Abstract
Aim of the study
A subgroup of cirrhotic patients undergoing therapeutic paracentesis develop acute kidney injury (AKI) despite adequate colloidal replacement.
The aim of the study was to determine the prevalence and predictors of paracentesis-induced AKI in cirrhotic patients with normal baseline renal parameters and adequate colloidal replacement.
Material and methods
This prospective, observational analytical study was undertaken between April 2015 and April 2017. All patients undergoing therapeutic paracentesis were enrolled as per inclusion and exclusion criteria. Based on Acute Kidney Injury Network (AKIN) criteria for AKI, comparative analysis was performed between those developing and not developing AKI for demography, renal parameters, frequency and quantity of paracentesis per session. Univariate and multivariate regression analyses were performed to determine the predictors of AKI.
Results
Altogether, 177 patients underwent 859 therapeutic paracenteses. Ninety-four paracentesis sessions resulted in an AKI (10.9%). The median number of paracenteses was 10 (range 1-25) and the median volume of fluid drained per paracentesis was 6 l (1-20 l). In univariate analysis, younger age (p < 0.02), higher MELD (Model For End-Stage Liver Disease) score (p < 0.0001), CTP (Child-Turcotte-Pugh) class C (p < 0.017) and prior history of renal dysfunction (p < 0.0001) were significantly associated with AKI. For each liter of fluid drained, the risk of AKI increased by 1.24 times. Frequency of paracentesis did not influence the AKI. In multivariate logistic regression, the significant predictors of AKI were past renal dysfunction, a higher MELD and volume of fluid tapped at paracentesis.
Conclusions
Post-paracentesis AKI occurs in 10.9% of cases, despite adequate colloid replacement. For each 1 l of fluid drained during paracentesis, the risk of AKI increased by 1.24 times.
Keywords: acute kidney injury, large volume paracentesis, cirrhosis, ascites
Introduction
Renal dysfunction is a common complication of liver cirrhosis and is a consequence of abnormal hemodynamics of systemic and splanchnic arterial vasodilatation and extra-hepatic vasoconstriction [1]. Acute kidney injury (AKI), predominantly prerenal and acute tubular necrosis, accounts for the majority of renal dysfunction [2]. Large volume paracentesis (LVP) is an important cause of AKI in cirrhosis with decompensation [3], and is due to an accelerated pathological hemodynamic change secondary to an intravascular contraction and decrease in renal perfusion [4].
Patients with massive ascites are initially managed by dietary restrictions (e.g., sodium and fluid restriction), abstinence from alcohol, and adjustment of dose of diuretics. In the absence of response to these measures, therapeutic paracentesis is recommended [5]. This is done to provide symptomatic relief while preventing complications such as paracentesis-induced circulatory dysfunction (PICD). This was first described in 1988 by Ginès et al. [6]. It follows large-volume paracentesis and results in rapid re-accumulation of ascites, hyponatremia, renal dysfunction (often hepatorenal syndrome), and poor survival [7]. Diagnosis of PICD involves measurement of plasma renin, which increases by more than 50% of baseline plasma renin activity to > 4 ng/ml/h on day 5 or 6 after paracentesis [7]. Based on this background, we decided to use acute kidney injury as a simple surrogate marker for post-paracentesis complication (a component of PICD). AKI in cirrhosis is usually multifactorial and carries a grim prognosis [8].
The present study prospectively aimed to determine the prevalence of AKI and risk factors contributing to AKI following large volume paracentesis in patients with decompensated liver cirrhosis.
Material and methods
This prospective, observational analytical study was undertaken at Gleneagles Global Health City, Chennai between April 2015 and April 2017. The study cohort included cirrhosis patients with decompensation, registered in the liver clinic and requiring one time or repeated paracentesis for relief of symptoms such as breathlessness or abdominal discomfort secondary to tense ascites. The selection of cases was consecutive and independent of CTP (Child-Turcotte-Pugh) and MELD (Model For End-Stage Liver Disease) score with a strict protocol for normal renal parameters at registration.
Adult cirrhotic patients aged 18 to 65 years, belonging to either gender and requiring paracentesis were included for the study. It was obligatory for inclusion that patients undergoing paracentesis had at baseline a normal urine output of > 500 ml/day and a serum creatinine of ≤ 1.5 mg/dl.
The diagnosis of decompensated liver cirrhosis was based on clinical presentation and radiological evidence of a shrunken liver with dilated portal vein, presence of ascites and collaterals. Patient details included age, gender, co-morbidity (diabetes mellitus, hypertension, coronary artery disease, dyslipidemia and hypothyroidism), etiology (alcohol, virus-related, cryptogenic, others), cirrhosis-related complications (past or present), CTP and MELD scores. All patients had baseline liver function tests, serum electrolytes and coagulation profile that included prothrombin time and INR.
Therapeutic paracentesis protocol
Patients and their attendants were explained about the need for paracentesis and procedure-related complications. After obtaining written informed consent, the procedure was performed under ultrasound guidance following the standard hospital protocol with close monitoring of pulse, blood pressure and respiratory rate. Fluid was removed at variable rates but not exceeding > 5 l per hour and the volume tapped was noted. 20% human albumin (20 γ for every 2.5 l of fluid drained) was replaced at the rate of 25 ml per hour. Paracentesis was terminated if the patient developed hemodynamic instability or when the fluid drain stopped.
All patients had a baseline ascitic fluid analysis and included protein, albumin cell count, culture and cytology. In those requiring repeated sessions of paracentesis, cell count alone was repeated during each session. Patients with culture-negative neutrocytic ascites or culture-positive spontaneous bacterial peritonitis (SBP) were excluded from the final analysis.
Pre- and post-paracentesis (48 hours) serum creatinine, serum sodium and urine output were checked.
Patients on nephrotoxic drugs, spontaneous bacterial peritonitis, grade III or IV hepatic encephalopathy, sepsis, non-cirrhotic or post-transplant ascites, hemodynamically unstable (due to gastrointestinal bleed or diarrhea) and severe cardiorespiratory distress were excluded.
Definitions
Large volume paracentesis
Removal of more than 5 l of ascitic fluid in one session [9].
Acute kidney injury
This is defined as an abrupt loss of kidney function that develops within 7 days [10]. Based on Acute Kidney Injury Network (AKIN) criteria, diagnosis of AKI is based on a percentage rise in serum creatinine > 50% from the baseline value or rise in absolute serum creatinine value by > 0.3 mg/dl in 48 hours. Only these two criteria were used to define post-paracentesis AKI in our cohort. Urine output measurement was not considered as a criterion in the present study as the majority of the paracenteses were done as a day care procedure and urine output was not monitored for 48 hours after paracentesis.
Statistical analysis
Occurrence of AKI was the primary outcome variable. Frequency of paracentesis and the volume of fluid drained during each paracentesis were primary explanatory variables. Demographic parameters such as age and gender, and clinical parameters such as CTP grading, etiology, and co-morbidities were considered as other explanatory variables.
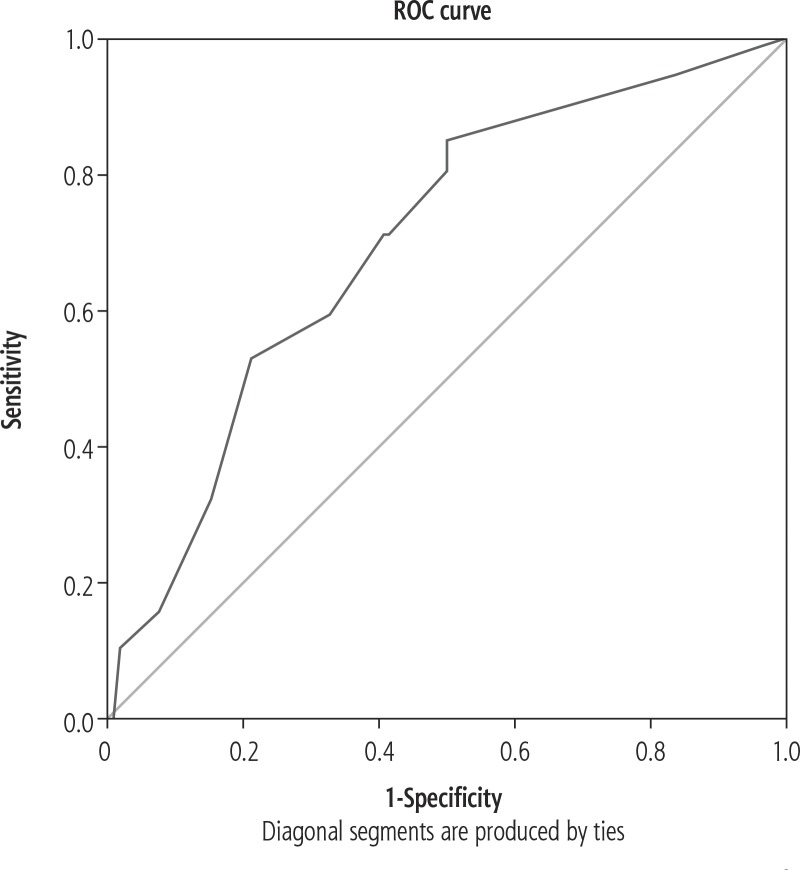
Quantitative variables were compared across AKI categories by mean values. The independent samples t-test was used to assess statistical significance. The chi square test was used to test statistical significance. Univariate binary logistic regression analysis was performed to test the association between the explanatory variables and outcome variables. Variables with statistical significance in univariate analysis were used to compute multivariate regression analysis. The utility of volume of drained fluid in predicting AKI was assessed by receiver operating curve (ROC) analysis (Fig. 1). A p value < 0.05 was considered statistically significant. IBM SPSS version 22 (IBM Corp Armonk, NY; 2013) was used for statistical analysis.
Fig. 1.
Receiver operating curve analysis showing good predictive validity of volume of fluid drained in predicting acute kidney injury (AKI)
The study was performed with ethical standards which conform with the Helsinki Declaration of 1975, as revised in 2000 and 2008, confirming human and animal rights.
Results
Altogether, 177 patients underwent 859 therapeutic paracenteses. Ninety-four paracentesis sessions resulted in an AKI in 31 patients (10.9%). Table 1 shows the baseline characteristics of the study cohort. The majority (87%) of patients were men and belonged to CTP C. Diabetes (p < 0.0001) and hypertension were the two most common co-morbid entities. The most common causes of cirrhosis were cryptogenic cirrhosis/non-alcoholic steatohepatitis and alcohol. Three fourths of the patients belonged to CTP class C with a median MELD score of 20 (range 8-40). Hepatic encephalopathy was the most common complication, followed by SBP.
Table 1.
Baseline demography of the cohort (177 patients)
| Age | Years (median) | 55 (21-84) |
|---|---|---|
| Gender | Male Female |
154 (87%) 23 (13%) |
| Comorbid conditions | Diabetes mellitus | 78 (44%) |
| Hypertension | 29 (16%) | |
| Coronary artery disease | 11 (6%) | |
| Hypothyroidism | 9 (5%) | |
| Combinations of above | 36 (20%) | |
| Others | 5 (2%) | |
| Etiology of cirrhosis | Alcohol | 65 (36%) |
| NASH/cryptogenic | 83 (46%) | |
| HBV, HCV | 26 (14%) | |
| Others | 3 (1.6%) | |
| CTP class | B | 40 (22%) |
| C | 137 (77%) | |
| MELD score | (median, range) | 20 (8-40) |
| Cirrhosis-related complications | SBP | 98 (55%) |
| Hepatic encephalopathy | 135 (76%) | |
| GI bleeding | 84 (47%) | |
| Sepsis/infection | 83 (47%) | |
| Renal dysfunction | 68 (38%) | |
| HCC | 3 (1.6%) |
CTP – Child-Turcotte-Pugh, MELD – Model For End-Stage Liver Disease, NASH – nonalcoholic steatohepatitis, HBV – hepatitis B virus, HCV – hepatitis C virus, SBP – spontaneous bacterial peritonitis, HCC – hepatocellular carcinoma
Patient-related factors predisposing to AKI
Baseline characteristics of 31 patients who developed AKI were compared with 146 patients who did not develop AKI. Patients who developed AKI had a significant renal dysfunction in the past, were significantly young, belonging to CTP C and with a high MELD score (median MELD 24 vs. 19) (Table 2).
Table 2.
Risk factors predisposing to acute kidney injury (AKI)
| Parameters | AKI group (n = 31) | Non-AKI group (n = 146) | p value |
|---|---|---|---|
| Age in years | 50 (21-77) | 55 (26-84) | 0.02 |
| Gender | 26 : 5 (83% : 17%) | 128 : 18 (91% : 9%) | 0.56 |
| Co morbid states | |||
| Diabetes mellitus | 12 (38%) | 66 (47%) | 0.67 |
| Hypertension | 5 (16%) | 24 (17%) | |
| Coronary artery disease | 1 (3.2%) | 10 (7%) | |
| Hypothyroidism | 3 (9.6%) | 6 (4.2%) | |
| Others | 0 (0%) | 5 (3.5%) | |
| Combined | 6 (19%) | 30 (21%) | |
| Etiology | |||
| Alcohol | 15 (48%) | 50 (35%) | 0.38 |
| NASH/cryptogenic | 11 (35%) | 72 (51%) | |
| Hepatotropic viruses | 4 (12%) | 22 (15%) | |
| Others | 1 (3.2%) | 2 (1.4%) | |
| CTP class | |||
| B | 2 (6.4%) | 38 (27%) | 0.017 |
| C | 29 (93%) | 108 (77%) | |
| MELD | |||
| MELD score | 24 (14-40) | 19 (8-40) | < 0.0001 |
| Baseline serum albumin (mean and SD) | |||
| Serum albumin (gm/dl) | 2.78 (0.45) | 2.73 (0.38) | 0.52 |
| Cirrhosis-related complications | |||
| SBP | 20 (64%) | 78 (55%) | 0.35 |
| HE | 26 (83%) | 109 (77%) | 0.46 |
| GI bleeding | 19 (61%) | 65 (46%) | 0.13 |
| Sepsis | 16 (51%) | 67 (47%) | 0.68 |
| Renal dysfunction | 22 (70%) | 46 (32%) | 0.0001 |
| HCC | 0 (0%) | 3 (2.1%) | Not applicable |
CTP – Child-Turcotte-Pugh, MELD – Model For End-Stage Liver Disease, NASH – nonalcoholic steatohepatitis, SBP – spontaneous bacterial peritonitis, HE – hepatic encephalopathy, GI – gastrointestinal, HCC – hepatocellular carcinoma
Paracentesis-related factors predisposing to AKI
The median number of paracenteses per patient was 10 (range 1-25) and volume of fluid drained per paracentesis was 6 l (range 1-20 l). Thirty (3.5%) and 7 (0.8%) of the 859 procedures resulted in hypotension and respiratory distress respectively. In univariate logistic regression, the risk of AKI was highest, that is 1.32 (p < 0.004), when the drained fluid was 8 l or more (Table 3).
Table 3.
Volume of paracentesis and risk for acute kidney injury (AKI)
| Fluid drained | Risk ratio | 95% CI | p value |
|---|---|---|---|
| ≤ 5 l | 0.69 | 0.38-1.25 | 0.22 |
| 5-8 l | 0.75 | 0.55-1.02 | 0.07 |
| > 8 l | 1.32 | 1.09-1.61 | 0.004 |
The utility of the amount of ascitic fluid tapped in determining the risk of AKI was assessed using AUROC. This was calculated to be 0.702 (95% CI: 0.649-0.755, p < 0.001), indicating good predictive validity (Fig. 1). In multivariate logistic regression, prior renal dysfunction (p < 0.001), paracentesis that exceeded 8 l and more (p < 0.001) and high MELD score (p < 0.0001) were significant predictors of AKI.
Discussion
Ascites and renal dysfunction are terminal events of circulatory dysfunction, characterized by marked splanchnic arterial vasodilatation and reduced effective arterial blood volume. The most common cause of renal dysfunction in cirrhosis is pre-renal injury, post-paracentesis being an important cause [11]. Large volume paracentesis exceeding 5 l causes intravascular volume depletion and pre-renal dysfunction. This is part of PICD and is also referred to as “postparacentesis syndrome”. This occurs in up to 80% of cases when a large-volume paracentesis is performed without additional therapeutic replacement. The incidence is reduced to 15% to 35% when volume expanders are used [12]. Supplementation with colloidal form of human albumin circumvents this complication to some extent and today is considered as the standard of care in management of these patients [13, 14]. Apart from maintenance of effective arterial blood volume and lowering rates of renal impairment after LVP, albumin has several other benefits. It maintains serum sodium, decreases activation of the renin-angiotensin-aldosterone system [15] and reduces overall morbidity and mortality [16].
However, a subgroup of cirrhotic patients with normal baseline renal function do develop AKI despite close monitoring and adequate colloid replacement. The present study has attempted to address some of these concerns.
Post-paracentesis AKI in our series occurred in 94 paracenteses in 31 patients (10.9%). A significant number of patients were young with a past history of renal dysfunction, and with more severe liver disease, as shown by advanced CTP and MELD scores. Our observations were similar to those of Nasr et al., who also reported PICD in the young [17]. The reason for higher incidence of PICD in younger individuals is unclear. One hypothesis is that there is a likelihood of blunting of the vasoactive response with advancing age [17].
AKI in the post-paracentesis state has been studied rarely. In a retrospective study of 935 paracentesis procedures from multiple centers in the United States, it was noted that the prevalence of AKI was 1.17% [18]. This is much lower than in our study. This may be related to lower MELD and a lower amount of ascitic fluid tapped on each occasion.
The most important predictor of adverse outcome after paracentesis in various studies has been MELD [18]. Our observations were also similar. Patients with ascites requiring therapeutic paracentesis, likely hepatorenal syndrome type 2, have greater impairment of circulatory function. This is demonstrated by lower arterial pressure and higher activation of vasoconstrictor factors. Moreover, the median survival in these patients with MELD > 20 is significantly lower [19]. Previous renal dysfunction, paracentesis exceeding 8 l (p < 0.001) and not the frequency of paracentesis were important predictors of AKI (p < 0.001). Tan et al. reported that paracentesis of less than 8 l of fluid at a single session at a slow rate with replacement of 6-8 γ of albumin per liter of fluid removed may prevent PICD-related renal dysfunction [20].
Limitations of the study
We did not exclude patients with diabetes or those on β-blockers; hence the prevalence of AKI in our cohort may be higher than that reported in world literature. Urine output measurement was not done as paracentesis was done as a day care procedure. Also misuse or a high dose of diuretics and failure to monitor AKI in between paracenteses are other drawbacks of this study. However, this is the real world scenario in decompensated cirrhotic patients who undergo periodic LVP.
Strengths of the study
We enrolled patients with normal baseline creatinine. We excluded patients with ongoing SBP, active gastrointestinal bleeding, diarrhea, hepatic encephalopathy (grade III-IV) and sepsis.
Disclosure
Authors report no conflict of interest.
References
- 1.Wong F. Recent advances in our understanding of hepatorenal syndrome. Nat Rev Gastroenterol Hepatol. 2012;9:382–391. doi: 10.1038/nrgastro.2012.96. [DOI] [PubMed] [Google Scholar]
- 2.Moreau R, Durand F, Poynard T, et al. Terlipressin in patients with cirrhosis and type 1 hepatorenal syndrome: a retrospective multicenter study. Gastroenterology. 2002;122:923–930. doi: 10.1053/gast.2002.32364. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Tsao G, Parikh Ch, Viola A. Acute kidney injury in cirrhosis. Hepatology. 2008;48:1–14. doi: 10.1002/hep.22605. [DOI] [PubMed] [Google Scholar]
- 4.Vila MC, Solà R, Molina L, et al. Hemodynamic changes in patients developing effective hypovolemia after total paracentesis. J Hepatol. 1998;28:639–645. doi: 10.1016/s0168-8278(98)80288-2. [DOI] [PubMed] [Google Scholar]
- 5.Kuiper JJ, de Man RA, van Buuren HR. Review article: Management of ascites and associated complications in patients with cirrhosis. Aliment Pharmacol Ther. 2007;26:183–193. doi: 10.1111/j.1365-2036.2007.03482.x. [DOI] [PubMed] [Google Scholar]
- 6.Ginès P, Titó L, Arroyo V, et al. Randomized comparative study of therapeutic paracentesis with and without intravenous albumin in cirrhosis. Gastroenterology. 1988;94:1493–1502. doi: 10.1016/0016-5085(88)90691-9. [DOI] [PubMed] [Google Scholar]
- 7.Ginès A, Fernández-Esparrach G, Monescillo A, et al. Randomized trial comparing albumin, dextran 70, and polygeline in cirrhotic patients with ascites treated by paracentesis. Gastroenterology. 1996;111:1002–1010. doi: 10.1016/s0016-5085(96)70068-9. [DOI] [PubMed] [Google Scholar]
- 8.Belcher JM, Parikh CR, Garcia-Tsao G. Acute kidney injury in patients with cirrhosis: perils and promise. Clin Gastroenterol Hepatol. 2013;11:1550–1558. doi: 10.1016/j.cgh.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Runyon BA. AASLD Practice Guidelines Committee Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087–2107. doi: 10.1002/hep.22853. [DOI] [PubMed] [Google Scholar]
- 10.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Critical Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sola-Vera J, Miñana J, Ricart E, et al. Randomized trial comparing albumin and saline in the prevention of paracentesis-induced circulatory dysfunction in cirrhotic patients with ascites. Hepatology. 2003;37:1147–1153. doi: 10.1053/jhep.2003.50169. [DOI] [PubMed] [Google Scholar]
- 12.Lindsay AJ, Burton J, Ray CE. Paracentesis-induced circulatory dysfunction: a primer for the interventional radiologist. Semin Intervent Radiol. 2014;31:276–278. doi: 10.1055/s-0034-1382799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gines A, Fernandez-Esparrach G, Monescillo A, et al. Randomized trial comparing albumin, dextran 70, and polygeline in cirrhotic patients with ascites treated by paracentesis. Gastroenterology. 1996;111:1002–1010. doi: 10.1016/s0016-5085(96)70068-9. [DOI] [PubMed] [Google Scholar]
- 14.Planas R, Gines P, Arroyo V, et al. Dextran-70 versus albumin as plasma expanders in cirrhotic patients with tense ascites treated with total paracentesis. Gastroenterology. 1990;99:1736–1744. doi: 10.1016/0016-5085(90)90481-f. [DOI] [PubMed] [Google Scholar]
- 15.Gines P, Tito L, Arroyo V, et al. Randomized study of therapeutic paracentesis with and without intravenous albumin in cirrhosis. Gastroenterology. 1988;94:1493–1502. doi: 10.1016/0016-5085(88)90691-9. [DOI] [PubMed] [Google Scholar]
- 16.Bernardi M, Caraceni P, Roberta N, et al. Albumin infusion in patients undergoing large-volume paracentesis. A meta-analysis of randomized trials. Hepatology. 2012;5:1172–1181. doi: 10.1002/hep.24786. [DOI] [PubMed] [Google Scholar]
- 17.Nasr G, Hasan A, Ahmed S, et al. Predictors of large volume paracantesis induced circulatory dysfunction in patients with massive hepatic ascites. J Cardiovasc Dis Res. 2010;1:136–144. doi: 10.4103/0975-3583.70914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson KB, Mueller JL, Simon TG, et al. Reduced albumin dosing during large-volume paracentesis is not associated with adverse clinical outcomes. Dig Dis Sci. 2015;60:2190–2195. doi: 10.1007/s10620-015-3578-z. [DOI] [PubMed] [Google Scholar]
- 19.Alessandria C, Ozdogan O, Guevara M, et al. MELD score and clinical type predict prognosis in hepatorenal syndrome: relevance to liver transplantation. Hepatology. 2005;41:1282–1289. doi: 10.1002/hep.20687. [DOI] [PubMed] [Google Scholar]
- 20.Tan HK, James PD, Wong F. Albumin may prevent the morbidity of paracentesis-induced circulatory dysfunction in cirrhosis and refractory ascites: a pilot study. Dig Dis Sci. 2016;61:3084–3092. doi: 10.1007/s10620-016-4140-3. [DOI] [PubMed] [Google Scholar]