Abstract
Background
Although the demand for esomeprazole is increasing in veterinary medicine, the pharmacokinetics (PK) and pharmacodynamics of esomeprazole have been described in only a few studies.
Objective
To determine the PK of 0.5 and 1 mg/kg esomeprazole administered IV q12h and to investigate its effects on intragastric pH in healthy dogs.
Animals
Six adult Beagles.
Methods
Open‐label, randomized, and crossover design. The dogs received 0.5 or 1 mg/kg esomeprazole IV q12h for 48 hours. Plasma concentrations of esomeprazole were measured by high‐performance liquid chromatography‐tandem mass spectrometry. Intragastric pH was determined using the Bravo pH monitoring system and recorded as mean percentage time (MPT) for which pH was ≥3 and ≥4 for 24 hours in each group.
Results
The peak plasma concentration and area under the curve from the time of dosing to the last measurable concentration in the 1 mg/kg group were higher than those in the 0.5 mg/kg group. However, when the dosage normalized, intergroup differences were not significant. The MPTs for which intragastric pH was ≥3 and ≥4 for 48 hours were 88% ± 7% and 81% ± 9% for the 0.5 mg/kg group and 90% ± 9% and 85% ± 11% for the 1 mg/kg group, respectively, with no significant intergroup differences.
Conclusions and Clinical Importance
The pharmacokinetic parameters and acid suppressant effect for 0.5 and 1 mg/kg esomeprazole were not significantly different. Furthermore, the efficacy of esomeprazole 0.5 mg/kg IV q12h was sufficient to increase intragastric pH in Beagles.
Keywords: Bravo pH monitoring system, canine, IV, S‐omeprazole, q12h
Abbreviations
- CL
clearance
- Cmax
peak plasma concentration
- ke
elimination rate constant
- MPT
mean percentage time
- PD
pharmacodynamics
- PK
pharmacokinetics
- PPIs
proton pump inhibitors
- RM anova
repeated‐measures analysis of variance
- T1/2
terminal elimination half‐life
- Tmax
time until maximum concentration
- Vss
steady‐state volume of distribution
1. INTRODUCTION
Esomeprazole is the S‐isomer of omeprazole and a potent proton pump inhibitor (PPI) that suppresses gastric acid secretion by specifically inhibiting H+/K+‐ATPase in gastric parietal cells.1 It has been found to be superior to other racemic PPIs for increasing gastric pH and maintaining gastric alkalization in humans.2, 3, 4 Oral and IV formulations of esomeprazole have been used widely in veterinary medicine.5 However, little information is available about the dosage and pharmacokinetics (PK) of esomeprazole in conscious dogs and other veterinary species. According to a study of IV esomeprazole and cisapride in anesthetized dogs, esomeprazole (1 mg/kg IV) alone did not significantly decrease the frequency of gastroesophageal reflux but increased gastric and esophageal pH for a few hours, while the effects of anesthesia persisted.6 The PK and acid suppressive efficacy of esomeprazole after IV, PO, and SC administration at a dosage of 1 mg/kg in healthy conscious Beagles have been reported in a recent study. The study showed that IV injections of esomeprazole q24h were not sufficient to increase intragastric pH.7 Furthermore, a study comparing the efficacy of IV pantoprazole (1 mg/kg q12h) and its coadministration with famotidine in healthy dogs showed that pantoprazole administered alone facilitated reaching clinical goals within a day after injection.8
Based on these previous results, we hypothesized that IV esomeprazole (1 mg/kg q12h) would have a greater effect with regard to increasing intragastric pH within a few days and would have adequate efficacy at lower dosages, because esomeprazole was found to be relatively more potent than pantoprazole in a study of humans.9
Thus, the objectives of our study were to evaluate the PK and pharmacodynamics (PD) of IV esomeprazole administered q12h and to compare the efficacy of 0.5 and 1 mg/kg dosages in healthy dogs.
2. MATERIALS AND METHODS
2.1. Dogs
The subjects were 6 healthy male Beagles from a research colony at the College of Veterinary Medicine of Chungnam National University. The dogs were aged 6‐8 years (median age, 7 years) and weighed 8.9‐11.1 kg (median weight, 9.48 kg). Physical examination, CBC, and serum biochemistry tests performed within 7 days before the experiment showed the animals to be healthy. No concerning clinical signs (anorexia, vomiting, diarrhea) were observed within a month before this study. The subjects were housed individually in cages and fed commercial dry dog food twice a day except before the PK study; water was offered ad libitum. This animal experiment was approved by the Institutional Animal Care and Use Committee at Chungnam National University (approval number, CNU‐00747). Complete anorexia over 24 hours and >20% weight loss were determined as humane end points during the study.
2.2. Study design
The PD study was performed with a randomized, open‐label, 2‐way crossover design. During each period, baseline intragastric pH was recorded for 24 hours before administering the drug. Six dogs were randomized into 2 groups with 3 dogs each, with the first group receiving 0.5 mg/kg and the second group receiving 1.0 mg/kg q12h IV for 48 consecutive hours followed by washout and crossover. The washout period was 10 days. Esomeprazole powder (Nexium Injection 40 mg; AstraZeneca AB, Södertälje, Sweden) was diluted with sterilized 0.9% saline to achieve a concentration of 8 mg/mL before administration. The drug was infused for 3 minutes through a cephalic venous catheter. Each treatment was performed at 8:30 am and 8:30 pm A ground diet mixed with water was given to all the dogs 30 minutes after administration. The PK study also was performed using a randomized, open‐label, 2‐way crossover design. All treatments were the same as those used in the PD study. Each treatment was administered in the morning after overnight fasting. Water was given 2 hours after treatment administration, and food was given after collecting the last blood sample during each treatment period.
The dogs in the experiment were not given any medication during the week before the experiment and during the experimental periods. Data on appetite, vomiting, number of defecations, and fecal consistency were obtained. A fecal scoring system (Nestle Purina, Vevey, Switzerland) was used to grade fecal consistency (on a scale of 1‐7) and a fecal score of ≥4 was defined as diarrhea.
2.3. Measurement of intragastric pH with the Bravo pH Monitoring System
On the day before the first administration (Day 0), the dogs were fasted for 12 hours and allowed to drink water until 2 hours before anesthesia. The dogs were anesthetized for gastroscopy‐assisted placement of the Bravo pH capsule (Given Imaging, Yoqne'am, Israel). Propofol (6 mg/kg IV, Provive 1% Injection; AFT Pharmaceuticals Ltd, Auckland, New Zealand) was used for anesthesia induction, which was maintained with isoflurane (I‐Fran Liquid; Hana Pharm Co., Ltd, Gyeonggi‐do, Korea) during the entire procedure. The Bravo pH capsules were calibrated with commercial buffer solutions before gastroscopy. The entire gastric mucosa was evaluated grossly before placement of the capsule. The capsule was attached to the fundic mucosa approximately 10 cm distal to the lower esophageal sphincter.10 The receivers of the Bravo pH capsules were placed on the cages of the animals in which the capsules had been placed.
Intragastric pH was continuously recorded at 6‐second intervals by the capsule. The 72‐hour recording data were obtained by the receiver. The raw data then were transferred to a computer at intervals of approximately 12 hours by using specific software (POLYGRAM NET; Medtronic, Fridley, Minnesota). The percentage of time that intragastric pH was ≥3 and ≥4 was determined, and the pH was categorized into 9 categories (pH 0‐1, 1‐2, 2‐3, 3‐4, 4‐5, 5‐6, 6‐7, 7‐8, and >8) from raw data using a spreadsheet program (Excel 2013; Microsoft Co., Redmond, Washington).
2.4. Blood collection and PK analysis
Blood samples for PK analysis were obtained from dogs that were treated using 2 dosage regimens for 12 hours. Each 1.5 mL sample of blood was collected by jugular venipuncture into heparinized tubes before dosing; at 0, 3, 5, 10, 20, and 40 minutes after drug administration; and 1, 1.5, 2, 3, 4, 6, 8, 10, and 12 hours after drug administration. At specific administration points (0 and 12 hours), blood collection preceded drug injection. Blood samples were centrifuged at 2500g for 10 minutes. Plasma was stored at −80°C until analysis. To determine esomeprazole concentrations in the plasma samples, a previously described high‐performance liquid chromatography‐tandem mass spectrometry (HPLC‐MS/MS)‐based method was used.1
2.5. PK analysis
To estimate the PK parameters of esomeprazole, the plasma concentration to time profiles were analyzed by a non‐compartmental model using WinNonlin software version 4.1 (Pharsight Corp., Mountain View, California).7 The elimination rate constant (ke) was determined by linear regression analysis of the log‐linear portion of the terminal phase.7 The terminal elimination half‐life (T1/2) was determined by dividing 0.693 by ke.7 To determine the elimination clearance (CL) and steady‐state volume of distribution (Vss) for esomeprazole, a moment analysis was performed.7 The area under the plasma esomeprazole concentration versus time curve from time zero to infinity and the area under the respective first moment time curve from time zero to infinity were determined by the linear trapezoidal rule and standard area extrapolation method using WinNonlin 4.1.7 The CL and Vss for esomeprazole were determined using the following equations7:
| (1) |
| (2) |
| (3) |
Peak plasma concentration and time until maximum concentration were obtained directly from the plasma concentration‐time curves.7
2.6. Statistical analysis
Independent t‐test and Mann‐Whitney test, paired t test, and Wilcoxon signed rank test were used to compare the percentage time intragastric pH was ≥3 and ≥ 4 between baseline and 2‐day treatment in each group and to assess the order effect of the crossover design. The Shapiro‐Wilk test was used to test for normality, and Levene's F test was used to test the assumption of homogeneity of variance. Repeated measures analysis of variance (RM anova) was used to compare the percent time intragastric pH was ≥3 and ≥4 between 2 regimen groups and among the time periods (an interval of 12 hours) within each group. When comparing the distribution of intragastric pH between the 2 treatment groups, RM anova also was used. Mauchly's sphericity test and multivariate test were performed as part of RM anova. The PK parameters were analyzed using an unpaired t‐test as a nonparametric Mann‐Whitney test. Commercial statistical software (IBM SPSS Statistics 22; IBM Co., New York) was used to analyze all data. The level of significance was set at P < .05.
3. RESULTS
3.1. Intragastric pH recordings
Twelve Bravo pH capsules were attached to the fundic mucosa; no complications were observed. The acid‐suppressive effect of PPI was evaluated based on the mean percentage time (MPT) for which intragastric pH was ≥3 and ≥4 over a 24‐hour period in each group.10, 11 These values are typical points of reference in both the human and veterinary medical literature. The values for MPT ± SD (SD) for which intragastric pH was ≥3 and ≥4 were 15% ± 11% and 6% ± 7% at baseline and 88% ± 7% and 81% ± 9% for the 2‐day treatment in the 0.5 mg/kg group, respectively. On the other hand, the values for MPT ± SD for which intragastric pH was ≥3 and ≥4 were 22% ± 16% and 13% ± 14% at baseline and 90% ± 9% and 85% ± 11% for the 2‐day treatment in the 1 mg/kg group, respectively.
Most data sets showed normal distribution, but normality and variance homogeneity were not satisfactory on Day 2 (24‐36 hours, 36‐48 hours). Moreover, because of small sample size, Mann‐Whitney and Wilcoxon signed rank tests were used for nonparametric analysis.
No difference was found in the values of MPT for which intragastric pH was ≥3 and ≥4 at baseline between the 2 groups (P = .58 and .81, respectively). Both treatment groups experienced a significant increase in intragastric pH after treatment compared to that at baseline (both P = .028). The MPTs for which intragastric pH was ≥3 and ≥4 were not significantly different between the 0.5 and 1 mg/kg groups from the first injection to 48 hours later; the interval period was 12 hours (P = .19 and .70, respectively; Figure 1). A comparison of the MPTs for which intragastric pH was ≥3 and ≥4 at baseline with those associated with each 12‐hour period after treatment showed that the baseline values were significantly lower than those 12‐48 hours after treatment (P < .05) within each group (Figure 1). No differences were found in the distribution of intragastric pH over pH categories 1‐9 between the 0.5 and 1 mg/kg groups (P = .49). The MPTs for which intragastric pH was ≥3 and ≥4 also were used to determine if there was an effect of the order of treatment or day of treatment on intragastric pH between or within a specific group. For all dogs, the order of treatment did not significantly affect the percentage time.
Figure 1.
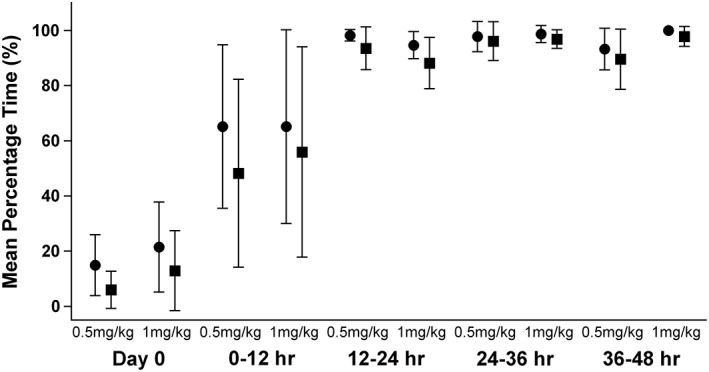
Comparison of the mean percentage time for which intragastric pH was ≥3 and ≥4 between the 0.5 and 1 mg/kg groups from the first injection to 48 hours during the treatment period; the period was divided into intervals of 12 hours. Circles represent the mean (±SD) percentage time for which intragastric pH was ≥3 and squares represent the mean (±SD) percentage time for which intragastric pH was ≥4
3.2. PK of esomeprazole
Plasma concentrations (mean ± SD) during the treatment period are shown in Figure 2. The PK parameters are shown in Table 1. No significant differences in T1/2, Vss, and CL values were found between groups. The peak plasma concentration (Cmax) and area under the curve from the time of dosing to the last measurable concentration values of the 1 mg/kg group were higher than those of the 0.5 mg/kg group. However, when the dosage was normalized, no significant intergroup differences were found.
Figure 2.
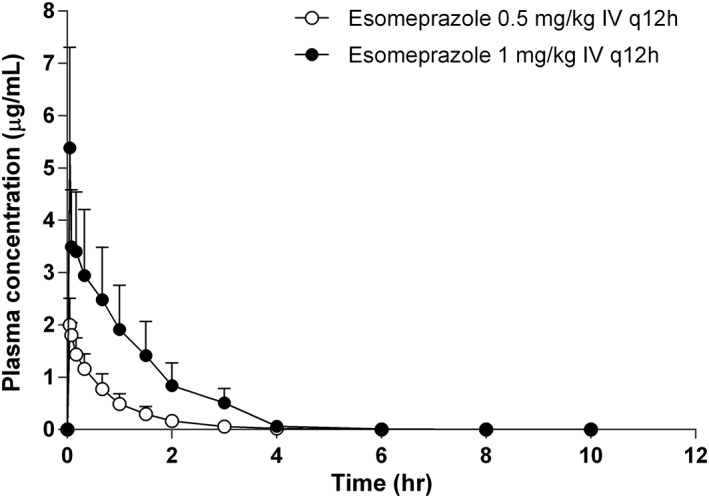
Esomeprazole plasma concentrations (mean ± SD) in 6 dogs treated with 0.5 mg/kg (black circle) or 1 mg/kg (open circle) for 12 hours from the time of the first administration of the drug
Table 1.
Pharmacokinetic parameters of 2 dosage groups, 0.5 and 1 mg/kg q12h, of esomeprazole in 6 dogs
| Parameters | Dosing regimens | ||
|---|---|---|---|
| 0.5 mg/kg (n = 6) | 1 mg/kg (n = 6) | P‐value | |
| Tmax (h) | 0.06 ± 0.02 | 0.05 ± 0.00 | .45 |
| T1/2 (h) | 0.70 ± 0.09 | 0.90 ± 0.32 | .50 |
| Cmax (μg/mL) | 2.03 ± 0.47 | 5.39 ± 1.92 | .002 |
| Cmax /dose | 4.07 ± 0.94 | 5.39 ± 1.92 | .18 |
| AUCinf (μg·h/mL) | 1.44 ± 0.51 | 5.05 ± 2.24 | .002 |
| AUClast (μg·h/mL) | 1.44 ± 0.51 | 5.05 ± 2.24 | .002 |
| AUCinf/dose | 2.89 ± 1.02 | 5.05 ± 2.24 | .06 |
| Vss (L/kg) | 0.30 ± 0.05 | 0.25 ± 0.10 | .21 |
| CL (L/h/kg) | 0.39 ± 0.14 | 0.24 ± 0.13 | .06 |
All parameters were calculated as the mean ± SD.
Abbreviations: AUCinf, area under the curve from zero to infinite; AUClast, area under the curve from the time of dosing to the last measurable concentration; CL, clearance; Cmax, peak plasma concentration; T1/2; half‐life; Tmax, time until maximum concentration; Vss, steady‐state volume of distribution.
3.3. Adverse effects of esomeprazole
Neither group experienced severe adverse effects. Three vomiting episodes in 2 dogs occurred. Two episodes of vomiting occurred in the same dog on the first day of treatment in the 0.5 and 1 mg/kg groups during the PK study, respectively. The other episode occurred on the second day of treatment in the 1 mg/kg group during the PD study. The mean fecal score was 2.8 ± 0.5 at baseline before the 0.5 mg/kg treatment, 2.7 ± 0.4 for 1‐2 days after the 0.5 mg/kg treatment, 3.0 ± 0.8 at baseline before the 1 mg/kg treatment, and 2.7 ± 0.4 for 1‐2 days after the 1 mg/kg treatment. No difference in fecal scores was found between treatments. In 4 instances, fecal score was ≥4 : 1 instance at baseline, 2 instances in the 0.5 mg/kg treatment group, and 1 instance in the 1 mg/kg treatment group.
4. DISCUSSION
Esomeprazole increases gastric pH more effectively than omeprazole in human patients with gastroesophageal reflux disease because its low first‐pass hepatic metabolism leads to a higher plasma concentration than that of omeprazole. Furthermore, it is sustained for a longer period in plasma and has better bioavailability than omeprazole.3, 4 Although no studies have compared esomeprazole with other PPIs in veterinary medicine, previous PD studies of esomeprazole showed that longer MPTs were achieved than those achieved with omeprazole, even with lower dosage treatment for a shorter time period in dogs.7, 12 In cases of critical illness, PPIs might not be administered to the affected individual PO, and the effect of histamine‐2 receptor antagonists at dosages of 0.5‐1 mg/kg q12h was not adequate to increase gastric pH in several studies.12, 13 Other routes of administration such as IV and SC were studied in a previous experiment. Although PO administration q24h effectively inhibited gastric acid production, IV administration increased MPT effectively only within the first 12 hours and decreased MPT during the remaining 12 hours.7
We evaluated 2 dosing regimens of IV esomeprazole (0.5 and 1 mg/kg q12h) to assess the acid‐suppressive effect of esomeprazole q12h during 48 hours of treatment in dogs. Additionally, the PK of IV esomeprazole were evaluated. No significant intergroup differences were found when comparing the MPT for which intragastric pH was ≥3 and ≥4 during the 48‐hour treatment period. Both dosing regimens met the following clinical goals in humans: intragastric pH ≥3 for 75% of the day while treating peptic ulcers and intragastric pH ≥4 for 67% of the day while treating gastroesophageal reflux disease.10, 11 Although a previous study of esomeprazole at 1 mg/kg IV q24h showed that this regimen was inadequate to achieve standard MPTs,7 q12h dosage increased intragastric pH enough to reach the clinical goal even with lower dosages such as 0.5 mg/kg, thus satisfying our hypothesis.
In a study comparing the efficacy of pantoprazole IV q12h and that of its combination with famotidine in healthy dogs, the MPTs for which intragastric pH was ≥3 and ≥4 were 53% ± 16% and 35% ± 19% in Beagle dogs on Day 2. The efficacy of esomeprazole IV was found to be greater than that of pantoprazole IV in Beagle dogs based on our study results (99% ± 2% and 97% ± 2%), which is consistent with the results of a study in humans.9 However, comparing baseline pH, response to treatment, and the MPTs for which intragastric pH is ≥3 and ≥4 between studies can be problematic because of the wide variability in gastric pH among dogs, especially in colony dogs.
In a study comparing 0.5 and 1 mg/kg IV q12h omeprazole in critically ill children, neither regimen afforded adequate acid suppression in the stomach during the first 24 hours.14 Although the 1 mg/kg dose had enough efficacy between 24 and 48 hours,14 in our study, therapeutic targets were met with both regimens after the first 12 hours of treatment. Although the information pertaining to the difference in efficacy between omeprazole and esomeprazole in veterinary medicine is inadequate, the difference could be attributable to the more potent characteristics of esomeprazole as compared to omeprazole in human medicine or differences in drug metabolism between healthy and critically ill individuals.9
After IV administration of 0.5 and 1 mg/kg, dosage normalized Cmax and area under the curve from zero to infinite (AUCinf) of esomeprazole showed a tendency to increase with dosage, indicating nonlinear PK. Similar nonlinear kinetics for omeprazole CL was observed in a study of humans and was considered to be caused by saturable metabolic elimination. However, no significant differences were found between the 2 dosages, which might have been caused by small sample size. The efficacies in both groups were almost the same in healthy conscious Beagles, apart from PK results. Considering esomeprazole IV q24h increased gastric pH only within 12 hours effectively in dogs,7 the effect of exposure time above a certain concentration on acid suppressant efficacy is believed to be greater than that of exposure at the initial high concentration.
Common adverse gastrointestinal effects such as vomiting and diarrhea were not observed in the 2 groups. As our study included only a small number of healthy Beagle dogs, future studies will be needed to investigate these effects in a large number of diverse dog breeds with or without disease to determine potential unknown adverse effects.
The main limitation of our study was its small sample size. The normality and homogeneity variance test were not satisfied, possibly due to small numbers of samples. Therefore, the MPTs and PK parameters were analyzed using nonparametric statistical methods. The acid suppressant effects between the 2 treatments groups might not have been significantly different because of small sample size and type II error as well. Further studies with larger sample sizes and in clinical settings are required.
In conclusion, the PK parameters for 0.5 and 1 mg/kg esomeprazole were not significantly different and acid suppressant effects of the 2 different dosages were effective in increasing intragastric pH.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
This manuscript is written based on the Master's thesis of author Do‐Hyun Seo.
Seo D‐H, Lee J‐B, Hwang J‐H, et al. Pharmacokinetics and pharmacodynamics of intravenous esomeprazole at 2 different dosages in dogs. J Vet Intern Med. 2019;33:531–535. 10.1111/jvim.15383
Do‐Huyn Seo and Jong‐Bok Lee contributed equally to this work.
Contributor Information
Tae‐Sung Koo, Email: kootae@cnu.ac.kr.
Kyoung‐Won Seo, Email: kwseo@cnu.ac.kr.
REFERENCES
- 1. Andersson T, Rohss K, Bredberg E, et al. Pharmacokinetics and pharmacodynamics of esomeprazole, the S‐isomer of omeprazole. Aliment Pharmacol Ther. 2001;15:1563‐1569. [DOI] [PubMed] [Google Scholar]
- 2. Iida H, Inamori M, Okuno K, et al. Early effects of oral administration of esomeprazole and omeprazole on the intragastric pH. Hepatogastroenterology. 2015;62:493‐496. [PubMed] [Google Scholar]
- 3. Hassan‐Alin M, Andersson T, Niazi M, Röhss K. A pharmacokinetic study comparing single and repeated oral doses of 20 mg and 40 mg omeprazole and its two optical isomers, S‐omeprazole (esomeprazole) and R‐omeprazole, in healthy subjects. Eur J Clin Pharmacol. 2005;60:779‐784. [DOI] [PubMed] [Google Scholar]
- 4. Scott LJ, Dunn CJ, Mallarkey G, Sharpe M. Esomeprazole: a review of its use in the management of acid‐related disorders in the US. Drugs. 2002;62:1091‐1118. [DOI] [PubMed] [Google Scholar]
- 5. Daure E, Ross L, Webster CR. Gastroduodenal ulceration in small animals: part 2. Proton pump inhibitors and histamine‐2 receptor antagonists. J Am Anim Hosp Assoc. 2017;53:11‐23. [DOI] [PubMed] [Google Scholar]
- 6. Zacuto AC, Marks SL, Osborn J, et al. The influence of esomeprazole and cisapride on gastroesophageal reflux during anesthesia in dogs. J Vet Intern Med. 2012;26:518‐525. [DOI] [PubMed] [Google Scholar]
- 7. Hwang JH, Jeong JW, Song GH, Koo TS, Seo KW. Pharmacokinetics and acid suppressant efficacy of esomeprazole after intravenous, oral, and subcutaneous administration to healthy Beagle dogs. J Vet Intern Med. 2017;31:743‐750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tolbert MK, Odunayo A, Howell RS, Peters EE, Reed A. Efficacy of intravenous administration of combined acid suppressants in healthy dogs. J Vet Intern Med. 2015;29:556‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kirchheiner J, Glatt S, Fuhr U, et al. Relative potency of proton‐pump inhibitors‐comparison of effects on intragastric pH. Eur J Clin Pharmacol. 2009;65:19‐31. [DOI] [PubMed] [Google Scholar]
- 10. Burget DW, Chiverton SG, Hunt RH. Is there an optimal degree of acid suppression for healing of duodenal ulcers? A model of the relationship between ulcer healing and acid suppression. Gastroenterology. 1990;99:345‐351. [DOI] [PubMed] [Google Scholar]
- 11. Bell NJ, Burget D, Howden CW, et al. Appropriate acid suppression for the management of gastro‐oesophageal reflux disease. Digestion. 1992;51(Suppl 1):59‐67. [DOI] [PubMed] [Google Scholar]
- 12. Tolbert K, Bissett S, King A, et al. Efficacy of oral famotidine and 2 omeprazole formulations for the control of intragastric pH in dogs. J Vet Intern Med. 2011;25:47‐54. [DOI] [PubMed] [Google Scholar]
- 13. Bersenas AM, Mathews KA, Allen DG, et al. Effects of ranitidine, famotidine, pantoprazole, and omeprazole on intragastric pH in dogs. Am J Vet Res. 2005;66:425‐431. [DOI] [PubMed] [Google Scholar]
- 14. Solana MJ, Lopez‐Herce J, Sanchez A, et al. 0.5 mg/kg versus 1 mg/kg of intravenous omeprazole for the prophylaxis of gastrointestinal bleeding in critically ill children: a randomized study. J Pediatr. 2013;162:776‐782. e771. [DOI] [PubMed] [Google Scholar]


