Abstract
Background
T‐zone lymphoma (TZL), an indolent disease in older dogs, comprises approximately 12% of lymphomas in dogs. TZL cells exhibit an activated phenotype, indicating the disease may be antigen‐driven. Prior research found that asymptomatic aged Golden Retrievers (GLDRs) commonly have populations of T‐zone‐like cells (phenotypically identical to TZL) of undetermined significance (TZUS).
Objective
To evaluate associations of inflammatory conditions, TZL and TZUS, using a case‐control study of GLDRs.
Animals
TZL cases (n = 140), flow cytometrically diagnosed, were identified through Colorado State University's Clinical Immunology Laboratory. Non‐TZL dogs, recruited through either a database of owners interested in research participation or the submitting clinics of TZL cases, were subsequently flow cytometrically classified as TZUS (n = 221) or control (n = 147).
Methods
Health history, signalment, environmental, and lifestyle factors were obtained from owner‐completed questionnaires. Odds ratios (ORs) and 95% confidence intervals (95% CIs) were estimated using multivariable logistic regression, obtaining separate estimates for TZL and TZUS (versus controls).
Results
Hypothyroidism (OR, 0.3; 95% CI, 0.1‐0.7), omega‐3 supplementation (OR, 0.3; 95% CI, 0.1‐0.6), and mange (OR, 5.5; 95% CI, 1.4‐21.1) were significantly associated with TZL. Gastrointestinal disease (OR, 2.4; 95% CI, 0.98‐5.8) had nonsignificantly increased TZL odds. Two shared associations for TZL and TZUS were identified: bladder infection or calculi (TZL OR, 3.5; 95% CI, 0.96‐12.7; TZUS OR, 5.1; 95% CI, 1.9‐13.7) and eye disease (TZL OR, 2.3; 95% CI, 0.97‐5.2; TZUS OR, 1.9; 95% CI, 0.99‐3.8).
Conclusions and Clinical Importance
These findings may elucidate pathways involved in TZUS risk and progression from TZUS to TZL. Further investigation into the protective association of omega‐3 supplements is warranted.
Keywords: dog, epidemiology, lymphosarcoma, noninfectious diseases, oncology, statistical modeling
Abbreviations
- CD45−
CD45‐negative
- CI
confidence interval
- CSU
Colorado State University
- df
degrees of freedom
- GLDR
Golden Retriever
- LPD
lymphoproliferative disorder
- LRT
likelihood ratio test
- NHL
non‐Hodgkin's lymphoma
- OR
odds ratio
- PTCL
peripheral T‐cell lymphoma
- TZL
T‐zone lymphoma
- TZUS
T‐zone‐like cells of undetermined significance
- UTI
urinary tract infection
1. INTRODUCTION
Peripheral T‐cell lymphomas (PTCLs) are a heterogeneous group of malignancies derived from mature T cells and natural killer cells.1 The etiology of most PTCL subtypes is poorly understood, largely because of their low incidence in people, which makes it difficult to study subtypes independently. T‐zone lymphoma (TZL), an indolent subtype of PTCL, accounts for approximately 12% of all lymphomas in dogs.1 T‐zone lymphoma can be readily diagnosed by histopathology or by identification of a homogeneous expansion of T cells lacking expression of the pan‐leukocyte surface marker CD45 using flow cytometry.2, 3, 4 To our knowledge, no studies have evaluated risk factors for TZL. However, this disease appears to have a striking predilection for Golden Retrievers (GLDRs), with over 40% of TZL cases occurring in this breed,2, 5 suggesting a genetic risk factor. We previously observed that >30% of GLDRs without lymphocytosis or lymphadenopathy have CD45‐negative (CD45−) T cells in their blood.6 The clinical relevance of these cells currently is undetermined. At the time of publication of the cited study, no dogs had progressed to overt TZL, with a median follow‐up of 1 year. We have adopted the term T‐zone‐like cells of undetermined significance (TZUS) for these dogs. This term mirrors the use in human literature, in which monoclonal gammopathy of undetermined significance occasionally progresses to hematopoietic neoplasms.7 Because the lack of CD45 expression may be associated with decreased apoptosis, antigen stimulation could lead to CD45− T cells undergoing unregulated division.8
Multiple studies have provided evidence for the plausibility of chronic antigen stimulation playing a role in the pathogenesis of TZL. First, TZL cells have an activated phenotype, expressing high levels of CD21 and CD25,2, 3, 9 suggesting the tumor arises from a T cell that has been activated by antigen. Second, epidemiologic evidence from the human population suggests that autoimmune disease and allergy are associated with PTCL.10, 11 Third, a recent study12 of PTCL in mice showed that the cells have a chronically activated phenotype and demonstrated decreased tumor growth by blocking antigen binding to the T cells. Thus, we suspect chronic inflammatory diseases persistently stimulate CD45− T cells to proliferate, predisposing them to progress to clinically apparent TZL. As such, our objective was to evaluate the association of inflammatory conditions, TZL and TZUS, using a case‐control study of GLDRs.
2. METHODS
2.1. Study subjects
We conducted a case‐control study of purebred GLDRs through the Colorado State University (CSU) Clinical Immunology Laboratory from October 2013 through March 2016. Golden Retrievers with TZL were identified through submissions from clinics throughout the United States (Figure 1). Two series of lymphoma‐free GLDRs, aged ≥9 years, were recruited as our comparison group: database‐recruited and clinic‐recruited. Dogs were restricted to age ≥9 years to allow reasonable time to develop TZL while mitigating risk of survival bias. Database‐recruited dogs were enrolled by email solicitation of a database of GLDR owners developed as part of the Canine Lifetime Health Project,13 which allowed owners to register with their dogs' breed and age to receive information about studies for which they may be eligible. Clinic‐recruited dogs were enrolled from the submitting clinic of TZL cases; veterinarians were asked to recruit their next 1‐2 GLDR patients that met our enrollment criteria (GLDR aged ≥9 years with no history or suspicion of a lymphoproliferative disorder [LPD]). Dogs in the comparison group subsequently were categorized as TZUS or control using flow cytometry, as described below. Our desired sample size was calculated using PS: Power and Sample Size Calculation (Nashville, Tennessee)14 to detect an odds ratio (OR) of 1.5‐2.5. Assuming an exposure percentage of 30%‐50% among controls, a 2:1 ratio of controls to TZL cases, an alpha of 0.05, and 80% power, we calculated an estimated 80 cases and 160 controls to detect an OR > 2.0. Because the TZUS population was discovered while conducting this study, they were not considered in our power calculations.
Figure 1.
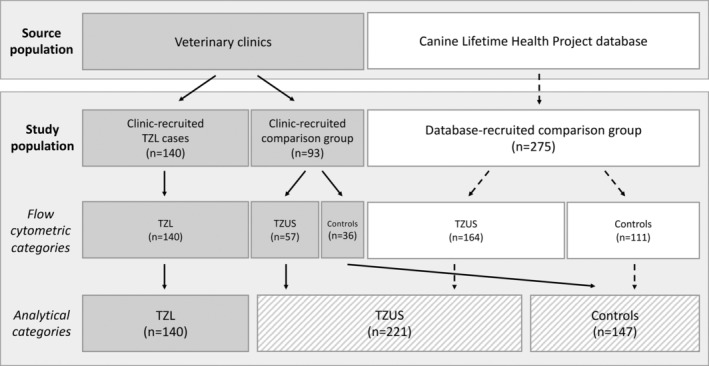
Diagram of recruitment and study population. TZL cases were recruited from veterinary clinics throughout the U.S. Lymphoma‐free GLDRs aged 9 years and older were recruited from both veterinary clinics and the Canine Lifetime Health Project database as the comparison group. All dogs recruited for the comparison group were tested via flow cytometry for designation as either TZUS or control. Recruitment strategies were subsequently combined based on flow cytometric categorization to create 3 groups: TZL, TZUS, and controls.
2.2. Disease definition
Golden Retrievers were classified as TZL, TZUS, or controls based on clinical presentation, flow cytometric analysis of peripheral blood samples, and CBC. Clinical presentation, including the presence of peripheral and visceral lymphadenopathy, was obtained from the veterinarian‐completed CSU Clinical Immunology Laboratory standard submission form. Complete blood counts were performed by the CSU Clinical Pathology Laboratory (Advia 120 Hematology Analyzer; Siemens, Tarrytown, NY) at the time of sample submission. Flow cytometry was conducted as previously described,2 and samples were analyzed with the antibody combinations listed in Supporting Information Table 1 using a 3‐laser cytometer (Coulter Gallios, Beckman Coulter Inc., Brea, California). T‐zone lymphoma definition included a homogeneous expansion of CD5+CD45− T cells and lymphocytosis (>5000 lymphocytes per microliter on CBC), lymphadenopathy (noted on veterinarian‐completed submission form), or both (Supporting Information Figure 1A); only incident TZL cases were included. Dogs with TZUS had no history (based on owner report at the time of enrollment) or clinical signs (no lymphadenopathy or lymphocytosis) of an LPD but had a small population of CD5+CD45− T cells on flow cytometry (>1% of total T cells; Supporting Information Figure 1B). Controls also had no history or clinical signs of an LPD and had no population of CD5+CD45− T cells identified by flow cytometry (≤1% of total T cells; Supporting Information Figure 1C).
2.3. Data collection and exposure assessment
We developed a comprehensive questionnaire for owners, based on the GLDR Lifetime Study questionnaire,13 to capture potential TZL risk factors and information about study population characteristics. Modifications to the existing questionnaire included altering phrasing to capture the dog's lifetime exposures (versus annual updates) and adjusting language about medications and health history for a lay audience (versus veterinarian). The resulting web‐based (SurveyMonkey, San Mateo, CA) questionnaire included 44 multipart questions (Supporting Information Figure 2). Information ascertained included the dog's health history (eg, cancer, infectious diseases), environmental exposures (eg, time in rural areas, exposure to smoke), medications (eg, antihistamines, corticosteroids, other anti‐inflammatory drugs), signalment (age, sex, timing of spay or neuter, location of residence), preventive care (flea and heartworm preventives, dental care, vaccinations), and diet. All disease history information was obtained based on age at diagnosis (categorized as <1 year of age, 1‐3 years, 4‐6 years, 7‐10 years, or >10 years), but was collapsed into ever/never diagnosed because of sparse data within categories. Health history variables were left as broad categories unless we had a specific hypothesis to evaluate a subcategory, as detailed below. Owners were instructed to complete the questionnaire and submit a blood sample within 3 months and were considered lost to follow‐up if they did not complete both in this time frame.
2.3.1. Health history
Specific factors of interest included infectious diseases, allergic disorders, autoimmune diseases, and inflammatory disorders that could cause chronic immune stimulation. Among infectious diseases, we were particularly interested in history of parasitic or nematode infection as a result of preliminary evidence from our laboratory that suggests TZL cells may be of Th2 origin; this information was gathered as a history of “worms” in the questionnaire. All other infectious diseases evaluated within this section of the questionnaire (depicted in Supporting Information Figure 2) were collapsed into a single category (ie, any infectious disease versus none). We evaluated bladder and ear infections separately from other infectious diseases because they were ascertained within different sections of the questionnaire (urinary/reproductive conditions and eye, ear, nose, and throat conditions, respectively).
Allergic and autoimmune disorders of interest included skin disease, vaccine reactions and hypothyroidism. Owing to the likely misclassification of skin disease categories using owner reporting, all skin diseases except mange were collapsed into 1 variable. Previous reports5 have indicated 10%‐50% of TZL cases present with demodectic mange,3, 5 leading to an a priori decision to evaluate mange separately from other skin diseases.
Other inflammatory disorders of interest included dental or oral disease, eye disease, cardiovascular disease, gastrointestinal disease, and orthopedic conditions. Uveitis was evaluated separately from other eye diseases because of the strong genetic component of this disease.15, 16, 17 In addition, orthopedic disease was divided into cruciate ligament rupture and “degenerative joint disease,” which included elbow and hip dysplasia, intervertebral disc disease, and osteoarthritis, because of differences in underlying disease processes.
2.3.2. Environmental exposures
We hypothesized that environmental exposures may stimulate an immune response. Average lifetime exposure to rural environments, parks, lawn chemicals, cigarette smoke, and household chemicals as well as frequency of swimming in irrigation water, ponds, or canals, lakes or streams, or the ocean were ascertained as daily, weekly, monthly, occasionally, or never. To avoid sparse cells, these categories were collapsed as “frequent” (daily, weekly, monthly exposure) versus “infrequent” (occasional or null exposure).
2.3.3. Medications
Certain medications dampen the immune response and therefore hypothetically could decrease TZL risk. Our main categories of interest included “ever” (versus never) use of antihistamines, nonsteroidal anti‐inflammatory medications, corticosteroids, antibiotics, immunosuppressive drugs (eg, cyclosporine, azathioprine), and nonprescription supplements. Supplementation with omega‐3 fatty acids was a hypothesized protective factor because of their anti‐inflammatory properties.18, 19, 20, 21 Thus, use of nonprescription supplements was divided into omega‐3 and “other.”
2.3.4. Signalment
We evaluated sex and age at spay or neuter, because multiple recent studies have indicated an association of early spay or neuter and lymphoma.22, 23, 24, 25 Age at spay or neuter was categorized as female spayed <1 year of age (used as the reference), female spayed >1 year of age (or intact), male neutered <1 year of age, and male neutered >1 year of age (or intact).
2.4. Statistical analysis
Inclusion criteria were rechecked based on survey results and non‐TZL dogs whose owners indicated they had been diagnosed with lymphoma or were <9 years old were excluded. In addition, dogs were excluded if only the signalment portion of the survey was completed. If >5% of questionnaires were blank for a given variable, a “missing” category was created and subsequently used in analyses. If <5% of questionnaires were blank for a given variable, those observations were removed from analyses involving those variables. Variables were summarized as frequency and percent or median and range as appropriate.
2.4.1. Evaluation of selection bias
To determine whether systematic differences existed between database‐ and clinic‐recruited dogs in the TZUS and control groups, we conducted univariable analyses to assess the association of recruitment strategy and each variable of interest between (1) TZUS and (2) controls. Fisher's exact tests were calculated for all variables; variables significantly associated with recruitment strategy (P < .05) between both TZUS and controls were noted as potential indicators of selection bias and thus included in multivariable modeling, as described below.
Because TZUS versus control status was not known at the time of enrollment and survey completion, we hypothesized that any bias in recruitment strategy should affect both TZUS and controls equally. Therefore, indicators of selection bias were not included in the TZUS versus control models. We conducted a chi‐square test to evaluate whether an indicator variable for recruitment strategy was associated with TZUS versus control status.
2.4.2. Modeling strategy
We implemented a data‐driven approach to evaluate variables that aligned with our hypothesized role of chronic antigen stimulation. Univariable associations for TZL versus control and TZUS versus control were calculated using logistic regression. We analyzed TZUS as a distinct group because we hypothesized their exposure levels would either be (1) similar to controls, under the hypothesis that a genetic risk exists for the presence of CD45− T cells and chronic antigen stimulation is needed for progression from TZUS to TZL, or (2) intermediate between controls and TZL, under the hypothesis of a dose‐response relationship for disease progression. Variables were selected for consideration in the multivariable model if they (1) had significant univariable association (using P < .25) with non‐near‐zero variance or (2) were a potential indicator of selection bias (TZL versus control analysis only). Variables that met the above criteria were included in a model and assessed for collinearity using variance decomposition proportions and condition indices26 before performing best subsets regression (Supporting Information Figure 3). Indicator variables were created for best subsets regression, and the Score test was used for subset optimization.27, 28 A consensus model then was built using variables deemed important by best subsets and their required constituents (ie, if only 1 indicator variable from a multilevel variable was chosen by best subsets, all categories were included in the consensus model). Our goal was to choose a consensus model with no more than 1 category per 10 participants in the smallest group (ie, no more than 14 categories/14 degrees of freedom (df) in a model assessing 140 TZL cases).
The consensus model was considered the “full” model, and variables were eliminated in a stepwise manner.26 At each step, (1) the variable with the highest, nonsignificant P‐value (>.05) was removed, (2) confounding was assessed based on a >10% change in OR estimates from the full model, and (3) a likelihood ratio test (LRT) was used to compare the reduced model to the prior model. This process was repeated until all variables were significant at P < .05, removal of a variable changed at least 1 OR by >10%, or the LRT suggested the prior model performed better than the reduced model.
2.4.3. Sensitivity analyses
For our final models, we conducted sensitivity analyses to evaluate the impact of age and non‐lymphoma cancers on our results. Age was restricted to ≥9 years among TZUS and control populations, but younger TZL cases were enrolled in the study. Thus, we first removed TZL cases that were<9 years of age and reanalyzed the TZL versus control final model. Non‐lymphoma cancers were highly represented in both our TZUS and controls because of recruitment from cancer specialty clinics and a vested interest among owners of dogs with cancer. For our second sensitivity analysis, we removed all dogs (TZL, TZUS, and controls) with diagnoses of non‐lymphoma cancer and reanalyzed both the TZL versus control and TZUS versus control models.
This study was conducted with prior approval from the CSU Institutional Animal Care and Use Committee (Protocol 13‐4473A). All data management and analyses were conducted using SAS 9.4 (Cary, North Carolina) and R version 3.2.2 was used to calculate near‐zero variance.29
3. RESULTS
3.1. Participants
Owners of 805 dogs expressed interest in our study. Of these, 46 did not meet eligibility criteria (37 had history of or concurrent non‐TZL LPDs, 6 were non‐TZL <9 years of age, 3 were not purebred GLDRs), 246 were lost to follow‐up (at least 12 dogs died before enrollment was completed), and 5 owners only completed the signalment portion of the survey. In total, 508 dogs (140 TZL, 221 TZUS, 147 controls) were eligible and completed enrollment (both a blood sample and owner‐completed questionnaire). These dogs represented at least 46 states (1 unknown) and Canada (Figure 2). Descriptive statistics for all variables, stratified by disease status, are shown in Table 1. The median age of the TZL population was 10 years (range, 7‐15), TZUS 11 years (range, 9‐18), and controls 10 years (range, 9‐15). Approximately 45% of the population was male, independent of disease status. The majority of the study population received multiple preventive care treatments, including flea and heartworm preventive medications (84% and 91% respectively) and regular rabies vaccinations (93%).
Figure 2.
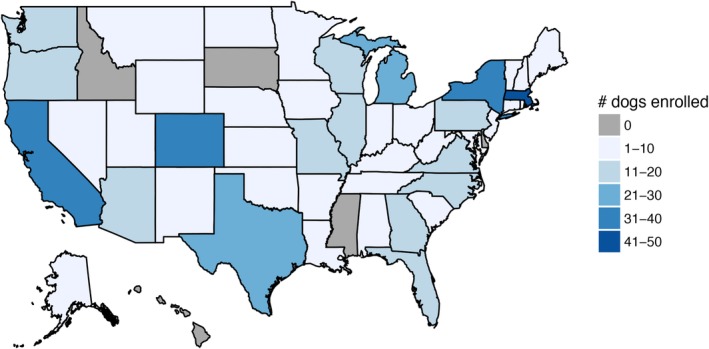
State of residence for 508 dogs enrolled in the study. Not depicted: 2 dogs from Canada, 1 with unknown zip code of residence, and 2 with invalid zip codes
Table 1.
Distribution of variables, stratified by disease status and recruitment strategy
| TZL | Controls | TZUS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All | All | Database | Clinic | All | Database | Clinic | |||
| n | (n = 140) | (n = 147) | (n = 111) | (n = 36) | (n = 221) | (n = 164) | (n = 57) | ||
| Missinga | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||
| Signalment | |||||||||
| Age (years) | Median (range) | 0 | 10 (7‐15) | 10 (9‐15) | 10 (9‐15) | 11 (9‐15) | 11 (9‐18) | 10 (9‐18) | 11 (9‐15) |
| Sex | Female (versus male) | 0 | 76 (54) | 85 (58) | 67 (60) | 18 (50) | 120 (54) | 96 (59) | 24 (42)* |
| Age at spay/neuter | Female < 1 year | 45 (32) | 32 (22) | 23 (21) | 9 (25)* | 47 (21) | 32 (20) | 15 (26)* | |
| Female > 1 year | 24 (17) | 48 (33) | 42 (38) | 6 (17) | 68 (31) | 61 (37) | 7 (13) | ||
| Male < 1 year | 35 (25) | 37 (25) | 26 (23) | 11 (31) | 36 (16) | 18 (11) | 18 (32) | ||
| Male > 1 year | 22 (16) | 23 (16) | 18 (16) | 5 (14) | 61 (28) | 47 (29) | 14 (25) | ||
| Missing | 14 (10) | 7 (5) | 2 (2) | 5 (14) | 9 (4) | 6 (4) | 3 (5) | ||
| Preventive care | |||||||||
| Flea preventives | Ever (versus never) | 9 | 116 (84) | 121 (83) | 92 (84) | 29 (83) | 180 (83) | 135 (84) | 45 (82) |
| Heartworm preventives | Ever (versus never) | 17 | 124 (91) | 130 (92) | 100 (93) | 30 (88) | 191 (90) | 145 (91) | 46 (87) |
| Rabies vaccination | As directed (versus not) | 4 | 128 (93) | 137 (94) | 104 (94) | 33 (94) | 205 (93) | 150 (91) | 55 (96) |
| DHPP vaccination | As directed (versus not) | 8 | 119 (88) | 100 (68) | 70 (63) | 30 (86)* | 145 (66) | 100 (62) | 45 (79)* |
| Bordetella vaccination | Frequent (versus not) | 80 (57) | 70 (48) | 52 (47) | 18 (50) | 113 (51) | 78 (48) | 35 (61) | |
| Missing | 13 (9) | 6 (4) | 3 (3) | 3 (8) | 10 (5) | 6 (4) | 4 (7) | ||
| Teeth cleaned | Ever (versus never) | 3 | 58 (42) | 64 (44) | 45 (41) | 19 (53) | 98 (45) | 70 (43) | 28 (49) |
| Infectious diseases | |||||||||
| Worms | Yes (versus no) | 0 | 17 (12) | 16 (11) | 12 (11) | 4 (11) | 28 (13) | 24 (15) | 4 (7) |
| Bladder infection or stones | Yes (versus no) | 0 | 10 (7) | 5 (3) | 4 (4) | 1 (3) | 30 (14) | 23 (14) | 7 (12) |
| Mange | Yes (versus no) | 0 | 16 (11) | 3 (2) | 1 (1) | 2 (6) | 5 (2) | 4 (2) | 1 (2) |
| Ear infection | Yes (versus no) | 1 | 65 (46) | 53 (36) | 41 (37) | 12 (34) | 102 (46) | 73 (45) | 29 (51) |
| Other infectious disease | Yes (versus no) | 0 | 25 (18) | 35 (24) | 31 (28) | 4 (11)* | 52 (24) | 41 (25) | 11 (19) |
| Inflammatory disorders | |||||||||
| Eye disease | Yes (versus no) | 1 | 21 (15) | 14 (10) | 10 (9) | 4 (11) | 39 (18) | 32 (20) | 7 (12) |
| Uveitis | Yes (versus no) | 1 | 7 (5) | 7 (5) | 3 (3) | 4 (11) | 10 (5) | 7 (4) | 3 (5) |
| Tooth or gum disease | Yes (versus no) | 17 | 22 (16) | 20 (14) | 15 (14) | 5 (14) | 27 (13) | 21 (13) | 6 (11) |
| Gastrointestinal disease | Yes (versus no) | 0 | 19 (14) | 12 (8) | 10 (9) | 2 (6) | 27 (12) | 18 (11) | 9 (16) |
| Cruciate ligament rupture | Yes (versus no) | 0 | 8 (6) | 7 (5) | 5 (5) | 2 (6) | 9 (4) | 5 (3) | 4 (7) |
| Degenerative joint disease | Yes (versus no) | 0 | 22 (16) | 23 (16) | 19 (17) | 4 (11) | 41 (19) | 31 (19) | 10 (18) |
| Non‐mange skin disease | Yes (versus no) | 0 | 60 (43) | 53 (36) | 38 (34) | 15 (42) | 89 (44) | 61 (37) | 28 (49) |
| Vaccine reaction | Ever (versus never) | 2 | 4 (3) | 12 (8) | 9 (8) | 3 (8) | 20 (9) | 16 (10) | 4 (7) |
| Other diseases | |||||||||
| Hypothyroidism | Yes (versus no) | 0 | 8 (6) | 24 (16) | 22 (20) | 2 (6) | 40 (18) | 33 (20) | 7 (12) |
| Other endocrine disorder | Yes (versus no) | 0 | 6 (4) | 2 (1) | 1 (1) | 1 (3) | 3 (1) | 2 (1) | 1 (2) |
| Cardiovascular disease | Yes (versus no) | 0 | 6 (4) | 10 (7) | 8 (7) | 2 (6) | 13 (6) | 11 (7) | 2 (4) |
| Non‐lymphoma cancer | Yes (versus no) | 0 | 21 (15) | 36 (24) | 25 (23) | 11 (31) | 55 (25) | 33 (20) | 22 (39)* |
| Medications | |||||||||
| Antihistamines | Ever (versus never) | 56 (40) | 63 (43) | 49 (44) | 14 (39) | 106 (48) | 81 (49) | 25 (44) | |
| Missing | 38 (27) | 35 (24) | 25 (23) | 10 (28) | 41 (19) | 29 (18) | 12 (21) | ||
| Anti‐inflammatories | Ever (versus never) | 67 (48) | 87 (59) | 72 (65) | 15 (42)* | 126 (57) | 94 (57) | 32 (56) | |
| Missing | 32 (23) | 21 (14) | 13 (12) | 8 (22) | 35 (16) | 24 (15) | 11 (19) | ||
| Steroids | Ever (versus never) | 50 (36) | 45 (31) | 34 (31) | 11 (31) | 87 (39) | 69 (42) | 18 (32) | |
| Missing | 40 (29) | 38 (26) | 28 (25) | 10 (28) | 44 (20) | 31 (19) | 13 (23) | ||
| Antibiotics | Ever (versus never) | 103 (74) | 118 (80) | 94 (85) | 24 (67)* | 173 (78) | 130 (79) | 43 (75) | |
| Missing | 25 (18) | 14 (10) | 6 (5) | 8 (22) | 29 (13) | 23 (14) | 6 (11) | ||
| Immunosuppressants | Ever (versus never) | 4 (3) | 8 (5) | 7 (6) | 1 (3) | 2 (1) | 2 (1) | 0 (0) | |
| Missing | 64 (46) | 52 (35) | 35 (32) | 16 (44) | 84 (38) | 58 (35) | 26 (46) | ||
| Omega‐3 supplements | Yes (versus no) | 14 (10) | 34 (23) | 28 (25) | 6 (17) | 66 (30) | 52 (32) | 14 (25) | |
| Missing | 30 (21) | 28 (19) | 21 (19) | 7 (19) | 32 (14) | 22 (13) | 10 (18) | ||
| Other supplements | Yes (versus no) | 38 (27) | 67 (46) | 55 (50) | 12 (33) | 98 (44) | 71 (43) | 27 (47) | |
| Missing | 30 (21) | 28 (19) | 21 (19) | 7 (19) | 32 (14) | 22 (13) | 10 (18) | ||
| Environmental exposures | |||||||||
| Lawn exposure | Frequent (versus not) | 6 | 56 (41) | 72 (49) | 57 (51) | 15 (43) | 108 (50) | 83 (52) | 25 (44) |
| Rural environment | Frequent (versus not) | 46 (33) | 72 (49) | 59 (53) | 13 (36) | 109 (49) | 85 (52) | 24 (42) | |
| Missing | 6 (4) | 10 (7) | 6 (5) | 4 (11) | 8 (4) | 5 (3) | 3 (5) | ||
| Parks | Frequent (versus not) | 54 (39) | 52 (35) | 40 (36) | 12 (33) | 54 (39) | 33 (20) | 21 (37)* | |
| Missing | 6 (4) | 5 (3) | 2 (2) | 3 (8) | 12 (5) | 8 (5) | 4 (7) | ||
| Tick on dog | Ever (versus never) | 4 | 77 (55) | 93 (64) | 72 (65) | 21 (58) | 133 (61) | 98 (60) | 35 (63) |
| Toxins | |||||||||
| Lawn chemicals | Frequent (versus not) | 10 | 17 (13) | 9 (6) | 4 (4) | 5 (14)* | 20 (9) | 15 (9) | 5 (9) |
| Cigarette smoke | Frequent (versus not) | 16 | 3 (2) | 10 (7) | 9 (8) | 1 (3) | 10 (5) | 8 (5) | 2 (4) |
| Paints/solvents | Frequent (versus not) | 19 | 6 (5) | 3 (2) | 2 (2) | 1 (3) | 7 (3) | 5 (3) | 2 (4) |
| Swimming | |||||||||
| Ocean | Frequent (versus not) | 4 (3) | 1 (1) | 1 (1) | 0 (0) | 6 (3) | 4 (3) | 2 (4) | |
| Missing | 35 (25) | 44 (30) | 35 (32) | 9 (25) | 75 (34) | 60 (37) | 15 (26) | ||
| Irrigation water | Frequent (versus not) | 5 (4) | 18 (12) | 17 (15) | 1 (3)* | 23 (10) | 16 (10) | 7 (12) | |
| Missing | 10 (7) | 6 (4) | 3 (3) | 3 (8) | 19 (9) | 12 (7) | 7 (12) | ||
| Lakes/streams | Frequent (versus not) | 12 (9) | 27 (19) | 24 (22) | 3 (8)* | 29 (13) | 22 (13) | 7 (12) | |
| Missing | 6 (4) | 8 (5) | 6 (5) | 2 (6) | 10 (5) | 6 (4) | 4 (7) | ||
p < .05 when comparing database‐recruited to clinic‐recruited by Fisher's exact test
For variables without a missing category.
3.2. Evaluation of selection bias
The distribution of most variables did not differ based on recruitment strategy (Table 1). Only 2 variables differed significantly among both controls and TZUS (age at spay or neuter and DHPP vaccination frequency) and thus were considered potential markers of selection bias in our TZL versus control multivariable models. Differences in age at spay or neuter appeared to be largely driven by a higher proportion of female database‐recruited dogs being spayed after 1 year of age (>35% among database‐recruited dogs versus <20% among clinic‐recruited dogs; P = .01). A higher proportion of information on timing of spay or neuter was missing among clinic‐recruited controls (14% versus 2% among database‐recruited dogs; P = .04). For DHPP vaccination frequency, database‐recruited dogs were less likely to be vaccinated as directed (62%‐63% among database‐recruited dogs versus 80‐86% among clinic‐recruited; P = .02). Database‐recruited owners were anecdotally more likely to use titers to dictate vaccination (versus a conventional vaccination schedule, as indicated by questionnaire free text options associated with vaccination questions), potentially driving this observed difference.
Once a final TZL versus control model was chosen, we removed these variables one‐by‐one to determine if confounding existed. When DHPP vaccination frequency was removed, ORs for spaying after 1 year of age, bladder infection or calculi, hypothyroidism, and gastrointestinal disease changed by >10%. When age at spay or neuter was removed, ORs for DHPP vaccination frequency, mange, and hypothyroidism changed by >10%. The LRTs suggested our chosen final model performed better than the models without DHPP vaccination frequency (P = .001) or age at spay or neuter (P = .02).
The proportion of TZUS and controls was equal for both recruitment strategies (60% of database‐recruited and 61% of clinic‐recruited dogs had TZUS), and the indicator variable for recruitment strategy was not significantly associated with TZUS versus control status (chi‐square P = .81).
3.3. Univariable results
Eight variables not suspected to be indicators of selection bias were significantly (P < .05) associated with TZL (versus control) (Figure 3). History of or concurrent mange was associated with increased TZL odds in the univariable setting (OR, 6.2; 95% CI, 1.8‐21.8). Variables associated with decreased TZL odds included history of or concurrent hypothyroidism (OR, 0.31; 95% CI, 0.13‐0.72), receiving nonprescription supplements (both omega‐3 supplements [OR, 0.37; 95% CI, 0.18‐0.73] and “other” supplements [OR, 0.41; 95% CI, 0.24‐0.70]), frequent exposure to rural environments (OR, 0.47; 95% CI, 0.29‐0.77), and frequent swimming in irrigation water (OR, 0.27; 95% CI, 0.10‐0.76) or lakes/streams (OR, 0.41; 95% CI, 0.20‐0.84). Four variables were significantly (P < .05) associated with TZUS (versus control). Bladder infection or calculi (OR, 4.5; 95% CI, 1.7‐11.8) and eye disease (OR, 2.0; 95% CI, 1.1‐3.9) were associated with increased TZUS odds; receiving immunosuppressants (OR, 0.16; 95% CI, 0.03‐0.78) and frequent exposures to parks (OR, 0.60; 95% CI, 0.38‐0.96) were associated with decreased TZUS odds.
Figure 3.
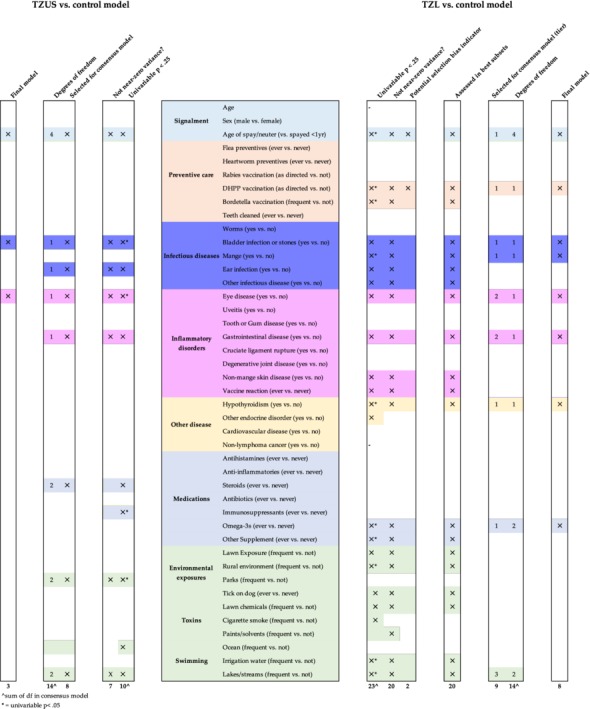
Flowchart of variables selected based on criteria to be assessed in multivariable modeling, best subsets, and backward selection
3.4. TZL versus control modeling
Variables included in each step of our modeling strategy are shown in Figure 3. Our full model included 9 variables and 14 df. From there, our elimination strategy removed frequency of swimming in lakes or streams, resulting in an 8‐variable final model (LRT P = .15).
Our final model identified 2 variables with significantly decreased TZL odds, including receiving omega‐3 supplements (OR, 0.29; 95% CI, 0.13‐0.63) and hypothyroidism (OR, 0.25; 95% CI, 0.10‐0.66) (Figure 4). History of or concurrent mange (OR, 5.5; 95% CI, 1.4‐21.1) was associated with increased TZL odds. Bladder infection or calculi (OR, 3.5; 95% CI, 0.96‐12.7), gastrointestinal disease (OR, 2.4; 95% CI, 0.98‐5.8), and eye disease (OR, 2.3; 95% CI, 0.97‐5.2) did not reach statistical significance (P‐value between .05 and .10). These generally were uncommon outcomes (mange: 11% TZL versus 2% controls; bladder infection or calculi: 7% versus 3%; gastrointestinal disease: 14% versus 8%; eye disease: 15% versus 10%). Two variables were included in the model as potential indicators of selection bias: timing of spay or neuter and DHPP vaccination frequency.
Figure 4.
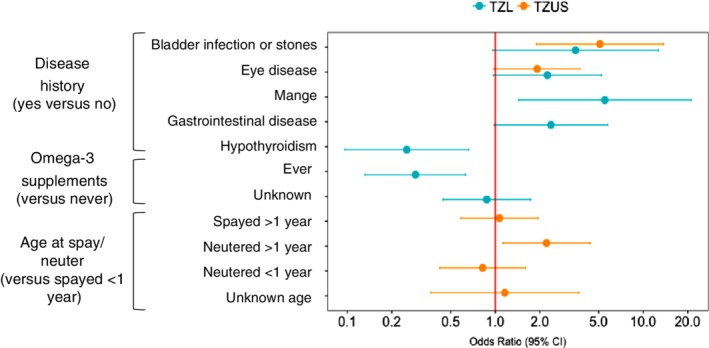
Final multivariable modeling results for TZL versus control model and TZUS versus control model. Odds ratios (ORs) and 95% confidence intervals (CIs) are shown in log scale. Age at spay or neuter and DHPP vaccination frequency were included in the TZL versus control model as indicators of selection bias, so their ORs are not shown in this figure
3.5. TZUS versus control modeling
The 8 variables that passed our screening step for the TZUS versus control model only accounted for 14 df, so we did not proceed with best subsets and instead included all variables in the initial full model (Figure 3). Our elimination strategy removed all variables except bladder infection or calculi, eye disease, and age at spay or neuter (Figures 3 and 4). Eye disease was borderline significant (OR, 1.9; 95% CI, 0.99‐3.75), but the model including eye disease performed better than the model without eye disease (LRT; P = .04). Dogs with history of or concurrent bladder infection or calculi had significantly increased TZUS odds (versus control) (OR, 5.1; 95% CI, 1.9‐13.7). Bladder infection or calculi and eye disease were relatively uncommon in TZUS, present in 14% and 18%, respectively (versus 3% and 10% of controls). The difference in age at spay or neuter for TZUS versus controls appeared to be driven by males neutered after 1 year of age (versus females spayed before 1 year) (OR, 2.2; 95% CI, 1.1‐4.4).
3.6. Sensitivity analyses
We removed 20 TZL cases <9 years of age and saw no appreciable differences in effect estimates or variable significance (Supporting Information Table 2). Removal of 112 dogs with non‐lymphoma cancers (21 TZL, 55 TZUS, 36 controls) led to model instability, evidenced by large confidence intervals (CIs) (Supporting Information Tables 2 and 3). However, effect estimates had a similar magnitude and direction of association.
4. DISCUSSION
The etiology of PTCL subtypes is not fully understood, but chronic antigen stimulation is suspected to play a role in some forms of this disease. We undertook a large case‐control study of TZL and TZUS in GLDRs to investigate the hypothesis that inflammatory conditions increase TZL risk. Our study found dogs that received omega‐3 supplements, which may decrease inflammation,20, 30, 31, 32 had decreased TZL odds. Hypothyroidism, which can arise through autoimmune mechanisms,33, 34 also was associated with decreased TZL odds. Consistent with prior reports,3, 5 we found a significant association of mange and TZL. In our multivariable models, bladder infection or calculi and eye disease had positive associations with both TZL and TZUS, although P‐values were between .05 and .10. We noted a similar increased TZL odds with borderline significance among dogs with gastrointestinal disease. No environmental exposures were significantly associated with TZL in the multivariable setting.
4.1. Omega‐3 supplements may protect against TZL
The use of omega‐3 supplements was associated with decreased TZL odds. Omega‐3 supplementation may have anti‐inflammatory and immunomodulatory effects,20, 21, 30, 31, 32 so it is possible they can mitigate the effects of inflammatory diseases. This finding is in agreement with previous studies suggesting omega‐3 supplementation is associated with a decreased risk of lymphoma in humans35, 36 and can delay onset of T‐cell lymphomas in mice.37 If corroborated in future studies, this observation suggests an inexpensive preventative strategy that can be used in high‐risk breeds such as GLDRs.2 Omega‐3 supplements are already recognized as adjunctive treatments for dogs with atopic skin disease38, 39 and osteoarthritis.40, 41 In future studies, detailed information about omega‐3 use, dose, duration of use, and composition (ie, ratio of eicosapentaenoic acid to docosahexaenoic acid) would be valuable to determine, because this information was not available in our data set. Doing so also would aid in the ability to evaluate effect modification of omega‐3 use and inflammatory disorders.
4.2. Variables associated with increased odds of TZL and TZUS
History of concurrent eye disease or bladder infection or calculi was associated with increased odds of both TZL and TZUS (Figure 4). The individual ORs for TZL and TZUS differed slightly, but the CIs had substantial overlap. The power to detect an association for bladder infection or calculi and eye disease was diminished because of small sample sizes, and thus the consistency of these associations for both TZL and TZUS supports the notion that these are biologically important associations. Because of our questionnaire design, we were unable to determine the frequency of urinary tract infections (UTIs) versus calculi in our data set. Owners may misreport bladder infection with underlying incontinence or sterile inflammation, limiting the conclusions we can draw from this finding. Bladder infections, more generally UTIs, are commonly bacterial in origin.42, 43 Bacterial UTIs are the most common infectious disease of dogs, affecting 14% of dogs during their lifetime.43, 44 Studies have shown asymptomatic bacteriuria may be present in 2%‐14% of dogs,45, 46 which may suggest that diagnosed UTIs represent underlying chronic inflammation. One study47 evaluated UTIs (categorized as cystitis, prostatitis, and pyelonephritis) as a risk factor for non‐Hodgkin's lymphoma (NHL) in humans and found that prostatitis increased risk of overall NHL but not T‐cell NHL. This may be a novel association for TZL or may reflect differences in UTI etiology between people and dogs. Although UTIs are most common in spayed female dogs,48 we believe bladder infection is an independent risk factor because our multivariable models adjusted for sex and neuter status. A study evaluating urinary calculi and cancer risk found an increased standardized incidence ratio for cancers of hematologic origin but did not specifically evaluate lymphoma.49 Urinary calculi can cause inflammation of the urinary tract and predispose dogs to UTIs, and thus an association with TZL is plausible based on our hypothesis.
The association of eye disease and TZL appears to be driven predominately by owner‐reported cataracts in both TZL and TZUS. Cataracts often are an inherited disease but can form as a result of trauma or secondary to diabetes mellitus.50, 51 Because lenticular sclerosis also causes a cloudy appearance to the eye,50, 51, 52 it may be misreported as cataracts by owners. Because our study relied on owner‐reported diagnoses, we were unable to differentiate true cataracts from lenticular sclerosis. Both diseases are common in older dogs (>9 years),50, 51, 52 and the mechanism by which they would influence TZUS or TZL odds is unclear. To our knowledge, no prior studies have assessed eye disease as a risk factor for lymphoma.
Because of the retrospective nature of our study design, we cannot assess the temporality of these associations. We hypothesized TZUS would either be similar to controls (assuming a genetic predisposition to developing CD45− T cells) or intermediate between controls and TZL (suggesting a dose‐response relationship for progression from normal to TZUS to TZL). However, the magnitude of the associations of eye disease and bladder infection or calculi was similar for TZUS and TZL. It is possible that these variables represent risk for developing CD45− T cells, whereas other factors mediate disease progression. Prospective analyses will be key to understanding the relationship between TZUS and TZL.
4.3. Variables associated with increased odds for TZL only
As with prior reports,3, 5 we found 11% of TZL cases (n = 16) had a recent or concurrent diagnosis of mange, compared to 3 controls (2%) and 5 TZUS (2%). Because diagnosis of mange and TZL is often concurrent, it is unclear whether mange is a risk factor for TZL or whether TZL causes immunosuppression, making dogs more susceptible to mange. We could not fully evaluate temporality in our data set because we had wide age categories that generally overlapped with age at diagnosis. A sensitivity analysis indicated TZL was significantly associated with diagnoses of mange at ≥7 years of age (P = .004) but not with diagnoses of mange at ≤6 years of age (P = .41), supporting the belief that TZL‐induced immunosuppression may precede mange diagnosis. Prospective studies would be beneficial in elucidating the natural history of TZL and mange.
Gastrointestinal disease had a borderline association with TZL (P‐value between .05 and .10). Gastrointestinal diseases were relatively evenly split across the specific disease categories (colitis, diarrhea, gastritis). These categories are nonspecific and often interrelated, and thus reliability of owner reporting may be poor. As with mange, we were unable to distinguish temporality and it is possible that TZL‐induced immunosuppression could lead to gastrointestinal disease. However, concurrent diagnosis of TZL and gastrointestinal disease is not a common finding in our laboratory submissions nor based on prior literature. Prior studies47, 53 have found no association of ulcerative colitis, inflammatory bowel disease, or gastroenteritis with NHL in humans, but the association may have been masked by using overall NHL instead of specific T‐cell subtypes. Certain long‐term gastrointestinal infections, including Helicobacter pylori and Campylobacter jejuni, have been associated with mucosa‐associated lymphoid tissue lymphomas in humans,54, 55, 56 highlighting the possibility that these may be subtype‐specific risk factors.
4.4. Variables associated with decreased TZL odds
In dogs, hypothyroidism generally is caused by destruction of the thyroid gland by either lymphocytic thyroiditis (likely immune‐mediated) or idiopathic atrophy.34 Overall, the breakdown of these 2 etiologies is believed to be 1:1, but some research indicates GLDRs may be more likely to have immune‐mediated disease (based on increased prevalence of autoantibodies to thyroid peroxidase and thyroglobulin).34, 57 Unfortunately, autoantibodies are not routinely measured and we do not know their frequency in the dogs in our study. Hypothyroidism was associated with decreased TZL odds, which is in contrast to previous studies that have found that autoimmune disorders increase risk for multiple NHL subtypes in humans, including PTCL.10, 53, 58, 59, 60 It is plausible that the positive metabolic and growth‐promoting effects of thyroid hormone could play a role in facilitating CD45− T‐cell proliferation. As such, lack of this hormone feasibly could decrease TZL risk. We were not able to confirm hypothyroidism diagnosis using the laboratory data in our data set. However, hypothyroidism is a common disease in GLDRs and owners in our study appear prudent about veterinary care, as evidenced by high use of preventive care measures. Overall, the potential mechanism by which hypothyroidism may decrease TZL odds is still unclear and may require future laboratory studies.
4.5. Variables that may be indicators of selection bias
The database‐recruited owners in our study were highly invested in GLDR health, and many showed or bred GLDRs or both. It is possible that this population is not representative of the TZL source population with respect to exposures of interest, thereby raising the concern of selection bias. We addressed this concern by conducting stratified analyses for all variables, and suspected selection bias for variables with significant differences between recruitment strategies (database versus clinic) in both TZUS and controls. Using this criteria, DHPP vaccination frequency and age at spay or neuter remain potential indicators of selection bias, and we believe controlling for these variables in our TZL versus control model decreased bias in our effect estimates. Our clinic‐recruited control series may better represent the TZL source population, but our sample size was too small to use only this control series for multivariable modeling. However, there was no difference in DHPP vaccination frequency or percent of females spayed after 1 year of age between TZL (88% received DHPP as directed, 17% spayed >1 year) and clinic‐recruited controls (86% received DHPP as directed, 17% spayed >1 year).
A potential role of sex hormones in TZL pathogenesis is plausible based on prior research that indicates late spay or neuter may decrease the incidence of many diseases, including lymphoma (nonsignificant reduction for females, significant reduction for males).22, 23 However, a sex hormone association has not been evaluated for specific lymphoma subtypes in dogs, and lymphoma research in humans only has identified a sex predilection for some subtypes.61, 62, 63, 64 Future studies may better evaluate subtype‐specific sex influences for lymphoma in dogs.
An association with vaccination status was not considered a priori. Among database‐recruited dogs, we observed increased use of titers to dictate vaccination schedules, reflected as decreased vaccination frequency. This may explain why only DHPP vaccination frequency was associated with TZL, and not rabies vaccination frequency, which is dictated by state laws, or Bordetella vaccination frequency, which depends on the dog's lifestyle and likelihood of exposure.
4.6. Variables not associated with TZL
Multiple variables that fit our inflammation hypothesis were not associated with TZL in our study, including infectious diseases, skin diseases other than mange, and environmental exposures. These variables had significant associations with TZL in univariable, but not multivariable, models which could suggest correlation with other variables, measurement error, or lack of statistical power. Alternatively, these variables may not be involved in TZL pathogenesis. Prior research has found inconsistent evidence for associations of eczema, atopic dermatitis, hay fever, and allergy with NHL and PTCL in humans,10, 65, 66 and thus it is unclear whether they have an etiologic role or not. Future investigations into these variables may be warranted because we cannot confidently differentiate measurement error from a lack of biologic significance in our study.
4.7. Strengths and limitations
Prior epidemiologic studies of PTCL have been limited by small sample sizes, requiring researchers to consider PTCL as a whole instead of evaluating subtype‐specific risk factors. Our study considers only 1 PTCL subtype among a relatively genetically homogeneous population, enhancing our ability to detect risk factors. In addition, we used a comprehensive questionnaire to evaluate disease history and environmental exposures, allowing us to assess a greater scope of risk factors than prior studies. To our knowledge, ours is the largest study assessing subtype‐specific risk factors for lymphoma in dogs.
Another strength of our study is the ability to rule out LPD in the control population using flow cytometric screening, thereby minimizing misclassification of disease status. It is still possible that our control dogs will develop TZL later in life, but we have minimized that likelihood by selecting older dogs and ruling out current disease. Because TZUS have not been described previously, there are no clear diagnostic criteria for this group. We chose a systematic flow cytometric cut point to minimize the number of TZUS in the control group, thereby maximizing specificity.
Measurement error is a concern with self‐reported questionnaires, especially because we asked owners to recall exposures and disease histories over the course of their dog's lifetime. Because the questionnaire was long and comprehensive and owners did not know our exposures of interest, we believe it is unlikely that there was extreme over‐(or under‐) reporting of our exposures of interest. However, measurement error likely arose through the collapsing of variables because of (1) sparseness of data and (2) our hypothesis that owners were unable to distinguish among certain diseases. In both these circumstances, collapsing variables resulted in an inability to evaluate distinct disease mechanisms individually, which likely attenuated effect estimates. Collapsing also limited incorporation of age at diagnosis in our analysis of health history variables, restricting temporality assessment. However, our study has provided a valuable foundation to inform further investigation into TZL pathogenesis. Future studies may be able to obtain larger sample sizes and more detailed information, including veterinarian‐confirmed diagnoses for the variables suggested to be important in our study.
5. CONCLUSIONS
Overall, our study contributes to the body of evidence that chronic immune stimulation may be important in TZL pathogenesis. Our findings raise the possibility that dampening inflammation using omega‐3 supplements may help prevent TZL. We also identified hypothyroidism as a potential protective factor, but the mechanism of this relationship is unclear.
The ability to identify TZUS poses a unique opportunity to evaluate factors involved in progression from TZUS to TZL. Although we cannot directly evaluate TZL pathogenesis in our study, we can gain insights from our results. We identified risk factors unique to TZL (mange and gastrointestinal disease). Although the temporality of these associations is not clear, they may reflect likelihood of progression from TZUS to TZL. We also identified factors that may be associated with likelihood of developing CD45− T cells (TZUS), including eye disease and bladder infection or calculi. Prospective studies that obtain detailed health and exposure histories throughout a dog's life will be paramount in furthering our understanding of the natural history of TZL.
CONFLICT OF INTEREST DECLARATION
Julia D. Labadie had salary support from the National Science Foundation (Grant No. DGE‐1450032) and from the Morris Animal Foundation.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Colorado Statue University Protocol 13‐4473A for canine blood samples.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Suppl. Table 1 Antibody panels used for immunophenotyping.
Suppl. Table 2. Modeling results for case versus control sensitivity analyses. Final model indicates the model reported as our main results (n = 140 cases, n = 147 controls). Age sensitivity analysis removed 20 cases that were < 9 years of age (n = 120 cases, n = 147 controls). Cancer sensitivity analysis removed both cases and controls with a history of or concurrent non‐lymphoma cancer (n = 119 cases, n = 111 controls). % change indicates the percent change in the OR estimate compared to the final model
Suppl. Table 3. Modeling results for TZUS versus control sensitivity analysis. Final model indicates the model reported as our main results (n = 221 TZUS, n = 147 controls). Cancer sensitivity analysis removed both TZUS and controls with a history of or concurrent non‐lymphoma cancer (n = 166 TZUS, n = 111 controls). % change indicates the percent change in the OR estimate compared to the final model.
Suppl. Figure 1. Flow cytometric analysis of peripheral blood samples. (A) Sample considered diagnostic for TZL due to homogeneous expansion of CD5+CD45− T cells (red cells; TZL). (B) Sample diagnosed as TZUS due to smaller population of CD5+CD45− T cells and absence of lymphocytosis or lymphadenopathy. (C) Sample considered a control; all T cells are CD5 + CD45+ (green cells; normal)
Suppl. Figure 2. Collapsing of variables from questionnaire for use in analysis. *Age at diagnosis was captured using the following categories: “<1 year”, “1‐3 years”, “4‐6 years”, “7‐10 years”, “Over 10 years”, and “Diagnosed with this condition, but can't remember age”.
Suppl. Figure 3 Flow chart of variable selection for multivariable modeling. All variables selected to be included in best subsets were evaluated for collinearity before proceeding with best subsets regression.
ACKNOWLEDGMENTS
We thank Morris Animal Foundation for the use of the Canine Lifetime Health Project database which was used for patient recruitment. This work had support of the Clinical Immunology Laboratory at Colorado State University.
Labadie JD, Magzamen S, Morley PS, Anderson GB, Yoshimoto J, Avery AC. Associations of environment, health history, T‐zone lymphoma, and T‐zone‐like cells of undetermined significance: A case‐control study of aged Golden Retrievers. J Vet Intern Med. 2019;33:764–775. 10.1111/jvim.15405
REFERENCES
- 1. Ito D, Frantz AM, Modiano JF. Canine lymphoma as a comparative model for human non‐Hodgkin lymphoma: recent progress and applications. Vet Immunol Immunopathol. 2014;159:192‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seelig DM, Avery P, Webb T, et al. Canine T‐zone lymphoma: unique immunophenotypic features, outcome, and population characteristics. J Vet Intern Med. 2014;28:878‐886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mizutani N, Goto‐Koshino Y, Takahashi M, Uchida K, Tsujimoto H. Clinical and histopathological evaluation of 16 dogs with T‐zone lymphoma. J Vet Med Sci. 2016;78:1237‐1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Valli VE, San Myint M, Barthel A, et al. Classification of canine malignant lymphomas according to the World Health Organization criteria. Vet Pathol. 2011;48:198‐211. [DOI] [PubMed] [Google Scholar]
- 5. Flood‐Knapik KE, Durham AC, Gregor TP, Sánchez MD, Durney ME, Sorenmo KU. Clinical, histopathological and immunohistochemical characterization of canine indolent lymphoma. Vet Comp Oncol. 2013;11(4):272‐286. [DOI] [PubMed] [Google Scholar]
- 6. Hughes KL, Labadie JD, Yoshimoto JA, Dossey JJ, Burnett RC, Avery AC. Increased frequency of CD45 negative T cells (T zone cells) in older Golden retriever dogs. Vet Comp Oncol. 2018;16(1):E109‐E116. [DOI] [PubMed] [Google Scholar]
- 7. Kyle RA, Rajkumar SV. Monoclonal gammopathy of undetermined significance. Br J Haematol. 2006;134:573‐589. [DOI] [PubMed] [Google Scholar]
- 8. Hernandez JD, Baum LG. Ah, sweet mystery of death! Galectins and control of cell fate. Glycobiology. 2002;12:127R‐136R. [DOI] [PubMed] [Google Scholar]
- 9. Mizutani N, Goto‐Koshino Y, Tsuboi M, et al. Evaluation of CD25‐positive cells in relation to the subtypes and prognoses in various lymphoid tumours in dogs. Vet Immunol Immunopathol. 2016;173:39‐43. [DOI] [PubMed] [Google Scholar]
- 10. Morton LM, Wang SS, Cozen W, et al. Etiologic heterogeneity among non‐Hodgkin lymphoma subtypes. Blood. 2008;112:5150‐5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang SS, Flowers CR, Kadin ME, et al. Medical history, lifestyle, family history, and occupational risk factors for peripheral T‐cell lymphomas: the InterLymph non‐Hodgkin lymphoma subtypes project. J Natl Cancer Inst Monogr. 2014;2014:66‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bachy E, Urb M, Chandra S, et al. CD1d‐restricted peripheral T cell lymphoma in mice and humans. J Exp Med. 2016;213:841‐857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guy MK, Page RL, Jensen WA, et al. The Golden Retriever Lifetime Study: establishing an observational cohort study with translational relevance for human health. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dupont WD, Plummer WD. Power and sample size calculations. A review and computer program. Control Clin Trials. 1990;11:116‐128. [DOI] [PubMed] [Google Scholar]
- 15. Holly VL, Sandmeyer LS, Bauer BS, Verges L, Grahn BH. Golden retriever cystic uveal disease: a longitudinal study of iridociliary cysts, pigmentary uveitis, and pigmentary/cystic glaucoma over a decade in western Canada. Vet Ophthalmol. 2016;19:237‐244. [DOI] [PubMed] [Google Scholar]
- 16. Ocular Disorders Presumed to Be Inherited in Purebred Dogs. 2nd ed. Phoenix, AZ: Genetic Committee of the American College of Veterinary Ophthalmologists; 1996. [Google Scholar]
- 17. Deehr AJ, Dubielzig RR. A histopathological study of iridociliary cysts and glaucoma in Golden retrievers. Vet Ophthalmol. 1998;1:153‐158. [DOI] [PubMed] [Google Scholar]
- 18. Maroon JC, Bost JW. Omega‐3 fatty acids (fish oil) as an anti‐inflammatory: an alternative to nonsteroidal anti‐inflammatory drugs for discogenic pain. Surg Neurol. 2006;65:326‐331. [DOI] [PubMed] [Google Scholar]
- 19. Smith WL. Cyclooxygenases, peroxide tone and the allure of fish oil. Curr Opin Cell Biol. 2005;17:174‐182. [DOI] [PubMed] [Google Scholar]
- 20. Calder PC. n‐3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83:1505S‐1519S. [DOI] [PubMed] [Google Scholar]
- 21. Calder PC. Omega‐3 fatty acids and inflammatory processes: from molecules to man. Biochem Soc Trans. 2017;45:1105‐1115. [DOI] [PubMed] [Google Scholar]
- 22. Torres de la Riva G, Hart BL, Farver TB, et al. Neutering dogs: effects on joint disorders and cancers in golden retrievers. PLoS One. 2013;8:e55937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hart BL, Hart LA, Thigpen AP, Willits NH. Long‐term health effects of neutering dogs: comparison of Labrador Retrievers with Golden retrievers. PLoS One. 2014;9:e102241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zink MC, Farhoody P, Elser SE, Ruffini LD, Gibbons TA, Rieger RH. Evaluation of the risk and age of onset of cancer and behavioral disorders in gonadectomized Vizslas. J Am Vet Med Assoc. 2014;244:309‐319. [DOI] [PubMed] [Google Scholar]
- 25. Hoffman JM, Creevy KE, Promislow DE. Reproductive capability is associated with lifespan and cause of death in companion dogs. PLoS One. 2013;8:e61082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kleinbaum DG, Klein M. Logistic Regression: A Self‐Learning Text. 3rd ed. New York: Springer‐Verlag; 2010. [Google Scholar]
- 27. Hosmer DW, Lemeshow S, Sturdivant RX. Applied Logistic Regression. 3rd ed. Hoboken, NJ: John Wiley & Sons, Inc.; 2013. [Google Scholar]
- 28. Furnival GM, Wilson RW. Regression by leaps and bounds. Dent Tech. 1974;16:499‐511. [Google Scholar]
- 29. caret: Classification and Regression Training R package version 6.0‐73 . 2016. Available at https://CRAN.R-project.org/package=caret.
- 30. Calder PC. Polyunsaturated fatty acids, inflammation, and immunity. Lipids. 2001;36:1007‐1024. [DOI] [PubMed] [Google Scholar]
- 31. Calder PC. Sir David Cuthbertson Medal Lecture. Immunomodulatory and anti‐inflammatory effects of n‐3 polyunsaturated fatty acids. Proc Nutr Soc. 1996;55:737‐774. [DOI] [PubMed] [Google Scholar]
- 32. Kinsella JE, Lokesh B, Broughton S, Whelan J. Dietary polyunsaturated fatty acids and eicosanoids: potential effects on the modulation of inflammatory and immune cells: an overview. Nutrition. 1990;6:24‐44. discussion 59‐62. [PubMed] [Google Scholar]
- 33. Gosselin SJ, Capen CC, Martin SL. Histologic and ultrastructural evaluation of thyroid lesions associated with hypothyroidism in dogs. Vet Pathol. 1981;18:299‐309. [DOI] [PubMed] [Google Scholar]
- 34. Graham PA, Refsal KR, Nachreiner RF. Etiopathologic findings of canine hypothyroidism. Vet Clin North Am Small Anim Pract. 2007;37:617‐631. [DOI] [PubMed] [Google Scholar]
- 35. Chang ET, Bälter KM, Torrång A, et al. Nutrient intake and risk of non‐Hodgkin's lymphoma. Am J Epidemiol. 2006;164:1222‐1232. [DOI] [PubMed] [Google Scholar]
- 36. Fritschi L, Ambrosini GL, Kliewer EV, Johnson KC, Group CCRER . Dietary fish intake and risk of leukaemia, multiple myeloma, and non‐Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2004;13:532‐537. [PubMed] [Google Scholar]
- 37. Johansson AS, Norén‐Nyström U, Larefalk A, Holmberg D, Lindskog M. Fish oil delays lymphoma progression in the TLL mouse. Leuk Lymphoma. 2010;51:2092‐2097. [DOI] [PubMed] [Google Scholar]
- 38. Logas D, Kunkle G. Double‐blinded cross‐over study with marine oil supplementation containing high‐dose eicosapenaenoic acid for the treatment of canine pruritic skin disease. Vet Dermatol. 1994;5:99‐104. [DOI] [PubMed] [Google Scholar]
- 39. Bond R, LLoyd D. Randomized single‐blind comparison of an evening primrose oil and fish oil combination and concentrates of these oils in the management of canine atopy. Vet Dermatol. 1992;3:215‐219. [PubMed] [Google Scholar]
- 40. Roush JK, Cross AR, Renberg WC, et al. Evaluation of the effects of dietary supplementation with fish oil omega‐3 fatty acids on weight bearing in dogs with osteoarthritis. J Am Vet Med Assoc. 2010;236:67‐73. [DOI] [PubMed] [Google Scholar]
- 41. Moreau M, Troncy E, Del Castillo JR, Bédard C, Gauvin D, Lussier B. Effects of feeding a high omega‐3 fatty acids diet in dogs with naturally occurring osteoarthritis. J Anim Physiol Anim Nutr (Berl). 2013;97:830‐837. [DOI] [PubMed] [Google Scholar]
- 42. Norris CR, Williams BJ, Ling GV, Franti CE, Johnson, Ruby AL. Recurrent and persistent urinary tract infections in dogs: 383 cases (1969‐1995). J Am Anim Hosp Assoc. 2000;36:484‐492. [DOI] [PubMed] [Google Scholar]
- 43. Ling GV, Norris CR, Franti CE, et al. Interrelations of organism prevalence, specimen collection method, and host age, sex, and breed among 8,354 canine urinary tract infections (1969‐1995). J Vet Intern Med. 2001;15:341‐347. [PubMed] [Google Scholar]
- 44. Ling GV. Therapeutic strategies involving antimicrobial treatment of the canine urinary tract. J Am Vet Med Assoc. 1984;185:1162‐1164. [PubMed] [Google Scholar]
- 45. McGhie JA, Stayt J, Hosgood GL. Prevalence of bacteriuria in dogs without clinical signs of urinary tract infection presenting for elective surgical procedures. Aust Vet J. 2014;92:33‐37. [DOI] [PubMed] [Google Scholar]
- 46. Wan SY, Hartmann FA, Jooss MK, Viviano KR. Prevalence and clinical outcome of subclinical bacteriuria in female dogs. J Am Vet Med Assoc. 2014;245:106‐112. [DOI] [PubMed] [Google Scholar]
- 47. Anderson LA, Atman AA, McShane CM, Titmarsh GJ, Engels EA, Koshiol J. Common infection‐related conditions and risk of lymphoid malignancies in older individuals. Br J Cancer. 2014;110:2796‐2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Brložnik M, Šterk K, Zdovc I. Prevalence and resistance patterns of canine uropathogens in regard to concurrent diseases. Berl Munch Tierarztl Wochenschr. 2016;129:340‐350. [PubMed] [Google Scholar]
- 49. Shih CJ, Chen YT, Ou SM, Yang WC, Chen TJ, Tarng DC. Urinary calculi and risk of cancer: a nationwide population‐based study. Medicine (Baltimore). 2014;93:e342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Slatter's Fundamentals of Veterinary Ophthalmology. St. Louis, MO: Elsevier Inc.; 2008. [Google Scholar]
- 51. Gelatt KN, Gilger BC, Kern TJ. Veterinary Ophthalmology. 5th ed. Hoboken, NJ: John Wiley & Sons, Inc.; 2013. [Google Scholar]
- 52. Donzel E, Arti L, Chahory S. Epidemiology and clinical presentation of canine cataracts in France: a retrospective study of 404 cases. Vet Ophthalmol. 2017;20:131‐139. [DOI] [PubMed] [Google Scholar]
- 53. Engels EA, Cerhan JR, Linet MS, et al. Immune‐related conditions and immune‐modulating medications as risk factors for non‐Hodgkin's lymphoma: a case‐control study. Am J Epidemiol. 2005;162:1153‐1161. [DOI] [PubMed] [Google Scholar]
- 54. Hussell T, Isaacson PG, Crabtree JE, Spencer J. Helicobacter pylori‐specific tumour‐infiltrating T cells provide contact dependent help for the growth of malignant B cells in low‐grade gastric lymphoma of mucosa‐associated lymphoid tissue. J Pathol. 1996;178:122‐127. [DOI] [PubMed] [Google Scholar]
- 55. Hussell T, Isaacson PG, Crabtree JE, Spencer J. The response of cells from low‐grade B‐cell gastric lymphomas of mucosa‐associated lymphoid tissue to helicobacter pylori. Lancet. 1993;342:571‐574. [DOI] [PubMed] [Google Scholar]
- 56. Lecuit M, Abachin E, Martin A, et al. Immunoproliferative small intestinal disease associated with campylobacter jejuni. N Engl J Med. 2004;350:239‐248. [DOI] [PubMed] [Google Scholar]
- 57. Skopek E, Patzl M, Nachreiner RF. Detection of autoantibodies against thyroid peroxidase in serum samples of hypothyroid dogs. Am J Vet Res. 2006;67:809‐814. [DOI] [PubMed] [Google Scholar]
- 58. Vanura K, Le T, Esterbauer H, et al. Autoimmune conditions and chronic infections in chronic lymphocytic leukemia patients at diagnosis are associated with unmutated IgVH genes. Haematologica. 2008;93:1912‐1916. [DOI] [PubMed] [Google Scholar]
- 59. Anderson LA, Gadalla S, Morton LM, et al. Population‐based study of autoimmune conditions and the risk of specific lymphoid malignancies. Int J Cancer. 2009;125:398‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fallah M, Liu X, Ji J, Försti A, Sundquist K, Hemminki K. Autoimmune diseases associated with non‐Hodgkin lymphoma: a nationwide cohort study. Ann Oncol. 2014;25:2025‐2030. [DOI] [PubMed] [Google Scholar]
- 61. Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992‐2001. Blood. 2006;107:265‐276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Smith A, Crouch S, Lax S, et al. Lymphoma incidence, survival and prevalence 2004‐2014: sub‐type analyses from the UK's Haematological Malignancy Research Network. Br J Cancer. 2015;112:1575‐1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sant M, Allemani C, Tereanu C, et al. Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood. 2010;116:3724‐3734. [DOI] [PubMed] [Google Scholar]
- 64. van Leeuwen MT, Turner JJ, Joske DJ, et al. Lymphoid neoplasm incidence by WHO subtype in Australia 1982‐2006. Int J Cancer. 2014;135:2146‐2156. [DOI] [PubMed] [Google Scholar]
- 65. Doody MM, Linet MS, Glass AG, et al. Leukemia, lymphoma, and multiple myeloma following selected medical conditions. Cancer Causes Control. 1992;3:449‐456. [DOI] [PubMed] [Google Scholar]
- 66. Legendre L, Barnetche T, Mazereeuw‐Hautier J, Meyer N, Murrell D, Paul C. Risk of lymphoma in patients with atopic dermatitis and the role of topical treatment: a systematic review and meta‐analysis. J Am Acad Dermatol. 2015;72:992‐1002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. Table 1 Antibody panels used for immunophenotyping.
Suppl. Table 2. Modeling results for case versus control sensitivity analyses. Final model indicates the model reported as our main results (n = 140 cases, n = 147 controls). Age sensitivity analysis removed 20 cases that were < 9 years of age (n = 120 cases, n = 147 controls). Cancer sensitivity analysis removed both cases and controls with a history of or concurrent non‐lymphoma cancer (n = 119 cases, n = 111 controls). % change indicates the percent change in the OR estimate compared to the final model
Suppl. Table 3. Modeling results for TZUS versus control sensitivity analysis. Final model indicates the model reported as our main results (n = 221 TZUS, n = 147 controls). Cancer sensitivity analysis removed both TZUS and controls with a history of or concurrent non‐lymphoma cancer (n = 166 TZUS, n = 111 controls). % change indicates the percent change in the OR estimate compared to the final model.
Suppl. Figure 1. Flow cytometric analysis of peripheral blood samples. (A) Sample considered diagnostic for TZL due to homogeneous expansion of CD5+CD45− T cells (red cells; TZL). (B) Sample diagnosed as TZUS due to smaller population of CD5+CD45− T cells and absence of lymphocytosis or lymphadenopathy. (C) Sample considered a control; all T cells are CD5 + CD45+ (green cells; normal)
Suppl. Figure 2. Collapsing of variables from questionnaire for use in analysis. *Age at diagnosis was captured using the following categories: “<1 year”, “1‐3 years”, “4‐6 years”, “7‐10 years”, “Over 10 years”, and “Diagnosed with this condition, but can't remember age”.
Suppl. Figure 3 Flow chart of variable selection for multivariable modeling. All variables selected to be included in best subsets were evaluated for collinearity before proceeding with best subsets regression.


