Abstract
Individuals may reduce competition by temporally partitioning their use of a shared resource. Behavioral differences between sexes in ungulates may encourage segregation as individuals attempt to avoid antagonistic interactions. However, dominant sex and age groups may reduce subordinates’ access to food resources, regardless of the subordinate’s sex. We hypothesized that white-tailed deer Odocoileus virginianus temporally segregated at supplemental feeding sites based on social rank (subordinate: yearling males and adult females; dominant: adult males) and that segregation was affected by phase of the breeding season and diel cycle. If deer temporally segregate according to social rank, we predicted that the resulting activity patterns would manifest in one social class being relatively more susceptible to hunter-induced mortality. We used a multi-state modeling approach to quantify temporal segregation and calculated the probability that a feeding site was in a particular state during diurnal and nocturnal hours for each of the 3 phases of the breeding season. We determined that transition probabilities differed by season and diel cycle and dominant and subordinate social classes clearly avoided each other, with <1% co-occurrence at feeding sites. During the pre-breeding season, the probability of a subordinate being present during diurnal hours was 3.0× more likely than a dominant being present, but did not differ during nocturnal hours. There was no difference for dominants and subordinates during diurnal or nocturnal hours during the breeding season. In the post-breeding season, subordinates were 1.7× more likely to occur at the feeding site than a dominant during diurnal hours but they did not differ during nocturnal hours. Our results indicate that dominance status influences temporal segregation at feeding sites and is affected by the phase of the breeding season. Therefore, the resulting activity patterns may increase subordinates’ risk to human predation during the pre-breeding and post-breeding seasons.
Keywords: competition; multistate modeling, predation risk; supplemental feeding sites; intraspecific temporal resource partitioning; white-tailed deer
Individuals may reduce competition by partitioning resources temporally and spatially (Schoener 1971; Ziv et al. 1993; Howerton and Mench 2014). Temporal resource partitioning, in particular, can be vital to the coexistence of species, or individuals, that share a common resource (Ziv et al. 1993). Mechanisms driving temporal resource partitioning are similar for inter- and intra-specific competition. Sex-specific behavioral differences in ungulate resource use increases segregation as individuals attempt to avoid antagonistic interactions (Bowyer 2004). However, interference competition from dominant sex-age classes may reduce subordinates’ access to food resources, regardless of sex (Appleby 1980; McGhee and Baccus 2006; Donohue et al. 2013). For example, subordinate male red deer Cervus elaphus may avoid using feeding sites when patch profitability does not outweigh the costs of agonistic interactions with dominant males (Schmidt et al. 1998). In addition, Grenier et al. (1999) reported that female and fawn white-tailed deer Odocoileus virginianus had greater access to food when adult males were not present.
Older, larger ungulate males are generally dominant over smaller males and females (Grenier et al. 1999; Côté 2000; Donohue et al. 2013), but dominance hierarchies are seasonally dynamic (Koutnik 1981; Grovenburg et al. 2009). Odocoileus spp. exhibit marked sexual segregation outside of the breeding season with males forming loose aggregations that consist of various age classes (Hirth 1977; Lagory 1986; Miller and Conner 2005). Female groups consist of a matriarchal female, multiple generations of her female offspring, and pre-dispersal males (Hirth 1977; Lagory 1986). Male dispersal typically occurs in their first spring or second autumn (Rosenberry et al. 1999). Therefore, during autumn, yearling males may be solitary or associate with either matrilineal or all-male groups. Regardless of their social affiliation, yearling males are competitively disadvantaged compared with adult males (Donohue et al. 2013).
Competitive asymmetries are common at concentrated resources (Donohue et al. 2013; Milner et al. 2014). It is possible that in populations with clear dominance hierarchies, competition drives temporal resource partitioning. Furthermore, if competitors temporally segregate their use of a shared resource, then subordinates may use the resource only when dominants are absent. Resulting activity patterns at the resource may enhance or diminish susceptibility to predation, depending on the suite of predators and their associated activity cycle.
Temporal resource partitioning has been studied in sympatric species of mammals (Kronfeld-Schor and Dayan 1999; Adams and Thibault 2006) and within cohorts of the same species (Taillon and Côté 2006, 2007). However, the effect of social rank on intraspecific temporal resource partitioning across sex-age classes has not been investigated in ungulates. We hypothesized that white-tailed deer would temporally segregate use of supplemental feed sites based on competitive status whereby adult females and yearling males would avoid encounters with adult males. Moreover, if we observed temporal resource partitioning based on competitive status, we hypothesized that activity patterns at the feeding sites would manifest in one social group being more at risk to hunter-induced mortality. We tested these hypotheses in a population of white-tailed deer offered supplemental feed during the hunting season.
Materials and Methods
Study area
We conducted this study on a 1619-ha property in Harris County, GA, USA (32.8023°N, −84.9049°W). Pine stands made up ∼983 ha (61%) of the land cover and were comprised primarily of loblolly Pinus taeda and shortleaf pine P. echinata. Hardwood stands constituted ∼582 ha (36%) of the study site and were dominated by oak (Quercus spp.), hickory (Carya spp.), tulip-poplar Liriodendron tulipifera, and sweetgum Liquidambar styraciflua. Open areas included pasture, fallow fields, row crops, and cultivated wildlife openings.
White-tailed deer hunting occurred on the property from 13 September–15 January, 2013 and from 14 September–15 January, 2014. The property received light hunting pressure and ∼10 deer (<1 deer per 160 ha) were harvested annually. Hunter effort data indicated that on average, one hunt (i.e., a single hunter from a fixed location) occurred every 2 days. Camera surveys (Jacobson et al. 1997) conducted in August 2013 and 2014 estimated the deer density was 0.16 and 0.19 deer per hectare, respectively. The buck:doe ratio was ∼1:1.1 in both years. Hunters typically harvested only mature males and adult females. Although hunting pressure on the site was relatively low during our study period, the site had been hunted for decades under varying harvest criteria and deer were not naïve to the threat of human predation.
Non-human predators on the site included bobcats Lynx rufus and coyotes Canis latrans. Although coyotes can affect fawn survival in some regions (Ballard 2011), they are not effective predators of adult deer in the southeastern United States (Chitwood et al. 2014). Where bobcats are sympatric with coyotes, food habit studies indicate that bobcats are not responsible for significant direct mortality on adult deer (Van Gilder 2008).
Study design
We established 16 feeding sites across the study site and used shelled corn as supplemental forage. Hunters were allowed to hunt at feeding sites and feed was provided ad libitum throughout the duration of the study, which began > 2 weeks prior to data collection to allow deer to acclimate to the feeding sites. From 13 September to 3 January in 2013 and 2014, we used infrared cameras (Reconyx Hyperfire HC550, Holmen, WI, USA) to observe visitation rates. We mounted cameras to a tree or post 3–4 m from the feed site and ∼75 cm from ground level. Cameras were triggered by motion and recorded photographs 24 h per day, with a 5-min delay between successive photographs. We assigned each deer observation to a sex-age class [adult male (≥2.5 years-old), yearling male (1.5 years-old), and adult female (≥1.5 years-old)] based on antler and body morphology (Richards and Brothers 2003), and recorded the time and date of the photograph. Adult male dominance at concentrated resources is well documented in white-tailed deer (Ozoga 1972; Donohue et al. 2013). Because both yearling males and adult females are subordinate to adult males during autumn, we pooled their observations. We excluded photographs when the sex-age class for all individuals in the photograph could not be determined.
Although adult males are dominant throughout autumn and early winter, social dynamics and the frequency of aggressive interactions change relative to the breeding season (Hirth 1977). Furthermore, physiological and behavioral changes related to breeding activity occur in males and could affect the degree of temporal partitioning. Therefore, we divided the study period into pre-breeding, breeding, and post-breeding seasons based on conception data from the study site (Stickles et al. 2015): 1) Pre-breeding—Weeks 1−6 (13 September−25 October); 2) Breeding—Weeks 7−11 (26 October−27 November); and 3) Post-breeding—Weeks 12−16 (28 November−3 January).
We sub-sampled photographs obtained for 3 days (Monday, Tuesday, and Wednesday) of each week because of the large number of photographs recorded at feeding sites. We binned each observation of a deer at a feeding site by hour of the day (0−23). We divided the diel cycle into diurnal and nocturnal hours based on legal hunting hours to determine the relative exposure of each social group to human predators. We used the hours in which legal hunting began and ended for our delineation of diurnal and nocturnal hours.
Models
To test our hypothesis that deer temporally segregated use of the feeding site based on social rank, we used a multistate modeling approach. In any given hour, the feeding site could have been in State 1 (no deer present), State 2 (subordinate present), State 3 (dominant present), or State 4 (both a dominant and subordinate present). We used all photographs of deer recorded during each hour to determine the state of the feeder, and did not consider photographs of other species in our analyses. If all of the photos collected during a given hour at a particular feeding site were of adult males and we collected no photos of adult females or yearling males during that hour, the state of the feeding site for that hour was State 3 (dominant present). Likewise, if an adult female and an adult male were photographed during a given hour at a particular feeding site, the state of the feeding site for that hour was State 4 (both a subordinate and a dominant present).
To quantify any potential temporal segregation between dominants and subordinates (segregation model), we used package “msm” (Jackson 2011) for R version 3.1.2 (R Core Team 2014) to calculate hazard ratios (HRs) and transition probabilities. Because feeding sites were available to all deer during all hours, we considered all transitions equally possible (see Figure 1 for transition diagram). Therefore, we assigned each transition an initial value of 0.25 in the transition intensity matrix. We estimated HRs and transition probabilities by season (pre-breed, breed, and post-breed) and time-of-day (diurnal or nocturnal), and used “state” as the response variable in our model. HR values of 1 indicate that the transition probabilities did not differ according to season or time-of-day (Jackson 2011).
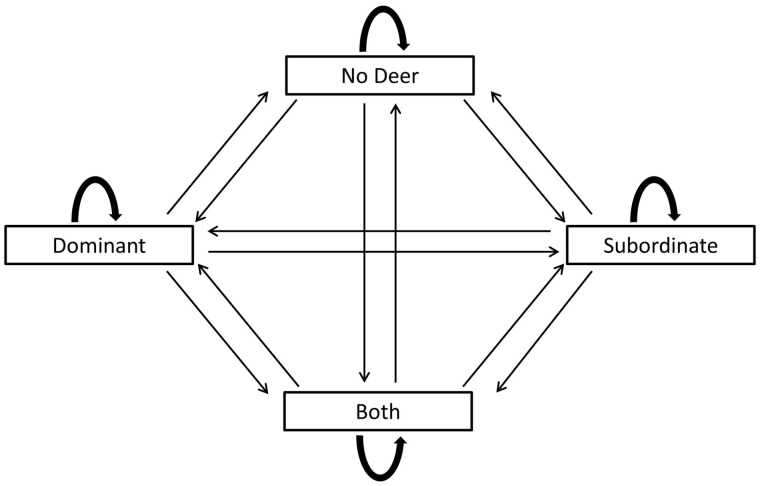
Figure 1.
Transition diagram for multistate model used to estimate probability of feeding sites transitioning from one state to another state, or the probability of the feeding site staying in the same state. There were 4 possible states (States 1–4) that a feeding site could be in during any given hour. State 1 = no deer present; State 2 = subordinate (adult females and yearling males) present; State 3 = dominant (adult males) present; State 4 = subordinate and a dominant present.
In addition, we estimated probabilities for going from one state at time t to a different state at time t + 1 during each season and period. Transition probabilities from State 1 (no deer present) at time t to any other state at time t + 1 are not affected by the presence of another social class occurring at a feeding site. Therefore, transition probabilities from State 1 (no deer present) to any other state are predicted to be equal (see Prediction 1, Table 1). Furthermore, if the presence of either social class at time t does not affect the probability of another social class occurring at the feeding site at time t + 1, we predict the transition probability from one social class to another to be equal to the probability of transitioning to State 1 (see Prediction 2, Table 1).
Table 1.
Predictions, state transitions, and interpretation for our hypothesis that white-tailed deer would temporally segregate use of supplemental feed sites based on competitive status
| Prediction | State transitions | Interpretation |
|---|---|---|
| 1a | 1–2 = 1–3 = 1–4 | No temporal segregation |
| 2 | 2–3 = 2–1 3–2 = 3–1 | No temporal segregation |
If we observed that state transitions were equal, we interpreted the results to indicate that there was no temporal segregation.
State 1 = no deer present; State 2 = subordinate present; State 3 = dominant present; State 4 = subordinate and dominant present.
To determine if temporal segregation between dominants and subordinates manifested in differential arrival times and use (i.e. nocturnal vs. diurnal), we implemented Markov chain Monte Carlo (MCMC) routines to fit multinomial response (States 1–4) generalized linear mixed models in package “MCMCglmm” (Hadfield 2010; hereafter, Diel Model). We modeled the response (state) by season, diel phase (diurnal or nocturnal hours), and their interactions. We treated the feeding site as a random effect.
Results
Camera trapping effort was 375, 411, and 402 camera days for the pre-breeding, breeding, and post-breeding seasons, respectively. We collected 6994 photographs containing images of 8469 white-tailed deer for which we could assign a sex-age class, and excluded fawns from our analyses. We recorded a total of 3192 adult male (47.1%), 1365 yearling male (20.1%), and 2225 (32.8%) adult female images. During the pre-breeding season, cameras collected 1164 (48.2%) adult male, 399 (16.5%) yearling male, and 850 (35.2%) adult female images. We observed a decline in images of all sex-age classes during the breeding season with 459 (36.4%) adult male, 177 (14.0%) yearling male, and 625 (49.6%) adult female images captured at feeding sites. During the post-breeding season, cameras collected 1569 (50.5%) adult male, 789 (25.4%) yearling male, and 750 (24.1%) adult female images. Our hourly bins resulted in 18 432 total state occurrences (Table 2) with only 195 occasions of the feeding site being in State 4. Furthermore, we detected only 54 (<1%) co-occurrences of subordinates and dominants in the same photograph.
Table 2.
Frequency of each state during nocturnal and diurnal hours of the pre-breeding, breeding, and post-breeding seasons
| State | Pre-breeding |
Breeding |
Post-breeding |
|||
|---|---|---|---|---|---|---|
| Nocturnal | Diurnal | Nocturnal | Diurnal | Nocturnal | Diurnal | |
| 1 | 2560 | 3788 | 2487 | 3029 | 2299 | 2600 |
| 2 | 135 | 95 | 64 | 40 | 197 | 147 |
| 3 | 234 | 45 | 75 | 45 | 265 | 132 |
| 4 | 47 | 8 | 14 | 6 | 71 | 49 |
State 1 = no deer present; State 2 = subordinate (adult females and yearling males) present; State 3 = dominant (adult males) present; State 4 = subordinate and a dominant present.
HRs from the segregation model indicated that transition probabilities varied according to season and the diel cycle. During the pre-breeding season, Transitions 1–2 (HR, 95% confidence interval; HR =1.61, CI = 1.25–2.08) and 1–3 (HR = 1.77, CI = 1.39–2.25) were more likely to occur than during the breeding season (Table 3). Transition 3–1 was less likely to occur in the pre-breeding (HR = 0.78, CI= 0.61–0.98) and post-breeding (HR = 0.75, CI = 0.60–0.94) seasons than in the breeding season (Tables 3 and 4). Compared with the breeding season, transition probabilities for Transitions 1–2 (HR = 2.91, CI = 2.28–3.71), 1–3 (HR = 2.93, CI = 2.32–3.68), and 1–4 (HR = 6.35, CI = 3.52–11.45) were greater during the post-breeding season (Table 4). During diurnal hours, transition probabilities for Transitions 1–2 (HR = 0.69, CI = 0.58–0.83), 1–3 (HR= 0.31, CI = 0.27–0.38), and 1–4 (HR = 0.48, CI = 0.33–0.70) were less likely to occur than at night (Table 5).
Table 3.
HRs and 95% confidence limits for transition probabilities at white-tailed deer supplemental feeding sites in Harris County, GA, USA (2013–2014) during the pre-breeding season (13 September−25 October), as compared with the breeding season
| Transitiona | HR | Lower | Upper |
|---|---|---|---|
| State 1–State 2 | 1.61 | 1.25 | 2.08 |
| State 1–State 3 | 1.77 | 1.39 | 2.25 |
| State 1–State 4 | 1.87 | 0.97 | 3.63 |
| State 2–State 1 | 0.88 | 0.68 | 1.15 |
| State 2–State 3 | 0.65 | 0.25 | 1.70 |
| State 2–State 4 | 1.82 | 0.39 | 8.57 |
| State 3–State 1 | 0.78 | 0.61 | 0.98 |
| State 3–State 2 | 1.81 | 0.39 | 8.43 |
| State 3–State 4 | 2.23 | 0.48 | 10.30 |
| State 4–State 1 | 1.11 | 0.50 | 2.47 |
| State 4–State 2 | 0.80 | 0.25 | 2.62 |
| State 4–State 3 | 0.82 | 0.28 | 2.38 |
aState 1 = no deer present; State 2 = subordinate present; State 3 = dominant present; State 4 = subordinate and dominant present.
Table 4.
HRs and 95% confidence limits for transition probabilities at white-tailed deer supplemental feeding sites in Harris County, GA, USA (2013–2014) during the post-breeding season (28 November−3 January) as compared with the breeding season
| Transitiona | HR | Lower | Upper |
|---|---|---|---|
| State 1–State 2 | 2.91 | 2.28 | 3.71 |
| State 1–State 3 | 2.93 | 2.32 | 3.68 |
| State 1–State 4 | 6.35 | 3.52 | 11.45 |
| State 2–State 1 | 0.86 | 0.67 | 1.10 |
| State 2–State 3 | 1.26 | 0.55 | 2.88 |
| State 2–State 4 | 2.44 | 0.56 | 10.64 |
| State 3–State 1 | 0.75 | 0.60 | 0.94 |
| State 3–State 2 | 3.60 | 0.85 | 15.23 |
| State 3–State 4 | 2.60 | 0.60 | 11.26 |
| State 4–State 1 | 1.33 | 0.64 | 2.78 |
| State 4–State 2 | 0.97 | 0.33 | 2.82 |
| State 4–State 3 | 0.69 | 0.26 | 1.83 |
State 1 = no deer present, State 2 = subordinate present, State 3 = dominant present, State 4 = subordinate and dominant present.
Table 5.
HRs and 95% confidence limits for transition probabilities at white-tailed deer supplemental feeding sites in Harris County, GA, USA (2013–2014) during diurnal hours as compared with nocturnal hours
| Transition | HR | L | U |
|---|---|---|---|
| State 1–State 2a | 0.69 | 0.58 | 0.83 |
| State 1–State 3 | 0.32 | 0.27 | 0.38 |
| State 1–State 4 | 0.48 | 0.33 | 0.70 |
| State 2–State 1 | 0.96 | 0.80 | 1.15 |
| State 2–State 3 | 0.89 | 0.49 | 1.62 |
| State 2–State 4 | 0.86 | 0.39 | 1.90 |
| State 3–State 1 | 1.11 | 0.93 | 1.33 |
| State 3–State 2 | 0.71 | 0.32 | 1.58 |
| State 3–State 4 | 1.18 | 0.53 | 2.63 |
| State 4–State 1 | 0.87 | 0.55 | 1.36 |
| State 4–State 2 | 0.88 | 0.43 | 1.83 |
| State 4–State 3 | 1.19 | 0.59 | 2.39 |
State 1 = no deer present; State 2 = subordinate present; State 3 = dominant present; State 4 = subordinate and dominant present.
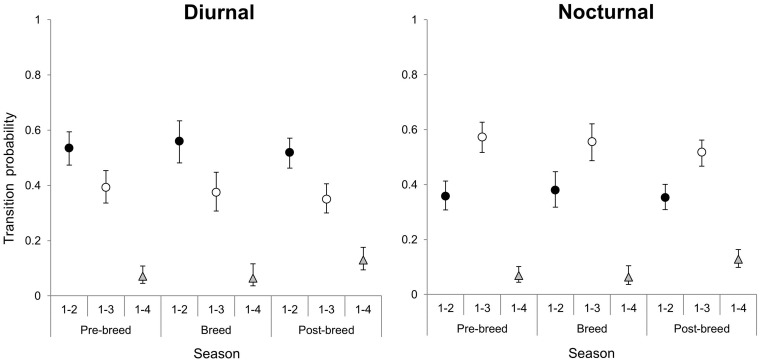
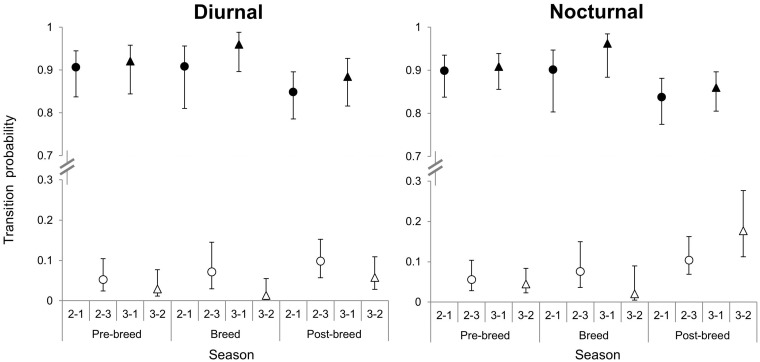
The probabilities for the next state to occur revealed marked temporal segregation at individual feeding sites. For Prediction 1 (Table 1), transition probabilities for Transitions 1–2 and 1–3 were greater than 1–4 during diurnal and nocturnal hours in all seasons (Figure 1). Interestingly, Transition probabilities for transition 1–2 were consistently greater than 1–3 during diurnal hours (Figure 1). During nocturnal hours, transition probabilities for Transitions 1–3 were greater than 1–2 during all seasons (Figure 1). For Prediction 2 (Table 1), Transitions 2–1 and 3–1 were greater than Transitions 2–3 and 3–2 in all seasons during both diurnal and nocturnal hours (Figure 2).
Figure 2.
Probability for a feeding site to transition from State 1–State 2 (solid circle), State 1–State 3 (hollow circle), and State 1–State 4 (triangle) during diurnal and nocturnal hours. State 1 = no deer present; State 2 = subordinate (adult females and yearling males) present; State 3 = dominant (adult males) present; State 4 = subordinate and a dominant present. Transition probability for transitions from State 1–State 2 was greater than State 1–State 3 and State 1–State 4 during diurnal hours in all seasons. During nocturnal hours, the transition probability for States 1–3 was greater than States 1–2 and State 1–4 in all seasons.
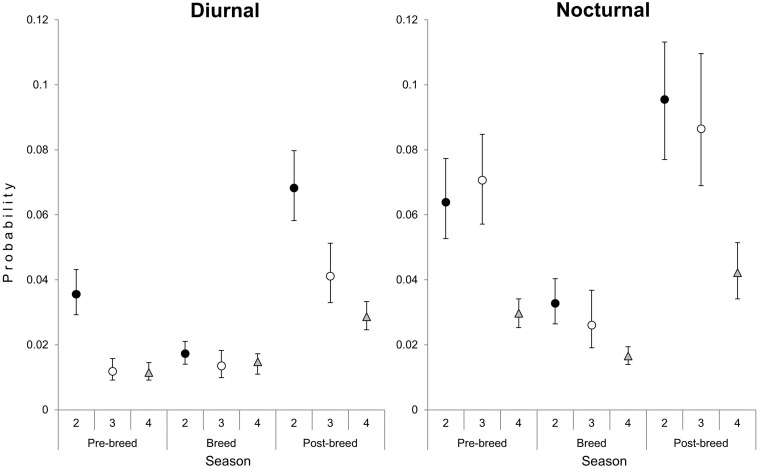
Results of the diel model indicated that the probability of the feeder being in a particular state differed by diel cycle and season (Table 6). During the pre-breeding season, the probability of a subordinate being present during day was 3.0× more likely than a dominant being present but did not differ at night (Figure 3). In addition, the probability of an adult male being present at the at a feeding site was 5.9× greater at night than day during the pre-breeding season (Figure 3). During the breeding season, there was no difference for dominants and subordinates during diurnal or nocturnal hours (Figure 3). In the post-breeding season, subordinates were 1.7× more likely to occur at the at a feeding site than a dominant during diurnal hours but there was no difference during nocturnal hours (Figure 3).
Table 6.
Estimates from MCMC generalized linear mixed model predicting the probability of a supplemental feeding site being in 1 of 4 states during the pre-breeding, breeding, and post-breeding seasons during diurnal and nocturnal hours
| Posterior mean | Lower CIa | Upper CIa | pMCMCb | |
|---|---|---|---|---|
| (Intercept) | −4.046 | −4.242 | −3.883 | <0.001 |
| State2: breed: diurnalc | −0.644 | −0.828 | −0.391 | <0.001 |
| State3: breed: diurnal | −0.944 | −1.317 | −0.553 | <0.001 |
| State4: breed: diurnal | −0.831 | −1.081 | −0.639 | <0.001 |
| State2: post: diurnal | 1.010 | 0.861 | 1.165 | <0.001 |
| State3: post: diurnal | 0.385 | 0.153 | 0.702 | <0.001 |
| State4: post: diurnal | −0.044 | −0.163 | 0.132 | 0.454 |
| State2: pre: diurnal | 0.212 | 0.067 | 0.389 | <0.001 |
| State3: pre: diurnal | −1.097 | −1.417 | −0.752 | <0.001 |
| State4: pre: diurnal | −1.128 | −1.318 | −0.831 | <0.001 |
| State2: breed: nocturnal | 0.112 | −0.084 | 0.307 | 0.328 |
| State3: breed: nocturnal | −0.170 | −0.515 | 0.210 | 0.430 |
| State4: breed: nocturnal | −0.694 | −0.837 | −0.559 | <0.001 |
| State2: post: nocturnal | 1.433 | 1.242 | 1.589 | <0.001 |
| State3: post: nocturnal | 1.302 | 1.047 | 1.620 | <0.001 |
| State4: post: nocturnal | 0.418 | 0.204 | 0.657 | <0.001 |
| State2: pre: nocturnal | 0.926 | 0.751 | 1.091 | <0.001 |
| State3: pre: nocturnal | 1.052 | 0.875 | 1.266 | <0.001 |
Represent lower and upper 95% credibility intervals.
Number of simulated cases that are >0 or <0 corrected for number of MCMC samples.
State 1 = no deer present; State 2 = subordinate (adult females and yearling males) present; State 3 = dominant (adult males) present; State 4 = subordinate and a dominant present. Reference class is State 1.
Figure 3.
Probability for a feeding site to transition from State 2–State 1 (solid circle), State 2–State 3 (hollow circle), State 3–State 1 (solid triangle), and State 3–State 2 (hollow triangle) during diurnal and nocturnal hours. State 1 = no deer present; State 2 = subordinate (adult females and yearling males) present; State 3 = dominant (adult males) present; and State 4 = subordinate and a dominant present. Transition probability for transitions from State 2 to State 1 was greater than State 2–State 3 during diurnal and nocturnal hours in all seasons. Transition probability for transitions from State 3 to State 1 was greater than State 3–State 2 during diurnal and nocturnal hours in all seasons.
Figure 4.
Probability that a feeding site was in State 2 (subordinate present), State 3 (dominant present), or State 4 (subordinate and dominant present) during diurnal and nocturnal hours in the pre-breeding, breeding, and post-breeding seasons. During the pre- and post-breeding seasons, subordinates were more likely than dominants to occur at a feeding site during diurnal hours. During all seasons, dominants and subordinates were equally likely to occur at a feeding site during nocturnal hours.
Discussion
Animals can reduce competitive interactions for a shared resource through temporal resource partitioning (Alanärä et al. 2001). However, how animals segregate along the temporal niche axis has been the focus of few studies, particularly in mammals (Adams and Thibault 2006). In this study, dominants and subordinates within the same species showed a clear pattern of avoidance of the opposing social class, with segregation varying according to season and the diel cycle.
Feeding site use was only similar for dominants and subordinates during the breeding season. However, feeding site use was also the lowest for both groups during this season. Adult males increase diurnal movements from pre-breeding to breeding season (Tomberlin 2007; Webb et al. 2010) and it is during this time that males switch their focus from foraging to mate-searching behaviors (Beier and McCullough 1990; Tomberlin 2007). Furthermore, many adult male ungulates exhibit hypophagia during the breeding season (Warren et al. 1981; Chin and Brown 1984; Brivio et al. 2010) and visit feeding sites less than during the pre- and post-breeding seasons (Ozoga and Verme 1982). As mating activities are prioritized over foraging during the breeding season, the breeding males’ activity budget is directed away from foraging activity (Pelletier et al. 2009). Younger males, however, do not invest as heavily in breeding activities allowing them to place a higher priority on growth to improve future opportunities for mating (Mysterud et al. 2008; Foley et al. 2015). Consequently, they are not as hypophagic as adult males during the breeding season (Mysterud et al. 2008).
Why subordinates also decreased feeding site visitation during the breeding season is unclear. With the lack of dominants at the sites, subordinates had greater access to the supplemental feed, but did not use it as heavily during the breeding season. Although yearling males do not exhibit the same intensity of search behavior as adult males, they do engage in mate-searching (Foley et al. 2015). Moreover, peak dispersal of yearling males coincides with the breeding season (Rosenberry et al. 1999). Therefore, some yearling males present during pre-breeding may have emigrated from the site, whereas immigrating yearling males would be naive to the feeding site locations.
Adult females of many species are often harassed by breeding males (Clutton-Brock et al. 1992; Reale et al. 1996; Holand et al. 2006). Harassment can lead to poorer body condition (Holand et al. 2006) and even direct mortality (Reale et al. 1996). Therefore, spatially and temporally avoiding breeding males is beneficial for females when not in estrus, which may explain why feeding site use was the lowest for females during the breeding season.
During the post-breeding season, males may be in poor physical condition, making abundant, high-energy food sources very profitable patches at which to forage. However, agonistic interactions are energetically expensive (Appleby 1980) and defending the feeding site from subordinates during this time would lessen the profitability of the resource. Likewise, testosterone levels are lower during the post-breeding season (Chin and Brown 1984; Miller et al. 1987) making aggressive behaviors less likely (Lincoln et al. 1972). Together, energy conservation and lowered aggression by adult males may explain why we observed less temporal segregation in the post-breeding season.
Dominance status clearly influenced temporal patterns in feeding site use. Adult male occurrences at the feeding sites were lower during diurnal periods, particularly during the pre-breeding season. The pattern of temporal partitioning whereby subordinates were more likely to occur at the feeding sites during diurnal hours has obvious implications for human-induced mortality. Although hunting pressure was relatively-low on our site, in most of the region hunting is responsible for the majority of deer mortality (Dusek et al. 1992), especially because most large carnivores have been extirpated. On our study site, predation risk from carnivores and hunters was low because hunting pressure was light and bobcats and coyotes are not efficient predators of adult deer. Previous research at the site demonstrated that no sex-age class altered vigilance levels at feeding sites according to the diel cycle (Stone et al. 2017). Therefore, deer may not have focused their anti-predator behaviors toward a specific predation threat.
Our study illustrates how patterns in temporal resource partitioning at feeding sites may influence relative risk to different sources of mortality. Subordinates used the shared resource at similar rates, but the pattern of temporal partitioning evidently would make subordinates more susceptible to human predation. Further research is needed to determine if an increase in hunting pressure acts to compress temporal partitioning at feeding sites and how the resulting temporal patterns affect predation risk for dominant and subordinate social classes.
Acknowledgments
Funding for this project was provided by the Georgia Wildlife Resources Division through the Wildlife Restoration Program, which derives monies through an excise tax on sporting arms and ammunition paid by hunters and recreational shooters and McIntire–Stennis Project GEOZ-0174-MS.
References
- Adams R, Thibault K, 2006. Temporal resource partitioning by bats at water holes. J Zool 270:466–472. [Google Scholar]
- Alanärä A, Burns MD, Metcalfe NB, 2001. Intraspecific resource partitioning in brown trout: The temporal distribution of foraging is determined by social rank. J Anim Ecol 70:980–986. [Google Scholar]
- Appleby MC, 1980. Social rank and food access in red deer stags. Behaviour 74:294–309. [Google Scholar]
- Ballard W, 2011. Predator-prey relationships. In: Hewitt DG. editor. Biology and Management of White-tailed Deer. Boca Raton: CRC Press; 251–286. [Google Scholar]
- Beier P, McCullough DR, 1990. Factors influencing white-tailed deer activity patterns and habitat use. Wildl Monogr 109:3–51. [Google Scholar]
- Bowyer RT, 2004. Sexual segregation in ruminants: definitions, hypotheses, and implications for conservation and management. J Mammal 85:1039–1052. [Google Scholar]
- Brivio F, Grignolio S, Apollonio M, 2010. To feed or not to feed? Testing different hypotheses on rut-induced hypophagia in a mountain ungulate. Ethol 116:406–415. [Google Scholar]
- Chin CC, Brown RD, 1984. Seasonal relationships of thyroid, sexual and adrenocortical hormones to nutritional parameters and climatic factors in white-tailed deer Odocoileus virginianus of south Texas. Comp Biochem Physiol A 77:299–306. [DOI] [PubMed] [Google Scholar]
- Chitwood MC, Lashley MA, Moorman CE, DePerno CS, 2014. Confirmation of coyote predation on adult female white-tailed deer in the southeastern United States. Southeast Nat 13:N30–N32. [Google Scholar]
- Clutton-Brock TH, Price OF, MacColl AD, 1992. Mate retention, harassment, and the evolution of ungulate leks. Behav Ecol 3:234–242. [Google Scholar]
- Côté SD, 2000. Determining social rank in ungulates: a comparison of aggressive interactions recorded at a bait site and under natural conditions. Ethol 106:945–955. [Google Scholar]
- Donohue RN, Hewitt DG, Fulbright TE, DeYoung CA, Litt AR. et al. 2013. Aggressive behavior of white-tailed deer at concentrated food sites as affected by population density. J Wildl Manage 77:1401–1408. [Google Scholar]
- Dusek GL, Wood AK, Stewart ST, 1992. Spatial and temporal patterns of mortality among female white-tailed deer. J Wildl Manage 56:645–650. [Google Scholar]
- Foley AM, DeYoung RW, Hewitt DG, Hellickson MW, Gee KL. et al. 2015. Purposeful wanderings: mate search strategies of male white-tailed deer. J Mammal 96:279–286. [Google Scholar]
- Grenier DC, Barrette C, Crête M, 1999. Food access by white-tailed deer Odocoileus virginianus at winter feeding sites in eastern Quebec. Appl Anim Behav Sci 63:323–337. [Google Scholar]
- Grovenburg TW, Jenks JA, Jacques CN, Klaver RW, Swanson CC, 2009. Aggressive defensive behavior by free-ranging white-tailed deer. J Mammal 90:1218–1223. [Google Scholar]
- Hadfield JH, 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J Stat Softw 33:1–22.20808728 [Google Scholar]
- Hirth DH, 1977. Social behavior of white-tailed deer in relation to habitat. Wildl Monogr 53:3–55. [Google Scholar]
- Holand Ø, Weladji RB, Røed KH, Gjøstein H, Kumpula J. et al. 2006. Male age structure influences females’ mass change during rut in a polygynous ungulate: the reindeer Rangifer tarandus. Behav Ecol Sociobiol 59:682–688. [Google Scholar]
- Howerton CL, Mench JA, 2014. Running around the clock: competition, aggression and temporal partitioning of running wheel use in male mice. Anim Behav 90:221–227. [Google Scholar]
- Jackson CH, 2011. Multi-state models for panel data: the msm package for R. J Stat Softw 38:1–29. [Google Scholar]
- Jacobson H, Kroll JC, Browning RW, Koerth BH, Conway MH, 1997. Infrared-triggered cameras for censusing white-tailed deer. Wildl Soc Bull 25:547–556. [Google Scholar]
- Koutnik DL, 1981. Sex-related differences in the seasonality of agonistic behavior in mule deer. J Mammal 62:1–11. [Google Scholar]
- Kronfeld-Schor N, Dayan T, 1999. The dietary basis for temporal partitioning: food habits of coexisting Acomys species. Oecologia 121:123–128. [DOI] [PubMed] [Google Scholar]
- Lagory KE, 1986. Habitat, group size, and the behaviour of white-tailed deer. Behaviour 98:168–179. [Google Scholar]
- Lincoln G, Guiness F, Short R, 1972. The way in which testosterone controls the social and sexual behavior of the red deer stag Cervus elaphus. Horm Behav 3:375–396. [Google Scholar]
- McGhee JD, Baccus JT, 2006. Behavioral interactions between axis and fallow deer at high-value food patches. Southwest Nat 51:358–367. [Google Scholar]
- Miller KV, Marchinton RL, Forand KJ, Johansen KL, 1987. Dominance, testosterone levels, and scraping activity in a captive herd of white-tailed deer. J Mammal 68:812–817. [Google Scholar]
- Miller MW, Conner MM, 2005. Epidemiology of chronic wasting disease in free-ranging mule deer: spatial, temporal, and demographic influences on observed prevalence patterns. J Wildl Dis 41:275–290. [DOI] [PubMed] [Google Scholar]
- Milner JM, Van Beest FM, Schmidt KT, Brook RK, Storaas T, 2014. To feed or not to feed? Evidence of the intended and unintended effects of feeding wild ungulates. J Wildl Manage 78:1322–1334. [Google Scholar]
- Mysterud A, Bonenfant C, Loe LE, Langvatn R, Yoccoz NG. et al. 2008. Age-specific feeding cessation in male red deer during rut. J Zool 275:407–412. [Google Scholar]
- Ozoga JJ, 1972. Aggressive behavior of white-tailed deer at winter cuttings. J Wildl Manage 36:861–868. [Google Scholar]
- Ozoga JJ, Verme LJ, 1982. Physical and reproductive characteristics of a supplementally-fed white-tailed deer herd. J Wildl Manage 46:281–301. [Google Scholar]
- Pelletie F, Mainguy J, Côté SD, 2009. Rut-induced hypophagia in male bighorn sheep and mountain goats: foraging under time budget constraints. Ethol 115:141–151. [Google Scholar]
- R Core Team, 2014. R: A language and environment for statistical computing [Internet]. Vienna (Austria): R Foundation for Statistical Computing [cited 2016 August 12]. Available from: http://www.r-project.org/. [Google Scholar]
- Reale D, Bousses P, Chapuis J, 1996. Female-biased mortality induced by male sexual harassment in a feral sheep population. Can J Zool 74:1812–1818. [Google Scholar]
- Richards D, Brothers A, 2003. Observing and Evaluating Whitetails In: Richards D. editor. Wilds of Texas Photography. Boerne (TX: ): Wilds of Texas Photography. [Google Scholar]
- Rosenberry CS, Lancia RA, Conner MC, 1999. Population effects of white-tailed deer dispersal. Wildl Soc Bull 27:858–864. [Google Scholar]
- Schmidt KT, Seivwright LJ, Hoi H, Staines BW, 1998. The effect of depletion and predictability of distinct food patches on the timing of aggression in red deer stags. Ecography 21:415–422. [Google Scholar]
- Schoener TW, 1971. Theory of feeding strategies. Annu Rev Ecol Syst 2:369–404. [Google Scholar]
- Stickles JH, Stone DB, Evans CS, Miller KV, Warren RJ. et al. , 2015. Using deer-vehicle collisions to map white-tailed deer breeding activity in Georgia. J Southeast Assoc Fish Wildl Agencies 2:202–207. [Google Scholar]
- Stone DB, Cherry MJ, Martin JA, Cohen BS, Miller KV, 2017. Breeding chronology and social interactions affect ungulate foraging behavior at a concentrated food resource. PLoS One 12:e0178477.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taillon J, Côté SD, 2006. The role of previous social encounters and body mass in determining social rank: an experiment with white-tailed deer. Anim Behav 72:1103–1110. [Google Scholar]
- Taillon J, Côté SD, 2007. Social rank and winter forage quality affect aggressiveness in white-tailed deer fawns. Anim Behav 74:265–275. [Google Scholar]
- Tomberlin JW, 2007. Movement, activity, and habitat use of adult male white-tailed deer at Chesapeake Farms, Maryland [MS Thesis]. Raleigh, North Carolina: USA North Carolina State University. [Google Scholar]
- Van Gilder CL, 2008. Coyote and bobcat food habits and the effects of an intensive predator removal on white-tailed deer recruitment in Northeastern Alabama [MS Thesis]. Athens, Georgia: University of Georgia. [Google Scholar]
- Warren RJ, Kirkpatrick RL, Oelschlaeger A, Scanlon PF, Gwazdauskus FC, 1981. Dietary and seasonal influences on nutritional indices of adult male white-tailed deer. J Wildl Manage 45:926–936. [Google Scholar]
- Webb SL, Gee KL, Strickland BK, Demarais S, DeYoung RW, 2010. Measuring fine-scale white-tailed deer movements and environmental influences using GPS collars. Int J Ecol 2010:1–12. [Google Scholar]
- Ziv Y, Abramsky Z, Kotler BP, Subach A, 1993. Interference competition and temporal and habitat partitioning in two gerbil species. Oikos 66:237–246. [Google Scholar]