Abstract
Background
Iatrogenic hypothyroidism might worsen the prognosis of cats with azotemic CKD after thyroidectomy. Varying thyroxine concentrations influence utility of creatinine in assessing renal function. Symmetric dimethylarginine (SDMA) has limited studies in cats with changing thyroid status.
Objectives
Thyroid status is stable 6 months post‐thyroidectomy. Symmetric dimethylarginine and creatinine are linearly associated without influence from total thyroxine concentration (tT4).
Animals
Electronic records of 2 first opinion practices were searched using the term “thyroidectomy” to include 81 client‐owned cats that had undergone bilateral thyroidectomy.
Methods
Retrospective cross‐sectional study assessing thyroid hormone concentrations of 68 cats within 6 months of surgery. A longitudinal study of thyroid status in 23 cats with >18 months follow‐up post‐thyroidectomy. A generalized estimating equation assessed the associations of bodyweight, tT4 and creatinine concentrations on SDMA concentration.
Results
Sixty‐eight cats had follow‐up within 6 months. Fifteen cats (22%) had persistent, or recurrent, hyperthyroidism and 33 cats (49%) were hypothyroid. Twenty‐three of the euthyroid/hypothyroid cats had long‐term follow‐up (595‐1955 days); 4 cats (17%) remained hypothyroid, 19 cats (83%) were euthyroid (often transiently), and 9 of 23 cats (44%) developed recurrent hyperthyroidism. Symmetric dimethylarginine and creatinine were linearly associated, but hyperthyroid cats had higher SDMA concentrations, relative to creatinine (P = .003).
Conclusions and Clinical Importance
Cats have changes in thyroid function for years after bilateral thyroidectomy, with a high incidence of recurrent hyperthyroidism. Both SDMA and creatinine are affected by thyroxine concentrations, and the effect is greater in hyperthyroid cats.
Keywords: azotemia, feline, hypothyroid, SDMA
Abbreviations
- BCS
body condition score
- RAI
radioactive iodine
- TSH
thyroid stimulating hormone
- tT4
total thyroxine concentration
- GEE
generalized estimating equation
- GFR
glomerular filtration rate
- USG
urine‐specific gravity
- SDMA
symmetric dimethylarginine
1. INTRODUCTION
Hyperthyroidism (caused by adenomatous hyperplasia)1 and chronic kidney disease are common in geriatric cats. Azotemia often develops when glomerular filtration rate (GFR) decreases after treatment for hyperthyroidism.2, 3 In 1 study, cats that were both hypothyroid and azotemic after treatment had shorter survival than nonazotemic hypothyroid cats.4 Reversal of hypothyroidism is associated with improvement in azotemia in most cats.5 Consequently “overtreatment” of hyperthyroidism is avoided, especially if cats are concurrently azotemic.6 However, irreversible treatment modalities (radioactive iodine [RAI] and surgery) cannot always avoid causing hypothyroidism.7, 8, 9
Thyroidectomy offers a potentially permanent cure for hyperthyroidism without requiring specialist equipment, making it widely available in general practice. Previous reports of thyroidectomy pre‐date interest in posttreatment hypothyroidism, focusing mainly on postoperative hypocalcaemia7, 8, 10, 11 or recurrence of hyperthyroidism.12, 13 Overall, 5 original studies report recurrence rates for hyperthyroidism of between 5% and 27%,7, 8, 10, 11, 12, 13 between 3 and 59 months after surgery. The highest rate of recurrence (3/11 cats) was in a report of unilateral surgery,7 with recurrence rates otherwise reported as 5%‐11%.8, 10, 12, 13 All were in referral practice and preoperative scintigraphy was used routinely in 2 studies,10, 12 or only in cats with recurrent disease.8, 13 Recurrence was identified at the surgical site, especially with intracapsular technique,8, 10 and ectopic foci.12, 13 Supplementation of levothyroxine after bilateral thyroidectomy was discontinued after 3‐6 months if no evidence of lethargy or haircoat change was seen.8 Cats undergoing unilateral thyroidectomy were not supplemented despite low total thyroxine (tT4) levels for several months after surgery.8 Low tT4 was noted in 25% (26/106) of cats undergoing bilateral thyroidectomy, and 13 (50%) of cats were given long‐term levothyroxine supplementation, but no clinical signs were seen.10 No study measured thyroid stimulating hormone (TSH), and long‐term only recurrence of hyperthyroidism, not hypothyroidism, was reported. 7, 8, 10, 11, 12, 13, 14
Assessment of renal function in geriatric cats with changing thyroid status poses many challenges. After treatment of cats with RAI, GFR decreased after 1 month and did not decrease further at 6 months, but creatinine increased for 3 months before stabilizing.3 A possible explanation is increasing muscle mass (and creatinine generation) in treated hyperthyroid cats.15 In addition, thyroxine increases renal blood flow and GFR by RAAS activation, increased sympathetic nervous activity, and decreased peripheral vascular resistance.16 Control of hyperthyroidism can therefore be expected to cause some increase in creatinine, as GFR decreases. Symmetric dimethylarginine (SDMA) correlates with GFR17 and creatinine.18, 19 Lean body mass had no effect on SDMA, in contrast to creatinine,20, 21 and so SDMA may provide a useful indicator of renal function in cats before and after treatment for hyperthyroidism.
The first, cross‐sectional, study (i) aimed to report the prevalence of hypothyroidism in cats in the first 6 months after bilateral thyroidectomy in first‐opinion practice. A second, longitudinal, study (ii) aimed to report long‐term follow‐up of a subset of cats to determine if postsurgical hypothyroidism is permanent or transient, and the rate of recurrence of hyperthyroidism. All samples from both of the initial studies were analyzed with (iii) the aim of assessing the influence of changing thyroid status on the relationship between SDMA and creatinine.
2. MATERIALS AND METHODS
2.1. Case selection
Cases were selected from geriatric feline clinics (recruiting cats over 9 years old) held at 2 first opinion practices (People's Dispensary for Sick Animals, Bow and the Beaumont Animal Hospital, Camden). Electronic records were searched using the term “thyroidectomy” during the time period from December 1, 1996, to August 30, 2015. Inclusion criteria were recorded dates for bilateral thyroidectomy surgery, performed either as 1 procedure or 2 staged unilateral procedures, and the availability of stored plasma samples for retrospective analysis of SDMA and TSH (and creatinine or tT4 if this had not been measured previously).
Inclusion in the cross‐sectional study, to determine the prevalence of hypothyroidism, required a single stored plasma sample, collected within 6 months of surgery. Inclusion criteria for the longitudinal study, of changes in thyroid and renal markers after surgery, required additional samples to be available for at least 18 months after surgery. Cats that developed recurrent hyperthyroidism were followed only up to the point that this developed, and the cat had additional treatment, usually with methimazole or carbimazole. The follow‐up period for the longitudinal study was determined by the available samples until the cats died, were lost to follow‐up or had reached the study end point (August 30, 2015).
Clinical data recorded included age, sex, breed, dates of thyroidectomy, tT4 and TSH concentrations, creatinine concentrations, urine‐specific gravity (USG), body condition score (BCS), and body weight. Cats were defined as hypothyroid if their tT4 was below the reference range (<10 nmol/L) with TSH above 0.15 ng/mL concurrently. This cutoff was established in the author's clinic previously, from 90 healthy cats over 9 years of age.22 Cats considered euthyroid had tT4 within the reference range (10‐55 nmol/L) and TSH <0.15 ng/mL. Cats with plasma tT4 above the laboratory reference range (>55 nmol/L) were considered hyperthyroid.
2.2. Blood sample analysis
Jugular venous blood samples and urine samples collected by cystocentesis (where performed) were obtained as part of the routine operation of the clinic and with the informed consent of the owner (CRERB 2013/1258). Blood samples were collected into heparinized tubes, refrigerated at 4°C and separated by centrifugation at 2016g for 10 minutes within 6 hours of collection. After separation, heparinized plasma samples were stored at −80°C, unless utilized for immediate analysis.
Analysis of all blood samples was by a single reference laboratory (IDEXX reference laboratory, Wetherby, United Kingdom). In most instances, measurement of tT4 and, on most occasions, creatinine was performed contemporaneously at the direction of the treating clinician, but occasionally this measurement was done retrospectively for the present study. Urine‐specific gravity was analyzed by in‐house refractometer at the time of sample collection.
Archived heparinized plasma samples were obtained from all cats (where possible) at a visit within 6 months of thyroidectomy. These were submitted for TSH and SDMA (and tT4 or creatinine if required) measurement at the same reference laboratory (IDEXX reference laboratory). Cats with long‐term follow‐up (more than 18 months) had, where possible, samples from 6‐month intervals submitted to the same external reference laboratory (IDEXX reference laboratory) for tT4, TSH, SDMA, and creatinine measurement. Total T4 was measured using a chemiluminescent assay, and TSH concentrations were measured by the Immulite canine TSH assay (Siemens, Camberley, United Kingdom) as previously validated.20
2.3. Data analysis
Graphical and statistical analysis were performed using commercial computer software (IBM SPSS Statistics Version 22 and GraphPad Prism 7 for Mac). Normality was assessed by examination of histogram and (where necessary) by Shapiro‐Wilk test. Numerical data are reported as median and range, and categorical data are reported as percentages. Statistical significance was defined as P < .05.
The time since thyroidectomy was calculated from the date of the second surgery for those cats that had staged procedures. Azotemia was defined as plasma creatinine measurement greater than the laboratory reference range (2 mg/dL, 177 μmol/L). This reference range was that used by IDEXX laboratories, United Kingdom, during the period of analysis. For the cross‐sectional study (i), chi‐square test was used to compare the proportion of cats that were hypothyroid after simultaneous (bilateral) and staged (unilateral) surgeries.
For the longitudinal study (ii) changes in tT4 and TSH were assessed by Kaplan‐Meier time‐to‐event analysis, with censoring if the cat died or was lost to follow‐up. Wilcoxon signed‐rank test was performed to assess whether the time for normalization of tT4 and TSH concentrations were significantly different.
For the SDMA study (iii), the relationship between SDMA and creatinine and other factors that might alter this relationship (body weight, BCS, and thyroid status) were assessed using both cross‐sectional and longitudinal data. Given that not all cats had longitudinal follow‐up, a generalized estimating equation (GEE) with working correlation matrix was used to account for correlation between those with repeated measures. For this part of the analysis, tT4 concentrations only were used to categorize cats at each time point using the IDEXX laboratory's reference range; hypothyroid (tT4 < 10 nmol/L), euthyroid (tT4 10‐55 nmol/L), or hyperthyroid (tT4 > 55 nmol/L). Thyroid stimulating hormone concentrations were not used to categorize cats in this analysis as the combination of tT4 and TSH in this postsurgical population resulted in many samples having uncategorizable thyroid status, often because the TSH remained elevated for a period of time after the tT4 normalized. In the univariable GEE analysis, SDMA was used as the dependent variable, a linear scaled response as the type of model, and the covariates/factors that were included were creatinine, body weight, BCS, and thyroid status (hypothyroid, euthyroid, and hyperthyroid). Only variables with P < .1 and their interactions (ThyroidStatus × Creatinine, ThyroidStatus × BodyWeight, and BodyWeight × Creatinine) were included in the multivariable analysis. Backward elimination was used to develop the final model.
3. RESULTS
3.1. Cross‐sectional study
Eighty‐one cats were identified as having undergone bilateral thyroidectomy. Three cats were excluded from the analysis as they had no recorded date for surgery, 3 cats were lost to follow‐up immediately after surgery, and a further 7 cats had no stored samples available. Sixty‐eight cats were therefore included in the cross‐sectional study. All cats were neutered, and no cats were of pedigree breeds. There were 29 male cats (41%) and 39 female cats (57%) included. The age at surgery was 14.9 (9.3‐20.4) years. The age of 4 cats was unknown. The cats' weight at surgery was 3.55 (2.1‐9.3) kilograms; preoperative weight was not recorded for 3 cats. In most instances (60/68, 88%), medical stabilization was performed (with varying success) before surgery. Total T4 measurement before surgery (within 2 months) was available for 45 cats and revealed that 24 cats were controlled (tT4 < 55 nmol/L), 12 of which had low tT4 on medication (tT4 < 10 nmol/L), with 21 cats still hyperthyroid (tT4 103 nmol/L, 56‐281 nmol/L) despite medication. Blood testing was not performed immediately before surgery in 23 cats, because the hyperthyroidism was overtly uncontrolled. This was based on the severity of clinical signs or, more often, as the owner was known not to be able to administer the medication. In all cases, hyperthyroidism was documented biochemically before treatment, but in some cats this was a considerable interval before surgery. Thyroidectomy was performed as a staged procedure in 40 of 68 cats with a median intraoperative interval of 258 (16‐2086) days.
In the first 6 months post‐surgery, hypothyroidism was documented in 33 of 68 (49%) cats, 15 of 68 (22%) cats continuing to be hyperthyroid and 13 of 68 (19%) cats euthyroid (Figure 1). In 7 cats, thyroid status was uncertain; in 3 cats, the uncertainty was because although tT4 was in the euthyroid range (16.7‐20.5 nmol/L), TSH was increased (0.8‐1.11 ng/mL) possibly consistent with subclinical hypothyroidism, and in 4 cats this was because tT4 was low (4‐8.8 nmol/L) but so was TSH concentration (0.04‐0.12 ng/mL) consistent with sick euthyroidism. The prevalence of overt hypothyroidism in the first 6 months was greater in cats that had both thyroid glands removed in a single surgical procedure (19/28, 67.9%) compared with cats having staged thyroidectomies (16/40, 40%, P = .04).
Figure 1.
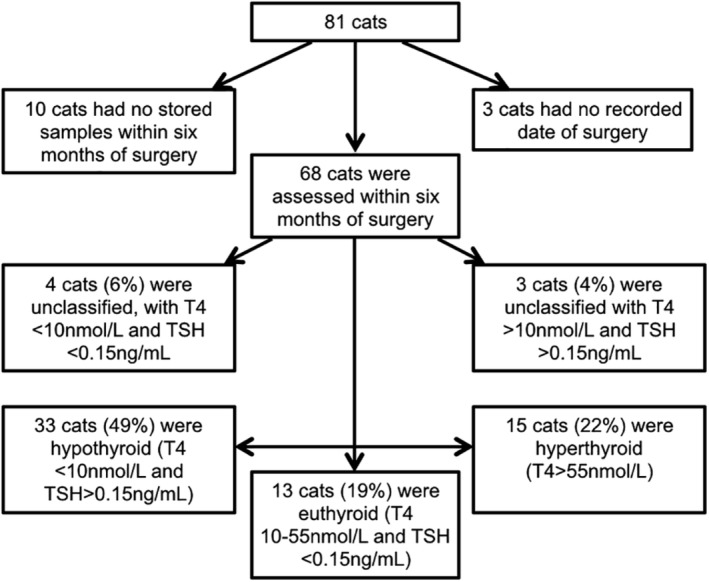
Flowchart of cats included in a cross‐sectional study evaluating thyroid status in the first 6 months after bilateral thyroidectomy in first opinion practice
3.2. Longitudinal study
Long‐term follow‐up was available for 23 cats (range 595‐1955 days). Of these cats, the cross‐sectional study had documented that 16 cats had low tT4 after surgery (2 of which were unclassified with normal TSH but low tT4), and 7 cats had normal tT4 (2 of which were unclassified with high TSH but normal tT4); Figure 2. Twelve of the cats (12/16, 75%) with low tT4 initially had normalization of tT4 concentrations (>10 nmol/L and <55 nmol/L), 234 days (193‐992 days) after surgery. Six (50%) of these cats had recurrence of hyperthyroidism. In 1 cat, tT4 increased from below 10 nmol/L to above 55 nmol/L when the cat was next presented (an interval of 476 days), but the other 5 cats were within the euthyroid range for between 168 and 637 days before hyperthyroidism recurred.
Figure 2.
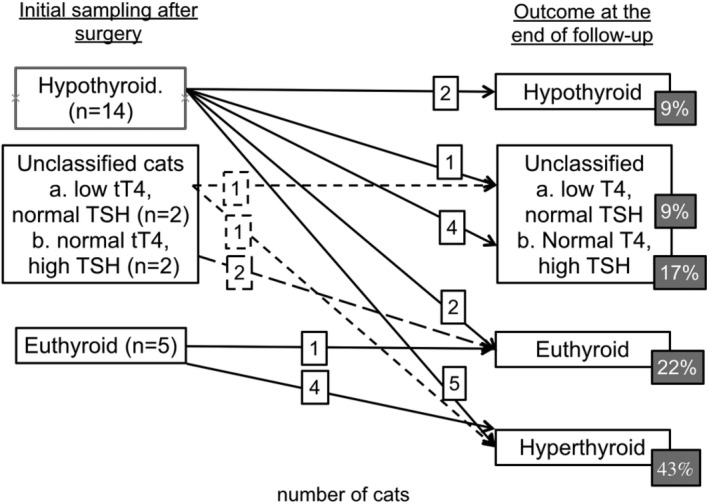
Longitudinal study outcome; classification of cats in the first 6 months after bilateral thyroidectomy surgery and their change in classification at the time of loss to follow‐up or death
In total, 10 of 23 cats became hyperthyroid after bilateral thyroidectomy (including 6 cats with a tT4 initially <10 nmol/L), giving a longer term recurrence rate of hyperthyroidism post‐surgery of 43.5%. The length of follow‐up to hyperthyroidism recurrence was 726 (208‐1049) days, and the follow‐up of cats without recurrence was 992 (607‐1955) days. Follow‐up was until death in 22 of 23 cats, only 1 was lost to follow‐up. Hyperthyroidism recurred at prolonged periods after surgery (1 cat at <1 year, 3 between 1 and 2 years, and 6 at >2 years after surgery).
Sixteen cats showed a pattern of TSH increase after surgery, with a prolonged time between restoration of normal T4 and resolution of increased TSH concentrations (Figure 3). The time for documentation of tT4 within reference range in all 23 cats was 412 days (range 21‐1955 days) and for TSH to return to reference range was 552 days (34‐1955 days); Figure 4. A Wilcoxon signed rank test confirmed a significant difference (P = .03).
Figure 3.
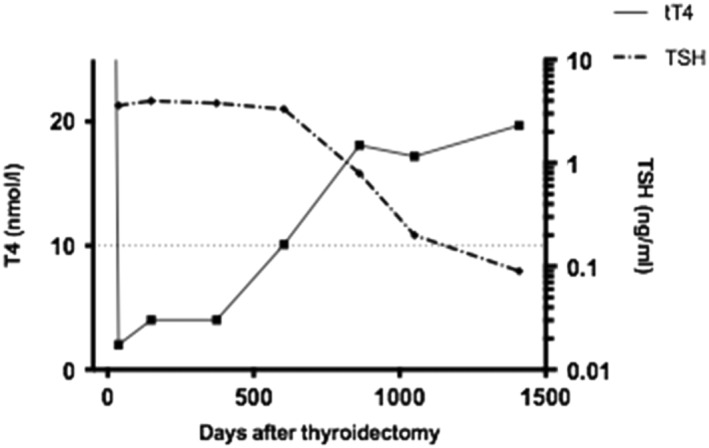
Illustrative changes in tT4 and TSH over time for 1 cat after bilateral thyroidectomy surgery in first opinion practice. The dotted line indicates the boundary of the reference ranges (tT4 over 10nmol/L, TSH <0.15ng/mL)
Figure 4.
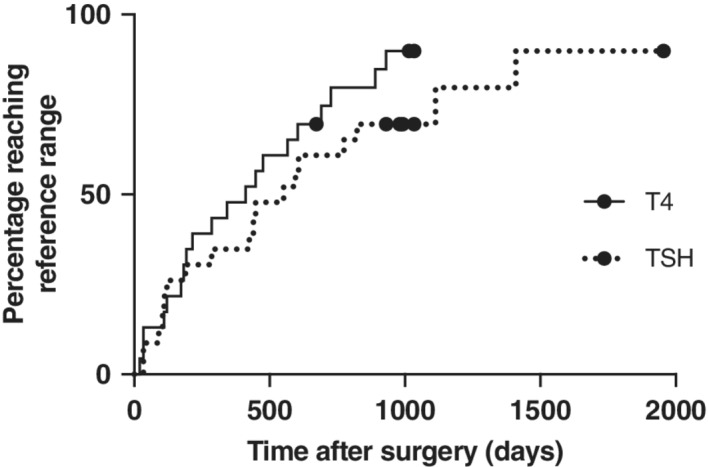
Kaplan‐Meier time‐to‐event analysis, demonstrating the first time at which normalization of tT4 (>10 nmol/L) and TSH (<0.15 ng/mL), respectively, was documented in 23 cats after bilateral thyroidectomy surgery. All cats had blood samples analyzed within 6 months of surgery and every 6 months afterwards where possible. Seven cats that died without reaching these levels were censored from the analysis, indicated by the circles on the graph. The 2 curves are not significantly different (P = .20) on log‐rank analysis
3.3. SDMA study
Symmetric dimethylarginine and creatinine were linearly associated (Figure 5), but the classification of renal status (azotemic/nonazotemic or normal/increased SDMA) was discordant in many cases. Symmetric dimethylarginine was above the reference range (>14 μg/dL) in 30% (45/151) of samples where creatinine was within reference range (≤2.0 mg/dL, ≤177 μmol/L). Urine‐specific gravity was available for 33 of these 45 samples, 6 of which (13%), were >1.035. In the 27 other samples, urine was ≤1.014 in 3 of 33, 1.015‐1.024 in 13 of 33, and 1.025‐1.034 in 11 of 33 samples. Four (9%) of the discordant samples were from hyperthyroid cats. In 5% (8/151) of samples, creatinine was increased (>2.0 mg/dL, >177 μmol/L) with normal SDMA (≤14 μg/dL). Urine‐specific gravity was measured concurrently in 5 of the 8 cats with increased creatinine and normal SDMA and was <1.035 in all of them (1.015‐1.024 in 4/5, 1.025‐1.034 in 1/5). All 8 cats had concurrent tT4 concentrations within or below the reference range.
Figure 5.
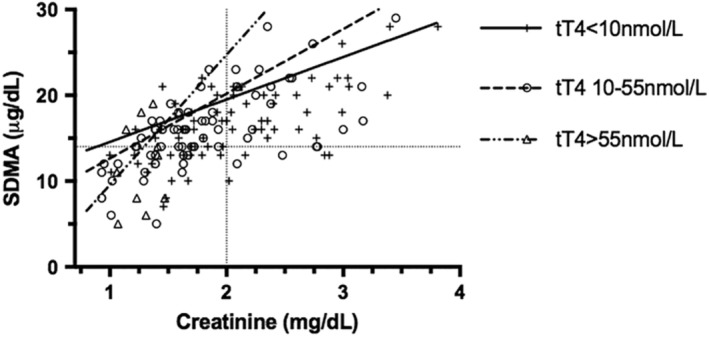
Samples from 68 cats that underwent treatment of hyperthyroidism by bilateral thyroidectomy, with symmetric dimethylarginine (SDMA) and creatinine measured concurrently both before and after surgery. In total, 151 data points plotted, some of which are repeated measures from the same cats. The dotted gridlines represent the reference ranges for creatinine (2.0 mg/dL, 177 μmol/L) and SDMA (14 μg/dL), respectively. Samples are categorized by tT4 concentration and lines of best fit plotted using intercept and slope generated from a generalized estimating equation
Analysis of variables influencing SDMA concentrations utilizing a GEE revealed significant influence on SDMA by thyroid status (P = .01), creatinine (P < .001), and their interaction (ThyroidStatus × Creatinine; P = .003). Bodyweight had a significant effect on SDMA alone (P = .008) but not as an interaction with other variables (ThyroidStatus × BodyWeight; P = .31, BodyWeight × Creatinine; P = .61). Body condition score was not significant in univariable analysis (P = .15). The effect of creatinine on SDMA concentration was greater in hyperthyroid cats than in cats that were euthyroid or hypothyroid (Table 1).
Table 1.
Analysis of the influence of creatinine and thyroid status on symmetric dimethylarginine concentrations, using a generalized estimating equation
| Variable | Coefficient | SE | P |
|---|---|---|---|
| Bodyweight | −0.67 | 0.25 | .008 |
| Thyroid status (intercept) | .01 | ||
| Hyperthyroid | −5.61 | 5.25 | |
| Euthyroid | 5.09 | 3.66 | |
| Hypothyroid | 9.62 | 1.79 | |
| Thyroid status × creatinine (regression coefficient of creatinine) | .003 | ||
| Hyperthyroid | 15.16 | 3.05 | |
| Euthyroid | 7.54 | 2.09 | |
| Hypothyroid | 4.95 | 0.50 | |
Data were obtained from 68 cats that had a bilateral thyroidectomy in first opinion practice; in total, 151 measurements were used. Creatinine, bodyweight, thyroid status, and an interaction term of creatinine × thyroid status were included in the final model.
4. DISCUSSION
Recurrent hyperthyroidism occurs in 5%‐11% of cats after bilateral thyroidectomy,8, 10, 12, 13 as compared to recurrence in our population of 22% in the short term (within 6 months of surgery), with a further 44% over the long‐term (up to 1049 days). Results of the present study are likely to be more representative of those achieved in first opinion practice than are previous reports. Recurrence is more frequently associated with a particular surgical (intracapsular) technique10 or in the presence of ectopic thyroid tissue.12 There was no consistent recording of the surgical technique used, and the experience of the surgeons was also variable, including relatively new graduates and experienced practitioners; and therefore, the higher recurrence rate is likely to be the result of multiple variables, preventing direct comparison. These cats also did not undergo presurgical screening (technetium scintigraphy) for ectopic tissue, estimated to affect 4%‐12% of cats, or carcinoma, estimated at 2%.23 These factors might have combined to result in a high incidence of postoperative hyperthyroidism in the present study. Radioiodine and surgery have both been portrayed as “permanent” cures for hyperthyroidism, often chosen to avoid long‐term medication, as difficulty in administering medication was a factor in the choice of radioiodine treatment for 43.9% of owners.24 If the present study can be considered representative of the results achieved in first opinion practice, then the notion that thyroidectomy offers a permanent “cure” for hyperthyroidism should perhaps be reconsidered. However, the recurrence of hyperthyroidism after thyroidectomy is a concern for only 11.5% of owners seeking radioiodine treatment.24 Interestingly, despite the anesthetic risk of surgery in geriatric cats, the population included here had a median age of 14.9 years and the oldest cat undergoing surgery was 20.4 years old, suggesting that perhaps this risk does not discourage owners as much as previously thought. This may reflect the expense of radioiodine in comparison to surgery, or a lack of awareness of nonsurgical options, demonstrated by 53.3% of hyperthyroid cat owners being unaware of RAI as an option.24
Many cats in the present study (40/68) had staged procedures (2 unilateral surgeries), in some cases with a prolonged time between them. This is despite the presence of bilateral disease occurring in the majority of cats (62.9‐66.1%).23, 25 The reason for staging of the primary procedure is thought to be due to concerns for iatrogenic hypoparathyroidism and subsequent hypocalcaemia after surgery. Staged bilateral thyroidectomy does reduce the incidence of hypocalcaemia, though the authors did not suggest that staged procedures should be performed routinely.7 Others have suggested a preference for staged surgery to allow resolution of damage to parathyroid function.26 A larger proportion of cats became hypothyroid after nonstaged bilateral procedures, which could reflect recovery of some function of the residual tissue after thyroidectomy occurring in the interim between procedures.14
Long term, this study documents restoration of euthyroidism in 39% of cats after bilateral thyroidectomy, although two‐thirds of these had previously had been hypothyroid. Success rates in the published literature are quoted as between 66% and 90%,10, 12, 13 but are difficult to compare, as the definitions of euthyroidism vary (with some studies including tT4 levels below the reference range), and follow‐up periods are generally short. Comparison of these figures with published follow‐up from radioiodine treatment, 1 of the main alternative options for owners, suggests that cats treated surgically in first opinion practice have a higher incidence of recurrent or persistent hyperthyroidism (compared to 1.5%‐9.5%) but comparable rates of hypothyroidism (between 4% and 36% depending on the study).16, 25, 27 However, our short‐term prevalence of hypothyroidism (49%) is based on a single time point for each cat and so could have been too soon or too long after surgery to document cats with transient hypothyroidism or those for which the increase in TSH after surgery is delayed.
Changes in thyroid status occurred at prolonged periods after surgery. This was true not just in cats that had recurrent hyperthyroidism (which could be explained by novel adenomatous transformation)14 but also in 3 cats in which normalization of tT4 was only seen after 604‐890 days of hypothyroidism. These 3 cats had prolonged follow‐up in their euthyroid state (2‐4 years), suggesting that it was not imminent hyperthyroidism causing the rise in tT4. Previous studies11, 16, 25 have mostly focused on follow‐up only in the initial 6‐12 months post‐treatment, but the present study suggests that longer term follow‐up of cats remains important. Thyroid function can, and frequently does, change dramatically at time periods far beyond 6 months after surgery. Unfortunately, it is not possible to confirm prevalence for the whole population, as some cats lacked long‐term follow‐up, but in total 25 of the 68 cats initially enrolled developed recurrent hyperthyroidism. As with many retrospective studies, our follow‐up has limitations; some cats were presented more regularly than others, and many cats had no follow‐up beyond 18 months, limiting the numbers enrolled and potentially introducing a selection bias in favor of those cats still symptomatic for their disease. In addition, it is likely that those cats undergoing thyroidectomy are not a random and representative sample of the affected population. The reason for choosing this treatment in the first place is frequently a failure to control their clinical signs medically, increasing the proportion of cats with more severe disease and potentially increasing recurrence rates.
Thyroid stimulating hormone declined rapidly to undetectable concentrations in hyperthyroid cats, often preceding development of hyperthyroidism (based on serum tT4) by 6‐12 months, in agreement with a previous finding of increased risk of hyperthyroidism in cats with undetectable TSH.22 Seven of 9 cats with undetectable TSH progressed to hyperthyroidism, with a further 1 having borderline thyroid status (tT4, 54.2 nmol/L) at the end of follow‐up. The remaining cat, however, had low or undetectable TSH concentrations (0.015‐0.04 ng/dL) for the whole follow‐up period (1014 days), with low tT4, and development of severe concurrent renal disease only late in follow‐up. Thyroid stimulating hormone suppression after radioiodine treatment in 7 of 8 cats in a previous study lasted for <3 months,4 and therefore the presence of persistent low TSH in this cat is difficult to explain. This variability in the relationship of T4 and TSH may pose a challenge when using TSH concentrations to characterize thyroid status after treatment, for diagnosis of nonthyroidal illness, occult hyperthyroidism, or subclinical hypothyroidism.28, 29, 30
The findings of the present study impact upon the timing of decisions to initiate levothyroxine supplementation, now a recommendation for all azotemic cats with low tT4.6 Lack of suppression of TSH concentrations, in cats with normal tT4, has been suggested to indicate subclinical hypothyroidism,31 therefore necessitating an increased dose of thyroid hormone. In cats reported here, TSH remained above our reference range22 for prolonged periods after normalization of tT4. If this sustained increase in TSH were to be ascribed to occult hypothyroidism, then some cats with normal T4 would also warrant supplementation. However, that abstract used 0.5 ng/dL as their cutoff for TSH, a higher value than that utilized here, and so cats would have fallen into this range more rapidly. In a further study, the reported reference range for TSH was 0.03‐0.3 ng/mL.32 However, the origin of that reference range is unclear, and the 131 clinically normal euthyroid cats in that study had TSH concentrations of 0.04 ng/mL (interquartile range, 0.02‐0.07 ng/mL), which suggests that a narrower reference range may be appropriate for healthy cats. Clearly these reference ranges used in various papers are difficult to reconcile, but the use of a broader range might be appropriate after treatment in hyperthyroid cats.
Symmetric dimethylarginine and creatinine showed good correlation, in agreement with previous reports.17, 18, 19 Our population showed a high number of discordant results, 35% of samples were classified differently based on 1 or the other measurement. We found that SDMA was more often increased with normal creatinine than the other way around, in agreement with a previous study.19 All the cats with increased SDMA and creatinine within the reference range were documented to have renal azotemia (elevated creatinine and USG <1.035) at a previous time point. However, 5% of samples in our population had increased creatinine (presumed to represent a reduced GFR) with SDMA within reference range, in contrast to a previous study that found the sensitivity of SDMA for a 30% reduction from median GFR to be consistently higher than that of creatinine.19 In part, this may stem from a lower reference interval for creatinine in the laboratory used in the present study than that used previously (ie, 2.0 mg/dL as opposed to 2.1 mg/dL).19 Closer inspection of the data for the discordant samples with high creatinine (and normal SDMA) revealed USG <1.035 in many of the cats, supporting the presence of renal azotemia. Although the influence of prerenal factors on creatinine cannot be discounted, this suggests that SDMA could be misleading in some cats with renal azotemia.
Assessment of SDMA and creatinine revealed a linear relationship. The GEE indicated that SDMA concentrations were related not only to creatinine concentrations but also to thyroid status. Specifically, the slope of the relationship between SDMA and creatinine was steeper (ie, SDMA was relatively higher) in hyperthyroid cats compared with euthyroid and hypothyroid cats. This is in contrast to the weak negative relationship of tT4 to both SDMA and creatinine found in a recent study.33 This could be because of the effect of thyroid hormones on muscle mass15, 20, 21 with a resultant decrease in creatinine production. However, only bodyweight, and not BCS, was found to be significant. Alternatively, thyroid hormones alter cellular metabolism and protein turnover,34 and the effect of low thyroid hormone levels in reducing endogenous production of creatinine has been previously reported.35 Thyroid hormones might also have direct effects on SDMA generation, as demonstrated for cystatin C in hyperthyroid cats,36 and in humans, SDMA concentrations have been found to be increased with either hyperthyroidism or hypothyroidism.37, 38 A relatively mild effect of bodyweight on the relationship of SDMA and creatinine was also present, in contrast to previous reports20, 21 with higher bodyweight associated with relatively lower SDMA concentrations compared to creatinine. The use of SDMA as a surrogate for GFR, therefore, has limitations, much as seen with the use of creatinine. It seems likely that thyroid status influences the concentrations of creatinine and SDMA, both relative to GFR and to 1 another. Unfortunately, for this retrospective study, gold standard assessment of renal function by GFR measurement was not available. Evaluation of BCS was recorded for only 92 of the 151 samples, and no record was made of muscle mass score. It is possible therefore that missing data could be the reason that there was an association with bodyweight but not BCS.
A major limitation of this retrospective study was the availability of samples, and the desired length of follow‐up, necessitating the use of samples that had been in storage for years (72/151 samples were stored for 10 years or more at −80°C) and some of which had undergone 1 or 2 freeze‐thaw cycles. Although data on the stability of feline biochemical markers and hormones is limited for this length of storage, there is evidence for stability of T4 in frozen canine samples over 5 days of storage.39 Thyroid stimulating hormone and fT4 are stable in frozen human serum for 13 months and for up to 29 years.40, 41 Stored samples have been used previously for analysis of SDMA in studies in 2014 and 2016,19, 42 although the length of time stored was not specified, the samples were noted to be from 2010 and after, suggesting some could have been 4‐6 years old. Symmetric dimethylarginine is stable over 3 freeze‐thaw cycles in canine samples.43 Further studies to assess the stability of measurement for hormone and renal markers in stored feline samples would be welcomed.
In summary, after thyroidectomy, a high percentage of cats can develop recurrent hyperthyroidism. Cats can experience recovery of thyroid function, or progression to hyperthyroidism, for years after surgery. Cats that regained normal thyroid function often showed a lag phase, where TSH remained increased but tT4 had normalized. Conversely, when TSH is undetectably low, it is frequently, although not always, a harbinger of recurrent hyperthyroidism.
Symmetric dimethylarginine and creatinine are strongly linearly related, as would be anticipated as they are both good estimates of GFR; however, they are also influenced by thyroid status. The association cannot be explained by changes in body weight or BCS. Further studies are required to evaluate the effect of hyperthyroidism on SDMA, ideally incorporating GFR estimation.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Covey HL, Chang Y, Elliott J, Syme HM. Changes in thyroid and renal function after bilateral thyroidectomy in cats. J Vet Intern Med. 2019;33:508–515. 10.1111/jvim.15450
REFERENCES
- 1. Peterson ME. Feline hyperthyroidism: an animal model for toxic nodular goitre. J Endocrinol. 2014;223:97‐114. [DOI] [PubMed] [Google Scholar]
- 2. Van Hoek I, Lefebvre HP, Peremans K, et al. Short and long term follow‐up of glomerular and tubular renal markers of kidney function in hyperthyroid cats after treatment with radioiodine. Domest Anim Endocrinol. 2009;36:45‐56. [DOI] [PubMed] [Google Scholar]
- 3. Boag AK, Neiger R, Slater L, et al. Changes in the glomerular filtration rate of 27 cats with hyperthyroidism after treatment with radioactive iodine. Vet Rec. 2007;161:711‐715. [DOI] [PubMed] [Google Scholar]
- 4. Williams TL, Elliott J, Syme HM. Association of iatrogenic hypothyroidism with azotaemia and reduced survival time in cats treated for hyperthyroidism. J Vet Intern Med. 2010;24:1086‐1092. [DOI] [PubMed] [Google Scholar]
- 5. Williams TL, Elliott J, Syme HM. Effect on renal function of restoration of euthyroidism in hyperthyroid cats with iatrogenic hypothyroidism. J Vet Intern Med. 2014;28:1251‐1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Daminet S, Kooistra HS, Fracassi F, et al. Best practice for the pharmacological management of hyperthyroid cats with antithyroid drugs. J Small Anim Pract. 2014;55(1):4‐13. [DOI] [PubMed] [Google Scholar]
- 7. Flanders JA, Harvey HJ, Erb HN. Feline thyroidectomy, a comparison of postoperative hypocalcaemia with three different surgical techniques. Vet Surg. 1987;16(5):362‐366. [DOI] [PubMed] [Google Scholar]
- 8. Birchard SJ, Peterson ME, Jacobson A. Surgical treatment of feline hyperthyroidism: results of 85 cases. J Am Anim Hosp Assoc. 1984;20:705‐709. [Google Scholar]
- 9. Peterson ME, Becker DV. Radioiodine treatment of 524 cats with hyperthyroidism. J Am Vet Med Assoc. 1995;207(11):1422‐1428. [PubMed] [Google Scholar]
- 10. Welches CD, Scavelli TD, Matthiesen DT, et al. Occurrence of problems after three techniques of bilateral thyroidectomy in cats. Vet Surg. 1989;18:392‐396. [DOI] [PubMed] [Google Scholar]
- 11. Flanders JA. Surgical options for the treatment of hyperthyroidism in the cat. J Feline Med Surg. 1999;1:127‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Naan EC, Kirpensteijn J, Kooistra HS, et al. Results of thyroidectomy in 101 cats with hyperthyroidism. Vet Surg. 2006;35:287‐293. [DOI] [PubMed] [Google Scholar]
- 13. Swalec KM, Birchard SJ. Recurrence of hyperthyroidism after thyroidectomy in cats. J Am Anim Hosp Assoc. 1990;26:433‐437. [Google Scholar]
- 14. Padgett S. Feline thyroid surgery. Vet Clin North Am Small Anim Pract. 2002;32(4):851‐859. [DOI] [PubMed] [Google Scholar]
- 15. Peterson ME, Castellano CA, Rishniw M. Evaluation of body weight, body condition and muscle condition in cats with hyperthyroidism. J Vet Intern Med. 2016;30:1780‐1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vaske HH, Schermerhorn T, Grauer GF. Effects of feline hyperthyroidism on kidney function: a review. J Feline Med Surg. 2016;18(2):55‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Braff J, Obare E, Yerramilli M, Elliott J, Yerramilli M. Relationship between serum symmetric dimethylarginine concentrations and glomerular filtration rate in cats. J Vet Intern Med. 2014;28:1699‐1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jepson RE, Syme HM, Vallance C, Elliott J. Plasma asymmetric dimethylarginine, symmetric dimethylarginine, L‐arginine, and nitrite/nitrate concentrations in cats with chronic kidney disease and hypertension. J Vet Intern Med. 2008;22:317‐324. [DOI] [PubMed] [Google Scholar]
- 19. Hall JA, Yerramilli M, Obare R, et al. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in cats with chronic kidney disease. J Vet Intern Med. 2014;28(6):1676‐1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hall JA, Yerramilli M, Obare E, Yerramilli M, Melendez LD, Jewell DE. Relationship between lean body mass and serum renal biomarkers in healthy dogs. J Vet Intern Med. 2015;29:808‐814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hall JA, Yerramilli M, Obare E, Yerramilli M, Yu S, Jewell DE. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in healthy geriatric cats fed reduced protein foods enriched with fish oil, L‐carnitine and medium chain triglycerides. Vet J. 2014;202:588‐596. [DOI] [PubMed] [Google Scholar]
- 22. Wakeling J, Elliott J, Syme HM. Evaluation of the predictors for the diagnosis of hyperthyroidism in cats. J Vet Intern Med. 2011;25:1057‐1065. [DOI] [PubMed] [Google Scholar]
- 23. Peterson ME, Broome MR. Thyroid scintigraphy findings in 2096 cats with hyperthyroidism. Vet Radiol Ultrasound. 2015;56(1):84‐95. 21. [DOI] [PubMed] [Google Scholar]
- 24. Boland LA, Murray JK, Bovens CP, Hibbert A. A survey of owners' perceptions and experiences of radioiodine treatment of feline hyperthyroidism in the UK. J Feline Med Surg. 2014;16(8):663‐670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nykamp SG, Nl D, Zarfoss MK, et al. Association of the risk of development of hypothyroidism after iodine 131 treatment with the pretreatment pattern of sodium pertechnetate Tc 99m uptake in the thyroid gland in cats with hyperthyroidism:165 cases (1990‐2002). J Am Vet Med Assoc. 2005;226(10):1671‐1675. [DOI] [PubMed] [Google Scholar]
- 26. Flanders JA. Surgical therapy of the thyroid. Vet Clin North Am Small Anim Pract. 1994;24:607‐621. [DOI] [PubMed] [Google Scholar]
- 27. Volckaert V, Vandermeulen E, Dobbeleir A, et al. Effect of thyroid volume on radioiodine outcome in hyperthyroid cats. J Feline Med Surg. 2015;18:144‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Williams TL, Peak KJ, Brodbelt D, Elliott J, Syme HM. Survival and the development of azotaemia in hyperthyroid cats. J Vet Intern Med. 2010;24:863‐869. [DOI] [PubMed] [Google Scholar]
- 29. Peterson ME, Gamble DA. Effect of nonthyroidal illness on serum thyroxine concentrations in cats. J Am Vet Med Assoc. 1990;197:1203‐1208. [PubMed] [Google Scholar]
- 30. Peterson ME, Graves TK, Cavanagh I. Serum hormone levels fluctuate in cats with hyperthyroidism. J Vet Intern Med. 1987;1(3):142‐146. [DOI] [PubMed] [Google Scholar]
- 31. Aldridge C, Behrend EN, Martin LG, et al. Evaluation of thyroid‐stimulating hormone, total thyroxine and free thyroxine in hyperthyroid cats receiving methimazole treatment. J Vet Intern Med. 2015;29(3):862‐868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Peterson ME, Guterl JN, Nichols R, Rishniw M. Evaluation of serum thyroid‐stimulating hormone concentration as a diagnostic test for hyperthyroidism in cats. J Vet Intern Med. 2015;29(5):1327‐1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peterson ME, Varela FV, Rishniw M, Polzin DJ. Evaluation of serum symmetric dimethylarginine concentration as a marker for masked chronic kidney disease in cats with hyperthyroidism. J Vet Intern Med. 2018;32(1):295‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Van Hoek I, Daminet S. Interactions between thyroid and kidney function in pathological conditions of these organ systems: a review. Gen Comp Endocrinol. 2009;160:205‐215. [DOI] [PubMed] [Google Scholar]
- 35. Panciera DL, Lefebvre HP. Effect of experimental hypothyroidism on glomerular filtration rate and plasma creatinine concentration in dogs. J Vet Intern Med. 2009;23(5):1045‐1050. [DOI] [PubMed] [Google Scholar]
- 36. Williams TL, Dillon H, Elliott J, Syme HM, Archer J. Serum cystatin C concentrations in cats with hyperthyroidism and chronic kidney disease. J Vet Intern Med. 2016;30:1083‐1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hermenegildo C, Medina P, Peiro M, et al. Plasma concentrations of asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, is elevated in hyperthyroid patients. J Clin Endocrinol Metab. 2002;87(12):5636‐5640. [DOI] [PubMed] [Google Scholar]
- 38. Arikan E, Karadag CH, Guldiken S. Asymmetric dimethylarginine levels in thyroid diseases. J Endocrinol Invest. 2007;30:186‐191. [DOI] [PubMed] [Google Scholar]
- 39. Behrend EN, Kemppainen RJ, Young DW. Effect of storage conditions on cortisol, total thyroxine and free thyroxine concentrations in serum and plasma of dogs. J Am Vet Med Assoc. 1998;212:1564‐1568. [PubMed] [Google Scholar]
- 40. Gislefoss RE, Grimsrud TK, Mørkrid L. Stability of selected serum hormones and lipids after long term storage in the Janus Serum Bank. Clin Biochem. 2015;48:364‐369. [DOI] [PubMed] [Google Scholar]
- 41. Brinc D, Khun Chan M, Venner AA, et al. Long term stability of biochemical markers in pediatric serum samples stored at ‐80°C: a CALIPER substudy. Clin Biochem. 2012;45:816‐826. [DOI] [PubMed] [Google Scholar]
- 42. Hall JA, Yerramilli M, Obare E, et al. Serum concentrations of symmetric dimethylarginine and creatinine in cats with kidney stones. PLoS One. 2017;12(4):e0174854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nabity MB, Lees GE, Boggess MM, et al. Symmetric dimethylarginine assay validation, stability and evaluation for the early detection of chronic kidney disease in dogs. J Vet Intern Med. 2015;29(4):1036‐1044. [DOI] [PMC free article] [PubMed] [Google Scholar]


