Abstract
Background
Tigecycline is regarded as a last resort treatment for carbapenem-resistant Enterobacter cloacae (CREC) infections, and increasing numbers of tigecycline- and carbapenem-resistant E. cloacae (TCREC) isolates have been reported in recent years. However, risk factors and clinical impacts of these isolates are poorly characterized.
Patients and methods
We conducted a retrospective case-case-control study of hospitalized patients with TCREC infection during the period 2012–2016 in Chongqing, China. Case patients with TCREC and those with CREC were compared to a control group with no E. cloacae infection. Multivariate logistic regression models were used to identify independent risk factors for acquiring TCREC and CREC.
Results
A total of 36 TCREC cases, 36 CREC cases, and 100 controls were enrolled in our study. Multivariable analysis indicated that nasal catheter (OR: 8.9; 95% CI: 1.1–75.2), exposure to penicillin (OR: 95.9; 95% CI: 8.9–1038.3), aminoglycosides (OR: 42.1; 95% CI: 2.1–830.6), and fluoroquinolones (OR: 18.6; 95% CI: 1.9–185.6) were independent predictors for acquiring TCREC. In addition, venous catheterization (OR: 12.2; 95% CI: 2.5–58.5), penicillin (OR: 30.8; 95% CI: 7.9–120.0), and broad-spectrum cephalosporin (OR: 5.0; 95% CI: 1.5–17.3) were independently associated with CREC acquisition.
Conclusion
Reasonable antibiotic stewardship programs and surveillance are necessary to control the tigecycline resistance among high-risk patients.
Keywords: carbapenem resistance, tigecycline, Enterobacter cloacae, risk factor
Introduction
Enterobacter cloacae has emerged worldwide as an important nosocomial opportunistic infection pathogen, which causes lower respiratory tract infections, urinary tract infections, wound infections, and meningitis.1,2 E. cloacae isolates usually exhibited intrinsic resistance to ampicillin, amoxicillin-clavulanate, and first- and second-generation cephalosporins.3 Carbapenem-resistant E. cloacae (CREC) has become the predominant multidrug resistant bacterium, followed by Klebsiella pneumoniae and Escherichia coli. Recent survey from China carbapenem-resistant Enterobacteriaceae (CRE) Network revealed that the incidence of CREC infection covering 25 tertiary hospitals in 14 provinces was about 7.1%.4 Furthermore, similar results were obtained when observing CREC isolates evaluated by the SENTRY surveillance program study from 18 European nations.5,6
Tigecycline, a novel glycylcycline antibiotic agent with a broad-spectrum of activity against a wide-range of clinically relevant nosocomial pathogens,7,8 was considered as a last resort available for the CRE.6,9,10 As recommended by the USFDA, tigecycline was considered for the treatment of some complicated and serious infections.11 Additionally, previous studies have reported that tigecycline can be used in the cancer patients with neutropenia,12 and some serious infections after chemotherapy.13,14 With the clinical utility of tigecycline, an escalation of tigecycline- and carbapenem-resistant E. cloacae (TCREC) has recently been discovered on a global scale. Previous studies have mainly focused on the resistance mechanisms of TCREC isolates. However, the clinical significance of these strains is currently unknown. To the best of our knowledge, this is the first study to identify the risk factors and clinical outcomes of TCREC infections by the case-case-control study.
Patients and methods
Study setting and design
We conducted a five-year retrospective case-case-control study to investigate the risk factors of TCREC at the First Affiliated Hospital of Chongqing Medical University, the surveillance center of antimicrobial resistance in Southwest China with a 3,200-bedded tertiary teaching hospital, between 2012 and 2016.
The case-case-control study was used to intercompare three groups more precisely than the ordinary case-control study, which excluded the interference of “susceptible phenotype”.15,16 The case 1 consisted of patients with tigecycline- and carbapenem-resistant E. cloacae; the case 2 had patients with tigecycline-susceptible and carbapenem-resistant E. cloacae; and the control consisted of tigecycline- and carbapenem-susceptible E. cloacae. Data of demographic characteristics, chronic underlying diseases, invasive procedures, antibiotic treatments, and clinical outcomes during hospitalization were retrieved from the hospital information system (HIS). Patients with incomplete medical data were excluded. The study was authorized by the First Affiliated Hospital of Chongqing Medical University Biomedical Ethics Committee.
Microbiological methods
Identification of E. cloacae was performed on VITEK 2 system (BioMérieux, Marcy-l’Étoile, France), which is one of the most advanced and automated microbiology identification and antibiotic susceptibility analysis systems. This system is highly specific, sensitive, and repeatable with the characteristics of simple operation and rapid detection. The identification of most bacteria can be obtained within 2–18 hours. Susceptibilities of tigecycline were determined with standard microdilution broth method using breakpoints from the European Committee on Antimicrobial Susceptibility Testing, while susceptibilities of other antibiotics were determined by VITEK 2 system and interpreted according to the guidelines of Clinical and Laboratory Standards Institute. E. cloacae isolates with tigecycline minimum inhibitory concentration (MIC) ≥4 mg/L were defined as non-susceptible.
Patients and variables
Of 166 CREC isolates collected from 2012 to 2016, 36 isolates were resistant to tigecycline. Thirty-six case patients were matched to 36 patients with CREC infection, and 100 patients without E. cloacae infection serving as controls were randomly matched to TCREC or CREC cases. In total, 172 patients were in the final study cohort. Data were obtained from the electronic medical records, routine inspection system, and clinical microbiology laboratory databases. We analyzed the following parameters as potential risk factors: 1) patient demographic characteristics (age and sex); 2) comorbidities (chronic hepatitis, coronary heart diseases, diabetes, gastrointestinal disease, hypertension, and kidney disease); 3) invasive operations (receipt of central venous catheterization, endotracheal intubation, mechanical ventilation, urinary catheterization, bladder irrigation, gastric catheter, and nasal catheter); 4) antibiotic therapy (penicillin, aminoglycosides, broad-spectrum cephalosporins, carbapenems, fluoroquinolones, glycopeptides, macrolides, and minocycline); and 5) hospitalization and clinical consequences (intensive care unit stay, total length of hospital stay, and mortality rate). The time range was determined to analyze risk factors at 4 weeks for invasive operations and 3 months for intensive care unit (ICU) stay and antibiotic exposure.17
Statistical analysis
All the statistic calculations were conducted by using the software SPSS v.22.0 (IBM Corporation, Armonk, NY, USA). Chi-squared test or Fisher’s exact test was used to analyze categorical variables, while Student’s t-test or the Wilcoxon rank sum test was used to compare continuous variables. Logistic regression was used to identify the independent risk factors for tigecycline resistance. The OR and their 95% CI were calculated to assess the strength of the association. Variables with P-value <0.1 in univariate analyses were enrolled into multivariate regression model in a backward manner. A two-tailed P-value of <0.05 was considered to be statistically significant. The Hosmer–Lemeshow test was used to evaluate the goodness fit of the logistic regression model. Ethical approval for the study was obtained from the research ethics committee at the First Affiliated Hospital of Chongqing Medical University.
Ethics approval
The data and the samples analyzed were approved by the Chongqing Medical University Institutional Review Board and Biomedical Ethics Committee. The ethics committee waived the need for written informed consent provided by participants due to the retrospective nature of the study. Because all patient data were analyzed in anonymity, no additional informed consent was required.
Results
Analysis of case I vs case II
During the five-year study period, 36 of the 166 CREC isolates (21.7%) were non-susceptible to tigecycline. The characteristics of three groups are shown in Table 1. Of the 36 patients with TCREC, 11 (31%) were male. The average age was 70 years. To identify risk factors of the acquisition of tigecycline resistance traits in patients with CREC, 36 patients with TCREC were firstly compared to 36 patients with tigecycline-susceptible CREC (Table 1). No significant difference was observed in the terms of gender, length of hospital stays, ICU admission, and underlying diseases. Univariate analysis revealed that urinary catheterization and prior exposure to fluoroquinolones and minocycline were significantly more frequent in patients with TCREC.
Table 1.
Univariate analysis of risk factors for the acquisition of tigecycline resistance in patients with carbapenem-resistant or -susceptible E. cloacae
| Variables | Patients (n=172) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Case 1 (n=36) | Case 2 B(n=36) | Control (n=100) | Case 1 vs Case 2 | Case 1 vs Control | Case 2 vs Control | ||||
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | ||||
| Age (years), median (IQR) | 70 (63, 83.8) | 60 (47, 76.8) | 60 (47, 75.8) | – | 0.01 | – | 0.001 | – | 0.838 |
| Length of stay(days), median (IQR) | 29 (14, 29.8) | 23 (9.3, 79) | 21 (17, 54) | – | 0.616 | – | 0.02 | – | 0.068 |
| Elderly (≥60 years), n (%) | 28 (77.8) | 20 (55.6) | 50 (50) | 2.1 (0.8–5.6) | 0.141 | 2.6 (1.1–6.0) | 0.021 | 1.25 (0.6–2.7) | 0.567 |
| ICU stay, n (%) | 18 (50) | 14 (39) | 24 (24) | 1.3 (0.5–3.3) | 0.578 | 3.2 (1.4–7.0) | 0.004 | 2 (0.9–4.5) | 0.088 |
| Male, n (%) | 11 (31) | 8 (22) | 52 (52) | 0.6 (0.2–1.6) | 0.279 | 2.5 (1.1–5.5) | 0.027 | 3.8 (1.6–9.1) | 0.002 |
| Number of deaths, n (%) | 1 (2.8) | 3 (8.3) | 0 (0) | 0.3 (0.03–3.2) | 0.303 | 1.0 (1.0–1.1) | 0.094 | 1.1 (1–1.2) | 0.004 |
| Comorbidities | |||||||||
| Chronic hepatitis, n (%) | 6 (16.7) | 5 (13.9) | 15 (15) | 1.2 (0.3–4.5) | 0.743 | 1.1 (0.4–3.2) | 0.812 | 0.9 (0.3–2.7) | 0.872 |
| Coronary heart diseases, n (%) | 7 (19.4) | 4 (11.1) | 10 (10) | 1.9 (0.5–6.9) | 0.335 | 2.2 (0.8–6.2) | 0.142 | 1.1 (0.3–3.8) | 0.851 |
| Diabetes, n (%) | 14 (38.9) | 7 (19.4) | 19 (19) | 1.2 (0.5–2.9) | 0.169 | 2.7 (1.2–6.3) | 0.017 | 1 (0.4–2.7) | 0.954 |
| Gastrointestinal disease, n (%) | 4 (11.1) | 4 (11.1) | 24 (24) | 1 (0.2–4.3) | 1 | 0.4 (0.1–1.2) | 0.101 | 0.4 (0.1–1.2) | 0.101 |
| Hypertension, n (%) | 17 (47.2) | 16 (44.4) | 24 (24) | 2.1 (0.7–5.9) | 0.761 | 2.8 (1.3–6.3) | 0.009 | 2.5 (1.1–5.6) | 0.021 |
| Kidney disease, n (%) | 4 (11.1) | 8 (22.2) | 14 (14) | 0.4 (0.1–1.6) | 0.206 | 0.7 (0.2–2.2) | 0.52 | 1.6 (0.6–4.1) | 0.373 |
| Invasive operation | |||||||||
| Central venous catheterization, n (%) | 15 (41.7) | 10 (27.8) | 4 (4) | 1.8 (0.7–4.6) | 0.242 | 17.1 (5.2–56.9) | <0.001 | 9.2 (2.7–31.8) | <0.001 |
| Endotracheal intubation, n (%) | 6 (16.7) | 13 (36.1) | 13 (13) | 0.3 (0.1–0.9) | 0.024 | 1.3 (0.5–3.8) | 0.586 | 3.8 (1.5–9.3) | 0.002 |
| Mechanical ventilation, n (%) | 11 (30.6) | 6 (16.7) | 13 (13) | 1.8 (0.6–5.4) | 0.312 | 2.9 (1.2–7.4) | 0.018 | 1.3 (0.5–3.8) | 0.586 |
| Urinary catheterization, n (%) | 25 (69.4) | 13 (36.1) | 36 (36) | 4.6 (1.8–12.2) | 0.001 | 4.0 (1.8–9.2) | 0.001 | 1 (0.5–2.2) | 0.99 |
| Bladder irrigation, n (%) | 9 (25) | 0 (0) | 3 (3) | – | 0.003 | 10.8 (2.7–42.6) | <0.001 | – | 0.565 |
| Nasogastric catheter, n (%) | 17 (47.2) | 10 (27.8) | 15 (15) | 1.8 (0.7–4.6) | 0.242 | 5.1 (2.2–12.0) | <0.001 | 2.2 (0.9–5.4) | 0.09 |
| Nasal catheter, n (%) | 18 (13.2) | 10 (27.8) | 7 (5.1) | 2.6 (1.0–7.0) | 0.05 | 13.3 (4.8–36.4) | <0.001 | 5.1 (1.7–14.7) | 0.001 |
| Antibiotic therapy | |||||||||
| Penicillin, n (%) | 18 (50) | 17 (47.2) | 8 (8) | 0.9 (0.4–2.3) | 0.846 | 11.5 (4.3–30.5) | <0.001 | 10.3 (3.9–27.2) | <0.001 |
| Aminoglycosides, n (%) | 9 (25) | 3 (8.3) | 3 (3) | 3 (0.7–12.1) | 0.111 | 10.8 (2.7–42.6) | <0.001 | 2.9 (0.6–15.3) | 0.181 |
| Broad-spectrum cephalosporins, n (%) | 13 (36.1) | 17 (47.2) | 28 (28) | 0.6 (0.2–1.5) | 0.291 | 1.4 (0.6–3.1) | 0.428 | 2.3 (1.1–5.1) | 0.036 |
| Carbapenems, n (%) | 10 (27.8) | 10 (27.8) | 10 (10) | 1 (0.4–2.8) | 0.947 | 3.5 (1.3–9.2) | 0.01 | 3.5 (1.3–9.2) | 0.01 |
| Fluoroquinolones, n (%) | 10 (27.8) | 3 (8.3) | 9 (9) | 4.4 (1.1–17.2) | 0.024 | 3.9 (1.4–10.6) | 0.005 | 0.9 (0.2–3.6) | 0.904 |
| Glycopeptides, n (%) | 6 (16.7) | 6 (16.7) | 6 (6) | 0.8 (0.2–2.8) | 0.765 | 0.4 (0.1–1.3) | 0.125 | 3.1 (1–10.4) | 0.053 |
| Macrolides, n (%) | 1 (2.8) | 0 (0) | 1 (1) | 0.5 (0.4–0.7) | 0.352 | 2.8 (0.2–46.4) | 0.447 | 0.9 (0.9–1.0) | 0.547 |
| Minocycline, n (%) | 8 (22.2) | 1 (2.8) | 1 (1) | 7 (0.8–60) | 0.044 | 28.3 (3.4–235.9) | <0.001 | 2.8 (0.2–46.4) | 0.447 |
Multivariate logistic regression finally identified that nasal catheter and prior exposure to fluoroquinolones were independently associated with the acquisition of tigecycline resistance in patients with CREC (Table 2). Of note, prior exposure to minocycline and carbapenems were not involved in the development of tigecycline resistance in patients with CREC. The Hosmer–Lemeshow test for the logistic regression model indicated a good fit for these data (χ2 =1.99; df =6; P=0.92).
Table 2.
Multivariate logistic regression analysis of risk factors for the acquisition of tigecycline resistance in patients with carbapenem-resistant or -susceptible E. cloacae
| Patients | ||||||
|---|---|---|---|---|---|---|
| Variables | Case 1 vs case 2 | Case 1 vs control | Case 2 vs control | |||
| Multivariate analysis | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value |
| Nasal catheter, n (%) | 6.0 (1.5–24.6) | 0.012 | 8.9 (1.1–75.2) | 0.044 | – | – |
| Central venous catheterization, n (%) | – | – | – | – | 12.2 (2.5–58.5) | 0.002 |
| Penicillin, n (%) | – | – | 95.9 (8.9–1,038.3) | <0.001 | 30.8 (7.9–120.0) | <0.001 |
| Broad-spectrum cephalosporin, n (%) | – | – | – | – | 5.0 (1.5–17.3) | 0.01 |
| Fluoroquinolones, n (%) | 9.1 (1.6–51.9) | 0.012 | 18.6 (1.9–185.6) | 0.013 | – | – |
| Aminoglycosides, n (%) | – | – | 42.1 (2.1–830.6) | 0.014 | – | – |
| Minocycline, n (%) | 13.7 (1–189.8) | 0.051 | – | – | – | – |
Notes: Case 1: patients with tigecycline- and carbapenem-resistant E. cloacae; case 2: patients with tigecycline-susceptible and carbapenem-resistant E. cloacae; and control: patients with tigecycline- and carbapenem-susceptible E. cloacae.
Analysis of case I vs controls
Since several tigecycline-resistant isolates were still susceptible to carbapenems,1 36 patients with TCREC were further compared to 100 patients with tigecycline- and carbapenem-susceptible E. cloacae to identify risk factors of harboring tigecycline traits in carbapenem-susceptible isolates (Table 1). Risk factors of TCREC patients were statistically significant in patients’ characteristics, including male, elderly, ICU stay, some underlying diseases (including diabetes and hypertension), some invasive procedures (including central venous catheter, mechanical ventilation, urinary catheterization, bladder irrigation, and nasogastric catheter), and prior exposure to some antibiotics (including penicillin, aminoglycosides, carbapenems, fluoroquinolones, and minocycline). In multivariate logistic regression, nasal catheter, prior exposure to penicillin, fluoroquinolones, and aminoglycosides were identified as the independent risk factors for the acquisition of tigecycline resistance traits in carbapenem-susceptible isolates. The Hosmer–Lemeshow test for the logistic regression model indicated a good fit for the data (χ2 =2.26; df =6; P=0.89).
Analysis of case 2 vs controls
The third group of tigecycline-susceptible CREC vs controls was compared to assess the potential risks. The results revealed that male gender, hypertension, central venous catheterization, endotracheal intubation, and exposure to penicillin, broad-spectrum cephalosporins, and carbapenems were statistically significant in the univariate analyses. Logistic regression identified central venous catheter and previous use of penicillin and broad-spectrum cephalosporins as the independent risk factors. The Hosmer–Lemeshow test for the logistic regression model indicated a good fit for the data (χ2 =4.88; df =5; P=0.43).
Comparing the two case groups with controls, we found that only exposure to penicillin was the independent risk factor in both TCREC and CREC patients. However, nasal catheter and previous aminoglycosides and fluoroquinolones therapies were unique to the TCREC cohort. In addition, we have found that fluoroquinolone-non-susceptible CREC isolates demonstrated significant higher tigecycline MICs than fluoroquinolone-susceptible ones in the 166 CREC isolates (P<0.0001; Figure 1).
Figure 1.
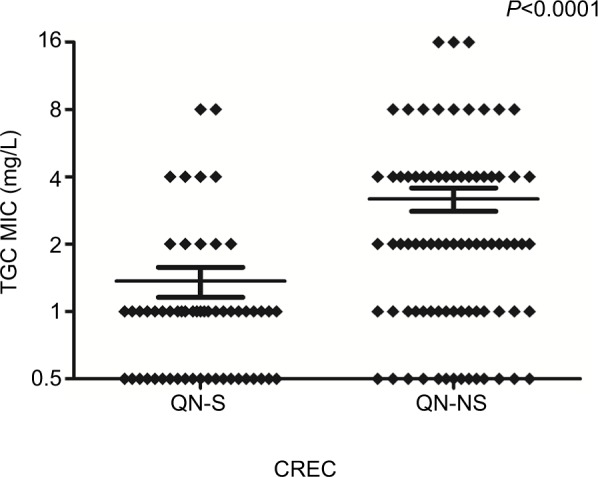
Comparison of tigecycline MICs in QN-S and QN-NS CREC isolates.
Abbreviations: CREC, carbapenem resistant E. cloacae; QN-S, fluoroquinolone-susceptible; QN-NS, fluoroquinolone-non-susceptible; TGC, tigecycline; MIC, minimum inhibitory concentration.
Clinical outcomes
During the study period, three (8.33%) patients died in CREC group, one patient (2.78%) died in TCREC group, while no one died in the control group. No significant difference of in-hospital mortality rate was observed between case 1 and case 2 (P=0.30) and case 1 and the control (P=0.09). Similarly, no significant difference of total length of stay was observed between case 1 and case 2 (P=0.62) and case 2 and the control (P=0.07).
A clinical case
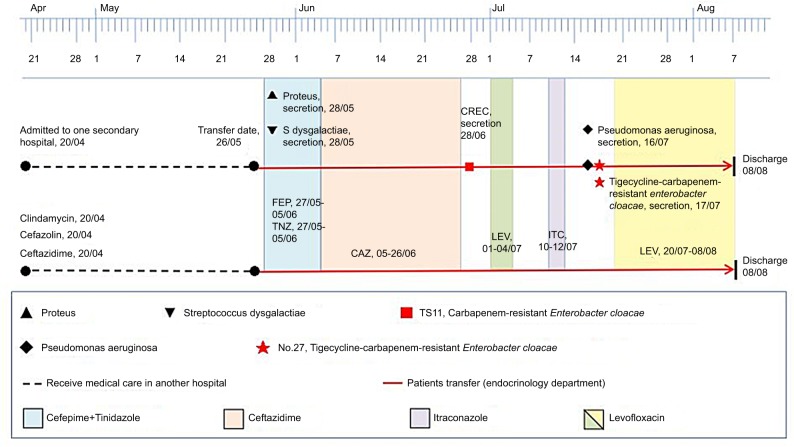
Two E. cloacae isolates (TS.11 and NO.27) were recovered, within an interval of 18 days, from foot tissues of the same patient who had undergone the operation of autologous skin flap grafting for diabetic foot infection after 4-day intravenous monotherapy of levofloxacin (600 mg every 24 hours). Both TS.11 and NO.27 strains were subjected to antimicrobial susceptibility and pulsed-field gel electrophoresis (PFGE). TS.11 showed susceptibility to levofloxacin and ciprofloxacin with the same MIC of 0.25 µg/mL, but resistance to ertapenem with a MIC of 2 µg/mL. NO.27 retained susceptibility to levofloxacin and ciprofloxacin with MICs of 1 and 0.25 µg/mL, but displayed increased resistance to ertapenem with a MIC of 16 µg/mL. The tigecycline MIC were 1 and 8 µg/mL, respectively. PFGE finally verified that TS.11 and NO.27 originated from the same clone. The timeline of the bacterial isolation process and antibiotics exposure are illustrated in Figure 2. This is the first case to report tigecycline resistance during levofloxacin monotherapy in diabetic foot infection by ertapenem-resistant E. cloacae.
Figure 2.
The timeline of the carbapenem-resistant E. cloacae TS.11 and the tigecycline- and carbapenem-resistant E. cloacae NO.27 isolated from the same patient.
Abbreviations: CAZ, ceftazidime; CREC, carbapenem resistant E. cloacae; FEP, cefepime; ITC, itraconazole; LEV, levofloxacin; TNZ, tinidazole.
Discussion
To the best of our knowledge, this is the first study to evaluate the potential risk factors and clinical outcomes for the isolation of tigecycline-resistant CREC by using the case-case-control study. In this work, we identified several particularly important findings. First, we found that some invasive operations appeared to be associated with TCREC or CREC acquisition. It is reasonable to assume that use of nasal catheter can damage the nasal mucosa, destroy the normal barrier, and lead to bacterial translocation. In addition, we showed that there was a significant difference in venous catheterization between CREC and the controls. Multiple factors could explain the association. Firstly, patients with venous catheterization are usually in serious condition and have poor immunity, so they are more likely to be infected with drug resistant strains. Secondly, nosocomial infection pathogens such as carbapenem-resistant E. cloacae may colonize on the catheter, entering into the blood circulation, and spreading across the body.18 Thirdly, it seems that the veins of the lower extremity are more likely to be propagated by bacteria because of the slowing blood flow in the veins.
Second, tigecycline resistance traits were more prone to be acquired under aminoglycosides exposure. However, aminoglycosides are not recommended to elder patients. In view that the median age of patients in this study was over 60 years, it is deduced that this significant correlation may have some bias of age. Moreover, in contrast to previous studies,19–22 carbapenem or tigecycline exposure was not relevant to the acquisition of tigecycline resistance traits in clinical E. cloacae isolates. Our case-case-control design may contribute to this irrelevance, since all these previous studies were subjected to case-control designs, which tended to overestimate antibiotic exposure.
Third, our current study identified that exposure to penicillin was the only common predictor in both TCREC and CREC groups compared to controls, while broad-spectrum cephalosporin was the independent risk factor of the CREC. This finding may be explained by the antibiotic pressure. As broad-spectrum antibiotics, penicillin and broad-spectrum cephalosporins were considered as the most common antibiotics for hospital and community-acquired infections. This might lead to the continuous propagation of resistant strains and the progress of resistance because of the antibiotic pressure.23 In the previous study, fluoroquinolones exposure was found to be an independent risk factor of tigecycline non-susceptibility in carbapenem-resistant K. pneumoniae.11,24 In the present investigation, this case-case-control study found that prior fluoroquinolone exposure was associated with the development of tigecycline resistance traits in either carbapenem-susceptible or -resistant E. cloacae isolates, revealing the need for surveillance of tigecycline resistance in regions with high consumption of fluoroquinolones. In addition, we have astonishingly found that fluoroquinolone-non-susceptibility may forecast tigecycline resistance. Prudent adoption of fluoroquinolones should be advocated to preclude the selection of tigecycline resistance. This present study reported the first clinical case of tigecycline resistance in CREC isolates, which occurred during levofloxacin monotherapy. The other potential risk factors included ICU stay, diabetes or hypertension, endotracheal intubation, mechanical ventilation, bladder irrigation, and use of carbapenems or minocycline. These findings either relate to the severity and complexity of underlying diseases, the use of invasive operation in the patients or may imply exposure to nosocomial microorganisms in medical environments and devices.
For clinical outcomes, the in-hospital mortality rate of patients with TCREC was relatively low, compared to those of tigecycline-non-susceptible K. pneumonia and Acinetobacter baumannii complex (41.9% and 36.4%, respectively).1,20,25 It is supposed that the sites and severity of infection should be responsible for this lower in-hospital mortality, because most frequent infections in this study were pneumonia instead of bacteremia.
This study has several limitations. Firstly, the results were derived from a single-center study and cautious interpretation of these data is suggested in other scenarios. Secondly, potential outbreaks may influence our results without analysis of resistance at the molecular level. Thirdly, we can evaluate only hospital records for risk factors, and did not assess other nonhospital information such as social antibiotic exposure or other factors that are not documented in hospital charts. Finally, although this is a five-year study, the sample size is also small. So, large sample studies should be analyzed to verify these conclusions.
Conclusion
The case-case-control study is conducted to assess independent risk factors associated with TCREC or CREC infection. We found that TCREC infection was associated with nasal catheter and prior antibiotics exposure of penicillin, fluoroquinolones, and aminoglycosides. Surveillance must be continued and antibiotics must be administered reasonably.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grant No.81572055) and Chongqing Science and Technology Commission Grant (cstc2016jcyjA0769). All authors meet the ICMJE authorship criteria.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Liu WY, Wong CF, Chung KM, Jiang JW, Leung FC. Comparative genome analysis of Enterobacter cloacae. PLoS One. 2013;12(9):e7448. doi: 10.1371/journal.pone.0074487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pérez A, Poza M, Aranda J, et al. Effect of transcriptional activators SoxS, RobA, and RamA on expression of multidrug efflux pump AcrAB-TolC in Enterobacter cloacae. Antimicrob Agents Chemother. 2012;56(12):6256–6266. doi: 10.1128/AAC.01085-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guérin F, Lallement C, Isnard C, Dhalluin A, Cattoir V, Giard J-C. Landscape of resistance-nodulation-cell division (RND)-type efflux pumps in Enterobacter cloacae Complex. Antimicrob Agents Chemother. 2016;60(4):2373–2382. doi: 10.1128/AAC.02840-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Wang Q, Yin Y, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae infections: report from China CRE network. Antimicrob Agents Chemother. 2018;62(2) doi: 10.1128/AAC.01882-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson BM, El Chakhtoura NG, Patel S, et al. Carbapenem-resistant Enterobacter cloacae in patients from the US veterans health administration, 2006–2015. Emerg Infect Dis. 2017;23(5):878–880. doi: 10.3201/eid2305.162034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sader HS, Castanheira M, Flamm RK, Mendes RE, Farrell DJ, Jones RN. Tigecycline activity tested against carbapenem-resistant Enterobacteriaceae from 18 European nations: results from the SENTRY surveillance Program (2010–2013) Diagn Microbiol Infect Dis. 2015;83(2):183–186. doi: 10.1016/j.diagmicrobio.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Zhong X, Xu H, Chen D, Zhou H, Hu X, Cheng G. First emergence of acrAB and oqxAB mediated tigecycline resistance in clinical isolates of Klebsiella pneumoniae pre-dating the use of tigecycline in a Chinese hospital. PLoS One. 2014;9(12):e115185. doi: 10.1371/journal.pone.0115185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montravers P, Dupont H, Bedos JP, Bret P, Tigecycline Group Tigecycline use in critically ill patients: a multicentre prospective observational study in the intensive care setting. Intensive Care Med. 2014;40(7):988–997. doi: 10.1007/s00134-014-3323-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ni W, Han Y, Liu J, et al. Tigecycline treatment for carbapenem-resistant Enterobacteriaceae infections: a systematic review and meta-analysis. Medicine. 2016;95(11) doi: 10.1097/MD.0000000000003126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marchaim D, Pogue JM, Tzuman O, et al. Major variation in MICs of tigecycline in gram-negative bacilli as a function of testing method. J Clin Microbiol. 2014;52(5):1617–1621. doi: 10.1128/JCM.00001-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sader HS, Castanheira M, Farrell DJ, Flamm RK, Mendes RE, Jones RN. Tigecycline antimicrobial activity tested against clinical bacteria from Latin American medical centres: results from SENTRY antimicrobial surveillance program (2011–2014) Int J Antimicrob Agents. 2016;48(2):144–150. doi: 10.1016/j.ijantimicag.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 12.Bucaneve G, Micozzi A, Picardi M, et al. Results of a multicenter, controlled, randomized clinical trial evaluating the combination of piperacillin/tazobactam and tigecycline in high-risk hematologic patients with cancer with febrile neutropenia. J Clin Oncol. 2014;32(14):1463–1471. doi: 10.1200/JCO.2013.51.6963. [DOI] [PubMed] [Google Scholar]
- 13.Chemaly RF, Hanmod SS, Jiang Y, et al. Tigecycline use in cancer patients with serious infections: a report on 110 cases from a single institution. Medicine. 2009;88(4):211–220. doi: 10.1097/MD.0b013e3181af01fc. [DOI] [PubMed] [Google Scholar]
- 14.Pugliese N, Salvatore P, Iula DV, et al. Ultrasonography-driven combination antibiotic therapy with tigecycline significantly increases survival among patients with neutropenic enterocolitis following cytarabine-containing chemotherapy for the remission induction of acute myeloid leukemia. Cancer Med. 2017;6(7):1500–1511. doi: 10.1002/cam4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teo J, Cai Y, Tang S, et al. Risk factors, molecular epidemiology and outcomes of ertapenem-resistant, carbapenem-susceptible Enterobacteriaceae: a case-case-control study. PLoS ONE. 2012;7(3):e34254. doi: 10.1371/journal.pone.0034254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaye KS, Harris AD, Samore M, Carmeli Y. The case-case-control study design: addressing the limitations of risk factor studies for antimicrobial resistance. Infect Control Hosp Epidemiol. 2005;26(4):346–351. doi: 10.1086/502550. [DOI] [PubMed] [Google Scholar]
- 17.Jia X, Ma W, Xu X, Yang S, Zhang L. Retrospective analysis of hospital-acquired linezolid-nonsusceptible enterococci infection in Chongqing, China, 2011–2014. Am J Infect Control. 2015;43(12):e101–e1061. doi: 10.1016/j.ajic.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 18.Soi V, Moore CL, Kumbar L, Yee J. Prevention of catheter-related bloodstream infections in patients on hemodialysis: challenges and management strategies. Int J Nephrol Renovasc Dis. 2016;9(9):95–103. doi: 10.2147/IJNRD.S76826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheng Z-K, Wang W, Guo Q, et al. Emergence of tigecycline- and carbapenem-nonsusceptible Klebsiella pneumoniae ST11 clone in patients without exposure to tigecycline. J Microbiol Immunol Infect. 2016;49(6):962–968. doi: 10.1016/j.jmii.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 20.Nigo M, Cevallos CS, Woods K, et al. Nested case-control study of the emergence of tigecycline resistance in multidrug-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother. 2013;57(11):5743–5746. doi: 10.1128/AAC.00827-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Duin D, Cober E, Richter SS, et al. Residence in skilled nursing facilities is associated with tigecycline nonsusceptibility in carbapenem-resistant Klebsiella pneumoniae. Infect Control Hosp Epidemiol. 2015;36(8):942–948. doi: 10.1017/ice.2015.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park GE, Kang C-I, Cha MK, et al. Bloodstream infections caused by Acinetobacter species with reduced susceptibility to tigecycline: clinical features and risk factors. Int J Infect Dis. 2017;62:26–31. doi: 10.1016/j.ijid.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 23.Baquero F, Negri MC, Morosini MI, Blázquez J. Antibiotic-selective environments. Clin Infect Dis. 1998;27(Suppl 1):S5–S11. doi: 10.1086/514916. [DOI] [PubMed] [Google Scholar]
- 24.Juan C-H, Huang Y-W, Lin Y-T, Yang T-C, Wang F-D. Risk factors, outcomes, and mechanisms of tigecycline-nonsusceptible Klebsiella pneumoniae bacteremia. Antimicrob Agents Chemother. 2016;60(12):7357–7363. doi: 10.1128/AAC.01503-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang YY, Liu YM, Liu CP, Kuo SC, Chen TL, ACTION study group Impact of reduced tigecycline susceptibility on clinical outcomes of Acinetobacter bacteremia. J Microbiol Immunol Infect. 2018;51(1):148–152. doi: 10.1016/j.jmii.2017.08.024. [DOI] [PubMed] [Google Scholar]