Abstract
Background
Blood typing for the A and B antigens is essential and crossmatching testing is generally recommended before transfusing blood to cats.
Objective
To evaluate 2 crossmatch (XM) tests.
Animals
Forty‐nine healthy domestic shorthair cats that had not received a blood transfusion.
Methods
Prospective study. Blood samples were typed for AB using immunochromatographic and flow cytometric techniques. A gel column (GC) and a feline antiglobulin‐enhanced gel column (AGC) XM tests were used for crossmatching.
Results
The population included 34 type A, 13 B, and 2 AB cats, with concordant results (r = 1, P < .005) by flow cytometry and immunochromatographic strip kit. The plasma from type A cats had either no or weak anti‐B alloantibodies. The plasma of 12 of 13 type B cats contained strong anti‐A alloantibodies. For crossmatching, plasma to RBC pairings were prepared using the GC (n = 446) and AGC (n = 630) tests. Both methods showed compatibilities in 329 and incompatibilities in 102 pairings including all A‐B mismatches. Additionally 15 pairings showed agglutination by the AGC but not GC method. Fourteen incompatibilities outside the expected A‐B mismatches were only revealed by AGC.
Conclusions and Clinical Importance
AB typing using immunochromatographic strip is as accurate as laboratory flow cytometry. The 2 XM methods had good agreement with additional incompatibilities being recognized by the AGC XM beyond A‐B incompatibilities. In clinic, feline AB typing and sensitive XM test kits are available and recommended before each transfusion, although the clinical implications of incompatible XM test results and clinical benefits of such crossmatching have not been documented.
Keywords: blood compatibility, blood type systems, blood typing, feline, hemolysis, transfusion
Abbreviations
- AGC
antiglobulin‐enhanced gel column
- FITC
fluorescein isothiocyanate
- GC
gel column crossmatch without antiglobulin
- Ig
immunoglobulin
- LISS
low ionic strength solution
- MFI
mean fluorescence intensity
- PBS
phosphate‐buffered saline
- PCV
packed cell volume
- pRBC
packed red blood cell
- RBC
red blood cell
- XM
crossmatch
1. INTRODUCTION
Humans and domestic animals have many blood group systems with or without naturally occurring alloantibodies. Domestic cats have one major blood group system, the AB system with types A, B, and AB. Type A cats have no or weak anti‐B alloantibodies, type B cats have strong anti‐A alloantibodies, and type AB cats have no naturally occurring alloantibodies.1 Although there are well‐recognized breed and geographical variations, type A is the most common type found followed by type B and then the extremely rare type AB.2 The A and B red blood cell (RBC) antigens represent sialic N‐glycolyl‐ and N‐acetyl‐neuraminic acids, respectively. The AB erythrocytes express both N‐glycolyl‐ and N‐acetyl‐neuraminic acids.3 Several genetic variants (mutations) have been identified in the Cytidine Monophosphate‐N‐Acetylneuramic acide hydrolase (CMAH) gene associated with type A, B, and AB, but the precise functional effects of these variants have not been determined, and genotyping assays have not been completely accurate until recently.3, 4, 5
In addition, Mik has been proposed as an additional feline blood group system with potentially naturally occurring alloantibodies in some Mik‐negative cats.6 Rarely other blood types are suspected based upon crossmatch (XM) incompatibilities pre‐ and post‐transfusion and/or acute hemolytic transfusion reactions despite A‐B matching.5, 7
A‐B typing of recipients and donors (as well as mates before breeding) is recommended to assure A‐B compatibility.5, 8, 9, 10 A‐B matched transfusions are required to be effective, and ignoring A‐B blood typing can result in serious acute hemolytic transfusion reactions.5, 10 A‐B mismatches can also induce neonatal isoerythrolysis. Although originally feline anti‐A and anti‐B antisera and lectins (Triticum vulgaris as anti‐B) have been used for typing,1 typing kits utilizing monoclonal anti‐A and anti‐B alloantibodies have been used in clinical practice for a couple of decades.11, 12, 13 Although canine blood as xenotransfusion is occasionally used, it leads to rapid destruction of canine RBCs in first few days (acute hemolytic transfusion reaction) and is fatal upon a second transfusion; therefore, this practice is not recommended.14, 15, 16
For decades, blood typing in cats has been recommended before the first transfusion and XM has been recommended before a second transfusion when given after more than 4 days.10 There is some evidence for the presence of other naturally occurring alloantibodies outside the AB blood group system, like anti‐Mik, but their importance at the time of a first transfusion has not been clearly defined clinically and experimentally.5, 17, 18 Some also perform crossmatching in addition to AB typing before a first transfusion due to the potential presence of naturally occurring alloantibodies outside of the AB system, such as anti‐Mik.5, 7, 18, 19 However, crossmatching cats is technically restricted by the difficulty to obtain blood samples from recipient and donor cat before transfusion, limited availability of standardized crossmatching protocols and expertise to perform elaborate XM assays, and lack of any in‐clinic kits for crossmatching cats.5, 10
The purpose of this prospective study was to detect naturally occurring alloantibodies by a new in‐clinic feline antiglobulin‐enhanced gel tube XM test in cats compared to a laboratory gel column card method to establish XM recommendations in cats not previously transfused. The second aim of the study was to assess the correlation between the two tests.
2. MATERIALS AND METHODS
2.1. Animals and blood sample collection
Male and female domestic shorthair cats, weighing at least 1 kg, presented for routine wellness examination and neutering (October 2016 and March 2017) to VetAgro Sup Campus Vétérinaire de Lyon, France (VetAgro Sup) were included in this prospective study. Cats were considered healthy based on history, physical examination, packed cell volume (PCV), total protein, and negative feline immunodeficiency antibody and leukemia virus antigen test results. Sick or previously transfused cats were excluded from the study. Approximately 3 mL blood were collected into EDTA from each cat for immediate routine preanesthetic diagnostic testing as well as for AB blood typing and crossmatching. This prospective study was approved by the Ethical Committee of VetAgro Sup (#1622), and written owner consent was obtained before enrollment of any cat into the study.
2.2. Laboratory methods
Aliquots of EDTA‐anticoagulated whole‐blood samples were used directly for AB blood typing by immunochromatographic strip kit (CHROM Method, Lab Test A + B; Alvedia, Limonest, France), and the remaining blood was centrifuged at 1000g for 10 minutes to prepare packed red blood cells (pRBCs) as well as plasma from each cat for AB typing with flow cytometry and XM testing using 2 different techniques. The pRBCs were stored at 4°C, and the remaining plasma was frozen at −20°C for XM testing.
2.2.1. Feline AB typing
AB typing was performed by 2 methods: a commercially available immunochromatographic strip kit and a flow cytometric AB typing technique. The immunochromatographic strip kit was used with 1‐ to 2‐day‐old EDTA‐anticoagulated whole blood as previously described11, 12 and according to the manufacturer's instructions. For flow cytometric AB typing, 10 μL of pRBCs (<1 week old) were washed 3 times with phosphate‐buffered saline (PBS), and the last pellet was mixed with 90 μL of PBS. Then, 10 μL of the 10% washed RBC suspension were mixed with 100 μL of a diluted monoclonal murine anti‐A or anti‐B immunoglobulin M (IgM) kappa antibody (Alvedia) and incubated at 37°C for 30 minutes. Thereafter, the RBC suspension was again washed with PBS, and 100 μL of a fluorescein isothiocyanate (FITC)‐conjugated monoclonal rat anti‐mouse IgM antibody solution (BD Pharmingen FITC Rat Anti‐Mouse IgM; BD Biosciences, San Jose, California; diluted 50‐fold in PBS) were added to the RBC pellet. The suspension was mixed and incubated at 37°C for 30 minutes, washed again in PBS, and the pellet was resuspended in 500 μL of PBS before flow cytometric analysis using an FACSCalibur (Becton Dickinson & Co, Franklin Lakes, New Jersey). Data were collected for 10,000 events through a gated region from each sample (CellQuest Pro software; Becton Dickinson & Co), and the mean fluorescence intensity (MFI) was obtained. The A, B, or both antigen RBC surface expression was designated as negative for an MFI < 10 and positive for any MFI ≥ 10. Incubation time, quantity of reagent, or blood samples were decided based upon experience from the laboratory and manufacturer instructions.1, 20
2.2.2. Gel column XM without antiglobulin
Crossmatch tests were performed and interpreted according to the manufacturer's instructions (Bio‐Rad, DiaMed GmbH, Cressier, Switzerland) and as previously described.11, 21 In a 3‐mL polystyrene test tube, 50 μL of 1% donor pRBCs in low ionic strength solution (LISS; Bio‐Rad, DiaMed GmbH) were added to 25 μL of recipient plasma, briefly mixed, and incubated at 22°C for 10 minutes. The RBC suspension was placed on top of the GC, filled with neutral gel (no antiglobulin). The GC cards were centrifuged (ID Centrifuge 6S; Bio‐Rad DiaMed GmbH) at 80g for 10 minutes, and the location of the migrated RBCs was recorded. In the absence of agglutination, the RBCs passed through the gel to the bottom, which was scored as “compatible,” whereas agglutination on the top of or within the gel was considered “incompatible.” Auto‐controls (using RBCs and plasma from same cat) and positive controls (using murine monoclonal anti‐A and anti‐B antibodies) were included for all XM tests performed.
2.2.3. Antiglobulin‐enhanced gel column XM kit
An antiglobulin‐enhanced gel technique routinely used in human medicine22, 23, 24 was adapted as a novel in‐clinic XM kit for cats6 with feline antiglobulin and an in‐clinic protocol as per manufacture. In a 3‐mL polystyrene test tube, 50 μL of 1% donor pRBCs in LISS (Bio‐Rad DiaMed GmbH) were added to 25 μL of recipient plasma, briefly mixed, and incubated at 22°C for 10 minutes. After incubation, the RBC suspension was placed on top of the gel test mini‐tube, filled with a gel containing a specific goat anti‐feline antiglobulin (VMRD, Pullman, Washington) binding only RBCs coated with feline IgG, IgM, complement C3, or all. The gel test tubes were centrifuged at 200g for 10 minutes (Jouan C4i Centrifuge; Thermo Fisher Scientific, Waltham, Massachusetts), and the location of the migrated RBCs was recorded. In the absence of agglutination, the RBCs passed through the gel to the bottom, which was scored as “compatible” (negative agglutination), whereas agglutination on the top of or within the gel was considered “incompatible” (positive). Auto‐controls were evaluated for each cat (using its own RBCs and plasma) and found to be all negative (no autoantibodies). For a positive and negative agglutination control test, we used murine monoclonal anti‐A or anti‐B antibodies.
For each XM test, the strength of the agglutination reaction was recorded as follows (Figure 1): 0 (negative), all RBCs were at the bottom of the tube; 1+ (positive), few RBCs' agglutinates were dispersed in the gel, but most of the RBCs were at the bottom of the tube; 2+ (positive), all RBCs' agglutinates were dispersed in the gel; 3+ (positive), some RBCs' agglutinates were dispersed in the upper part of the gel, most of the RBCs form a red line on the surface of the gel; and 4+ (positive), all RBCs were agglutinated and form a red line on the surface of the gel.
Figure 1.
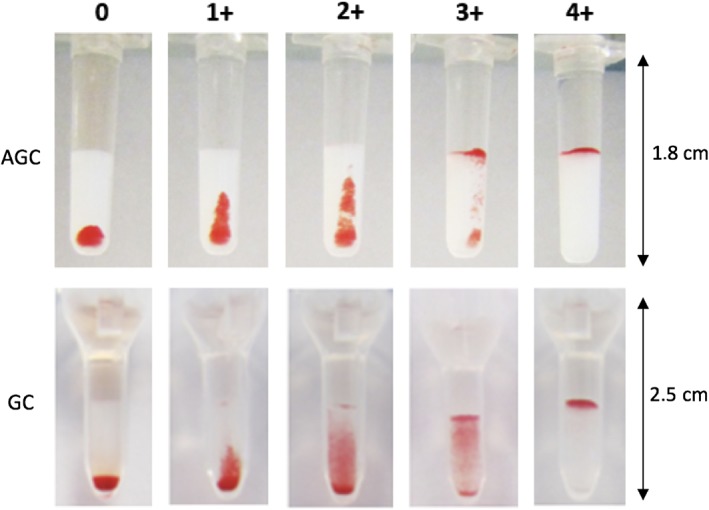
Interpretation of agglutination reaction results of crossmatch tests performed with antiglobulin‐enhanced gel column kit (AGC) crossmatch test and gel column card without antiglobulin (GC). The strength of the agglutination reaction is described as: 0 (negative), all red blood cells (RBCs) are at the bottom of the tube; 1+ (positive), few RBCs' agglutinates are dispersed in the gel, but most of the RBCs are at the bottom of the tube; 2+ (positive), all RBCs' agglutinates are dispersed in the gel; 3+ (positive), some RBCs' agglutinates are dispersed in the upper part of the gel, most of the RBCs form a red line on the surface of the gel; and 4+ (positive), all RBCs are agglutinated and form a red line on the surface of the gel
2.3. Statistical analysis
Descriptive data are presented as median, range, and percentage. The XM test results were compared using Wilcoxon's test and Spearman rho's test. The statistical analyses were performed using a commercially available statistical program (GraphPad Prism 7; GraphPad Software, Inc., La Jolla, California), and a P ≤ .05 was considered significant.
3. RESULTS
3.1. AB typing results
Forty‐nine domestic shorthair cats from Lyon (France) were included in the study over a 6‐month period. There were 28 males and 21 females (Supporting Information Table S1). Their median age was 7 months (range, 6‐51 months), and their median PCV was 46% (range, 38%‐50%). According to both flow cytometry and immunochromatographic strip kit methods for AB typing, 34 (69%) cats had type A, 13 (27%) had type B, and 2 (4%) had type AB blood (CAT48, CAT49) (Supporting Information Table S1). The MFIs for the A and B antigens were clearly above the background (MFI < 10) ranging from 126 to 497 for type A blood, and 15 to 67 for type B blood. One AB cat (CAT48) had a stronger expression of the A (MFI = 35) than B (MFI = 16) antigen. The second AB cat (CAT49) had a weaker expression of the A (MFI = 12) than B (MFI = 19) antigen.
3.2. Crossmatch results
Plasma from 10 of 35 A cats, 9 of 13 B cats, and the 2 AB cats were tested with pRBCs from 24 A cats, 5 B cats, and 1 AB cat. For the two cats, CAT45 (type B) and CAT49 (type AB), we used both plasma and pRBCs. Using both the GC and AGC method, 446 XM tests were performed, and 184 additional XMs were done solely by AGC method (totaling 630 XM tests) depending on the available volume of plasma and/or RBCs from each cat (Table 1). There was a strong correlation (r = 0.938, P < .005) between the GC XM techniques with and without antiglobulin. Compatibilities were detected in 329 of 446 (74%) pairings using both XM methods. Incompatibilities were found in 102 of 446 (23%) pairings using both XM methods. Seventy‐five of those 102 pairings (73%) showed similar strength of agglutination reactions, whereas 27 of these 102 pairings (27%) showed stronger strength reactions using the AGC compared to the GC XM method (Table 2, Figure 2). There was not a single pairing with a negative AGC and a positive GC test result.
Table 1.
Number of crossmatch tests performed with antiglobulin‐enhanced gel column crossmatch test (AGC) and gel column card without antiglobulin crossmatch test (GC)
| Red blood cells | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| A (n = 24) | B a (n = 5) | AB a (n = 1) | Total | ||||||
| AGC | GC | AGC | GC | AGC | GC | AGC | GC | ||
| Plasma | A (n = 10) | 240 | 193 | 50 | 31 | 10 | 4 | 300 | 228 |
| B a (n = 9) | 216 | 124 | 45 | 28 | 9 | 6 | 270 | 158 | |
| AB a (n = 2) | 48 | 48 | 10 | 10 | 2 | 2 | 60 | 60 | |
| Total | 504 | 365 | 105 | 69 | 21 | 12 | 630 | 446 | |
Plasma and RBCs were used from 1 type B (CAT45) and 1 AB cat (CAT49).
Table 2.
Comparison of grading obtained with antiglobulin‐enhanced gel column crossmatch test (AGC) and gel column card without antiglobulin crossmatch test (GC) in 446 plasma‐pRBCs pairings tested with both tests
| AGC | GC | Both | |
|---|---|---|---|
| 0 | 329 | 344 | 329 |
| 1+ | 6 | 7 | 3 |
| 2+ | 10 | 10 | 5 |
| 3+ | 14 | 19 | 1 |
| 4+ | 87 | 66 | 66 |
Figure 2.
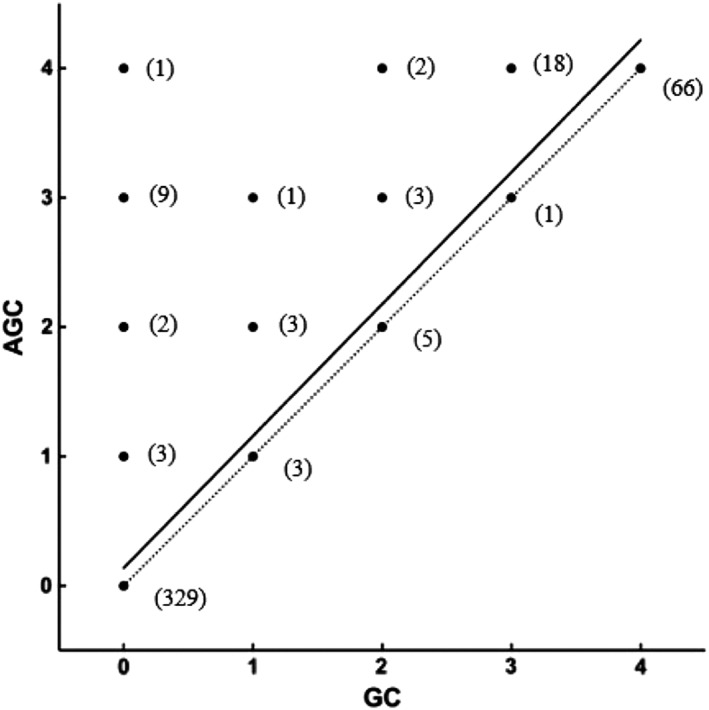
Linear regression (continuous line) of agglutination grading results between 446 antiglobulin‐enhanced gel column crossmatch test (AGC) and gel column card without antiglobulin crossmatch test (GC) pairings. Numbers in bracket represent the number of tested pairings with their respective results with GC (abscises) and AGC (ordinates) methods. Equation of the linear regression is AGC = 1.02 × GC + 0.1372 (P < .0001). R‐square 0.8866
Specifically, all but 1 A‐B mismatches were detected based upon the presence of naturally occurring alloantibodies in type B cats. Plasma from 8 of 9 type B cats strongly agglutinated type A RBCs by both XM methods. Surprisingly, no agglutination reaction was detected between the plasma from 1 B cat (CAT45) and all 24 type A RBCs again by both methods suggesting a lack of anti‐A alloantibodies (Figure 3). This cat was followed 6 months later after the study completion, and blood type B was again confirmed using both flow cytometry and immunochromatography. Further GC and AGC XMs were both compatible between this cat's plasma and pRBCs from 7 type A cats, different from those previously included in this study. Furthermore, the RBCs from 1 AB cat (CAT49) reacted with plasma from 7 B cats but with none of the 10 tested plasma samples from A cats. This cat's typing results showed a stronger type A than type B band and MFI for the A antigen (Supporting Information Table S1).
Figure 3.
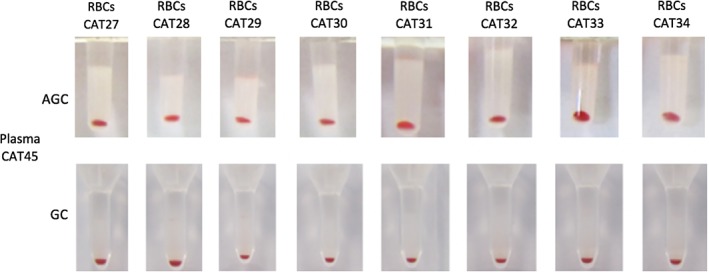
Crossmatch test results using antiglobulin‐enhanced gel tube (AGC) and gel column card (GC) methods. Type B cat plasma CAT45 did not react with 8 type A cat red blood cells (RBCs). All agglutination reactions were negative
There were discordant results between methods with samples involving type A, B, and AB cats, which will be further detailed below. These additional incompatibilities were only detected with AGC XM method in 15 of 446 pairings, whereas the GC XM test results indicated compatibility (Table 2, Figure 2). Plasma from 1 type B cat (CAT36) was incompatible with RBCs from 1 type AB cat (CAT49; with a weaker A vs B expression). Plasma from 1 type A cat (CAT2) was incompatible with RBCs from 8 of the 13 type A cats tested (CAT11, CAT12, CAT13, CAT15, CAT17, CAT18, CAT19, and CAT22) as well as 1 of the 2 type B tested cats (CAT47) (Figure 4). Furthermore, the plasma from 1 type A cat (CAT10) was incompatible with RBCs from 1 of the 24 type A cats (CAT11) and 3 of the 5 type B tested cats (CAT43, CAT45, and CAT47). Neither of these 2 type A cats' plasmas (CAT2 and CAT10) caused agglutination of RBCs from the type AB cat (CAT 49). Moreover, plasma from 1 type AB cat (CAT48) was incompatible with RBCs from 1 type A cat (CAT21).
Figure 4.
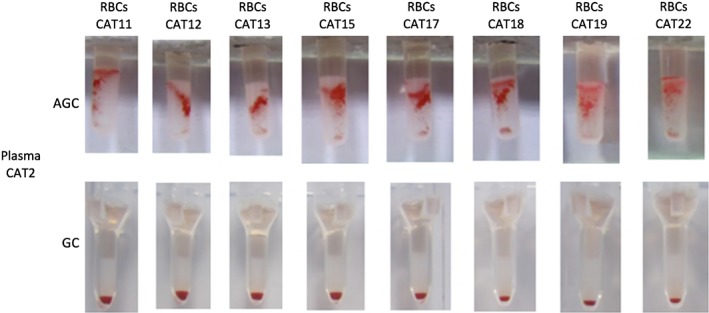
Incompatible antiglobulin‐enhanced gel tube (AGC) and compatible gel column card (GC) crossmatch test results with plasma from one type A cat (CAT2) against red blood cells (RBCs) from 8 type A cats
Among the 184 XM tests exclusively done with the AGC method, 5 were found to be incompatible. Plasma from 1 type A cat (CAT2) was incompatible with RBCs from 4 of 11 tested type A cats (CAT24, CAT26, CAT28, and CAT32). In addition, AGC revealed 1 additional incompatibility between the plasma of CAT2 and RBCs from 1 of 3 tested type B pRBCs cats (CAT47).
Fourteen incompatibilities outside the expected A‐B mismatches were only revealed by AGC representing a prevalence of 2.2% of all AGC XM. Plasma from 1 type AB cat was incompatible with pRBCs from 1 type A pRBCs. Plasma from 2 of the 10 type A cats (CAT2 and CAT10) had incompatibilities with pRBCs from 13 type A cats. The prevalence for having an incompatible AGC XM between plasma from type A cats and pRBCs from type A cats was 5.4%.
4. DISCUSSION
Although extensive AB blood typing surveys have been reported in not previously transfused domestic cats of different breeds and geographic regions, XM test results are scarce.7, 17, 18, 25, 26 Our prospective study shows completely concordant AB blood typing results by flow cytometry and immunochromatographic strip kit. The plasma from type A cats had either no or weak anti‐B alloantibodies. The plasma of type B cats contained strong anti‐A alloantibodies (except CAT45). Both methods showed concordant compatibilities (73%) and incompatibilities (23%) with few discordant results (4%) showing agglutination only by the AGC but not GC method suggesting that the AGC method could be more sensitive than the GC XM test.
In human medicine, antiglobulin‐enhanced XM tests are generally used before the first and any following transfusion events,27 but veterinary transfusion practice is limited by the availability of efficient and reliable in‐clinic XM tests particularly those with an antiglobulin‐phase. In this prospective study, we document that AB typing and sensitive XM tests are available to detect mismatches and incompatibilities in feline clinical medicine. In addition, this study supports the presence of natural occurring alloantibodies outside the AB blood group system and importance of using an antiglobulin‐enhanced gel tube XM test to reveal incompatibilities not detected by usual methods.6, 17, 18
There are several typing kits available for the feline AB blood system in clinical settings including agglutination card, immunochromatographic strip, and cartridge techniques.11, 12, 13 Recent reports compared them with each other and indicated that immunochromatographic strip method showed better performance than agglutination card.11, 12 Furthermore, flow cytometry is a blood typing method used in research laboratories available to settle discordant typing results by other techniques.1 This study compared immunochromatographic strip method to the laboratory flow cytometry technique, both utilizing the same monoclonal antibodies for feline AB typing, giving completely concordant results for all type A (69%), B (27%), and AB (4%) blood samples.
Discordant typing results have been reported previously with various methods and mostly related to the AB blood type. It has been recommended to confirm any AB blood type result by another method and perform also back typing, that is, checking for the presence of alloantibodies. Moreover, molecular genetic approaches could be applied to further define the A, B, and AB blood type, those have been described for type AB for the Ragdoll breed and recently for A‐B blood group system in domestic cats.3, 4 Similarly, it is also recommended to confirm the type B blood with a second method and back typing or XM in clinical practice.5
Before any transfusion, XM incompatibilities6, 18, 19 and transfusion reactions outside the AB system14 have been reported in cats and suggested the existence of naturally occurring alloantibodies against other blood antigens. A novel RBC antigen labeled Mik antigen through crossmatching after transfusion of type A blood to type A recipient cats and acute hemolytic transfusion reactions outside the AB system were reported.6 Furthermore, naturally occurring alloantibodies against the Mik antigen were found, whereas a recent study in the United Kingdom about transfusion‐naive cats revealed no major XM incompatibilities on 112 XMs performed.7 In addition, another recent study does not support the use of the major XM test to increase efficacy of, and to decrease adverse events associated with RBC transfusion in AB blood typed transfusion‐naive cats.26
Those differences could be explained by the lack of XM test sensitivity and method standardization; the XM methods used in those studies did not use antiglobulin‐enhanced assays. Also cats with a different health status and coming from other countries may influence these compatibility test results. In 2014, a retrospective study showed that administering XM compatible RBCs to cats as compared to non‐crossmatched led to a greater increase in the post‐transfusion PCV, but the groups were small and the non‐crossmatched group was considered to be too sick to wait for a XM and thus were at higher risk of dying and were not comparable to the crossmatch group.18 The establishment of a more feasible and reliable XM test, as described in our study, appears of growing importance to prevent acute hemolytic transfusion reactions due to blood type incompatibilities in either naive and previously transfused cats as per XM test results. The clinical importance of the detected alloantibodies outside the AB (and Mik‐ ) system still needs to be determined.
In human transfusion medicine, alloimmunization is a common posttransfusion sequelae and has been also described in dogs21, 28 and recently for cats.5, 17 Recently, development of alloantibodies against erythrocyte antigens outside the AB system has been observed in 25% of an anemic cat population after the first transfusion event.17 It has, therefore, been recommended to introduce crossmatching into routine pretransfusion testing protocols and encouraged the development of in‐clinic feline XM tests.6, 17 Moreover, as in human transfusion medicine, clerical errors may occur and result in life‐threatening acute hemolytic transfusion reactions.5, 8, 14 Thus, a second level of compatibility testing besides A‐B matching can be further assuring.
In veterinary medicine, several XM tests are available. The first methods reported in cats were slide and tube methods, but problems with these techniques included labor‐intensiveness, required technical expertise, rouleaux confused for agglutination, lack of standardization of methods, weakly false‐positive and false‐negative results particularly in anemic cats, and interobserver variation in interpretation. The standard laboratory XM technique (tube) was considered the gold standard method but requires expertise, is time consuming, and its reliability has been shown to be very operator dependent.29 To address these problems in human medicine, the GC method was developed in the late 1980s and is easy to use, requires small blood samples, is more sensitive and easier to grade, thereby helping standardization. This GC technique has also been used in companion animals (dogs, cats, and horses) and shown to be effective (without using antiglobulins).5, 10, 14
Moreover, human studies showed that using XM test with species specific antiglobulin improves the sensitivity of the test for detecting potentially clinically important alloantibodies coating RBCs23, 24 This prospective study in cats compared gel column XM test kits with (AGC) and without (GC) the addition of feline antiglobulin in non‐transfused cats with type A, B, and AB blood samples. Both techniques detected any A‐B incompatibilities as well as other incompatibilities. Furthermore, the novel AGC test kit is simple and rapid to perform and does not require prior RBC washing.
Regarding type A cats, not only our study did confirm the weakness of their anti‐B alloantibodies, but we did not find any A cats' plasma that reacted with all type B RBCs. Moreover, agglutinations between A cats' plasma and type B RBCs were detected only with the AGC XM test. The absence of agglutination reactions could result from a lack of anti‐B alloantibodies in type A cats or a weak expression of the type B antigen on the surface of RBCs or both. Our results confirm that type B cats in this study, which were at least 5 months of age, have naturally occurring alloantibodies as previously shown in larger surveys.8, 9 Surprisingly, in this survey, one adult type B cat had no naturally occurring anti‐A alloantibodies, which has not been reported before. This cat's blood type and lack of anti‐A alloantibodies was confirmed 6 months later. Moreover, additional GC and AGC XMs were compatible between this cat's plasma and 7 type A cats pRBCs. May be this cat could be AB type with some type A antigen of very low expression and not recognized by the typing reagents. The use of not washed pRBCs may have allowed the presence of soluble antigens that could have inhibited the antigen‐antibody agglutination reaction.30 Severe hypogammaglobulinemia (ie, sever IgM deficit) has been reported in human medicine to cause blood typing discrepancy.31 The apparently healthy condition of this type B cat did not support this hypothesis. To authors' knowledge, no further explanations are available.
Finally, XM incompatibilities outside the major AB blood group system were detected only with antiglobulin‐enhanced XM test. Plasma samples from 2 type A cats and 1 type AB cat showed unexpected incompatibilities with other type A RBCs. Human studies reported that using XM test with antiglobulin improves the sensitivity of the test for detecting potentially clinically important antibodies coating RBCs.22, 23, 24 False‐positive reactivity has been described with gel methods and has been partially explained by the low‐ionic wash solution used commercially in acid‐elution kits.24 In this study, we used the same LISS in both GC and AGC. The AGC test showed concordant results with GC test but AGC test detected more A‐B and non‐A‐B system incompatibilities. Moreover, all auto‐controls were negative, which do not support the existence of false‐positive results. Furthermore, performing blood transfusion between XM‐incompatible cats would represent the only way to look for false‐positive results, which was not performed (unethical). These results confirm the presence of naturally occurring alloantibodies not only against type A or B RBCs but also yet to be defined antigens. Although serious transfusion reactions have been reported due to A‐B incompatibilities,8 the clinical importance of these other alloantibodies was not determined. These findings yet confirm the importance of using antiglobulin‐enhanced XM technique to increase the sensitivity in detecting potentially important alloantibodies.
Limitations of our study are the small cohort on animals despite the large number of XM tests, the lack of a definitive reference XM method, and the fact that all laboratory tests were performed by two well‐trained medical technologists. Although they did not know of the immunochromatographic strip typing results when performing the flow cytometric evaluations, they selected the pairings based upon the typing results and were aware of the blood types in each pairing.
In conclusion, feline AB typing with the immunochromatographic strip kit is as accurate as the laboratory flow cytometry. The 2 XM test results showed good agreement, but using an antiglobulin‐enhanced XM kit revealed additional incompatibilities outside the AB blood group system. The associated RBCs antigens and clinical importance of these other naturally occurring alloantibodies remain to be determined. Based upon this prospective study as well as prior publications on naturally occurring alloantibodies in type B and to a lesser degree in type A cats as well as potentially naturally occurring anti‐Mik and other alloantibodies, it is recommended to not only type cats for AB compatibility but also to XM cats before a first transfusion.
CONFLICT OF INTEREST DECLARATION
M. Guidetti, S. Bourgeois and B. Chaprier were employed, and I. Goy‐Thollot and U. Giger have been scientific advisors to Dianov. Reagents were received for these studies from Alvedia which is commercially offering typing and crossmatch kits. However, the design and execution of the study, data analysis, and writing of the manuscript have been done independently. Urs Giger is the director of PennGen at the University of Pennsylvania which is a not‐for‐profit laboratory offering blood typing and compatibility testing and is supported compatibility by the National Institutes of Health (OD 010939).
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
This prospective study was approved by the Ethical Committee of VetAgro Sup (#1622), and written owner consent was obtained before enrollment of any cat into the study.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Table S1: Cat population of the study with article number, sex, age, group and packed cell volume for each cat. These 49 cats were all domestic shorthair seronegative for FIV and FeLV. DSH = Domestic shorthair
ACKNOWLEDGMENT
The authors thank the nursing staff in the Intensive Care Unit at VetAgro Sup for their assistance with blood sampling and typing and Dr Maxime Cambournac for statistical analysis. Presented in part at the International Veterinary Emergency and Critical Care Symposium, Nashville, Tennessee, September 2017. This work was performed in the Intensive Care Unit of VetAgro Sup campus vétérinaire de Lyon, Marcy l'Etoile, France and Dianov Laboratories, Limonest, France.
Goy‐Thollot I, Nectoux A, Guidetti M, et al. Detection of naturally occurring alloantibody by an in‐clinic antiglobulin‐enhanced and standard crossmatch gel column test in non‐transfused domestic shorthair cats. J Vet Intern Med. 2019;33:588–595. 10.1111/jvim.15381
REFERENCES
- 1. Griot‐Wenk ME, Callan MB, Casal ML, et al. Blood type AB in the feline AB blood group system. Am J Vet Res. 1996;57:1438‐1442. [PubMed] [Google Scholar]
- 2. Malik R, Griffin DL, White JD, et al. The prevalence of feline A/B blood types in the Sydney region. Aust Vet J. 2005;83:38‐44. [DOI] [PubMed] [Google Scholar]
- 3. Kehl A, Heimberger K, Langbein‐Detsch I, et al. Molecular characterization of blood type A, B, and C (AB) in domestic cats and a CMAH genotyping scheme. PLoS ONE. 2018;13:e0204287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gandolfi B, Grahn RA, Gustafson NA, et al. A novel variant in CMHA is associated with blood type AB in ragdoll cats. PLoS One. 2016;11:e0154973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Giger U. Blood typing and crossmatching to ensure blood compatibility In: Bonagura JD, Twedt DC, eds. Current Veterinary Therapy. St. Louis: Missouri; 2014:e143. [Google Scholar]
- 6. Weinstein NM, Blais MC, Harris K, Oakley DA, Aronson LR, Giger U. A newly recognized blood group in domestic shorthair cats: the Mik red cell antigen. J Vet Intern Med. 2007;21:287‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tasker S, Barker EN, Day MJ, Helps CR. Feline blood genotyping versus phenotyping, and detection of non‐AB blood type incompatibilities in UKcats. J Small Anim Pract. 2014;55:185‐189. [DOI] [PubMed] [Google Scholar]
- 8. Giger U, Akol KC. Acute hemolytic transfusion reaction in an abyssinian cat with blood type B. J Vet Intern Med. 1990;4:315‐316. [DOI] [PubMed] [Google Scholar]
- 9. Bücheler J, Giger U. Alloantibodies against A and B blood types in cats. Vet Immunol Immunopathol. 1993;38:283‐295. [DOI] [PubMed] [Google Scholar]
- 10. Abrams‐Ogg ACG. Feline recipient screening In: Yagi K, Holowaychuk M, eds. Manual of Veterinary Transfusion Medicine and Blood Banking. Ames, Iowa: John Wiley & Sons Inc; 2016:322‐375. [Google Scholar]
- 11. Seth M, Jackson KV, Giger U. Comparison of five blood‐typing methods for the feline AB blood group system. Am J Vet Res. 2011;72:203‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spada E, Proverbio D, Baggiani L, Bagnagatti de Giorgi G, Perego R, Ferro E. Evaluation of an immunochromatographic test for feline AB system blood typing. J Vet Emerg Crit Care. 2016;26:137‐141. [DOI] [PubMed] [Google Scholar]
- 13. Hourani L, Weingart C, Kohn B. Evaluation of a novel feline AB blood typing device. J Feline Med Surg. 2014;16:826‐831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Euler CC, Raj K, Mizukami K, et al. Xenotransfusion of anemic cats with blood compatibility issues: pre‐ and posttransfusion laboratory diagnostic and crossmatching studies. Vet Clin Pathol. 2016;45:244‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ron L, Bruchim Y, Klainbart S, Kelmer E. Ultrasound‐guided intracardiac xenotransfusion of canine packed red blood cells and epinephrine to the left ventricule of a severely anemic cat during cardiopulmonary resuscitation. J Vet Emerg Crit Care. 2017;27:218‐223. [DOI] [PubMed] [Google Scholar]
- 16. Priolo V, Masucci M, Spada E, et al. Naturally occuring antibodies in cats against dog erythrocyte antigens and vice versa. J Feline Med Surg. 2018;20:690–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hourani L, Weingart C, Kohn B. Alloimmunisation in transfused patients: serial cross‐matching in a population of hospitalised cats. J Feline Med Surg. 2017;19:1231‐1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weltman JG, Fletcher DJ, Rogers C. Influence of cross‐match on posttransfusion packed cell volume in feline packed red blood cell transfusion. J Vet Emerg Crit Care. 2014;24:429‐436. [DOI] [PubMed] [Google Scholar]
- 19. Specht AJ, Hepinstall RA, Crawford PC. Incompatible crossmatch results related to factors other than A, B, and AB blood types in cats. J Vet Emerg Crit Care. 2017;S10. [Google Scholar]
- 20. Acierno M, Rai K, Giger U. DEA 1 expression on dog erythrocytes analyzed immunochromatographic and flow cytometrc techniques. J Vet Intern Med. 2014;28:592‐598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goy‐Thollot I, Giger U, Boisvineau C, et al. Pre‐ and post‐transfusion alloimmunisation in dogs characterized by two antiglobulin‐enhanced crossmatch tests. J Vet Intern Med. 2017;31:1420‐1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lapierre Y, Rigal D, Adam J, et al. The gel test: a new way to detect red cell antigen‐antibody reactions. Transfusion. 1990;30:109‐113. [DOI] [PubMed] [Google Scholar]
- 23. Novaretti MC, Silveira EJ, Filho EC, Dorlhiac‐Llacer PE, Chamone DA. Comparison of tube and gel techniques for antibody identification. Immunohematology. 2000;16:138‐141. [PubMed] [Google Scholar]
- 24. Novaretti MC, Jens E, Pagliarini T, et al. Comparison of conventional tube test technique and gel microcolumn assay for direct antiglobulin test: a large study. J Clin Lab Anal. 2004;18:255‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tocci LJ, Ewing PJ. Increasing patient safety in veterinary transfusion medicine: an overview of pretransfusion testing. J Vet Emerg Crit Care. 2009;19:66‐73. [DOI] [PubMed] [Google Scholar]
- 26. Sylvane B, Prittie J, Hohenhaus AE, Tozier E. Effect of cross‐match on packed cell volume after transfusion of packed red blood cells in transfusion‐naïve anemic cats. J Vet Intern Med. 2018;00:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lamba DS, Mittal K, Sood T, Bedi RK, Kaur P, Kaur G. Antibody screening in multitransfused patients: a prerequisite before each transfusion. Transfus Apher Sci. 2014;51:132‐133. [DOI] [PubMed] [Google Scholar]
- 28. Callan MB, Jones LT, Giger U. Hemolytic transfusion reactions in a dog with an alloantibody to a common antigen. J Vet Intern Med. 1995;9:277‐279. [DOI] [PubMed] [Google Scholar]
- 29. Davidow B. Transfusion medicine in small animals. Vet Clin N Am Small. 2013;43:735‐756. [DOI] [PubMed] [Google Scholar]
- 30. Vos GM, Moores PP. Hemagglutination inhibition studies of water soluble blood group substance recovered from the erythrocytes of classical Bombay Oh subjects. Transfusion. 1976;16:421‐426. [DOI] [PubMed] [Google Scholar]
- 31. Jung CL, Cha MK, Jun BH, Hong KS. A case of IgM deficiency with B cell deficiency detected by ABO discrepancy in a patient with acute osteomyelitis. Ann Lab Med. 2013;33:208‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Cat population of the study with article number, sex, age, group and packed cell volume for each cat. These 49 cats were all domestic shorthair seronegative for FIV and FeLV. DSH = Domestic shorthair


