Abstract
Background
Due to their chemical constituents and biological properties, plants have long been used to control life-threatening diseases. The flora of Lebanon includes many plants that have already been demonstrated to have medicinal value, and other species, such as Pentapera sicula libanotica, that are yet to be characterized. The present study characterized the chemical composition, anti-oxidant, anti-inflammatory, and anti-proliferative potential of aqueous, ethanol, and methanol extracts derived from the leaves of the Lebanese Pentapera plant.
Material/Methods
High-performance liquid chromatography (HPLC) was used to determine the chemical composition. Gas chromatography (GC) coupled with mass spectrometry (MS) was applied to determine the content of essential oil. DPPH radical scavenging assay was performed to evaluate the anti-oxidant potential. The anti-inflammatory potential was assessed using quantitative real-time PCR (qRT-PCR) by measuring TNF-α, IL-6, and CCL4 mRNA levels, and we assessed Cox-2 and iNOS proteins levels using Western blot (WB) analysis. MTT assay was carried out to determine the anti-proliferative potential.
Results
We identified, mainly in the alcoholic (methanol and ethanol) extracts, distinct bioactive compounds with pharmacological relevance. In parallel, with their phytochemical content, these 2 extracts showed significant anti-oxidant, anti-inflammatory and anti-proliferative capacities.
Conclusion
Pentapera sicula libanotica appears to be a promising pharmacological tool.
MeSH Keywords: Anti-Inflammatory Agents, Antioxidants, Cell Survival
Background
When present in moderate levels, reactive oxygen species (ROS) such as superoxide ion (O2−) and hydrogen peroxide (H2O2), and reactive nitrogen species (RNS) such as nitric oxide (NO) and nitric dioxide (NO2), which are byproducts of normal cellular metabolism, play important roles in distinct biological mechanisms and can regulate cellular physiology and behavior by acting as second messenger signaling [1–4]. However, aberrant ROS and RNS generation causes harmful processes such as oxidative stress and inflammation [4–]. In humans, ROS and RNS can be neutralized by: (1) endogenous enzymatic systems such as superoxide dismutases (SOD), glutathione peroxidase (GPX), and catalase, (2) non-enzymatic endogenous antioxidants such as glutathione and melatonin, and (3) exogenous antioxidants such as vitamins C and E [8,9]. It is widely described that plants, due to their phytochemicals such carbohydrates, lipids, phenolics, flavonoids, alkaloids, and terpenoids, can exert important anti-oxidant, anti-inflammatory, anti-viral, anti-microbial, and/or anti-proliferative activities, and are thus valuable pharmacological tools [10–13].
Oxidative stress, a phenomena capable of damaging all biological macromolecules and cell structures, is considered as a risk factor associated with distinct serious pathologies, including cancer, autoimmune disorders, and neurodegenerative diseases [9,14]. Inflammation is a local tissue defensive process triggered by pathogens and/or injury, and leads to: activation of immune cells (mainly macrophages); and release of pro-inflammatory mediators and cytokines such as IL-1, IL-6, and tumor necrosis factor-α (TNF-α), chemokines such as CCL3 and CCL4, and soluble factors such as nitric oxide (NO), and prostaglandin E2 (PGE2) [15,16]. Despite its role in restoration of tissue structure and function, untreated inflammation can trigger critical health problems such as autoimmune disorders and cancer. Accordingly, characterization of plants with anti-inflammatory and anti-oxidant properties is of great medicinal value.
Lebanese Pentapera (also referred to as Pentapera sicula libanotica), a Lebanese plant threatened with extinction, grows in Jabal Moussa along the Naher Ibrahim region and occurs as a small shrub (30–50 cm) having 4 whorled leaves, pink to pink-purple corolla, 5 terminal and lateral flowers in the umbel, and 10 stamens. There have been no published reports on the chemical composition and biological potential of this plant. Here, we analyzed the phytochemical components of Pentapera and characterized the anti-oxidant, anti-inflammatory, and anti-proliferative capacity of 3 extracts from its leaves.
Material and Methods
Collection of plant and preparation of powders
Fresh leaves were collected from Ibrahim River at 400 m altitude in spring season (March 2018), and the biological authentication was performed by Professor George Tohme (president of C.N.R.S of Lebanon). Leaves were then washed, cut into small pieces, and dried in the shade (at room temperature). Leaves were then crushed and ground to obtain homogeneous fine powder and then conserved at room temperature (in a dark place) until use.
Chemicals and apparatus
The chemicals used were all of analytical grade. Absolute ethanol, methanol, n-hexane, sodium hydroxide, ethyl acetate, and dichloromethane were purchased from BDH England. Aluminium chloride, FeSO4·7H2O, and silica gel were obtained from Merck Germany. Sodium carbonate and hydrogen peroxide were obtained from Unichem India. DPPH were obtained from Sigma Aldrich, USA. PBS was obtained from Gibco, UK. MS spectra were recorded on an Agilent series device, and MSMS spectra were recorded on a Shimadzu series device.
Crude extracts preparation using aqueous, ethanol, and methanol as solvents
Powdered leaves (100 g) were put in a flask with 500 ml of the solvent (distilled water, ethanol, or methanol). Following maceration and stirring (1 week at room temperature), the macerate was isolated and filtered. After that, extracts were concentrated by a rotary evaporator at 40°C under reduced pressure (in the case of ethanol and methanol extracts). The aqueous extract was prepared as described for the alcoholic extracts, except that the temperature of the extraction step was 60°C and the filtrates were frozen prior to lyophilization to obtain powders.
Gas chromatography-mass spectrometry (GC/MS) analysis
Agilent 7890A-GCMS was used to perform the GC/MS analysis. During separation and identification by GC/MS technique, components were identified based on the retention time and spectral index from the NIST and WILEY library. Tables 1 and 2 summarize the instrument specifications and analysis conditions.
Table 1.
The instrument specifications and analysis conditions for water extracts.
| GC program | |
|---|---|
| Oven maximum temperature | 325°C |
| Hold time | 1 min |
| Post run | 50 c |
| Program | 8 c/min – 290 c – 11 min |
| Equilibration time | 3 min |
| Injection volume | 1 μl |
| Front SS inlet mode | Split |
| Injector temperature | 280°C |
| Pressure | 52.76 psi |
| Total flow | 6 ml/min |
| Split ratio | 5: 1 |
| Split flow | 5 ml/min |
| Column | DB-5MS: 30 m×250 μm ×0.25 μm |
| MS source | 230 c maximum 250 c |
| MS quad | 150 c maximum 200 c |
| Acquisition mode | Scan |
| Solvent delay | 2.5 min |
| Low mass | 33 |
| High mass | 500 |
Table 2.
The instrument specifications and analysis conditions for methanol and ethanol extracts.
| GC program | |
|---|---|
| Oven temperature set point | 35°C |
| Hold time | 2 min |
| Post run | 60 c |
| Program | 3 c/min – 320 c – 1 min |
| Equilibration time | 0.5 min |
| Injection volume | 1 μl |
| Back SS inlet mode | Split |
| Injector temperature | 300°C |
| Pressure | 11.192 psi |
| Total flow | 504 ml/min |
| Split ratio | 500: 1 |
| Split flow | 500 ml/min |
| Column | TG-5MS: 30 m×250 μm ×0.25 μm |
| MS source | 230 c maximum 250 c |
| MS quad | 150 c maximum 200 c |
| Acquisition mode | Scan |
| Solvent delay | 2 min |
| Low mass | 1.6 |
| High mass | 450 |
Liquid chromatography-mass spectrometry (LC/MS/MS) analysis
Shimadzu-AB Sciex LCMSMS was used for the LC/MS/MS analysis. During separation and identification by LC/MS/MS technique, components were identified based on the retention time and mass spectral characteristics. Table 3 summarizes the instrument specifications and analysis conditions.
Table 3.
The instrument specifications and analysis conditions.
| HPLC/Pump | Shimadzu/LC20AD |
|---|---|
| Mass spectrometer | API 4000/AB Sciex instruments |
| Component name | Triple Quadrupole LC/MS/MS Mass Spectrometer |
| Source temperature (at set point) | 300°C |
| LC system equilibration time | 2 min |
| LC system injection volume | 10 μl |
| Pumping mode | Low pressure gradient: Time (min) Module Events Parameter 0.01 Pumps ACN+ 0.1% Formic acid 0.0 0.10 Pumps ACN+ 0.1% Formic acid 20 6.00 Pumps ACN+ 0.1% Formic acid 90 9.00 Pumps ACN+ 0.1% Formic acid 90 9.50 Pumps ACN+ 0.1% Formic acid 0 12.00 System Controller Stop |
| Total flow | 0.3 ml/min |
| Autosampler model | SIL-20A/HT |
| Column | C18 (15 cm×0.2 mm×3.5 um) |
DPPH radical scavenging assay
The anti-oxidant potential was assessed according to the method of Farhan et al. [17] using free radical DPPH. We prepared 5, 50, 100, 150, and 200 μg/ml concentrations of extracts. For each extract, we added 1 ml of each prepared dilution to 1 ml of DPPH reagent [0.135 mM]. Following incubation in the dark for 30 min at room temperature, the absorbance was measured at 517 nm using a Gene Quant 1300 UV-Vis spectrophotometer. The DPPH scavenging ability was calculated according to the following equation:
Control samples were made of 1 ml DPPH and 1 ml of selected solvent.
The blank control corresponded to 1 ml of the selected solvent.
Anti-inflammatory activity
RAW 264.7, a murine monocyte/macrophage cell line, was cultivated in DMEM supplemented with 10% defined FBS and 1% penicillin G-streptomycin in an atmosphere containing 5% CO2 and 95% air at 37°C. Cells were seeded in 6-well plates (1×106 cells/well) using fresh medium. Following preincubation for 24 h, cells were treated with LPS (10 ng/ml) and 2 different concentrations of the extracts (100 μg/ml and 200 μg/ml) in DMEM without FBS for 24 h.
Cell viability
MCF7 and MDA- MB 231, corresponding to breast cancer cell lines, were seeded in 96-well plates (5×103 cells/well). Cells were then treated with the different extracts at different concentrations ranging from 50 to 500 μg/ml for 24, 48, and 72 h and viability was assayed using Cell Proliferation Assay (MTT, Sigma, USA). The proliferation test is based on the color reaction of mitochondrial dehydrogenases from living cells with MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide). At the end of the treatment period, MTT (final concentration 0.5 mg/mL) was added to each well, which was then incubated at 37°C in 5% CO2 for 3 h. The colored crystals of produced formazan were dissolved in DMSO (dimethyl sulfoxide) (Sigma, USA). The absorbance at 570 nm was measured using an EL×800 Microplate Reader (Bio-Tek Instruments). The effect of Pentapera sicula libanotica extracts on cell viability was calculated as the effect (%) of individual extract dose vs. control (untreated cells).
Quantitative real-time PCR
Total RNA was extracted using Trizol reagent following the manufacturer’s guidelines (Invitrogen, Merelbeke, Belgium) and first-strand cDNAs were synthesized by reverse transcription (Superscript First-strand Synthesis System for RT-PCR kit; Invitrogen, Merelbeke, Belgium). Quantitative mRNA expression was assessed by real-time PCR with the PRISM 7900 sequence detection system (Applied Biosystems, Gent, Belgium), and the SYBR Green Master mix kit with β-actin mRNA used as an internal control. Table 4 summarizes the primers used for the amplification of each of the tested genes. The program used for amplification was: 10 min at 95°C followed by 40 cycles of 15 s at 95°C, 1 min at 60°C. All qPCR reactions were performed in triplicate. The expression levels (2−ΔΔCt) of mRNAs were calculated as described previously [18].
Table 4.
List of primers used in this study.
| Primers | Sequence (5′-3′) |
|---|---|
| IL-6-FO | GAGGATACCACTCCCAACAGACC |
| IL-6-RE | AAGTGCATCATCGTTGTTCATACA |
| CCL4-FO | AAAACCTCTTTGCCACCAATACC |
| CCL4-RE | GAGAGCAGAAGGCAGCTACTAG |
| COX2-FO | CAGACAACATAAACTGCGCCTT |
| COX2-RE | GATACACCTCTCCACCAATGACC |
| iNOS-FO | GCAGAATGTGACCAT CATGG |
| iNOS-RE | ACAACCTTG GTGTTGAAG GC |
Western blotting
Protein samples were loaded into the wells of stacking gel and run until bromophenol blue reached the bottom of the gel. Gels were then transferred to nitrocellulose membranes (4°C at 80 V for 1 h). The membrane was then blocked in 3% BSA (bovine serum albumin, Sigma A2153), prepared in Tween PBS, for 2 h at room temperature. Detection of the protein of interest was achieved by probing the membrane with the primary antibody of interest. Monoclonal antibodies that can detect Cox-2 (Abcam USA, ab15191), iNOS (Abcam USA, ab3523) and beta-actin (Abcam USA, ab20272) were used.
Statistical analysis
The data are shown as means ±SEM of at least 3 independent experiments. Normality of the data was checked using the Kolmogorov-Smirnov test and analysis was performed using the t test. The Statistical Package for Social Sciences (IBM SPSS), version 21 was used for statistical analysis. P values <0.05 (*), <0.01 (**), <0.001 (***) were considered significant.
Results
GC/MS analysis of different Lebanese Pentapera leaf extracts
The GC spectrum analysis of the aqueous, ethanolic, and methanolic extracts are shown in Figures 1–3, respectively. Using the chromatographic method with the help of NIST and WILEY library, as shown in Tables 5–7, respectively, a total of 2 compounds were identified in the aqueous extract, 5 compounds were detected in the ethanolic extract and 8 compounds were detected in the methanolic extract. In the case of aqueous extract, compound NORUNS-12-ENE showed the highest concentration (10.8%), followed by the second compound, NOROLEAN-12-ENE (9.39%) (Table 5). In the case of ethanolic extract, SQUALENE was the most prominent component (5.5%) followed by vitamin E (2.97%), ethyl (9z, 12z)-9, 12-octadecadienoate (1.87%), linoleic acid ethyl ester (1.06%), and hexadecanoic acid, ethyl ester (0.89%) (Table 6). In the case of methanolic extract, guanosine was most abundant (12.74%), followed by alpha-tocopherol-beta-d-mannoside (6.50%), linolenic acid (3.24%), palmitic acid (2.25%), solanesol (1.89%), 1,2,3-propanetriol monoacetate (1.66%), 2,3-dihydro-benzofuran (0.66%), and syringol (0.63%) (Table 7).
Figure 1.
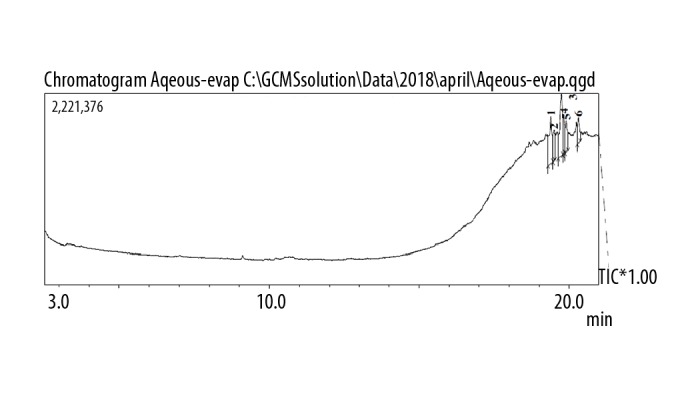
GC chromatogram of the aqueous extract of Pentapera sicula libanotica leaf.
Figure 2.
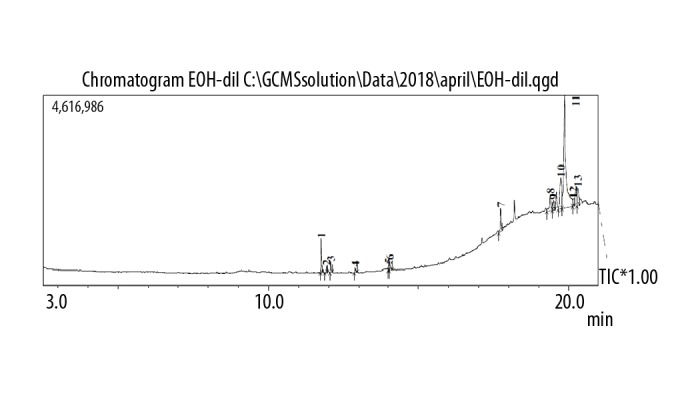
GC chromatogram of the ethanol extract of Pentapera sicula libanotica leaf.
Figure 3.
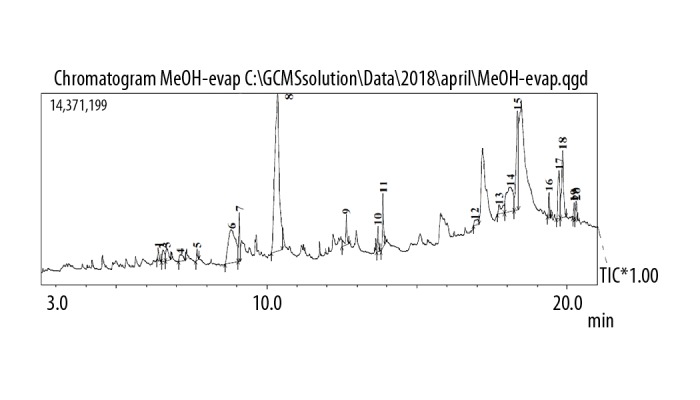
GC chromatogram of the methanol extract of Pentapera sicula libanotica leaf.
Table 5.
Results of the GC-MS analysis of the aqueous extract of the Pentapera sicula libanotica leaf.
| Peak# | RT | NAME | MW | Structure | Molecular formula | Area % |
|---|---|---|---|---|---|---|
| 1 | 19.488 | NOROLEAN-12-ENE | 410.686 |
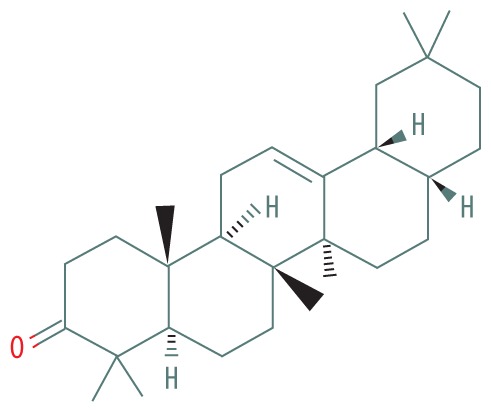
|
C29H46O | 9.39 |
| 2 | 19.808 | NORUNS-12-ENE | 472.71 |
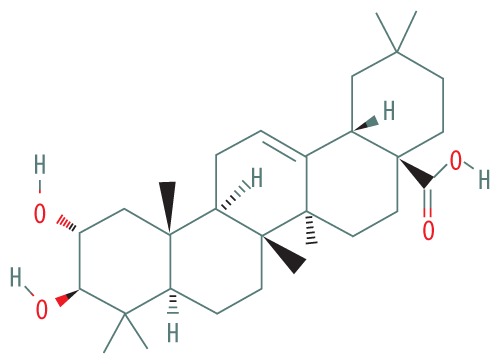
|
C30H48O4 | 10.80 |
Table 6.
Results of the GC-MS analysis of the ethanol extract of Pentapera sicula libanotica leaf.
| Peak# | RT | NAME | MW | Structure | Molecular formula | Area % |
|---|---|---|---|---|---|---|
| 1 | 12.882 | Hexadecanoic acid, ethyl ester | 300.477 |
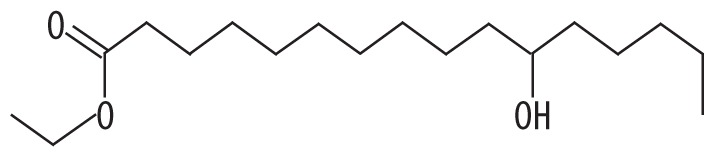
|
C18H36O3 | 0.89 |
| 2 | 14.016 | Linoleic acid ethyl ester | 308.499 |
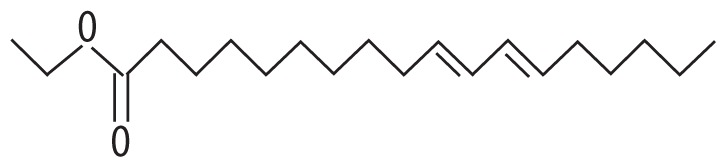
|
C20H36O2 | 1.06 |
| 3 | 14.056 | Ethyl (9Z,12Z)-9,12-octadecadienoate | 308.506 |
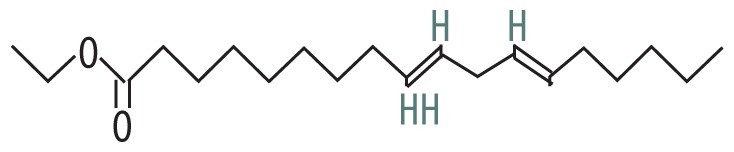
|
C20H36O2 | 1.87 |
| 4 | 17.736 | Squalene | 410.718 |
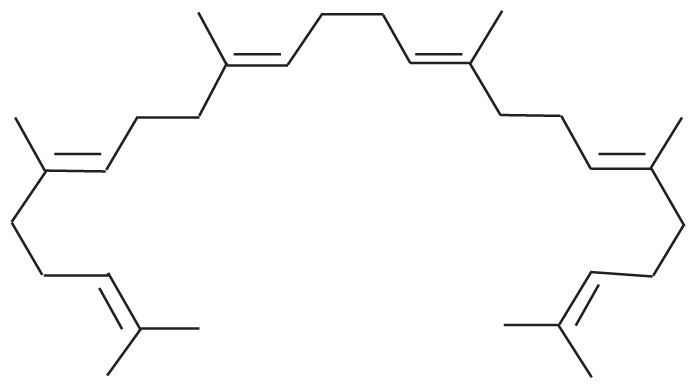
|
C30H50 | 5.05 |
| 5 | 19.850 | Vitamin E | 430.717 |
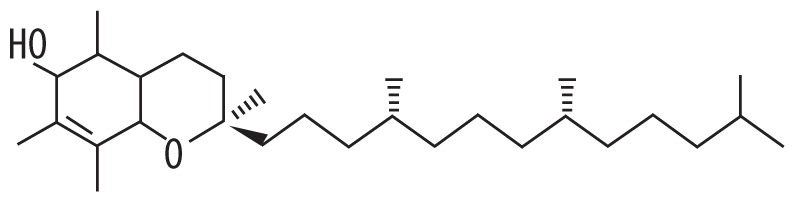
|
C29H50O2 | 2.97 |
Table 7.
Results of the GC-MS analysis of the methanol extract of Pentapera sicula libanotica leaf.
| Peak# | RT | NAME | MW | Structure | Molecular formula | Area % |
|---|---|---|---|---|---|---|
| 1 | 6.401 | 2,3-dihydro-Benzofuran | 120.151 |
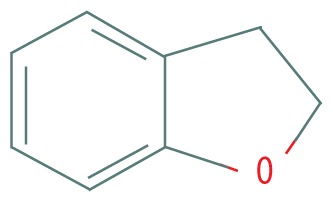
|
C8H8O | 0.66 |
| 2 | 6.667 | 1,2,3-Propanetriol, monoacetate | 134.13 |
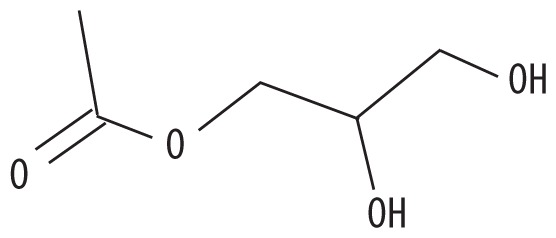
|
C5H10O4 | 1.66 |
| 3 | 7.673 | Syringol | 154.165 |
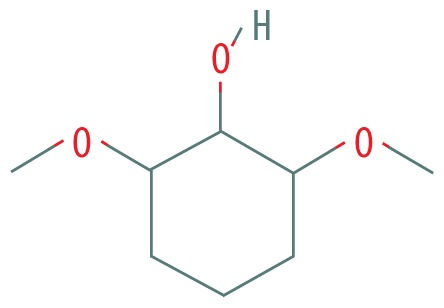
|
C8H10O3 | 0.63 |
| 4 | 8.831 | Guanosine | 283.241 |
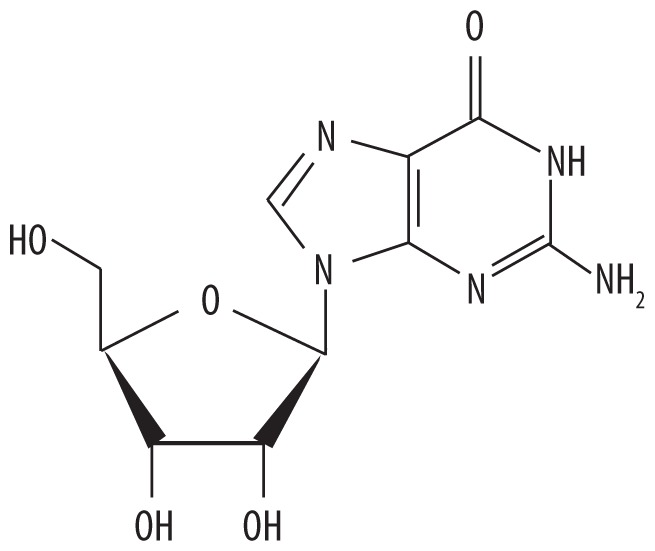
|
C10H13N5O5 | 12.74 |
| 5 | 12.649 | Palmitic acid | 256.4 |
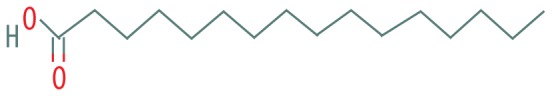
|
C16H32O2 | 2.25 |
| 6 | 13.874 | Linolenic acid | 280.452 |
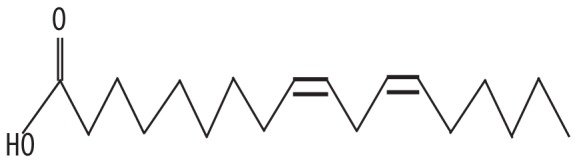
|
C18H30O2 | 3.24 |
| 7 | 17.733 | Solanesol | 631.086 |
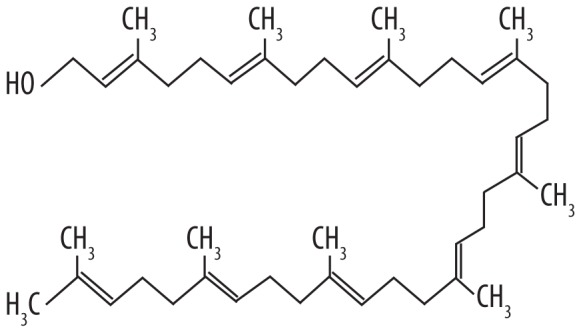
|
C45H74O | 1.89 |
| 8 | 19.846 | .alpha.-Tocopherol-.beta.-D-mannoside | 592.846 |
|
C35H60O7 | 6.50 |
The LC/MS/MS analysis for Pentapera sicula libanotica leaf extracts
The LC spectrum results of the Pentapera sicula libanotica leaf extracts are shown in Table 8. A total of 3 compounds (isorhamentin, hyperoside, and quercetin) were detected in the aqueous, ethanolic, and methanolic extracts using the chromatographic method based on the retention time and mass characteristics.
Table 8.
Results of LC/MS/MS technique of Pentapera sicula libanotica leaf.
| Pentapera sicula libanotica | Compounds names | Retention time | Q1 mass (Da) | Q3 mass (Da) | CE | DP (V) |
|---|---|---|---|---|---|---|
| Water | Isorhamentin | 7.49 | 431 | 341 | −30 | −40 |
| Hyperoside | 6.10 | 463 | 301 | −38 | −40 | |
| Quercetin | 7.31 | 301 | 179 | −35 | −40 | |
| Ethanol | Isorhamentin | 7.80 | 431 | 341 | −30 | −40 |
| Hyperoside | 6.11 | 463 | 301 | −38 | −40 | |
| Quercetin | 7.23 | 301 | 179 | −35 | −40 | |
| Methanol | Isorhamentin | 7.78 | 431 | 341 | −30 | −40 |
| Hyperoside | 6.14 | 463 | 301 | −38 | −40 | |
| Quercetin | 7.28 | 301 | 179 | −35 | −40 |
Anti-oxidant activity of Pentapera sicula libanotica leaf extracts
DPPH free radical scavenging assay was used to determine the anti-oxidant potential of the Pentapera leaf-derived aqueous, ethanolic, and methanolic extracts. Antioxidants can react with the violet-colored stable free radical DPPH and convert it into a yellow-colored α,α-diphenil-β-picrylhydrazine; therefore, quantification of the change of the reaction mixture color was used as a readout of the scavenging ability. The different extracts showed different anti-oxidant activities with methanolic extract (IC50=48.27±3.52 μg/ml) >ethanolic extract (IC50=52.14±5.6 μg/ml) >aqueous extracts (75.26±5.7) (Table 9).
Table 9.
DPPH free scavenging capacity (IC50, μg/ml) of aqueous, methanol, or ethanol extracts derived from fresh leaves of Pentapera sicula libanotica. IC50 value correspond to the concentration of sample required to scavenge DPPH radical by 50%. Each value represents a mean ±SD (N=3).
| DPPH assay | ||
|---|---|---|
| Extract | IC50, μg/ml | |
| Fresh Leaves | Aqueous | 75.26±5.7 |
| Methanol | 52.14±5.6 | |
| Ethanol | 48.27±3.52 |
Anti-inflammatory activity of Pentapera sicula libanotica leaf extracts
Inflammation is a reaction by the host against invading pathogens and can be triggered by distinct microbial components such as lipopolysaccharide (LPS) [19]. LPS can stimulate different immune cells, mainly macrophages, which in turn generate increased levels of pro-inflammatory cytokines (such as TNF-α and IL-6) and chemokines (including CCL3 and CCL4) [19], in addition to other pro-inflammatory components such as nitric oxide (NO) and prostaglandin E2 (PGE2) that are produced by the inflammation-inducible isoforms of NO synthase (iNOS) and cyclooxygenase-2 (COX-2) enzymes, respectively [20,21].
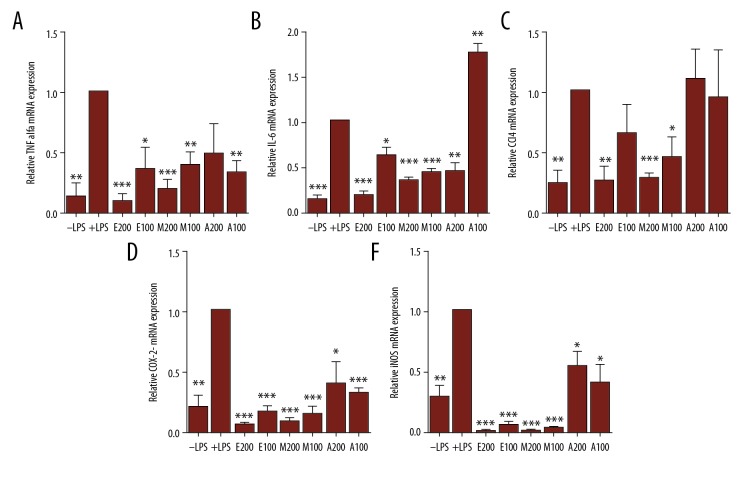
In a first step, quantitative real-time PCR (qRT-PCR) was used to determine relative TNF-α, IL-6, CCL4, COX-2, and iNOS mRNA levels in LPS + extract-treated RAW264.7 cells versus LPS-treated cells.
In the case of TNF-α, 100 μg/ml of either extract significantly reduced TNF-α transcription levels (Figure 4A). Interestingly, treating cells with 200 μg/ml of either ethanol or methanol extract lead to more striking inhibitory effect, with the ethanol extract being more potent that the methanolic one (Figure 4A). On the other hand, 200 μg/ml of aqueous extract exerted no significant negative effect on TNF-α mRNA levels (Figure 4A). In the case of IL-6, treating cells with 100 μg/ml of either ethanol or methanol extract significantly reduced IL-6 mRNA levels. No inhibitory effect was observed upon treating cells with 100 μg/ml of aqueous extract (Figure 4B). Notably, treating cells with 200 μg/ml of ethanolic extract dramatically reduced IL-6 mRNA levels to an extent lower than that exhibited by cells treated by 200 μg/ml of methanolic or aqueous extract (Figure 4B). In the case of CCL4, treating cells with 100 μg/ml of methanol extract significantly lowered CCL4 mRNA levels (Figure 4C). However, no significant inhibitory effect was detected after treating cells with 100 μg/ml of ethanolic or aqueous extract. On the other hand, 200 μg/ml of ethanol or methanol extract strikingly reduced CCL4 transcription levels (Figure 4C). In the case of COX-2 and iNOS, treating cells with either 100 or 200 μg/ml of aqueous extract significantly lowered the mRNA levels (Figure 4D, 4E). Interestingly, a dramatic decrease in COX-2 and iNOS mRNA levels was observed upon treating cells with 100 or 200 μg/ml of either methanolic or ethanolic extract (Figure 4D, 4E).
Figure 4.
Effect of Pentapera sicula libanotica leaf extracts on LPS-induced TNF-α, IL-6 CCL4, COX-2, and iNOS mRNA levels in RAW 264.7 cells. Cells were treated for 24 h with 100 ng/ml LPS in the absence or presence of 100 or 200 μg/ml of either ethanol (E), methanol (M), or aqueous (A) extract. Total RNA was prepared and qRT-PCR was performed to quantify the mRNA levels of TNF-α (A), IL-6 (B), CCL4 (C), COX-2 (D), and iNOS (E). The data represent the relative mRNA levels (values obtained in: RAW 264.7 cells treated with both LPS and extract/RAW 264.7 cells treated with only LPS). Presented values correspond to the averages ±SEM of 3 independent experiments (n=3) each done in triplicate. * p<0.05; ** p<0.01, *** p<0.001 vs. control untreated cells (t test).
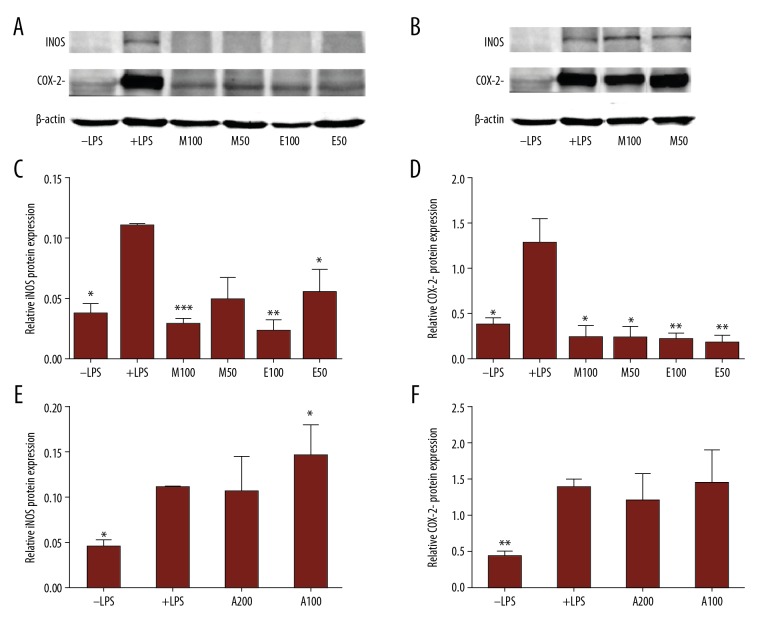
In a second step, Western blot analysis was carried out to assess iNOS and Cox2 protein levels in LPS + extract-treated RAW264.7 cells vs. LPS-treated cells (Figure 5). Interestingly, the induced protein levels of both iNOS and Cox2, in response to LPS treatment, were strongly reduced after treating cells with either 50 or 100 μg/ml of ethanol or methanol extract (Figure 5A, 5C, 5D), but no significant repressive effect on either iNOS or Cox2 protein levels was detected after treating cells with the aqueous extract (Figure5B, 5E, 5F).
Figure 5.
Effect of Pentapera sicula libanotica leaf extracts on LPS-Induced iNOS and Cox-2 protein levels in RAW 264.7 cells. Cells were treated for 24 h with 100 ng/ml LPS in the absence or presence of 100 or 50 μg/ml of either methanol (M), ethanol (E), or aqueous (A) extract. Total protein was extracted and Western blot analysis was performed to assess the protein levels of Cox-2 and iNOS. A representative Western blot result is shown (A, B). Levels of iNOS (C, E) and Cox-2 (D, F) were quantified by densitometer and normalized to GAPDH.
Anti-proliferative activity of Pentapera sicula libanotica leaf extracts
To assess the ability of Pentapera sicula libanotica leaf extracts to alter cell viability, MTT assay was performed. This is a colorimetric assay based on the conversion of the yellow substrate MTT to a highly-colored formazan product by succinate dehydrogenase enzymes in metabolically active cells. This occurs only in viable cells; therefore, the amount of the produced formazan is proportional to viable cells in the sample.
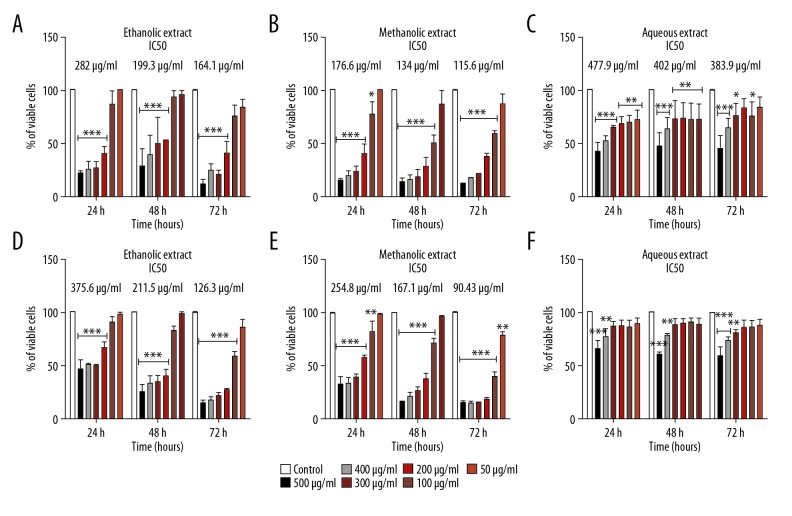
MCF7 and MDA-MB-231 breast cancer cells were treated with varying concentrations (50, 100, 200, 300, 400, and 500 μg/mL) of either aqueous, ethanolic, or methanolic crude extracts for different time periods (24, 48, or 72 h).
In the case of MCF7 cells, the ethanolic and methanolic extracts exerted dose- and time-dependent inhibitory effects (Figure 6A, 6B). For the ethanolic extract, the IC50 value (dose required to inhibit cell growth by 50%) corresponded to 282 μg/ml after 24 h, 199.3 μg/ml after 48 h, and 164.1 μg/ml after 72 h (Figure 6A). For the methanolic extract, the IC50 value was 176.6 μg/ml after 24 h, 134 μg/ml after 48 h and 115.6 μg/ml after 72 h (Figure 6B). The aqueous extract exerted a less striking effect, with IC50 value of 477.9 μg/ml after 24 h, 402 μg/ml after 48 h, and 383.9 μg/ml after 72 h (Figure 6C).
Figure 6.
Effects of Pentapera sicula libanotica leaf extracts on MCF-7 and MDA-MB-231 cancer cell viability. Cells were treated with various concentrations (0, 50, 100, 200, and 300 μg/ml) of extracts for 24, 48, and 72 h, and cell viability was measured by MTT assay. Each value corresponds to a mean ±SEM for 3 independent experiments (n=3), each done in triplicate. Ethanol extract-treated MCF-7 cells (A), Methanol extract-treated MCF-7 cells (B), Aqueous extract-treated MCF-7 cells (C), Ethanol extract-treated MDA-MB-231 cells (D), Methanol extract-treated MDA-MB-231 cells (E), Aqueous extract-treated MDA-MB-231 cells (F). * p<0.05; ** p<0.01, *** p<0.001 vs. control untreated cells (t test).
In the case of MDA-MB-231, a dose and time-dependent inhibitory effect was also detected in ethanol extract- and methanol extract-treated cells (Figure 6D, 6E). For the ethanolic extract, the IC50 was 375.6 μg/ml after 24 h, 211.5 μg/ml after 48 h, and 126.3 μg/ml after 72 h (Figure 6D). For the methanolic extract, the IC50 value exhibited 254.8 μg/ml after 24 h, 167.1 μg/ml after 48 h, and 90.43 μg/ml after 72 h (Figure 6E). The aqueous extract showed no inhibitory effect on cell viability during the time course (Figure 6F).
Discussion
Pentapera sicula libanotica is one of many Lebanese plants that are still not characterized in terms of their chemical composition and pharmacological potential. Therefore, we assessed the essential oil content, bioactive components, anti-oxidant, anti-inflammatory, and anti-proliferative potential of the aqueous, ethanol, and methanol extracts derived from the leaves of this plant.
Although the 3 extracts appeared to contain isorhamentin, hyperoside, and quercetin, those extracts were dissimilar in terms of their oil content, with the alcoholic ones (ethanol and methanol extracts) being richer than the aqueous extract. Despite the identified bioactive components, the Pentapera sicula libanotica content of bioactive compounds appears to be much more striking. Various limitations accounted for this limited number of identified components: (1) the output of the GC/MS analysis, used to detect essential oils, is highly affected by the type of the solvent used during the extraction method; (2) LC/MS/MS analysis was targeted towards only 11 flavonoid compounds, and many other components were not analyzed; and (3) the chemical content of this plant is sensitive to different environmental and geographical factors such as the year and the season of harvest, exposure to sunlight, the elevation and the region in which the plants were harvested.
Oxidative stress, chronic inflammation, and uncontrolled cell proliferation are deleterious to human health; therefore, identifying new natural components that can suppress these events is of great importance. In this study, we showed that Pentapera sicula libanotica has significant anti-oxidant, anti-inflammatory, and anti-proliferative effects. In fact, the aqueous extract negatively affected, even to lower extent than alcoholic extracts, TNF-α, IL-6, iNOS and Cox-2 mRNA levels, as well as MCF-7 viability. This anti-inflammatory and anti-proliferative capacity could be attributed to quercetin, which exerts anti-inflammatory and anti-proliferative effects [22,23]. Despite the negative effect of the aqueous extract on iNOS and Cox-2 mRNA levels, the protein levels of these 2 components were not affected, indicating that the aqueous extract can impair gene transcription but not translation.
Remarkably, the anti-oxidant, anti-inflammatory, and anti-proliferative properties were more striking in the alcohol extracts, which is in line with the better chemical content of the alcohol extracts than the aqueous extract. For instance, the ethanol extract appeared to contain squalene and vitamin E. Squalene is a triterpene (an intermediate in the cholesterol biosynthesis pathway), which is characterized by different biological and pharmacological activities [24]. Squalene is known for its anti-oxidant properties, acting as a highly effective oxygen-scavenging agent [25]. Moreover, squalene has been reported to effectively inhibit tumorigenesis [26]. Vitamin E is a potent bioactive component with a wide variety of biological activities [27]. In fact, vitamin E can efficiently suppress cancer cell proliferation. Moreover, vitamin E is well known for its anti-inflammatory potential via suppressing the expression of TNF-α, IL-1, IL-6, IL-8, iNOS, and COX-2, all of which are mediators of the inflammatory response. Furthermore, vitamin E has important anti-oxidant activities via induction of anti-oxidant enzymes [27]. The methanol extract included guanosine and linolenic acid. Guanosine, a purine nucleoside, plays important roles in cell metabolism and has significant neuroprotective properties, especially by reducing inflammation and oxidative stress in different components of the nervous system [28,29]. Linolenic acid has a variety of biological effects, such as anti-cancer, anti-oxidation, and anti-inflammatory activity, that are relevant to human health [30].
It is noteworthy that the observed anti-cancer potential could be attributed to the fact that the chemical components of the studied plant can induce cell necrosis or activate components of the apoptotic signaling pathway, thus leading to cell death. In parallel, the observed anti-inflammatory effect could be due to the ability of the plant constituents to inhibit the NF-κB pathway, which usually ensures the expression of pro-inflammatory elements. Characterization of these molecular events and signaling pathways will be addressed in our future studies.
Conclusions
In conclusion, the present study shows that Pentapera sicula libanotica contains important bioactive compounds and has significant anti-oxidant, anti-inflammatory, and anti-proliferative effects. Pentapera sicula libanotica could, therefore, hold promise for designing novel drugs against oxidative stress, chronic inflammatory responses, or uncontrolled cell proliferation.
Acknowledgments
We thank Professor George Tohme for his valuable help in the identification of this plant, the Association for the Protection of Jabal Moussa (the managing body of Jabal Moussa UNESCO Biosphere Reserve) for their help collecting the plant.
Footnotes
Source of support: Biodiagnostic Company (Beirut, Lebanon)
Conflict of interest.
None.
References
- 1.Dowling DK, Simmons LW. Reactive oxygen species as universal constraints in life-history evolution. Proc Biol Sci. 2009;276:1737–45. doi: 10.1098/rspb.2008.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scherz-Shouval R, Elazar Z. Regulation of autophagy by ROS: Physiology and pathology. Trends Biochem Sci. 2011;36:30–38. doi: 10.1016/j.tibs.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 4.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valko M, Izakovic M, Mazur M, et al. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem. 2004;266:37–56. doi: 10.1023/b:mcbi.0000049134.69131.89. [DOI] [PubMed] [Google Scholar]
- 6.Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Acharya A, Das I, Chandhok D, Saha T. Redox regulation in cancer: A double-edged sword with therapeutic potential. Oxid Med Cell Longev. 2010;3:23–34. doi: 10.4161/oxim.3.1.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valko M, Rhodes CJ, Moncol J, et al. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci. 2008;4:89–96. [PMC free article] [PubMed] [Google Scholar]
- 10.Liu C-Y, Lin Y-C, Deng J-S, et al. Antioxidant, anti-inflammatory, and antiproliferative activities of Taxillus sutchuenensis. Am J Chin Med. 2012;40:335–48. doi: 10.1142/S0192415X12500267. [DOI] [PubMed] [Google Scholar]
- 11.Choi H-S, Seo HS, Kim SR, et al. Anti-inflammatory and anti-proliferative effects of Rhus verniciflua Stokes in RAW264.7 cells. Mol Med Rep. 2014;9:311–15. doi: 10.3892/mmr.2013.1775. [DOI] [PubMed] [Google Scholar]
- 12.Choi H-S, Seo HS, Kim SR, et al. Anti-inflammatory and anti-proliferative effect of herbal medicines (APR) in RAW264.7 cells. Mol Med Rep. 2014;9:1569–74. doi: 10.3892/mmr.2014.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talhouk RS, Karam C, Fostok S, et al. Anti-inflammatory bioactivities in plant extracts. J Med Food. 2007;10:1–10. doi: 10.1089/jmf.2005.055. [DOI] [PubMed] [Google Scholar]
- 14.Fransen M, Nordgren M, Wang B, Apanasets O. Role of peroxisomes in ROS/RNS-metabolism: Implications for human disease. Biochim Biophys Acta. 2012;1822:1363–73. doi: 10.1016/j.bbadis.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Ashley NT, Weil ZM, Nelson RJ. Inflammation: Mechanisms, costs, and natural variation. Annu Rev Ecol Evol Syst. 2012;43:385–406. [Google Scholar]
- 16.Rankin JA. Biological mediators of acute inflammation. AACN Clin Issues. 2004;15:3–17. doi: 10.1097/00044067-200401000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Farhan H, Rammal H, Hijazi A, et al. In vitro antioxidant activity of ethanolic and aqueous extracts from crude Malva parviflora L. grown in Lebanon. Asian J Pharm Clin Res. 2012;5:234–38. [Google Scholar]
- 18.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–18. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 19.Khan FA, Khan MF. Inflammation and acute phase response. Int J Appl Biol Pharm Technol Page. 2010;1:312–21. [Google Scholar]
- 20.Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006;55:115–22. doi: 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenhough A, Smartt HJM, Moore AE, et al. The COX-2/PGE2 pathway: Key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30:377–86. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- 22.Kleemann R, Verschuren L, Morrison M, et al. Anti-inflammatory, anti-proliferative and anti-atherosclerotic effects of quercetin in human in vitro and in vivo models. Atherosclerosis. 2011;218:44–52. doi: 10.1016/j.atherosclerosis.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 23.Alvarado-Sansininea J, Sánchez-Sánchez L, López-Muñoz H, et al. Quercetagetin and patuletin: Antiproliferative, necrotic and apoptotic activity in tumor cell lines. Molecules. 2018;23 doi: 10.3390/molecules23102579. pii: E2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang Z-R, Lin Y-K, Fang J-Y. Biological and pharmacological activities of squalene and related compounds: Potential uses in cosmetic dermatology. Molecules. 2009;14:540–54. doi: 10.3390/molecules14010540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohno Y, Egawa Y, Itoh S, et al. Kinetic study of quenching reaction of singlet oxygen and scavenging reaction of free radical by squalene in n-butanol. Biochim Biophys Acta. 1995;1256:52–56. doi: 10.1016/0005-2760(95)00005-w. [DOI] [PubMed] [Google Scholar]
- 26.Smith TJ. Squalene: Potential chemopreventive agent. Expert Opin Investig Drugs. 2000;9:1841–48. doi: 10.1517/13543784.9.8.1841. [DOI] [PubMed] [Google Scholar]
- 27.Ahsan H, Ahad A, Iqbal J, Siddiqui WA. Pharmacological potential of tocotrienols: A review. Nutr Metab (Lond) 2014;11:52. doi: 10.1186/1743-7075-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bettio LEB, Gil-Mohapel J, Rodrigues ALS. Guanosine and its role in neuropathologies. Purinergic Signal. 2016;12:411–26. doi: 10.1007/s11302-016-9509-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanznaster D, Dal-Cim T, Piermartiri TCB, Tasca CI. Guanosine: A neuromodulator with therapeutic potential in brain disorders. Aging Dis. 2016;7:657–79. doi: 10.14336/AD.2016.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hur SJ, Park GB, Joo ST. Biological activities of conjugated linoleic acid (CLA) and effects of CLA on animal products. Livest Sci. 2007;110:221–29. [Google Scholar]