Abstract
Alcohol abuse is a major public health problem worldwide. Understanding the molecular mechanisms that control regular drinking may help to reduce hazards of alcohol consumption. While immunological mechanisms have been related to alcohol drinking, most studies reported changes in immune function that are secondary to alcohol use. In this report, we analyse how the gene “TRAF family member-associated NF-κB activator” (TANK) affects alcohol drinking behavior. Based on our recent discovery in a large GWAS dataset that suggested an association of TANK, SNP rs197273, with alcohol drinking, we report that SNP rs197273 in TANK is associated both with gene expression (P = 1.16 × 10−19) and regional methylation (P = 5.90 × 10−25). A tank knock out mouse model suggests a role of TANK in alcohol drinking, anxiety-related behavior, as well as alcohol exposure induced activation of insular cortex NF-κB. Functional and structural neuroimaging studies among up to 1896 adolescents reveal that TANK is involved in the control of brain activity in areas of aversive interoceptive processing, including the insular cortex, but not in areas related to reinforcement, reward processing or impulsiveness. Our findings suggest that the cortical neuroimmune regulator TANK is associated with enhanced aversive emotional processing that better protects from the establishment of alcohol drinking behavior.
Keywords: alcohol, anxiety, drinking, insular cortex, NF-κB, TANK
Introduction
Alcohol is a toxic substance that is regularly consumed virtually worldwide with the risk of developing an alcohol addiction. Non-addictive consumption is integrated in many cultural activities (Heath 2000; Müller and Schumann 2011). However, even non-addicted regular alcohol drinking is causing major public health problems (WHO 2014) with the burden of alcohol associated diseases being largely related to the level of alcohol consumption in the general population (Rehm et al. 2009). It has long been recognized that small shifts in the mean of a continuously distributed behavior such as alcohol drinking can have major public health benefits. For example, a shift from heavy to moderate drinking could have beneficial effects on cardiovascular disease risk (Rose 1981). Understanding the mechanisms regulating regular alcohol consumption could, therefore, lead to public health benefits from reducing regular alcohol consumption.
Alcohol drinking is a heritable behavior, with heritability estimates ranging from 24% to 75% (Heath et al. 1991). While the genetic mechanisms of alcohol addiction are emerging (Treutlein et al. 2009), very few mechanisms have been identified so far for non-addicted alcohol consumption (Stacey et al. 2012) or alcohol instrumentalization (Müller and Kornhuber 2017; Müller et al. 2017; Ahmed et al. 2018). While immunological mechanisms have been related to alcohol drinking, most studies reported changes in immune function that are secondary to alcohol use. In a recent genome-wide association study (GWAS) meta-analysis of alcohol intake, we identified a suggestive signal for the TRAF family member-associated NF-κB activator (TANK) gene SNP rs197273 (A/G) with the minor A allele associated with reduced drinking (discovery sample: P = 1.02 × 10−8) (Schumann et al. 2016). This effect of the minor A allele was slightly higher in men (beta = −0.017, P = 4.4 × 10−5) than women (beta = −0.01, P = 0.002). This finding supported a GWAS study on dietary macronutrient intake that also identified a signal for TANK SNP rs197273 (Chu et al. 2013), suggesting a potential overlap in the genetic regulation of macronutrient choice and alcohol consumption (Talukdar et al. 2016).
TANK is localized on chromosome 2q24 and encodes a scaffolding protein involved in immune receptor signaling. TANK was identified as an intracellular protein that interacts with tumor necrosis factor receptors (TNFR)-associated factors (TRAF) (Cheng and Baltimore 1996). It links receptors of the IL-1R/Toll and TNF receptor family to signaling cascades, leading to the activation of NF-κB and mitogen-activated protein kinases (ERK1/2) (Nomura et al. 2000). TANK plays an important role in Toll-like receptor signaling (Wajant et al. 2001), innate immunity (Kawai and Akira 2007), bone turnover (Maruyama et al. 2012), and autoimmune nephritis (Kawagoe et al. 2009). In neuronal tissue, it is involved in sensory neuron repair, survival and neurite growth (Salerno et al. 2013) and degeneration (Pottier et al. 2015). Although it is also expressed in the brain, its role in behavioral control is unknown. However, recent studies have suggested Toll-like receptors and innate immune signaling in the brain contribute to alcohol drinking (Crews et al. 2017). Here we asked what functional consequences the rs197273 TANK polymorphism has for the regulation of alcohol drinking and addiction related behavioral dimensions (Schumann et al. 2014) and how these effects are mediated in the brain. For this purpose, we investigated the effects of TANK rs197273 on gene expression and its mediation by DNA methylation in humans. In order to estimate the impact of this SNP on brain functions related to drug abuse, we evaluated how rs197273 affects fMRI brain processing during reward learning, impulsivity and emotional responses in humans. In order to confirm this role of TANK in alcohol consumption and emotional behavior experimentally, we tested tank knock out (KO) mice. They were also used to identify a potential mechanism of how TANK might act in a key cortical brain structure involved in emotional responses as identified in the human fMRI study.
Material and Methods
Framingham Heart Study population description
To investigate if rs197273 in TANK has an effect on gene expression, we investigated 2 independent cohorts for TANK RNA expression. In 1971, the offspring and their spouses (Offspring cohort, N = 5124) of the Original cohort participants were recruited and have been examined approximately every 4 years (except 8 years between the first and the second examination). From 2002 to 2005, the adult children (Third Generation cohort, N = 4095) of the Offspring cohort participants were recruited and were examined for the first time (Splansky et al. 2007). The examination 2 of the Third Generation was begun in May of 2008 and was completed in February 2011.
In Framingham Heart Study, the information of alcohol consumption was collected by asking questions to the participants on drinking of beer, wine and spirits (80 proof) in the past year at each examination cycle. A drink was defined as having 12 oz of beer (in bottle, can, or glass, which was equivalent to ~14 g of alcohol), 5 oz of wine (~15 g of alcohol), or 1.5 oz of liquor (~14 g of alcohol). The continuous g/day was derived from numbers of drinks consumed. “Current non-drinkers” were subjects with no alcohol consumption (i.e., g/day = 0). “Current light drinkers” were subjects who consumed 0 < g/day ≤ 28 in men and 0 < g/day ≤ 14 in women. “Current at risk-drinkers” were subjects who consumed 28<g/day < 42 in men and 14 < g/day < 28 in women. “Current heavy drinkers” were subjects who consumed ≥42 g/day in men and ≥28 g/day in women.
Gene Expression Profiling in the Framingham Heart Study
The blood samples of a total of 5626 participants from the Offspring (N = 2446) at examination 8 and the Third Generation (N = 3180) at examination 2 were collected for gene expression profiling (Huan et al. 2015). Fasting peripheral whole-blood samples (2.5 mL) were collected in PAXgene™ tubes (PreAnalytiX, Hombrechtikon, Switzerland). RNA expression profiling was conducted using the Affymetrix Human Exon Array ST 1.0 (Affymetrix, Inc., Santa Clara, CA) for samples that passed the RNA quality control. The expression values for ~18 000 transcripts were obtained from the total 1.2 million core probe sets. Quality control procedures for transcripts were described previously (Joehanes et al. 2013). All data used herein are available online in dbGaP (http://www.ncbi.nlm.nih.gov/gap; accession number: phs000007).
Gene Expression Profiling in the IMAGEN Study
The IMAGEN consortium (www.imagen-europe.com), “Reinforcement behaviour in normal brain function and psychopathology” has recruited n = 2090 participants from 4 European countries assessed at baseline at an age of 14 years, with current follow-up assessments (Schumann et al. 2010). In the IMAGEN study, total RNA of 592 individuals was extracted from whole-blood cells using the PAXgene Blood RNA Kit (QIAGEN Inc., Valencia, CA). Following quality control of the total RNA extracted, labeled complementary RNA (cRNA) was generated using the Illumina® TotalPrep™ RNA Amplification kit (Applied Biosystems/Ambion, Austin, TX). cRNA was purified and quantified using a Qubit® 2.0 Fluorometer (Invitrogen, Paisly, UK). The size distributions of cRNA was determined through Bioanalyzer (Agilent Technologies, Santa Clara, CA) using the Eukaryotic mRNA Assay with smear analysis. Gene expression profiling was performed using Illumina HumanHT-12 v4 Expression BeadChips (Illumina Inc., San Diego, CA). Expression data was normalized using the loess method.
DNA Methylation Quantification in the Framingham Heart Study
To investigate if rs197273 in TANK has an effect on DNA methylation, we quantified DNA methylation in participants from the Framingham Heart Study. The DNA samples were obtained from the peripheral whole blood of 2846 Offspring who provided consent for genetic study at examination 8 using the Puregene DNA extraction kit (Qiagen, Venlo, Netherlands). Bisulfite conversion of genomic DNA was performed with the EZ DNA Methylation kit (Zymo Research, Irvine, CA). DNA methylation was quantified after whole genome amplification, fragmentation, array hybridization, and single-base pair extension. The measured DNA methylation was processed using the DASEN methodology implemented in the wateRmelon R package (6) (version 3.0.2, http://www.bioconductor.org/packages/release/bioc/html/wateRmelon.html). The methylated and unmethylated fluorescent intensities (M and U) and technical variations were adjusted. Then, quantile normalization of the M and U values were performed with consideration of 2 types of probe technologies. Beta values were then derived as the ratio of methylated probe intensity to the overall intensity. For quality control purposes, we compared genotypes of the 65 SNPs obtained in methylation and in previous genotyping efforts. We excluded samples with a probe missing rate >1% (n = 45), poor SNP matching to the 65 SNP control probe locations (n = 79), and outliers by multi-dimensional scaling techniques (n = 73). At the probe level, we excluded those with missing rate >20% at P < 0.01 (n = 466 from laboratory 1 and n = 366 from Laboratory 2), as well as probes previously identified to map to multiple locations or have an underlying SNP (minor allele frequency >5% in European ancestry (EUR) 1000 genomes project data) at the CpG site or ≤10 bp of the single-base extension (n = 42 251). After quality control, 2651 (522 in S1 and 2129 in S2) samples with ~440 000 CpG probes were used for subsequent analyses.
Genotyping and Imputation in the Framingham Heart Study
Genotyping was performed using The Affymetrix 500 K mapping array and the Affymetrix 50 K gene-focused MIP array after quality control procedures. MACH (version 1.0.15) was used to impute all autosomal SNPs genotyped in the 1000 Genomes Project using the publicly available reference set (Sanger version). The single-nucleotide polymorphisms (SNPs) that were within 100 kb of the significant CpGs were selected (minor allele frequency >5% and imputation quality score (r2 > 0.5) from 1000 G imputation data).
cis-Expression Quantitative Trait Loci Analysis in the Framingham Heart Study
To investigate if rs197273 in TANK has potential biological functions, we performed cis-eQTL analysis. The SNP in each gene was used as the independent variable in association analysis with the transcript of TANK that was measured using whole-blood samples in the Framingham Heart Study (n = 5236). Age, sex, BMI, batch effects, and blood cell differentials were included as covariates in the association analysis. Linear mixed model was used to account for familial correlation in association analysis.
cis-Methylation Quantitative Trait Loci Analysis in the Framingham Heart Study
To investigate if the significant SNP is also mQTLs for DNA methylation sites of TANK, we performed association analysis using the SNPs as the independent variables and the top principal components (PC) of methylations as the outcome variables in the Framingham Heart Study (n = 2427). Twenty CpG sites are in or ±5 kb of the TANK gene. To account for multiple testing, we first performed PCA using the methylation site in or ±10 kb of a gene. 3 top PC that can explain at least 5% of the variance in all CpGs of each gene were retained. The top PC were used as the outcome variables in association analysis with the SNPs adjusting for age, sex, BMI, batch effects, and cell differentials. A linear mixed model was used to account for familial structure (Schumann et al. 2016).
Association of TANK Gene Expression with Alcohol Consumption in the Framingham Heart Study
We performed an association analysis between TANK gene expression level and categorical alcohol consumption. The mean TANK gene expression level was compared between current heavy alcohol drinkers/moderate alcohol drinkers/light drinkers versus current non-drinkers, adjusting for age, sex, BMI, and family structure.
cis-Methylation and Gene Expression in the NIMH Brain Tissue Collection
Probes mapping all different gene variants were selected when analysing mQTL in the brain in the NIMH Brain Tissue Collection (Colantuoni et al. 2011). Raw gene expression 2-color microarray (Illumina Human 49 K Oligo array (HEEBO-7 set)) intensity data from postmortem dorsolateral prefrontal cortex (DLPFC) were downloaded (available at GSE30272) and, among them, data from individuals free from psychiatric and/or neurologic diagnoses and substance abuse according to DSM-IV were loess-normalized as described previously (Colantuoni et al. 2011). Processed and normalized DNA methylation data generated by the Illumina HumanMethylation450 microarray on samples that had both DNA methylation and expression data were also collected as described before (Jaffe et al. 2016). Expression probes were re-annotated to the hg19 genome on these samples by using the Gemma tool (Zoubarev et al. 2012) as described previously (Jaffe et al. 2016). For methylation–expression associations, we first performed PCA using the methylation site in or ±10 kb of a gene, and the PC explaining >5% of the variance were used in association analysis with the corresponding gene expression by linear regression model.
Animals
Mice and respective controls were tested for their alcohol consumption. Tank was isolated from genomic DNA extracted from embryonic stem cells (GSI-I) by PCR. The targeting vector was constructed by replacement of a 2.0-kb fragment encoding the tank open reading frame with a neomycin-resistance gene cassette; the gene encoding herpes simplex virus thymidine kinase driven by the promoter of the gene encoding phosphoglycerate kinase was inserted into the genomic fragment to facilitate negative selection. After transfection of the targeting vector into embryonic stem cells, colonies doubly resistant to the aminoglycoside G418 and ganciclovir were selected, screened by PCR and further confirmed by Southern blot analysis. Homologous recombinants were microinjected into blastocysts from C57BL/6 female mice, and heterozygous F1 progenies were inter-crossed to obtain tank–/– (tank KO) mice. Tank KO mice on the 129 Sv × C57BL/6 background and their controls were used (Kawagoe et al. 2009). Littermate mice were individually housed for alcohol drinking tests or group housed for emotional behavior tests and immunohistochemistry, provided with food and water ad libitum, and kept on a 12:12 h light: dark cycle (lights on at 1.00 pm). Room temperature was maintained between 19 °C and 22 °C at a humidity of 55% (±10%). tank KO mice develop at an age of 6 month severe glomerulonephritis with renal failure as the most likely reason for an enhanced mortality after that age (Kawagoe et al. 2009). In our study, we tested mice only at younger age (<3 month) and did not observe abnormal spontaneous behavior or enhanced lethality. All experiments were carried out in accordance with the Animal Protection Law of the Federal Republic of Germany and the European Communities Council 2010 Directive (2010/63/EU). They were all approved by the local authority “Regierung von Mittelfranken”.
Alcohol Drinking and Withdrawal Effects
Alcohol drinking was tested in naïve male mutant and wild-type (WT) control mice (8-weeks-old) using a 2-bottle free-choice drinking paradigm. In this test, male mice were used since we observed the stronger TANK association with alcohol drinking in human males. Each cage was equipped with 2 bottles constantly available, one of which contained tap water the other bottle contained alcohol in various concentrations. After an acclimatization period to establish a drinking baseline, animals received alcohol at increasing concentrations of 2, 4, 8, 12, and 16 vol.%. Mice were exposed to each concentration of alcohol for 4 days. Bottles were changed and weighed daily. The consumed amount of alcohol relative to body weight and the preference versus water were measured (Stacey et al. 2012; Easton et al. 2013).
Alcohol Deprivation Effect
Thereafter, ethanol concentration was maintained at 16 vol.% and animals were allowed to drink for 2 weeks. In order to measure the ethanol deprivation effect, baseline consumption of 16 vol.% ethanol was measured. Ethanol was removed for 3 weeks (both bottles containing tap water) before it was re-introduced for 4 days. This procedure was repeated one more time. Bottles were weighed daily and their positions were changed every other day. The consumed amount of ethanol relative to body weight and the preference versus water were measured and corrected for fluid loss (Stacey et al. 2012; Easton et al. 2013).
Taste Preference Test
Alcohol experienced animals were used for this test. Sucrose (0.5% and 5%) and quinine (2 and 20 mg/dL) preference was measured in a 2-bottle free-choice test versus water. Each dose was offered for 3 days with the position of the bottles being changed and weighed daily (Stacey et al. 2012; Easton et al. 2013). A new batch of mice was tested for saccharine preference in a 2-bottle free-choice test versus water. Saccharin was offered for 3 days, respectively, at concentration of 0.5% and 1%.
Blood Alcohol Determination
Naïve mice received an i.p. alcohol injection (3.5 g/kg) and 20-μL blood samples were obtained from the submandibular vein 1, 2, and 3 h after alcohol injection. The blood samples were subjected to enzymatic alcohol determination using the alcohol dehydrogenase method as described elsewhere (Zheng et al. 2016; Müller et al. 2017).
Emotional Behavior
Naïve male and female tank KO and WT mice were tested for their emotional behavior. Open Field: Each mouse was placed in a square gray acrylic arena (50 × 50 cm), facing an outer wall, for 20 min (parameters were measured per 5-min blocks and summarized) and allowed to freely explore the arena. White light of 25 lx was evenly distributed across the arena during testing. Video recordings were taken and analysed using Biobserve Viewer III (Biobserve GmbH, Germany). A virtual square of equal distance from the periphery (36 × 36 cm) was defined as the “central zone” in order to determine the number of entries, and time (s) spent in the central zone. Distance moved in the outer and central zones (cm), number of entries and time spent in the central zone were registered (Easton et al. 2011; Mielenz et al. 2018). Elevated Plus Maze: The elevated plus maze was constructed from gray opaque acrylic with gray lining on the floor, each arm measuring 30 × 5 cm and the central platform 5 × 5 cm. One set of arms, opposing one another, were enclosed completely by a wall of opaque acrylic, 15-cm high, while the other set was open. The maze was elevated 50 cm from the ground. Each mouse was placed on the central platform, facing towards a closed arm, and allowed to freely explore the maze for 5 min. Videomot2 tracking software (TSE Systems GmbH, Germany) was used to record locomotor activity during the test (distance moved in the open and closed arms), and the number of entries into the closed and open arms and time spent in them, recorded from the center of the animal position (Easton et al. 2011; Mielenz et al. 2018). Novelty-suppressed feeding: Animals were deprived from food for 24 h before novelty-suppressed feeding test. After deprivation each mouse was put in the corner of a square gray acrylic arena (50 × 50 × 50 cm), facing an outer wall. White light of 25 lx was evenly distributed across the arena during testing. A piece of food was placed in the center of the arena. Video recordings were taken and analysed using Videomot2. The time (s) before a mouse began eating after the fasting period (24-h) and the distance moved before eating were registered (Easton et al. 2011; Mielenz et al. 2018). Since we did not observe sex effects on these parameters, data were collapsed for analysis.
Immunohistochemistry
Naïve male and female tank KO and WT mice were treated i.g. with ethanol (5 g/kg, i.g., 25% ethanol, w/v), or with equal volume water (control), daily for 10 days. Mice were then injected intraperitoneally with saline (control) or lipopolysaccharide (LPS; 3 mg/kg, i.p.; strain O111:B4; Calbiochem, San Diego, CA, in saline) 24 h after the last dose of ethanol. One hour after LPS injection, mice were anesthetized with sodium pentobarbital and transcardially perfused with phosphate buffered saline (PBS) followed by 4% (w/v) paraformaldehyde in PBS. Coronal sections (40 μm) were cut on a sliding microtome (MICROM HM450, ThermoScientific, Austin, TX), and sections were sequentially collected into 24-well plates and stored at −20°C in a cryoprotectant solution (30% glycol/30% ethylene glycol in PBS) for immunohistochemistry. Free floating anterior insula (AI) sections were processed for immunostaining as previously described (Qin and Crews 2012). The sections were washed in PBS and antigen retrieval was performed by incubation in Citra solution (BioGenex, San Ramon, CA) for 1 h at 70°C. Following incubation in blocking solution, slides were processed in primary antibody p-NF-κB p65 (1:200; p-NFκB p65 (Ser 276) (SC-101749) rabbit polyclonal IgG, Santa Cruz Biotechnology, Inc., Dallas, TX) overnight at 4°C, and the secondary antibody, biotinylated goat anti-rabbit IgG (H + L; Vector Laboratories Inc., Burlingame, CA) was used at a 1:200 dilution for 1 h. The immune labeling was visualized using nickel-enhanced 3,3′-diaminobenzidine (DAB). Negative control for non-specific binding was conducted on separate sections employing the above-mentioned procedures with the exception that the primary antibody was omitted. For the assessment of p-NF-κB p65 positive immunoreactivity (p-NF-κB p65+IR), a modified method was used to quantify cells within regions of interest. p-NF-κB p65 +IR was assessed in 3 randomly selected region of AI per hemisphere, for a total of 6 AI regions per animal. BioQuant Nova Advanced Image Analysis (R&M Biometrics Inc., Nashville, TN) was used for image capture and quantification. The number of p-NF-κB p65 + IR cells was counted within the AI regions. Since we did not observe sex effects on these parameters, data were collapsed for analysis.
Neuroimaging Analyses
Data were acquired from 1896 14-year-old adolescents from the IMAGEN project. The recruitment procedures employed in the IMAGEN project and demographic information have been described previously (Schumann et al. 2010; 2016). Data from facial emotion processing task (FT) was available from 1456 participants, data from the successful inhibition trials of the stop signal task (SST) was available from 1286 participants and data from reward anticipation task (MID) was available from 1327 participants.
Standard Operating Procedures
The standard operating procedures for the IMAGEN project are available at http://www.imagen-europe.com/en/Publications_and_SOP.php and contain details on ethics, recruitment, neuropsychological tests, instructions for the SST (French, English, and German), and for blood collection and storage.
Emotional Faces Processing Task
Emotion processing was investigated using the “faces task” (FT). Participants were exposed to a sequence of stimuli which consisted of short (2–5 s) black-and-white video clips showing male and female faces with varying facial expressions. Stimuli showed human faces which started with the expression of a neutral expression and then either turned angry or displayed a neutral movement without a particular emotional content (for example, twitching the nose). Stimuli were arranged in 18 s blocks, each block including 4–7 video clips depicting faces of the same emotion (either angry or neutral). Altogether, there were 5 blocks of neutral faces and 5 blocks containing angry faces. In between 2 blocks of face clips, an 18 s non-biological control video clip was presented. The control stimuli consisted of expanding and contracting black-and-white concentric circles of various contrasts, roughly matching the contrast and motion characteristics of the faces clips.
Stop Signal Task
Participants also performed an event-related SST designed to study neural responses to successful and unsuccessful inhibitory control. The task was composed of Go trials and Stop trials. During Go trials (83%; 480 trials) participants were presented with arrows pointing either to the left or to the right. During these trials, subjects were instructed to make a button response with their left or right index finger corresponding to the direction of the arrow. In the unpredictable Stop trials (17%; 80 trials), the arrows pointing left or right were followed (on average 300 ms later) by arrows pointing upwards; participants were instructed to inhibit their motor responses during these trials. A tracking algorithm changes the time interval between Go signal and Stop signal onsets according to each subject’s performance on previous trials (average percentage of inhibition over previous Stop trials, recalculated after each Stop trial), resulting in 50% successful, and 50% unsuccessful inhibition trials. The inter-trial interval was 1800 ms. The tracking algorithm of the task ensured that subjects were successful on 50% of Stop trials and worked at the edge of their own inhibitory capacity.
Monetary Incentive Delay Task
The participants performed a modified version of the Monetary Incentive Delay (MID) task to study neural responses to reward anticipation and reward feedback18. This event-related task consisted of 66 10-s trials. In each particular trial, participants were presented with one of 3 cue shapes (cue, 250 ms) denoting whether a target (a white square) would subsequently appear on the left or right side of the screen and whether 0, 2, or 10 points could be won in that particular trial. After a variable delay (4000–4500 ms) of fixation on a white crosshair, participants were instructed to respond by pressing a button with their left or right index finger as soon as the target appeared. Feedback on whether and how many points were won during the trial was presented for 1450 ms after the response. Using a tracking algorithm, task difficulty (i.e., target duration varied between 100 and 300 ms) was individually adjusted such that each participant successfully responded on ~66% of trials. Participants had first completed a practice session outside the scanner (for ~5 min), during which they were instructed that for each 5 points won they would receive one food snack in the form of small chocolate candies. The current study used the contrast ‘anticipation high win versus no win’. Only successful trials were included in analysis.
Neuroimaging Acquisition and Pre-processing
Full details of the MRI acquisition protocols and quality checks have been described previously, including an extensive period of standardization across MRI scanners (Schumann et al. 2010). Effect of MRI site was controlled by adding it as a nuisance covariate in all statistical analyses. MRI data were processed using SPM8 (Statistical Parametric Mapping, http://www.fil.ion.ucl.ac.uk/spm/). Time series data were corrected for slice timing, then for movement, non-linearly warped onto MNI space using a custom EPI template, and Gaussian-smoothed at 5-mm full-width half maximum. Estimated movement (3 translations, 3 rotations, 3 translations shifted one volume acquisition before, and 3 translations shifted one volume acquisition later) parameters were added as nuisance variables. Each fMRI time series underwent automatic spike detection and any artifactual time points were regressed out of each subject’s data. For the FT, BOLD responses during “angry faces” versus BOLD responses during “control” stimuli were contrasted. For the SST, BOLD responses during “successful stop” versus BOLD responses during “successful go” were contrasted. For the MID, BOLD responses during “high win” versus BOLD responses during “no win” were contrasted. For each contrast, individual contrast images were then taken to group-level analyses.
Genotyping and Association Analysis
DNA purification and genotyping of the IMAGEN sample were performed by the Centre National de Génotypage in Paris. DNA was purified from whole-blood samples (~10 mL) preserved in BD Vacutainer EDTA tubes (Becton, Dickinson and Company, Oxford, UK) using the Gentra Puregene Blood Kit (Qiagen, Manchester, UK) according to the manufacturer’s instructions. A total of 705 and 1382 individuals were genotyped with the Illumina (Little Chesterford, UK) Human610-Quad Beadchip and Illumina Human660-Quad Beadchip, respectively. For each genotyping platform, the following quality control was performed separately. Single-nucleotide polymorphisms (SNPs) with call rates <95%, minor allele frequency <5%, deviation from the Hardy–Weinberg equilibrium (P ≤ 10−3) and non-autosomal SNPs were excluded from the analyses. Individuals with excessive missing genotypes (failure rate >5%) were also excluded. Population homogeneity was examined with the Structure software using HapMap populations as reference groups. Individuals with divergent ancestry (from Utah residents with ancestry from northern and western Europe) were excluded. Identity-by-state clustering and multi-dimensional scaling were used to estimate cryptic relatedness for each pair of individuals using the PLINK software and closely related individuals were eliminated from the subsequent analysis. We applied PCA to remove remaining outliers, defined as individuals located at more than 4 SD of the mean PCA scores on one of the first 20 dimensions. Finally, the integrated genotypes from both Illumina Human610-Quad BeadChip and Human660-Quad BeadChip were combined and platform-specific SNPs were removed. After the quality control measures, we obtained a total of 466 125 SNPs in 1834 individuals. The imputation was done by minimac, and the reference is EUR 1000 Genomes (Phase 1 version 3; November 2010).
For association analysis between genetic risk for alcohol consumption and brain activation, we first computed a gene score based on local SNPs. We assigned to the TANK gene in the genome a set of SNPs that lie within 100-kb upstream and 50-kb downstream of the gene’s most extreme transcript boundaries. We thus captured signals from potential causal variants affecting regulatory elements as well as variants affecting amino acid sequence (non-synonymous variants). We then carried out LD clumping on these SNPs with r2 = 0.02. Each gene is then assigned a score as a summation of their SNPs (Supplementary Table S4), weighted by effect sizes estimated from GWAS of alcohol drinking.
Statistical Analysis
All data are expressed as means ± SEM. Statistical analysis between the 2 groups was performed by unpaired 2-tailed Student’s t-test using Excel or GraphPad Prism (GraphPad Software, Inc.). For multiple comparisons, analysis of variance (ANOVA) with pre-planned Bonferroni-corrected LSD tests or post hoc Tukey was done when appropriate, using SPSS.
Results
Association of rs197273 with TANK Gene Expression in Humans
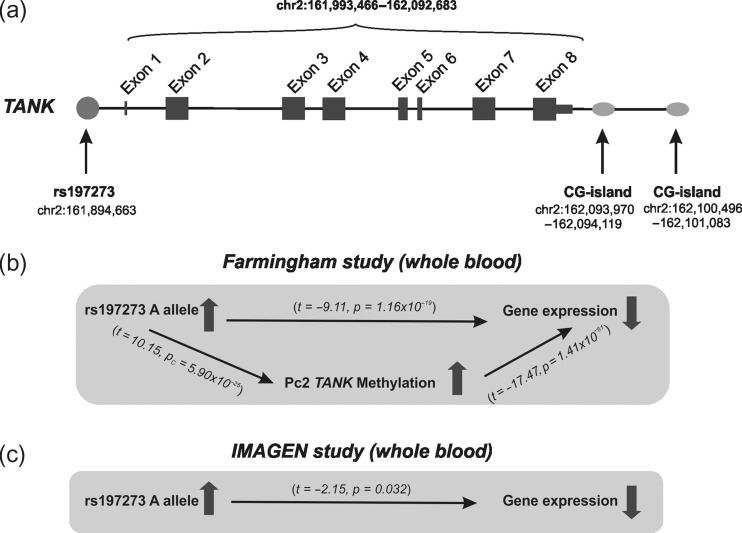
We investigated whether SNPs rs197273 is associated with gene expression of TANK in humans. We first tested the relation of genotype and gene expression in peripheral blood of 5236 participants of the Framingham study (Splansky et al. 2007). For demographic characteristics of the sample, see: Table 1. We found an association of the minor A-allele of rs197273 with TANK gene expression (P = 1.16 × 10−19) (Fig. 1A–B; Supplementary Table S1A). We replicated this association among 592 14-year old participants of the IMAGEN study (Schumann et al. 2010) (P = 0.032) (Fig. 1C; Supplementary Table S2A), a group that has not yet started drinking substantial amounts of alcohol, suggesting that the allele specific effect on gene expression is not secondary to long term alcohol abuse. In silico analyses of data from the discovery sample (Schumann et al., 2016) revealed an inverse association of the A-allele of rs197273 with gene expression in liver tissue (P = 0.015), and direct association in the brain (thalamus) (P = 0.015), but no association in adipose tissue (Supplementary Table S3). For demographic characteristics of this sample, see: Table 2. These findings suggest an association of the TANK SNP rs197273 with TANK gene expression at adolescent as well as at adult age.
Table 1.
Demographics for gene expression and methylation in peripheral blood in the Framingham Heart Study
| Phenotypes/covariates | Offspring Cohort (examination cycle 8:2005-2008) | Third Generation Cohort (examination cycle 2:2008-2011) |
|---|---|---|
| Gene Expression Analysis | n = 2222 | n = 3014 |
| Female (%) | 1221 (54.95) | 1603 (53.10) |
| Age (years), mean (SD) | 66.41 (8.95) | 46.88 (8.79) |
| BMI (kg/m2), mean (SD) | 28.04 (5.87) | 28.31 (5.53) |
| Methylation Analysis | n = 2062 | |
| Female (%) | 1140 (55.28) | |
| Age (years), mean (SD) | 66.30 (8.96) | |
| BMI (kg/m2), mean (SD) | 28.35 (5.31) |
BMI: Body mass index.
Figure 1.
Schematic diagrams of the TANK gene structure. (a) Possible mechanisms linking the TANK single-nucleotide polymorphism rs197273 to TANK gene expression through DNA methylation in the (b) Framingham and (c) in the IMAGEN study.
Table 2.
Demographics for gene expression in peripheral blood in the IMAGEN study
| N | % | |
|---|---|---|
| Female | 980 | 51.7 |
| Right handed | 1683 | 88.8 |
| Mean | SD | |
| Age | 14.6 | 0.45 |
| Verbal IQ | 110.7 14.85 | 14.9 |
| Performance IQ | 107.6 | 14.8 |
rs197273 Effects on Gene Expression are Related to DNA Methylation
Since rs197273 is localized in close proximity to several CpG sites, we investigated whether the effect of this SNP on gene expression is mediated by DNA methylation. We analysed in the Framingham offspring study (n = 2427) methylation data from CpG sites ±5 kb of the gene locus using the Illumina 450k chip (Table 1). A principal component analysis (PCA) of the 20 probes in this region revealed 3 principal components (PC) explaining more than 5% of total variance in methylation. The second principal component (PC2), which maps the shelves of 2 CpG islands adjacent to TANK 3′UTR accounts for 14% of the explained variance, and is significantly associated with rs197273 (P = 5.90 × 10−25) (Fig. 1B; Supplementary Table S1B). The PC2 is also associated with TANK gene expression in peripheral blood (P = 1.4 × 10−61) and in the DLPFC (P = 5.0×10−19) (Fig. 1B; Supplementary Table S1C, S1D). The effect of rs197273 on gene expression in peripheral blood is mediated by PC2 (Supplementary Table S1E).
TANK Gene Expression and Alcohol Consumption
About 25.2% of Framingham Heart Study participants were current non-drinkers, 57.2% were current light-drinkers, 11.0% were current at-risk drinkers, and 6.6% of individuals were heavy drinkers. The mean level of TANK gene expression is higher in current heavy drinkers compared with current non-drinkers. However, this association was borderline significant (beta/SE = 0.029/0.016, P = 0.07). The mean level of TANK gene expression did not show different in current light drinkers (P = 0.70) or in current mild drinkers (P = 0.89) compared with current non-drinkers.
TANK Affects Alcohol Drinking in Mice
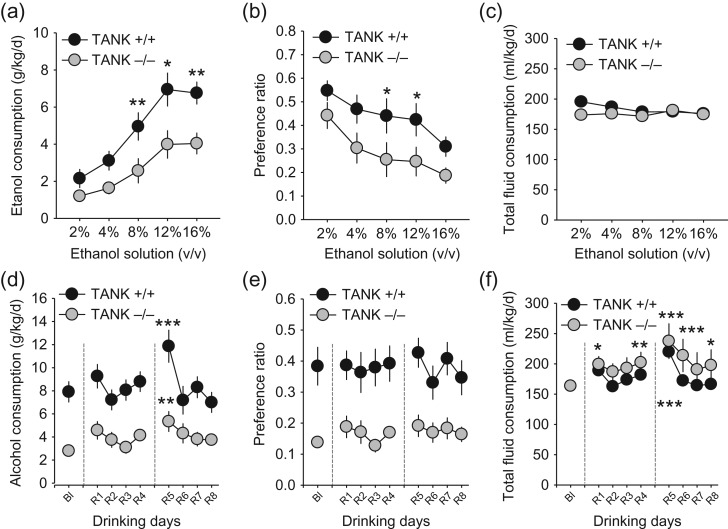
Next, we investigated alcohol drinking in tank KO mice. We measured ethanol intake and preference using a 2-bottle free-choice alcohol drinking test. Tank KO mice showed significantly lower alcohol consumption (F1,105 = 28.2771, P < 0.0001) than WT controls at the 8 vol.% (P < 0.01), 12 vol.% (P < 0.05), and 16 vol.% alcohol (P < 0.01) (Fig. 2A). Also, the preference of alcohol versus water was significantly reduced (F1,105 = 15.9561, P < 0.0002) at 8 vol.% (P < 0.031) and 12 vol.% (P < 0.039) alcohol (Fig. 2B). However, total fluid intake, indicative of general drinking activity was not affected by the lack of TANK (P > 0.05; Fig. 1C).
Figure 2.
TANK is required to establish normal alcohol drinking in mice. tank KO (n = 11) and wild-type (WT; n = 12) mice were tested in a free-choice 2-bottle drinking paradigm for their alcohol consumption. (a) Amount of alcohol consumed at different concentrations of the drinking fluid. (b) Preference of alcohol versus water. (c) Total fluid consumption during testing (pre-planned Bonferroni-corrected LSD test; *P < 0.05, **P < 0.01; vs. WT). (d) Consumption of 16 vol.% alcohol after 2 weeks of drinking and alcohol deprivation effect (ADE) in tank KO (n = 11) and WT (n = 12) mice. After continuous drinking, animals were withdrawn from alcohol for 2 times 3 weeks (dotted lines) and reinstated (R) for 4 days, respectively, (e) alcohol preference and (f) total fluid consumption during ADE (pre-planned Bonferroni-corrected LSD test; *P < 0.05, **P < 0.01; ***P < 0.001 vs. baseline (Bl)).
Thereafter, animals were left undisturbed with water and 16 vol.% alcohol available for 14 days, during which the reduced consumption was maintained in the tank KO mice (F1,21 = 17.4186, P < 0.0005). When animals were repeatedly withdrawn from alcohol drinking for 3 weeks each time, and then reinstated, they maintained a genotype effect (F1,8 = 18.6691, P < 0.0003) and showed an alcohol deprivation effect (ADE) which became evident by a temporarily restricted increase of consumption (Day: F8,168 = 8.1122, P < 0.0001; genotype × day interaction: F8,168 = 2.2879, P < 0.0237). Results showed a significant increase in alcohol consumption in the WT mice on the first day after second withdrawal versus the day before withdrawal (P < 0.0001). Also, the tank KO mice developed an ADE, which was significant after the second withdrawal (P < 0.0072). However, this effect was significantly reduced compared with WT mice (P < 0.0001) (Fig. 2D). Also, alcohol preference was significantly attenuated in the tank KO mice during withdrawal testing (genotype: F1,8 = 12.4997, P < 0.0019; Day: F8,168 = 2.2905, P < 0.0236; interaction: P > 0.05), but did not reach significance versus baseline at single day level (P > 0.05; Fig. 2E). Total fluid consumption during withdrawal was not affected by the genotype (P > 0.05), but was enhanced by the alcohol deprivation in both groups to a comparable degree (Day: F8,168 = 10.8922, P < 0.0001; interaction: P > 0.05, Fig. 2F). In the WT mice, this was significant on Day 1 of the second reinstatement (P < 0.0001). In the tank KO mice, this was significant on Day 1 (P < 0.0144) and Day 4 (P < 0.0064) of the first reinstatement, as well as on Days 1 and 2 (P < 0.0001) and Day 4 (P < 0.0232) of the second reinstatement. These findings suggest reduced alcohol consumption and preference, as well as an attenuated ADE in mice lacking tank. Given that total fluid consumption was comparable between genotypes, this effect may not be the consequence of reduced or impaired locomotor activity in the tank KO mice.
We found that the avoidance of a bitter tasting quinine solution was not altered in tank KO mice compared with WT mice (P > 0.05; Fig. 3A). However, tank KO mice showed a significantly lower preference for sweet tasting sucrose solution comparted with WT mice with 0.5% (P < 0.002) and 5% sucrose (P < 0.006). We tested animals for their preference for saccharin solution, which is sweet tasting, but devoid of carbohydrate calories, and found also here a lower preference in tank KO mice (F1,70 = 4.453; P = 0.0384) which was most evident for the 1% concentration (P < 0.034) (Fig. 3B). The tank KO had no effect on alcohol bioavailability in mice (P > 0.05; Fig. 3C). Altogether, these findings suggest that TANK contributes to sweetness preference independent of the nutritional content of the food.
Figure 3.

TANK controls preference for sweet taste in a 2-bottle free-choice paradigm in tank KO (n = 11) and wild-type (WT; n = 12) mice. (a) Sucrose (sweet) preference and quinine (bitter) avoidance test in a free-choice 2-bottle drinking paradigm indicates no difference between tank KO (n = 11) and WT mice (n = 12) in the avoidance of bitter tasting quinine, but a reduced preference for sucrose (pre-planned Bonferroni-corrected LSD test; ##P < 0.01). (b) A saccharine preference test in a free-choice 2-bottle drinking paradigm indicates a reduced preference for sweet tasting, but caloric neutral saccharin in naïve tank KO mice (n = 12) versus WT (n = 12) (pre-planned Bonferroni-corrected LSD test; #P < 0.05). (c) TANK has no effect on alcohol bioavailability in mice. Blood alcohol concentration in tank KO (n = 10) and WT mice (n = 10) after alcohol injection (3.5 g/kg, i.p.). Over the 3-h tested, there was no difference in alcohol bioavailability between genotypes (P > 0.05).
No Role of rs197273 in Reward Processing and Impulsivity
We investigated single SNPs and gene scores of TANK association with brain activity indexed by blood oxygenation-level dependent (BOLD) signal, during reinforcement-related neuroimaging tasks relevant to alcohol drinking in up to 1456 14-year-old adolescents of the IMAGEN study (Schumann et al. 2010; Table 2). We calculated a polygenic risk score as a summation of the effects of the common variants, weighted by effect sizes from our quantitative GWAS data on alcohol intake (Huan et al. 2015; Schumann et al. 2016) (Supplementary Table S4). The MID task was used as a measure of reward processing. For each voxel, we calculated the test statistic for the association between SNP or gene score and activation (Euesden et al. 2015). We used a significance threshold of P ≤ 0.001 after a conservative family wise error correction. We did not find a relationship between TANK and altered reward processing (P > 0.05).
Differences in alcohol consumption may also be accounted for by altered impulsivity. Brain activation with the orbitofrontal cortex as region of interested was measured during the SST in the same adolescents. Also for the SST, neither whole-brain analysis nor region of interest analyses exceeded the significance threshold. Moreover, no associations were found with intracranial brain volume or gray matter thickness (P > 0.05). These data suggest that TANK SNP rs197273 does not affect the incentive properties of a rewarding stimulus or impulsivity.
rs197273 Predicts Cortical Response to Emotional Stimuli
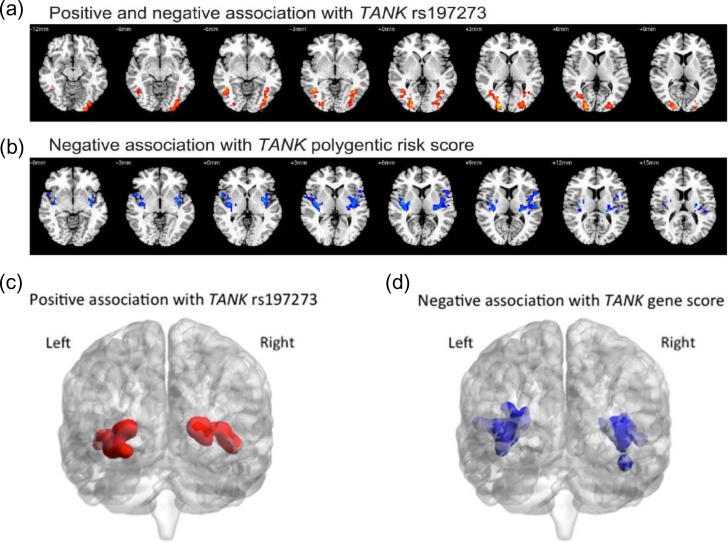
Humans and animals may consume alcohol in order to cope with anxiety and stress (Müller and Schumann 2011). We investigated single SNPs and the gene scores of TANK for associations with brain activity during the viewing of angry faces—designed to assess processing of negative emotions that are related to emotional stress, and involving a social component in up to 1456 14-year-old adolescents of the IMAGEN study (Schumann et al. 2010; Table 2). We used a significance threshold of P ≤ 0.001 after a conservative family wise error correction. In this analysis, the angry FT exceeded this significance threshold (Fig. 4; Table 3). The “protective” A-allele of TANK rs197273 was associated with a stronger BOLD response in the right inferior temporal gyrus (P = 0.001, cluster size = 229 mm3, peak T value = 4.44) and with a stronger response in a cluster involving the left middle temporal gyrus and the left inferior occipital gyrus (P = 9.15 × 10−4, cluster size = 232 mm3, peak T value = 3.71; Fig. 4A and 4C; Table 3). For the TANK gene score, we found the strongest association of the brain response to angry faces (vs. control stimulus) in a cluster in the left insula and the inferior frontal gyrus (P = 1.1×10−5, cluster size = 375 mm3, peak T value = 3.92). For an allele specific analysis, see: Supplementary information; Supplementary Figures S1–S3. An additional cluster was detected in the right insula (P = 5.2 × 10−4, cluster size = 249 mm3, peak T value = 4.63; Fig. 4B and 4D; Table 3; Supplementary Figs S4–S6), a region involved in interoceptive processing and drug craving (Contreras et al. 2007). These findings suggest that TANK is associated with cortical emotion processing, in particular in the insular cortex.
Figure 4.
Association of whole-brain activity during social emotional processing with the tank gene. Brain regions associated with (a/c) TANK rs197273 and (b/d) TANK gene score during angry faces task. Red and blue colors represent a positive association and a negative association, respectively.
Table 3.
Whole-brain association analysis with SNPs and polygenetic risk scores (PRS) for TANK in “angry face” processing
| Peak T value’s MNI co-ordinates | Peak T value | Cluster Size | FWE-corrected P-value | |
|---|---|---|---|---|
| Positive association with TANK rs197273 | ||||
| Right Inferior Temporal gyrus | 21,−91,4 | 4.44 | 229 | 0.001 |
| Left Middle Temporal gyrus, Left Inferior Occipital gyrus | −42,−61,1 | 3.71 | 232 | 9.15 × 10−4 |
| Negative association with TANK rs197273 | ||||
| Right Paracentral gyrus, Right Middle cingulum gyrus | 12,−40,52 | 4.18 | 194 | 0.0034 |
| Negative association with TANK PRS | ||||
| Right Insula | 42,−1,−17 | 4.63 | 249 | 5.20 × 10−4 |
| Right Paracentral gyrus, Right Middle cingulum gyrus | 12,−40,52 | 4.51 | 124 | 0.048 |
| Left Insula, Left Inferior frontal gyrus | −36,−4,−2 | 3.92 | 375 | 1.11 × 10−5 |
| Right Parahppocampus, Right Fusiform gyrus, Right Cerebelum | 24,−43,−5 | 3.86 | 163 | 0.011 |
TANK Affects Anxiety in Mice
In order to explore how TANK affects emotional behavior, we used naïve tank KO mice and tested them in behavioral paradigms measuring anxiety and depression (Easton et al. 2011; Mielenz et al. 2018). In the elevated plus maze, tank KO mice showed higher levels of anxiety-related behaviors compared with WT. This was evident by less time (P < 0.0284) and locomotion (P < 0.0223) spent on the open arms of the maze, and more time spent in the closed arms (P = 0.01; Fig. 5A–B). This was confirmed by an analysis of relative open arm time (t = 2.098, P < 0.0482), which suggests that the anxiogenic effect is not locomotor dependent (Fig. 5C).
Figure 5.
TANK controls anxiety-related behavior in mice. Tank KO mice display more anxiety-related behavior in a novel environment than wild-type (WT) mice. Enhanced anxiety in the elevated plus maze test (EPM) by tank KO (n = 12) vs. WT (n = 11) mice shown by (a) reduced time spent and (b) less distance moved on open arms (P < 0.05) and (c) less relative time in open arms. (d) No effect on depression-related behavior in the novelty-suppressed feeding test in tank KO mice. (e) In the open field (OF) test, tank KO mice (n = 12) spend less time in the anxiogenic center of the maze than WT mice (n = 11). (f) The number of OF center entries is reduced in tank KO mice. Locomotor activity of tank KO mice is reduced in (g) the center of the maze, but also (h) when total locomotion is considered (pre-planned Bonferroni-corrected LSD test; #P < 0.05; $P < 0.001 vs. WT).
The open field (OF) test further supported more anxiety-related behavior in tank KO mice in a novel environment. This was evident by a significant reduction in the time (P < 0.0394) and number of entries (P < 0.0271) to the center of the OF in the first 5 min of testing (Fig. 5E–F). The locomotion in the center (F1,21 = 23.9442; P < 0.0001) as well as total locomotion (F1,21 = 45.9935; P < 0.0001) was lower in tank KO mice during the whole test period, suggesting a general reduction in locomotor activity in a novel environment (Fig. 5G–H). There was no difference in depression-related behavior in the novelty-suppressed feeding test (P > 0.05; Fig. 5D). Altogether, these data suggest that a lack of TANK enhances anxiety-related behavior towards novel stimuli.
TANK Regulates Alcohol-Induced NF-κB in the Insular Cortex
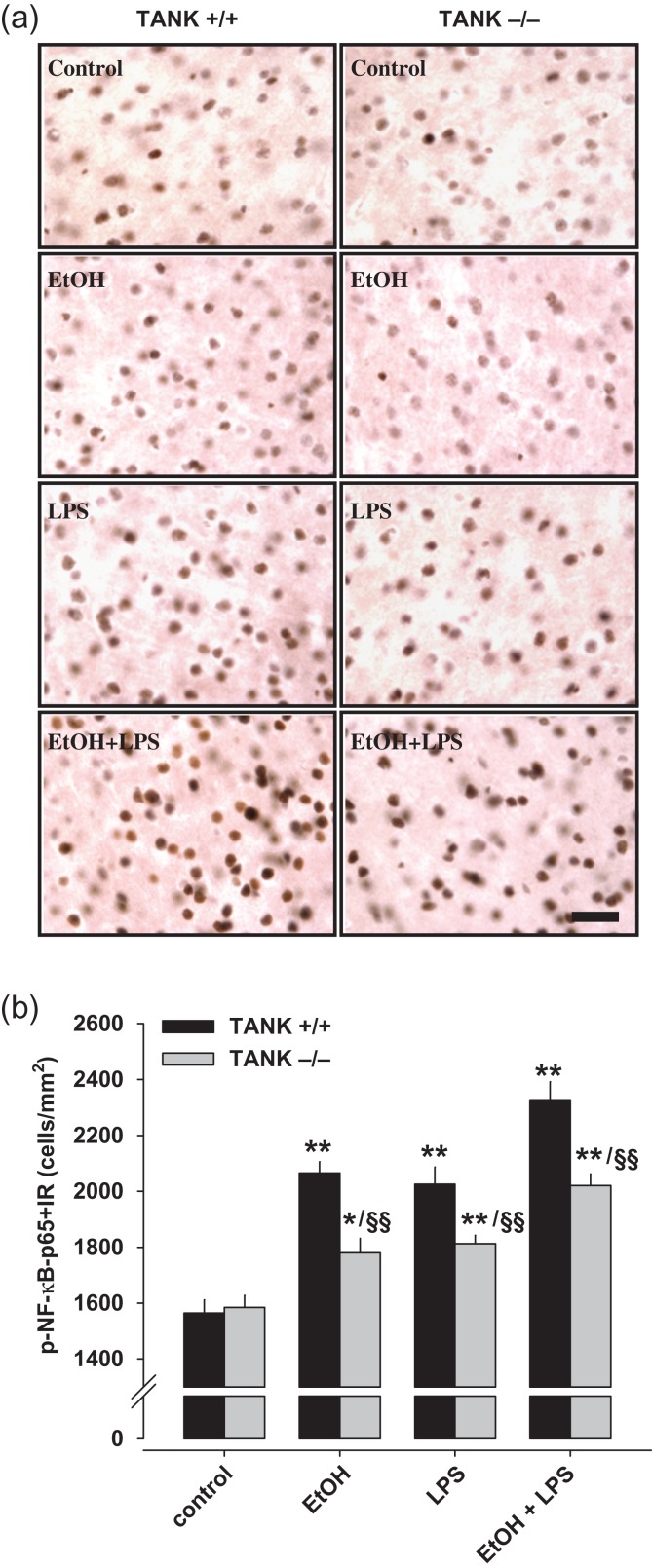
TANK signaling is linked to activation, i.e., expression, of the transcription factor NF-κB that increases neuroimmune signaling, which can regulate drinking and anxiety by activation of TANK-binding kinase 1 (TBK1) (Crews et al. 2017). NF-κB is activated by alcohol in the brain (Zou and Crews 2010) inducing innate immune responses, which can further promote alcohol drinking in rodents and humans (Edenberg et al. 2008). Such an increase can be driven by a pro-inflammatory event, e.g., LPS, or by alcohol itself (Blednov et al. 2011). The insula was found to be a critical brain region for the control of aversion-resistant alcohol consumption (Seif et al. 2013). We identified the insular cortex as a brain area whose activity differs during emotional processing dependent upon the specific TANK alleles. Here, we asked whether alcohol induces NF-κB activation in the insular cortex by TANK activation. We treated tank KO mice sub-chronically with i.g. alcohol or water. In addition, animals received an injection with LPS or vehicle. Using immunohistochemistry, we found that alcohol and LPS enhanced p-NF-κB immunoreactivity in the AI of the mice (alcohol vs. control: P < 0.01 (WT), P < 0.05 (tank KO); LPS vs. control: P < 0.01 (WT and KO)). This effect was potentiated when both treatments were combined (P < 0.01). All effects on p-NF-κB were significantly attenuated in tank KO mice (P < 0.01; Fig. 6). These findings suggest that TANK contributes to chronic alcohol- and LPS-induced NF-κB activation in the insula.
Figure 6.
TANK controls neuroimmune activation after alcohol and/or lipopolysaccharide (LPS) in the insular cortex of mice. (a) Images of p-NFκB-p65+immunoreactive cells (+IR) of the anterior insular cortex of tank KO and WT mice after alcohol (5 g/kg, i.g., 10 days) and LPS (3 mg/kg, i.p.) treatment (scale bar = 30 μm). (b) Quantification of p-NFκB-p65+IR cells. BioQuant image analysis shows that cell number of p-NFκB-p65+IR was significantly increased after 10 days of alcohol (i.g.) or LPS alone. Pretreatment of alcohol significantly enhances LPS-induced p-NFκB-p65 immunoreactivity in both WT and tank KO mice (ANOVA: P < 0.01). Anterior insular cortex of tank KO mice shows significantly decreased p-NFκB-p65+IR cells after alcohol and/or LPS compared with WT mice (t-test; *P < 0.05, **P < 0.01 vs. control treatment; §§P < 0.01 vs. WT mice with same treatment).
Discussion
This study aimed to identify a mechanism which may explain how TANK exerts its control on alcohol consumption. In contrast to a number of previously described genetic mechanisms that all affect functions of the reward system in the brain and alcohol effects thereupon (Stacey et al., 2012; Easton et al., 2013; Schumann et al. 2011, 2016), here we describe a genetic mechanisms that acts via neuroimmune modulation of cortical emotional processing. The TANK gene SNP rs197273 was identified as a possible locus for alcohol drinking and macronutrient choice in large GWAS meta-analyzes in humans (Chu et al. 2013; Schumann et al. 2016). Here, we report that the minor A-allele of SNP rs197273 is associated with reduced TANK gene expression in humans. We identified an enhanced CpG methylation of TANK as a potential mediating mechanism in minor A-allele carriers. The minor A-allele of SNP rs197273, which leads to reduced expression of TANK, is associated with lower alcohol consumption. Experimental tests in young adult mice confirmed that a lack of TANK leads to a reduced alcohol consumption and attenuates withdrawal-induced escalation of consumption. Adolescent humans of the IMAGEN sample with the rs197273 TANK A-allele drink less alcohol (Schumann et al., 2016) and have more negative affective responses in the insular cortex (this study). Together, this may suggest a role of TANK particularly in the acquisition and early phase of alcohol drinking. Our findings in mice support a role for TANK in regulating alcohol activation of neuroimmune signaling in the insula, an area that is crucial for emotional responses. Blunted alcohol-induced cortical neuroimmune activation in this region may limit alcohol consumption in individuals with reduced TANK expression.
Based on a previous association of the minor A-allele of TANK SNP rs197273 with alcohol consumption in humans (Schumann et al., 2016), we sought to explore mechanisms of this association. Here, we report in 2 independent samples that the minor A-allele of SNP rs197273 is associated with a reduction in TANK gene expression. This may be explained by the increase in TANK (PC2) methylation that was found to be associated with the minor A-allele of SNP rs197273 as well as with gene expression.
The human findings have been associative by nature, and only suggest, but do not prove a causal involvement. This can only be achieved when TANK function is experimentally manipulated. In a translational approach, we tested the role of TANK in alcohol drinking in mice. Herein, we confirmed the human phenotype, in that reduced TANK availability leads to a reduced consumption and preference of alcohol in mice. Also, the effects of repeated withdrawal on alcohol drinking were attenuated in mice lacking TANK. Importantly, we did not observe general reduction in drinking behavior, i.e., in total fluid consumption, which suggests that locomotor activity required for drinking is basically preserved. These data may confirm the human phenotype and suggest a role for TANK in alcohol drinking.
Mice lacking TANK showed a reduced preference for a usually preferred sucrose solution. Since TANK was implicated in macronutrient choice, in particular for carbohydrates (Chu et al. 2013), we asked whether the caloric content or the sweet taste might be responsible for the change in taste preference. This test showed also a reduced preference for the sweet tasting, but caloric neutral saccharin solution. These findings may confirm a role of TANK in carbohydrate choice, which is mainly guided by its hedonic taste properties, rather than by the caloric content. Since changes in sweet taste perception may affect mainly alcohol consumption at low concentrations of alcohol, the effects on taste perception may not account for the observed attenuation in alcohol consumption, which is most striking at high alcohol concentrations.
We did not find an effect of rs197273 on reward processing in the brain reward pathway during the MID task. However, the “protective” A-allele of TANK rs197273 was associated with stronger activation of the right inferior temporal gyrus and with stronger activation of a cluster involving the left middle temporal gyrus and the left inferior occipital gyrus. These brain regions have been linked to psychological and physiological stress reactions, to alcohol craving, and alcohol-addiction related behaviors (Cooper et al. 1995; Seif et al. 2013; Sutherland et al. 2013). Using a task where negative emotions are processed, we discovered that the TANK gene score was associated with activation of a cluster in the insula. The insula is a key region for dynamic interoceptive processing that integrates somatosensory information with emotional salience of aversive as well as pleasant stimuli (Craig 2009; Hassanpour et al. 2018). It is also involved in risk prediction and risk adjustment during decision making (Bossaerts 2010). During risky decision making, insula activation was associated with better harm avoidance in healthy individuals (Paulus et al. 2003). It was shown that problematic substance use is associated with reduced responsiveness of the insula to non-drug related aversive cues (Paulus and Stewart 2014; Stewart et al. 2014). Pleasant cues, however, may be processed with higher sensitivity in substance abusers (Stewart, May et al. 2015). Alcohol use disorder and neuroticism were associated with a lower cortical thickness of the insula in humans (Zhao et al. 2017). In non-addicts, alcohol consumption is controlled by the aversive side effects of the consumption, which can also be cue-conditioned (Riley 2011). Aversive cues and memories can suppress alcohol consumption in non-addicts (Limpens et al. 2014; Labots et al. 2018). In that, a strong processing of aversive interoceptive signals may counteract risky behaviors such as drug abuse (Paulus et al., 2009). A loss of responses to aversive cues and of conditioned suppression of drug consumption are considered as a classical indicators for alcohol addiction in humans (Kim et al. 2014) and animal models (Vendruscolo et al. 2012; Seif et al. 2013). It was suggested that an attenuated processing of aversive interoceptive states, such as induced by aversive memories of drug side effects, may put an individual at higher risk for drug dependence. Our findings may thus be interpreted in a way that the TANK polymorphism enhances responsiveness to aversive interoceptive cues in the insula, and, by that way, provides resilience to the establishment of risky alcohol consumption.
After the human findings suggested a role of TANK in aversive emotional processing, we sought to verify a role in a translational approach. Using 2 tests of (state) anxiety, we found that a lack of TANK leads to enhanced levels of anxiety-related behaviors in both, the EPM as well as the OF test. A test of depression-like behavior, however, did not suggest a role of TANK. In both, the EPM and OF, but not in the NSF test, tank KO mice showed less locomotion that WT controls. This effect could, however, be separated from the anxiogenic action. Altogether, these tests may suggest a role of TANK in anxiety related, but not in depression-related behavior.
TANK is involved in inflammatory processes (Kawagoe et al. 2009). An inflammation, induced by LPS in healthy volunteers was shown to enhance AI cortex responses and cortical connectivity after an acute aversive pain stimulus (Karshikoff et al. 2016; Lekander et al. 2016). In a molecular analysis in mice, we found that TANK is required for the alcohol- and/or inflammation-induced increase of NF-κB in the insular cortex, which can drive escalation of alcohol consumption (Edenberg et al. 2008). These findings suggest a unique influence of TANK on insular cortex processing. TANK is associated with alcohol drinking, in a way which limits the risk of alcohol-induced neuroimmune activation and its driving of drinking escalation. The present findings might implicate that a stronger cortical activation during negative emotions, possibly related to negative interospective memories of consumption episodes (Müller 2013), may facilitate a proper risk assessment for alcohol consumption and, thus, limit alcohol consumption in non-addictive drinking.
This study has some limitations that need to be considered when estimating the importance of TANK for alcohol abuse and emotion. First, the strong association in the discovery sample reached only a nominal significance in an independent replication sample (replication: P = 0.04; discovery and replication combined: P = 7.4 × 10−8) (Schumann et al. 2016). Thus, TANK effects may be limited to distinct populations. Second, the association refers to general alcohol consumption that is distinct from addiction. It should be noted that genetic and brain mechanisms of controlled drug use are largely distinct from those mediating the transition to addiction (Müller and Homberg 2015). In this study, we have used an animal model with a constitutive KO of tank to translate human associations into an experimental approach in animals. While this model translates the human situation of a live long reduction in TANK function in a whole body mouse KO, it does not allow extracting the most effective brain area(s) responsible for local TANK action in mice. It may also slightly over-estimate the effect size that the human SNP may possibly have. Furthermore, the alcohol drinking study measured only male mice and confirmed the human association in males. While a somewhat weaker association between TANK and alcohol consumption was also observed in human females (Schumann et al. 2016), this still has to be tested in female mice separately. No such limitation applies to emotional behavior and neuroimmune responses, were we did not observe sex effects of TANK.
In summary, our findings suggest that the cortical neuroimmune regulator TANK, for which a naturally occurring mutation was associated with alcohol drinking in humans, is involved in cortical aversive emotion processing and related control of alcohol drinking behavior.
Supplementary Material
Notes
Conflict of Interest: None declared.
Funding
This work was supported by the Interdisciplinary Center for Clinical Research Erlangen, Project E22, and by grant MU2789/8-2 from the Deutsche Forschungsgemeinschaft (C.P.M.). This work received support from the following sources: the European Union-funded FP6 Integrated Project IMAGEN (Reinforcement-related behavior in normal brain function and psychopathology) (LSHM-CT- 2007-037286), the Horizon 2020 funded ERC Advanced Grant “STRATIFY” (Brain network based stratification of reinforcement-related disorders) (695313), ERANID (Understanding the Interplay between Cultural, Biological and Subjective Factors in Drug Use Pathways) (PR-ST-0416-10004), BRIDGET (JPND: BRain Imaging, cognition Dementia and next generation GEnomics) (MR/N027558/1), the FP7 projects IMAGEMEND(602450; IMAging GEnetics for MENtal Disorders) and MATRICS (603016), the Innovative Medicine Initiative Project EU-AIMS (115300-2), the Medical Research Council Grant “c-VEDA” (Consortium on Vulnerability to Externalizing Disorders and Addictions) (MR/N000390/1), the Swedish Research Council FORMAS, the Medical Research Council, the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, the Bundesministeriumfür Bildung und Forschung (BMBF grants 01GS08152; 01EV0711; eMED SysAlc01ZX1311A; Forschungsnetz AERIAL), the Deutsche Forschungsgemeinschaft (DFG grants SM 80/7-1, SM 80/7-2, SFB 940/1). Further support was provided by grants from: ANR (project AF12-NEUR0008-01—WM2NA, and ANR-12-SAMA-0004), the Fondation de France, the Fondation pour la Recherche Médicale, the Mission Interministérielle de Lutte-contre-les-Drogues-et-les-Conduites-Addictives (MILDECA), the Fondation pour la Recherche Médicale (DPA20140629802), the Fondation de l’Avenir, Paris Sud University IDEX 2012; the National Institutes of Health, Science Foundation Ireland (16/ERCD/3797), USA (Axon, Testosterone and Mental Health during Adolescence; RO1 MH085772-01A1), and by NIH Consortium grant U54 EB020403, supported by a cross-NIH alliance that funds Big Data to Knowledge Centres of Excellence.
The Framingham Heart Study is funded by National Institutes of Health contract N01-HC-25195. The laboratory work for DNA methylation and gene expression was funded by the Division of Intramural Research, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD. The analytical component of statistical analyses in Framingham Heart Study was funded by the Division of Intramural Research, National Heart, Lung, and Blood Institute, and the Center for Information Technology, National Institutes of Health, Bethesda, MD.
References
- Ahmed S, Badiani A, Miczek KM, Müller CP. 2018. Non-pharmacological factors that determine drug use and addiction. Neurosci Biobehav Rev. 10.1016/j.neubiorev.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Geil C, Perra S, Morikawa H, Harris RA. 2011. Activation of inflammatory signalling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain Behav Immun. 25(Suppl 1):S92–S105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossaerts P. 2010. Risk and risk prediction error signals in anterior insula. Brain Struct Funct. 214:645–653. [DOI] [PubMed] [Google Scholar]
- Cheng G, Baltimore D. 1996. TANK, a co-inducer with TRAF2 of TNF- and CD 40L-mediated NF-kappaB activation. Genes Dev. 10:963–973. [DOI] [PubMed] [Google Scholar]
- Chu AY, Workalemahu T, Paynter NP, Rose LM, Giulianini F, Tanaka T, Ngwa JS, CHARGE Nutrition Working Group , Qi Q, Curhan GC, et al. 2013. Novel locus including FGF21 is associated with dietary macronutrient intake. Hum Mol Genet. 22:1895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colantuoni C, Lipska BK, Ye T, Hyde TM, Tao R, Leek JT, Colantuoni EA, Elkahloun AG, Herman MM, Weinberger DR, et al. 2011. Temporal dynamics and genetic control of transcription in the human prefrontal cortex. Nature. 478:519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras M, Ceric F, Torrealba F. 2007. Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science. 318:655–658. [DOI] [PubMed] [Google Scholar]
- Cooper ML, Frone MR, Russell M, Mudar P. 1995. Drinking to regulate positive and negative emotions: a motivational model of alcohol use. J Pers Soc Psychol. 69:990–1005. [DOI] [PubMed] [Google Scholar]
- Craig AD. 2009. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci. 10:59–70. [DOI] [PubMed] [Google Scholar]
- Crews FT, Walter TJ, Coleman LG Jr, Vetreno RP. 2017. Toll-like receptor signalling and stages of addiction. Psychopharmacology (Berl). 234(9-10):1483–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton AC, Lucchesi W, Schumann G, Giese KP, Müller CP, Fernandes C. 2011. alphaCaMKII autophosphorylation controls exploratory activity to threatening novel stimuli. Neuropharmacology. 61:1424–1431. [DOI] [PubMed] [Google Scholar]
- Easton AC, Lucchesi W, Lourdusamy A, Lenz B, Solati J, Golub Y, Lewczuk P, Fernandes C, Desrivieres S, Dawirs RR, et al. 2013. alphaCaMKII autophosphorylation controls the establishment of alcohol drinking behaviour. Neuropsychopharmacology. 38:1636–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ, Xuei X, Wetherill LF, Bierut L, Bucholz K, Dick DM, Hesselbrock V, Kuperman S, Porjesz B, Schuckit MA, et al. 2008. Association of NFKB1, which encodes a subunit of the transcription factor NF-kappaB, with alcohol dependence. Hum Mol Genet. 17(7):963–970. [DOI] [PubMed] [Google Scholar]
- Euesden J, Lewis CM, O’Reilly PF. 2015. PRSice: polygenic risk score software. Bioinformatics. 31:1466–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanpour MS, Simmons WK, Feinstein JS, Luo Q, Lapidus RC, Bodurka J, Paulus MP, Khalsa SS. 2018. The insular cortex dynamically maps changes in cardiorespiratory interoception. Neuropsychopharmacology. 43:426–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath DB. 2000. Drinking Occasions: Comparative Perspectives on Alcohol and Culture. Philadelphia: Brunner/Mazel. [Google Scholar]
- Heath AC, Meyer J, Jardine R, Martin NG. 1991. The inheritance of alcohol consumption patterns in a general population twin sample: II. Determinants of consumption frequency and quantity consumed. J Stud Alcohol. 52:425–433. [DOI] [PubMed] [Google Scholar]
- Huan T, Liu C, Joehanes R, Zhang X, Chen BH, Johnson AD, Yao C, Courchesne P, O’Donnell CJ, Munson PJ, et al. 2015. A systematic heritability analysis of the human whole blood transcriptome. Hum Genet. 134:343–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe AE, Gao Y, Deep-Soboslay A, Tao R, Hyde TM, Weinberger DR, Kleinman JE. 2016. Mapping DNA methylation across development, genotype and schizophrenia in the human frontal cortex. Nat Neurosci. 19:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joehanes R, Ying S, Huan T, Johnson AD, Raghavachari N, Wang R, Liu P, Woodhouse KA, Sen SK, Tanriverdi K, et al. 2013. Gene expression signatures of coronary heart disease. Arterioscler Thromb Vasc Biol. 33:1418–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karshikoff B, Jensen KB, Kosek E, Kalpouzos G, Soop A, Ingvar M, Olgart Höglund C, Lekander M, Axelsson J. 2016. Why sickness hurts: a central mechanism for pain induced by peripheral inflammation. Brain Behav Immun. 57:38–46. [DOI] [PubMed] [Google Scholar]
- Kawagoe T, Takeuchi O, Takabatake Y, Kato H, Isaka Y, Tsujimura T, Akira S. 2009. TANK is a negative regulator of Toll-like receptor signalling and is critical for the prevention of autoimmune nephritis. Nat Immunol. 10:965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. 2007. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 13:460–469. [DOI] [PubMed] [Google Scholar]
- Kim SM, Han DH, Min KJ, Kim BN, Cheong JH. 2014. Brain activation in response to craving- and aversion-inducing cues related to alcohol in patients with alcohol dependence. Drug Alcohol Depend. 141:124–131. [DOI] [PubMed] [Google Scholar]
- Labots M, Cousijn J, Jolink LA, Kenemans JL, Vanderschuren LJMJ, Lesscher HMB. 2018. Age-related differences in alcohol intake and control over alcohol seeking in rats. Front Psychiatry. 9:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekander M, Karshikoff B, Johansson E, Soop A, Fransson P, Lundström JN, Andreasson A, Ingvar M, Petrovic P, Axelsson J, et al. 2016. Intrinsic functional connectivity of insular cortex and symptoms of sickness during acute experimental inflammation. Brain Behav Immun. 56:34–41. [DOI] [PubMed] [Google Scholar]
- Limpens JH, Schut EH, Voorn P, Vanderschuren LJ. 2014. Using conditioned suppression to investigate compulsive drug seeking in rats. Drug Alcohol Depend. 142:314–324. [DOI] [PubMed] [Google Scholar]
- Maruyama K, Kawagoe T, Kondo T, Akira S, Takeuchi O. 2012. TRAF family member-associated NF-kappaB activator (TANK) is a negative regulator of osteoclastogenesis and bone formation. J Biol Chem. 287:29114–29124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielenz D, Reichel M, Jia T, Quinlan EB, Stöckl T, Mettang M, Zilske D, Kirmizi-Alsan E, Schönberger P, Praetner M, et al. 2018. EFhd2/ Swiprosin-1 is a common genetic determinator for sensation seeking/ low anxiety and alcohol addiction. Mol Psychiatry. 23(5):1303–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller CP. 2013. Episodic memories and their relevance for psychoactive drug use and addiction. Front Behav Neurosci. 7:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller CP, Kalinichenko LS, Tiesel J, Witt M, Stöckl T, Sprenger E, Fuchser J, Beckmann J, Praetner M, Huber SE, et al. 2017. Paradoxical antidepressant effects of alcohol are related to acid sphingomyelinase and its control of sphingolipid homeostasis. Acta Neuropathol. 133(3):463–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller CP, Homberg J. 2015. The role of serotonin in drug use and addiction. Behav Brain Res. 277C:146–192. [DOI] [PubMed] [Google Scholar]
- Müller CP, Kornhuber J. 2017. Biological evidence for paradoxical improvement of psychiatric disorder symptoms by addictive drugs. Trends Pharmacol Sci. 38(6):501–502. [DOI] [PubMed] [Google Scholar]
- Müller CP, Schumann G. 2011. Drugs as instruments: a new framework for non-addictive psychoactive drug use. Behav Brain Sci. 34:293–310. [DOI] [PubMed] [Google Scholar]
- Nomura F, Kawai T, Nakanishi K, Akira S. 2000. NF-kappaB activation through IKK-i-dependent I-TRAF/TANK phosphorylation. Genes Cells. 25:191–202. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. 2003. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage. 19:1439–1448. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stewart JL. 2014. Interoception and drug addiction. Neuropharmacology. 76(Pt B):342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schulteis G. 2009. The role of interoception and alliesthesia in addiction. Pharmacol Biochem Behav. 94:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottier C, Bieniek KF, Finch N, van de Vorst M, Baker M, Perkersen R, Brown P, Ravenscroft T, van Blitterswijk M, Nicholson AM, et al. 2015. Whole-genome sequencing reveals important role for TBK1 and OPTN mutations in frontotemporal lobar degeneration without motor neuron disease. Acta Neuropathol. 130:77–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Crews FT. 2012. Chronic ethanol increases systemic TLR3 agonist-induced neuroinflammation and neurodegeneration. J Neuroinflammation. 9:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. 2009. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 373:2223–2233. [DOI] [PubMed] [Google Scholar]
- Riley AL. 2011. The paradox of drug taking: the role of the aversive effects of drugs. Physiol Behav. 103(1):69–78. [DOI] [PubMed] [Google Scholar]
- Rose G. 1981. Strategy of prevention: lessons from cardiovascular disease. Br Med J (Clin Res Ed). 282:1847–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salerno KM, Jing X, Diges CM, Davis BM, Albers KM. 2013. TRAF family member-associated NF-kappa B activator (TANK) expression increases in injured sensory neurons and is transcriptionally regulated by Sox11. Neuroscience. 231:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann G, Loth E, Banaschewski T, Barbot A, Barker G, Büchel C, Conrod PJ, Dalley JW, Flor H, Gallinat J, et al. 2010. The IMAGEN study: reinforcement-related behaviour in normal brain function and psychopathology. Mol Psychiatry. 15:1128–1139. [DOI] [PubMed] [Google Scholar]
- Schumann G, Coin LJ, Lourdusamy A, Charoen P, Berger KH, Stacey D, Desrivières S, Aliev FA, Khan AA, Amin N, et al. 2011. Genome-wide association and genetic functional studies identify AUTS2 in the regulation of alcohol consumption. Proc Natl Acad Sci USA. 108(17):7119–7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann G, Binder EB, Holte A, de Kloet ER, Oedegaard KJ, Robbins TW, Walker-Tilley TR, Bitter I, Brown VJ, Buitelaar J, et al. 2014. Stratified medicine for mental disorders. Eur Neuropsychopharmacol. 24(1):5–50. [DOI] [PubMed] [Google Scholar]
- Schumann G, Liu C, O'Reilly P, Gao H, Song P, Xu B, Ruggeri B, Amin N, Jia T, Preis S, et al. 2016. KLB is associated with alcohol drinking, and its gene product β-Klotho is necessary for FGF21 regulation of alcohol preference. Proc Natl Acad Sci USA. 13:14372–14377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif T, Chang SJ, Simms JA, Gibb SL, Dadgar J, Chen BT, Harvey BK, Ron D, Messing RO, Bonci A, et al. 2013. Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat Neurosci. 16(8):1094–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D'Agostino RB Sr, Fox CS, Larson MG, Murabito JM, et al. 2007. The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 165:1328–1335. [DOI] [PubMed] [Google Scholar]
- Stacey D, Bilbao A, Maroteaux M, Jia T, Easton AC, Longueville S, Nymberg C, Banaschewski T, Barker GJ, Büchel C, et al. 2012. RASGRF2 regulates alcohol-induced reinforcement by influencing mesolimbic dopamine neuron activity and dopamine release. Proc Natl Acad Sci USA. 109:21128–21133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, Juavinett AL, May AC, Davenport PW, Paulus MP. 2015. Do you feel alright? Attenuated neural processing of aversive interoceptive stimuli in current stimulant users. Psychophysiology. 52:249–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, May AC, Poppa T, Davenport PW, Tapert SF, Paulus MP. 2014. You are the danger: attenuated insula response in methamphetamine users during aversive interoceptive decision-making. Drug Alcohol Depend. 142:110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, May AC, Tapert SF, Paulus MP. 2015. Hyperactivation to pleasant interoceptive stimuli characterizes the transition to stimulant addiction. Drug Alcohol Depend. 154:264–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, Carroll AJ, Salmeron BJ, Ross TJ, Stein EA. 2013. Insula’s functional connectivity with ventromedial prefrontal cortex mediates the impact of trait alexithymia on state tobacco craving. Psychopharmacology (Berl). 228:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukdar S, Owen BM, Song P, Hernandez G, Zhang Y, Zhou Y, Scott WT, Paratala B, Turner T, Smith A, et al. 2016. FGF21 regulates sweet and alcohol preference. Cell Metab. 23:344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutlein J, Cichon S, Ridinger M, Wodarz N, Soyka M, Zill P, Maier W, Moessner R, Gaebel W, Dahmen N, et al. 2009. Genome-wide association study of alcohol dependence. Arch Gen Psychiatry. 66:773–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW Jr, Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, et al. 2012. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci. 32:7563–7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajant H, Henkler F, Scheurich P. 2001. The TNF-receptor-associated factor family: scaffold molecules for cytokine receptors, kinases and their regulators. Cell Signal. 13:389–400. [DOI] [PubMed] [Google Scholar]
- WHO 2014. Global status report on alcohol and health 2014.
- Zhao Y, Zheng ZL, Castellanos FX. 2017. Analysis of alcohol use disorders from the Nathan Kline Institute-Rockland Sample: correlation of brain cortical thickness with neuroticism. Drug Alcohol Depend. 170:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, Puppel A, Huber S, Link AS, Müller CP, Alzheimer C. 2016. Activin controls ethanol potentiation of inhibitory synaptic transmission through GABAA receptors and concomitant behavioural sedation. Neuropsychopharmacology. 41:2024–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Crews F. 2010. Induction of innate immune gene expression cascades in brain slice cultures by ethanol: key role of NF-κB and proinflammatory cytokines. Alcohol Clin Exp Res. 34(5):777–789. [DOI] [PubMed] [Google Scholar]
- Zoubarev A, Hamer KM, Keshav KD, McCarthy EL, Santos JR, Van Rossum T, McDonald C, Hall A, Wan X, Lim R, et al. 2012. Gemma: a resource for the reuse, sharing and meta-analysis of expression profiling data. Bioinformatics. 28:2272–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.