Abstract
Background
Indoxyl sulfate (IS) has been reported not only to increase with the severity of impaired renal function, but also possibly to be a factor associated with bone abnormalities linked to fibroblast growth factor‐23 (FGF‐23) in humans with chronic kidney disease (CKD). It is not yet known whether this correlation between IS and FGF‐23 holds true for cats with CKD.
Hypothesis
Accumulation of IS is related to FGF‐23 secretion in cats with CKD.
Animals
Twenty clinically healthy cats and 73 cats with CKD cases were evaluated retrospectively.
Methods
The concentrations of IS and FGF‐23 in plasma were determined by high‐performance liquid chromatography and ELISA, respectively. Progression was defined as an increment of 0.5 mg/dL of serum creatinine concentration within 3 months.
Results
Plasma IS and FGF‐23 concentrations were significantly increased concurrently with decreasing renal function. Higher concentration of FGF‐23 was significantly associated with higher concentration of IS after adjusting for various confounding factors including creatinine and phosphate. Furthermore, the correlation between IS and phosphate was higher than that between FGF‐23 and phosphate. When the renal progression group was compared with the non‐progression group, both IS and FGF‐23 were found to be significantly increased (P < .05). In addition, the area under receiver operator curve of the combination of IS and FGF‐23 predicted renal progression at a level >0.9.
Conclusions and Clinical Importance
Both FGF‐23 and IS are associated with phosphate metabolism and CKD progression.
Keywords: azotemia, feline renal diseases, phosphate regulator, progression, uremic toxin
Abbreviations
- AUROC
area under ROC
- BUN
blood urea nitrogen
- CKD
chronic kidney disease
- CV
coefficient of variation
- FGF‐23
fibroblast growth factor‐23
- GFR
glomerular filtration rate
- HCT
hematocrit
- Hgb
hemoglobin
- HPLC
high‐performance liquid chromatography
- IRIS
International Renal Interest Society
- IS
indoxyl sulfate
- ROC
receiver operating characteristics
- UPC
urine protein‐to‐creatinine ratio
- WBC
white blood cells
1. INTRODUCTION
Indoxyl sulfate (IS) is a protein‐bound uremic toxin derived from dietary protein and mainly eliminated by renal excretion.1 It has been reported to increase with the severity of impaired renal function in humans2 and laboratory animals,3 specifically dogs and cats.4, 5 Additionally, increased IS also has been found to be related to prognosis in humans with chronic kidney disease (CKD)6 and to be associated with progression in cats with CKD.4 Some studies in animals have indicated that IS also plays a role in the inhibition of endothelial proliferation and osteoblast function.7 These findings suggest that accumulation of IS may be factor involved in the bone abnormalities found in human CKD patients in whom it may alter calcium and phosphate concentrations and affect their regulation.
Fibroblast growth factor‐23 (FGF‐23), which is secreted by osteocytes and osteoblasts in response to increases serum phosphate concentration, acts with parathyroid hormone to regulate serum phosphate concentration, calcitriol, and phosphate homeostasis.8 The concentration of FGF‐23 also was reported to increase with the severity of impaired renal function in humans9 and laboratory animals10 and results in the deposition of calcium phosphate within the body.11 Studies also have shown that FGF‐23 increases with the severity of CKD in both cats12 and dogs.13 Furthermore, an increased FGF‐23 concentration was found to be associated with progression of CKD in people9 and shorter survival time in cats with CKD.14
Indoxyl sulfate in humans has been reported to correlate independently with FGF‐23 and to be involved in mineral metabolism.15 Chronic kidney disease is a common disease with a high prevalence in cats, and both IS and FGF‐23 have been reported to be related to severity and outcome but it is not yet known whether or not an association of IS with FGF‐23 exists in cats with CKD. Therefore, our aims were to: (1) elucidate whether serum FGF‐23 concentrations are correlated with IS concentrations and other clinical variables across the various stages of CKD in cats and (2) evaluate the possibility of using IS and FGF‐23 as predictive markers for CKD progression in cats.
2. MATERIALS AND METHODS
2.1. Patient and sample collection
Plasma and urine samples were collected from cats that attended National Taiwan University Veterinary Hospital and stored at −80°C until ELISA and high‐performance liquid chromatography (HPLC). All cases that formed the control group lacked any clinical signs of CKD and also met our inclusion criteria, namely they had CBC, serum biochemistry analysis, and had blood urea nitrogen (BUN) and serum creatinine concentrations within normal limits (ie, BUN concentration <30 mg/dL and serum creatinine concentration <1.6 mg/dL). These cats also had urinalysis performed and ultrasound examination of their kidneys and these findings also were within normal limits. Cats with abnormal serum biochemistry analysis (serum creatinine concentration ≥1.6 mg/dL) and showing 1 of the following conditions: a history of clinical signs (eg, polyuria and polydispsia), abnormal urinalysis (urine specific gravity <1.030 or persistent proteinuria based on urine protein‐to‐creatinine ratio [UPC] >0.5 on 3 occasions and >2 weeks apart), with or without abnormal ultrasonographic findings (small irregular kidneys, decreased corticomedullary distinction) for at least 3 months were enrolled in the CKD group. Patients were excluded from the study if they had 1 of the following problems: acute kidney disease or acute worsening azotemia (>0.3 mg/dL increase in serum creatinine concentration within 7 days) or when they had concurrent hyperthyroidism, lower urinary tract disease, or any clinical signs of other nonrenal diseases (hepatic, cardiac, infectious, or neoplastic disease). All cats with CKD were further grouped using the International Renal Interest Society (IRIS) staging system. In accordance with IRIS CKD staging, these patients were classified into stages 1, 2, 3, or 4. Eligible CKD patients were classified into a progression group and a non‐progression group. Progression was defined as a 0.5 mg/dL increment in serum creatinine concentration or death, including those that were euthanized for progression within the 3‐month period.
2.2. Measurement of IS by HPLC
Total IS concentration was determined using an HPLC system equipped with a pump (Hitachi L‐7100, Hitachi, Tokyo, Japan), fluorescence detector (Hitachi F‐1050, Hitachi, Tokyo, Japan) and reversed phase HPLC column (Mightysil RP‐18 GP, 4.6 × 250 mm, 5 μm). Indoxyl sulfate potassium salt was dissolved in methanol as a standard solution and stored at −20°C and protected from light. One‐hundred microliters of plasma were mixed with 360 μL of acetonitrile and vortexed for 30 seconds before the mixture was centrifuged at 8160g for 5 minutes at 4°C. The supernatant then was filtered through a 0.22 μm polyvinylidene difluoride syringe filter before HPLC was performed.
The analytical conditions were similar to those previously described.5 Briefly, the mobile phase consisted of sodium acetate buffer (pH 4.5) and acetonitrile (10:90, V/V). The flow rate was 1 mL/min and the injection volume was 10 μL. The excitation and emission wavelengths were set at 280 and 375 nm, respectively. Under these conditions, the retention time of IS was 2.34 ± 0.01 minutes. The limit of detection and limit of quantification were 0.05 and 0.2 mg/L, respectively. Intraday and interday assay variations were determined at both 1 and 10 mg/L. The coefficients of variation (CVs) of the intraday assays were 3.11 and 0.63% and the CVs of interday assays were 7.94 and 9.47% for 1 and 10 mg/L, respectively. The recoveries of IS at 1, 5, and 10 mg/L of spiked plasma were 89.9 ± 3.7, 85.8 ± 3.4, and 89.5 ± 0.6%, respectively.
2.3. Measurement of FGF‐23 by commercial feline FGF‐23 ELISA kit
Feline FGF‐23 concentrations were measured in plasma samples using a commercial quantitative sandwich ELISA Kit (MyBioSource, San Diego, California) for the detection of intact FGF‐23 by following the manufacturer's instructions. Each sample was performed in duplicate and the final concentration is presented as the mean of the 2 concentrations. All chemicals and reagents were supplied by the kit (MyBioSource), including 6 vials of standard solutions: 1000, 500, 250, 125, 62.5, and 31.2 pg/mL, sample diluent, horseradish peroxidase‐conjugate reagent, washing solution, chromogen solution A, chromogen solution B, and stop solution. The sensitivity of this kit is 5.0 pg/mL, the linear detection range is 31.2‐1000 pg/mL, and both the intraassay CV and interassay CV are <15% (Supporting Information S1).
2.4. Statistical analysis
Statistical analysis was performed using SPSS software (v.20). The normality of each data set was tested using the Kolmogorov‐Smirnov test. Based on these results, either the Mann‐Whitney U test or the Student's t test was used to assess differences between groups. Differences in categorical variables were analyzed by either the Chi‐square test or Fisher's exact test. Receiver operator characteristic (ROC) curves were applied to predict renal progression and to yield the best cutoff. Kaplan‐Meier curves were used to assess 90‐day CKD progression. Spearman correlation coefficients, Pearson correlation coefficients, and linear regression were calculated to determine the associations between different variables. Logistic regression was applied to evaluate the correlation between variables and CKD progression and to calculate odds ratios. Multivariate logistic regression was applied to construct a covariate set for adjustment of the variables. The probability of the F value to be entered and probability of the F value to be removed during multiple regression analysis were set at F ≤0.05 and F ≥0.1, respectively.
3. RESULTS
Based on IRIS stage, 38 cats were in stage 2, 23 cats were in stage 3, and 12 cats were in stage 4, and these cats then were enrolled. In addition, 20 clinically healthy cats also were enrolled. Demographic findings and clinical characteristics of the cats are summarized in Table 1. The results showed that CKD cats were significantly older and had lower hematocrits (HCT), hemoglobin (Hgb) concentrations, and serum albumin concentrations than did the control group. Serum creatinine, phosphate, and BUN concentrations, the product of calcium and phosphate (tCa ×Pi), UPC, FGF‐23, and IS all were significantly increased in the advanced CKD groups. The results of the univariate and multiple regression analysis for FGF‐23 as a dependent variable are shown in Table 2. In the simple regression analysis, FGF‐23 was found to be significantly correlated with HCT, Hgb, albumin, BUN, creatinine, phosphate, sodium, chloride, and IS. However, after pooling the large number of variables together using stepwise multiple regression analysis, IS was the only independent factor associated with the FGF‐23 concentration (P = .04). Table 3 shows that there were direct correlations among IS, FGF‐23, and phosphate, but after adjusting for IS using partial correlation analysis, FGF‐23 and phosphate did not show a significant correlation.
Table 1.
Clinical characteristics and baseline biochemical parameters of the studied patients
| Variables | Control (n = 20) | CKD classification (n = 73) | ||
|---|---|---|---|---|
| Stage 2 | Stage 3 | Stage 4 | ||
| Sex (male %)a | 50%, n = 20 | 55.3%, n = 38 | 47.8%, n = 23 | 50%, n = 12 |
| Age (year)b | 4 (3‐5.75), n = 20 | 10 (5.8‐15)c, n = 38 | 12 (8‐15)c, n = 23 | 8 (5.5‐9.5)c, n = 12 |
| BUN (mg/dL)b | 22.5 (19‐27), n = 20 | 28.5 (24‐42)c, n = 38 | 52 (38.5‐64.5)c , d, n = 23 | 106.5 (77.8‐130.3)c , d , e, n = 12 |
| Creatinine (mg/dL)b | 1.4 (1.3‐1.5), n = 20 | 2.2 (2‐2.5)c, n = 38 | 3.7 (3.3‐4.1)c , d, n = 23 | 6.45 (5.2‐7.9)c , d , e, n = 12 |
| HCT (%)b | 41 (37.5‐48.5), n = 4 | 36.2 (30.6‐42.3)c, n = 30 | 27.2 (23.2‐33.5)c , d, n = 19 | 24.8 (21.3‐28.4)c , d, n = 12 |
| Hemoglobin (g/L)b | 15.9 (12‐17), n = 4 | 12.2 (10.7‐14), n = 30 | 10.3 (9‐11.6)c , d, n = 19 | 8.7 (6.3‐10)c , d , e, n = 12 |
| WBC (/μL)b | 6735 (5812.5‐8542.5), n = 4 | 8165 (6400‐10 600), n = 30 | 6600 (5550‐13 285), n = 19 | 12 325 (7400‐27 330), n = 12 |
| Albumin (g/dL)b | 4 (3.3‐4), n = 20 | 3.4 (3‐3.7)c, n = 34 | 3.3 (3.1‐3.5)c, n = 22 | 3 (2.9‐3.3)c, n = 11 |
| Ionized calcium (mmol/L)b | 1.19 (1.16‐1.23), n = 6 | 1.27 (1.21‐1.32)c, n = 14 | 1.26 (1.2‐1.3)c, n = 17 | 1.25 (1.15‐1.29)c, n = 10 |
| Total calcium (mg/dL)b | 9.7 (9.2‐10), n = 20 | 9.9 (9.8‐10.9), n = 20 | 10.2 (8.9‐11.2), n = 9 | 9.4 (7.9‐10), n = 6 |
| Phosphate (mg/dL)b | 4 (4‐4.9), n = 20 | 4 (3.8‐5.2), n = 38 | 4.9 (4.1‐7)c , d, n = 23 | 8 (7.1‐12)c , d , e, n = 12 |
| tCa × P (mg2/dL2)b | 42.2 (35.6‐46.5), n = 20 | 37.3 (32.7‐46.6), n = 18 | 69.4 (49.6‐101.2)c , d, n = 9 | 69.3 (52.9‐141)c , d, n = 5 |
| Sodium (mEq/L)b | 154.5 (152‐155.8), n = 20 | 153.9 (152‐155.83), n = 38 | 155.1 (153‐157), n = 23 | 155.2 (153.3‐159.8), n = 12 |
| Potassium (mEq/L)b | 4 (3.85‐4.65), n = 20 | 3.9 (3.5‐4), n = 38 | 4 (3.6‐4.5), n = 23 | 4 (5.5), n = 12 |
| Chloride (mEq/L)b | 119.5 (116.3‐122.8), n = 20 | 118 (115.7‐119.3), n = 38 | 118 (114‐121), n = 23 | 120.6 (116.1‐128), n = 12 |
| AST (U/L)b | 22.5 (18.5‐30.8), n = 18 | 34 (21‐59), n = 23 | 33.5 (21.8‐38.3), n = 14 | 26 (20.8‐52.5), n = 8 |
| ALT (U/L)b | 54.5 (44‐74.8), n = 20 | 54 (42‐114.8), n = 26 | 69 (34‐81), n = 15 | 44 (34.5‐86), n = 9 |
| ALP (U/L)b | 42 (31.3‐46.3), n = 20 | 39 (34.5‐55), n = 25 | 32 (27‐48), n = 15 | 27 (19.5‐53), n = 9 |
| UPCb | 0.00 (0‐0.02), n = 18 | 0.1 (0‐0.9)c, n = 21 | 0.5 (0.167‐0.8)c, n = 8 | 0.73 (0.4‐1.7)c, n = 6 |
| FGF‐23 (pg/mL)b | 121.05 (71.85), n = 20 | 163.05 (126.93), n = 38 | 270.8 (167.5)c , d, n = 23 | 728.25 (793.48)c , d , e, n = 12 |
| IS (mg/L)b | 1.67 (1‐2), n = 20 | 2.91 (2‐4.8)c, n = 38 | 5.8 (3.1‐10)c , d, n = 23 | 25.7 (21‐30)c , d , e, n = 12 |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CKD, chronic kidney disease; FGF‐23, fibroblast growth factor‐23; HCT, hematocrit; IS, indoxyl sulfate; tCa × P, calcium‐phosphate product; UPC, urine protein‐to‐creatinine ratio; WBC, white blood cell.
Chi‐square test: represented as percentage (%).
Kruskal‐Wallis test: represented as the median (interquartile range).
Achieved significance with control group.
Achieved significance with stage 2 group.
Achieved significance with stage 3 group.
Table 2.
Simple and stepwise multiple linear regression analysis for fibroblast growth factor‐23 (FGF‐23) as a dependent variable
| Simple regression analysis | Stepwise multiple regression analysis | |||||
|---|---|---|---|---|---|---|
| Variables | Adjustment β | t | P * | Adjustment β | t | p |
| Age | 0.027 | 0.230 | .82 | – | – | – |
| SAP | 0.134 | 0.884 | .38 | – | – | – |
| Hematocrit | −0.386 | 3.499 | .001 | – | – | – |
| Hemoglobin | −0.380 | −3.154 | .003 | – | – | – |
| RBC | −0.316 | −2.557 | .01 | – | – | – |
| Albumin | −0.339 | −2.907 | .005 | – | – | – |
| BUN | 0.449 | 4.232 | <.001 | – | – | – |
| Creatinine | 0.399 | 3.672 | <.001 | – | – | – |
| Ionized calcium | 0.020 | 0.153 | .88 | – | – | – |
| Phosphate | 0.388 | 3.545 | .001 | – | – | – |
| tCa × Pi | 0.331 | 1.921 | .06 | – | – | – |
| Sodium | 0.247 | 2.150 | .03 | – | – | – |
| Potassium | 0.104 | 0.879 | .38 | – | – | – |
| Chloride | 0.300 | 2.653 | .01 | – | – | – |
| AST | −0.150 | −0.995 | .32 | – | – | – |
| ALT | −0.121 | −0.845 | .40 | – | – | – |
| ALP | −0.250 | −1.769 | .08 | – | – | – |
| USG | 0.289 | 1.955 | .06 | – | – | – |
| UPC | 0.237 | 1.403 | .17 | – | – | – |
| IS | 0.466 | 4.432 | <.001 | 0.466 | 2.169 | .04 |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; HCT, hematocrit; IS, indoxyl sulfate; RBC, red blood cell; SAP, systolic arterial pressure; t, t statistic; tCa × P, calcium‐phosphate product; UPC, urine protein‐to‐creatinine ratio; USG, urine specific gravity; WBC, white blood cell.
P < .05 as significant.
Table 3.
Spearman correlation and partial correlation results among indoxyl sulfate (IS), fibroblast growth factor‐23 (FGF‐23), and phosphate
| Variables | Spearman correlation analysis | Partial correlation analysis | ||
|---|---|---|---|---|
| P | r | P | Partial r | |
| FGF‐23 and phosphate | .008 | 0.310 | .14 | 0.175 |
| Phosphate and IS | <.001 | 0.530 | <.001 | 0.462 |
| FGF‐23 and IS | <.001 | 0.480 | .005 | 0.326 |
The progression and non‐progression groups of cats with CKD were composed of 33 and 40 cases, respectively (Table 4) and median progression was 28 days (interquartile range [IQR], 14‐57 days). No cat died or was euthanized during the study. The cats with renal progression had significantly lower HCT and WBC counts and higher BUN, phosphate, tCa × Pi, UPC, FGF‐23, and IS than did the non‐progression group. However, serum creatinine concentration did not reach statistical significance between groups. Table 5 shows the serum creatinine, phosphate, IS, and FGF‐23 concentrations of CKD cats at the different stages within the progression and non‐progression groups. Because the number of stage 4 CKD cases was low, only CKD stage 2 and stage 3 groups were enrolled for evaluation of progression. Both IS and FGF‐23 concentrations in the progression group remained significantly higher than in the non‐progression group at the same stage. The univariate logistic regression analysis identified that higher serum FGF‐23, IS, age, BUN, phosphate, tCa × Pi, UPC, and lower HCT, Hgb, and albumin concentrations were significantly associated with progression of CKD (Table 6). In the multivariate logistic regression analysis, the predictive roles of IS and FGF‐23 remained independently significant after adjustment for the various different models (Table 7), except for the adjustment for UPC in both the IS and FGF‐23 models, as well as for tCa × Pi in the FGF‐23 model. In addition, using a final model of adjustment for various covariates (BUN, creatinine, HCT, Hgb, albumin, phosphate and FGF‐23, model 11a), IS was found to be an independent progression factor for CKD. However, when the model was adjusted with the same set of covariates, FGF‐23 did not achieve significance when IS was added (model 11b). Moreover, the correlations between UPC and IS, as well as UPC and FGF‐23, both were calculated. Only IS was correlated with UPC (r of Spearman's correlation, 0.366; P = .05).
Table 4.
Clinical characteristics and baseline biochemical parameters of all feline chronic kidney disease (CKD) patients, divided into the progression group and non‐progression group
| Variables | Progression | Non‐progression | P |
|---|---|---|---|
| Sex (male %)a | 60.6, n = 33 | 45, n = 40 | .18 |
| Age (year)b | 11 (8‐15), n = 33 | 8 (5‐15), n = 40 | .09 |
| SAP (mmHg)b | 162.5 (146‐182), n = 21 | 150 (140‐170), n = 24 | .333 |
| BUN (mg/dL)b | 58 (38.5‐74.5), n = 33 | 35 (25.3‐51.3), n = 40 | .001 |
| Creatinine (mg/dL)b | 3.3 (2.3‐4.7), n = 33 | 2.6 (2‐3.7), n = 40 | .19 |
| HCT (%)b | 30 (25.6‐33.5), n = 33 | 34.5 (25.4‐41.4), n = 39 | <.001 |
| Hemoglobin (g/L)b | 10.1 (9.1‐11.9), n = 29 | 12.6 (9.2‐14), n = 32 | .06 |
| WBC (103/μL)b | 6700 (5850‐13 130), n = 29 | 9510 (5755‐24070), n = 32 | .05 |
| Albumin (g/dL)b | 3.4 (3‐3.7), n = 29 | 3.3 (3‐3.5), n = 38 | .06 |
| Ionized calcium (mmol/L)b | 1.20 (1.19‐1.25), n = 17 | 1.27 (1.25‐1.3), n = 20 | .41 |
| Total calcium (mg/dL)b | 10.8 (10.1‐11.8), n = 14 | 10.2 (9.3‐11), n = 21 | .19 |
| Phosphate (mg/dL)b | 5.9 (4.4‐7), n = 33 | 4.1 (3.6‐7), n = 40 | .01 |
| tCa × P (mg2/dL2)b | 60.4 (48.3‐80.3), n = 12 | 45.9 (36.2‐66), n = 20 | .002 |
| Sodium (mEq/L)b | 156.5 (152.8‐160.9), n = 33 | 153.8 (152‐155.6), n = 40 | .19 |
| Potassium (mEq/L)b | 4 (3.7‐4.4), n = 33 | 3.92 (3.4‐4), n = 40 | .67 |
| Chloride (mEq/L)b | 119.3 (116.4‐125), n = 33 | 118 (115.5‐120.5), n = 40 | .27 |
| AST (U/L)b | 36 (22‐62.3), n = 20 | 27 (20‐45), n = 25 | .42 |
| ALT (U/L)b | 73 (50‐106.5), n = 21 | 45 (35‐85.5), n = 29 | .15 |
| ALP (U/L)b | 30 (25.5‐56.7), n = 21 | 37 (26.5‐54), n = 28 | .95 |
| UPCb | 0.735 (0.54‐1.88), n = 10 | 0.1 (0.1‐0.7), n = 25 | .02 |
| FGF‐23 (pg/mL)b | 245 (201.5‐491.5), n = 33 | 165 (121.5‐255.4), n = 40 | <.001 |
| IS (mg/L)b | 9.89 (4.2‐25.4), n = 33 | 2.83 (2.1‐5.4), n = 40 | <.001 |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; FGF‐23, fibroblast growth factor‐23; HCT, hematocrit; IS, indoxyl sulfate; SAP systolic arterial pressure; tCa × P, calcium‐phosphate product; UPC, urine protein‐to‐creatinine ratio; WBC, white blood cell.
Chi‐square test: represented as percentage (%).
Mann‐Whitney test: represented as the median (interquartile range).
Table 5.
Creatinine, phosphate, indoxyl sulfate (IS), and fibroblast growth factor‐23 (FGF‐23) concentrations of chronic kidney disease (CKD) cats with different stage in the progression group and non‐progression group
| IRIS stage | Progression group (n = 27) | Non‐progression group (n = 34) | P a | |
|---|---|---|---|---|
| Creatinine (mg/dL) | 2 | 2.25 (1.8‐2.6), n = 14 | 2.1 (2‐2.5), n = 24 | .86 |
| 3 | 4 (3.5‐4.1), n = 13 | 3.5 (3.3‐3.8), n = 10 | .29 | |
| Phosphate (mg/dL) | 2 | 5.1 (3.9‐5.9), n = 14 | 4 (3.6‐4.4), n = 24 | .04* |
| 3 | 6.6 (4.5‐9.1), n = 13 | 4.55 (4‐5), n = 10 | .09 | |
| IS (mg/L) | 2 | 5.12 (3.74‐7.47), n = 14 | 2.24 (1.63‐2.86), n = 24 | <.001* |
| 3 | 8.42 (5.45‐15.12), n = 13 | 3.73 (2.82‐5.87), n = 10 | .008* | |
| FGF‐23 (pg/mL) | 2 | 247.3 (205.8‐292.9), n = 14 | 128.7 (113.5‐166.9), n = 24 | .001* |
| 3 | 354 (246.9‐510.2), n = 13 | 229.95 (133.7‐281.8), n = 10 | .03* |
Abbreviation: IRIS, International Renal Interest Society.
Mann‐Whitney test: represented as the median (interquartile range).
Significant difference between progression group and non‐progression group (P < .05).
Table 6.
Univariate logistic regression analysis for the various different variables associated with chronic kidney disease (CKD) progression in cats
| Variables | OR | 95% CI for OR | P |
|---|---|---|---|
| IRIS stage (1 stage) | 0.449 | 0.156‐1.289 | .14 |
| Age (year) | 1.131 | 1.004‐1.274 | .04* |
| BUN (mg/dL) | 1.072 | 1.029‐1.116 | .001* |
| Creatinine (mg/dL) | 1.653 | 0.908‐3.010 | .1 |
| Hematocrit (%) | 0.871 | 0.802‐0.946 | .001* |
| Hemoglobin (g/L) | 0.764 | 0.576‐0.995 | .05* |
| Albumin (g/dL) | 0.201 | 0.048‐0.839 | .03* |
| Phosphate (mg/dL) | 1.874 | 1.167‐3.010 | .009* |
| tCa × P (mg2/dL2) | 1.090 | 1.017‐1.169 | .01* |
| UPC | 3.781 | 1.124‐12.715 | .03* |
| FGF‐23 (pg/mL) | 1.009 | 1.003‐1.015 | .002* |
| IS (mg/L) | 2.176 | 1.409‐3.361 | <.001* |
Abbreviations: BUN, blood urea nitrogen; CI, confidence interval; FGF‐23, fibroblast growth factor‐23; IRIS, International Renal Interest Society; IS, indoxyl sulfate; OR, odds ratio; tCa × P, calcium‐phosphate product; UPC, urine protein‐to‐creatinine ratio.
Significant correlation with CKD progression (P < .05).
Table 7.
Multivariate logistic regression analysis for the various different variables associated with renal progression in cats
| IS | FGF‐23 | |||||
|---|---|---|---|---|---|---|
| Models | Odds ratio | 95% CI | P | Odds ratio | 95% CI | P |
| Unadjusted | 2.176 | 1.409‐3.361 | <.001* | 1.009 | 1.003‐1.015 | .002* |
| Model 1 | 2.590 | 1.509‐4.444 | <.001* | 1.009 | 1.003‐1.015 | .004* |
| Model 2 | 2.147 | 0.864‐1.207 | .001* | 1.008 | 1.002‐1.014 | .005* |
| Model 3 | 3.455 | 1.046‐11.405 | .04* | 1.038 | 0.999‐1.079 | .05 |
| Model 4 | 1.713 | 0.962‐3.050 | .07 | 1.006 | 0.999‐1.014 | .09 |
| Model 5 | 2.203 | 1.245‐3.896 | .007* | 1.010 | 1.003‐1.017 | .006* |
| Model 6a | 1.901 | 1.228‐2.942 | .004* | – | – | – |
| Model 6b | – | – | – | 1.006 | 1.000‐1.012 | .05* |
| Model 7a | 3.81 | 1.404‐10.33 | .009* | – | – | – |
| Model 7b | 1.011 | 0.998‐1.023 | .11 | |||
Model 1 was adjusted for International Renal Interest Society (IRIS) stage; Model 2 was adjusted for age and gender; Model 3 was adjusted for tCa × P, calcium‐phosphate product (tCa × P); Model 4 was adjusted for urine protein‐to‐creatinine (UPC); Model 5 was adjusted for systolic arterial pressure (SAP); Model 6a was adjusted for fibroblast growth factor‐23 (FGF‐23); Model 6b was adjusted for indoxyl sulfate (IS); Model 7a was adjusted for blood urea nitrogen (BUN), creatinine, hematocrit, hemoglobin, albumin, phosphate, and FGF‐23. Model 7b was adjusted for BUN, creatinine, hematocrit, hemoglobin, albumin, phosphate, and IS.
Significant correlation with CKD progression (P < .05).
Figure 1 shows the ROC curve analysis for evaluation of the predictive ability of IS, FGF‐23, and a combination of both, for progression in the CKD cats. The combined predictive values (P combination) of FGF‐23 and IS were calculated by multivariate logistic regression: log (odds of progression) = 0.642 * IS + 0.006 * FGF‐23. The following equation was derived: P combination = IS (mg/L) + (0.006/0.642) * FGF‐23 (pg/mL). The area under ROC (AUC) values for the combination score, IS, and FGF‐23 to predict progression were 0.921, 0.850, and 0.806, respectively. The optimal cutoff for the combination was 5.02, with a sensitivity of 96.3% and specificity of 73.5% (P < .001); the optimal cutoff for IS was 3.41 mg/L, with a sensitivity of 92.6% and specificity of 76.5% (P < .001); and the optimal cutoff for FGF‐23 was 200 pg/mL, with a sensitivity of 85.2% and specificity of 70.6% (P < .001). Based on these cutoffs, the cats then were divided into a higher value group and a lower value group. The cats with IS concentration >3.41 mg/L (P < .001) and FGF‐23 concentration >200 pg/mL (P < .001) both showed significantly faster renal progression (Figure 2A,B).
Figure 1.
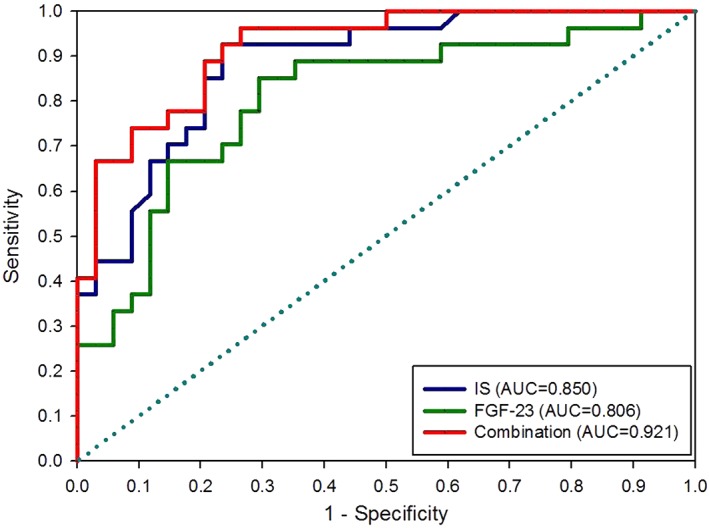
Receiver operator characteristic (ROC) curve of indoxyl sulfate (IS), fibroblast growth factor‐23 (FGF‐23), and combination of both (IS + (0.006/0.642) × FGF‐23) to predict renal progression in cats with chronic kidney disease (CKD). Area under ROC (AUC): combination (0.921) > IS (0.850) > FGF‐23 (0.806)
Figure 2.
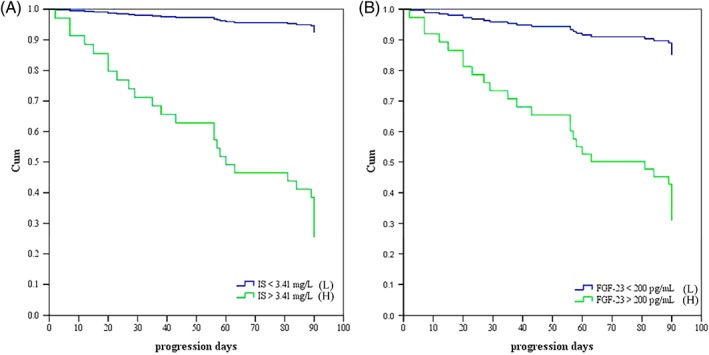
A comparison of the Kaplan‐Meier curves using the indoxyl sulfate (IS) levels (A) and fibroblast growth factor‐23 (FGF‐23) (B). High‐value group (H): IS > 3.41 mg/L; low‐value group (L): IS < 3.41 mg/L; high‐value group (H): FGF‐23 > 200 pg/mL; low‐value group (L): FGF‐23 < 200 pg/mL. Both higher levels showed significantly faster progressions. (P < .001, log‐rank test)
4. DISCUSSION
Although both IS and FGF‐23 have been reported to be biomarkers for the severity of CKD and to be predictors of progression, to the best of our knowledge, ours is the first study to investigate the relationship in cats with CKD between IS and FGF‐23 and to evaluate them simultaneously along with other factors. Similar to other studies,4, 14 IS and FGF‐23 increased with the severity of CKD and were able to act as predictors of CKD progression in cats. Interestingly, multivariate logistic regression showed that IS and FGF‐23 were independently associated with CKD progression after adjustment for other reported progression‐related variables, such as HCT, Hgb, and phosphate.16 This result confirms the importance of IS and FGF‐23 having association with the progression of CKD, and these findings are similar to previous studies in human medicine6 and veterinary medicine.4, 14 However, we also used both of these factors to construct an equation that has a higher AUC when predicting progression, and thus using the 2 factors together is a more precise measure overall.
Similar to a previous study in humans with CKD,15 our results also show that FGF‐23 and IS not only show a significant correlation but also have a significant linear relationship with each other. Noticeably, after pooling together other variables that are believed to affect FGF‐23 concentration, such as serum creatinine and phosphate concentrations, IS was the only variable that cannot be removed with the stepwise procedures and has significant correspondence with FGF‐23. Furthermore, both plasma IS and FGF‐23 concentrations are significantly correlated with serum phosphate concentration. Fibroblast growth factor‐23 has been shown to increase in parallel to serum phosphate concentration in humans9 with CKD and cats12 with CKD. Hyperphosphatemia is thought to be the physiological stimulus for FGF‐23 secretion and the increase in FGF‐23 is a response that is aimed at correcting the abnormal serum phosphate concentration; it does so by suppressing renal tubule sodium‐dependent phosphate transporter expression and inhibiting the production of calcitriol.17
In our study, surprisingly, IS showed a higher correlation with phosphate than FGF‐23 did. Additionally, when IS was controlled for, the significant correlation between FGF‐23 and phosphate became insignificant. In contrast, IS and phosphate do maintain a significant correlation when FGF‐23 is controlled for. A recent study indicated that IS is a potential factor in the induction of secondary renal hyperparathyroidism via oxidative stress and impairment of osteoblast function.7 Osteocytes and osteoblasts are the primary sources of FGF‐23 synthesis and secretion.8 Moreover, 1 study also has determined that administration of IS to rats with CKD decreases renal expression of klotho, a cofactor in FGF‐23 receptor interactions that enhances its phosphate‐lowering function.18 These findings imply that IS may have a deleterious effect on mineral metabolism, which occurs via a disturbance of the phosphate‐lowing function of FGF‐23. Furthermore, our results also suggest that IS in cats with CKD also may be a potential factor affecting FGF‐23 concentrations and be involved in phosphate metabolism. However, the cross talk among IS, FGF‐23, and phosphate warrants further study.
In our study, the IS was significantly correlated with UPC, but the predictive power of both IS and FGF‐23 regarding CKD progression was decreased to insignificant by adjusting for UPC. Similarly, proteinuria, as an important risk factor, also has been reported to be associated with CKD progression in cats.16 It is well known that IS leads to glomerular sclerosis through oxidative stress18 and can injure the glomerular podocytes,19 which then results in defects in the glomerular filtration barrier. Eventually, excessive filtering of protein may overload the proximal tubular cells. Additionally, IS might cause damage to renal tubular cells1 and lead to decreased reabsorption of albumin and finally to increased proteinuria. Consequently, this proteinuria might further damage tubular cells resulting in the accumulation of IS. In combination, the detrimental effects of both proteinuria and IS on renal tubules might be the reason why the predictive power of IS was affected by UPC. However, although the current design prevented us from evaluating these speculations, further studies are warranted. Higher concentrations of FGF‐23 have been reported to be related to proteinuria in humans with CKD.20 Oxidative stress was related to deficient expression of klotho and eventually will cause both FGF‐23 resistance21 and proteinuria.22 Therefore, the similar effects of proteinuria and increased FGF‐23 concentrations on renal tubules might be the reason that UPC is able to affect the predictive power of FGF‐23. However, FGF‐23 was not significantly correlated with UPC in our study (data not shown), and further investigations are needed to explore this issue.
In our study, the FGF‐23 concentrations of late stage cats were different from those obtained in previous studies.12, 14 The ELISA kit we used was designed for the detection of feline intact FGF‐23 (Supporting Information S1), and it was different from the previously reported kit, which was designed to detect human FGF‐23. Although this kit was used successfully for studies in cats, whether the response level (cross reactivity) was similar for human and for feline samples remains to be confirmed by the manufacturer. One possible explanation for the difference in the response level is the sample dilution effect (in which after the response goes beyond the linear range of the assay, the sample must be further diluted) and might start to exhibit a disproportionate increase in response.23 In addition, our study focused on the difference between the progression and non‐progression groups, in which some data came from younger and less severe CKD cats that could have brought the median FGF‐23 concentration lower and mostly within the assay, linear range. We believe that both sets of data are legitimate and stand on their own, given the fact that different validated ELISAs were employed. However, further studies are warranted in the future to decipher whether different ELISA kits show differences in specificity and response intensity in different animal species.
Our study had some limitations. First, because FGF‐23 and IS results were derived from previously banked plasma samples, not all of the blood and urine variables could be obtained for all cases. Second, although there was no statistically significant difference, CKD stage 4 cats seemed to be younger than CKD stage 2 and 3 cats, which may be considered controversial, given that age can be a factor leading to a later stage of CKD.24 Although the sample size, especially the CKD stage 4 cats, was small and may not be representative of the larger feline population, it is possible that different kidney diseases that may compromise renal function also coexisted in these cats. Without biopsies and further examinations, the definite cause of CKD cannot be ascertained and might limit the application of our results to other types of kidney disease. Third, the expression of klotho, the cofactor of FGF‐23, was not evaluated at the same time, which limits our ability to explain the mechanism of the interaction between IS and FGF‐23. Fourth, serial measurements were not available for comparison of the continued change of these uremic toxins in CKD progression. Sixth, because the duration of follow‐up was not fixed, the calculation of progression days might not be precise. Therefore, further studies are required to answer these questions.
5. CONCLUSIONS
In conclusion, IS is independently correlated with FGF‐23, suggesting that IS may be an important factor in the regulation of mineral metabolism of cats with CKD. Not only FGF‐23, but also IS play important roles in phosphate metabolism, and they are independent factors in relation to renal function. Thus, they should be considered simultaneously when examining phosphate control and mineral metabolism in cats. Both IS and FGF‐23 appear to be promising renal biomarkers for the early detection of progression in cats with CKD.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
This study got IACUC approval (NTU‐EL‐00078) June 2016.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGMENTS
The authors thank all the doctors at National Taiwan University Veterinary Hospital for providing the cases and collecting the samples.
Liao Y‐L, Chou C‐C, Lee Y‐J. The association of indoxyl sulfate with fibroblast growth factor‐23 in cats with chronic kidney disease. J Vet Intern Med. 2019;33:686–693. 10.1111/jvim.15457
Liao and Chou contributed equally to this study.
Funding information Ministry of Science and Technology, Taiwan, Grant/Award Number: MOST 107‐2313‐B‐002‐053
REFERENCES
- 1. Motojima M, Hosokawa A, Yamato H, Muraki T, Yoshioka T. Uremic toxins of organic anions up‐regulate PAI‐1 expression by induction of NF‐kappaB and free radical in proximal tubular cells. Kidney Int. 2003;63:1671‐1680. [DOI] [PubMed] [Google Scholar]
- 2. Lin CJ, Chen HH, Pan CF, et al. p‐Cresylsulfate and indoxyl sulfate level at different stages of chronic kidney disease. J Clin Lab Anal. 2011;25:191‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Owada S, Goto S, Bannai K, Hayashi H, Nishijima F, Niwa T. Indoxyl sulfate reduces superoxide scavenging activity in the kidneys of normal and uremic rats. Am J Nephrol. 2008;28:446‐454. [DOI] [PubMed] [Google Scholar]
- 4. Chen CN, Chou CC, Tsai PSJ, Lee YJ. Plasma indoxyl sulfate concentration predicts progression of chronic kidney disease in dogs and cats. Vet J. 2018;232:33‐39. [DOI] [PubMed] [Google Scholar]
- 5. Cheng FP, Hsieh MJ, Chou CC, Hsu WL, Lee YJ. Detection of indoxyl sulfate levels in dogs and cats suffering from naturally occurring kidney diseases. Vet J. 2015;205:399‐403. [DOI] [PubMed] [Google Scholar]
- 6. Wu IW, Hsu KH, Lee CC, et al. p‐Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol Dial Transplant. 2011;26:938‐947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim YH, Kwak KA, Gil HW, Song HY, Hong SY. Indoxyl sulfate promotes apoptosis in cultured osteoblast cells. BMC Pharmacol Toxicol. 2013;14:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bergwitz C, Juppner H. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu Rev Med. 2010;61:91‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fliser D, Kollerits B, Neyer U, et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) study. J Am Soc Nephrol. 2007;18:2600‐2608. [DOI] [PubMed] [Google Scholar]
- 10. Chen XJ, Chen X, Wu WJ, Zhou Q, Gong XH, Shi BM. Effects of FGF‐23‐mediated ERK/MAPK signaling pathway on parathyroid hormone secretion of parathyroid cells in rats with secondary hyperparathyroidism. J Cell Physiol. 2018;233:7092‐7102. [DOI] [PubMed] [Google Scholar]
- 11. Jimbo R, Kawakami‐Mori F, Mu S, et al. Fibroblast growth factor 23 accelerates phosphate‐induced vascular calcification in the absence of klotho deficiency. Kidney Int. 2014;85:1103‐1111. [DOI] [PubMed] [Google Scholar]
- 12. Geddes RF, Finch NC, Elliott J, Syme HM. Fibroblast growth factor 23 in feline chronic kidney disease. J Vet Intern Med. 2013;27:234‐241. [DOI] [PubMed] [Google Scholar]
- 13. Harjes LM, Parker VJ, Dembek K, et al. Fibroblast growth factor‐23 concentration in dogs with chronic kidney disease. J Vet Intern Med. 2017;31:784‐790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Geddes RF, Elliott J, Syme HM. Relationship between plasma fibroblast growth factor‐23 concentration and survival time in cats with chronic kidney disease. J Vet Intern Med. 2015;29:1494‐1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin CJ, Pan CF, Chuang CK, et al. Association of indoxyl sulfate with fibroblast growth factor 23 in patients with advanced chronic kidney disease. Am J Med Sci. 2014;347:370‐376. [DOI] [PubMed] [Google Scholar]
- 16. Chakrabarti S, Syme HM, Elliott J. Clinicopathological variables predicting progression of azotemia in cats with chronic kidney disease. J Vet Intern Med. 2012;26:275‐281. [DOI] [PubMed] [Google Scholar]
- 17. Saito H, Kusano K, Kinosaki M, et al. Human fibroblast growth factor‐23 mutants suppress Na+‐dependent phosphate co‐transport activity and 1alpha,25‐dihydroxyvitamin D3 production. J Biol Chem. 2003;278:2206‐2211. [DOI] [PubMed] [Google Scholar]
- 18. Adijiang A, Shimizu H, Higuchi Y, Nishijima F, Niwa T. Indoxyl sulfate reduces klotho expression and promotes senescence in the kidneys of hypertensive rats. J Ren Nutr. 2011;21:105‐109. [DOI] [PubMed] [Google Scholar]
- 19. Ichii O, Otsuka‐Kanazawa S, Nakamura T, et al. Podocyte injury caused by indoxyl sulfate, a uremic toxin and aryl‐hydrocarbon receptor ligand. PLoS One. 2014;9:e108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vervloet MG, van Zuilen AD, Heijboer AC, et al. Fibroblast growth factor 23 is associated with proteinuria and smoking in chronic kidney disease: an analysis of the MASTERPLAN cohort. BMC Nephrol. 2012;13:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lim K, Lu TS, Molostvov G, et al. Vascular klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation. 2012;125:2243‐2255. [DOI] [PubMed] [Google Scholar]
- 22. Hage V, Pelletier S, Dubourg L, et al. In chronic kidney disease, serum alpha‐klotho is related to serum bicarbonate and proteinuria. J Ren Nutr. 2014;24:390‐394. [DOI] [PubMed] [Google Scholar]
- 23. Thakur K, Sharma S, Prabhakar S, Gupta P, Anand A. Revisiting the dilution factor as vital parameter for sensitivity of ELISA assay in CSF and plasma. Ann Neurosci. 2015;22:37‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brown CA, Elliott J, Schmiedt CW, Brown SA. Chronic kidney disease in aged cats: clinical features, morphology, and proposed pathogeneses. Vet Pathol. 2016;53:309‐326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information


