Abstract
Background
Factors indicative of a negative prognosis for appendicular osteosarcoma (OSA) in dogs are visible metastatic disease, location, and size of lesion. In human medicine maximum standard uptake value (SUVmax), as measured on a fluorine18 flourodeoxyglucose positron emission tomography/computed tomography (18F‐FDG PET/CT), is prognostic for survival for several tumor types.
Objective
Determine if SUVmax is associated with progression‐free interval (PFI) and determination of survival in dogs with appendicular OSA.
Animals
Sixty‐two dogs with untreated appendicular OSA that had been staged with 18F‐FDG PET/CT.
Methods
Retrospective analysis of the 18F‐FDG PET/CT was performed. Dogs were excluded from the study if they did not receive definitive intent treatment for their primary OSA and adjuvant chemotherapy with carboplatin, or had visible metastatic disease on initial imaging. A region of interest (ROI) was created around the primary tumor to measure SUVmax. Univariable and multivariable Cox proportional hazards analysis was performed to evaluate for associations between variables including SUVmax and outcome of PFI and overall survival (OS).
Results
Maximum standard uptake value of the primary tumor was significantly associated with the OS (P = .04) with adjustment for treatment type and monocyte count. The overall median survival time (OST) was 284 days (range, 39‐1293 days) with the OST of dogs having an SUVmax of ≥7.4 of 254 days (range, 98‐428 days) and dogs with an SUVmax of <7.4 of 680 days (range, 108‐811 days, P = .01).
Conclusions and Clinical Importance
Maximum standard uptake value as measured via 18F‐FDG PET/CT is significantly associated with survival in dogs with appendicular OSA with a high SUVmax being an indicator of a negative prognosis.
Keywords: avidity, cancer, canine, MST, SUVmax
Abbreviations
- ALP
alkaline phosphatase
- CI
confidence interval
- CSU‐VTH
Colorado State University Veterinary Teaching Hospital
- DFI
disease‐free interval
- F18‐FDG
fluorine18 flourodeoxyglucose
- HR
hazard ratio
- MI
mitotic index
- MST
median survival time
- OSA
osteosarcoma
- OST
overall survival time
- PET/CT
positron emission tomography/computed tomography
- PFI
progression‐free interval
- ROI
region of interest
- RT
radiation therapy
- SRT
stereotactic radiation therapy
- SUVmax
maximum standard uptake value
1. INTRODUCTION
Osteosarcoma (OSA) is an osseous neoplasm that affects the appendicular skeleton of dogs. It accounts for approximately 85% of all bone tumors.1, 2 Historically, the standard of care treatment has involved amputation of the affected limb followed by adjuvant chemotherapy.3, 4, 5 Recent advancements in surgery and radiation therapy (RT) have allowed for salvage of the affected limb with reasonable rates of local control and similar survival times to amputation.6, 7, 8, 9 Definitive treatment (ie, amputation, surgical limb spare, or high‐dose RT) of the primary appendicular OSA in combination with adjuvant chemotherapy provides a median survival time (MST) of approximately 10‐12 months, with most dogs dying of metastatic disease.6, 8, 9, 10, 11, 12, 13, 14
There are multiple prognostic factors that are associated with a reduction in anticipated survival times in dogs with OSA, including pulmonary metastatic lesions visible on radiographs,15 osseous or lymph node metastasis,16, 17 elevated serum alkaline phosphatase (ALP) activity,18 elevated blood monocyte count,19 and primary tumor location.20, 21, 22 Any of these factors identified on initial staging tests can indicate a change in prognosis and therefore influence therapeutic decisions. For example, the MST of dogs with visible pulmonary metastatic disease is reported as only 59 days.15 As prognostic factors that negatively affect outcome can play such an important role in decision making for both the clinician and the owner, accurate staging before undertaking definitive treatment is vital.
Fluorine18 flourodeoxyglucose positron emission tomography/computed tomography (18F‐FDG PET/CT) is an imaging modality that combines the sensitivity of nuclear medicine with the resolution of advanced 3D imaging. Flourodeoxyglucose is a glucose analog that is taken up by metabolically active cells. These cells could represent a normal variant (such as uptake in the heart, salivary glands, and brain23) or a disease process such as neoplasia or inflammation. As many cancer cells have an increased need for glucose for uncontrolled growth, neoplastic lesions have the potential to exhibit increased uptake of FDG (described as increased avidity).24 Analysis of avidity on an 18F‐FDG PET/CT includes evaluation of a unitless semiquantitative factor called a standard uptake value (SUV). Maximum SUV (SUVmax) is the most commonly reported value and is indicative of the highest pixel of metabolic activity in a tumor. Maximum standard uptake value is proportional to the growth rate of a tumor cell, as reflected by proliferative measures such as Ki‐67 labeling index.25 By extension, SUVmax might be reflective of the aggressiveness of certain cancers and SUVmax of a lesion in a variety of tumors (including appendicular OSA,26 lymphoma,27 chondrosarcoma,28 non‐small cell lung cancer,29 and glioblastoma30 in humans) is prognostic for survival.
Fluorine18 flourodeoxyglucose positron emission tomography/computed tomography is a relatively new staging method in veterinary medicine. There are multiple case series evaluating this modality31, 32, 33 but only a few studies have examined the effectiveness of 18F‐FDG PET/CT as a staging method to evaluate for metastasis or concurrent neoplasia.34, 35, 36, 37 Larger scale investigation into the utility of 18F‐FDG PET/CT staging has not been previously reported in veterinary medicine.
This study was undertaken to determine if SUVmax, as measured by pretreatment 18F‐FDG PET/CT, has an association with outcome in dogs presenting for definitive intent treatment of appendicular OSA.
2. MATERIALS AND METHODS
A retrospective medical record search at the Colorado State University Veterinary Teaching Hospital (CSU‐VTH) was performed. Dogs were included in this study from a population of privately owned pet dogs that presented to the CSU‐VTH for definitive intent treatment (stereotactic RT [SRT], amputation, or surgical limb spare) of an appendicular OSA between November 1, 2009 and June 30, 2017. Other inclusion criteria were cytologically or histologically confirmed appendicular OSA, 18F‐FDG PET/CT that was negative for confirmed metastatic disease before initiation of treatment, and intent to treat with a standard of care chemotherapy protocol that consisted of 4‐6 doses of carboplatin chemotherapy given every 3 weeks at a dose of 300 mg/m2 IV after local treatment.
Dogs underwent 18F‐FDG PET/CT imaging with a Philips Gemini TF Big Bore 16‐slice PET/CT scanner (Philips North America, Andover, Massachusetts) under general anesthesia according to a protocol previously reported.38 Dogs were administered 18F‐FDG IV at a dose of 170 μCi/kg. After allowing for 1 hour of uptake of 18F‐FDG, a whole‐body CT before and after administration of contrast was performed followed immediately by a whole‐body PET.
Image assessment was originally performed by a board‐certified radiologist or radiology resident on a dedicated Extended Brilliance Workstation (Philips Medical System, Nederland BV, Best, the Netherlands). Scans were evaluated for characteristics of the primary lesion and the presence of any potential metastases or other abnormalities that would impact treatment decisions utilizing maximum intensity projections and dorsal, sagittal, and transverse imaging planes of the CT with an 18F‐FDG PET/CT overlay. Primary tumor SUVmax were automatically measured by region‐of‐interest (ROI) analysis. Manually defined ROIs were centered over the primary lesion and expanded in a 3D fashion to encompass the area of concern. The reported SUVmax was retrospectively collected to allow for analysis.
For the purposes of this retrospective study the location of primary lesion, SUVmax of the primary lesion, type of local treatment (amputation, surgical limb spare, or SRT), ALP levels, and monocyte count at initial presentation were recorded. In addition, medical records were evaluated to determine how many had histological analysis of the primary tumor at or around (within 7 days) the time of the 18F‐FDG PET/CT. Mitotic index (MI) counted in 10 high‐power fields was recorded for analysis. These data were not collected if dogs underwent any therapeutic intervention between the time of imaging and biopsy. Medical records were reviewed to determine how many dogs had confirmed metastatic disease at the time of 18F‐FDG PET/CT imaging. When available, the date of progression of the primary disease or disease recurrence, date of development of metastasis and date of death or euthanasia or last follow‐up, and the cause of death or euthanasia was recorded.
2.1. Statistical analysis
Continuous data were assessed for normality using skewness, kurtosis, and Shapiro‐Wilk tests. If the data were normally distributed, the mean and SD were used for description. The median and range were used if the data were non‐normally distributed. Frequencies and percentages were used to describe any categorical variables.
The progression‐free interval (PFI) was calculated as the number of days from the date of imaging to the date of initial detection of metastasis, local recurrence, or local progression. The overall survival time (OST) was calculated for all cause and disease‐specific survival. For all cause survival, OST was calculated as the number of days from date of 18F‐FDG PET/CT to death because of all causes. For the disease‐specific survival, OST was calculated as the number of days from F18‐FDG PET/CT to death because of OSA or treatment. Dogs were censored in the PFI analysis if they did not have any metastases or local recurrence/progression documented at the time of last follow‐up or at the time of death. Dogs were censored in the all‐cause OST analysis if they were alive at last follow‐up or were lost to follow‐up. Dogs were censored in the disease‐specific OST analysis if they were alive at last follow‐up or were lost to follow‐up or died because of causes other than OSA or treatment.
Kaplan‐Meier methods were used to calculate median PFI and OST with 95% confidence intervals (CI). Cox proportional hazards regression analysis was used to evaluate for associations between SUVmax and other variables (proximal humeral tumor site, ALP, monocyte count, and treatment type) with outcome variables PFI, all‐cause OST, and disease‐specific OST. Additionally, a log‐rank test was used to compare survival in dogs with SUVmax < 7.4 and dogs with SUVmax ≥ 7.4. An SUVmax of 7.4 was used as a cutoff value after it was established as the median measurement on all dogs. Multivariable Cox proportional hazards regression was performed to allow adjustment of other variables including first‐order interaction terms and assess association of SUVmax with PFI, and all cause and disease‐specific OST. Variables were entered into the model if they had a univariate P < .2 or if they could be a confounding variable. Backward selection was used for model creation with variables being retained in the model if P < .05.
Statistical significance was set at α = 0.05 and the statistical analysis was performed by using commercially available software (SAS software, version 9.4. Cary, North Carolina).
3. RESULTS
Seventy‐seven dogs were identified that met the initial inclusion criteria. Of these, 11 dogs were excluded as carboplatin chemotherapy was not given and 4 were excluded when metastatic disease was confirmed at the time of initial imaging, leaving a total of 62 dogs included in the statistical analysis. The baseline characteristics of dogs are presented in Tables 1 and 2.
Table 1.
Characteristics of study population (n = 62)
| (Outcome = PFI) | (Outcome = OST any cause) | (Outcome = OST cause by disease) | |||
|---|---|---|---|---|---|
| Variable | Category | n (%) | P value | P value | P value |
| Treatment type | Amputation | 15 (24%) | .98 | .59 | .84 |
| Limb spare | 12 (19%) | ||||
| Stereotactic radiation | 35 (57%) | ||||
| Proximal humeral site | Yes | 15 (24%) | .75 | .62 | .88 |
| No | 47 (76%) | ||||
| ALP (IU/L) (median, range) | 71.5 (15.0‐418) | .21 | .08 | .02* | |
| Monocyte count (×103/uL) (median, range) | 0.4 (0.0‐1.3) | .51 | .08 | .03* | |
| SUVmax (median, range) | 7.4 (1.8‐25.6) | .24 | .04* | .02* | |
Abbreviations: ALP, alkaline phosphatase; OST, overall survival time; PFI, progression‐free interval; SUVmax, maximum standard uptake value.
Table 2.
Dog characteristics separated by treatment type
| SRT | Limb spare | Amputation | Totals | |
|---|---|---|---|---|
| Number of dogs | 35 | 12 | 15 | 62 |
| SUVmax (median, range) | 5.8 (1.8‐25.6) | 7.3 (3.1‐20.2) | 11.0 (5.5‐23.2) | 7.4 (1.8‐25.6) |
| OST (median, 95% CI) (number of censored from evaluation) | 284 (216‐422) (n = 5) | 315 (98‐811) (n = 3) | 236 (128‐857) (n = 4) | 284 (233‐420) (n = 12) |
| Monocyte count (/uL)(median, range) | 400 (0‐1100) | 442 (1001300) | 65 (27‐252) | 400 (0‐1300) |
| Serum ALP activity (IU/L) (median, range) | 78 (15‐418) | 63.5 (27‐215) | 67 (27‐252) | 82.5 (15‐418) |
Abbreviations: ALP, alkaline phosphatase; CI, confidence interval; OST, overall survival time; SRT, stereotactic radiation therapy; SUVmax, maximum standard uptake value.
For all dogs, the median PFI and all‐cause OST were 320 and 284 days (95% CI, 219‐378 days and 233‐420 days, respectively); the median disease‐specific OST was 434 days (95% CI, 279‐811 days). Thirty‐one and 15 dogs were censored from PFI and OST analysis, respectively. The median follow‐up time in censored dogs was 304 days (range, 57‐1199 days). Thirty‐four of 62 dogs (52%) eventually developed confirmed metastatic disease and 2/62 dogs (3%) had progression at the site of their primary lesion.
The median SUVmax was 7.4 (range, 1.8‐25.6). Dogs with an SUVmax of <7.4 had an MST of 680 days (95% CI, 434‐* upper end of CI could not be calculated), whereas dogs with an SUVmax of ≥7.4 had an MST of 254 days (95% CI, 216‐428 days) (P = .01; Figures 1 and 2). The maximum standard uptake value was significantly associated with overall survival for all causes of death and overall survival cause by disease (P = .04 and .02, respectively).
Figure 1.

Kaplan‐Meier graph of survival time with dogs having a maximum standard uptake value (SUVmax) <7.4 having an overall survival time (OST) of 680 days (95% confidence interval [CI] 434‐*; * upper end of CI cannot be calculated) and dogs with an SUVmax ≥ 7.4 having an OST of 254 days (95% CI, 216‐428 days) (P = .01)
Figure 2.
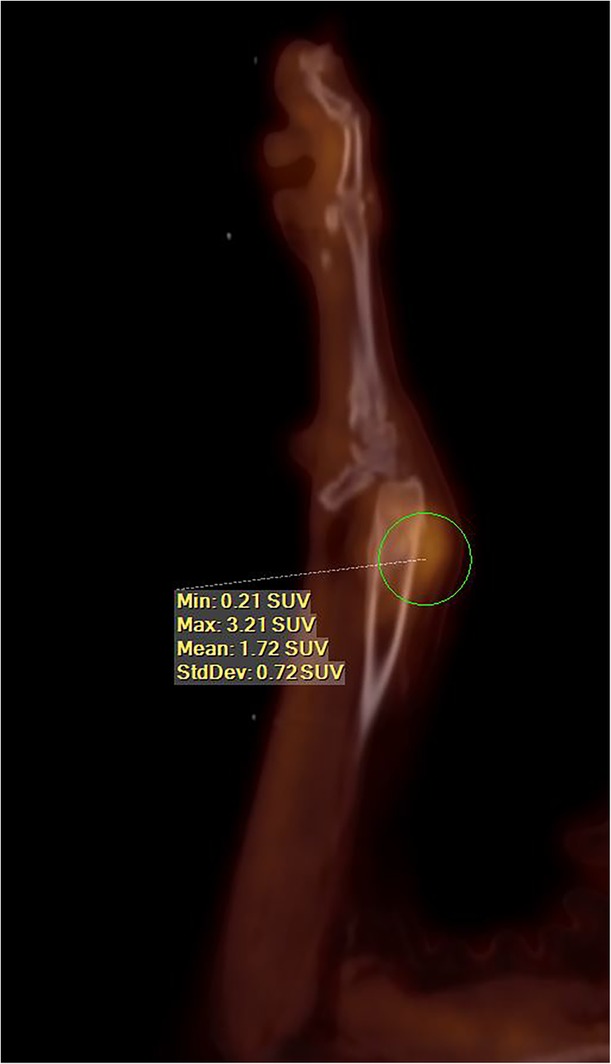
Sagittal image from a fluorine18 flourodeoxyglucose (F18–FDG) positron emission tomography/computed tomography (PET/CT) of a dog with a radial osteosarcoma (maximum standard uptake value [SUVmax] of 3.21). Survival time for this dog after 3 fractions of stereotactic radiation therapy (SRT) was 900 days, at which point the dog was euthanized because of “old age” as per the owner
Figure 3.
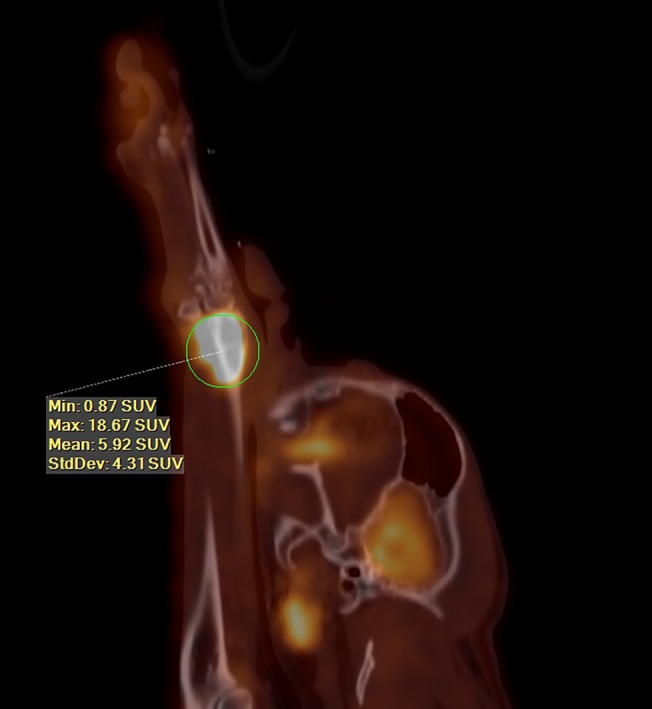
Sagittal image from a fluorine18 flourodeoxyglucose (F18–FDG) positron emission tomography/computed tomography (PET/CT) of a dog with a distal radial osteosarcoma (OSA) (maximum standard uptake value [SUVmax] of 18.67). Survival time for this dog was 117 days after a surgical limb spare, at which point the dog was euthanized because of mentation issues, etiology unknown
There were 26/62 (42%) dogs that had histologic samples of the primary tumor available for analysis that met the inclusion criteria. The median MI was 12.0 (range, 0‐70). Mitotic index had no correlation with SUVmax (Pearson correlation coefficient = 0.10). Greater MI was significantly associated with increased risk of death cause by disease (hazard ratio [HR], 1.04; 95% CI, 1.00‐1.09; P = .05) but not significantly associated with recurrence or metastatic disease (HR, 1.02; 95% CI, 0.99‐1.06; P = .18) or death because of all causes (HR, 1.02; 95% CI, 0.99‐1.06; P = 0.21).
The univariate analysis results are presented in Table 1. The anatomic location of the primary tumor (proximal humerus versus any other location) and the type of treatment were not associated with any of the outcomes (PFI, all‐cause OST, or disease specific). There were no variables including SUVmax significantly associated with PFI. Serum ALP activity and blood monocyte count were both significantly associated with OST for all cause and OST cause by disease.
Multivariable results are presented in Tables 3 and 4. Maximum standard uptake value of the primary tumor was significantly associated with the all cause survival (P = .001) with adjustment for treatment type and monocyte count. Monocyte count was independently associated with death cause by disease and death because of all causes (HR, 5.8 [95% CI, 1.6‐21.5] and 4.4 [95% CI, 1.4‐13.7], respectively). Stereotactic radiation therapy resulted in a greater hazard of death because of all causes compared to amputation (HR, 2.8 [95% CI, 1.3‐6.4], P = .01) with adjustment for SUVmax and monocyte count. In addition, SUVmax of the primary tumor was also significantly associated (HR, 1.10 [95% CI, 1.03‐1.17], P = .007) with death cause by disease with adjustment for monocyte count.
Table 3.
Results of multivariable cox proportional hazards regression model assessing variables relationship with outcome of death because of any cause
| Variable | Hazard ratio (95% CI) | P value | |
|---|---|---|---|
| Treatment type | Limbsparing | 1.8 (0.67‐4.9) | .24 |
| SRT | 2.8 (1.3‐6.4) | .01 | |
| SUVmax | 1.10 (1.04‐1.16) | .001 | |
| Monocyte count | 4.4 (1.4‐13.7) | .009 |
Abbreviations: CI, confidence interval; SRT, stereotactic radiation therapy; SUVmax, maximum standard uptake value.
Table 4.
Results of multivariable cox proportional hazards regression model assessing variables relationship with outcome of death cause by disease
| Variable | Hazard ratio (95% CI) | P value |
|---|---|---|
| SUVmax | 1.10 (1.03‐1.17) | .007 |
| Monocyte count | 5.8 (1.6‐21.5) | .008 |
Abbreviations: CI, confidence interval; SUVmax, maximum standard uptake value.
4. DISCUSSION
This study has shown that SUVmax of the untreated appendicular OSA primary lesion in dogs is significantly associated with OST (all cause and disease specific), despite adjustment for other variables including treatment type and blood monocyte count before treatment. Increasing SUVmax was associated with increased risk of death because of all causes and disease‐specific death (HR, 1.10 [1.04‐1.16] and 1.10 [1.03‐1.17], respectively).
Maximum standard uptake value is a measure of FDG uptake and is reflective of the metabolic requirements and proliferative ability of a tumor.25 In veterinary medicine, histological grade is consistently prognostic in multiple tumor types, including mast cell tumor, soft tissue sarcoma, and mammary tumors.39, 40, 41 The relationship between grade of a disease and prognosis in dogs with OSA is unclear.42, 43 The more aggressive a tumor, the higher the SUVmax in humans and animals.25, 27, 38, 44, 45 Although we cannot draw conclusions on grade or other proliferative indices such as Ki67 in this study as we lack histology and immunohistochemistry on many of the primary lesions, we postulate that higher SUVmax is indicative of a more aggressive form of OSA, leading to a shorter survival time. Further studies with a larger number of dogs to prospectively correlate histologic grading and SUVmax measured from an 18F‐FDG PET/CT could be performed to assess this potential relationship in appendicular OSA. Mitotic index was not significantly associated with SUVmax in this study. This is partially because of innate tumor heterogeneity as most OSAs exhibit a high degree of pleomorphism.47 There is conflicting evidence regarding the relationship between MI and prognosis for OSAs. There is no relationship to survival of dogs with mandibular OSA and MI.48 In other cases, MI influences disease‐free interval (DFI) but not survival time of dogs with OSA, which is contrary to the finding of this study in which increase MI was associated with disease‐specific OST but not DFI or all‐cause OST.43 In humans with malignant gastrointestinal stromal tumors, MI is significantly related to SUVmax.49
Maximum standard uptake value was not significantly associated with PFI. Because of the retrospective nature of this study, the exact medical histories were not available for all dogs, creating limitations to this portion of the analysis. In addition, there was no standardization of timing on restaging dogs, with some having regular rechecks and others having further imaging only when clinical signs developed. As such, we had only 32/62 dogs with a definitive date on development of metastasis or progression of local disease.
Analysis revealed that when controlled for SUVmax and pretreatment monocyte count, dogs treated with SRT (35/62) had a 2.8 times greater hazard of death (because of all causes) compared to dogs treated with amputation. There were no first‐order interactions between variables that were found to be significant. This finding of increased hazard of death for dogs undergoing SRT could be because most dogs treated with SRT procedures are dogs that are unable to tolerate amputation because of other orthopedic or neurological diseases, or because of owner perception and biases. These comorbidities could influence owner perception of quality of life, thus impacting the timing of euthanasia and survival. Additionally, 1 of the primary complications of SRT for the treatment of primary appendicular OSA lesions in dogs is fracture of the affected limb.10 It could be that the dogs treated with SRT were euthanized if a pathologic fracture developed, well before signs of metastatic disease, which would likely be the primary cause of death in the dogs treated with amputation. Only 3/35 dogs treated with SRT were confirmed as having a pathologic fracture develop, but the cause of death was unknown in 10/35 of these dogs.
Tumor location was not significantly associated with survival in this study in spite of some previous studies stating that proximal humeral lesions have a poorer prognosis.22 Other studies, however, have indicated that location does not impact survival, making the finding in this study consistent with previously reported outcomes.7 In addition, serum ALP levels before treatment was not a prognostic factor for survival as an independent variable in the multivariable analysis. Given the retrospective nature of this study, there are some differences in the timeline for when these values were obtained, with preanesthetic bloodwork being considered suitable for up to a month before anesthesia for imaging. It is unknown if this may have influenced these results.
Retrospective studies are limited because it is difficult to control for all variables. In order to keep this study as accurate as possible, we excluded all dogs that did not pursue some form of definitive treatment, did not receive carboplatin, had confirmed metastatic disease at the time of imaging, or did not have a definitive cytologic or histologic diagnosis of OSA. A possible limitation is the lack of histology in all of the included population. Although several of the dogs had definitive histology reports from bone samples obtained either before treatment via a bone biopsy, after amputation, or after euthanasia, there were multiple dogs that had only a cytologic diagnosis of OSA. Bone biopsy and cytology have a relatively similar accuracy in diagnosis of bone lesions in dogs.50 In addition, several of the cytologic samples did have additional ALP staining, which increases the accuracy of this test. Sensitivity and specificity of ALP staining for OSA is 88% and 94%, respectively, with possible false‐positive results from melanomas with osteoid production, GISTs, and collision tumors.51 Given the definitive cytologic diagnosis of sarcoma in the dogs, as well as the increased prevalence of OSA versus other primary malignant bone tumors, lesion location, and imaging characteristics, it is likely that the dogs included in this study had primary appendicular OSA.
It should be stated that the cutoff of 7.4 SUVmax for calculation of prognosis is the median based on the present data set and cannot be translated to 18F‐FDG PET/CT data obtained at other institutions. Maximum standard uptake value is a measure based upon the ratio of radioactivity and injected FDG dose normalized to body weight. It is variably dependent upon blood pooling, image acquisition timing, and protocol as well as influenced by software programs. Heterogeneity within the tumor itself should also be considered as a confounding factor.
In conclusion, this retrospective study suggests that SUVmax, as measured by an 18F‐FDG PET/CT performed before treatment, may be prognostic for survival in dogs with appendicular OSA. This provides another valid reason for utilizing this staging method in dogs before making therapeutic decisions.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENTS
The preliminary results of this paper were presented at the 2018 Veterinary Society of Surgical Oncologists meeting, Maui, Hawaii. An abstract for the 2018 Veterinary Cancer Society meeting, Louisville, Kentucky, has been submitted.
Griffin LR, Thamm DH, Brody A, Selmic LE. Prognostic value of fluorine18 flourodeoxyglucose positron emission tomography/computed tomography in dogs with appendicular osteosarcoma. J Vet Intern Med. 2019;33:820–826. 10.1111/jvim.15453
This paper is dedicated to the memory of Mary Lafferty whose assistance with the data collection was invaluable.
REFERENCES
- 1. Ling GV, Morgan JP, Pool RR. Primary bone rumors in the dog: a combined clinical, radiographic, and histologic approach to early diagnosis. J Am Vet Med Assoc. 1974;165:55‐67. [PubMed] [Google Scholar]
- 2. Withrow S, Vail D, Page R. Withrow & MacEwen's Small Animal Clinical Oncology. 5th ed St. Louis, MO: Elsevier Saunders; 2013. [Google Scholar]
- 3. Phillips B, Powers BE, Dernell WS, et al. Use of single‐agent carboplatin as adjuvant or neoadjuvant therapy in conjunction with amputation for appendicular osteosarcoma in dogs. J Am Anim Hosp Assoc. 2009;45:33‐38. [DOI] [PubMed] [Google Scholar]
- 4. Berg J, Weinstein MJ, Springfield DS, Rand WM. Results of surgery and doxorubicin chemotherapy in dogs with osteosarcoma. J Am Vet Med Assoc. 1995;206:1555‐1560. [PubMed] [Google Scholar]
- 5. Bailey D, Erb H, Williams L, Ruslander D, Hauck M. Carboplatin and doxorubicin combination chemotherapy for the treatment of appendicular osteosarcoma in the dog. J Vet Intern Med. 2003;17:199‐205. [DOI] [PubMed] [Google Scholar]
- 6. Covey JL, Farese JP, Bacon NJ, et al. Stereotactic radiosurgery and fracture fixation in 6 dogs with appendicular osteosarcoma. Vet Surg. 2014;43:174‐181. [DOI] [PubMed] [Google Scholar]
- 7. Culp WTN, Olea‐Popelka F, Sefton J, et al. Evaluation of outcome and prognostic factors for dogs living greater than one year after diagnosis of osteosarcoma: 90 cases (1997‐2008). J Am Vet Med Assoc. 2014;245:1141‐1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mitchell KE, Boston SE, Kung M, et al. Outcomes of limb‐sparing surgery using two generations of metal endoprosthesis in 45 dogs with distal radial osteosarcoma. A Veterinary Society of Surgical Oncology retrospective study. Vet Surg. 2016;45:36‐43. [DOI] [PubMed] [Google Scholar]
- 9. Seguin B, O'Donnell MD, Walsh PJ, et al. Long‐term outcome of dogs treated with ulnar rollover transposition for limb‐sparing of distal radial osteosarcoma: 27 limbs in 26 dogs. Vet Surg. 2017;46:1017‐1024. [DOI] [PubMed] [Google Scholar]
- 10. Kubicek L, Vanderhart D, Wirth K, et al. Association between computed tomographic characteristics and fractures following stereotactic radiosurgery in dogs with appendicular osteosarcoma. Vet Radiol Ultrasound. 2016;57:321‐330. [DOI] [PubMed] [Google Scholar]
- 11. Straw RC, Withrow SJ. Limb‐sparing surgery versus amputation for dogs with bone tumors. Vet Clin North Am Small Anim Pract. 1996;26:135‐143. [DOI] [PubMed] [Google Scholar]
- 12. Selmic LE, Burton JH, Thamm DH, Withrow SJ, Lana SE. Comparison of carboplatin and doxorubicin‐based chemotherapy protocols in 470 dogs after amputation for treatment of appendicular osteosarcoma. J Vet Intern Med. 2014;28:554‐563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Skorupski KA, Uhl JM, Szivek A, Allstadt Frazier SD, Rebhun RB, Rodriguez CO Jr. Carboplatin versus alternating carboplatin and doxorubicin for the adjuvant treatment of canine appendicular osteosarcoma: a randomized, phase III trial. Vet Comp Oncol. 2016;14:81‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liptak JM, Dernell WS, Lascelles BD, et al. Intraoperative extracorporeal irradiation for limb sparing in 13 dogs. Vet Surg. 2004;33:446‐456. [DOI] [PubMed] [Google Scholar]
- 15. Boston SE, Ehrhart NP, Dernell WS, Lafferty M, Withrow SJ. Evaluation of survival time in dogs with stage III osteosarcoma that undergo treatment: 90 cases (1985‐2004). J Am Vet Med Assoc. 2006;228:1905‐1908. [DOI] [PubMed] [Google Scholar]
- 16. Hillers KR, Dernell WS, Lafferty MH, Withrow SJ, Lana SE. Incidence and prognostic importance of lymph node metastases in dogs with appendicular osteosarcoma: 228 cases (1986‐2003). J Am Vet Med Assoc. 2005;226:1364‐1367. [DOI] [PubMed] [Google Scholar]
- 17. Jankowski MK, Steyn PF, Lana SE, et al. Nuclear scanning with 99mTc‐HDP for the initial evaluation of osseous metastasis in canine osteosarcoma. Vet Comp Oncol. 2003;1:152‐158. [DOI] [PubMed] [Google Scholar]
- 18. Garzotto CK, Berg J, Hoffmann WE, Rand WM. Prognostic significance of serum alkaline phosphatase activity in canine appendicular osteosarcoma. J Vet Intern Med. 2000;14:587‐592. [DOI] [PubMed] [Google Scholar]
- 19. Sottnik JL, Rao S, Lafferty MH, et al. Association of blood monocyte and lymphocyte count and disease‐free interval in dogs with osteosarcoma. J Vet Intern Med. 2010;24:1439‐1444. [DOI] [PubMed] [Google Scholar]
- 20. Baines SJ, Lewis S, White RA. Primary thoracic wall tumours of mesenchymal origin in dogs: a retrospective study of 46 cases. Vet Rec. 2002;150:335‐339. [DOI] [PubMed] [Google Scholar]
- 21. Hammer AS, Weeren FR, Weisbrode SE, Padgett SL. Prognostic factors in dogs with osteosarcomas of the flat or irregular bones. J Am Anim Hosp Assoc. 1995;31:321‐326. [DOI] [PubMed] [Google Scholar]
- 22. Kuntz CA, Asselin TL, Dernell WS, et al. Limb salvage surgery for osteosarcoma of the proximal humerus: outcome in 17 dogs. Vet Surg. 1998;27:417‐422. [DOI] [PubMed] [Google Scholar]
- 23. Randall E, Loeber S, Kraft S. Physiologic variants, benign processes, and artifacts from 106 canine and feline FDG‐PET/computed tomography scans. Vet Radiol Ultrasound. 2014;55:213‐226. [DOI] [PubMed] [Google Scholar]
- 24. Koppenol WH, Bounds PL, Dang CV. Otto Warburg's contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11:325‐337. [DOI] [PubMed] [Google Scholar]
- 25. Vesselle H, Schmidt RA, Pugsley JM, et al. Lung cancer proliferation correlates with [F‐18]fluorodeoxyglucose uptake by positron emission tomography. Clin Cancer Res. 2000;6:3837‐3844. [PubMed] [Google Scholar]
- 26. Im HJ, Zhang Y, Wu HY, et al. Prognostic value of metabolic and volumetric parameters of FDG PET in pediatric osteosarcoma: a hypothesis‐generating study. Radiology. 2018;287:303‐312. [DOI] [PubMed] [Google Scholar]
- 27. Okada J, Yoshikawa K, Imazeki K, et al. The use of FDG‐PET in the detection and management of malignant‐lymphoma ‐ correlation of uptake with prognosis. J Nucl Med. 1991;32:686‐691. [PubMed] [Google Scholar]
- 28. Brenner W, Conrad EU, Eary JF. FDG PET imaging for grading and prediction of outcome in chondrosarcoma patients. Eur J Nucl Med Mol Imaging. 2004;31:189‐195. [DOI] [PubMed] [Google Scholar]
- 29. Im HJ, Pak K, Cheon GJ, et al. Prognostic value of volumetric parameters of F18‐FDG PET in non‐small‐cell lung cancer: a meta‐analysis. Eur J Nucl Med Mol Imaging. 2015;42:241‐251. [DOI] [PubMed] [Google Scholar]
- 30. Holzer T, Herholz K, Jeske J, et al. FDG‐PET as a prognostic indicator in radiochemotherapy of glioblastoma. J Comput Assist Tomogr. 1993;17:681‐687. [DOI] [PubMed] [Google Scholar]
- 31. Hansen AE, McEvoy F, Engelholm SA, et al. FDG PET/CT imaging in canine cancer patients. Vet Radiol Ultrasound. 2011;52:201‐206. [DOI] [PubMed] [Google Scholar]
- 32. LeBlanc AK, Jakoby BW, Townsend DW, et al. 18FDG‐PET imaging in canine lymphoma and cutaneous mast cell tumor. Vet Radiol Ultrasound. 2009;50:215‐223. [DOI] [PubMed] [Google Scholar]
- 33. Leblanc AK, Miller AN, Galyon GD, et al. Preliminary evaluation of serial (18) FDG‐PET/CT to assess response to toceranib phosphate therapy in canine cancer. Vet Radiol Ultrasound. 2012;53:348‐357. [DOI] [PubMed] [Google Scholar]
- 34. Seiler SM, Baumgartner C, Hirschberger J, et al. Comparative oncology: evaluation of 2‐deoxy‐2‐[18F]fluoro‐D‐glucose (FDG) positron emission tomography/computed tomography (PET/CT) for the staging of dogs with malignant tumors. PLoS One. 2015;10:e0127800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Borgatti A, Winter AL, Stuebner K, et al. Evaluation of 18‐F‐fluoro‐2‐deoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) as a staging and monitoring tool for dogs with stage‐2 splenic hemangiosarcoma ‐ a pilot study. PLoS One. 2017;12:e0172651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Song SH, Park NW, Eom KD. Positron emission tomography/computed tomography imaging features of renal cell carcinoma and pulmonary metastases in a dog. Can Vet J. 2014;55:466‐470. [PMC free article] [PubMed] [Google Scholar]
- 37. Randall EK, Kraft SL, Yoshikawa H, LaRue SM. Evaluation of 18F‐FDG PET/CT as a diagnostic imaging and staging tool for feline oral squamous cell carcinoma. Vet Comp Oncol. 2016;14:28‐38. [DOI] [PubMed] [Google Scholar]
- 38. Griffin LR, Thamm DH, Selmic LE, Ehrhart EJ, Randall E. Pilot study utilizing fluorine‐18 fluorodeoxyglucose‐positron emission tomography/computed tomography for glycolytic phenotyping of canine mast cell tumors. Vet Radiol Ultrasound. 2018;59:461‐468. [DOI] [PubMed] [Google Scholar]
- 39. Pena L, De Andres PJ, Clemente M, et al. Prognostic value of histological grading in noninflammatory canine mammary carcinomas in a prospective study with two‐year follow‐up: relationship with clinical and histological characteristics. Vet Pathol. 2013;50:94‐105. [DOI] [PubMed] [Google Scholar]
- 40. Dennis MM, McSporran KD, Bacon NJ, et al. Prognostic factors for cutaneous and subcutaneous soft tissue sarcomas in dogs. Vet Pathol. 2011;48:73‐84. [DOI] [PubMed] [Google Scholar]
- 41. Kiupel M, Webster JD, Bailey KL, et al. Proposal of a 2‐tier histologic grading system for canine cutaneous mast cell tumors to more accurately predict biological behavior. Vet Pathol. 2011;48:147‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schott CR, Tatiersky LJ, Foster RA, Wood GA. Histologic grade does not predict outcome in dogs with appendicular osteosarcoma receiving the standard of care. Vet Pathol. 2018;55:202‐211. [DOI] [PubMed] [Google Scholar]
- 43. Kirpensteijn J, Kik M, Rutteman GR, Teske E. Prognostic significance of a new histologic grading system for canine osteosarcoma. Vet Pathol. 2002;39:240‐246. [DOI] [PubMed] [Google Scholar]
- 44. Benz MR, Dry SM, Eilber FC, et al. Correlation between glycolytic phenotype and tumor grade in soft‐tissue sarcomas by 18F‐FDG PET. J Nucl Med. 2010;51:1174‐1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Binderup T, Knigge U, Loft A, Federspiel B, Kjaer A. 18F‐fluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin Cancer Res. 2010;16:978‐985. [DOI] [PubMed] [Google Scholar]
- 46. Tsunemi T, Nagoya S, Kaya M, et al. Postoperative progression of pulmonary metastasis in osteosarcoma. Clin Orthop Relat Res. 2003;407:159‐166. [DOI] [PubMed] [Google Scholar]
- 47. Loukopoulos P, Robinson WF. Clinicopathological relevance of tumour grading in canine osteosarcoma. J Comp Pathol. 2007;136:65‐73. [DOI] [PubMed] [Google Scholar]
- 48. Straw RC, Powers BE, Klausner J, et al. Canine mandibular osteosarcoma: 51 cases (1980‐1992). J Am Anim Hosp Assoc. 1996;32:257‐262. [DOI] [PubMed] [Google Scholar]
- 49. Yoshikawa K, Shimada M, Kurita N, et al. Efficacy of PET‐CT for predicting the malignant potential of gastrointestinal stromal tumors. Surg Today. 2013;43:1162‐1167. [DOI] [PubMed] [Google Scholar]
- 50. Sabattini S, Renzi A, Buracco P, et al. Comparative assessment of the accuracy of cytological and histologic biopsies in the diagnosis of canine bone lesions. J Vet Intern Med. 2017;31:864‐871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ryseff JK, Bohn AA. Detection of alkaline phosphatase in canine cells previously stained with Wright‐Giemsa and its utility in differentiating osteosarcoma from other mesenchymal tumors. Vet Clin Pathol. 2012;41:391‐395. [DOI] [PubMed] [Google Scholar]


