Abstract
Immune‐mediated hemolytic anemia (IMHA) is an important cause of morbidity and mortality in dogs. IMHA also occurs in cats, although less commonly. IMHA is considered secondary when it can be attributed to an underlying disease, and as primary (idiopathic) if no cause is found. Eliminating diseases that cause IMHA may attenuate or stop immune‐mediated erythrocyte destruction, and adverse consequences of long‐term immunosuppressive treatment can be avoided. Infections, cancer, drugs, vaccines, and inflammatory processes may be underlying causes of IMHA. Evidence for these comorbidities has not been systematically evaluated, rendering evidence‐based decisions difficult. We identified and extracted data from studies published in the veterinary literature and developed a novel tool for evaluation of evidence quality, using it to assess study design, diagnostic criteria for IMHA, comorbidities, and causality. Succinct evidence summary statements were written, along with screening recommendations. Statements were refined by conducting 3 iterations of Delphi review with panel and task force members. Commentary was solicited from several professional bodies to maximize clinical applicability before the recommendations were submitted. The resulting document is intended to provide clinical guidelines for diagnosis of, and underlying disease screening for, IMHA in dogs and cats. These should be implemented with consideration of animal, owner, and geographical factors.
Keywords: comorbidity, Delphi survey, direct antiglobulin test, erythrocyte, evidence, flow cytometry, hemolysis, iceberg model, spherocyte, veterinary and comparative clinical immunology society
Abbreviations
- AIHA
autoimmune hemolytic anemia
- C
confidence of comorbidity diagnosis score
- CI
confidence interval
- D
study design score
- DAT
direct antiglobulin test
- FeLV
feline leukemia virus
- FIP
feline infectious peritonitis
- FIV
feline immunodeficiency virus
- I
confidence of IMHA diagnosis score
- IME
integrated metric of evidence
- IMHA
immune‐mediated hemolytic anemia
- L
likelihood of a causal link between comorbidity and IMHA score
- N
number of patients with a given comorbidity
- PCR
polymerase chain reaction
- Q
study quality score
- SAT
saline agglutination test
- VCCIS
Veterinary and Comparative Clinical Immunology Society
1. INTRODUCTION
Immune‐mediated hemolytic anemia (IMHA) in dogs and cats is associated with high morbidity and mortality.1, 2, 3, 4, 5 Pathogenic autoantibodies target erythrocyte membrane epitopes,6, 7 providing a mechanism for fraction crystallizable receptor‐mediated extravascular hemolysis mediated by macrophages.8 Complement can interact with antibodies bound to erythrocytes, facilitating extravascular hemolysis or causing intravascular hemolysis by formation of the membrane attack complex. An expeditious diagnosis that distinguishes IMHA from other causes of anemia is critical to the rapid institution of appropriate treatment. Various criteria for the diagnosis of IMHA have been described in the literature based on the documentation of immune‐mediated erythrolysis or proxy markers for this phenomenon,9, 10, 11, 12 but little consensus exists on which criteria are required for definitive diagnosis. Furthermore, the differentiation of spontaneous IMHA from disease associated with putative trigger factors is an important first step in the diagnostic evaluation, because removal of trigger factors whenever possible is a crucial component of treatment. However, no guidelines exist for the diagnostic assessment of trigger factors, and formal assessment of the evidence for their implication in IMHA is lacking. An evidence summary would allow clinicians to better gauge the likelihood of a given comorbidity being implicated in the pathogenesis of IMHA, and would help to guide on which diagnostic tests should be performed in individual patients.
The objective of this Consensus Statement is therefore to present guidelines on both the fundamental diagnosis of IMHA and tests to screen for putative trigger factors, based on evidence, inferences from parallel data in human medicine, and expert opinion. Work contributing to this Consensus Statement was completed by members of the Consensus Panel and additional members of the relevant Veterinary and Comparative Clinical Immunology Society (VCCIS) task forces established in 2015.
2. MATERIALS AND METHODS
2.1. Literature review
We searched 2 databases (Medline and Web of Science) for relevant references in April 2016 and March 2018. Standard Boolean search terms allowing lemmatization were adopted. References captured by the algorithm {(anemia OR anaemia) AND (dog OR cat) AND (immun*)}, hereafter denoted by A1, were imported into reference management software (Mendeley, Elsevier, New York; EndNote X8, Clarivate Analytics, Philadelphia), before manual screening on the basis of inclusion criteria outlined in Supporting Information S1. The reference lists of papers also were examined to capture references not cited on Medline or Web of Science. Pathogen‐specific searches were conducted to capture additional references (Supporting Information S1).
2.2. Curation of records
A total of 723 papers were captured by the search algorithm A1. Abstracts of all papers were reviewed by OAG, LK, UJ, ALM, SB, BG, RG, and JS, leading to the rejection of 475 papers because they failed to meet inclusion criteria. A further 67 duplicate papers were excluded, yielding 181 unique papers. Of these, an additional 118 papers were excluded because they did not include information on patients with potential trigger factors. Of the remaining 63 papers, 52 contained information of relevance to infectious disease, including 14 genera of microbes infecting dogs and 8 genera of microbes infecting cats. A pathogen‐specific search on the basis of these genera yielded 11 additional papers meeting inclusion criteria. A search performed in March 2018, using both A1 and the pathogen‐specific algorithms, yielded another 6 papers. An important paper published before the advent of online archiving was added to the list. Data therefore were extracted from 81 papers (Figure 1).
Figure 1.
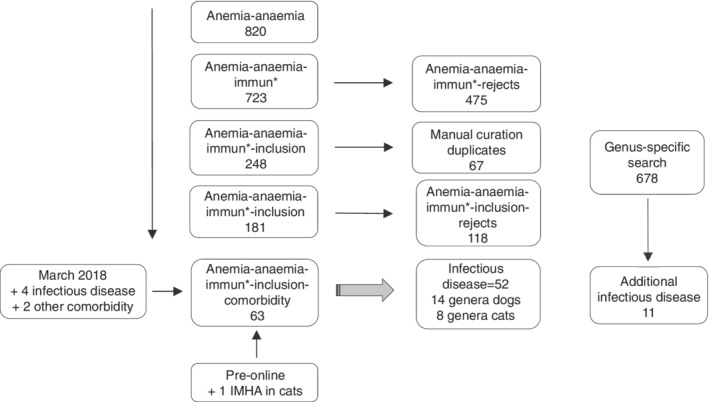
Curation of records. Papers captured by a search algorithm for anemia that met inclusion criteria (n = 248) were manually curated to remove duplicates (n = 67), after which remaining papers were screened to assess whether they mentioned comorbidities, yielding 63 papers; an additional 6 were added in March 2018. An independent search for infectious agents yielded an additional 11 papers of relevance. One additional paper was identified by examining reference lists of the captured papers. IMHA, immune‐mediated hemolytic anemia
2.3. Quality assessment
We designed a novel quality assessment and data extraction tool, which included domains to capture information on study design (D) and quality (Q), confidence of comorbidity diagnosis (C), likelihood of a causal link between comorbidity and IMHA (L), confidence of IMHA diagnosis (I), and the number of patients with a given comorbidity (N). For the purposes of this study, the term “comorbidity” included exposure to drugs, toxins, and vaccines. Additional domains captured detailed information on each of the comorbidities, including statistical inferences when available. Comorbidities were summarized in 5 broad categories: infectious disease, cancer, inflammatory disease, drugs and toxins, and vaccines. Panel members and non‐panel VCCIS task force members were assigned to random pairings for the purpose of data extraction and quality assessment, dividing the total number of papers equally among all pairs. Concordance among the pairs was sought if individual members disagreed on specific observations, and all observations relating to quality assessment were confirmed by LK and OAG.
For each comorbidity identified in a paper, an integrated metric of evidence (IME) was computed as the sum of the normalized scores, weighted according to our assessment of relative importance to evidence rating, so long as only that comorbidity was present in individual patients, hence IME = 2D + Q + C + 2L + I + N. If >1 comorbidity was present in individual patients, including those infected with >1 agent, an IME value was not calculated. Reference to the patients nevertheless was made in the narrative if they yielded insight. Score D was assessed after positing the question: Does the study ask whether a comorbidity induces (or is associated with) IMHA as part of its hypothesis or specific aims, or is the question that a comorbidity induces (or is associated with) IMHA answered by study design? If the answer was yes, a D score was assigned; if the answer was no, the study was designated “Descriptive Association Only” for that comorbidity and assigned an arbitrary D score of 1 (the lowest possible) out of a maximum of 7. A Q score was not computed for comorbidities assigned Descriptive Association Only, because general study quality in those cases was irrelevant to the question of the causal relationship between comorbidity and IMHA; Q scores in those cases were therefore 0. The maximum normalized score for each criterion was 1, yielding a maximum IME value of 8 and a minimum of <1. An IME value of 0 was applied when a study presented evidence against a comorbidity being associated with IMHA. For each comorbidity within a paper, the most conservative score for each of the IME criteria was applied for the relevant cohort of patients, to avoid exaggeration of evidence.
Threshold IME values were computed to allow comorbidities to be designated as negligible, low, intermediate, or high evidence for a causal relationship with IMHA. The threshold between negligible and low evidence was taken to be a hypothetical Descriptive Association Only study, with intermediate C, L, and I scores, and 1 positive case (IME = 2.95). The threshold between low and intermediate evidence was taken to be a hypothetical cross‐sectional study, with a Q score of 28, intermediate C, L, and I scores, and 2‐5 positive cases (IME = 4.37). Finally, the threshold between intermediate and high evidence was taken to be a hypothetical prospective cohort/case‐control study, with a Q score of 28, high C score, intermediate L score, high I score (mechanistically based), and 2‐5 positive cases (IME = 5.78).
The quality assessment and data extraction tool is shown in Supporting Information S2; Supporting Information S3 shows the derivation of each of the scores D, Q, C, L, I, and N.
2.4. Delphi process
Each panel member drafted recommendations in assigned areas. The draft recommendations then were subjected to 3 rounds of Delphi review, during which each panel member was able to give written feedback on every recommendation using an online questionnaire (Survey Monkey, San Mateo, California). At the end of each round, suggestions were incorporated into the working template by 1 facilitator, and only recommendations for which consensus had not yet been reached were included in the subsequent Delphi cycle. After 3 rounds of review, unanimous consensus had been reached on the majority of recommendations. Where differences of opinion remained, these are indicated in the text of the Consensus Statement.
2.5. Production of the consensus statement
OAG, LK, UJ, AMM, and SB were assigned ≥1 sections of the working document for further editing and incorporation of comments generated during oral presentation. These sections then were edited by the co‐Chairs, before submission to ACVIM for review by all members. The draft Consensus Statement also was submitted to the European College of Veterinary Internal Medicine, American College of Veterinary Emergency and Critical Care, European College of Veterinary Emergency and Critical Care, the American College of Veterinary Pathology, and the European College of Veterinary Clinical Pathology for solicitation of comments from members. Feedback from these specialist colleges was used by the panel members to produce the final Consensus Statement.
3. DIAGNOSIS OF IMMUNE‐MEDIATED HEMOLYTIC ANEMIA
No agreed diagnostic gold (criterion) standard exists for IMHA in veterinary patients, similar to the situation in humans. The British Society for Hematology's guidelines for diagnosis of autoimmune hemolytic anemia (AIHA) recommend the direct antiglobulin test (DAT) in patients with hemolysis, but also emphasize that the test is neither specific nor 100% sensitive for AIHA.13 The diagnostic tests described below therefore should be interpreted in combination with the results of other diagnostic tests and response to immunosuppression. The lack of a gold (criterion) standard also presents challenges when interpreting the literature. For example, although sensitivities and specificities are discussed here (either as reported directly in the referenced studies, or calculated by the panel using data within the reference), the criteria used to define cases as positive or negative for IMHA vary. Variability also exists in how tests are performed and validated, and therefore performance in 1 study may not generalize to others.
Cognizant of these caveats, 7 of 8 panel members considered the algorithm in Figure 2 appropriate for diagnosis of primary or secondary IMHA, complemented by the following explanatory notes. (One panel member considered that markers of immune‐mediated disease should be assessed with caution in the absence of hemolysis.) This discussion is restricted to immune‐mediated destruction of circulating erythrocytes (ie, precursor‐targeted immune‐mediated anemia or pure red cell aplasia are not discussed). Readers requiring additional information on immune‐mediated differentiation failure or destruction at the level of the bone marrow are referred elsewhere.14, 15, 16, 17
Figure 2.
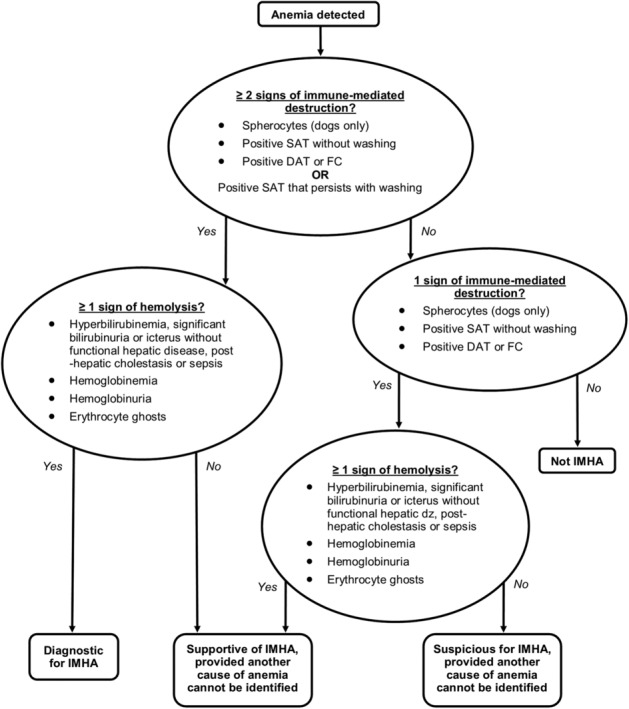
Diagnostic algorithm for immune‐mediated hemolytic anemia (IMHA). Having identified anemia in a patient, biomarkers of immune‐mediated destruction should next be assessed, including the saline agglutination test (SAT), direct antiglobulin test (DAT), and/or flow cytometry (FC); at least 2 should be present, or a positive SAT that persists with washing, to make a firm diagnosis of IMHA. Signs of hemolysis should then be assessed, at least 1 of which should be present for a firm diagnosis. Variations on this theme would yield a supportive or suspicious diagnosis, provided another cause of anemia is not identified. Additional abbreviations: ≥, at least; dz, disease
3.1. Anemia
A spun PCV is suggested because calculated hematocrit may be unreliable when agglutination is present.18, 19, 20 However, studies in humans have reported increased plasma trapping by abnormal erythrocytes (eg, spherocytes), which could introduce inaccuracy into the spun PCV.21 To the best of our knowledge, the effect of agglutination or spherocytosis on spun PCV has not been investigated in veterinary species. Lack of regeneration (as assessed by reticulocyte count) does not eliminate IMHA because approximately 30% of dogs have non‐regenerative anemia at presentation.5, 22
3.2. Signs of immune‐mediated destruction
3.2.1. Prominent spherocytosis
Spherocytes should be used as a diagnostic criterion only in dogs because feline erythrocytes do not consistently display central pallor. Interpreting spherocytes after blood transfusion should be done cautiously, because stored blood products may contain high proportions of spherocytes23 and spherocytes have been documented in human patients with hemolytic transfusion reactions.24, 25 Spherocytosis should be assessed in the monolayer of a well‐made blood smear, because spherocyte‐like artifacts arise toward the feathered edge and in thick areas.26 In anemic animals, spherocytes should be confirmed in the deeper monolayer to avoid artifacts in thin areas. Spherocytosis also can induce increased osmotic fragility,27, 28 but because osmotic fragility testing is influenced by other factors (eg, hyperlipidemia,27 erythrocyte age29), the panel does not advocate its routine use in the diagnosis of IMHA.
Reported causes of non‐immune‐mediated spherocytes, or morphologically similar pyknocytes, should be eliminated, including oxidative damage (eg, zinc30, 31 and acetaminophen32), envenomation,33, 34, 35, 36, 37 hypersplenism (eg, hepatosplenic lymphoma),38 pyruvate kinase deficiency,39 disorders associated with erythrocyte fragmentation (eg, endocarditis,40 microangiopathic hemolytic disorders including hemangiosarcoma,41 or hemolytic uremic syndrome),42 and dyserythropoiesis.43 Hereditary spectrin deficiency also potentially may give rise to spherocytes if smears are made from blood stored >24 hours.44 The percentage of spherocytes on blood smears from human patients with mutations causing hereditary spherocytosis is variable, but can be high.45 A literature search for canine hereditary spherocytosis did not identify any cases with marked spherocytosis.
In a single study, ≥5 spherocytes/×100 oil immersion field yielded 63% sensitivity (95% confidence interval [CI], 39%‐84%) (when 95% CIs were not provided by the authors, the online MedCalc Diagnostic test evaluation calculator [https://www.medcalc.org/calc/diagnostic_test.php] was used to calculate them) and 95% specificity (95% CI, 76%‐100%) for IMHA in dogs,27 compared with 74% sensitivity (95% CI, 49%‐91%) and 81% specificity (95% CI, 59%‐95%)c for ≥3/×100 oil immersion field.27 A threshold of ≥5 spherocytes/×100 oil immersion field therefore could be considered supportive of a diagnosis of IMHA, but 3‐4 spherocytes/×100 oil immersion field also may be consistent with IMHA provided no other cause of spherocytosis is identified. These thresholds are similar to the criteria for 1+ spherocytosis in a proposed semiquantitative grading system.46 Where spherocyte numbers are low (versus their typical abundance in extravascular IMHA), variability among fields could be an issue: calculating mean count over several fields (eg, 10) could help establish the true extent of spherocytosis. For enrollment of cases in IMHA research, only high‐quality blood smears should be used, and given the pitfalls of spherocyte recognition, examination of the smears by a board‐certified clinical pathologist is advantageous.
3.2.2. Positive saline agglutination test
Although evaluation of dried blood smears or hematology instrument scatter plots20 can suggest agglutination, the panel does not consider these techniques adequate to confirm agglutination based on the possibility of overlapping rouleaux on blood smears and the potential for other causes of macrocytes on scattergram evaluation.47 Saline agglutination testing performed by mixing 4 drops of saline with 1 drop of blood has a reported specificity of 100% (95% CI, 95%‐100%)c for IMHA in dogs.27 Mixing blood and saline 1:1 yielded a specificity of 95% (95% CI, 88%‐99%)c based on 85 dogs without IMHA, or 85% (95% CI, 65%‐96%)c when only anemic dogs were considered.11 Agglutination that persists after mixing 1 drop of blood with 4 drops of saline therefore is considered adequate evidence for agglutination in most cases.27 Considerably higher dilution ratios can aid the microscopic identification of agglutination. To decrease false positives, confirming that agglutination persists after washing erythrocytes 3 times in a 1:4 ratio with saline11 is recommended for animals with equivocal results (eg, rare small erythrocyte clumps in an otherwise negative test), markedly increased total protein (eg, leishmaniasis, multiple myeloma, and feline infectious peritonitis [FIP]) or fibrinogen concentrations,48 or strong rouleaux formation on blood smear examination. Based on reports of agglutination of washed erythrocytes from normal dogs at 4°C, we suggest that the saline solution should be between room temperature and 37°C.49
3.2.3. Demonstration of anti‐erythrocyte antibodies
Five panel members preferred the direct Coombs' test (DAT) and 3 considered flow cytometry and DAT to be equally useful. Supporting Information S4 provides recommendations for performance of these tests. If the DAT cannot be performed because agglutination persists after washing, the combination of anemia, hemolysis, and persistent agglutination is sufficient for diagnosis of IMHA. Immunochromatography offers an alternative to conventional DAT or flow cytometry, but confirming negative results by conventional DAT may be advisable because of frequent weak positive test strips in DAT‐positive dogs.11
For DAT, sensitivity ranged from 61 to 82% for dogs27, 50 and 82% for cats51 for studies reported between 2006 and 2016 that did not rely on DAT alone for the diagnosis of IMHA and reported sensitivity or sufficient information for its calculation. Specificity for DAT was 94%‐100% for dogs11, 27, 50, 52 and 95%‐100% for cats9, 51, 53 for studies published between 2006 and 2016 that reported specificity or sufficient information for its calculation. Although small experimental studies have reported sensitivities of up to 100% for flow cytometry,54, 55, 56, 57 sensitivity was 67% (95% CI, 53%‐79%)c in a larger study reporting results of routine clinical testing.58 For studies including clinically ill negative controls, specificity for flow cytometry was 87.5% (95% CI, 47%‐100%)c 54 to 92% (95% CI, 88%‐95%)c.56
Reports of sample handling effects on flow cytometry are lacking. Storage of samples at 4°C for up to 7 days before DAT testing is acceptable unless the laboratory advises otherwise.11 Current data, although limited, suggest that although immunosuppression does not immediately result in a negative DAT,11, 59 interindividual variability exists in the time required to become negative DAT after initiation of treatment.11, 60 For flow cytometry, anecdotal reports suggest that immunosuppression decreases the percentage of antibody‐positive erythrocytes.56 Therefore, where possible, we recommend collection of samples for DAT or flow cytometry before initiation of treatment. Large‐scale studies of the effect of prior blood transfusion on DAT or flow cytometry are lacking. Based on reports of DAT‐negative results for 21 dogs posttransfusion,11 prior blood transfusion is not an absolute contraindication for testing. However, a positive DAT has been reported in a dog without signs of IMHA but with a history of multiple transfusions.61 Furthermore, delayed serological or hemolytic transfusion reactions with positive DAT are reported in humans.62, 63, 64 Therefore, where possible, we recommend collection of samples for DAT before blood transfusion.
A suggested advantage of flow cytometry compared with DAT is the generation of a more quantitative result, potentially allowing monitoring of therapeutic success. Statistical associations are reported between laboratory or clinical features and the percentage of antibody‐positive erythrocytes.56, 58 However, the clinical value of the percentage of positive erythrocytes has not been evaluated rigorously.56
3.3. Evidence of hemolysis
3.3.1. Spherocytosis
In dogs, spherocytes (assessed as described) can provide evidence of hemolysis, consistent with evidence of this phenomenon in human erythrocytes.65, 66 The increased rigidity of spherocytes results in entrapment within the spleen and subsequent extravascular hemolysis.28, 67, 68
3.3.2. Hyperbilirubinemia
In the absence of decreased functional hepatic mass, obstructive cholestasis, or sepsis, hyperbilirubinemia may represent evidence of hemolysis. At least 1 of the following is considered sufficient evidence for hyperbilirubinemia: icterus, total serum or plasma bilirubin concentration above reference interval, bilirubinuria in cats, or ≥2+ bilirubin on a urine reagent strip in dogs. Bilirubin reported for hemolyzed samples should be interpreted in combination with information regarding the likely impact of hemolysis on the assay.69
3.3.3. Hemoglobinemia/Hemoglobinuria
Hemoglobinemia can be detected by visual examination of plasma or measurement of cell‐free hemoglobin. When using instrument‐based indicators of hemolysis, limitations of the individual method should be considered. For example, spectrophotometric hemolytic indices rely on manufacturer‐specific methods and algorithms that are not directly comparable among different instruments.70 Similarly, discrepancy between mean cell hemoglobin concentration and cellular hemoglobin concentration provided by ADVIA hematology instruments may reflect hemolysis or other sample characteristics, such as lipemia.71
Hemoglobinemia should only be interpreted as evidence of hemolysis after eliminating artifactual hemolysis. Common causes of in vitro hemolysis include, but are not limited to, traumatic venipuncture,72, 73, 74, 75 freezing, storage, and (based on studies in humans) sampling via an IV catheter72, 76, 77 or post‐collection injection of samples into vacutainers.78 The likelihood of in vitro hemolysis is increased if factors that increase erythrocyte fragility are present (eg, lipemia).79 Provided causes of myoglobinuria are absent, hemoglobinuria is considered present if urine is red and discoloration is not cleared by centrifugation, or if a positive heme reaction on urine dipstick is present in the absence of intact erythrocytes on microscopic sediment examination. Assessment of hemoglobinuria should be performed using a fresh urine sample, and anecdotally the likelihood of erythrocyte lysis in urine is increased in alkaline or poorly concentrated or hyposthenuric urine samples.
3.3.4. Erythrocyte ghosts
Ghost cells provide evidence of intravascular hemolysis if seen on a smear made immediately after blood collection.3, 80
4. PUTATIVE TRIGGER FACTORS
4.1. Infectious disease
Recent evidence suggests that any infection can trigger immune dysregulation, loss of immune tolerance, and development of immune‐mediated disease in an individual patient with a genetic, epigenetic, or susceptible microenvironmental milieu at the time of infection.81, 82, 83, 84, 85, 86 However, certain organisms may cause specific immune‐mediated diseases. Examples in people include Mycoplasma pneumoniae infection causing IMHA and Helicobacter pylori causing immune‐mediated thrombocytopenia.87, 88 Other mechanisms such as circulating immune complex deposition and activation of immune cells through fraction crystallizable receptor engagement or delivery of immunoglobulin‐bound nucleic acid to Toll‐like receptors also occur during some infections.89 Damage to target cells is another mechanism that may make a pathogen particularly likely to induce autoimmunity by increased exposure of self‐epitopes that normally are sequestered or inefficiently presented to immune cells.81, 90 This proposed mechanism for the development of IMHA91 contributes to the accelerated clearance of erythrocytes during Plasmodium infection in people and mice.92 It also may occur during Babesia gibsoni infection in dogs and Mycoplasma haemofelis infection in cats.93, 94, 95, 96, 97 Antibody‐mediated removal is part of normal erythrocyte senescence.98 Organisms thus may cause IMHA by amplifying normal antibody‐mediated removal of aged or damaged erythrocytes.
4.1.1. Consensus Summary Statement
Organisms identified in this review with a high and an intermediate level of evidence as a cause of IMHA are likely to induce disease by a mechanism that can trigger immune‐mediated erythrocyte destruction in many patients. Further study is required to determine the role of other infections in IMHA. We emphasize that, for most studies, a low level of evidence represents a lack of studies designed to answer whether an infection is associated with IMHA, rather than studies that specifically demonstrated a lack of evidence. In addition, environmental, genetic, and epigenetic factors play a role in whether immune‐mediated disease occurs in an individual patient. Therefore, clinicians should consider the possibility that any identified recent or recurrent infection may contribute to the development of IMHA. Furthermore, eliminating the possibility of infection is prudent before immunosuppressive treatment.
4.1.2. Summary of evidence
Overall, 66 manuscripts were reviewed.3, 9, 10, 12, 27, 51, 57, 94, 95, 96, 97, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151, 152, 153 The IME values were calculated for 27 infectious agents or types of infection (Figures 3, 4, 5), but could not be calculated for many infectious agents either because of the way data were summarized (eg, the number of individual patients with IMHA and an infection could not be discerned) or because an individual patient with IMHA had >1 comorbidity (Supporting Information S2). In addition, most investigators did not specifically ask whether infection causes IMHA. Consensus Summary Statements are presented here for all genera of organisms for which at least 1 study had an intermediate or higher level of evidence that infection induced IMHA. All additional organisms are discussed in Supporting Information S5.
Figure 3.
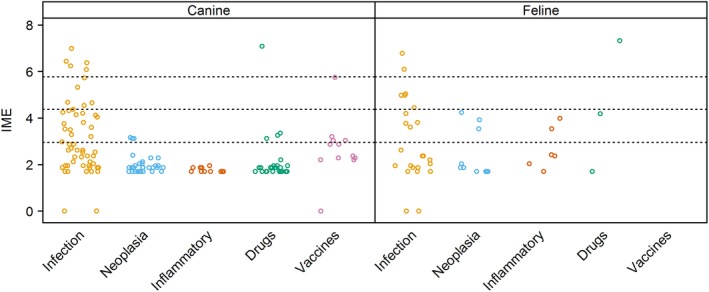
Integrated metric of evidence (IME) summary for all comorbidity categories. Horizontal dotted lines indicate the threshold IME values between negligible and low (2.95), low and intermediate (4.37), and intermediate and high (5.78) levels of evidence. Most IME values fell within the negligible and low zones of evidence, while a small number were 0, indicating evidence against that comorbidity inducing immune‐mediated hemolytic anemia (IMHA). No studies of relevance to vaccination as a potential trigger for IMHA in cats could be found
Figure 4.
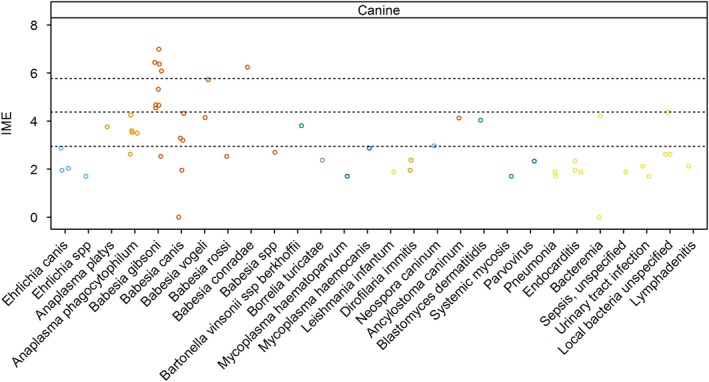
Integrated metric of evidence (IME) values for infectious agents and diseases in dogs. Horizontal dotted lines indicate the threshold IME values between negligible and low (2.95), low and intermediate (4.37), and intermediate and high (5.78) levels of evidence. spp, species (plural term)
Figure 5.
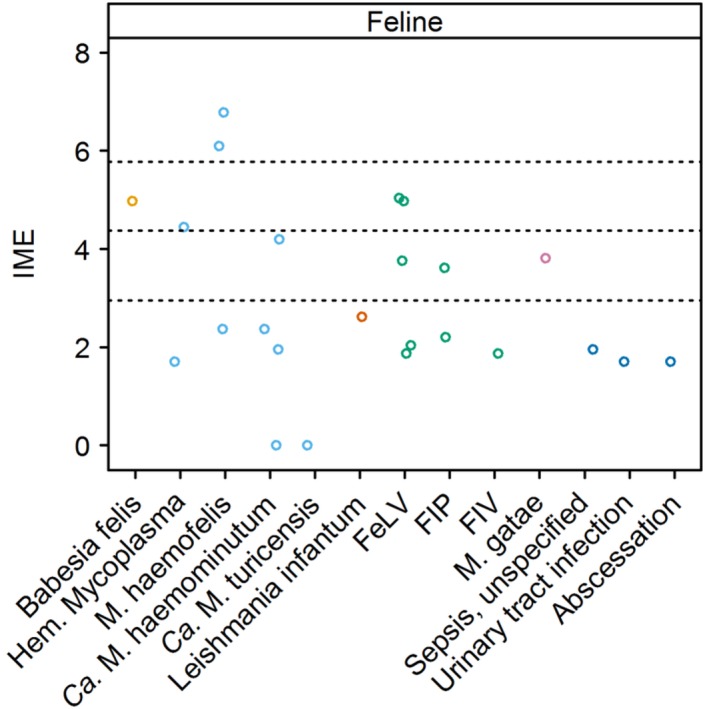
Integrated metric of evidence (IME) values for infectious agents and diseases in cats. Horizontal dotted lines indicate the threshold IME values between negligible and low (2.95), low and intermediate (4.37), and intermediate and high (5.78) levels of evidence. Ca., Candidatus; FeLV, feline leukemia virus; FIP, feline infectious peritonitis; FIV, feline immunodeficiency virus; Hem., hemotropic; M., Mycoplasma
4.2. Infections in dogs
4.2.1. Piroplasms
Seventeen studies documented 103 cases of IMHA in Babesia‐infected dogs.57, 93, 94, 95, 96, 99, 102, 103, 104, 111, 120, 122, 128, 134, 141, 147, 151 The IME values for Babesia species as a whole ranged from 0.00 to 6.99, with a median of 4.55. Fifty‐three percent (10/19) of the IME values demonstrated an intermediate or high level of evidence that Babesia causes IMHA. For 3 additional studies, the number of dogs with Babesia and IMHA could not be determined.105, 125, 136
There is a high level of evidence that immune‐mediated destruction of erythrocytes contributes to anemia in dogs infected with B. gibsoni. Immune‐mediated hemolytic anemia was documented in 69 dogs in 9 studies,93, 94, 95, 96, 99, 102, 103, 111, 151 with an additional study (in which the number of infected dogs with IMHA could not be determined) providing useful mechanistic insight.125 The median IME value was 5.32, ranging from 2.54 to 6.99. For this Babesia species, 88% (8/9) of the studies showed intermediate (4) or high (4) IME values. Four were studies of dogs experimentally infected with B. gibsoni, yielding a median IME value of 6.41 and range of 6.08‐6.99.94, 95, 96, 99 Natural infection with B. gibsoni occurs most commonly in fighting breeds.102, 125 However, mixed breed dogs used in experimental studies also develop IMHA, suggesting that the immune‐mediated pathogenesis is largely driven by the parasite.99
Whether other species of Babesia cause IMHA in dogs remains unclear. One study documented IMHA in 2 chronically infected splenectomized mixed breed dogs experimentally infected with what was thought to be B. gibsoni, but later characterized as Babesia conradae,147 yielding an IME value of 6.25. Five studies documented 13 cases of IMHA in dogs infected with Babesia canis, with a median IME value of 3.20 and range of 0‐4.32.57, 104, 111, 128, 134 Babesia vogeli was documented in 2 studies of 5 dogs with IMHA, with IME values of 5.73 and 4.14. Five cases of IMHA were documented in a study of Babesia rossi‐infected dogs, although the authors presumed a Babesia species based on cytological examination of blood smears and geographic locale.120 The IME value was 2.56. In an additional study, the Babesia species was not specified, but again was likely to be B. rossi.122 Nine dogs with IMHA were documented in that study, with an IME value of 2.70. Thus, the evidence for large Babesia species causing IMHA is lower than that for B. gibsoni, attributed in part to the fact that most studies were not designed to determine if an association between IMHA and infection existed. Nevertheless, differences also may exist in pathogenicity among Babesia species that influence the risk of IMHA. For example, 1 study found that the majority of anemic B. vogeli‐infected dogs had IgM and IgG bound to erythrocytes, but these antibodies were not detected in dogs infected with B. canis.57 In this study, eccentrocytosis, suggesting oxidative damage, was more common in B. canis‐infected dogs. The IME value for B. canis in this study was 0, whereas it was 5.73 for B. vogeli.57
The mechanism of immune‐mediated erythrocyte destruction during B. gibsoni infection has been explored. Because Babesia species infect erythrocytes, antibodies appropriately targeting the organisms could result in “immune‐mediated” erythrocyte destruction without targeting self‐antigen. However, antibodies produced during infection also appear to target erythrocyte membranes. Oxidative injury may play a role in anti‐erythrocyte antibody formation.96 Activated macrophages cause oxidative damage to uninfected as well as infected erythrocytes during B. gibsoni infection, a factor that may contribute to the severity of IMHA in some dogs.96 In addition to oxidative damage, sialic acid residue removal is required to expose epitopes that are targeted by antibody.94 Interestingly, anti‐erythrocyte antibodies that developed in dogs experimentally infected with B. gibsoni did not attach to undamaged red blood cells in dogs that had recovered from clinical infection.93 Furthermore, in vitro studies have shown that Babesia‐induced antibody reactivity against erythrocytes is higher for aged and oxidized than for fresh erythrocytes.95 Taken together, these data suggest that ongoing damage to the red cell membrane and increased exposure of epitopes that are usually “hidden” facilitates immune‐mediated erythrocyte destruction. Once infection is controlled, the drive for immune‐mediated destruction stops.
Like Babesia species, Rangelia and Theileria species are protozoan parasites that infect erythrocytes in dogs. A study of dogs experimentally infected with Rangelia vitelli demonstrated that a regenerative anemia suspicious for IMHA developed in infected dogs.149 Treatment of infection resolved the anemia without immunosuppression. A retrospective case series of dogs naturally infected with Theileria spp. developed IMHA.137 Dogs were treated with combined immunosuppression and imidocarb dipropionate. The authors reported resolution of hematological abnormalities during an unspecified study period. The IME values could not be calculated, because the total number of dogs with IMHA could not be discerned.
Consensus Summary Statement
The evidence that piroplasms, and in particular B. gibsoni, cause IMHA is intermediate to high. For B. gibsoni, evidence suggests that during infection, antibodies target host erythrocyte antigens exposed as a consequence of transient oxidative damage or sialic acid residue removal. Further study is needed to determine if differences in pathogenicity among species, host factors, or both mediate risk of development of IMHA in infected dogs. What is known about the mechanism of erythrocyte destruction suggests that immunosuppression should not be necessary to resolve immune‐mediated erythrocyte destruction in most cases.
4.2.2. Anaplasma species
Nine dogs with IMHA in 5 studies were infected with, or exposed to, Anaplasma phagocytophilum.101, 108, 126, 129, 150 The median IME value was 3.53, with a range of 2.62‐4.25. In addition to IMHA, platelet‐bound antibodies were documented in some dogs with concurrent thrombocytopenia.101, 108 Although most dogs in these reports were treated concurrently with doxycycline and immunosuppressive corticosteroid treatment, 1 dog responded to doxycycline treatment alone,150 whereas another dog had prednisone discontinued after 2 days.108 One retrospective case series documented 2 dogs with acute Anaplasma platys infections with concurrent IMHA.100 Both dogs had spherocytosis and positive saline agglutination and Coombs' test results supporting the diagnosis of IMHA. The IME value for this study was 3.76.
Consensus Summary Statement
The evidence that A. phagocytophilum causes IMHA is low. However, most studies reporting A. phagocytophilum in dogs with IMHA were limited to case reports or retrospective studies, and were not designed to investigate a causal relationship. The presence of IMHA and other immune‐mediated conditions concurrent with this infection suggests that prospective controlled studies to examine a possible causal relationship between A. phagocytophilum and IMHA in dogs are warranted. The evidence that A. platys induces IMHA in dogs is low, but data are limited to a single retrospective case series. Further prospective, controlled studies are required to document a possible causal relationship between A. platys and IMHA in dogs.
Evidence that other vector‐borne agents (including Dirofilaria immitis, Ehrlichia spp. Borrelia spp., hemotropic Mycoplasma spp., Bartonella spp., and Leishmania infantum), non‐vector‐borne protozoal pathogens (including Neospora caninum), and other bacterial infections induce IMHA was negligible to low (Figure 4), or could not be quantified based on how results were reported (Supporting Information S5).105, 109, 125, 137, 145 For some of these organisms, such as Leishmania spp., D. immitis, and Bartonella spp., Coombs' test‐positive anemia is observed commonly with infection.105, 109, 145 Therefore, from a clinical perspective, it is still important to eliminate infection with these agents in a dog in which IMHA is a differential diagnosis.
4.3. Infections in cats
4.3.1. Babesia felis
Immune‐mediated hemolytic anemia was documented in 9 of 56 cats infected with B. felis in 1 study from South Africa. Six of the 9 cats were coinfected with feline leukemia virus (FeLV).138 Treatment for B. felis without immunosuppression resolved IMHA, yielding an IME value of 4.98.
Consensus Summary Statement
Although studies are limited, an intermediate level of evidence was found that B. felis causes IMHA in cats, and that treatment resolves IMHA without immunosuppression.
4.3.2. Hemotropic Mycoplasma species
Seven studies documenting IMHA in 21 cats infected with hemotropic Mycoplasma spp. yielded IME values.3, 51, 97, 110, 130, 140, 153 The median IME value was 2.37, but values differed widely among hemotropic Mycoplasma species, ranging from 0 to 6.78. Overall, a high level of evidence exists for M. haemofelis inducing IMHA in cats. Immune‐mediated hemolytic anemia was documented in 15 cats in 3 studies for this species.3, 97, 140 The median IME value was 6.10, with a range of 2.37‐6.78. Two of the 3 studies provided high evidence97, 140; the other study was not designed to answer whether infection causes IMHA.3 In a study of cats experimentally infected with M. haemofelis, severe macrocytic Coombs' test‐positive anemia and persistent autoagglutination of erythrocytes developed. In contrast, these findings did not occur when cats were infected with the less pathogenic Candidatus (Ca.) Mycoplasma haemominutum and Ca. Mycoplasma turicensis species.140 The target of anti‐erythrocyte antibody that develops during M. haemofelis infection in cats was investigated in 1 study.97 Serum from cats infected with M. haemofelis agglutinated infected and neuraminidase‐treated erythrocytes but not normal erythrocytes, suggesting that, as in babesiosis, damage to erythrocytes and unmasking of antigens contribute to the pathogenesis of IMHA.97 Ca. M. haemominutum infection was documented in 3 cats with IMHA over 4 studies (1 study showing no association with IMHA),3, 51, 130, 140 yielding a median IME value of 2.16 and range of 0‐4.2.
Consensus Summary Statement
A high level of evidence was found that M. haemofelis causes IMHA in cats. Negligible to low level of evidence was found that the less pathogenic species Ca. M. haemominutum causes IMHA, and no evidence was found that Ca. M. turicensis induces IMHA. Whether coinfection and host immune status play roles in development of IMHA in cats infected with different hemotropic Mycoplasma species requires further study.
4.3.3. Viral infections
Feline leukemia virus
Seven studies meeting inclusion criteria were identified.3, 9, 110, 119, 138, 153, 154 However, other comorbidities such as erythroleukemia, myeloproliferative disease, chronic interstitial nephritis, glomerulonephritis and splenic amyloidosis, and drug administration were documented in some infected cats, precluding IME calculation.110, 138, 153, 154 The median IME value for the others was 3.77, with a range of 1.87‐5.04.3, 9, 110, 119, 153, 154
Consensus Summary Statement
Collectively, the evidence that FeLV infection induces IMHA is low. The observation that some FeLV‐positive cats also have Coombs' test‐positive anemia should prompt further investigation into whether immune‐mediated erythrocyte destruction can contribute to anemia in FeLV‐positive cats.
Summaries of the evidence for other infections that have been documented in cats with IMHA, including FIP, feline immunodeficiency virus (FIV), L. infantum, Mycoplasma gatae, M. haemofelis, soft tissue infection, and urinary tract infection are provided in Supporting Information S6.
5. CANCER
5.1. Cancer in dogs
Immune‐mediated hemolytic anemia is a recognized paraneoplastic syndrome in people.155, 156, 157, 158 Chronic lymphocytic leukemia is a well‐established cause of IMHA in people.155, 156 Other neoplasms have been associated with IMHA in humans, but the causal mechanisms remain elusive.157, 158 The IME values could be calculated for 13 studies (Figure 6). The global median IME value was 1.87, with a range from 1.70 to 3.12, thus representing levels of evidence that were negligible (29 IME values; 70 patients) or low (3 IME values; 3 patients). The generally low level of evidence reflects the fact that the majority of the published studies did not specifically ask whether cancer is associated with canine IMHA, or if they did, were associated with low Q scores.
Figure 6.
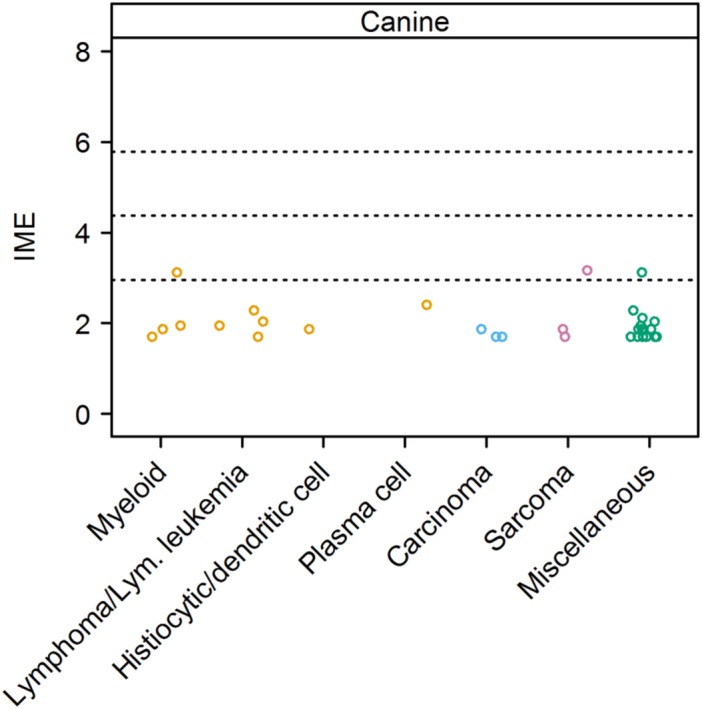
Integrated metric of evidence (IME) values for cancer types in dogs. Horizontal dotted lines indicate the threshold IME values between negligible and low (2.95), low and intermediate (4.37), and intermediate and high (5.78) levels of evidence. Miscellaneous cancer types included pheochromocytoma, unspecified abdominal, adrenal, bladder, cardiac, mediastinal, and splenic masses, other hematopoietic tumors, and unspecified neoplasia. Lym., lymphoid; Myeloid, myeloid leukemia or myeloproliferative disease
Two individual studies yielded low level evidence. The first described a mast cell tumor in a dog with IMHA, with an IME value of 3.12, and a pheochromocytoma in a dog with concurrent IMHA and immune‐mediated thrombocytopenia, with an IME value of 3.12.116 The second, a case report of an undifferentiated sarcoma in a Flat‐Coated Retriever with IMHA, postulated that the sarcoma was a trigger for the IMHA, with an IME value of 3.17.159 Mycoplasma haemocanis was identified in a splenectomized dog with cytological and clinical characteristics of acute lymphocytic leukemia.139 Although the development of IMHA in this dog was attributed to the hemotropic Mycoplasma infection, involvement of the leukemia could not be excluded. A canine IMHA patient with a duodenal leiomyosarcoma had an IME value of 1.70.10 Three further patients with hemangiosarcoma and IMHA were described, for which the IME value was 1.87.2 A number of other papers yielded a negligible level of evidence for neoplasia as a cause of IMHA. These documented the presence of carcinomas,2, 10, 12 malignant histiocytosis, and other hematopoietic tumors,134 myeloid neoplasia,2, 10, 132, 134, 160 multiple myeloma,161 sarcomas,2, 10 and miscellaneous undefined tumors2, 10, 134, 142, 143, 162, 163 in dogs with IMHA.
Consensus Summary Statement
Evidence of a causal link between cancer and IMHA in dogs currently is lacking in the veterinary literature, largely reflecting the fact that the majority of the published studies did not specifically ask whether cancer was associated with IMHA in dogs. Further studies are needed to determine if such an association exists. Although no evidence for a causal link exists, cancer cannot be eliminated as a potential trigger for this disease.
5.2. Cancer in cats
Five studies reported 21 cats with neoplasia and IMHA (Figure 7). These studies provide negligible evidence for a causal link between neoplasia and IMHA, yielding a median IME value of 1.87 and a range of 1.7‐4.4. No study specifically addressed this hypothesis. In a single retrospective study of 107 cats with IMHA, concurrent neoplasia was present in 16 (15%) cats.3
Figure 7.
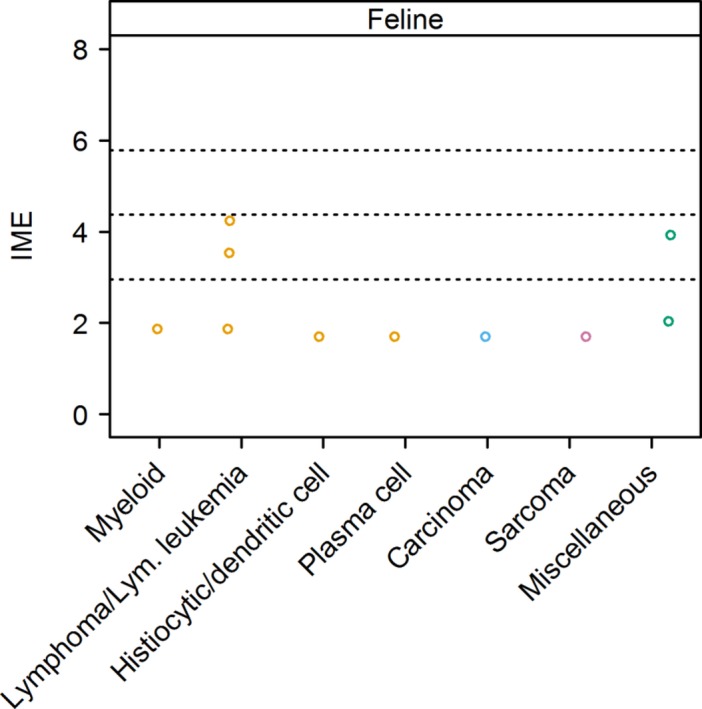
Integrated metric of evidence (IME) values for cancer types in cats. Horizontal dotted lines indicate the threshold IME values between negligible and low (2.95), low and intermediate (4.37), and intermediate and high (5.78) levels of evidence. Miscellaneous cancer types included gastrointestinal and uncharacterized neoplasia. Lym., lymphoid; Myeloid, myeloid leukemia or myeloproliferative disease
5.2.1. Hematopoietic and lymphoid neoplasia
Eight cats for which an IME value could be calculated for lymphoma and IMHA were identified.3, 9, 164 Two of the 3 studies reporting these cases did not demonstrate a causal association between IMHA and lymphoma,3, 9 and 1 study was considered to partially report or suggest causality.164 The latter reports 2 sibling specific pathogen‐free experimental cats. For both cats, lymphoma/lymphocytic leukemia was diagnosed on histological review after necropsy. However, interpretation of the histology in both cats was equivocal. The histological pattern was described as multicentric T‐lymphoblastic infiltration with associated B‐lymphocyte proliferation, which the authors concluded was most likely a lymphoproliferative disorder, but they did not eliminate an aberrant immune response. For 1 cat, the diagnosis of lymphoma/lymphocytic leukemia was made within 3 weeks of the onset of IMHA, and no other potential trigger for secondary IMHA was described. For the second cat, 2 episodes of IMHA were described, 1 potentially associated with an experimental herpes virus infection and the other potentially associated with experimental FeLV infection. For the other 2 studies, neither the method of diagnosis nor the subtype of lymphoma was specified. The evidence for a causal association between IMHA and lymphoma is low, with a median IME value of 3.54 and a range of 1.87‐4.24.
A single cat with multiple myeloma and IMHA was identified.3 This study did not show a causal association between neoplasia and IMHA, and the method of diagnosis of neoplasia was unclear. The evidence for an association between IMHA and multiple myeloma was negligible, with an IME value of 1.70.
Three cats with erythroleukemia3, 153 and 3 cats with non‐specified myeloproliferative disease110 and IMHA were identified. No study demonstrated a causal association between IMHA and neoplasia. The report of 2 of the cats with erythroleukemia suggests that diagnosis was based on bone marrow cytological or histological review, or both.3 The method of diagnosis of neoplasia was not described for the third cat with erythroleukemia.153 For the cats with non‐specified myeloproliferative disease, the diagnosis was based on bone marrow examination, but details are limited.110 All cats with non‐specified myeloproliferative disease were FeLV positive.110 The evidence for a causal association between erythroleukemia and IMHA is negligible, with an IME value for the 1 study in which it could be assigned of 1.87.3 Other studies of erythroleukemia or unspecified myeloproliferarative disease did not yield IME values because of the presence of comorbidities.
A single cat with histiocytic sarcoma and IMHA was identified.3 This study did not show a causal association between IMHA and neoplasia, and the method of diagnosis of neoplasia was not described, yielding an IME value of 1.70.
5.2.2. Solid tumors
A single case of pancreatic carcinoma110 and a single case of anaplastic sarcoma3 with giant cells in cats with IMHA were identified. Neither study showed a causal association between IMHA and neoplasia. The evidence for a causal association between IMHA and carcinoma, and sarcoma, was negligible, with an IME value of 1.70 in each case.
5.2.3. Miscellaneous and minimally described neoplasia
One cat with IMHA and uncharacterized gastrointestinal neoplasia51 and 6 cats with IMHA and uncharacterized masses3 were identified. A causal association between IMHA and these lesions was not identified. The method of diagnosis for the presumed neoplastic lesions was not described. The evidence for a causal association between IMHA and uncharacterized gastrointestinal neoplasia is low, with an IME value of 3.92, and negligible for uncharacterized masses, with an IME value of 2.04.
Consensus Summary Statement
Currently, no strong evidence exists for a causal link between cancer and IMHA in cats; further studies are needed to determine if such an association exists. Nevertheless, retrospective evidence suggests a relatively high prevalence of concurrent cancer in cats with IMHA.
6. INFLAMMATORY DISEASE
6.1. Pancreatitis in dogs and cats
Inflammation that often occurs with IMHA could indirectly lead to pancreatitis by activation of neutrophils or formation of thromboemboli. Subsequent oxidative damage, ischemic events, or both then may directly damage the pancreas. Alternatively, inflammation associated with pancreatitis could lead to IMHA by indirectly inducing autoantibodies to form against erythrocytes. Autoantibodies that bind to epitopes on both exocrine pancreatic epithelium and erythrocytes also may be generated.165 To date, none of these hypotheses has been confirmed in veterinary species. Observation of concurrent pancreatitis and IMHA has been reported in an 8‐year‐old female Cocker Spaniel166 and in some retrospective studies of dogs with IMHA (Figure 8). Studies that evaluated groups of dogs with IMHA indicate that the prevalence of concurrent pancreatitis is low, between 1% (1/93 IMHA dogs142) and 5% (1/19 dogs10). Both of these studies yielded an IME value for pancreatitis of 1.70.10, 142 The Cocker Spaniel in the case report had both IMHA and pancreatitis.166 However, cholestasis and renal failure also were present. Although cholestasis and renal failure can be complications of pancreatitis, primary organ disease could not be eliminated, precluding calculation of an IME value.
Figure 8.
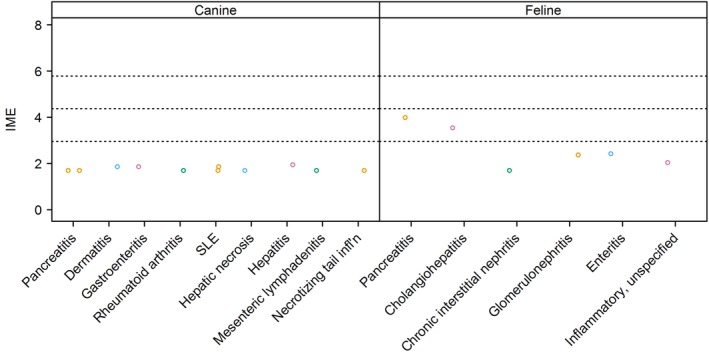
Integrated metric of evidence (IME) values for inflammatory diseases. Horizontal dotted lines indicate the threshold IME values between negligible and low (2.95), low and intermediate (4.37), and intermediate and high (5.78) levels of evidence. infl'n, inflammation; SLE, systemic lupus erythematosus
A recent study of 11 cats with IMHA showed that 3 of these cats (3/11; 27.3%) had pancreatitis,165 yielding an IME value of 3.99. This study was designed to answer the question of whether IMHA is associated with pancreatitis.165 Another large study of cats with IMHA indicated that 6/107 (5.6%) had concurrent cholangitis, pancreatitis, or both.3 An IME value could not be calculated because information regarding the number of cats with pancreatitis alone was not specified.
Consensus Summary Statement
The evidence for pancreatitis causing IMHA is negligible in dogs and negligible to low in cats. Additional studies would be required to establish a causal relationship.
6.2. Necrosis in dogs
One study attributed secondary IMHA to concurrent liver necrosis12 and another to concurrent necrotizing inflammation of the tail,2 both in single patients; both studies yielded an IME value of 1.70. Further evaluation of these patients was not pursued.
Consensus Summary Statement
The evidence for necrosis as a cause of IMHA is negligible in dogs and is not reported in cats.
6.3. Other sources of inflammation in dogs and cats
Reports of other inflammatory processes were identified in several reports. Two studies reported 4 dogs with IMHA that were diagnosed with systemic lupus erythematosus.2, 167 Other reported inflammatory diseases in dogs with IMHA included 3 dogs with gastroenteritis, 2 dogs with dermatitis,2 and 1 dog each with hepatitis,143 rheumatoid arthritis,10 and mesenteric lymphadenitis.2 Negligible evidence was found for these inflammatory conditions inducing IMHA, with a median IME value of 1.70 and a range of 1.70‐1.95. Seven of 107 (6.5%) cats with IMHA had clinical evidence of inflammation or infection that was not further classified, yielding an IME value of 2.04.2
Consensus Summary Statement
Anecdotal reports suggest that generalized inflammatory processes induce IMHA in dogs and cats, but direct evidence is lacking. Well‐designed studies to determine whether non‐infectious inflammatory processes cause IMHA are warranted.
7. DRUGS AND TOXINS
7.1. Dogs
Seventeen studies described dogs with IMHA that had been exposed to drugs or toxins,1, 10, 12, 27, 34, 80, 116, 127, 135, 142, 143, 160, 168, 169, 170, 171, 172 but only 11 reported cases with sufficient primary data for the calculation of an IME value.10, 12, 27, 34, 80, 142, 143, 168, 169, 170, 172 The majority of cases (35/36) were dogs exposed to antimicrobial drugs.10, 80, 142, 143, 168, 170 For these cases, IME values ranged from 1.70 to 7.09, with a median of 1.87 (Figure 9). The highest level of evidence, with an IME value of 7.09, came from 1 unblinded, randomized, prospective clinical trial in which 6 of 14 dogs given escalating doses of cefazedone acquired anti‐erythrocyte antibodies.168 The remaining reported cases were associated with low or negligible evidence to support other drugs or toxins as a cause for IMHA in dogs (Figure 9).
Figure 9.
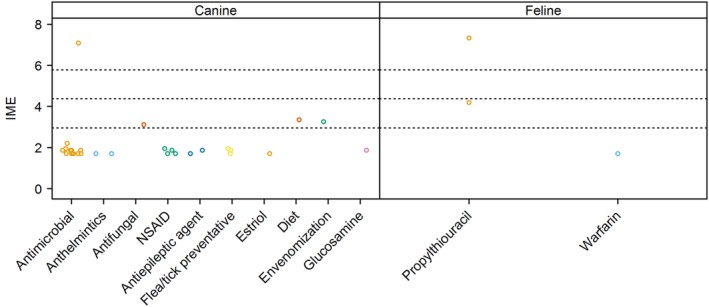
Integrated metric of evidence (IME) values for drugs. Horizontal dotted lines indicate the threshold IME values between negligible and low (2.95), low and intermediate (4.37), and intermediate and high (5.78) levels of evidence. The single antimicrobial drug yielding high‐level evidence in dogs was cefazedone. NSAID, non‐steroidal anti‐inflammatory drug
7.2. Cats
Two papers describe the development of IMHA after administration of propylthiouracil to cats, with respective IME values of 7.33 and 4.19. In the first study of 105 cats, 7 cats with hyperthyroidism treated with propylthiouracil developed immune‐mediated drug reactions.173 This finding was followed by a prospective, un‐blinded, non‐randomized trial in which 17 healthy cats were given the same drug, causing 9 to develop Coombs' test‐positive anemia.174 One additional case report describes warfarin exposure in a cat with IMHA, with an IME value of 1.70.110
Consensus Summary Statement
The prevalence of drug‐induced IMHA in dogs and cats is either rare or underreported. However, a lack of evidence does not preclude the possibility of a drug or toxin triggering IMHA.
8. VACCINES
8.1. Dogs
The most effective vaccines elicit robust immune responses only against the pathogen of interest. However, vaccines also may elicit unfavorable immune responses resulting from mechanisms such as molecular mimicry, bystander cell activation, or downregulation of self‐tolerance, which contribute to autoimmunity.175, 176 For dogs, 32 papers mentioned that vaccines could be a trigger for IMHA, of which only 12 papers describe 79 clinical cases with documented temporal associations of ≤30 days between vaccine administration and IMHA.5, 22, 80, 107, 116, 143, 170, 177, 178, 179, 180, 181 The types of vaccines given to each patient were not consistently recorded. Seven papers provide negligible primary case data linking vaccination and IMHA.22, 80, 116, 143, 170, 180, 181 Three papers provide evidence for a link between vaccination and IMHA in the low range, with IME values between 2.95 and 4.37.5, 177, 178 No publications that provide high levels of evidence to support an association between vaccination and IMHA were found. Two studies reported intent to evaluate an association between vaccines and IMHA. In 1 retrospective study, associated with an IME value of 5.76, a difference was found in the frequency of IMHA cases diagnosed within the first month after vaccination and those diagnosed at subsequent months, whereas a similar temporal distribution was not identified in a control group.179 A subsequent study had a similar Q score, but showed no difference in the number of cases with recent vaccination history between the IMHA and control groups, and therefore was awarded an IME value of 0.107
Other papers we reviewed excluded patients with a recent vaccination history, with the intent of describing only dogs with idiopathic IMHA.182, 183, 184, 185 In addition, some papers did not include the vaccination status of dogs with IMHA.56, 101, 161, 186, 187 Other data we excluded from analysis included studies with an uncertain diagnosis of IMHA,188, 189 case studies in which alternative causes of IMHA were possible,171, 190 and cases from studies in which the timing of vaccination was not specified.16 Only 1 study prospectively investigated a link between vaccination and autoimmune disease in 5 dogs, demonstrating the presence of autoantibodies after vaccine administration. However, these dogs only were followed for 21 days after vaccination and did not meet the criteria for diagnosis of IMHA.191 With only 2 papers in the veterinary literature aiming to evaluate a link between vaccination and IMHA, and each of these respectively supporting179 or refuting107 an association, the question of whether vaccines trigger IMHA in dogs remains unanswered. Similarly, insufficient evidence is available to determine whether vaccination triggers autoimmune disease in people.192 No reports of an association between vaccine administration and IMHA in cats were found.
Consensus Summary Statement
Considering the wide practice of vaccination and lack of conclusive evidence of an association with IMHA, current vaccination strategies generally are safe. Patients should be individually assessed for their own risks and benefits before vaccination. Further studies are needed to determine if and when vaccine‐associated IMHA occurs in dogs and cats, and to develop better methods for the diagnosis of vaccine‐associated disease.
9. GLOBAL SCREENING RECOMMENDATIONS
9.1. Optimal minimum database (dogs and cats)
9.1.1. Consensus Summary Statement
A thorough history documenting vaccination, travel, exposure to fleas and ticks, flea and tick prevention, and heartworm testing and prevention is recommended. A thorough physical examination including retinal examination should be performed. Laboratory screening should include a CBC, blood film examination by a board‐certified clinical pathologist (or equivalently trained hematologist), serum biochemical profile, and routine urinalysis. Urine culture and fecal flotation with centrifugation also should be considered. Abdominal radiographs are important to eliminate hemolysis caused by zinc toxicity. Imaging and other diagnostic tests to screen for cancer remain a reasonable component of a diagnostic evaluation for IMHA in dogs and cats, performed at the discretion of the attending clinician on the basis of the likelihood of cancer in the individual patient. Routine testing for pancreatitis in dogs and cats with IMHA is not recommended, unless clinical presentation suggests that it is a credible differential diagnosis.
9.1.2. Rationale
History will help assess the likely risk of certain infections. A thorough physical examination and diagnostic imaging will help identify any potential nidus of infection or the presence of neoplastic lesions. Patterns of abnormalities identified on the CBC, serum biochemistry, and urinalysis can increase the index of suspicion for specific infectious agents that may be associated with IMHA.193, 194, 195, 196, 197, 198, 199, 200, 201, 202 This minimum database also can identify additional pathological processes (eg, proteinuria) that may require specific treatment. Although insensitive, blood smear examination can be useful in identifying the presence of vector‐borne disease agents. Although the evidence associated with urinary tract infection as a cause of IMHA is negligible, identification and treatment of infection before immunosuppression is prudent. We refer the reader to the ACVIM consensus statement on the treatment of IMHA (in press) for further recommendations and additional discussion on the specific circumstance of treating subclinical bacteriuria in an immunosuppressed patient. The evidence that gastrointestinal parasites cause IMHA in dogs is low, but rapid resolution of IMHA with treatment and minimal immunosuppression has been described (Supporting Information S5).121 Imaging will help identify neoplasia or a nidus of infection.
9.2. Testing for infectious agents in dogs
9.2.1. Consensus Summary Statement
Dogs with IMHA should be screened for infection with Babesia spp. using combined testing with serology and polymerase chain reaction (PCR). Repeat testing by means of PCR should be performed in all dogs originally testing negative but with a high risk of infection based on breed or exposure risk. The sensitivity of PCR and serological testing may vary depending on the laboratory and test design. Infection with other piroplasms, including Rangelia and Theileria species, should be eliminated in endemic areas. Because D. immitis infection is associated with anemia and positive Coombs' test results, all dogs should be screened for D. immitis in endemic areas or when travel to such areas has occurred. Further study to determine how and if other vector‐borne disease agents cause IMHA is required before definitive screening recommendations can be made for additional organisms. However, screening for additional vector‐borne pathogens, in particular Anaplasma spp., Bartonella spp., Ehrlichia spp., and, in endemic areas, Leishmania spp., should be strongly considered. Potential foci of other infections identified during initial screening should be further investigated at the discretion of the attending clinician.
9.2.2. Rationale
The evidence that Babesia spp. induce IMHA is intermediate to high. Infection with B. gibsoni should be ruled out. Transmission of B. gibsoni in fighting breeds is through bite wounds and vertical transmission.102, 203 However, tick transmission by Haemaphysalis spp. and possibly Rhipicephalus sanguineus can occur, and experimental infection of mixed breed dogs results in IMHA.99, 204, 205, 206, 207, 208 Therefore, screening for B. gibsoni in all breeds with IMHA is prudent. B. vogeli should be ruled out in dogs with a history of exposure to R. sanguineus. Retired racing Greyhound dogs are at increased risk of infection because of the common occurrence of R. sanguineus infestations in racing kennels.102, 193, 209 Testing for B. canis and B. rossi by means of serology and PCR should be performed in endemic areas. Dogs living in California and Coyote hunting dogs specifically should be screened for B. conradae by means of PCR (no serological test is available).210, 211 Although evidence of causation is lacking, Coombs' test‐positive anemia is commonly documented in dogs with heartworm disease, bartonellosis, and leishmaniosis (Supporting Information S5).105, 109, 145 General principles for optimal use of serology and PCR in diagnosing vector‐borne disease are summarized in Supporting Information S7. Generally, combining PCR with serological testing enhances sensitivity.212, 213, 214 Repeat testing, including repeating PCR on the same or additional samples, and pairwise serological testing to demonstrate a 4‐fold change between acute and convalescent titers, also are necessary to document infection in many cases.212, 213, 214, 215, 216, 217
9.3. Testing for infectious agents in cats
9.3.1. Consensus Summary Statement
Polymerase chain reaction testing for B. felis should be performed in cats from endemic areas and in those with suggestive clinical signs. Serological testing was not available at the time of writing, but combined testing would be optimal based on studies of Babesia species infecting dogs. Polymerase chain reaction testing for M. haemofelis should be performed in all cats with IMHA. Further studies are needed to determine whether infection with other hemotropic Mycoplasma species is associated with IMHA in immunosuppressed or coinfected cats. Testing for all 3 species is preferred when possible. All sick cats should be tested for FeLV and FIV infection, according to American Association of Feline Practitioners retrovirus management guidelines (https://www.catvets.com/guidelines/practice-guidelines/retrovirus-management-guidelines), screening all cats with IMHA for FeLV using antigen ELISA. Proviral FeLV DNA quantitative PCR testing may be helpful as a confirmatory test. Routine testing for feline coronavirus and non‐hemotropic Mycoplasma spp. in cats with IMHA is not recommended, but appropriate diagnostic tests should be considered in cats with compatible clinical signs.
9.3.2. Rationale
Identification of Babesia spp. by light microscopy of blood smears is considered insensitive for screening in cats. Polymerase chain reaction to identify parasitic DNA or RNA is recommended.218 A high level of evidence was found that M. haemofelis causes IMHA in cats. Coinfection and host immune status may play a role in the development of IMHA in cats infected with the less pathogenic hemotropic Mycoplasma spp. In addition, coinfection with multiple hemotropic Mycoplasma species is common.51, 144 Therefore, infection with a less pathogenic species may signal that repeat testing for M. haemofelis is warranted. Non‐hemotropic Mycoplasma infection only has been described in 1 cat with IMHA.148 However, M. pneumoniae causes cold agglutinin hemolytic anemia in people, and infection with Mycoplasma cynos was associated with development of cold agglutinins in a dog.135 Therefore, it should be considered as a possible trigger in cats with IMHA and other findings compatible with infection. The evidence for FeLV in association with IMHA in cats is negligible to intermediate. Polymerase chain reaction testing for proviral DNA could be considered as part of infectious disease screening. The overall evidence that FIP induces IMHA is negligible. However, given the immune mechanisms underlying effusive FIP, testing for FIP in cats with compatible clinical and laboratory findings is judicious.
9.4. Drug and vaccine administration in dogs and cats
9.4.1. Consensus Summary Statement
There is insufficient evidence to recommend withholding necessary medications for dogs and cats with IMHA. However, all medications, particularly those previously implicated in immune‐mediated diseases, should be used with caution in patients with IMHA. Every patient should ideally have a complete history recorded, which includes all vaccines and drugs administered, the doses, dates, frequency, duration, and route of their administration, and information about the products being used such as manufacturer, indications, specific lot, and any adverse events. Exposure to toxins should also be documented in any dog or cat with IMHA.
9.4.2. Rationale
Evidence for cefazedone in dogs168 and propylthiouracil in cats173, 174 suggests that >1 class of drugs may be associated with IMHA in small animals. For most commonly prescribed medications, the evidence is negligible. Specific documentation of vaccine histories and long‐term prospective studies may help determine whether vaccines can trigger IMHA. To date, approximately 8% of dogs with a diagnosis of IMHA and vaccination histories had been vaccinated within 30 days of IMHA diagnosis. However, studies comparing this prevalence to adequate controls are limited and inconclusive. Animals with IMHA are at risk for recurrence of anemia, making careful decisions about the risks and benefits of revaccinating important in every case. Animals receiving immunosuppressive treatment are less likely to mount protective immunity after routine vaccination.
10. ICEBERG MODEL AND PROPOSED NEW NOMENCLATURE
Based on the data analyzed here, we propose a unified model for the pathogenesis of IMHA and a new system of nomenclature, in which the disease is categorized as “non‐associative” and “associative” rather than “primary” and “secondary,” respectively (Figure 10A,B). This clarification is needed because the word “primary” implies that all triggers have been definitively ruled out, whereas “secondary” implies causation. We propose that the term “associative” be used when a comorbidity is identified. In some cases, the comorbidity might have caused the IMHA (secondary IMHA), whereas in others it might be coincidental (primary IMHA). “Non‐associative” IMHA cases are those in which comorbidities are not identified in the diagnostic evaluation, and include primary (“idiopathic”) and cryptogenic cases. The latter implies that an underlying cause was not identified, perhaps because the underlying pathomechanisms are not currently understood, or the comorbidity could not be detected using available testing.
Figure 10.
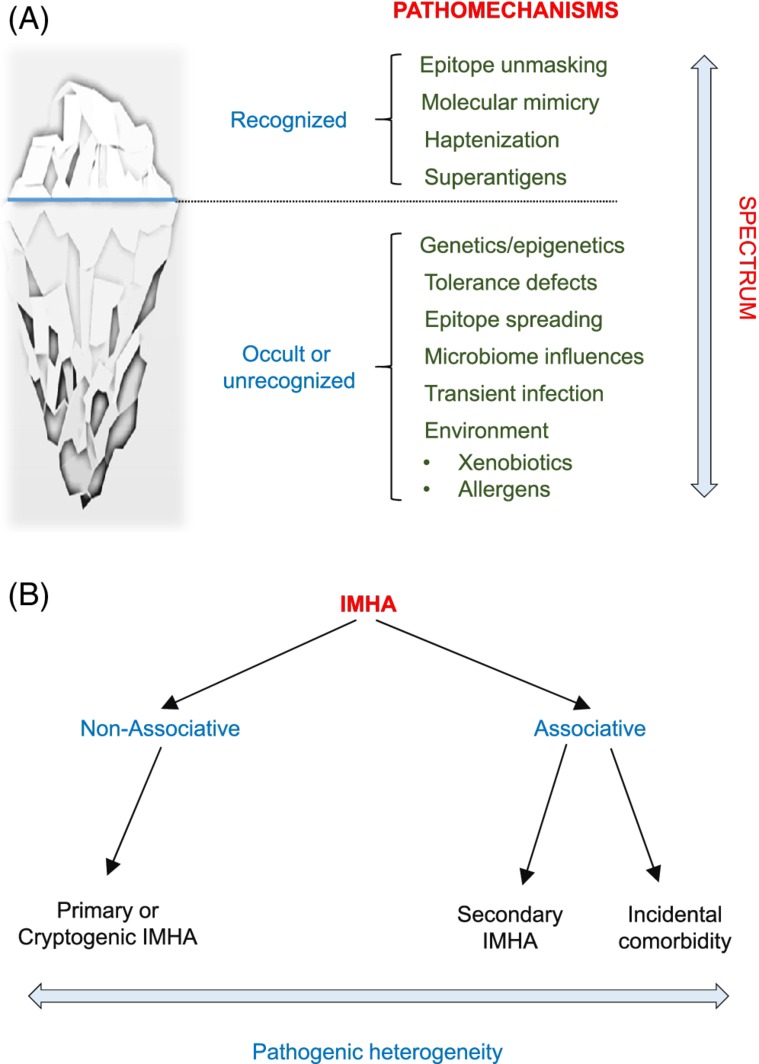
Iceberg model and proposed new nomenclature for immune‐mediated hemolytic anemia (IMHA). A, The iceberg model posits that pathomechanisms underlying IMHA fall on a spectrum, both recognized (above the water level) and currently unrecognized or occult (concealed), the latter postulated to be the majority. Hypothetical occult pathomechanisms are listed. B, We propose a new nomenclature for IMHA to better reflect the heterogeneity in pathomechanisms underlying IMHA
11. FUTURE RESEARCH DIRECTIONS
When the VCCIS task forces were formed, we began by identifying a focused question that represented an important problem in veterinary immunology, namely “What is the evidence that infection, neoplasia, drugs, vaccines, and other comorbidities cause IMHA in dogs and cats?” Our original intent was to perform a systematic review of the literature to answer this question. However, it quickly became apparent that very few studies in the veterinary literature were designed to determine if a given comorbidity causes IMHA, hence an expanded approach was used to evaluate the evidence presented in our review. There is a critical need for well‐designed, prospective, case‐controlled clinical studies that directly ask the question of whether infections, neoplasia, drugs, and vaccines cause IMHA. Some comorbidities are likely to cause IMHA in a large number of affected patients, such as an infection that expresses an epitope mimicking an erythrocyte antigen widely expressed in a population, or an organism that causes transient expression of normally hidden epitopes. Others might induce IMHA only in patients with epigenetic and genetic predisposition, or a given inflammatory context. Studies that investigate how individual comorbidities trigger IMHA, and the role of genetics and epigenetics, will help identify what diseases to screen for in all patients, and what diseases to screen for in selected patients that may be at increased risk of developing IMHA from any trigger. Mechanistic studies also will determine which comorbidities, when treated, will lead to resolution of IMHA without the need for immunosuppression. Stringent criteria for the diagnosis of IMHA and definitive diagnosis of a comorbidity must be integrated into study design in order to make meaningful observations.
CONFLICT OF INTEREST DECLARATION
Jonathan Fogle has been paid by Merial for speaking engagements and continuing education. Linda Kidd has been a paid speaker for IDEXX and Zoetis and has occasionally consulted for IDEXX, Zoetis and Merck. All other authors had no conflicts of interest to declare.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Appendix S1 Supporting information
Appendix S2 Supporting information
Appendix S3 Supporting information
Appendix S4 Supporting information
Appendix S5 Supporting information
Appendix S6 Supporting information
Appendix S7 Supporting information
ACKNOWLEDGMENTS
The authors thank Sandy LaMonaca, Peggy Alfarano, and Victoria Cramer for their invaluable administrative assistance. The authors are also very grateful to the members of the various specialty listservs who kindly offered their expert feedback on the draft manuscript, thus improving the quality of the finished product. Finally, the authors extend their sincere thanks to Ivy Leventhal of ACVIM, who managed the submission process to the various listservs and the final manuscript to Journal of Veterinary Internal Medicine. The consensus statement was presented at the 2018 ACVIM Forum in Seattle, Washington.
Garden OA, Kidd L, Mexas AM, et al. ACVIM consensus statement on the diagnosis of immune‐mediated hemolytic anemia in dogs and cats. J Vet Intern Med. 2019;33:313–334. 10.1111/jvim.15441
Consensus Statements of the American College of Veterinary Internal Medicine (ACVIM) provide the veterinary community with up‐to‐date information on the pathophysiology, diagnosis, and treatment of clinically important animal diseases. The ACVIM Board of Regents oversees selection of relevant topics, identification of panel members with the expertise to draft the statements, and other aspects of assuring the integrity of the process. The statements are derived from evidence‐based medicine whenever possible and the panel offers interpretive comments when such evidence is inadequate or contradictory. A draft is prepared by the panel, followed by solicitation of input by the ACVIM membership which may be incorporated into the statement. It is then submitted to the Journal of Veterinary Internal Medicine, where it is edited prior to publication. The authors are solely responsible for the content of the statements.
Oliver A. Garden and Linda Kidd are joint first authors.
Adam Birkenheuer, Simona Buoncompagni, Julien R. S. Dandrieux, Antonio Di Loria, Claire L. Fellman, Barbara Glanemann, Robert Goggs, Jennifer L. Granick, Dana N. LeVine, Claire R. Sharp, Saralyn Smith‐Carr, James W. Swann, and Balazs Szladovits contributed equally to this study and were all members of the relevant Veterinary and Comparative Clinical Immunology Society task forces. OAG, LK, AMM, YMC, UJ, SB, JEF, ALM, and GL were all members of the IMHA Diagnosis Consensus Statement Panel.
REFERENCES
- 1. Piek CJ. Canine idiopathic immune‐mediated haemolytic anaemia: a review with recommendations for future research. Vet Q. 2011;31:129‐141. [DOI] [PubMed] [Google Scholar]
- 2. Piek CJ, van Spil WE, Junius G, Dekker A. Lack of evidence of a beneficial effect of azathioprine in dogs treated with prednisolone for idiopathic immune‐mediated hemolytic anemia: a retrospective cohort study. BMC Vet Res. 2011;7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Swann JW, Szladovits B, Glanemann B. Demographic characteristics, survival and prognostic factors for mortality in cats with primary immune‐mediated hemolytic anemia. J Vet Intern Med. 2016;30:147‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Swann JW, Skelly BJ. Evaluation of immunosuppressive regimens for immune‐mediated haemolytic anaemia: a retrospective study of 42 dogs. J Small Anim Pract. 2011;52:353‐358. [DOI] [PubMed] [Google Scholar]
- 5. Weinkle TK, Center SA, Randolph JF, Warner KL, Barr SC, Erb HN. Evaluation of prognostic factors, survival rates, and treatment protocols for immune‐mediated hemolytic anemia in dogs: 151 cases (1993‐2002). J Am Vet Med Assoc. 2005;226:1869‐1880. [DOI] [PubMed] [Google Scholar]
- 6. Barker RN, Gruffydd‐Jones TJ, Stokes CR, Elson CJ. Autoimmune haemolysis in the dog: relationship between anaemia and the levels of red blood cell bound immunoglobulins and complement measured by an enzyme‐linked antiglobulin test. Vet Immunol Immunopathol. 1992;34:1‐20. [DOI] [PubMed] [Google Scholar]
- 7. Barker RN, Elson CJ. Red blood cell glycophorins as B and T‐cell antigens in canine autoimmune haemolytic anaemia. Vet Immunol Immunopathol. 1995;47:225‐238. [DOI] [PubMed] [Google Scholar]
- 8. Berentsen S, Sundic T. Red blood cell destruction in autoimmune hemolytic anemia: role of complement and potential new targets for therapy. Biomed Res Int. 2015;2015:363278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kohn B, Weingart C, Eckmann V, Ottenjann M, Leibold W. Primary immune‐mediated hemolytic anemia in 19 cats: diagnosis, therapy, and outcome (1998‐2004). J Vet Intern Med. 2006;20:159‐166. [DOI] [PubMed] [Google Scholar]
- 10. Warman SM, Murray JK, Ridyard A, Eastwood J, Silva S, Day MJ. Pattern of Coombs' test reactivity has diagnostic significance in dogs with immune‐mediated haemolytic anaemia. J Small Anim Pract. 2008;49:525‐530. [DOI] [PubMed] [Google Scholar]
- 11. Caviezel LL, Raj K, Giger U. Comparison of 4 direct Coombs' test methods with polyclonal antiglobulins in anemic and nonanemic dogs for in‐clinic or laboratory use. J Vet Intern Med. 2014;28:583‐591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Engelbrecht R, Kohn B, Leibold W, et al. Clinical findings, diagnostics and treatment results in primary and secondary immune‐mediated hemolytic anemia in the dog. Kleintierpraxis. 2002;47:265‐278. [Google Scholar]
- 13. Hill QA, Stamps R, Massey E, et al. The diagnosis and management of primary autoimmune haemolytic anaemia. Br J Haematol. 2017;176:395‐411. [DOI] [PubMed] [Google Scholar]
- 14. Lucidi CA, de Rezende CLE, Jutkowitz LA, et al. Histologic and cytologic bone marrow findings in dogs with suspected precursor‐targeted immune‐mediated anemia and associated phagocytosis of erythroid precursors. Vet Clin Pathol. 2017;46:401‐415. [DOI] [PubMed] [Google Scholar]
- 15. Means RT Jr. Pure red cell aplasia. Blood. 2016;128:2504‐2509. [DOI] [PubMed] [Google Scholar]
- 16. Stokol T, Blue JT, French TW. Idiopathic pure red cell aplasia and nonregenerative immune‐mediated anemia in dogs: 43 cases (1988‐1999). J Am Vet Med Assoc. 2000;216:1429‐1436. [DOI] [PubMed] [Google Scholar]
- 17. Weiss DJ. Bone marrow pathology in dogs and cats with non‐regenerative immune‐mediated haemolytic anaemia and pure red cell aplasia. J Comp Pathol. 2008;138:46‐53. [DOI] [PubMed] [Google Scholar]
- 18. Bessman JD, Banks D. Spurious macrocytosis, a common clue to erythrocyte cold agglutinins. Am J Clin Pathol. 1980;74:797‐800. [DOI] [PubMed] [Google Scholar]
- 19. Rojas‐Temahuay G, Crain S, Benson C, Sharkey L, Nothnagel G. Cold agglutinin activity in 2 dogs. Vet Clin Pathol. 2014;43:330‐336. [DOI] [PubMed] [Google Scholar]
- 20. Zandecki M, Genevieve F, Gerard J, Godon A. Spurious counts and spurious results on haematology analysers: a review. Part II: white blood cells, red blood cells, haemoglobin, red cell indices and reticulocytes. Int J Lab Hematol. 2007;29:21‐41. [DOI] [PubMed] [Google Scholar]
- 21. Furth FW. Effect of spherocytosis on volume of trapped plasma in red cell column of capillary and Wintrobe hematocrits. J Lab Clin Med. 1956;48:421‐430. [PubMed] [Google Scholar]
- 22. Klag AR, Giger U, Shofer FS. Idiopathic immune‐mediated hemolytic anemia in dogs: 42 cases (1986‐1990). J Am Vet Med Assoc. 1993;202:783‐788. [PubMed] [Google Scholar]
- 23. Sierra FD, Melzak KA, Janetzko K, et al. Flow morphometry to assess the red blood cell storage lesion. Cytom Part A. 2017;91:874‐882. [DOI] [PubMed] [Google Scholar]
- 24. Mollison PL, Newlands M. Unusual delayed haemolytic transfusion reaction characterised by slow destruction of red cells. Vox Sang. 1976;31:54‐57. [DOI] [PubMed] [Google Scholar]
- 25. Rao KR, Patel AR. Delayed hemolytic transfusion reactions in sickle cell anemia. South Med J. 1989;82:1034‐1036. [DOI] [PubMed] [Google Scholar]
- 26. Lanaux TM, Rozanski EA, Simoni RS, et al. Interpretation of canine and feline blood smears by emergency room personnel. Vet Clin Pathol. 2011;40:18‐23. [DOI] [PubMed] [Google Scholar]
- 27. Paes G, Paepe D, Meyer E, et al. The use of the rapid osmotic fragility test as an additional test to diagnose canine immune‐mediated haemolytic anaemia. Acta Vet Scand. 2013;55:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Safeukui I, Buffet PA, Deplaine G, et al. Quantitative assessment of sensing and sequestration of spherocytic erythrocytes by the human spleen. Blood. 2012;120:424‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wen ZY, Song LC, Yan ZY, et al. An animal model to study erythrocyte senescence with a narrow time window of erythrocyte production: alterations in osmotic fragility and deformability of erythrocytes during their life span. Clin Hemorheol Microcirc. 1998;19:299‐306. [PubMed] [Google Scholar]
- 30. Gurnee CM, Drobatz KJ. Zinc intoxication in dogs: 19 cases (1991‐2003). J Am Vet Med Assoc. 2007;230:1174‐1179. [DOI] [PubMed] [Google Scholar]
- 31. Bexfield N, Archer J, Herrtage M. Heinz body haemolytic anaemia in a dog secondary to ingestion of a zinc toy: a case report. Vet J. 2007;174:414‐417. [DOI] [PubMed] [Google Scholar]
- 32. Schlesinger DP. Methemoglobinemia and anemia in a dog with acetaminophen toxicity. Can Vet J. 1995;36:515‐517. [PMC free article] [PubMed] [Google Scholar]
- 33. Masserdotti C. Unusual "erythroid loops" in canine blood smears after viper‐bite envenomation. Vet Clin Pathol. 2009;38:321‐325. [DOI] [PubMed] [Google Scholar]
- 34. Noble SJ, Armstrong PJ. Bee sting envenomation resulting in secondary immune‐mediated hemolytic anemia in two dogs. J Am Vet Med Assoc. 1999;214(1026–1027):1021. [PubMed] [Google Scholar]
- 35. Waddell LS, Drobatz KJ. Massive envenomation by Vespula spp. in two dogs. J Vet Emerg Crit Car. 1999;9:67‐71. [Google Scholar]
- 36. Walton RM, Brown DE, Hamar DW, Meador VP, Horn JW, Thrall MA. Mechanisms of echinocytosis induced by Crotalus atrox venom. Vet Pathol. 1997;34:442‐449. [DOI] [PubMed] [Google Scholar]
- 37. Wysoke JM, Bland van‐den Berg P, Marshall C. Bee sting‐induced haemolysis, spherocytosis and neural dysfunction in three dogs. J S Afr Vet Assoc. 1990;61:29‐32. [PubMed] [Google Scholar]
- 38. Fry MM, Vernau W, Pesavento PA, Brömel C, Moore PF. Hepatosplenic lymphoma in a dog. Vet Pathol. 2003;40:556‐562. [DOI] [PubMed] [Google Scholar]
- 39. Chapman BL, Giger U. Inherited erythrocyte pyruvate‐kinase deficiency in the West Highland white terrier. J Small Anim Pract. 1990;31:610‐616. [Google Scholar]
- 40. Breitschwerdt EB, Kordick DL, Malarkey DE, Keene B, Hadfield TL, Wilson K. Endocarditis in a dog due to infection with a novel Bartonella subspecies. J Clin Microbiol. 1995;33:154‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ng CY, Mills JN. Clinical and haematological features of haemangiosarcoma in dogs. Aust Vet J. 1985;62:1‐4. [DOI] [PubMed] [Google Scholar]
- 42. Dell'Orco M, Bertazzolo W, Pagliaro L, Roccabianca P, Comazzi S. Hemolytic‐uremic syndrome in a dog. Vet Clin Pathol. 2005;34:264‐269. [DOI] [PubMed] [Google Scholar]
- 43. Holland CT, Canfield PJ, Watson ADJ, Allan GS. Dyserythropoiesis, polymyopathy, and cardiac disease in three related English Springer Spaniels. J Vet Intern Med. 1991;5:151‐159. [DOI] [PubMed] [Google Scholar]
- 44. Slappendel RJ, van Zwieten R, van Leeuwen M, et al. Hereditary spectrin deficiency in Golden Retriever dogs. J Vet Intern Med. 2005;19:187‐192. [DOI] [PubMed] [Google Scholar]
- 45. Mariani M, Barcellini W, Vercellati C, et al. Clinical and hematologic features of 300 patients affected by hereditary spherocytosis grouped according to the type of the membrane protein defect. Haematologica. 2008;93:1310‐1317. [DOI] [PubMed] [Google Scholar]
- 46. Weiss DJ. Uniform evaluation and semiquantitative reporting of hematologic data in veterinary laboratories. Vet Clin Pathol. 1984;13:27‐31. [DOI] [PubMed] [Google Scholar]
- 47. Conrado FO, Weeden AL, Speas AL, Leissinger MK. Macrocytosis secondary to hydroxyurea therapy. Vet Clin Pathol. 2017;46:451‐456. [DOI] [PubMed] [Google Scholar]
- 48. Rampling MW. The binding of fibrinogen and fibrinogen degradation products to the erythrocyte membrane and its relationship to haemorheology. Acta Biol Med Ger. 1981;40:373‐378. [PubMed] [Google Scholar]
- 49. Slappendel RJ. The diagnostic significance of the direct antiglobulin test (DAT) in anemic dogs. Vet Immunol Immunop. 1979;1:49‐59. [DOI] [PubMed] [Google Scholar]
- 50. Overmann JA, Sharkey LC, Weiss DJ, Borjesson DL. Performance of 2 microtiter canine Coombs' tests. Vet Clin Pathol. 2007;36:179‐183. [DOI] [PubMed] [Google Scholar]
- 51. Tasker S, Murray JK, Knowles TG, Day MJ. Coombs', haemoplasma and retrovirus testing in feline anaemia. J Small Anim Pract. 2010;51:192‐199. [DOI] [PubMed] [Google Scholar]
- 52. Blais MC, Rozanski EA, Hale AS, Shaw SP, Cotter SM. Lack of evidence of pregnancy‐induced alloantibodies in dogs. J Vet Intern Med. 2009;23:462‐465. [DOI] [PubMed] [Google Scholar]
- 53. Tritschler C, Mizukami K, Raj K, Giger U. Increased erythrocytic osmotic fragility in anemic domestic shorthair and purebred cats. J Feline Med Surg. 2016;18:462‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wilkerson MJ, Davis E, Shuman W, Harkin K, Cox J, Rush B. Isotype‐specific antibodies in horses and dogs with immune‐mediated hemolytic anemia. J Vet Intern Med. 2000;14:190‐196. [DOI] [PubMed] [Google Scholar]
- 55. Quigley KA, Chelack BJ, Haines DM, Jackson ML. Application of a direct flow cytometric erythrocyte immunofluorescence assay in dogs with immune‐mediated hemolytic anemia and comparison to the direct antiglobulin test. J Vet Diagn Invest. 2001;13:297‐300. [DOI] [PubMed] [Google Scholar]
- 56. Morley P, Mathes M, Guth A, Dow S. Anti‐erythrocyte antibodies and disease associations in anemic and nonanemic dogs. J Vet Intern Med. 2008;22:886‐892. [DOI] [PubMed] [Google Scholar]
- 57. Carli E, Tasca S, Trotta M, Furlanello T, Caldin M, Solano‐Gallego L. Detection of erythrocyte binding IgM and IgG by flow cytometry in sick dogs with Babesia canis canis or Babesia canis vogeli infection. Vet Parasitol. 2009;162:51‐57. [DOI] [PubMed] [Google Scholar]
- 58. Harkin KR, Hicks JA, Wilkerson MJ. Erythrocyte‐bound immunoglobulin isotypes in dogs with immune‐mediated hemolytic anemia: 54 cases (2001‐2010). J Am Vet Med Assoc. 2012;241:227‐232. [DOI] [PubMed] [Google Scholar]
- 59. Day MJ. Serial monitoring of clinical, haematological and immunological parameters in canine autoimmune haemolytic anaemia. J Small Anim Pract. 1996;37:523‐534. [DOI] [PubMed] [Google Scholar]
- 60. Mason N, Duval D, Shofer FS, Giger U. Cyclophosphamide exerts no beneficial effect over prednisone alone in the initial treatment of acute immune‐mediated hemolytic anemia in dogs: a randomized controlled clinical trial. J Vet Intern Med. 2003;17:206‐212. [DOI] [PubMed] [Google Scholar]
- 61. Mills JN, Day MJ, Shaw SE, et al. Autoimmune haemolytic anaemia in dogs. Aust Vet J. 1985;62:121‐123. [DOI] [PubMed] [Google Scholar]
- 62. Aygun B, Padmanabhan S, Paley C, Chandrasekaran V. Clinical significance of RBC alloantibodies and autoantibodies in sickle cell patients who received transfusions. Transfusion. 2002;42:37‐43. [DOI] [PubMed] [Google Scholar]
- 63. Winters JL, Richa EM, Bryant SC, Tauscher CD, Bendix BJ, Stubbs JR. Polyethylene glycol antiglobulin tube versus gel microcolumn: influence on the incidence of delayed hemolytic transfusion reactions and delayed serologic transfusion reactions. Transfusion. 2010;50:1444‐1452. [DOI] [PubMed] [Google Scholar]
- 64. Ness PM, Shirey RS, Thoman SK, Buck SA. The differentiation of delayed serologic and delayed hemolytic transfusion reactions: incidence, long‐term serologic findings, and clinical significance. Transfusion. 1990;30:688‐693. [DOI] [PubMed] [Google Scholar]
- 65. Da Costa L, Mohandas N, Sorette M, et al. Temporal differences in membrane loss lead to distinct reticulocyte features in hereditary spherocytosis and in immune hemolytic anemia. Blood. 2001;98:2894‐2899. [DOI] [PubMed] [Google Scholar]
- 66. LoBuglio AF, Cotran RS, Jandl JH. Red cells coated with immunoglobulin G: binding and sphering by mononuclear cells in man. Science. 1967;158:1582‐1585. [DOI] [PubMed] [Google Scholar]
- 67. Iolascon A, Andolfo I, Barcellini W, et al. Recommendations regarding splenectomy in hereditary hemolytic anemias. Haematologica. 2017;102:1304‐1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wiley JS. Red cell survival studies in hereditary spherocytosis. J Clin Invest. 1970;49:666‐672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shull BC, Lees H, Li PK. Mechanism of interference by hemoglobin in the determination of total bilirubin. I. Method of Malloy‐Evelyn. Clin Chem. 1980;26:22‐25. [PubMed] [Google Scholar]
- 70. Dolci A, Panteghini M. Harmonization of automated hemolysis index assessment and use: is it possible? Clin Chim Acta. 2014;432:38‐43. [DOI] [PubMed] [Google Scholar]
- 71. Yoo G, Kim J, Uh Y, Yoon KR, Park SD, Yoon KJ. Scoring system for detecting spurious hemolysis in anticoagulated blood specimens. Ann Lab Med. 2015;35:341‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Grant MS. The effect of blood drawing techniques and equipment on the hemolysis of ED laboratory blood samples. J Emerg Nurs. 2003;29:116‐121. [DOI] [PubMed] [Google Scholar]
- 73. Dugan L, Leech L, Speroni KG, Corriher J. Factors affecting hemolysis rates in blood samples drawn from newly placed IV sites in the emergency department. J Emerg Nurs. 2005;31:338‐345. [DOI] [PubMed] [Google Scholar]
- 74. Garcia‐Pereira BL, Scott MA, Koenigshof AM, Brown AJ. Effect of venipuncture quality on thromboelastography. J Vet Emerg Crit Care. 2012;22:225‐229. [DOI] [PubMed] [Google Scholar]
- 75. Wollowitz A, Bijur PE, Esses D, John Gallagher E. Use of butterfly needles to draw blood is independently associated with marked reduction in hemolysis compared to intravenous catheter. Acad Emerg Med. 2013;20:1151‐1155. [DOI] [PubMed] [Google Scholar]
- 76. Kennedy C, Angermuller S, King R, et al. A comparison of hemolysis rates using intravenous catheters versus venipuncture tubes for obtaining blood samples. J Emerg Nurs. 1996;22:566‐569. [DOI] [PubMed] [Google Scholar]
- 77. Lippi G, Cervellin G, Mattiuzzi C. Critical review and meta‐analysis of spurious hemolysis in blood samples collected from intravenous catheters. Biochem Med. 2013;23:193‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fang L, Fang SH, Chung YH, Chien ST. Collecting factors related to the haemolysis of blood specimens. J Clin Nurs. 2008;17:2343‐2351. [DOI] [PubMed] [Google Scholar]
- 79. Dimeski G, Mollee P, Carter A. Increased lipid concentration is associated with increased hemolysis. Clin Chem. 2005;51:2425. [DOI] [PubMed] [Google Scholar]
- 80. Goggs R, Boag AK, Chan DL. Concurrent immune‐mediated haemolytic anaemia and severe thrombocytopenia in 21 dogs. Vet Rec. 2008;163:323‐327. [DOI] [PubMed] [Google Scholar]
- 81. Barros MM, Blajchman MA, Bordin JO. Warm autoimmune hemolytic anemia: recent progress in understanding the immunobiology and the treatment. Transfus Med Rev. 2010;24:195‐210. [DOI] [PubMed] [Google Scholar]
- 82. Cavalcante P, Galbardi B, Franzi S, et al. Increased expression of Toll‐like receptors 7 and 9 in myasthenia gravis thymus characterized by active Epstein‐Barr virus infection. Immunobiology. 2016;221:516‐527. [DOI] [PubMed] [Google Scholar]
- 83. Iberg CA, Jones A, Hawiger D. Dendritic cells as inducers of peripheral tolerance. Trends Immunol. 2017;38:793‐804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Liu GY, Fairchild PJ, Smith RM, Prowle JR, Kioussis D, Wraith DC. Low avidity recognition of self‐antigen by T cells permits escape from central tolerance. Immunity. 1995;3:407‐415. [DOI] [PubMed] [Google Scholar]
- 85. Netea MG, Wijmenga C, O'Neill LA. Genetic variation in Toll‐like receptors and disease susceptibility. Nat Immunol. 2012;13:535‐542. [DOI] [PubMed] [Google Scholar]
- 86. Rivera‐Correa J, Rodriguez A. Divergent roles of antiself antibodies during infection. Trends Immunol. 2018;39:515‐522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Frydman GH, Davis N, Beck PL, Fox JG. Helicobacter pylori eradication in patients with immune thrombocytopenic purpura: a review and the role of biogeography. Helicobacter. 2015;20:239‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Khan FY, Ay M. Mycoplasma pneumoniae associated with severe autoimmune hemolytic anemia: case report and literature review. Braz J Infect Dis. 2009;13:77‐79. [DOI] [PubMed] [Google Scholar]
- 89. Hirako IC, Gallego‐Marin C, Ataide MA, et al. DNA‐containing immunocomplexes promote inflammasome assembly and release of pyrogenic cytokines by CD14+CD16+CD64highCD32low inflammatory monocytes from malaria patients. MBio. 2015;6:e01605‐e01615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chung CY, Ysebaert D, Berneman ZN, et al. Dendritic cells: cellular mediators for immunological tolerance. Clin Dev Immunol. 2013;2013:972865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Barker RN, Vickers MA, Ward FJ. Controlling autoimmunity–lessons from the study of red blood cells as model antigens. Immunol Lett. 2007;108:20‐26. [DOI] [PubMed] [Google Scholar]
- 92. Fernandez‐Arias C, Rivera‐Correa J, Gallego‐Delgado J, et al. Anti‐self phosphatidylserine antibodies recognize uninfected erythrocytes promoting malarial anemia. Cell Host Microbe. 2016;19:194‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Adachi K, Tateishi M, Horii Y, et al. Elevated erythrocyte‐bound IgG value in dogs with clinical Babesia gibsoni infection. J Vet Med Sci. 1994;56:757‐759. [DOI] [PubMed] [Google Scholar]
- 94. Adachi K, Tateishi M, Horii Y, et al. Reactivity of serum anti‐erythrocyte membrane antibody in Babesia gibsoni‐infected dogs. J Vet Med Sci. 1994;56:997‐999. [DOI] [PubMed] [Google Scholar]
- 95. Morita T, Saeki H, Imai S, Ishii T. Reactivity of anti‐erythrocyte antibody induced by Babesia gibsoni infection against aged erythrocytes. Vet Parasitol. 1995;58:291‐299. [DOI] [PubMed] [Google Scholar]
- 96. Otsuka Y, Yamasaki M, Yamato O, Maede Y. The effect of macrophages on the erythrocyte oxidative damage and the pathogenesis of anemia in Babesia gibsoni‐infected dogs with low parasitemia. J Vet Med Sci. 2002;64:221‐226. [DOI] [PubMed] [Google Scholar]
- 97. Zulty JC, Kociba GJ. Cold agglutinins in cats with haemobartonellosis. J Am Vet Med Assoc. 1990;196:907‐910. [PubMed] [Google Scholar]
- 98. Badior KE, Casey JR. Molecular mechanism for the red blood cell senescence clock. IUBMB Life. 2018;70:32‐40. [DOI] [PubMed] [Google Scholar]
- 99. Adachi K, Yoshimoto A, Hasegawa T, et al. Anti‐erythrocyte membrane antibodies detected in sera of dogs naturally infected with Babesia gibsoni . J Vet Med Sci. 1992;54:1081‐1084. [DOI] [PubMed] [Google Scholar]
- 100. Antognoni MT, Veronesi F, Morganti G, Mangili V, Fruganti G, Miglio A. Natural infection of Anaplasma platys in dogs from Umbria region (Central Italy). Vet Ital. 2014;50:49‐56. [DOI] [PubMed] [Google Scholar]
- 101. Bexfield NH, Villiers EJ, Herrtage ME. Immune‐mediated haemolytic anaemia and thrombocytopenia associated with Anaplasma phagocytophilum in a dog. J Small Anim Pract. 2005;46:543‐548. [DOI] [PubMed] [Google Scholar]
- 102. Birkenheuer AJ, Correa MT, Levy MG, Breitschwerdt EB. Geographic distribution of babesiosis among dogs in the United States and association with dog bites: 150 cases (2000‐2003). J Am Vet Med Assoc. 2005;227:942‐947. [DOI] [PubMed] [Google Scholar]
- 103. Birkenheuer AJ, Levy MG, Savary KC, Gager RB, Breitschwerdt EB. Babesia gibsoni infections in dogs from North Carolina. J Am Anim Hosp Assoc. 1999;35:125‐128. [DOI] [PubMed] [Google Scholar]
- 104. Bouzouraa T, Cadore JL, Chene J, et al. Implication, clinical and biological impact of vector‐borne haemopathogens in anaemic dogs in France: a prospective study. J Small Anim Pract. 2017;58:510‐518. [DOI] [PubMed] [Google Scholar]
- 105. Breitschwerdt EB, Blann KR, Stebbins ME, et al. Clinicopathological abnormalities and treatment response in 24 dogs seroreactive to Bartonella vinsonii (berkhoffii) antigens. J Am Anim Hosp Assoc. 2004;40:92‐101. [DOI] [PubMed] [Google Scholar]
- 106. Bunch SE, Metcalf MR, Crane SW, Cullen JM. Idiopathic pleural effusion and pulmonary thromboembolism in a dog with autoimmune hemolytic anemia. J Am Vet Med Assoc. 1989;195:1748‐1753. [PubMed] [Google Scholar]
- 107. Carr AP, Panciera DL, Kidd L. Prognostic factors for mortality and thromboembolism in canine immune‐mediated hemolytic anemia: a retrospective study of 72 dogs. J Vet Intern Med. 2002;16:504‐509. [DOI] [PubMed] [Google Scholar]
- 108. Chirek A, Silaghi C, Pfister K, Kohn B. Granulocytic anaplasmosis in 63 dogs: clinical signs, laboratory results, therapy and course of disease. J Small Anim Pract. 2018;59:112‐120. [DOI] [PubMed] [Google Scholar]
- 109. Ciaramella P, Oliva G, Luna RD, et al. A retrospective clinical study of canine leishmaniasis in 150 dogs naturally infected by Leishmania infantum . Vet Rec. 1997;141:539‐543. [DOI] [PubMed] [Google Scholar]
- 110. Dunn JK, Searcy GP, Hirsch VM. The diagnostic significance of a positive direct antiglobulin test in anemic cats. Can J Comp Med. 1984;48:349‐353. [PMC free article] [PubMed] [Google Scholar]
- 111. Farwell GE, LeGrand EK, Cobb CC. Clinical observations on Babesia gibsoni and Babesia canis infections in dogs. J Am Vet Med Assoc. 1982;180:507‐511. [PubMed] [Google Scholar]
- 112. Feldman B. Demographics of canine immune‐mediated haemolytic anaemia in the southeastern United States. Comp Haematol Int. 1996;6:42‐45. [Google Scholar]
- 113. Foster AP, Sturgess CP, Gould DJ, Iwasaki T, Day MJ. Pemphigus foliaceus in association with systemic lupus erythematosus, and subsequent lymphoma in a cocker spaniel. J Small Anim Pract. 2000;41:266‐270. [DOI] [PubMed] [Google Scholar]
- 114. Fry DR, McSporran KD, Ellis JT, Harvey C. Protozoal hepatitis associated with immunosuppressive therapy in a dog. J Vet Intern Med. 2009;23:366‐368. [DOI] [PubMed] [Google Scholar]
- 115. Holloway SA, Meyer DJ, Mannella C. Prednisolone and danazol for treatment of immune‐mediated anemia, thrombocytopenia, and ineffective erythroid regeneration in a dog. J Am Vet Med Assoc. 1990;197:1045‐1048. [PubMed] [Google Scholar]
- 116. Jackson ML, Kruth SA. Immune‐mediated hemolytic anemia and thrombocytopenia in the dog: a retrospective study of 55 cases diagnosed from 1979 through 1983 at the Western College of Veterinary Medicine. Can Vet J. 1985;26:245‐250. [PMC free article] [PubMed] [Google Scholar]
- 117. Kidd L, Geddings J, Hisada Y, et al. Procoagulant microparticles in dogs with immune‐mediated hemolytic anemia. J Vet Intern Med. 2015;29:908‐916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Klein MK, Dow SW, Rosychuk RA. Pulmonary thromboembolism associated with immune‐mediated hemolytic anemia in dogs: ten cases (1982‐1987). J Am Vet Med Assoc. 1989;195:246‐250. [PubMed] [Google Scholar]
- 119. Limlenglert P, Rungsipipat A, Pusoonthornthum R. Application of flow cytometry for early diagnosis and monitoring in cats with immune‐mediated hemolytic anemia. Thai J Vet Med. 2011;41:185‐191. [Google Scholar]
- 120. Lobetti R. Changes in the serum urea: creatinine ratio in dogs with babesiosis, haemolytic anaemia, and experimental haemoglobinaemia. Vet J. 2012;191:253‐256. [DOI] [PubMed] [Google Scholar]
- 121. Lobetti RG, Schoeman T. Immune‐mediated haemolytic anaemia: possible association with Ancylostoma caninum infection in three dogs. J S Afr Vet Assoc. 2001;72:52‐54. [DOI] [PubMed] [Google Scholar]
- 122. Lobetti R, Dvir E, Pearson J. Cardiac troponins in canine babesiosis. J Vet Intern Med. 2002;16:63‐68. [DOI] [PubMed] [Google Scholar]
- 123. Magana A, Sanchez F, Villa K, et al. Systemic neosporosis in a dog treated for immune‐mediated thrombocytopenia and hemolytic anemia. Vet Clin Pathol. 2015;44:592‐596. [DOI] [PubMed] [Google Scholar]
- 124. Marcos R, Santos M, Malhao F, et al. Pancytopenia in a cat with visceral leishmaniasis. Vet Clin Pathol. 2009;38:201‐205. [DOI] [PubMed] [Google Scholar]
- 125. Matsuu A, Kawabe A, Koshida Y, et al. Incidence of canine Babesia gibsoni infection and subclinical infection among Tosa dogs in Aomori Prefecture, Japan. J Vet Med Sci. 2004;66:893‐897. [DOI] [PubMed] [Google Scholar]
- 126. Mazepa AW, Kidd LB, Young KM, Trepanier LA. Clinical presentation of 26 Anaplasma phagocytophilum‐seropositive dogs residing in an endemic area. J Am Anim Hosp Assoc. 2010;46:405‐412. [DOI] [PubMed] [Google Scholar]
- 127. Mellor PJ, Roulois AJ, Day MJ, et al. Neutrophilic dermatitis and immune‐mediated haematological disorders in a dog: suspected adverse reaction to carprofen. J Small Anim Pract. 2005;46:237‐242. [DOI] [PubMed] [Google Scholar]
- 128. Mathe A, Voros K, Papp L, et al. Clinical manifestations of canine babesiosis in Hungary (63 cases). Acta Vet Hung. 2006;54:367‐385. [DOI] [PubMed] [Google Scholar]
- 129. Kjelgaard‐Hansen M, Jensen AL, Houser GA, Jessen LR, Kristensen AT. Use of serum C‐reactive protein as an early marker of inflammatory activity in canine type II immune‐mediated polyarthritis: case report. Acta Vet Scand. 2006;48:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Merten N, Weingart C, Kohn B. Causes, diagnostics and course of disease in 194 cats with anemia. Kleintierpraxis. 2015;60:625‐636. [PubMed] [Google Scholar]
- 131. Miller SA, Hohenhaus AE, Hale AS. Case‐control study of blood type, breed, sex, and bacteremia in dogs with immune‐mediated hemolytic anemia. J Am Vet Med Assoc. 2004;224:232‐235. [DOI] [PubMed] [Google Scholar]
- 132. Mitchell KD, Kruth SA, Wood RD, Jefferson B. Serum acute phase protein concentrations in dogs with autoimmune hemolytic anemia. J Vet Intern Med. 2009;23:585‐591. [DOI] [PubMed] [Google Scholar]
- 133. Nel M, Lobetti RG, Keller N, Thompson PN. Prognostic value of blood lactate, blood glucose, and hematocrit in canine babesiosis. J Vet Intern Med. 2004;18:471‐476. [DOI] [PubMed] [Google Scholar]
- 134. Piek CJ, Teske E, van Leeuwen MW, Day MJ. Good agreement of conventional and gel‐based direct agglutination test in immune‐mediated haemolytic anaemia. Acta Vet Scand. 2012;54:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Pinkos AC, Friedrichs KR, Monaghan KN, Sample SH, Trepanier LA. Transient cold agglutinins associated with Mycoplasma cynos pneumonia in a dog. Vet Clin Pathol. 2015;44:498‐502. [DOI] [PubMed] [Google Scholar]
- 136. Reyers F, Leisewitz AL, Lobetti RG, Milner RJ, Jacobson LS, van Zyl M. Canine babesiosis in South Africa: more than one disease. Does this serve as a model for falciparum malaria? Ann Trop Med Parasitol. 1998;92:503‐511. [PubMed] [Google Scholar]
- 137. Rosa CT, Pazzi P, Nagel S, et al. Theileriosis in six dogs in South Africa and its potential clinical significance. J S Afr Vet Assoc. 2014;85:1114. [DOI] [PubMed] [Google Scholar]
- 138. Schoeman T, Lobetti RG, Jacobson LS, Penzhorn BL. Feline babesiosis: signalment, clinical pathology and concurrent infections. J S Afr Vet Assoc. 2001;72:4‐11. [DOI] [PubMed] [Google Scholar]
- 139. Sykes JE, Bailiff NL, Ball LM, et al. Identification of a novel hemotropic mycoplasma in a splenectomized dog with hemic neoplasia. J Am Vet Med Assoc. 2004;224:1946‐1951, 1930‐1931. [DOI] [PubMed] [Google Scholar]
- 140. Tasker S, Peters IR, Papasouliotis K, et al. Description of outcomes of experimental infection with feline haemoplasmas: copy numbers, haematology, Coombs' testing and blood glucose concentrations. Vet Microbiol. 2009;139:323‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Solano‐Gallego L, Trotta M, Carli E, Carcy B, Caldin M, Furlanello T. Babesia canis canis and Babesia canis vogeli clinicopathological findings and DNA detection by means of PCR‐RFLP in blood from Italian dogs suspected of tick‐borne disease. Vet Parasitol. 2008;157:211‐221. [DOI] [PubMed] [Google Scholar]
- 142. Wang A, Smith JR, Creevy KE. Treatment of canine idiopathic immune‐mediated haemolytic anaemia with mycophenolate mofetil and glucocorticoids: 30 cases (2007 to 2011). J Small Anim Pract. 2013;54:399‐404. [DOI] [PubMed] [Google Scholar]
- 143. Warman SM, Helps CR, Barker EN, et al. Haemoplasma infection is not a common cause of canine immune‐mediated haemolytic anaemia in the UK. J Small Anim Pract. 2010;51:534‐539. [DOI] [PubMed] [Google Scholar]
- 144. Weingart C, Tasker S, Kohn B. Infection with haemoplasma species in 22 cats with anaemia. J Feline Med Surg. 2016;18:129‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Werner LL, Halliwell RE, Jackson RF, et al. An investigation of the role of immunologic factors in anemia associated with canine heartworm disease. Vet Immunol Immunopathol. 1984;7:285‐292. [DOI] [PubMed] [Google Scholar]
- 146. Whitney MS, Schwan TG, Sultemeier KB, McDonald PS, Brillhart MN. Spirochetemia caused by Borrelia turicatae infection in 3 dogs in Texas. Vet Clin Pathol. 2007;36:212‐216. [DOI] [PubMed] [Google Scholar]
- 147. Wozniak EJ, Barr BC, Thomford JW, et al. Clinical, anatomic, and immunopathologic characterization of Babesia gibsoni infection in the domestic dog (Canis familiaris). J Parasitol. 1997;83:692‐699. [PubMed] [Google Scholar]
- 148. Zeugswetter F, Hittmair KM, de Arespacochaga AG, Shibly S, Spergser J. Erosive polyarthritis associated with Mycoplasma gateae in a cat. J Feline Med Surg. 2007;9:226‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Franca RT, Da Silva AS, Costa MM, et al. Hematologic and bone marrow changes in dogs experimentally infected with Rangelia vitalii. Vet Clin Pathol. 2013;42:31‐39. [DOI] [PubMed] [Google Scholar]
- 150. Khatat SE, Defauw P, Marynissen S, et al. Exposure to Anaplasma phagocytophilum in two dogs in Belgium. Vlaams Diergeneeskundig Tijdschrift. 2015;84:39‐46. [Google Scholar]
- 151. Lee MJ, Yu DH, Yoon JS, et al. Epidemiologic and clinical surveys in dogs infected with Babesia gibsoni in South Korea. Vector Borne Zoonotic Dis. 2009;9:681‐686. [DOI] [PubMed] [Google Scholar]
- 152. van Geffen C. Coinfection with Mycoplasma haemofelis and 'Candidatus Mycoplasma haemominutum' in a cat with immune‐mediated hemolytic anemia in Belgium. Vlaams Diergeneeskundig Tijdschrift. 2012;81:224‐228. [Google Scholar]
- 153. Fathi EA, Atyabi N, Sharifi YH, Nasiri SM. Immune‐mediated hemolytic anemia in cats referring to Veterinary Teaching Hospital of Tehran (2006‐2007). Iran J Vet Res. 2009;10:373‐377. [Google Scholar]
- 154. Scott DW, Schultz RD, Post JE, et al. Autoimmune hemolytic anemia in the cat. J Am Anim Hosp Assoc. 1973;9:530‐539. [Google Scholar]
- 155. Hall AM, Vickers MA, McLeod E, Barker RN. Rh autoantigen presentation to helper T cells in chronic lymphocytic leukemia by malignant B cells. Blood. 2005;105:2007‐2015. [DOI] [PubMed] [Google Scholar]
- 156. Tsang M, Parikh SA. A concise review of autoimmune cytopenias in chronic lymphocytic leukemia. Curr Hematol Malig Rep. 2017;12:29‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Ugoeke N, Onweni C, Treece J, et al. Inflammatory breast cancer and warm antibody autoimmune hemolytic anemia: a rare paraneoplastic syndrome. J Investig Med High Impact Case Rep. 2017;5:2324709617740905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Yu H, Fu R, Wang H, Liu H, Shao Z. Paraneoplastic Evans syndrome in a patient with adenocarcinoma of the lung: a case report. Thorac Cancer. 2017;8:57‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Mellanby RJ, Holloway A, Chantrey J, Herrtage ME, Dobson JM. Immune‐mediated haemolytic anaemia associated with a sarcoma in a flat‐coated retriever. J Small Anim Pract. 2004;45:21‐24. [PubMed] [Google Scholar]
- 160. Cuq B, Blois SL, Mathews KA. Anti‐thymocyte serum as part of an immunosuppressive regimen in treating haematological immune‐mediated diseases in dogs. J Small Anim Pract. 2017;58:348‐354. [DOI] [PubMed] [Google Scholar]
- 161. Day MJ, Penhale WJ. Immune‐mediated disease in the old English Sheepdog. Res Vet Sci. 1992;53:87‐92. [DOI] [PubMed] [Google Scholar]
- 162. Fenty RK, Delaforcade AM, Shaw SE, et al. Identification of hypercoagulability in dogs with primary immune‐mediated hemolytic anemia by means of thromboelastography. J Am Vet Med Assoc. 2011;238:463‐467. [DOI] [PubMed] [Google Scholar]
- 163. Goggs R, Wiinberg B, Kjelgaard‐Hansen M, Chan DL. Serial assessment of the coagulation status of dogs with immune‐mediated haemolytic anaemia using thromboelastography. Vet J. 2012;191:347‐353. [DOI] [PubMed] [Google Scholar]
- 164. Gunn‐Moore DA, Day MJ, Graham ME, et al. Immune‐mediated haemolytic anaemia in two sibling cats associated with multicentric lymphoblastic infiltration. J Feline Med Surg. 1999;1:209‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Zoia A, Drigo M. Association between pancreatitis and immune‐mediated haemolytic anaemia in cats: a cross‐sectional study. J Comp Pathol. 2017;156:384‐388. [DOI] [PubMed] [Google Scholar]
- 166. Guadarrama‐Olhovich M, Ortuno LEG, Remolina JAR, et al. Acute pancreatitis, azotaemia, cholestasis and haemolytic anaemia in a dog: a case report. Vet Med. 2013;58:44‐49. [Google Scholar]
- 167. Malik R, Zunino P, Hunt GB. Complete heart block associated with lupus in a dog. Aust Vet J. 2003;81:398‐401. [DOI] [PubMed] [Google Scholar]
- 168. Bloom JC, Thiem PA, Sellers TS, Lewis HB, Deldar A. Cephalosporin‐induced immune cytopenia in the dog: demonstration of erythrocyte‐, neutrophil‐, and platelet‐associated IgG following treatment with cefazedone. Am J Hematol. 1988;28:71‐78. [DOI] [PubMed] [Google Scholar]
- 169. DeLong D, Gunther R, Manning PJ. Immune mediated hemolytic anemia associated with antilymphocyte globulin therapy in dogs. Lab Anim Sci. 1990;40:415‐418. [PubMed] [Google Scholar]
- 170. Goggs R, Dennis SG, Di Bella A, et al. Predicting outcome in dogs with primary immune‐mediated hemolytic anemia: results of a multicenter case registry. J Vet Intern Med. 2015;29:1603‐1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Ong HM, Witham A, Kelers K, Boller M. Presumed secondary immune‐mediated haemolytic anaemia following elapid snake envenomation and its treatment in four dogs. Aust Vet J. 2015;93:319‐326. [DOI] [PubMed] [Google Scholar]
- 172. Smith AJ, Stenske KA, Bartges JW, Kirk CA. Diet‐associated hepatic failure and immune‐mediated hemolytic anemia in a Weimaraner. J Vet Emerg Crit Car. 2006;16(S1):S42‐S47. [Google Scholar]
- 173. Peterson ME, Hurvitz AI, Leib MS, Cavanagh PG, Dutton RE. Propylthiouracil‐associated hemolytic anemia, thrombocytopenia, and antinuclear antibodies in cats with hyperthyroidism. J Am Vet Med Assoc. 1984;184:806‐808. [PubMed] [Google Scholar]
- 174. Aucoin DP, Peterson ME, Hurvitz AI, et al. Propylthiouracil‐induced immune‐mediated disease in the cat. J Pharmacol Exp Ther. 1985;234:13‐18. [PubMed] [Google Scholar]
- 175. Segal Y, Shoenfeld Y. Vaccine‐induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell Mol Immunol. 2018;15:586‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Toussirot E, Bereau M. Vaccination and induction of autoimmune diseases. Inflamm Allergy Drug Targets. 2015;14:94‐98. [DOI] [PubMed] [Google Scholar]
- 177. Burgess K, Moore A, Rand W, Cotter SM. Treatment of immune‐mediated hemolytic anemia in dogs with cyclophosphamide. J Vet Intern Med. 2000;14:456‐462. [DOI] [PubMed] [Google Scholar]
- 178. Dodds WJ. Immune‐mediated diseases of the blood. Adv Vet Sci Comp Med. 1983;27:163‐196. [PubMed] [Google Scholar]
- 179. Duval D, Giger U. Vaccine‐associated immune‐mediated hemolytic anemia in the dog. J Vet Intern Med. 1996;10:290‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Kidd L, Rasmussen R, Chaplow E, Richter K, Hill S, Slusser PG. Seasonality of immune‐mediated hemolytic anemia in dogs from southern California. J Vet Emerg Crit Care. 2014;24:311‐315. [DOI] [PubMed] [Google Scholar]
- 181. Reimer ME, Troy GC, Warnick LD. Immune‐mediated hemolytic anemia: 70 cases (1988‐1996). J Am Anim Hosp Assoc. 1999;35:384‐391. [DOI] [PubMed] [Google Scholar]
- 182. Weiss DJ, Brazzell JL. Detection of activated platelets in dogs with primary immune‐mediated hemolytic anemia. J Vet Intern Med. 2006;20:682‐686. [DOI] [PubMed] [Google Scholar]
- 183. Ishihara M, Fujino Y, Setoguchi A, et al. Evaluation of prognostic factors and establishment of a prognostic scoring system for canine primary immune‐mediated hemolytic anemia. J Vet Med Sci. 2010;72:465‐470. [DOI] [PubMed] [Google Scholar]
- 184. Horgan JE, Roberts BK, Schermerhorn T. Splenectomy as an adjunctive treatment for dogs with immune‐mediated hemolytic anemia: ten cases (2003‐2006). J Vet Emerg Crit Care. 2009;19:254‐261. [DOI] [PubMed] [Google Scholar]
- 185. Orcutt ES, Lee JA, Bianco D. Immune‐mediated hemolytic anemia and severe thrombocytopenia in dogs: 12 cases (2001‐2008). J Vet Emerg Crit Care. 2010;20:338‐345. [DOI] [PubMed] [Google Scholar]
- 186. Sinnott VB, Otto CM. Use of thromboelastography in dogs with immune‐mediated hemolytic anemia: 39 cases (2000‐2008). J Vet Emerg Crit Care. 2009;19:484‐488. [DOI] [PubMed] [Google Scholar]
- 187. Piek CJ, Junius G, Dekker A, Schrauwen E, Slappendel RJ, Teske E. Idiopathic immune‐mediated hemolytic anemia: treatment outcome and prognostic factors in 149 dogs. J Vet Intern Med. 2008;22:366‐373. [DOI] [PubMed] [Google Scholar]
- 188. Dodds WJ. Estimating disease prevalence with health surveys and genetic screening. Adv Vet Sci Comp Med. 1995;39:29‐96. [DOI] [PubMed] [Google Scholar]
- 189. Frana TS, Clough NE, Gatewood DM, Rupprecht CE. Postmarketing surveillance of rabies vaccines for dogs to evaluate safety and efficacy. J Am Vet Med Assoc. 2008;232:1000‐1002. [DOI] [PubMed] [Google Scholar]
- 190. Yuki M. A case of non‐regenerative immune‐mediated anemia treated by combination therapy of human immune globulin and mycophenolate mofetil in a dog. Open Vet J. 2011;1:46‐49. [PMC free article] [PubMed] [Google Scholar]
- 191. Hogenesch H, Azcona‐Olivera J, Scott‐Moncrieff C, et al. Vaccine‐induced autoimmunity in the dog. Adv Vet Med. 1999;41:733‐747. [DOI] [PubMed] [Google Scholar]
- 192. Wraith DC, Goldman M, Lambert PH. Vaccination and autoimmune disease: what is the evidence? Lancet. 2003;362:1659‐1666. [DOI] [PubMed] [Google Scholar]
- 193. Breitschwerdt EB, Malone JB, MacWilliams P, Levy MG, Qualls CW Jr, Prudich MJ. Babesiosis in the Greyhound. J Am Vet Med Assoc. 1983;182:978‐982. [PubMed] [Google Scholar]
- 194. Gianopoulos A, Mylonakis ME, Theodorou K, Christopher MM. Quantitative and qualitative leukocyte abnormalities in dogs with experimental and naturally occurring acute canine monocytic ehrlichiosis. Vet Clin Pathol. 2016;45:281‐290. [DOI] [PubMed] [Google Scholar]
- 195. Hess PR, English RV, Hegarty BC, Brown GD, Breitschwerdt EB. Experimental Ehrlichia canis infection in the dog does not cause immunosuppression. Vet Immunol Immunopathol. 2006;109:117‐125. [DOI] [PubMed] [Google Scholar]
- 196. Plier ML, Breitschwerdt EB, Hegarty BC, Kidd LB. Lack of evidence for perinatal transmission of canine granulocytic anaplasmosis from a bitch to her offspring. J Am Anim Hosp Assoc. 2009;45:232‐238. [DOI] [PubMed] [Google Scholar]
- 197. Qurollo BA, Davenport AC, Sherbert BM, Grindem CB, Birkenheuer AJ, Breitschwerdt EB. Infection with Panola Mountain Ehrlichia sp. in a dog with atypical lymphocytes and clonal T‐cell expansion. J Vet Intern Med. 2013;27:1251‐1255. [DOI] [PubMed] [Google Scholar]
- 198. Ravnik U, Bajuk BP, Lusa L, Tozon N. Serum protein profiles, circulating immune complexes and proteinuria in dogs naturally infected with Anaplasma phagocytophilum . Vet Microbiol. 2014;173:160‐165. [DOI] [PubMed] [Google Scholar]
- 199. Slade DJ, Lees GE, Berridge BR, Clubb FJ, Kuczynski LA, Littman MP. Resolution of a proteinuric nephropathy associated with Babesia gibsoni infection in a dog. J Am Anim Hosp Assoc. 2011;47:e138‐e144. [DOI] [PubMed] [Google Scholar]
- 200. Ullal T, Birkenheuer A, Vaden S. Azotemia and proteinuria in dogs infected with Babesia gibsoni . J Am Anim Hosp Assoc. 2018;54:156‐160. [DOI] [PubMed] [Google Scholar]
- 201. Whittemore JC, Hawley JR, Radecki SV, Steinberg JD, Lappin MR. Bartonella species antibodies and hyperglobulinemia in privately owned cats. J Vet Intern Med. 2012;26:639‐644. [DOI] [PubMed] [Google Scholar]
- 202. Zygner W, Gojska‐Zygner O, Wedrychowicz H. Strong monovalent electrolyte imbalances in serum of dogs infected with Babesia canis . Ticks Tick Borne Dis. 2012;3:107‐113. [DOI] [PubMed] [Google Scholar]
- 203. Fukumoto S, Suzuki H, Igarashi I, Xuan X. Fatal experimental transplacental Babesia gibsoni infections in dogs. Int J Parasitol. 2005;35:1031‐1035. [DOI] [PubMed] [Google Scholar]
- 204. Chao LL, Liao HT, Ho TY, Shih CM. First detection and molecular identification of Babesia gibsoni from Rhipicephalus sanguineus ticks. Acta Trop. 2017;166:356‐362. [DOI] [PubMed] [Google Scholar]
- 205. Groves MG, Dennis GL. Babesia gibsoni: field and laboratory studies of canine infections. Exp Parasitol. 1972;31:153‐159. [DOI] [PubMed] [Google Scholar]
- 206. Higuchi S, Izumitani M, Hoshi H, et al. Development of Babesia gibsoni in the midgut of larval tick, Rhipicephalus sanguineus . J Vet Med Sci. 1999;61:689‐691. [DOI] [PubMed] [Google Scholar]
- 207. Higuchi S, Kuroda H, Hoshi H, et al. Development of Babesia gibsoni in the midgut of the nymphal stage of the tick, Rhipicephalus sanguineus . J Vet Med Sci. 1999;61:697‐699. [DOI] [PubMed] [Google Scholar]
- 208. Jongejan F, Su BL, Yang HJ, et al. Molecular evidence for the transovarial passage of Babesia gibsoni in Haemaphysalis hystricis (Acari: Ixodidae) ticks from Taiwan: a novel vector for canine babesiosis. Parasit Vectors. 2018;11:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Taboada J, Harvey JW, Levy MG, Breitschwerdt EB. Seroprevalence of babesiosis in Greyhounds in Florida. J Am Vet Med Assoc. 1992;200:47‐50. [PubMed] [Google Scholar]
- 210. Dear JD, Owens S, Biondo A, Marcondes M, Sykes J. Efficacy of azithromycin and atovaquone for treatment of Babesia conradae and hemoplasma infections in coyote hunting dogs infected with multiple blood‐borne pathogens. Paper presented at: Second International Society for Companion Animal Infectious Diseases Symposium; 2012.
- 211. Di Cicco MF, Downey ME, Beeler E, et al. Re‐emergence of Babesia conradae and effective treatment of infected dogs with atovaquone and azithromycin. Vet Parasitol. 2012;187:23‐27. [DOI] [PubMed] [Google Scholar]
- 212. Kidd L, Maggi R, Diniz PP, et al. Evaluation of conventional and real‐time PCR assays for detection and differentiation of Spotted Fever Group Rickettsia in dog blood. Vet Microbiol. 2008;129:294‐303. [DOI] [PubMed] [Google Scholar]
- 213. Kidd L, Qurollo B, Lappin M, et al. Prevalence of vector‐borne pathogens in Southern California dogs with clinical and laboratory abnormalities consistent with immune‐mediated disease. J Vet Intern Med. 2017;31:1081‐1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Maggi RG, Birkenheuer AJ, Hegarty BC, Bradley JM, Levy MG, Breitschwerdt EB. Comparison of serological and molecular panels for diagnosis of vector‐borne diseases in dogs. Parasit Vectors. 2014;7:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Nair AD, Cheng C, Ganta CK, et al. Comparative experimental infection study in dogs with Ehrlichia canis, E. chaffeensis, Anaplasma platys and A. phagocytophilum . PLoS One. 2016;11:e0148239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Starkey LA, Barrett AW, Beall MJ, et al. Persistent Ehrlichia ewingii infection in dogs after natural tick infestation. J Vet Intern Med. 2015;29:552‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Starkey LA, Barrett AW, Chandrashekar R, et al. Development of antibodies to and PCR detection of Ehrlichia spp. in dogs following natural tick exposure. Vet Microbiol. 2014;173:379‐384. [DOI] [PubMed] [Google Scholar]
- 218. Bosman AM, Venter EH, Penzhorn BL. Occurrence of Babesia felis and Babesia leo in various wild felid species and domestic cats in Southern Africa, based on reverse line blot analysis. Vet Parasitol. 2007;144:33‐38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting information
Appendix S2 Supporting information
Appendix S3 Supporting information
Appendix S4 Supporting information
Appendix S5 Supporting information
Appendix S6 Supporting information
Appendix S7 Supporting information


