Abstract
Background
Acute pancreatitis (AP) is associated with a high death rate in dogs, but accurate predictors of early death are still lacking.
Objectives
To develop a scoring system for prediction of short‐term case fatality in dogs with AP.
Animals
One hundred sixty‐nine dogs with AP including 138 dogs in the training cohort and 31 dogs in the validation cohort.
Methods
Multicenter, retrospective cohort study. Survival analysis was used to assess the associations with short‐term death (within 30 days after admission). Independent predictors of death were identified by a stepwise selection method and used for the score calculation.
Results
Death rate within 30 days after admission was 33% in the training cohort. Four independent risk factors for short‐term death were identified in the training cohort: presence of systemic inflammatory response syndrome, coagulation disorders, increased creatinine and ionized hypocalcemia. Canine Acute Pancreatitis Severity (CAPS) score was developed to predict short‐term death, integrating these 4 factors in a weighted way. A simplified version of CAPS score (sCAPS) including respiratory rate instead of SIRS was also assessed. The area under the receiver‐operating characteristic curve (AUC) of CAPS and sCAPS scores was 0.92 in the training cohort with an optimal cutoff of 11 (sensitivity, 89%; specificity, 90%) and 6 (sensitivity, 96%; specificity, 77%), respectively. CAPS and sCAPS score were validated in the validation cohort with respective AUC of 0.91 and 0.96.
Conclusions and Clinical Importance
We propose 2 scoring systems that allow early and accurate prediction of short‐term death in dogs with AP.
Keywords: creatinine, coagulation disorders, hypocalcemia, prognosis, severity, systemic inflammatory response syndrome
Abbreviations
- aHR
adjusted HR
- AP
acute pancreatitis
- AUC
area under the curve
- BIC
Bayesian Information Criteria
- CAPS
Canine Acute Pancreatitis Severity
- CI
confidence interval
- cPL
canine pancreatic lipase
- CRC
C‐reactive protein
- HR
hazard ratio
- NVSA
National Veterinary School of Alfort
- NVST
National Veterinary School of Toulouse
- ROC
receiver operating curve
- RR
respiratory rate
- sCAPS
simplified Canine Acute Pancreatitis Severity
- SIRS
systemic inflammatory response syndrome
- Spec cPL
specific canine pancreatic lipase
- WBC
white blood cell
1. INTRODUCTION
Acute pancreatitis (AP) is common in dogs, but its pathogenesis is not completely understood.1, 2 Mild pancreatitis, which is characterized by moderate clinical signs (anorexia, abdominal pain, vomiting, and lethargy) generally results in full recovery with appropriate medical treatment. In severe pancreatitis, however, acute pancreatic necrosis results in more severe clinical signs and multisystem complications such as systemic inflammatory response syndrome (SIRS), multiple organ dysfunction syndrome, or disseminated intravascular coagulation.3, 4 In human medicine, there is an association between the presence of SIRS within the first 48 hours of severe AP and death.5, 6
To date, the diagnosis of AP and the assessment of its severity remain challenging. Recently developed diagnostic tests have allowed improvements in the diagnosis of AP. However, the combined sensitivity and specificity of the available tests is still imperfect, and diagnosis is usually established on the basis of consistent history, clinical, laboratory, and ultrasonographic findings.7, 8, 9, 10, 11 Currently, there is no blood test to differentiate mild from severe disease in dogs with AP.12, 13 In human medicine, early identification of severe AP is considered mandatory to initiate appropriate treatment as early as possible to decrease death rate.5, 14 Several scoring systems including Ranson, Glasgow, APACHE II (Acute Physiology and Chronic Health Evaluation II), BISAP (Bedside Index of Severity in Acute Pancreatitis), and recently the PANC 3 score have been shown to predict the severity of AP in people.15, 16, 17, 18, 19
Despite an increased knowledge about AP, the death rate in dogs remains high, ranging from 27% to 58%, contrasting with 5% to 15% death rate reported in human medicine.20, 21, 22 Consequently, severity assessment of AP in dogs appears as a major challenge to implement adequate treatment and reduce death rate.13, 20 Two scoring systems have been proposed in veterinary medicine to assess the severity of AP in dogs.12, 23 The first was based on the evaluation of organ system dysfunction.12 In this study, diagnosis of AP was based on high serum amylase or lipase activity as this study was conducted well before the routine availability of specific lipase immunoassays or high‐resolution abdominal ultrasonography.7, 24 More recently, a clinical severity index correlated with outcome has been proposed.23 In this study, a significant association with outcome was restricted to 4 body systems dysfunction (cardiac and respiratory systems, intestinal integrity, and vascular force).23 Although informative, this study lacked a multivariable analysis. Finally, neither of these 2 scoring systems has been validated in an independent external cohort of patients.
The aim of this study was to identify independent predictors of short‐term death in dogs with AP, to develop a scoring system to predict outcome, and, finally, to validate this scoring system in an independent external population of dogs with AP.
2. MATERIALS AND METHODS
2.1. Case selection
Medical records of dogs presented at the National Veterinary School of Alfort (NVSA) and the National Veterinary School of Toulouse (NVST) from January 2008 to December 2015 and diagnosed with AP were retrospectively reviewed. Dogs from NVSA were used for the development of a scoring system model (training cohort). The scoring system was subsequently validated for reliability on an independent population composed of dogs from NVST (validation cohort).
2.2. Diagnostic criteria of AP
Diagnosis of AP was based on the following inclusion criteria: acute onset of at least 2 compatible clinical signs (vomiting, anorexia, abdominal pain, or lethargy) and specific canine pancreatic lipase (Spec cPL) concentration > 400 μg/L associated or not with ultrasonographic findings consistent with AP (thickened hypoechoic pancreas with blurred margins, surrounded by hyperechoic adipose tissue) or Spec cPL concentration between 200 and 400 μg/L, abnormal SNAP cPL result (color intensity of the sample spot equal to or more intense than the control spot, corresponding to a cPL ≥ 200 μg/L), or both, associated with ultrasonographic findings consistent with AP. Dogs were excluded from analysis if clinical signs were present for more than 7 days or if a previous episode of AP was reported.
2.3. Data collection
Data extracted from the electronic medical records included signalment, history, physical examination findings at admission, CBC, plasma biochemistry profile, electrolytes, coagulation profile, and abdominal ultrasonography results obtained at the earliest after admission (within a maximum delay of 48 hours post‐admission). The outcome defined as death within 30 days of admission (ie, short‐term death) was recorded. A 30‐day cutoff was selected to appreciate short‐term death related to AP. Dogs dead within 30 days of admission for causes unrelated to AP or euthanized for financial reasons were right censored at the date of death. Dogs still alive 30 days of admission were right censored at the corresponding date (ie, date of admission +30 days). Dogs were excluded if outcome data were not available. The survival time was defined as the interval from admission to either the outcome (ie, death resulting from AP within 30 days of admission) or censoring. Cause and date of death were obtained in medical records or via telephone contact with owners. The study protocol was approved by the Ethics Committee of NVSA. During the study period, informed consents for privacy and personal data use were not obtained at time of dogs' admission given that data protection regulation was not applicable. Therefore, an oral consent was obtained afterwards when telephone contact was needed.
2.4. Laboratory findings
CBC, biochemistry, and coagulation profile were performed using routine methods at the diagnostic laboratories of NVSA and NVST. Spec cPL concentration was measured in a commercial laboratory (Idexx Laboratories). SNAP cPL test (Idexx Laboratories) was performed in house according to manufacturer's instruction.
2.5. Selection of variables
The selection of variables tested as potential risk factors for death in this study was based on previous data describing complications of AP in dogs or on variables already associated with poor prognosis in AP in dogs.3, 4, 12, 23, 25 Eleven binary variables were considered: (1) SIRS defined by the presence of at least 2 of the following criteria: heart rate > 120 beats per minute, respiratory rate (RR) > 20 movements per minute, body temperature <38.1°C or >39.2°C, white blood cell (WBC) count < 6000/mm3 or >16 000/mm3;26 (2) coagulation disorders defined by the presence of at least 1 of the following criteria: thrombocytopenia (platelet count <63 000/mm3),27 prothrombin time, activated partial thromboplastin time prolonged by more than 25% of the upper end of the reference interval, or both; (3) metabolic acidosis considered when blood pH < 7.35 and bicarbonate concentration <15 mEq/L; (4) hepatic injury defined by the presence of increased alanine aminotransferase (>133 U/L), alkaline phosphatase (>459 U/L) (ie, >3‐fold increase of the upper end of the reference interval), or both; (5) serum creatinine was considered increased when >1.6 mg/dL; (6) hyperkalemia (serum potassium >5.3 mEq/L); (7) hyperglycemia (serum glucose >150 mg/dL); (8) ionized hypocalcemia (ionized calcium <4.4 mg/dL); (9) hypoalbuminemia (serum albumin <2.6 g/dL); (10) marked elevation of Spec cPL concentrations (>1000 μg/L), and (11) age (in years).
2.6. Statistical analysis
Construction of the scoring system was based on a previously described model.28
The identification of risk factors for short‐term death was performed in 2 steps. First, a univariable analysis was performed to identify potential risk factors, that is, exposures associated with the outcome with a P‐value <.20 (using Kaplan‐Meier curves and log‐rank test). Second, for each potential risk factor identified in step 1, a multivariable model was constructed including the identified potential risk factors, the age of the dog (forced variable), as well as variables associated with both the exposure of interest and the outcome with a P‐value <.20 (potential confounders). Univariable associations among the above mentioned 11 variables were assessed using chi‐square test (or Fisher exact test when appropriate). If more than 2 potential confounders were identified, only the 2 with the lowest P‐value testing the association with the exposure of interest were selected, to have no more than 4 variables included into each multivariable model.29 If a potential risk factor remained significantly associated with the outcome in its multivariable model (P ≤ .05), it was considered as a risk factor for the outcome. Continuous variables identified as risk factors were categorized into 4 classes according to the quartiles or as binary variables (as previously defined). Identified risk factors were finally included into a new Cox model; if these identified risk factors were continuous, they were taken into account either as categorical (according to quartiles) or binary variables. The Cox models were then compared by using the Bayesian Information Criteria (BIC).30 The Cox model with the lowest BIC value was selected to perform the scoring methods. The value of the hazard ratio (HR) for each risk factor estimated from the selected Cox model was used, as weighting factors, to calculate scores for each dog. Scores were calculated through 2 scoring methods. For the first scoring method, the weighting factor was the value of the HR corresponding to the score was the value of the HR rounded to the first digit after the decimal point. For the second scoring method, the weighting factor was the value of the HR rounded to its integer value; when the fractional part was 0.5 (for instance, 7.5), the value was rounded to the closest higher integer (ie, 8). For each scoring method, the score was calculated for each dog by adding the weighting factors based on their individual profile on the identified risk factors. Receiver‐operating characteristic (ROC) analysis was performed to evaluate the effectiveness of the score for distinguishing dogs that died within 30 days of admission from those that remained alive both in the training and validation cohorts.31, 32 The areas under the curve (AUC) were calculated to quantify this discriminatory power. The optimal cutoffs of the score were calculated based on the Youden index.33 Different stages of Canine Acute Pancreatitis Severity (CAPS) score establishment and validation are presented by means of a flow diagram (Figure 1).
Figure 1.
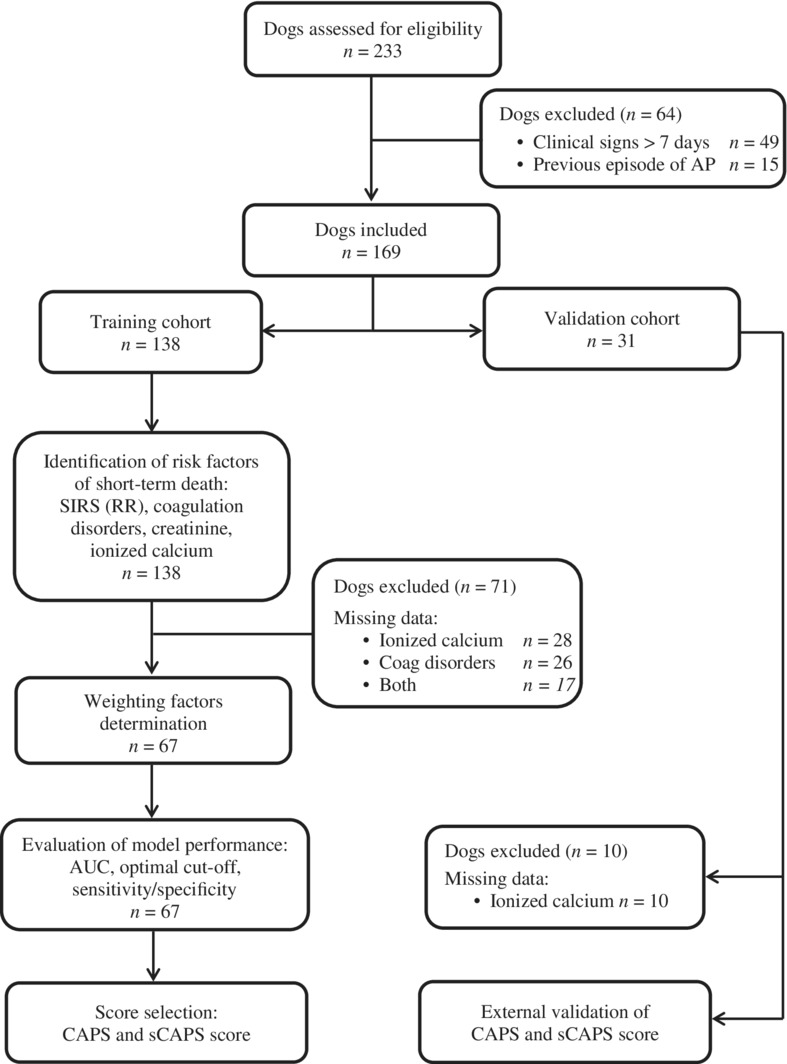
Flow diagram showing different stages of CAPS and sCAPS score establishment and validation. AUC, area under the curve; CAPS, Canine Acute Pancreatitis Severity; Coag, coagulation; sCAPS, simplified Canine Acute Pancreatitis Severity; SIRS, systemic inflammatory response syndrome
In this study, some biological variables (eg, calcium, coagulation times, bicarbonate concentration) were missing. Biological variables measured at the time of admission of the dog were chosen at the discretion of the attending clinician and may be limited owing to financial restriction of the owner, which is independent of the subsequent outcome. Therefore, data were considered missing at random. The identification of the risk factors was performed on all dogs (to take into account confounders more efficiently); the analyses regarding score establishment and validation were performed only in dogs with nonmissing data of the identified risk factors. No imputation has been performed. An association was considered as significant if P‐value ≤.05. All statistical analyses were performed using a commercially available software (SAS version 9.3; SAS Institute Inc., Cary, North Carolina).
3. RESULTS
3.1. Population demographic and clinicopathological data
A total of 233 dogs were included in this study. Among them, 49 dogs were excluded because of duration of clinical signs >7 days and 15 dogs were excluded because a previous episode of AP was reported. Therefore, the final study population was composed of 169 dogs including 138 dogs for the training cohort and 31 dogs for the validation cohort.
The training cohort was composed of 72 males including 23 castrated males and 66 females including 44 spayed females. Mean age of the dogs at time of AP diagnosis was 10 years (range 1‐20 years). Thirty‐seven breeds were represented including crossbreed dogs (n = 24, 17%), Yorkshire Terrier (n = 18, 13%), Jack Russell Terrier (n = 12, 9%), Poodle (n = 11, 8%), French Bulldog (n = 9, 7%), English Cocker Spaniels (n = 8, 6%), Brittany Spaniels (n = 5, 4%), Shih Tzu (n = 4, 3%), West Highland Terrier, (n = 4, 3%), Boxer (n = 3, 2%), American Staffordshire (n = 3, 2%), Rottweiler (n = 3, 2%), and 25 other breeds represented with 1 or 2 dogs. Clinical signs consistent with AP reported at admission were lethargy (n = 122, 88%), anorexia (n = 119, 86%), vomiting (n = 115, 83%), and abdominal pain (n = 81, 59%). One hundred twenty‐five dogs (91%) had a Spec cPL concentration >400 μg/L. Thirteen (9%) dogs presented a Spec cPL concentration between 200 and 400 μg/L associated with ultrasonographic signs of AP. Abdominal ultrasonography was performed in 127 of 138 dogs (92%). Findings consistent with AP were observed in 90 of 127 dogs (71%, distributed as follows: 61% in dogs with Spec cPL > 400 μg/L and 10% with Spec cPL 200‐400 μg/L).
The validation cohort was composed of 13 males including 3 castrated males and 18 females including 13 spayed females. The mean age was 10 years (range 1‐16 years). Ten breeds were represented with crossbreed dogs (n = 10, 32%), Labrador Retriever (n = 5, 19%), and Yorkshire (n = 4, 13%); each of the other 7 breeds were represented by 1 or 2 dogs. Clinical signs reported at admission were lethargy (n = 26, 84%), vomiting (n = 25, 81%), anorexia (n = 19, 62%), and abdominal pain (n = 18, 58%). For 27 dogs (87%), abnormal SNAP cPL result, in association with abdominal ultrasonographic findings of AP was used for diagnosis. Spec cPL concentration was available for 4 dogs (greater than 400 μg/L for 2 dogs; between 200 and 400 μg/L for the other 2). Abdominal ultrasonography was performed in 30 of 31 dogs (97%) and was consistent with AP in 29 of 30 dogs (97%). Spec cPL concentration was greater than 400 μg/L in the 2 dogs for whom the diagnosis of AP was not supported by abdominal ultrasonography.
3.2. Outcome
The case fatality rate 30 days after admission (ie, short‐term death) was 33% (46/138 dogs) in the training cohort. Among them, 29 dogs were euthanized (13 during the hospitalization period and 16 after discharge). In the validation cohort, the case fatality rate 30 days after admission was 35% (11/31 dogs). Among them, 7 dogs were euthanized (3 during the hospitalization and 4 after discharge). For all dogs in both cohorts, death within the 30 days after admission was related to AP. Therefore, no dog was censored because of death unrelated to AP.
3.3. Identification of risk factors for short‐term death
The 11 variables were initially tested for potential association with short‐term death. Among them, presence of SIRS at admission, coagulation disorders, metabolic acidosis, increased creatinine, hyperkalemia, ionized hypocalcemia, hyperglycemia, hypoalbuminemia, and Spec cPL concentrations >1000 μg/L were associated with short‐term death with a P‐value <.20 on univariable analyses (Table 1) and were therefore selected for the multivariable analysis.
Table 1.
Univariable association between variables and short‐term death in dogs with acute pancreatitis in training cohort
| Variable | Total n/N0 | Outcome at 30 days after‐admission | P‐value | Crude hazard ratio (95% CI) | |
|---|---|---|---|---|---|
| Survivors n/N1 (%) | Nonsurvivors n/N2 (%) | ||||
| SIRS | 72/138 | 38/92 (41) | 34/46 (74) | <.001 | 5.0 (2.3‐10.8) |
| Coagulation disorders | 13/97 | 4/63 (6) | 9/34 (26) | .006 | 2.9 (1.4‐6.4) |
| Metabolic acidosis | 32/85 | 12/51 (24) | 20/34 (59) | <.001 | 3.2 (1.6‐6.4) |
| Hepatic injury | 67/124 | 46/88 (52) | 21/36 (58) | .51 | 1.3 (0.7‐2.6) |
| Increased creatinine | 64/138 | 19/92 (21) | 27/46 (59) | <.001 | 3.7 (2.0‐6.7) |
| Hyperkalemia | 15/130 | 5/85 (6) | 10/45 (22) | .020 | 2.4 (1.0‐5.7) |
| Ionized hypocalcemia | 26/96 | 8/62 (13) | 18/34 (53) | <.001 | 4.7 (2.3‐9.4) |
| Hyperglycemia | 16/122 | 7/84 (8) | 9/38 (24) | .011 | 2.7 (1.3‐5.6) |
| Hypoalbuminemia | 62/101 | 41/71 (58) | 21/30 (70) | .048 | 1.9 (1.0‐4.1) |
| Spec‐cPL > 1000 μg/L | 41/138 | 22/92 (24) | 19/46 (41) | .054 | 1.8 (1.0‐3.2) |
| Age (years) | 138/138 | 11.3 (range, 1‐16) | 9.3 (range, 2.3‐20) | .67 | 1.3 (0.7‐2.4) |
Abbreviations: CI, confidence interval; N, number of dogs for which the abnormality was investigated (N0 total, N1 survivor, N2 nonsurvivor); n, number of dogs presenting the laboratory or clinical abnormality; SIRS, systemic inflammatory response syndrome
The multivariable analysis identified SIRS (adjusted HR [aHR], 5.8; 95% confidence interval [CI], 2.2‐15.4; P < .01), coagulation disorders (aHR, 3.8; 95% CI, 1.5‐9.7; P < .01), increased creatinine (aHR, 2.9; 95% CI, 1.3‐6.5; P < .01), and ionized hypocalcemia (aHR, 4.5; 95% CI, 1.6‐12.8; P < .01) as independent risk factors of short‐term death (Table 2). At this stage, plasma concentrations of creatinine and ionized calcemia were also considered as categorical according to the quartiles. Comparison of Cox models using the BIC value did not show superiority of quartile‐specific categorical considerations of these variables over the binary considerations. Therefore, further analyses were performed considering creatinine and ionized calcium as binary variables (see Supporting Information).
Table 2.
Results from multivariable Cox proportional hazard models
| Variables | Two most pertinent variables with lower P‐value (≤.2)a | Adjusted hazard ratio (95% CI) | P‐value | |
|---|---|---|---|---|
| SIRS | Increased creatinine | Ionized hypocalcemia | 5.8 (2.2–15.4) | <.001 |
| Coagulation disorders | Spec‐cPL > 1000 μg/L | Ionized hypocalcemia | 3.8 (1.5‐9.7) | .005 |
| Metabolic acidosis | Ionized hypocalcemia | Increased creatinine | 1.5 (0.5‐4.4) | .44 |
| Increased creatinine | SIRS | Metabolic acidosis | 2.9 (1.3–6.5) | .008 |
| Hyperkalemia | Increased creatinine | / | 1.6 (0.7‐4.0) | .29 |
| Ionized hypocalcemia | Hypo‐albuminemia | Hyperglycemia | 4.5 (1.6–12.8) | .005 |
| Hyperglycemia | Ionized hypocalcemia | / | 1.2 (0.4‐4.0) | .73 |
| Hypo‐albuminemia | Ionized hypocalcemia | Coagulation disorders | 2.0 (0.8‐5.0) | .16 |
| Spec‐cPL > 1000 μg/L | Coagulation disorders | Ionized hypocalcemia | 1.8 (0.8‐4.2) | .17 |
Variables significantly associated with the short‐term death in dogs with acute pancreatitis (P ≤ .05) are considered as risk factors of short‐term death in the training cohort.
Abbreviations: CI, confidence interval; SIRS, systemic inflammatory response syndrome; Spec‐cPL, specific canine pancreatic lipase.
In the multivariable model, only age (forced variable) and the 2 variables associated with the variable of interest with the lowest P‐value among variable with P ≤ .2 in the univariable analysis (considered as most pertinent confounding variable) were included as potential confounders.
In order to simplify the score, each variable defining SIRS (heart rate, RR, body temperature, and WBC count) were evaluated separately as categorical variables according to the quartiles. Only RR was associated with short‐term death after crude and adjusted analysis. In a Cox model containing creatinine, ionized calcemia, and coagulation disorders, RR was included considering the first quartile (RR < 24 mpm) as the reference category, and results showed similar HR for the 3 quartile‐specific categories. Consequently, these last 3 categories were combined and RR was considered as a binary variable (RR < 24 mpm versus ≥24 mpm).
3.4. Scoring system development
Identified risk factors in the previous analyses were simultaneously included into a single multivariable Cox proportional hazard model to determine weighting factors of the score for each of the 4 variables. Model A represented the Cox model including creatinine, ionized calcium, coagulation disorders, and SIRS. Model B represented the Cox model including creatinine, ionized calcium, coagulation disorders, and RR. BIC value of Model A and Model B was 184.1 and 176.0, respectively, suggesting superiority of Model B over Model A. Consequently, both models were kept into consideration for further analysis.
Then 4 scores were calculated: score Ad, score Ai, score Bd, and score Bi, respectively, for scores from Model A with weighting factors rounded after the decimal point (“d” for “decimal”), from Model A with weighting factors rounded to the closest integer (“i” for “integer”), from Model B with weighting factors rounded after the decimal point and from Model B with weighting factors rounded to the closest integer (Table 3). All the 4 scores Ad, Ai, Bd, and Bi presented the same AUC of 0.92 and the same 95% CI (0.85‐0.99; Figure 2). Cutoff values, corresponding sensitivities and specificities, and Youden index were determined for each score and are presented in Table 4. The optimal cutoff value of scores Ad, Ai, Bd, and Bi yielded sensitivity/specificity of 89%/90%, 89%/90%, 96%/77%, and 96%/77%, respectively.
Table 3.
Variables and corresponding weighting factors for scores Ad, Ai, Bd, and Bi
| Weighting factorsa | |||||
|---|---|---|---|---|---|
| Variables | Score Ad | Score Ai | Score Bd | Score Bi | |
| Creatinine (mg/dL) | ≥1.6 | 4.0 | 4 | 3.9 | 4 |
| <1.6 | 0 | 0 | 0 | 0 | |
| Ionized calcium (mg/dL) | <4.4 | 3.2 | 3 | 2.7 | 3 |
| ≥4.4 | 0 | 0 | 0 | 0 | |
| Coagulation disorder | Yes | 2.5 | 3 | 3.1 | 3 |
| No | 0 | 0 | 0 | 0 | |
| SIRS | Yes | 7.5 | 8 | / | / |
| No | 0 | 0 | |||
| Respiratory rate (mpm) | ≥24 | / | / | 2.7 | 3 |
| <24 | 0 | 0 | |||
Abbreviation: SIRS, systemic inflammatory response syndrome.
Weighting factors was assigned based on calculated hazard ratio rounded after the decimal point for scores Ad and Bd (“d” for “decimal”) or rounded to the closest integer value for scores Ai and Bi (“i” for “integer”).
Figure 2.
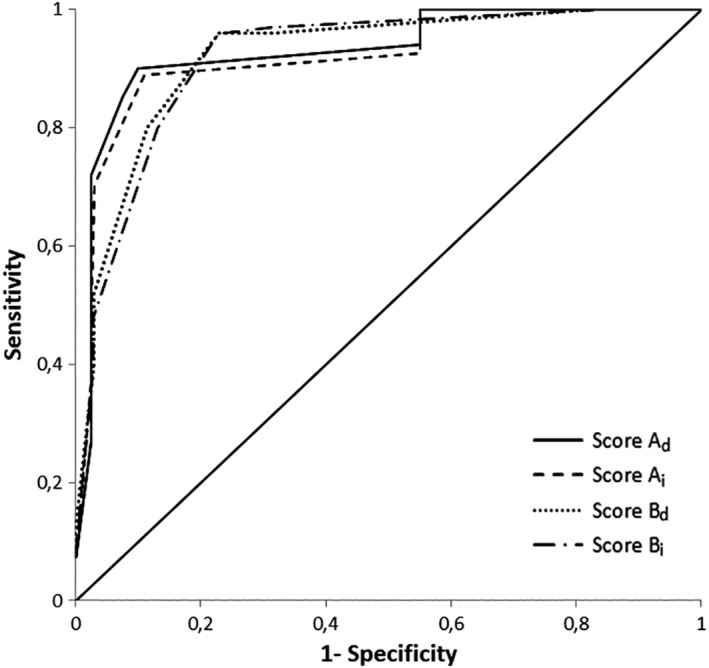
Receiver operator characteristic (ROC) curve for prediction of short‐term death in the training cohort for scores Ad, Ai, Bd, and Bi. Each score has an area under the curve (AUC) of 0.92
Table 4.
Performance characteristics for short‐term death prediction of scores Ad, Ai, Bd and Bi on training cohort
| Score | Optimal cutoff | Sensitivity (%) | Specificity (%) | Youden index | AUC | 95% CI for AUC |
|---|---|---|---|---|---|---|
| Ad | 10.0 | 89 | 90 | 0.79 | 0.92 | 0.85‐0.99 |
| Ai | 11 | 89 | 90 | 0.79 | 0.92 | 0.85‐0.99 |
| Bd | 5.4 | 96 | 77 | 0.73 | 0.92 | 0.85‐0.99 |
| Bi | 6 | 96 | 77 | 0.73 | 0.92 | 0.85‐0.99 |
Score Ad, from model A with weighting factors rounded after the decimal point.
Score Ai, from model A with weighting factors rounded to the closest integer.
Score Bd, from model B with weighting factors rounded after the decimal point.
Score Bi from model B with weighting factors rounded to the closest integer.
Abbreviations: AUC, area under the ROC curve; CI, Confidence Interval.
Considering the similar performance of the 2 scoring methods (same Youden indexes), Scores Ai and Bi were selected to facilitate score calculation using integer values for weighting factors. Score Ai was renamed as the “Canine Acute Pancreatic Severity (CAPS) score” and score Bi was renamed as the “simplified Canine Acute Pancreatic Severity (sCAPS) score”. Formula of CAPS score was 8 × (1 if SIRS, 0 otherwise) + 3 × (1 if coagulation disorders, 0 otherwise) + 4 × (1 if increased creatinine, 0 otherwise) + 3 × (1 if ionized hypocalcemia, 0 otherwise). Formula of sCAPS score was 3 × (1 if RR ≥ 24 mpm, 0 otherwise) + 3 × (1 if coagulation disorders, 0 otherwise) + 4 × (1 if increased creatinine, 0 otherwise) + 3 × (1 if ionized hypocalcemia, 0 otherwise).
3.5. Validation of the scoring system
CAPS and sCAPS scores were validated in 21 of the 31 dogs of the validation cohort for which all data for score calculation were available. The AUC of the ROC curve for CAPS and sCAPS scores were 0.91 (95% CI, 0.77‐1.00) and 0.96 (95% CI, 0.88‐1.00), respectively (Table 5). Application of the CAPS and sCAPS scores and their respective cutoff values in the validation cohort provided sensitivity/specificity of 86%/92% and 100%/85%, respectively (Table 5), and correctly classified short‐term outcome in 89% and 92% of dogs, respectively.
Table 5.
Performance characteristics for short‐term death prediction of CAPS and sCAPS scores on validation cohort
| Model | Optimal cutoff | Sensitivity (%) | Specificity (%) | Youden index | Correctly classified (%) | AUC | 95% CI for AUC |
|---|---|---|---|---|---|---|---|
| CAPS score | 11 | 86 | 92 | 0.79 | 89 | 0.91 | 0.77‐1.00 |
| sCAPS score | 6 | 100 | 85 | 0.73 | 92 | 0.96 | 0.88‐1.00 |
CAPS score corresponding to score Ai; sCAPS score corresponding to Score Bi.
Abbreviations: AUC, area under the ROC curve; CAPS, Canine Acute Pancreatitis Severity; CI, confidence interval; sCAPS, simplified Canine Acute Pancreatitis Severity.
4. DISCUSSION
In this study, we identified 4 independent risk factors for short‐term death in dogs with AP: presence of SIRS, coagulation disorders, increased creatinine, and ionized hypocalcemia. We also proposed 2 scoring systems able to predict short‐term death in dogs with AP: CAPS score, developed using the 4 risk factors identified, and a simplified severity score (sCAPS score), determined for a faster calculation. Both of them permitted accurate identification of dogs at risk of short‐term death to initiate appropriate management and monitoring of severe form of AP. These severity scores fulfill the criteria of a good scoring system which should be applicable at the time of diagnosis, easy to use, and accurate.34 CAPS and sCAPS scores allow specific evaluation of AP severity, whereas recently developed illness severity score such as APPLE score is intended for dogs admitted in an intensive care unit regardless of the underlying disease.35, 36
These scoring systems were developed in a large cohort of dogs diagnosed with AP. Demographic data and clinical presentations in the study population were in accordance to previously published data.1, 10, 12, 23 In this study, short‐term death rates (33% for training cohort and 35% for validation cohort) are similar to death rates previously reported for AP in dogs (27%‐58%).20, 21 In the training cohort, abdominal ultrasonography revealed findings suggestive of AP in 70% of the cases. This result is consistent with the previously reported sensitivity (68%) of abdominal ultrasonography for diagnosis of AP in dogs.11 In the other cases, the abdominal ultrasound findings were not suggestive of AP but allowed exclusion of other diseases.
The CAPS score is based on 4 prognostic factors selected after multivariable statistical analysis: presence of SIRS at admission, coagulation disorders, increased creatinine concentration, and ionized hypocalcemia. These variables are usually recorded within the first 24 hours of hospitalization, allowing an early evaluation of the patient risk for short‐term death. Moreover, CAPS score proposed good performance with AUC of the ROC curve of 0.92 and 0.91 in the training and the validation cohort, respectively.
Although presence of SIRS had already been described as a negative prognostic factor in critical dogs, this study reveals that SIRS is statistically associated with short‐term death in dogs with AP.37 In human AP, an association between SIRS and adverse outcome has also been reported and SIRS is included in the BISAP severity score.6, 18 Systemic inflammatory condition could have been evaluated through C‐reactive protein (CRP) measurement especially because CRP has been associated with outcome prediction in dogs with AP.23, 25, 38 Unfortunately, because of the retrospective design of this study and the time frame during which the dogs were included, few dogs had CRP measured to consider this variables in those selected.
As in previous studies, our study identified that increased coagulation times or thrombocytopenia are associated with poor prognosis in dogs with AP.25, 39 These findings are consistent with a consumptive coagulopathy, a well‐recognized complication of AP in dogs.3, 40
Identification of increased creatinine as a prognostic factor in dogs with AP is in agreement with previous published studies in veterinary literature as well as in human literature.12, 41, 42, 43 Acute kidney injury is a complication described in dogs with AP.1 In humans, it is a well‐described complication of AP.42, 43, 44 Although the exact mechanism of acute kidney injury associated with AP remains unclear, chronic kidney disease has been associated with a higher risk of death in humans diagnosed with AP.42, 44
Finally, ionized hypocalcemia was identified as a risk factor for short‐term death in this study. Hypocalcemia has already been associated with poor outcome in humans and cats with AP but not yet in dogs.45, 46, 47
However, ionized calcium measurement and coagulation profile are not always available in all clinical practices, making it less accessible at admission time for all veterinarians. Moreover, evaluation of SIRS may be time consuming, considering the fact that it requires evaluation of 4 systems. Therefore, a simplified score including RR instead of SIRS was proposed. The sCAPS score presents good performance characteristics with AUC of the ROC curve of 0.92 and 0.96 in the training and the validation cohort, respectively, and allows faster calculation. Moreover, its good sensitivity (96%) allows accurate identification of dogs necessitating intensive care. However, the user must be aware of its lower specificity (77%) when compared to CAPS score specificity of 90%. This finding may be explained by the lack of specificity of an increased RR, which can occur secondary to nondisease conditions such as stress. Considering this limit, sCAPS score should never be used for prognostic evaluation, for which the use of CAPS score seems more appropriate due to a higher specificity. Consequently, CAPS score remains recommended to optimize predictive accuracy.
Limitations of this study include the lack of ultrasonography in 11 dogs (6.5%) for which Spec cPL concentration was above 400 μg/L. For these dogs, misdiagnosis could not be excluded, as up to 23% of dogs with acute abdominal disease not related to AP might present Spec cPL results consistent with AP.48
Secondly, the retrospective design of the study explains the lack of data for several dogs. Therefore, variables associated with death were possibly falsely excluded because of insufficient statistical power (eg, metabolic acidosis). Also, some risk factors were not tested and could have proven useful in the score determination. For example, cardiovascular injury was not assessed because relevant data necessary to determine this variable were lacking in many cases. Likewise, concomitant diseases such as hyperadrenocorticism or hypothyroidism have not been investigated as a potential risk factor for death because this information was not always available at the time of admission or investigated during AP management. In the training cohort, only 9 dogs were known to have a concomitant disease at the time of diagnosis. We did not include this variable in the univariable analysis as the proportion of dogs affected was probably underestimated.
Thirdly, outcome defined as death within 30 days after admission included naturally dead and euthanized dogs. In veterinary medicine, the prevalence of euthanasia poses a unique challenge to all scores based on death outcome. Inclusion of euthanized dogs represents a bias as clinician perception of clinical signs or clinicopathological data during the follow‐up of the dog probably influence the owner's decision of euthanasia. Although we excluded dogs euthanized for reasons unrelated to AP, we cannot exclude that false‐positive death association occurred. In the same way, misclassification errors on the event (ie, wrongly considering as a death secondary to AP) may have occurred. Such errors lead to misclassification bias, which reduces the strength of the association between the exposure and the outcome. We therefore might have not identified risk factors for death from AP, because they were not significantly associated with the study outcome. However, if any, these errors should be few considering that details of the death were available for most of the dogs.
Finally, a 30‐day period after admission was chosen for the evaluation of outcome drawing on previous study dealing with survival in acute diseases.28, 49, 50, 51 By considering the 30‐day outcome, we wanted to identify dogs at risk of short‐term death requiring early and appropriate management. We cannot exclude that some dogs died few days after the 30‐day period. Therefore, our results cannot be generalized to middle (ie, >30 days after admission) or long‐term death due to AP.
Considering these limitations, the quality of this scoring system can certainly be improved by a prospective validation. Furthermore, it would be interesting to prospectively assess the evolution of variables over time. In human medicine, assessment of variables at admission and within the first 48 hours post‐admission are used for outcome prediction of AP.15, 18 In veterinary medicine, serial CRP concentration measurements were also proven as potential prognostic risk factor in AP.25 The retrospective nature of our study did not allow us to assess the change of laboratory variables as outcome predictors; we thus suggest this investigation in a future, prospective study.
Finally, CAPS and sCAPS scores might prove useful to demonstrate effective randomization of dogs in clinical trial. One of its objectives is to guide appropriate management of severe AP and its usefulness should be confirmed in a study evaluating different therapeutic strategies.
In conclusion, this study proposes 2 scoring systems applicable early after admission to help clinicians identifying dogs with AP at high‐risk of short‐term death and thus undertake appropriate management.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Appendix S1 Supplementary Information.
ACKNOWLEDGMENT
Part of this work was presented as an abstract at the 2016 ECVIM‐CA congress in Goteborg, Sweden.
Fabrès V, Dossin O, Reif C, et al. Development and validation of a novel clinical scoring system for short‐term prediction of death in dogs with acute pancreatitis. J Vet Intern Med. 2019;33:499–507. 10.1111/jvim.15421
REFERENCES
- 1. Mansfield CS. Acute pancreatitis in dogs: advances in understanding, diagnostics, and treatment. Top Companion Anim Med. 2012;27:123‐132. [DOI] [PubMed] [Google Scholar]
- 2. Watson PJ. Pancreatitis in dogs and cats: definitions and pathophysiology. J Small Anim Pract. 2015;56:3‐12. [DOI] [PubMed] [Google Scholar]
- 3. Ruaux C. Pathophysiology of organ failure in severe acute pancreatitis in dogs. Compend Contin Educ Vet. 2000;22:531‐542. [Google Scholar]
- 4. Mansfield CS. Pathophysiology of acute pancreatitis: potential application from experimental models and human medicine to dogs. J Vet Intern Med. 2012;26:875‐887. [DOI] [PubMed] [Google Scholar]
- 5. Tenner S, Baillie J, DeWitt J, Vege SS, American College of Gastroenterology . American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400‐1415. [DOI] [PubMed] [Google Scholar]
- 6. Mofidi R, Duff MD, Wigmore SJ, Madhavan KK, Garden OJ, Parks RW. Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis. Br J Surg. 2006;93:738‐744. [DOI] [PubMed] [Google Scholar]
- 7. Trivedi S, Marks SL, Kass PH, et al. Sensitivity and specificity of canine pancreas‐specific lipase (cPL) and other markers for pancreatitis in 70 dogs with and without histopathologic evidence of pancreatitis. J Vet Intern Med. 2011;25:1241‐1247. [DOI] [PubMed] [Google Scholar]
- 8. McCord K, Morley PS, Armstrong J, et al. A multi‐institutional study evaluating the diagnostic utility of the spec cPL™ and SNAP® cPL™ in clinical acute pancreatitis in 84 dogs. J Vet Intern Med. 2012;26:888‐896. [DOI] [PubMed] [Google Scholar]
- 9. Kook PH, Kohler N, Hartnack S, Riond B, Reusch CE. Agreement of serum spec cPL with the 1,2‐o‐dilauryl‐rac‐glycero glutaric acid‐(6′‐methylresorufin) ester (DGGR) lipase assay and with pancreatic ultrasonography in dogs with suspected pancreatitis. J Vet Intern Med. 2014;28:863‐870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Steiner JM. Diagnosis of pancreatitis. Vet Clin North Am Small Anim Pract. 2003;33:1181‐1195. [DOI] [PubMed] [Google Scholar]
- 11. Hess RS, Saunders HM, Van Winkle TJ, et al. Clinical, clinicopathologic, radiographic, and ultrasonographic abnormalities in dogs with fatal acute pancreatitis: 70 cases (1986–1995). J Am Vet Med Assoc. 1998;213:665‐670. [PubMed] [Google Scholar]
- 12. Ruaux CG, Atwell RB. A severity score for spontaneous canine acute pancreatitis. Aust Vet J. 1998;76:804‐808. [DOI] [PubMed] [Google Scholar]
- 13. Mansfield CS, Jones BR, Spillman T. Assessing the severity of canine pancreatitis. Res Vet Sci. 2003;74:137‐144. [DOI] [PubMed] [Google Scholar]
- 14. Williams M, Simms HH. Prognostic usefulness of scoring systems in critically ill patients with severe acute pancreatitis. Crit Care Med. 1999;27:901‐907. [DOI] [PubMed] [Google Scholar]
- 15. Ranson JHC. Etiological and prognostic factors in human acute pancreatitis. Arch Surg. 1993;128:586‐590.8489394 [Google Scholar]
- 16. Blamey SL, Imrie CW, O'Neill J, Gilmour WH, Carter DC. Prognostic factors in acute pancreatitis. Gut. 1984;25:1340‐1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818‐829. [PubMed] [Google Scholar]
- 18. Singh VK, Wu BU, Bollen TL, et al. A prospective evaluation of the bedside index for severity in acute pancreatitis score in assessing mortality and intermediate markers of severity in acute pancreatitis. Am J Gastroenterol. 2009;104:966‐971. [DOI] [PubMed] [Google Scholar]
- 19. Brown A, James‐Stevenson T, Dyson T, Grunkenmeier D. The panc 3 score: a rapid and accurate test for predicting severity on presentation in acute pancreatitis. J Clin Gastroenterol. 2007;41:855‐858. [DOI] [PubMed] [Google Scholar]
- 20. Cook AK, Breitschwerdt EB, Levine JF, Bunch SE, Linn LO. Risk factors associated with acute pancreatitis in dogs: 101 cases (1985–1990). J Am Vet Med Assoc. 1993;203:673‐679. [PubMed] [Google Scholar]
- 21. Charles J. Pancreas In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. 5th ed. Edinburgh: Saunders Elsevier; 2007:389‐423. [Google Scholar]
- 22. Al Mofleh IA. Severe acute pancreatitis: Pathogenetic aspects and prognostic factors. World J Gastroenterol. 2008;14:675‐684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mansfield CS, James FE, Robertson ID. Development of a clinical severity index for dogs with acute pancreatitis. J Am Vet Med Assoc. 2008;233:936‐944. [DOI] [PubMed] [Google Scholar]
- 24. Steiner JM, Newman S, Xenoulis P, et al. Sensitivity of serum markers for pancreatitis in dogs with macroscopic evidence of pancreatitis. Vet Ther. 2008;9:263‐273. [PubMed] [Google Scholar]
- 25. Sato T, Ohno K, Tamamoto T, et al. Assessment of severity and changes in C‐reactive protein concentration and various biomarkers in dogs with pancreatitis. J Vet Med Sci. 2017;79:35‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hauptman JG, Walshaw R, Olivier NB. Evaluation of the sensitivity and specificity of diagnostic criteria for sepsis in dogs. Vet Surg. 1997;26:393‐397. [DOI] [PubMed] [Google Scholar]
- 27. Bourgès‐Abella N, Geffré A, Concordet D, Braun JP, Trumel C. Canine reference intervals for the Sysmex XT‐2000iV hematology analyzer. Vet Clin Pathol. 2011;40:303‐315. [DOI] [PubMed] [Google Scholar]
- 28. Segev G, Kass PH, Francey T, Cowgill LD. A novel clinical scoring system for outcome prediction in dogs with acute kidney injury managed by hemodialysis. J Vet Intern Med. 2008;22:301‐308. [DOI] [PubMed] [Google Scholar]
- 29. Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and cox regression. Am J Epidemiol. 2007;165:710‐718. [DOI] [PubMed] [Google Scholar]
- 30. Schwarz G. Estimating the dimension of a model. Ann Statist. 1978;6:461‐464. [Google Scholar]
- 31. Sweig MH, Campbell G. Receiver‐operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561‐577. [PubMed] [Google Scholar]
- 32. Greiner M, Gardner IA. Epidemiologic issues in the validation of veterinary diagnostic tests. Prev Vet Med. 2000;45:3‐22. [DOI] [PubMed] [Google Scholar]
- 33. Schisterman EF, Perkins NJ, Liu A, Bondell H. Optimal cut‐point and its corresponding Youden index to discriminate individuals using pooled blood samples. Epidemiology. 2005;16:73‐81. [DOI] [PubMed] [Google Scholar]
- 34. Gunning K, Rowan K. ABC of intensive care: outcome data and scoring systems. BMJ. 1999;319:241‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hayes G, Mathews K, Doig G, et al. The acute patient physiologic and laboratory evaluation (APPLE) score: a severity of illness stratification system for hospitalized dogs. J Vet Intern Med. 2010;24:1034‐1047. [DOI] [PubMed] [Google Scholar]
- 36. Giunti M, Troia R, Bergamini PF, Dondi F. Prospective evaluation of the acute patient physiologic and laboratory evaluation score and an extended clinicopathological profile in dogs with systemic inflammatory response syndrome. J Vet Emerg Crit Care. 2015;25:226‐233. [DOI] [PubMed] [Google Scholar]
- 37. Okano S, Yoshida M, Fukushima U, Higuchi S, Takase K, Hagio M. Usefulness of systemic inflammatory response syndrome criteria as an index for prognosis judgement. Vet Rec. 2002;150:245‐246. [DOI] [PubMed] [Google Scholar]
- 38. Gommeren K, Desmas I, Garcia A, et al. Inflammatory cytokine and C‐reactive protein concentrations in dogs with systemic inflammatory response syndrome. J Vet Emerg Crit Care. 2018;28:9‐19. [DOI] [PubMed] [Google Scholar]
- 39. Pápa K, Máthé A, Abonyi‐Tóth Z, et al. Occurrence, clinical features and outcome of canine pancreatitis (80 cases). Acta Vet Hung. 2011;59:37‐52. [DOI] [PubMed] [Google Scholar]
- 40. Watson P. Pancreatitis in the dog: dealing with a spectrum of disease. In Pract. 2004;26:64‐77. [Google Scholar]
- 41. Marchetti V, Gori E, Lippi I, Luchetti E, Manca ML, Pierini A. Elevated serum creatinine and hyponatraemia as prognostic factors in canine acute pancreatitis. Aust Vet J. 2017;95:444‐447. [DOI] [PubMed] [Google Scholar]
- 42. Kes P, Vucicevic Z, Ratkovic‐Gusic I, et al. Acute renal failure complicating severe acute pancreatitis. Ren Fail. 1996;18:621‐628. [DOI] [PubMed] [Google Scholar]
- 43. Kumar R, Pahwa N, Jain N. Acute kidney injury in severe acute pancreatitis: an experience from a tertiary care center. Saudi J Kidney Dis Transpl. 2015;26:56‐60. [DOI] [PubMed] [Google Scholar]
- 44. Tran DD, Oe PL, de Fijter CW, van der Meulen J, Cuesta MA. Acute renal failure in patients with acute pancreatitis: prevalence, risk factors and outcome. Nephrol Dial Transplant. 1993;8:1079‐1084. [PubMed] [Google Scholar]
- 45. Jacobs ML, Daggett WM, Civette JM, et al. Acute pancreatitis: analysis of factors influencing survival. Ann Surg. 1977;185:43‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kimmel SE, Washabau RJ, Drobatz KJ. Incidence and prognostic value of low plasma ionized calcium concentration in cats with acute pancreatitis: 46 cases (1996‐1998). J Am Vet Med Assoc. 2001;219:1105‐1109. [DOI] [PubMed] [Google Scholar]
- 47. Dias C, Carreira LM. Serum ionised calcium as a prognostic risk factor in the clinical course of pancreatitis in cats. J Feline Med Surg. 2015;17:984‐990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Haworth MD, Hosgood G, Swindells KL, Mansfield CS. Diagnostic accuracy of the SNAP and Spec canine pancreatic lipase tests for pancreatitis in dogs presenting with clinical signs of acute abdominal disease. J Vet Emerg Crit Care (San Antonio). 2014;24:135‐143. [DOI] [PubMed] [Google Scholar]
- 49. Lee YJ, Chang CC, Chan JP, et al. Prognosis of acute kidney injury in dogs using RIFLE (risk, injury, failure, loss and end‐stage renal failure)‐like criteria. Vet Rec. 2011;168:264. [DOI] [PubMed] [Google Scholar]
- 50. Gredal H, Toft N, Westrup U, et al. Survival and clinical outcome of dogs with ischaemic stroke. Vet J. 2013;196:408‐4013. [DOI] [PubMed] [Google Scholar]
- 51. Goggs R, Dennis SG, Di Bella A, et al. Predicting outcome in dogs with primary immune‐mediated hemolytic anemia: results of a multicenter case registry. J Vet Intern Med. 2015;29:1603‐1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supplementary Information.


