Abstract
Background
Ureteroceles are a rare condition in dogs in which conventional treatments can result in substantial morbidity. Cystoscopic and fluoroscopic‐guided laser ablation (CLA) of ureteroceles can successfully relieve obstruction.
Objectives
To describe the technique and outcomes of attempting CLA for treatment of ureteroceles in dogs.
Animals
Thirteen client‐owned dogs that underwent CLA for treatment of ureteroceles.
Methods
Retrospective multicentered study. Medical records were reviewed in all dogs that underwent CLA for ureterocele(s). A laser was used to extend the opening of the ureteral orifice (UO) unless surgical conversion was necessary. Data collected included signalment, clinicopathologic data, imaging, procedural findings, complications, and short‐ and long‐term outcome.
Results
Thirteen dogs with 13 ureteroceles associated with 14 UOs resulting in ureteral obstruction were included. One ureterocele extended bilaterally. Treatment was initiated via retrograde cystoscopy (7 females), percutaneous perineal urethrocystoscopy (4 males), or percutaneous antegrade cystoscopy (2 males). Surgical conversion was necessary in 2 males. Ten of 14 (71%) UOs associated with the ureteroceles were ectopic. Thirteen of 14 had stenotic or imperforate UOs. No postoperative complications were noted. Preoperative incontinence or pollakiuria was present in 9 of 13 and 3 of 13 dogs and resolved in 8 of 9 and 3 of 3 dogs, respectively. Follow‐up imaging showed resolution of all ureteroceles and improved ureteral/renal pelvic dilatation. Median follow‐up time was 27 months (range, 3‐96 months).
Conclusions and Clinical Importance
Cystoscopic‐guided laser ablation was effective for the treatment of ureteroceles(s) in 11 of 13 dogs.
Keywords: canine, interventional endoscopy, interventional radiology
Abbreviations
- APC
antegrade percutaneous cystourethroscopy
- CLA
cystoscopic‐guided ureteral ablation
- CT
computed tomography
- Hol:YAG
holmium, yttrium, aluminum, and garnet laser
- PPAC
percutaneous perineal access cystourethroscopy
- UO
ureteral orifice
- UTI
urinary tract infection
- UVJ
ureterovesicular junction
1. INTRODUCTION
A ureterocele is a cystic dilatation of the terminal ureter and is a rare condition in dogs.1, 2 Ureteroceles are classified as orthotopic if the associated ureteral orifice (UO) is in a normal position or ectopic if the UO is distal to the normal position in the trigone.3 Ureteroceles can be clinically silent or clinical signs can develop secondary to chronic ureterovesicular junction (UVJ) obstruction, urinary tract infections (UTIs), urethral or trigonal outflow tract obstructions, or from altered urine flow dynamics.2 Ureterovesicular junction obstruction or UTIs accompanying ureteroceles can result in secondary changes including hydroureter, hydronephrosis, and progressive renal dysfunction.2
Goals for management include prevention of renal damage associated with UVJ obstruction, vesicoureteral reflux, and UTIs, as well as promotion of urinary continence and minimization of morbidity.2 In human medicine, treatment options include endoscopic ureterocele decompression via incision, puncture, or balloon dilatation;4, 5, 6 laparoscopic or robotic reimplantation;7, 8, 9, 10 or various surgical techniques.2, 5, 11 Endoscopic decompression for single‐system ureteroceles is considered to be standard of care for initial intervention in human medicine.2, 3, 12, 13
In veterinary medicine, a small number of published reports exist of ureteroceles in dogs.14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30 Renal‐sparing interventions were performed in only 15 of 21 cases.14, 15, 17, 20, 22, 23, 24, 25, 27, 29, 30 Historically, surgical management of ureteroceles in dogs has been the mainstay of treatment.14, 17 Techniques include ureteronephrectomy, neoureterocystostomy, ureterocelectomy, and intravesicular incision via electrocautery or a scalpel blade.23 Complications include ureteral dehiscence, cystic dehiscence, ureteral stricture, uroabdomen, and incisional infections.2, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 31 Because of procedural morbidity associated with surgical techniques, additional investigation into novel techniques is warranted.14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28
Cystoscopic and fluoroscopic‐guided laser ablation (CLA) is reported as a therapeutic modality for ureteroceles in dogs.3, 15, 29, 30 However, to date, a single case report exists describing the use of CLA for treatment of unilateral orthotopic ureterocele in a female dog with follow‐up.29 This case report documented resolution of pollakiuria and ultrasonographic resolution of hydroureter with persistent mild pyelectasia; however, only 6 weeks of follow‐up was available.29 An additional case series describes CLA for the treatment of congenital distal UO stenosis in 16 dogs that included 4 dogs with ureteroceles.30 However, outcomes for dogs with ureteroceles were not separated from those lacking ureteroceles.30
To the authors' knowledge, no published series exist evaluating long‐term outcomes for dogs specifically with ureteroceles treated with any therapeutic modality. Thorough descriptions are lacking of CLA for male dogs with ureteroceles and for female dogs with ectopic ureteroceles. Standardized long‐term outcomes like continence scores, assessment of renal function, and imaging are also lacking.3, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 29, 30 In this study, we describe the technique and clinical outcome of attempted CLA for the treatment of ureteroceles in dogs. The hypothesis is that CLA for the treatment of ureteroceles in dogs is safe, effective, and associated with minimal morbidity.
2. MATERIALS AND METHODS
2.1. Case selection
A retrospective review of medical records from the Animal Medical Center of New York City and the Small Animal Hospital of Purdue University was performed for all dogs diagnosed with a ureterocele and with CLA attempted from March 2008 to April 2017. Each ureterocele was identified preoperatively via ultrasound, computed tomography (CT), or both modalities, and definitively diagnosed cystoscopically, fluoroscopically, or via laparotomy for inclusion in the study.
2.2. Medical records review
Data collected included signalment, clinical signs, physical examination findings, continence scores, diagnostic imaging findings, laboratory biochemical, hematologic, and microbiologic findings, procedural report, length of the procedure, and complications. Preoperative, intraoperative, and postoperative (short‐term [<30 days], long‐term [>30 days]) were recorded. Incontinence was defined as any persistent or intermittent involuntary leakage of urine. Continence was graded on a scale of 1 through 10,32 where 1 was minimally continent (leaking all the time), 5 was moderately continent (leaking when lying down or when the bladder is full but able to hold urine between urinations and to produce a normal stream and volume of urine), and 10 was perfectly continent (no leakage at all). If omitted from the record, continence scores were assigned retrospectively. Follow‐up information was obtained via medical record or telephone interview with the owner of each dog or primary veterinarian. Ultrasound reports were generated by radiologists, internists, and general practitioners. Computed tomography reports were generated by radiologists. Images were not retrospectively reviewed. Information regarding presence of a ureterocele, renal architecture, renal pelvic diameter, ureteral diameter, and ureteral ectopia was recorded.
2.3. Procedures
Cystoscopic laser procedures were performed by the authors A. C. Berent or L. G. Adams. Female dogs had retrograde cystourethroscopy with a rigid cystoscope (2.7 or 4 mm) (rigid endoscope, 2.7 mm 30° lens with 14 fr integrated sheath, Richard Wolf, Vernon Hills, Illinois; rigid endoscope, 2.7 mm 30° lens, Karl Storz Endoscopy‐America, Culver City, California; Cystoscope‐urethroscope sheath, 14.5 fr, Karl Storz Endoscopy‐America; rigid endoscope, 4.0 mm 30° lens, Karl Storz Endoscopy‐America; cystoscope‐urethroscope sheath, 19 fr, Karl Storz Endoscopy‐America) with a 30° angle performed to evaluate the vestibule, vagina, urethra, bladder, and each UVJ as previously described.32 Male dogs had retrograde cystoscopy performed with a flexible cystoscope (flexible endoscope, Flex X2, 2.7 mm, Karl Storz Endoscopy‐America; flexible endoscope, Flex XC, 2.7 mm, Karl Storz Endoscopy‐America) to evaluate the urethra, bladder, and UVJs followed by either retrograde percutaneous perineal access cystourethroscopy (PPAC) or antegrade percutaneous cystourethroscopy (APC) with a rigid cystoscope (2.7 mm; rigid endoscope, 2.7 mm 30° lens with 14 fr integrated sheath, Richard Wolf, Vernon Hills, Illinois; rigid endoscope, 2.7 mm 30° lens, Karl Storz Endoscopy‐America; Cystoscope‐urethroscope sheath, 14.5 fr, Karl Storz Endoscopy‐America).33, 34, 35 The decision to perform PPAC or APC for male dogs was based on size with an approximately 10 kg cutoff to accommodate a 14 or 16 fr peel‐away introducer sheath (14 or 16 fr peel‐away introducer sheath, Cook Medical, Bloomington, Indiana) in the pelvic urethra.33, 35 Retrograde ureteropyelography with concurrent cystourethrography was performed to determine if ectopic ureters were intramural or extramural.32 Intramural ectopic tracts were corrected with the laser.32
A UO with an associated ureterocele was identified (Figure 1). If the UO was stenotic but able to be catheterized with an angle‐tipped hydrophilic guide wire (0.025″ or 0.035″) (Weasel Wire 0.025‐ or 0.035‐in. hydrophilic angle‐tipped guidewire; Infiniti Medical LLC, Menlo Park, California) and an open‐ended ureteral catheter (4 or 5 f. open‐ended ureteral catheter; Cook Medical Inc), the ureterocele was lasered via a central linear incision with an end‐fire laser fiber (diode [600‐μm diode laser fiber and 25‐W diode laser; Lumenis Inc, Santa Clara, California] or holmium, yttrium, aluminum, and garnet laser [Hol:YAG; 550‐μm Hol:YAG SlimLine laser fiber, Boston Scientific Corp, Natick, Massachusetts; VersaPulse Power Suite Holmium 20 W, Lumenis]) over the catheter/wire combination32 (Figure 1). If the ureterocele was unable to be catheterized (imperforate) (Figure 2), laser puncture was performed before catheterization. The cystoscope was deflected toward the urethral lumen to angle the laser fiber tip toward the medial aspect of the ureterocele, avoiding the lateral aspect of the bladder or urethral wall. The medial aspect of the ureterocele was cut in a linear continuous manner by use of the diode laser at 5‐25 W (600‐μm diode laser fiber and 25‐W diode laser; Lumenis Inc) or a pulsed manner by the use of the Hol:YAG laser (20 Hz and 0.5 J at a pulse width of 350 milliseconds; 550‐μm Hol:YAG SlimLine laser fiber, Boston Scientific Corp; VersaPulse Power Suite Holmium 20 W, Lumenis). When the neo‐UO was within the urinary bladder lumen (for ectopic ureteroceles) or subjectively open enough to allow a ureteral lumen to be visualized accepting the guide wire and catheter, laser ablation was terminated. Retrograde contrast urethrocystography and ureteropyelography were performed to ensure that there was no contrast extravasation, no ureteral obstruction, and that the flow of contrast to the urinary bladder was appropriate. This also documented the new location of the UO relative to the urethrovesicular junction.32, 34 (Figure 2).
Figure 1.
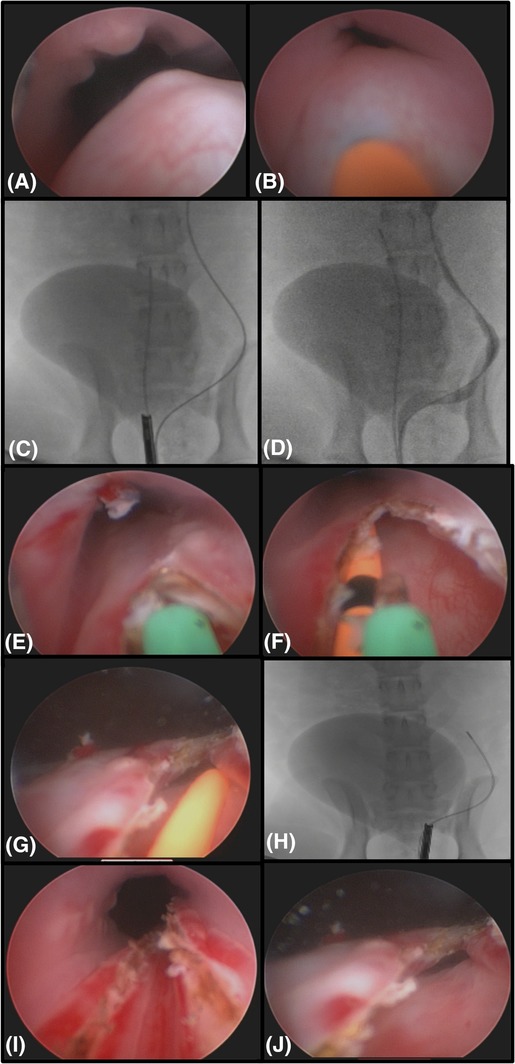
Endoscopic and fluoroscopic images of a left ectopic ureterocele in a 6‐month‐old female intact Labrador Retriever with bilateral ureteral ectopia. The dog is positioned in dorsal recumbency. A, Endoscopic image of the fluid filled intramural ectopic ureteral tunnel causing the ureterocele within the urethral lumen. B, A catheter visualized inside the stenotic orifice of the ectopic ureteral opening within the urethra. C, Fluoroscopic imaging of the dog with a guide wire and catheter advanced up the left ureter with a cystoscope at the trigone of the urinary bladder. There is contract material in the urinary bladder. D, A retrograde ureteropyelogram of this dog through the ureteral catheter. Notice the ureteral tunnel entering the bladder appropriately but traveling distally down the urethra. This is an intramural ectopic ureter with severe hydroureter. E, Endoscopic image of a diode laser fiber ablating the stenosis to open the ureteral orifice. F, The laser fiber (green) is cutting the intramural medial wall of the ectopic ureter with a ureteral catheter (orange) placed inside the ureteral lumen. Notice the orifice is now open and the fluid filled structure is gone. G, After completion of laser ablation, the ureteral catheter (orange) is seen exiting the neoureteral orifice within the urinary bladder. H, A fluoroscopic image of the neoureteral orifice, marked by the tip of the cystoscope showing it is now in front of the urethrovesicular junction. I, An endoscopic image of the urethra after complete laser ablation of the ureteral tunnel. J. Final image of the neoureteral orifice after ablation is complete in the urinary bladder
Figure 2.
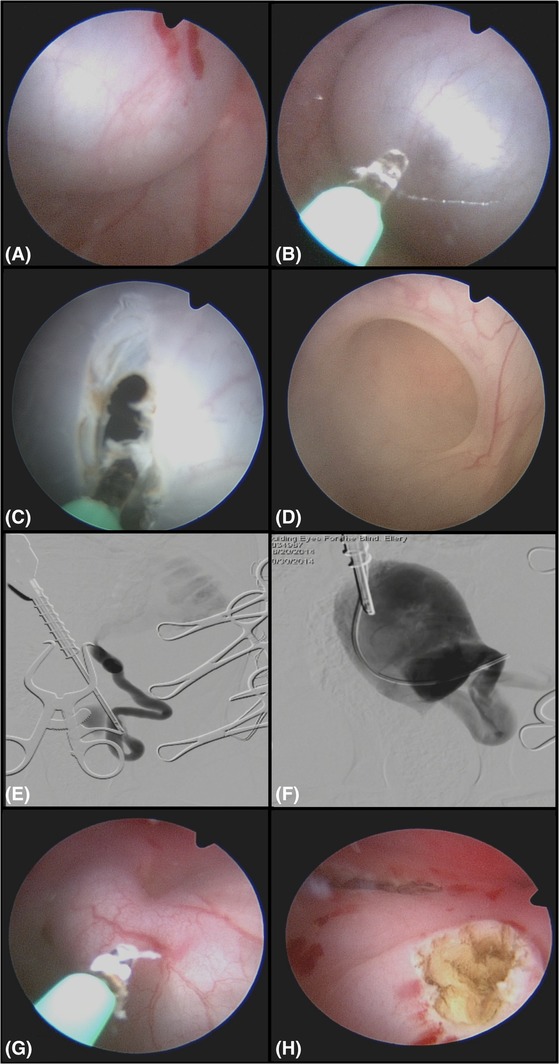
Endoscopic and fluoroscopic images of a 2‐month‐old male intact Labrador Retriever with severe left hydroureter and hydronephrosis and an imperforate orthotopic ureterocele on the left and an ectopic ureter on the right. This procedure was performed from an antegrade cystoscopy approach owing to the small size of the canine pelvic urethra limiting the ability to perform percutaneous perineal access cystourethroscopy. The dog is in dorsal recumbency. A, A large fluid‐filled structure covering the left ureteral orifice. There is no orifice seen. B, A diode laser is used to puncture the fluid‐filled structure. C, The orifice in the structure is expanded with the laser and then the scope is used to explore the structure (D). D, The ureteral opening is seen inside this fluid‐filled structure and is largely dilated. E, A digital subtraction contrast study is done using as a retrograde ureterogram up the ureteral orifice showing the severe hydroureter and hydronephrosis. F, Contrast is injected into the cyst after it is opened using a ureteral catheter, and the cyst and wall can be seen inside the urinary bladder as contrast refluxes retrograde up the ureteral orifice. G, Laser ablation of the contralateral ectopic ureter from an antegrade approach as the ureteral orifice could not be cannulated retrograde owing to the small size of the canine penis at this young age. H, Endoscopic image after both orifices are open and the filled cystic structure was ablated
After completion of ureterocele CLA in females, the paramesonephric remnant, if present, was laser ablated.32, 36 Contralateral ectopic ureter CLA was performed if necessary.32
3. RESULTS
3.1. Population
Review of medical records revealed 13 dogs that had CLA attempted. Eight dogs were seen at the Animal Medical Center (2009‐2017) and 5 dogs at the Small Animal Hospital of Purdue University (2008‐2017). None of these dogs were excluded from the study. Three dogs from the Animal Medical Center had been previously included in a prior publication describing treatment of a series of dogs with UO obstruction.30 The focus of that paper was not on ureteroceles, so these individual dogs were not thoroughly described and were therefore included in this study.30
3.2. Preoperative findings
3.2.1. Signalment
Six male dogs (4 neutered and 2 sexually intact) and 7 female dogs (4 neutered and 3 sexually intact) were included, with a median age of 1 year (range, 2 months to 9 years) and weight of 23 kg (range, 7.88‐45.2 kg). Dogs included 7 Labrador Retrievers, 2 Golden Retrievers, and 1 of each of the following Basset Hound, Chow Chow, Cocker Spaniel, and mixed breed.
3.2.2. Clinical signs
Data regarding clinical signs was available for all dogs. Nine of 13 dogs were incontinent and 3 of 13 dogs were pollakiuric. One dog had vomiting and difficulty rising. No dogs were both incontinent and pollakiuric. Duration of presenting clinical signs was available for 12 dogs. Median duration of clinical signs was 6 months (range, 2 weeks‐3 years). One dog had a history of struvite cystolithiasis. One dog had previously undergone episioplasty surgery. Nine of 13 dogs presented for incontinence, and 3 of 13 dogs presented for pollakiuria. One dog presented for vomiting and difficulty rising.
3.3. Physical examination
Vital signs were within normal limits for all animals. One dog was painful on abdominal palpation. One dog had referred upper airway sounds on thoracic auscultation and was diagnosed with an elongated soft palate and hiatal hernia. One dog had perivulvar urine scald. None of the dogs had an abnormal rectal examination. None of the females had digital vaginal palpation performed.
3.4. Continence scoring
Data regarding continence was available for all dogs (Appendix 1). The median preprocedure continence score was 5 (range, 1‐10). Nine of 13 were noted to be incontinent and 4 continent, preoperatively. The 4 dogs noted to be continent were pollakiuric (n = 3) and had vomiting/difficulty standing (n = 1). Two of the incontinent dogs were being treated with continence medications preoperatively but remained incontinent. One dog was receiving phenylpropanolamine (0.63 mg/kg PO q12h; Proin, PRN Pharmacal, Pensacola, Florida), another was receiving phenylpropanolamine (1.4 mg/kg PO q12h; Proin, PRN Pharmacal), diethylstilbestrol (0.03 mg/kg PO once weekly; diethylstilbestrol, compounding pharmacy), and imipramine (0.28 mg/kg PO q12h; Tofranil; Mallinckrodt Inc, St. Louis, Missouri).
3.5. Clinicopathologic data
CBC and serum biochemical profiles within 14 days before CLA were available for all the dogs. Preoperative azotemia was present in 2 dogs. One dog had values of creatinine 1.3 mg/dL (reference: 0.3‐1.2 mg/dL) and BUN 29 mg/dL(reference range, 7–29 mg/dL) with a USG of 1.015. The other had values of creatinine 2.2 mg/dL and BUN 30 mg/dL with a USG of 1.019. One dog had hypoalbuminemia 2.1 g/dL (reference range, 2.5‐4.0 g/dL) preoperatively. No additional CBC or serum biochemical abnormalities were noted preoperatively.
Preoperative urine bacteriologic culture and sensitivity was performed in 9 of 13 dogs (69%) within 60 days before the procedure (median, 15.5 days; range, 5‐57 days). Urine was obtained via cystocentesis. Six cultures were negative for bacterial growth. However, 3 of 6 dogs with cultures negative for growth were on antibiotics for previously diagnosed bacterial cystitis at the time of the culture. Three cultures were positive for growth including Alpha‐hemolytic streptococcus (1), Escherichia coli (1), and a combination of E. coli and Proteus mirabilis (1).
3.6. Diagnostic imaging
Pretreatment abdominal ultrasound was performed in 12 of 13 (92%) dogs. Ureteroceles were identified ultrasonographically in 8 of 12 (67%) dogs.
Ten of 12 (83%) ultrasound reports described a hydroureter (ipsilateral [5 dogs] or bilateral [5 dogs]). Hydroureter measurements were provided for 4 dogs (median 10 mm; range, 5‐40 mm). One ultrasound report described unilateral ureterocele associated with ureters lacking dilatation. Eleven of 12 (92%) ultrasound reports described the architecture of 12 renal units associated with ureteroceles. Ten of 12 (83%) renal units were noted to have preoperative renal pelvic dilatation, most of which was characterized as severe. Only 2 renal pelvis sizes were quantified at 4 and 2 mm in the transverse plane, respectively.
Multiple ultrasound reports were available for review in the dog with previous ectopic ureter reimplantation. Two weeks post‐neoureterocystostomy, cystitis with a solitary urinary bladder polyp and granulation tissue at the level of both neoureterostomy sites were described. Nine months later, a report described a prostatic cyst with severe cystitis. Drainage of the cyst and aerobic culture and sensitivity showed no growth with resolution of the pollakiuria. The lesion recurred 3 months later; it was thought to be a ureterocele from the prior ureteral remnant of the previously repaired ectopic ureter.
Only one dog had excretory urography performed with fluoroscopy revealing dilatation of the distal ureteral extremity consistent with a ureterocele. Three dogs had preoperative CT scans performed. One CT report was not available other than a conclusion of diagnosis of a ureterocele. One CT involved an intravenous pyeloureterogram and urethrogram in the dog with previous ectopic ureter reimplantation. This revealed a fluid‐filled cystic structure communicating with the urethra, suspect right ureterocele. All 3 CT scans positively identified the ureteroceles, which were confirmed cystoscopically or surgically.
3.7. Intraoperative findings
3.7.1. Diagnostic cystourethroscopy
All ureteroceles were confirmed using cystourethroscopy. Eight of 13 ureteroceles were confirmed cystoscopically to be right sided, 4 were left sided, and 1 ureterocele extended bilaterally, with an orthotopic centrally located cyst‐like structure encompassing both of the canine UOs.
Ten of 14 (71%) UOs associated with the ureteroceles were ectopic. One additional dog with an orthotopic ureteroceles had a contralateral ectopic UO. This resulted in 11 of 13 (85%) dogs with ectopia. Ectopic ureteroceles were present in 10 of 13 (77%) dogs. Bilateral ectopia was present in 8 of 11 (73%) dogs. Two of 13 dogs lacked ectopia.
Of the 11 dogs with an ectopic ureterocele or an ectopic contralateral ureter, 8 were incontinence, 2 were pollakiuric, and 1 had vomiting and difficulty rising. Of the 2 dogs lacking ectopia, 1 was pollakiuric and the other was incontinent (the dog with a bilateral ureterocele).
Each ureterocele was associated with either a stenotic but patent UO or a completely imperforate UO cystoscopically. Six of 12 (50%) unilateral ureteroceles were noted to end in blind sacs with completely imperforate or unidentifiable UO. Six of 12 (50%) unilateral ureteroceles had stenotic but patent UOs. Two unilateral ureteroceles noted to have stenotic UOs also had contralateral UO stenosis. Both these dogs only had unilateral hydronephrosis on the side of the ureterocele. Ureteropyelogram revealed hydronephrosis and hydroureter ipsilateral to the ureterocele in all cases other than the dog with a prior ureteral reimplantation. Ureteropyelography was not performed on the contralateral kidney/ureter in all cases, unless there was an associated hydroureter, hydronephrosis, or both conditions present. Ureteropyelogram results were not at all consistent with ultrasound findings.
Of the 7 Labrador Retrievers, 2 (29%) had completely imperforate or unidentifiable UOs, 3 (43%) had patent but stenotic UOs, and 2 did not have data regarding patency of the UOs. One Labrador Retriever and 1 mixed breed dog had bilateral UO stenosis. All ectopic ureters and ectopic ureteroceles were intramural.
3.8. Procedures
Treatment was accomplished via retrograde cystoscopy (7 females), APC (2 males), or PPAC (4 males).
All 7 females successfully underwent standard retrograde cystourethroscopy and no surgical conversion was necessary. Surgical duration was available for 4 female dogs with a median of 58 minutes (range, 35‐150 minutes). For the 6 male dogs, 2 underwent an APC approach and 4 underwent a PPAC approach. APC was performed in male dogs weighing 10.8 and 7.8 kg. Percutaneous perineal access cystourethroscopy dogs weighed a median of 27.2 kg (range, 19‐45 kg). Two male dogs required surgical conversion. One dog undergoing APC approach required surgical conversion because of failure to endoscopically identify the orifice to the ureterocele. The dog had bilateral intramural ectopic ureters surgically reimplanted with distal tract ligation 2 years prior. A ureterocele of the distal ligated intramural ectopic ureteral tract developed as the tunnel resulted in an infected, inspissated intramural ureterocele causing a urethral obstruction. A ureteroceleostomy and ureterocele omentalization was performed.23, 31
In another dog, a PPAC approach was performed and a ureterocele was noted in the central trigone with unidentifiable orifices. A blind incision was made into the ureterocele with a 600‐μm diode laser (Lumenis Inc). No urine was noted coming from incision; there was concern for contrast extravasation from this incision, so the procedure was converted to a laparotomy. No leakage was confirmed upon opening. The ureterocele was resected with a 600‐μm diode laser (Lumenis Inc) during antegrade transurethral cystoscopy that was performed concurrently during a laparotomy to assist in gaining access to the remnant ureteral lumen. Because the orifice within the urethra was imperforate, access was unable to be obtained with cystourethroscopy alone. This approach also allowed external ureteral resection surgically and intraluminal orifice extension endoscopically, without the need for a urethrotomy. Closure of the urinary bladder and resection of the distal ureter was routine. Contrast cystography showed no leakage from the bladder after closure.
Duration of the procedure was available for 5 of 6 male dogs and was a median of 88 minutes (range, 79‐95 minutes) when CLA of ureteroceles was performed. In the 2 cases that required surgical conversion, duration of procedure was 210 and 110 minutes, respectively. Seven of 13 dogs had additional surgical procedures performed, which were not distinguished in surgical times from ureterocele ablation. Procedures included staphylectomy (n = 1), CLA of persistent paramesonephric septal remnants (n = 2), CLA of contralateral ectopic ureters (n = 7), and a bilateral closed neuter (n = 1).31, 32, 35, 36
Five cases used a 400‐μm Hol:YAG (550‐μm Hol:YAG SlimLine laser fiber, Boston Scientific Corp) laser fiber, 6 cases used a 600‐μm diode laser fiber (Lumenis Inc), 1 case that was converted to a laparotomy used a combination of 600‐μm diode laser fiber (Lumenis Inc) and a scalpel blade to puncture the ureterocele, and 1 case that was converted to a laparotomy used monopolar cautery for ureterocele puncture.
3.9. Microbiologic Testing
Urine bacteriologic culture and sensitivity at the time of the procedure was performed in 10 of 13 (77%) dogs; urine was obtained via the cystoscope. Three dogs had urine cultures positive for the growth of Enterococcus (1), Staphylococcus pseudointermedius (1), and Pseudomonas (1). The 3 dogs that did not have urine cultures submitted at the time of the procedure had urine cultures negative for bacterial growth 2‐4 weeks preoperatively.
3.10. Postoperative findings
3.10.1. Short‐term outcomes
All dogs survived to discharge. No complications were noted in the short‐term postoperatively from the CLA procedure.
3.11. Clinical signs
Short‐term follow‐up data was available for 12 of 13 dogs. Eleven of 12 (92%) dogs had continence scores of 10/10; these dogs had a median continence score of 5 (range, 1‐10) preoperatively. One dog scored 7.5/10, increased from 5/10 preoperatively. Two of the 11 fully continent dogs remained on continence medications indefinitely (1 dog was receiving phenylpropanolamine and estriol and 1 was receiving phenylpropanolamine and diethylstilbestrol, doses unknown), and it was unclear whether they had been attempted to be weaned off from the history, records, or client interview. These 2 dogs had scores of 5 and 1 preoperatively. The 2 of 3 dogs with pollakiuria before CLA had resolution of the pollakiuria; the final dog had resolution with initiation of doxycycline 11.6 mg/kg PO q24h (Merrem, Pfizer, New York City, New York).
3.12. Clinicopathologic data
Short‐term aerobic urine cultures were performed via cystocentesis on 4 dogs (median, 14 days; range, 14‐22 days postoperatively). All were negative for bacterial growth. Three dogs were not on antibiotics and 1 dog was on enrofloxacin (Baytril; Bayer, Shawness Mission, Kansas) 12.4 mg/kg PO q24h targeting the sensitivity for a previously cultured Staphylococcus pseudointermedius.
3.13. Long‐term outcomes
The median long‐term follow‐up for all dogs was 27 months (range, 3‐96 months). No long‐term complications were noted from the CLA procedure.
3.14. Clinical signs
The median postoperative continence score at the time of last follow‐up was 10 (range, 3‐10). Twelve of 13 dogs were fully continent at last follow‐up. The dog with a continence score of 7.5 two weeks postoperatively became fully continent within weeks of discharge. One dog with a continence score of 1 preoperatively and no 2‐week follow‐up score available remained incontinent. This dog was estimated to have a continence score of 2‐4 long term despite continence medications (medications and dose unknown) 2 years postoperatively. This was based on referring veterinarian communications, because the owner was deceased. After treatment, incontinence resolved in 8 of 9 (89%) dogs.
For the 3 dogs who presented with pollakiuria, all had long‐term resolution.
3.15. Clinicopathologic data
Long‐term aerobic urine cultures were performed on 8 of 13 dogs at a median of 3 months (range, 45 days to 24 months) postoperatively. Only 2 female dogs had urine cultures positive for bacterial growth, both with growth of E. coli. Both these dogs had positive growth of this organism preoperatively. After a 6‐week course of meropenem (12 mg/kg SC q12h; Baytril, Bayer) one of these dogs had urine cultures negative for growth at 3, 4, and 10 months postoperatively. The second dog with E. coli growth did not have additional urine culture data available.
Serum biochemistry results were available for 7 dogs at a median of 2 months (range, 2‐24 months) postoperatively. Only 1 dog was azotemic postoperatively. This was a dog with preoperative creatinine and BUN of 2.2 and 30 mg/dL, respectively. The azotemia had progressed to values of creatinine 2.4 mg/dL and BUN 28 mg/dL, 2 months postoperatively and then to creatinine 5.4 mg/dL and BUN 64 mg/dL 18 months postoperatively. This dog was suspected to have renal dysplasia based on the bilaterally dysplasic renal architecture noted on preoperative and postoperative ultrasonography.
3.16. Diagnostic imaging
Postoperative ultrasound reports were available at a median of 4 months (range, 36 days −18 months) postoperatively in 6 dogs. Postoperative ultrasound reports described the ureteral diameters of 5 dogs. Only 1 of 5 dogs described a persistent, but improved, hydroureter. The diameter decreased from 40 mm preoperatively to 4 mm postoperatively. Three postoperative ultrasound reports described static hydroureter measurements over a 2.5‐year period. The remaining 4 ureters were noted to be of normal diameter and thickness, indicating resolution of hydroureter in 4 of 5 dogs.
Six postoperative ultrasound reports commented on renal pelvic diameter. All 6 reports had described preoperative pyelectasia. Three of 6 postoperative reports described ultrasonographic resolution of pyelectasia on the side of the ureterocele, whereas pyelectasia persisted in 3 of 6 dogs. One dog with persistent pyelectasia had ultrasonographic changes consistent with renal dysplasia, which were noted both preoperatively and 6 months postoperatively. Hydroureter or pyelectasia was not noted to worsen postoperatively in any of these reports.
3.17. Long‐term Outcomes
One dog was euthanized due to diffuse hepatic and splenic neoplasia 2 years post‐CLA. One dog had a 1.5 cm apical bladder mass diagnosed 2 years post‐CLA. A urothelial carcinoma, grade 2, was completely surgically excised. Postoperative chemotherapy was pursued, and the dog was followed up for 538 days until death from cause unknown.
4. DISCUSSION
Results of the present study suggest that the use of a minimally invasive CLA procedure is a safe and effective treatment for ureteroceles in most dogs. This technique can avoid the need for more invasive surgery in most cases, especially in female dogs. It also suggests that ureteroceles in dogs are most commonly associated with a stenotic UO which can be associated with an ectopic ureter. They typically cause at least partial ureteral obstruction and should be treated to prevent renal injury. When successful, this procedure enabled a rapid and effective decompression of the ureterocele with low morbidity. In this population, 12 of 13 dogs (5/6 males, 7 females) had cystourethroscopy to diagnose a ureterocele. Eleven of 13 were able to be successfully approached using endoscopy alone, with the other 2 dogs being diagnosed with a UO stenosis using endoscopy, which then subsequently required surgical assistance for fixation. In all dogs, clinical assessments after fixation of the ureterocele were consistent with resolution of ureterocele. Surgical conversion was needed in 2 males. No postoperative complications were noted. Resolution of pollakiuria was noted in all cases and resolution of urinary incontinence was noted in most cases.
Of the 11 dogs with ectopia, 8 were incontinent (73%), 2 were pollakiuric, and 1 had vomiting/difficulty rising. Of the 2 dogs lacking ectopia, 1 had pollakiuria and 1 was incontinent. This is comparable to previous reports where 9 of 10 (90%) dogs with ectopic ureteroceles were incontinent and only 2 of 7 (29%) dogs with orthotopic ureteroceles were incontinent.14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29 This suggests that not all ectopic dogs are incontinent and not all orthotopic dogs are continent, but if incontinence is present it can be more likely that the dog is concurrently ectopic.
The UO pathology associated with the ureteroceles is suggestive of a congenital etiology and is supported by the early age (median age 1 year) of diagnosis in this population. In previously described ureterocele cases, only 4 of 20 dogs were Labrador Retrievers. 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30 In this series, Labrador Retrievers seemed subjectively overrepresented (7/13; 54%). Labrador Retrievers have been previously described as a breed documented to have congenital UVJ stenosis30 but most commonly without a ureterocele. Ten of 14 (71%) UOs associated with the ureteroceles were ectopic in this study. This is similar to historic rates of ectopic ureteroceles (59%).14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29 Ectopia is thought to be of a congenital etiology,32 further supporting a congenital basis for ureterocele formation in dogs.
In the human literature, there is controversy regarding etiology of ureteroceles identified in adulthood; it is thought that trauma or inflammation to the UO can result in stenosis and subsequent dilatation of the intramural distal ureter, appearing as an intravesicular cystic structure.37, 38, 39 Based on the history of this dog, this was considered a remnant ureterocele that was intermittently infected owing to stagnant urine accumulation. This study included 4 dogs older than 2 years, suggesting that an acquired pathogenesis cannot be excluded. One of the ureteroceles was suspected to be acquired secondary to surgical ligation of intramural ectopic ureters. Formation of a ureterocele after intramural ectopic ureter anastomosis and continued reflux of urine into the intramural ureter has been reported.25 Additional research is needed to understand etiology of ureteroceles in dogs.
In this case series, incontinence and pollakiuria resolved in 8 of 9 and 3 of 3 dogs after treatment long‐term. Because of the small number of cases reported with ureteroceles in both the surgical and interventional literature, it is difficult to say whether CLA provides an advantage in postoperative continence rates, compared with that of traditional surgery. The median surgical time of 58 minutes in female dogs coupled with the 0% surgical conversion rate and 89% continence rate would support that this is a highly successful modality to consider for the treatment of this condition.
The main complications that could occur with CLA are iatrogenic ureteral, bladder, or urethral wall perforation with the laser, leading to urinary leakage. Urine extravasation was suspected in 1 male dog that was treated using PPAC. Due to the thickness of the wall of the ureterocele and failure to cannulate from complete stenosis of the orifice, blind ablation was attempted. Surgical conversion was then pursued. No postoperative complications were noted as a result of conversion or from urine extravasation. Difficulty visualizing extraluminal structures is also a potential complication. In 1 dog who had previous reimplantation surgery, an open approach was elected for visualization and to remove a permanent suture that had been previously placed around an intramural ectopic ureter extraluminally, as this could be a source of the abscessed intramural ureteral lumen. Omentalization was also performed. None of the dogs required a second procedure to address persistent clinical signs postoperatively, in contrast to previous surgical reports.21, 23 Bleeding, stricture, vaginal tears, and ureterocele recanalization are other theoretical risks36, 40 which were not documented in any of these dogs.
Abdominal ultrasound examinations were performed preoperatively in 12 of 13 dogs. Only 8 of 12 (67%) dogs had ureteroceles positively identified on ultrasound. Preoperative ultrasound described 10 of 12 (83%) hydroureters, yet intraoperative ureteropyelography revealed hydroureter ipsilateral to the ureterocele in all cases other than the dog with a prior ureteral reimplantation. In a human study of the sensitivity of ultrasound for hydroureter resulting from nephrolithiasis and ureterolithiasis, the sensitivity for detection of hydroureter was only 50%.41 In this study, these differences could have been due to differences in operator skill and variation in dog conformation. Human studies similarly cite the sensitivity and specificity of ultrasound for the detection of hydronephrosis as being highly dependent on hydration status with values of 100% and 84%, respectively, for hydrated patients and 35%‐73% and 74%, respectively, for nonhydrated patients.42, 43 Hydration status of the dogs could have affected ultrasonographic identification of hydroureter and hydronephrosis in this study. The lack of bladder or urethral distention might have made the imaging difficult. Computed tomographic scan positively identified all ureteroceles; however, only 3 dogs underwent this modality. All but 1 ureterocele were cystoscopically confirmed, the orifice location identified and treatment attempted at the same time. Because of this, cystourethroscopy is the authors' diagnostic test of choice for the diagnosis and simultaneous treatment of both ectopic ureters and ureteroceles, avoiding the need for more expensive imaging modalities. In 1 case, the ureterocele's communication with the bladder was unable to be identified cystoscopically. Ultrasound‐guided percutaneous injection of a radiopaque or colored contrast agent could have facilitated fluoroscopic and endoscopic identification.
On preoperative ultrasound reports, 10 of 12 dogs had hydroureter and a dilated renal pelvis. When reevaluated postoperatively, 1 of 5 had some hydroureter, but it was improved, and 3 of 6 had some evidence of persistent renal pelvic dilatation. Persistence of the hydroureter or hydronephrosis could have been due to the chronic nature of the obstructive lesion leading to stretch, the presence of additional congenital abnormalities in the ureters (megaureter), stricture formation at the CLA site, or fibrosis from chronic obstruction.
The previously reported clinical findings of pollakiuria, bacterial cystitis, and urinary incontinence in dogs with ureteroceles,42 were consistent with findings in the 12 of 13 dogs in this study, with 1 dog presenting for vomiting/difficulty standing. In the latter dog, the ureteroceles was likely an incidental finding to gastroenteritis that resolved with supportive care. In the human literature, the treatment of clinically silent ureteroceles is recommended due to the potential risk of UTI, persistent ureteral outflow tract obstruction, and the loss of renal function.12 The treatment of asymptomatic ureteroceles in veterinary medicine should also be considered.
Azotemia was present in only 2 of 13 dogs preoperatively and 1 of 7 dogs postoperatively. The dog with progressive persistent azotemia was noted to have dysplastic architecture of the kidneys on preoperative and postoperative ultrasound imaging. However, without obtaining renal biopsies, the exact etiology is unknown. Hydroureter has been reported to predispose to increased hydrostatic pressure and permanent renal damage.15 Additional research would be required to understand if the progressive disease was primary renal dysplasia, progressive renal damage from chronic ureteral obstruction, or vesiculoureteral reflux from a large UO after fixation.
In this study, a single laser incision was utilized. No evidence of vesicoureteral reflux was appreciated on postoperative imaging, but follow‐up contrast retrograde cystourethrography to test for ureterovesicular reflux was not performed. Assessing outcomes of multiple, solitary punctures creating a “watering‐can” appearance44 versus a single laser incision in the cystoscopic treatment of ureteroceles in dogs is another potential area of research, but with the positive outcomes shown here, this might be unnecessary.
Evaluation for long‐term ureterovesicular reflux using voiding cystourethrograms would be helpful to determine if there is concern. Because of the excessively long ureter seen in dogs, compared to people, the development of clinically relevant UVJ reflux is highly unlikely. Clinically, with the resolution of ureteral dilatation, improvement of renal pelvic dilatation, improvement in urinary incontinence, and pollakiuria, along with the improved rate of UTIs, and failure of progressive azotemia, the likelihood of reflux creating a clinical problem is low.
The limitations of this study include its retrospective nature and lack of standardized follow‐up. However, these results represent a standardized cystoscopic approach performed by 2 institutions with specialists trained in endourology. Both institutions had comparable outcomes. Differential glomerular filtration rate (GFR) studies were not performed to evaluate renal function before and after CLA. Urethral pressure profilometry was not performed to assess concurrent urethral sphincter mechanism incompetence; it is possible that urethral sphincter mechanism incompetence contributed to clinical signs. However, given the high resolution of incontinence with CLA, this was not clinically impactful. Given the rare incidence of ureteroceles in dogs, this case series possesses the largest number of cases when compared to the current literature and can help form the basis for further comparison studies.
In conclusion, ureteroceles are a rare condition in dogs but should be considered as a differential for dysuria or incontinence, with concurrent hydroureter. Cystoscopic‐guided ureteral ablation was a safe, effective, and minimally invasive technique for treating UO stenosis associated ureteroceles in most dogs. Ureteroceles are commonly associated with ureteral ectopia but can be orthotopic as well. They are uncommonly associated with azotemia. Dogs with ureteroceles should be considered to have a good to excellent prognosis and renal sparing treatments should be encouraged. Prospective studies are warranted to assess short‐ and long‐term outcomes, the presence of ureterovesicular reflux, and to compare laser ablation to other surgical techniques.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENTS
Presented in abstract form at the Veterinary Interventional Radiology and Interventional Endoscopy Conference, Cabo San Lucas, June 2017.
Urinary continence score (1‐10).
1 = Leaking when walking, running, playing, and sleeping, never urinates a full bladder.
5 = Leaking mainly when laying down; minimally when walking or playing; leak immediately before or after urination.
7.5 = Only leaking when sleeping
9 = Notice urine on fur a few times per week.
10 = No leaking known at all.
Rogatko CP, Berent AC, Adams LG, Weisse CW, Bagley D. Endoscopic laser‐ablation for the treatment of orthotopic and ectopic ureteroceles in dogs: 13 cases (2008‐2017). J Vet Intern Med. 2019;33:670–679. 10.1111/jvim.15424
Present address Cleo P. Rogatko, The New England Animal Medical Center, West Bridgewater, MA
REFERENCES
- 1. Merlini E, Lelli Chiesa P. Obstructive ureterocele—an ongoing challenge. World J Urol. 2004;22:107‐114. [DOI] [PubMed] [Google Scholar]
- 2. Timberlake MD, Corbett ST. Minimally invasive techniques for management of the ureterocele and ectopic ureter. Urol Clin North Am. 2015;42:61‐76. [DOI] [PubMed] [Google Scholar]
- 3. Ettinger S. Textbook of Veterinary Internal Medicine: Diseases of the Dog and Cat. St. Louis, MO: Elsevier; 2017. [Google Scholar]
- 4. Castagnetti M, El‐Ghoneimi A. Management of duplex system ureteroceles in neonates and infants. Nat Rev Urol. 2009;6:307‐315. [DOI] [PubMed] [Google Scholar]
- 5. Husmann D et al. Management of ectopic ureterocele associated with renal duplication: a comparison of partial nephrectomy and endoscopic decompression. J Urol. 1999;162:1406‐1409. [PubMed] [Google Scholar]
- 6. Patel RC, Newman RC. Ureteroscopic management of ureteral and ureteroenteral strictures. Urol Clin North Am. 2004;31:107‐113. [DOI] [PubMed] [Google Scholar]
- 7. Van Batavia JP, Casale P. Robotic surgery in pediatric urology. Curr Urol Rep. 2014;15(402):1‐7. [DOI] [PubMed] [Google Scholar]
- 8. Leavitt DA, Rambachan A, Haberman K, DeMarco R, Shukla AR. Robot‐assisted laparoscopic ipsilateral ureteroureterostomy for ectopic ureters in children: description of technique. J Endourol. 2012;26:1279‐1283. [DOI] [PubMed] [Google Scholar]
- 9. McLeod DJ, Alpert SA, Ural Z, Jayanthi VR. Ureteroureterostomy irrespective of ureteral size or upper pole function: a single center experience. J Pediatr Urol. 2014;10:616‐619. [DOI] [PubMed] [Google Scholar]
- 10. Lee RS, Retik AB, Borer JG, Diamond DA, Peters CA. Pediatric retroperitoneal laparoscopic partial nephrectomy: comparison with an age matched cohort of open surgery. J Urol. 2005;174:708‐711; discussion 712. [DOI] [PubMed] [Google Scholar]
- 11. Chertin B, Ben‐Chaim J, Landau EH, et al. Pediatric transperitoneal laparoscopic partial nephrectomy: comparison with an age‐matched group undergoing open surgery. Pediatr Surg Int. 2007;23:1233‐1236. [DOI] [PubMed] [Google Scholar]
- 12. Di Benedetto V, Morrison‐Lacombe G, Bagnara V, Monfort G. Transurethral puncture of ureterocele associated with single collecting system in neonates. J Pediatr Surg. 1997;32:1325‐1327. [DOI] [PubMed] [Google Scholar]
- 13. Conlin MJ, Skoog SJ, Tank ES. Current management of ureteroceles. Urology. 1995;45:357‐362. [DOI] [PubMed] [Google Scholar]
- 14. McLoughlin MA, Hauptman JG, Spaulding K. Canine ureteroceles: a case report and literature review. J Am Anim Hosp Assoc. 1989;25:699‐706. [Google Scholar]
- 15. Stiffler KS, McCrackin Stevenson MA, Mahaffey MB, Howerth EW, Barsanti JA. Intravesical ureterocele with concurrent renal dysfunction in a dog: a case report and proposed classification system. J Am Anim Hosp Assoc. 2002;38:33‐39. [DOI] [PubMed] [Google Scholar]
- 16. Stowater JL, Springer AL. Ureterocele in a dog (a case report). Vet Med Small Anim Clin. 1979;74:1753‐1756. [PubMed] [Google Scholar]
- 17. Smith AL, Radlinsky MG, Rawlings CA. Cystoscopic diagnosis and treatment of ectopic ureters in female dogs: 16 cases (2005–2008). J Am Vet Med Assoc. 2010;237:191‐195. [DOI] [PubMed] [Google Scholar]
- 18. Pearson H, Gibbs C. Urinary tract abnormalities in the dog. J Small Anim Pract. 1971;12:67‐84. [DOI] [PubMed] [Google Scholar]
- 19. Scott RC, Greene RW, Patnaik AK. Unilateral ureterocele associated with hydronephrosis in a dog. J Am Anim Hosp Assoc. 1974;10:126‐132. [Google Scholar]
- 20. Hoffman S, Metropolitan VH, Ferguson HR. Ureterocele in a dog: case study. J Am Anim Hosp Assoc. 1991;27:93‐95. [Google Scholar]
- 21. Lautzenhiser SJ, Bjorling DE. Urinary incontinence in a dog with an ectopic ureterocele. J Am Anim Hosp Assoc. 2002;38:29‐32. [DOI] [PubMed] [Google Scholar]
- 22. Takiguchi M, Yasuda J, Ochiai K, Morita Y, Hashimoto A. Ultrasonographic appearance of orthotopic ureterocele in a dog. Vet Radiol Ultrasound. 1997;38:398‐399. [DOI] [PubMed] [Google Scholar]
- 23. Tattersall JA, Welsh E. Ectopic ureterocele in a male dog: a case report and review of surgical management. J Am Anim Hosp Assoc. 2006;42:395‐400. [DOI] [PubMed] [Google Scholar]
- 24. Ross LA, Lamb CR. Reduction of hydronephrosis and hydroureter associated with ectopic ureters in two dogs after ureterovesical anastomosis. J Am Vet Med Assoc. 1990;196:1497‐1499. [PubMed] [Google Scholar]
- 25. Martin RA, Harvey HJ, Flanders JA. Bilateral ectopic ureters in a male dog: a case report. J Am Anim Hosp Assoc. 1985;21:80‐84. [Google Scholar]
- 26. Secrest S, Britt L, Cook C. Imaging Diagnosis‐ Bilateral Orthotopic Ureteroceles in a dog. Vet Radiol Ultrasound. 2011;52:448‐450. [DOI] [PubMed] [Google Scholar]
- 27. Green TA, Arble JB, Chew DJ, Dudley RM. Diagnosis and management of ureteroceles in two female dogs. J Am Anim Hosp Assoc. 2011;47:138‐144. [DOI] [PubMed] [Google Scholar]
- 28. Colopy SA, Dennison SE, Kerwin‐Lucchi LJ, Danielson KC. What Is your diagnosis? J Am Vet Med Assoc. 2011;238:293‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Auger M, Bua A‐S, Carmel ÉN, Dunn M. Use of cystoscopic‐guided laser ablation for treatment of unilateral ureterovesicular stenosis and secondary orthotopic ureterocele in a female dog. J Am Vet Med Assoc. 2018;253:463‐469. [DOI] [PubMed] [Google Scholar]
- 30. Meler E, Berent AC, Weisse C, Dunn M. Treatment of congenital distal ureteral orifice stenosis by endoscopic laser ablation in dogs: 16 cases (2010–2014). J Am Vet Med Assoc. 2018;253:452‐462. [DOI] [PubMed] [Google Scholar]
- 31. Tobias K, Spencer J. Veterinary Surgery: Small Animal. Vol 2 St.Louis, MO: Elsevier; 2011. [Google Scholar]
- 32. Berent AC, Weisse C, Mayhew PD, Todd K, Wright M, Bagley D. Evaluation of cystoscopic‐guided laser ablation of intramural ectopic ureters in female dogs. J Am Vet Med Assoc. 2012;240:716‐725. [DOI] [PubMed] [Google Scholar]
- 33. Runge JJ, Berent AC, Mayhew PD, Weisse C. Transvesicular percutaneous cystolithotomy for the retrieval of cystic and urethral calculi in dogs and cats: 27 cases (2006–2008). J Am Vet Med Assoc. 2011;239:344‐349. [DOI] [PubMed] [Google Scholar]
- 34. Berent AC, Mayhew PD, Porat‐Mosenco Y. Use of cystoscopic‐guided laser ablation for treatment of intramural ureteral ectopia in male dogs: four cases (2006‐2007). J Am Vet Med Assoc. 2008;232:1026‐1034. [DOI] [PubMed] [Google Scholar]
- 35. Tong K, Weisse C, Berent AC. Rigid urethrocystoscopy via a percutaneous fluoroscopic‐assisted perineal approach in male dogs: 19 cases (2005–2014). J Am Vet Med Assoc. 2016;249:918‐925. [DOI] [PubMed] [Google Scholar]
- 36. Burdick S, Berent AC, Weisse C, Langston C. Endoscopic‐guided laser ablation of vestibulovaginal septal remnants in dogs: 36 cases (2007–2011). J Am Vet Med Assoc. 2014;244:944‐949. [DOI] [PubMed] [Google Scholar]
- 37. Coplen DE, Duckett JW. The modern approach to ureteroceles. J Urol. 1995;153:166‐171. [DOI] [PubMed] [Google Scholar]
- 38. Sumfest JM, Burns MW, Mitchell ME. Pseudoureterocele: potential for misdiagnosis of an ectopic ureter as a ureterocele. Br J Urol. 1995;75:401‐405. [DOI] [PubMed] [Google Scholar]
- 39. Clinical Urography: An Atlas and Textbook of Urological Imaging. Philadelphia, PA: Saunders; 1990. [Google Scholar]
- 40. Whitacre MD, Tate LP, Estill CT, Van Camp SD. Transendoscopic Nd:YAG laser ablation of vaginal septa in a bitch. Vet Surg. 1991;20:257‐259. [DOI] [PubMed] [Google Scholar]
- 41. Ather MH, Jafri AH, Sulaiman MN. Diagnostic accuracy of ultrasonography compared to unenhanced CT for stone and obstruction in patients with renal failure. BMC Med Imaging. 2004;4(2):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dalla Palma L, Stacul F, Bazzocchi M, Pagnan L, Festini G, Marega D. Ultrasonography and plain film versus intravenous urography in ureteric colic. Clin Radiol. 1993;47:333‐336. [DOI] [PubMed] [Google Scholar]
- 43. Dalla Palma L, Pozzi‐Mucelli R, Stacul F. Present‐day imaging of patients with renal colic. Eur Radiol. 2001;11:4‐17. [DOI] [PubMed] [Google Scholar]
- 44. Haddad J, Meenakshi‐Sundaram B, Rademaker N, et al. “Watering can” ureterocele puncture technique leads to decreased rates of de novo vesicoureteral reflux and subsequent surgery with durable results. Urology. 2017;108:161‐165. [DOI] [PubMed] [Google Scholar]


