Abstract
The gut microbiota has recently been recognized as a major environmental factor in the pathophysiology of several human diseases. The anatomical and functional association existing between the gut and the liver provides the theoretical basis to assume that the liver is a major target for gut microbes. In the last decades, many studies have reported an altered composition of gut microbiota in patients with chronic liver diseases and liver cirrhosis, suggesting a progressively marked dysbiosis to be related to worsening of the liver disease. Modifications of microbiota result in alteration in providing signals through the intestine and bacterial products, as well as hormones produced in the bowel that affect metabolism at different levels including the liver. There is increasing evidence for a correlation between intestinal microbiota, bacterial translocation and hepatic steatosis. Intestinal microbiota affects nutrient absorption and energy homeostasis. Altered intestinal permeability may favor the passage of bacteria derived compounds into the systemic circulation, causing a systemic inflammatory state, characteristic of the metabolic syndrome. At present, an increasing number of studies indicate a close relationship between dysbiosis, defined as abnormal composition and the amount of intestinal bacteria (gut microbiota), intestinal permeability and some metabolic, inflammatory, degenerative and even psychiatric diseases. Microbiota pharmacological modulation seems to be a promising tool for a new therapeutic approach to non-alcoholic fatty liver disease and in prevention of cirrhosis.
The following study aims to briefly discuss the role of microbiota disorder (dysbiosis), and in particular small intestinal bacterial overgrowth (SIBO), in the pathogenesis of nonalcoholic fatty liver disease (NAFLD).
Keywords: nonalcoholic fatty liver disease, small intestinal bacterial overgrowth, microbiota, insulin resistance, obesity
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in developed countries. The incidence of small intestinal bacterial overgrowth (SIBO) due to the variety of symptoms is difficult to estimate accurately. At present, the gut microbiota has been recognized as a major environmental factor in the pathophysiology of several human diseases [1, 2]. Many studies have reported an altered composition or amount of intestinal bacteria (dysbiosis) in patients with chronic liver diseases and liver cirrhosis, suggesting a progressively marked dysbiosis to be related to worsening of the liver disease. An increasing number of studies have also indicated a close relationship between dysbiosis and some metabolic, inflammatory, degenerative and even psychiatric diseases. SIBO-related diseases include type 2 diabetes (T2DM), metabolic syndrome (MS), obesity and NAFLD, whose common link is insulin resistance (IR) and oxidative stress, and in most cases a chronic, low-grade inflammatory process.
Modifications of microbiota result in alteration in providing signals through the intestine and bacterial products, as well as hormones produced in the bowel that affect metabolism at different levels including the liver. There is increasing evidence for a correlation between intestinal microbiota, bacterial translocation and hepatic steatosis. Intestinal microbiota affects nutrient absorption and energy homeostasis. Altered intestinal permeability may favor the passage of bacteria derived products into the systemic circulation, causing a systemic inflammatory state, characteristic of the metabolic syndrome [3].
Microbiota pharmacological modulation seems to be a promising tool for a new therapeutic approach to NAFLD and in prevention of cirrhosis and its complications.
The following study aims to briefly discuss the role of microbiota disorders (dysbiosis), and in particular SIBO in the pathogenesis of NAFLD and its progression.
Small intestinal bacterial overgrowth
Small intestinal bacterial overgrowth is a disease entity associated with an increase in the small intestine of the amount of bacteria typical for the large intestine. In the physiological state there are a number of natural mechanisms to prevent aberrant bacterial colonization in the small intestine. These include acidic pH in the stomach, pancreatic enzymes, intestinal immune system, small intestine peristalsis, ileocecal valve and correct intestinal barrier resulting from the construction of the intestinal wall and its proper renewal. If one of these mechanisms is disturbed, SIBO may develop. The most common symptoms of SIBO reported by patients are abdominal pain, diarrhea, flatulence, and abdominal overflow. The symptoms are usually not very specific and diverse, depending on the patient and the cause that led to SIBO. There have also been cases of patients without typical intestinal symptoms, but with weight loss, neuropathy, megaloblastic anemia, peripheral edema, erythema nodosum, and osteomalacia. Due to the wide variety of symptoms and often overlapping disease entities, precise data on the prevalence of SIBO are not available. The disease is more common in older people, which is probably associated with an increase in the number of drugs taken, including proton pump inhibitors, peristaltic disorders, coexistence of other diseases, especially diabetes, decreased gastric acid secretion and endocrine disorders.
The overgrowth of altered gut microbiota in the small intestine can affect the absorption and metabolism of carbohydrates, proteins, fats and vitamins. Damage to the intestinal villi, impaired digestive enzyme production and intestinal barrier dysfunction lead to malabsorption and increased loss of nutrients. In addition, the increased presence of undigested nutrients such as sorbitol or lactose enhances bacterial fermentation in the gut. Deconjugation of bile salts is responsible for impaired digestion of fats and absorption of fat-soluble vitamins. Anaerobic bacteria increase the consumption of vitamin B12, which may lead to the development of megaloblastic anemia. The level of folic acid is not lowered, and can even be slightly raised due to the synthesis of intestinal bacteria [1, 2].
Currently, the basic test for diagnosing SIBO is a hydrogen breath test with glucose or lactulose [4-7].
There are different methods of preparation, performance and interpretation of the breath test, so it is connected with heterogeneity between centers and doctors. Due to the lack of clear consensus how to perform this test we should treat it with caution. Before the test antibiotics should be avoided for 4 weeks, promotility drugs and laxatives for one week. It is not clear whether probiotics should be avoided before this test. Based on the North American consensus proton pump inhibitors are not forbidden [4-7]. Due to costs, difficulties in proper performance and invasiveness, the quantitative assessment of the small intestine aspirate is not used [4, 7]. Treatment is primarily antibiotic therapy. The first-choice drug is rifaximin at a dose of 3-4 × 400 mg for 14 days, together with dietary treatment and, if possible, removal of SIBO risk factors [4].
Nonalcoholic fatty liver disease
Nonalcoholic fatty liver disease is a liver disease that affects about 25-30% of the population in developed countries, with even higher incidence in obese, overweight and T2DM [8]. It covers a wide spectrum of disorders from simple steatosis by nonalcoholic steatohepatitis (NASH) to liver cirrhosis and hepatocellular carcinoma (HCC) [9]. It should be mentioned here that the risk of HCC in the course of NAFLD is already increasing in the early stages of the disease, in which advanced fibrosis and increased inflammation are not yet present [10]. Currently, almost half of HCC diagnoses in patients with NAFLD are made before the appearance of advanced fibrosis (bridging fibrosis and cirrhosis), of which about 20% are made before the occurrence of significant fibrosis (periportal fibrosis). The increase in the prevalence of NAFLD is in parallel with the prevalence of obesity, T2DM and MS. NAFLD involves, among other things, an increased risk of premature cardiovascular disease, kidney disease and the development of many cancers, including colorectal cancer [11]. The increase in cancer risk is particularly influenced by the emergence of obesity and/or T2DM. At the root of the pathogenesis of development and progression of NAFLD there are many factors, the role of which is described by the “multiple parallel hits hypothesis”. The development of NAFLD is significantly influenced by genetic and environmental factors [12]. Recently, more and more evidence points to a close relationship between the composition of microbiota and the development and progression of NAFLD and its complications [3].
The relationship between small intestinal bacterial overgrowth and non-alcoholic fatty liver disease, the importance of the proper microbiota
The human microbiota consists of about 1014 cells of mainly anaerobic bacteria, of which 60-90% belong to two types: Bacteroidetes and Firmicutes [13]. The intestinal microbiota is influenced by diet, gastrointestinal infections, drugs used, mainly antibiotics, age, body weight, past surgery and coexisting diseases [14].
The intestinal microbiota is involved in the metabolism of the host by the secretion of bioactive metabolites, which affects the immune system and permeability of the mucosal barrier. It takes part in the fermentation of undigested food residues, the synthesis of vitamin K and B group vitamins and in the synthesis of short-chain fatty acids (SCFAs), which are the source of energy for intestinal cells. Intestinal bacteria contribute to the absorption of polyphenols that are contained in the diet, through interaction in the transformation from inactive to active form. The intestinal microbiota also participates in the metabolism of xenobiotics and drugs [15].
The portal vein supplies the liver with blood rich in digestive products, but also microbiological components derived from the microbiota. The liver is first exposed to contact with products such as bacterial endotoxins, mainly lipopolysaccharide (LPS), unmethylated DNA sequences that, when released into the liver, may trigger an inflammatory reaction that affects the course of some hepatic disorders [14].
Mechanisms leading to the development of NAFLD with coexisting SIBO
In 1921, B. Hoefert first drew attention to significant changes in the composition of the intestinal microbiota in patients with chronic liver disease [16, 17]. In recent years, the relationship between gut microbiota disorders and some liver diseases has not been in doubt. Attention was also drawn to the effect of SIBO on the severity and course of liver disease and the increased risk of complications, especially ascites, hepatic encephalopathy, spontaneous bacterial peritonitis and portal hypertension, which accompany decompensated liver cirrhosis [16].
The gut microbiota may affect all risk factors for the development of NAFLD by disturbing energy homeostasis, enhancing IR, increasing oxidative stress, developing inflammation, and evoking alteration of bile acids and choline levels.
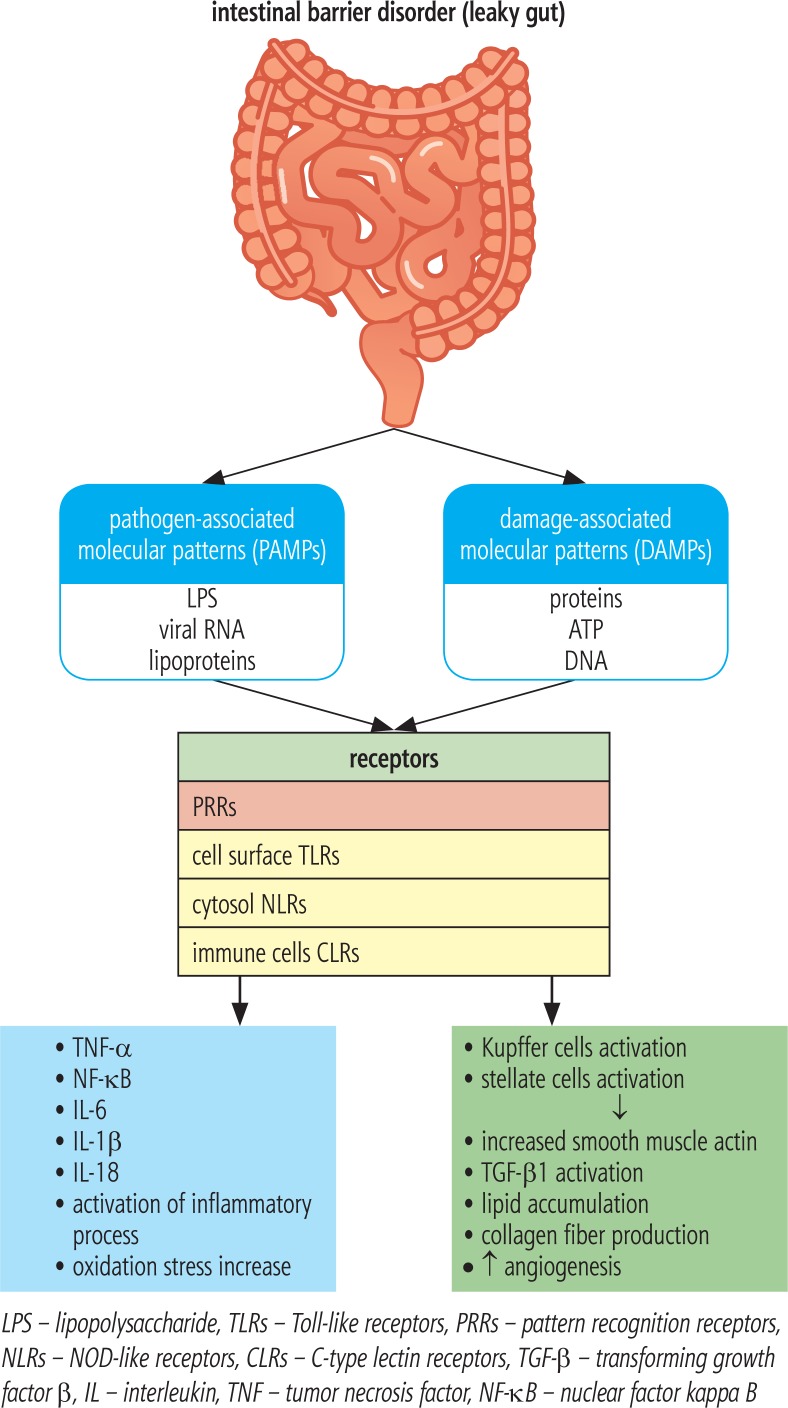
In the case of SIBO, the permeability of the intestinal barrier increases, which promotes bacterial translocation together with bacterial products, especially LPS [18]. Kapil et al. observed in patients with NAFLD and SIBO an increase in the level of endotoxins, CD14 mRNA, nuclear factor kappa B (NF-kB) and Toll-like receptor 4 (TLR-4). Endotoxemia in SIBO patients is likely to activate the TLR-4 and CD14 receptor by stimulating the expression of NF-kB, which mediates the production of proinflammatory cytokines such as tumor necrosis factor α (TNF-α), interleukin (IL)-1β, IL-6 and IL-8. Overproduction of these cytokines contributes to the development of inflammation and insulin resistance and may be essential in the pathogenesis of NASH, liver fibrosis and HCC [19]. The association between SIBO and activation of TLRs is shown in Figure 1. In addition, the up-regulation of TNF-α may be associated with an increase in IR, which is a well known trigger of hepatic fibrosis development and progression [18].
Fig. 1.
Relationship between dysbiosis and pattern recognition receptors activation
The traditional theory of two hits in the pathogenesis of NAFLD is currently being developed and replaced with the multiple parallel hits hypothesis, describing NAFLD as a result of the action of many factors acting simultaneously [3, 12]. Increase in IR is associated with hyperinsulinemia, which is particularly deleterious for the liver. Insulin resistance leads to an increase in levels of free fatty acids (FFAs) by up-regulation of their hepatic synthesis and enhancement of adipose tissue lipolysis. When the level of FFAs exceeds the possibility of their transformation and transport to peripheral tissues (as very low density lipoprotein – VLDL), oxidative stress occurs, which leads to alteration in the adipokine profile, increased production of proinflammatory cytokines (TNF-α, IL-6) and, consequently, to the development of fatty liver [12, 20].
The inflammasome is an intracellular protein pro-inflammatory complex consisting of caspases and molecules derived from fragments of pathogens and damaged cells, which is responsible for the activation of the pro-inflammatory cascade. The inflammasome is a specific sensor for host particles formed during necrosis or apoptosis of cells, mainly hepatocytes (damage-associated molecular patterns – DAMPs) and pathogens derived from intestinal microorganisms (pathogen-associated molecular patterns – PAMPs), which include LPS, lipoproteins, peptidoglycans, bacterial polysaccharides, viral RNA, and bacterial wall proteins. PAMPs act on pattern recognition receptors (PRRs), which include TLRs located on the surface of the cell, and nucleotide-binding oligomerization domain-like receptors (NOD-like receptors – NLRs) localized in the cytosol and C-type lectin receptors (CLRs) present in immune cells. TLRs are very well known, especially TLR-4 recognizing mainly the Gram-negative bacterial LPS, TLR-5 – bacterial flagellin, and TLR-9 – bacterial double-stranded DNA [21]. In the development of NAFLD, activation of TLR-4 and TLR-9 receptors is most important, whereas TLR-2 activation may exert even hepatoprotective effects. Despite the lack of expression in hepatic cells, activation of TLR-5 in studies in mice fed a high-calorie diet was probably associated with disturbed gut microbiota and probably as a consequence it caused an increased tendency to obesity, IR and hepatic steatosis. Activation of the above-mentioned PRRs induces activation of inflammasomes, which up-regulate secretion of proinflammatory cytokines such as IL-1β and IL-18 [22, 23]. Levels of inflammasome-associated proteins such as NLRP3, proIL-1β, and pro-IL-18 have been found to be significantly higher in NASH patients. The inflammasome also directly affects hepatic stellate cells (HSCs) and macrophages (Kupffer cells) by stimulating the production of smooth muscle actin, transforming growth factor β (TGF-β) and collagen fibers, which promotes the development and progression of fibrosis [12]. Kupffer cells play an important role in the pathogenesis of NASH. They can occur in two forms: pro-inflammatory, called M1, and M2, responsible for the production of anti-inflammatory cytokines. Bacterial products such as LPS activate the proinflammatory M1 form. The disturbed balance between the forms M1 and M2 plays a key role in the progression of NAFLD. M1 macrophages through increased release of IL-1β stimulate hepatic production of triglycerides and activate HSCs [23]. The complex display describing the role of PRRs activation is presented in Figure 1.
On the basis of some research, the lack of certain components of the inflammasome may be associated with protective action in the development of NAFLD. However, other studies revealed that this may even be associated with a more aggressive course of the disease [22]. Animal studies also showed a close association between inflammasome component dysfunction and gut microbiota composition. Recent studies found a relationship between a high-fat, high-carbohydrate diet and a lack of NLRP3 inflammasome with exacerbated liver damage and increased number of Proteobacteria and Verrucomicrobia. These bacteria facilitate damage to the mucosa and promote bacterial translocation [22].
Adipose tissue, mainly abdominal, is a very active endocrine organ. It produces a number of factors with auto- and paracrine effects, called adipokines (adipocytokines). The adipokine family is constantly growing. The best known are leptin and adiponectin. Adiponectin improves insulin sensitivity, and exerts anti-inflammatory, anti-atherosclerotic and anti-steatotic effects. In contrast, leptin has pro-inflammatory and pro-angiogenic activity, stimulates fibrosis progression, and promotes the development of HCC. Adipose tissue of obese individuals produces a low amount of adiponectin and an elevated amount of leptin. This disturbed leptin and adiponectin release results in increased steatosis, activation of the inflammatory process and development of fibrosis within the liver [12]. More and more evidence confirms a significant impact of other “newly discovered” adipokines in the pathogenesis of chronic liver disease, including NAFLD [24-27].
Bacteria using glycoside hydrolase and polysaccharide lyase metabolize undigested polysaccharides to monosaccharides and short chain fatty acids (SCFAs). Monosaccharides transported to the liver with portal circulation activate factors responsible for increasing the transcription of proteins involved in hepatic lipogenesis. Short-chain fatty acids are material for lipo- and gluconeogenesis, which cover up to 5-10% of the host’s energy requirement [20]. SCFAs act on FFA3 and FFA2 receptors. Activation of the FFA3 receptor stimulates enteroendocrine cells to increase production of the peptide YY (PYY), which reduces intestinal motility and increases the absorption of SCFAs. Activation of the FFA2 receptor in intestinal neutrophils inhibits lipolysis. The presence of these receptors in neutrophils may be important in increasing inflammation and permeability of the intestinal barrier, and thus in the pathogenesis of NASH [20]. SCFAs, however, along with its influence on the development of obesity and fatty liver, by acting on the GPR43 receptor, reduce inflammation within the intestinal mucosa, thus improving the intestinal barrier status and reducing its permeability to bacterial products [28]. A special positive role is played by butyric acid produced by the normal gut microbiota. Microbiota impaired composition results in a reduced amount of butyric acid in the intestine [29]. SCFAs also affect glucose metabolism by regulating the secretion of incretin hormones such as the glucagon-like peptide-1 peptide (GLP-1) [22]. These hormones increase insulin secretion after a meal. GLP-1 also delays gastric emptying and reduces appetite, preventing weight gain. GLP-2, which is secreted with GLP-1, helps maintain the integrity of the intestinal barrier, improves the absorption of nutrients, and delays gastric emptying [22].
Intestinal bacteria may also have an inhibitory effect on intestinal expression of the fasting-induced adipose factor (FIAF), which is an inhibitor of lipoprotein lipase. Microbiota can increase lipoprotein lipase activity in adipose tissue in this way, which leads to the intensification of the delivery of adipocyte-derived triglycerides and to the hepatic accumulation of triacylglycerols [14].
Zhu et al. in their study linked intestinal bacteria, in particular Escherichia coli, with increased production of endogenous alcohol in patients with NASH, which in normal conditions in healthy patients with normal microbiota is almost immediately removed by alcohol dehydrogenase in the liver. Increased alcohol level and its metabolism is associated with damage and increased permeability of the intestinal barrier, endotoxemia, increased proinflammatory cytokines, increased oxidative stress, and consequently the development of inflammation in the liver, which may also have a role in the pathogenesis of this disease [30, 31].
Bile acids are formed in the liver, and the classical pathway which begins with the conversion of cholesterol into 7a-hydroxycholesterol under the influence of cholesterol 7’-hydroxylase, leading to the formation of cholic acid (CA) and chenodeoxycholic acid (CDCA). The alternative pathway leads to the conversion of cholesterol to 27-hydroxycholesterol under the influence of sterol 27-hydroxylase. It is not entirely clear what determines the choice of bile acid pathway. Perhaps the classical process takes place under physiological conditions, and an alternative in the situation of the existing liver pathology [32]. Primary bile acids formed in the classical pathway are conjugated with glycine and taurine and then stored in the gallbladder and secreted into the duodenum in response to food intake. In the digestive tract under the influence of gut microbiota deconjugation occurs and secondary bile acids are formed – lithocholic acid (LCA) and deoxycholic acid (DCA). The bile acids are then reabsorbed in the ileum, and almost 95% return to the liver via enterohepatic circulation [33]. The reuptake processes in the liver depend on the liver status and the amount of bile acids. The farnesoid X receptor (FXR), by regulating gene expression, plays an important role in regulating many important processes in the body. These receptors are predominantly present in the ileum and liver, and are activated by bile acids, mainly by CDCA, followed by decreasing DCA, CA and LCA affinities, respectively. Their activation reduces bile acid synthesis and potentiates their secretion from the liver, influences the regulation of lipid metabolism and gluconeogenesis, and is important in the regulation of inflammatory processes, regeneration, fibrosis and carcinogenesis in the liver [33]. Another receptor involved in regulating the amount of bile acids is the TGR5 receptor (the G protein-coupled receptor) found mainly in the liver, gallbladder and bile ducts, adipose tissue, small intestine, kidneys and spleen. Its activation affects, inter alia, the distribution of bile acids, modulates the inflammatory response in the liver, improves insulin sensitivity and regulates glucose metabolism, and also diminishes the risk of atherosclerosis. It is activated by bile acids in a different order than in the case of FXR, i.e. mainly through LCA, followed by DCA, CDCA and the worst by CA [33, 34]. Intestinal bacteria, through the influence on the modification of the composition of bile acids, affect the activation of the above-mentioned receptors with corresponding consequences. Patients with NASH have a change in the composition of bile acids with a predominance of secondary (lithocholic and deoxycholic) bile acids [20, 35, 36]. On the other hand, bile acids prevent bacterial overgrowth in the intestine. This effect is achieved due to detergent properties of bile acids, but also by the FXR receptor, consequently reducing bacterial proliferation in the small intestine [22]. Intestinal bacteria can also affect choline metabolism. Choline is delivered with food and is synthesized in the host. It takes part in the synthesis of the neurotransmitter acetylcholine and in the metabolism of lipids in the liver. The relationship between choline deficiency and the accumulation of hepatic lipids has been known for many years; however, there is not much information related to the application and usefulness of choline in the prevention and treatment of NAFLD. Gut microbiota is involved in the intestinal choline metabolism, which may lead to a decrease in its bioavailability and result in a deficiency of choline. Intestinal bacteria are also responsible for the conversion of choline and phosphatidylcholine to trimethylamine, which on the one hand reduces choline levels, predisposing to NAFLD, and on the other raises the level of trimethylamine, which is associated with an increased risk of cardiovascular disease [37].
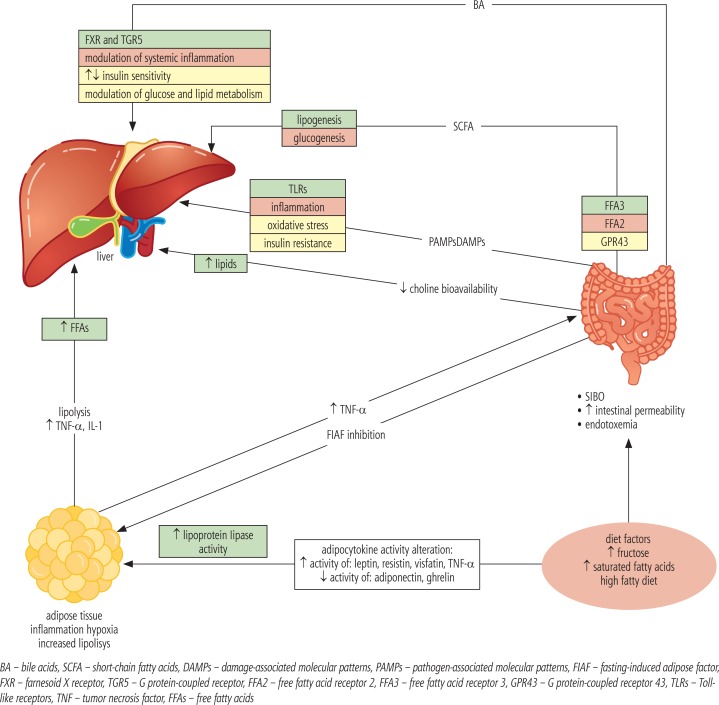
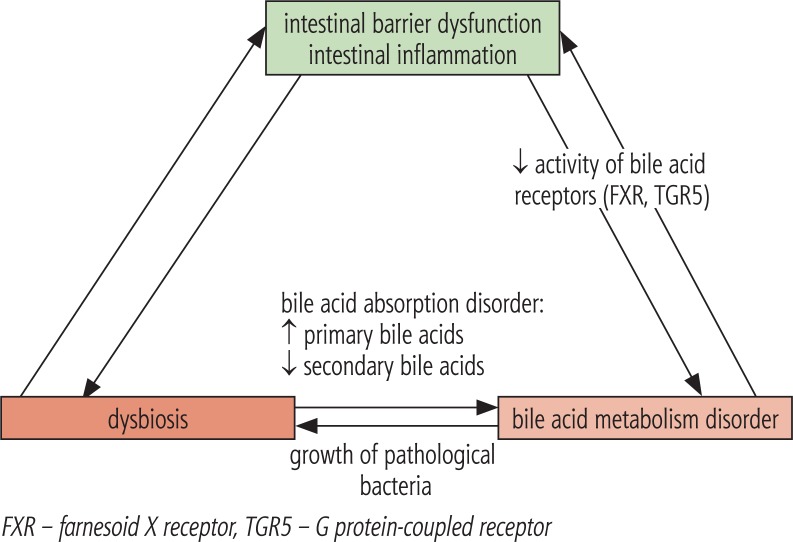
In studies in mice, it was found that even a small amount of intestinal endotoxins may have an effect on increased sensitivity to leptin, exacerbating the inflammatory response and fibrosis progression [12]. Complex relationships between SIBO and NAFLD is shown in Figure 2, while relationship between alterations in intestinal microbiota composition and bile acid metabolism is presented in Figure 3.
Fig. 2.
Relationships between SIBO and NAFLD
Fig. 3.
Relationship between alterations in intestinal microbiota composition and bile acid metabolism
Gut microbiota disorders in patients with NAFLD
Much research has been done on mice that suggested a significant relationship between the gut microbiota and NAFLD. The first such association in humans was described in 1982 by Drenic et al. Patients who underwent bariatric surgery to produce gastric bypass due to morbid obesity developed NASH and SIBO. Steatosis resolved or decreased after treatment with antibiotics [38]. In 2001, Wigg et al. presented a study strongly confirming that SIBO is much more prevalent among NASH patients compared to those without liver disease (50% vs. 22%) [39].
Various studies show not entirely consistent data on the bacteria that predominate in gut microbiota [28]. Among patients with obesity, a decrease in the number of bacteria was found from the phylum Bacteroidetes, and the rise of Firmicutes. The reduction of body weight led to the improvement of these disorders. Similarly, a reduced percentage of Bacteroidetes was observed in patients with NASH when compared to those with simple staetosis or without liver disease [17]. Another paper by Boursier et al., in which 57 patients with biopsy proven NAFLD were examined, confirmed a definite increase in the number of Bacteroides (Bacteroidetes) in patients with NASH and the growth of Ruminococcus bacteria (phylum Firmicutes) in patients with existing liver fibrosis and reduction of Prevotella bacteria (Bacteroidetes type) in patients with NASH [38]. However, there were also studies in which no significant differences in the number of individual types of bacteria were found [20].
A lot of attention is devoted to the bacterium Akkermansia muciniphila (type Verrucomicrobia). It is an anaerobic bacterium found in the gastrointestinal tract in about 80% of people, constituting up to 4% of all bacterial cells in the healthy human stool. This bacterium consumes glycosylated intestinal mucosa proteins for the production of acetates and propionates, which are an important source of energy for intestinal cells. This significantly influences the condition of the intestinal barrier [40]. Its level decreases clearly in obese mice and combines with disorders such as an increase in glucose and triglyceride levels and an increase in insulin resistance. Increasing the amount of A. muciniphila in mice is associated with improvement of the integrity of the intestinal barrier, resulting in better glucose control and reduction of circulating pro-inflammatory LPS [41, 42].
The increase in the number of Bacteroides may influence the development of NASH through several mechanisms. There was an increase in deoxycholic acid, D-pinitol, choline, raffinose and stachyose, and a decrease in SCFAs and amino acids in stool with an increased amount of Bacteroides. Most of these components contribute to the pathogenesis of NAFLD, and especially NASH [43]. It should be noted, however, that the vast majority of tests carried out base their data on the examination of the stool microbiota composition, which consists mainly of bacteria from the large intestine, not small. Initially, it was based on the cultivation of bacteria. However, it is known that over 60% of bacteria cannot be grown in vitro. Thanks to the development of genetic engineering, a much more accurate assessment is now possible [28]. The studies carried out concerned patients with NAFLD and elevated BMI, as obesity had already been repeatedly associated in studies with microbiota disorders. It is not clear what the situation looks like in patients with normal BMI.
Treatment of small intestinal bacterial overgrowth and its impact on the course of NAFLD
Antibiotics
Currently, antibiotics that work only in the gastrointestinal tract, primarily rifaximin at a dose of 1200-1600 mg/day (or metronidazole 750 mg/day) for 10-14 days, are used. Antibiotic therapy can be repeated at the relapse of the disease. Rifaximin is an antibiotic with broad-spectrum activity against Gram positive and Gram negative aerobic and anaerobic bacteria. Additionally, rifaximin exerts bile acid-dependent solubility, and therefore becomes more effective in the small intestine while colonic bacteria are poorly inhibited [44]. Indeed, changes in the colonic gut microbiota composition are progressively but completely reversed after rifaximin interruption, differently from the effects on duodenal bacteria, which appear to be more stable [45]. In addition to the bactericidal and bacteriostatic activity, which is typical for an antibiotic, rifaximin can also exert a non-traditional effect through positive modulation of microbiota composition and interactions [4, 46]. Rifaximin can down-regulate the inflammatory response due to inhibition of proinflammatory cytokines and down-regulation of the NF-κB via pregnane X receptor (PXR) activation [47, 48]. The beneficial effect of rifaximin in the course of severe liver disease, in the secondary prevention of hepatic encephalopathy, has been proven, and the drug has been introduced into daily practice. This antibiotic also works well as secondary prevention of spontaneous bacterial peritonitis (SBP) and an adjuvant with b-blockers, in order to reduce the pressure in the portal vein system, which significantly reduces the risk of bleeding from esophageal varices [49, 50]. Less available are data on the efficacy of rifaximin in the treatment of NAFLD. Gangarapu et al. used rifaximin 1200 mg daily for 28 days in 42 patients with NAFLD confirmed by histopathological examination. In this group, 27 patients were diagnosed with NASH. After administration of rifaximin, there was a significant decrease in aspartate transaminase (AST) and γ-glutamyl transpeptidase (GGTP) and endotoxemia in NASH patients [51].
Probiotics
The experiments conducted on animals with VSL # 3 (Streptococcus thermophilus, Bifidobacterium longum, Bifidobacterium breve, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus paracasei, Lactobacillus delbrueckii ssp. Bulgaricus) used in the NAFLD showed promising results, favorably influencing the severity of histopathological changes and decreasing the activity of aminotransferases [28]. However, the results in humans are not so obvious and unequivocal. Loguercio et al. used VSL # 3 in 22 patients with NAFLD and 20 patients with alcoholic cirrhosis, resulting in a decrease in serum oxidative stress factors [52]. Malaguarmera et al. divided a group of 66 from NASH into two subgroups. Both groups were recommended lifestyle modifications, and one group was additionally treated with Bifidobacterium longum and fructooligosaccharides. Among patients receiving the prebiotic, a significant decrease in TNF-α, CRP, LDL cholesterol and endotoxin concentrations, AST activity and HOMA-IR, NAFLD activity score and steatosis grade was observed [53]. Wong et al. administered to 16 NASH patients Lactobacillus and Bifidobacterium spp., obtaining a reduction in steatosis after treatment, but without any changes in the remaining morphological parameters [54]. However, as previously reported, the data on the amount of individual bacteria in patients with NAFLD/NASH are not consistent, and additionally the administered probiotics did not contain bacterial strains, the greatest deficiency of which is observed in these diseases.
Prebiotics are non-digestible nutrients that have beneficial effects on the host organism, among others, by improving the composition of the gut microbiota and regeneration of the intestinal epithelium. The role of probiotics in the treatment of NAFLD is mainly an improvement in lipid metabolism, and by influencing the change in the composition of microbiota, improving the intestinal barrier, reducing its permeability and lowering the level of proinflammatory cytokines, which results, inter alia, from reduced bacterial translocation. There are not enough studies that could provide convincing evidence of the efficacy of probiotic treatment, but one can consider using it in patients without satisfactory improvement after a typical treatment [55].
It seems that a low-calorie diet with a reduced content of carbohydrates and fats, and increased choline content, should have a beneficial effect on improving the composition of the intestinal microbiota. However, such studies are not available [28]. It is highly probable that such a diet positively affects the development of NAFLD and should be recommended for patients with this disease.
Conclusions
The studies available in the literature confirm the relationship between SIBO and NAFLD. There is increasing evidence for a correlation between intestinal microbiota, bacterial translocation and hepatic steatosis. The pathogenesis of liver changes associated with gut microbiota alteration is still not entirely clear. Altered intestinal permeability may favor the passage of bacteria derived products into the systemic circulation, causing a systemic inflammatory state. On the other hand, the effect of hepatic dysfunction on gut intestinal bacteria is also poorly explained. These aspects require further research, but at the current stage of knowledge, patients with diagnosed SIBO should be examined for NAFLD and vice versa. This will allow earlier use of available therapeutic tools, including therapy with non-absorbed antibiotics and lifestyle modification of patients, and thus diminish the risk of progression of liver disease and its complications, reduce or eliminate symptoms typical of SIBO, and may prevent the occurrence of other metabolic disorders.
Disclosure
Authors report no conflict of interest.
References
- 1.Gasbarrini A, Lauritano EC, Gabrieli M, et al. Small intestinal bacterial overgrowth: diagnosis and treatment. Dig Dis. 2007;25:237–240. doi: 10.1159/000103892. [DOI] [PubMed] [Google Scholar]
- 2.Fan X, Sellin JH. Review article: small intestinal bacterial overgrowth, bile acid malabsorption and gluten intolerance as possible causes of chronic watery diarrhea. Aliment Pharmacol Ther. 2009;29:1069–1077. doi: 10.1111/j.1365-2036.2009.03970.x. [DOI] [PubMed] [Google Scholar]
- 3.Lonardo A, Nascimbeni F, Maurantonio M, et al. Nonalcoholic fatty liver disease: evolving paradigms. World J Gastroenterol. 2017;23:6571–6592. doi: 10.3748/wjg.v23.i36.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pietrzak AM. Rifaximin in the treatment of small intestinal bacterial overgrowth dependent diseases. Forum Zakażeń. 2017;8:269–279. [Google Scholar]
- 5.Quigley EM. Small intestinal bacterial overgrowth: what it is and what it is not. Curr Opin Gastroenterol. 2014;30:141–146. doi: 10.1097/MOG.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 6.Rezaie A, Buresi M, Lembo A, et al. Hydrogen and methane-based breath testing in gastrointestinal disorders: the north american consensus. Am J Gastroenterol. 2017;112:775–784. doi: 10.1038/ajg.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Usai-Satta P, Giannetti C, Oppia F, et al. The North American consensus on breath testing: the controversial diagnostic role of lactulose in SIBO. Am J Gastroenterol. 2018;113:440. doi: 10.1038/ajg.2017.392. [DOI] [PubMed] [Google Scholar]
- 8.Araujo AR, Rosso N, Bedogni G, et al. Global epidemiology of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: What we need in the future. Liver Int. 2018;38:47–51. doi: 10.1111/liv.13643. [DOI] [PubMed] [Google Scholar]
- 9.Ziolkowski A, Wylezol M, Kukla M, et al. The comparison of scoring scales in morbidly obese patients undergoing bariatric surgery. Obes Surgery. 2005;15:1309–1314. doi: 10.1381/096089205774512582. [DOI] [PubMed] [Google Scholar]
- 10.Piscaglia F, Svegliati-Baroni G, Barchetii, et al. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology. 2016;63:827–838. doi: 10.1002/hep.28368. [DOI] [PubMed] [Google Scholar]
- 11.Sanna C, Rosso C, Marietti M, et al. Non-alcoholic fatty liver disease and extra-hepatic cancers. Int J Mol Sci. 2016 doi: 10.3390/ijms17050717. available from: https://dx.doi.org/10.3390%2Fijms17050717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) Metabolism. 2016;65:1038–1048. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65–80. doi: 10.1053/j.gastro.2008.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferolla SM, Armiliato GNA, Couto CA, et al. The role of intestinal bacteria overgrowth in obesity-related nonalcoholic fatty liver disease. Nutrients. 2014;6:5583–5599. doi: 10.3390/nu6125583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jandhyala SM, Talukdar R, Subramanyam C, et al. Role of the normal gut microbiota. World J Gastroenterol. 2015;21:8787–8803. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quigley EM, Stanton C, Murphy E. The gut microbiota and the liver. Pathophysiological and clinical implications. J Hepatol. 2013;58:1020–1027. doi: 10.1016/j.jhep.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 17.Xuyun He, Guang J, Houkai L, et al. Gut microbiota and nonalcoholic fatty liver disease: insights on mechanism and application of metabolomics. Int J Mol Sci. 2016;17:300. doi: 10.3390/ijms17030300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fialho A, Fialho A, Thota P, et al. Small intestinal bacterial overgrowth is associated with nonalcoholic fatty liver disease. J Gastrointestin Liver Dis. 2016;25:159–165. doi: 10.15403/jgld.2014.1121.252.iwg. [DOI] [PubMed] [Google Scholar]
- 19.Kapil S, Duseja A, Sharma BK, et al. Small intestinal bacterial overgrowth and toll-like receptor signaling in patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2016;31:213–221. doi: 10.1111/jgh.13058. [DOI] [PubMed] [Google Scholar]
- 20.Ghetti F, Oliveira DG, Oliveira JM. Influence of gut microbiota on the development and progression of nonalcoholic steatohepatitis. Eur J Nutr. 2018;57:861–876. doi: 10.1007/s00394-017-1524-x. [DOI] [PubMed] [Google Scholar]
- 21.Majewska M, Szczepanik M. The role of toll-like receptors(TLR) in innate and adaptive immune responses and their function in immune response regulation. Postępy Hig Med Dosw. 2006;60:52–63. [PubMed] [Google Scholar]
- 22.Marra F, Svegliati-Baroni G. Lipotoxity and the gut-liver axis in NASH pathogenesis. J Hepatol. 2017 doi: 10.1016/j.jhep.2017.11.014. available from: https://doi.org/10.1016/j.hep 2017.11.0014. [DOI] [PubMed] [Google Scholar]
- 23.Marra F, Lotersztajn S. Pathophysiology of NASH: perspectives for a targeted treatment. Curr Pharm Des. 2013;19:5250–5269. doi: 10.2174/13816128113199990344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kukla M, Mazur W, Bułdak RJ, et al. Potential role of leptin, adiponectin and three novel adipokines visfatin, chemerin and vaspin in chronic hepatitis. Mol Med. 2011;17:1397–1410. doi: 10.2119/molmed.2010.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kukla M, Berdowska A, Gabriel A, et al. Association between hepatic angiogenesis and serum adipokine profile in non-obese chronic hepatitis C patients. Pol J Pathol. 2011;62:218–228. [PubMed] [Google Scholar]
- 26.Waluga M, Kukla M, Żorniak M, et al. Vaspin mRNA levels in the liver of morbidly obese women with nonalcoholic fatty liver disease. Pol J Pathol. 2017;68:128–137. doi: 10.5114/pjp.2017.69688. [DOI] [PubMed] [Google Scholar]
- 27.Kajor M, Kukla M, Waluga M, et al. Hepatic chemerin mRNA in morbidly obese patients with nonalcoholic fatty liver disease. Pol J Pathol. 2017;68:117–127. doi: 10.5114/pjp.2017.69687. [DOI] [PubMed] [Google Scholar]
- 28.Zhu L, Baker D, Baker S. Gut microbiome and nonalcoholic fatty liver disease. Pediatr Res. 2015;77:245–251. doi: 10.1038/pr.2014.157. [DOI] [PubMed] [Google Scholar]
- 29.Riviere A, Selak M, Lantin D, et al. Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front Microbiol. 2016;7:979. doi: 10.3389/fmicb.2016.00979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu L, Baker S, Gill C, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601–609.. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 31.Rahmatollah R, Mahboobeh B, Fereshteh R, et al. Liver disease symptoms in non-alcoholic fatty liver disease and small intestinal bacterial overgrowth. Rom J Intern Med. 2017 doi: 10.1515/rjim-2017-0042. Available from: [DOI] [PubMed] [Google Scholar]
- 32.Hebanowska A. Bile acid biosynthesis and its regulation. Postępy His Med Dosw. 2010;64:544–554.. [PubMed] [Google Scholar]
- 33.Yuan L, Bambha K. Bile acid receptors and nonalcoholic fatty liver disease. World J Hepatol. 2015;7:2811–2818. doi: 10.4254/wjh.v7.i28.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panek-Jeziorna M, Mulak A. The role of bile aids in the pathogenesis of bowel diseases. Postępy Hig Med Dosw. 2017;71:737–746. doi: 10.5604/01.3001.0010.3852. [DOI] [PubMed] [Google Scholar]
- 35.Torres J, Palmela C, Brito H, et al. The gut microbiota, bile acids and their correlation in primary sclerosing cholangitis associated with inflammatory bowel disease. United European Gastroenterol J. 2018;6:112–122. doi: 10.1177/2050640617708953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mouzaki M, Wang AY, Bandsma R, et al. Bile acids and dysbiosis in non-alcoholic fatty liver disease. PLoS One. 2016;11:e0151829. doi: 10.1371/journal.pone.0151829. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sherriff JL, O’Sullivan T, Properzi C, et al. Choline, its potential role in nonalcoholic fatty liver disease and the case for human and bacterial genes. Adv Nutr. 2016;7:5–13. doi: 10.3945/an.114.007955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drenick EJ, Fisler J, Johnson D. Hepatic steatosis after intestinal bypass – prevention and reversal by metronidazole, irrespective of protein-calorie malnutrition. Gastroenterology. 1982;82:535–548. [PubMed] [Google Scholar]
- 39.Wigg AJ, Roberts-Thomson IC, Dymock RB, et al. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48:206–211. doi: 10.1136/gut.48.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xianfeng G, Shenghui L, Jiachun ZI, et al. Genome sequencing of 39 Akkermansia muciniphila isolates reveals its population structure, genomic and functional diversity and global distribution in mammalian gut microbiotas. BMC Genomics. 2017;18:800. doi: 10.1186/s12864-017-4195-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mithieux G. Gut microbiota and host metabolism: what relationship? Neuroendocrinology. 2018;106:352–356. doi: 10.1159/000484526. [DOI] [PubMed] [Google Scholar]
- 42.El Hage R, Hernandez-Sanabria E, Van de Wiele T. Emerging trends in “smart probiotics”: Functional consideration for the development of novel health and industrial applications. Front Microbiol. 2017;8:1889. doi: 10.3389/fmicb.2017.01889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boursier J, Mueller O, Diehl AM, et al. The severity of NAFLD is associated with gut symbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;68:764–775. doi: 10.1002/hep.28356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Darkoh C, Lichtenberger LM, Ajami N, et al. Bile acids improve the antimicrobial effect of rifaximin. Antimicrob Agents Chemother. 2010;54:3618–3624. doi: 10.1128/AAC.00161-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim MS, Morales W, Hani AA, et al. The effect of rifaximin on gut flora and Staphylococcus resistance. Dig Dis Sci. 2013;58:1676–1682. doi: 10.1007/s10620-013-2675-0. [DOI] [PubMed] [Google Scholar]
- 46.Ponziani FR, Zocco MA, Gasbarrini A. Eubiotic properties of rifaximin: Disruption of the traditional concepts in gut microbiota modulation. World J Gastroenterol. 2017;23:4491–4499. doi: 10.3748/wjg.v23.i25.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mencarelli A, Migliorati M, Barbanti M, et al. Pregnane-X-receptor mediates the anti-inflammatory activities of rifaximin on detoxification pathways in intestinal epithelial cells. Biochem Pharmacol. 2010;80:1700–1707. doi: 10.1016/j.bcp.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 48.Hirota SA. Understanding the molecular mechanisms of rifaximin in the treatment of gastrointestinal disorders-a focus on the modulation of host tissue function. Mini Rev Med Chem. 2015;16:206–217. doi: 10.2174/1389557515666150722105705. [DOI] [PubMed] [Google Scholar]
- 49.Fan X, Sellin JH. Review article: small intestinal bacterial overgrowth, bile acid malabsorption and gluten intolerance as possible causes of chronic watery diarrhea. Aliment Pharmacol Ther. 2009;29:1069–1077.. doi: 10.1111/j.1365-2036.2009.03970.x. [DOI] [PubMed] [Google Scholar]
- 50.Juszczyk J. Eubiotic rifaximin: new approaches in prophylaxis and treatment of cirrhosis of the liver and hepatic encephalopathy. Gastroenterologia Praktyczna. 2017;3:16–23. [Google Scholar]
- 51.Gangarapu V, Ince AT, Baysal B, et al. Efficacy of rifaximin on circulating endotoxins and cytokines in patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2015;27:840–845.. doi: 10.1097/MEG.0000000000000348. [DOI] [PubMed] [Google Scholar]
- 52.Loguercio C, Federico A, Tuccillo C, et al. Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver disease. J Gastroenterol. 2005;39:540–543. doi: 10.1097/01.mcg.0000165671.25272.0f. [DOI] [PubMed] [Google Scholar]
- 53.Malaguarnera M, Vacante M, Antic T, et al. Bifidobacterium longum with fructo-oligosaccharides in patients with nonalcoholic steatohepatitis. Dig Dis Sci. 2012;57:545–553. doi: 10.1007/s10620-011-1887-4. [DOI] [PubMed] [Google Scholar]
- 54.Wong VW, Tse CH, Lam TT, et al. Molecular characterization of faecal microbiota in patients with nonalcoholic steatohepatitis – a longitudinal study. PLoS One. 2013;8:e62885. doi: 10.1371/journal.pone.0062885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oldfield EC, Dong RZ, Johnson DA. Nonalcoholic fatty liver disease and the gut microbiota: Exploring the connection. J Gastrointest Dig Syst. 2014 doi: 10.4172/2161-069X.1000245. [DOI] [Google Scholar]