Abstract
Many animal species migrate over long distances, but the physiological challenges of migration are poorly understood. It has recently been suggested that increased molecular oxidative damage might be one important challenge for migratory animals. We tested the hypothesis that autumn migration imposes an oxidative challenge to bats by comparing values of 4 blood-based markers of oxidative status (oxidative damage and both enzymatic and nonenzymatic antioxidants) between Nathusius’ bats Pipistrellus nathusii that were caught during migration flights with those measured in conspecifics after resting for 18 or 24 h. Experiments were carried out at Pape Ornithological Station in Pape (Latvia) in 2016 and 2017. Our results show that flying bats have a blood oxidative status different from that of resting bats due to higher oxidative damage and different expression of both nonenzymatic and enzymatic antioxidants (glutathione peroxidase). The differences in oxidative status markers varied between sampling years and were independent from individual body condition or sex. Our work provides evidence that migratory flight might impose acute oxidative stress to bats and that resting helps animals to recover from oxidative damage accrued en route. Our data suggest that migrating bats and birds might share similar strategies of mitigating and recovering from oxidative stress.
Keywords: antioxidants, mammals, oxidative damage, stopover, stress
Migration is a fascinating behavior regularly performed by a wide range of animal species varying largely in taxon (from insects to mammals), body size and in the habitats they use (Dingle 2014). Seasonally changing resources are the main reason for almost all of the known animal migrations. Fluctuations in resource abundance force animals to move from areas with insufficient supplies of critical resources to more favorable areas. Owing to these ecological constraints, billions of animals are driven to move between a summer area, in which they breed, to a wintering habitat and back, some spanning thousands of miles (Dingle 2014). Many terrestrial bat and bird migrants make use of powered, soaring and gliding flight to cover long distances. Indeed, flapping flight involves very high metabolic rates during migration, which needs to be powered by appropriate oxidative fuels when animals are en route (McWilliams et al. 2004; McGuire and Guglielmo 2009; Voigt et al. 2012a; Avgar et al. 2014).
While migrating, animals face a number of endogenous challenges for homeostasis, which may force animals to solve trade-off situations. For example, the energy budget of a journey has to be spent optimally by either uptake and storage of sufficient nutrients before migration or by refuelling while en route. Negative energy balances will eventually lead to nutrient sparing and catabolism or inflammation (McWilliams et al. 2004; McGuire and Guglielmo 2009; Avgar et al. 2014). In recent years, it has been proposed that regulation of oxidative balance might be one additional key challenge for migrating animals (Costantini et al. 2007; Jenni-Eiermann et al. 2014; Skrip et al. 2015; Birnie-Gauvin et al. 2017; Eikenaar et al. 2017). For example, intense and prolonged physical activity may lead to an increased production of pro-oxidant chemicals (e.g., free radicals) in skeletal muscle and blood (Sureda et al. 2009; Nikolaidis et al. 2012). Intense physical activity may also cause inflammation and activation of immune cells (Matson et al. 2012) that release pro-oxidants into the bloodstream (Sorci and Faivre 2009). Pro-oxidants such as reactive oxygen species interact with critical signaling molecules to stimulate a broad variety of cellular processes (Halliwell and Gutteridge 2015). However, they are also prone to attack proteins, lipids, and nucleic acids, causing chemical modifications referred to as oxidative damage (Costantini 2014; Halliwell and Gutteridge 2015). Organisms may control the activity of pro-oxidants relying on a large variety of antioxidant mechanisms and molecules (Costantini 2014; Halliwell and Gutteridge 2015).
Thus far, studies on the link between migration and oxidative stress were restricted to birds and fishes. For example, Costantini et al. (2007) found in two passerine species caught at a stopover site that plasma nonenzymatic antioxidants were higher in individuals in better body condition. Jenni-Eiermann et al. (2014) found that blood oxidative damage and activity of glutathione peroxidase (antioxidant enzyme) were significantly higher in European robins Erithacus rubecula caught during their nocturnal flight than in conspecifics caught during the day while resting. Similarly, Skrip et al. (2015) found that birds at stopover sites were capable of recovering from the oxidative damage they have accrued during migration, since plasma oxidation levels decreased with the length of the stopover stay. In contrast, while energy expenditure increased in flying Northern bald ibis Geronticus eremite compared with that measured before flying, two markers of oxidative status were not affected by flight effort (Bairlein et al. 2015). Finally, Birnie-Gauvin et al. (2017) and Eikenaar et al. (2017) found that, as compared with conspecific resident individuals, migrating individuals had upregulated antioxidant defences in brown trout (Salmo trutta) and blackbird Turdus merula, respectively.
Bats are another group capable of long-distance migration, but, compared with birds, they have received less attention in terms of physiological adaptations to migratory flight and trade-offs involved (McGuire and Guglielmo 2009; Popa-Lisseanu and Voigt 2009). Although balancing time and energy budgets is important for any migrating animal, bats and birds have disparate adaptations with respect to their physiology during migration. For example, conversely to birds that derive much energy from fatty acids accumulated in adipose tissue, bats also fuel their long migrations using nutrients derived from insects eaten while on migratory transit flights (Voigt et al. 2012a). Moreover, most birds are incapable of doing extended periods of torpor during the migration (Fleming and Eby 2003; McGuire et al. 2014). Yet, migratory birds seem to have a better aerodynamic efficiency than bats (Muijres et al. 2012).
In this study, we compared values of 4 blood-based markers of oxidative status measured in bats that were caught during the phase of high flight activity with those measured in bats after resting. We carried out the experiments using Nathusius’ bats Pipistrellus nathusii, which migrate seasonally from their north-eastern breeding range to the southwest of Europe where they hibernate, covering up to approximately 2,000 km twice during the year (Pētersons 2004). Conversely to birds, migration and breeding are not temporally isolated in bats during the spring migration because it coincides with pregnancy. Thus, we carried out the study during the autumn migration (i.e., after the reproductive season) to isolate migration from potential effects of pregnancy on stress physiology.
Materials and Methods
Study area and sampling
Experiments were carried out at Pape Bird Ringing Station in Pape, Latvia, (568090 N 218030 E) between 20 and 22 August 2016 and between 26 and 27 August 2017 under the licenses Nr. 31/2016-E from 06.07.2016 and Nr. 33/2017-E from 19.07.2017 issued by the Latvian Nature Conservation agency and under the licence Nr. 92/2017 issued by Latvian Food and Veterinary service. All methods were carried out in accordance with the relevant guidelines and regulations. The study period included the peak migration season for P. nathusii in Latvia (Pētersons 2004). Meteorological conditions were similar in the two sampling years (Table 1). Migratory bats were captured between 2200 and 0200 h using a Helgoland funnel trap. The Helgoland trap was manned continuously for trapping bats. Once a bat was caught, it was bled as soon as possible at the site of capture. The time elapsed from capture to bleeding was below 5 min. In 2016 and 2017, we collected blood samples from 31 (11 males and 20 females) and 12 (5 males and 7 females) bats, respectively, right after bats were captured during migratory transit flights. We also collected blood from other 10 (4 males and 6 females) and 19 (6 males and 13 females) bats in 2016 and 2017, respectively, after having rested for either 18 or 24 h in cages in groups of maximum 4 individuals with food (mealworms) and water provided ad libitum. Wooden cages in which bats were kept temporarily simulated natural roosts in tree hollows. The dimensions were 28 × 16 × 13 cm (l × w × h). Holes in the front door enabled air circulation. Bats in cages were housed in a wooden hut at PBRS, which is specifically used for keeping bats in dark, temperature-stable, and quiet conditions. Bats kept for a day stopover go into torpor during the day subsequent of the night of capture. Bats were never caught in rainy days. The blood was collected using heparinized capillaries after puncturing the vein in the tail membrane (previously sterilized) with a sterile needle (Sterican® Gr. 1, G 20 × 1 1/2”/ø 0.90 × 40 mm). Then the blood was transferred into an Eppendorf tube, which was centrifuged straightaway to separate plasma from blood cells. Both samples of plasma and blood cells were stored in a dryshipper under liquid nitrogen straightaway. The body mass of bats kept in cages was recorded with an electronic balance (accuracy 0.01 g; Kern CM 150-1N, Germany) before feeding. All individuals used in both years of the study were identified as adults, that is, they were at least one year old according to the closure of the epiphyseal gap.
Table 1.
Meteorological conditions according to the global forecast system
| Sampling day | Air temperature (°C) | Wind speed (km/h) | Humidity (%) |
|---|---|---|---|
| 20-08-2016 | 18.3 | 15 | 63 |
| 21-08-2016 | 17.0 | 5 | 97 |
| 22-08-2016 | 15.9 | 9 | 92 |
| 26-08-2017 | 14.5 | 9 | 86 |
| 27-08-2017 | 14.0 | 6 | 91 |
Note: Avaialble at http://www.emc.ncep.noaa.gov/via earth nullschool model at 23: 00.
Molecular analyses
We measured one marker of plasma oxidative damage, one marker of plasma nonenzymatic antioxidant capacity and two markers of red blood cell antioxidant enzymes using standard methods for vertebrates (for more technical details see e.g., Costantini et al. 2013; Schneeberger et al. 2013). Briefly, the d-ROMs assay (Diacron International, Grosseto, Italy) was used to measure the reactive oxygen metabolites in 4 µl of plasma, which include primary oxidative damage products (e.g., organic hydroperoxides, endoperoxides). Values were expressed as mM H2O2 equivalents. The OXY-Adsorbent test (Diacron International) was used to quantify the ability of nonenzymatic antioxidant compounds (e.g., vitamins, thiols) present in the plasma (diluted 1: 100 with distilled water) to cope with the in vitro oxidant action of hypochlorous acid (HOCl, an endogenously produced oxidant). Values were expressed as mM of HOCl neutralized. The Ransel assay (RANDOX Laboratories, Crumlin, UK) was used to measure the activity of the enzyme glutathione peroxidase (gpx) in erythrocytes. The activity was expressed as units of gpx per mg of proteins. The Ransod assay (RANDOX Laboratories, Crumlin, UK) was used to measure the activity of the enzyme superoxide dismutase (sod) in erythrocytes. The activity was expressed as units of sod per mg of proteins. Quality controls with known concentrations of the markers were included in all assays performed. The Bradford protein assay (Bio-Rad Laboratories, Hercules, USA) with bovine albumin as a reference standard was used to quantify the concentration of proteins in samples. All analyses were carried out within a few months from collection. The between year coefficient of variation of quality controls included in all assays was below 10%, indicating that the differences of the oxidative status marker values between sampling years were not due to assay performance.
Statistics
Generalized linear models were used to assess relationships between each oxidative status marker and the predictor variables experimental group (flying vs. resting) and sampling year (2016 vs. 2017), as well as their interaction. A normal error function and an identity-link function were applied to models of nonenzymatic antioxidant capacity and gpx. A gamma error function and an identity-link function were applied to models of reactive oxygen metabolites and sod. Generalized linear models were also used to assess relationships between a body condition index (body mass as dependent variable and forearm length as covariate; e.g., García-Berthou 2001; Pearce et al. 2008; Reynolds et al. 2009; Jonasson and Willis 2011) and the predictor variables experimental group and sampling year, as well as their interaction. A normal error function and an identity-link function were applied. The Tukey test was used for post-hoc comparisons when the interaction was significant. Preliminary analyses did not reveal any significant differences between males and females between experimental groups and years (experimental group × sex × sampling year, all P values ≥ 0.36) for each metric analyzed. Thus sex was not retained in the analyses in order to minimize the number of variables that went into the full models. Outcomes were similar when sex was retained in full models. Data on oxidative status markers for both males and females are reported in Table 2. Finally, the Pearson correlation was used to test whether there was a correlation between the time blood was sampled and each oxidative status marker in those bats blood-sampled straightaway from their capture (i.e., flying bats). For the analysis of body condition index in flying bats, we used partial correlation with body mass as dependent variable and both time of blood sampling and forearm length (index of body size) as independent variables (García-Berthou 2001). Analyses were run pooling data from 2016 and 2017, as well as for each year, separately. We also used partial correlation to assess whether any of the oxidative status markers was correlated with body condition index (body mass and forearm length both included as independent variables) in flying birds. All analyses were performed using the software STATISTICA 10 (StatSoft. Inc., Tulsa, OK, USA).
Table 2.
Descriptive statistics (mean ± standard error) of markers of oxidative status in both males and females
| Study year | Sex | ROMs | OXY | GPX | SOD |
|---|---|---|---|---|---|
| 2016 | Male | 0.26 ± 0.08 (14) | 195 ± 11 (15) | 0.48 ± 0.03 (15) | 0.62 ± 0.08 (15) |
| 2016 | Female | 0.34 ± 0.06 (24) | 207 ± 8 (25) | 0.48 ± 0.03 (26) | 0.66 ± 0.06 (26) |
| 2017 | Male | 1.56 ± 0.09 (11) | 268 ± 13 (11) | 0.23 ± 0.04 (11) | 1.85 ± 0.09 (11) |
| 2017 | Female | 1.42 ± 0.06 (20) | 277 ± 9 (20) | 0.26 ± 0.03 (20) | 1.58 ± 0.07 (20) |
Notes: ROMs, reactive oxygen metabolites (mM H2O2 equivalents); OXY, nonenzymatic antioxidant capacity (mM HOCl neutralised); GPX, glutathione peroxidase (units/mg proteins); SOD, superoxide dismutase (units/mg proteins). Sample sizes are reported between brackets.
Finally, the compute.es package (Del Re 2013) in R (R Core Team 2013) was used to calculate the standardized effect size Hedges’ g from test statistics of oxidative status markers. The forestplots function of the metafor package in R was used to visualize values of effect size and 95% confidence interval. Effect sizes were considered to be small (Hedges g = 0.2, explaining 1% of the variance), intermediate (g = 0.5, explaining 9% of the variance), or large (g = 0.8, explaining 25% of the variance) according to Cohen (1988).
Results
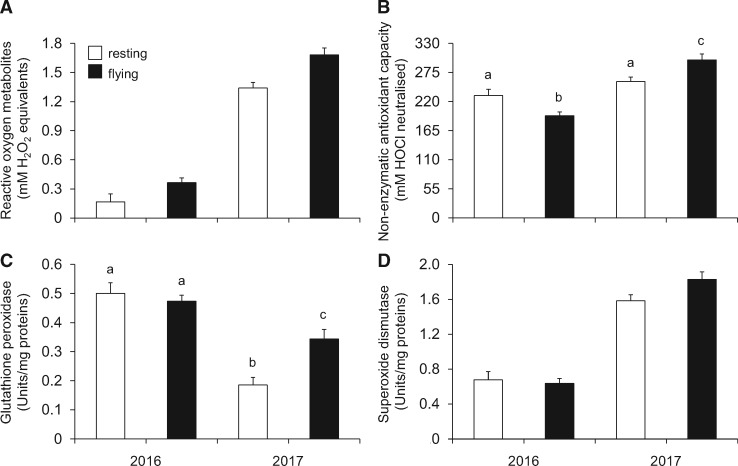
In both study years, flying bats had significantly higher reactive oxygen metabolites than resting bats (Table 3, Figure 1A). Reactive oxygen metabolites were also significantly higher in 2017 than in 2016 (Table 3, Figure 1A). Resting bats had significantly lower plasma nonenzymatic antioxidant capacity than flying bats in 2016, while they had significantly higher plasma nonenzymatic antioxidant capacity than flying bats in 2017 (Table 3, Figure 1B). Glutathione peroxidase did not differ between flying and resting bats in 2016, while it was significantly higher in flying than resting bats in 2017 (Table 3, Figure 1C). Superoxide dismutase was significantly higher in 2017 than 2016 and it did not differ between flying and resting bats in both sampling years (Table 3, Figure 1D). Body condition index did not differ between flying and resting bats in both sampling years (Table 3; see also Table 4 for values of body mass, forearm length and body condition index).
Table 3.
Outcomes of generalized linear models used to detect the significant predictors of blood oxidative status markers and of body condition index
| Variable | Factor | Wald | P |
|---|---|---|---|
| Reactive oxygen metabolites | Experimental group | 4.73 | 0.030 |
| Sampling year | 101.36 | <0.001 | |
| Experimental group × sampling year | 0.34 | 0.559 | |
| Nonenzymatic antioxidant capacity | Experimental group | 0.02 | 0.876 |
| Sampling year | 47.24 | <0.001 | |
| Experimental group × sampling year | 17.00 | <0.001 | |
| Glutathione peroxidase | Experimental group | 5.15 | 0.023 |
| Sampling year | 59.01 | <0.001 | |
| Experimental group × sampling year | 10.13 | 0.002 | |
| Superoxide dismutase | Experimental group | 0.89 | 0.346 |
| Sampling year | 91.69 | <0.001 | |
| Experimental group × sampling year | 1.69 | 0.194 | |
| Body mass | Experimental group | 1.73 | 0.189 |
| Sampling year | 2.52 | 0.112 | |
| Forearm length | 16.79 | <0.001 | |
| Experimental group × sampling year | 0.23 | 0.632 |
Notes: Significant P-values are shown in bold. The removal of the interaction when nonsignificant did not change the outcomes.
Figure 1.
Differences of plasma reactive oxygen metabolites (A), plasma nonenzymatic antioxidant capacity (B), erythrocyte glutathione peroxidase (C) and erythrocyte superoxide dismutase (D) between flying and resting bats in the sampling years 2016 and 2017. Resting duration was 18 and 24 h in 2016 and 2017, respectively. Means that are not sharing a same superscript (i.e., letters a, b or c) are significantly different from each other (Tukey test, P < 0.05). Note that post-hoc tests were performed only for nonenzymatic antioxidant capacity and glutathione peroxidase because their respective models detected a significant interaction between experimental group and sampling year. Values are shown as least square means ± standard error.
Table 4.
Descriptive statistics of body mass (grams), forearm length (mm), and body condition index
| Experimental group | Study year | Body mass | Forearm length | Body condition |
|---|---|---|---|---|
| Resting | 2016 | 7.77 ± 0.17 | 34.65 ± 0.31 | 7.67 ± 0.19 |
| Flying | 2016 | 7.97 ± 0.12 | 34.41 ± 0.18 | 7.94 ± 0.11 |
| Resting | 2017 | 7.99 ± 0.16 | 34.35 ± 0.22 | 7.98 ± 0.14 |
| Flying | 2017 | 7.93 ± 0.19 | 33.70 ± 0.28 | 8.11 ± 0.18 |
Notes: Values of body mass and forearm length are reported as means and standard error. Values of body condition index are reported as least square means and standard error generated by generalized linear models (i.e., body mass standardized by the covariate forearm length).
There was no significant correlation between time of blood sampling and values of all oxidative status markers analyzed or body condition index in flying bats (r-values ranging from −0.53 to 0.46, P ≥ 0.08). There was also no significant correlation between each oxidative status marker and body condition index (r-values ranging from −0.19 to 0.20, P ≥ 0.22), There was also no difference in blood sampling time (t-test, P = 0.82; Levene test, P = 0.73) between 2016 (average and range: 00: 03, 22: 00-02: 00) and 2017 (average and range: 23: 58, 22: 20-01: 22).
Estimates of effect size for reactive oxygen metabolites, nonenzymatic antioxidants and gpx measured in 2017 were large and their 95% confidence intervals did not include zero (Figure 2). Estimates of effect size for gpx in 2016 and for sod were small and their 95% confidence intervals included zero (Figure 2).
Figure 2.
Estimates of effect size and 95% confidence interval calculated from all test statistics. Estimates are positive when values of a given marker are higher in flying than resting bats.
Discussion
Our work provides strong evidence that performing migratory flights may result in increased blood oxidative stress and that resting (e.g., during a stopover) may help the animal to detoxify cells from damage accumulated while en route or to reduce generation of oxidative damage. Effect size estimates of significant differences between resting and flying animals were large (Hedges’ g ≥ 0.8), thus explaining more than 25% of the variance (Cohen 1988). It has been suggested that intermediate effect sizes are biologically meaningful because average proportions of variance explained in ecological, evolutionary, and physiological studies is usually below 7% (Møller and Jennions 2002).
In both study years, bats that were kept resting had lower plasma reactive oxygen metabolites (early derivatives of oxidative damage) than flying bats irrespective of whether they rested for either 18 (year 2016) or 24 h (year 2017). Our results suggest that bats share with birds the capability of recovering from oxidative damage accrued during migratory flight by performing short stopover periods (Jenni-Eiermann et al. 2014; Skrip et al. 2015). It is very likely, although not yet supported by empirical data, that Nathusius’ bats go into torpor on a daily basis during migration, similar to North American migratory bats, which would enable bats to save energy (McGuire et al. 2014) and, possibly, to recover from oxidative stress. We do not know to what extent such reduction is due to either activation of detoxification pathways or reduction in generation of metabolites. The lower glutathione peroxidase activity that we observed in resting bats in 2017 compared with flying bats suggests that this enzyme, which detoxifies cells from peroxides and organic hydroperoxides (Halliwell and Gutteridge 2015), might have been downregulated. Similarly, prior work on migrating birds found a decreased glutathione peroxidase activity in resting birds (Jenni-Eiermann et al. 2014). This result suggests that migrating bats and birds might not need to keep expression of certain enzymes up during the whole duration of migration, rather they upregulate certain enzymes on demand, such as during a strenuous flight. Conversely, the nonenzymatic antioxidant component of plasma showed different patterns between study years. In 2016, it was lower in flying bats, while in 2017 it was lower in resting bats. Previous work on birds did not find a link between resting at a stopover site and plasma nonenzymatic antioxidant capacity (Skrip et al. 2015). An important component of plasmatic nonenzymatic antioxidants comes from diet or from dietary antioxidants accumulated in key body tissues like fat (e.g., Metzger and Bairlein 2011). It might be that the amount or type (e.g., insects richer or poorer in antioxidant content) of prey while en route differed between the two study years or mobilization of stored antioxidants into the bloodstream was stronger in 2017 because of a higher demand. Also, varying meteorological factors bats were facing before passing our field site, that is direction of wind and wind speed, could have created differences in the amount of physical stress to the individuals in both years. The reasons and mechanisms underlying these results remain unanswered.
Irrespective of the mechanism, a reduction of oxygen metabolites might bring the organism some benefits. First, reactive oxygen metabolites may be broken down into several very reactive free radicals that can propagate further the oxidative damage to fundamental biomolecules like lipids, DNA or proteins (Lajtha et al. 2009; Halliwell and Gutteridge 2015). Second, some reactive oxygen metabolites (lipid hydroperoxides) are precursors of end products of lipid peroxidation, such as malondialdehyde, hydroxynonenal, and isoprostanes, which can be toxic at high concentrations and may cause damage to proteins (e.g., carbonylation) (Lajtha et al. 2009; Halliwell and Gutteridge 2015). Previous work found that high plasma reactive oxygen metabolites may be associated with shorter telomeres (Geiger et al. 2012; Hau et al. 2015) or reduced survival (Costantini and Dell’Omo 2015; Herborn et al. 2016). Thus, bats might also need to optimize their migratory effort to avoid any potential detrimental carry-over effects of accumulated oxidative damage. In this context, it will be important to measure additional markers of oxidative damage (e.g., to DNA) to assess whether migrating animals recover from any kind of damage while resting.
Our study also found significant differences in markers between the two study years. In 2016, bats had lower damage, nonenzymatic antioxidants and activity of superoxide dismutase and higher activity of glutathione peroxidase than bats in 2017, respectively. The lower amount of reactive oxygen metabolites might be explained to some extent by the higher activity of glutathione peroxidase, an enzyme that detoxifies cells from peroxides and organic hydroperoxides. The lower nonenzymatic antioxidants and activity of superoxide dismutase (which detoxifies cells from the histolesive superoxide free radical) might indicate a lower need of keeping antioxidant defenses high compared with 2017. Overall these results might suggest that (1) animals caught in the two years represented different cohorts of the population or (2) the migratory flight was less demanding (in terms of time spent or energy needed to conduct it), and thus the production of free radicals lower, in 2016. The first explanation does not appear plausible because bats caught in the two years were similar in terms of body traits (Table 4). Results of body condition index also do not appear to support the first explanation because body condition would be expected to decline with migratory effort because of a higher consumption of body energy reserves, such as fat. However, bats can use a mixed strategy to fuel flight, relying on both recently ingested nutrients and endogenous fat stores (Welch et al. 2008; Voigt et al. 2010, 2012a).
Finally, our work did not detect any significant differences between males and females. In Nathusius’ bat, males migrate slightly later than females at our study site (Pētersons 2004). Thus it might be that the cohorts of males and females included in this study were not representative of the overall intra-sexual variation whether oxidative status or individual quality are linked to the migration phenology. However, sexual differences in oxidative status markers are usually low in mammals (Costantini 2018). Sexual differences in oxidative status might emerge stronger during the spring migration because females engage into migration while being pregnant. Thus females would be exposed to stringent trade-offs in resource allocation during that time.
In conclusion, we found the first evidence that increased oxidative stress might be a consequence of migratory flight in a bat species. We also observed a role of resting as a way for recovering from oxidative stress. These results also indicate that any potential stress due to restraint in a cage was negligible given the lower values of oxidative damage in resting than flying bats. Future work will be needed to clarify whether migratory flight imposes an oxidative challenge comparable or different to that of other important activities like foraging flight or reproduction. It will also be important to assess whether, as previously suggested for birds (Skrip et al. 2015; Eikenaar et al. 2017), bats may employ the physiological strategy of building prophylactic antioxidant capacity or upregulating key antioxidant or repair enzymes before starting a migratory flight. Given the capacity of bats of recovering from oxidative stress while resting (i.e., a one-day stopover), one important avenue for research will be understanding whether bats adjust torpor duration at stopover sites and/or length of stopover to the oxidative damage accrued during the migratory flight. Finally, Nathusius’ bats suffer significant mortality during migration because of the collision with wind turbines (Voigt et al. 2012b, 2016). Migratory stress might be a concurring factor in determining increased probability of mortality or reduced reproductive fitness, thus further jeopardizing the conservation status of this bat species.
Acknowledgments
We thank Donāts Spalis, Oskars Keišs and the Pape ornithology team for their help with field installations. Ilze Brila and Viesturs Vintulis supported trapping of animals. We thank two anonymous reviewers for their valuable comments that helped us to improve the presentation of the results.
Funding
D.C. was supported by the Alexander-von-Humboldt-Foundation; O.L. was supported by the Berlin Funding for Graduates (Elsa-Neumann-Stipendium).
Conflicts of Interest
None declared.
References
- Avgar T, Street G, Fryxell JM, 2014. On the adaptive benefits of mammal migration. Can J Zool 92: 481–490. [Google Scholar]
- Bairlein F, Fritz J, Scope A, Schwendenwein I, Stanclova G. et al. 2015. Energy expenditure and metabolic changes of free-flying migrating northern bald ibis. PLoS ONE 10: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnie-Gauvin K, Peiman KS, Larsen MH, Baktoft H, Aarestrup K. et al. 2017. Oxidative stress and partial migration in a Salmonid fish. Can J Zool 95: 829–835. [Google Scholar]
- Cohen J, 1988. Power Analysis for the Behavioural Sciences. Hillsdale: Erlbaum. [Google Scholar]
- Costantini D, 2014. Oxidative Stress and Hormesis in Evolutionary Ecology and Physiology: A Marriage between Mechanistic and Evolutionary Approaches. Berlin, Heidelberg: Springer; 348. [Google Scholar]
- Costantini D, 2018. Meta-analysis reveals that reproductive strategies are associated with sexual differences in oxidative balance across vertebrates. Curr Zool 64: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini D, Cardinale M, Carere C, 2007. Oxidative damage and anti-oxidant capacity in two migratory bird species at a stop-over site. Comp Biochem Physiol Part C 144: 363–371. [DOI] [PubMed] [Google Scholar]
- Costantini D, Dell’Omo G, 2015. Oxidative stress predicts long-term resight probability and reproductive success in the Scopoli’s shearwater Calonectris diomedea. Conserv Physiol 3: cov024.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini D, Monaghan P, Metcalfe N, 2013. Loss of integration is associated with reduced resistance to oxidative stress. J Exp Biol 216: 2213–2220. [DOI] [PubMed] [Google Scholar]
- Del Re AC, 2013. Compute.es: compute effect sizes. R package version 0.2–2. Available from: http://cran.r–project.org/web/packages/compute.es.
- Dingle H, 2014. Migration: The Biology of Life on the Move. USA: Oxford University Press. [Google Scholar]
- Eikenaar C, Källstig E, Andersson MN, Herrera-Dueñas A, Isaksson C, 2017. Oxidative challenges of avian migration: a comparative field study on a partial migrant. Physiol Biochem Zool 90: 223–229. [DOI] [PubMed] [Google Scholar]
- Fleming TH, Eby P, 2003. Ecology of bat migration In: Kunz TH, Fenton MB, editors. Bat Ecology. Chicago: University of Chicago Press; 156–208. [Google Scholar]
- García-Berthou E, 2001. On the misuse of residuals in ecology: testing regression residuals vs. the analysis of covariance. J Anim Ecol 70: 708–711. [Google Scholar]
- Geiger S, Le Vaillant M, Lebard T, Reichert S, Stier A. et al. 2012. Catching-up but telomere loss: half-opening the black box of growth and ageing trade-off in wild king penguin chicks. Mol Ecol 21: 1500–1510. [DOI] [PubMed] [Google Scholar]
- Halliwell BH, Gutteridge JMC, 2015. Free Radicals in Biology and Medicine. Oxford: Oxford University Press. [Google Scholar]
- Hau M, Haussmann MF, Greives TJ, Matlack C, Costantini D. et al. 2015. Repeated stressors in adulthood increase the rate of biological ageing. Front Zool 12: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herborn KA, Daunt F, Heidinger BJ, Granroth-Wilding HMV, Burthe SJ. et al. 2016. Age, oxidative stress exposure and fitness in a long-lived seabird. Funct Ecol 30: 913–921. [Google Scholar]
- Jenni-Eiermann S, Jenni L, Smith S, Costantini D, 2014. Oxidative stress in endurance flight: an unconsidered factor in bird migration. PLoS ONE 9: e97650.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonasson KA, Willis CKR, 2011. Changes in body condition of hibernating bats support the thrifty female hypothesis and predict consequences for populations with white-nose syndrome. PLoS ONE 6: e21061.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajtha A, Tettamanti G, Goracci G, 2009. Handbook of Neurochemistry and Molecular Neurobiology. 3rd edn. Neural Lipids. New York: Springer Science. [Google Scholar]
- Matson KD, Horrocks NP, Tieleman BI, Haase E, 2012. Intense flight and endotoxin injection elicit similar effects on leukocyte distributions but dissimilar effects on plasma-based immunological indices in pigeons. J Exp Biol 215: 3734–3741. [DOI] [PubMed] [Google Scholar]
- McGuire LP, Guglielmo CG, 2009. What can birds tell us about the migration physiology of bats?. J Mammal 90: 1290–1297. [Google Scholar]
- McGuire LP, Jonasson KA, Guglielmo CG, 2014. Bats on a budget: torpor-assisted migration saves time and energy. PloS ONE 9: e115724.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliams SR, Guglielmo C, Pierce B, Klaassen M, 2004. Flying, fasting, and feeding in birds during migration: a nutritional and physiological ecology perspective. J Avian Biol 35: 377–393. [Google Scholar]
- Metzger BJ, Bairlein F, 2011. Fat stores in a migratory bird: a reservoir of carotenoid pigments for times of need?. J Comp Physiol B 181: 269–275. [DOI] [PubMed] [Google Scholar]
- Muijres FT, Johansson LC, Bowlin MS, Winter Y, Hedenström A, 2012. Comparing aerodynamic efficiency in birds and bats suggests better flight performance in birds. PLoS ONE 7: e37335.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller A, Jennions MD, 2002. How much variance can be explained by ecologists and evolutionary biologists?. Oecologia 132: 492–500. [DOI] [PubMed] [Google Scholar]
- Nikolaidis MG, Kyparos A, Spanou C, Paschalis V, Theodorou AA. et al. 2012. Redox biology of exercise: an integrative and comparative consideration of some overlooked issues. J Exp Biol 215: 1615–1625. [DOI] [PubMed] [Google Scholar]
- Pearce RD, O’Shea TJ, Wunder BA, 2008. Evaluation of morphological indices and total body electrical conductivity to assess body composition in big brown bats. Acta Chiropterol 10: 153–159. [Google Scholar]
- Pētersons G, 2004. Seasonal migrations of north-eastern populations of Nathusius' bat Pipistrellus nathusii (Chiroptera). Myotis 40–41: 29–56. [Google Scholar]
- Popa-Lisseanu AG, Voigt CC, 2009. Bats on the move. J. Mammal 90: 1283–1289. [Google Scholar]
- R Core Team, et al. 2013. R: A language and environment for statistical computing. R Foundation for Statistical Computing Vienna, Austria. Available from: https://www.R-project.org/.
- Reynolds DS, Sullivan JC, Kunz TH, 2009. Evaluation of total body electrical conductivity to estimate body composition of a small mammal. J Wildl Manag 73: 1197–1206. [Google Scholar]
- Schneeberger K, Czirjak GA, Voigt CC, 2013. Inflammatory challenge increases measures of oxidative stress in a free-ranging, long-lived mammal. J Exp Biol 216: 4514–4519. [DOI] [PubMed] [Google Scholar]
- Skrip MM, Bauchinger U, Goymann W, Fusani L, Cardinale M. et al. 2015. Migrating songbirds on stopover prepare for, and recover from, oxidative challenges posed by long-distance flight. Ecol Evol 5: 3198–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorci G, Faivre B, 2009. Inflammation and oxidative stress in vertebrate host-parasite systems. Philos Trans Roy Soc 364: 71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sureda A, Ferrer MD, Tauler P, Romaguera D, Drobnic F. et al. 2009. Effects of exercise intensity on lymphocyte H2O2 production and antioxidant defences in soccer players. Br J Sports Med 43: 186–190. [DOI] [PubMed] [Google Scholar]
- Voigt CC, Lindecke O, Schönborn S, Kramer-Schadt S, Lehmann D, 2016. Habitat use of migratory bats killed during autumn at wind turbines. Ecol Appl 26: 771–783. [DOI] [PubMed] [Google Scholar]
- Voigt CC, Popa-Lisseanu AG, Niermann I, Kramer-Schadt S, 2012b. The catchment area of wind farms for European bats: a plea for international regulations. Biol Cons 153: 80–86. [Google Scholar]
- Voigt CC, Sörgel K, Dechmann DKN, 2010. Refuelling while flying: foraging bats combust food rapidly and directly to fuel flight. Ecology 91: 2908–2917. [DOI] [PubMed] [Google Scholar]
- Voigt CC, Sörgel K, Śuba J, Keišs O, Pētersons G, 2012a. The insectivorous bat Pipistrellus nathusii uses a mixed-fuel strategy to power autumn migration. Proc R Soc Lond B 279: 3772–3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch KC Jr, Herrera M LG, Suarez RK, 2008. Dietary sugar as a direct fuel for flight in the nectarivorous bat Glossophaga soricina. J Exp Biol 211: 310–316. [DOI] [PubMed] [Google Scholar]