Abstract
Background
Proteinuria in dogs with kidney disease can contribute to protein‐energy wasting and malnutrition. Little is known about amino acid (AA) status in dogs with protein‐losing nephropathy (PLN).
Objectives
The purpose of our study was to further elucidate AA status in PLN dogs, with the hypothesis that PLN dogs would have altered AA status as compared to healthy dogs.
Animals
Thirty client‐owned PLN dogs were compared to 10 healthy control dogs.
Methods
Prospective observational study. Dogs with PLN that were presented to the teaching hospital were enrolled. Plasma AA profiles were measured using an automated high‐performance liquid chromatography AA analyzer.
Results
Compared to control dogs, PLN dogs had significantly lower concentrations of leucine, threonine, histidine, glycine, proline, asparagine, tyrosine, o‐hydroxyproline, and serine, as well as sums of both essential and nonessential AA (P < .05). Dogs with PLN had significantly lower ratios of tyrosine‐to‐phenylalanine and glycine‐to‐serine (P < .05), and a significantly greater ratio of valine‐to‐glycine (P < .05).
Conclusions and Clinical Importance
Dogs with PLN have altered AA status compared to healthy dogs. These findings could have therapeutic implications in determining optimal management of PLN dogs, such as providing AA supplementation along with other standard treatment.
Keywords: essential, kidney disease, nonessential, proteinuria
Abbreviations
- AA
amino acids
- BCS
body condition score
- CKD
chronic kidney disease
- EAA
essential amino acids
- GFR
glomerular filtration rate
- MCS
muscle condition score
- NEAA
nonessential amino acids
- OSU‐VMC
Ohio State University Veterinary Medical Center
- PEW
protein‐energy wasting
- PLN
protein‐losing nephropathy
- SSA
sulfasalicylic acid
- UPC
urine protein : creatinine
1. INTRODUCTION
Proteinuria is both a contributor to and a complication of chronic kidney disease (CKD), and it is associated with progression of disease and decreased survival in dogs and cats with kidney disease.1, 2 Whereas dietary protein restriction is not recommended for early stages of CKD, reducing dietary protein is almost invariably recommended in nonazotemic proteinuric dogs to decrease proteinuria.3, 4, 5, 6 Therefore, dogs with proteinuria are particularly predisposed to malnutrition, and optimal methods of nutritional management specific for this form of renal disease are needed. In people, both proteinuria and the inflammation associated with chronic disease can contribute to protein‐energy wasting (PEW), resulting in accelerated loss of lean body mass that can also adversely affect morbidity and mortality.7
Amino acid (AA) profiles are altered in dogs, cats, and humans with CKD.8, 9, 10 Nonessential amino acids (NEAA) increase while essential amino acids (EAA) decrease in CKD, as compared to healthy control subjects.10, 11, 12 Without adequate concentrations of EAA, animals cannot meet physiologic demands for protein synthesis, thus contributing to decreased albumin concentrations, impaired immune function, and loss of lean muscle mass. In addition to measuring absolute plasma concentrations, ratios comparing EAA and NEAA have been used in people to assess AA status.12, 13 The alterations in AA in CKD are because of alterations in nitrogen metabolism, tubular filtration, reabsorption, and excretion as well as increased inflammation.12, 14
Information on AA status in dogs with protein‐losing nephropathy (PLN) is lacking in the literature, yet it might impact optimal management of dogs with PLN. The goal of our study was to characterize plasma AA profiles in dogs with PLN. We hypothesize that dogs with PLN will have altered plasma AA profiles as compared to healthy dogs.
2. MATERIALS AND METHODS
2.1. Case selection criteria
Client‐owned dogs diagnosed with renal proteinuria were prospectively recruited from the dogs referred to The Ohio State University Veterinary Medical Center (OSU‐VMC) between January 2014 and July 2015. Causes of prerenal and postrenal proteinuria such as hyperadrenocorticism, cystoliths, or urinary neoplasia were excluded to the best of the attending clinicians' assessment. All dogs had a urine culture performed. Abdominal ultrasound examination was performed in most dogs (n = 21). Proteinuria was quantified by a urine protein : creatinine (UPC) ratio using turbidimetric (protein) and Jaffe (creatinine) methods, respectively. Dogs with a UPC > 1.5 were included. Dogs <1 year of age and dogs diagnosed with concurrent diseases known to affect AA status or proteinuria such as protein‐losing enteropathy, hyperadrenocorticism, or urinary tract infection were excluded. Dogs that had clinical signs or ultrasonographic changes consistent with hyperadrenocorticism were excluded. Specific infectious disease testing was determined by the managing clinician.
Dogs enrolled as controls were deemed healthy on the basis of a physical examination, CBC, serum biochemistry profile, and urinalysis. The OSU‐VMC Institutional Animal Care and Use Committee approved the protocol, and all owners signed a consent form before dogs were enrolled in the study.
2.2. Study design
Each dog had a physical examination performed, including body weight, body condition score (BCS; on a 1‐9 scoring system) and muscle condition score (MCS; using normal, mild, moderate, or severe scores).15 All BCS and MCS scoring were done by 1 author (V. J. Parker). A Doppler systolic blood pressure was measured. Blood was collected via jugular venipuncture and urine was collected via cystocentesis for CBC, serum biochemistry profile, urinalysis, urine culture, and UPC.
Heparinized plasma was collected for AA analysis. After collection, the plasma was mixed in a 1 : 1 ratio with 6% sulfasalicylic acid and sat for 20 minutes. This mixture was then centrifuged at 14 000 rpm (22 132 rcf) for 25 minutes. The supernatant was removed and frozen at −80°C.
2.3. AA analysis
Plasma AA concentrations were measured with an automated high‐performance liquid chromatography AA analyzer (Biochrom 30; Biochrom Ltd, Holliston, Massachusetts) using a method described elsewhere (Kim 1995).16
2.4. Data analysis
The nonparametric Wilcoxon rank sum test was used to compare the AA profiles of PLN and control dogs. The Holm‐Bonferroni method was used to control the type I error at a familywise rate of 5%. Data are presented as median and range. P‐values <.05 were considered significant. Sums of EAA and NEAA were calculated. Ratios of EAA‐to‐NEAA, valine‐to‐glycine, tyrosine‐to‐phenylalanine, and glycine‐to‐serine were compared between PLN and control dogs. Statistical analysis was performed using R 3.4.4 (R Core Team [2018]; Vienna, Austria).
3. RESULTS
Thirty dogs with PLN and 10 control dogs were enrolled. Median age for PLN dogs was 9.4 years (range, 3.1‐14.3 years). Median age for control dogs was 4.3 years (range, 1.4‐10.3 years). Dogs with PLN were significantly older than control dogs (P < .001). Breeds most represented among PLN dogs were mixed breed (n = 5), Labrador Retriever (n = 3), Fox Terrier (n = 3), Yorkshire Terrier (n = 3). There were 2 Cocker Spaniels, 2 Shetland Sheepdogs, 2 Miniature Schnauzers, 1 each of the following breeds: Akita, Australian Shepherd, Bichon Frise, Chihuahua, Cavalier King Charles Spaniel, Doberman, Golden Retriever, Malamute, Soft Coated Wheaten Terrier, and Welsh Terrier. Eleven castrated male and 19 spayed female dogs were included. Control dogs included mixed breed (n = 4), American Pit Bull Terrier (n = 3), German Shepherd (n = 2), and Rottweiler (n = 1). Six dogs were castrated males and 4 were spayed females.
Median body weight of PLN dogs was 12.9 kg (range, 2.0‐70.3 kg). With the 9‐point scoring system, median BCS was 7 (range, 4‐9). Five dogs had an ideal BCS (4, 5), and 25 dogs were overconditioned (BCS > 5), with 8 dogs characterized as obese (BCS 8‐9). The MCS was assessed to be normal in 24 dogs. Muscle loss was noted to be mild in 4 dogs and moderate in 2 dogs. Median body weight of control dogs was 26.2 kg (range, 13.5‐47.0 kg). Median BCS was 6 (range, 4.5‐8). All control dogs had normal MCS.
Pertinent laboratory values from the PLN and control cohorts are presented in Table 1. According to the IRIS CKD staging system, dogs were classified as stage 1 (n = 23), stage 2 (n = 1), stage 3 (n = 6). Median UPC was 4.8 (range, 1.7‐27.5). Eleven PLN dogs had hypoalbuminemia (<2.9 g/dL), and the median UPC of these dogs was 7.1 (range, 4.2‐27.5). None of the control dogs was proteinuric or hypoalbuminemic. One dog had an increased systolic blood pressure of 180 mm Hg. No specific underlying etiology was determined to account for this canine hypertension.
Table 1.
Laboratory variables of dogs with protein‐losing nephropathy (PLN) and healthy dogs
| Laboratory variable and reference range | PLN (n = 30) | Control (n = 10) | P‐value |
|---|---|---|---|
| BUN (5‐20 mg/dL) | 21 (6‐58) | 19 (14‐23) | .98 |
| Creatinine (0.6‐1.6 mg/dL) | 0.8 (0.5‐2.7) | 1.0 (0.8‐1.2) | .82 |
| Phosphorus (3.2‐8.1 mg/dL) | 3.9 (2.5‐8.1) | 4.0 (2.7‐5.6) | .98 |
| Albumin (2.9‐4.2 g/dL) | 3.2 (0.9‐4.1) | 3.7 (3.0‐4.0) | .06 |
| USG | 1.018 (1.007‐1.065) | 1.043 (1.031‐1.053) | <.001 |
| UPC | 4.8 (1.7‐27.5) | 0.1 (0.1‐0.1) | <.001 |
| BP (mm Hg) | 155 (120‐240) | 137 (100‐180) | .15 |
Results are presented as median (range).
Abbreviations: BUN, blood urea nitrogen; UPC, urine protein : creatinine; USG, urine‐specific gravity.
All PLN dogs were eating commercial diets with a wide range of dietary protein concentrations. Only 4 dogs were eating a diet specifically designed for dogs with kidney disease. Eighteen dogs were receiving medications to inhibit the renin‐angiotensin‐aldosterone system. Eight dogs were receiving fish oil supplements. Five dogs were also receiving glucosamine/chondroitin.
3.1. AA profiles
Results of complete AA profiles are in Table 2. Compared to control dogs, PLN dogs had significantly lower concentrations of several EAA (leucine, threonine, histidine; Figure 1) and NEAA (glycine, proline, asparagine, tyrosine, o‐hydroxyproline, and serine; Figure 2). Sums of both EAA and NEAA were significantly lower in PLN dogs as compared to control dogs. The AA concentrations were not significantly different between dogs based on serum albumin concentrations or severity of proteinuria.
Table 2.
Amino acid profiles of dogs with protein‐losing nephropathy (PLN) and control dogs
| Plasma amino acid (nMol/mL) | PLN (n = 30) | Control (n = 10) | P‐value |
|---|---|---|---|
| EAA | |||
| Threonine | 201 (81‐351) | 277 (200‐477) | <.01 |
| Valine | 186 (113‐407) | 224 (200‐423) | .13 |
| Methionine | 64 (27‐141) | 89 (61‐230) | .13 |
| Isoleucine | 71 (37‐171) | 80 (73‐144) | .33 |
| Leucine | 138 (79‐327) | 243 (206‐287) | <.01 |
| Phenylalanine | 80 (4‐129) | 76 (63‐98) | .90 |
| Lysine | 210 (97‐422) | 201 (126‐329) | .90 |
| Histidine | 83 (52‐246) | 102 (80‐143) | <.05 |
| Tryptophan | 59 (2‐116) | 87 (63‐189) | .05 |
| Arginine | 105 (73‐230) | 142 (130‐287) | .13 |
| Sum of EAA | 1244 (747‐2282) | 1558 (1338‐2370) | <.01 |
| NEAA | |||
| Taurine | 126 (1‐263) | 111 (100‐158) | 0.99 |
| Aspartic acid | 13 (6‐19) | 13 (9‐19) | 0.99 |
| Serine | 113 (57‐214) | 178 (129‐268) | .02 |
| Asparagine | 58 (32‐126) | 96 (75‐146) | <.001 |
| Glutamic acid | 54 (26‐84) | 51 (36‐100) | 0.99 |
| Glutamine | 785 (441‐1352) | 829 (628‐1349) | 0.99 |
| Glycine | 198 (125‐322) | 372 (322‐490) | <.001 |
| Alanine | 521 (174‐1207) | 587 (480‐702) | 0.99 |
| Citrulline | 81 (25‐218) | 77 (53‐126) | 0.99 |
| Cystine | 39 (6‐80) | 39 (23‐54) | 0.99 |
| Tyrosine | 40 (8‐88) | 67 (49‐85) | <.01 |
| Homocysteine | 2 (0‐10) | 2 (0‐3) | 0.99 |
| Ornithine | 22 (8‐40) | 22 (15‐34) | 0.99 |
| 1‐Methylhistidine | 20 (6‐95) | 20 (7‐29) | 0.99 |
| 3‐Methylhistidine | 22 (6‐69) | 12 (11‐20) | .11 |
| O‐Hydroxyproline | 12 (0‐71) | 58 (9‐110) | <.01 |
| Proline | 185 (82‐381) | 408 (324‐560) | <.001 |
| Sum of NEAA | 2317 (1552‐23 860) | 2928 (2507‐3742) | .02 |
| Ratios | |||
| Valine : glycine | 0.93 (0.42‐2.46) | 0.60 (0.45‐1.13) | <.01 |
| Tyrosine : phenylalanine | 0.52 (0.34‐0.83) | 0.92 (0.76‐1.02) | <.01 |
| Glycine : serine | 1.61 (1.01‐2.87) | 2.13 (1.63‐2.67) | .03 |
| Sum EAA : sum NEAA | 0.52 (0.39‐0.81) | 0.54 (0.45‐0.65) | .79 |
Results are presented as median (range). Holm‐Bonferroni adjusted P‐values reported.
Abbreviations: EAA, essential amino acids; NEAA, nonessential amino acids; PLN, protein‐losing nephropathy.
Figure 1.
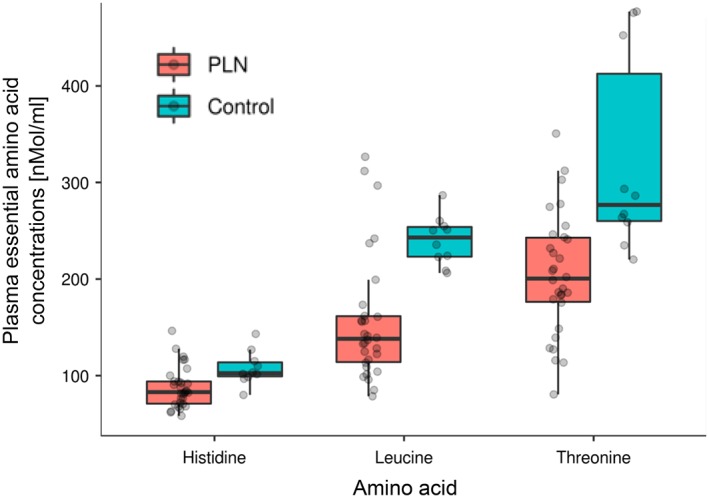
Selected EAA in PLN and control dogs. Each dot represents a dog. The boxes represent the 25th and 75th percentiles, and the central lines in the boxes represent the median values. The whiskers extend up to 1.5× IQR below and above the 25th and 75th percentiles, respectively. Points above and below the whiskers are indications for outlier values. The PLN dogs had lower concentrations (P < .05) of EAA compared to the control dogs. EAA, essential amino acids; IQR, interquartile range; PLN, protein‐losing nephropathy
Figure 2.
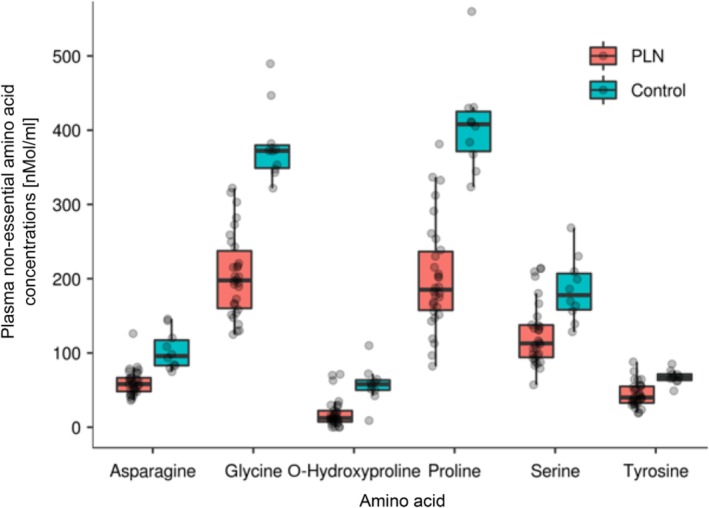
Selected NEAA in dogs with PLN and control dogs. Each dot represents a dog. The boxes represent the 25th and 75th percentiles, and the central lines in the boxes represent the median values. The whiskers extend up to 1.5× IQR below and above the 25th and 75th percentiles, respectively. Points above and below the whiskers are indications for outlier values. The dogs with PLN had lower NEAA concentrations (P < .05). IQR, interquartile range; NEAA, nonessential amino acids; PLN, protein‐losing nephropathy
Dogs with PLN had significantly lower ratios of tyrosine‐to‐phenylalanine (P < .001) and glycine‐to‐serine (P < .05) and a significantly greater ratio of valine‐to‐glycine (P < .01). (Figure 3) There was no significant difference of EAA‐to‐NEAA ratios between cohorts.
Figure 3.
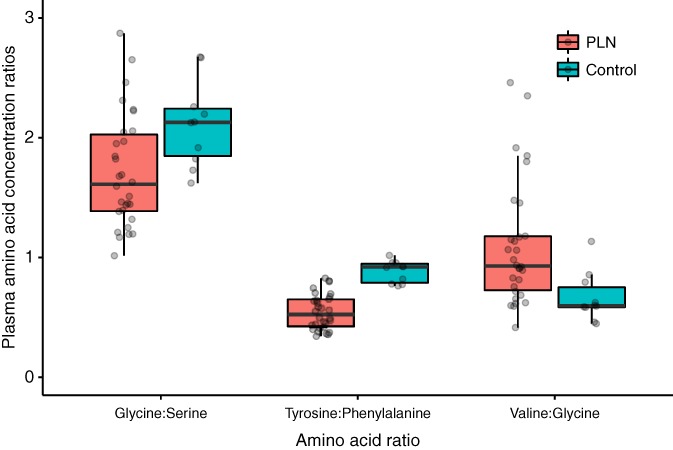
Ratios of amino acids in dogs with PLN and control dogs. Each dot represents a dog. The boxes represent the 25th and 75th percentiles, and the central lines in the boxes represent the median values. The whiskers extend up to 1.5× IQR below and above the 25th and 75th percentiles, respectively. Points above and below the whiskers are indications for outlier values. The dogs with PLN had lower glycine‐to‐serine (P < .05) and tyrosine‐to‐phenylalanine ratios (P < .05 and P < 0.01, respectively) and higher valine‐to‐glycine ratios (P < .001). IQR, interquartile range; PLN, protein‐losing nephropathy
4. DISCUSSION
We found that PLN dogs have altered AA status as compared to healthy dogs, with reduced plasma concentrations of several EAA and NEAA. The results from this study are similar to other studies in dogs and cats with various forms of kidney disease. The 30 PLN dogs from this study and 9 dogs with nonproteinuric CKD from a previous study had reduced plasma serine and proline concentrations.8 Our PLN group and another group of dogs (n = 11) with induced proteinuric kidney disease17 both had lower plasma leucine and tyrosine concentrations, as compared to their respective control groups. Similar to cats with CKD, the dogs with PLN in this study had significantly lower plasma concentrations of glycine, proline, tyrosine, and o‐hydroxyproline, and significantly higher concentrations of 3‐methylhistidine.9 Finding increased plasma 3‐methylhistidine concentrations in these PLN dogs contrasts lower concentrations in dogs with experimentally induced kidney disease.17
Similar to what has been described in other studies of human CKD, dogs with PLN had significantly lower leucine, tyrosine, and threonine concentrations, as compared to healthy controls.11, 12 However, there were several dissimilarities between our findings and these studies, as well as people with nephrotic syndrome in which there are significantly higher concentrations of NEAA, glutamic acid, hydroxyproline and alanine.13 In people with CKD, low histidine concentrations are associated with PEW, inflammation, oxidative stress, and decreased survival.18
The kidneys express the enzyme phenylalanine hydroxylase which is responsible for conversion of phenylalanine to tyrosine.19 Decreased expression of this enzyme likely explains our finding of lower plasma tyrosine concentration and tyrosine‐to‐phenylalanine ratio in the dogs with PLN. For this reason, tyrosine has been proposed to be a conditionally EAA in people with kidney disease.19
Other AA ratios that have been described to have clinical importance in humans with kidney disease are glycine : serine and valine : glycine. Both these ratios correlate with glomerular filtration rate (GFR) in humans.20 The glycine : serine ratio increases and the valine : glycine ratio decreases as GFR decreases.20 Because the kidney is a major site for conversion of glycine to serine, it is not unexpected that dogs with kidney disease can have both higher glycine concentrations and glycine : serine ratios.20 However, we found the dogs with PLN in this study had lower plasma glycine concentrations and glycine : serine ratios than the control dogs. This might be due in part to an effect of age as glycine concentrations have been shown to decrease with age in dogs.21
In humans with CKD, plasma valine concentrations and the valine : glycine ratio decrease with more advanced CKD.20, 22 Many of the dogs with PLN in this study had IRIS stage 1 disease, characterized by good urine concentrating ability and normal serum creatinine concentrations, suggesting minimal reduction in GFR, although neither GFR nor symmetric dimethylarginine was measured in these dogs. The lack of difference in plasma valine concentrations between dogs with PLN and control dogs is likely explained by the predominance of early IRIS stage dogs with PLN. This, in combination with the lower plasma glycine concentrations, can explain why dogs with PLN had higher valine : glycine ratios.
Certainly, decreased protein intake and malnutrition could contribute to altered AA metabolism. It was difficult to determine what effect diet had on these results, and dogs were eating diets that had variable protein concentrations. None of the dogs was assessed to be underweight. Interestingly, despite all dogs having adequate fat stores, 6/30 (20%) of the dogs were assessed to have either mild or moderate muscle atrophy. There were 2 dogs each with a BCS of 4, 5, and 6 of 9.
The results of this study have potential implications in nutritional management of PLN. The goal of nutritional modification in dogs with PLN is to reduce dietary protein enough to decrease proteinuria but not so much as to contribute to lean body mass loss. Theoretically, this concept seems simple; however, clinically it is more challenging to accomplish. One possible approach to optimize dietary restriction of protein without contributing to muscle loss is to strategically supplement AA. In humans with PLN, feeding low protein diets with concurrent supplementation of keto acids or EAA is advantageous as it can improve protein status while minimizing nitrogen load.23, 24 This concept is related to the ability of keto acids to stimulate protein synthesis while inhibiting protein breakdown. This diet regimen improves hypoalbuminemia and reduces proteinuria.23 Additionally, in 1 study in humans with CKD, those who received an AA supplement had lower C‐reactive protein concentrations than those who did not.18
Oral EAA supplementation for 4‐8 weeks in dogs with renal proteinuria results in increased serum albumin concentrations and body weight with no effect on UPC.25 Ultimately, it remains unclear whether the lack of decrease in UPC is clinically as important as the increase in albumin and body weight.
There were a few limitations to note. The study population was not a totally homogenous group, and renal biopsy was not performed to specifically identify type of kidney disease present. We initially set out to include dogs only with stage 1 and 2 CKD to reduce the effect that more advanced azotemia might have on AA status, as cats with CKD had more pronounced AA alterations with disease progression;9 however, given timing of dog recruitment, some dogs with stage 3 CKD were enrolled.
Many dogs did have abdominal ultrasound examinations performed, but dogs were not required to have this test performed. Although specific infectious disease testing was not required, there is anecdotally a low rate of tick‐borne infections seen at this teaching hospital. Unfortunately, not all dogs were fasted before sample collection, which might influence AA concentrations. None of the samples were noted to be overtly hemolyzed.
The PLN dogs did differ in age and size from the control dogs, which might bias results. We did not see significant evidence of relationship between AA levels and age or weight; nevertheless, the assumption for the absence of relationship between the AA and age and weight is needed. One study that compared plasma AA from 19 different breeds (not specified) revealed that plasma AA did change from birth to young adulthood (1‐5 years), but changes were variable, and dogs >5 years were not assessed.21
In conclusion, we found that dogs with PLN have altered AA status as compared to healthy control dogs. It remains to be determined whether these findings correlate with disease progression, PEW, or survival.
CONFLICT OF INTEREST DECLARATION
Andrea J. Fascetti is the Scientific Director of the Amino Acid Laboratory at the University of California Davis that provides amino acid analysis on a fee for service basis. This did not lead to any conflict of interest or influenced the collection or interpretation of results.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The IACUC and Ohio State University internal clinical research committee approved the protocol.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENTS
The authors thank the Clinical Trials Office (CTO) at the College of Veterinary Medicine for coordinating sample collection, storage and handling. The authors thank Dr. Zengshou Yu for technical assistance. The project described was supported by Award Number Grant UL1TR001070 from the National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Parker VJ, Fascetti AJ, Klamer BG. Amino acid status in dogs with protein‐losing nephropathy. J Vet Intern Med. 2019;33:680–685. 10.1111/jvim.15436
Funding information American Academy of Veterinary Nutrition; Waltham Foundation ; National Center for Advancing Translational Sciences, Grant/Award Number: UL1TR001070
This article was published online on 19 February 2019. The errors were subsequently identified. This notice is included in the online version to indicate that has been corrected 21 March 2019.
REFERENCES
- 1. Jacob F, Polzin DJ, Osborne CA, et al. Evaluation of the association between initial proteinuria and morbidity rate or death in dogs with naturally occurring chronic renal failure. J Am Vet Med Assoc. 2005;226:393‐400. [DOI] [PubMed] [Google Scholar]
- 2. King JN, Tasker S, Gunn‐Moore DA, Strehlau G. Prognostic Factors in Cats with Chronic Kidney Disease. J Vet Intern Med. 2007;21:906‐916. [PubMed] [Google Scholar]
- 3. Burkholder WJ, Lees GE, LeBlanc AK, et al. Diet modulates proteinuria in heterozygous female dogs with X‐linked hereditary nephropathy. J Vet Intern Med. 2004;18:165‐175. [DOI] [PubMed] [Google Scholar]
- 4. Parker VJ, Freeman LM. Nutritional management of protein‐losing nephropathy in dogs. Compendium. 2012;July;E1‐E5. [PubMed] [Google Scholar]
- 5. Polzin DJ. Evidence‐based step‐wise approach to managing chronic kidney disease in dogs and cats. J Vet Emerg Crit Care. 2013;23:205‐215. [DOI] [PubMed] [Google Scholar]
- 6. Brown S, Elliott J, Francey T, et al. Consensus recommendations for standard therapy of glomerular disease in dogs. J Vet Intern Med. 2013;27 Suppl 1:S27‐S43. [DOI] [PubMed] [Google Scholar]
- 7. Kovesdy CP, Kopple JD, Kalantar‐Zadeh K. Management of protein‐energy wasting in non‐dialysis‐dependent chronic kidney disease: reconciling low protein intake with nutritional therapy. Am J Clin Nutr. 2013;97:1163‐1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hansen B, DiBartola SP, Chew DJ, Brownie C, Berrie HK. Amino acid profiles in dogs with chronic renal failure fed two diets. Am J Vet Res. 1992;53:335‐341. [PubMed] [Google Scholar]
- 9. Goldstein RE, Marks SL, Cowgill LD, Kass PH, Rogers QR. Plasma amino acid profiles in cats with naturally acquired chronic renal failure. Am J Vet Res. 1999;60:109‐113. [PubMed] [Google Scholar]
- 10. Tizianello A, Deferrari G, Garibotto G, et al. Amino acid imbalance in patients with chronic renal failure. Contrib Nephrol. 1989;75:185‐193. [DOI] [PubMed] [Google Scholar]
- 11. Flugel‐Link RM, Jones MR, Kopple JD. Red cell and plasma amino acid concentrations in renal failure. JPEN J Parenter Enteral Nutr. 1983;7:450‐456. [DOI] [PubMed] [Google Scholar]
- 12. Suliman ME, Qureshi AR, Stenvinkel P, et al. Inflammation contributes to low plasma amino acid concentrations in patients with chronic kidney disease. Am J Clin Nutr. 2005;82:342‐349. [DOI] [PubMed] [Google Scholar]
- 13. el‐Gayar A, Sobh M, el‐Kholy A, Sallam S, Wafa E. Alterations of plasma free amino acids in nephrotic syndrome. Int Urol Nephrol. 1994;26:707‐712. [DOI] [PubMed] [Google Scholar]
- 14. Cano NJ, Fouque D, Leverve XM. Application of branched‐chain amino acids in human pathological states: renal failure. J Nutr. 2006;136:299S‐307S. [DOI] [PubMed] [Google Scholar]
- 15. Baldwin KBJ, Buffington T, Freeman LM, Grabow M, Legred J, Ostwald D. AAHA nutritional assessment guidelines for dogs and cats. J Am Anim Hosp Assoc. 2010;46:285‐296. [DOI] [PubMed] [Google Scholar]
- 16. Kim SW, Morris JG, Rogers QR. Dietary soybean protein decreases plasma taurine in cats. J Nutr. 1995;125:2831‐2837. [DOI] [PubMed] [Google Scholar]
- 17. Fukuda S, Kopple JD. Chronic uremia syndrome in dogs induced with uranyl nitrate. Nephron. 1980;25:139‐143. [DOI] [PubMed] [Google Scholar]
- 18. Watanabe M, Suliman ME, Qureshi AR, et al. Consequences of low plasma histidine in chronic kidney disease patients: associations with inflammation, oxidative stress, and mortality. Am J Clin Nutr. 2008;87:1860‐1866. [DOI] [PubMed] [Google Scholar]
- 19. Kopple JD. Phenylalanine and tyrosine metabolism in chronic kidney failure. J Nutr. 2007;137:1586S‐1590S. discussion 1597S‐1598S. [DOI] [PubMed] [Google Scholar]
- 20. Laidlaw SA, Berg RL, Kopple JD, Naito H, Walker WG, Walser M. Patterns of fasting plasma amino acid levels in chronic renal insufficiency: results from the feasibility phase of the modification of diet in Renal Disease Study. Am J Kidney Dis. 1994;23:504‐513. [DOI] [PubMed] [Google Scholar]
- 21. Blazer‐Yost B, Jezyk PF. Free amino acids in the plasma and urine of dogs from birth to senescence. Am J Vet Res. 1979;40:832‐838. [PubMed] [Google Scholar]
- 22. Ceballos I, Chauveau P, Guerin V, et al. Early alterations of plasma free amino acids in chronic renal failure. Clin Chim Acta. 1990;188:101‐108. [DOI] [PubMed] [Google Scholar]
- 23. Walser M, Hill S, Tomalis EA. Treatment of nephrotic adults with a supplemented, very low‐protein diet. Am J Kidney Dis. 1996;28:354‐364. [DOI] [PubMed] [Google Scholar]
- 24. Aparicio M, Chauveau P, De Précigout V, et al. Nutrition and outcome on renal replacement therapy of patients with chronic renal failure treated by a supplemented very low protein diet. J Am Soc Nephrol. 2000;11:708‐716. [DOI] [PubMed] [Google Scholar]
- 25. Zatelli A, D'Ippolito P, Roura X, et al. Short‐term effects of dietary supplementation with amino acids in dogs with proteinuric chronic kidney disease. Can Vet J. 2017;58:1287‐1293. [PMC free article] [PubMed] [Google Scholar]


