Abstract
Habitat use and preferences may be subject to spatial and temporal changes. However, long-term studies of species–habitat relationships are the exception. In the present research, long-term trends in habitat use by an alpine ungulate, the Tatra chamois Rupicapra rupicapra tatrica, were analyzed. We examined how environmental changes attributable to climate change, removal of sheep, and habituation to hikers, which took place over the last half-century have changed the spatial distribution of animals. Data on the localities of groups sighted between 1957 and 2013 during autumnal population surveys were used to evaluate habitat associations: these were correlated with year, group size, population size, and climatic conditions. The results indicate that the Tatra chamois is tending, over the long term, to lower its altitude of occurrence, reduce its average distance to hiking trails, and stay less often on slopes with a southerly aspect. These trends are independent of group size, population size, and the weather conditions prevailing during observations, though not for altitude, where increases in air temperature are related to finding chamois at higher elevations. The proportion of alpine meadows and slope in the places used by chamois is correlated with population size, while the proportion of areas with trees and/or shrubs is correlated with group size and air temperature, though long-term changes were not evident for these variables. To the best of our knowledge, this work is the first to document long-term trends in habitat use by ungulates. It shows that a species’ ecology is influenced by human-induced changes: abandonment of pasturage, high-mountain tourism, and climate changes, which constitute the most probable reasons for this aspect of behavioral evolution in the Tatra chamois.
Keywords: habitat selection, long-term study, population ecology, protected area, ruminant
Habitat use and preferences depend on the needs of animals and the advantages accruing from their occupation of a particular place (Krebs 2009; Macandza et al. 2012). However, these uses and preferences depend, in turn, on accessibility of habitats, the distribution, and quality of key resources within them (Lott 1990; Araújo et al. 2011; Robertson et al. 2015) and also inter- and intraspecific interactions (Richard et al. 2010; Kiszka et al. 2012; Beest et al. 2014). Factors affecting habitat use by herbivores include weather conditions (e.g., temperature and precipitation), temporal and spatial changes in resource distribution, abundance and quality (due to vegetation phenology and vegetation dynamics), accessibility of places of rest and concealment, individual features (e.g., sex, age, and condition), and types of behavior (e.g., neophobia and neophilia) (Clobert et al. 2012; Brivio et al. 2016; Ofstad et al. 2016). Other influences include the presence of predators (Sergio et al. 2003; Lone et al. 2014) and intraspecific competition (Beest et al. 2014, 2016).
Needs and advantages, and hence habitat use and preferences are not, however, a constant feature and may change in both time and space (Iversen et al. 2014). Short-term changes in the choice and use of habitats are attributable mainly to the various facets of the growing season (Fynn et al. 2014; Thompson et al. 2014) or depend on the reproductive cycle of herbivores (Nicholson et al. 1997; Rolandsen et al. 2017). In contrast, long-term changes in habitat selection and use are associated with an animal’s life cycle and individual needs, which are governed mainly by changes in condition, body size, the ability to reproduce, experience (Forchhammer et al. 2001; Long et al. 2016), or may be the result of its adaptation to changing habitat conditions (Lister 2004; Kawecki 2008).
Most studies to date on changes in habitat preferences and use have been carried out in the short-term context, chiefly in terms of the seasonality of habitat conditions (winter–summer) or changes in age cohorts (juveniles versus adults) over a small number of consecutive years (Brambilla et al. 2006; Nesti et al. 2010), exceptionally decades (Garel et al. 2007; Shenbrot et al. 2010). A review of papers on habitat-related research from top-ranked ecology journals for the past 10 years shows that of 84 articles dealing with this subject, only 15% provide data for periods longer than 7 years, and barely 5% concern long-term analyses of the nature of such changes (Uboni et al. 2015). There are a number of reasons why so few long-term studies have been carried out, even though they are fundamental to understanding the ways in which animals adapt to their natural environment: such studies require long-term commitments from scientific institutions, permanent funding, and above all, untrammelled access to study areas. This final criterion often means having to establish an uneasy working relationship with the owner or administrator of the land in question (Schradin and Hayes 2017).
The choice and use of optimal habitat present a challenge to montane ungulates living in extreme conditions. The occurrence and survival of herbivore populations is determined by the availability of natural resources, which are strongly influenced by dynamically changing climatic factors like precipitation and snow cover, often varying widely from season to season (Shackleton and Bunnell 1987). The present analysis of the long-term changes in habitat use by a montane ungulate is based on a long-term data set for Tatra chamois Rupicapra rupicapra tatrica. During the last 50 years this animal has been subjected to intense pressure owing to mass tourism (Blazejczyk 2002; Skawiński 2010): the spatial distribution of its population may have been affected by this. At the same time, protection of the Tatra Mountains in the form of a National Park has led to the gradual elimination of pasturage from this area; it also guarantees the complete protection of the chamois population. Climate change (M. Ciach and Ł. Pęksa, submitted for publication), as well as the dynamic changes in the size of chamois population — a dramatic fall from the 1950s until the turn of the century followed by the subsequent rebuilding of the population (Jamrozy and Pęksa 2004) — could have affected habitat use as well. This paper analyses long-term changes in habitat use by the Tatra chamois and attempts to identify the factors governing this. To do so, a unique and exceptionally long (57 years) series of data is used to track the changes in habitat use by these animals, providing an opportunity to assess the direction of changes in the ecology of the Tatra chamois. As far as we are aware, this material is one of the longest sequences of data for evaluating trends in habitat use by ungulates (see Festa-Bianchet et al. 2017).
Materials and Methods
Study area
The Tatras are the only mountains in central and eastern Europe of an alpine character. They cover an area of some 800 km2, around 20% of which lies in Poland, and the remainder in Slovakia. The elevation difference is 1,755 m, with the tallest peak, the Gerlach, 2,655 m amsl. The Tatras are protected in their entirety in the form of national parks: the Tatranský Národný Park (TANAP) in Slovakia (formed in 1949) and the Tatrzański Narodowy Park (tatra national park - TNP) in Poland (formed in 1954). In addition, the Tatras have been declared a Man and Biosphere Reserve and are included in the Natura 2000 network of protected areas in Europe.
The Tatras are characterized by a vertical zonation of climate and vegetation. The tree line lies at 1,500 m; above this, there are alpine meadows and the subnival zone. This altitude corresponds to the cool, moderately cold, and cold climatic zones, with features including low air temperatures and low atmospheric pressure (Hess 1996). The mean annual temperature is –0.7°C, and the coldest month is February. On average there are 188 winter days, that is, when the mean daily temperature (Tmean) is <0°C, whereas a thermal summer (when Tmean >15°C) does not occur at all. The growing season (when Tmean >5°C) lasts for just under 100 days. Snow typically covers the ground from December to April. In the alpine zone of the mountains snow persists well into May and June, for an average of 221 days of snow cover in the year, hindering access to grazing areas and exacerbating the risk of triggering avalanches. In the highest elevation regions, the snow layer is no more than 50 cm thick only from mid-June to early September; on occasion, snow falls in July and August. The mean annual precipitation is nearly 1,800 mm. The thermal conditions prevailing in the Tatras, expressed as air temperature, resemble those of the Alps (Niedźwiedź 2006).
The Tatras are built of granitoids, metamorphic, and limestone rocks. The rock formations in the zone inhabited by chamois can be divided into non-calcareous ones, from which acidic soils are formed, and calcareous ones, which give rise to soils with a neutral pH (Komornicki and Skiba 1985). The habitats in the zone occupied by chamois are associated with the plants Oreochloa disticha, Juncus trifidus, Festuca versicolor, and Sesleria tatrae (Jamrozy et al. 2007). Above the tree line there is dwarf pine Pinus mugo scrub: this is quite dense at lower altitudes, but gradually thins out with increasing elevation, becoming patchy and forming clumps, before disappearing entirely at around 1,800 m. As a result of the abandonment of pasturage there has been regeneration of the woodland communities on mid-forest meadows, which lie at lower altitudes, far below the range of occurrence of the Tatra chamois. Only locally, forest regeneration was recorded near the tree line and in the dwarf pine zone (Czajka et al. 2012). However, comparison of aerial photographs from 1955 and 2004 shows that the course of the tree line in the Tatras has not changed over the long term (Guzik 2008) and no major changes in vegetation structure of the alpine zone have been recorded.
The zone occupied by chamois in the Tatras covers the area above the tree line and reaches up to the summits of the highest peaks (Jamrozy et al. 2007). The areas above the tree line, lying in their entirety within the two National Parks, are protected: hunting there is strictly prohibited and incidents of poaching are exceptional (Jamrozy et al. 2007). The Tatra chamois population is completely isolated from other mountain areas, so neither emigration nor immigration are possible. A feature unique to the Tatra chamois population is the lack of vertical migrations meaning that the animals spend the whole year in habitats above the tree line (Jamrozy et al. 2007). Within its distribution range, moreover, the Tatra chamois has no serious competition from other ungulates with regard to habitat and food resource use. The red deer Cervus elaphus and roe deer Capreolus capreolus inhabiting the Tatras graze in the open (alpine) areas above the tree line only rarely and/or periodically (Jamrozy et al. 2007). Sheep grazing, once common all over the Tatras, gradually declined following the creation of the National Park, and since the 1980s has been restricted to mid-forest meadows, which lie at lower altitudes, far below the range of occurrence of the Tatra chamois (Mirek 1996).
Material and data sources
The changes in habitat use by Tatra chamois were analyzed on the basis of counts done by the Tatra National Park (TNP) between 1957 and 2013. The counts were carried out in November (exceptionally in early December, if the weather conditions so dictated). The counting methodology was based on the one suggested by Jozef Müller in 1932 (Chudík 1969). The entire area of the Tatras inhabited by chamois was divided into counting areas that included all potential chamois habitats. Originally, the TNP was divided into 17 such areas (14 in the High Tatras and 2 in the Western Tatras). Later, up to 1988, their number was gradually increased to 30, their areas being reduced at the same time. In the history of these counts, there were years when none were undertaken (1979, 1981, and 1997) or the results (record cards), for some unknown reason, not deposited in the TNP archives (1958 and 2000). However, the main assumptions of inventories remain unchanged since their introduction (Chovancová et al. 2006) and the official Tatra chamois population size in the TNP is based on the data acquired from these annual inventories.
Each TNP counting area was patrolled during two days by a team of at least two people, who recorded the numbers of chamois in the groups encountered and the places where they had been observed (Chovancová et al. 2006). The results were recorded on cards, subsequently deposited in the TNP archives. Due to the legal restrictions (high regime of protection) and the conservation needs (low population size), the animals remained unmarked. However, the groups recorded by individual observers in a given counting season were checked by the coordinator in order to prevent the multiplication of records of the same groups moving about and noted by different recorders. Based on the sex, age, number, and direction of movement multiplied records referring to the same group were excluded. During the count period the recorders, the coordinators summarizing the annual counts, and technical details of recording changed. For the purposes of this analysis, therefore, only those records of chamois were used (N = 2,425) that enabled the precise localization of groups (data include location on map, coordinates, or precise description of the location) and that did not refer to the same groups recorded multiple times.
The data on animal observations were digitized to produce a layer with the autumn positions of the chamois and an attribute describing group size. Six habitat parameters were measured for each location where chamois were recorded: altitude above mean sea level (m amsl), percentage of terrain with a southerly aspect (%), slope (degrees), percentage of alpine meadows (%), percentage of habitats with trees and/or shrubs (%), and distance to the nearest hiking trail (m). The spatial analysis was carried out in ArcINFO software from the ArcGIS package (ESRI 2005) using the 20 × 20 cm resolution numerical terrain model (NTM) in the possession of the TNP. On the basis of NTM, the following parameters were established for each chamois group location: altitude (accurate to 1 m), aspect (accurate to 1°), and the slope of the terrain. The habitat variables were established for a circle of radius 100 m around the observation location. This radius was chosen because of the difficulty in defining an observation place as a point, because the chamois would often be on the move as they were being observed (including while they were grazing), and in the case of larger groups because of the scatter of the animals within. To calculate the value of a given variable the weighted mean was used by multiplying the value of a pixel by their number in the circle. The slope layers and aspects were generated using functions available in the expanded Spatial Analyst (ESRI 2005). Aspect was expressed in eight classes (N, NE, E, SE, S, SW, W, and NW). For this analysis a variable, calculated as the summed area of pixels with S, SE, and SW aspects, was used. The assumption was that slopes with a southerly aspect usually have a longer growing season, thus providing the chamois with richer and more attractive grazing in autumn, and also that the autumnal snow cover would be thinner and lie on the ground for a shorter period, not hindering access to food.
A vegetation cover map for the areas lying above the tree line was also used. It was generated by means of an analysis of a satellite image taken by the Ikonos satellite in August 2004, and the areas above the tree line were allocated to 20 habitat classes (Guzik 2008). Variables were used to define the overall proportions of alpine meadows (areas covered by grasses, sedges, herbaceous vegetation) and the overall proportion of shrubby and/or wooded habitats (patches of dwarf pine, single trees, or clumps of trees growing among dwarf pines or in open terrain).
Distance to the nearest hiking trail was defined as the distance between the position of the center of a group and the nearest trail. For this, the hiking trail layer (a total length of 275 km) in the possession of the TNP was used. The network of hiking trails in the Tatras was mainly determined in the period preceding the establishment of the national park. The course of the trails, with minor modifications and periodic exclusion from use of selected trails, remained relatively stable during the entire research period. Information on mean air temperatures, total precipitation, and snow cover thickness in November 1957–2013 was provided by the Institute of Meteorology and Water Management’s high-mountain observatory at the summit of the Kasprowy Wierch mountain (altitude 1,987 m amsl), situated in the central part of the Tatra massif, in the center of the altitudinal zone inhabited by chamois. Overall population size of Tatra chamois was based on the data acquired from annual inventories (Chudík 1969, see above for details) and represent official population estimates provided by national park authorities. It is based on total sum of all individuals recorded during inventory (excluding the groups recorded multiple times). In favorable conditions (rain-free and fog-free weather and cloud-base height above summits) and with a large number of observers densely covering surveyed area this method assumes over 90% of the total population to be counted (Chudík 1969). However, the true detection probability of the method applied and, therefore, its accuracy have not been tested at the time of its implementation and throughout the entire monitoring period (Chovancová et al. 2006). Since, the number of animals in a given year could be underestimated when reported based on counting, these estimates should be considered to represent the minimum population size.
Data analysis
Descriptive statistics (mean ± SD, median with quartiles, and range values) of all six habitat variables — altitude above mean sea level, percentage of terrain with a southerly aspect, slope, percentage of alpine meadows, percentage of habitats with trees and/or shrubs, and distance to the nearest hiking trail — recorded in the places of observation of Tatra chamois were calculated. In the first approach (group level), the habitat parameters for the chamois record localities (N = 2,425) were taken to be dependent variables, and observation year and group size were explanatory variables. Multiple regression analysis was used to evaluate the correlation between year, group size, and each of six habitat variables.
In the second approach (yearly means level), within-year mean values of each habitat variable were calculated (N = 52; excluding 5 years with missing data, see the “Material and data sources” section) and considered to be a new set of dependent variables. This approach was associated with the kind of explanatory variable used in the analysis: their values characterized the whole study area and were not attributed to any particular group. The observation year, the overall size of the chamois population estimated in a given year, and the weather conditions in November (the month when counting took place) in a given year, that is, temperature, precipitation, and thickness of snow cover, were treated as explanatory variables. Multiple regression analysis was used to evaluate the correlation between these explanatory variables and mean values of each of the six habitat variables.
The strength of each predictor relying on effect sizes was estimated using z-transformed Pearson’s product-moment correlation coefficients. To describe the effect sizes of statistically significant explanatory variables, the criteria listed by Cohen (1988) for small (r = 0.1, explaining 1% of the variance), intermediate (r = 0.3, explaining 9% of the variance), or large effect sizes (r = 0.5, explaining 25% of the variance) were adopted. To visualize long-term trends in habitat use, least squares regression lines were fitted to within-year means of each habitat variable with a significant trend. The statistical procedures were performed using Statistica 12.0 software (StatSoft Inc. 2014). All tests were considered significant with P < 0.05.
Results
The mean altitude at which chamois were recorded in the TNP from 1957 to 2013 was 1,899 ± 180.0 m amsl (Table 1). The mean percentage of terrain with a southerly aspect in the areas where the autumnal observations were carried out was 27.5 ± 25.9%, and these localities had a mean slope of 38.3 ± 8.4°. The mean percentage of alpine meadows in these localities was 37.5 ± 31.3%, and the mean percentage of terrain with shrubby and/or woody vegetation was 6.3± 16.1%. The mean distance between the points of observation of groups and hiking trails was 293.1 ± 292.0 m (Table 1).
Table 1.
Descriptive statistics for all habitat variables analyzed at the Tatra chamois Rupicapra rupicapra tatrica observation sites and group size characteristics (TNP; for parameters, see the “Materials and Methods” section; N = 2,425)
| Variable | Mean | SD | Median | Quartile range | Range |
|---|---|---|---|---|---|
| Altitude (m amsl) | 1,899.3 | 180.0 | 1,930.9 | 1,796.9–2,034.3 | 1,294.7–2,332.1 |
| Southerly aspect (%) | 27.5 | 25.9 | 20.9 | 6.7–39.7 | 0.0–100.0 |
| Slope (degrees) | 38.3 | 8.4 | 39.0 | 33.4–44.1 | 12.7–61.2 |
| Meadows (%) | 37.5 | 31.3 | 28.4 | 9.7–64.0 | 0.0–100.0 |
| Trees and shrubs (%) | 6.3 | 16.1 | 0.0 | 0.0–1.3 | 0.0–98.2 |
| Distance to trail (m) | 293.1 | 292.0 | 185.2 | 43.3–485.4 | 0.1–1,001.0 |
| Group size (individuals) | 3.6 | 3.6 | 2.0 | 1–5 | 1–50 |
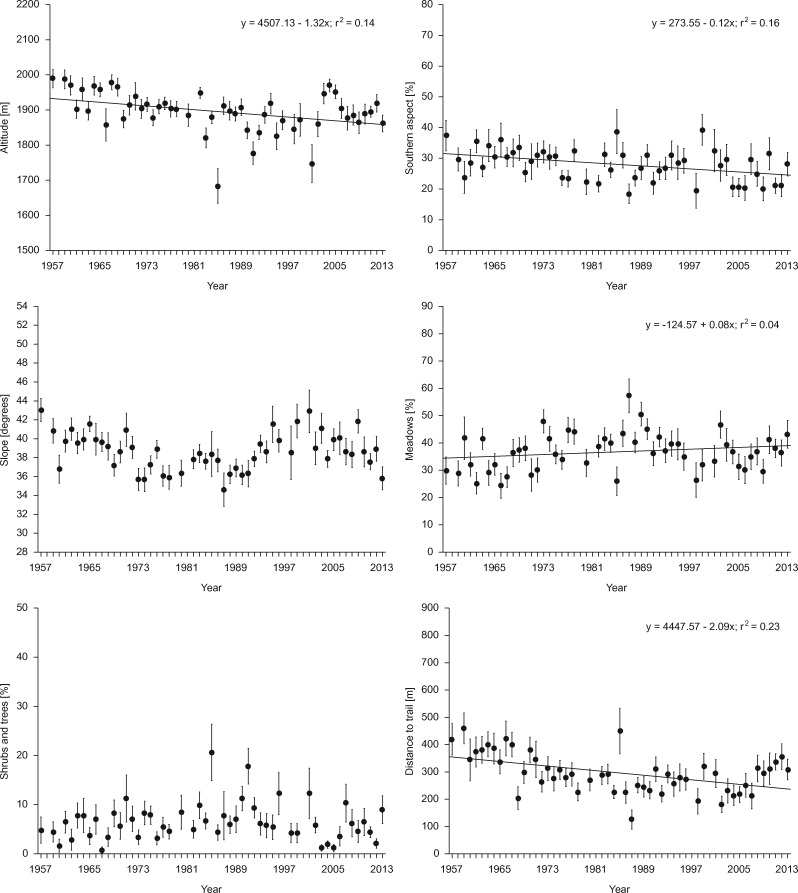
There were significant long-term changes in habitat parameters at the autumn observation sites with respect to the following variables: altitude (F1,2417 = 21.44, P = 0.000), southerly aspect (F1,2417 = 17.85, P = 0.000), percentage of alpine meadows (F1,2417 = 4.48, P = 0.034), and distance to hiking trails (F1,2417 = 22.08, P = 0.000) (Table 2 and Figure 1). Group size was unrelated to any of the habitat parameters except the habitats with shrubby and/or woody vegetation (F1,2417 = 4.18, P = 0.041) — groups tended to be larger in areas with a larger proportion of habitat containing patches of dwarf pine and/or clumps of trees (Table 2). The effect size of statistically significant explanatory variables was small, indicating a high level of variation in habitat use among Tatra chamois groups (Table 2).
Table 2.
The results of multiple regression analysis testing for long-term changes in habitat use by the Tatra chamois R. rupicapra tatrica: correlation between year, group size, and habitat variables recorded at the group sighting location (TNP; for parameters, see the “Materials and Methods” section; N = 2,425)
| Dependent variable | Effect | Estimate | SE | −95% CL | +95% CL | t | P | Effect size |
|---|---|---|---|---|---|---|---|---|
| Altitude | ||||||||
| Intercept | 3,996.45 | 452.76 | 3,108.62 | 4,884.28 | 8.83 | 0.000 | ||
| Year | −1.06 | 0.23 | −1.50 | −0.61 | −4.63 | 0.000 | 0.094 | |
| Group size | 0.08 | 1.00 | −1.89 | 2.05 | 0.08 | 0.936 | 0.002 | |
| Southerly aspect | ||||||||
| Intercept | 305.55 | 65.59 | 176.93 | 434.18 | 4.66 | 0.000 | ||
| Year | −0.14 | 0.03 | −0.20 | −0.07 | −4.22 | 0.000 | 0.087 | |
| Group size | −0.24 | 0.15 | −0.53 | 0.04 | −1.67 | 0.095 | 0.037 | |
| Slope | ||||||||
| Intercept | 63.87 | 21.16 | 22.37 | 105.37 | 3.02 | 0.003 | ||
| Year | −0.01 | 0.01 | −0.03 | 0.01 | −1.19 | 0.234 | 0.026 | |
| Group size | −0.08 | 0.05 | −0.17 | 0.01 | −1.71 | 0.087 | 0.036 | |
| Meadows | ||||||||
| Intercept | −129.70 | 79.29 | −285.18 | 25.77 | −1.64 | 0.102 | ||
| Year | 0.08 | 0.04 | 0.01 | 0.16 | 2.12 | 0.034 | 0.042 | |
| Group size | −0.18 | 0.18 | −0.52 | 0.17 | −1.01 | 0.315 | 0.019 | |
| Trees and shrubs | ||||||||
| Intercept | −2.10 | 40.93 | −82.36 | 78.16 | −0.05 | 0.959 | ||
| Year | 0.00 | 0.02 | −0.04 | 0.04 | 0.19 | 0.851 | 0.005 | |
| Group size | 0.19 | 0.09 | 0.01 | 0.36 | 2.04 | 0.041 | 0.042 | |
| Distance to trail | ||||||||
| Intercept | 3,763.59 | 736.48 | 2,319.39 | 5,207.80 | 5.11 | 0.000 | ||
| Year | −1.74 | 0.37 | −2.47 | −1.02 | −4.70 | 0.000 | 0.097 | |
| Group size | −2.65 | 1.63 | −5.85 | 0.55 | −1.62 | 0.105 | 0.036 | |
Notes: Effect size is the z-transformed Pearson product-moment correlation coefficient. Statistically significant terms (P < 0.05) are shown in bold.
Figure 1.
Long-term changes in habitat use by the Tatra chamois Rupicapra rupicapra tatrica (TNP; for parameters, see the “Materials and Methods” section; N = 2,425); dots and whiskers represent means and standard errors, respectively; and regression lines are shown for variables with a significant trend (P < 0.05; see Table 2).
The long-term declining trend in the mean altitude of chamois observations (F1,46 = 10.48, P = 0.002) was counterbalanced by the weather conditions at the time of observations (F1,46 = 9.63, P = 0.003): higher temperatures in November meant that chamois could be seen at higher altitudes (Table 3). November temperatures were also significantly correlated with the percentage of shrubby and/or woody vegetation (F1,46 = 6.87, P = 0.012): as temperatures fell, the chamois’ use of these habitats rose (Table 3). The chamois population size in the TNP and weather conditions in November were not significantly correlated with the proportion of terrain with a southerly aspect or with distance from hiking trails. This was indicative of the diminishing use of areas with a southerly aspect (F1,46 = 9.09, P = 0.004) and closeness to hiking trails (F1,46 = 14.66, P = 0.000) over the long term (Table 3). Slope (F1,46 = 17.91, P = 0.000) and the proportion of alpine meadows (F1,46 = 4.73, P = 0.035) at the chamois observation sites depended on population size: when the total number of individuals in TNP was larger, chamois were more likely to use areas with larger proportions of alpine meadows and gentler slopes (Table 3). The effect size of the statistically significant explanatory variables was intermediate or large, indicating that they explain a considerable proportion of the variance in inter-annual variation documented (Table 3).
Table 3.
The results of multiple regression analysis testing for long-term changes in habitat use by the Tatra chamois R. rupicapra tatrica: correlation between year, population size, climatic conditions (temperature, precipitation, and snow depth), and within-a-year mean values of habitat variables recorded at the group sighting location (TNP; for parameters, see the “Materials and Methods” section; N = 52)
| Dependent variable | Effect | Estimate | SE | −95% CL | +95% CL | t | P | Effect size |
|---|---|---|---|---|---|---|---|---|
| Altitude (mean) | ||||||||
| Intercept | 4,707.94 | 852.26 | 2,992.44 | 6,423.45 | 5.52 | 0.000 | ||
| Year | −1.39 | 0.43 | −2.26 | −0.53 | −3.24 | 0.002 | 0.395 | |
| Population size | −0.09 | 0.12 | −0.34 | 0.16 | −0.72 | 0.478 | 0.096 | |
| Temperature | 13.64 | 4.39 | 4.79 | 22.48 | 3.10 | 0.003 | 0.404 | |
| Precipitation | 0.05 | 0.19 | −0.34 | 0.43 | 0.25 | 0.803 | 0.126 | |
| Snow depth | 0.06 | 0.37 | −0.68 | 0.79 | 0.16 | 0.873 | 0.161 | |
| Southerly aspect (mean) | ||||||||
| Intercept | 274.92 | 81.84 | 110.18 | 439.65 | 3.36 | 0.002 | ||
| Year | −0.12 | 0.04 | −0.21 | −0.04 | −3.01 | 0.004 | 0.420 | |
| Population size | 0.00 | 0.01 | −0.03 | 0.02 | −0.42 | 0.679 | 0.140 | |
| Temperature | −0.25 | 0.42 | −1.10 | 0.60 | −0.59 | 0.558 | 0.159 | |
| Precipitation | −0.02 | 0.02 | −0.06 | 0.02 | −1.17 | 0.248 | 0.061 | |
| Snow depth | 0.04 | 0.04 | −0.03 | 0.11 | 1.25 | 0.219 | 0.169 | |
| Slope (mean) | ||||||||
| Intercept | 46.25 | 28.90 | −11.92 | 104.42 | 1.60 | 0.116 | ||
| Year | 0.00 | 0.01 | −0.03 | 0.03 | −0.15 | 0.884 | 0.052 | |
| Population size | −0.02 | 0.00 | −0.03 | −0.01 | −4.23 | 0.000 | 0.614 | |
| Temperature | 0.22 | 0.15 | −0.08 | 0.52 | 1.49 | 0.142 | 0.106 | |
| Precipitation | 0.00 | 0.01 | −0.01 | 0.01 | 0.11 | 0.912 | 0.075 | |
| Snow depth | 0.00 | 0.01 | −0.02 | 0.03 | 0.40 | 0.693 | 0.138 | |
| Meadows (mean) | ||||||||
| Intercept | −114.53 | 110.03 | −336.01 | 106.96 | −1.04 | 0.303 | ||
| Year | 0.07 | 0.06 | −0.04 | 0.19 | 1.33 | 0.191 | 0.200 | |
| Population size | 0.03 | 0.02 | 0.00 | 0.07 | 2.17 | 0.035 | 0.387 | |
| Temperature | −0.35 | 0.57 | −1.50 | 0.79 | −0.62 | 0.535 | 0.039 | |
| Precipitation | 0.01 | 0.02 | −0.04 | 0.06 | 0.26 | 0.794 | 0.135 | |
| Snow depth | −0.05 | 0.05 | −0.14 | 0.05 | −0.98 | 0.331 | 0.221 | |
| Trees and shrubs (mean) | ||||||||
| Intercept | −36.77 | 60.72 | −158.99 | 85.45 | −0.61 | 0.548 | ||
| Year | 0.02 | 0.03 | −0.04 | 0.08 | 0.66 | 0.514 | 0.048 | |
| Population size | 0.01 | 0.01 | −0.01 | 0.02 | 0.63 | 0.533 | 0.118 | |
| Temperature | −0.82 | 0.31 | −1.45 | −0.19 | −2.62 | 0.012 | 0.348 | |
| Precipitation | 0.02 | 0.01 | −0.01 | 0.05 | 1.44 | 0.157 | 0.184 | |
| Snow depth | −0.05 | 0.03 | −0.10 | 0.00 | −1.88 | 0.067 | 0.026 | |
| Distance to trail (mean) | ||||||||
| Intercept | 4,557.58 | 1,104.35 | 2,334.65 | 6,780.52 | 4.13 | 0.000 | ||
| Year | −2.13 | 0.56 | −3.25 | −1.01 | −3.83 | 0.000 | 0.527 | |
| Population size | −0.08 | 0.16 | −0.40 | 0.24 | −0.49 | 0.627 | 0.105 | |
| Temperature | 4.28 | 5.69 | −7.18 | 15.74 | 0.75 | 0.456 | 0.078 | |
| Precipitation | −0.13 | 0.25 | −0.63 | 0.37 | −0.52 | 0.604 | 0.046 | |
| Snow depth | 0.21 | 0.47 | −0.74 | 1.16 | 0.44 | 0.659 | 0.025 | |
Notes: Effect size is the z-transformed Pearson product-moment correlation coefficient. Statistically significant terms (P < 0.05) are shown in bold.
Discussion
This analysis of a 57-year-long series of data derived from autumn counts of chamois shows that habitat use by these animals has changed over the long term. The results indicate that chamois tend to be found at lower altitudes than they formerly did, they are seen closer to hiking trails and do not use slopes with a southerly aspect as often as before. These trends are independent of group size, population size, and weather conditions during the observation period (except with respect to altitude, where higher temperatures in November were associated with an increase in the altitude at which chamois were observed).
The greater number of chamois records at lower altitudes is probably due to the gradual abandonment of the large-scale sheep pasturage in the areas where chamois are found (Mirek 1996). Competition with sheep is thought to be the main factor limiting the Tatra chamois’ living space in lower-altitude alpine meadows (Jamrozy et al. 2007). A study in the Alps has shown that chamois avoid places where sheep graze, instead adapting their spatial distribution to the presence of the sheep and the accompanying sheepdogs (Chirichella et al. 2013). This study has also shown that the long-term reduction in the altitude of chamois observation localities was moderated by the temperatures prevailing during the observation period: in warmer years the chamois moved to higher elevations. Such movements in response to weather conditions are typical of animals in Arctic and high-mountain environments: their intolerance of higher temperatures forces them to migrate to cooler areas (Aublet et al. 2009). In the case of the Tatra chamois, this implies movements toward the cooler alpine zone. The influence of high temperatures on chamois migrations to higher (cooler) mountain regions is, however, regarded as relatively insignificant compared with movements that are the consequence of flushing by sheep and shepherds (Mason et al. 2014).
This study demonstrates a long-term decline in the proportion of areas with a southerly aspect where chamois were counted in the autumn. Areas with such an aspect have a milder climate and thus a longer growing season (Toftegaard et al. 2016), which ensures more abundant food resources for a longer period (Seydack et al. 2012). However, the global rise in average temperature (IPCC 1996) is gradually prolonging the growing season (Kullman 2004), as a result of which, areas with a southerly aspect are losing their advantage as grazing places in autumn, since growing seasons on slopes with other aspects are also getting longer.
Habitat use by Tatra chamois may be limited by extensive human pressure (Pęksa and Ciach 2015). At present the TNP is visited by 3 million people every year; as a consequence, the chamois living there have had to adapt to the presence of human beings. The flight distance of the Tatra chamois from humans on hiking trails is less than 100 m (Jamrozy and Pęksa 2004), which is less than that of the Alpine chamois Rupicapra r. rupicapra, for which the flight distance was found to vary from 103 to 180 m (Gander and Ingold 1997). Our long-term data confirm that the distance separating Tatra chamois and hiking trails has systematically fallen over the last half-century, indicating a gradually increasing tolerance of the almost constant presence of large numbers of people on the Tatra hiking trails. Even though the influence of hikers is largely restricted to the hiking trails, it is worth noting that 96% of the TNP lies within 1 km of a hiking trail (Blazejczyk 2002; Skawiński 2010). The intensity of human pressure is exceptionally great around the Kasprowy Wierch mountain. In the peak summer season, the cable car carries 1200–1300 people up to the summit daily, and a further 2000–3000 people arrive there on foot (Pęksa and Ciach 2015). Such crowds of people are extremely stressful for the chamois, leading to reactions measurable at both the physiological (elevated stress hormone levels; Zwijacz-Kozica et al. 2013) and behavioral levels (break-up of large chamois groups into smaller ones as a result of their being flushed; Jamrozy et al. 2007).
This study reveals that slope and the proportion of alpine meadows at the chamois recording locations are correlated with population size: the higher the number of animals, the more often they are observed in habitats with a greater proportion of alpine meadows and on terrain with a gentler slope. Chamois move onto steep slopes in order to minimize attacks by predators, which move far more slowly on steep slopes than on flatter ground (Fox and Krausman 1994). Utilizing steep slopes at times when overall numbers of Tatra chamois are low can thus reduce the effectiveness of predatory attacks. When numbers are high, on the other hand, individual chamois groups are larger, and the animals use open habitats with a gentler slope more often (Hebblewhite and Pletscher 2002): presumably owing to the dilution effect, but also because heightened vigilance lowers the hunting success of the predator (Lima and Dill 1990). The changes in habitat use with increasing population size could also be related to increasing intraspecific competition.
The results of this research indicate that when temperatures are low, chamois are more often found in habitats with larger proportions of trees and/or shrubs. This may be because this taller vegetation offers better shelter from inclement weather, especially strong winds. However, these greater proportions of areas with trees and/or shrubs are also associated with group size: the larger the group, the more frequently it is found in such habitats. The antipredator benefits of group living, including dilution, satiation and confusion effects, vigilance, and selfish herding (Lehtonen and Jaatinen 2016) may explain such relationship. A larger group size improves the chances of a potential threat being detected early, thereby reducing the risk of predation (Pérez-Barbería and Nores 1994). The predators hunting chamois in the Tatras include the wolf Canis lupus, brown bear Ursus arctos, and lynx Lynx lynx (Jamrozy et al. 2007). The latter predator, which is known to be an important natural threat to chamois (Molinari-Jobin et al. 2002), can use scrub for concealment, from which an effective attack can be launched. In such cases, the larger the number of herbivores in a group, the better they are able to monitor their surroundings and the earlier they can perceive a threat (Roberts 1996; Kluever et al. 2008; Beauchamp 2017). This allows them to utilize habitat with a potentially greater risk of predation. Although group size generally increases with habitat openness in large mammalian herbivores (Gerard and Loisel 1995), chamois co-occurring with lynx may adopt the opposite strategy. In open areas they likely have an advantage over their primary predators, since this type of habitats overlap with steep, rocky slopes, which are less suitable for successful hunting than habitats partially covered with dense forest vegetation.
At present, habitat modification and fragmentation are among the main challenges facing animal populations (Fischer and Lindenmayer 2007). With their unique ecological adaptations, high-mountain species are particularly vulnerable to habitat change (Case et al. 2015). The effects of human activities in high-mountain regions, be they local ones like sheep grazing and tourism, or global ones like climate change, can affect how animals use their habitats and bring about long-term changes in their behavior. Although animals can adapt to functioning in dynamically changing habitats, changes in species’ ecology are usually of a long-term nature and therefore hard to predict on the basis of short-term studies. This work, the first documentation of long-term changes in habitat use by ungulates, demonstrates that changes in the ecology of a species can be induced by human activities: the abandonment of pasturage, high-mountain tourism, and climate change, over the time frame of our study, have been the principal drivers of behavioral evolution in the Tatra chamois. The present study also highlights the fact that knowledge about habitat use and preferences gained during short-term studies can only provide a fleeting and incomplete image of a species’ ecology: extrapolating this over time is likely to impose error and will result in increasingly unreliable conclusions as the intensity of ongoing changes increases.
Author contributions
M.C. formulated the idea and analyzed the data. Ł.P. provided the data. M.C. and Ł.P. wrote the manuscript.
Acknowledgments
Autumnal population surveys of Tatra chamois were conducted by employees of the Polish and Slovakian TNPs. This work is dedicated to all the participants of the annual chamois inventory. We thank James Hare, Marco Festa-Bianchet, and two anonymous reviewers for critical and valuable comments on this paper.
Funding
This work was financially supported by the Polish Ministry of Science and Higher Education by statutory funds to M. Ciach.
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
References
- Araújo MS, Bolnick DI, Layman CA, 2011. The ecological causes of individual specialisation. Ecol Lett 14:948–958. [DOI] [PubMed] [Google Scholar]
- Aublet JF, Festa-Bianchet M, Bergero D, Bassano B, 2009. Temperature constraints on foraging behaviour of male Alpine ibex Capra ibex in summer. Oecologia 159:237–247. [DOI] [PubMed] [Google Scholar]
- Beauchamp G, 2017. Disentangling the various mechanisms that account for the decline in vigilance with group size. Behav Process 136:59–63. [DOI] [PubMed] [Google Scholar]
- Beest FM, McLoughlin PD, Mysterud A, Brook RK, 2016. Functional responses in habitat selection are density dependent in a large herbivore. Ecography 39:515–523. [Google Scholar]
- Beest FM, McLoughlin PD, Vander Wal E, Brook RK, 2014. Density-dependent habitat selection and partitioning between two sympatric ungulates. Oecologia 175:1155–1165. [DOI] [PubMed] [Google Scholar]
- Blazejczyk A, 2002. Some problems of tourist activity in the Tatra National Park In: Monitoring and Management of Visitor Flows in Recreational and Protected Areas. Conference Proceedings. Vienna: Institute for Landscape Architecture and Landscape Management, 417–420. [Google Scholar]
- Brambilla P, Bocci A, Ferrari C, Lovari S, 2006. Food patch distribution determines home range size of adult male chamois only in rich habitats. Ethol Ecol Evol 18:185–193. [Google Scholar]
- Brivio F, Bertolucci C, Tettamanti F, Filli F, Apollonio M. et al. , 2016. The weather dictates the rhythms: alpine chamois activity is well adapted to ecological conditions. Behav Ecol Sociobiol 70:1291–1304. [Google Scholar]
- Case MJ, Lawler JJ, Tomasevic JA, 2015. Relative sensitivity to climate change of species in northwestern North America. Biol Conserv 187:127–133. [Google Scholar]
- Chirichella R, Ciuti S, Apollonio M, 2013. Effects of livestock and non-native mouflon on use of high-elevation pastures by Alpine chamois. Mammal Biol 78:344–350. [Google Scholar]
- Chovancová B, Zięba F, Zwijacz-Kozica T, 2006. Polish and Slovakian counting of chamois: assumptions, methods and sources of errors In: Krzan Z, editor. Tatrzański Park Narodowy Na Tle Innych Górskich Terenów Chronionych, Tom II. Zakopane: TPN, 47–51. [Google Scholar]
- Chudík I, 1969. Ursachen der Verluste und der Einfluss der grossen Raubtiere auf die Population des Schalenwildes im Tatra-Nationalpark. Folia Venatoria 4:69–84. [Google Scholar]
- Clobert J, Baguette M, Benton TG, Bullock JM, 2012. Dispersal Ecology and Evolution .Oxford: Oxford University Press. [Google Scholar]
- Cohen J, 1988. Statistical Power Analysis for the Behavioral Sciences. 2nd edn. Hillsdale: Lawrence Erlbaum. [Google Scholar]
- Czajka B, Kaczka RJ, Guzik M, 2012. Zmiany morfometrii szlaków lawinowych w Dolinie Kościeliskiej od utworzenia Tatrzańskiego Parku Narodowego. Prace Wydz Nauk Ziemi Uniw Śląskiego 77:126–135. [Google Scholar]
- ESRI, 2005. ArcGIS. Ver. 9.1. Redlands: ESRI Inc. [Google Scholar]
- Festa-Bianchet M, Douhard M, Gaillard J-M, Pelletier F, 2017. Successes and challenges of long-term field studies of marked ungulates. J Mammal 98:612–620. [Google Scholar]
- Fischer J, Lindenmayer DB, 2007. Landscape modification and habitat fragmentation: a synthesis. Glob Ecol Biogeogr 16:265–280. [Google Scholar]
- Forchhammer MC, Clutton-Brock TH, Lindström J, Albon SD, 2001. Climate and population density induce long-term cohort variation in a northern ungulate. J Anim Ecol 70:721–729. [Google Scholar]
- Fox KB, Krausman PR, 1994. Fawning habitat of desert mule deer. Southwest Nat 39:269–275. [Google Scholar]
- Fynn RW, Chase M, Röder A, 2014. Functional habitat heterogeneity and large herbivore seasonal habitat selection in northern Botswana. South Afr J Wildl Res 44:1–15. [Google Scholar]
- Gander H, Ingold P, 1997. Reactions of male alpine chamois Rupicapra r. rupicapra to hikers, joggers and mountain bikers. Biol Conserv 79:107–109. [Google Scholar]
- Garel M, Cugnasse JM, Maillard D, Gaillard JM, Hewison AJ. et al. , 2007. Selective harvesting and habitat loss produce long-term life history changes in a mouflon population. Ecol Appl 17:1607–18. [DOI] [PubMed] [Google Scholar]
- Gerard J-F, Loisel P, 1995. Spontaneous emergence of a relationship between habitat openness and mean group size and its possible evolutionary consequences in large herbivores. J Theor Biol 176:511–522. [Google Scholar]
- Guzik M, 2008. Analiza wpływu czynników naturalnych i antropogenicznych na kształtowanie się zasięgu lasu i kosodrzewiny w Tatrach. PhD thesis. Kraków: Katedra Botaniki Leśnej i Ochrony Przyrody, Uniwersytet Rolniczy.
- Hebblewhite M, Pletscher DH, 2002. Effects of elk group size on predation by wolves. Can J Zool 80:800–809. [Google Scholar]
- Hess MT, 1996. Klimat In: Mirek Z, editor. Przyroda Tatrzańskiego Parku Narodowego. Kraków-Zakopane: TPN-PAN, 53–68. [Google Scholar]
- IPCC (Intergovernmental Panel on Climate Change), 1996. Climate Change 1995: The Science of Climate Change: Contribution of Working Group I to the Second Assessment Report of the IPCC. New York: Cambridge University Press. [Google Scholar]
- Iversen M, Fauchald P, Langeland K, Ims RA, Yoccoz NG. et al. , 2014. Phenology and cover of plant growth forms predict herbivore habitat selection in a high latitude ecosystem. PLoS One 9:e100780.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamrozy G, Pęksa Ł, 2004. Numbers, distribution and population changes of the Tatra chamois Rupicapra rupicapra tatrica Blahout, 1971. Nat Conserv 60:63–73. [Google Scholar]
- Jamrozy G, Pęksa Ł, Urbanik Z, Gąsienica Byrcyn W, 2007. Kozica Tatrzańska Rupicapra rupicapra tatrica. Zakopane: TPN. [Google Scholar]
- Kawecki TJ, 2008. Adaptation to marginal habitats. Annu Rev Ecol Evol Syst 39:321–342. [Google Scholar]
- Kiszka J, Simon-Bouhet B, Gastebois C, Pusineri C, Ridoux V, 2012. Habitat partitioning and fine scale population structure among insular bottlenose dolphins Tursiops aduncus in a tropical lagoon. J Exp Mar Biol Ecol 416:176–184. [Google Scholar]
- Kluever BM, Breck SW, Howery LD, Krausman PR, Bergman DL, 2008. Vigilance in cattle: the influence of predation, social interactions, and environmental factors. Rangeland Ecol Manag 61:321–328. [Google Scholar]
- Komornicki T, Skiba S, 1985. Mapa gleb In: Trafas K, editor. Atlas Tatrzańskiego Parku Narodowego. Kraków-Zakopane: TPN-PAN. [Google Scholar]
- Krebs CJ, 2009. Ecology: The Experimental Analysis of Distribution and Abundance. 6th edn. San Francisco: Benjamin Cummings. [Google Scholar]
- Kullman L, 2004. Long-term geobotanical observations of climate change impacts in the Scandes of West-Central Sweden. Nord J Bot 24:445–467. [Google Scholar]
- Lehtonen J, Jaatinen K, 2016. Safety in numbers: the dilution effect and other drivers of group life in the face of danger. Behav Ecol Sociobiol 70:449–458. [Google Scholar]
- Lima SL, Dill LM, 1990. Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640. [Google Scholar]
- Lister AM, 2004. The impact of Quaternary Ice Ages on mammalian evolution. Philos Trans R Soc B 359:221–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lone K, Loe LE, Gobakken T, Linnell JD, Odden J. et al. , 2014. Living and dying in a multi-predator landscape of fear: roe deer are squeezed by contrasting pattern of predation risk imposed by lynx and humans. Oikos 123:641–651. [Google Scholar]
- Long RA, Bowyer RT, Porter WP, Mathewson P, Monteith KL. et al. , 2016. Linking habitat selection to fitness-related traits in herbivores: the role of the energy landscape. Oecologia 181:709–720. [DOI] [PubMed] [Google Scholar]
- Lott FD, 1990. Intraspecific Variation in the Social Systems of the Wild Vertebrates. Cambridge: Cambridge University Press. [Google Scholar]
- Macandza VA, Owen-Smith N, Cain JW III, 2012. Habitat and resource partitioning between abundant and relatively rare grazing ungulates. J Zool 287:175–185. [Google Scholar]
- Mason TH, Stephens PA, Apollonio M, Willis SG, 2014. Predicting potential responses to future climate in an alpine ungulate: interspecific interactions exceed climate effects. Glob Change Biol 20:3872–3882. [DOI] [PubMed] [Google Scholar]
- Mirek Z, 1996. Antropogeniczne zagrożenia i przekształcenia środowiska przyrodniczego In: Mirek Z, editor. Przyroda Tatrzańskiego Parku Narodowego. Kraków-Zakopane: TPN-PAN, 595–617. [Google Scholar]
- Molinari-Jobin A, Molinari P, Breitenmoser-Würsten C, Breitenmoser U, 2002. Significance of lynx Lynx lynx predation for roe deer Capreolus capreolus and chamois Rupicapra rupicapra mortality in the Swiss Jura Mountains. Wildl Biol 8:109–115. [Google Scholar]
- Nesti I, Posillico M, Lovari S, 2010. Ranging behaviour and habitat selection of Alpine chamois. Ethol Ecol Evol 22:215–231. [Google Scholar]
- Nicholson MC, Bowyer RT, Kie JG, 1997. Habitat selection and survival of mule deer: tradeoffs associated with migration. J Mammal 78:483–504. [Google Scholar]
- Niedźwiedź T, 2006. Zmienność temperatury powietrza w Tatrach w porównaniu z Karpatami Południowymi i Alpami In: Kotarba A, Borowiec W, editors. Przyroda Tatrzańskiego Parku Narodowego a Człowiek. Zakopane: Tatrzański Park Narodowy, 9–17. [Google Scholar]
- Ofstad EG, Herfindal I, Solberg EJ, Sæther B-E, 2016. Home ranges, habitat and body mass: simple correlates of home range size in ungulates. Proc R Soc B 283:20161234.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pęksa Ł, Ciach M, 2015. Negative effects of mass tourism on high mountain fauna: the case of the Tatra chamois Rupicapra rupicapra tatrica. Oryx 49:500–505. [Google Scholar]
- Pérez-Barbería FJ, Nores C, 1994. Seasonal variation in group size of Cantabrian chamois in relation to escape terrain and food. Acta Theriol 39:295–305. [Google Scholar]
- Richard E, Gaillard JM, Saïd S, Hamann JL, Klein F, 2010. High red deer density depresses body mass of roe deer fawns. Oecologia 163:91–97. [DOI] [PubMed] [Google Scholar]
- Roberts G, 1996. Why individual vigilance declines as group size increases. Anim Behav 51:1077–1086. [Google Scholar]
- Robertson A, McDonald RA, Delahay RJ, Kelly SD, Bearhop S, 2015. Resource availability affects individual niche variation and its consequences in group-living European badgers Meles meles. Oecologia 178:31–43. [DOI] [PubMed] [Google Scholar]
- Rolandsen CM, Solberg EJ, Sæther B-E, Moorter BV, Herfindal I. et al. , 2017. On fitness and partial migration in a large herbivore: migratory moose have higher reproductive performance than residents. Oikos 126:547–555. [Google Scholar]
- Schradin C, Hayes LD, 2017. A synopsis of long-term field studies of mammals: achievements, future directions, and some advice. J Mammal 98:670–677. [Google Scholar]
- Sergio F, Pedrini P, Marchesi L, 2003. Spatio-temporal shifts in gradients of habitat quality for an opportunistic avian predator. Ecography 26:243–255. [Google Scholar]
- Seydack AH, Grant CC, Smit IP, Vermeulen WJ, Baard J. et al. , 2012. Large herbivore population performance and climate in a South African semi-arid savanna. Koedoe 54:1–20. [Google Scholar]
- Shackleton DM, Bunnell FL, 1987. Natural factors affecting productivity of mountain ungulates: a risky existence? In: Lovari S, editor. Reintroduction of predators in protected areas, proceedings of the workshop on the reintroduction of predators in protected areas. Regione Piemonte, Torino, Italy, 46–57. [Google Scholar]
- Shenbrot G, Krasnov B, Burdelov S, 2010. Long-term study of population dynamics and habitat selection of rodents in the Negev Desert. J Mammal 91:776–786. [Google Scholar]
- Skawiński P, 2010. Zarządzanie ruchem turystycznym w Tatrzańskim Parku Narodowym. Folia Turistica 22:25–34. [Google Scholar]
- StatSoft Inc., 2014. Statistica. Version 12. Tulsa, Oklahoma.
- Thompson ID, Wiebe PA, Mallon E, Rodgers AR, Fryxell JM. et al. , 2014. Factors influencing the seasonal diet selection by woodland caribou Rangifer tarandus tarandus in boreal forests in Ontario. Can J Zool 93:87–98. [Google Scholar]
- Toftegaard T, Posledovich D, Navarro-Cano JA, Wiklund C, Gotthard K. et al. , 2016. Variation in plant thermal reaction norms along a latitudinal gradient: more than adaptation to season length. Oikos 125:622–628. [Google Scholar]
- Uboni A, Smith DW, Mao JS, Stahler DR, Vucetich JA, 2015. Long- and short-term temporal variability in habitat selection of a top predator. Ecosphere 6:1–16. [Google Scholar]
- Zwijacz-Kozica T, Selva N, Barja I, Silván G, Martínez-Fernández L. et al. , 2013. Concentration of fecal cortisol metabolites in chamois in relation to tourists pressure in Tatra National Park (South Poland). Acta Theriol 58:215–222. [Google Scholar]