Abstract
Successful application of potent antibody-based T-cell engaging immunotherapeutic strategies is currently limited mainly to hematological cancers. One major reason is the lack of well-characterized antigens on solid tumors with sufficient cancer specific expression. Aberrantly O-glycosylated proteins contain promising cancer-specific O-glycopeptide epitopes suitable for immunotherapeutic applications, but currently only few examples of such antibody epitopes have been identified. We previously showed that chimeric antigen receptor T-cells directed towards aberrantly O-glycosylated MUC1 can control malignant growth in a mouse model. Here, we present a discovery platform for the generation of cancer-specific monoclonal antibodies targeting aberrant O-glycoproteins. The strategy is based on cancer cell lines engineered to homogeneously express the truncated Tn O-glycoform, the so-called SimpleCells. We used SimpleCells of different cancer origin to elicit monoclonal antibodies with selectivity for aberrant O-glycoproteins. For validation we selected and characterized one monoclonal antibody (6C5) directed to a Tn-glycopeptide in dysadherin (FXYD5), known to be upregulated in cancer and promote metastasis. While dysadherin is widely expressed also in normal cells, we demonstrated that the 6C5 epitope is specifically expressed in cancer.
Keywords: CRISPR-CAS9, dysadherin, FXYD5, GalNAc-T7, SimpleCell
Introduction
Classical cancer immunotherapy relies on monoclonal antibodies that can differentially or exclusively target cancer cells (Hendriks et al. 2017). Potent immunotherapeutic strategies are currently available with antibodies in drug-loaded formats (Pastan et al. 2006) and with immune cell engaging strategies such as chimeric antigen receptors (CAR) (Kalos and June 2013) and bispecific T-cell engaging antibodies (BiTE) (Baeuerle et al. 2009). These recent developments have imposed demands for antibodies targeting truly cancer-specific antigens due to risks of targeting normal cells (Steentoft et al. 2018). CAR T-cells have been very successful in treatments of hematologic malignancies targeting lineage-specific antigens such as CD19 on dispensable cells (Brentjens et al. 2013; Grupp et al. 2013; Davila et al. 2014; Maude et al. 2014), while application of CAR T-cells for the treatment of solid tumors face a number of challenges including lack of safe antigen targets (Maus and June 2016; Newick et al. 2017). This is in particular due to the finding that few if any proteins are exclusively expressed by tumor cells, and even low levels of expression in normal tissues may result in severe toxicity or even death (Morgan et al. 2010). Somatic cancer-specific mutations, often single nucleotide changes (Monach et al. 1995), in a cancer cell may introduce cancer-specific epitopes. These mutations are often unique for a given patient’s cancer and rarely shared by different cancer types, and they most commonly arise from mutations in genes encoding intracellular proteins that need to be recognized by T-cell receptors as mutant peptide–MHC complexes on the cancer cell surface. Currently therefore such epitopes are difficult to target, although recent developments in RNA encoded personal mutation vaccines suggest that personal mutations can be a feasible immunotherapy target in the future (Sahin et al. 2017).
Aberrant posttranslational modifications are common features of cancer and these may induce cancer-associated neoantigens. In particular aberrant O-glycosylation leading to exposure of truncated immature O-glycans that are not found on normal cells have attracted substantial attention (Posey et al. 2016; RodrÍguez et al. 2018; Steentoft et al. 2018). These truncated O-glycans include Tn (GalNAcα1-O-Ser/Thr), STn (NeuAcα2-6GalNAcα1-O-Ser/Thr) and T (Galβ1-3GalNAcα1-O-Ser/Thr) (Figure 1), and their expression may result in the exposure of immunodominant O-glycopeptide epitopes that are highly restricted to cancer cells (Springer 1984; Schietinger et al. 2006; Sørensen et al. 2006; Tarp et al. 2007; Tarp and Clausen 2008). The monoclonal antibody (mAb) 5E5 is a prominent example of an antibody targeting such an immunodominant Tn-glycopeptide epitope in the cancer-associated mucin MUC1 (Sørensen et al. 2006; Tarp et al. 2007). MAb 5E5 was originally generated by immunization with a chemoenzymatically produced glycopeptide (60-mer Tn-MUC1) coupled to Keyhole limpet hemocyanin (KLH), and it exhibits high-affinity binding to Tn-MUC1 (KD ~2 nM) (Lavrsen et al. 2013). Several other mAbs to aberrant O-glycosylated MUC1 have been generated (Taylor-Papadimitriou et al. 2018) including PankoMAb (Danielczyk et al. 2006), and MY.1E12 (Yamamoto et al. 1996; Takeuchi et al. 2002). Interestingly, spontaneous IgG antibodies to the Tn-MUC1 5E5 epitope may be found in cancer patients (Wandall et al. 2010; Pedersen et al. 2014), and recently we demonstrated that T-cells genetically engineered to express a CAR based on the mAb 5E5 were able to induce target-specific cytotoxicity and control malignant growth in xenograft mouse models of leukemia and pancreatic cancer (Posey et al. 2016).
Fig. 1.
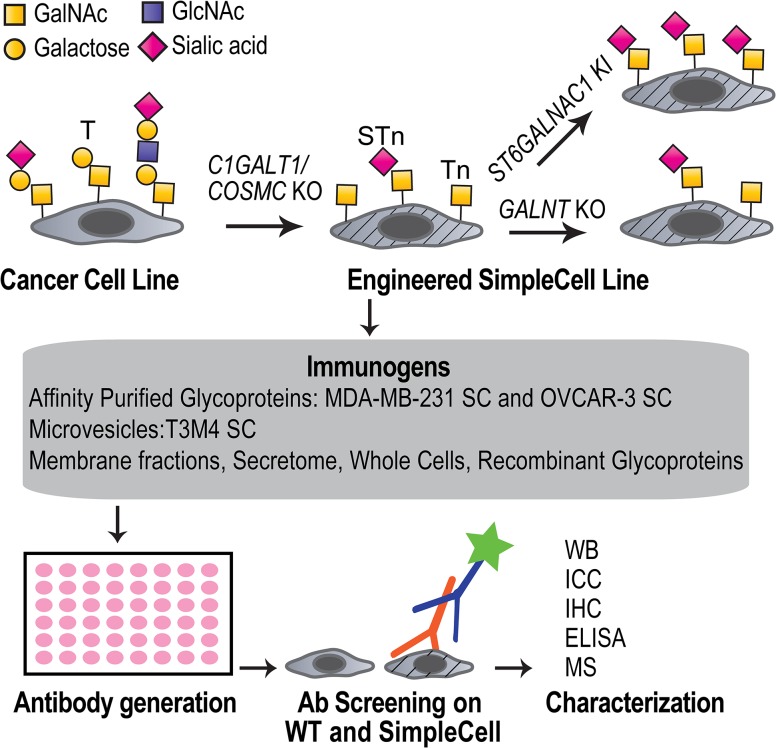
Depiction of the strategy to generate mAbs to aberrant O-glycoproteins. Cancer cell lines express a heterogeneous repertoire of O-glycan structures on the cell surface. SimpleCells are generated by targeted KO of COSMC/C1GALT1, which blocks the O-glycan elongation pathway and results in homogenous expression of the cancer-associated Tn and/or STn antigens, depending on the expression of ST6GALNAC1. The O-glycan site occupancy is controlled by the repertoire of GalNAc-Ts in the selected cell line and can also be engineered by targeted KO or KI of different GALNTs. The SCs provide an unlimited source of immunogens with homogenous cancer-associated O-glycans either in the format of different cell extracts or recombinant expressed and purified glycoproteins. O-glycopeptide specific antibodies can be selected by screening on WT and corresponding isogenic SC and further characterized. Monosaccharide symbols follow the CFG recommended symbol nomenclature (Varki et al. 2015).
In general the immature truncated Tn and STn O-glycan haptens are considered promising immunotherapeutic targets as they are widely expressed in cancers (Itzkowitz et al. 1991; Springer 1997; Chia et al. 2016; RodrÍguez et al. 2018), their expression correlate with poor prognosis and low overall survival (Springer 1984; Itzkowitz et al. 1990), and truncation of O-glycosylation directly induce oncogenic features including increased proliferation and invasion (Radhakrishnan et al. 2014). O-glycosylation is initiated in the Golgi by a family of 20 polypeptide GalNAc-transferase isoenzymes (GalNAc-Ts), and the elongated mature O-glycan structures found in normal cells are generated by successive actions of multiple glycosyltransferases located in the Golgi stacks (Tarp and Clausen 2008). The most abundant normal O-glycans are based on the core1 (Galβ1-3GalNAcα1-O-Ser/Thr) and core2 (Galβ1-3(GlcNAcβ1-6) GalNAcα1-O-Ser/Thr) structures that both rely on the core1 synthase, C1GalT1. C1GalT1 is unique among glycosyltransferases as it requires a private chaperone, Cosmc, for folding and activity (Wang et al. 2010). A number of different mechanisms appear to underlie the characteristic aberrant truncated O-glycosylation found in cancer cells, including changes in the expression, organization and location of the responsible glycosyltransferases (Burchell et al. 1999; Marcos et al. 2004; Sewell et al. 2006; Gill et al. 2010, 2013), somatic mutations or hypermethylation of the COSMC gene (Ju and Cummings 2002, 2005, 2010; Ju et al. 2008; Radhakrishnan et al. 2014), as well as changes in cellular pH (Axelsson et al. 2001; Hassinen et al. 2011). Antibodies to the truncated O-glycan haptens are detectable in healthy individuals and are the basis of polyagglutinability with desialylated red blood cells (Dahr et al. 1975; Dausset et al. 1959) and titers of these increase in cancer patients (Springer 1984). The affinity of glycan hapten antibodies are generally in the low mM range (Haji-Ghassemi et al. 2015) which is in striking contrast to the high affinity achievable for mAbs targeting glycopeptide epitopes (Steentoft et al. 2018).
Here, we present a versatile strategy for discovery and generation of mAbs targeting cancer-specific truncated O-glycopeptide epitopes. The strategy employs glycoengineered cancer SimpleCell (SC) lines (Steentoft et al. 2011) displaying homogenous truncated O-glycans as a consequence of COSMC knockout (KO). We demonstrate a wide discovery potential of the strategy by using engineered breast, ovarian and pancreatic cancer SCs to generate six novel mAbs designated 6C5, 4B7, 2E2, 6G10, 1D5 and 5D10. We selected mAb 6C5 for detailed characterization and demonstrate how the epitope of such glycopeptide specific mAbs can be deciphered by combinatorial gene engineering using KO as well as knock-in (KI) in relevant cell models. MAb 6C5 was shown to be directed to a Tn-glycopeptide epitope in the cancer-associated dysadherin (FXYD5) cell membrane glycoprotein with dependence of a particular repertoire of GalNAc-Ts including the GalNAc-T7 isoform.
Results
SCs with homogenous O-glycoproteomes
We previously generated isogenic cancer SC lines with truncated O-glycans by KO of COSMC in human cell lines using precise gene editing strategies (Zinc finger nucleases (ZFN) or CRISPR/Cas9). The SCs express homogeneous Tn and/or STn O-glycans depending on the expression of ST6GALNAC1 in individual cell lines (Steentoft et al. 2011, 2014; Steentoft, Bennett, et al. 2013; Steentoft, Vakhrushev, et al. 2013). Here, we applied the SCs to develop a comprehensive strategy for the generation of cancer-specific mAbs towards aberrantly glycosylated O-glycoproteins. Gene engineering was used to investigate the effects of glycosites by KO of GALNT genes, and we engineered SCs with STn O-glycoproteomes by KI of ST6GALNAC1 (Figure 1).
Generation of mAbs
Two different classes of immunogens can be generated from engineered SCs: (i) endogenous glycoproteins presented as whole fixed cells, membrane extracts, microvesicles, secretomes or affinity purified glycoproteins; and (ii) recombinantly expressed O-glycoproteins (Figure 1). In the current study we used affinity enriched Tn-glycoproteins derived from cell lysates from breast (MDA-MB-231) and ovarian (OVCAR-3) cancer SCs (Steentoft, Vakhrushev, et al. 2013), as well as microvesicles purified from a pancreatic cancer (T3M4) SC (Steentoft et al. 2011). Affinity-enriched lysates were isolated by Triton X-100 extraction followed by lectin chromatography using the GalNAc-binding lectin Vicia villosa (VVA) and elution with GalNAc. While MDA-MB-231 SC only express the Tn-glycoform (Steentoft, Vakhrushev, et al. 2013), OVCAR-3 SC express a mixture of STn and Tn, and we therefore included pretreatment with neuraminidase prior to lectin binding. On the contrary, the purified microvesicles were not neuraminidase treated, which allowed for discovery of STn glycopeptide epitopes. Mice were immunized with these preparations and the obtained hybridoma supernatants were screened by immunocytochemistry on trypsinized acetone-fixed isogenic WT cells and SCs. Antibodies with preferred SC reactivity were selected for further analysis (Figure 1). We used secondary anti-IgG to deselect common IgM antibodies to truncated O-glycan haptens (Takahashi et al. 1988). When using microvesicles as the immunogen we obtained a significant number of hybridomas producing IgG Abs with strong binding to the T3M4 SC, however, only one of these clones, 5D10, did not react with T3M4 WT cells. When immunizing with affinity enriched Tn-glycoproteins, we obtained fewer reactive mAbs, however, multiple clones with preferred SC reactivity were isolated (Figure 2 and Supplementary data, Figures S1 and S2). While several reactive hybridomas were lost during subcloning we cloned and stabilized the following six mAbs: 6C5, 6G10, 4B7, 2E2 (MDA-MB-231 SC), 1D5 (OVCAR-3 SC) and 5D10 (T3M4 SC). All six mAbs were IgG1 and exhibited strong binding to the respective SC without or with very weak reactivity with the corresponding WT cells (Figure 2A and Supplementary data, Figures S1 and S2). MAbs 5D10 and 1D5 did not react by Western blot analysis suggesting involvement of non-linear epitopes (unpublished data). In contrast, mAb 4B7 and 2E2 appeared to react with the same two bands around 100 kDa in total cell lysates of MDA-MB-231 SC, mAb 6G10 reacted with a 62 kDa band (Supplementary data, Figure S2), and mAb 6C5 recognized a band migrating around 45 kDa (Figure 2). mAb 6C5 was selected for further characterization to serve as an illustrative example of how the binding specificity of glycopeptide mAbs can be elucidated by genetic engineering.
Fig. 2.
Generation and characterization of the mAb 6C5. (A) mAb 6C5 was characterized by immunocytochemistry staining on MDA-MB-231 SC and WT grown on cover slide using anti-Tn (mAb 1E3) as control. (B) Additional characterization was performed on a panel of acetone-fixed SCs including Colo-205, IMR-32, MCF7 and HepG2 with and without neuraminidase treatment. (C) IP was performed with mAb 6C5 on MDA-MB-231 SC lysates and analyzed by Western blotting staining with either mAb 6C5, the secondary anti-Ig-HRP alone or the anti-Tn lectin VVA.
Characterization of mAb 6C5
First we used mAb 6C5 to stain a panel of SCs from various tissue origins including HEK293, T3M4, HeLa, Colo-205, IMR-32, MCF7 and HepG2 (Steentoft et al. 2011; Steentoft, Vakhrushev, et al. 2013) with and without pretreatment with neuraminidase. While most SC lines were stained strongly, MCF7 SC displayed very faint staining and HepG2 SC was negative (Figure 2B), suggesting that mAb 6C5 recognized a specific Tn-glycoprotein. Neuraminidase enhanced the staining of 6C5 indicating that the preferred glycoform is Tn. Lack of STn reactivity by mAb 6C5 was confirmed by staining of HEK293 SC with KI of ST6GALNAC1 that predominantly expresses the STn glycoform (Supplementary data, Figure S3). Western blot analysis with MDA-MB-231 SC cell lysates showed that the 45 kDa band bound by 6C5 was also reactive with the Tn-binding lectin VVA. Moreover, mAb 6C5 immunoprecipitated (IP) the 45 kDa band (Figure 2C).
Mab 6C5 exhibits cancer-specific reactivity
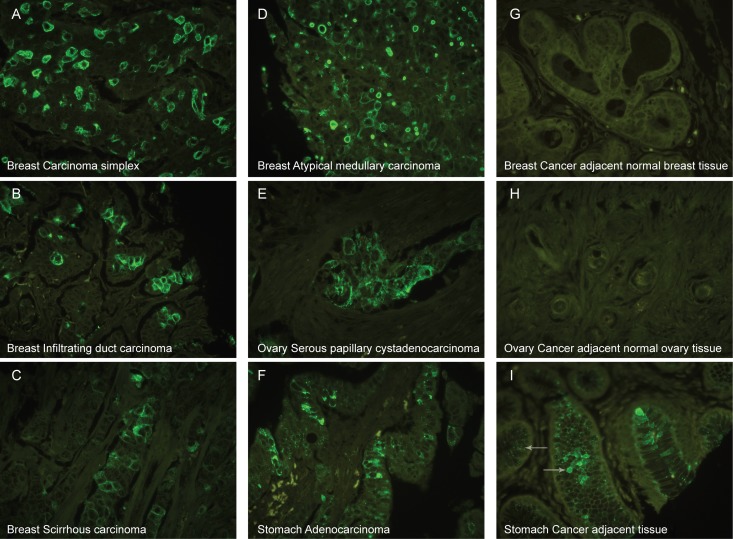
We performed a pilot immunohistological study using tissue microarrays with paraffin-embedded cores from four different types of breast cancer, three different types of ovarian cancer and adenocarcinomas of stomach as well as normal appearing tissue adjacent to cancer. The results are summarized in Table I and representative images displayed in Figure 3. mAb 6C5 was reactive with all three cancers (Figure 3A–F) with breast cancer having the highest number of positive cores (carcinoma simplex: 14/25, atypical medullary carcinoma: 6/13, infiltrating duct carcinoma: 6/13 and scirrhous carcinoma: 7/12). Ovarian cancer had fewer positive cores (serous papillary cyst adenomas: 10/47, mucinous carcinomas: 3/6 and endometrioid adenocarcinomas: 4/7). In stomach 7/22 adenocarcinomas were stained. The percentage of positive cells in all the tested tumors varied from less than 30% to more than 60% (Table I). The staining was mainly membraneous and cytoplasmic, although a subset of the cancer cores only showed a weak punctuate granular intracellular staining (Table I). In a few cancer cores mAb 6C5 labeled vascular endothelium and single dispersed cells possibly representing immune cells or detached cancer cells.
Table I.
Summary of immunohistology with mAb 6C5
| Negative | <30% | 30–60% | >60% | ||
|---|---|---|---|---|---|
| Tissues | Cases | #(%) | #(%) | #(%) | #(%) |
| Breast | |||||
| Cancer | 68 | 30 (44%)a | 21 (31%) | 10 (15%) | 7 (10%) |
| Normal | 8 | 8 (100%)b | 0 (0%) | 0 (0%) | 0 (0%) |
| Ovary | |||||
| Cancer | 59 | 39 (66%)c | 18 (31%) | 1 (2%) | 1 (2%) |
| Normal | 10 | 10 (100%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Stomach | |||||
| Cancer | 22 | 15 (68%)d | 3 (14%) | 2 (9%) | 2 (9%) |
| Normal | 45 | 45 (100%)e | 0 (0%) | 0 (0%) | 0 (0%) |
The tissue microarray cores were classified according to apparent cell surface membrane staining.
aOne core had granular intracellular staining.
bTwo cores had granular intracellular staining.
c11 Cores had granular intracellular staining.
dFour cores had granular intracellular staining.
e15 Cores had strong Golgi-like staining of mucous producing cells. Four of those also had homogeneous staining throughout the cytoplasm (Figure 3).
Fig. 3.
Immunohistochemistry with mAb 6C5. Tissue microarrays of breast cancer tissues (A–D), ovarian cancer (E), stomach cancer (F), and the corresponding tumor adjacent normal tissue (G–I) stained with mAb 6C5. MAb 6C5 showed cell surface immunofluorescence in four types of breast cancers, i.e. carcinoma simplex (A), infiltrating duct carcinoma (B), scirrhous carcinoma (C), atypical medullary carcinoma (D) as well as serous papillary cystadenocarcinoma from ovary (E) and adenocarcinoma from stomach (F). Cancer adjacent normal breast (G) and ovary tissue (H) did not react with mAb 6C5, whereas cancer adjacent normal appearing stomach tissue presented with strong intracellular granular staining in most mucous producing cells (left pointing arrow) and a few of those also gave a more homogenous pattern throughout the cell (right pointing arrow) (I).
Eight normal breast cores were examined, six of which were completely negative (Figure 3G), while two cores showed a very faint granular intracellular staining in few cells with mAb 6C5. Normal appearing ovarian tissues were completely negative (10/10) (Figure 3H). In normal appearing stomach strong intracellular Golgi-like staining was observed with mAb 6C5 in mucous producing cells (15/41), and in 4 of those cores a small fraction of mucous producing cells stained homogenously throughout the cytoplasm (Figure 3I). No vascular endothelium staining was observed in any of the normal tissue cores.
The 6C5 glycopeptide epitope on FXYD5 requires the function of GalNAc-T7
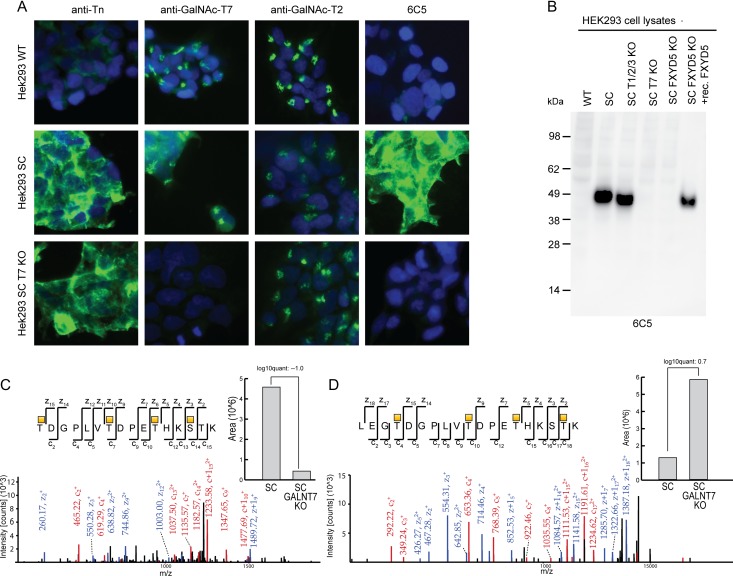
To further identify the O-glycoprotein and epitope recognized by mAb 6C5, we screened a panel of engineered isogenic HEK293 SC with different repertoire of GalNAc-Ts (Narimatsu et al., in preparation), and surprisingly found that reactivity was almost abolished in HEK293 SC with KO of GALNT7 (Figure 4A). This finding was confirmed by Western blot analysis where the ~45 kDa immunoreactive band was undetected in lysates from HEK293 SC with KO of GALNT7 as well as in HEK293 WT cells with elongated O-glycans. Triple KO of the most abundant and broadly active GALNTs (GALNT1, T2 and T3) merely resulted in a slight reduction in the size of the immunoreactive band (Figure 4B).
Fig. 4.
The mAb 6C5 epitope is dependent on GalNAc-T7 glycosylation. KO of GALNT7 in HEK293 SC (SC T7 KO) removed mAb 6C5 staining as shown in panel (A) using anti-Tn (mAb 5F4), anti-GalNAc-T7 (mAb 8B8) and anti-GalNAc-T2 (mAb 4C4) as controls. (B) The lack of 6C5 reactivity upon GALNT7 KO was confirmed by Western blot on HEK293 cell lysates were triple KO of GALNT1, GALNT2 and GALNT3 (SC T1/2/3 KO) did not affect 6C5 reactivity. (C, D) MS spectra from a quantitative differential glycoproteome analysis of HEK293 SC and HEK293 SC GALNT7 KO. The figure depicts the two spectra obtained for FXYD5 with the largest values for quantification difference.
We have in a separate study (Narimatsu et al., in preparation) performed differential O-glycoproteome analysis of HEK293 SC with and without the GaNAc-Ts known to be expressed in HEK293, including GalNAc-T7, using dimethyl labeled glycoproteins, lectin affinity chromatography and mass spectrometry (MS) sequencing as previously described (Schjoldager et al. 2015). The differential O-glycoproteome identified a number of GalNAc-T7 specific substrates, and one of these glycoproteins, FXYD5 (also known as dysadherin), was previously reported to migrate at 50 kDa by SDS-PAGE Western blot analysis (Akopov et al. 2006). Specific FXYD5 glycopeptides, e.g. T*DGPLVT*DPET*HKS*TK (*GalNAc) (Figure 4C) and T*DGPLVT*DPET*HKST*K showed minor differences in relative abundance after KO of GALNT7 (Figure 4D), however, when analyzing all the FXYD5 glycopeptides quantified by MS we considered these within the technical variability of the method, making it impossible to predict the specific glycopeptide epitope recognized by 6C5. We used an anti-FXYD5 mAb (NCC-M53) (Ino et al. 2002; Shimamura et al. 2003) to confirm by Western blot analysis (Figure 5A) and flow cytometry (Figure 5B) the expression of FXYD5 and demonstrate that the expression was unaffected by KO of GALNT7 in HEK293 SC. Both mAb 6C5 and NCC-M53 immunoprecipitated the 45 kDa band from HEK293 SC, however, the ability of 6C5 to immunoprecitate the 45 kDa band was selectively lost by KO of GALNT7 (Figure 5C).
Fig. 5.
mAb 6C5 specifically recognizes a GalNAc-T7 dependent epitope on FXYD5. (A) Western blot of cell lysates of HEK293 WT, HEK293 SC and HEK293 SC with either combinatorial KO of GALNT1, GALNT2 and GALNT3 (SC T1/2/3 KO) or HEK293 SC with KO of GALNT7 (SC T7 KO) or FXYD5 (SC FXYD5 KO) stained with an anti-FXYD5 mAb (NCC-MC53) or mAb 6C5. An anti-GAPDH mAb was included as loading control. (B) Flow cytometry of HEK293 WT, HEK293 SC, HEK293 SC GALNT7 KO and HEK293 SC FXYD5 KO stained with NCC-MC53 or mAb 6C5. (C) IP using NCC-MC53 or mAb 6C5 and HEK293 SC or HEK293 SC GALNT7 KO cell lysates as input and detection with NCC-MC53 or mAb 6C5. (D) HEK293 SC and HEK293 FXYD5 KO stained by immunocytochemistry using NCC-MC53, mAb 6C5 and an anti-Tn lectin (VVA). (E) A full length FXYD5 construct was expressed in the HEK293 SC FXYD5 KO cells and IP was performed on cell lysate from either HEK293 SC or HEK293 SC FXYD5 KO with recombinant FXYD5 and detected with NCC-M53, 6C5 or anti-Tn (VVA). (F) A 30-mer peptide covering aa 81–110 of FXYD5 (TDGPLVTDPETHKSTKAAHPTDDTTTLSER) was purchased and in vitro glycosylated with either GalNAc-T1 or GalNAc-T1 and T7 in combination. Peptides and glycopeptides were tested for 6C5 and anti-Tn reactivity (VVA) by ELISA including a MUC1 20mer 3Tn glycopeptide (AHGVTSAPDTRPAPGSTAPP) as control.
To confirm the binding specificity of mAb 6C5 for FXYD5 we next targeted the FXYD5 gene by KO in HEK293 SC. The FXYD5 KO was validated by DNA sequencing as well as immunocytochemistry (Figure 5D), flow cytometry (Figure 5B), and Western blot analysis (Figure 5A). While KO of FXYD5 completely abolished 6C5 staining as evaluated by immunochytochemistry and Western blot analysis, we did observe weak residual reactivity by flow cytometry (Figure 5B). We could fully restore mAb 6C5 reactivity by expression of a full length FXYD5 expression construct in HEK293 SC with KO of FXYD5 (Figures 4B and 5E). Surprisingly, overexpression of FXYD5 resulted in two major bands reactive with NCC-M53 mAb, while mAb 6C5 only reacted with the upper 45 kDa band. We therefore tested reactivity of these bands with the anti-Tn lectin VVA and found that only the upper band was reactive with VVA indicating that the lower band represented an unglycosylated form of FXYD5.
The calculated size of the protein core of FXYD5 is <20 kDa, however, a significantly larger size (50–55 kDa) has been reported in human cancer cells (Ino et al. 2002; Gabrielli et al. 2011). The larger size is likely related to the dense O-glycosylation of FXYD5 (Tokhtaeva et al. 2016), in agreement with our findings here. We did find though that the lower molecular weight form without apparent O-glycans migrated at ~32 kDa, suggesting the presence of other posttranslational modifications (Lubarski Gotliv 2016) or simple abnormal electrophoretic mobility of FXYD5 (Lubarski et al. 2005).
To further narrow down the binding epitope of mAb 6C5 we predicted that the epitope could be placed in a sequence of FXYD5 (FXYD581–110: TDGPLVTDPETHKSTKAAHPTDDTTTLSER) where we identified multiple O-glycosylation sites by MS. We used the FXYD581–110 peptide as substrate for in vitro enzyme assays monitored by MALDI-TOF. We tested the three ubiquitous expressed and dominant GalNAc-T1, -T2 and -T3 isoenzymes alone as well as in combination with the GalNAc-T7. The GalNac-T7 isoenzyme is known to function as an follow-up enzyme and requiring initial GalNAc residues attached in order to function (Bennett, Hassan, Hollingsworth, et al. 1999). We observed no glycosylation of the peptide with GalNAc-T7 alone, while GalNAc-T1 incorporated multiple GalNAc residues (Supplementary data, Figure S4), GalNAc-T2 a single residue and GalNAc-T3 none (not shown). Glycosylation with the combination of GalNAc-T1 and GalNAc-T7 resulted in a heterogeneous mixture of GalNAc-glycopeptides with 0–8 GalNAc residues incorporated (Supplementary data, Figure S4). The MS spectrum of this heterogeneous mixture was difficult to unambiguously interpret and could not define the specific glycosites contributed by GalNAc-T7. We therefore tested 6C5 reactivity in ELISA with purified glycopeptides from the reaction mixtures (Figure 5F), and while mAb 6C5 did not react with unglycosylated FXYD581–110 peptide or the peptide glycosylated with GalNAc-T1 alone, it showed strong reactivity towards the FXYD581–110 peptide glycosylated with both GalNAc-T1 and T7. Thus, narrowing the epitope of 6C5 down to the 81–110 region of FXYD5 and requiring complex regulated O-glycosylation involving GalNAc-T7 in agreement with the studies of HEK293 cells with KO of GALNTs (Figure 4). MAb 6C5 showed weak reactivity with the control Tn-MUC1 glycopeptide, which may represent cross-reactivity with the Tn-hapten structure at high concentrations as also found for other Tn-glycopeptide specific mAbs (Sørensen et al. 2006).
Discussion
Here, we present a strategy to generate cancer-specific mAbs directed to truncated O-glycopeptide epitopes using gene edited cancer cells displaying homogeneous truncated O-glycoforms. The strategy can be applied to any desired cancer cell line, designed to target the three most cancer-associated O-glycans (Tn, STn, T) as well as different patterns of O-glycans by manipulation of the GalNAc-T repertoire. A broad selection of immunogens, ranging from whole cells to purified recombinant glycoproteins, can be extracted from the engineered cells and used for immunization and screening. The strategy provides pairs of isogenic cells suitable for rapid screening and selection of antibodies targeting aberrant O-glycopeptide epitopes. We applied the strategy with different types of complex immunogens and obtained six IgG1 mAbs with selective reactivity to cancer cells displaying homogeneous Tn/STn-glycoforms of their proteomes. Importantly, by screening only for IgG mAbs the common natural IgM Tn-hapten antibodies are avoided (Takahashi et al. 1988; Wandall et al. 2010).
Given the potential of truncated O-glycopeptide epitopes as high-affinity cancer-specific immune targets, it may be surprising that antibodies targeting these have been elusive after decades of attempts to produce mAbs to cancer cells. The major explanation likely lies in the great heterogeneity in O-glycosylation both with respect to glycosylation sites and structures as well as technical difficulties in characterization and validation of antibodies targeting such epitopes (Steentoft et al. 2018). Only recently it has become possible to produce recombinant O-glycoproteins with a homogenous Tn glycoform (Yang et al. 2014), and in vitro chemoenzymatic generation of glycopeptide immunogens are still not practical for proteome-wide discoveries. Moreover, antibodies directed to aberrant O-glycopeptide epitopes may often be deselected in screening because of difficulties in validating the protein target. Thus, previous studies using unmodified cancer cell lines have mainly generated low affinity antibodies to O-glycan haptens (Hirohashi et al. 1985), although exceptions exist (Matsuura and Hakomori 1985). There are still only a few examples of well-characterized mAbs with restricted specificities for Tn-glycopeptide epitopes (Matsuura and Hakomori 1985; Sørensen et al. 2006; Brooks et al. 2010; Steentoft et al. 2018). In a study using the Jurkat lymphoblastoid cell line, which is a spontaneous COSMC mutant, several Tn-hapten mAbs as well as two mAbs with apparent O-glycopeptide specificity were generated after multiple complicated screening steps (Blixt et al. 2011). Originally, in an attempt to develop a synthetic Tn-hapten vaccine we used a highly clustered Tn-glycopeptide based on the MUC2 tandem repeat sequence, however, we exclusively found antibodies directed to Tn-MUC2 glycopeptide as exemplified by the PMH1 mAb (Reis et al. 1998). This led us to develop a panel of mAbs to Tn-MUC1 using defined glycopeptides coupled to KLH as immunogens (Sørensen et al. 2006; Tarp et al. 2007), and the same strategy was used to develop cancer-specific IgG Tn-MUC1 responses in humans (Sabbatini et al. 2007; Wandall et al. 2010). Other mAbs with truncated O-glycopeptide specificities include MY.1E12 to the ST glycoform of MUC1 (Takeuchi et al. 2002), PankoMAb to Tn/T glycoforms of MUC1 (Danielczyk et al. 2006), and UN1 to Tn/T glycoforms of CD43 (Tassone et al. 1994; de Laurentiis et al. 2011). The mAb 237 directed to a Tn-glycopeptide in murine podoplanin was discovered in a mouse spontaneous fibrosarcoma model as a result of a mutation in Cosmc (Schietinger et al. 2006), and the Tn-glycopeptide epitope was shown to be immunodominant in mice eliciting a high-affinity IgG response specific to the Tn-glycopeptide (Steentoft et al. 2010). The mAb 237 has furthermore shown promising results in a CAR and BiTE format (Stone et al. 2012). A mAb towards sialylated human podoplanin has been reported (Kaneko et al. 2017) with apparent binding to the orthologous region of the epitope in the murine protein reactive with mAb 237 (Steentoft et al. 2010). One of the first O-glycopeptide specific mAbs, FDC-6, was developed already in 1985 to an oncofetal form of fibronectin (Matsuura and Hakomori 1985), and only after extensive work shown to react specifically with a Tn-hexapeptide located in the III CS variable region of fibronectin (Matsuura et al. 1988). Almost 10 years later it was shown that this O-glycopeptide epitope is specifically induced by de novo expression of the closely related GalNAc-T isoenzymes, GalNAc-T3 and GalNAc-T6 (Wandall et al. 1997; Bennett, Hassan, Mandel, et al. 1999). The expression of truncated O-glycopeptide epitopes thus not only depends on changes in O-glycan elongation but may also depend on the O-glycosylation sites occupied and require a specific GalNAc-T repertoire, as also demonstrated here for mAb 6C5. This clearly complicates discovery and validation of these epitopes and antibodies, but it also provides for a larger epitome space and increased cell and protein-specific regulation for this class of mAbs.
All current mAbs to Tn-glycopeptide epitopes exhibit characteristic cancer-associated or cancer-specific reactivity, and their discoveries were largely serendipitous in nature. There is little information guiding searches for such immunogenic aberrant O-glycopeptide epitopes, and the approach described here using isogenic SCs now provides an open discovery platform for mining the cancer truncated O-glycoproteome. The 6C5 Tn-glycopeptide epitope identified in dysadherin/FXYD5 illustrates the power of the strategy. FXYD5 is a type I membrane protein, part of the FXYD family of which all seven members are known to interact and affect the kinetic properties of the Na+/K+-ATPase (Garty and Karlish 2006). The extracellular domain of FXYD5 is extensively glycosylated, and while the calculated molecular weight is ~20 kDa, FXYD5 has been reported to migrate at 50–55 kDa in human cancer cells (Ino et al. 2002; Gabrielli et al. 2011). FXYD5 was initially named dysadherin due to its ability to reduce cell adhesion and promote metastasis (Ino et al. 2002; Tsuiji et al. 2003), through E-cadherin dependent (Ino et al. 2002; Tamura et al. 2005) as well as independent mechanisms (Nam et al. 2006; Tokhtaeva et al. 2016). Several clinical studies have correlated FXYD5 upregulation with cancer progression and poor patient outcome (Ino et al. 2002; Sato et al. 2003; Shimamura et al. 2003; Nakanishi et al. 2004; Shimada, Hashimoto, et al. 2004; Shimada, Yamasaki, et al. 2004; Wu et al. 2004; Batistatou et al. 2005, 2006; Nishizawa et al. 2005; Nam et al. 2007; Mitselou et al. 2010; Maehata et al. 2011; Park et al. 2011; Lee et al. 2012), and FXYD5 overexpression has been associated with oncogene activation or tumor suppressor mutations (Robinson et al. 2003; Bild et al. 2006), altogether suggesting that FXYD5 could be a promising target for cancer immunotherapy. However, FXYD5 is also widely expressed in normal tissue (Lubarski et al. 2005), and traditional antibodies targeting the core protein would be expected to result in adverse effects. In contrast, mAb 6C5 developed here targeting a cancer-specific Tn-glycoform of FXYD5 may provide a safe therapeutic target even with potent T-cell engaging strategies.
The finding that the epitope of mAb 6C5 requires the specific function of the GalNAc-T7 isoform introduces the additional question of expression of GALNT7 in normal tissues and cancer. Several GalNAc-T isoforms have been shown to be dysregulated in cancer (Sutherlin et al. 1997; Gomes et al. 2009), and studies have implicated some isoforms in oncogenic processes (Taniuchi et al. 2011; Matsumoto et al. 2012; Lavrsen et al. 2018), however, little is known about GalNAc-T7. One study in human melanoma cells proposed that GalNAc-T7 is targeted by a microRNA, miR-30b/d, and that expression of miR-30b/d, and the consequent reduction of GalNAc-T7, results in increased metastatic behavior (Gaziel-Sovran et al. 2011). On the contrary other studies have suggested that miR-214 targets GalNAc-T7 and decreases cell migration and invasion in cervical and esophageal cancer (Peng et al. 2012; Lu et al. 2016). MAb 6C5 stained more than 50% of all the breast cancer cores tested in this study, but interestingly the number of positive cells varied from a few single cells to more than 60% of the tumor (Figure 3 and Table I). A more thorough histopathological study would be required to determine if 6C5 positive cells correlate with FXYD5 expression, differentiation status or metastatic potential as well as the relation to expression of GalNAc-T7. To verify surface expression in tissue, a confocal microscopy study should be performed, although the data presented here using FACS on unpermeabilized cells clearly indicate surface expression of the 6C5 antigen. Nevertheless, mAb 6C5 exhibited no binding with most normal tissue cores tested, apart from a strong intracellular Golgi-localized staining in normal stomach tissue and a few mucous producing cells that were stained homogenously throughout the cytoplasm (Figure 3). This pattern of reactivity is comparable to what we have observed with similar Tn-glycopeptide specific mAbs including mAb 5E5 to Tn-MUC1, and a key question for the future is to determine safety of these mAbs with potent immunotherapeutic measures. We have though demonstrated that use of mAb 5E5 as a CAR T-cell did not induce lysis of normal human primary cells including renal epithelial cells and pulmonary endothelial cells (Posey et al. 2016). Thus, the strategy presented for generation of mAbs targeting aberrant O-glycopeptide epitopes may become a valuable tool for meeting the need for safe cancer-specific immune targets.
Material and methods
Cell line engineering
Human cell lines and corresponding COSMC KO (SC) and GALNT7 KO were cultured and generated by Zink Finger Nuclease based engineering as previously described (Steentoft et al. 2011; Steentoft, Bennett, et al. 2013; Steentoft, Vakhrushev, et al. 2013). KO of GALNT1, GALNT2, GALNT3 and FXYD5 in HEK293 SC were made using the CRISPR-Cas9 system as described elsewhere (Lonowski et al. 2017; Narimatsu et al. 2018). The gRNA targeting FXYD5: 5′-TCGTTGGCCTGATTCTCCCC-3′ were designed by a Desktop Genetics-Horizon Discovery algorithm. KO clones with frame-shift mutations were identified by Indel Detection by Amplicon Analysis (IDAA) (Yang et al. 2015) and subsequent sequencing using the following primers; Fw:5′-GCCAGAGGTTTTTGCTCAGG-3′, Rv:5′-CAGGACAACGTTCACACGG-3′. We used a modified ObLiGaRe targeted KI strategy as previously described (Pinto et al. 2017), utilizing two inverted ZFN binding sites flanking the ST6GALNAC1 full open reading frame in donor plasmid. The ST6GALNAC1 donor plasmid was transfected into HEK293 SC. The KI clones were screened by the immunocytochemistry staining with anti-STn (mAb 3F1) and then confirmed the targeted integration into the AAVS1 site with 5′ and 3′ junction PCR (Narimatsu et al., in preparation).
Immunogen preparation
Cell lysis of cell pellets (4× T175 MDA-MB-231 SC or 2× T175 OVCAR-3 SC) were made in 1% Triton X-100 in lectin buffer (20 mM Tris–HCl pH 7.4, 150 mM NaCl, 1 M Urea, 1 mM CaCl2, MgCl2, MnCl2 and ZnCl2) and protease inhibitor (cOmplete, EDTA-free (Roche)). The lysates were diluted with lectin buffer to a final concentration of 0.1% Triton X-100 and the OVCAR-3 SC lysate neuraminidase treated 1.5 h, 37°C with 0.01 U/mL neuraminidase (Clostridium perfringens, type VI (Sigma)). Samples were passed over 500 μL VVA coupled agarose beads (Vector Laboratories), preequilibrated with lectin buffer containing 0.1% Triton X-100. The beads were subsequently washed and eluted with 2 × 1 mL 0.4 M GalNAc in 20 mM Tris–HCl pH 7.4, 150 mM NaCl and 0.1% Triton X-100. Glycoprotein enrichment was confirmed by western blot by VVA detection and eluted glycoproteins were used for immunization.
Microvesicles were purified from 500 mL of serum free (48 h) cell culture medium from T3M4 SC by five centrifugation steps (10′ 150 × g, 30′ 300 × g, 30′ 850 × g, 30′ 10,000 × g, 1 h 100,000 × g all at 4°C). The obtained pellet was re-dissolved in PBS and used for immunization.
Immunization and mAb generation
Wild-type BALB/c mice were immunized with a single intraperitoneal injection of 20–40 μg protein of VVA-enriched glycoproteins (MDA-MB-231 SC and OVCAR-3 SC) or with a subcutaneous injection of microvesicle fraction (T3M4 SC) in a total volume of 200 μL (1:1 mix with Freunds adjuvant) three times, 3 weeks apart and finally an intraperitoneal boost without adjuvant. Three days after the fourth immunization splenocytes from one mouse were fused with NS1 myeloma cells as described previously (Vester-Christensen et al. 2013). Hybridoma supernatants were screened by immunocytochemistry on trypsinized and acetone-fixed cells (Steentoft, Bennett et al. 2013) after 10–12 days of culture using goat antimouse IgG-FITC (SouthernBiotech) as secondary antibody. Hybridomas producing Abs with significant reactivity to SC and not to WT cells were subjected to at least two limiting dilutions. Six mAb producing clones were finally selected for further characterization: 6C5, 2E2, 4B7, 6G10 (MDA-MB-231 SC), 1D5 (OVCAR-3 SC) and 5D10 (T3M4 SC) and all six were found to secrete IgG1.
Antibody characterization
For immunocytochemistry, cells were seeded on sterile coverslips coated with type I collagen (0.4 mg/mL) and cultivated for 2–3 days. The cells were then washed in cold PBS and fixed in cold 4% PFA for 10 min. After washing in cold PBS, the primary antibody (1 μg/mL (NCC-M53 established at National Cancer Center, Tokyo, Japan), 0.5 μg/mL VVA-biotin, 10 or 100 × diluted hybridoma supernatant (6C5), undiluted hybridoma supernatant (5F4, 1E3, 4C4, 8B8) was added ON 4°C. After washing, the secondary antibody was added (anti-Mouse Ig-FITC (Dako)), 1:400 dilution in 2.5% BSA in PBS or 1:2000 strepdavidin-Alexa488 (Life technologies)) and incubated for 45 min, washed and mounted with ProLong® Gold Antifade Mountant with DAPI (Life Technologies).
For immunohistochemistry, formalin fixed paraffin embedded tissue microarrays (Biomax) or were heated at 60°C 30 min, dewaxed, rehydrated and subjected to antigen retrieval by microwave treatment at pH 6 (citrate buffer). Sections were blocked with 10% calf serum, incubated overnight at 4°C with primary antibody (undiluted supernatant), rinsed and incubated with anti-Mouse Ig-FITC, 1:200 dilution in 2.5% BSA/PBS and incubated for 45 min. Slides were washed and mounted in Vectashield (Vector Laboratories, Inc). All images were acquired using Zeiss Axioscope 2 plus with an AxioCam MRc (40×).
For flow cytometry, cells were harvested by trypsin, washed in PBS, blocked in FACS buffer (2% Fetal bovine serum in PBS) and antibody added 45 min on ice (1 μg/mL (NCC-MC53), 1:100 × diluted hybridoma supernatant (6C5)). Cells were washed in FACS buffer, incubated 35 min on ice with anti-Mouse Ig-FITC, washed and analyzed on a FACS Calibur (Becton-Dickinson, Franklin Lakes, USA).
Cell lysis and subsequent IP was performed following a modified protocol (Marcon et al. 2015). Cells were lysed in 1× high salt lysis buffer (10 mM, Tris–HCL, 420 mM NaCl, 0.1% NP-40), and protease inhibitor cocktail (cOmplete, EDTA-free (Roche)), subjected to 3× freeze–thaw cycles and sonication. The lysate was centrifuged, 13,000 rpm 10 min, and the protein concentration was measured by 280 nm absorption on nanodrop. For IP 800 μg of lysate was incubated with 1 μg of antibody (or 50 μL hybridoma supernatant) and incubated for 4 h 4°C. The 15 μL of DynabeadsTM Protein G (Invitrogen) were washed 3× in low salt lysis buffer (10 mM Tris–HCL, 100 mM NaCl, 0.1% NP-40), added to the lysate-Ab mix and incubated ON 4°C. Beads were washed with 4× low salt lysis buffer and eluted in 60 μL 0.5 M ammonium hydroxide or Novex NuPAGE LDS Sample Buffer with 20 mM DTT.
For western blot, samples were mixed to a final concentration of 1x Novex NuPAGE LDS Sample Buffer and 20 mM DTT. After denaturation at 96°C for 10 min, the samples were loaded into a NuPAGE Bis-Tris 4–12% gel (Invitrogen) and electrophoresis was carried out at 200 V for 35 min. The proteins were transferred onto a nitrocellulose membrane at 30 V for 90 min and the membrane blocked in 5% skim milk (for antibody detection) or 1% polyvinylpyrrolidone (for VVA detection) in TBS-T. The membrane was incubated with primary antibody (1 μg/mL (NCC-MC53, VVA-biotin), 0.1 μg/mL (anti-GAPDH, FL-335 Santa cruise biotechnology), 10× diluted hybridoma supernatant (6C5)) in blocking buffer at 4°C overnight, washed and incubated with the secondary layer at room temperature for 1 h (rabbit antimouse Ig-HRP, goat anti-rabbit Ig-HRP or streptavidin-HRP (Dako, 1:4000 dilution)) and developed using the Thermo Scientific Pierce ECL Western Blotting Substrate kit.
GalNAc-T7 differential O-glycoproteome
The differential O-glycoproteome for GALNT7 KO cells were prepared and analyzed as previously described (Schjoldager et al. 2015). Briefly, reduced and alkylated tryptic digest of HEK293 SC or HEK293 SC GALNT7 KO were labeled with light and medium isotopomeric dimethyl. The labeled digests were mixed in 1:1 ratio and glycopeptides were enriched on a VVA lectin agarose column (Steentoft et al. 2011), followed by isoelectic focusing fractionation (Vakhrushev et al. 2013), and LC–MS and HCD/ETD–MS/MS analysis. Detailed methods are presented in (Narimatsu et al., in preparation).
FXYD5 recombinant protein and glycopeptides
Full length FXYD5 with a C-terminal Myc-tag was cloned into the PTT5 vector. HEK293 SC FXYD5 KO cells were transfected with 1 μg of DNA using lipofectamin® 3000 and cells were harvested after 48 h. Cell lysis, IP and western blot were performed as described above.
A 30-mer peptide, FXYD581–110, (TDGPLVTDPETHKSTKAAHPTDDTTTLSER) (SynPeptide) covering the identified glycosylation sites of FXYD5 was used for in vitro glycosylation using recombinant glycosyltransferases expressed as purified soluble secreted truncated proteins in insect cells, as previously described (Tarp et al. 2007; Pedersen et al. 2011). Glycosylation of the peptide was performed in a reaction mixture (100 μL) containing 25 mM cacodylate buffer (pH 7.4), 10 mM MnCl2, 0.25% Triton X-100, 0.4 mg/mL FXYD581–110 peptide, 12 μg/mL GalNAc-Ts and 2 mM UDP-GalNAc. The reactions were incubated at 37°C, and product development was monitored by a time-course MALDI-TOF MS (Bruker, Autoflex), as previously described (Kong et al. 2015).
For ELISA assays, 100 μg of peptide was glycosylated with either GalNAc-T1 alone or in combination with GalNAc-T7, and resulting glycopeptides purified by C18 (Phenomenex, Jupiter, 5 μm, 300 Å, 250 mm) UHPLC (Thermo, Ultimate™ 3000). 96-well plates (MaxiSorp, Nunc) were coated overnight with peptides or glycopeptides diluted in coating buffer (Na2CO3-buffer pH 9.6) in the given concentration at 4°C ON. Plates were blocked with 150 μL/well PLI-P-buffer (PO4-buffer pH 7.4, Na/K, 1% Triton-X, 1% BSA) for 1 h at room temperature and incubated 1 h with the primary layer (0.5 μg/mL, VVA-biotin or 6C5). After 1 h of incubation with secondary layer (1:4000 Streptavidin-HRP or anti-IgG-HRP), the plates were developed with TMB+ chromogen (Dako) and stopped with 0.5 M H2SO4 and read at 450 nm. All washing steps were performed with PBS-T (PBS-buffer pH 7.4, 0.05% Tween-20).
Supplementary Material
Acknowledgements
We would like to thank Yoshinori Ino, MD, PhD and late Setsuo Hirohashi, MD, PhD, (National Cancer Center Research Institute, Tokyo, Japan) for providing the NCC-M53 mAb.
Abbreviations
- BiTE
bispecific T-cell engaging
- CAR
chimeric antigen receptor
- GalNAc-T
N-acetylgalactosamine transferase
- IP
immunoprecipitation
- KI
knock-in
- KLH
Keyhole limpet hemocyanin
- KO
knock-out
- mAb
monoclonal antibody
- MS
mass spectrometry
- PTM
post-translation modification
- SC
SimpleCell
- STn
NeuAcα2-6GalNAcα1-O-Ser/Thr
- T
Galβ1-3GalNAcα1-O-Ser/Thr
- Tn
GalNAcα1-O-Ser/Thr
- VVA
Vicia villosa lectin
- ZFN
Zinc finger nuclease.
Conflict of interest statement
Catharina Steentoft, Ulla Mandel and Yoshiki Narimatsu have filed a patent application (WO 2017194699 A1) related to the use of Glycosyltransferase engineering and mAb discovery.
Funding
The Danish Research Councils [DFF – 4004-00397B]; The Danish National Research Foundation [DNRF107]; Lundbeck Foundation [R223-2016-563]; and National Institutes of Health [R01-CA37156].
References
- Akopov SB, Ruda VM, Batrak VV, Vetchinova AS, Chernov IP, Nikolaev LG, Bode J, Sverdlov ED. 2006. Identification, genome mapping, and CTCF binding of potential insulators within the FXYD5-COX7A1 locus of human chromosome 19q13.12. Mamm Genome. 17:1042–1049. [DOI] [PubMed] [Google Scholar]
- Axelsson MA, Karlsson NG, Steel DM, Ouwendijk J, Nilsson T, Hansson GC. 2001. Neutralization of pH in the Golgi apparatus causes redistribution of glycosyltransferases and changes in the O-glycosylation of mucins. Glycobiology. 11:633–644. [DOI] [PubMed] [Google Scholar]
- Baeuerle PA, Kufer P, Bargou R. 2009. BiTE: Teaching antibodies to engage T-cells for cancer therapy. Curr Opin Mol Ther. 11:22–30. [PubMed] [Google Scholar]
- Batistatou A, Charalabopoulos AK, Scopa CD, Nakanishi Y, Kappas A, Hirohashi S, Agnantis NJ, Charalabopoulos K. 2006. Expression patterns of dysadherin and E-cadherin in lymph node metastases of colorectal carcinoma. Virchows Arch. 448:763–767. [DOI] [PubMed] [Google Scholar]
- Batistatou A, Scopa CD, Ravazoula P, Nakanishi Y, Peschos D, Agnantis NJ, Hirohashi S, Charalabopoulos KA. 2005. Involvement of dysadherin and E-cadherin in the development of testicular tumours. Br J Cancer. 93:1382–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett EP, Hassan H, Hollingsworth MA, Clausen H. 1999. A novel human UDP-N-acetyl-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase, GalNAc-T7, with specificity for partial GalNAc-glycosylated acceptor substrates. FEBS Lett. 460:226–230. [DOI] [PubMed] [Google Scholar]
- Bennett EP, Hassan H, Mandel U, Hollingsworth MA, Akisawa N, Ikematsu Y, Merkx G, van Kessel AG, Olofsson S, Clausen H. 1999. Cloning and characterization of a close homologue of human UDP-N-acetyl-alpha-D-galactosamine:Polypeptide N-acetylgalactosaminyltransferase-T3, designated GalNAc-T6. Evidence for genetic but not functional redundancy. J Biol Chem. 274:25362–25370. [DOI] [PubMed] [Google Scholar]
- Bild AH, Yao G, Chang JT, Wang Q, Potti A, Chasse D, Joshi MB, Harpole D, Lancaster JM, Berchuck A et al. 2006. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature. 439:353–357. [DOI] [PubMed] [Google Scholar]
- Blixt O, Lavrova OI, Mazurov DV, Cló E, Kračun SK, Bovin NV, Filatov AV. 2011. Analysis of Tn antigenicity with a panel of new IgM and IgG1 monoclonal antibodies raised against leukemic cells. Glycobiology. 22:529–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska M et al. 2013. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 5:177ra138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks CL, Schietinger A, Borisova SN, Kufer P, Okon M, Hirama T, Mackenzie CR, Wang LX, Schreiber H, Evans SV. 2010. Antibody recognition of a unique tumor-specific glycopeptide antigen. Proc Natl Acad Sci USA. 107:10056–10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchell J, Poulsom R, Hanby A, Whitehouse C, Cooper L, Clausen H, Miles D, Taylor-Papadimitriou J. 1999. An alpha2,3 sialyltransferase (ST3Gal I) is elevated in primary breast carcinomas. Glycobiology. 9:1307–1311. [DOI] [PubMed] [Google Scholar]
- Chia J, Goh G, Bard F. 2016. Short O-GalNAc glycans: Regulation and role in tumor development and clinical perspectives. Biochim Biophys Acta. 1860:1623–1639. [DOI] [PubMed] [Google Scholar]
- Dahr W, Uhlenbruck G, Gunson HH, Van Der Hart M. 1975. Molecular basis of Tn-polyagglutinability. Vox Sang. 29:36–50. [DOI] [PubMed] [Google Scholar]
- Danielczyk A, Stahn R, Faulstich D, Loffler A, Marten A, Karsten U, Goletz S. 2006. PankoMab: A potent new generation anti-tumor MUC1 antibody. Cancer Immunol Immunother. 55:1337–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dausset J, Moullec J, Bernard J. 1959. Acquired hemolytic anemia with polyagglutinability of red blood cells due to a new factor present in normal human serum (Anti-Tn). Blood. 14:1079–1093. [PubMed] [Google Scholar]
- Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska M et al. 2014. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 6:224ra225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Laurentiis A, Gaspari M, Palmieri C, Falcone C, Iaccino E, Fiume G, Massa O, Masullo M, Tuccillo FM, Roveda L et al. 2011. Mass spectrometry-based identification of the tumor antigen UN1 as the transmembrane CD43 sialoglycoprotein. Mol Cell Proteomics. 10:M111.007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielli NM, Veiga MF, Matos ML, Quintana S, Chemes H, Blanco G, Vazquez-Levin MH. 2011. Expression of dysadherin in the human male reproductive tract and in spermatozoa. Fertil Steril. 96:554–561.e552. [DOI] [PubMed] [Google Scholar]
- Garty H, Karlish SJD. 2006. Role of FXYD proteins in ion transport. Annu Rev Physiol. 68:431–459. [DOI] [PubMed] [Google Scholar]
- Gaziel-Sovran A, Segura MF, Di Micco R, Collins MK, Hanniford D, de Miera EV-S, Rakus JF, Dankert JF, Shang S, Kerbel RS. 2011. miR-30b/30d regulation of GalNAc transferases enhances invasion and immunosuppression during metastasis. Cancer Cell. 20:104–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill DJ, Chia J, Senewiratne J, Bard F. 2010. Regulation of O-glycosylation through Golgi-to-ER relocation of initiation enzymes. J Cell Biol. 189:843–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill DJ, Tham KM, Chia J, Wang SC, Steentoft C, Clausen H, Bard-Chapeau EA, Bard FA. 2013. Initiation of GalNAc-type O-glycosylation in the endoplasmic reticulum promotes cancer cell invasiveness. Proc Natl Acad Sci USA. 110:E3152–E3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes J, Marcos NT, Berois N, Osinaga E, Magalhães A, Pinto-de-Sousa J, Almeida R, Gärtner F, Reis CA. 2009. Expression of UDP-N-acetyl-D-galactosamine: Polypeptide N-acetylgalactosaminyltransferase-6 in gastric mucosa, intestinal metaplasia, and gastric carcinoma. J Histochem Cytochem. 57:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF et al. 2013. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 368:1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haji-Ghassemi O, Blackler RJ, Martin Young N, Evans SV. 2015. Antibody recognition of carbohydrate epitopes. Glycobiology. 25:920–952. [DOI] [PubMed] [Google Scholar]
- Hassinen A, Pujol FM, Kokkonen N, Pieters C, Kihlstrom M, Korhonen K, Kellokumpu S. 2011. Functional organization of Golgi N- and O-glycosylation pathways involves pH-dependent complex formation that is impaired in cancer cells. J Biol Chem. 286:38329–38340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks D, Choi G, de Bruyn M, Wiersma VR, Bremer E. 2017. Antibody-based cancer therapy: Successful agents and novel approaches. Int Rev Cell Mol Biol. 331:289–383. [DOI] [PubMed] [Google Scholar]
- Hirohashi S, Clausen H, Yamada T, Shimosato Y, Hakomori S. 1985. Blood group A cross-reacting epitope defined by monoclonal antibodies NCC-LU-35 and -81 expressed in cancer of blood group O or B individuals: Its identification as Tn antigen. Proc Natl Acad Sci USA. 82:7039–7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ino Y, Gotoh M, Sakamoto M, Tsukagoshi K, Hirohashi S. 2002. Dysadherin, a cancer-associated cell membrane glycoprotein, down-regulates E-cadherin and promotes metastasis. Proc Natl Acad Sci USA. 99:365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzkowitz SH, Bloom EJ, Kokal WA, Modin G, Hakomori S-I, Kim YS. 1990. Sialosyl-Tn. A novel mucin antigen associated with prognosis in colorectal cancer patients. Cancer. 66:1960–1966. [DOI] [PubMed] [Google Scholar]
- Itzkowitz S, Kjeldsen T, Friera A, Hakomori S, Yang US, Kim YS. 1991. Expression of Tn, sialosyl Tn, and T antigens in human pancreas. Gastroenterology. 100:1691–1700. [DOI] [PubMed] [Google Scholar]
- Ju T, Cummings RD. 2002. A unique molecular chaperone Cosmc required for activity of the mammalian core 1 beta 3-galactosyltransferase. Proc Natl Acad Sci USA. 99:16613–16618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju T, Cummings RD. 2005. Protein glycosylation: Chaperone mutation in Tn syndrome. Nature. 437:1252. [DOI] [PubMed] [Google Scholar]
- Ju T, Cummings RD. 2010. Functional assays for the molecular chaperone cosmc. Methods Enzymol. 479:107–122. [DOI] [PubMed] [Google Scholar]
- Ju T, Lanneau GS, Gautam T, Wang Y, Xia B, Stowell SR, Willard MT, Wang W, Xia JY, Zuna RE et al. 2008. Human tumor antigens Tn and sialyl Tn arise from mutations in Cosmc. Cancer Res. 68:1636–1646. [DOI] [PubMed] [Google Scholar]
- Kalos M, June CH. 2013. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity. 39:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko MK, Nakamura T, Honma R, Ogasawara S, Fujii Y, Abe S, Takagi M, Harada H, Suzuki H, Nishioka Y et al. 2017. Development and characterization of anti-glycopeptide monoclonal antibodies against human podoplanin, using glycan-deficient cell lines generated by CRISPR/Cas9 and TALEN. Cancer Med. 6:382–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Y, Joshi HJ, Schjoldager KT, Madsen TD, Gerken TA, Vester-Christensen MB, Wandall HH, Bennett EP, Levery SB, Vakhrushev SY et al. 2015. Probing polypeptide GalNAc-transferase isoform substrate specificities by in vitro analysis. Glycobiology. 25:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavrsen K, Dabelsteen S, Vakhrushev SY, Levann AMR, Haue AD, Dylander A, Mandel U, Hansen L, Frodin M, Bennett EP et al. 2018. De novo expression of human polypeptide N-acetylgalactosaminyltransferase 6 (GalNAc-T6) in colon adenocarcinoma inhibits the differentiation of colonic epithelium. J Biol Chem. 293:1298–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavrsen K, Madsen CB, Rasch MG, Woetmann A, Odum N, Mandel U, Clausen H, Pedersen AE, Wandall HH. 2013. Aberrantly glycosylated MUC1 is expressed on the surface of breast cancer cells and a target for antibody-dependent cell-mediated cytotoxicity. Glycoconj J. 30:227–236. [DOI] [PubMed] [Google Scholar]
- Lee YK, Lee SY, Park JR, Kim RJ, Kim SR, Roh KJ, Nam JS. 2012. Dysadherin expression promotes the motility and survival of human breast cancer cells by AKT activation. Cancer Sci. 103:1280–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonowski LA, Narimatsu Y, Riaz A, Delay CE, Yang Z, Niola F, Duda K, Ober EA, Clausen H, Wandall HH et al. 2017. Genome editing using FACS enrichment of nuclease-expressing cells and indel detection by amplicon analysis. Nat Protoc. 12:581–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Xu L, Li C, Yuan Y, Huang S, Chen H. 2016. miR-214 inhibits invasion and migration via downregulating GALNT7 in esophageal squamous cell cancer. Tumor Biol. 37:14605–14614. [DOI] [PubMed] [Google Scholar]
- Lubarski I, Pihakaski-Maunsbach K, Karlish SJ, Maunsbach AB, Garty H. 2005. Interaction with the Na,K-ATPase and tissue distribution of FXYD5 (related to ion channel). J Biol Chem. 280:37717–37724. [DOI] [PubMed] [Google Scholar]
- Lubarski Gotliv I. 2016. FXYD5: Na(+)/K(+)-ATPase regulator in health and disease. Front Cell Dev Biol. 4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehata Y, Hirahashi M, Aishima S, Kishimoto J, Hirohashi S, Yao T, Takashima H, Tsuneyoshi M, Oda Y. 2011. Significance of dysadherin and E-cadherin expression in differentiated-type gastric carcinoma with submucosal invasion. Hum Pathol. 42:558–567. [DOI] [PubMed] [Google Scholar]
- Marcon E, Jain H, Bhattacharya A, Guo HB, Phanse S, Pu SY, Byram G, Collins BC, Dowdell E, Fenner M et al. 2015. Assessment of a method to characterize antibody selectivity and specificity for use in immunoprecipitation. Nat Methods. 12:725–U747. [DOI] [PubMed] [Google Scholar]
- Marcos NT, Pinho S, Grandela C, Cruz A, Samyn-Petit B, Harduin-Lepers A, Almeida R, Silva F, Morais V, Costa J et al. 2004. Role of the human ST6GalNAc-I and ST6GalNAc-II in the synthesis of the cancer-associated sialyl-Tn antigen. Cancer Res. 64:7050–7057. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Zhang Q, Akita K, Nakada H, Hamamura K, Tokuda N, Tsuchida A, Matsubara T, Hori T, Okajima T et al. 2012. pp-GalNAc-T13 induces high metastatic potential of murine Lewis lung cancer by generating trimeric Tn antigen. Biochem Biophys Res Commun. 419:7–13. [DOI] [PubMed] [Google Scholar]
- Matsuura H, Hakomori S. 1985. The oncofetal domain of fibronectin defined by monoclonal antibody FDC-6: Its presence in fibronectins from fetal and tumor tissues and its absence in those from normal adult tissues and plasma. Proc Natl Acad Sci USA. 82:6517–6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura H, Takio K, Titani K, Greene T, Levery SB, Salyan ME, Hakomori S. 1988. The oncofetal structure of human fibronectin defined by monoclonal antibody FDC-6. Unique structural requirement for the antigenic specificity provided by a glycosylhexapeptide. J Biol Chem. 263:3314–3322. [PubMed] [Google Scholar]
- Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF et al. 2014. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 371:1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maus MV, June CH. 2016. Making better chimeric antigen receptors for adoptive T-cell therapy. Clin Cancer Res. 22:1875–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitselou A, Batistatou A, Nakanishi Y, Hirohashi S, Vougiouklakis T, Charalabopoulos K. 2010. Comparison of the dysadherin and E-cadherin expression in primary lung cancer and metastatic sites. Histol Histopathol. 25:1257–1267. [DOI] [PubMed] [Google Scholar]
- Monach PA, Meredith SC, Siegel CT, Schreiber H. 1995. A unique tumor antigen produced by a single amino acid substitution. Immunity. 2:45–59. [DOI] [PubMed] [Google Scholar]
- Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. 2010. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 18:843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi Y, Akimoto S, Sato Y, Kanai Y, Sakamoto M, Hirohashi S. 2004. Prognostic significance of dysadherin expression in tongue cancer: Immunohistochemical analysis of 91 cases. Appl Immunohistochem Mol Morphol. 12:323–328. [DOI] [PubMed] [Google Scholar]
- Nam JS, Hirohashi S, Wakefield LM. 2007. Dysadherin: A new player in cancer progression. Cancer Lett. 255:161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam JS, Kang MJ, Suchar AM, Shimamura T, Kohn EA, Michalowska AM, Jordan VC, Hirohashi S, Wakefield LM. 2006. Chemokine (C-C motif) ligand 2 mediates the prometastatic effect of dysadherin in human breast cancer cells. Cancer Res. 66:7176–7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narimatsu Y, Joshi HJ, Yang Z, Gomes C, Chen YH, Lorenzetti FC, Furukawa S, Schjoldager KT, Hansen L, Clausen H et al. 2018. A validated gRNA library for CRISPR/Cas9 targeting of the human glycosyltransferase genome. Glycobiology. 28:295–305. [DOI] [PubMed] [Google Scholar]
- Newick K, O’Brien S, Moon E, Albelda SM. 2017. CAR T cell therapy for solid tumors. Annu Rev Med. 68:139–152. [DOI] [PubMed] [Google Scholar]
- Nishizawa A, Nakanishi Y, Yoshimura K, Sasajima Y, Yamazaki N, Yamamoto A, Hanada K, Kanai Y, Hirohashi S. 2005. Clinicopathologic significance of dysadherin expression in cutaneous malignant melanoma: Immunohistochemical analysis of 115 patients. Cancer. 103:1693–1700. [DOI] [PubMed] [Google Scholar]
- Park JR, Kim RJ, Lee YK, Kim SR, Roh KJ, Oh SH, Kong G, Kang KS, Nam JS. 2011. Dysadherin can enhance tumorigenesis by conferring properties of stem-like cells to hepatocellular carcinoma cells. J Hepatol. 54:122–131. [DOI] [PubMed] [Google Scholar]
- Pastan I, Hassan R, FitzGerald DJ, Kreitman RJ. 2006. Immunotoxin therapy of cancer. Nat Rev Cancer. 6:559–565. [DOI] [PubMed] [Google Scholar]
- Pedersen JW, Bennett EP, Schjoldager KT, Meldal M, Holmer AP, Blixt O, Clo E, Levery SB, Clausen H, Wandall HH. 2011. Lectin domains of polypeptide GalNAc transferases exhibit glycopeptide binding specificity. J Biol Chem. 286:32684–32696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen JW, Gentry-Maharaj A, Nostdal A, Fourkala EO, Dawnay A, Burnell M, Zaikin A, Burchell J, Papadimitriou JT, Clausen H et al. 2014. Cancer-associated autoantibodies to MUC1 and MUC4—A blinded case-control study of colorectal cancer in UK collaborative trial of ovarian cancer screening. Int J Cancer. 134:2180–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng R-Q, Wan H-Y, Li H-F, Liu M, Li X, Tang H. 2012. MicroRNA-214 suppresses growth and invasiveness of cervical cancer cells by targeting UDP-N-acetyl-α-d-galactosamine:polypeptide N-Acetylgalactosaminyltransferase 7. J Biol Chem. 287:14301–14309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto R, Hansen L, Hintze J, Almeida R, Larsen S, Coskun M, Davidsen J, Mitchelmore C, David L, Troelsen JT et al. 2017. Precise integration of inducible transcriptional elements (PrIITE) enables absolute control of gene expression. Nucleic Acids Res. 45:e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posey AD Jr., Schwab RD, Boesteanu AC, Steentoft C, Mandel U, Engels B, Stone JD, Madsen TD, Schreiber K, Haines KM et al. 2016. Engineered CAR T cells targeting the cancer-associated tn-glycoform of the membrane mucin MUC1 control adenocarcinoma. Immunity. 44:1444–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan P, Dabelsteen S, Madsen FB, Francavilla C, Kopp KL, Steentoft C, Vakhrushev SY, Olsen JV, Hansen L, Bennett EP et al. 2014. Immature truncated O-glycophenotype of cancer directly induces oncogenic features. Proc Natl Acad Sci USA. 111:E4066–E4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis CA, Sorensen T, Mandel U, David L, Mirgorodskaya E, Roepstorff P, Kihlberg J, Hansen JE, Clausen H. 1998. Development and characterization of an antibody directed to an alpha-N-acetyl-D-galactosamine glycosylated MUC2 peptide. Glycoconj J. 15:51–62. [DOI] [PubMed] [Google Scholar]
- Robinson M, Jiang P, Cui J, Li J, Wang Y, Swaroop M, Madore S, Lawrence TS, Sun Y. 2003. Global genechip profiling to identify genes responsive to p53-induced growth arrest and apoptosis in human lung carcinoma cells. Cancer Biol Ther. 2:406–415. [DOI] [PubMed] [Google Scholar]
- RodrÍguez E, Schetters STT, van Kooyk Y. 2018. The tumour glyco-code as a novel immune checkpoint for immunotherapy. Nat Rev Immunol. 18:204–211. [DOI] [PubMed] [Google Scholar]
- Sabbatini PJ, Ragupathi G, Hood C, Aghajanian CA, Juretzka M, Iasonos A, Hensley ML, Spassova MK, Ouerfelli O, Spriggs DR et al. 2007. Pilot study of a heptavalent vaccine-keyhole limpet hemocyanin conjugate plus QS21 in patients with epithelial ovarian, fallopian tube, or peritoneal cancer. Clin Cancer Res. 13:4170–4177. [DOI] [PubMed] [Google Scholar]
- Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Lower M, Bukur V, Tadmor AD, Luxemburger U, Schrors B et al. 2017. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 547:222–226. [DOI] [PubMed] [Google Scholar]
- Sato H, Ino Y, Miura A, Abe Y, Sakai H, Ito K, Hirohashi S. 2003. Dysadherin: Expression and clinical significance in thyroid carcinoma. J Clin Endocrinol Metab. 88:4407–4412. [DOI] [PubMed] [Google Scholar]
- Schietinger A, Philip M, Yoshida BA, Azadi P, Liu H, Meredith SC, Schreiber H. 2006. A mutant chaperone converts a wild-type protein into a tumor-specific antigen. Science. 314:304–308. [DOI] [PubMed] [Google Scholar]
- Schjoldager KT, Joshi HJ, Kong Y, Goth CK, King SL, Wandall HH, Bennett EP, Vakhrushev SY, Clausen H. 2015. Deconstruction of O-glycosylation—GalNAc-T isoforms direct distinct subsets of the O-glycoproteome. EMBO Rep. 16:1713–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell R, Backstrom M, Dalziel M, Gschmeissner S, Karlsson H, Noll T, Gatgens J, Clausen H, Hansson GC, Burchell J et al. 2006. The ST6GalNAc-I sialyltransferase localizes throughout the Golgi and is responsible for the synthesis of the tumor-associated sialyl-Tn O-glycan in human breast cancer. J Biol Chem. 281:3586–3594. [DOI] [PubMed] [Google Scholar]
- Shimada Y, Hashimoto Y, Kan T, Kawamura J, Okumura T, Soma T, Kondo K, Teratani N, Watanabe G, Ino Y et al. 2004. Prognostic significance of dysadherin expression in esophageal squamous cell carcinoma. Oncology. 67:73–80. [DOI] [PubMed] [Google Scholar]
- Shimada Y, Yamasaki S, Hashimoto Y, Ito T, Kawamura J, Soma T, Ino Y, Nakanishi Y, Sakamoto M, Hirohashi S et al. 2004. Clinical significance of dysadherin expression in gastric cancer patients. Clin Cancer Res. 10:2818–2823. [DOI] [PubMed] [Google Scholar]
- Shimamura T, Sakamoto M, Ino Y, Sato Y, Shimada K, Kosuge T, Sekihara H, Hirohashi S. 2003. Dysadherin overexpression in pancreatic ductal adenocarcinoma reflects tumor aggressiveness: Relationship to e-cadherin expression. J Clin Oncol. 21:659–667. [DOI] [PubMed] [Google Scholar]
- Springer GF. 1984. T and Tn, general carcinoma autoantigens. Science. 224:1198–1206. [DOI] [PubMed] [Google Scholar]
- Springer GF. 1997. Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. J Mol Med. 75:594–602. [DOI] [PubMed] [Google Scholar]
- Steentoft C, Bennett EP, Clausen H. 2013. Glycoengineering of human cell lines using zinc finger nuclease gene targeting: SimpleCells with homogeneous GalNAc O-glycosylation allow isolation of the O-glycoproteome by one-step lectin affinity chromatography. Methods Mol Biol. 1022:387–402. [DOI] [PubMed] [Google Scholar]
- Steentoft C, Bennett EP, Schjoldager KT, Vakhrushev SY, Wandall HH, Clausen H. 2014. Precision genome editing: A small revolution for glycobiology. Glycobiology. 24:663–680. [DOI] [PubMed] [Google Scholar]
- Steentoft C, Migliorini D, King TR, Mandel U, June CH, Posey AD Jr. 2018. Glycan-directed CAR-T cells. Glycobiology. 28:656–669. [DOI] [PubMed] [Google Scholar]
- Steentoft C, Schjoldager KT, Clo E, Mandel U, Levery SB, Pedersen JW, Jensen K, Blixt O, Clausen H. 2010. Characterization of an immunodominant cancer-specific O-glycopeptide epitope in murine podoplanin (OTS8). Glycoconj J. 27:571–582. [DOI] [PubMed] [Google Scholar]
- Steentoft C, Vakhrushev SY, Joshi HJ, Kong Y, Vester-Christensen MB, Schjoldager KT, Lavrsen K, Dabelsteen S, Pedersen NB, Marcos-Silva L et al. 2013. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J. 32:1478–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steentoft C, Vakhrushev SY, Vester-Christensen MB, Schjoldager KT, Kong Y, Bennett EP, Mandel U, Wandall H, Levery SB, Clausen H. 2011. Mining the O-glycoproteome using zinc-finger nuclease-glycoengineered SimpleCell lines. Nat Methods. 8:977–982. [DOI] [PubMed] [Google Scholar]
- Stone JD, Aggen DH, Schietinger A, Schreiber H, Kranz DM. 2012. A sensitivity scale for targeting T cells with chimeric antigen receptors (CARs) and bispecific T-cell Engagers (BiTEs). Oncoimmunology. 1:863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherlin ME, Nishimori I, Caffrey T, Bennett EP, Hassan H, Mandel U, Mack D, Iwamura T, Clausen H, Hollingsworth MA. 1997. Expression of three UDP-N-acetyl-alpha-D-galactosamine:polypeptide GalNAc N-acetylgalactosaminyltransferases in adenocarcinoma cell lines. Cancer Res. 57:4744–4748. [PubMed] [Google Scholar]
- Sørensen AL, Reis CA, Tarp MA, Mandel U, Ramachandran K, Sankaranarayanan V, Schwientek T, Graham R, Taylor-Papadimitriou J, Hollingsworth MA et al. 2006. Chemoenzymatically synthesized multimeric Tn/STn MUC1 glycopeptides elicit cancer-specific anti-MUC1 antibody responses and override tolerance. Glycobiology. 16:96–107. [DOI] [PubMed] [Google Scholar]
- Takahashi HK, Metoki R, Hakomori S. 1988. Immunoglobulin G3 monoclonal antibody directed to Tn antigen (tumor-associated alpha-N-acetylgalactosaminyl epitope) that does not cross-react with blood group A antigen. Cancer Res. 48:4361–4367. [PubMed] [Google Scholar]
- Takeuchi H, Kato K, Denda-Nagai K, Hanisch FG, Clausen H, Irimura T. 2002. The epitope recognized by the unique anti-MUC1 monoclonal antibody MY.1E12 involves sialyl alpha 2-3galactosyl beta 1-3N-acetylgalactosaminide linked to a distinct threonine residue in the MUC1 tandem repeat. J Immunol Methods. 270:199–209. [DOI] [PubMed] [Google Scholar]
- Tamura M, Ohta Y, Tsunezuka Y, Matsumoto I, Kawakami K, Oda M, Watanabe G. 2005. Prognostic significance of dysadherin expression in patients with non-small cell lung cancer. J Thorac Cardiovasc Surg. 130:740–745. [DOI] [PubMed] [Google Scholar]
- Taniuchi K, Cerny RL, Tanouchi A, Kohno K, Kotani N, Honke K, Saibara T, Hollingsworth MA. 2011. Overexpression of GalNAc-transferase GalNAc-T3 promotes pancreatic cancer cell growth. Oncogene. 30:4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarp MA, Clausen H. 2008. Mucin-type O-glycosylation and its potential use in drug and vaccine development. Biochim Biophys Acta. 1780:546–563. [DOI] [PubMed] [Google Scholar]
- Tarp MA, Sørensen AL, Mandel U, Paulsen H, Burchell J, Taylor-Papadimitriou J, Clausen H. 2007. Identification of a novel cancer-specific immunodominant glycopeptide epitope in the MUC1 tandem repeat. Glycobiology. 17:197–209. [DOI] [PubMed] [Google Scholar]
- Tassone P, Bond H, Bonelli P, Tuccillo F, Valerio G, Petrella A, Lamberti A, Cecco L, Turco MC, Cerra M et al. 1994. UN1, a murine monoclonal antibody recognizing a novel human thymic antigen. Tissue Antigens. 44:73–82. [DOI] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J, Burchell JM, Graham R, Beatson R. 2018. Latest developments in MUC1 immunotherapy. Biochem Soc Trans. 46:659–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokhtaeva E, Sun H, Deiss-Yehiely N, Wen Y, Soni PN, Gabrielli NM, Marcus EA, Ridge KM, Sachs G, Vazquez-Levin M et al. 2016. The O-glycosylated ectodomain of FXYD5 impairs adhesion by disrupting cell-cell trans-dimerization of Na,K-ATPase beta1 subunits. J Cell Sci. 129:2394–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuiji H, Takasaki S, Sakamoto M, Irimura T, Hirohashi S. 2003. Aberrant O-glycosylation inhibits stable expression of dysadherin, a carcinoma-associated antigen, and facilitates cell-cell adhesion. Glycobiology. 13:521–527. [DOI] [PubMed] [Google Scholar]
- Vakhrushev SY, Steentoft C, Vester-Christensen MB, Bennett EP, Clausen H, Levery SB. 2013. Enhanced mass spectrometric mapping of the human GalNAc-type O-glycoproteome with SimpleCells. Mol Cell Proteomics. 12:932–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A, Cummings RD, Aebi M, Packer NH, Seeberger PH, Esko JD, Stanley P, Hart G, Darvill A, Kinoshita T et al. 2015. Symbol nomenclature for graphical representations of glycans. Glycobiology. 25:1323–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vester-Christensen MB, Bennett EP, Clausen H, Mandel U. 2013. Generation of monoclonal antibodies to native active human glycosyltransferases. Methods Mol Biol. 1022:403–420. [DOI] [PubMed] [Google Scholar]
- Wandall HH, Blixt O, Tarp MA, Pedersen JW, Bennett EP, Mandel U, Ragupathi G, Livingston PO, Hollingsworth MA, Taylor-Papadimitriou J et al. 2010. Cancer biomarkers defined by autoantibody signatures to aberrant O-glycopeptide epitopes. Cancer Res. 70:1306–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandall HH, Hassan H, Mirgorodskaya E, Kristensen AK, Roepstorff P, Bennett EP, Nielsen PA, Hollingsworth MA, Burchell J, Taylor-Papadimitriou J et al. 1997. Substrate specificities of three members of the human UDP-N-acetyl-alpha-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase family, GalNAc-T1, -T2, and -T3. J Biol Chem. 272:23503–23514. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ju T, Ding X, Xia B, Wang W, Xia L, He M, Cummings RD. 2010. Cosmc is an essential chaperone for correct protein O-glycosylation. Proc Natl Acad Sci USA. 107:9228–9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Qiao Y, Kristensen GB, Li S, Troen G, Holm R, Nesland JM, Suo Z. 2004. Prognostic significance of dysadherin expression in cervical squamous cell carcinoma. Pathol Oncol Res. 10:212–218. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Bhavanandan VP, Nakamori S, Irimura T. 1996. A novel monoclonal antibody specific for sialylated MUC1 mucin. Jpn J Cancer Res. 87:488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Halim A, Narimatsu Y, Jitendra Joshi H, Steentoft C, Schjoldager KT, Alder Schulz M, Sealover NR, Kayser KJ, Paul Bennett E et al. 2014. The GalNAc-type O-glycoproteome of CHO cells characterized by the SimpleCell strategy. Mol Cell Proteomics. 13:3224–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Steentoft C, Hauge C, Hansen L, Thomsen AL, Niola F, Vester-Christensen MB, Frodin M, Clausen H, Wandall HH et al. 2015. Fast and sensitive detection of indels induced by precise gene targeting. Nucleic Acids Res. 43:e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.