Abstract
Equine metabolic syndrome (EMS) is a widely recognized collection of risk factors for endocrinopathic laminitis. The most important of these risk factors is insulin dysregulation (ID). Clinicians and horse owners must recognize the presence of these risk factors so that they can be targeted and controlled to reduce the risk of laminitis attacks. Diagnosis of EMS is based partly on the horse's history and clinical examination findings, and partly on laboratory testing. Several choices of test exist which examine different facets of ID and other related metabolic disturbances. EMS is controlled mainly by dietary strategies and exercise programs that aim to improve insulin regulation and decrease obesity where present. In some cases, pharmacologic aids might be useful. Management of an EMS case is a long‐term strategy requiring diligence and discipline by the horse's carer and support and guidance from their veterinarians.
Keywords: EMS, endocrinopathic, insulin, laminitis
Abbreviations
- BCS
body condition score
- BM
body mass
- BPM
beats per minute
- CGIT
combined glucose insulin test
- DMI
dry matter intake
- EDC
endocrine disputing chemicals
- ELISA
enzyme linked immunosorbent assay
- EMS
equine metabolic syndrome
- GIP
gastric inhibitory polypeptide
- GLP‐1
glucagon‐like peptide 1
- HMW
high molecular weight
- HR
heart rate
- ID
insulin dysregulation
- IGF‐1
insulin‐like growth factor 1
- IR
insulin resistance
- IRT
insulin response test
- MAP kinase
mitogen‐activated protein kinase
- NSC
nonstructural carbohydrates
- OGT
oral glucose test
- OST
oral sugar test
- PPID
pituitary pars intermedia dysfunction
- RIA
radioimmunoassay
- SEL
secondary epidermal lamellae
- SGLT2
sodium‐glucose co‐transporter 2
- TRH
thyrotropin‐releasing hormone
1. INTRODUCTION
Experimental studies in the 1980's recognized an association between insulin dysregulation (ID) and laminitis in ponies.1, 2 Equine metabolic syndrome (EMS) was more thoroughly described subsequently3 and, in 2010, an American College of Veterinary Internal Medicine consensus statement on EMS was published.4 The advantage of recognizing EMS is to identify animals with increased risk of laminitis and to allow implementation of evidence‐based prevention strategies. The aim of this ECEIM consensus statement is to summarize and appraise more recent scientific evidence in order to optimize recommendations on how to recognize and manage the syndrome in practice.
2. DEFINITIONS
Equine Metabolic Syndrome is not a disease per se but rather a collection of risk factors for endocrinopathic laminitis. The key central and consistent feature of EMS is ID.5 The term ID is used to indicate disturbance of the balanced interrelationship among plasma concentrations of insulin, glucose, and lipids. Insulin dysregulation can manifest in several ways including 1 or more of basal hyperinsulinemia; an excessive or prolonged hyperinsulinemic response to oral or IV carbohydrate challenge, with or without an excessive or prolonged hyperglycemia (“glucose intolerance”), and tissue insulin resistance (IR). Hypertriglyceridemia can also be a consequence of IR (Figure 1).
Figure 1.
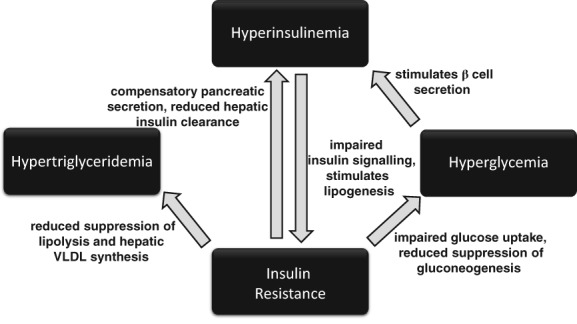
The interrelated components of insulin dysregulation
Obesity is defined as increased adiposity that has a negative impact on the health of the affected individual. This may be manifest as 1 or more of generalized or regionally excessive fat accumulation6, 7 a predisposition to weight gain and resistance to weight loss.8 EMS is usually associated with obesity, although exceptions occur.9 Further inconsistent features of EMS comprise cardiovascular changes including increased blood pressure, heart rate (HR) and cardiac dimensions9, 10, and adipose dysregulation manifesting as abnormal plasma adipokine concentrations including hypoadiponectinemia and hyperleptinemia.7, 11
Laminitis is the primary clinical consequence of EMS. However, horses with EMS might also be at risk of additional problems including hyperlipemia and critical care‐associated metabolic derangements including hyperglycemia and hypertriglyceridemia. Additional clinical concerns including preputial and mammary edema, mesenteric lipoma, inappropriate lactation, and subfertility in mares and stallions were also considered by the panel although it was concluded, pending further evidence, that these might simply be obesity‐related rather than associated with EMS.
3. DIFFERENTIAL DIAGNOSIS
Laminitis associated with ID can also arise in association with glucocorticoid administration and pituitary pars intermedia dysfunction (PPID). Additionally, nonendocrinopathic causes of laminitis can arise in association with systemic inflammatory response syndrome and excessive weight bearing. However, it should be remembered that EMS can serve as a contributory factor in laminitis resulting from other causes.
Adiposity is not inextricably linked with ID, and it is possible for equids to have EMS in association with a lean phenotype or to have excessive fat depots without the concurrent presence of ID or EMS. Thus, it is vital to demonstrate the presence of ID in an overweight animal before a diagnosis of EMS is made.
4. EPIDEMIOLOGY
There is little epidemiological data relating to the prevalence of EMS although the prevalence of its components has been evaluated by some studies. The prevalence of hyperinsulinemia in populations of horses has been reported in a few publications with 27% of ponies being hyperinsulinemic in an Australian study,12 22% of horses in a US study,13 and 18% of healthy, nonlaminitic horses in another US study.14 Published cases of EMS largely involve British native breeds,6, 7, 9 and cases of primary endocrinopathic laminitis were more likely to occur in British native ponies compared to Nordic ponies, cold‐blooded horses, and warm‐ and hot‐blooded horses.15 Breed differences in insulin sensitivity can also occur, as was demonstrated with ponies and Andalusian horses showing reduced insulin sensitivity compared to Standardbred horses.16
Equine metabolic syndrome appears to be more common in physically inactive animals, perhaps because of a beneficial effect of exercise on insulin regulation as well as decreased adiposity via increased energy expenditure.17, 18, 19 Additionally, certain predisposed breeds such as Shetland ponies, donkeys, and miniature horses are frequently not exercised as much as many other breeds as part of standard management. Studies have generally found insulin concentrations to be significantly higher in older versus younger horses and ponies.12, 20, 21, 22 Circulating adiponectin concentrations are also lower in older animals compared to younger animals, consistent with an age association with ID.23 Sex does not appear to influence most markers of ID in normal or laminitic ponies and horses.20, 24, 25, 26
The prevalence of obesity in various equine populations ranges from 21% to 45% in the United Kingdom.27, 28, 29, 30, 31 In other countries, obesity occurs in 10% of mature Icelandic horses in Denmark,32 8%‐29% of horses in Canada,33, 34 24.5% of Australian pleasure horses and ponies,35 and 51% of mature light‐breed horses in the United States.36 Regional adiposity, based on a cresty neck score >3/5, was present in 33% of horses in southwest England.37 Draught‐type, cob‐type, British native and Welsh breeds,29 Shetland ponies,35 Rocky Mountain Horses, Tennessee Walking Horses, Quarter Horses, Warmbloods, and mixed‐breed horses were all more likely to be obese compared to Thoroughbreds.36 Animals described as “good doers,” animals used for pleasure riding, nonridden animals, and more dominant individuals were more likely to be obese.29, 38 Finally, in Sweden, professional equine establishments and premises in more rural regions had fewer problems with overweight animals, whereas premises that kept other species for meat production were more likely to have overweight horses.39
The effects of season on components of EMS remain quite unclear with several studies having contrasting findings for regional and generalized adiposity,28, 33, 37, 39 insulin,9, 20, 40, 41, 42, 43 adiponectin,24, 44 leptin,45 and triglycerides.9, 46 Pregnancy‐associated ID occurs in pregnant mares,42, 47, 48 although hyperinsulinemia does not always occur.42, 49
Endocrine disrupting chemicals (EDCs) are associated with metabolic syndrome in humans.50 Preliminary data demonstrated that horses from farms within 30 miles of EDC disposal sites were more likely to have had laminitis, and had laboratory evidence of ID, suggesting EDC exposure might be an EMS risk factor.51
5. PATHOPHYSIOLOGY
5.1. Insulin dysregulation
Insulin dysregulation plays a central role in EMS and hyperinsulinemia is probably the most important pathophysiologic feature of ID in horses.52 Although hyperinsulinemia might often be simply a compensatory response to systemic IR, resting and postprandial hyperinsulinemia can occur to a greater extent than explained by IR or even occur independently of IR.52, 53
Oral administration of glucose stimulates a greater insulin secretion than IV administration of glucose in ponies52, 54 indicating that the gastrointestinal tract releases factors that augment glucose‐induced pancreatic insulin secretion. At least 3 hormonal factors, or incretins, are known to exist in the horse, namely gastric inhibitory polypeptide (GIP) and glucagon‐like peptides 1 and 2 (GLP‐1, GLP‐2). The relative contribution of these incretins to insulin production varies considerably among species and, in horses, an estimated influence of glucose, GLP‐1 and GIP on insulin response to oral glucose administration was 76%, 23%, and 2% respectively.52 Active GLP‐1 clearly stimulates insulin secretion in horses,52, 55 although its role in ID remains unclear.55, 56, 57 Glucagon‐Like Peptide 2 is a further incretin with no direct effect on insulin secretion, although it increases glucose bioavailability via gastrointestinal effects. Its role in EMS is also unclear.58
It has generally been found that chronic feeding of a diet high in nonstructural carbohydrates (NSCs) will decrease insulin sensitivity and adiponectin concentrations compared to forage or fat‐rich diets.17, 59, 60, 61, 62 However, the association of dietary NSC intake and IR is not straightforward, and more recent work suggests adaptation to high NSC diets can actually improve glucose tolerance and tissue insulin sensitivity, but induce an exaggerated postprandial insulinemic response.53, 63
Decreased hepatic clearance of insulin might also contribute to hyperinsulinemia. More than 70% of the insulin secreted by pancreatic beta cells is normally cleared from the portal blood by the liver in horses, with evidence of decreased hepatic insulin clearance in horses with obesity and IR.64
5.2. Development of laminitis
Hyperinsulinemia induces laminitis in horses.65, 66, 67 Field‐based observations have shown an association between hyperinsulinemia, or other components of ID, and laminitis,6, 7, 11, 68, 69 and clinical laminitis can be induced by 48‐72 hours of insulin infusion in euglycemic ponies65 and horses66 (both studies mean insulin 1036 μIU/mL). Laminitis lesions, but not lameness, were experimentally induced by 48 hours of glucose infusion inducing hyperglycemia (mean 11 mmol/L) and endogenous hyperinsulinemia (mean 208 μIU/mL67), giving an indication of the threshold degree and duration of hyperinsulinemia required to trigger laminitis.
The resultant lesions in endocrinopathic laminitis are initially found in the secondary epidermal lamellae (SEL) including lengthening and narrowing, developing tapered tips and with SELs angled more acutely to the primary epidermal lamellar axis.70 Stretching of the lamellar epithelial cells is the earliest histological change observed after 6 hours of hyperinsulinemia and subsequent cellular changes include an accelerated cell death (apoptosis) and proliferation cycle.71, 72 This cellular stretching indicates cytoskeletal disruption,70 and deformation of the lamellae also explains how divergent laminitic rings can often appear before lameness.69
It is less well established how hyperinsulinemia causes laminitis, with various theories having been disproven including glucose deprivation,73 glucotoxicity,74 matrix metalloprotease upregulation,75 and increased blood flow‐induced hyperthermia.76 Current plausible theories include changes in intracellular insulin signaling resulting in endothelial dysfunction and mechanisms involving insulin‐like growth factor 1 (IGF‐1). In healthy animals, stimulation of insulin receptors initiates intracellular signaling processes via the so‐called metabolic pathway, which result in nitric oxide‐mediated vasodilation and GLUT4 translocation.77 Blockade of this metabolic pathway in IR favors the alternative so‐called mitogenic or mitogen‐activated protein (MAP) kinase (or Ras ERK 1/2) pathway, which results in endothelin 1‐mediated vasoconstriction, upregulation of cellular adhesion modules, and mitogenesis.77 Support for a potential vascular mechanism linking IR and laminitis comes from studies of horses where an abnormal contractile response to insulin was observed in digital vessels and lamellar arteries in the presence of IR,78, 79, 80 and also increased endothelin‐1 has been measured after insulin infusion in an ex vivo perfused limb model.81 However, research on vascular endothelial dysfunction provides a limited view of the intracellular events that occur during hyperinsulinemia. It is likely that multiple hormones, cytokines, or signaling molecules play a role and that the roles might be different in the lamellar epithelial cells and vascular endothelium. The IGF‐1 receptor has received attention owing to its similarity with the insulin receptor and the ability of high concentrations of insulin to activate it resulting in intracellular signaling through the MAP kinase pathway.82 The IGF‐1 receptor is found on lamellar epithelial cells as well as endothelia, unlike the insulin receptor that is primarily located only on endothelial cells.83 Studies of horses have shown clear evidence of upregulation of intracellular signaling downstream from the IGF‐1 receptor both in response to dietary carbohydrate challenge and insulin infusion.84 Of particular relevance was the observed activation of the downstream molecule RPS6, which disrupts cytoskeletal regulation resulting in a loss of cell stiffness or integrity that might explain the observed cellular elongation or stretching.84
5.3. Role of microbiome, diet, and gut homeostasis
The intestine generates different types of messages via hormones and afferent nerves that inform peripheral organs (liver, adipose tissue, brain) of nutritional status, which in response generate signals to modify energy storage or expenditure.85 Factors such as changes in diet or EDCs leading to disruption of gastrointestinal homeostasis can therefore influence the metabolic status of the whole body.
The gut microbiota has been suggested as a driving force in the pathogenesis of metabolic disease and obesity in humans and other species.86, 87 A small study of fecal microbiota in EMS and control horses showed less diversity in EMS horses with an increase in Verrucomicrobia members,88 which has been suggested as microbial biomarkers for progression of glucose intolerance in humans.89 Additionally, small changes in carbohydrate content in different cuttings of hay elicited measurable changes in equine hindgut streptococci 4 hours after meal feeding,90 and supplementation with short‐chain fructo‐oligosaccharides alters microbial populations in different parts of the intestine.91
5.4. Role of obesity
Obesity is no longer seen as a sole cause of EMS but rather a commonly associated feature that, when present, can exacerbate ID.92, 93 A possible consequence of increased fat mass is dysregulation of adipokines, including leptin and adiponectin, as well as inflammatory mediators, which might impact glycemic control, inflammation, and cardiovascular function.94
Adipocytes are the main site of leptin synthesis and fat mass appears to be the primary determinant of serum leptin concentration in the horse.95 Leptin serves to relay signals to the brain indicating fat status and to maintain body condition by appetite suppression and increased energy expenditure during energy excess.96 In some horses, leptin concentrations exceed those expected based on the amount of fat due to the presence of obesity‐related leptin resistance, which might act to worsen the existing degree of obesity.95, 96 Increased leptin concentrations correlate with hyperinsulinemia in ponies.12
Adiponectin is a further adipokine secreted exclusively from adipose tissue, which improves insulin sensitivity and reduces inflammation.97 Adiponectin concentration is inversely related to fat mass and IR.95 Decreased adiponectin concentrations were associated with mildly increased serum amyloid A and development of ID in horses fed a cereal‐rich diet, although horses fed a fat‐rich diet showed no evidence of ID and had normal adiponectin concentrations.63
Several studies in horses have examined a possible role of adipose tissue in generating inflammation by releasing various pro‐inflammatory cytokines. However, findings have been inconsistent, possibly reflecting differences between fat depots and individual variability, and the association of inflammation with ID and generalized or regional obesity remains unclear.24, 93, 98, 99, 100, 101, 102, 103, 104, 105
5.5. Role of genetics
The expression of EMS is the result of a complex interaction between genetics and the environment. Although environmental factors including excessive nutrition have been linked to EMS, high planes of nutrition or changes in the pasture do not result in metabolic derangements and laminitis in all horses indicating the importance of underlying genetic influences.92 Horses with EMS are often considered “good doers” or “easy keepers,” and genes contributing to this phenotype are likely to be advantageous for survival in the wild during periods of famine by enhancing feed efficiency.106 Initial research into the genetic components of EMS in a group of ponies found that the prevalence of laminitis was consistent with the action of a major gene or genes expressed dominantly, but with reduced penetrance attributable to sex‐mediated factors, age of onset, and further epigenetic factors.6 More recently, genome wide association studies have been undertaken using Arabian horses, Welsh ponies, and Morgan horses, and several candidate genes were identified associated with relevant traits including height and insulin, triglyceride, and adiponectin concentrations.106, 107, 108 Nutritional status and body condition of the mare during pregnancy influences the foal in terms of reproductive, orthopedic, and metabolic responses later in life, probably related to epigenetic changes in utero.109, 110
6. LABORATORY DIAGNOSIS
Among the various testing protocols for assessing laboratory markers of ID, analysis of blood samples to determine insulin concentration is a common feature. Several studies have reported marked differences in equine insulin values determined by different laboratory methods.111, 112, 113, 114, 115 Therefore, cutoff values generated in particular studies cannot be applied universally, and care should be taken to only directly compare results generated using the same insulin assay. Samples for glucose assay should be collected into tubes designed for preserving plasma glucose such as oxalate fluoride or sodium fluoride and, ideally, be chilled.
Different test procedures provide diverse information regarding aspects of ID (Figure 1), which might partly explain discordant results when various test procedures are compared.116, 117, 118, 119, 120, 121 Knowledge about physiological principles of the test procedure is important to interpret results and to identify which component or components of ID are affected. In general, a complete assessment of ID can be achieved in a step‐wise manner by combining multiple diagnostic tests.
6.1. Pretest considerations
Acute stress can affect insulin and glucose concentrations via activation of the adrenocortical axis and by catecholamine release. It is advisable to minimize stress from transportation, environmental and feeding management changes, pain from active laminitis, or excessive starvation before testing.122, 123, 124, 125 Although the absence of feed intake might be expected to decrease insulin concentrations, fasting for as little as 6 hours can cause IR in horses and donkeys.122, 126 Furthermore, postprandial glucose or insulin responses will be affected by gastric emptying, which in turn will be influenced by the presence or absence of ingesta in the stomach and small intestine and therefore feeding status should always be considered.
6.2. Basal testing
6.2.1. Insulin
An indication of ID can be based on finding resting (or basal) hyperinsulinemia.4 However, the use of fasting before measuring basal insulin is no longer recommended owing to low sensitivity for ID as well as the possible confounding effects of secondary IR induced by the fasting protocol. Measurement of insulin within 4‐5 hours of grain feeding is also not recommended owing to lack of standardization of responses. Serum insulin concentrations measured in ponies 1‐3 hours after coming off pasture could differentiate laminitis‐prone individuals using a cutoff for insulin of 20 μIU/mL using the Coat‐a‐Count radioimmunoassay (RIA).6, 7 Resting insulin can also be measured under forage‐fed conditions, although the effects of different forages and rates of consumption will influence results. In 112 ponies with free access to hay, a reference interval of 2.0‐21.1 μIU/mL was established with the Immulite 2000 chemiluminescent assay.127 In another study, 6 normal ponies fed 0.25% BM forage as dry matter intake (DMI) had peak insulin values of <3.9 μIU/mL when fed soaked hay and <19.8 μIU/mL when fed dry hay using the same assay (Immulite 2000).128 Haylage feeding was associated with higher peak insulin values in these normal ponies (up to 66.0 μIU/mL).128 When forage of unknown quality is being fed, mild increases in resting insulin concentrations (20‐50 μIU/mL) should be considered suspicious for ID and dynamic testing using OGT or OST is recommended (Equine Endocrinology Group: https://sites.tufts.edu/equineendogroup/. Accessed May 6, 2018).
6.2.2. Glucose
Measuring resting plasma glucose in horses with suspected ID is generally uninformative although warrants inclusion for identification of occasional cases of diabetes mellitus.129
6.2.3. Triglycerides
Horses and ponies affected by EMS often suffer from dyslipidemia,12, 130 and hypertriglyceridemia has been found to be a significant predictor of laminitis in ponies with cutoff values from 0.64 to 1.06 mmol/L (57‐94 mg/dL).6, 7 Given the relatively mild and inconsistent changes, however, this has not proved to be a reliable diagnostic test.
6.2.4. Adipokines
Adiponectin is an adipose‐derived hormone with antiinflammatory and insulin‐sensitizing actions that is synthesized as monomers and secreted into the circulation as trimers, low‐molecular‐weight hexamers, and high‐molecular‐weight (HMW) multimers.131 In humans, HMW adiponectin is generally regarded as a more sensitive marker of metabolic disease than total adiponectin concentration,132 although the importance of total adiponectin is also supported.133 In horses, both HMW and total adiponectin are associated with obesity and ID,104, 134 and low total concentrations are identified as a risk factor for future laminitis11. An immunoturbidimetric assay has recently been validated for total adiponectin in equine samples.135 Leptin concentrations are useful in the prediction of laminitic episodes in ponies7 although they appear better associated with fat mass rather than ID in horses and therefore have the potential to mislead in EMS investigation.26, 63, 95
6.2.5. C‐peptide
C‐peptide is co‐secreted with insulin in equimolar amounts.136 In contrast to insulin, C‐peptide is unaffected by the hepatic first pass effect and therefore provides a more accurate assessment of pancreatic secretion of insulin.137 Increased C‐peptide concentrations in response to oral and IV administered glucose have been described in horses using RIA.64, 138 A commercially available ELISA is also described, which performed acceptably in horses.138
6.2.6. Incretins
Studies of the diagnostic usefulness of plasma incretin concentrations thus far have not offered adequate support for their employment as markers for ID and EMS, although some encouraging findings are reported for active GLP‐152, 55, 56, 139 and GLP‐2.58
6.3. Dynamic testing
Dynamic tests provide additional information on the relationship between insulin and glucose and are generally more sensitive than basal testing for identification of ID. Tests measuring responses to PO administered NSC more closely mimic the more complete sequence of glucose and insulin dynamics following food intake, whereas tests measuring responses to IV insulin, glucose, or both focus on tissue insulin sensitivity and beta cell responsiveness. In experimental and research settings, tests including the frequently sampled IV glucose tolerance test62, 129, 140 and the euglycemic hyperinsulinemic clamp116, 118 are often used to investigate insulin and glucose regulation but are too complex and costly for routine clinical use.
6.3.1. Oral challenge tests
Oral glucose test
In‐feed oral glucose tests (OGTs) can be performed by offering glucose (or dextrose) powder mixed in small, low‐glycemic meals,2, 141, 142 and post‐glucose insulin concentration has been shown to be associated with the risk of developing laminitis in ponies being fed a high‐NSC diet.142 Fasting overnight is recommended before the test, both to increase compliance with ingesting the test dose and to facilitate gastric glucose transit. Most frequently 0.5 or 1.0 g/kg body mass (BM) glucose powder is used. Peak plasma glucose occurs between 60 and 120 minutes after consuming the meal.117 The main limitation of the protocol is that palatability of the large glucose dose can be poor, and test results may be affected by ingestion time, gastric emptying, and intestinal absorption.143, 144 However, a recently published dosage of 0.75 g/kg BM resulted in satisfactory results and increased acceptance by ponies.144 Insulin concentrations regarded as indicative of ID, when measured at 120 minutes post‐feeding, are >68 μIU/mL for 0.5 g/kg BM glucose and >80‐90 μIU/mL for 1.0 g/kg BM glucose with Immulite 1000 chemiluminescent analysis (Equine Endocrinology Group: https://sites.tufts.edu/equineendogroup/. Accessed May 6, 2018).141, 145 In a study where 14 of 37 ponies developed laminitis while receiving a high‐NSC diet, no pony developed laminitis that had resting insulin <8.5 μIU/mL and insulin <65.5 μIU/mL at 120 minutes following 1.0 g/kg glucose using the ADVIA Centaur chemiluminescent assay.142
As an alternative, the OGT can be performed with 1.0 g/kg BM glucose dissolved in 2 L of water and administered via nasogastric tube as long as sedation or stressful restraint are not required.146 The suggested cutoff value for this test protocol is 110 μIU/mL insulin at 120 minutes, using the equine optimized insulin ELISA.146
Oral sugar test
The oral sugar test (OST) uses a specific corn syrup (Karo Light Corn Syrup; ACH Food Companies, Inc, Oakbrook Terrace, IL) at a dosage of 0.15 mL/kg BM PO via dosing syringe.119 Although pretest fasting has not always been found to affect results,147 it is advised that horses should be fasted for between 3 and 12 hours before the OST.122, 123 This might be accomplished by offering a reduced amount of hay (eg, 0.2‐0.4 kg per 100 kg BM) the night before the test. Blood sampling to measure insulin response is recommended at between 60 and 90 minutes after corn syrup (Karo Light Corn Syrup; ACH Food Companies, Inc) administration. Insulin concentrations >60 μIU/mL post‐dosing were initially used to indicate ID using RIA (Millipore Human RIA),141, 148 although a lower cutoff of >45 μIU/mL was recommended more recently using the same assay (Equine Endocrinology Group: https://sites.tufts.edu/equineendogroup/. Accessed May 6, 2018). A higher dosage of 0.25 mL/kg BM corn syrup (Karo Light Corn Syrup; ACH Food Companies, Inc) was suggested to improve diagnostic value with a cutoff of >30 μIU/mL between 60 and 90 minutes using Coat‐A‐Count RIA.149 An even higher dosage of 0.45 mL/kg BW corn syrup (Karo Light Corn Syrup; ACH Food Companies, Inc) could provide higher sensitivity for ID with a value of >110 μIU/mL measured by RIA (Insulin CT, MP Biomedical) at 60 minutes successfully differentiating 5 previously laminitic ponies from 3 ponies with no history of laminitis.147 Cutoff values have also been determined for insulin concentrations measured with other assays, and results from the higher dose OST in 35 ponies with no history of laminitis indicated cutoffs between 60 and ‐90 minutes post‐dosing of 40 μIU/mL using the Immulite 1000 and 63 μIU/mL using the Immulite 2000xpi chemiluminescent assays (A. E. Durham, unpublished data).
A further OST using commercially available Scandinavian glucose syrup (Dan Sukker Glykossirap; Nordic Sugar A/S, Copenhagen, Denmark) has been described.150 A dosage of 0.2 mL/kg BM is comparable to corn syrup at 0.15 mL/kg, although reference ranges have not been established to date.
Finally, horse compliance might be improved by using a grain‐based diet as a carbohydrate challenge rather than glucose, with comparable insulin responses and prediction of laminitis risk.142, 144 However, the type of grain, consumption time, variable gastric transit, and absorption could still confound test results.
6.3.2. Intravenous challenge tests
Parenteral delivery of exogenous glucose, insulin, or both has the advantage of decreasing variability associated with gastrointestinal transit and absorption but also omits some possibly important elements of ID including incretin effects. Nevertheless, such tests allow focus on specific elements of ID such as IR and have been successfully used to diagnose and monitor EMS in clinical and research settings.151, 152
Insulin response test (IRT)
The insulin response test (IRT) measures the glycemic response to exogenous insulin, directly assessing insulin‐dependent glucose uptake and insulin sensitivity. Forage is made freely available during the test to reduce the risk of clinical hypoglycemia.153 In normal horses, blood glucose will decrease to <50% of the baseline concentration within 20‐30 minutes after 0.02‐0.125 IU/kg BM neutral (regular) insulin IV and then return back to baseline levels within 2 hours.154 In horses with IR, the blood glucose concentration will remain higher and return to baseline sooner. An abbreviated protocol is described with glucose measured before and 30 minutes after 0.1 IU/kg BM neutral insulin IV, followed by dextrose administration, or a small feed, after 30 minutes to prevent hypoglycemia.155
Combined glucose insulin test
The combined glucose insulin test (CGIT) is based on injection of 150 mg/kg BM glucose IV, directly followed by 0.1 IU/kg BM neutral (regular) insulin IV.156 In a shortened protocol, both insulin and glucose are measured in the basal sample and after 45 and 75 minutes.152 Typical healthy horses show a biphasic blood glucose curve starting with hyperglycemia, followed by a negative phase in which glucose drops below the initial baseline value. Glucose should normally then increase back to baseline concentrations within 45 minutes. Insulin concentration should be under 20 μIU/mL both at baseline and at 75 minutes, and remain <100 μIU/mL at 45 minutes when analyzed with the Coat‐A‐Count RIA.156 Hyperinsulinemia and a delayed return to baseline glucose indicate ID. The CGIT is generally well tolerated although hypoglycemia can occur and tested horses should be closely monitored and additional glucose injections or a small feed should be readily available.
6.4. Testing for PPID
There are similarities between EMS and a subset of animals with PPID in terms of predisposition to laminitis and the presence of ID157, 158 and, furthermore, the 2 conditions can coexist. Thus, testing for PPID using basal ACTH concentrations or ACTH response to exogenous thyrotropin‐releasing hormone (TRH) should be considered in horses with ID, laminitis, or both and especially those that are greater than 10 years of age.159 Combined testing for PPID and ID with the TRH stimulation test and OST has been recently studied. When TRH stimulation testing is performed after PO administration of corn syrup, post‐stimulation ACTH concentrations are significantly lower and, therefore, this order of combination testing is not recommended.160
6.5. Key laboratory testing recommendations
The specific methodology used to quantify analytes such as insulin has a marked effect on results and should always be considered, especially when considering diagnostic criteria from published studies.
The most useful and practical tests for routine assessment of the various manifestations of ID include resting (not fasting) insulin, the OGT or OST, and the IRT or CGIT.
Interpretation of the test results requires a good understanding of the test selected.
A subset of animals with PPID have ID, and EMS and PPID can coexist, thus concurrent testing for PPID should be considered in all horses over 10 years of age.
7. MANAGEMENT
7.1. Dietary energy restriction
Nutritional strategies for management of obesity primarily rely on energy restriction through limiting the total replace with DMI. In general, grains or cereal‐based complementary feeds, fruit, or vegetables such as carrots, apples, or treats should be excluded from the diet because of their high NSC content. Likewise, high‐fat feeds should be avoided in obese horses because of their high energy contents.
Progressive BM losses by energy restriction improve insulin regulation in obese and ID horses and ponies.8, 68, 104, 152, 161, 162, 163, 164 There is debate about the optimal degree of restriction owing to concerns about not meeting minimum roughage requirements.165 In general, daily allowance of a mixed species grass hay‐based diet of 1.25%‐1.5% of actual BM as DMI, or 1.4%‐1.7% of actual BM as fed, is recommended, typically corresponding to a digestible energy (DE) intake of 64%‐94% of maintenance requirements.8, 104, 165, 166
There is considerable individual variability in proportional weight losses among horses subject to dietary restriction independently of variables such as their diet, ID, morphometric measures, or body fat content, leading to the categorization of individuals as either sensitive or resistant to weight loss.8 In horses resistant to weight loss, a further forage restriction to as little as 1.0% actual BM as DMI, or 1.15% actual BM as fed, should be considered if appropriately monitored.
The nutrient composition of the forage should be determined where possible,167 although this seems to be performed rarely in practice.168 Hays with low NSC content (<10%) are recommended to limit insulin responses,169 although moderate (12%) or high (18%) NSC content in hay had no long‐term effect on serum insulin concentrations in obese Arabian geldings in one study.170 Haylage is often presumed to have low NSC owing to fermentation, although insulinemic responses to haylage are greater than to hay of an equivalent NSC content.128 This, along with frequently greater palatability of haylage, means that it is not recommended for feeding horses with ID.
Soaking reduces the glycemic and insulinemic responses to hay and haylage fed to both normal and ID ponies128, 171 and is recommended for horses with ID, especially those demonstrated to have abnormal responses to oral carbohydrate challenges. Soaking hay for 7‐16 hours at ambient temperature decreases nutrients including water‐soluble carbohydrates by 24%‐43% and is associated with more than double the losses of BM compared to feeding the equivalent amount of dry hay.165 However, there are concerns over microbial growth during a long soaking process in warm or tepid water unless the hay is subsequently steamed172 and therefore limiting to a 1‐ to 2‐hour soak is recommended in warm conditions. As forages can be low in protein, and mineral and vitamin leaching occurs after soaking, these nutrients must be balanced by supplements to cover requirements.165, 173 Increases in plasma creatinine was found in one study of dietary restriction as a result of muscle catabolism due to a deficient protein intake.164
Dry matter intake is increased on pasture, at least when offered ad libitum,174 and high NSC pasture has been shown to exacerbate hyperinsulinemia in ponies.6 Restricting grazing time alone has a limited effect on DMI and can result in rapid consumption with over 0.9% BM as DMI having been recorded in just 3 hours of time at pasture.175, 176 Grazing muzzles reduce DMI to 77%‐83% of grazing without a muzzle177 and can have the added benefits of increasing foraging time and exercise. During the initial 6‐12 weeks of dietary restriction in animals with ID, pasture access should be prevented as even partial access is difficult to quantify in terms of DMI. However, successful long‐term management of EMS cases can still include some grazing provided that ID is under control. In this respect, ID is best assessed by the insulin response to PO administration of carbohydrates or after grazing.152
The use of dietary supplements to facilitate BM losses or to improve ID is popular, but their efficacy remains questionable or unproven. Supplements including magnesium, chromium, and short‐chain fructo‐oligosaccharides have received support based on some evidence of improved insulin regulation,178, 179, 180 although other studies have not confirmed these positive effects.151, 181 Little benefit has been shown for other supplements including cinnamon,182 l‐carnitine,183 and Psyllium,184 although some promising effects are reported for a feed enriched with Spirulina platensis algae185 and the wheat bran protein aleurone.186
7.2. Welfare considerations
Safe rates of BM loss are not well defined. Although abrupt feed restriction resulting in loss of approximately 1% BM weekly was not associated with hypertriglyceridemia in a study of Welsh ponies,164 more rapid BM losses have been associated with increases in serum triglycerides and non‐esterified fatty acid concentrations, reflecting increased lipolysis.166, 183 Furthermore, quite considerable variation in individual sensitivity or resistance to weight loss can occur, in addition to effects of variation in hay quality.8, 165 Consequently, energy restriction should be targeted toward achieving 0.5%‐1.0% BM losses per week and carefully monitored during restriction to ensure targets are reached but not exceeded. There is rarely a need to institute abrupt diet restriction, and this can generally be phased in over a week or 2.
Attempts to extend feeding time during feed restriction are ideal for the animal's health and welfare.164 As straw has a lower energy content than hay, DMI can be increased to >1.25% BM, thereby increasing feeding time. In one study, a mixture of alfalfa, hay, and straw was offered without any negative clinical consequences during a 17‐week weight loss program in ponies.163 Although other studies are lacking, gradual introduction of straw in exchange for hay at a rate of up to 30% of total forage can be recommended to allow increased DMI with little effect on DE intake. Monitoring fecal output to detect early signs of colon impaction is advisable after introduction of straw.
During feed restriction to a DMI of 1.0% BM in horses and ponies, feeding from a hay net compared to feeding from the stall floor resulted in extension of hay intake time from 120 to 193 minutes.175 Dynamic feeding systems have been designed by applying time‐dependent cycles with controlled hay access to provide a combination of exercise and extension of feed intake while improving BM losses.187 A possible consequence of restricting DMI is ingestion of bedding such as straw, wood shavings, or paper,188 and consequently, rubber matting in stables might be considered in some cases during energy restriction. The impact of dietary restriction on the incidence of gastric ulceration and behavior should also be considered. Both these concerns were examined in a study of 16 weeks' dietary restriction in Shetland ponies where no effect on gastric ulceration was found.166 The same study suggested an increase in coprophagy during the period of dietary restriction, whereas other studies have found feed restriction to be associated with lethargy.189, 190
7.3. Key dietary recommendations
An ideal target for weight loss in obese horses is between 0.5% and 1.0% BM losses weekly.
This may be achieved with a forage‐based ration totaling 1.4%‐1.7% BM as fed, or in exceptional cases that appear weight loss resistant, as little as 1.15% BM as fed.
Forage with NSC <10% is recommended to limit postprandial insulin responses in horses with ID and soaking may be required to achieve this in many instances.
Ensuring adequate protein, vitamins, and minerals is important via a ration balancer supplement.
7.4. Exercise programs
Exercise improves insulin sensitivity and reduces inflammation in people, even in the absence of weight loss.191, 192 A similar antiinflammatory effect has also been observed in ponies undergoing low intensity training (5 minutes trotting per day for 14 days) with a reduction in serum amyloid A and normalization of serum haptoglobin in previously laminitic ponies.193 However, based on the evidence from human studies, exercise intensities required for improving insulin sensitivity may be higher than are often undertaken, which may explain the variable success of programs that used lower intensities of exercise.194 Low‐intensity exercise in young‐ to middle‐aged horses and ponies should be producing HRs of 110‐150 beats per minute (BPM) (50%‐70% HRmax) and moderate intensity 150‐190 BPM (70%‐90% HRmax). As a guide, for inactive to lightly exercised horses, fast trotting on a lunge elicits HRs of around 100‐110 BPM and canter 120‐160 BPM whether ridden or unridden.195
An increase in insulin sensitivity has been found for several days following 7 days of endurance training in healthy Standardbred horses at 55% VO2max (70% HRmax or 150 BPM) for 45 minutes17 with similar beneficial findings for low‐to moderate‐intensity exercise in other studies of healthy and obese horses.18, 19 Swimming healthy Thoroughbred horses in a moderate intensity exercise program for a month (HRs 160‐180 BPM for 60 minutes per day) decreased basal and dynamic plasma insulin concentrations.196
However, not all studies have found exercise to improve basal insulin or insulin dynamics,197 and lower levels of exercise have frequently had disappointing results. Only short‐term improvements in insulin sensitivity were found after 7 days of 30 minutes light exercise (mean HR 130 BPM) in obese mares,198 and only a marginal improvement in OGT responses was seen after 6 weeks of training obese ponies (5 days a week for 9 minutes trot and canter, HR <140 BPM), although their improvements were maintained over 6 weeks deconditioning.161 Insulin sensitivity was not improved during an exercise program of 2 weeks of 30 minutes slow trotting for 4 days/week and 2 weeks of 30 minutes trotting for 2 days per week and 20 minutes slow cantering plus 10 minutes trotting for 2 days/week.197
7.5. Key exercise recommendations
Exercise recommendations are opinions extrapolated from medical research and clinical experience; relative gait and HR ranges are based on horse and pony exercise physiology research.
Exercise recommendations will depend on lamellar stability and cannot be applied to horses with current or recent laminitis. All exercise should be increased gradually based on the horse's baseline fitness level and with careful monitoring in case of deterioration of lamellar stability especially in horses with previous laminitis.
In nonlaminitic horses with ID, minimum recommendations are low‐to moderate‐intensity exercise (canter to fast canter, ridden or unridden; or HRs 130‐170 bpm) for >30 minutes, >5 times per week.
In previously laminitic horses with recovered and stable hoof lamellae, minimum exercise recommendations are low‐intensity exercise on a soft surface (fast trot to canter unridden; or HRs 110‐150 bpm) for >30 minutes, >3 times per week, while carefully monitoring for signs of lameness.
8. PHARMACOLOGIC AIDS
Metformin hydrochloride is commonly prescribed for managing ID in equids although it is only licensed for use in humans. Dosages range from 15 to 30 mg/kg q8‐12h PO, and the drug should ideally be administered 30‐60 minutes before feeding. An initial study administered metformin (15 mg/kg PO q12h) to 18 ponies and horses with ID and reported that basal insulin concentrations decreased over time.199 However, the oral bioavailability of metformin is poor in equids,200 and it does not have systemic effects on insulin sensitivity.201 Instead, metformin has a direct effect on the enterocyte in other species,202 and decreases enteric glucose absorption and consequently the insulin response to an OGT in horses and rats,203, 204 providing a possible explanation for the observed beneficial actions of metformin on insulin concentrations in the absence of systemic absorption. Indeed, response to metformin can be evaluated by performing an OST or OGT 30‐60 minutes after drug administration to assess the benefits in the individual patient,203 or by comparing insulin concentrations 2 and 4 hours after feeding with and without medication.
Levothyroxine might be prescribed for equids with increased adiposity to accelerate weight loss through increasing the metabolic rate, but diet and exercise changes should ideally be implemented at the same time. Administration of levothyroxine to healthy horses of 500 kg BM at dosages between 24 and 96 mg per day PO for up to 48 weeks was well tolerated, BM decreased, and insulin sensitivity increased.205 Levothyroxine treatment in horses with EMS has beneficial effects on BM and neck circumference after 48 mg levothyroxine/day PO for 6 months and then 72 mg levothyroxine/day for 3 months.206
There is a product available for use in horses in the United States (Thyro L; LLOYD, Inc, Shenandoah, Iowa) at a dosage of 0.1‐0.15 mg/kg levothyroxine sodium once daily PO until an appropriate body condition is attained or for a maximum of 6 months. Horses are weaned off levothyroxine at the end of the treatment period by decreasing the dose by half for 2 weeks and then a quarter for 2 weeks. In Europe, use of levothyroxine in equids requires prescription of products licensed in other species.
Pioglitazone is a thiazolidinedione antidiabetic drug that is prescribed for the management of type 2 DM in humans. This drug is not routinely used for managing ID in horses, as results from early equine studies were largely disappointing207, 208; however, findings from a recent study examining effects of pioglitazone (2 mg/kg PO q24 h for 28 days) on OST results and serum HMW adiponectin concentrations in healthy horses and ponies are more encouraging.209
Pergolide is a dopamine receptor agonist licensed in several countries for the treatment of PPID in horses (Prascend; Boehringer Ingelheim Vetmedica, Inc, Ingelheim, Germany). Medical management of PPID with pergolide typically improves insulin regulation in those horses affected by ID and it has been further proposed that pergolide might have an independent effect on insulin dynamics in horses. One study treated horses with clinical signs of EMS with 1 mg pergolide/day for 8 weeks and found improved OST results (D. McFarlane, personal communication), although it is difficult to rule out early PPID in those horses with EMS, so additional studies are required to confirm any specific effect of pergolide on ID. Several dopamine agonists improve insulin regulation and suppress insulin concentrations in other species,210 although no beneficial effect of cabergoline on ID in horses has been found.211
Sodium‐glucose co‐transporter 2 (SGLT2) inhibitors target receptors in the proximal tubule of the kidney to increase glucose loss in the urine.212 Preliminary studies have been performed to assess the safety and efficacy in horses, but further work should be done before more widespread use.213 In humans, SGLT2 inhibitors are administered once daily as tablets and hypoglycemia and urinary tract infections are the most common adverse events reported.
Sweet taste receptor (T1R2/3) inhibitors have been used to reduce the insulin and glucose responses to oral carbohydrates in other species, and evidence of some minor beneficial effects has been shown recently in horses as well.214
8.1. Key pharmacologic recommendations
Drug treatment of EMS should never be used as a substitute for diet and exercise interventions.
Because metformin appears to blunt postprandial increases in blood glucose and insulin, it may be useful for horses with severe ID while management changes are implemented, and in animals that remain insulin dysregulated even when managed optimally.
Levothyroxine may be used in weight loss‐resistant cases alongside dietary control and exercise.
Where PPID has been diagnosed, pergolide treatment is recommended to minimize the effects of PPID on ID.
9. MONITORING AND PREVENTION
9.1. Laminitis detection
All horses with EMS must be monitored frequently for the earliest clinical signs of laminitis, which are often seen when the horse is turned in tight circles on a hard surface or transitions from soft to hard ground. Digital pulse intensity increases as laminitis progresses. However, some equids have hoof changes consistent with lamellar damage without exhibiting lameness.69 In these cases, divergent hoof rings (“founder lines”) might be seen on the hoof wall or widening of the white line on the solar surface. Third phalanx remodeling, displacement, or rotation might be detected in the same horses when radiographs are taken of the feet. Ideally, evaluations should be performed every 6‐12 months to reassess the risk of laminitis. The minimum database for each visit includes an updated diet and exercise history, physical examination and body condition score, visual inspection of hooves for signs of laminitis, and endocrine tests (eg, OST). It is also crucial that preventive and corrective farriery is coordinated alongside veterinary care.215
9.2. Obesity control
Although diet and exercise recommendations might appear relatively straightforward, they can often be limited by poor compliance from horse owners and other practical barriers to implementation.216 Key factors in EMS management are both the tailoring of the program to individual owners' situations and monitoring the effect.217 A tendency for owners of obese horses to underestimate their horse's condition has been identified by several studies.27, 30, 32 Various reasons have been proposed to explain the apparent lack of recognition of obesity including lack of owner education.39 However, it should also be acknowledged that the high prevalence of equine obesity can lead to habituation and acceptance of the status as apparently normal. Additionally, perceived criticism and peer pressure from owners of overweight horses stabled on the same premises can present a barrier to other owners wishing to implement fat loss programs.216 Continual overt veterinary support and education are crucial to promote the success of advised management changes.
Body mass monitoring on a weighbridge is ideal although tapes or commercial applications, which consider belly, girth, and rump width are useful alternatives.164, 218 Body condition scoring to monitor success has some limitations because of the nonlinear relationship of total body fat with BCS when BCS is greater than 7/9,219 and variable reported changes in BCS after 6‐12 weeks of energy restriction.104, 151, 164 In the first week of energy restriction, higher BM losses are expected because of reduced gut fill after transition to restricted DMI164, 183 and beyond the first 12 weeks of dietary restriction, the same level of restriction, even if adjusted for BM, might not have the same effect because of adaptive suppression of resting metabolic rate.8 Where this is the case, the level of dietary restriction may need to be increased or metabolic rate increased, for example, by implementing an exercise program.
9.3. Endocrine monitoring
Body mass reduction is expected to improve ID,104, 152, 164 and laboratory testing is recommended to support the weight loss program and to confirm the degree of success in improving ID. The indication for dietary control should always be based on the degree of ID as well as BM, especially when used to guide return to pasture. For example, a horse with rapid improvements in ID might be able to resume restricted grazing relatively quickly and be maintained on long‐term minor restrictions with some seasonal changes in BCS permitted. In contrast, a horse with continued marked ID might require low NSC feeding and maintenance irrespective of changes in BM. Ideally, monitoring should include an OST or OGT or simply the insulin response to the prescribed feed (eg, an insulin sample taken 2 hours after the morning feed or 2 hours after being turned out on to pasture) to allow evaluation of the requirement for continued low NSC intake. The regular monitoring of serum TG concentrations during the weight loss program helps to detect hyperlipemia owing to a negative energy balance, especially in pregnant animals or in miniature breeds, Shetland ponies, and donkeys.
9.4. Key monitoring recommendations
Monitoring and support are crucial elements of EMS management because of poor compliance rates.
Initial veterinary reexaminations should be performed monthly after starting dietary restriction and then less frequently (3‐12 monthly intervals) once good progress is made.
Success should be judged on the basis of retesting for ID (especially using oral carbohydrate challenge tests) and not simply BM losses.
CONFLICT OF INTEREST DECLARATION
The authors provide the following declarations:
A.E. Durham is employed by the Liphook Equine Hospital that offers a commercial diagnostic service including endocrine testing of horses and has received travel expenses from Boehringer Ingelheim Vetmedica for meetings related to Equine Metabolic Syndrome.
N. Frank has received financial compensation for consultation on study design for Boehringer‐Ingelheim Vetmedica, Inc. and Kindred Biosciences, Inc. Boehringer‐Ingelheim Vetmedica, Inc. has also supported travel and accommodation expenses for Equine Endocrinology Group meetings.
C.M. McGowan is employed by the University of Liverpool which has received funding for research projects and consultancy related to Equine Metabolic Syndrome from Boehringer Ingelheim Vetmedica.
K. Feige is employed by the University of Veterinary Medicine, Hannover which has received funding for research grants related to Equine Metabolic Syndrome from Boehringer Ingelheim Vetmedica.
K. Fey is employed by the Justus‐Liebig‐University in Giessen/Germany and has received fees for consultancy, presentations as well as travel expenses from Boehringer Ingelheim Vetmedica for subjects related to equine endocrine diseases.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Durham AE, Frank N, McGowan CM, et al. ECEIM consensus statement on equine metabolic syndrome. J Vet Intern Med. 2019;33:335–349. 10.1111/jvim.15423
Consensus Statements of the European College of Equine Internal Medicine (ECEIM) provide the veterinary community with up‐to‐date information on the pathophysiology, diagnosis, and treatment of clinically important animal diseases. The ECEIM Board oversees selection of relevant topics, identification of panel members for each topic with the expertise to draft the statements, and other aspects of assuring the integrity of the process. The statements are derived from evidence‐based medicine whenever possible and the panel offers interpretive comments when such evidence is inadequate or contradictory. A draft is prepared by the panel, followed by solicitation of input by the ECEIM membership which may be incorporated into the statement. It is then submitted to the Journal of Veterinary Internal Medicine, where it is edited prior to publication. The authors are solely responsible for the content of the statements.
REFERENCES
- 1. Coffman JR, Colles CM. Insulin tolerance in laminitic ponies. Can J Comp Med. 1983;47(3):347‐351. [PMC free article] [PubMed] [Google Scholar]
- 2. Jeffcott LB, Field JR, McLean JG, O'Dea K. Glucose tolerance and insulin sensitivity in ponies and Standardbred horses. Equine Vet J. 1986;18(2):97‐101. [DOI] [PubMed] [Google Scholar]
- 3. Johnson PJ. The equine metabolic syndrome peripheral Cushing's syndrome. Vet Clin North Am Equine Pract. 2002;18(2):271‐293. [DOI] [PubMed] [Google Scholar]
- 4. Frank N, Geor RJ, Bailey SR, Durham AE, Johnson PJ, American College of Veterinary Internal Medicine . Equine metabolic syndrome. J Vet Intern Med. 2010;24(3):467‐475. [DOI] [PubMed] [Google Scholar]
- 5. Frank N, Tadros EM. Insulin dysregulation. Equine Vet J. 2014;46(1):103‐112. [DOI] [PubMed] [Google Scholar]
- 6. Treiber KH, Kronfeld DS, Hess TM, Byrd BM, Splan RK, Staniar WB. Evaluation of genetic and metabolic predispositions and nutritional risk factors for pasture‐associated laminitis in ponies. J Am Vet Med Assoc. 2006;228(10):1538‐1545. [DOI] [PubMed] [Google Scholar]
- 7. Carter RA, Treiber KH, Geor RJ, et al. Prediction of incipient pasture‐associated laminitis from hyperinsulinaemia, hyperleptinaemia and generalised and localised obesity in a cohort of ponies. Equine Vet J. 2009;41(2):171‐178. [DOI] [PubMed] [Google Scholar]
- 8. Argo CM, Curtis GC, Grove‐White D, et al. Weight loss resistance: a further consideration for the nutritional management of obese Equidae. Vet J. 2012;194(2):179‐188. [DOI] [PubMed] [Google Scholar]
- 9. Bailey SR, Habershon‐Butcher J, Ransom K, et al. Hypertension and insulin resistance in a mixed‐breed population of ponies predisposed to laminitis: further characterizing pre‐laminitic metabolic syndrome. Am J Vet Res. 2008;69(1):122‐129. [DOI] [PubMed] [Google Scholar]
- 10. Heliczer N, Gerber V, Bruckmaier R, van der Kolk JH, de Solis CN. Cardiovascular findings in ponies with equine metabolic syndrome. J Am Vet Med Assoc. 2017;250(9):1027‐1035. [DOI] [PubMed] [Google Scholar]
- 11. Menzies‐Gow NJ, Harris PA, Elliott J. Prospective cohort study evaluating risk factors for the development of pasture‐associated laminitis in the United Kingdom. Equine Vet J. 2017;49(3):300‐306. [DOI] [PubMed] [Google Scholar]
- 12. Morgan RA, McGowan TW, McGowan CM. Prevalence and risk factors for hyperinsulinaemia in ponies in Queensland, Australia. Aust Vet J. 2014;92(4):101‐106. [DOI] [PubMed] [Google Scholar]
- 13. Muno J, Gallatin L, Geor R, et al. Prevalence and risk factors for hyperinsulinaemia in clinically normal horses in central ohio. J Vet Intern Med. 2009;23(3):721. [Google Scholar]
- 14. Pleasant RS, Suagee JK, Thatcher CD, Elvinger F, Geor RJ. Adiposity, plasma insulin, leptin, lipids, and oxidative stress in mature light breed horses. J Vet Intern Med. 2013;27(3):576‐582. [DOI] [PubMed] [Google Scholar]
- 15. Karikoski NP, Horn I, McGowan TW, McGowan CM. The prevalence of endocrinopathic laminitis among horses presented for laminitis at a first‐opinion/referral equine hospital. Domest Anim Endocrinol. 2011;41(3):111‐117. [DOI] [PubMed] [Google Scholar]
- 16. Bamford NJ, Potter SJ, Harris PA, Bailey SR. Breed differences in insulin sensitivity and insulinemic responses to oral glucose in horses and ponies of moderate body condition score. Domest Anim Endocrinol. 2014;47:101‐107. [DOI] [PubMed] [Google Scholar]
- 17. Stewart‐Hunt L, Geor RJ, McCutcheon LJ. Effects of short‐term training on insulin sensitivity and skeletal muscle glucose metabolism in standardbred horses. Equine Vet J Suppl. 2006;36:226‐232. [DOI] [PubMed] [Google Scholar]
- 18. McCutcheon LJ, Geor RJ, Hinchcliff KW. Changes in skeletal muscle GLUT4 content and muscle membrane glucose transport following 6 weeks of exercise training. Equine Vet J Suppl. 2002;34:199‐204. [DOI] [PubMed] [Google Scholar]
- 19. Liburt NR, Fugaro MN, Wunderlich EK, et al. The effect of exercise training on insulin sensitivity and fat and muscle tissue cytokine profiles of old and young standardbred mares. J Equine Vet Sci. 2011;31:237‐238. [Google Scholar]
- 20. Hart KA, Wochele DM, Norton NA, McFarlane D, Wooldridge AA, Frank N. Effect of age, season, body condition, and endocrine status on serum free cortisol fraction and insulin concentration in horses. J Vet Intern Med. 2016;30(2):653‐663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nielsen BD, O'Connor‐Robison CI, Spooner HS, Shelton J. Glycemic and insulinemic responses are affected by age of horse and method of feed processing. J Equine Vet Sci. 2010;30:249‐258. [Google Scholar]
- 22. Rapson JL, Schott HC 2nd, Nielsen BD, et al. Effects of age and diet on glucose and insulin dynamics in the horse. Equine Vet J. 2018;50(5):690‐696. [DOI] [PubMed] [Google Scholar]
- 23. Kawasumi K, Yamamoto M, Koide M, et al. Aging effect on plasma metabolites and hormones concentrations in riding horses. Open Vet J. 2015;5(2):154‐157. [PMC free article] [PubMed] [Google Scholar]
- 24. Wray H, Elliott J, Bailey SR, Harris PA, Menzies‐Gow NJ. Plasma concentrations of inflammatory markers in previously laminitic ponies. Equine Vet J. 2013;45(5):546‐551. [DOI] [PubMed] [Google Scholar]
- 25. Cartmill JA, Thompson DL Jr, Del Vecchio RP, et al. Leptin secretion in horses: effects of dexamethasone, gender, and testosterone. Domest Anim Endocrinol. 2006;31(2):197‐210. [DOI] [PubMed] [Google Scholar]
- 26. Buff PR, Dodds AC, Morrison CD, et al. Leptin in horses: tissue localization and relationship between peripheral concentrations of leptin and body condition. J Anim Sci. 2002;80(11):2942‐2948. [DOI] [PubMed] [Google Scholar]
- 27. Stephenson HM, Green MJ, Freeman SL. Prevalence of obesity in a population of horses in the UK. Vet Rec. 2011;168(5):131. [DOI] [PubMed] [Google Scholar]
- 28. Giles SL, Rands SA, Nicol CJ, Harris PA. Obesity prevalence and associated risk factors in outdoor living domestic horses and ponies. PeerJ. 2014;2:e299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Robin CA, Ireland JL, Wylie CE, Collins SN, Verheyen KLP, Newton JR. Prevalence of and risk factors for equine obesity in Great Britain based on owner‐reported body condition scores. Equine Vet J. 2015;47(2):196‐201. [DOI] [PubMed] [Google Scholar]
- 30. Wyse CA, McNie KA, Tannahil VJ, et al. Prevalence of obesity in riding horses in Scotland. Vet Rec. 2008;162(18):590‐591. [DOI] [PubMed] [Google Scholar]
- 31. Harker IJ, Harris PA, Barfoot CF. The body condition score of leisure horses competing at an unaffiliated championship in the UK. J Equine Vet Sci. 2011;31:253‐254. [Google Scholar]
- 32. Jensen RB, Danielsen SH, Tauson AH. Body condition score, morphometric measurements and estimation of body weight in mature Icelandic horses in Denmark. Acta Vet Scand. 2016;58(Suppl 1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Christie JL, Hewson CJ, Riley CB, Mcniven MA, Dohoo IR, Bate LA. Demographics, management, and welfare of nonracing horses in Prince Edward Island. Can Vet J. 2004;45(12):1004‐1011. [PMC free article] [PubMed] [Google Scholar]
- 34. Kosolofski HR, Gow SP, Robinson KA. Prevalence of obesity in the equine population of Saskatoon and surrounding area. Can Vet J. 2017;58(9):967‐970. [PMC free article] [PubMed] [Google Scholar]
- 35. Potter SJ, Bamford NJ, Harris PA, Bailey SR. Prevalence of obesity and owners' perceptions of body condition in pleasure horses and ponies in south‐eastern Australia. Aust Vet J. 2016;94(11):427‐432. [DOI] [PubMed] [Google Scholar]
- 36. Thatcher CD, Pleasant RS, Geor RJ, Elvinger F. Prevalence of overconditioning in mature horses in southwest Virginia during the summer. J Vet Intern Med. 2012;26(6):1413‐1418. [DOI] [PubMed] [Google Scholar]
- 37. Giles SL, Nicol CJ, Rands SA, Harris PA. Assessing the seasonal prevalence and risk factors for nuchal crest adiposity in domestic horses and ponies using the Cresty Neck Score. BMC Vet Res. 2015;11:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Giles SL, Nicol CJ, Harris PA, Rands SA. Dominance rank is associated with body condition in outdoor‐living domestic horses (Equus caballus). Appl Anim Behav Sci. 2015;166:71‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hitchens PL, Hultgren J, Frossling J, et al. Prevalence and risk factors for overweight horses at premises in Sweden assessed using official animal welfare control data. Acta Vet Scand. 2016;58(Suppl 1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Place NJ, McGowan CM, Lamb SV, et al. Seasonal variation in serum concentrations of selected metabolic hormones in horses. J Vet Intern Med. 2010;24(3):650‐654. [DOI] [PubMed] [Google Scholar]
- 41. Borer‐Weir KE, Menzies‐Gow NJ, Bailey SR, Harris PA, Elliott J. Seasonal and annual influence on insulin and cortisol results from overnight dexamethasone suppression tests in normal ponies and ponies predisposed to laminitis. Equine Vet J. 2013;45(6):688‐693. [DOI] [PubMed] [Google Scholar]
- 42. George LA, Staniar WB, Cubitt TA, Treiber KH, Harris PA, Geor RJ. Evaluation of the effects of pregnancy on insulin sensitivity, insulin secretion, and glucose dynamics in Thoroughbred mares. Am J Vet Res. 2011;72(5):666‐674. [DOI] [PubMed] [Google Scholar]
- 43. Schreiber CM, Stewart AJ, Kwessi E, et al. Seasonal variation in results of diagnostic tests for pituitary pars intermedia dysfunction in older, clinically normal geldings. J Am Vet Med Assoc. 2012;241(2):241‐248. [DOI] [PubMed] [Google Scholar]
- 44. Selim S, Elo K, Jaakkola S, et al. Relationships among body condition, insulin resistance and subcutaneous adipose tissue gene expression during the grazing season in mares. PLoS One. 2015;10(5):e0125968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Buff PR, Messer NT, Cogswell AM, et al. Seasonal and pulsatile dynamics of thyrotropin and leptin in mares maintained under a constant energy balance. Domest Anim Endocrinol. 2007;33(4):430‐436. [DOI] [PubMed] [Google Scholar]
- 46. Robie SM, Janson CH, Smith SC, O'Connor JT Jr. Equine serum lipids: serum lipids and glucose in Morgan and Thoroughbred horses and Shetland ponies. Am J Vet Res. 1975;36(12):1705‐1708. [PubMed] [Google Scholar]
- 47. Fowden AL, Comline RS, Silver M. Insulin secretion and carbohydrate metabolism during pregnancy in the mare. Equine Vet J. 1984;16(4):239‐246. [DOI] [PubMed] [Google Scholar]
- 48. Watson TD, Burns L, Packard CJ, Shepherd J. Effects of pregnancy and lactation on plasma lipid and lipoprotein concentrations, lipoprotein composition and post‐heparin lipase activities in Shetland pony mares. J Reprod Fertil. 1993;97(2):563‐568. [DOI] [PubMed] [Google Scholar]
- 49. Smith S, Marr CM, Dunnett C, Menzies‐Gow NJ. The effect of mare obesity and endocrine function on foal birthweight in Thoroughbreds. Equine Vet J. 2017;49(4):461‐466. [DOI] [PubMed] [Google Scholar]
- 50. Petrakis D, Vassilopoulou L, Mamoulakis C, et al. Endocrine disruptors leading to obesity and related diseases. Int J Environ Res Public Health. 2017;14(10):E1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Durward‐Akhurst SA, Norton EM, Schultz NS, et al. 2017. The association between endocrine disrupting chemicals and equine metabolic syndrome. Dorothy Russell Havemeyer Foundation Equine Endocrinology Symposium, Miami, FL, p. 34.
- 52. de Laat MA, McGree JM, Sillence MN. Equine hyperinsulinemia: investigation of the enteroinsular axis during insulin dysregulation. Am J Physiol Endocrinol Metab. 2016;310(1):E61‐E72. [DOI] [PubMed] [Google Scholar]
- 53. Jacob SI, Geor RJ, Weber PSD, Harris PA, McCue ME. Effect of age and dietary carbohydrate profiles on glucose and insulin dynamics in horses. Equine Vet J. 2018;50(2):249‐254. [DOI] [PubMed] [Google Scholar]
- 54. Duhlmeier R, Deegen E, Fuhrmann H, et al. Glucose‐dependent insulinotropic polypeptide (GIP) and the enteroinsular axis in equines (Equus caballus). Comp Biochem Physiol A Mol Integr Physiol. 2001;129(2–3):563‐575. [DOI] [PubMed] [Google Scholar]
- 55. Bamford NJ, Baskerville CL, Harris PA, Bailey SR. Postprandial glucose, insulin, and glucagon‐like peptide‐1 responses of different equine breeds adapted to meals containing micronized maize. J Anim Sci. 2015;93(7):3377‐3383. [DOI] [PubMed] [Google Scholar]
- 56. Chameroy KA, Frank N, Elliott SB, Boston RC. Comparison of plasma active glucagon‐like peptide 1 concentrations in normal horses and those with equine metabolic syndrome and in horses placed on a high‐grain diet. J Equine Vet Sci. 2016;40:16‐25. [Google Scholar]
- 57. Frank N, Walsh DM. Repeatability of oral sugar test results, glucagon‐like peptide‐1 measurements, and serum high‐molecular‐weight adiponectin concentrations in horses. J Vet Intern Med. 2017;31(4):1178‐1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. de Laat MA, Fitzgerald DM, Sillence MN, Spence RJ. Glucagon‐like peptide‐2: a potential role in equine insulin dysregulation. Equine Vet J. 2018;50(6):842‐847. [DOI] [PubMed] [Google Scholar]
- 59. Bamford NJ, Potter SJ, Baskerville CL, Harris PA, Bailey SR. Effect of increased adiposity on insulin sensitivity and adipokine concentrations in different equine breeds adapted to cereal‐rich or fat‐rich meals. Vet J. 2016;214:14‐20. [DOI] [PubMed] [Google Scholar]
- 60. Treiber KH, Boston RC, Kronfeld DS, Staniar WB, Harris PA. Insulin resistance and compensation in Thoroughbred weanlings adapted to high‐glycemic meals. J Anim Sci. 2005;83(10):2357‐2364. [DOI] [PubMed] [Google Scholar]
- 61. Quinn RW, Burke AO, Hartsock TG, et al. Insulin sensitivity in Thoroughbred geldings: effect of weight gain, diet, and exercise on insulin sensitivity in Thoroughbred geldings. Equine Vet Sci. 2008;28:728‐738. [Google Scholar]
- 62. Hoffman RM, Boston RC, Stefanovski D, Kronfeld DS, Harris PA. Obesity and diet affect glucose dynamics and insulin sensitivity in Thoroughbred geldings. J Anim Sci. 2003;81:2333‐2342. [DOI] [PubMed] [Google Scholar]
- 63. Bamford NJ, Potter SJ, Harris PA, Bailey SR. Effect of increased adiposity on insulin sensitivity and adipokine concentrations in horses and ponies fed a high fat diet, with or without a once daily high glycaemic meal. Equine Vet J. 2016;48(3):368‐373. [DOI] [PubMed] [Google Scholar]
- 64. Toth F, Frank N, Martin‐Jimenez T, et al. Measurement of C‐peptide concentrations and responses to somatostatin, glucose infusion, and insulin resistance in horses. Equine Vet J. 2010;42(2):149‐155. [DOI] [PubMed] [Google Scholar]
- 65. Asplin KE, Sillence MN, Pollitt CC, McGowan CM. Induction of laminitis by prolonged hyperinsulinaemia in clinically normal ponies. Vet J. 2007;174(3):530‐535. [DOI] [PubMed] [Google Scholar]
- 66. de Laat MA, McGowan CM, Sillence MN, Pollitt CC. Equine laminitis: induced by 48 h hyperinsulinaemia in Standardbred horses. Equine Vet J. 2010;42(2):129‐135. [DOI] [PubMed] [Google Scholar]
- 67. de Laat MA, Sillence MN, McGowan CM, Pollitt CC. Continuous intravenous infusion of glucose induces endogenous hyperinsulinaemia and lamellar histopathology in Standardbred horses. Vet J. 2012;191(3):317‐322. [DOI] [PubMed] [Google Scholar]
- 68. Walsh DM, McGowan CM, McGowan T, et al. Correlation of plasma insulin concentration with laminitis score in a field study of equine cushing's disease and equine metabolic syndrome. J Equine Vet Sci. 2009;29(2):87‐94. [Google Scholar]
- 69. Karikoski NP, McGowan CM, Singer ER, et al. Pathology of natural cases of equine endocrinopathic laminitis associated with hyperinsulinemia. Vet Pathol. 2015;52(5):945‐956. [DOI] [PubMed] [Google Scholar]
- 70. Patterson‐Kane JC, Karikoski NP, McGowan CM. Paradigm shifts in understanding equine laminitis. Vet J. 2018;231:33‐40. [DOI] [PubMed] [Google Scholar]
- 71. de Laat MA, Patterson‐Kane JC, Pollitt CC, Sillence MN, McGowan CM. Histological and morphometric lesions in the pre‐clinical, developmental phase of insulin‐induced laminitis in Standardbred horses. Vet J. 2013;195(3):305‐312. [DOI] [PubMed] [Google Scholar]
- 72. Karikoski NP, Patterson‐Kane JC, Asplin KE, et al. Morphological and cellular changes in secondary epidermal laminae of horses with insulin‐induced laminitis. Am J Vet Res. 2014;75(2):161‐168. [DOI] [PubMed] [Google Scholar]
- 73. Asplin KE, Curlewis JD, McGowan CM, et al. Glucose transport in the equine hoof. Equine Vet J. 2011;43(2):196‐201. [DOI] [PubMed] [Google Scholar]
- 74. de Laat MA, Kyaw‐Tanner MT, Sillence MN, McGowan CM, Pollitt CC. Advanced glycation endproducts in horses with insulin‐induced laminitis. Vet Immunol Immunopathol. 2012;145(1–2):395‐401. [DOI] [PubMed] [Google Scholar]
- 75. de Laat MA, Kyaw‐Tanner MT, Nourian AR, McGowan CM, Sillence MN, Pollitt CC. The developmental and acute phases of insulin‐induced laminitis involve minimal metalloproteinase activity. Vet Immunol Immunopathol. 2011;140(3–4):275‐281. [DOI] [PubMed] [Google Scholar]
- 76. de Laat MA, Pollitt CC, Walsh DM, McGowan CM, Sillence MN. Persistent digital hyperthermia over a 48 h period does not induce laminitis in horses. Vet J. 2012;192(3):435‐440. [DOI] [PubMed] [Google Scholar]
- 77. Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113(15):1888‐1904. [DOI] [PubMed] [Google Scholar]
- 78. Venugopal CS, Eades S, Holmes EP, Beadle RE. Insulin resistance in equine digital vessel rings: an in vitro model to study vascular dysfunction in equine laminitis. Equine Vet J. 2011;43(6):744‐749. [DOI] [PubMed] [Google Scholar]
- 79. Wooldridge AA, Waguespack RW, Schwartz DD, Venugopal CS, Eades SC, Beadle RE. Vasorelaxation responses to insulin in laminar vessel rings from healthy, lean horses. Vet J. 2014;202(1):83‐88. [DOI] [PubMed] [Google Scholar]
- 80. Morgan RA, Keen JA, Walker BR, Hadoke PW. Vascular dysfunction in horses with endocrinopathic laminitis. PLoS One. 2016;11(9):e0163815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gauff F, Patan‐Zugaj B, Licka TF. Hyperinsulinaemia increases vascular resistance and endothelin‐1 expression in the equine digit. Equine Vet J. 2013;45(5):613‐618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. de Laat MA, Pollitt CC, Kyaw‐Tanner MT, McGowan CM, Sillence MN. A potential role for lamellar insulin‐like growth factor‐1 receptor in the pathogenesis of hyperinsulinaemic laminitis. Vet J. 2013;197(2):302‐306. [DOI] [PubMed] [Google Scholar]
- 83. Burns TA, Watts MR, Weber PS, Mccutcheon LJ, Geor RJ, Belknap JK. Distribution of insulin receptor and insulin‐like growth factor‐1 receptor in the digital laminae of mixed‐breed ponies: an immunohistochemical study. Equine Vet J. 2013;45(3):326‐332. [DOI] [PubMed] [Google Scholar]
- 84. Lane HE, Burns TA, Hegedus OC, et al. Lamellar events related to insulin‐like growth factor‐1 receptor signalling in two models relevant to endocrinopathic laminitis. Equine Vet J. 2017;49(5):643‐654. [DOI] [PubMed] [Google Scholar]
- 85. Geurts L, Neyrinck AM, Delzenne NM, Knauf C, Cani PD. Gut microbiota controls adipose tissue expansion, gut barrier and glucose metabolism: novel insights into molecular targets and interventions using prebiotics. Benef Microbes. 2014;5(1):3‐17. [DOI] [PubMed] [Google Scholar]
- 86. Kovatcheva‐Datchary P, Arora T. Nutrition, the gut microbiome and the metabolic syndrome. Best Pract Res Clin Gastroenterol. 2013;27(1):59‐72. [DOI] [PubMed] [Google Scholar]
- 87. Backhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet‐induced obesity in germ‐free mice. Proc Natl Acad Sci U S A. 2007;104(3):979‐984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Elzinga SE, Weese JS, Adams AA. Comparison of the fecal microbiota in horses with equine metabolic syndrome and metabolically normal controls fed a similar all‐forage diet. J Equine Vet Sci. 2016;44:9‐16. [Google Scholar]
- 89. Zhang X, Shen D, Fang Z, et al. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS One. 2013;8(8):e71108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Staniar WB, Neuendorf LE, Brooks SA. Preliminary investigation of the changes in fecal streptococcal population due to diet and time of day in horses. J Equine Vet Sci. 2016;46:18‐23. [Google Scholar]
- 91. Respondek F, Goachet A‐G, Rudeaux F, Julliand V. Effects of short‐chain fructo‐oligosaccharides on the microbial and biochemical profile of different segments of the gastro‐intestinal tract in horses. Pferdeheilkunde. 2007;23(2):146‐150. [Google Scholar]
- 92. McCue M, Geor RJ, Schultz N. Equine metabolic syndrome: a complex disease influenced by genetics and the environment. J Equine Vet Sci. 2015;35(5):367‐375. [Google Scholar]
- 93. Selim S, Elo K, Jaakkola S, et al. Relationships among body condition, insulin resistance and subcutaneous adipose tissue gene expression during the grazing season in mares. PLoS One. 2015;10(5):e0125968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Radin MJ, Sharkey LC, Holycross BJ. Adipokines: a review of biological and analytical principles and an update in dogs, cats, and horses. Vet Clin Pathol. 2009;38(2):136‐156. [DOI] [PubMed] [Google Scholar]
- 95. Kearns CF, McKeever KH, Roegner V, et al. Adiponectin and leptin are related to fat mass in horses. Vet J. 2006;172(3):460‐465. [DOI] [PubMed] [Google Scholar]
- 96. Van Weyenberg S, Buyse J, Kalmar ID, et al. Voluntary feed intake and leptin sensitivity in ad libitum fed obese ponies following a period of restricted feeding: a pilot study. J Anim Physiol Anim Nutr (Berl). 2013;97(4):624‐631. [DOI] [PubMed] [Google Scholar]
- 97. Whitehead JP, Richards AA, Hickman IJ, Macdonald GA, Prins JB. Adiponectin—a key adipokine in the metabolic syndrome. Diabetes Obes Metab. 2006;8(3):264‐280. [DOI] [PubMed] [Google Scholar]
- 98. Basinska K, Marycz K, Sieszek A, Nicpon J. The production and distribution of IL‐6 and TNF‐a in subcutaneous adipose tissue and their correlation with serum concentrations in Welsh ponies with equine metabolic syndrome. J Vet Sci. 2015;16(1):113‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Vick MM, Adams AA, Murphy BA, et al. Relationships among inflammatory cytokines, obesity, and insulin sensitivity in the horse. J Anim Sci. 2007;85(5):1144‐1155. [DOI] [PubMed] [Google Scholar]
- 100. Suagee JK, Corl BA, Crisman MV, Hulver MW, McCutcheon LJ, Geor RJ. Effects of acute hyperinsulinemia on inflammatory proteins in horses. Vet Immunol Immunopathol. 2011;142(3–4):141‐146. [DOI] [PubMed] [Google Scholar]
- 101. Waller AP, Kohler K, Burns TA, Mudge MC, Belknap JK, Lacombe VA. Naturally occurring compensated insulin resistance selectively alters glucose transporters in visceral and subcutaneous adipose tissues without change in AS160 activation. Biochim Biophys Acta. 2011;1812(9):1098‐1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Waller AP, Huettner L, Kohler K, Lacombe VA. Novel link between inflammation and impaired glucose transport during equine insulin resistance. Vet Immunol Immunopathol. 2012;149(3–4):208‐215. [DOI] [PubMed] [Google Scholar]
- 103. Burns TA, Geor RJ, Mudge MC, McCutcheon LJ, Hinchcliff KW, Belknap JK. Proinflammatory cytokine and chemokine gene expression profiles in subcutaneous and visceral adipose tissue depots of insulin‐resistant and insulin‐sensitive light breed horses. J Vet Intern Med. 2010;24(4):932‐939. [DOI] [PubMed] [Google Scholar]
- 104. Ungru J, Bluher M, Coenen M, et al. Effects of body weight reduction on blood adipokines and subcutaneous adipose tissue adipokine mRNA expression profiles in obese ponies. Vet Rec. 2012;171(21):528. [DOI] [PubMed] [Google Scholar]
- 105. Bruynsteen L, Erkens T, Peelman LJ, et al. Expression of inflammation‐related genes is associated with adipose tissue location in horses. BMC Vet Res. 2013;9:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lewis SL, Holl HM, Streeter C, et al. Genomewide association study reveals a risk locus for equine metabolic syndrome in the Arabian horse. J Anim Sci. 2017;95(3):1071‐1079. [DOI] [PubMed] [Google Scholar]
- 107. Norton EM, Aliva F, Schultz NS, et al. Identification of a genetic locus associated with height and metabolic traits in Welsh ponies. J Equine Vet Sci. 2017;52:73‐74. [Google Scholar]
- 108. Schultz N, McCue M, Martinson N, et al. Characterization of the equine metabolic syndrome phenotype—part I: biochemistry, morphometrics and breed comparisons. J Vet Intern Med. 2012;26:767. [Google Scholar]
- 109. Robles M, Gautier C, Mendoza L, et al. Maternal nutrition during pregnancy affects testicular and bone development, glucose metabolism and response to overnutrition in weaned horses up to two years. PLoS One. 2017;12(1):e0169295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Robles M, Nouveau E, Gautier C, et al. Maternal obesity increases insulin resistance, low‐grade inflammation and osteochondrosis lesions in foals and yearlings until 18 months of age. PLoS One. 2018;13(1):e0190309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Warnken T, Huber K, Feige K. Comparison of three different methods for the quantification of equine insulin. BMC Vet Res. 2016;12(1):196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Carslake HB, Pinchbeck GL, McGowan CM. Evaluation of a chemiluminescent immunoassay for measurement of equine insulin. J Vet Intern Med. 2017;31(2):568‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Tinworth KD, Wynn PC, Boston RC, et al. Evaluation of commercially available assays for the measurement of equine insulin. Dom Anim Endocrinol. 2011;41(2):81‐90. [DOI] [PubMed] [Google Scholar]
- 114. Borer‐Weir KE, Bailey SR, Menzies‐Gow NJ, Harris PA, Elliott J. Evaluation of a commercially available radioimmunoassay and species‐specific ELISAs for measurement of high concentrations of insulin in equine serum. Am J Vet Res. 2012;73(10):1596‐1602. [DOI] [PubMed] [Google Scholar]
- 115. Banse HE, McCann J, Yang F, Wagg C, McFarlane D. Comparison of two methods for measurement of equine insulin. J Vet Diagn Invest. 2014;26(4):527‐530. [DOI] [PubMed] [Google Scholar]
- 116. Pratt SE, Geor RJ, McCutcheon LJ. Repeatability of 2 methods for assessment of insulin sensitivity and glucose dynamics in horses. J Vet Intern Med. 2005;19(6):883‐888. [DOI] [PubMed] [Google Scholar]
- 117. Smith S, Harris PA, Menzies‐Gow NJ. Comparison of the in‐feed glucose test and the oral sugar test. Equine Vet J. 2016;48(2):224‐227. [DOI] [PubMed] [Google Scholar]
- 118. Pratt‐Phillips SE, Geor RJ, McCutcheon LJ. Comparison among the euglycemic‐hyperinsulinemic clamp, insulin‐modified frequently sampled intravenous glucose tolerance test, and oral glucose tolerance test for assessment of insulin sensitivity in healthy Standardbreds. Am J Vet Res. 2015;76(1):84‐91. [DOI] [PubMed] [Google Scholar]
- 119. Schuver A, Frank N, Chameroy KA, Elliott SB. Assessment of insulin and glucose dynamics by using an oral sugar test in horses. J Equine Vet Sci. 2014;34(4):465‐470. [Google Scholar]
- 120. Dunbar LK, Mielnicki KA, Dembek KA, Toribio RE, Burns TA. Evaluation of four diagnostic tests for insulin dysregulation in adult light‐breed horses. J Vet Intern Med. 2016;30(3):885‐891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Banse HE, McFarlane D. Comparison of three methods for evaluation of equine insulin regulation in horses of varied body condition score. J Equine Vet Sci. 2014;34(6):742‐748. [Google Scholar]
- 122. Bertin FR, Taylor SD, Bianco AW, Sojka‐Kritchevsky JE. The effect of fasting duration on baseline blood glucose concentration, blood insulin concentration, glucose/insulin ratio, oral sugar test, and insulin response test results in horses. J Vet Intern Med. 2016;30(5):1726‐1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Knowles EJ, Harris PA, Elliott J, Menzies‐Gow NJ. Use of the oral sugar test in ponies when performed with or without prior fasting. Equine Vet J. 2017;49(4):519‐524. [DOI] [PubMed] [Google Scholar]
- 124. Toth B, Auth A, Rompos L, Bakos Z. Effect of feed deprivation on selected parameters of lipid mobilisation and hepatic function in healthy Akhal Teke horses. Equine Vet J. 2018;50(1):98‐103. [DOI] [PubMed] [Google Scholar]
- 125. Brojer J, Lindase S, Hedenskog J, et al. Repeatability of the combined glucose‐insulin tolerance test and the effect of a stressor before testing in horses of 2 breeds. J Vet Intern Med. 2013;27(6):1543‐1550. [DOI] [PubMed] [Google Scholar]
- 126. Forhead AJ, Dobson H. Plasma glucose and cortisol responses to exogenous insulin in fasted donkeys. Res Vet Sci. 1997;62(3):265‐269. [DOI] [PubMed] [Google Scholar]
- 127. Köller G, Bassewitz K, Schusser GF. Referenzbereiche von Insulin, insulinähnlichem Wachstumsfaktor 1 (IGF‐1) und adrenokortikotropem Hormon bei Ponys. Tierärztl Prax. 2016;44(1):19‐25. [DOI] [PubMed] [Google Scholar]
- 128. Carslake HB, Argo CM, Pinchbeck GL, et al. Insulinaemic and glycaemic responses to three forages in ponies. Vet J. 2018;235:83‐89. [DOI] [PubMed] [Google Scholar]
- 129. Durham AE, Hughes KJ, Cottle HJ, et al. Type 2 diabetes mellitus with pancreatic β cell dysfunction in 3 horses confirmed with minimal model analysis. Equine Vet J. 2009;41(9):924‐929. [DOI] [PubMed] [Google Scholar]
- 130. Frank N, Elliott SB, Brandt LE, Keisler DH. Physical characteristics, blood hormone concentrations, and plasma lipid concentrations in obese horses with insulin resistance. J Am Vet Med Assoc. 2006;228(9):1383‐1390. [DOI] [PubMed] [Google Scholar]
- 131. Waki H, Yamauchi T, Kamon J, et al. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278(41):40352‐40363. [DOI] [PubMed] [Google Scholar]
- 132. Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116(7):1784‐1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Acharya SD, Evans RW, Brooks MM, Linkov F, Burke LE. Total and high‐molecular‐weight adiponectin levels in relation to insulin resistance among overweight/obese adults. Cent Asian J Glob Health. 2013;2(2):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Wooldridge AA, Edwards HG, Plaisance EP, et al. Evaluation of high‐molecular weight adiponectin in horses. Am J Vet Res. 2012;73(8):1230‐1240. [DOI] [PubMed] [Google Scholar]
- 135. Menzies‐Gow NJ, Knowles EJ, Rogers I, Rendle DI. Validity and application of immunoturbidimetric and enzyme‐linked immunosorbent assays for the measurement of adiponectin concentration in ponies. Equine Vet J. 2018;51(1):33‐37. [DOI] [PubMed] [Google Scholar]
- 136. Wilcox G. Insulin and insulin resistance. Clin Biochem Rev. 2005;26(2):19‐39. [PMC free article] [PubMed] [Google Scholar]
- 137. Kjems LL, Christiansen E, Vølund A, et al. Validation of methods for measurement of insulin secretion in humans in vivo. Diabetes. 2000;49(4):580‐588. [DOI] [PubMed] [Google Scholar]
- 138. de Laat MA, van Haeften JJ, Sillence MN. The effect of oral and intravenous dextrose on C‐peptide secretion in ponies. J Anim Sci. 2016;94(2):574‐580. [DOI] [PubMed] [Google Scholar]
- 139. Chameroy K, Frank N, Schuver A. Plasma glucagon‐like peptide 1 (Glp‐1) concentrations in response to an oral sugar test in healthy and insulin‐resistant horses. J Vet Intern Med. 2010;24:780‐780. [Google Scholar]
- 140. Bailey SR, Menzies‐Gow NJ, Harris PA, et al. Effect of dietary fructans and dexamethasone administration on the insulin response of ponies predisposed to laminitis. J Am Vet Med Assoc. 2007;231(9):1365‐1373. [DOI] [PubMed] [Google Scholar]
- 141. Frank N, Geor R. Current best practice in clinical management of equine endocrine patients. Equine Vet Educ. 2014;26(1):6‐9. [Google Scholar]
- 142. Meier AD, de Laat MA, Reiche DB, et al. The oral glucose test predicts laminitis risk in ponies fed a diet high in nonstructural carbohydrates. Domest Anim Endocrinol. 2018;63:1‐9. [DOI] [PubMed] [Google Scholar]
- 143. Kronfeld DS, Treiber KH, Geor RJ. Comparison of nonspecific indications and quantitative methods for the assessment of insulin resistance in horses and ponies. J Am Vet Med Assoc. 2005;226(5):712‐719. [DOI] [PubMed] [Google Scholar]
- 144. de Laat MA, Sillence MN. The repeatability of an oral glucose test in ponies. Equine Vet J. 2017;49(2):238‐243. [DOI] [PubMed] [Google Scholar]
- 145. Bertin FR, de Laat MA. The diagnosis of equine insulin dysregulation. Equine Vet J. 2017;49(5):570‐576. [DOI] [PubMed] [Google Scholar]
- 146. Warnken T, Delarocque J, Schumacher S, Huber K, Feige K. Retrospective analysis of insulin responses to standard dosed oral glucose tests (OGTs) via naso‐gastric tubing towards definition of an objective cut‐off value. Acta Vet Scand. 2018;60(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Jocelyn NA, Harris PA, Menzies‐Gow NJ. Effect of varying the dose of corn syrup on the insulin and glucose response to the oral sugar test. Equine Vet J. 2018;50(6):836‐841. [DOI] [PubMed] [Google Scholar]
- 148. Frank N. Equine metabolic syndrome. Vet Clin North Am Equine Pract. 2011;27(1):73‐92. [DOI] [PubMed] [Google Scholar]
- 149. Manfredi JM 2016. Identifying breed differences in insulin dynamics, skeletal muscle and adipose tissue histology and biology. Comparative Medicine and Integrative Biology—Doctor of Philosophy, Michigan State University.
- 150. Lindase S, Nostell K, Brojer J. A modified oral sugar test for evaluation of insulin and glucose dynamics in horses. Acta Vet Scand. 2016;58(Suppl 1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. McGowan CM, Dugdale AH, Pinchbeck GL, Argo CM. Dietary restriction in combination with a nutraceutical supplement for the management of equine metabolic syndrome in horses. Vet J. 2013;196(2):153‐159. [DOI] [PubMed] [Google Scholar]
- 152. Morgan RA, Keen JA, McGowan CM. Treatment of equine metabolic syndrome: a clinical case series. Equine Vet J. 2016;48(4):422‐426. [DOI] [PubMed] [Google Scholar]
- 153. Caltabilota TJ, Thompson DL, Clavier SE, et al. Evaluation of the combined intravenous glucose and insulin test for assessing insulin sensitivity. J Equine Vet Sci. 2009;29(5):409‐410. [Google Scholar]
- 154. Caltabilota TJ, Earl LR, Thompson DL Jr, Clavier SE, Mitcham PB. Hyperleptinemia in mares and geldings: assessment of insulin sensitivity from glucose responses to insulin injection. J Anim Sci. 2010;88(9):2940‐2949. [DOI] [PubMed] [Google Scholar]
- 155. Bertin FR, Sojka‐Kritchevsky JE. Comparison of a 2‐step insulin‐response test to conventional insulin‐sensitivity testing in horses. Domestic Anim Endocrinol. 2013;44(1):19‐25. [DOI] [PubMed] [Google Scholar]
- 156. Eiler H, Frank N, Andrews FM, Oliver JW, Fecteau KA. Physiologic assessment of blood glucose homeostasis via combined intravenous glucose and insulin testing in horses. Am J Vet Res. 2005;66(9):1598‐1604. [DOI] [PubMed] [Google Scholar]
- 157. McGowan TW, Pinchbeck GP, McGowan CM. Prevalence, risk factors and clinical signs predictive for equine pituitary pars intermedia dysfunction in aged horses. Equine Vet J. 2013;45(1):74‐79. [DOI] [PubMed] [Google Scholar]
- 158. McGowan CM, Frost R, Pfeiffer DU, Neiger R. Serum insulin concentrations in horses with equine Cushing's syndrome: response to a cortisol inhibitor and prognostic value. Equine Vet J. 2004;36(3):295‐298. [DOI] [PubMed] [Google Scholar]
- 159. Durham AE, McGowan CM, Fey K, et al. Pituitary pars intermedia dysfunction: diagnosis and treatment. Equine Vet Educ. 2014;26(4):216‐223. [Google Scholar]
- 160. Restifo MM, Frank N, Hermida P, Sanchez‐Londoño A. Effects of withholding feed on thyrotropin‐releasing hormone stimulation test results and effects of combined testing on oral sugar test and thyrotropin‐releasing hormone stimulation test results in horses. Am J Vet Res. 2016;77(7):738‐748. [DOI] [PubMed] [Google Scholar]
- 161. Freestone JF, Beadle R, Shoemaker K, et al. Improved insulin sensitivity in hyperinsulinaemic ponies through physical conditioning and controlled feed intake. Equine Vet J. 1992;24(3):187‐190. [DOI] [PubMed] [Google Scholar]
- 162. Buff PR, Spader BR, Morrison CD, Keisler DH. Endocrine responses in mares undergoing abrupt changes in nutritional management. J Anim Sci. 2006;84(10):2700‐2707. [DOI] [PubMed] [Google Scholar]
- 163. Van Weyenberg S, Hesta M, Buyse J, Janssens GP. The effect of weight loss by energy restriction on metabolic profile and glucose tolerance in ponies. J Anim Physiol Anim Nutr (Berl). 2008;92(5):538‐545. [DOI] [PubMed] [Google Scholar]
- 164. Dugdale AH, Curtis GC, Cripps P, et al. Effect of dietary restriction on body condition, composition and welfare of overweight and obese pony mares. Equine Vet J. 2010;42(7):600‐610. [DOI] [PubMed] [Google Scholar]
- 165. Argo CM, Dugdale AH, McGowan CM. Considerations for the use of restricted, soaked grass hay diets to promote weight loss in the management of equine metabolic syndrome and obesity. Vet J. 2015;206(2):170‐177. [DOI] [PubMed] [Google Scholar]
- 166. Bruynsteen L, Moons CPH, Janssens GPJ, et al. Level of energy restriction alters body condition score and morphometric profile in obese Shetland ponies. Vet J. 2015;206(1):61‐66. [DOI] [PubMed] [Google Scholar]
- 167. Harris PA, Ellis AD, Fradinho MJ, et al. Review: Feeding conserved forage to horses: recent advances and recommendations. Animal. 2017;11(6):958‐967. [DOI] [PubMed] [Google Scholar]
- 168. Taylor D, Sperandeo A, Schumacher J, et al. Clinical outcome of 14 obese, laminitic horses managed with the same rehabilitation protocol. J Equine Vet Sci. 2014;34(4):556‐564. [Google Scholar]
- 169. Borgia L, Valberg S, McCue M, Watts K, Pagan J. Glycaemic and insulinaemic responses to feeding hay with different non‐structural carbohydrate content in control and polysaccharide storage myopathy‐affected horses. J Anim Physiol Anim Nutr (Berl). 2011;95(6):798‐807. [DOI] [PubMed] [Google Scholar]
- 170. Shepherd ML, Pleasant RS, Crisman MV, Werre SR, Milton SC, Swecker Jr WS. Effects of high and moderate non‐structural carbohydrate hay on insulin, glucose, triglyceride, and leptin concentrations in overweight Arabian geldings. J Anim Physiol Anim Nutr (Berl). 2012;96(3):428‐435. [DOI] [PubMed] [Google Scholar]
- 171. Müller C, Nostell K, Bröjer J. Methods for reduction of water soluble carbohydrate content in grass forages for horses. Livest Sci. 2016;186:46‐52. [Google Scholar]
- 172. Moore‐Colyer MJ, Lumbis K, Longland A, Harris P. The effect of five different wetting treatments on the nutrient content and microbial concentration in hay for horses. PLoS One. 2014;9(11):e114079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Mack SJ, Dugdale AH, Argo CM, Morgan RA, McGowan C. Impact of water‐soaking on the nutrient composition of UKhays. Vet Rec. 2014;174(18):452. [DOI] [PubMed] [Google Scholar]
- 174. Chavez SJ, Siciliano PD, Huntington GB. Intake estimation of horses grazing tall fescue (Lolium arundinaceum) or fed tall fescue hay. J Anim Sci. 2014;92(5):2304‐2308. [DOI] [PubMed] [Google Scholar]
- 175. Glunk EC, Hathaway MR, Grev AM, Lamprecht ED, Maher MC, Martinson KL. The effect of a limit‐fed diet and slow‐feed hay nets on morphometric measurements and postprandial metabolite and hormone patterns in adult horses. J Anim Sci. 2015;93(8):4144‐4152. [DOI] [PubMed] [Google Scholar]
- 176. Ince J, Longland AC, Newbold JC, et al. Changes in proportions of dry matter intakes by ponies with access to pasture and haylage for 3 and 20 hours per day respectively, for six weeks. J Equine Vet Sci. 2011;31:283. [Google Scholar]
- 177. Longland AC, Barfoot C, Harris PA. The effect of wearing a grazing muzzle vs not wearing a grazing muzzle on pasture dry matter intake by ponies. J Equine Vet Sci. 2011;31:282‐283. [Google Scholar]
- 178. Vervuert I, Osswald B, Cuddeford D, Coenen M. Effects of chromium yeast supplementation on postprandial glycaemic and insulinaemic responses in insulin‐resistant ponies and horses. Pferdeheilkunde. 2010;26(2):245‐250. [Google Scholar]
- 179. Respondek F, Myers K, Smith TL, Wagner A, Geor RJ. Dietary supplementation with short‐chain fructo‐oligosaccharides improves insulin sensitivity in obese horses. J Anim Sci. 2011;89(1):77‐83. [DOI] [PubMed] [Google Scholar]
- 180. Winter JC, Liertz S, Merle R, Aschenbach JR, Gehlen H. Oral supplementation of magnesium aspartate hydrochloride in horses with equine metabolic syndrome. Pferdeheilkunde. 2016;32(4):372‐377. [Google Scholar]
- 181. Chameroy KA, Frank N, Elliott SB, Boston RC. Effects of a supplement containing chromium and magnesium on morphometric measurements, resting glucose, insulin concentrations and insulin sensitivity in laminitic obese horses. Equine Vet J. 2011;43(4):494‐499. [DOI] [PubMed] [Google Scholar]
- 182. Earl LR, Thompson DL, Mitcham PB. Factors affecting the glucose response to insulin injection in mares: epinephrine, short‐ and long‐term prior feed intake, cinnamon extract, and omega‐3 fatty acid supplementation. JEquine Vet Sci. 2012;32(1):15‐21. [Google Scholar]
- 183. Schmengler U, Ungru J, Boston R, Coenen M, Vervuert I. Effects of l‐carnitine supplementation on body weight losses and metabolic profile in obese and insulin‐resistant ponies during a 14‐week body weight reduction programme. Livest Sci. 2013;155(2):301‐307. [Google Scholar]
- 184. Moreaux SJJ, Nichols JL, Bowman JGP, Hatfield PG. Psyllium lowers blood glucose and insulin concentrations in horses. J Equine Vet Sci. 2011;31(4):160‐165. [Google Scholar]
- 185. Nawrocka D, Kornicka K, Smieszek A, Marycz K. Spirulina platensis improves mitochondrial function impaired by elevated oxidative stress in adipose‐derived mesenchymal stromal cells (ASCs) and intestinal epithelial cells (IECs), and enhances insulin sensitivity in equine metabolic syndrome (EMS) horses. Mar Drugs. 2017;15(8):237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Boshuizen B, Van Nieuwerburgh F, Deforce D, et al. 2017. Effect of aleurone on equine fecal microbiome & glucose metabolism in non‐training horses. 10th European College of Equine Internal Medicine Congress, Budapest, Germany.
- 187. de Laat MA, Hampson BA, Sillence MN, Pollitt CC. Sustained, low‐intensity exercise achieved by a dynamic feeding system decreases body fat in ponies. J Vet Intern Med. 2016;30(5):1732‐1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Curtis GC, Barfoot CF, Dugdale AH, et al. Voluntary ingestion of wood shavings by obese horses under dietary restriction. Br J Nutr. 2011;106(Suppl 1):S178‐S182. [DOI] [PubMed] [Google Scholar]
- 189. Brinkmann L, Gerken M, Riek A. Effect of long‐term feed restriction on the health status and welfare of a robust horse breed, the Shetland pony (Equus ferus caballus). Res Vet Sci. 2013;94(3):826‐831. [DOI] [PubMed] [Google Scholar]
- 190. Gupta AK, Pal Y, Mamta YMP. Effect of feed deprivation on biochemical indices in equids. J Equine Sci. 1999;10(2):33‐38. [Google Scholar]
- 191. Karstoft K, Pedersen BK. Exercise and type 2 diabetes: focus on metabolism and inflammation. Immunol Cell Biol. 2016;94(2):146‐150. [DOI] [PubMed] [Google Scholar]
- 192. Bird SR, Hawley JA. Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport Exerc Med. 2016;2(1):e000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Menzies‐Gow NJ, Wray H, Bailey SR, Harris PA, Elliott J. The effect of exercise on plasma concentrations of inflammatory markers in normal and previously laminitic ponies. Equine Vet J. 2014;46(3):317‐321. [DOI] [PubMed] [Google Scholar]
- 194. Mann S, Beedie C, Balducci S, et al. Changes in insulin sensitivity in response to different modalities of exercise: a review of the evidence. Diabetes Metab Res Rev. 2014;30(4):257‐268. [DOI] [PubMed] [Google Scholar]
- 195. Harris P, Marlin DJ, Davidson H, Rodgerson J, Gregory A, Harrison D. Practical assessment of heart rate response to exercise under field conditions. Equine Comp Ex Physiol. 2007;4(1):15‐21. [Google Scholar]
- 196. Bonelli F, Sgorbini M, Meucci V, Sighieri C, Baragli P. How swimming affects plasma insulin and glucose concentration in Thoroughbreds: a pilot study. Vet J. 2017;226:1‐3. [DOI] [PubMed] [Google Scholar]
- 197. Carter RA, McCutcheon LJ, Valle E, et al. Effects of exercise training on adiposity, insulin sensitivity, and plasma hormone and lipid concentrations in overweight or obese, insulin‐resistant horses. Am J Vet Res. 2010;71(3):314‐321. [DOI] [PubMed] [Google Scholar]
- 198. Powell DM, Reedy SE, Sessions DR, Fitzgerald BP. Effect of short‐term exercise training on insulin sensitivity in obese and lean mares. Equine Vet J Suppl. 2002;34:81‐84. [DOI] [PubMed] [Google Scholar]
- 199. Durham AE, Rendle DI, Newton JE. The effect of metformin on measurements of insulin sensitivity and beta cell response in 18 horses and ponies with insulin resistance. Equine Vet J. 2008;40(5):493‐500. [DOI] [PubMed] [Google Scholar]
- 200. Hustace JL, Firshman AM, Mata JE. Pharmacokinetics and bioavailability of metformin in horses. Am J Vet Res. 2009;70(5):665‐668. [DOI] [PubMed] [Google Scholar]
- 201. Tinworth KD, Boston RC, Harris PA, Sillence MN, Raidal SL, Noble GK. The effect of oral metformin on insulin sensitivity in insulin‐resistant ponies. Vet J. 2012;191:79‐84. [DOI] [PubMed] [Google Scholar]
- 202. Bailey CJ, Mynett KJ, Page T. Importance of the intestine as a site of metformin‐stimulated glucose utilization. Br J Pharmacol. 1994;112(2):671‐675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Rendle DI, Rutledge F, Hughes KJ, Heller J, Durham AE. Effects of metformin hydrochloride on blood glucose and insulin responses to oral dextrose in horses. Equine Vet J. 2013;45(6):751‐754. [DOI] [PubMed] [Google Scholar]
- 204. Sakar Y, Meddah B, Faouzi MA, Cherrah Y, Bado A, Ducroc R. Metformin‐induced regulation of the intestinal D‐glucose transporters. J Physiol Pharmacol. 2010;61(3):301‐307. [PubMed] [Google Scholar]
- 205. Frank N, Sommardahl CS, Eiler H, Webb LL, Denhart JW, Boston RC. Effects of oral administration of levothyroxine sodium on concentrations of plasma lipids, concentration and composition of very‐low‐density lipoproteins, and glucose dynamics in healthy adult mares. Am J Vet Res. 2005;66(6):1032‐1038. [DOI] [PubMed] [Google Scholar]
- 206. Chameroy K, Frank N, Elliott S. Effects of levothyroxine sodium on body condition, blood measures of metabolic status, and glucose dynamics in horses with equine metabolic syndrome (EMS). J Vet Intern Med. 2010;24:780. [Google Scholar]
- 207. Suagee JK, Corl BA, Wearn JG, et al. Effects of the insulin‐sensitizing drug pioglitazone and lipopolysaccharide administration on insulin sensitivity in horses. J Vet Intern Med. 2011;25(2):356‐364. [DOI] [PubMed] [Google Scholar]
- 208. Wearn JM, Crisman MV, Davis JL, et al. Pharmacokinetics of pioglitazone after multiple oral dose administration in horses. J Vet Pharmacol Ther. 2011;34(3):252‐258. [DOI] [PubMed] [Google Scholar]
- 209. Legere RM, Ruffin‐Taylor D, Bello K, Parker C, Judd RL & Wooldridge AA (2018) Pioglitazone in equids increases high‐molecular‐weight adiponectin concentrations and decreases insulin response after oral sugar (Abstract E10). Proceedings of the ACVIM Forum, Seattle, WA, p. 507.
- 210. Lopez Vicchi F, Luque GM, Brie B, Nogueira JP, Garcia Tornadu I, Becu‐Villalobos D. Dopaminergic drugs in type 2 diabetes and glucose homeostasis. Pharmacol Res. 2016;109:74‐80. [DOI] [PubMed] [Google Scholar]
- 211. Arana Valencia N, Thompson DL, Oberhaus EL, Gilley RM. Long‐term treatment of insulin‐insensitive mares with cabergoline: effects on prolactin and melanocyte stimulating hormone responses to sulpiride and on indices of insulin sensitivity. J Equine Vet Sci. 2014;34(5):680‐686. [Google Scholar]
- 212. Carlson CJ, Santamarina ML. Update review of the safety of sodium‐glucose cotransporter 2 inhibitors for the treatment of patients with type 2 diabetes mellitus. Expert Opin Drug Saf. 2016;15(10):1401‐1412. [DOI] [PubMed] [Google Scholar]
- 213. Meier A, Reiche D, de Laat M, Pollitt C, Walsh D, Sillence M. The sodium‐glucose co‐transporter 2 inhibitor velagliflozin reduces hyperinsulinemia and prevents laminitis in insulin‐dysregulated ponies. PLoS One. 2018;13(9):e0203655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. de Laat MA, Kheder MH, Pollitt CC, Sillence MN. Sweet taste receptor inhibitors: potential treatment for equine insulin dysregulation. PLoS One. 2018;13(6):e0200070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. O'Grady SE. Farriery for chronic laminitis. Vet Clin North Am Equine Pract. 2010;26(2):407‐423. [DOI] [PubMed] [Google Scholar]
- 216. Furtado T, McGowan C, Perkins E, et al. Concerns around equine weight management: an analysis of internet fora. Equine Vet J. 2017;49:29‐29. [Google Scholar]
- 217. Morgan R, Keen J, McGowan C. Equine metabolic syndrome. Vet Rec. 2015;177(7):173‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Carroll CL, Huntington PJ. Body condition scoring and weight estimation of horses. Equine Vet J. 1988;20(1):41‐45. [DOI] [PubMed] [Google Scholar]
- 219. Dugdale AH, Grove‐White D, Curtis GC, et al. Body condition scoring as a predictor of body fat in horses and ponies. Vet J. 2012;194(2):173‐178. [DOI] [PubMed] [Google Scholar]


