beneficial to characterize the nature and extent of the risk they represent, with the aim of limiting their transmission among horses, but also to other species including humans and to the environment.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENTS
The work was presented in:
M de Lagarde, C Larrieu, K Praud, N Lallier, A Trotereau, C Schouler, G Sallé, B Doublet, J Arsenault, JM Fairbrother. “Prevalence and characterization of multidrug resistant and ESBL/AmpC producing E. coli in the healthy equine population in France and Quebec.” Poster at the 15th symposium of the WEVA, Beijing, China, April 2018.
M de Lagarde, C Larrieu, K Praud, N Lallier, A Trotereau, C Schouler, G Sallé, J Arsenault, JM Fairbrother, B Doublet. “Inclure l'espèce équine dans un programme d'antibiosurveillance: une nécessité manifeste.” Oral presentation for the AVEQ Symposium 2018, Saint Hyacinthe, Canada, February 2018.
M de Lagarde, C Larrieu, K Praud, N Lallier, A Trotereau, C Schouler, G Sallé, J Arsenault, JM Fairbrother, B Doublet. “Inclure l'espèce équine dans un programme d'antibiosurveillance: une nécessité manifeste.” Oral presentation at the 6th day of Antibiosurveillance, Saint Hyacinthe, Canada, November 2017.
M de Lagarde, J Arsenault, JM Fairbrother. “Le microbiote intestinal des chevaux, au Québec, est‐il un réservoir de gènes d'antibiorésistance?.” Oral presentation at the 85th Symposium of the ACFAS, Montreal, Canada, May 2017.
M de Lagarde, C Larrieu, K Praud, N Lallier, A Trotereau, C Schouler, G Sallé, J Arsenault, JM Fairbrother, B Doublet. “Le microbiote intestinal des chevaux, en France, est‐il un réservoir de gène d'antibiorésistance? Caractérisation des souches de E. coli productrices de BLSE/AmpC en 2015” Oral presentation at the 43rd day of Equine research, Paris, France March 2017.
M de Lagarde, C Larrieu, K Praud, N Lallier, A Trotereau, C Schouler, G Sallé, J Arsenault, JM Fairbrother, B Doublet. “Le microbiote intestinal des chevaux, en France, est‐il un réservoir de gènes d'antibiorésistance? Caractérisation des souches de E. coli productrices de BLSE/AmpC en 2015”. Oral presentation and poster at the 7th symposium of the CIFMA, Liege, Belgium, March 2017.
de Lagarde M, Larrieu C, Praud K, et al. Prevalence, risk factors, and characterization of multidrug resistant and extended spectrum β‐lactamase/AmpC β‐lactamase producing Escherichia coli in healthy horses in France in 2015. J Vet Intern Med. 2019;33:902–911. 10.1111/jvim.15415
Funding information Institut national de recherche agro‐alimentaire (INRA); l’Institut Francais du cheval et de l’équitation (IFCE); OIE Reference Laboratory for E. coli
REFERENCES
- 1. World Health Organization . Antimicrobial resistance: global report on surveillance. Switzerland: World Health Organization; 2014. [Google Scholar]
- 2. van Spijk J, Schmitt S, Schoster A. Infections caused by multidrug‐resistant bacteria in an equine hospital (2012–2015). Equine Vet Educ. 2017. (Epub). [Google Scholar]
- 3. Jokisalo J, Bryan J, Legget B, Abbott Y, Katz LM. Multiple‐drug resistant Acinetobacter baumannii bronchopneumonia in a colt following intensive care treatment. Equine Vet Educ. 2010;22:281‐286. [Google Scholar]
- 4. Maddox TW, Clegg PD, Diggle PJ, et al. Cross‐sectional study of antimicrobial‐resistant bacteria in horses. Part 1: prevalence of antimicrobial‐resistant Escherichia coli and methicillin‐resistant Staphylococcus aureus . Equine Vet J. 2012;44:289‐296. [DOI] [PubMed] [Google Scholar]
- 5. Rubin JE, Pitout JD. Extended‐spectrum beta‐lactamase, carbapenemase and AmpC producing Enterobacteriaceae in companion animals. Vet Microbiol. 2014;170:10‐18. [DOI] [PubMed] [Google Scholar]
- 6. Chevance A, Moulin G. Suivi des ventes de médicaments vétérinaires contenant des antibiotiques en France en 2013. Volumes et estimation de l'exposition des animaux aux antibiotiques 2014.
- 7. European Food Safety Authority EFSA, European Centre for Disease Prevention and Control ECDC . The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2015. EFSA Journal. 2017;15:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Toombs‐Ruane LJ, Riley CB, Kendall AT, Hill KE, Benschop J, Rosanowski SM. Antimicrobial susceptibility of bacteria isolated from neonatal foal samples submitted to a New Zealand veterinary pathology laboratory (2004 to 2013). N Z Vet J. 2016;64:107‐111. [DOI] [PubMed] [Google Scholar]
- 9. Dunowska M, Morley PS, Traub‐Dargatz JL, Hyatt DR, Dargatz DA. Impact of hospitalization and antimicrobial drug administration on antimicrobial susceptibility patterns of commensal Escherichia coli isolated from the feces of horses. J Am Vet Med Assoc. 2006;228:1909‐1917. [DOI] [PubMed] [Google Scholar]
- 10. Ewers C, Bethe A, Semmler T, Guenther S, Wieler LH. Extended‐spectrum beta‐lactamase‐producing and AmpC‐producing Escherichia coli from livestock and companion animals, and their putative impact on public health: a global perspective. Clin Microbiol Infect. 2012;18:646‐655. [DOI] [PubMed] [Google Scholar]
- 11. Schmiedel J, Falgenhauer L, Domann E, et al. Multiresistant extended‐spectrum beta‐lactamase‐producing Enterobacteriaceae from humans, companion animals and horses in Central Hesse, Germany. BMC Microbiol. 2014;14:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ewers C, Bethe A, Stamm I, et al. CTX‐M‐15‐D‐ST648 Escherichia coli from companion animals and horses: another pandemic clone combining multiresistance and extraintestinal virulence? J Antimicrob Chemother. 2014;69:1224‐1230. [DOI] [PubMed] [Google Scholar]
- 13. Cantón R, González‐Alba J, Galán J. CTX‐M enzymes: origin and diffusion. Front Microbiol. 2012;3 (Epub). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mathers AJ, Peirano G, Pitout JD. The role of epidemic resistance plasmids and international high‐risk clones in the spread of multidrug‐resistant Enterobacteriaceae . Clin Microbiol Rev. 2015;28:565‐591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carattoli A. Plasmids in gram negatives: molecular typing of resistance plasmids. Int J Med Microbiol. 2011;301:654‐658. [DOI] [PubMed] [Google Scholar]
- 16. Huijbers PM, de Kraker M, Graat EA, et al. Prevalence of extended‐spectrum beta‐lactamase‐producing Enterobacteriaceae in humans living in municipalities with high and low broiler density. Clin Microbiol Infect. 2013;19:E256‐E259. [DOI] [PubMed] [Google Scholar]
- 17. Yang Y, Zhang A, Lei C, et al. Characteristics of plasmids coharboring 16S rRNA methylases, CTX‐M, and virulence factors in Escherichia coli and Klebsiella pneumoniae isolates from chickens in China. Foodborne Pathog Dis. 2015;12:873‐880. [DOI] [PubMed] [Google Scholar]
- 18. Debroy C, Roberts E, Jayarao BM, Brooks JW. Bronchopneumonia associated with extraintestinal pathogenic Escherichia coli in a horse. J Vet Diagn Invest. 2008;20:661‐664. [DOI] [PubMed] [Google Scholar]
- 19. Van Duijkeren E, Van Asten A, Gaastra W. Characterization of Escherichia coli isolated from adult horses with and without enteritis. Vet Q. 2000;22:162‐166. [DOI] [PubMed] [Google Scholar]
- 20. Dolejska M, Duskova E, Rybarikova J, et al. Plasmids carrying bla CTX‐M‐1 and qnr genes in Escherichia coli isolates from an equine clinic and a horseback riding Centre. J Antimicrob Chemother. 2011;66:757‐764. [DOI] [PubMed] [Google Scholar]
- 21. Salle G, Cortet J, Bois I, et al. Risk factor analysis of equine strongyle resistance to anthelmintics. Int J Parasitol Drugs Drug Resist. 2017;7:407‐415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maddox TW, Pinchbeck GL, Clegg PD, et al. Cross‐sectional study of antimicrobial‐resistant bacteria in horses. Part 2: risk factors for faecal carriage of antimicrobial‐resistant Escherichia coli in horses. Equine Vet J. 2012;44:297‐303. [DOI] [PubMed] [Google Scholar]
- 23. Traub WH, Raymond EA, Linehan J. Identification of Enterobacteriaceae in the clinical microbiology laboratory. Appl Microbiol. 1970;20:303‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martins MT, Rivera IG, Clark DL, Stewart MH, Wolfe RL, Olson BH. Distribution of uidA gene sequences in Escherichia coli isolates in water sources and comparison with the expression of beta‐glucuronidase activity in 4‐methylumbelliferyl‐beta‐D‐glucuronide media. Appl Environ Microbiol. 1993;59:2271‐2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jahanbakhsh S, Kabore KP, Fravalo P, Letellier A, Fairbrother JM. Impact of medicated feed along with clay mineral supplementation on Escherichia coli resistance to antimicrobial agents in pigs after weaning in field conditions. Res Vet Sci. 2015;102:72‐79. [DOI] [PubMed] [Google Scholar]
- 26. Vounba P, Yaghouba K, Ndiaye C, et al. Molecular characterization of Escherichia coli isolated from chickens with Colibacillosis in Senegal. Foodborne Pathog Dis. 2018;15:517‐525. [DOI] [PubMed] [Google Scholar]
- 27. Maluta RP, Fairbrother JM, Stella AE, Rigobelo EC, Martinez R, Ávila FA. Potentially pathogenic Escherichia coli in healthy, pasture‐raised sheep on farms and at the abattoir in Brazil. Vet Microbiol. 2014;169:89‐95. [DOI] [PubMed] [Google Scholar]
- 28. Mataseje L, Bryce E, Roscoe D, et al. Carbapenem‐resistant gram‐negative bacilli in Canada 2009–10: results from the Canadian nosocomial infection surveillance program (CNISP). Journal of Antimicrob Chemother. 2012;67:1359‐1367. [DOI] [PubMed] [Google Scholar]
- 29. Borgogna TR, Borgogna JL, Mielke JA, et al. High diversity of CTX‐M extended‐spectrum beta‐lactamases in municipal wastewater and urban wetlands. Microb Drug Resist. 2016;22:312‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug‐resistant, extensively drug‐resistant and pandrug‐resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268‐281. [DOI] [PubMed] [Google Scholar]
- 31. Agerso Y, Aarestrup FM, Pedersen K, Seyfarth AM, Struve T, Hasman H. Prevalence of extended‐spectrum cephalosporinase (ESC)‐producing Escherichia coli in Danish slaughter pigs and retail meat identified by selective enrichment and association with cephalosporin usage. J Antimicrob Chemother. 2012;67:582‐588. [DOI] [PubMed] [Google Scholar]
- 32. Hammerum AM, Larsen J, Andersen VD, et al. Characterization of extended‐spectrum beta‐lactamase (ESBL)‐producing Escherichia coli obtained from Danish pigs, pig farmers and their families from farms with high or no consumption of third‐ or fourth‐generation cephalosporins. J Antimicrob Chemother. 2014;69:2650‐2657. [DOI] [PubMed] [Google Scholar]
- 33. Prescott JF, Dowling PM. Antimicrobial Therapy in Veterinary medicine. Switzerland: John Wiley & Sons; 2013. [Google Scholar]
- 34. Adams RJ, Kim SS, Mollenkopf DF, et al. Antimicrobial‐resistant Enterobacteriaceae recovered from companion animal and livestock environments. Zoonoses Public Health. 2018;Aug;65:519‐527. [DOI] [PubMed] [Google Scholar]
- 35. Jahanbakhsh S, Letellier A, Fairbrother JM. Circulating of CMY‐2 beta‐lactamase gene in weaned pigs and their environment in a commercial farm and the effect of feed supplementation with a clay mineral. J Appl Microbiol. 2016;121:136‐148. [DOI] [PubMed] [Google Scholar]
- 36. Dierikx CM, van Duijkeren E, Schoormans AH, et al. Occurrence and characteristics of extended‐spectrum‐beta‐lactamase‐ and AmpC‐producing clinical isolates derived from companion animals and horses. J Antimicrob Chemother. 2012;67:1368‐1374. [DOI] [PubMed] [Google Scholar]
- 37. Robert J, Cambau E, Grenet K, et al. Trends in quinolone susceptibility of Enterobacteriaceae among inpatients of a large university hospital: 1992‐98. Clin Microbiol Infect. 2001;7:553‐561. [DOI] [PubMed] [Google Scholar]
- 38. Andersson DI, Hughes D. Antibiotic resistance and its cost: is it possible to reverse resistance? Nat Rev Microbiol. 2010;8:260‐271. [DOI] [PubMed] [Google Scholar]
- 39. Damborg P, Marskar P, Baptiste KE, Guardabassi L. Faecal shedding of CTX‐M‐producing Escherichia coli in horses receiving broad‐spectrum antimicrobial prophylaxis after hospital admission. Vet Microbiol. 2012;154:298‐304. [DOI] [PubMed] [Google Scholar]
- 40. Johns I, Verheyen K, Good L, Rycroft A. Antimicrobial resistance in faecal Escherichia coli isolates from horses treated with antimicrobials: a longitudinal study in hospitalised and non‐hospitalised horses. Vet Microbiol. 2012;159:381‐389. [DOI] [PubMed] [Google Scholar]


