Abstract
Background
In clinical practice, histopathological diagnosis of chronic intestinal disease is challenging because of difficulty in obtaining adequate duodenal samples. At present, no studies have investigated the influence of biopsy forceps size on sample quality in cats.
Objectives
Duodenal biopsy using larger biopsy forceps (2.4 mm) will provide higher quality samples.
Animals
Fifty client‐owned cats underwent endoscopy of the upper gastrointestinal tract for evaluation of chronic gastrointestinal signs, with inflammatory bowel disease (IBD) or intestinal lymphoma as differential diagnoses.
Methods
For each cat, duodenal biopsy specimens were obtained using both small (1.8 mm) and large (2.4 mm) forceps and evaluated for adequacy, orientation, the presence of artifacts, villi morphology, the presence of inflammation, and neoplastic infiltration.
Results
The percentage of adequate and evaluable biopsy specimens obtained using the larger forceps was significantly higher than that collected using the smaller forceps. Agreement between the forceps was variable for histological features and substantial in the case of lymphoma. However, in case of disagreement, the proper diagnosis usually was achieved only with the larger biopsy forceps.
Conclusions and Clinical Importance
Use of a larger biopsy forceps allows collection of a higher percentage of adequate and evaluable biopsy specimens compared to the commonly used smaller forceps and indirectly decreases the percentage of artifacts and increases the percentage of samples with evaluable villi. The use of a larger forceps could be helpful to obtain high‐quality samples and improve diagnostic accuracy.
Keywords: biopsy forceps, cats, endoscopy, gastrointestinal diseases, inflammatory bowel disease, lymphoma
Abbreviations
- IBD
inflammatory bowel disease
- CI95% 95%
confidence intervals
- OR
odds ratio
1. INTRODUCTION
The proper clinical approach in a cat with chronic intestinal disease includes appropriate antihelminthic, dietary, and antimicrobial treatment, but also often requires endoscopic examination of the upper and lower gastrointestinal tract to obtain targeted biopsy specimens for histological confirmation of the clinical diagnosis.
Standardized endoscopic procedure, quality of the samples (e.g., adequacy, proper orientation, and the absence of artifacts), correct laboratory processing and accurate histopathological interpretation by a board‐certified pathologist, all play essential roles in achieving an accurate diagnosis.1, 2, 3, 4 The first essential step for diagnostic pathology in enteric disease is obtaining an adequate tissue sample. A duodenal sample has been described as being adequate when it includes at least 3 intact villi with complete lamina propria, extending to the muscolaris mucosae (included or not); a sample is defined as marginal when it includes at least 1 intact villus with at least part of the lamina propria, not clearly extending to the muscularis mucosa. Finally, a sample is considered inadequate when it includes only the villi or the lamina propria but not both of them.3
Sample quality can be influenced by several factors such as the skills of the endoscopist (inadequate sampling can be as high as 26% in the case of inexperienced operators) and the type of equipment used, which in turn influence the number of samples necessary to obtain high diagnostic accuracy.5, 6, 7
In cats, the main differential diagnoses for severe chronic gastrointestinal disease are inflammatory bowel disease (IBD) and intestinal lymphoma. Some of the histological features considered to be diagnostic for intestinal lymphoma (eg, depth of infiltration by lymphoid cells) require adequate well‐oriented tissue samples to be evaluated.8 Similarly, in IBD, clinical decisions depend on the severity of inflammation, and also grading of the inflammation requires well‐oriented adequate biopsy specimens.4
Many studies have examined some of the factors influencing the efficacy of the biopsy procedure;7 however, to the best of our knowledge, none of these studies has investigated the sampling method or biopsy specimen processing technique in cats. Conversely, in human medicine, the influence of the shape and size of the biopsy forceps on the quality of the sample is well documented.9, 10, 11, 12, 13 In veterinary medicine, because of the size of the patient, when a tissue sample is taken from the intestine of a cat during endoscopy, a 1.8‐mm biopsy forceps is commonly used.7
We hypothesized that the use of a larger forceps when performing duodenal biopsies in cats could increase the quality of samples and, consequently, improve diagnostic accuracy. Thus, the aim of our study was to evaluate whether the use of differently sized biopsy forceps could significantly influence the quality of duodenal biopsy specimens in cats.
2. MATERIALS AND METHODS
2.1. Selection of samples and equipment
We performed a prospective study and collected duodenal samples from 50 cats with chronic gastrointestinal signs. Age, breed, and sex were not criteria for inclusion. All cats were clinically evaluated before endoscopy, and causes of gastrointestinal diseases such as parasites, food‐responsive enteropathy, or metabolic diseases were ruled out. Therefore, all cats included in the study had IBD or lymphoma as the principal differential diagnosis for their disease. Endoscopically obtained duodenal biopsy specimens were collected by a only skilled endoscopist (Enrico Bottero) using 2 differently sized biopsy forceps: the smaller forceps had a diameter of 1.8 mm (PE2‐OVAL‐18‐160 biopsy forceps, oval, fenestrated cups with spike, tapered Endo‐technik) and required a working channel of 2 mm, whereas the larger forceps had a diameter of 2.4 mm (PE2‐OVAL‐24‐160 biopsy forceps, oval, fenestrated cups with spike, tapered Endo‐technik) and required a working channel of 2.5 mm (Figure 1). Consequently, 2 different endoscopes were used: a trans‐nasal gastroscope (Fujinon EG 270 N‐5 diameter 5.9 mm, working channel 2 mm) and a standard gastroscope (Silver Scope Karl Storz 60719 PKS/NKS diameter 7.9 mm, working channel 2.8 mm). The biopsies were performed by positioning the forceps valves perpendicular to the mucosal surface and exerting a suitable pressure to be able to view, after sampling, the pale translucent aspect of the submucosa (a combination of avulsion and push‐off techniques).
Figure 1.

Comparison of the dimensions of the smaller (left, 1.8 mm) and larger (right, 2.4 mm) biopsy forceps
The study was designed to obtain 5‐6 duodenal biopsy specimens from each forceps, as previously described.3 The biopsy specimens subsequently were put on a 0.8‐μm porosity cellulose nitrate paper (Endofilters Bio‐optica, code 08‐8600) to maintain proper orientation of the biopsy specimens.14, 15
2.2. Histology
The biopsy specimens obtained with the 2 forceps were placed in 2 different tubes, fixed in 10% buffered formalin and sent to the Veterinary Pathology Service (Department of Veterinary Medicine, University of Perugia, Italy). To perform a blinded evaluation, a random number (1 or 2) was assigned to each pair of tubes using the web site http://www.random.org by a first operator. Afterward, the biopsy specimens were routinely processed and stained with hematoxylin and eosin and evaluated by light microscopy by a second operator, following a random order and without knowing any details about the slides. All of the slides were evaluated by a board‐certified pathologist (Elvio Lepri) to eliminate subjective variability. When assessing the quality of biopsy specimens, the following aspects were evaluated: adequacy (adequate, marginal, or inadequate) of each biopsy specimen, as previously described,3 their orientation toward the cellulose nitrate paper, the presence of mechanical artifacts (squeezing, streaming chromatin, roll‐over) and their severity (percentage of sections affected by artifacts) for each cat as previously described,16 and the ability to evaluate villi and their morphology (e.g. blunting, distortion, fusion). With regard to orientation, we considered the following conditions: properly oriented (when biopsy specimens were placed with the anti‐luminal surface in contact with the cellulose nitrate paper), backward orientation (when the villi were placed in contact with the cellulose nitrate paper), and inadequately oriented (when it was not possible to recognize the orientation of the biopsy specimens, or when only the superficial portion of villi or the crypt mucosa was observed, but not both of them). Consequently, we classified marginal biopsy specimens as those that had at least 1 villus but did not have the full thickness lamina propria, as previously described,3 but also wide biopsy specimens in which only superficial lamina propria or crypt mucosa was seen as a consequence of inadequate orientation (“marginal because of the orientation”). Only adequate and marginal biopsy specimens, regardless of orientation, were considered appropriate (evaluable) to assess the following histological features: composition of inflammatory infiltrates (lymphocytes, plasma cells, eosinophils, neutrophils) and severity of inflammation (mild, moderate, severe); structural changes (villous atrophy, mucosal fibrosis) according to World Small Animal Association guidelines;1 and neoplastic infiltration (small, medium, or large cell lymphoma). Finally, the pathologist gave a final diagnosis for all samples (n = 100).
2.3. Statistical analysis
We evaluated data and obtained absolute frequencies of the features described above. Because the number of biopsies performed with each forceps was slightly different, we used relative frequencies for descriptive statistics of adequacy and orientation. The statistical significance of these frequencies was determined using the arcsine square root transformed values to allow parametric‐based methods in comparisons. A paired t test was performed to compare samples obtained using the 2 forceps sizes regarding total number of biopsy specimens, total number of evaluable biopsy specimens (samples useful for diagnosis obtained by the sum of adequate and marginal samples), adequacy (total number of adequate, marginal, and inadequate biopsy specimens), orientation (total number of properly, backward, and inadequately oriented samples), and quantity of artifacts; 95% confidence intervals (CI95%) for the difference were reported. We used ordinal logistic regression to assess the effect of the forceps size on biopsy specimen adequacy (0 = inadequate, 1 = marginal, 2 = adequate) and orientation (0 = inadequate, 1 = backward, 2 = proper), including the individual effect of each cat in the model. To compare the presence of artifacts and evaluable villi in samples obtained using the 2 forceps, we performed a chi‐square (χ2) test of independence; using logistic regression, we also evaluated the effect that forceps size, total number of biopsy specimens, and number of adequate and of marginal biopsy specimens properly oriented collected in each cat on the presence of artifacts and of evaluable villi. In addition, we performed ordinal logistic regression to assess the influence of forceps size on the percentage of artifacts, categorizing them in classes (0 = 0%, 1 = 0%‐5%, 2 = 6%‐10%, 3 = 11%‐20%, 4 = 21%‐30%, 5 = >30%). Histological evaluations were analyzed by Cohen's kappa test (interpreted according as previously described)17 and McNemar's χ2 test with continuity correction to assess measurement agreement between the 2 biopsy forceps.
Data were analyzed using the software R (R version 3.3.2);18 P values ≤.05 were considered statistically significant.
3. RESULTS
3.1. Adequacy and orientation
We prospectively collected duodenal biopsy specimens from 50 cats using both the smaller and larger forceps (Figure 2A): the number of biopsy specimens collected per cat (mean ± SD) was 5.04 ± 1.62 and 5.36 ± 1.54 with the smaller and larger forceps, respectively. Although the total number of biopsy specimens per forceps size was slightly different, for technical reasons, it did not differ significantly between the smaller and the larger forceps (P = .19), which was expected. Descriptive statistics concerning the quality of biopsy specimens collected using the smaller and larger forceps are summarized in Table 1 and Figure 3.
Figure 2.
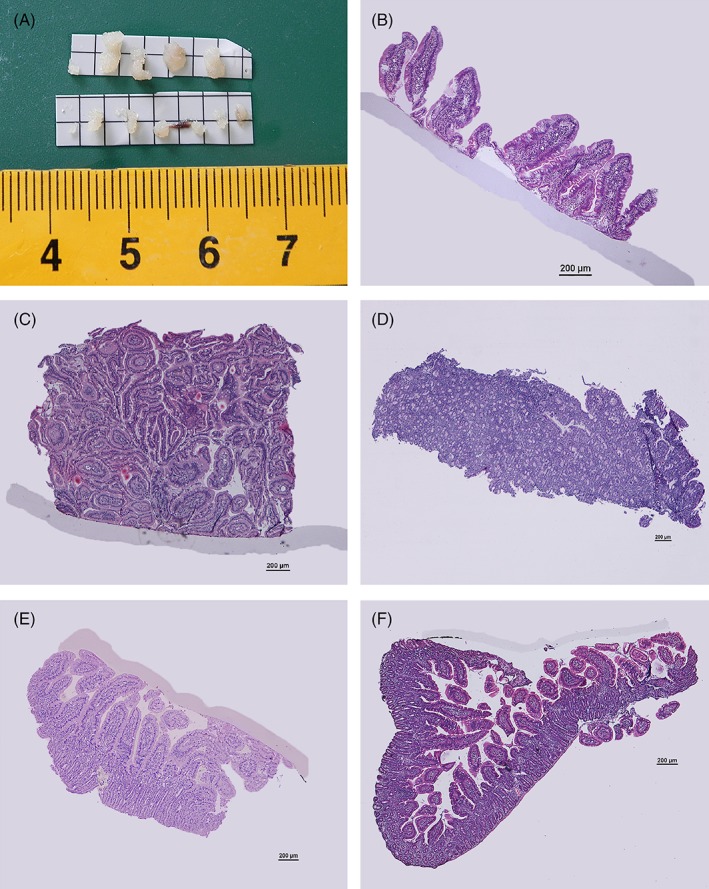
(A) Comparison of the gross dimensions of the biopsy performed with the smaller (bottom) and the larger forceps (top). (B‐F) Duodenum, hematoxylin and eosin stain, 4×. Scale bar = 200 μm. (B) Marginal sample. (C, D) Samples marginal because of the orientation: only the superficial lamina propria (C) or only the cryptal mucosa is present (D). (E) Backwardly oriented sample. (F) Roll over artifact
Table 1.
Biopsy adequacy and orientation
| Mean ± SD | CI95% | t‐test | ||
|---|---|---|---|---|
| L | S | P‐value | ||
| Total biopsies (n) | 5.36 ± 1.54 | 5.04 ± 1.62 | −0.17 to 0.81 | .19 |
| Adequacy (%) | ||||
| Evaluable | 82.09 ± 24.13 | 70.03 ± 31.93 | 0.37 to 20.52 | .04 |
| Adequate | 39.77 ± 27.1 | 21.7 ± 24.08 | 8.53 to 24.65 | .0001 |
| Marginal | 16.02 ± 23.72 | 25.67 ± 26.6 | −18.73 to −1.17 | .03 |
| Marginal for orientation | 26.63 ± 31.14 | 22.66 ± 26.9 | −6.71 to 12.1 | .57 |
| Inadequate | 17.24 ± 23.92 | 29.24 ± 32.06 | −20.32 to −0.01 | .05 |
| Orientation (%) | ||||
| Proper | 35.27 ± 31.7 | 42.01 ± 34.84 | −17.94 to 5.12 | .27 |
| Backwards | 22.59 ± 23.18 | 19.73 ± 28.37 | −4.16 to 12.12 | .33 |
| Inadequate | 42.14 ± 36.07 | 38.26 ± 35.63 | −9.92 to 12.93 | .79 |
Abbreviations: CI95%, 95% confidence interval of the difference; L, larger forceps; S, smaller forceps.
Values in bold are statistically significant (P ≤ .05)
Figure 3.

Boxplots of adequacy and orientation with the larger (L) and smaller (S) forceps. The symbol indicates a significant difference at P ≤ .05 (*), P ≤ .01 (**), or P ≤ .001 (***)
The percentage of evaluable biopsy specimens obtained with the larger forceps was significantly higher than that obtained for the smaller forceps (P = .04; CI95% = 0.37‐20.52); we found the same significant difference regarding the percentage of adequate biopsy specimens (P ≤ .001; CI95% = 8.53‐24.65).
The marginal samples were divided into 2 subgroups: “true” marginal as defined previously3 and those marginal because of orientation (Figure 2B‐D). The percentage of “true” marginal biopsy specimens was significantly lower for the larger forceps (P = .03; CI95% = −18.73 to −1.17), whereas no statistically significant difference was found in the percentage of marginal biopsy specimens because of orientation when comparing the smaller and larger forceps (P = .57). Finally, the percentage of inadequate biopsy specimens obtained using the larger forceps was significantly lower than that for the smaller forceps (P = .05; CI95% = −20.32 to −0.01).
Ordinal logistic regression showed that the adequacy of the biopsy specimens was significantly influenced by forceps size (P ≤ .001). For a single biopsy specimen, the probability of being in the next higher class of adequacy (from inadequate [0] to marginal [1] and from marginal [1] to adequate [2]) was decreased by 57% when it was collected with the smaller forceps (odds ratio [OR] = 0.43; CI95% = 0.30‐0.61).
As expected, there also was an effect of the patient on the adequacy of the biopsy specimens: in 4 of 50 cats, it was significantly easier to obtain adequate biopsy specimens, whereas in 1 cat, the sampling was significantly more difficult.
No statistically significant differences were found between the 2 differently sized forceps in any of the types of orientation toward the cellulose nitrate paper (inadequate, backwards, proper; Table 1 and Figure 2E). However, ordinal logistic regression indicated that the orientation of biopsy specimens was significantly influenced by forceps size (P = .03). For a single biopsy specimen, the probability of being in the next higher class of orientation (from inadequately [0] to backwardly oriented [1] and from backwardly [1] to adequately oriented [2]) was increased by 60% when the sample was collected using the smaller forceps (OR = 1.6; CI95% = 1.04‐2.43). Furthermore, as already noted for adequacy, there was an effect of the individual patient on the orientation of the biopsy specimen. In 8 of 50 cats, it was easier to obtain a better oriented sample, whereas in 1 cat, it was more difficult.
Artifacts were present in 63.7% of the evaluable samples (60/95); the results are summarized in Table 2. The χ2 test identified no association between the presence of artifacts and forceps size (P = .41). However, logistic regression showed that the presence of artifacts was negatively influenced by adequacy (P = .04). For every adequate biopsy, the probability of having artifacts decreased by 28% (OR = 0.72; CI95% = 0.52‐0.99). No significant difference was found between the percentage of artifacts in biopsy specimens collected using the smaller or the larger forceps (paired t test, P = .85). Nevertheless, ordinal logistic regression showed that the percentage of artifacts was negatively influenced by adequacy (P ≤ .001). For every adequate biopsy, the probability of being in the next higher class of percentage of artifacts decreased by 33% (OR = 0.67; CI95% = 0.50‐0.90).
Table 2.
Biopsy artifacts and villi morphology
| Present | Absent | Testa | |||
|---|---|---|---|---|---|
| L | S | L | S | P‐value | |
| Artifacts (%)b | |||||
| Presence of artifacts | 29/49 (60%) | 31/46 (67%) | 20/49 (40%) | 15/46 (33%) | .41 |
| Artifact quantity (mean) | 8.32% (±11.38) | 8.64% (±11.73) | NA | NA | .85 |
| Compression artifact | 14/49 (29%) | 12/46 (26%) | 35/49 (71%) | 34/46 (74%) | .79 |
| Streaming chromatin | 19/49 (39%) | 23/46 (50%) | 30/49 (61%) | 23/46 (50%) | .27 |
| Roll‐over artifact | 11/49 (22%) | 5/46 (11%) | 38/49 (78%) | 41/46 (89%) | .13 |
| Villi (%) | |||||
| Evaluable villi | 44/50 (88%) | 39/50 (78%) | 6/50 (12%) | 11/50 (22%) | .18 |
| Blunting | 6/44 (14%) | 3/39 (0.08%) | 38/44 (86%) | 36/39 (99.02%) | .38 |
| Fusion | 1/44 (0.02%) | 1/39 (0.03%) | 43/44 (99.08%) | 38/39 (99.07%) | .93 |
| Distortion | 4/44 (0.09%) | 3/39 (0.08%) | 40/44 (99.01%) | 36/39 (99.02%) | .82 |
Abbreviations: L, larger forceps; NA, not applicable; S, smaller forceps.
t‐test for mean difference of quantity of artifact; χ2 test for association of forceps size with artifact types.
Not applicable = 5.
The villi could be evaluated for morphological changes (blunting, distortion, fusion) in 83% of cases (83/100), specifically in 78% (39/50) of the samples obtained using the smaller forceps and in 88% (44/50) of those obtained using the larger forceps. This difference however was not significant (P = .18). Logistic regression analysis indicated that the possibility of evaluating the villi increased depending on the total number of biopsy specimens per cat (OR = 1.66; CI95% = 1.10‐2.51; P = .015), the number of adequate biopsy specimens (OR = 3.9; CI95% =1.73‐8.79; P = .001), and the number of marginal biopsy specimens properly oriented (OR = 3.50; CI95% = 1.94‐6.31; P ≤ .001).
3.2. Histological features
Samples subsequently were evaluated for histological features, and data are summarized in Table 3. Agreement between the 2 forceps was variable, ranging from poor to substantial depending on the histological feature, and it was poorer when the severity of inflammation was considered. Regarding the identification of the presence of histological lesions (inflammation or neoplasia versus no lesions), agreement was achieved in 41 of 45 cases (in 5 cases, a diagnosis was not possible regardless of the forceps used; κ = −0.034). However, McNemar's χ2 test did not identify a significant difference in diagnosis between the use of the smaller and larger forceps (P = .61); moderate agreement was achieved with the assessment of non‐neoplastic lesions (κ = 0.56). The agreement was substantial (κ = 0.78) when the diagnosis was lymphoma, but in 3 of 45 cases, there was no agreement and, in 2 of these cases, lymphoma was identified only using the larger forceps. In particular, the agreement was perfect (κ = 1) in the case of large cell lymphoma, whereas the diagnosis of small cell lymphoma was more challenging (κ = 0.66).
Table 3.
Agreement on histological features
| Forceps | McNemar χ2 test | ||||
|---|---|---|---|---|---|
| L | S | κ‐test | CI95% a | P‐value | |
| Single lesions | |||||
| LP | 39/49 (79.59%) | 35/46 (76.09%) | 0.57 | 0.21 to 0.83 | 1 |
| Eosinophilic infiltration | 15/49 (30.61%) | 13/46 (28.26%) | 0.68 | 0.38 to 0.88 | 1 |
| Neutrophilic infiltration | 16/49 (32.65%) | 9/46 (19.57%) | 0.52 | 0.20 to 0.78 | .29 |
| Atrophy | 0/49 (0%) | 1/46 (2.17%) | NA | NA | NA |
| Fibrosis | 3/49 (6.12%) | 4/46 (8.7%) | −0.08 | −0.14 to 0 | 1 |
| Lymphoma | 9/49 (18.37%) | 8/46 (17.39%) | 0.78 | 0.42 to 1 | 1 |
| Small cell lymphoma | 7/49 (14.29%) | 7/46 (15.22%) | 0.66 | 0.23 to 0.92 | 1 |
| Medium cell lymphoma | 1/49 (2.04%) | 0/46 (0%) | NA | NA | NA |
| Large cell lymphoma | 1/49 (2.04) | 1/46 (2.17%) | 1 | NA | NA |
| Main diagnosis | |||||
| Inflammation/neoplasia versus others | 48/49 (97.96%) | 43/46 (93.48%) | −0.03 | −0.13 to 0 | .62 |
| Neoplastic versus non‐neoplastic | 10/49 (79.59%) | 11/46 (76.09%) | 0.56 | 0.23 to 0.83 | 1 |
| LP versus small cell lymphoma versus others | |||||
| LP | 39/49 (79.59%) | 35/46 (76.09%) | 0.53 | 0.23 to 0.79 | .50 |
| Small cell lymphoma | 7/49 (14.29%) | 7/46 (15.22%) | |||
| Others | 3/49 (6.12%) | 4/46 (8.70%) | |||
Abbreviations: L, larger forceps; NA, not applicable; S, smaller forceps; LP, lymphoplasmacytic infiltration.
95% confidence interval of κ based on bootstrapping (10 000 replicates).
There was moderate agreement in the distinction between lymphoplasmacytic inflammation and small cell lymphoma, or other features between the smaller and larger forceps (κ = 0.53). Specifically, in 4 of 45 cases, lymphoplasmacytic inflammation was identified only with 1 forceps, whereas with the other forceps, different histological features were seen and, in 3 of these cases, the lymphoplasmacytic infiltration was identified only with the larger forceps. In 3 of 45 cases, small cell lymphoma was diagnosed with 1 forceps and lymphoplasmacytic inflammation was identified with the other; in 2 of these cases, small cell lymphoma was identified only with the larger forceps. Finally, in 1 case with the smaller forceps, the final diagnosis made was small cell lymphoma, whereas with the larger forceps, severe lymphoplasmacytic infiltration was identified.
4. DISCUSSION
In cats with gastrointestinal disease, because of the size of the animals, small biopsy forceps usually are used for the collection of duodenal samples. In a previous study, inadequate samples decreased the possibility of finding some lesions, which, in turn, affected the sensitivity of the diagnostic technique.3 However, different from dogs, in cats, a few adequate samples obtained using a smaller forceps (1.8 mm) are sufficient to find even severe infiltrative lesions. It was suggested that cats have a thin intestinal wall, and hence, it would be easier for the endoscopist to obtain adequate biopsie specimens.3 However, our study addresses the difficulties faced in clinical practice obtaining adequate duodenal biopsy specimens with the small forceps routinely used in cats and, consequently, in achieving an accurate final diagnosis.
Our results highlight the usefulness of larger biopsy forceps when performing duodenal biopsies. We found that it is indeed possible to collect a higher percentage of adequate and evaluable tissue samples and a lower percentage of marginal and inadequate samples when compared to the samples obtained using smaller biopsy forceps. Moreover, the ordinal logistic regression confirmed that forceps size significantly influenced the adequacy of the biopsy specimen, because the probability of obtaining an adequate sample decreased by 57% with the smaller forceps. Similar to previous studies, we found that 10% of the samples (5 of 50 cases) were not evaluable despite the use of the 2 differently sized forceps and the same experienced endoscopist (Enrico Bottero). These data suggest that in some patients, it is more difficult to obtain adequate samples, independently of the biopsy forceps used or the operator. This individual peculiarity was highlighted in our study by ordinal logistic regression because a single patient was shown to influence the quality of the biopsy specimen. The sampling technique, the forceps type, and the experience of the endoscopist, all can influence the adequacy of the sample obtained. However, in our case, the biopsies were performed by a single board‐certified endoscopist (Enrico Bottero) using the same technique and the same forceps type (oval fenestrated cups with spike, different only in size) for each patient; therefore, we believe that these variables have only a minimal influence on the quality of biopsy specimens. It would be valuable to conduct further studies comparing different sampling techniques, forceps types, and operators.
Considering all the types of orientation of the sample toward the cellulose nitrate paper, we did not find any significant difference between the percentages of the samples obtained with the 2 differently sized biopsy forceps, but ordinal logistic regression showed that the probability of obtaining a properly oriented sample increased by 60% when the smaller forceps was used. This result was unexpected and may be because of the difficulty in identifying the submucosal side of the biopsy specimens when placing it on the cellulose nitrate paper. Furthermore, this manual skill is even more complicated with a larger biopsy specimen, which tends to be curved when taken from the cups of the needle forceps (Figure 2F). This difficulty also is reflected in the high percentage of marginal biopsy specimens because of the orientation of the biopsy specimens collected with the larger forceps.
Artifacts were present in a high proportion of the evaluable samples (64%), but both the presence and percentage of artifacts were not associated with forceps size. Nonetheless, in agreement with the literature, in our study, the adequacy of samples negatively influenced the percentage of artifacts and ordinal logistic regression indicated that the percentage of artifacts decreased by 28% when the number of adequate biopsy specimens increased.
No significant difference was observed in the percentages of evaluable villi in biopsy specimens obtained using the 2 differently sized biopsy forceps. However, by logistic regression, we found that the probability of obtaining biopsy specimens with evaluable villi was positively influenced by the total number of biopsies, the number of adequate samples, and the number of marginal samples that were properly oriented. Consequently, because we clearly demonstrated that the use of the larger biopsy forceps increased the number of adequate samples obtained compared to the smaller forceps, we can suggest, indirectly, that their use positively influences the possibility of evaluating villi for morphological changes and of obtaining a lower percentage of artifacts. Furthermore, in our study, the size of the forceps did not directly affect either biopsy specimen orientation or the presence of artifacts. These results emphasize that the biopsy forceps is not the only element that directly affects the quality of the biopsy specimens. Rather, it emphasizes the role of the ability and experience of the endoscopist, both of which greatly influence these 2 variables, independent of the type of biopsy forceps used.
The agreement between the 2 forceps in the assessment of histological features was variable, ranging from poor to substantial, and it was even poorer when the severity of inflammation was evaluated. Moderate agreement was found between the smaller and larger biopsy forceps in distinguishing lymphoplasmacytic inflammation from small cell lymphoma or other histological features. Particularly regarding the diagnosis of lymphoma, although agreement between the 2 forceps was substantial, in 3 of 45 cases, disagreement occurred and, in 2 of these cases, the diagnosis was achieved only with the larger forceps. Actually, some of the features used to diagnose small cell lymphoma (eg, intraepithelial lymphocytes forming plaques or nests)8 also are visible in small, inadequately oriented samples, whereas others (eg, architecture distortion and villous fusion or invasion of the deep lamina propria) are only appreciated in large, well‐oriented biopsy specimens. Moreover, as a general rule, neoplastic lymphocytes are very fragile, and small samples are at risk of being masked, entirely or in part, by mechanical artifacts (eg, nuclear stripes, cell lysis) that consequently could interfere with diagnosis. We thus suggest that the use of a larger forceps can lead to better evaluation of the biopsy specimens, which is especially crucial in the case of small cell lymphoma because its diagnosis is challenging. However, in our study, too few cases of lymphoma were available to evaluate this aspect properly. Moreover, the diagnosis of small cell low‐grade lymphoma generally is complex and may require the use of ancillary techniques such as immunohistochemistry or PCR.8 Consequently, further studies considering a larger cohort of lymphoma cases are needed to confirm this hypothesis.
In conclusion, our study demonstrated the usefulness of a larger biopsy forceps for performing endoscopic duodenal biopsy sampling in cats, because it allows the collection of a higher percentage of adequate and evaluable biopsy specimens compared to the commonly used smaller forceps. In turn, collection of a higher number of adequate samples significantly decreases the percentage of artifacts and increases the percentage of samples with evaluable villi, thus making assessment of the histological sample easier.
In our experience, use of the larger biopsy forceps, even when not oriented perfectly perpendicular to the mucosal surface, allows collection of a sufficient amount of tissue. Conversely, use of the smaller forceps, if incorrectly positioned, only guarantees superficial sampling, hence, only marginal or inadequate biopsy specimens can be obtained and the proper histological diagnosis can be more difficult to achieve.
The individual patient also plays an important role, because in some cats, it was more difficult to obtain high‐quality samples. However, to the best of our knowledge, no studies have investigated the reasons for low‐quality samples in some patients and whether it could be influenced by signalment (breed, sex, or age) or pathological condition (eg, chronic disease, type of inflammation). Consequently, further studies are needed to address this issue.
Although the agreement between the 2 differently sized biopsy forceps was substantial for the diagnosis of lymphoma, in the majority of cases, the proper diagnosis was achieved only with the larger biopsy forceps. Because the distinction between lymphoplasmacytic inflammation and lymphoma is challenging, we suggest that use of a larger forceps could be helpful to obtain high‐quality samples and a more accurate diagnosis. However, because only a few cases were histologically classified as lymphoma, subsequent studies would be needed to understand whether the use of a large biopsy forceps can substantially increase diagnostic sensitivity in the case of low‐grade lymphoma in cats. Finally, we speculate that a different number of samples could be taken depending on the forceps size used, but this hypothesis requires further focused studies.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Bottero E, Mussi E, Pieramati C, De Lorenzi D, Silvestri S, Lepri E. Comparison of 2 differently sized endoscopic biopsy forceps in the evaluation of intestinal disease in cats. J Vet Intern Med. 2019;33:523–530. 10.1111/jvim.15356
REFERENCES
- 1. Day MJ, Bilzer T, Mansell J, et al. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsies from the dog and the cat: a report from the world small animal veterinary association gastrointestinal standardization groups. J Comp Pathol. 2008;137:S1‐S43. [DOI] [PubMed] [Google Scholar]
- 2. Ruiz GC, Reyes Gomez E, Mansell EJ, et al. Comparison of 3 handling techniques for endoscopically obtained gastric and duodenal biopsy specimen: a prospective study I dog and cat. J Vet Intern Med. 2016;30(4):1014‐1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Willard MD, Mansell J, Fosgate GT, et al. Effect of sample quality on the sensitivity of endoscopic biopsy for detecting gastric and duodenal lesions in dogs and cats. J Vet Intern Med. 2008;22:1084‐1089. [DOI] [PubMed] [Google Scholar]
- 4. Willard MD, Moore GE, Denton BD, et al. Effect of tissue processing on assessment of endoscopic intestinal biopsies in dogs and cats. J Vet Intern Med. 2010;24:84‐89. [DOI] [PubMed] [Google Scholar]
- 5. Goutal‐Landry CM, Mansell J, Ryan KA, Gaschen FP. Effect of endoscopic forceps on quality of duodenal mucosal biopsy in healthy dogs. J Vet Intern Med. 2013;27:456‐461. [DOI] [PubMed] [Google Scholar]
- 6. Van der Gaag I, Happe RP. The histological appearance of peroral small intestinal biopsies in clinically healthy dogs and cats with chronic diarrhea. Zentralbl Veterinarmed B. 1990;3:73‐78. [PubMed] [Google Scholar]
- 7. Willard MD, Overing SL, Cohen ND, et al. Quality of tissue specimens obtained endoscopically from the duodenum of dogs and cats. J Am Vet Med Assoc. 2001;219:474‐479. [DOI] [PubMed] [Google Scholar]
- 8. Kupiel M, Smedley RC, Xie Y, et al. Diagnostic algorithm to differentiate lymphoma from inflammation in feline small intestinal biopsy sample. Vet Pathol. 2011;48(1):212‐222. [DOI] [PubMed] [Google Scholar]
- 9. Danesh BJZ, Burke M, Newman J, et al. Comparison of weight, depth, and diagnostic adequacy of specimens obtained with 16 different biopsy forceps designed for upper gastrointestinal endoscopy. Gut. 1985;26:227‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Woods KL, Anand BS, Cole RA, et al. Influence of endoscopic biopsy forceps characteristic on tissue specimens: result of a prospective randomized study. Gastrointest Endosc. 1999;49:177‐183. [DOI] [PubMed] [Google Scholar]
- 11. Yang R, Ng S, Nichol M, Laine L. Cost and performance evaluation of disposable and re‐usable biopsy forceps in G. I. Endoscopy. Gastrointest Endosc. 2000;51:266‐270. [DOI] [PubMed] [Google Scholar]
- 12. Fantin AC, Neuweiler J, Binek JS, Suter WR, Meyenberger C. Diagnostic quality of biopsy specimens: comparison between a conventional biopsy forceps and multi‐bite forceps. Gastrointest Endosc. 2001;54:600‐604. [DOI] [PubMed] [Google Scholar]
- 13. Padda S, Shaha I, Ramirez FC. Adequacy of mucosal sampling with the two‐bite forceps technique: a prospective, randomized, blinded study. Gastrointest Endosc. 2003;57:170‐173. [DOI] [PubMed] [Google Scholar]
- 14. Bilzer T. Istopatologia In: Steiner JM, ed. Gastroenterologia del cane e del gatto. Milano: Elsevier; 2009:93‐96. [Google Scholar]
- 15. Jergens AE. Endoscopy: gastricendoscopy In: Washabau RJ, Day MJ, eds. Canine and Feline Gastroenterology. St. Louis, Missouri: ElsevierSaunders; 2013:276‐281. [Google Scholar]
- 16. Jergens AE, Moore FM. Endoscopic biopsy specimen collection and histopathologic considerations In: Tams TR, ed. Small Animal Endoscopy. 2nd ed St. Louis, Missouri: Mosby; 1999:323‐340. [Google Scholar]
- 17. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159‐174. [PubMed] [Google Scholar]
- 18. R Core Team . A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. Available from https://www.R-project.org/. [Google Scholar]


