Abstract
Background
Focal seizures with fear as a primary ictal manifestation, their diagnostic challenges, and impact on quality of life are well described in human medicine. Reports focusing on ictal fear‐like behavior in animals are scarce.
Objective
To describe the clinical and histopathological characteristics of a novel focal epilepsy in Boerboel dogs.
Animals
Five client‐owned Boerboel littermates presented for evaluation of sudden episodes of severe fear‐related behavior.
Methods
Clinical examination, complete blood cell count, routine blood biochemistry, and urinalysis were performed in all dogs. Magnetic resonance imaging (MRI) scans of the brain were performed in 3 affected Boerboels. In addition, in 2 affected Boerboels, metabolic screening, cerebrospinal fluid (CSF) analysis, and necropsy were performed.
Results
Onset of signs was 3 months of age in all affected Boerboels. All Boerboels howled loudly, had an extremely fearful facial expression and trembled during seizures. All affected Boerboels also had autonomic or motor signs. Results of laboratory investigations, diagnostic imaging, and metabolic screening were generally unremarkable. Histopathology showed moderate numbers of single large vacuoles in the perikaryon of neurons throughout the brain, specifically in the deeper cerebral cortical regions. Family history, pedigree analysis, and the homogenous phenotype were suggestive of autosomal recessive inheritance.
Conclusions and Clinical Importance
The observed paroxysmal fear‐related behavior represents a newly recognized hereditary focal epilepsy in dogs with distinctive clinical and histopathologic features. Veterinarians should be aware that sudden episodes of unusual behavior can represent focal epilepsy.
Keywords: anxiety, canine, neuronal vacuoles, seizures
Abbreviations
- CSF
cerebrospinal fluid
- EEG
electroencephalography
- H&E
hematoxylin and eosin
- MRI
magnetic resonance imaging
- T1w‐SE
T1‐weighted spin echo
- T2w‐TSE
T2‐weighted turbo spin echo
- TSE
transmissible spongiform encephalopathy
1. INTRODUCTION
In focal epilepsy, abnormal excessive neuronal discharges in specific regions of the brain can result in episodic behavioral signs such as anxiety or unexplainable fear. These signs can occur in isolation or evolve to focal seizures with impaired awareness or to generalized seizures.1, 2 Up to 15% of human patients with focal seizures experience fear during an aura.3, 4, 5 The association between anxiety and epilepsy has increasingly become a point of focus in human epilepsy research.1, 4, 5, 6, 7, 8, 9
Focal onset epilepsy evolving into generalized seizures is the most common type of epilepsy observed in dogs.2 A strong resemblance between seizure semiology in people and dogs with focal epilepsy has been reported.2, 10 Paroxysms of sudden, stereotypical behavior occur in 80% of dogs with focal epilepsy whether or not these signs evolve into generalized seizures and often are interpreted by owners as fear or anxiety.2
Publications on seizure‐related fear or anxiety are scarce in veterinary medicine, but a considerable increase in interictal fear and anxiety‐related behavior has been reported after onset of idiopathic epilepsy in dogs.11 Paroxysmal behavioral abnormalities, including extreme irrational fear, have been observed in Bull Terriers with focal seizures with impaired awareness.12
Here, we report a newly recognized juvenile‐onset focal epilepsy in Boerboel dogs with ictal fear‐related behavior as the most prominent sign. The aim of our study was to characterize the clinicopathological, diagnostic, and histopathological features of the disorder and to determine if this unique condition has a hereditary component.
2. MATERIALS AND METHODS
2.1. Dogs and clinical studies
Five Boerboel littermates (2 intact females and 3 intact males) were presented to the Neurology Division of the Utrecht University Clinic for Companion Animals, the Netherlands, for evaluation of acute episodes of abnormal behavior, referred to by the owners as “panic attacks.” The dogs came from a single large litter of 19 Boerboel pups (including 3 stillborn) that were born after an uncomplicated pregnancy. At the time of 1st presentation, the ages of the dogs were 4 months, 4 months, 5 months, 5 months, and 3 years. A comprehensive history was taken, and all dogs underwent general physical and neurological examination, blood testing (CBC and biochemical profile including urea, creatinine, alkaline phosphatase, alanine aminotransferase, fasting bile acids, ammonia, total protein, albumin, glucose, potassium, sodium, and calcium), and urinalysis.
Cerebrospinal fluid (CSF) was collected by cisternal puncture in 2 affected Boerboels. The CSF was analyzed for total protein concentration, nucleated cell count, and red blood cell count. In addition, cytological examination was performed.
Physical examination, blood testing, and urinalysis were repeated in one of the female Boerboels after 1 year. Another female and 1 male littermate were closely monitored from the time of the 1st visit until they were euthanized and submitted for pathological examination (at ages 3 years and 10 years, respectively) with clinical re‐evaluations at 1, 2, and 4 months after the initial visit, and physical examinations and laboratory blood testing at least once a year in the dog that lived for 10 years.
2.2. Diagnostic imaging
Magnetic resonance imaging (MRI) of the brain was performed with the animal in sternal recumbency in 3 of the affected Boerboel littermates, aged 5 months, 1 year, and 3 years, respectively, using a 0.2‐Tesla open magnet (Magnetom Open Viva, Siemens N.V., The Hague, The Netherlands). The protocol included transverse T2‐weighted Turbo Spin Echo (T2w‐TSE) and T1‐weighted Spin Echo (T1w‐SE) sequences. After IV administration of 0.1 mmol/kg contrast medium (Dotarem (0.5 mmol/mL gadoterate meglumiane; Guerbet, Roissy CdG Cedex, France), transverse T1w‐SE, T1‐weighted spoiled Gradient Echo, and fluid attenuation inversion recovery sequences also were obtained in a dorsal or transverse plane or both. All of the MRI studies were evaluated by a board‐certified veterinary radiologist.
2.3. Metabolic research
Urine samples and heparinized plasma from 2 affected Boerboels and 7 healthy control dogs of different breeds, including 1 Boerboel, were collected. In addition, CSF samples from 1 affected Boerboel and 1 control dog also were used for the metabolic studies. The study was approved by the Utrecht University Animal Experiments Committee as required under Dutch legislation (Experiments on Animals Act Wod. 2014, European Directive 2010/63/EU). All samples were immediately stored at −20°C until analysis at the Department of Genetics of the University Medical Centre Utrecht, the Netherlands.
Amino acid concentrations were determined by ion‐exchange chromatography with post‐column ninhydrin derivatization on a 30‐amino acid analyzer (Biochrom 30; Biochrom Ltd, Cambridge, UK) for all urine, plasma, and CSF samples. In addition, organic acids, purines, and pyrimidines were measured in the urine samples. Organic acids were analyzed by an in‐house method as oxime‐trimethylsilyl derivatives using gas chromatography–mass spectrometry (Supporting Information Supplemental File 1). Purines and pyrimidines were analyzed by ultra‐performance liquid chromatography–tandem mass spectrometry by an in‐house method (Supporting Information Supplemental File 2). In all urine samples, creatinine was quantified colorimetrically, and results of the aforementioned urinary analyses were related to the urinary creatinine concentration. All analytical methods currently are in use as diagnostic platforms and were validated according to the guidelines of ISO15189.
Urinary creatinine measurement was repeated for one of the affected dogs 2 months after initial measurement, because a spurious result had been found at that time. The results of the metabolic analyses of the affected dogs were compared to those of the control dogs.
2.4. Pathological examination
Two of the Boerboel dogs that were previously examined at the Utrecht University Clinic were available for necropsy immediately after death. The 1st dog was euthanized at 3 years of age because of refractory seizures. The 2nd dog was euthanized for reasons unrelated to the epilepsy at the age of 10 years. A comprehensive necropsy was performed, and brain tissue was fixed by immersion in 10% neutral‐buffered formalin for histopathological evaluation. After fixation, representative sections of the frontal, parietal, temporal, and occipital lobes, as well as the hippocampus region, brainstem, medulla oblongata, and cerebellum were trimmed and paraffin embedded. Four‐micrometer tissue sections were routinely stained with hematoxylin and eosin (H&E) and cresyl violet (Nissl stain) to visualize Nissl substance in the neurons. In addition, small unfixed tissue samples from different regions of the brain were snap frozen by immersion in a container with isopentane that was chilled by placing it in a Dewar flask with liquid nitrogen. The samples were stored at −70°C until examination and subsequently routinely processed to frozen tissue sections that were stained with Oil‐Red‐O for visualization of lipids.
2.5. Pedigree analysis
The breeder reported that typical “panic attacks” also had been observed in 3 other littermates and in several litters from the same breeding line. These dogs were not available for clinical research because they had been euthanized because of the seizures or owners either declined to participate or could not be located. To identify a possible mode of inheritance, pedigree data of the examined littermates, both parents and the suspected cases were combined and analyzed.
3. RESULTS
3.1. Clinical description
The episodes of abnormal behavior started at 3 months of age in all 5 affected dogs. They were consistently characterized by a facial expression interpreted by the owners as reflecting extreme fear (Supporting Information Supporting Video 1), sudden loud vocalizations (eg, crying and “howling like a wolf”, Supporting Information Supporting Video 2) and generalized trembling in all dogs. Other behavioral signs, occurring sometimes during a seizure, were trying to hide (4/5), cowering (3/5), and freezing (2/5). All dogs also exhibited at least 1 of the following ictal autonomic signs: salivating or foaming from the mouth (3/5, Supporting Information Supporting Videos 1 and 3), urinary incontinence (2/5), and fecal incontinence (2/5). In addition, ataxia (3/5), jerking of limb muscles (2/5, Supporting Information Supporting Video 3), lateralized facial muscles spasms (2/5, Supporting Information Supporting Video 3), dystonia of limbs (1/5), and falling over (1/5) were reported. Most dogs (4/5) did not respond when the owner called them during an episode.
All owners reported a mean seizure frequency of 1 or 2 per week, but seizure frequency varied over time in individual dogs. Clusters of seizures (>1 in 24 hours) were observed in 2 Boerboels. The median duration of seizures was 1 minute (range, 20 seconds to 10 minutes).
All owners reported predisposing factors: all examined Boerboels were prone to having seizures when waking up, and 2 of them during excitement or if startled.
Four Boerboels were treated with phenobarbital (starting dosage 2‐2.5 mg/kg PO q12h), and subsequently seizure frequency decreased by >50% in the 4 months after starting the treatment, the duration of seizures shortened, and clinical signs became milder in all treated dogs. However, the efficacy of treatment diminished over time, and dosage had to be increased based on clinical signs and phenobarbital blood concentrations in all treated dogs. Eventually, potassium bromide was added in 3 of the 4 treated dogs because of inadequate seizure control despite blood phenobarbital concentrations approaching 35 mg/L, and combination treatment resulted in a decrease in the seizure frequency in all 3 dogs. Nevertheless, seizure frequencies waxed and waned over the course of time.
According to the breeder, the onset of “panic attacks” in the 3 aforementioned allegedly affected littermates, and other suspected cases developed at approximately 3‐4 months of age. All dogs suspected to be affected howled loudly, trembled, and had facial expressions interpreted as reflecting extreme fear during the episodes.
3.2. Clinical examinations and diagnostic imaging
General physical and neurological examinations were unremarkable in all examined dogs, both at the initial consultation and at follow‐up consultations. The results of CBC and blood biochemistry analysis were within age‐adjusted reference intervals at the time of the 1st and 2nd visits except for some small inconsistent fluctuations. Alkaline phosphatase activity was slightly increased (129 U/L) in 1 Boerboel already receiving phenobarbital, an antiepileptic drug well known for increasing alkaline phosphatase activity.13 Serum alkaline phosphatase and alanine aminotransferase activities increased substantially over time (up to 1215 and 240 U/L, respectively) in the Boerboel that received phenobarbital for >9 years. However, fasting bile acid and ammonia concentrations were within reference ranges in all dogs. Analysis of the CSF of 2 affected Boerboels was unremarkable.
Magnetic resonance imaging of the brain of 3 affected Boerboels did not yield abnormal findings.
3.3. Metabolic studies
The creatine/creatinine ratio in the urine of 1 affected Boerboel (1015 mmol/mol) was very high compared to the ratios in the control dogs (mean, 21 ± 25; range, 6‐77 mmol/mol). However, the ratios in a 2nd urine sample from the same dog, collected 2 months later, and another affected Boerboel were within the previously mentioned range of the control dogs. Results of the other metabolic analyses were unremarkable.
3.4. Pathological examination
Postmortem examination and histopathological findings of the brain were largely similar in the 2 affected Boerboels aged 3 and 10 years at the time of necropsy. No obvious abnormalities were identified on gross examination of the central nervous system. Microscopic evaluation of the brains identified a variable number of single large, optically empty, vacuoles in neurons throughout the whole cerebrum, but predominantly in the deeper cortical regions of the parietal, temporal, and occipital lobes (Figure 1A and C). The vacuoles were present in the perikaryon of individual or groups of neurons and varied in size, regularly compressing and displacing the nucleus to the periphery of the cell body (Figure 1B,D). Nissl staining identified the presence of Nissl substance in the cytoplasm of the cells containing the vacuoles and thus confirmed that they were neurons. However, because of the large size of the vacuoles, Nissl substance was present only in the small rim of cytoplasm surrounding the vacuoles (Supporting Information Supplemental Figure 1). The vacuolar changes were not associated with morphological changes in the surrounding neuropil. Neuronal vacuolation was not seen in the amygdala, mesial temporal lobe, or interthalamic adhesion. Histopathology of the cerebellum and brainstem was unremarkable. Unfortunately, the vacuoles in the cerebral cortex were not visible in the frozen tissue sections stained with Oil‐Red‐Oil. The pathological examination was performed by a board‐certified veterinary pathologist.
Figure 1.
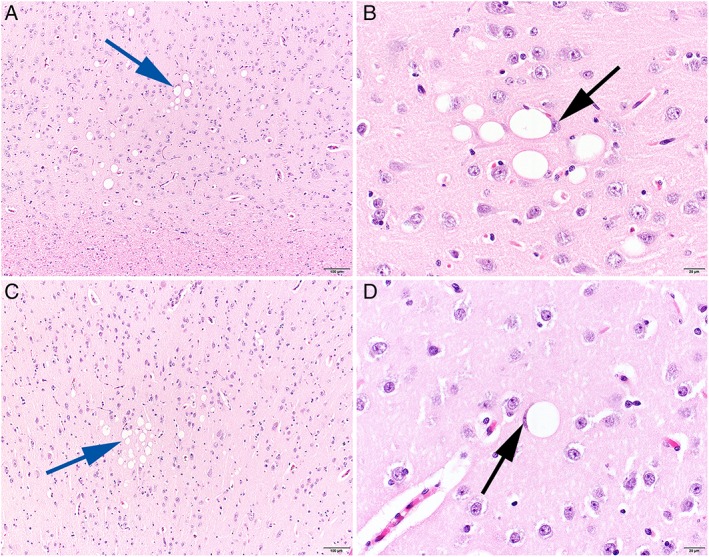
Histopathological changes in the cerebral cortex of a 3‐year‐old (A and B) and a 10‐year‐old (C and D) Boerboel with focal epilepsy. Individual and clusters of neurons reveal single, variably sized optically empty vacuoles in the perikaryon (blue arrows). Regularly compression and displacement of the nucleus are visible (black arrows). H&E stain, size bar 100 μm (A and C) and 20 μm (B and D)
3.5. Pedigree analysis
Pedigree analysis disclosed short inbreeding loops among the parents of 3 litters with affected individuals (Figure 2). Both males and females were affected. The parents of all patients were clinically normal and shared a common ancestor.
Figure 2.
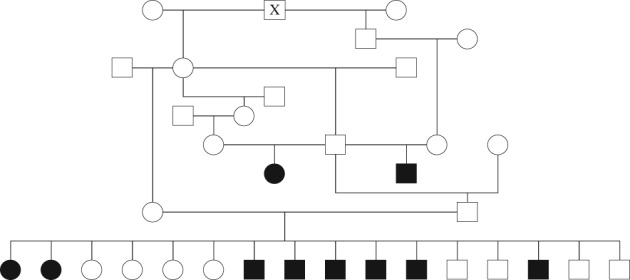
Pedigree showing the relationship among affected Boerboels. Black symbols indicate affected and unfilled symbols indicate unaffected dogs. Squares represent male and circles represent female dogs. Stillborn puppies and dogs with unknown phenotype are not displayed. All parents of affected dogs share a common ancestor (X)
4. DISCUSSION
Our study is the 1st report of familial juvenile onset epilepsy in dogs with fear‐related behavior as the main ictal feature and unique histopathological findings, characterized by neuronal vacuolation in the cerebral cortex.
Fear and anxiety can be related to epilepsy in different ways: (1) in the pre‐ictal phase, possibly as a result of a decrease in synchronization of neurons, (2) as an ictal sign of a focal seizure, (3) in the post‐ictal phase because of transitory seizure‐induced modifications in neuronal function or (4) as an interictal sign caused by the same underlying neuropathology as the seizures.6, 8, 9, 14 High prevalences of comorbid anxiety disorders, often with profound impact on the quality of life, frequently have been documented in human patients with epilepsy.9, 15, 16, 17, 18, 19 Increased anxiety‐related behavior after onset of epilepsy also has been described in dogs.11
Ictal fear is the most common psychic clinical sign in people with focal epilepsy.5, 6 It can be extremely difficult to distinguish ictal fear from a primary anxiety disorder, and numerous studies report focal seizures being misdiagnosed as panic attacks, resulting in incorrect or delayed treatment, sometimes with devastating consequences for the patients.6, 8, 20, 21, 22, 23, 24 In 1 study, it was postulated that in dogs, focal ictal signs, other than motor signs, probably often are not recognized as epileptic seizures.2
Definitive confirmation that fear is an ictal sign requires a patient to express fear during ictal video electroencephalography (EEG), provided that a sufficient amount of brain tissue is involved in seizure activity to evoke epileptiform EEG abnormalities.5 However, only a small percentage of focal aware seizures have an EEG correlate; even in human medicine, efforts to confirm ictal fear by EEG often are not successful.5, 25, 26 Therefore, in clinical practice, the differentiation between ictal fear and anxiety disorders typicallly is based on systematic and detailed evaluation of the history and clinical signs. A limitation of our study is that we were not able to make EEG recordings in the affected Boerboels. Still, the stereotypical semiology along with autonomic signs (eg, salivating) in all affected dogs and motor signs (eg, facial automatisms, jerking of limb muscles) in most affected dogs strongly suggest that the sudden episodes of abnormal behavior represent focal epilepsy.20 In humans, paroxysmal salivation is pathognomonic for focal seizures.8 Moreover, the beneficial effect of anticonvulsant drugs in all treated dogs is a strong indication of epilepsy. However, because seizure frequency waxed and waned over time in most affected dogs, it cannot definitely be excluded that the observed decrease in the numbers of seizures was caused by the natural course of the disease rather than a treatment effect.
All owners of the affected Boerboels interpreted the paroxysmal behavioral changes as intense fear. Evidently, we cannot objectively determine what dogs experience during seizures and therefore can only assume that the Boerboels in our study were indeed fearful. However, in analogy to people with epilepsy, it is conceivable that the observed signs reflect severe fear. In animals, distinguishing psychic ictal signs from psychological or behavioral disorders is likely even more challenging than in people, and misdiagnosis may result in incorrect treatment or even euthanasia. Veterinarians and veterinary behavior specialists therefore should be aware that sudden episodes of abnormal behavior or paroxysmal behavior atypical for an individual animal can be manifestations of focal epilepsy.
Metabolic screening tests for known inborn errors of metabolism did not detect consistent abnormalities in the Boerboels. However, these negative results do not exclude the possibility of an underlying metabolic storage disease. The single high creatine/creatinine ratio in 1 affected Boerboel most likely is the result of transient muscle breakdown, possibly as a result of a seizure.
Consistent with the MRI findings, gross pathology was not abnormal. However, histopathology identified the presence of solitary large vacuoles in the perikaryon of a variable number of neurons throughout the brain, specifically in the deeper cortical regions of the cerebrum of the affected Boerboels. A small number of large isolated neuronal vacuoles occasionally have been observed as incidental findings in specific brain regions of apparently healthy aged cattle and sheep but not in dogs.27 Numerous single or small clusters of large neuronal vacuoles in brain tissue are a distinctive histologic feature of transmissible spongiform encephalopathies (TSE) such as scrapie, bovine spongiform encephalopathy, and Creutzfeldt‐Jakob disease. However, canids appear to be resistant to prion diseases, and no cases of TSE in dogs have been published.28 Large cytoplasmic vacuoles in neurons have sporadically been described in non‐TSE conditions in animals, including rabies29, 30, 31 and disorders of unknown etiology.32, 33, 34 They often are associated with progressive neurological disease and a poor prognosis.
Few instances of vacuoles in the perikaryon have been described in dogs. Mutations in the Warburg syndrome gene RAB3GAP1 cause intracytoplasmic neuronal vacuolation characterized by large vacuoles, a few in number per cell, predominantly involving cerebellar roof nuclei, selected brainstem nuclei, thalamus, and spinal cord in Alaskan Huskies, Black Russian Terriers, and Rottweilers. Prominent clinical features include severe progressive ataxia, paresis, ocular anomalies, and laryngeal paralysis.35, 36, 37, 38, 39 A neurodegenerative storage disease characterized by progressive cerebellar ataxia and profound vacuolization of neuronal cytoplasm affecting both the peripheral and central nervous system (especially the cerebellar cortex) has been described in Lagotto Romagnolo dogs. The disease is associated with a missense variant in the autophagy‐related ATG4D gene.40 Lastly, abundant small neuronal cytoplasmic vacuoles have been reported in animals with viral infections, intoxications, and lysosomal storage diseases such as fucosidosis and GM1‐gangliosidosis.27, 41, 42 However, to the best of our knowledge, histopathologic lesions of the nature and distribution as described in the affected Boerboels have not been reported previously in dogs.
Because several litters were affected and the described psychomotor seizures occurred only in Boerboels, it is likely that the observed histological abnormalities and clinical features were caused by a genetic metabolic disorder rather than an infectious agent or toxin. All 5 parents of affected dogs from the 3 litters are closely related and share an ancestor within 3 generations. These findings in combination with the homogenous clinical features are consistent with an autosomal monogenetic recessive mode of inheritance, although the fraction of affected dogs in the litter of the examined cases is much larger than would be expected. A dominant monogenetic mode of inheritance is less likely because all of the parents were clinically normal. However, an autosomal dominant condition with incomplete penetrance cannot be excluded.
Genetic studies such as genome‐wide association studies or whole‐genome sequencing may help identify a causative metabolic defect. Furthermore, characterization of the contents of the vacuoles might aid in understanding the nature of the neuropathologic changes. Failure to visualize the vacuoles in the frozen tissue sections stained with Oil‐Red‐Oil is probably the result of the relatively small size of the frozen samples and the relatively low number of affected neurons. Unfortunately, no non‐paraffinized formalin‐fixed tissue was available for further evaluation of the vacuoles.
Ictal fear has typically been associated with the involvement of the mesial temporal lobe, particularly the amygdala.8, 20, 22, 43 The role of this region in evoking fear‐related behavior has been confirmed by intracerebral electrical stimulation studies.44 In addition, ictal fear has been reported in seizures originating from other brain areas such as the frontal, parietal, and occipital lobes.5, 7, 8, 45, 46 Most likely, this finding is the result of early spread of epileptic discharges from the epileptogenic onset area to a symptomatogenic zone in the amygdala.43, 46 The localization of the histological abnormalities in the brain of the examined Boerboels (temporal, occipital, and parietal lobes) corresponds to the aforementioned regions related to ictal fear in humans.
Although the pathological pathways have not yet been elucidated, we propose to classify this familial epilepsy in Boerboels under structural epilepsy, rather than under idiopathic epilepsy,47 because histopathological examination identified structural changes in the brain.
In conclusion, we describe a newly recognized hereditary juvenile onset epilepsy in dogs with distinctive clinical and histopathologic characteristics. Additional studies are required to identify the genetic basis of this disorder and further elucidate its etiology.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The study was approved by the Utrecht University Animal Experiments Committee as required under Dutch legislation (Experiments on Animals Act Wod. 2014, European Directive 2010/63/EU).
Supporting information
Appendix s1: Supplementary Material
Appendix s2: Supplementary Material
Supplemental Figure 1 Staining of the Nissl substance (rough endoplasmic reticulum) in the cytoplasm of neurons in the cerebral cortex of a 6‐year‐old Boerboel with focal epilepsy. Nissl stain, Obj 80.
Supporting Video 1 (https://vimeo.com/254271770/c17623497f). A Boerboel displaying signs of ictal fear and foaming at the mouth.
Supporting Video 2 (https://vimeo.com/254271713/e1a422622f). The same Boerboel as in Video 1, howling at the end of a focal seizure.
Supporting Video 3 (https://vimeo.com/254271707/828e65292f). A Boerboel (the same dog as in Videos 1 and 2) with lateralized facial muscles spasms, jerking of limb muscles and foaming at the mouth during a focal seizure.
ACKNOWLEDGMENTS
We are grateful to breeders, owners, and referring veterinarians who contributed to the research. We thank Dr Ellen Meyer and Tammy Belle for each adopting an affected Boerboel. Dr Hans Kooistra and Prof Jan Willem Hesselink are appreciatively acknowledged for critical appraisal of our article. We also thank Dr Valerie Jonckheer for editing the manuscript. The work was performed at Utrecht University, Utrecht, The Netherlands. Part of the paper was presented at the ESVN‐ECVN Symposium 2012.
Stassen QEM, Grinwis GCM, van Rhijn NC, Beukers M, Verhoeven‐Duif NM, Leegwater PAJ. Focal epilepsy with fear‐related behavior as primary presentation in Boerboel dogs. J Vet Intern Med. 2019;33:694–700. 10.1111/jvim.15346
REFERENCES
- 1. Kasper BS, Kasper EM, Pauli E, Stefan H. Phenomenology of hallucinations, illusions, and delusions as part of seizure semiology. Epilepsy Behav. 2010;18:13‐23. [DOI] [PubMed] [Google Scholar]
- 2. Berendt M, Gredal H, Alving J. Characteristics and phenomenology of epileptic partial seizures in dogs: similarities with human seizure semiology. Epilepsy Res. 2004;61:167‐173. [DOI] [PubMed] [Google Scholar]
- 3. Torta R, Keller R. Behavioral, psychotic, and anxiety disorders in epilepsy: etiology, clinical features, and therapeutic implications. Epilepsia. 1999;40(Suppl 10):S2‐S20. [DOI] [PubMed] [Google Scholar]
- 4. Chiesa V, Gardella E, Tassi L, et al. Age‐related gender differences in reporting ictal fear: analysis of case histories and review of the literature. Epilepsia. 2007;48:2361‐2364. [DOI] [PubMed] [Google Scholar]
- 5. Chong DJ, Dugan P, EPGP Investigators . Ictal fear: associations with age, gender, and other experiential phenomena. Epilepsy Behav. 2016;62:153‐158. [DOI] [PubMed] [Google Scholar]
- 6. Goldstein MA, Harden CL. Epilepsy and anxiety. Epilepsy Behav. 2000;1:228‐234. [DOI] [PubMed] [Google Scholar]
- 7. Biraben A, Taussig D, Thomas P, et al. Fear as the main feature of epileptic seizures. J Neurol Neurosurg Psychiatry. 2001;70:186‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kanner AM. Ictal panic and interictal panic attacks: diagnostic and therapeutic principles. Neurol Clin. 2011;29:163‐175. ix. [DOI] [PubMed] [Google Scholar]
- 9. Kimiskidis VK, Valeta T. Epilepsy and anxiety: epidemiology, classification, aetiology, and treatment. Epileptic Disord. 2012;14:248‐256. [DOI] [PubMed] [Google Scholar]
- 10. Licht BG, Licht MH, Harper KM, et al. Clinical presentations of naturally occurring canine seizures: similarities to human seizures. Epilepsy Behav. 2002;3:460‐470. [DOI] [PubMed] [Google Scholar]
- 11. Shihab N, Bowen J, Volk HA. Behavioral changes in dogs associated with the development of idiopathic epilepsy. Epilepsy Behav. 2011;21:160‐167. [DOI] [PubMed] [Google Scholar]
- 12. Dodman NH, Knowles KE, Shuster L, Moon‐Fanelli AA, Tidwell AS, Keen CL. Behavioral changes associated with suspected complex partial seizures in Bull Terriers. J Am Vet Med Assoc. 1996;208:688‐091. [PubMed] [Google Scholar]
- 13. Gaskill CL, Hoffmann WE, Cribb AE. Serum alkaline phosphatase isoenzyme profiles in phenobarbital‐treated epileptic dogs. Vet Clin Pathol. 2004;33:215‐222. [DOI] [PubMed] [Google Scholar]
- 14. Mormann F, Kreuz T, Andrzejak RG, David P, Lehnertz K, Elger CE. Epileptic seizures are preceded by a decrease in synchronization. Epilepsy Res. 2003;53:173‐185. [DOI] [PubMed] [Google Scholar]
- 15. Johnson EK, Jones JE, Seidenberg M, Hermann BP. The relative impact of anxiety, depression, and clinical seizure features on health‐related quality of life in epilepsy. Epilepsia. 2004;45:544‐550. [DOI] [PubMed] [Google Scholar]
- 16. Beyenburg S, Mitchell AJ, Schmidt D, Elger CE, Reuber M. Anxiety in patients with epilepsy: systematic review and suggestions for clinical management. Epilepsy Behav. 2005;7:161‐171. [DOI] [PubMed] [Google Scholar]
- 17. Tellez‐Zenteno JF, Patten SB, Jette N, Williams J, Wiebe S. Psychiatric comorbidity in epilepsy: a population‐based analysis. Epilepsia. 2007;48:2336‐2344. [DOI] [PubMed] [Google Scholar]
- 18. Bragatti JA, Torres CM, Londero RG, et al. Prevalence of psychiatric comorbidities in temporal lobe epilepsy: the value of structured psychiatric interviews. Epileptic Disord. 2010;12:283‐291. [DOI] [PubMed] [Google Scholar]
- 19. Rai D, Kerr MP, McManus S, Jordanova V, Lewis G, Brugha TS. Epilepsy and psychiatric comorbidity: a nationally representative population‐based study. Epilepsia. 2012;53:1095‐1103. [DOI] [PubMed] [Google Scholar]
- 20. Young GB, Chandarana PC, Blume WT, McLachlan R, Muñoz DG, Girvin JP. Mesial temporal lobe seizures presenting as anxiety disorders. J Neuropsychiatry Clin Neurosci. 1995;7:352‐357. [DOI] [PubMed] [Google Scholar]
- 21. Meyer MA, Zimmerman AW, Miller CA. Temporal lobe epilepsy presenting as panic attacks: detection of interictal hypometabolism with positron emission tomography. J Neuroimaging. 2000;10:120‐122. [DOI] [PubMed] [Google Scholar]
- 22. Sazgar M, Carlen PL, Wennberg R. Panic attack semiology in right temporal lobe epilepsy. Epileptic Disord. 2003;5:93‐100. [PubMed] [Google Scholar]
- 23. Beletsky V, Mirsattari SM. Epilepsy, mental health disorder, or both? Epilepsy Res Treat. 2012;2012:163731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Akiyama M, Kobayashi K, Inoue T, Akiyama T, Yoshinaga H. Five pediatric cases of ictal fear with variable outcomes. Brain Dev. 2014;36:758‐763. [DOI] [PubMed] [Google Scholar]
- 25. Devinsky O, Sato S, Theodore WH, Porter RJ. Fear episodes due to limbic seizures with normal ictal scalp EEG: a subdural electrographic study. J Clin Psychiatry. 1989;50:28‐30. [PubMed] [Google Scholar]
- 26. Devinsky O, Kelley K, Porter RJ, Theodore WH. Clinical and electroencephalographic features of simple partial seizures. Neurology. 1988;38:1347‐1352. [DOI] [PubMed] [Google Scholar]
- 27. Grant Maxie M. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. Vol 1 St. Louis, Missouri: Elsevier; 2016:2554. [Google Scholar]
- 28. Polymenidou M, Trusheim H, Stallmach L, et al. Canine MDCK cell lines are refractory to infection with human and mouse prions. Vaccine. 2008;26:2601‐2614. [DOI] [PubMed] [Google Scholar]
- 29. Charlton KM, Casey GA, Webster WA, Bundza A. Experimental rabies in skunks and foxes. Pathogenesis of the spongiform lesions. Lab Invest. 1987;57:634‐645. [PubMed] [Google Scholar]
- 30. Foley GL, Zachary JF. Rabies‐induced spongiform change and encephalitis in a heifer. Vet Pathol. 1995;32:309‐311. [DOI] [PubMed] [Google Scholar]
- 31. Scott CA, Rossiter JP, Andrew RD, Jackson AC. Structural abnormalities in neurons are sufficient to explain the clinical disease and fatal outcome of experimental rabies in yellow fluorescent protein‐expressing transgenic mice. J Virol. 2008;82:513‐521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hamir AN, Fischer KA. Neuronal vacuolation in raccoons from Oregon. J Vet Diagn Invest. 1999;11:303‐307. [DOI] [PubMed] [Google Scholar]
- 33. Hamir AN, Habecker P, Jenny A, et al. Idiopathic disseminated intracytoplasmic neuronal vacuolation in a neonatal Holstein calf born in the USA. J Vet Diagn Invest. 2001;13:349‐351. [DOI] [PubMed] [Google Scholar]
- 34. Hamir AN, Miller JM, Yaeger MJ. Neuronal vacuolation in an adult ferret. Can Vet J. 2007;48:389‐391. [PMC free article] [PubMed] [Google Scholar]
- 35. Kortz GD, Meier WA, Higgins RJ, et al. Neuronal vacuolation and spinocerebellar degeneration in young Rottweiler dogs. Vet Pathol. 1997;34:296‐302. [DOI] [PubMed] [Google Scholar]
- 36. van den Ingh TS, Mandigers PJ, van Nes JJ. A neuronal vacuolar disorder in young Rottweiler dogs. Vet Rec. 1998;142:245‐247. [DOI] [PubMed] [Google Scholar]
- 37. Wiedmer M, Oevermann A, Borer‐Germann SE, et al. A RAB3GAP1 SINE insertion in Alaskan huskies with polyneuropathy, ocular abnormalities, and neuronal vacuolation (POANV) resembling human Warburg micro syndrome 1 (WARBM1). G3 (Bethesda). 2015;6:255‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mhlanga‐Mutangadura T, Johnson GS, Ashwini A, et al. A homozygous RAB3GAP1:C.743delC mutation in Rottweilers with neuronal vacuolation and spinocerebellar degeneration. J Vet Intern Med. 2016;30:813‐818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mhlanga‐Mutangadura T, Johnson GS, Schnabel RD, et al. A mutation in the Warburg syndrome gene, RAB3GAP1, causes a similar syndrome with polyneuropathy and neuronal vacuolation in Black Russian Terrier dogs. Neurobiol Dis. 2016;86:75‐85. [DOI] [PubMed] [Google Scholar]
- 40. Kyostila K, Syrja P, Jagannathan V, et al. A missense change in the ATG4D gene links aberrant autophagy to a neurodegenerative vacuolar storage disease. PLoS Genet. 2015;11:e1005169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kondagari GS, Ramanathan P, Taylor R. Canine fucosidosis: a neuroprogressive disorder. Neurodegener Dis. 2011;8:240‐251. [DOI] [PubMed] [Google Scholar]
- 42. Muller G, Alldinger S, Moritz A, et al. GM1‐gangliosidosis in Alaskan Huskies: clinical and pathologic findings. Vet Pathol. 2001;38:281‐290. [DOI] [PubMed] [Google Scholar]
- 43. Cendes F, Andermann F, Gloor P, et al. Relationship between atrophy of the amygdala and ictal fear in temporal lobe epilepsy. Brain. 1994;117(Pt 4):739‐746. [DOI] [PubMed] [Google Scholar]
- 44. Meletti S, Tassi L, Mai R, Fini N, Tassinari CA, Russo GL. Emotions induced by intracerebral electrical stimulation of the temporal lobe. Epilepsia. 2006;47(Suppl 5):47‐51. [DOI] [PubMed] [Google Scholar]
- 45. Inutsuka M, Ogino T, Yoshinaga H, Ohtsuka Y, Oka E. A child with ictal fear as the primary epileptic manifestation. No To Hattatsu. 2003;35:336‐341. [PubMed] [Google Scholar]
- 46. Oehl B, Schulze‐Bonhage A, Lanz M, Brandt A, Altenmuller DM. Occipital lobe epilepsy with fear as leading ictal symptom. Epilepsy Behav. 2012;23:379‐383. [DOI] [PubMed] [Google Scholar]
- 47. Berendt M, Farquhar RG, Mandigers PJ, et al. International veterinary epilepsy task force consensus report on epilepsy definition, classification and terminology in companion animals. BMC Vet Res. 2015;11:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix s1: Supplementary Material
Appendix s2: Supplementary Material
Supplemental Figure 1 Staining of the Nissl substance (rough endoplasmic reticulum) in the cytoplasm of neurons in the cerebral cortex of a 6‐year‐old Boerboel with focal epilepsy. Nissl stain, Obj 80.
Supporting Video 1 (https://vimeo.com/254271770/c17623497f). A Boerboel displaying signs of ictal fear and foaming at the mouth.
Supporting Video 2 (https://vimeo.com/254271713/e1a422622f). The same Boerboel as in Video 1, howling at the end of a focal seizure.
Supporting Video 3 (https://vimeo.com/254271707/828e65292f). A Boerboel (the same dog as in Videos 1 and 2) with lateralized facial muscles spasms, jerking of limb muscles and foaming at the mouth during a focal seizure.


