Abstract
Protein‐losing enteropathy, or PLE, is not a disease but a syndrome that develops in numerous disease states of differing etiologies and often involving the lymphatic system, such as lymphangiectasia and lymphangitis in dogs. The pathophysiology of lymphatic disease is incompletely understood, and the disease is challenging to manage. Understanding of PLE mechanisms requires knowledge of lymphatic system structure and function, which are reviewed here. The mechanisms of enteric protein loss in PLE are identical in dogs and people, irrespective of the underlying cause. In people, PLE is usually associated with primary intestinal lymphangiectasia, suspected to arise from genetic susceptibility, or “idiopathic” lymphatic vascular obstruction. In dogs, PLE is most often a feature of inflammatory bowel disease (IBD), and less frequently intestinal lymphangiectasia, although it is not proven which process is the true driving defect. In cats, PLE is relatively rare. Review of the veterinary literature (1977‐2018) reveals that PLE was life‐ending in 54.2% of dogs compared to published disease‐associated deaths in IBD of <20%, implying that PLE is not merely a continuum of IBD spectrum pathophysiology. In people, diet is the cornerstone of management, whereas dogs are often treated with immunosuppression for causes of PLE including lymphangiectasia, lymphangitis, and crypt disease. Currently, however, there is no scientific, extrapolated, or evidence‐based support for an autoimmune or immune‐mediated mechanism. Moreover, people with PLE have disease‐associated loss of immune function, including lymphopenia, severe CD4+ T‐cell depletion, and negative vaccinal titers. Comparison of PLE in people and dogs is undertaken here, and theories in treatment of PLE are presented.
Keywords: crypt, hypoalbuminemia, lymphangiectasia, lymphatic, panhypoproteinemia, PLE
Abbreviations
- APCs
antigen presenting cells
- ATIII
antithrombin III
- BECs
blood endothelial cells
- CCECAI
canine chronic enteropathy clinical activity index
- CD
Crohn's disease
- CE
chronic enteropathy
- CIBDAI
canine IBD activity index
- CRP
C‐reactive protein
- CT/MRI
computerized tomography/magnetic resonance imaging
- EFA
essential fatty acid
- α1 PI
alpha 1 protease inhibitor
- FISH
fluorescence in situ hybridization
- GALT
gut‐associated lymphoid tissue
- GI
gastrointestinal
- GL
granulomatous lymphangitis
- HS
heparan sulfate
- IBD
inflammatory bowel disease
- IL
intestinal lymphangiectasia
- IMPDH
inosine monophosphate dehydrogenase
- IP
immunophenotyping
- LCFA
long‐chain fatty acids
- LECs
lymphatic endothelial cells
- LN
lymph node
- MCFA
medium‐chain fatty acids
- MCT
medium‐chain triglycerides
- PARR/PCR
PCR for antigen‐receptor rearrangements
- PIL
primary intestinal lymphangiectasia
- PLE
protein‐losing enteropathy
- PLN
protein‐losing nephropathy
- RER
resting energy requirement
- PTE
pulmonary thromboembolism
- SCFA
short‐chain fatty acids
- SCWT
Soft Coated Wheaten Terrier
- SI
small intestinal
- TE
thromboembolism
- UC
ulcerative colitis
- UFH
unfractionated heparin sulfate
- YT
Yorkshire Terrier
- YT‐PLE
Yorkshire Terrier protein‐losing enteropathy
1. COMPARATIVE PATHOPHYSIOLOGY OF PROTEIN‐LOSING ENTEROPATHY
In dogs and people, protein‐losing enteropathy (PLE) is associated with numerous related or unrelated diseases (Table 1), but there are a limited number of mechanisms by which plasma protein is lost through the gastrointestinal (GI) tract1, 2, 3:
Physical or functional lymphatic obstruction leading to “overflow” lymph leak, for example, congenital or acquired lymphatic disease.
Release of cellular mediators affecting vascular permeability and causing fluid egress into tissues, for example, widespread mast cell activation, eosinophilic gastroenteropathy.
Mucosal inflammation (nonerosive or erosive/ulcerative), for example, inflammatory bowel disease (IBD).
Table 1.
Causes of PLE in people and dogs
| People | Dogs |
|---|---|
| 1. Mucosal injury | |
| a. Erosive | |
| Inflammatory bowel diseases (Crohn's disease, ulcerative colitis) | Inflammatory bowel diseases (lymphoplasmacytic, eosinophilic, granulomatous) |
| Infections: Giardia, Clostridium, Campylobacter, Salmonella, rotavirus, Whipple's disease, intestinal tuberculosis | Infections: Parvovirus, Clostridium, Campylobacter, Salmonellosis, Histoplasmosis, Schistosomiasis (Heterobilharzia americana) |
| Neoplasia | Neoplasia |
| Nonsteroidal enteropathy | Nonsteroidal enteropathy |
| b. Non‐Erosive | |
| Menetrier's disease (hypertrophic gastritis) | Diet‐induced enteropathy |
| Eosinophilic gastritis | Immunoproliferative enteropathy |
| Celiac disease | Hypoadrenocorticism |
| Lactose or other food intolerance | Intestinal crypt disease |
| Systemic lupus erythematosus | |
| Intestinal crypt disease | |
| 2. Infectious | |
| Lymphatic filariasis | Lymphatic filariasis (RARE) |
| Hookworms | |
| Strongyloides stercoralis | |
| 3. Lymphatic disease | |
| Idiopathic primary IL (Waldmann's disease) | Idiopathic primary IL |
| Secondary IL: Crohn's disease, neoplasia sarcoidosis, congestive heart failure, restrictive pericarditis | Secondary IL: IBD, neoplasia, lymphatic infections, right‐sided congestive cardiac failure |
| Fontan surgery | Lymphangitis (granulomatous/inflammatory) |
| Genetic: Lymphodysplasia (Hennekam's syndrome) | |
| Lymphangitis | |
Abbreviations: IBD, inflammatory bowel disease; IL, intestinal lymphangiectasia.
In each mechanism, protein‐rich fluid accumulates in the interstitium and passes into the GI tract via mucosal tight junctions, without requiring mucosal epithelial disruption or ulceration (Figure 1).3, 4 Albumin is the most affected protein in people because of its slow turnover rate, which is why protein loss in PLE is diagnosed by detection of hypoalbuminemia.5 As a water‐soluble protein in dogs and people, albumin maintains plasma oncotic pressure and transports hormones, fatty acids, ions, and bilirubin. In health, enteric losses in people occur as sloughed enterocytes and normal secretions, and account for around only 1‐2% of the entire protein pool and less than 10% of total albumin.6 In steady state, albumin broken down approximates albumin synthesized in people, but in PLE, protein wasting can amount to 60% of the total protein pool.7, 8 The most severely diminished are long half‐life proteins with limited ability to respond rapidly—albumin, immunoglobulins, and ceruloplasmin.5 Hepatic synthesis in PLE is normally accelerated so that proteins with shorter half‐lives such as IgE, clotting factors, prealbumin, and transferrin are preserved near normal.1, 5
Figure 1.
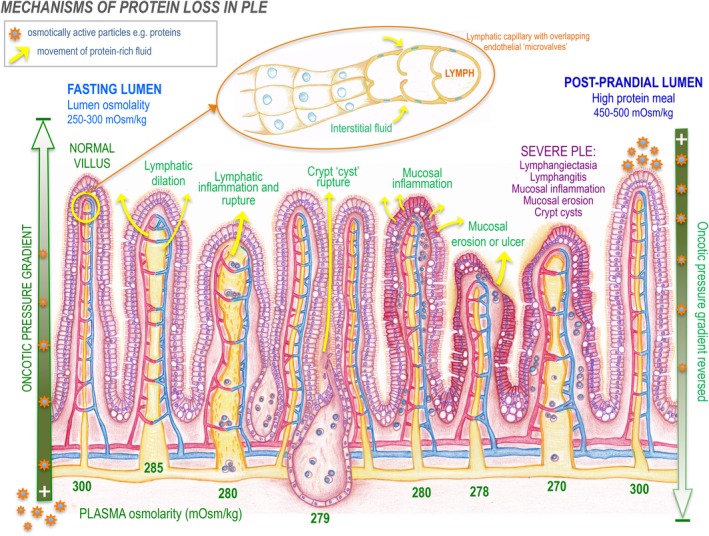
Diagrammatic representation of “PLE syndrome” diseases in dogs (lymphangiectasia, lymphangitis, crypt lesions, IBD, mucosal ulceration) and the effect of Starling's forces on intestinal osmolality and the interstitial‐lumenal oncotic gradient. Protein leaks into the small intestine passively, via an oncotic gradient from the interstitium to the lumen. If osmotic pressures equilibrate, a reduction in passive leak is expected. When the intestinal lumen has a higher oncotic pressure than the interstitium, such as postprandially, the gradient may be reversed, depending on the underlying pathology. Top: Magnification of lymphatic capillary structure, showing overlapping endothelial cells that act as flap‐like doors allowing interstitial fluid to enter while preventing lymph exit. IBD, inflammatory bowel disease; PLE, protein‐losing enteropathy
Protein‐losing enteropathy can be asymptomatic but can also present as a life‐threatening condition with effusions, lymphedema, and in people, severe disfigurement. Immune defenses are disrupted because of loss of lymphocytes, globulins, iron, calcium, and other serum components (lipids, fat soluble vitamins).2, 4, 5, 7, 8
The immunological deficits documented in PLE are numerous in people, including impaired antibody function, B‐cell depletion, diminished IgG, IgA, and IgM, and T‐cell depletion, especially CD4+ T cells.2 Despite profoundly low CD4+ counts, opportunistic infection appears to be uncommon in people.9, 10 Similar studies are lacking in dogs, but depletion in these key components is to be similarly expected.
Sudden death caused by thromboembolism (TE) occurs in PLE and can manifest in dogs that have clinically “silent” disease.11 The mechanism is not well defined, but suggested causes of PLE‐associated thrombosis include systemic inflammation, altered vitamin K absorption, loss of antithrombin III (ATIII), hyperaggregation of platelets, hyperfibrinogenemia, and vascular compromise.11, 12, 13
2. LYMPHATIC STRUCTURE AND FUNCTION
The lymphatics are a unidirectional vascular network forming an extensive drainage system throughout the body with roles of extracellular fluid homeostasis, fat absorption and transport, and immune system function.3 Running parallel with the venous system, the lymphatic system is broadly divided into lymphatic capillaries (where interstitial fluid enters), prenodal and postnodal vessels, and collecting vessels.3 Collecting vessels drain to the thoracic duct via discrete regional lymph nodes (LNs) before joining the confluences of large veins.3, 10 Specialized lymphatic endothelial cells (LECs), with structural similarity to blood endothelial cells (BECs), form lymphatic capillary walls. Derived embryologically from the outbranching of cardinal vein BECs, LECs are under control of genes such as Prospero Homeobox Protein 1, Lymphatic Vessel Endothelial Receptor 1, Podoplanin, and Vascular Endothelial Growth Factor 3.14, 15, 16, 17, 18
2.1. Lymphatic capillaries
The LECs of capillary walls are not tightly joined, but only the cell edges overlap, forming “flap‐like” valves (Figure 1).19 Lymphatic endothelial cells have no support (mural) cells but attach to the extracellular matrix via fine collagen filaments that anchor to surrounding tissues. This allows LECs to operate like swinging doors, receiving interstitial fluid in a strictly 1‐way system.3 When pressure in the lymphatic capillary exceeds interstitial pressure, the endothelial “door” flap remains shut, preventing lymph from leaving.3, 20 But when interstitial pressure exceeds lymphatic capillary pressure, the “doors” flap open and interstitial fluid moves to the lymph system.20 Not only fluid but also other substances such as proteins and pathogens, inflammatory and neoplastic cells can join lymph in this manner.21, 22 Interstitial proteins enter lymph capillaries easily, despite being too large to enter blood capillaries.22
2.2. Collecting lymphatics and lymphangions
Lymphatic capillaries merge and drain into precollecting vessels. The spindle‐shaped LECs of precollectors are continuously adherent, with “zipper”‐like junctions.23 Intraluminal valves prevent backflow of lymph, and smooth muscle cells enable intrinsic contractility.20, 21, 24 Precollectors merge into larger collecting vessels, made up of a series of subunits called “lymphangions” (the section between 2 intraluminal valves).24, 25 The collecting vessel wall is organized into a layer of continuously adherent LECs (intima), a basement membrane comprising laminin and type IV collagen, and circumferential smooth muscle (media).26, 27 Lymphangions act individually and in groups, forming contractile units that propel lymph via intraluminal valves made of connective tissue covered with LECs.23, 25, 26
2.3. Intestinal lymphatic networks
The microanatomy of the intestinal lymphatics is described in ultrastructural studies, and in dogs and people there are 3 layers: central villus lymphatics (lacteals), submucosal lymphatics, and smooth muscle lymphatics.19, 27 The lacteals connect to the submucosal lymphatic network with abundant interconnections. Injection of tracers shows free flow of fluid throughout the submucosal network and into adjacent villi, suggesting a valveless system lacking in smooth muscle and spontaneous contraction.20 The lymphatic vessels draining the muscle wall form a separate and noncommunicating network. Both networks transport lymph to collecting lymphatics24 with 1‐way valves and spontaneous contractions propelling lymph away from the intestine to the mesentery.3, 20
2.4. Tissue fluid homeostasis
Lymphatic capillaries maintain tissue fluid homeostasis by absorbing and transporting extravasated fluid to the venous circulation. Starling demonstrated that lymphatics play an important role in the regulation of hydrostatic and oncotic pressure throughout the entire body.28 From the capillary blood, fluid filters continuously into the interstitium and a dynamic equilibrium is reached between filtration, reabsorption, and tissue fluid clearance by lymphatics.3 The extravasation of fluid is dictated by Starling forces, and in people there can accumulate up to 8 L during 24 hours.21 Traditionally, venous reabsorption was thought to clear extravasated fluid, but there is now comprehensive published scientific evidence21, 22, 24, 28 that venous reabsorption is nonexistent in most vascular beds during steady‐state conditions.21, 22 Lymph transport is both passive and active. Extrinsic factors such as arterial pulsation, skeletal muscle contraction, and respiration cause passive compression and expansion of the small capillary lymphatics. Intrinsic spontaneous contractions are generated in larger vessels, and in people intraluminal lymph pressure can reach up to 100 mm Hg.21 Lymphatic vessel contractions are influenced by many different stimuli: humoral, neuronal, pressure, temperature, and shear stress.21, 24 They show reactivity, either inhibitory or stimulatory, to a vast number of vasoactive substances such as substance P, nitric oxide, noradrenaline, histamine, endothelin, and prostaglandins.21, 24, 25 Despite evidence of lymphatic innervation, most consistently described as sympathetic, the exact role of the nerve supply in lymphatic function is unclear.3
The drainage function of lymphatic vessels is crucial for resolving inflammatory reactions in the interstitium, and during inflammation, lymphatics can proliferate through lymphangiogenesis to remove debris directly or via phagocytic cells.3, 17, 21 Development and proliferation of lymphatic vessels is primarily mediated by vascular endothelial growth factor C in pathological states.29
2.5. Lymph nodes
The LNs, spleen, and other secondary lymphoid organs (eg, intestinal Peyer's patches) are anatomically located to capture foreign antigen and antigen presenting cells (APCs) for immune surveillance.30 Afferent lymphatic vessels traverse the fibrous capsule of the LN and drain lymph into the subcapsular sinus and cortex. The cortex has a paracortex, populated by T cells (for antigen presentation by dendritic cells), and a germinal center, comprised B cells (for humoral immune responses). Lymph drains through the cortex via trabecular sinuses and into the central medullary region via medullary sinuses. The systemic blood circulation interacts with the LN via high endothelial venules in the cortex.25, 31 Lymph then drains from the medulla toward the hilus and exits the LN via an efferent lymph vessel, effectively clearing and filtering extravascular fluid from tissues throughout the entire body.
2.6. Immune surveillance
Lymphatic vessels function in immune surveillance as the major conduit of antigens and immune cells from the periphery, through the tissues of the immune system. The highly organized microarchitecture of LNs hosts peripheral APCs to meet and inform naive T cells.25, 32 The largest immunologic organ is the GI tract, where immunologic barrier function plays a vital role in maintaining homeostasis. The gut‐associated lymphoid tissue (GALT) consists of the intestinal lamina propria cells, intraepithelial lymphocytes, Peyer's patches, and mesenteric LNs.33 Lymphocytes continuously recirculate from the bloodstream into intestinal lymph through GALT, to exert immune control in almost all tissues.34 Mature lymphocytes migrate from blood to tissues, and back to the bloodstream via lymph, once or twice a day, until the cell either finds its antigen or is removed by apoptosis.35
2.7. Intestinal lymphatics
Dietary fat is absorbed into lacteals (Figure 1) and transported as chyle to the bloodstream. Resin casts of canine small intestinal (SI) lymphatics have been produced using methacrylate resin and imaging by scanning electron microscopy.19 The casts show the lymphatic canal of the jejunum as a single, blind‐ending, central tubule, with circular constrictions at the villus tip, and oval indentations corresponding to impressions by endothelial nuclei (Figure 2). The central lacteals of the ileum in dogs are smaller, slender, leaf‐like shapes and drain to the lymphatic plexus, above the muscularis layer.19
Figure 2.
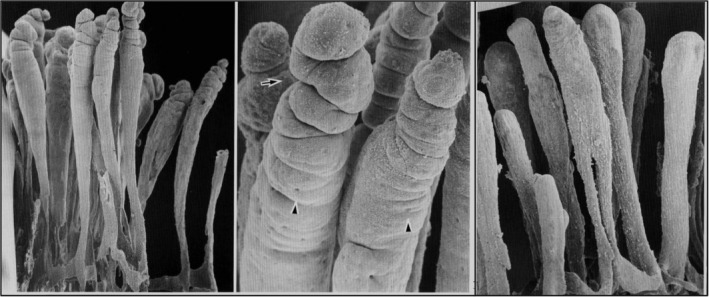
A scanning electron microscopy view (left) of a corrosion cast of central lacteals in canine jejunum. A higher magnification (center) showing circular constrictions (arrow) near the top of the lacteals. Arrowheads show imprints of endothelial nuclei. Central lacteals in the ileum (right) are slender leaf‐like forms. ×110. From Yamanaka et al.,23 with permission
2.8. Fat absorption
The lacteals absorb essential fatty acids (EFAs) from the bowel. Most ingested fat in a normal diet is taken up by the lacteals and transported to the venous circulation.36 Fatty acids are classified as short‐chain, (SCFAs, <6 carbons), medium‐chain, (MCFAs, 6‐12 carbons), or long‐chain fatty acids (LCFAs, 12 carbons).36 Most dietary fat is in the form of LCFAs and is absorbed by the enterocyte to form chylomicrons, which enter lymph via the lacteal. In addition to lymphatic absorption, medium‐chain triglycerides (MCTs) are water soluble and can pass through enterocytes into the portal blood, bypassing the lymphatics.37 In people and dogs, MCTs are more efficiently digested and absorbed in this way, and the resulting MCFAs are transported directly to the liver to be metabolized.36, 37, 38, 39
2.8.1. PLE in people
In people, PLE is usually caused by primary intestinal lymphangiectasia (PIL),3 described in 1961 by Dr. Thomas A. Waldmann's 18 cases of “idiopathic hypercatabolic hypoproteinemia.”4 These patients had edema associated with hypoproteinemia, low serum albumin, and serum gammaglobulins. The total exchangeable albumin pool, assessed with radiolabeled 131I‐albumin, was low in all patients, and daily fecal excretion of 131I was twice that of healthy controls. Intestinal histology showed dilatation of lymph vessels in the mucosa and submucosa and the authors proposed the term “intestinal lymphangiectasia” (IL). Known since as Waldmann's disease, PIL can also be asymptomatic.40
The tortuous and dilated lacteals of the mucosa and submucosa in PIL continually leak protein‐rich lymph into the bowel. Patients usually present in childhood with edema, weight loss, diarrhea, and lymphopenia with no obvious cause. Edema is pitting because oncotic pressure is low, and moderate serous effusions (peritoneal, pleural, pericardial) are common.40, 41 The worldwide incidence of PIL in people is unknown, but familial forms are rare and there is no predilection for sex or race.2, 40 Pathophysiology is unclear, but theories include genetic anomaly and lymphatic obstruction.42 Lymphatic obstruction theory says that lymphatic hypoplasia or idiopathic malformation causes obstruction and pressure‐induced rupture of cystically dilated lymphatic vessels.42 Genetic theory implicates genes with vital roles in lymphogenesis, that is, VEGF3, prospero‐related homeobox‐transcriptional factor, forkhead transcriptional factor, and SOX18.43 Mutation of CCBE1, a gene that produces a protein with functions in extracellular matrix remodeling and migration, is known to cause generalized lymphatic dysplasia (lymphedema and lymphangiectasia) in Hennekam syndrome, an inherited autosomal recessive disorder.44
Secondary IL occurs when a primary disease process obstructs lymphatic vessels or when increased venous pressure induces lymphatic hypertension (Table 1). In people, secondary IL has been associated with constrictive pericarditis, lymphoma, Whipple's disease, sarcoidosis, intestinal tuberculosis, and Crohn's disease (CD).2, 40 In CD, numerous studies describe lymphangitis, lymphangiectasia, lymphatic bacterial infiltration, and LN infection.45 Some authors suggest that the core driving pathology in CD is lymphatic disease, with secondary vasculitis and transmural inflammation.46, 47, 48 In other diseases where PLE occurs, lymphatics are not the root cause and protein is lost via mucosal epithelium by intercellular leak or exudation,2 for example, eosinophilic gastroenteropathy, Menetrier's disease, autoimmune enteropathy, and systemic lupus erythematosus.40
2.8.2. PLE in dogs
In contrast to people, PLE in dogs is usually associated with lymphoplasmacytic enteritis (LPE) rather than PIL (Table 1). In the last 30 years, published data on PLE includes 23 articles, spanning from 1977 to 2018 (Table 2).12, 13, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69 Taken together, they describe in total 469 dogs of 61 different breeds, most prevalent the Yorkshire Terrier (YT), Border Collie, German Shepherd, and Rottweiler (Figures 3 and 4). Historical features commonly described are ascites, vomiting, diarrhea, weight loss, polyuria and polydipsia, anorexia, weight loss, and lethargy. Clinical examination findings commonly include abdominal distension (ascites), cachexia, muscle wasting, weakness, depression, dyspnea/tachypnea, and abdominal pain. Clinical scoring systems including CIBDAI70 (canine IBD activity index) and CCECAI71 (canine chronic enteropathy clinical activity index) were compared in 8 studies and did not always concur: CIBDAI scores indicated “mild IBD” (score 4‐5) or “severe IBD” (score ≥ 9), whereas CCECAI scores indicated “clinically insignificant disease,” (score 0‐3) “moderate disease”(score 6‐8) or “very severe disease” (score ≥ 12).
Table 2.
Types of data presented in published papers on PLE in dogs (single case reports excluded)
| First author | Study n | Deaths due to PLE (%) | CIBDAI • and CCECAI * | Clinical history | Clinical exam | Clinical pathology | Imaging | Endoscopic description | Endoscopic score | Histology description | WSAVA score | Diet | Treatment | Survival |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Equilino 201559 | 29 | 17 (74) |
•
* |
‐ | ‐ | • | • | ‐ | ‐ | • | ‐ | • | • | • |
| Okanishi 201463 | 24 | 3 (13.6) | * | ‐ | ‐ | • | ‐ | • | ‐ | • | • | • | • | • |
| Simmerson 201464 | 30 | 11 (40.7) | ‐ | • | • | • | • | ‐ | ‐ | • | • | • | • | • |
| Goodwin 201113 | 15 | 10 (66.7) | * | • | • | • | ‐ | ‐ | ‐ | • | ‐ | ‐ | ‐ | ‐ |
| Willard a 200365 | 6 | 4 (66.7) | ‐ | • | • | • | ‐ | • | ‐ | • | ‐ | • | • | ‐ |
| Kull 200112 | 17 | 6 (35.3) | ‐ | • | • | • | • | ‐ | ‐ | • | ‐ | ‐ | ‐ | ‐ |
| Dandrieux 201367 | 27 | 15 (55.6) | ‐ | ‐ | ‐ | • | ‐ | ‐ | ‐ | ‐ | • | • | • | • |
| Nakashima 201568 | 92 | 53 (60.0) |
•
* |
‐ | ‐ | • | ‐ | ‐ | ‐ | ‐ | • | • | • | • |
| Kimmel 200050 | 5 | 2 (40) | ‐ | • | • | • | • | • | ‐ | • | ‐ | • | • | • |
| Littman 200051 | 76 | 51 (72.9) | ‐ | • | ‐ | • | ‐ | ‐ | ‐ | • | ‐ | • | ‐ | • |
| Van Kruiningen 198455 | 4 | 2 (66.7) | ‐ | • | • | • | ‐ | ‐ | ‐ | • | ‐ | ‐ | ‐ | ‐ |
| Watson 200856 | 6 | 1 (20.0) | ‐ | • | ‐ | • | • | ‐ | ‐ | • | ‐ | • | • | • |
| Gianella 201757 | 59 | 26 (44.1) | * | • | • | • | ‐ | ‐ | ‐ | • | • | • | • | • |
| Allenspach 201760 | 43 | 22 (51.2) | * | ‐ | ‐ | • | ‐ | ‐ | ‐ | ‐ | ‐ | • | • | • |
| Rudinsky 201758 | 11 | 4 (44.4) | * | • | ‐ | • | • | ‐ | ‐ | • | ‐ | • | • | • |
| Willard b 200361 | 2 | 1 (50) | ‐ | • | • | • | • | • | ‐ | • | ‐ | • | • | • |
| Djikstra 201062 | 17 | 11 (64.7) | • | • | ‐ | • | ‐ | ‐ | • | • | • | ‐ | • | • |
indicates data present, ‐ indicates data absent.
Abbreviations: CCECAI, canine chronic enteropathy clinical activity index; CIBDAI, canine IBD activity index; PLE, protein‐losing enteropathy; WSAVA, World Small Animal Veterinary Association.
Figure 3.
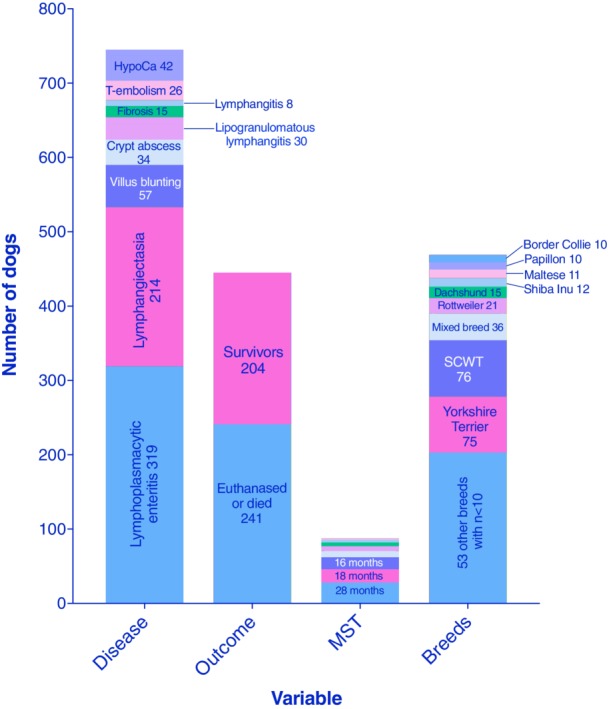
Cumulative data from 23 PLE articles spanning from 1977 to 2017, involving a total of 469 dogs. HypoCa, hypocalcemia; MST, median survival time; PLE, protein‐losing enteropathy; SCWT, Soft Coated Wheaten Terrier; T‐embolism, thromboembolism12, 13, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69
Figure 4.

Breed frequencies represented across canine PLE publications; numbers on bars indicate the number of dogs within the breed.12, 13, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69 PLE, protein‐losing enteropathy
The cumulative median serum albumin level, reported in 21 of 23 studies, was 1.49 g/dL, globulins 1.95 g/dL, and total serum proteins 3.35 g/dL (Figure 5), and low serum cobalamin was reported in 8 of 23 studies.13, 52, 54, 56, 64, 66, 67, 69 Small bowel diseases were LPE, in 319 of 469 dogs (68%) and lymphangiectasia in 214 dogs (58%, Figure 3). Inflammatory lymphatic disease, including lipogranulomatous foci and lymphangitis, was present in 38 dogs (8%). Crypt disease was present in 34 dogs (7.2%). Histology was scored according to the standards of the World Small Animal Veterinary Association (WSAVA) GI Standardization Group72 in 6 studies, with detailed scores published in 3 (Figure 6).
Figure 5.
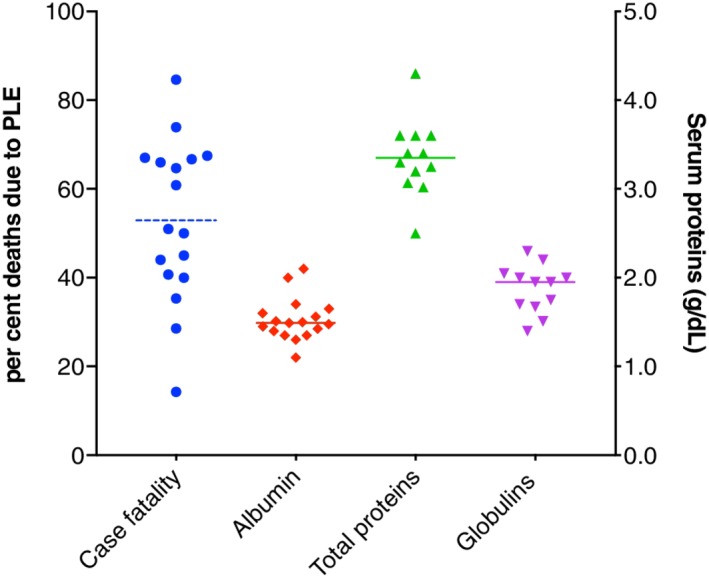
Cumulative disease‐associated deaths and serum proteins reported in PLE publications (solid line median, dotted line mean).12, 13, 49, 50, 51, 52, 53, 54, 55, 56, 57, 61, 64, 65, 66, 67, 69 PLE, protein‐losing enteropathy
Figure 6.
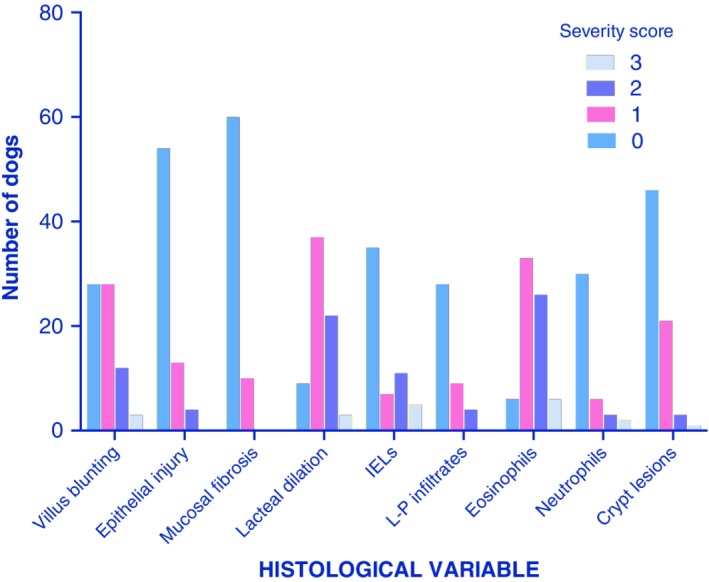
Cumulative histology scores from 3 canine studies of PLE, per the WSAVA GI standardization group scoring system.48, 53, 65GI, gastrointestinal; PLE, protein‐losing enteropathy. Numbers on bars are the number of dogs
Treatment was described in 16 of 23 reports.49, 51, 52, 53, 54, 55, 56, 57, 59, 61, 62, 64, 65, 66, 67, 69 An immune‐mediated cause was nearly always presumed, as shown by the use of immune suppressive medication in 15 of 16 publications (Figure 7).49, 51, 52, 53, 54, 55, 56, 57, 59, 61, 62, 64, 65, 66, 67, 69 Prednisolone was most frequently used (15 studies) followed by azathioprine (9 studies), cyclosporine (9 studies), chlorambucil (4 studies), and methotrexate (1). Metronidazole and other antimicrobials were each prescribed in 8 studies. Dietary change to low‐fat, limited antigen, hydrolyzed, or home‐cooked food was implemented in 14 studies and MCT provision in 2.
Figure 7.
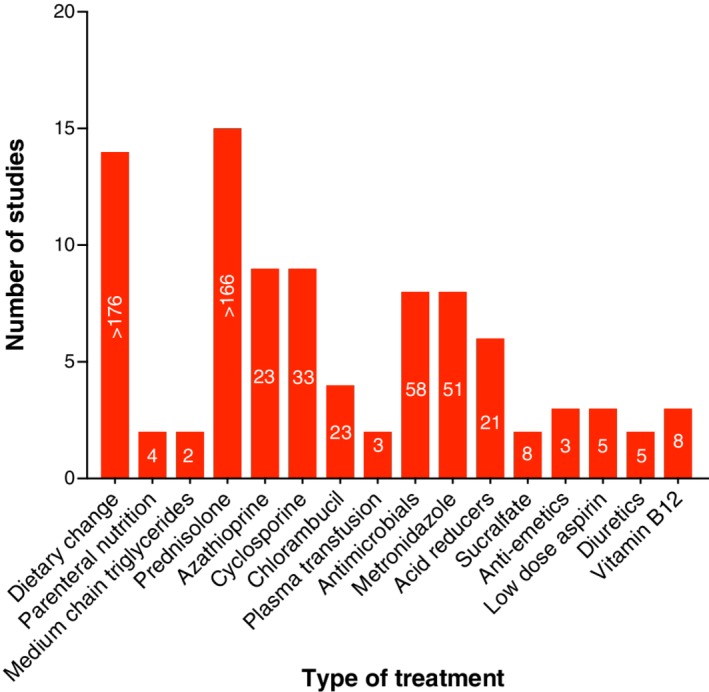
Cumulative treatment data in PLE across studies; white numbers on each bar indicate the minimum number of dogs that received each specific treatment.49, 51, 52, 53, 54, 55, 56, 57, 61, 64, 65, 66, 67, 69 PLE, protein‐losing enteropathy
Median survival times, reported in 8 studies, were highly variable (months: 1.0, 2.2, 5, 5, 8.4, 16, 18, and 28). Complicating factors included TE in 26 dogs and hypocalcemia in 42 dogs. Follow‐up information was available for 445 of 469 cases (94.8%) from 23 publications, and disease‐associated death occurred in 54.2%, (204 survivors and 241 dead or euthanized because of PLE). Various negative prognostic factors were reported in 6 separate studies and are listed in Table 3. Factors repeatedly identified include medium bodyweight (2 studies),65, 69 altered serum urea (3 studies),52, 56, 57 and decreased serum albumin (4 studies).52, 56, 57, 69
Table 3.
Negative prognostic indicators and other findings from published reports of PLE in dogs (1977–2017)
| First author | Study n | Prognostic indicators | Other findings |
|---|---|---|---|
| Equilino 201559 | 29 | NEGATIVE:
|
|
| Okanishi 201463 | 24 | Serum CRP level did not differ significantly between pre‐treatment and 1 or 2 months post‐treatment in responders and non‐responders. | |
| Simmerson 201464 | 30 | Significant predictors of death within 4 months of diagnosis were:
|
|
| Dandrieux 201367 | 27 |
|
Duration of primary treatment was shorter with chlorambucil‐prednisolone treatment than azathioprine‐prednisolone (P = 0.004). |
| Nakashima 201568 | 92 |
|
|
| Kimmel 200050 | 5 | Yorkshire Terriers with PLE were 9.2 times more likely to develop hypomagnesemia and hypocalcemia than other breeds. | |
| Gianella 201757 | 59 |
|
All short‐term survivors (19/59) were unresponsive to immune suppression. |
| Allenspach 201760 | 43 | NEGATIVE:
POSITIVE:
|
|
In summary, published reports to date show that PLE in dogs is associated with lymphangiectasia in around 50% of cases and LPE in approximately 66%. Lymphangitis and crypt disease each occur in <10%, and other types of IBD (granulomatous, eosinophilic) are rare causes of PLE. Immune suppressive treatment is often prescribed, usually corticosteroids, and sometimes 2 or 3 immune suppressive agents concurrently. Dietary modification is frequently but not always implemented. Although prolonged survival can occur, over half of dogs (54.2%) with PLE die from their disease or from pulmonary thromboembolism (PTE) as a complicating factor. It is interesting to note that, in contrast, disease‐associated deaths from 2 published outcome studies of IBD in dogs71, 73 were considerably lower at 13%73 and 18%,71 suggesting that the PLE underlying disease mechanisms are not merely a more severe continuum of IBD spectrum pathophysiology. Perhaps, this is not unexpected when considering the functions of lymph fluid in immune defense, and the role of albumin in systemic immunomodulatory and anti‐inflammatory activities, antioxidant properties, and endothelial stabilization.74
2.8.3. Lymphangiectasia in dogs
Primary IL, a diagnosis of exclusion, is a relatively rare problem of unknown etiology in dogs. Breed predispositions support a genetic susceptibility in the Soft Coated Wheaten Terrier (SCWT), Norwegian Lundehund, Yorkshire and Maltese Terriers, and Shar‐Pei.11 Studies of PIL with scanning electron microscopy in dogs show grossly dilated lacteals that rupture and leak protein‐rich fluid into the lumen and interstitium (Figure 1).19 Lymph is supposedly a local tissue irritant, causing inflammation and granuloma formation,46 which presents a unique challenge in the histological determination of primary versus secondary IL, also lymphangitis and vasculitis. Other potential diagnostic pitfalls are the similar appearance of blood and lymphatic endothelium on routine stains, and that the drainage function of lymphatics during inflammation stimulates the appearance of new vessels (lymphangiogenesis).3, 75, 76 Consequently, diagnosis of IL is not straightforward, especially without the use of differentiative histological markers. The immunohistochemical markers Prox‐1 and CD31 separate lymphatic from blood capillary endothelium.75 In the SCWT, lymphatic dilatation (17% prevalence) is reported to be strongly associated with lymphangitis (35% prevalence), and less so with IBD, whereas the presence of all 3 lesions concurrently was rare.49
Secondary IL results from direct blockade to lymphatics, for example, neoplasia, or an indirect block, for example, dense inflammatory reaction and impaired drainage. It is reported that IL exists in a high proportion of endoscopic biopsies (44/83; 53%) from dogs with chronic GI disease, and in this patient population, there was a significant association of IL with hypoalbuminemia (28/37; 76%).77 In close agreement, on full thickness biopsy, 59% (38/64) of dogs with chronic GI disease were shown to have IL.78
Published evidence showing an immune‐mediated etiology of IL in dogs (and people) is totally lacking.11, 79 The obvious place to seek an anomaly is in the lymphatic endothelium or in the fine collagen fibers that anchor lymphatic capillaries to surrounding connective tissue, but none has ever been described.
2.8.4. Granulomatous lymphangitis
Lymphangitis and granuloma formation are rare in dogs with PLE.11 Clinical presentation is typical for PLE but can also include fever and abdominal pain.51 The histological description is of transmural intestinal granulomas containing large “multinucleate giant cells,” neutrophils, macrophages, and lymphoplasmacytic infiltrates surrounding lymphatics.50, 80 The lesions of granulomatous lymphangitis (GL) are not always localized to the bowel. In some cases involvement of the entire mesentery, LNs, and intrathoracic lymphatics goes undetected until laparatomy or necropsy because lymphatic vascular imaging and magnetic resonance imaging (MRI) are not routine procedures.50, 80 Across species, granulomatous bowel disease is particularly associated with infectious agents, for example, Whipple's Disease in people and Tropheryma whipplei, Mycobacterium paratuberculosis in Johne's disease of cattle, and Escherichia coli in Granulomatous Colitis of the Boxer dog. In GL, an etiology is rarely found but could include infectious, parasitic, and neoplastic causes.11, 51 An immune‐mediated basis is often also cited but not proven. A report of 10 dogs with GL uncovered no evidence of a bacterial cause; however unusual bacteria can evade (fluorescence in situ hybridization; FISH) detection.80
2.8.5. Intestinal crypt pathology
Crypt disease is increasingly recognized as a cause of PLE in dogs although by an unknown mechanism. The YT is overrepresented in the United States and Europe for PLE,56, 64, 81, 82 and is particularly susceptible to crypt pathology.56 The lesions are described as dilated cystic crypts containing sloughed epithelial cells, debris, and leucocytes67 and are often called “abscess” by pathologists but are not known to be associated with a specific pathogen (Figures 1 and 8). Their histological appearance, packed with proteinaceous and cellular debris, seems inconsistent, however, with simple cystic malformation. Parallels drawn with parvovirus infection, that is, villus collapse and fusion and crypt distension, led researchers to undertake intestinal immunostaining for parvovirus antigen in 2 dogs, which was negative.67
Figure 8.

Histology of endoscopic mucosal biopsies showing small intestinal crypt lesions from a Yorkshire Terrier with PLE (H&E stain; Aperio Digital Scan, 10×, left). Florescence in situ hybridization microscopic analysis (right, magnification ×200) with eubacterial probe (red), non‐eubacterial (green), and DAPI staining nuclear structures (blue) showing no evidence of intralesional or mucosally adherent bacteria.81 PLE, protein‐losing enteropathy
In people, crypt lesions are reported to be highly predictive of ulcerative colitis (UC).83 Increased numbers of neutrophils and mucosa‐associated bacteria are found to colonize crypts in UC, suggesting an opportunist role for bacteria in “cryptitis” or crypt “abscess” formation.83, 84 Investigation for a similar etiology in YT crypt PLE (YT‐PLE) has been undertaken.81 In this work, involving 12 YT (median age 8 years) presented for investigation of hypoalbuminemia (median albumin 1.6 g/dL), duodenal biopsies were evaluated (endoscopic n = 7 and surgical n = 5). Histology was dominated by large crypt lesions (median severity score 2.5/3), alongside lymphangiectasia (median score 2/3) and lymphoplasmacytic plus eosinophilic inflammation (median score 2.5/3) Analysis of intestinal biopsies using FISH with probes targeting “all bacteria” revealed no evidence of bacteria within or adjacent to crypt cysts, and the mucosal surface was infrequently colonized by bacteria (Figure 8). Most dogs were treated with immune suppressive prednisolone/dexamethasone (11/12) or azathioprine (2/12), antimicrobials (9/12), and dietary change. Within 3 months of diagnosis, 7 of 12 (58%) had died or were euthanized due to PLE (4/7) and suspected TE (3/7). Longer term survival occurred in 3 of 12 dogs, (10, 24, and 36 months) before death or euthanasia because of PLE. Two dogs were alive at 24 and 26 months after diagnosis, and 1 was still hypoalbuminemic (albumin 1.4‐1.9 mg/dL). The authors concluded that crypt lesions in YT‐PLE do not contain bacteria (aseptic abscess or “cyst”) and found no support for localized intestinal dysbiosis in the initiation of crypt lesions. The material contained within crypts was not precisely determined but appeared to be sloughed cells and mucus/debris. The high frequency of cysts in the biopsies examined would represent a vast number if present throughout the SI, and their rupture to the lumen could plausibly cause overwhelming protein loss. A disease histologically similar to YT‐PLE is “tufting enteropathy” of people, a form of epithelial dysplasia characterized by partial villus atrophy, crypt hyperplasia/pseudocyst formation, and epithelial disorganization. Mutations in the gene encoding epithelial cell adhesion molecule are associated with tufting enteropathy.85
2.8.6. Lymphoplasmacytic enteritis
In dogs, IBD is currently thought to arise from immune system dysfunction, adverse reaction to dietary components, intestinal bacteria (dysbiosis), and genetic susceptibility.86, 87 In PLE, IBD (“IBD‐PLE”) causes protein loss either via leaky intercellular junctions or severe mucosal exudation (Figure 1). Although inflammatory processes can alone increase protein turnover, this would not usually cause hypoproteinemia unless very severe and extensive. Secondary IL can accompany IBD, although defining primary versus secondary IL is problematic.46, 47 Now that antibodies to lymphatics are available, it might become possible to better define primary lymphatic disease versus IBD as the driving force in IBD‐PLE.
There are numerous published accounts of IBD‐PLE in dogs that describe the absence of lymphatic disease.11, 77, 88 It is unclear whether lymphatic changes are interlinked with IBD severity; however, the idea that IBD‐PLE could lack a lymphatic response deserves careful consideration when structural studies have confirmed that every villus contains a thin‐walled lymphatic capillary connected to a valveless vascular network (Figures 1 and 2). The bowel is a huge organ, and in most cases the ileum and majority of the jejunum are never sampled, so that mucosal biopsies collected from approximately 10% to 15% of intestinal surface area inevitably falsely assume the full picture of pathology. Because many (>50%) dogs with PLE die caused by their disease soon after diagnosis, it becomes important to consider all possible confounding mechanisms when managing IBD‐PLE. A safe assumption is a multifactorial process with varying degrees of lymphatic and intercellular losses and mucosal exudation. Targeted treatment would ideally encompass each contributing aspect, that is, cellular restoration and protection, mucosal barrier repair, resolving lymphatic obstruction, stasis and hypertension, and guarding against bacterial translocation across damaged mucosa.
3. DIAGNOSIS OF PLE
Differential diagnosis of moderate to severe hypoalbuminemia in dogs includes hepatic failure, protein‐losing nephropathy (PLN), and GI disease. In hypoalbuminemic dogs with normal preprandial and postprandial bile acids and normal urine protein : creatinine ratio, PLE is probable. Ultrasound appearance of mucosal thickening and duodenal striations/speckling are suggestive but not specific for IBD/lymphoma and lymphatic disease, respectively.89 Presently there are no noninvasive tests for definitive diagnosis of PLE, which necessitates intestinal biopsy. The exception is lymphoma which can occasionally be diagnosed by ultrasound‐guided fine needle aspiration cytology of bowel mucosa, abdominal LNs, and liver/spleen. Routine tests in PLE include serum chemistry, CBC, fecal culture and parasitology, urinalysis, abdominal and thoracic imaging (for PTE and comorbidities), and GI endoscopy and biopsy.11 In dogs, PLE involving severe hypoalbuminemia and effusions can occur in hypoadrenocorticism, and measuring basal serum cortisol concentration is also a consideration.90, 91, 92
3.1. Noninvasive “biomarker” tests
Various potential biomarkers of IBD and PLE have been studied, such as cobalamin, C‐reactive protein (CRP), perinuclear anti‐neutrophil cytoplasmic antibodies, S100A12 (calgranulin‐C), calprotectin, and fecal alpha‐1 protease inhibitor (α1 PI).11, 87, 93, 94, 95 Cobalamin, CRP, and α1 PI are the only commercially available tests at this time. Hypocobalaminemia is an inconsistent finding, reported in 7 of 19,96 8 of 13,13 4 of 18,69and 15 of 20 dogs65 with PLE in separate studies but was associated with a negative outcome.71 CRP was reported to be a strongly correlated marker for systemic inflammatory activity in dogs with IBD97 but in later studies was increased in only 43% of cases98 and did not correlate with disease severity by histologic grade or clinical scoring.99, 100 More recently it was found that a high CRP concentration is a negative prognostic indicator in PLE (P = .004),65 but in other work, there was no difference between responders and nonresponders.55 There is no clear consensus on the diagnostic utility of CRP, and as a nonspecific inflammatory marker, its validity across studies may depend on factors such as geographical region (endemic disease, parasitism, animal welfare standards), and laboratory methodology. More recently, fecal α1 PI, a measurable serum protease inhibitor lost into the bowel in PLE, was reported to distinguish dogs with moderate to severe intestinal crypt disease and lacteal dilatation (n = 54) from those without (n = 66) with sensitivity of 44%‐74% and specificity of 57%‐93%.100 Being a noninvasive surveillance tool for screening PLE‐susceptible breeds, α1 PI is also helpful to detect PLE in animals with concurrent PLN or hepatic insufficiency.
Fecal calgranulin‐C (S100A12) is a noninvasive marker of IBD in people.101, 102 A calcium‐binding protein, S100A12, is secreted by activated neutrophils, a cell type abundant in the intestinal mucosa of IBD in people. When tested and validated for accuracy in distinguishing dogs with IBD from healthy controls, fecal S100A12 showed a sensitivity of 65% and specificity of 84%, with a misclassification rate of 20%.100 In another study, serum S100A12 did not identify dogs with PLE; however, reference range serum S100A12 and serum calprotectin concentrations (P = .04 and P = .03, respectively), were found to be negative prognostic indicators in dogs with PLE. In people, calprotectin is another useful IBD marker,103 corresponding to a neutrophil cytosol protein with resistance to bacterial degradation. In dogs, serum calprotectin ≥296 μg/L showed sensitivity of 82.4% and specificity 68.4% for IBD detection, although it did not correlate with CIBDAI, CRP, or histology.104, 105, 106 The “gold standard” biomarkers for IBD and PLE in dogs have yet to be realized. The dissimilarities in histological appearance of human and canine diseases, for example, neutrophilic/granulomatous inflammation (people) versus lymphoplasmacytic inflammation (dogs and cats), and transmural (people) versus mucosal (dogs and cats) distribution, imply probable species specificity in a high performing biomarker.
3.2. Intestinal biopsy in PLE
Definitive diagnosis of PLE necessitates histological evaluation of intestinal biopsies. Endoscopic biopsy carries lower procedural risk than surgical biopsy owing to specific comorbidities: (1) PLE is a risk for thrombosis, a “silent” killer, with risk of clot embolization or extension during anesthesia and surgical manipulations; (2) hypovitaminosis D and hypocalcemia have known potential adverse effects on wound healing, intestinal repair, cardiac and vascular function107; (3) hypoalbuminemia was significantly associated with death or euthanasia in 2 separate IBD outcome studies in dogs,71, 73 and 4 studies of PLE.52, 56, 57, 69 Logically, posing increased physiologic demands for protein (intraoperative hemorrhage and wound healing) might be reasonably expected to increase the albumin‐associated risk of negative outcome. When, however, a focal intestinal mass is detected, (eg, lipogranulomatous lymphangitis, lymphoma), endoscopic reach can be a limiting factor in achieving a noninvasive definitive diagnosis, wherein surgical biopsy (with/without mass resection) might be required to facilitate an accurate diagnosis (assuming that fine needle aspirates were nondiagnostic).50, 51, 80, 108
Historically, it has been queried whether endoscopic biopsy is adequate for diagnosis of IL because of failure to sample submucosal lymph vessels.77, 78, 87, 88, 96 More recently, histological diagnosis of IL was reported as equivalent by endoscopic (44/83; 53%) and surgical biopsy (38/64; 59%).77, 78 The latter report, describing 38 dogs with IL, found transmural lymphatic pathology in 29 of 38 (76%) cases, suggesting that mucosal biopsies would usually be of adequate depth. Furthermore, the interconnected structure of the “web‐like” and valveless intestinal network implies that IL would not usually be confined to submucosal lymphatics without some degree of transference of lymph capillary hypertension to the lacteal. Diagnostic accuracy of endoscopic biopsy inevitably depends on sample quality88 and 6 (high quality) to 15 (moderate quality) duodenal samples are advised in dogs, except for diagnosis of crypt lesions, which reportedly requires 13‐20 biopsies. The sensitivity of endoscopy for lymphoma diagnosis was >90%, regardless of biopsy quality.88
The gross appearance of the duodenal mucosa and particularly the presence of white villus tips are inadequate to accurately predict IL, with reported sensitivity of 68% and specificity of 42% for endoscopic appearance alone in IL diagnosis.96
The endoscopic appearance of PIL in people reveals obvious, creamy yellow villi corresponding to marked dilatation of intestinal lymphatics.40 Histology confirms the presence of dilated mucosal and submucosal lymphatics, from a few millimeters to centimeters. Lacteals can be dilated in many villi or only a few. Mucosal abnormalities vary, but the absence of villous atrophy, significant mucosal/submucosal defect, and microorganisms, is key to diagnosis. Endoscopy can be negative when intestinal lesions are segmental or localized. In these cases, video capsule endoscopy or enteroscopy can be diagnostic.40 Ultrasonography can show dilatation of intestinal loops, wall thickening, severe mesenteric edema, and ascites.109 Contrast computerized tomography (CT) identifies localized IL, showing typically diffuse, nodular, small bowel thickening with or without dilatation, and a “halo sign” due to bowel swelling and edema.42, 110 Magnetic resonance imaging has been successfully applied in people with IL to evaluate for localized and systemic disease.111 The use of CT and MRI for the purpose of PLE diagnosis in dogs is not reported.
4. SPECIAL TESTS
4.1. Mucosal biopsy FISH and culture
A pathogenic role of the intestinal microbiome is not recognized in PLE; however, intestinal dysbiosis with a Gram‐negative shift is emerging as a contributor to IBD pathophysiology. It should not be forgotten that the loss of immune system components in people with PLE depletes immune barrier function,79 and so potentially, intestinal dysbiosis could be of greater severity and detriment in IBD‐PLE.83, 112, 113, 114, 115 Disrupted mucosal immunity and dysbiosis in PLE are further implied by the observation that mucosal neutrophils were significantly more often present (P = .003) in dogs with chronic enteropathy (CE) and hypoalbuminemia (17/37, 46%) than CE‐normoalbuminemia (7/46, 15%).77 In dogs at greater risk of mucosal immune compromise, for example, lymphangiectasia, ulceration, granulomatous or predominantly neutrophilic inflammation, and chronic intussusception,116, 117 analysis by FISH can be applied to search mucosae for bacterial adherence/invasion. Used in molecular microbiology to identify bacteria within formalin fixed tissues, FISH is commercially offered, at Cornell University. Where histology or FISH reveal intramucosal or mucosally adherent bacteria, intestinal specimens can be submitted for culture and sensitivity (biopsies can be stored frozen in Luria‐Bertani culture medium).
4.2. Mucosal biopsy immunophenotyping and polymerase chain reaction for antigen‐receptor rearrangements
When routine histology fails to differentiate a benign, polyclonal lymphoid proliferation from a monoclonal, neoplastic process, clarification may be achieved using immunophenotyping (IP) for lymphocyte markers118 or polymerase chain reaction (PCR) to detect clonal B and T cell populations (PCR for antigen‐receptor rearrangements).118 Both methods utilize the paraffin wax‐embedded histology block, without need for further sampling. The consensus of the WSAVA International GI Standardization Group is that IP retains precedence over clonality testing as a diagnostic procedure.72
5. MANAGEMENT OF PLE
In treating PLE, defining the precise nature of protein loss, that is, inflammatory, exudative, or structural (lymphatic or crypt distension) determines the selection of appropriate therapeutics. To optimize recovery rates, an otherwise safe assumption is that the processes of lymph fluid loss, active protein exudation, and leaky cell junctions could be present at some juncture in every affected bowel.
5.1. Diet
A low‐fat diet with supplementary MCT is the cornerstone of PLE management in people, proven to be the most effective, most widely prescribed approach and with minimal adverse effects.40, 119 Desai et al. showed that within a few weeks, a high‐protein, low‐fat diet with MCT not only improves clinical signs but also reduces mortality in people.37 The need for dietary control in people appears to be permanent, because clinical and biochemical findings reappear after low‐fat diet withdrawal.40 Dietary fat restriction prevents engorgement of intestinal lymphatics with chyle, preventing rupture of grossly distended lacteals. Medium‐chain triglycerides are directly absorbed into the portal venous circulation and provide nutrient fat without lacteal engorgement.40 The benefit of MCT should not be assumed to be restricted to lymphatic bypass. Medium‐chain triglyceride digestion is rapid and simple, without stimulating cholecystokinin secretion or utilizing the actions of bile and lipase for absorption. Absorption of MCT occurs via passive diffusion along the GI tract into the portal system bound to albumin. No further packaging or modification of MCT molecules is required. Moreover, MCTs are not dependent on the carnitine acyltransferase system for transport into the mitochondria for β‐oxidation. This provides the ability for more rapid metabolism of MCTs and improved utilization even in states of protein deficiency.120
Suggested dietary proportions for dogs with PLE are calories from carbohydrate 55‐60%, total fat calories 10‐15%, and protein calories 25‐30%, using a highly digestible protein (>87%).36 The canine diet must always contain LCFAs for provision of EFAs, and supplementation of fat‐soluble vitamins (A, D, E, K) is particularly important when dietary fat content is low. Available low‐fat commercial diets are listed in Table 4; however, the choice of diet in PLE cases is often dictated by patient appetite and preference. Many (especially YT) are historically fussy eaters (perhaps reflecting more chronic subclinical GI disease), and are frequently anorexic. Nutritional management in PLE is paramount and if not effectively implemented, the cycle of protein leak and catabolism will continue until eventually death is inevitable. In selective eaters or those with unreliable appetite, the authors routinely place an esophagostomy tube (E‐tube) during the endoscopy anesthesia. This step seems to be the key to success in PLE management for both patient (particularly YT) and owner. Often very unwell and with critically low serum proteins, these patients need every modicum of support. The pet‐owner bond can be disrupted when owners force their pet to take medications and a specific diet, and life can become unbearably stressful and anxious for both. The authors' experience is that in retrospect, the feeding tube was not infrequently a life‐saving measure. When tube feeding is initiated, a commercial low fat, blenderized canned food is supplemented up to 30% protein calories (with whey or pea protein powder) and divided into 6‐8 meals per day (resting energy requirement, RER, in kcal/day = 70 × [ideal weight in kg]0.75). It should be recognized that multiple daily feedings might not be possible in every clinical scenario. If feeding is well accepted, in underweight dogs, a gradually increasing amount over the RER may be possible. In tube fed dogs, oral alimentation is encouraged, for example, lean protein (cooked turkey/fish), low fat prescription foods, and tube feeding is adjusted according to appetite. Free amino acid preparations (eg, Vivonex) can be useful during critical stages but should not replace or delay provision of complete nutritional support. To supplement caloric density, MCT can be given in the form of mixed C8 and C10 triglycerides to provide up to 30% of total fat calories (better achieved via an E‐tube, due to variable palatability; higher levels can be ketogenic).
Table 4.
Range of currently available lower fat, hydrolyzed, and assisted feeding commercial diets for dogs at the time of writing
| Diet | Description | Form | Protein source | Protein (%) | Fat (%) | Kcal/kg | Fiber (%) | Protein kcal (%) | Fat kcal (%) |
|---|---|---|---|---|---|---|---|---|---|
| Royal Canin GI low fat | Fat restricted | Dry | Chicken by‐product meal | AF 20 | AF 4.5‐8.5 | AF 3237 | AF 1.8 | 24 | 17 |
| DM 24.2 | DM 7.1 | DM 3473 | DM 3.6 | ||||||
| Wet | Pork liver, pork by‐products, fish oil | 6.0 | 1.0‐2.5 | 895 | 2.5 | 29 | 16 | ||
| 28.8 | 6.5 | 5.2 | |||||||
| Royal Canin hypoallergenic | Hydrolyzed | Dry | Hydrolyzed soy protein, chicken fat, fish oil | 19‐21.5 | 10.5‐17 | 3491 | 1.2 | 23 | 34 |
| 26.2 | 17.5 | 3805 | 3.1 | ||||||
| Wet | Peas, hydrolyzed soy, hydrolyzed chicken liver | 24.5 | 15.1 | 1126 | 6.0 | 22 | 34 | ||
| Royal Canin Anallergenic | Hydrolyzed | Dry | Hydrolyzed poultry by‐products aggregate, fish oil | 17 | 14.5 | 3688 | 4.2 | 17 | 38 |
| 19.3 | 17.7 | 3859 | |||||||
| Royal Canin recovery | Assisted feeding | Wet | Chicken, chicken liver, fish oil | 9.5 | 5.7 | 1112 | 2.6 | 37 | 59 |
| 47.2 | 17.5 | 7.3 | |||||||
| Hill's i/d | Fat restricted | Wet | Pork, turkey, egg | 6.7 | 13.9 | 1016 | 0.6 | 23 | 32 |
| 24.6 | 14.8 | 3893 | 2.7 | ||||||
| Dry | Chicken, chicken meal | 24.5 | 13.7 | 3615 | 1.3 | 24 | 32 | ||
| 25.7 | 14.8 | 3951 | 1.6 | ||||||
| Hill's i/d low fat | Low fat | Dry | Chicken by‐product meal, pork fat | 23.7 | 6.8 | 3360 | 1.6 | 25 | 17 |
| 25.9 | 7.4 | 3672 | 1.7 | ||||||
| Wet | Pork liver and by‐products, turkey liver, egg whites | 6.5 | 2.2 | 948 | 0.6 | 24 | 20 | ||
| 25.1 | 8.5 | 3660 | 2.3 | ||||||
| Hill's a/d | Assisted feeding | Wet | Turkey liver, pork, chicken, egg | 10.6 | 7.3 | 1173 | 0.4 | 33 | 55 |
| 44.0 | 33.2 | 4796 | 1.3 | ||||||
| Hill's z/d | Hydrolyzed | Wet | Chicken liver hydrolysate | 4.9 | 3.5 | 948 | 1.2 | 18 | 31 |
| 20.0 | 14.1 | 3862 | 5.0 | ||||||
| Dry | Chicken liver hydrolysate | 17.6 | 13.3 | 950 | 4.0 | 17 | 32 | ||
| 19.1 | 14.4 | 3569 | 4.4 | ||||||
| Purina EN | GI low fat | Dry | Poultry by‐product meal, animal digest, fish oil | 26.5 | 11.9 | 3826 | 1.5 | 26.5 | 28.9 |
| 29.1 | 13.0 | 1.6 | |||||||
| Wet | Liver, soy, animal by‐products, turkey, salmon | 10.5 | 4.8 | 1088 | 0.2 | 33.4 | 36.5 | ||
| 39.5 | 17.8 | 0.6 | |||||||
| Purina EN low fat | GI lower fat | Dry | Chicken meal, animal fats | 26 | 6.2 | 3550 | 1.1 | 28.3 | 16.4 |
| 28.4 | 6.8 | 1.2 | |||||||
| Wet | Meat by‐products, chicken liver, soy protein | 11.7 | 2.3 | 959 | 0.9 | 43.1 | 20.4 | ||
| 44 | 8.6 | 3.5 | |||||||
| Purina HA | Hydrolyzed | Dry | Soy protein hydrolysate | 19.3 | 9.5 | 3723 | 1.4 | 20.1 | 24.0 |
| 21.3 | 10.5 | 1.6 |
Note that different countries may have differently formulated and named products and these may also change in content over time or be renamed, (eg, Royal Canin Anallergenic in Europe is called Royal Canin Ultamino in the United States).
Abbreviations: AF, as fed (bold text); DM: dry matter (gray text); GI, gastrointestinal.
A measurable response should be apparent promptly if nutritional intake is appropriate in content and proportion. In severely hypoalbuminemic dogs in particular (albumin <1.5 g/dL), if serum albumin has not begun to increase after 3 days, the protein proportion can be increased to 35%, aiming to achieve positive protein balance. If there is no increase after 5‐7 days, the authors switch to a different protein source, in case it should (on case‐by‐case basis) be more easily digested and absorbed, and better (immunogenically) tolerated. In some cases (author observation), animals achieve normalization of serum albumin but remain hypoproteinemic because of persistently low serum globulins and can respond further after supplementation with oral immunoglobulins, for example, bovine colostrum powder. Importantly, if no or only a partial diet response is initially apparent, the dog should not immediately be categorized as “food unresponsive,” without further trial of other low fat, restricted antigen, or hydrolyzed diets or home‐cooked food. It is feasible to begin fat restriction and dietary treatment at the animal's first presentation with GI signs and before biopsy retrieval, so that the histological picture reflects the dog's food responsiveness. This patient‐centered approach can usefully inform the clinician's decisions regarding prognosis and other treatments.53, 55, 64
5.2. Drug treatment
5.2.1. Immune modification
Immune suppressive agents are infrequently utilized in people for the treatment of most types of PLE.2, 40, 41, 42 In contrast, in dogs an immune‐mediated etiology of PLE is usually presumed11; however, there is no scientific or convincing volume of case‐based evidence that IL (also lymphangitis and crypt disease) is autoimmune or primarily immune‐mediated. Rather, the immune system can suffer the loss of components9 such that the induction of immune suppression becomes potentially a precarious step. Lymphatic disease is a particularly unknown entity in dogs, and it is interesting to note the differing numbers of disease‐associated deaths in PLE (~54%) versus IBD (<20%),71, 73 when in both diseases immune suppression with corticosteroids is the mainstay of treatment worldwide. The adverse effects of corticosteroid treatment in dogs, that is, muscle protein catabolism, thrombosis,121, 122, 123 and hyperlipidemia124 (with potential for lymphatic expansion) are particularly disadvantageous in PLE, and their use is somewhat counterintuitive. Recently, immunosuppressive treatment of PLE was significantly associated with a negative outcome (P < .001, 2/21 dogs alive), versus those treated with dietary modification alone (13/22 dogs alive).53 Clinical response to diet alone (various foods) is also reported in in 10 of 11 YT with PLE64 Interestingly, in a recent report52 all “short‐term” survivors (19 of 59 dogs with PLE) appeared unresponsive to immune suppressive treatment, which brings forth enquiry on how best to rationalize their use in this setting.
Immune suppression is often suggested to be reserved as “last resort” treatment, for IBD87 and some animals with moderate to severe change are reported to clinically respond completely to dietary management alone.71, 87, 125 The clinical severity of IBD does not always correlate with the histological severity score,72 and neither variable is demonstrated to predict the requirement for immune suppressive treatment.71, 73 Commonly used immune suppressive medications in dogs are prednisolone, cyclosporine, azathioprine, chlorambucil, and mycophenolate. In a small study, improved survival of dogs with PLE was associated with the use of prednisolone plus chlorambucil (10/14 alive) versus prednisolone plus azathioprine (2/13 alive, P = .004).69 Consideration of the type of inflammation present and agent pharmacodynamics should help to guide drug choice. Mycophenolate, chlorambucil, and azathioprine can all cause severe GI toxicosis and myelosuppression. Azathioprine is a purine antimetabolite that inhibits nucleic acid synthesis causing chromosomal breaks and disruption of cellular metabolism. Azathioprine is poorly absorbed PO and clinical response can take up to 6 weeks.126, 127 Chlorambucil is a cytotoxic alkylating agent with delay of up to 4 weeks before clinical response is appreciated.126, 127 It is well absorbed PO and highly bound to plasma proteins, which is of note when used in hypoproteinemic animals. Cyclosporine suppresses cell‐mediated immunity and might be more efficacious in suppressing T‐cell‐macrophage interactions in granulomatous inflammation.127, 128 However, it is variably absorbed PO and can be less well absorbed in the diseased bowel.129 Mycophenolate mofetil, a prodrug of mycophenolic acid, is an inhibitor of inosine monophosphate dehydrogenase (IMPDH), a rate‐limiting enzyme in nucleotide synthesis.130 Lymphocytes are particularly dependent on IMPDH, unlike other cell types (eg, neutrophils) that recycle nucleotides via a salvage pathway. Mycophenolate mofetil is thought to suppress proliferation specifically of lymphocytes130 and has many desirable properties in treating IBD: absorbed PO, “neutrophil‐sparing,” and without extensive time delay.127 However, it causes significant GI irritation, and in people has a “black box” warning (serious or life‐threatening risk) for lymphoma.126 Regarding corticosteroids, budesonide is a preparation with topical activity in the GI tract but has not been specifically evaluated for PLE treatment.127 Some practitioners use parenteral dexamethasone due to concerns that prednisolone is insufficiently PO absorbed, but in studies of people with Crohn's disease, this theory is not well supported.131, 132
5.3. Heparan sulfate
A common histological feature of PLE in people is loss of heparan sulfate (HS) from the basolateral surface of intestinal epithelial cells (in numerous seemingly unrelated PLE etiologies).133 A complex proteoglycan, HS is a large, highly sulfated glycosaminoglycan composed of alternating units of α‐N‐acetylglucosamine and α‐glucuronic acid. Heparan sulfate functions as an anticoagulant by binding ATIII and is a component of basement membranes in numerous organs, including intestinal mucosa. In the kidney, HS molecules are an important barrier against protein leak and are deficient in the glomerulus of PLN (nephrotic syndrome). Interestingly, in children born with severe PLE, deficiency of HS has been discovered in the basolateral surface of the enterocyte.134 Exogenous heparin treatment to these children reversed enteric protein loss at doses that are considered subtherapeutic for anticoagulation in people (5000 U/day SC).135 The precise mechanism by which HS relieves protein loss across the mucosa is unknown, but the effect has been reported in several other PLE‐associated diseases.135, 136, 137 The ability of HS treatment to reverse PLE in dogs is hopeful but has not yet been evaluated (see antithrombotics).
5.4. Octreotide
Octreotide is a long‐acting somatostatin analogue that suppresses GI motility and hormone secretion in the pituitary gland, pancreas, and intestine. In 1998, the efficacy of octreotide in 1 human PIL patient was first reported, and it has since, as monotherapy with MCT diet, been shown to lead to long‐term clinical, biochemical, and histological remission of PLE of differing etiologies in people.138 The mechanism of action of octreotide in diminishing protein loss through the GI tract is unclear. Somatostatin receptors exist in normal gut lymphoid tissue and on veins and venules.139 Both somatostatin and octreotide normally decrease splanchnic blood flow via vasoconstriction and also decrease intestinal motility, gastric emptying, gallbladder contraction, pancreatic secretion, and triglyceride absorption.140, 141 Theorized mechanisms of octreotide's action in PIL include decreased intestinal fat absorption, inhibition of GI vasoactive peptides, and stimulation of the autonomic nervous system.138 Octreotide is expensive and administered parenterally, and in responsive patients discontinuation usually causes PLE recurrence.142 Reported adverse effects in human clinical trials were diarrhea, abdominal pain, nausea, headache, cholelithiasis, hyperglycemia, and constipation.40, 119, 140, 143 Octreotide has been used in dogs for the treatment of insulinoma.144
5.5. Antithrombotics
The published data described here suggests that TE occurs in around 6% of PLE cases but is almost certainly underestimated, being a “silent” killer.13, 145, 146Prophylaxis with antithrombotic medication could prevent death and is usually well tolerated, inexpensive, and with minimal adverse effect. There is no consensus on optimal antithrombotic treatment in PLE, and options include unfractionated heparin sulfate (UFH), low‐molecular‐weight heparin, clopidogrel, and low‐dose aspirin. In consideration of the markedly beneficial effect on PLE in people, UFH would be a logical choice but is poorly tolerated by painful SQ injection q8h. For years, UFH was thought to be minimally absorbed PO, supported by minimal changes in plasma APTT levels after oral administration, but sublingual and oral UFH absorption has since been demonstrated in numerous species including dogs.147, 148, 149 There is now published evidence of reliable oral UFH absorption in healthy dogs, evidenced by ATIII inhibition and anti‐Xa activity.150 Recovery of UFH from both plasma and urine in people and dogs also supports that UFH administered PO is absorbed and widely distributed.148, 150 In dogs, no negative effects were observed to indicate UFH‐induced hemorrhage, excessive ATIII consumption, or thrombocytopenia during oral heparin administration.150 Further studies determining optimal oral dosage in dogs, and clinical benefit in disease states has not yet emerged. Clopidogrel and low‐dose aspirin are frequently used as antiplatelet agents in dogs with PLE, either alone or in combination.145 The optimal combination, dosages, and required duration of treatment is unknown; however, dogs in complete clinical remission of PLE can die suddenly from TE,145 which suggests a benefit of prolonged treatment.
5.5.1. Other
Other medications used empirically to treat IBD and PLE are antimicrobials (metronidazole, tylosin, doxycycline), gut protectants (acid reducers, sucralfate), antiemetics, and probiotics.11, 36 The use of gut protectants might help to maintain a lumenal environment that favors ongoing repair of the mucosal barrier. If mucosal ulceration or neutrophilic inflammation are present, broad spectrum antimicrobial treatment might be warranted, for example, doxycycline, amoxycillin‐clavulanate, cephalexin, and FISH analysis alongside culture data would also serve to guide antimicrobial treatment. Cobalamin deficiency has been associated with a negative prognosis,93 and supplementation by oral and parenteral routes is described.151 Although rare, folate deficiency can be corrected by oral supplementation. Corrections of deficits in calcium and magnesium in PLE have been described by Kimmel et al.,61 by administration of “constant rate infusions of magnesium sulfate (1.0 mEq/kg/d [0.5 mEq/lb/d], IV) and calcium gluconate solution (10 mg/kg/h, IV) until correction of the electrolyte abnormalities was achieved.” Specific dosages and preparations of fat soluble vitamins A, D, E, and K in PLE are not described; however, it is unlikely that the use of a reputable veterinary supplement at the recommended daily dosage would result in vitamin toxicosis. Diagnosis of specific vitamin deficiencies and their resolution after supplementation would necessitate individual quantification of vitamin levels. Addressing concurrent problems such as nausea/emesis (eg, maropitant, ondansetron), abdominal pain (eg, tramadol, oral buprenorphine, cautious paracetamol), intestinal dysmotility (eg, metoclopramide, cisapride) is also important. The use of appetite stimulants in animals with GI disease is controversial. In dogs, reduced appetite is nearly always an indication of another problem such as nausea, pain, dysmotility, and use of appetite stimulants can mask a debilitating untreated problem. Antioxidant treatment and reduction of environmental stressors might also be helpful.152, 153, 154, 155 Complications of peripheral limb edema can be avoided by using support wraps, vigilant monitoring for skin breakdown, gentle grooming and cleaning, frequent ambulation, and massage. A deep, conforming mattress will provide greater comfort when ascites and limb edema are present. Diuretics are not generally useful because the edema relates to a reduction in plasma oncotic pressure.41
5.5.2. Oncotic support
Measures to increase plasma oncotic pressure such as the use of hydroxyethyl starch (Hetastarch 6%, HS), plasma transfusion, and purified canine albumin are only transiently helpful in PLE. Proteins administered IV are leaked into the GI tract, and this can in itself be detrimental due to the potential for aggravated loss of lymph and immune system components. However, this can also be life‐saving in management of critically unwell patients and facilitates anesthesia and diagnostic procedures. Plasma volume expansion produced by HS approximates that of 5% albumin, for 24‐36 hours. When TE is suspected, HS or purified canine albumin is preferable over plasma transfusion to avoid thrombus extension. The provision of parenteral nutrition via a central venous catheter is another means of increasing plasma oncotic pressure while providing nutritional support, with the unfortunate caveat of higher risks of sepsis, local infection/abscess, and thrombosis.13, 74, 156, 157
5.6. Theories on PLE management
5.6.1. Stopping the leak
The lymphatic circulation is devoid of any central pump. To achieve continuous lymph flow external compression is necessary, via intestinal peristalsis, movement of body parts, arterial pulsations, and tissue compression by external forces (eg, gravitational, weight bearing, massage). When lacteals are grossly dilated, the overlapping LECs might not be able to maintain their door‐like function to ensure the unidirectional flow of interstitial fluid into lymphatic capillaries (Figure 1). Without this 1‐way system, the external compressive “pumps” could merely contribute to lymph entering interstitial tissues and the bowel lumen, so that an unrelenting cycle of lymph leakage, local irritation, protein loss, and edema formation develops. Anterograde lymph flow then becomes largely dependent on passive (Starling) forces. A steady state could be reached by catabolism of interstitial tissue proteins or lymphangiogenesis to restore lymph drainage, until these compensatory mechanisms are also exhausted.158 Animals that present with panhypoproteinemia and effusions are frequently in a late stage of a dangerous “PLE cycle” disease.
It follows that diminishing the protein leak might be achieved by (1) emphasis on Starling forces (2) by modifying intestinal peristalsis, and (3) bypass of the affected intestinal area, if PLE is confirmed to be segmental.
5.6.2. Emphasis on Starling forces
Osmolality of the human duodenum in fasted state is 200‐250 mOsm/kg, versus serum osmolality, 290‐300 mOsm/kg.159 In healthy people, the SI environment changes on a time‐after‐food basis. The effect of different “fed states” on SI intraluminal osmolality was evaluated by administering the nutritional drink Ensure Plus.159 Fasted state osmolality values were in general hypo‐osmotic, with overall median value of 224 mOsm/kg, whereas hyperosmotic values (as high as 600 mOsm/kg, median 285 mOsm/kg) were found in fed states during the first 3 hours of digestion.159 In dogs, fasted intestinal lumen osmolality is around 200‐250 mOsm/kg and serum osmolality 290‐310 mOsm/kg.160
Applying Starling's forces to maintain an osmotic gradient between the bowel lumen and the interstitium should be helpful in PLE management. The bowel is continually leaking across a large surface area, with no means to “plug” the leak. But by continually matching intestinal intraluminal with interstitial osmolality, an iso‐osmotic oncotic gradient develops and the rate of protein loss by passive Starling forces must slow or stop. Depending on the underlying pathology (eg, lymphatic obstruction versus functional stasis), maintaining a mildly hyperosmolar lumen reverses the oncotic pressure gradient to stimulate anterograde lymph flow and protein absorption (Figure 1). It is not practicable to continually monitor lumen and serum osmolality, but an informative baseline could be measured at endoscopy. An iso‐ or mildly hyperosmotic lumen could theoretically be achieved by feeding small, frequent, high‐protein/low‐fat meals every 2‐3 hours so that the bowel is in the fed state more of the time. Protein requirements in people are normally 0.6‐0.8 g/kg/day, and in PLE this value may increase 5‐fold (3.0‐4.0 g/kg/day) before a positive protein balance is reached.42 The optimal level of protein supplementation in dogs with PLE has never been determined. A warmed, liquid consistency, low‐fat diet appears logically advantageous over dried or dense wet food to reduce GI “work” and to optimize enterocyte contact and surface tension for maximal nutrient absorption.
5.6.3. Modifying intestinal peristalsis
Intestinal peristalsis passively facilitates lymph transport and a hypomotile bowel can develop lymph stasis. Studies of GI motility in PLE are lacking; however, bowel swelling and edema might alter GI transit times. Motility can be assessed radiographically using barium impregnated polyethylene spheres, barium fluoroscopy, radionuclide transit, or video capsule endoscopy. When dysmotility is present, prokinetic treatment, for example, metoclopramide (0.2‐0.5 mg/kg PO q8h) or cisapride (0.1‐0.5 mg/kg PO q8h), should be considered, with careful monitoring for signs of worsening hypoproteinemia or deteriorating clinical condition. Enhanced bowel contractility could also serve to accelerate protein and lymph loss and is likely to be more safely initiated alongside a well implemented feeding plan.
5.6.4. Bypass of segmental PLE
Enterectomy for localized areas of lymphangiectasia has been curative in people with segmental PLE.161 The reasons for disease localization to a particular anatomical area are unknown, but improvement can be only transient because of disease recurrence. Segmental PLE has been diagnosed using (99 m) Tc‐labeled albumin scintigraphy.162, 163 Segmental presentations in dogs and breed‐specific anatomical localization are not recognized so far but are unexplored phenomena. Serial monitoring using scintigraphy (with radiolabeled canine albumin) could help to identify dogs with lesion localization that might be safely amenable to surgical treatment.
6. CONCLUSIONS
In dogs, PLE is most frequently associated with lymphangiectasia and IBD and is managed empirically with immune suppressive agents, most frequently prednisolone, azathioprine, cyclosporine, and chlorambucil, as well as dietary change and antimicrobials. The use of immunosuppressive agents remains controversial in any of these disorders and is not utilized in people for the treatment of most types of PLE. Lifelong dietary management is the cornerstone of treatment, and recent studies in dogs support this approach.53, 64 Current treatment is associated with >50% fatality, which logically drives management toward the safest approach until pathophysiology is better understood and specific, nonempirical treatment can be formulated. The investigative history of E. coli‐associated granulomatous colitis in the Boxer dog (GC) highlights exceptionally well that the rationale for exerting immune suppression, albeit as a “last resort” treatment, invites careful consideration. Historically, for around a half century, GC was often treated with immune suppression and with usually fatal outcome. When clinical observations with antimicrobials drove the discovery of an infectious etiology, GC became largely curable.164, 165 An excellent relationship with the internist and a high level of owner support are also a crucial aspect of managing PLE.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
Craven MD, Washabau RJ. Comparative pathophysiology and management of protein‐losing enteropathy. J Vet Intern Med. 2019;33:383–402. 10.1111/jvim.15406
REFERENCES
- 1. Braamskamp MJAM, Dolman KM, Tabbers MM. Clinical practice. Protein‐losing enteropathy in children. Eur J Pediatr. 2010;169(10):1179‐1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Freeman HJ, Nimmo M. Intestinal lymphangiectasia in adults. World J Gastrointest Oncol. 2011;3(2):19‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rovenská E, Rovenský J. Lymphatic vessels: structure and function. Isr Med Assoc J. 2011;13(12):762‐768. [PubMed] [Google Scholar]
- 4. Waldmann TA, Steinfeld JL, Dutcher TF, Davidson JD, Gordon RS. The role of the gastrointestinal system in idiopathic hypoproteinemia. Gastroenterology. 1961;41:197‐207. [PubMed] [Google Scholar]
- 5. Takeda H, Ishihama K, Fukui T, et al. Significance of rapid turnover proteins in protein‐losing gastroenteropathy. Hepatogastroenterology. 2003;50(54):1963‐1965. [PubMed] [Google Scholar]
- 6. Schmidt PN, Blirup‐Jensen S, Svendsen PJ, Wandall JH. Characterization and quantification of plasma proteins excreted in faeces from healthy humans. Scand J Clin Lab Invest. 1995;55(1):35‐45. [DOI] [PubMed] [Google Scholar]
- 7. Wochner RD, Weissman SM, Waldmann TA, Houston D, Berlin NI. Direct measurement of the rates of synthesis of plasma proteins in control subjects and patients with gastrointestinal protein loss. J Clin Invest. May 1, 1968;47(5):971‐982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Waldmann TA, Morell AG, Wochner RD, Strober W, Sternlieb I. Measurement of gastrointestinal protein loss using ceruloplasmin labeled with copper. J Clin Invest. 1967;46(1):10‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Magdo HS, Stillwell TL, Greenhawt MJ, et al. Immune abnormalities in Fontan protein‐losing enteropathy: a case‐control study. J Pediatr. 2015;167(2):331‐337. [DOI] [PubMed] [Google Scholar]
- 10. Winkler S, Linke A, Adams V, Schuler G, Erbs S. How to improve endothelial repair mechanisms: the lifestyle approach. Expert Rev Cardiovasc Ther. 2010;8(4):573‐580. [DOI] [PubMed] [Google Scholar]
- 11. Dossin O, Lavoué R. Protein‐losing enteropathies in dogs. Vet Clin North Am Small Anim Pract. 2011;41(2):399‐418. [DOI] [PubMed] [Google Scholar]
- 12. Kull PA, Hess RS, Craig LE, Saunders HM, Washabau RJ. Clinical, clinicopathologic, radiographic, and ultrasonographic characteristics of intestinal lymphangiectasia in dogs: 17 cases (1996‐1998). J Am Vet Med Assoc. 2001;219(2):197‐202. [DOI] [PubMed] [Google Scholar]
- 13. Goodwin LV, Goggs R, Chan DL, Allenspach K. Hypercoagulability in dogs with protein‐losing Enteropathy. J Vet Intern Med. 2011;25(2):273‐277. [DOI] [PubMed] [Google Scholar]
- 14. Harvey NL, Srinivasan RS, Dillard ME, et al. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult‐onset obesity. Nat Genet. 2005;37(10):1072‐1081. [DOI] [PubMed] [Google Scholar]
- 15. Johnson NC, Dillard ME, Baluk P, et al. Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes Dev. 2008;22(23):3282‐3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Srinivasan RS, Dillard ME, Lagutin OV, et al. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev. 2007;21(19):2422‐2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oliver G, Alitalo K. The lymphatic vasculature: recent progress and paradigms. Annu Rev Cell Dev Biol. 2005;21(1):457‐483. [DOI] [PubMed] [Google Scholar]
- 18. Birke K, Drecoll E, Kerjaschki D, Birke MT. Expression of podoplanin and other lymphatic markers in the human anterior eye segment. Investig Opthalmol Vis Sci. 2010;51(1):344. [DOI] [PubMed] [Google Scholar]
- 19. Yamanaka Y, Araki K, Ogata T. Three‐dimensional organization of lymphatics in the dog small intestine: a scanning electron microscopic study on corrosion casts. Arch Histol Cytol. 1995;58(4):465‐474. [DOI] [PubMed] [Google Scholar]
- 20. Unthank JL, Bohlen HG. Lymphatic pathways and role of valves in lymph propulsion from small intestine. Am J Physiol. 1988;254(3 Pt 1):G389‐G398. [DOI] [PubMed] [Google Scholar]
- 21. Adamczyk LA, Gordon K, Kholová I, et al. Lymph vessels: the forgotten second circulation in health and disease. Virchows Arch. 2016;469:3‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ikomi F, Hunt J, Hanna G, Schmid‐Schönbein GW. Interstitial fluid, plasma protein, colloid, and leukocyte uptake into initial lymphatics. J Appl Physiol. 1996;81(5):2060‐2067. [DOI] [PubMed] [Google Scholar]
- 23. Baluk P, Fuxe J, Hashizume H, et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204(10):2349‐2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miller MJ, McDole JR, Newberry RD. Microanatomy of the intestinal lymphatic system. Ann N Y Acad Sci. 2010;1207:E21‐E28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weitman E, Cuzzone D, Mehrara BJ. Tissue engineering and regeneration of lymphatic structures. Future Oncol. 2013;9(9):1365‐1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Määttä M, Liakka A, Salo S, Tasanen K, Bruckner‐Tuderman L, Autio‐Harmainen H. Differential expression of basement membrane components in lymphatic tissues. J Histochem Cytochem. 2004;52(8):1073‐1081. [DOI] [PubMed] [Google Scholar]
- 27. Ohtani O, Ohtani Y. Organization and developmental aspects of lymphatic vessels. Arch Histol Cytol. 2008;71:1‐22. [DOI] [PubMed] [Google Scholar]
- 28. Starling EH. On the absorption of fluids from the connective tissue spaces. J Physiol. 1896;19(4):312‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nurmi H, Saharinen P, Zarkada G, Zheng W, Robciuc MR, Alitalo K. VEGF‐C is required for intestinal lymphatic vessel maintenance and lipid absorption. EMBO Mol Med. 2015;7(11):1418‐1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol. 2006;7(4):344‐353. [DOI] [PubMed] [Google Scholar]
- 31. von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3(11):867‐878. [DOI] [PubMed] [Google Scholar]
- 32. Randolph GJ, Angeli V, Swartz MA. Dendritic‐cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5(8):617‐628. [DOI] [PubMed] [Google Scholar]
- 33. Garden OA. Gastrointestinal Immunology In: Washabau RJ, Day MJ, eds. Canine & Feline Gastroenterology. St Louis, MO: Elsevier Saunders; 2013:42‐53. [Google Scholar]
- 34. Young AJ, Hay JB. Rapid turnover of the recirculating lymphocyte pool in vivo. Int Immunol. 1995;7(10):1607‐1615. [DOI] [PubMed] [Google Scholar]
- 35. Salmi M, Jalkanen S. How do lymphocytes know where to go: current concepts and enigmas of lymphocyte homing. Adv Immunol. 1997;64(8):139‐218. [DOI] [PubMed] [Google Scholar]
- 36. Sanderson SL. Nutritional strategies in GI disease In: Washabau RJ, Day MJ, eds. Canine and Feline Gastroenterology. St Louis, MO: Elsevier Saunders; 2013:409‐428. [Google Scholar]
- 37. Desai AP, Guvenc BH, Carachi R. Evidence for medium chain triglycerides in the treatment of primary intestinal lymphangiectasia. Eur J Pediatr Surg. 2009;19(4):241‐245. [DOI] [PubMed] [Google Scholar]
- 38. Alfano V, Tritto G, Alfonsi L, Cella A, Pasanisi F, Contaldo F. Stable reversal of pathologic signs of primitive intestinal lymphangiectasia with a hypolipidic, MCT‐enriched diet. Nutrition. 2000;16(4):303‐304. [DOI] [PubMed] [Google Scholar]
- 39. Beynen AC, Kappert HJ, Lemmens AG, Van Dongen AM. Plasma lipid concentrations, macronutrient digestibility and mineral absorption in dogs fed a dry food containing medium‐chain triglycerides. J Anim Physiol Anim Nutr (Berl). 2002;86(9–10):306‐312. [DOI] [PubMed] [Google Scholar]
- 40. Vignes S, Bellanger J. Primary intestinal lymphangiectasia (Waldmann's disease). Orphanet J Rare Dis. 2008;3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Umar SB, DiBaise JK. Protein‐losing enteropathy: case illustrations and clinical review. Am J Gastroenterol. 2010;105(1):43‐49. [DOI] [PubMed] [Google Scholar]
- 42. Siddeswari R, Manohar S, Reddy T, Suryanarayana B, Abhilash T, Devender. Primary intestinal lymphangiectasia. Indian Acad Clin Med. 2016;17(1):60‐63. [Google Scholar]
- 43. Hokari R, Kitagawa N, Watanabe C, et al. Changes in regulatory molecules for lymphangiogenesis in intestinal lymphangiectasia with enteric protein loss. J Gastroenterol Hepatol. 2008;23(7 PT2):e88‐e95. [DOI] [PubMed] [Google Scholar]
- 44. Alders M, Hogan BM, Gjini E, et al. Mutations in CCBE1 cause generalized lymph vessel dysplasia in humans. Nat Genet. 2009;41(12):1272‐1274. [DOI] [PubMed] [Google Scholar]
- 45. Von Der Weid P‐Y, Rehal S, Ferraz JG. Role of the lymphatic system in the pathogenesis of Crohn's disease. Pathology. 2002;34(6):335‐341. [DOI] [PubMed] [Google Scholar]
- 46. Sura R, Colombel J‐F, Van Kruiningen HJ. Lymphatics, tertiary lymphoid organs and the granulomas of Crohn's disease: an immunohistochemical study. Aliment Pharmacol Ther. 2011;33(8):930‐939. [DOI] [PubMed] [Google Scholar]
- 47. Van Kruiningen HJ, J‐FJ‐F C. The forgotten role of lymphangitis in Crohn's disease. Gut. 2008;57(1):1‐4. [DOI] [PubMed] [Google Scholar]
- 48. Van Kruiningen HJ, Hayes AW, Colombel J‐F. Granulomas obstruct lymphatics in all layers of the intestine in Crohn's disease. APMIS. 2014;122(11):1125‐1129. [DOI] [PubMed] [Google Scholar]
- 49. Yuki M, Sugimoto N, Takahashi K, et al. A case of protein‐losing enteropathy treated with methotrexate in a dog. J Vet Med Sci. 2006;68(4):397‐399. [DOI] [PubMed] [Google Scholar]
- 50. Kimmel SE, Waddell LS, Michel KE. Hypomagnesemia and hypocalcemia associated with protein‐losing enteropathy in Yorkshire Terriers: five cases (1992‐1998). J Am Vet Med Assoc. 2000;217(5):703‐706. [DOI] [PubMed] [Google Scholar]
- 51. Littman MP, Dambach DM, Vaden SL, Giger U. Familial protein‐losing enteropathy and protein‐losing nephropathy in soft coated wheaten terriers: 222 cases (1983‐1997). J Vet Intern Med. 2000;14(1):68‐80. [DOI] [PubMed] [Google Scholar]
- 52. Lane IF, Miller E, Twedt DC. Parenteral nutrition in the management of a dog with lymphocytic‐plasmacytic enteritis and severe protein‐losing enteropathy. Can Vet J. 1999;40(10):721‐724. [PMC free article] [PubMed] [Google Scholar]
- 53. Olson NC, Zimmer JF. Protein‐losing enteropathy secondary to intestinal lymphangiectasia in a dog. J Am Vet Med Assoc. 1978;173(3):271‐274. [PubMed] [Google Scholar]
- 54. Milstein M, Sanford SE. Intestinal lymphangiectasia in a dog. Can Vet J. 1977;18(5):127‐130. [PMC free article] [PubMed] [Google Scholar]
- 55. Van Kruiningen HJ, Lees GE, Hayden DW, Meuten DJ, Rogers WA. Lipogranulomatous lymphangitis in canine intestinal lymphangiectasia. Vet Pathol. 1984;21(4):377‐383. [DOI] [PubMed] [Google Scholar]
- 56. Watson VE, Hobday MM, Durham AC. Focal intestinal lipogranulomatous lymphangitis in 6 dogs (2008‐2011). J Vet Intern Med. 2014;28(1):48‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gianella P, Lotti U, Bellino C, et al. Clinicopathologic and prognostic factors in short‐ and long‐term surviving dogs with protein‐losing enteropathy. Schweiz Arch Tierheilkd. 2017;159(3):163‐169. [DOI] [PubMed] [Google Scholar]
- 58. Rudinsky AJ, Howard JP, Bishop MA, Sherding RG, Parker VJ, Gilor C. Dietary management of presumptive protein‐losing enteropathy in Yorkshire terriers. J Small Anim Pract. 2017;58(2):103‐108. [DOI] [PubMed] [Google Scholar]
- 59. Equilino M, Théodoloz V, Gorgas D, et al. Evaluation of serum biochemical marker concentrations and survival time in dogs with protein‐losing enteropathy. J Am Vet Med Assoc. 2015;246(1):91‐99. [DOI] [PubMed] [Google Scholar]
- 60. Allenspach K, Rizzo J, Jergens AE, Chang YM. Hypovitaminosis D is associated with negative outcome in dogs with protein losing enteropathy: a retrospective study of 43 cases. BMC Vet Res. 2017;13(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Willard MD, Zenger E, Mansell JL. Protein‐losing enteropathy associated with cystic mucoid changes in the intestinal crypts of two dogs. J Am Anim Hosp Assoc. 2003;39(2):187‐191. [DOI] [PubMed] [Google Scholar]
- 62. Dijkstra M, Kraus JS, Bosje JT, Den Hertog E. Protein‐losing enteropathy in Rottweilers. Tijdschr Diergeneeskd. May 15, 2010;135(10):406‐412. [PubMed] [Google Scholar]
- 63. Okanishi H, Yoshioka R, Kagawa Y, Watari T. The clinical efficacy of dietary fat restriction in treatment of dogs with intestinal lymphangiectasia. J Vet Intern Med. 2014;28(3):809‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Simmerson SM, Armstrong PJ, Wünschmann A, Jessen CR, Crews LJ, Washabau RJ. Clinical features, intestinal histopathology, and outcome in protein‐losing enteropathy in Yorkshire Terrier dogs. J Vet Intern Med. 2014;28(2):331‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Willard MD, Helman G, Fradkin JM, et al. Intestinal crypt lesions associated with protein‐losing enteropathy in the dog. 14(3):298‐307. [DOI] [PubMed] [Google Scholar]
- 66. Tamura Y, Ohta H, Kashiide T, et al. Case report: protein‐losing enteropathy caused by Mesocestoides vogae (syn. M. corti) in a dog. Vet Parasitol. 2014;205(1–2):412‐415. [DOI] [PubMed] [Google Scholar]
- 67. Dandrieux JRS, Noble P‐JM, Scase TJ, Cripps PJ, German AJ. Comparison of a chlorambucil‐prednisolone combination with an azathioprine‐prednisolone combination for treatment of chronic enteropathy with concurrent protein‐losing enteropathy in dogs: 27 cases (2007‐2010). J Am Vet Med Assoc. 2013;242(12):1705‐1714. [DOI] [PubMed] [Google Scholar]
- 68. Nakashima K, Hiyoshi S, Ohno K, et al. Prognostic factors in dogs with protein‐losing enteropathy. Vet J. 2015;205(1):28‐32. [DOI] [PubMed] [Google Scholar]
- 69. Parker VJ, Jergens AE, Whitley EM, Frana TS. Isolation of Cokeromyces recurvatus from the gastrointestinal tract in a dog with protein‐losing enteropathy. J Vet Diagn Invest. 2011;23(5):1014‐1016. [DOI] [PubMed] [Google Scholar]
- 70. Jergens AE. Clinical assessment of disease activity for canine inflammatory bowel disease. J Am Anim Hosp Assoc. 2004;40(6):437–45. [DOI] [PubMed] [Google Scholar]
- 71. Allenspach K, Wieland B, Gröne A, Gaschen F. Chronic enteropathies in dogs: evaluation of risk factors for negative outcome. J Vet Intern Med. 2007;21(4):700‐708. [DOI] [PubMed] [Google Scholar]
- 72. Washabau RJ, Day MJ, Willard MD, et al. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J Vet Intern Med. 2010;24(1):10‐26. [DOI] [PubMed] [Google Scholar]
- 73. Craven M, Simpson JW, Ridyard AE, Chandler ML. Canine inflammatory bowel disease: retrospective analysis of diagnosis and outcome in 80 cases (1995‐2002). J Small Anim Pract. 2004;45(7):336‐342. [DOI] [PubMed] [Google Scholar]
- 74. Ferrer R, Mateu X, Maseda E, et al. Non‐oncotic properties of albumin. A multidisciplinary vision about the implications for critically ill patients. Expert Rev Clin Pharmacol. 2018;11(2):125‐137. [DOI] [PubMed] [Google Scholar]
- 75. Sleeckx N, Van Brantegem L, Fransen E, et al. Evaluation of immunohistochemical markers of lymphatic and blood vessels in canine mammary tumours. J Comp Pathol. 2013;148(4):307‐317. [DOI] [PubMed] [Google Scholar]
- 76. Van den Eynden GG, Van der Auwera I, Van Laere SJ, et al. Distinguishing blood and lymph vessel invasion in breast cancer: a prospective immunohistochemical study. Br J Cancer. 2006;94(11):1643‐1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wennogle SA, Priestnall SL, Webb CB. Histopathologic characteristics of intestinal biopsy samples from dogs with chronic inflammatory enteropathy with and without hypoalbuminemia. J Vet Intern Med. 2017;31(2):371‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kleinschmidt S, Meneses F, Nolte I, Hewicker‐Trautwein M. Retrospective study on the diagnostic value of full‐thickness biopsies from the stomach and intestines of dogs with chronic gastrointestinal disease symptoms. Vet Pathol. 2006;43(6):1000‐1003. [DOI] [PubMed] [Google Scholar]
- 79. Strober W, Fuss IJ. Protein‐losing Enteropathies Mucosal Immunology. 4th ed. Boston, MA: Elsevier Inc.; 2015:1667‐1694. [Google Scholar]
- 80. Lecoindre A, Lecoindre P, Cador JL, et al. Focal intestinal lipogranulomatous lymphangitis in 10 dogs. J Small Anim Pract. 2016;57(9):465‐471. [DOI] [PubMed] [Google Scholar]
- 81. Craven MD, Duhamel GE, Sutter NB, McDonough SP, Simpson KW. Absence of bacterial association in Yorkshire terriers with protein‐losing enteropathy and cystic intestinal crypts. J Vet Intern Med. 2009;23:757. [Google Scholar]
- 82. Peterson PB, Willard MD. Chapter 58: protein‐losing enteropathies. Vet Clin North Am Small Anim Pract. 2003;33(5):1061‐1082. [DOI] [PubMed] [Google Scholar]
- 83. Sokol H. Crypt abscess‐associated microbiota in inflammatory bowel disease and acute self‐limited colitis. World J Gastroenterol. 2010;16(5):583‐587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Swidsinski A, Loening‐Baucke V, Vaneechoutte M, Doerffel Y. Active Crohn's disease and ulcerative colitis can be specifically diagnosed and monitored based on the biostructure of the fecal flora. Inflamm Bowel Dis. 2008;14(2):147‐161. [DOI] [PubMed] [Google Scholar]
- 85. Sivagnanam M, Mueller JL, Lee H, et al. Identification of EpCAM as the gene for congenital tufting Enteropathy. Gastroenterology. 2008;135(2):429‐437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Jergens AE. Inflammatory bowel disease. Current perspectives. Vet Clin North Am Small Anim Pract. 1999;29(2):501‐521. vii. [PubMed] [Google Scholar]
- 87. Simpson KW, Jergens AE. Pitfalls and progress in the diagnosis and management of canine inflammatory bowel disease. Vet Clin North Am Small Anim Pract. 2011;41(2):381‐398. [DOI] [PubMed] [Google Scholar]
- 88. Willard MD, Mansell J, Fosgate GT, et al. Effect of sample quality on the sensitivity of endoscopic biopsy for detecting gastric and duodenal lesions in dogs and cats. J Vet Intern Med. 2008;22(5):1084‐1089. [DOI] [PubMed] [Google Scholar]
- 89. Sutherland‐Smith J, Penninck DG, Keating JH, Webster CRL. Ultrasonographic intestinal hyperechoic mucosal striations in dogs are associated with lacteal dilation. Vet Radiol Ultrasound. 2007;48(1):51‐57. [DOI] [PubMed] [Google Scholar]
- 90. Lyngby JG, Sellon RK. Hypoadrenocorticism mimicking protein‐losing enteropathy in 4 dogs. Can Vet J. 2016;57(7):757‐760. [PMC free article] [PubMed] [Google Scholar]
- 91. Lennon EM, Boyle TE, Hutchins RG, et al. Use of basal serum or plasma cortisol concentrations to rule out a diagnosis of hypoadrenocorticism in dogs: 123 cases (2000–2005). J Am Vet Med Assoc. 2007;231(3):413‐416. [DOI] [PubMed] [Google Scholar]
- 92. Wakayama JA, Furrow E, Merkel LK, Armstrong PJ. A retrospective study of dogs with atypical hypoadrenocorticism: a diagnostic cut‐off or continuum? J Small Anim Pract. 2017;58(7):365‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Allenspach K, Culverwell C, Chan D. Long‐term outcome in dogs with chronic enteropathies: 203 cases. Vet Rec. 2016;178(15):368. [DOI] [PubMed] [Google Scholar]
- 94. Ruaux CG, Steiner JM, Williams DA. Protein‐losing enteropathy in dogs is associated with decreased fecal proteolytic activity. Vet Clin Pathol. 2004;33(1):20‐22. [DOI] [PubMed] [Google Scholar]
- 95. Allenspach K. Diagnosis of small intestinal disorders in dogs and cats. Vet Clin North Am Small Anim Pract. 2013;43(6):1227‐1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Larson RNN, Ginn JAA, Bell CMM, Davis MJJ, Foy DSS. Duodenal endoscopic findings and histopathologic confirmation of intestinal lymphangiectasia in dogs. J Vet Intern Med. 2012;26(5):1087‐1092. [DOI] [PubMed] [Google Scholar]
- 97. Jergens AE, Schreiner CA, Frank DE, et al. A scoring index for disease activity in canine inflammatory bowel disease. J Vet Intern Med. 2003;17(3):291‐297. [DOI] [PubMed] [Google Scholar]
- 98. Jergens AE, Crandell J, Morrison JA, et al. Comparison of oral prednisone and prednisone combined with metronidazole for induction therapy of canine inflammatory bowel disease: a randomized‐controlled trial. J Vet Intern Med. 2010;24(2):269‐277. [DOI] [PubMed] [Google Scholar]
- 99. McCann TM, Ridyard AE, Else RW, Simpson JW. Evaluation of disease activity markers in dogs with idiopathic inflammatory bowel disease. J Small Anim Pract. 2007;48(11):620‐625. [DOI] [PubMed] [Google Scholar]
- 100. Heilmann RM, Parnell NK, Grützner N, et al. Serum and fecal canine α1‐proteinase inhibitor concentrations reflect the severity of intestinal crypt abscesses and/or lacteal dilation in dogs. Vet J. 2016;207:131‐139. [DOI] [PubMed] [Google Scholar]
- 101. Foell D, Kucharzik T, Kraft M, et al. Neutrophil derived human S100A12 (EN‐RAGE) is strongly expressed during chronic active inflammatory bowel disease. Gut. 2003;52(6):847‐853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kaiser T, Langhorst J, Wittkowski H, et al. Faecal S100A12 as a non‐invasive marker distinguishing inflammatory bowel disease from irritable bowel syndrome. Gut. 2007;56(12):1706‐1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Tibble JA, Bjarnason I. Non‐invasive Investigation of Inflammatory Bowel Disease. World JGastroenterol. 2001;7:460‐465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Heilmann RM, Suchodolski JS, Steiner JM. Purification and partial characterization of canine calprotectin. Biochimie. 2008;90(9):1306‐1315. [DOI] [PubMed] [Google Scholar]
- 105. Heilmann RM, Jergens AE, Ackermann MR, Barr JW, Suchodolski JS, Steiner JM. Serum calprotectin concentrations in dogs with idiopathic inflammatory bowel disease. Am J Vet Res. 2012;73(12):1900‐1907. [DOI] [PubMed] [Google Scholar]
- 106. Heilmann RM, Berghoff N, Mansell J, et al. Association of fecal calprotectin concentrations with disease severity, response to treatment, and other biomarkers in dogs with chronic inflammatory enteropathies. J Vet Intern Med. 2018;32(2):679‐692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Mukherjee K, Kavalukas SL, Barbul A. Nutritional aspects of gastrointestinal wound healing. Adv Wound Care. 2016;5(11):507‐515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Meschter CL, Rakich PM, Tyler DE. Intestinal lymphangiectasia with lipogranulomatous lymphangitis in a dog. J Am Vet Med Assoc. 1987;190(4):427‐430. [PubMed] [Google Scholar]
- 109. Maconi G, Molteni P, Manzionna G, Parente F, Bianchi Porro G. Ultrasonographic features of long‐standing primary intestinal lymphangiectasia. Eur J Ultrasound. 1998;7(3):195‐198. [DOI] [PubMed] [Google Scholar]
- 110. Mazzie JP, Maslin PI, Moy L, Price AP, Katz DS. Congenital intestinal lymphangiectasia: CT demonstration in a young child. Clin Imaging. 2003;27(5):330‐332. [DOI] [PubMed] [Google Scholar]
- 111. Liu NF, Lu Q, Wang CG, Zhou JG. Magnetic resonance imaging as a new method to diagnose protein losing enteropathy. Lymphology. 2008;41(3):111‐115. [PubMed] [Google Scholar]
- 112. Darfeuille‐Michaud A, Boudeau J, Bulois P, et al. High prevalence of adherent‐invasive Escherichia coli associated with ileal mucosa in Crohn's disease. Gastroenterology. 2004;127(2):412‐421. [DOI] [PubMed] [Google Scholar]
- 113. Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134(2):577‐594. [DOI] [PubMed] [Google Scholar]
- 114. Mann E a. Gastrointestinal infection as a trigger for inflammatory bowel disease. Curr Opin Gastroenterol. 2011;10:1‐6. [DOI] [PubMed] [Google Scholar]
- 115. Vázquez‐baeza Y, Hyde ER, Suchodolski JS, Knight R. Dog and human inflammatory bowel disease rely on overlapping yet distinct dysbiosis networks. Nat Microbiol. 2016;1(October):1‐5. [DOI] [PubMed] [Google Scholar]
- 116. Jergens AE, Pressel M, Crandell J, et al. Fluorescence in situ hybridization confirms clearance of visible helicobacter spp. associated with gastritis in dogs and cats. J Vet Intern Med. 2009;23(1):16‐23. [DOI] [PubMed] [Google Scholar]
- 117. Kornreich BG, Craven M, McDonough SP, et al. Fluorescence in‐situ hybridization for the identification of bacterial species in archival heart valve sections of canine bacterial endocarditis. J Comp Pathol. 2012;146(4):298‐307. [DOI] [PubMed] [Google Scholar]
- 118. Vernau W, Moore PF. An immunophenotypic study of canine leukemias and preliminary assessment of clonality by polymerase chain reaction. Vet Immunol Immunopathol. 1999;69(2–4):145‐164. [DOI] [PubMed] [Google Scholar]
- 119. Troskot R, Jurčić D, Bilić A, Palčić MG, Težak S, Brajković I. How to treat an extensive form of primary intestinal lymphangiectasia? World J Gastroenterol. 2015;21(23):7320‐7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Shah ND, Limketkai BN. Nutrition issues in gastroenterology, series #160. The Use of Medium‐Chain Triglycerides in Gastrointestinal Disorders.
- 121. Rose LJ, Dunn ME, Allegret V, Bédard C. Effect of prednisone administration on coagulation variables in healthy beagle dogs. Vet Clin Pathol. 2011;40(4):426‐434. [DOI] [PubMed] [Google Scholar]
- 122. Van Winkle TJ, Bruce E. Thrombosis of the portal vein in eleven dogs. Vet Pathol. 1993;30(1):28‐35. [DOI] [PubMed] [Google Scholar]
- 123. O'Kell AL, Grant DC, Panciera DL, Troy GC, Weinstein NM. Effects of oral prednisone administration with or without ultralow‐dose acetylsalicylic acid on coagulation parameters in healthy dogs. Am J Vet Res. 2012;73(10):1569‐1576. [DOI] [PubMed] [Google Scholar]
- 124. Xenoulis PG, Steiner JM. Lipid metabolism and hyperlipidemia in dogs. Vet J. 2010;183:12‐21. [DOI] [PubMed] [Google Scholar]
- 125. Jergens AE, Simpson KW. Inflammatory bowel disease in veterinary medicine. Front Biosci (Elite Ed). 2012;4:1404‐1419. [DOI] [PubMed] [Google Scholar]
- 126. Plumb Donald C. Plumb's Veterinary Drug Handbook. Eighth ed. Hoboken, New Jersey: Blackwell Wiley; 2015. [Google Scholar]
- 127. Viviano KR. Update on immununosuppressive therapies for dogs and cats. Vet Clin North Am Small Anim Pract. 2013;43(5):1149‐1170. [DOI] [PubMed] [Google Scholar]
- 128. Archer TM, Boothe DM, Langston VC, Fellman CL, Lunsford KV, Mackin AJ. Oral cyclosporine treatment in dogs: a review of the literature. J Vet Intern Med. 2014;28(1):1‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Archer TM, Fellman CL, Stokes JV, et al. Pharmacodynamic monitoring of canine T‐cell cytokine responses to Oral cyclosporine. J Vet Intern Med. 2011;25(6):1391‐1397. [DOI] [PubMed] [Google Scholar]
- 130. Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000;47:85‐118. [DOI] [PubMed] [Google Scholar]
- 131. Shaffer JA, Williams SE, Turnberg LA, Houston JB, Rowland M. Absorption of prednisolone in patients with Crohn's disease. Gut. 1983;24(3):182‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Tanner AR, Halliday JW, Powell LW. Serum prednisolone levels in Crohn's disease and coeliac disease following oral prednisolone administration. Digestion. 1981;21(6):310‐315. [DOI] [PubMed] [Google Scholar]
- 133. Bode L, Eklund EA, Murch S, Freeze HH. Heparan sulfate depletion amplifies TNF‐induced protein leakage in an in vitro model of protein‐losing enteropathy. AJP Gastrointest Liver Physiol. May 1, 2005;288(5):G1015‐G1023. [DOI] [PubMed] [Google Scholar]
- 134. Murch SH, Winyard PJ, Koletzko S, et al. Congenital enterocyte heparan sulphate deficiency with massive albumin loss, secretory diarrhoea, and malnutrition. Lancet. May 11, 1996;347(9011):1299‐1301. [DOI] [PubMed] [Google Scholar]
- 135. Donnelly JP, Rosenthal A, Castle VP, Holmes RD. Reversal of protein‐losing enteropathy with heparin therapy in three patients with univentricular hearts and Fontan palliation. J Pediatr. 1997;130(3):474‐478. [DOI] [PubMed] [Google Scholar]
- 136. Kelly AM, Feldt RH, Driscoll DJ, Danielson GK. Use of heparin in the treatment of protein‐losing enteropathy after fontan operation for complex congenital heart disease. Mayo Clin Proc. 1998;73:777‐779. [DOI] [PubMed] [Google Scholar]
- 137. Westphal V, Murch S, Kim S, et al. Reduced heparan sulfate accumulation in enterocytes contributes to protein‐losing enteropathy in a congenital disorder of glycosylation. Am J Pathol. 2000;157(6):1917‐1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Ballinger AB, Farthing MJ. Octreotide in the treatment of intestinal lymphangiectasia. Eur J Gastroenterol Hepatol. 1998;10(8):699‐702. [PubMed] [Google Scholar]
- 139. Reubi JC, Laissue JA, Waser B, Steffen DL, Hipkin RW, Schonbrunn A. Immunohistochemical detection of somatostatin sst2a receptors in the lymphatic, smooth muscular, and peripheral nervous systems of the human gastrointestinal tract: facts and artifacts. J Clin Endocrinol Metab. 1999;84(8):2942‐2950. [DOI] [PubMed] [Google Scholar]
- 140. Al Sinani S, Rawahi YA, Abdoon H. Octreotide in Hennekam syndrome‐associated intestinal lymphangiectasia. World J Gastroenterol. 2012;18(43):6333‐6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Sari S, Baris Z, Dalgic B. Primary intestinal lymphangiectasia in children: is octreotide an effective and safe option in the treatment? J Pediatr Gastroenterol Nutr. 2010;51(4):454‐457. [DOI] [PubMed] [Google Scholar]
- 142. Kuroiwa G, Takayama T, Sato Y, et al. Primary intestinal lymphangiectasia successfully treated with octreotide. J Gastroenterol. 2001;36(2):129‐132. [DOI] [PubMed] [Google Scholar]
- 143. Bac DJ, Van Hagen PM, Postema PTE, ten Bokum AMC, Zondervan PE, van Blankenstein M. Octreotide for protein‐losing enteropathy with intestinal lymphangiectasia. Lancet. 1995;345:1639. [DOI] [PubMed] [Google Scholar]
- 144. Robben JH, van den Brom WE, Mol JA, van Haeften TW, Rijnberk A. Effect of octreotide on plasma concentrations of glucose, insulin, glucagon, growth hormone, and cortisol in healthy dogs and dogs with insulinoma. Res Vet Sci. 2006;80(1):25‐32. [DOI] [PubMed] [Google Scholar]
- 145. Jacinto AML, Ridyard AE, Aroch I, et al. Thromboembolism in dogs with protein‐losing Enteropathy with non‐neoplastic chronic small intestinal disease. J Am Anim Hosp Assoc. 2017;53(3):185‐192. [DOI] [PubMed] [Google Scholar]
- 146. Futterman LG, Lemberg L. A silent killer‐‐often preventable. Am J Crit Care. 2004;13(5):431‐436. [PubMed] [Google Scholar]
- 147. Gonze MD, Manord JD, Leone‐Bay A, et al. Orally administered heparin for preventing deep venous thrombosis. Am J Surg. 1998;176:176‐178. [DOI] [PubMed] [Google Scholar]
- 148. Hiebert LM, Wice SM, Ping T. Increased plasma anti‐Xa activity and recovery of heparin from urine suggest absorption of orally administered unfractionated heparin in human subjects. J Lab Clin Med. 2005;145(3):151‐155. [DOI] [PubMed] [Google Scholar]
- 149. Ueno M, Nakasaki T, Horikoshi I, Sakuragawa N. Oral administration of liposomally‐entrapped heparin to beagle dogs. Chem Pharm Bull (Tokyo). 1982;30(6):2245‐2247. [DOI] [PubMed] [Google Scholar]
- 150. Erickson M, Hiebert LM, Carr AP, Stickney JD. Effect of oral administration of unfractionated heparin (UFH) on coagulation parameters in plasma and levels of urine and fecal heparin in dogs. Can J Vet Res. 2014;78(3):193‐201. [PMC free article] [PubMed] [Google Scholar]
- 151. Toresson L, Steiner JM, Suchodolski JS, Spillmann T. Oral cobalamin supplementation in dogs with chronic enteropathies and hypocobalaminemia. J Vet Intern Med. 2016;30(1):101‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Marks SL, Laflamme DP, McAloose D. Dietary trial using a commercial hypoallergenic diet containing hydrolyzed protein for dogs with inflammatory bowel disease. Vet Ther. 2002;3(2):109‐118. [PubMed] [Google Scholar]
- 153. Rubio CP, Martínez‐Subiela S, Hernández‐Ruiz J, Tvarijonaviciute A, Cerón JJ, Allenspach K. Serum biomarkers of oxidative stress in dogs with idiopathic inflammatory bowel disease. Vet J. 2017;221:56‐61. [DOI] [PubMed] [Google Scholar]
- 154. Xu J, Verbrugghe A, Lourenço M, et al. Does canine inflammatory bowel disease influence gut microbial profile and host metabolism? BMC Vet Res. 2016;12(1):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Cerquetella M, Spaterna A, Laus F, et al. Inflammatory bowel disease in the dog: differences and similarities with humans. World J Gastroenterol. 2010;16(9):1050‐1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Lippert AC, Fulton RB, Parr AM. A retrospective study of the use of Total parenteral nutrition in dogs and cats. J Vet Intern Med. 1993;7:52‐64. [DOI] [PubMed] [Google Scholar]
- 157. Queau Y, Larsen JA, Kass PH, Glucksman GS, Fascetti AJ. Factors associated with adverse outcomes during parenteral nutrition administration in dogs and cats. J Vet Intern Med. 2011;25(3):446‐452. [DOI] [PubMed] [Google Scholar]
- 158. Olszewski WL. Lymph Stasis. Boca Raton: CRC press; 1991. [Google Scholar]
- 159. Clarysse S, Tack J, Lammert F, Duchateau G, Reppas C, Augustijns P. Postprandial evolution in composition and characteristics of human duodenal fluids in different nutritional states. J Pharm Sci. 2009;98(3):1177‐1192. [DOI] [PubMed] [Google Scholar]
- 160. Eade MN, Pybus J, Ready J. No evidence of a countercurrent multiplier in the intestinal villus of the dog. Gastroenterology. 1990;98(1):3‐10. [DOI] [PubMed] [Google Scholar]
- 161. Kim NR, Lee S‐K, Suh Y‐L. Primary intestinal lymphangiectasia successfully treated by segmental resections of small bowel. J Pediatr Surg. 2009;44(10):e13‐e17. [DOI] [PubMed] [Google Scholar]
- 162. Engelmann N, Ondreka N, von Pückler K, Mohrs S, Sicken J, Neiger R. Applicability of 99m Tc‐labeled human serum albumin scintigraphy in dogs with protein‐losing Enteropathy. J Vet Intern Med. 2017;31(2):365‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Chiu N‐T, Lee B‐F, Hwang S‐J, Chang J‐M, Liu G‐C, Yu H‐S. Protein‐losing enteropathy: diagnosis with 99m Tc‐labeled human serum albumin scintigraphy. Radiology. 2001;219(1):86‐90. [DOI] [PubMed] [Google Scholar]
- 164. Hostutler RA, Luria BJ, Johnson SE, et al. Antibiotic‐responsive histiocytic ulcerative colitis in 9 dogs. J Vet Intern Med. 2004;18(4):499‐504. [DOI] [PubMed] [Google Scholar]
- 165. Simpson KW, Dogan B, Rishniw M, et al. Adherent and invasive Escherichia coli is associated with granulomatous colitis in boxer dogs. Infect Immun. 2006;74(8):4778‐4792. [DOI] [PMC free article] [PubMed] [Google Scholar]


