Abstract
Background
The south-west insular territories of the Indian Ocean have recently received attention concerning the diversity of arthropods of medical or veterinary interest. While a recent study highlighted the circulation of Culicoides-borne viruses, namely bluetongue and epizootic hemorrhagic disease, with clinical cases in Mayotte (comprising two islands, Petite-Terre and Grand-Terre), Comoros Archipelago, no data have been published concerning the species diversity of Culicoides present on the two islands.
Results
A total of 194,734 biting midges were collected in 18 sites, covering two collection sessions (April and June) in Mayotte. Our study reports for the first time livestock-associated Culicoides species and recorded at least 17 described Afrotropical species and one undescribed species. The most abundant species during the April collection session were C. trifasciellus (84.1%), C. bolitinos (5.4%), C. enderleini (3.9%), C. leucostictus (3.3%) and C. rhizophorensis (2.1%). All other species including C. imicola represented less than 1% of the total collection. Abundance ranged between 126–78,842 females with a mean and median abundance of 14,338 and 5111 individuals/night/site, respectively. During the June collection, the abundance per night was low, ranging between 6–475 individuals. Despite low abundance, C. trifasciellus and C. bolitinos were still the most abundant species. Culicoides sp. #50 is recorded for the first time outside South Africa.
Conclusions
Our study reports for the first time the Culicoides species list for Mayotte, Comoros Archipelago, Indian Ocean. The low abundance and rare occurrence of C. imicola, which is usually considered the most abundant species in the Afrotropical region, is unexpected. The most abundant and frequent species is C. trifasciellus, which is not considered as a vector species so far, but its role needs further investigation. Further work is needed to describe Culicoides sp. #50 and to carry on faunistic investigations on the other islands of the archipelago as well as in neighboring countries.
Electronic supplementary material
The online version of this article (10.1186/s13071-019-3379-x) contains supplementary material, which is available to authorized users.
Keywords: Culicoides, Diversity, Spatial distribution, Afrotropical region, Mayotte
Background
The south-west insular territories of the Indian Ocean have recently received attention concerning the diversity of arthropods of medical or veterinary interest [1–9]. This has been motivated by the recent epidemiological situation in the area. Even though the malaria morbidity and mortality has declined on some islands [10, 11], the region has faced major outbreaks of chikungunya [12–15] and endemic circulation of dengue virus [16, 17], West Nile virus [18, 19], Rift valley fever virus [20–26], bluetongue virus (BTV) and epizootic hemorrhagic disease virus (EHDV) [27–30] among others.
Culicoides are small biting midges (Diptera: Ceratopogonidae) distributed worldwide and implicated in the transmission of important viruses to ruminants (BTV, EHDV, Akabane virus), and equids (African horse sickness virus, AHSV) [31, 32]. The study of Afrotropical fauna started long ago [33–40] and recent work (starting in the 1990s) has tremendously updated these records [7, 41–51]. To date, the number of Culicoides species in the Afrotropical region is estimated to be around 190 species [50] with at least 120 species reported in the Southern African region [49].
In the south-west insular territories of the Indian Ocean, recent records mentioned five Afrotropical Culicoides species on La Reunion Island (C. imicola, C. enderleini, C. bolitinos, C. grahamii and C. kibatiensis) [7], where outbreaks of BTV and EHDV are regularly observed [30], and two Afrotropical species on Mauritius (C. imicola and C. enderleini) [52]. The faunistic inventory in Madagascar is probably largely incomplete as only 14 species have been recorded [48] and the precise identification of species related to C. schultzei needs further investigation [48, 53]. The Seychelles fauna for the genus Culicoides was investigated at three different times and three species were recorded (C. leucosticus, an Afrotropical species; C. kusaiensis, an Oriental species; and C. adamskii, reported only on a small Seychelles atoll) [54, 55]. Recent local reports highlighted the circulation of BTV and EHDV with some clinical cases in Mayotte, Comoros Archipelago [56]. Interestingly, no data are published concerning the Culicoides species present on this island.
Herein we report on a survey of Culicoides biting midges conducted in Mayotte, in the context of previous BTV and EHDV clinical cases [56]. A recent serosurvey on the island showed active circulation of both viruses throughout the island, with at least five BTV serotypes and one EHDV serotype [56]. Our survey is the first to address the Culicoides species diversity for the island and the whole archipelago. Our field survey covered different livestock breeding and production present on the island. Our specific objective was to describe the species diversity of Culicoides in Mayotte, to assess the abundance of dominant species and to map their spatial distribution to provide important insight to the epidemiology of the Culicoides-borne viruses on the island. Together with other published checklists for Culicoides in the region (South Africa, Kenya, La Réunion, Seychelles, Mauritius, Zimbabwe), we analyzed the species-area relationship (i.e. number of species in areas of different size irrespective of the identity of the species within the areas) to estimate the species richness in Madagascar.
Methods
Mayotte is an overseas department of France in the south-west part of the Indian Ocean, located in the northern Mozambique Channel. The island, constituted of a main island (Grande-Terre) and a smaller one (Petite-Terre), belongs geographically to the Comoros Archipelago (Fig. 1). The soil type is mostly related to the volcanic origin of the island with massive soil erosion caused by heavy tropical rainfall on unprotected and deforested areas. The latest survey (2010) totaled 5700 cattle farms with 17,150 heads of cattle (less than 5 heads of cattle per farm on average), and 2200 sheep and goat farms with 12,600 animals (less than 6 animals per farm on average), highlighting the importance of smallholder farming. Breeding practices are mostly traditional with tethered cattle (72%) and small ruminants (51%) of local breeds. There is a single horse-riding center on Grande-Terre.
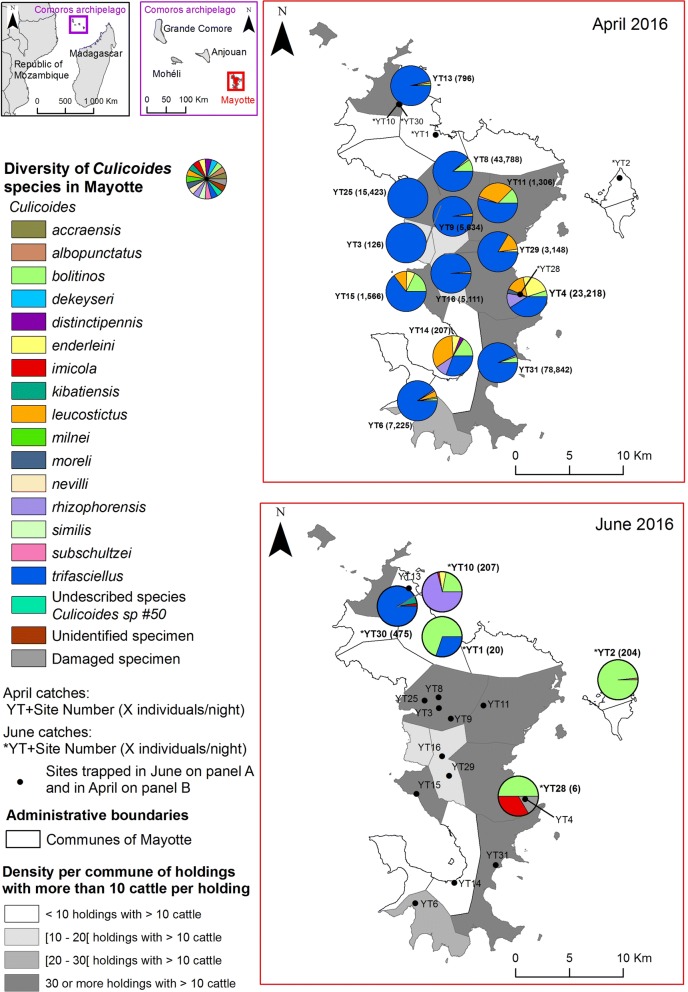
Fig. 1.
Diversity of Culicoides species recorded in Mayotte in two seasons, April 2016 and June 2016. The map was generated using ArcGIS 10.3 (ESRI). Administrative layers for Mayotte were extracted from Diva Gis (http://www.diva-gis.org/gData) and the GADM database (http://www.gadm.org, version 2.5, July 2015)
Thirteen sites were prospected, and collections were made from single night collections from 4th to 11th of April 2016, and 5 sites (YT1, YT2, YT10, YT28, YT30) from 20th to 27th of June 2016 (Fig. 1, see Additional file 1: Table S1). The timeframe in April was chosen to match the end of the rainy season when Culicoides populations are supposed to be the highest (dry season from April to November and rainy season from December to March). Selected sites were cattle (YT2, YT4, YT8, YT10, YT14, YT15, YT25, YT29, YT31), mixed farms with cattle and sheep and/or goats (YT1, YT6, YT11, YT13), sheep farms (YT9), goat farms (YT3, YT30) and the unique horse-riding center (YT28) to represent the different ruminants or equids present on the island. Culicoides trapping was done using a black light suction trap (Onderstepoort Veterinary Institute design, powered with a 12 V car battery) set up from before dusk until after sunrise, and positioned in the vicinity of the animal holdings (inside the shelter, along the fence, the closest tree for tethered animals) (Additional file 1: Table S1).
Specimens of Culicoides were stored in 70% alcohol until identification. Large samples were subsampled following a modified procedure described by Van Ark & Meiswinkel [57]. For each large sample, a 3 ml subsample was entirely sorted out and used to calculate the estimated total catch. All individuals were identified to the species level and sexed using a stereomicroscope. Morphological identification was performed using the available literature for the Afrotropical region [44, 49, 58, 59] and the expertise of KL. Biting midge specimens are deposited in the collection of Cirad, UMR ASTRE, Ste Clotilde, La Reunion, France (accession code: YT), and are available upon request to CG. Maps were generated using ArcGis® software (version 10.3).
To determine the sampling efficiency at the end of the rainy season, species accumulation curves were plotted according to a randomization procedure using the R vegan package version 2.5-1 and by fixing the number of permutations to 100 [60]. A species accumulation curve derives as a plot of cumulative number of species discovered as a function of sampling effort. Each species is considered regardless of its abundance or rarity. The number of non-sampled species was extrapolated by estimating different richness indices (Chao, Chao bias-corrected, first order jackknife, second order jackknife and bootstrap estimators).
One of the reasons why islands are important in ecology and biogeography is that they are relatively isolated areas and therefore excellent natural laboratories to study the relationship between area and species diversity [61]. To estimate Madagascar’s Culicoides species diversity, we plotted the area-species curve of the south-west Indian Ocean using literature, our dataset for Mayotte and official island sizes. All analyses were performed using R (https://www.r-project.org) [62].
Results
During the two collection sessions, 17 farms were prospected for 18 collection sites. Thirteen sites were prospected in April and 5 sites were prospected in June 2016. In one of the farms prospected in June, collections were carried out in two sites because of the presence of cattle (YT10) and goats (YT30) on two separate fields. The other sites prospected in June were the unique horse-riding center (YT28), one isolated goat and cattle farm in the north of Grande-Terre (YT1) and one site on Petite Terre (YT2) (Fig. 1).
At least 17 species were recorded during the two sessions (Table 1). One damaged specimen (absence of wings) collected in site YT28 was not identified. In site YT3, DNA of one specimen which could not be identified based on morphological features was extracted and the cox1 gene amplified. Unfortunately, the amplification failed. Out of the 17 species, 16 were known species distributed in the Afrotropical region (Table 2) and one was an undescribed species named Culicoides sp. #50 [49, 63, 64]. This latter species was collected in 9 sites (Fig. 1, Table 1). Ten species were collected both in April and in June: Culicoides albopunctatus, C. bolitinos, C enderleini, C. imicola, C. leucostictus, C. rhizophorensis, C. similis, C. subschultzei, C. trifasciellus and the undescribed species. Four species were collected only in April: C. accraensis, C. distinctipennis, C. milnei and C. moreli. Three species were only collected in June: C. dekeyseri, C. kibatiensis and C. nevilli plus one unidentified specimen (Fig. 1, Table 1). The species accumulation curve highlighted that the collection effort was sufficient to cover the species richness on Mayotte at the end of the rainy season (Fig. 2). Moreover, the different indices used to calculate extrapolated richness and compare it to our dataset showed that we could have missed from 1 (bootstrap) to 5 species (second order jackknife). Overall, this indicates that the inventory for the rainy season was notably robust and comprehensive.
Table 1.
Number of Culicoides individuals (one-night collections) collected for the 18 sites. Thirteen sites were sampled from 3rd to 12th of April 2016, and 5 sites (YT1, YT2, YT10, YT28, YT30) from 20th to 27th of June 2016
| Species | YT3 | YT4 | YT6 | YT8 | YT9 | YT11 | YT13 | YT14 | YT15 | YT16 | YT25 | YT29 | YT31 | YT1 | YT2 | YT10 | YT28 | YT30 | Positive sites | Total (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C. trifasciellus | 122 | 9396 | 6451.5 | 38,160 | 5491 | 693 | 759 | 63.5 | 1000 | 4235 | 15,201 | 3066 | 72,191 | 6 | 1 | 431 | 16 | 157,267 (83.96) | ||
| C. bolitinos | 1 | 1140 | 153 | 4240 | 160 | 18 | 33.5 | 276 | 12 | 6 | 3982 | 14 | 198 | 44 | 3 | 15 | 10,280.5 (5.49) | |||
| C. enderleini | 5316 | 76.5 | 747 | 4 | 5 | 13.5 | 104 | 117 | 47 | 6 | 735 | 1 | 11 | 3 | 14 | 7186 (3.84) | ||||
| C. leucostictus | 1 | 3480 | 365.5 | 293 | 139 | 413 | 12 | 70 | 168 | 712 | 81 | 51 | 271 | 2 | 14 | 6058.5 (3.23) | ||||
| C. rhizophorensis | 2687 | 93.5 | 27 | 20 | 4 | 1044 | 143 | 7 | 4018.5 (2.15) | |||||||||||
| C. moreli | 870 | 240 | 47 | 3 | 1157 (0.62) | |||||||||||||||
| C. albopunctatus | 34 | 1 | 2 | 12 | 348 | 3 | 6 | 400 (0.21) | ||||||||||||
| C. imicola | 116 | 27 | 2 | 116 | 1 | 3 | 2 | 11 | 8 | 278 (0.15) | ||||||||||
| C. distinctipennis | 1 | 174 | 27 | 13 | 6.5 | 23 | 12 | 6 | 8 | 262.5 (0.14) | ||||||||||
| Undescribed species Culicoides sp. #50 | 42.5 | 27 | 1 | 2 | 23 | 13 | 116 | 1 | 2 | 9 | 227.5 (0.12) | |||||||||
| C. subschultzei | 8.5 | 6 | 12 | 39 | 2 | 5 | 67.5 (0.04) | |||||||||||||
| C. milnei | 39 | 1 | 39 (0.02) | |||||||||||||||||
| C. accraensis | 27 | 1 | 27 (0.01) | |||||||||||||||||
| C. kibatiensis | 26 | 1 | 26 (0.01) | |||||||||||||||||
| C. similis | 2 | 1 | 2 | 3 (<0.01) | ||||||||||||||||
| C. dekeyseri | 1 | 1 | 1 (<0.01) | |||||||||||||||||
| C. nevilli | 1 | 1 | 1 (<0.01) | |||||||||||||||||
| Unidentified specimen | 1 | 1 | 1 (<0.01) | |||||||||||||||||
| Damaged specimen | 1 | 1 | 1 (<0.01) | |||||||||||||||||
| No. of species | 5 | 9 | 8 | 9 | 3 | 5 | 6 | 6 | 10 | 6 | 7 | 6 | 9 | 2 | 5 | 9 | 3 | 6 | ||
| Total | 126 | 23,218 | 7225 | 43,788 | 5634 | 1306 | 796 | 207 | 1566 | 5111 | 15,423 | 3148 | 78,842 | 20 | 204 | 207 | 6 | 475 | 187,302 |
Table 2.
Species list of Culicoides recorded in Mayotte, with systematic affiliations, published bionomics and current known distribution (review based on [49, 50])
| Species | Systematic affiliation following Borkent’s catalogue | Systematic affiliation following [49] | Bionomics | Known distribution |
|---|---|---|---|---|
| C. accraensis Carter, Ingram & Macfie, 1920 | Subgenus Synhelea | No subgenus affiliation known, Accraensis Group | Breeds primarily in tree holes, although there are several records from other habitat types. The host-feeding preference of C. accraensis is probably large mammals based on the low number of antennal sensilla. In Nigeria, adults were most numerous in the forest zone near livestock pens; abundance in relation to rainy season in Senegal. | Angola, Cameroon, Congo, Democratic Republic of Congo, Gambia, Ghana, Kenya, Nigeria, Senegal, Uganda, Zimbabwe |
| C. albopunctatus Clastrier, 1960 | Subgenus Synhelea | No subgenus affiliation known, Accraensis Group | Tree-hole breeder species. In South Africa, rarely collected species and usually with low abundance. | Congo, Democratic Republic of Congo, South Africa, Tanzania |
| C. bolitinos Meiswinkel, 1989 | Subgenus Avaritia | Subgenus Avaritia | Widespread distribution in South Africa. This species is described as breeding in the dung of cattle and buffaloes. In Zimbabwe, the species is abundant in regions characterized by low rainfall and high environmental temperatures, although in South Africa C. bolitinos has been reported to be more abundant in the cooler mountainous regions. | Botswana, Ivory Coast, Kenya, Lesotho, Malawi, Mauritius, Nigeria, Reunion, Senegal, South Africa, Zimbabwe |
| C. dekeyseri Clastrier, 1958 | No subgenus affiliation known, Dekeyseri Group | No subgenus affiliation known, Dekeyseri Group | Not a common species in South Africa but apparently widespread in Nigeria in both forest and savanna areas. | Angola, Gambia, Mali, Nigeria, Senegal, South Africa, Uganda |
| C. distinctipennis Austen, 1912 | Subgenus Meijerehelea | Subgenus Meijerehelea | Widespread and common in Nigeria. Recorded as primarily a bird-feeder but also collected feeding on humans in Zaire and Senegal. Breeding sites described as moist soil and mud taken from boggy ground, edges of pools or lakes, puddles and streams. | Angola, Cameroon, Congo, Egypt, Gambia, Ghana, Guinea, Kenya, Madagascar, Mali, Nigeria, Democratic Republic of Congo, Sao Tome, Senegal, Sierra Leone, South Africa, Sudan, Tanzania, Uganda, Zimbabwe |
| C. enderleini Cornet & Brunhes, 1994 | Subgenus Remmia | Subgenus Remmia | Widespread and common species. | Ethiopia, Gambia, Kenya, Madagascar, Nigeria, Reunion Island, Senegal, South Africa, Tanzania, Uganda, Zimbabwe |
| C. imicola Kieffer, 1913 | Subgenus Avaritia | Subgenus Avaritia | Very widely distributed and common species. Can be extremely abundant under ideal conditions. Historically recognized as the Afrotropical vector species of AHS and BT viruses. | Algeria, Angola, Burkina Faso, Cameroon, Chad, Congo, Democratic Republic of Congo, Egypt, Ethiopia, Gambia, Ghana, Guinea, Ivory Coast, Kenya, Madagascar, Mauritius, Morocco, Nigeria, Reunion, Sao Tome, Senegal, South Africa, Sudan, Tanzania, Uganda, Zimbabwe |
| C. kibatiensis Goetghebuer, 1935 | Subgenus Avaritia | Subgenus Avaritia | Widespread and common species. | Kenya, Nigeria, Democratic Republic of Congo, Tanzania, Uganda |
| C. leucostictus Kieffer, 1911 | Subgenus Meijerehelea | Subgenus Meijerehelea | – | Angola, Cameroon, Congo, Democratic Republic of Congo, Egypt, Ethiopia, Gambia, Ghana, Guinea, Kenya, Madagascar, Mali, Nigeria, Sao Tome, Senegal, Sierra Leone, South Africa, Sudan, Tanzania, Uganda, Zimbabwe |
| C. milnei Austen, 1909 | Milnei Group | No subgenus affiliation known, Milnei Group | In South Africa, rarely collected species while widespread in the eastern part. | Democratic Republic of Congo, Madagascar, Nigeria, Senegal, South Africa, Uganda |
| C. moreli Clastrier, 1959 | Milnei Group | No subgenus affiliation known, Milnei Group | Regularly collected in high numbers from KwaZulu-Natal in South Africa while rare elsewhere in the country. | Gambia, Ivory Coast, Kenya, Madagascar, Nigeria, Senegal, South Africa, Sudan, Uganda |
| C. nevilli Cornet & Brunhes, 1994 | Subgenus Remmia | Subgenus Remmia | Widespread and common species in South Africa. | Democratic Republic of Congo, Kenya, Madagascar, Nigeria, Senegal, South Africa, Zimbabwe |
| C. rhizophorensis Khamala & Kettle, 1971 | Subgenus Remmia | Subgenus Remmia | Rare species, collected along the eastern and southern coastal area near swampy and saline areas in South Africa. | Comoros Islands, Madagascar, Kenya, South Africa |
| C. similis Carter, Ingram & Macfie, 1920 | Subgenus Synhelea | No subgenus affiliation known, Similis Group | Widespread and common in the Afrotropical region, abundant in Senegal during the dry season, breeding sites in mud from the edges of temporary pools, puddles or ponds, and rotting banana stems or other vegetation | Burkina Faso, Egypt, Ethiopia, Gambia, Ghana, Kenya, Mali, Nigeria, Senegal, South Africa, Sudan, Tanzania, Zimbabwe |
| C. subschultzei Cornet & Brunhes, 1994 | Subgenus Remmia | Subgenus Remmia | Widespread and common species. Could be locally abundant. | Ethiopia, Kenya, Senegal, South Africa, Zimbabwe |
| C. trifasciellus Goetghebuer, 1935 | Subgenus Avaritia | Subgenus Avaritia | In Kenya, species collected from high altitude forest and grass-land and reported as major human biting species. Reported primarily as diurnal with peaks in morning hours and in the late evening. | Democratic Republic of Congo, Kenya, Senegal, Tanzania, South Africa |
Fig. 2.
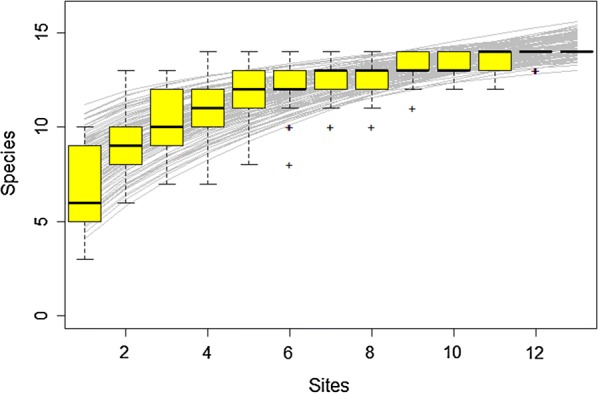
Species accumulation curve for the species observed in Mayotte. Boxplots mark standard deviations, crosses represent outlier points and grey curves represent the different simulations of richness indexes (Chao, Chao bias-corrected, first order jackknife, second order jackknife and bootstrap estimators)
A total of 194,734 individuals were collected in the 18 sites during 20 collection nights (Table 1). Indeed, due to social protests resulting in road barricades, two sites (YT6 and YT14) could not be reached on the morning after the trap was set up but only the day after. As the lights and fans of these two traps were still running correctly, we assumed that the mean of the total catch of the two nights of each trap best represented single night catch estimates. Overall, 98.29% of individuals were females (191,401) and 1.71% were males (3333). Taking into account 18 collection nights, a total of 187,302 individuals were identified during the two sessions (mean and median catch per night 10,406 and 1436, respectively) with 98.25% of females (184,026.5) and 1.75% of males (3275.5).
As expected, over 99.5% of the individuals (186,390 individuals) were caught in the 13 sites sampled in April and only 912 individuals were collected in June. Percentage of females in April was 98.26 in June (183,137.5 females) and 97.48 in April (889 females).
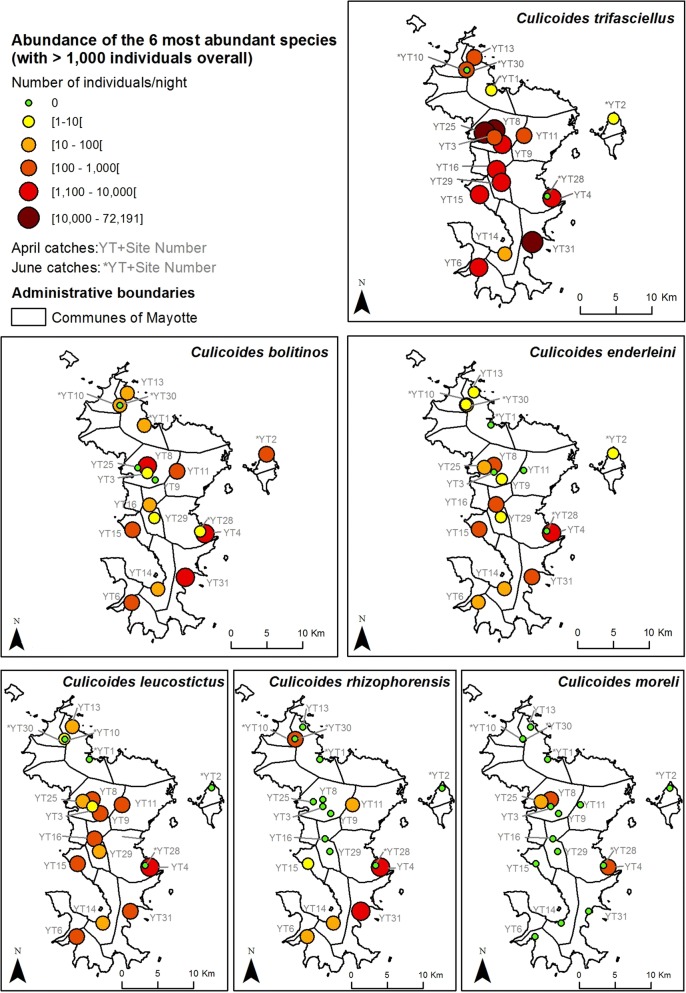
Considering only the April collection session, the most abundant species were C. trifasciellus (84.1% of collection), C. bolitinos (5.4%), C. enderleini (3.9%), C. leucostictus (3.3%) and C. rhizophorensis (2.1%) (Figs. 1, 3). All other species including C. imicola represented less than 1% of catches (Table 1, Fig. 4). Three to ten species were collected per site (Table 1). Four sites (YT31, YT8, YT4 and YT25) represented 86.5% of the total catches. Abundance ranged from 126 to 78,842 females with a mean and median abundance of 14,338 and 5111 individuals/night/site, respectively. Culicoides trifasciellus was present in all 13 sites sampled in April and was the most abundant species in all but one site, YT14, where C. leucostictus was the most abundant (Table 1, Fig. 3).
Fig. 3.
Abundance maps for the six most abundant species. The map was generated using ArcGIS 10.3 (ESRI). Administrative layers for Mayotte were extracted from Diva Gis (http://www.diva-gis.org/gData) and the GADM database (http://www.gadm.org, version 2.5, July 2015)
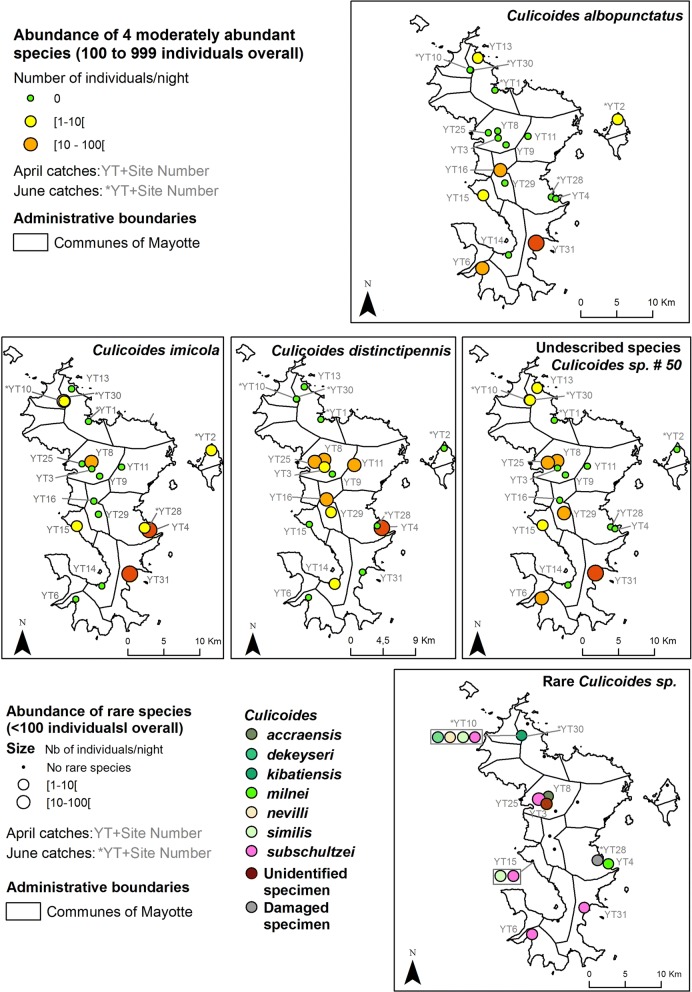
Fig. 4.
Abundance maps for the species with moderate and rare occurrence. The map was generated using ArcGIS 10.3 (ESRI). Administrative layers for Mayotte were extracted from Diva Gis (http://www.diva-gis.org/gData) and the GADM database (http://www.gadm.org, version 2.5, July 2015)
During the June collection session, the abundance per night was low, ranging between 6 and 475 individuals. Mean and median abundance were 182 and 204 individuals/night/site, respectively. Despite low abundance, C. trifasciellus and C. bolitinos were still the most abundant species, representing 48.0 and 28.4% of catches, respectively; C. rhizophorensis represented 15.7% of catches and all other species represented less than 1% of catches (Table 1, Fig. 3). Culicoides bolitinos was the most abundant species in 3 out of the 5 sites; C. rhizophorensis and C. trifasciellus were the most abundant in YT10 and YT30, respectively. Culicoides trifasciellus was also present in 2 other sites but in low numbers (1 and 6 individuals in YT1 and YT2, respectively). The number of species collected per site varied between 2 and 9 (Fig. 1, Table 1).
The area-species curve was plotted using species lists previously published [7, 44, 47, 49, 52, 54, 55] and our dataset for Mayotte (Fig. 5). The correlation was relatively high (R2 = 0.797) and allowed to predict 71 species for Madagascar.
Fig. 5.
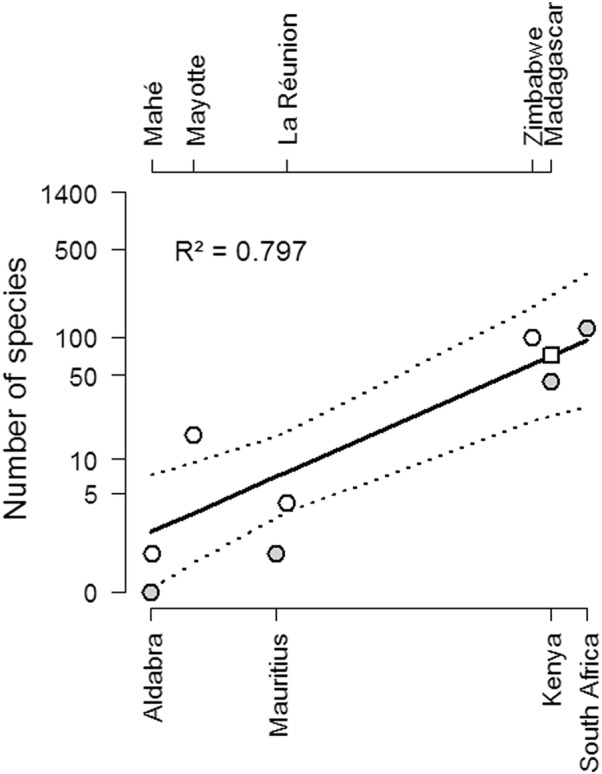
Species-area relationship in the south-west Indian Ocean region. Circle symbols represent the intersection of surface and the know number of species. Grey symbols refer to the lower X-axis. The square symbol represents the intersection of Madagascar’s surface and the regression line (black line, with 95% confidence interval in dashed lines). The X-axis represents the size of the island or country
Discussion
Faunistic inventory of mosquitoes was recently made in the south-western islands of the Indian Ocean [5, 6] but no such work has been done before for Culicoides species. Yet, completing a species checklist is the first fundamental step before any further epidemiological studies on vector species can be launched. Indeed, tropical oceanic islands are highly appropriate for surveys to attempt to complete a species checklist for a given group as they are isolated and endemism may be rampant [61].
Our study reports for the first time livestock-associated Culicoides species and records at least 17 described Afrotropical species with one undescribed species (Culicoides sp. #50). The reference trap for Culicoides collection was used because it can catch the highest diversity and abundance regardless of the season [65]. However, as it is a light-trap, species with diurnal activities may have been missed. Traps were localized in farms with cattle or small ruminants to investigate the species in close contact with hosts for BTV and EHDV. Therefore, we could have missed species breeding in other ecosystems such as sandy beaches, mangroves or sylvatic species. However, the species accumulative curve plot allows to consider our study as a good picture of the diversity of livestock-associated species at the supposed abundance peak (end of the rainy season). As the statistical analysis was carried out on species richness at the end of the rainy season, this work could be completed by another inventory during the dry season (only five sites were prospected in June).
Considering the size of Mayotte (374 km2) and its volcanic origin, the species diversity is high (Reunion island, five species for 2512 km2; Mauritius, two species for 1865 km2). All the species recorded in Mayotte had been previously found in South Africa [49], while the species presence and diversity composition was quite different from that recorded on neighboring island territories. In Mauritius, the last update inventory mentioned two species, C. imicola and C. enderleini [52], which needs to be confirmed with a large-scale survey. Different surveys recorded five species on La Réunion island [7, 66] and allow to consider this figure as robust. Unfortunately, the Culicoides diversity is clearly underestimated in Madagascar [53] and unknown on the other islands of the Comoros Archipelago. Our estimation of species number in Madagascar reached 71 species (Fig. 5). Although entomological surveys were undertaken in the Seychelles early in the past century [54], only two inventories were published for the islands. The oldest one [54] reported two species, C. leucosticus, an Afrotropical species, and C. kusaiensis, an Australasian species extending to Southeast Asia: Melanesia; Micronesia; Australia (Queensland) [67]; Thailand [68]; China [67]; and Malaysia [54]. This species list was further completed with a description of a new species from the Aldabra island, Seychelles, named C. adamskii [55]. All these collections in the different territories are incomplete and certainly require updating. The high species diversity recorded in Mayotte might be explained by the close geographical connection to the African continent which may facilitate Culicoides dispersal [69]. Indeed, the Comoros Archipelago has strong links to the African continent through current and past trade and human migrations.
Culicoides imicola was collected in eight sites and with a maximum abundance of 116 individuals per night. This is an unexpectedly low abundance and patchy distribution of C. imicola. Culicoides imicola is usually the most frequent and/or abundant species in the fringe of the African continent (Mediterranean basin and south of Africa where the climate is characterized by dry summers and rainy winters) [49, 65, 70, 71], while less abundant or frequent in other regions of the continent [42, 43, 72]. This could be related to soil type or other environmental variables. In our survey, this could be explained by the limited number of hosts and low cattle density and abundant sylvatic environment around the sites. The most abundant and frequent species was C. trifasciellus. This species has often been mentioned in Central Africa [37] or Kenya [73] studies and recently as a cave breeding species in Gabon [74]. It has been reported as an anthropophilic species [37, 49, 73] but no such nuisance was noticed during our fieldwork or reported by farmers. Moreover, C. trifasciellus is the vector species of Onchocerca gutturosa, a cattle microfilaria. We have no data attesting the presence of this parasite on the island, but it has been recorded on the African continent [75, 76].
To our knowledge and based on literature, there are no data on the vector role of C. trifasciellus for BTV or EHDV (Table 3). Culicoides bolitinos and C. enderleini, the 2nd and 3rd most abundant species respectively found in Mayotte, are known for their vector role [49]. The vector competence of C. bolitinos has been demonstrated in the laboratory for several viruses [77–80] and its host preference towards horses and ruminants has been documented [42, 58, 81, 82] which makes this species a major vector species for BTV, AHS and EHD virus in the Afrotropical region. Culicoides enderleini is highly suspected to be implicated in BTV transmission based on laboratory susceptibility studies and isolations of BTV in South African Culicoides populations [78, 80].
Table 3.
| Species | Host-vector contact | Detection of viral genome | Detection of parasites | Vector competence in the laboratory | |||
|---|---|---|---|---|---|---|---|
| Bovine | Equine | Sheep/goat | Other | ||||
| C. accraensis | + | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown |
| C. albopunctatus | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown |
| C. bolitinos | +++ | +++ | +++ | Unknown | BTV, AHSV, unidentified virus | Unknown | AHSV, BTV, EEV, EHDV |
| C. dekeyseri | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown |
| C. distinctipennis | Unknown | Unknown | Unknown | Birds, humans | Unknown | Unknown | Unknown |
| C. enderleini | + | + | + | Poultry | BTV | Unknown | |
| C. imicola | +++ | +++ | +++ | AHSV, Akabane virus, BTV, BEDV, EEV, Letsitele virus, Nyabira virus, Sabo virus, Shamonda virus, Simbu virus, unidentified virus | Unknown | AHSV, BTV, EEV, EHDV | |
| C. kibatiensis | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown |
| C. leucostictus | − | − | − | Birds, poultry | Unknown | Unknown | EHDV |
| C. milnei | Akabane virus, BTV | Unknown | |||||
| C. moreli | Unknown | Unknown | |||||
| C. nevilli | + | + | + | Poultry | EHDV | Unknown | |
| C. rhizophorensis | Unknown | Unknown | |||||
| C. similis | Unknown | Unknown | |||||
| C. subschultzei | Unknown | Unknown | |||||
| C. trifasciellus | Humans | Unknown | Onchocerca gutturosa | ||||
Key: +, positive association; −, negative association
Culicoides leucostictus and C. rhizophorensis were frequently and abundantly collected in our survey. In a study by Venter et al. [78], BTV isolation was successful from one pool of C. leucostictus while it is a common and widespread species in South Africa, being dominant near birds [49, 83, 84]. Indeed, the species was not attracted by horse or sheep baits in a recent host vector contact study in Senegal [81]. Both species are reported to breed in swampy, saline areas and salt-marshes environment in South Africa, like those created by periodical flooding with sea water due to tidal activity [49, 83]. Indeed, C. rhizophorensis was notably collected in our survey in farms close to the coast.
Culicoides sp. #50 is reported for the first time outside its known distribution range, i.e. South Africa [49, 63]. This species was mentioned for the first time in the Kruger National Park in South Africa reared from the dung of elephant and plains zebras [64]. Our record updates the known species distribution and its biology as no big wild mammals are present in Mayotte.
The assumption that competence for orbiviruses might be widespread in the genus Culicoides encourages further assessment of the role of each species in relation to its abundance and seasonality [78]. Meanwhile, the potential involvement of numerous species in virus transmission, each exhibiting different bionomics and phenology, greatly increases the complexity of the epidemiology of Culicoides-borne viruses. Because of the limited number of livestock on the island and low ruminant density, species usually associated with livestock farming in the Afrotropical region were either collected in small numbers (C. imicola, C. bolitinos and C. milnei) or absent (C. kingi). We could also not exclude the assumption that local ecological conditions (soil composition) are not favorable for these species. The relatively large number of C. leucostictus and C. rhizophorensis might be due to the presence of natural larval habitats around the prospected farms. Overall, no clear spatial pattern was observed regarding species diversity or abundance.
Culicoides species delimitation is commonly known to be complicated by large morphological variations [85]. Recently, systematics and taxonomy of the Afrotropical species of Culicoides using molecular tools [41, 48, 86] or morphological characters [49] confirmed the existence of tentative undescribed new species for the region. Molecular data could provide more resolution of the species diversity collected in Mayotte. Furthermore, C. trifasciellus has a close undescribed taxa named Culicoides sp. #20 [86]. In light of these ongoing changes, one needs to be careful with the species list that reflects our taxonomic knowledge at the time of identification.
Conclusions
Our study reports for the first time the Culicoides species list for Mayotte, Comoros Archipelago, Indian Ocean. Further work is needed to describe Culicoides sp. #50 and to carry on faunistic investigations on the other islands of the archipelago as well as in neighboring countries. The role of the most abundant species, C. trifasciellus, in the transmission of pathogens requires further investigation.
Additional file
Additional file 1: Table S1. Description of study sites and trap localization.
Acknowledgements
The authors acknowledge the farmers for allowing to collect Culicoides on their farms and Coopadem for its hospitality and logistical help during the fieldwork.
Funding
This study was funded by UMR 117 ASTRE, Cirad and RITA Mayotte Defi Animal project.
Availability of data and materials
All samples are available upon request to CG. Representative biting midge specimens are deposited in the collection of Cirad, UMR ASTRE, Ste Clotilde, La Reunion, France (accession code: YT).
Authors’ contributions
CG, HG, LD and EC designed the study. CG, LD, MB and HG contributed to the collection of Culicoides. KL identified all Culicoides specimens. CG, HG, TB and FM analysed the data. KL, LD, AB, TB, FM, MTB and EC contributed to the manuscript after its first draft by CG and HG. All authors read, commented on, and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- BTV
bluetongue virus
- EHDV
epizootic hemorrhagic disease virus
- AHSV
African horse sickness virus
Contributor Information
Claire Garros, Email: claire.garros@cirad.fr.
Karien Labuschagne, Email: labuschagnek@arc.agric.za.
Laure Dommergues, Email: l.dommergues@gmail.com.
M’sa Ben, Email: Ali.ben@eleveurs-de-mayotte.fr.
Thomas Balenghien, Email: Thomas.balenghien@cirad.fr.
Facundo Muñoz, Email: facundo.munoz@cirad.fr.
Mame Thierno Bakhoum, Email: thierno.bakhoum@gmail.com.
Eric Cardinale, Email: eric.cardinale@cirad.fr.
Hélène Guis, Email: helene.guis@cirad.fr.
References
- 1.Bagny L, Delatte H, Elissa N, Quilici S, Fontenille D. Aedes (Diptera: Culicidae) vectors of arboviruses in Mayotte (Indian Ocean): distribution area and larval habitats. J Med Entomol. 2009;46:198–207. doi: 10.1603/033.046.0204. [DOI] [PubMed] [Google Scholar]
- 2.Robert V, Rocamora G, Julienne S, Goodman SM. Why are anopheline mosquitoes not present in the Seychelles? Malar J. 2011;10:31. doi: 10.1186/1475-2875-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bousses P, Dehecq JS, Brengues C, Fontenille D. Updated inventory of mosquitoes (Diptera: Culicidae) of the island of La Reunion, Indian Ocean. Bull Soc Pathol Exot. 2013;106:113–125. doi: 10.1007/s13149-013-0288-7. [DOI] [PubMed] [Google Scholar]
- 4.Delatte H, Toty C, Boyer S, Bouetard A, Bastien F, Fontenille D. Evidence of habitat structuring Aedes albopictus populations in Reunion Island. PLoS Negl Trop Dis. 2013;7:e2111. doi: 10.1371/journal.pntd.0002111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Goff G, Brengues C, Robert V. Stegomyia mosquitoes in Mayotte, taxonomic study and description of Stegomyia pia n. sp. Parasite. 2013;20:31. doi: 10.1051/parasite/2013030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Goff G, Goodman SM, Elguero E, Robert V. Survey of the mosquitoes (Diptera: Culicidae) of Mayotte. PLoS One. 2014;9:e100696. doi: 10.1371/journal.pone.0100696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desvars A, Grimaud Y, Guis H, Esnault O, Allene X, Gardes L, et al. First overview of the Culicoides Latreille (Diptera: Ceratopogonidae) livestock associated species of Reunion Island, Indian Ocean. Acta Trop. 2015;142:5–19. doi: 10.1016/j.actatropica.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 8.Tantely ML, Le Goff G, Boyer S, Fontenille D. An updated checklist of mosquito species (Diptera: Culicidae) from Madagascar. Parasite. 2016;23:20. doi: 10.1051/parasite/2016018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tantely LM, Cetre-Sossah C, Rakotondranaivo T, Cardinale E, Boyer S. Population dynamics of mosquito species in a West Nile virus endemic area in Madagascar. Parasite. 2017;24:3. doi: 10.1051/parasite/2017005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kassim SA, James PB, Alolga RN, Assanhou AG, Kassim SM, Bacar A, et al. Major decline in malaria morbidity and mortality in the Union of Comoros between 2010 and 2014. The effect of a combination of prevention and control measures. S Afr Med J. 2016;106:709–714. doi: 10.7196/SAMJ.2016.v106i7.10902. [DOI] [PubMed] [Google Scholar]
- 11.Maillard O, Lernout T, Olivier S, Achirafi A, Aubert L, Lepere JF, et al. Major decrease in malaria transmission on Mayotte Island. Malar J. 2015;14:323. doi: 10.1186/s12936-015-0837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flahault A, Aumont G, Boisson V, de Lamballerie X, Favier F, Fontenille D, et al. An interdisciplinary approach to controlling chikungunya outbreaks on French islands in the south-west Indian ocean. Med Trop (Mars). 2012;72:66–71. [PubMed] [Google Scholar]
- 13.Gauzere BA, Aubry P. History of human epidemic and endemic diseases in the southwest Indian Ocean. Med Sante Trop. 2013;23:145–157. doi: 10.1684/mst.2013.0183. [DOI] [PubMed] [Google Scholar]
- 14.Savini H, Gautret P, Gaudart J, Field V, Castelli F, Lopez-Velez R, et al. Travel-associated diseases, Indian Ocean Islands, 1997–2010. Emerg Infect Dis. 2013;19:1297–1301. doi: 10.3201/eid1908.121739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dellagi K, Salez N, Maquart M, Larrieu S, Yssouf A, Silai R, et al. Serological evidence of contrasted exposure to arboviral infections between islands of the Union of Comoros (Indian Ocean) PLoS Negl Trop Dis. 2016;10:e0004840. doi: 10.1371/journal.pntd.0004840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lernout T, Giry C, Binder P, Zumbo B, Durquety E, Lajoinie G, et al. Emergence of dengue virus serotype 3 on Mayotte Island, Indian Ocean. East Afr J Public Health. 2011;8:155–156. [PubMed] [Google Scholar]
- 17.Lustig Y, Wolf D, Halutz O, Schwartz E. An outbreak of dengue virus (DENV) type 2 Cosmopolitan genotype in Israeli travellers returning from the Seychelles, April 2017. Euro Surveill. 2017;22:30563. doi: 10.2807/1560-7917.ES.2017.22.26.30563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tantely ML, Goodman SM, Rakotondranaivo T, Boyer S. Review of West Nile virus circulation and outbreak risk in Madagascar. Entomological and ornithological perspectives. Parasite. 2016;23:49. doi: 10.1051/parasite/2016058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cardinale E, Bernard C, Lecollinet S, Rakotoharinome VM, Ravaomanana J, Roger M, et al. West Nile virus infection in horses, Indian ocean. Comp Immunol Microbiol Infect Dis. 2017;53:45–49. doi: 10.1016/j.cimid.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Balenghien T, Cardinale E, Chevalier V, Elissa N, Failloux AB, Jean Jose Nipomichene TN, et al. Towards a better understanding of Rift Valley fever epidemiology in the south-west of the Indian Ocean. Vet Res. 2013;44:78. doi: 10.1186/1297-9716-44-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roger M, Beral M, Licciardi S, Soule M, Faharoudine A, Foray C, et al. Evidence for circulation of the rift valley fever virus among livestock in the union of Comoros. PLoS Negl Trop Dis. 2014;8:e3045. doi: 10.1371/journal.pntd.0003045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cavalerie L, Charron MV, Ezanno P, Dommergues L, Zumbo B, Cardinale E. A stochastic model to study Rift Valley fever persistence with different seasonal patterns of vector abundance: new insights on the endemicity in the tropical island of Mayotte. PLoS One. 2015;10:e0130838. doi: 10.1371/journal.pone.0130838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Metras R, Cavalerie L, Dommergues L, Merot P, Edmunds WJ, Keeling MJ, et al. The epidemiology of Rift Valley fever in Mayotte: insights and perspectives from 11 years of data. PLoS Negl Trop Dis. 2016;10:e0004783. doi: 10.1371/journal.pntd.0004783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lancelot R, Beral M, Rakotoharinome VM, Andriamandimby SF, Heraud JM, Coste C, et al. Drivers of Rift Valley fever epidemics in Madagascar. Proc Natl Acad Sci USA. 2017;114:938–943. doi: 10.1073/pnas.1607948114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metras R, Fournie G, Dommergues L, Camacho A, Cavalerie L, Merot P, et al. Drivers for Rift Valley fever emergence in Mayotte: a Bayesian modelling approach. PLoS Negl Trop Dis. 2017;11:e0005767. doi: 10.1371/journal.pntd.0005767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olive MM, Grosbois V, Tran A, Nomenjanahary LA, Rakotoarinoro M, Andriamandimby SF, et al. Reconstruction of Rift Valley fever transmission dynamics in Madagascar: estimation of force of infection from seroprevalence surveys using Bayesian modelling. Sci Rep. 2017;7:39870. doi: 10.1038/srep39870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Breard E, Sailleau C, Hamblin C, Zientara S. Bluetongue virus in the French Island of Reunion. Vet Microbiol. 2005;106:157–165. doi: 10.1016/j.vetmic.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 28.Sailleau C, Zanella G, Breard E, Viarouge C, Desprat A, Vitour D, et al. Co-circulation of bluetongue and epizootic haemorrhagic disease viruses in cattle in Reunion Island. Vet Microbiol. 2012;155:191–197. doi: 10.1016/j.vetmic.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Andriamandimby SF, Viarouge C, Ravalohery JP, Reynes JM, Sailleau C, Tantely ML, et al. Detection in and circulation of bluetongue virus among domestic ruminants in Madagascar. Vet Microbiol. 2015;176:268–273. doi: 10.1016/j.vetmic.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Cetre-Sossah C, Roger M, Sailleau C, Rieau L, Zientara S, Breard E, et al. Epizootic haemorrhagic disease virus in Reunion Island: evidence for the circulation of a new serotype and associated risk factors. Vet Microbiol. 2014;170:383–390. doi: 10.1016/j.vetmic.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 31.Carpenter S, Groschup MH, Garros C, Felippe-Bauer ML, Purse BV. Culicoides biting midges, arboviruses and public health in Europe. Antiviral Res. 2013;100:102–113. doi: 10.1016/j.antiviral.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 32.Carpenter S, Mellor PS, Fall AG, Garros C, Venter GJ. African horse sickness virus: history, transmission, and current status. Annu Rev Entomol. 2017;62:343–358. doi: 10.1146/annurev-ento-031616-035010. [DOI] [PubMed] [Google Scholar]
- 33.Clastrier J. Notes on the Ceratopogonidae. IV. Ceratopogonidae of French West Africa. Arch Inst Pasteur Alger. 1958;36:192–258. [PubMed] [Google Scholar]
- 34.Clastrier J. Notes on Ceratopogoninae. VII. Ceratopogoninae of French West Africa. Arch Inst Pasteur Alger. 1959;37:340–383. [PubMed] [Google Scholar]
- 35.Auriault M. Contribution à l’étude biologique et écologique de Culicoides grahamii (Austen) 1909 (Diptera: Ceratopogonidae) Cahiers ORSTOM série Entomol Med Parasitol. 1979;17:77–79. [Google Scholar]
- 36.Fiedler OGH. The South African biting midges of the genus Culicoides (Diptera: Ceratopogonidae) Onderstepoort J Vet Res. 1951;21:3–33. [Google Scholar]
- 37.Itoua A, Cornet M, Vattier-Bernard G, Trouillet J. The Culicoides (Diptera: Ceratopogonidae) of Central Africa. Cahiers ORSTOM série Entomol Med Parasitol. 1987;25:127–134. [Google Scholar]
- 38.Khamala CPM, Kettle DS. The Culicoides Latreille (Diptera: Ceratopogonidae) of East Africa. Trans R Entomol Soc Lond. 1971;123:1–95. doi: 10.1111/j.1365-2311.1971.tb00840.x. [DOI] [Google Scholar]
- 39.Boorman J, Dipeolu OO. A taxonomic study of adult Nigerian Culicoides Latreille (Diptera: Ceratopogoaidae) species. Occ Publ Ent S Nigeria. 1979;22:1–121. [Google Scholar]
- 40.Colçao TF. Some Culicoides of the Transvaal. An Inst Med Trop. 1946;2:235–266. [Google Scholar]
- 41.Bakhoum MT, Fall M, Fall AG, Bellis GA, Gottlieb Y, Labuschagne K, et al. First record of Culicoides oxystoma Kieffer and diversity of species within the Schultzei group of Culicoides Latreille (Diptera: Ceratopogonidae) biting midges in Senegal. PLoS One. 2013;8:e84316. doi: 10.1371/journal.pone.0084316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diarra M, Fall M, Fall AG, Diop A, Seck MT, Garros C, et al. Seasonal dynamics of Culicoides (Diptera: Ceratopogonidae) biting midges, potential vectors of African horse sickness and bluetongue viruses in the Niayes area of Senegal. Parasit Vectors. 2014;7:147. doi: 10.1186/1756-3305-7-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fall M, Diarra M, Fall AG, Balenghien T, Seck MT, Bouyer J, et al. Culicoides (Diptera: Ceratopogonidae) midges, the vectors of African horse sickness virus - a host/vector contact study in the Niayes area of Senegal. Parasit Vectors. 2015;8:39. doi: 10.1186/s13071-014-0624-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glick JI. Culicoides biting midges (Diptera: Ceratopogonidae) of Kenya. J Med Entomol. 1990;27:85–195. doi: 10.1093/jmedent/27.2.85. [DOI] [PubMed] [Google Scholar]
- 45.Meiswinkel R, Braack LE. African horsesickness epidemiology: five species of Culicoides (Diptera: Ceratopogonidae) collected live behind the ears and at the dung of the African elephant in the Kruger National Park, South Africa. Onderstepoort J Vet Res. 1994;61:155–170. [PubMed] [Google Scholar]
- 46.Venter GJ, Meiswinkel R, Nevill EM, Edwardes M. Culicoides (Diptera: Ceratopogonidae) associated with livestock in the Onderstepoort area, Gauteng, South Africa as determined by light-trap collections. Onderstepoort J Vet Res. 1996;63:315–325. [PubMed] [Google Scholar]
- 47.Musuka GN, Meiswinkel R, Baylis M, Kelly PJ, Mellor PS. Prevalence of Culicoides imicola and other species (Diptera: Ceratopogonidae) at eight sites in Zimbabwe. J S Afr Vet Assoc. 2001;72:62–63. [PubMed] [Google Scholar]
- 48.Augot D, Randrianambinintsoa FJ, Gasser A, Depaquit J. Record of two species of Culicoides (Diptera, Ceratopogonidae) new for Madagascar and molecular study showing the paraphylies of the subgenus Oecacta and the Schultzei group. Bull Soc Pathol Exot. 2013;106:201–205. doi: 10.1007/s13149-013-0302-4. [DOI] [PubMed] [Google Scholar]
- 49.Labuschagne K. The Culicoides Latreille (Diptera: Ceratopogonidae) species of South Africa. Pretoria: Faculty of Natural and Agricultural Science, University of Pretoria; 2016. [Google Scholar]
- 50.Bakhoum MT. Ecologie et taxonomie intégrative des moucherons piqueurs du genre Culicoides Latreille (Diptera: Ceratopogonidae) en région afrotropicale. Montpellier: AgroParisTech; 2017. [Google Scholar]
- 51.Becker E, Venter GJ, Labuschagne K, Greyling T, van Hamburg H. The effect of anthropogenic activity on the occurrence of Culicoides species in the South-Western Khomas Region, Namibia. Vet Ital. 2013;49:277–284. doi: 10.12834/VetIt.1011.10. [DOI] [PubMed] [Google Scholar]
- 52.Jori F, Roger M, Baldet T, Delecolle JC, Sauzier J, Jaumally MR, Roger F. Orbiviruses in Rusa deer, Mauritius, 2007. Emerg Infect Dis. 2011;17:312–313. doi: 10.3201/eid1702.101293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rabeantoandro Z. Etude des Cératopogonidae de Madagascar: Taxonomie, biogéographie et écologie. Antananarivo: Université d’Antananarivo, Faculté des Sciences; 2012. [Google Scholar]
- 54.Wirth WW. Notes on the biting midges of the Seychelles. Proc Entomol Soc Wash. 1975;79:293–309. [Google Scholar]
- 55.Wirth WW. The biting midges of Aldabra atoll, Indian Ocean (Diptera: Ceratopogonidae) Proc Entomol Soc Wash. 1990;92:230–247. [Google Scholar]
- 56.Dommergues L, Viarouge C, Métras R, Youssoufi C, Sailleau C, Zientara S, et al. Evidence of bluetongue and epizootic haemorrhagic disease circulation on the island of Mayotte. Acta Trop. 2019;191:24–28. doi: 10.1016/j.actatropica.2018.12.037. [DOI] [PubMed] [Google Scholar]
- 57.Van Ark H, Meiswinkel R. Subsampling of large light trap catches of Culicoides (Diptera: Ceratopogonidae) Onderstepoort J Vet Res. 1992;59:183–189. [PubMed] [Google Scholar]
- 58.Meiswinkel R. Afrotropical Culicoides: a redescription of C. (Avaritia) imicola Kieffer, 1913 (Diptera: Ceratopogonidae) with description of the closely allied C. (A.) bolitinos sp. nov. reared from the dung of the African buffalo, blue wildebeest and cattle in South Africa. Onderstepoort J Vet Res. 1989;56:23–39. [PubMed] [Google Scholar]
- 59.Meiswinkel R, Dyce AL. Afrotropical Culicoides: Synhelea Kieffer, 1925, resurrected as subgenus to embrace 10 species (Diptera: Ceratopogonidae) Onderstepoort J Vet Res. 1989;56:147–164. [PubMed] [Google Scholar]
- 60.Ugland KI, Gray JS, Ellingsen KE. The species-accumulation curve and estimation of species richness. J Anim Ecol. 2003;72:888–897. doi: 10.1046/j.1365-2656.2003.00748.x. [DOI] [Google Scholar]
- 61.MacArthur RH, Wilson OE. The theory of island biogeography. Princeton: Princeton University Press; 1967. [Google Scholar]
- 62.R Development Core Team . R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2012. [Google Scholar]
- 63.Meiswinkel R. Afrotropical Culicoides: biosystematics of the Imicola group, subgenus Avariatia (Diptera: Ceratopogonidae) with species reference to the epidemiology of African horse Sickness. South Africa: University of Pretoria; 1995. [Google Scholar]
- 64.Dyce AL, Marshall BD. An early record of Culicoides species (Diptera: Ceratopogonidae) developing in the dung of game animals in southern Africa. Onderstepoort J Vet Res. 1989;56:85–86. [PubMed] [Google Scholar]
- 65.Venter GJ, Labuschagne K, Hermanides KG, Boikanyo SN, Majatladi DM, Morey L. Comparison of the efficiency of five suction light traps under field conditions in South Africa for the collection of Culicoides species. Vet Parasitol. 2009;166:299–307. doi: 10.1016/j.vetpar.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 66.Grimaud Y, Guis H, Boucher F, Chiroleu F, Tran A, Rakotoarivony I, et al. Modelling temporal dynamics of Culicoides populations on Reunion Island (Indian Ocean) vectors of viruses of veterinary importance. In: 21st E-SOVE (European Society for Vector Ecology) Palermo, Italy. 2018.
- 67.Bellis GA. Studies on the taxonomy of Australasian species of Culicoides Latreille (Diptera: Ceratopogonidae) Queensland: The University of Queensland; 2013. [Google Scholar]
- 68.Thepparat A, Bellis GA, Ketavan C, Ruangsittichai J, Sumruayphol S, Apiwathnasorn C. Ten species of Culicoides Latreille (Diptera: Ceratopogonidae) newly recorded from Thailand. Zootaxa. 2015;4033:48–56. doi: 10.11646/zootaxa.4033.1.2. [DOI] [PubMed] [Google Scholar]
- 69.Jacquet S, Huber K, Pages N, Talavera S, Burgin LE, Carpenter S, et al. Range expansion of the bluetongue vector, Culicoides imicola, in continental France likely due to rare wind-transport events. Sci Rep. 2016;6:27247. doi: 10.1038/srep27247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Venail R, Balenghien T, Guis H, Tran A, Setier-Rio ML, Delecolle JC, et al. Assessing diversity and abundance of vector populations at a national scale: example of Culicoides surveillance in France after bluetongue virus emergence. Par Res Monographs. 2012;3:77–102. doi: 10.1007/978-3-642-28842-5_4. [DOI] [Google Scholar]
- 71.Baylis M, el Hasnaoui H, Bouayoune H, Touti J, Mellor PS. The spatial and seasonal distribution of African horse sickness and its potential Culicoides vectors in Morocco. Med Vet Entomol. 1997;11:203–212. doi: 10.1111/j.1365-2915.1997.tb00397.x. [DOI] [PubMed] [Google Scholar]
- 72.Gordon SJG, Bolwell C, Rogers C, Musuka GN, Kelly PJ, Labuschagne K, et al. The occurence of Culicoides species, the vectors of arboviruses at selected trap sites in Zimbabwe. Onderstepoort J Vet Res. 2015;82:900. doi: 10.4102/ojvr.v82i1.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khamala CPM. Breeding habitats and biting activities of Culicoides (Diptera: Ceratopogonidae) at Lake Nakuru National Park, Kenya, with special reference to C. trifasciellus Goetghebuer. East Afr Med J. 1975;52:405–412. [PubMed] [Google Scholar]
- 74.Obame-Nkoghe J, Rahola N, Ayala D, Yangari P, Jiolle D, et al. Exploring the diversity of blood-sucking Diptera in caves of Central Africa. Sci Rep. 2017;7:250. doi: 10.1038/s41598-017-00328-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vassiliades G, Delbove P, Bain O. Onchocercoses bovines au Sénégal. Note préliminaire. Rev Elev Med Vet Pays Trop. 1983;36:351–353. [PubMed] [Google Scholar]
- 76.Wahl G, Achu-Kwi MD, Mbah D, Dawa O, Renz A. Bovine onchocercosis in North Cameroon. Vet Parasitol. 1994;52:297–311. doi: 10.1016/0304-4017(94)90121-X. [DOI] [PubMed] [Google Scholar]
- 77.Paweska JT, Venter GJ, Hamblin C. A comparison of the susceptibility of Culicoides imicola and C. bolitinos to oral infection with eight serotype of epizootic haemorrhagic disease virus. Med Vet Entomol. 2005;19:200–207. doi: 10.1111/j.0269-283X.2005.00560.x. [DOI] [PubMed] [Google Scholar]
- 78.Venter GJ, Wright IM, Del Rio R, Lucientes J, Miranda M. The susceptibility of Culicoides imicola and other South African livestock-associated Culicoides species to infection with bluetongue virus serotype 8. Med Vet Entomol. 2011;25:320–326. doi: 10.1111/j.1365-2915.2010.00931.x. [DOI] [PubMed] [Google Scholar]
- 79.Venter GJ, Paweska JT, Van Dijk AA, Mellor PS, Tabachnick WJ. Vector competence of Culicoides bolitinos and C. imicola for South African bluetongue virus serotypes 1, 3 and 4. Med Vet Entomol. 1998;12:378–385. doi: 10.1046/j.1365-2915.1998.00116.x. [DOI] [PubMed] [Google Scholar]
- 80.Venter GJ, Mellor PS, Paweska JT. Oral susceptibility of South African stock-associated Culicoides species to bluetongue virus. Med Vet Entomol. 2006;20:329–334. doi: 10.1111/j.1365-2915.2006.00635.x. [DOI] [PubMed] [Google Scholar]
- 81.Fall M, Fall AG, Seck MT, Bouyer J, Diarra M, Lancelot R, et al. Host preferences and circadian rhythm of Culicoides (Diptera: Ceratopogonidae), vectors of African horse sickness and bluetongue viruses in Senegal. Acta Trop. 2015;149:239–245. doi: 10.1016/j.actatropica.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 82.Venter GJ, Meiswinkel R. The virtual absence of Culicoides imicola (Diptera: Ceratopogonidae) in a light-trap survey of the colder, high-lying area of the eastern Orange Free State, South Africa, and implications for the transmission of arboviruses. Onderstepoort J Vet Res. 1994;61:327–340. [PubMed] [Google Scholar]
- 83.Nevill H, Nevill EM. A survey of the Culicoides (Diptera: Ceratopogonidae) of the Umlalazi Natiire Reserve in Zululand, South Africa, with notes on two species biting man. Onderstepoort J Vet Res. 1995;62:51–58. [PubMed] [Google Scholar]
- 84.Meiswinkel R, Nevill EM, Venter GJ. Vectors: Culicoides spp. In: Coetzer JAW, Thomson GR, Tustin RC, editors. Infectious diseases of livestock with special reference to southern Africa. Cape Town: Oxford University Press; 1994. pp. 68–89. [Google Scholar]
- 85.Harrup LE, Bellis GA, Balenghien T, Garros C. Culicoides Latreille (Diptera: Ceratopogonidae) taxonomy: current challenges and future directions. Infect Genet Evol. 2015;30:249–266. doi: 10.1016/j.meegid.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bakhoum MT, Labuschagne K, Huber K, Fall M, Mathieu B, Venter GJ, et al. Phylogenetic relationships and molecular delimitation of Culicoides Latreille (Diptera: Ceratopogonidae) species in the Afrotropical region: interest for the subgenus Avaritia. Syst Entomol. 2017;43:355–371. doi: 10.1111/syen.12279. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Description of study sites and trap localization.
Data Availability Statement
All samples are available upon request to CG. Representative biting midge specimens are deposited in the collection of Cirad, UMR ASTRE, Ste Clotilde, La Reunion, France (accession code: YT).