New Delhi metallo-β-lactamase (NDM) is a metallo-β-lactamase able to hydrolyze almost all β-lactams. Twenty-four NDM variants have been identified in >60 species of 11 bacterial families, and several variants have enhanced carbapenemase activity.
KEYWORDS: Acinetobacter, Enterobacteriaceae, NDM, carbapenem resistance, carbapenemase, metalloenzymes
SUMMARY
New Delhi metallo-β-lactamase (NDM) is a metallo-β-lactamase able to hydrolyze almost all β-lactams. Twenty-four NDM variants have been identified in >60 species of 11 bacterial families, and several variants have enhanced carbapenemase activity. Klebsiella pneumoniae and Escherichia coli are the predominant carriers of blaNDM, with certain sequence types (STs) (for K. pneumoniae, ST11, ST14, ST15, or ST147; for E. coli, ST167, ST410, or ST617) being the most prevalent. NDM-positive strains have been identified worldwide, with the highest prevalence in the Indian subcontinent, the Middle East, and the Balkans. Most blaNDM-carrying plasmids belong to limited replicon types (IncX3, IncFII, or IncC). Commonly used phenotypic tests cannot specifically identify NDM. Lateral flow immunoassays specifically detect NDM, and molecular approaches remain the reference methods for detecting blaNDM. Polymyxins combined with other agents remain the mainstream options of antimicrobial treatment. Compounds able to inhibit NDM have been found, but none have been approved for clinical use. Outbreaks caused by NDM-positive strains have been reported worldwide, attributable to sources such as contaminated devices. Evidence-based guidelines on prevention and control of carbapenem-resistant Gram-negative bacteria are available, although none are specific for NDM-positive strains. NDM will remain a severe challenge in health care settings, and more studies on appropriate countermeasures are required.
INTRODUCTION
New Delhi metallo-β-lactamase (NDM) is a type of metallo-β-lactamase (MBL) able to hydrolyze most β-lactams (including carbapenems) but not monobactams (1, 2). NDM has poor activity against amdinocillin, an extended-spectrum penicillin antibiotic of the amidinopenicillin family (3). Carbapenems are the mainstay antimicrobial agents of choice for treating severe infections caused by many Gram-negative bacteria (4, 5). The hydrolysis of β-lactams by NDM enzymes cannot be prevented by clinically available β-lactamase inhibitors, including avibactam, clavulanate, sulbactam, and tazobactam. NDM-1 was first identified in a Klebsiella pneumoniae strain isolated from a Swedish patient who had been hospitalized in New Delhi, India, in 2008 (2). Since then, NDM-1 has been found in various species of the Enterobacteriaceae, Acinetobacter, and Pseudomonas, and 24 variants of NDM have been identified. NDM-positive strains are usually resistant to most antimicrobial agents in addition to β-lactams due to the coexistence of other resistance mechanisms (1). NDM-positive strains cause a variety of infections that have been reported to be associated with high mortality rates (6). NDM-positive strains have been found worldwide, representing a significant challenge for clinical management and public health (7, 8).
NDM, A SUBCLASS B1 MBL
β-Lactamases are divided into the A, B, C, and D classes based on amino acid sequence identity (9–11). Class A, C, and D enzymes contain a serine residue at the active site of the β-lactamase, while class B enzymes contain one or two zinc ions and are therefore termed MBLs. MBLs have been further subdivided into three subclasses (B1, B2, and B3) based on amino acid sequence identities (12, 13). The subclass B1 and B3 enzymes have two zinc ions at the active site and exhibit a broad-spectrum substrate profile, including penicillins, cephalosporins, and carbapenems (9, 12, 14). In contrast, subclass B2 enzymes have one active zinc ion, while the binding of the second zinc ion inhibits their catalysis activity (15). The subclass B2 enzymes exhibit a narrow-spectrum substrate profile, including carbapenems but not penicillins and cephalosporins (15, 16). A few MBLs belong to subclass B2, including CphA from Aeromonas hydrophila, Sfh-1 from Serratia fonticola, and ImiS from Aeromonas veronii. Similarly, a few subclass B3 MBLs have been identified, such as L1 from Stenotrophomonas maltophilia, AIM-1 from Pseudomonas aeruginosa, and GOB-1 from Elizabethkingia meningoseptica (formerly Chryseobacterium septicum). The majority of MBLs that have been identified so far belong to subclass B1 (9, 17). The three most common MBLs seen in clinical isolates, IMP (imipenemase), VIM (Verona integron-encoded metallo-β-lactamase), and NDM, are subclass B1 enzymes (17). Genes encoding IMP, VIM, and NDM are largely plasmid borne and can be transferred between bacterial strains, meaning that they are of particular significance in health care settings. NDM enzymes have low amino acid sequence identity with the other subclass B1 MBLs; for instance, the amino acid identity between NDM-1 and IMP-1 or VIM-2 is only 34% or 35%. It has been proposed that subclass B1 be further divided into two clades (B1a and B1b), with NDM belonging to clade B1b and the remaining subclass B1 enzymes belonging to clade B1a (9). Zinc ions play the key role in the function of NDM-1 (18, 19). The interaction between NDM-1 and the substrate is through zinc ions bound in the active site (20). The zinc ions also activate a water molecule, which donates a proton to generate a new active hydroxide for the hydrolysis of the β-lactam ring by attacking the carbon atom of the β-lactam carbonyl group (20, 21).
Of note, the cellular localization of NDM is different from that of all other MBLs. NDM is a lipoprotein that anchors to the outer membrane in Gram-negative bacteria, which has been attributed to the presence of a canonical lipidation amino acid sequence, LSGC (lipobox), at end of the signal peptide of NDM (22, 23). In contrast, all other MBLs are soluble periplasmic proteins (24). Membrane anchoring significantly enhances the stability of NDM under conditions of zinc deprivation, which occurs at the infection site as large amounts of the metal-chelating protein calprotectin are released as a response of host immunity. The resulting zinc deprivation can interfere with the function of MBLs such as NDM. Membrane anchoring also facilitates the secretion of this enzyme in outer membrane vesicles (OMVs) (23, 25). OMVs containing NDM can protect neighboring bacterial populations from the action of β-lactams, and OMVs can carry both NDM and blaNDM (23, 25). As a result, membrane anchoring, an important feature of NDM, may therefore contribute to the wide distribution of NDM-positive strains in health care settings.
NDM VARIANTS
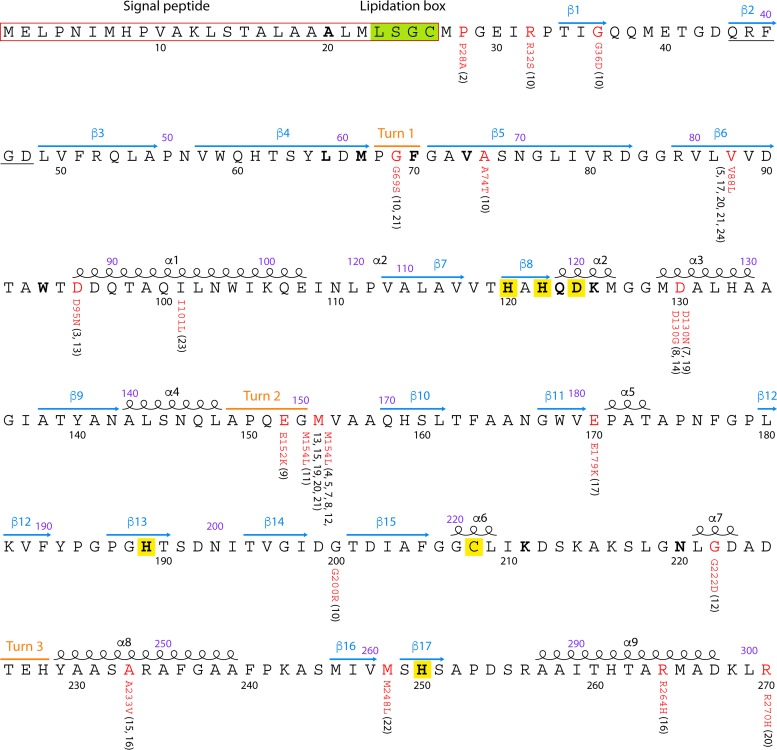
NDM enzymes are composed of 270 amino acids, containing two zinc ions at the active site, where the hydrolysis of β-lactams takes place. The secondary structure of NDM enzymes contains 9 α-helices, 17 β-strands, and 3 turns (Fig. 1). Substitutions have been observed at 17 of the 270 amino acids, resulting in 24 distinct NDM variants. The M154L substitution is the most common and is observed in 10 of the 24 distinct NDM variants. NDM variants commonly contain between 1 and 5 amino acid substitutions compared to NDM-1. NDM-18 is an exception in that it is identical to NDM-1, with the exception of a tandem repeat of 5 amino acids (QRFGD, amino acid positions 44 to 48 of NDM-1). None of the amino acid substitutions observed in NDM variants occur within the active site (Fig. 1), but some variants have been reported to exhibit altered activities against β-lactams (see Table S1 in the supplemental material). Caution is required when interpreting the impact of amino acid substitutions on the carbapenemase activity of NDM variants due to the inconsistency of phenotypic susceptibility results, heterogeneity in experiment methodologies (e.g., the use of different promoters, cloning vectors, and strains), and the fact that different parameters (e.g., MICs or kinetics) have been used for comparing the activities of NDM variants across different studies. Nonetheless, variants containing the V88L substitution (NDM-5, -17, -20, and -21) (Table S1) have repeatedly been reported to exhibit enhanced carbapenemase activity (26–29). MICs of ertapenem against strains producing NDM-5 or NDM-20 are 4- or 8-fold higher than those against strains producing NDM-1 (26, 28), while NDM-17 and NDM-21 have the same carbapenemase activity as NDM-5 (27, 29). This suggests that such a substitution may have a significant impact on enzyme activity despite not being located at the active site, and the mechanism of action of enhanced activity remains unclear. Variants containing V88L also have other substitutions, and a recent study, using the natural promoter of blaNDM-1 for cloning, failed to report any difference in carbapenem MICs between strains producing NDM-5 or NDM-17 (V88L-containing variants) and a strain producing NDM-1 (30). A variant exists which contains solely the V88L substitution, NDM-24, and the true ability of this substitution to enhance activity will be known when the phenotypic characteristics of this variant are reported. M154L (NDM-4) and D130G (NDM-14) substitutions have also been reported to result in enhanced carbapenemase activity (31, 32). However, MICs of carbapenems against Escherichia coli TOP10 strains harboring the recombinant plasmid pNDM-4 or pNDM-1, expressing NDM-4 and NDM-1, respectively, have no significant changes in MIC (31). NDM-8, which contains both M154L and D130G, does not exhibit increased carbapenemase activity (31). Of note, the media used in these experiments, such as Mueller-Hinton (MH) broth and LB, are rich in zinc. However, under conditions of zinc deprivation, the M154L (NDM-4), A233V (NDM-6), and E152K (NDM-9) substitutions in NDM enzymes enhance resistance to cefotaxime by improving metal affinity (M154L) or by improving the stability of NDM enzymes (A233V and E152K) (25). The D95N (NDM-3) and D130G (NDM-14) substitutions also enhance resistance to cefotaxime under conditions of zinc starvation, but their mechanisms remain unclear (25). In contrast, R264H (NDM-16), M154V (NDM-11), and P28A (NDM-2) have no significant impact on NDM function under zinc-restricting conditions (25). The stress imposed by zinc deprivation has therefore been proposed to be a major driver of the evolution of NDM enzymes (25). Unfortunately, MICs of carbapenems against strains producing different NDM variants have not been determined under conditions of zinc deprivation. The carbapenemase activity of new NDM variants is required to be characterized by a standardized assay under both zinc-rich and zinc-restricting conditions to fully elucidate the phenotypic importance of the emergence and evolution of novel substitutions.
FIG 1.
NDM-1 amino acid sequence and NDM variants. The annotation of the NDM amino acid sequence is adopted from data reported under UniProt accession no. C7C422. Signal peptides of NDM-1 are framed with red lines. α-Helices, β-strands, and turns are indicated as black spirals and blue and orange lines, respectively. Amino acids at active sites of NDM-1 are highlighted in boldface type, and the zinc binding residues are highlighted in yellow. The lipidation box is highlighted in green. Two numbering systems for the amino acids are shown: numbering according to the standard number scheme of MBLs is shown in purple above the amino acid sequence, while numbering from the translation of NDM enzymes is shown in black below the sequence. Amino acid substitutions compared with NDM-1 are labeled in red, with the variant names shown in parentheses. NDM-18 has a tandem repeat of 5 amino acids (QRFGD), which is underlined.
EPIDEMIOLOGY OF NDM-POSITIVE STRAINS
Distribution and Prevalence of NDM-Positive Strains in Health Care Settings
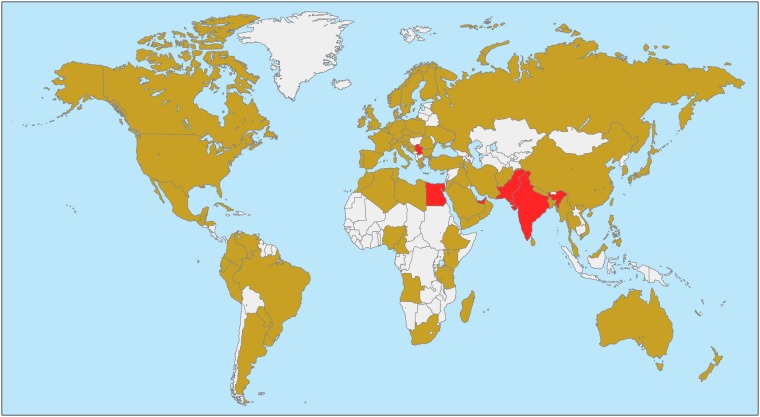
After the initial discovery of NDM-1, a follow-up study revealed the widespread existence of blaNDM-1 in the Indian subcontinent, including India, Pakistan, and Bangladesh (33). Since then, NDM-positive strains have been shown to be globally distributed, with virtually all countries conducting epidemiological searches detecting NDM-positive strains (Fig. 2; a complete list of countries with documented NDM-positive strains is available in Appendix S1 in the supplemental material).
FIG 2.
Worldwide distribution of NDM-positive strains of the Enterobacteriaceae. Countries (Egypt, India, Pakistan, Serbia, and UAE) with evidence showing a prevalence of NDM-positive strains among the Enterobacteriaceae of ≥5% are indicated in red, while countries with reports of NDM-positive strains but without evidence of a ≥5% prevalence are shown in light brown. Countries without reports or data on NDM-positive strains are indicated in white.
The worldwide distribution of NDM-positive strains appears to be heterogeneous with regard to prevalence. The SMART global surveillance program collected 103,960 isolates of Enterobacteriaceae in 55 countries from 2008 to 2014 and demonstrated that 290 strains (0.28% of all strains) were NDM positive, suggesting a relatively low prevalence (34). In the SMART program, the prevalence of NDM-positive strains varied significantly across countries: up to 5.01% in the United Arab Emirates (UAE), 6.15% in Egypt, 6.22% in India, and 6.26% in Serbia (34). This supports the observation that NDM-positive strains are more highly prevalent in South Asia, the Balkans, North Africa, and the Middle East. The high prevalence of NDM-positive strains in the Middle East has been proposed to be a result of population exchange with the Indian subcontinent (8). INFORM is another large-scale multinational study, which collected 38,266 Enterobacteriaceae isolates and 8,010 P. aeruginosa strains from 40 countries between 2012 and 2014 (35). The proportions of NDM-positive strains were 0.19% (72/38,266) in Enterobacteriaceae and 0.04% (3/8,010) in P. aeruginosa strains (35), consistent with the relatively low prevalence revealed by the SMART project. Unfortunately, the prevalence of NDM-positive strains in individual countries was not provided in that study (35). Other than the SMART and INFORM global surveillance programs, there are very few appropriately designed large-scale studies to determine the true prevalence of NDM carriage in given species or genera at a national level. In Pakistan, 18.5% of hospitalized patients at two military hospitals were found to carry NDM-positive Enterobacteriaceae (36). In China, a study of 1,162 clinical isolates of Enterobacteriaceae and Acinetobacter spp. collected at multiple sites reported that 3.9% were NDM positive, but the proportion of NDM-positive strains among the Enterobacteriaceae remains unclear, as the exact number of isolates of Enterobacteriaceae was not given (37). More large-scale surveillance studies, in particular on strains collected after 2014, are required to reveal changing trends and changes in the prevalence of NDM-positive strains.
In the SMART global surveillance program, blaNDM was the third most common carbapenemase-encoding gene and accounted for 19.42% of carbapenemase positivity, after blaKPC (53.18%) and the blaOXA-48-like gene (20.09%) (34). In China, which was not included in the SMART program, 31% of 1,105 carbapenem-resistant Enterobacteriaceae (CRE) strains were NDM positive (38). The EuSCAPE survey across Europe revealed that 7.7% of carbapenem-resistant K. pneumoniae (n = 1,203) and 10.3% of carbapenem-resistant E. coli (n = 194) isolates were NDM positive (39).
In addition to clinical samples, blaNDM genes have also been detected in hospital sewage in several countries, including China (40, 41), India (42), and Lebanon (43). Some NDM-positive strains recovered from hospital sewage belong to the Enterobacteriaceae (42, 43), which may reflect intestinal carriage of NDM-positive strains among the population within health care settings. However, NDM-positive strains of various Acinetobacter species have also been recovered from hospital sewage (40, 41). This suggests that sewage may be a reservoir of blaNDM- and NDM-positive strains. The links between hospital sewage and the spread of blaNDM are yet to be established and require more studies. Nonetheless, hospital sewage should be properly treated according to existing guidelines and regulations (44).
Spread of NDM-Positive Strains and International Travel
The rapid spread of NDM from its initial emergence in India to all continents is significantly associated with global travel (33). The initial discovery of blaNDM-1 in India, Pakistan, and the United Kingdom showed that almost all United Kingdom cases were associated with travel to the Indian subcontinent (33). Following the first report, the incidence of NDM cases rose sharply, with countries in the Mediterranean region of Europe showing the largest increase (45). A detailed study of the first reported cases of NDM in Europe showed that 57% of all cases were associated with previous hospitalization in the Indian subcontinent or Balkans region (46). The first reported outbreak in Europe was in Italy in 2011. The blaNDM-1 gene was detected in both Klebsiella and E. coli strains isolated from clinical infections in a hospital in Bologna, Italy, with the index case being a patient initially treated for an infection due to NDM-positive bacteria in New Delhi, India, before traveling to Bologna, where further treatment was required (47). This initial seeding led to Italy having some of the highest rates of NDM cases in Europe by 2017 (39). By 2014, Greece was reporting sustained cases of hospital-associated infections caused by K. pneumoniae sequence type 11 (ST11) strains carrying blaNDM-1 (48), believed to be first introduced via travel from the eastern Balkans region. International travel has also been associated with the movement of NDM into North America, with direct patient transfer from India to Canada resulting in the introduction of blaNDM-1-carrying P. aeruginosa ST654 (49) as well as cases of blaNDM-1-carrying E. coli and K. pneumoniae (50). Direct travel from India and then Iran was also implicated in the first cases of NDM isolation in the United States (51–53).
While the role of travel in observed clinical cases of infection with blaNDM-1-carrying bacteria is clear, less is known of the role that travel may play in introducing NDM into the wider community. A number of high-quality studies have been performed, showing that travel to regions of endemicity, such as India and Southeast Asia, leads to significant levels of intestinal colonization by bacteria carrying extended-spectrum β-lactamase (ESBL) genes (54–56). However, to date, there has been only one study examining the risk of asymptomatic colonization by blaNDM-1-carrying bacteria during travel (57). This small study of French travelers to India identified intestinal colonization by a blaNDM-1-carrying E. coli strain and reported that colonization lasted for only 1 month, compared to as long as 8 months for ESBL-carrying E. coli (55). This suggests that there may be intrinsic differences in the abilities of blaNDM-carrying bacteria to successfully outcompete intestinal microbiota and colonize the human intestinal tract, an area that merits full and intensive study.
Host Species of NDM
To obtain a comprehensive picture of the distribution of NDM across bacteria, we retrieved all bacterial genome sequences containing blaNDM from GenBank (n = 766; accessed on 8 January 2018) (see Data Set S1 in the supplemental material), in addition to reviewing the available literature. blaNDM genes have been found in species belonging to 11 bacterial families (Aeromonadaceae, Alcaligenaceae, Cardiobacteriaceae, Enterobacteriaceae, Moraxellaceae, Morganellaceae, Neisseriaceae, Pseudomonadaceae, Shewanellaceae, Vibrionaceae, and Xanthomonadaceae) of the class Gammaproteobacteria (Table 1). A blaNDM gene has also been identified in the genome sequence (GenBank accession no. JPNZ00000000) of a strain of Bacillus subtilis, which is a Gram-positive bacterium of the family Bacillaceae. This is very unusual, and resequencing of this strain would be advised to exclude any possibility of sequence read contamination. According to the literature, the Enterobacteriaceae are the major hosts of blaNDM, among which K. pneumoniae is the most common species, accounting for just over half of all isolates, followed by E. coli and the Enterobacter cloacae complex (Table 2). The Enterobacteriaceae are able to cause a variety of community-onset or hospital-acquired infections, such as abscesses, bloodstream infection, intra-abdominal infection, meningitis, pneumonia, and urinary tract infection (58).
TABLE 1.
Bacterial species having NDM variants
| Type | Family | Species | Reference(s) |
|---|---|---|---|
| NDM-1 | Enterobacteriaceae | Cedecea lapagei, Citrobacter braakii,a Citrobacter freundii, Citrobacter koseri, Citrobacter portucalensis,a Citrobacter sedlakii, Citrobacter werkmanii,a Enterobacter asburiae,a E. cloacae, E. hormaechei, Enterobacter kobei,a E. coli, Klebsiella aerogenes, Klebsiella michiganensis, Klebsiella oxytoca, K. pneumoniae, Klebsiella quasipneumoniae,a Klebsiella variicola,a Leclercia adecarboxylata, Lelliottia nimipressuralis,a Pantoea agglomerans, Pseudocitrobacter faecalis,a Raoultella ornithinolytica, Raoultella planticola, Salmonella enterica, Serratia marcescens, Shigella boydii | 2, 86, 102, 279, 320, 347–358 |
| Morganellaceae | Morganella morganii, Providencia rettgeri, Providencia stuartii | 359–361 | |
| Moraxellaceae | A. baumannii, Acinetobacter baylyi, Acinetobacter beijerinckii, Acinetobacter bereziniae, Acinetobacter calcoaceticus, Acinetobacter defluvii, Acinetobacter dijkshoorniae,a Acinetobacter guillouiae, Acinetobacter haemolyticus, Acinetobacter johnsonii, Acinetobacter junii, Acinetobacter lwoffii, Acinetobacter nosocomialis, Acinetobacter pittii, Acinetobacter radioresistens,a Acinetobacter schindleri, Acinetobacter soli, Acinetobacter towneri, Acinetobacter variabilisa | 104, 107, 362–374 | |
| Pseudomonadaceae | P. aeruginosa, Pseudomonas oryzihabitans, Pseudomonas putida, Pseudomonas pseudoalcaligenes | 86, 375, 376 | |
| Xanthomonadaceae | Stenotrophomonas maltophiliab | 377 | |
| Aeromonadaceae | Aeromonas caviae | 86 | |
| Vibrionaceae | Vibrio parahaemolyticus, Vibrio fluvialis, V. cholerae | 256, 378, 379 | |
| Cardiobacteriaceae | Suttonella indologenes | 86 | |
| Neisseriaceae | Kingella denitrificans | 86 | |
| Alcaligenaceae | Achromobacter spp. | 86 | |
| Shewanellaceae | Shewanellaceae spp.a | ||
| Bacillaceae | Bacillus subtilisa,b | ||
| NDM-2 | Moraxellaceae | A. baumannii | 380 |
| NDM-3 | Enterobacteriaceae | E. coli | 37 |
| Moraxellaceae | A. baumannii | 381 | |
| NDM-4 | Enterobacteriaceae | E. cloacae, E. coli, K. pneumoniae | 95, 382, 383 |
| NDM-5 | Enterobacteriaceae | C. freundii, Citrobacter europaeus,a E. coli, K. michiganensis, K. pneumoniae, K. quasipneumoniae,a S. enterica | 37, 355, 384, 385 |
| Morganellaceae | P. mirabilis | 386 | |
| NDM-6 | Enterobacteriaceae | E. coli, K. aerogenesa | 387 |
| NDM-7 | Enterobacteriaceae | C. freundii, K. pneumoniae, E. cloacae, E. hormaechei, E. coli, S. entericaa | 35, 95, 384 |
| NDM-8 | Enterobacteriaceae | E. coli | 388 |
| NDM-9 | Enterobacteriaceae | Cronobacter sakazakii, E. coli, K. pneumoniae, K. variicola, S. entericaa | 134, 389–391 |
| NDM-10 | Enterobacteriaceae | K. pneumoniae | 392 |
| NDM-11 | Enterobacteriaceae | E. coli | 393 |
| NDM-12 | Enterobacteriaceae | E. coli | 384 |
| NDM-13 | Enterobacteriaceae | E. coli | 135 |
| NDM-14 | Moraxellaceae | A. lwoffii | 32 |
| NDM-15 | Enterobacteriaceae | E. colia | |
| NDM-16 | Enterobacteriaceae | K. pneumoniae | 35 |
| NDM-17 | Enterobacteriaceae | E. coli | 27 |
| NDM-18 | Enterobacteriaceae | E. colia | |
| NDM-19 | Enterobacteriaceae | E. coli,a K. pneumoniaea | |
| NDM-20 | Enterobacteriaceae | E. coli | 28 |
| NDM-21 | Enterobacteriaceae | E. coli | 29 |
| NDM-22 | Enterobacteriaceae | E. cloacaea | |
| NDM-23 | Enterobacteriaceae | K. pneumoniaea | |
| NDM-24 | Morganellaceae | P. stuartiia | |
NDM-positive strains of the species have not been reported in literature but have genomes available in GenBank (see Data Set S1 in the supplemental material).
This is unusual and needs to be verified to exclude contamination.
TABLE 2.
Species distribution of NDM-positive Enterobacteriaceae strainsb
| Location | Yr(s) | NDM-positive strain | No. of isolates |
Reference | |||
|---|---|---|---|---|---|---|---|
| E. coli | K. pneumoniae | E. cloacae | Other | ||||
| South Africa | 2012–2015 | 469 | 11 | 325 | 31 | 102 | 394 |
| China | 2014–2015 | 343 | 81 | 121 | 81 | 60 | 38 |
| UK | 2008–2013 | 326 | 80 | 180 | 31 | 35 | 64 |
| Global (n = 55)a | 2008–2014 | 290 | 57 | 169 | 40 | 24 | 34 |
| South Korea | 2010–2015 | 146 | 34 | 69 | 27 | 16 | 89 |
| India (Mumbai) | 2012 | 106 | 30 | 43 | 29 | 4 | 395 |
| Total | 1,680 | 293 | 907 | 239 | 241 | ||
The SMART Global Surveillance Program collected strains of the Enterobacteriaceae only in 55 countries.
Studies with at least 100 strains are included.
Acinetobacter spp. are also frequently identified as hosts of blaNDM (41). Acinetobacter strains carrying blaNDM have been found in at least 25 countries in Africa (Algeria, Libya, Morocco, Egypt, Ethiopia, Kenya, and Tunisia), the Americas (Argentina, Brazil, Cuba, Honduras, and Paraguay), Asia (Bangladesh, China, India, Israel, South Korea, Lebanon, Malaysia, Palestine, and Thailand), and Europe (Croatia, Denmark, Greece, and Slovenia). Acinetobacter baumannii is a notorious opportunistic pathogen associated with hospital-acquired infections and pneumonia in particular (59). Surprisingly, blaNDM is also found in at least 18 other Acinetobacter species, most of which have been recovered from sewage and are rarely associated with human diseases (Table 1). This suggests that Acinetobacter may play a vital role in the dissemination of NDM-encoding genes and raises the question of whether Acinetobacter species could be the origin of blaNDM. This wide species distribution may also be due to multiple mechanisms facilitating the transfer of blaNDM across bacterial populations. In addition to conjugation, which is the major mode of horizontal transfer of blaNDM in the Enterobacteriaceae, it has been found that OMVs of A. baumannii are able to mediate the intra- and interspecies transfer of blaNDM plasmids at high transformation frequencies (10−5 to 10−6 transformants in the total cell count [CFU per milliliter]) (23, 60). General transduction facilitated by prophages present in the chromosome can also mediate the horizontal transfer of blaNDM between A. baumannii strains (61). More studies are required on this remarkable diversity of Acinetobacter strains carrying blaNDM and its relevance to the successful emergence of NDM across Gram-negative bacteria.
Among the 24 NDM variants, NDM-1 has the widest host spectrum identified so far and has been found in a number of species belonging to 11 bacterial families. Publicly available genome sequences also reveal that most NDM-positive Acinetobacter species, Enterobacter species, and K. pneumoniae isolates have NDM-1, while NDM-5 is most common in E. coli. NDM is rare in P. aeruginosa, with VIM being the most common MBL in this species (35).
Clonal Background of NDM-Positive Strains
There is a limited number of large-scale studies that have examined the clonal background of NDM-producing strains. These include one study involving multiple nations (62), one study across three countries (63), three studies conducted at a national level (38, 64, 65), and two municipal-level studies (66, 67) (see Table S2 in the supplemental material). While studying genomes deposited in GenBank can result in inherent bias, it is also the most comprehensive approach available to provide additional insights into the clonal background of NDM-positive strains. Among the 766 NDM-positive bacterial genomes available in GenBank, E. coli (n = 305), K. pneumoniae (n = 214), Acinetobacter spp. (n = 84), and Enterobacter spp. (n = 67) are the most common (Data Set S1). NDM-positive E. coli and K. pneumoniae strains are distributed across more than 40 STs for each species. NDM-positive Enterobacter and Acinetobacter strains are also distributed across various species and multiple STs (Data Set S1). This suggests heterogeneous clonal backgrounds of NDM-positive strains and multiple acquisitions of blaNDM genes across bacterial species. Despite this heterogeneity, a small number of STs of the Enterobacteriaceae and A. baumannii have been identified to be the most common carriers of blaNDM (see below). These STs warrant further investigation to identify high-risk clones mediating the international spread of blaNDM genes as well as determine underpinning factors that may make a clone more likely to emerge as a successful multidrug-resistant (MDR) pathogen.
NDM-positive E. coli strains belong to a variety of STs, and there are no predominant STs. NDM has been found in strains of E. coli ST131 (68–70), the pandemic clone mediating the global spread of the ESBL gene blaCTX-M-15 (71, 72). However, NDM-positive ST131 strains remain uncommon (Table S2 and Data Set S1). In contrast, ST167 is relatively common among NDM-positive E. coli strains (Table S2 and Data Set S1) and accounted for 14.4% (44/305) of the 305 NDM-positive E. coli genomes available (Data Set S1). ST167 has been detected in multiple countries (India, Niger, South Africa, South Korea, Switzerland, and the United States) and throughout China and has been predominantly recovered from humans (Data Set S1). ST617 and ST410 are two other types of E. coli strains seen in multiple countries, although they are less common than ST167 (Data Set S1), and both types have sequenced isolates recovered from animals in addition to humans. In a multiple-site study in China, ST167 was the most common type of NDM-positive E. coli strain, followed by ST410 (38). Our previous study also suggested that E. coli ST167 and ST617 appear to be two major types of globally disseminated, NDM-positive E. coli strains (73). More studies are required to investigate whether ST167, ST410, and ST617 are international epidemic clones of NDM-positive E. coli.
NDM-positive K. pneumoniae strains are also distributed across a large number of STs, with no predominant lineages, suggesting that there are no obvious high-risk clones of NDM-positive K. pneumoniae. This is in contrast to KPC-positive K. pneumoniae, whose global spread is largely due to clonal complex 258 comprising ST258 and ST11 (74, 75). ST11, ST14, ST15, and ST147 strains are relatively common NDM-positive K. pneumoniae lineages and have been found in multiple countries across several continents, almost all of which were isolated from humans (Data Set S1). In the literature, ST14 is repeatedly reported as one of the most common types of NDM-positive K. pneumoniae strains (63–65). ST11 is another common type in multiple studies (38, 63–65). Of note, ST11 is the predominant ST of carbapenem-resistant K. pneumoniae in China but mainly carries KPC-2 rather than NDM (76). Although the currently available evidence is insufficient to demonstrate that ST11, ST14, ST15, and ST147 are truly epidemic clones mediating the international spread of blaNDM, their distribution in multiple countries warrants further study. A multiple-site study reported that ST23 is the most common type of NDM-positive K. pneumoniae strain in China (38). However, ST23 is rarely seen in other countries, and there is only one ST23 genome in GenBank, isolated in China. This suggests that ST23 may be largely restricted to China (38). The well-known international epidemic carbapenem-resistant K. pneumoniae ST258, which carries blaKPC-2 or blaKPC-3, has not yet been found to carry blaNDM (77).
Most NDM-positive strains of Enterobacter spp. are either Enterobacter xiangfangensis or Enterobacter hormaechei strains (Data Set S1). ST78 and ST171 have been reported as two emerging lineages of carbapenem-resistant Enterobacter spp., but strains of these lineages usually produce KPC rather than NDM (78). There are only three NDM-positive ST78 (belonging to E. hormaechei) and three NDM-positive ST171 (belonging to E. xiangfangensis) genomes in GenBank (Data Set S1). ST114 (belonging to E. hormaechei) strains have been found in multiple countries (Data Set S1). A study on an international collection of carbapenem-resistant Enterobacter strains demonstrated that ST114 is the most common type of NDM-positive Enterobacter strain, although it accounts for only a minority of all strains due to the very diverse clonal background of NDM-positive Enterobacter strains (62). Therefore, no obvious international epidemic clones of NDM-positive Enterobacter strains have been identified at present.
NDM-positive A. baumannii strains are very genetically diverse with respect to ST lineage (67), with ST85 being the most commonly isolated (11 genomes in GenBank) (Data Set S1) across several countries. NDM has also been found in P. aeruginosa in multiple countries (35, 49, 79, 80). Whole-genome sequences exist for a small number of P. aeruginosa strains from lineage ST308, all isolated in Singapore (Data Set S1). NDM-positive ST308 P. aeruginosa has also been detected in neighboring Malaysia (81), suggesting that this type of NDM-positive P. aeruginosa strain may be circulating in the region.
Plasmids Carrying blaNDM
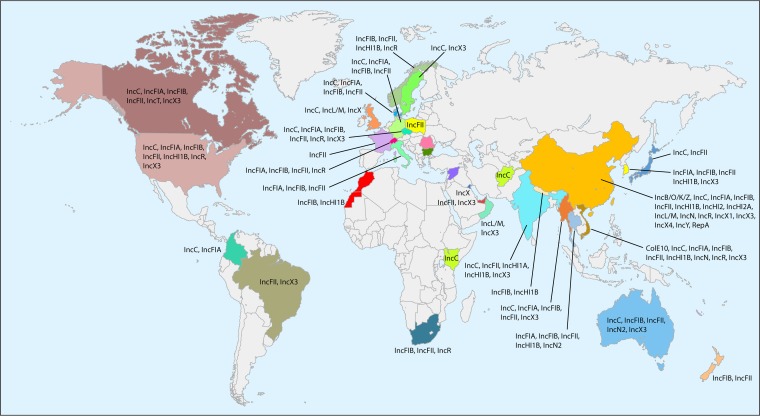
Although blaNDM has been found on bacterial chromosomes (82–84), the vast majority of carriage occurs on plasmids, which play a vital role in dissemination. blaNDM has been reported to be carried on plasmids with a variety of replicon types (33, 82, 85–104). There are a total of 355 blaNDM-carrying plasmids with complete sequences available in GenBank (accessed on 8 January 2018) (see Data Set S2 in the supplemental material). We determined their replicon types using PlasmidFinder (https://cge.cbs.dtu.dk/services/PlasmidFinder/). There are 20 replicon types of blaNDM-carrying plasmids in the Enterobacteriaceae, including IncC, IncB/O/K/Z, IncFIA, IncFIB, IncFIC, IncFIII, IncHI1, IncHI2, IncHI3, IncN, IncN2, IncL/M, IncP, IncR, IncT, IncX1, IncX3, IncX4, IncY, and ColE10 (Table 3) (e.g., see references 33, 82, and 85–104). This suggests multiple acquisitions of blaNDM by various plasmids and also highlights that the horizontal transfer of blaNDM is mediated by multiple plasmids. The global distribution of the replicon types of blaNDM-carrying plasmids is shown in Fig. 3.
TABLE 3.
Replicon types of blaNDM-carrying plasmids in the Enterobacteriaceae
| Type | Replicon type(s)b | Reference(s) |
|---|---|---|
| NDM-1 | A/C, ColE,a FIA, FIB, FII, HI1, HI3, HIB, L/M, N2, P, R, T,a X1,a X3, Ya | 33, 82, 85–104, 396 |
| NDM-3 | A/Ca | |
| NDM-4 | FII, HI2, X3 | 95, 397 |
| NDM-5 | B/O/K/Z,a FUA,a FIB,a FIC,a FII, X3, X4,a Ya | 82, 89, 385, 398–400 |
| NDM-6 | A/C, FIB, FII, R,a X3a | 387, 399 |
| NDM-7 | A/C, FIC, FII, X3 | 89, 133, 399, 401, 402 |
| NDM-8 | FII | 393 |
| NDM-9 | B/O/K/Z, FIA,a FII, HI2, N,a Ra | 134, 389, 390 |
| NDM-10 | FII | 392 |
| NDM-11 | FII | 393 |
| NDM-12 | F | 403 |
| NDM-13 | X3 | 135 |
| NDM-17 | X3 | 27 |
| NDM-19 | X3a | |
| NDM-20 | X3 | 28 |
| NDM-21 | X3 | 29 |
Replicon types have not been reported in the literature but have plasmid sequences available in GenBank (see Data Set S2 in the supplemental material).
Common replicon types (≥10 plasmid sequences in GenBank) are highlighted in boldface type.
FIG 3.
Worldwide distribution of the replicon types of blaNDM-carrying plasmids in Enterobacteriaceae. Detailed information about the distribution of the replicon types of blaNDM-carrying plasmids is available in Table 3 and Data Set S2 in the supplemental material.
IncX3 appears to be the most common type of plasmid carrying blaNDM. Among the 355 blaNDM-carrying plasmids available in GenBank, 117 (about one-third) had the IncX3 replicon, including 112 plasmids with IncX3 alone and 5 with IncX3 plus other replicons. IncX3 plasmids are narrow-host-range plasmids and have so far been seen only in the Enterobacteriaceae. Most of the IncX3 plasmids in GenBank (67/117; 57.3%) were present in E. coli, followed by K. pneumoniae (20/117; 17.1%). Although blaNDM-carrying IncX3 plasmids have been found in Europe and North America, most of the plasmids deposited in GenBank (80/117; 68.4%) have been recovered in China and neighboring countries, such as South Korea (n = 7), Vietnam (n = 2), and Myanmar (n = 3). This suggests that IncX3 plasmids may be a major vehicle in mediating the dissemination of blaNDM in East Asia, particularly in China. Variants of blaNDM, including blaNDM-1, blaNDM-4, blaNDM-5, blaNDM-6, blaNDM-7, blaNDM-13, blaNDM-17, blaNDM-19, blaNDM-20, and blaNDM-21, have also been found on IncX3 plasmids (Table 3). This highlights that IncX3 plasmids may serve as one of the major platforms on which blaNDM genes are evolving with the generation of new NDM variants.
There are 99 blaNDM-carrying plasmids containing an IncFII replicon, alone or in combination with other types of replicons, commonly IncFIB in GenBank. A replicon sequence typing (RST) scheme is available for IncF plasmids (105). We performed RST for these plasmids using the pMLST tool (https://cge.cbs.dtu.dk/services/pMLST/). It is evident that IncFII plasmids of the FIA−:FIB36:FIIY4 allele type (n = 27) or the FIA−:FIB−:FII2 type (n = 21) are particularly common and have been found in various species of the Enterobacteriaceae from multiple countries in several continents (Data Set S2). These two IncFII plasmids have mainly been found in strains from human samples (Data Set S2).
Another common type of blaNDM-carrying plasmid (53/355; 14.9%) is IncC (also incorrectly known as IncA/C2) (106). IncC plasmids carrying blaNDM have a worldwide distribution and are found on all continents except Antarctica (Data Set S2). IncA/C has a broad host range, and IncC plasmids carrying blaNDM have been found in the Morganellaceae and the Vibrionaceae in addition to the Enterobacteriaceae. A plasmid multilocus sequence typing (pMLST) scheme exists for IncA/C plasmids (https://pubmlst.org/plasmid/). Almost all IncC plasmids carrying blaNDM belong to either ST1 (39/53) or ST3 (12/53) (Data Set S2). However, there are only 13 STs of IncA/C plasmids in the database, suggesting low diversity of these plasmids or low resolution of the scheme for typing such plasmids. A core genome pMLST (cgPMLST) scheme also exists for IncA/C plasmids (https://pubmlst.org/plasmid/). IncC plasmids of cgST1.2 are particularly common (n = 17) (Data Set S2) and have been found in several species of the Enterobacteriaceae and Morganellaceae from all continents except Antarctica. Plasmids of the above-mentioned IncX3 type, FIA−:FIB36:FIIY4 and FIA−:FIB−:FII2 types, and cgST1.2 of IncC warrant further investigation to understand their epidemiology, their true contribution to the NDM problem, and the mechanisms mediating their wide spread.
Plasmids carrying blaNDM have been well documented in Acinetobacter (e.g., see references 107–110). A replicon typing scheme for plasmids of A. baumannii has been developed (111), but the replicon types of blaNDM-carrying plasmids in Acinetobacter have rarely been reported, and the scheme has not been incorporated into PlasmidFinder or any other commonly used plasmid typing tools. Therefore, the replicon types of blaNDM-carrying plasmids in Acinetobacter remain largely unknown. These plasmids vary significantly in size from 1,634 bp to 354,308 bp (Data Set S2), suggesting that multiple plasmids are involved in the spread of blaNDM in Acinetobacter.
Genetic Contexts of blaNDM
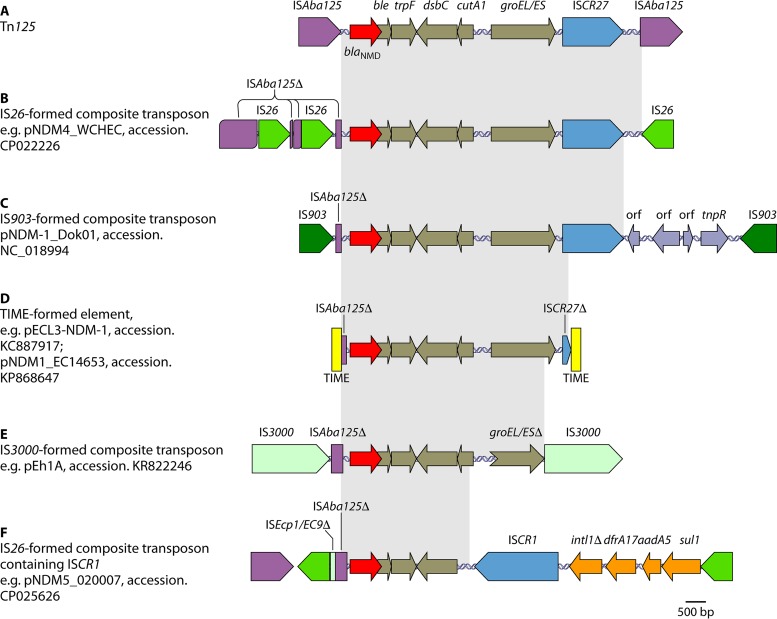
Mobile genetic elements, such as insertion sequences, transposons, and integrons, can mobilize antimicrobial resistance genes. This mobilization can be between different plasmids as well as between plasmids and the chromosome. blaNDM has been found in a variety of genetic contexts, which suggests that multiple mechanisms have been involved in the mobilization of blaNDM. The genetic contexts of blaNDM share two common features. The insertion sequence ISAba125 (intact or truncated) is always upstream of blaNDM, while a bleomycin resistance gene, bleMBL, is always downstream. Further downstream of bleMBL, there is usually a complete or remnant form of a set of several genes, including trpF (encoding a phosphoribosylanthranilate isomerase), dsbC (also called tat, encoding a twin-arginine translocation pathway signal sequence domain protein), cutA1 (also called dct, encoding a periplasmic divalent cation tolerance protein), and groES-groEL (encoding chaperonin), and the insertion sequence ISCR27. ISAba125 provides the −35 region of a promoter for the expression of blaNDM-1 (112, 113). Another ISAba125 element has been found downstream of ISCR27 in Acinetobacter, and the two ISAba125 elements form a composite transposon carrying blaNDM-1, termed Tn125 (Fig. 4A). It appears that the genetic components within Tn125 have different origins, as the groES-groEL-ISCR27 section may originate from Xanthomonas spp. (114, 115). The exact origin of blaNDM-1 remains unknown. Careful examination of the genetic context and sequence of blaNDM-1 reveals that blaNDM-1 is a chimeric gene. The first 19 bp of nucleotide sequence (encoding the first 6 amino acids of NDM-1) originate from an aminoglycoside resistance gene, aphA6. The remaining nucleotide sequence originates from a yet-to-be-identified preexisting MBL gene (116). As ISAba125 and aphA6 are widespread in Acinetobacter spp. (117, 118), it is very likely that the fusion of genes to form blaNDM-1 occurred in Acinetobacter (116). ISCR elements are known to acquire and accumulate genetic components via a rolling-circle mechanism (119, 120). As an ISCR element, ISCR27 may have initially acquired the progenitor gene of blaNDM-1 (115, 116, 121) and mobilized the gene together with bleMBL, trpF, dsbC, cutA1, and groES-groEL into aphA6 (downstream of a copy of ISAba125), allowing fusion and the formation of blaNDM-1 (115, 116). The second copy of ISAba125 then inserted downstream of ISCR27 to form the ISAba125-based composite transposon Tn125 (116).
FIG 4.
Examples of genetic contexts and mobilization mechanisms of blaNDM. (A) Tn125 formed by two copies of ISAba125. (B) Composite transposon formed by two copies of IS26. (C) Composite transposon formed by two copies of IS903. orf, open reading frame. (D) Element formed by two copies of the TIME. (E) Composite transposon formed by two copies of IS3000. (F) Genetic contexts containing ISCR1. This element is also flanked by two copies of IS26. The plasmid names and GenBank accession numbers are shown. Δ represents truncated genes or elements.
Acinetobacter spp. serve as the intermediate source for the mobilization of blaNDM into the Enterobacteriaceae (116, 122). The blaNDM-1-carrying element Tn125 has been interrupted or truncated in Enterobacteriaceae, to generate a variety of complex genetic contexts for blaNDM. This interruption is largely due to the insertion of many other mobile genetic elements (e.g., IS1, IS5, IS26, IS903, ISEc33, and ISKpn14, etc.) and recombination. The flanking sequences of various remnants of Tn125 have also formed mechanisms involved in the mobilization of blaNDM-1. These mechanisms included a number of composite transposons formed by two copies of the same insertion sequence, such as IS26 (123) (Fig. 4B), IS903 (124) (Fig. 4C), and IS3000 (termed Tn3000) (Fig. 4F) (125). The duplication of blaNDM on the same plasmid is due to the action of an IS26-formed composite transposon (94). The mobilization of blaNDM may be associated with another ISCR element, ISCR1 (Fig. 4E) (95, 126). Two tandem copies of blaNDM-1 genes have been found on the chromosomes of both an ST167 E. coli strain in China (127) and a P. aeruginosa strain in Serbia (128). In both cases, the tandem copies of blaNDM-1 are associated with ISCR1, which uses a rolling-circle mechanism of transposition and may generate a tandem duplication of its mobilized sequence via homologous recombination (119). Tn3-derived inverted-repeat transposable elements (TIMEs), which were previously described as miniature inverted-repeat transposable elements (MITEs) (94, 129), have also been found to mobilize blaNDM (Fig. 4D) (95). TIMEs are a type of mobile genetic element bounded by 38-bp inverted repeats characteristic of the Tn3 family but lacking the transposase gene tnpA and, usually, the resolvase gene tnpR (130). Two copies of the TIME can form a composite transposon-like element, which is able to mobilize the intervening genetic components in the presence of the external Tn3-like transposase (94, 129).
Genetic contexts similar to those of blaNDM-1 are shared in other blaNDM variants, which are commonly associated with ISAba125 (intact or truncated) upstream and bleMBL downstream (29, 95, 123, 131–135). This suggests that other blaNDM variants emerged from blaNDM-1 via nucleotide mutations. Due to the highly mobile nature of blaNDM, the gene can also be lost by bacterial cells. Loss can be due to the deletion of DNA fragments as a result of insertion sequence and transposon activity, such as recombination (136, 137), or the complete loss of the blaNDM-carrying plasmids (138).
DETECTION METHODS
Detection of NDM is essential for informing therapeutic decisions. Detection of NDM also provides critical information in investigating outbreaks, guiding infection control, and tracking the global and local epidemiology of NDM-positive strains.
Detection of Carbapenemase Activity
New methods and tools are continuously being introduced for the detection of carbapenemase activity. A number of phenotypic methods to detect carbapenemase activity have been developed. These include the modified Hodge test (MHT) (139), the Carba NP (CNP) test (140) and its variants, the β-Carba test (141, 142), the carbapenem inactivation method (CIM) (143), the modified carbapenem inactivation method (mCIM) (144), matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) (145), isothermal titration calorimetry (ITC) (146), and UV spectrophotometry (147). The MHT, CNP, and mCIM have been extensively evaluated, and the latter two are currently recommended by the CLSI for detecting carbapenemases in carbapenemase-producing Enterobacteriaceae (CPE) and carbapenemase-producing P. aeruginosa (148). The MHT had been recommended by the CLSI for epidemiological or infection control purposes since 2009 (149), but the recommendation was removed in 2018 due to the availability of newer tests (CNP and mCIM) with higher accuracy (148). None of these phenotypic tests are specific for NDM, as they are designed to detect all carbapenemases, including class A (e.g., KPC) and class D (e.g., OXA-48) carbapenemases and other MBLs (e.g., IMP and VIM). We do not review these methods in detail here but refer the reader to the latest review on phenotypic methods for detecting carbapenemases (150).
CNP.
The CNP test is based on the in vitro hydrolysis of imipenem and has been extensively evaluated for detecting carbapenemases in Enterobacteriaceae (140) and Pseudomonas (151). The test has both excellent specificity (84% to 100%) and sensitivity (93.3% to 100%) for detecting carbapenemases, including NDM in Enterobacteriaceae (152–154) and P. aeruginosa (155). However, CNP is relatively labor-intensive, as reagents need to be prepared in-house, and some have short shelf lives (e.g., 72 h) (156). It has also been reported that CNP may miss some mucoid NDM-positive Enterobacteriaceae strains (157). Many variants of CNP have been developed with minor modifications or are based on the same principle but with simplified procedures. A modified CNP test has been developed, with a short turnaround time (<2 h) from the isolation of single colonies (144). This test has 84.9% to 100% sensitivity and 100% specificity for detecting carbapenemases in Enterobacteriaceae (152–154, 158). The BYG (Bogaerts-Yunus-Glupczynski) Carba test uses an electrochemical method to detect enzymatic hydrolysis of carbapenems (159), while GoldNano Carb employs gold nanoparticles to visualize carbapenemase activity (160). The detection of carbapenemases in Acinetobacter by CNP is more challenging due to its intrinsic low permeability (161). The CarbAcineto NP test is a modified CNP protocol, using modified lysis conditions and an increased bacterial inoculum to detect all types of carbapenemases, with 88.9% to 94.7% sensitivity and 100% specificity for Acinetobacter (162, 163). Several variants of CNP with simplified procedures have become commercially available, including Rapidec Carba NP (bioMérieux) (141, 164, 165), Neo-Rapid Carb (Rosco Diagnostica) (153, 164), Rapid Blue Carb (Rosco Diagnostica) (166), and β-Carba (Bio-Rad) (141, 142). Rapidec Carba NP and Neo-Rapid Carb have overall comparable sensitivity and specificity compared with CNP (141, 153, 164, 165), while it has been reported that Rapid Blue Carb (166) and β-Carba (141, 142) may be slightly less sensitive.
CIM and mCIM.
The CIM is based on the enzymatic hydrolysis of meropenem (143). The pooled sensitivity and specificity of this test are 85.7% to 95.1% and 94.4% to 95.7%, respectively, for detecting carbapenemases in Enterobacteriaceae (157, 167, 168). CIM is less expensive than CNP (<$1 per test compared to $2 to $16) (143, 150). An mCIM with a longer incubation period (4 h rather than 2 h) has been developed and demonstrated increases in both sensitivity and specificity to 100% for detecting carbapenemases in Enterobacteriaceae (144, 169–171). The mCIM has been included in CLSI guidelines for detecting carbapenemases in Enterobacteriaceae for epidemiological or infection control purposes since 2017 (172). A rapid carbapenem inactivation method (rCIM) for detecting carbapenemases within 3 h has recently been developed and has exhibited excellent sensitivity (99%) and specificity (100%) (173). A multisite evaluation found that the mCIM is accurate for detection of carbapenemases in P. aeruginosa, with 86.7% to 100% sensitivity and 93.3% to 100% specificity. Detection of carbapenemase-producing Acinetobacter with this method is more problematic, with 36.3% to 95.7% sensitivity and 28.6% to 100% specificity (155). Another study has shown mCIM to have low sensitivity (45.1%) for the detection of carbapenemases in Acinetobacter and Pseudomonas (174). A new method, termed CIMTris, has been developed to detect carbapenemases in Acinetobacter and Pseudomonas. The method is modified by extracting carbapenemases from bacteria with 0.5 M Tris hydrochloride and has 97.6% sensitivity and 92.6% specificity (174).
MALDI-TOF MS.
MALDI-TOF MS platforms are well established for genus or species identification for bacterial strains. MALDI-TOF MS has the potential to detect carbapenemases, as the method can detect carbapenem degradation products when bacterial protein extracts are incubated with carbapenems. MALDI-TOF MS has 77% to 100% sensitivity and 94% to 100% specificity when tested for detecting carbapenemases (145, 150, 175–177). MALDI-TOF MS works well to detect carbapenemases in Acinetobacter (178). MALDI-TOF MS has an objective endpoint for result interpretation (179), but no standardized in-house protocol for carbapenemase detection is available (150, 176, 180). A commercially available MBT Star-Carba IVD kit (Bruker) was introduced very recently and was evaluated with 96.1% to 100% sensitivity and 89.0% to 99.9% specificity (181).
ITC.
ITC is an approach to measure heat change during binding between ligands and their targets (182) and has been developed to study kinetics and inhibition of β-lactamases (146). It can also be used to readily detect (<10 min) the activity of carbapenemases by monitoring the change in thermal power after the exposure of bacterial cells to carbapenems (146). Although the sensitivity and specificity of ITC for detecting carbapenemases have not been established, it has the potential to be used for screening the production of carbapenemases in bacterial strains.
Detection of MBLs
Disc- or strip-based inhibition methods.
A number of tests have been developed for detecting MBLs in bacterial strains, mainly based on the combination of MBL inhibitors and carbapenems. The combined disc test (CDT), the double-disc synergy test (DDST), and the modified Etest have been widely used for detecting MBLs, including NDM (183, 184). Although the Etest MBL test is simple, it is costly, lacks the sensitivity to detect weak MBL activities (185), and can generate false-positive results in the presence of certain OXA-type enzymes (e.g., OXA-23) (186). The CDT compares the inhibition zones of carbapenem discs with or without MBL inhibitors (commonly EDTA), while the DDST is based on the difference of the inhibition zones of a β-lactam (commonly a carbapenem) disc in the presence of a disc containing MBL inhibitors (EDTA, dipicolinic acid, or 2-mercaptopropionic acid). Both the CDT and DDST are inexpensive, simple, and convenient and have good sensitivity and specificity for the detection of MBLs in Enterobacteriaceae. One study found that the imipenem-EDTA CDT correctly detected all 27 tested NDM-positive Enterobacteriaceae strains (187). Another study shows that the sensitivity and specificity of DDSTs using EDTA magnesium disodium salt tetrahydrate for 75 MBL producers (including 2 NDM producers) and 25 non-MBL producers were 96.0% and 100%, respectively (188). CDTs and DDSTs incorporating MBL inhibitors are unable to detect carbapenemases other than MBLs. MAST-Carba plus (MAST group) is a variant of the CDT containing multiple CDT discs and has been developed to detect major types of carbapenemases, including MBLs. It correctly detected all NDM-positive Enterobacteriaceae in small bacterial collections (189, 190). EDTA is a commonly used MBL inhibitor and may generate nonspecific (false-positive) results for nonfermenting bacteria, in particular Acinetobacter, as it increases the permeability of the bacterial outer cell membrane (147, 191). It has also been reported that some other MBL inhibitors, such as sodium mercaptoacetic acid (SAM), may provide poor performance in DDSTs (190).
MBL-targeted mCIM.
The mCIM alone is unable to differentiate MBLs from other carbapenemases, but the addition of MBL inhibitors can be used to specifically detect MBLs following a positive result with the mCIM. The combination of SAM and the mCIM (SAM-mCIM) has demonstrated 100% sensitivity and specificity for detecting MBLs in a small collection of CRE strains (n = 55) (169). The EDTA-modified mCIM (eCIM) was recommended by the CLSI in 2018 for detecting MBLs in CRE for epidemiological or infection control purposes as a tandem test following a positive mCIM result (148). The eCIM combined with the mCIM have a >95% sensitivity and a >92% specificity (148). Strains with a coexistence of MBLs and non-MBL carbapenemases (e.g., KPC and OXA-48) have been increasingly reported (51, 94, 192). In such cases, the eCIM and SAM-mCIM may generate false-negative results.
Carb NP test II.
Carb NP test II is a derivate of CNP which incorporates tazobactam for detection of KPC and EDTA for detection of MBLs. It has been reported to exhibit 100% sensitivity and specificity (193). However, in a study on a small collection of isolates, Carb NP test II failed to detect three (Providencia or Proteus) out of four NDM-positive strains (194). More studies are clearly required to validate Carb NP test II.
Modified MALDI-TOF MS.
Protocols for MALDI-TOF MS have been modified by the addition of carbapenemase inhibitors for detecting MBLs. The addition of phenylboronic acid (an inhibitor of class A carbapenemases) or dipicolinic acid (an MBL inhibitor) with ertapenem allows differentiation between MBLs and class A carbapenemases (195).
ITC with MBL inhibitors.
In addition to detecting carbapenemases, ITC has also been used to detect the activity of MBLs by comparing the change in thermal power after the exposure of bacterial cells to carbapenems in the absence and presence of MBL inhibitors such as EDTA (146, 196). However, the sensitivity and specificity of ITC need to be established.
Detection of NDM Enzymes
Lateral flow immunoassays.
The lateral flow immunoassay (LFIA) is an antibody-based method developed to detect different types of carbapenemases, which has been validated. It allows the specific detection of NDM enzymes in singleplex (for NDM only) (197) or multiplex (for NDM and other major types of carbapenemases) (198, 199) assays by using specific antibodies. These assays are easy to perform and have a short turnaround time, as they yield results from cultured colonies within 15 min. These tests have also been shown to have 100% sensitivity and specificity (197, 200). A multiplex LFIA has also been developed for the rapid detection of NDM, KPC, and OXA-48 carbapenemases directly from positive blood cultures (201). The LFIA has been shown to detect NDM-1, -2, -3, -4, -5, -6, -7, and -9 (199, 202), but any amino acid substitutions occurring in the epitope that is used to generate antibodies for detecting NDM may generate false-negative results. However, the limited diversity of amino acid sequences within the NDM family allows universal antibodies to be designed to detect all known NDM variants. Several commercially available multiplex LFIAs have been developed and evaluated. These assays include Resist-3 O.K.N. (OXA-48-like, KPC, and NDM; Coris BioConcept) (203), the O.K.N. K-SeT assay (OXA-48-like, KPC, and NDM; Coris BioConcept) (200), the O.K.N.V. K-Set assay (OXA-48-like, KPC, NDM, and VIM; Coris BioConcept) (204), and the NG-Test Carba 5 assay (OXA-48-like, KPC, NDM, VIM, and IMP; NG Biotech) (199, 202). These assays exhibit nearly 100% sensitivities and specificities for detection of NDM.
Detection of NDM-Encoding Genes
Molecular techniques are the reference methods for detecting carbapenemase genes, including blaNDM, due to their excellent sensitivity and specificity and robust performance (187, 205). Molecular techniques are mostly based on PCR, but whole-genome sequencing (WGS) is being increasingly used. The main limitations of molecular technologies are the high costs and the requirement for trained technicians (184, 206).
PCR.
PCR can be singleplex, multiplex, or real time, and validated protocols are available to allow convenient and robust detection of blaNDM (184). Many in-house singleplex and multiplex conventional and real-time PCR assays for detecting blaNDM have been developed (e.g., see references 2 and 207–213). Commercially available real-time PCR approaches for detecting multiple carbapenemase genes, including blaNDM, also exist. These include Xpert Carba-R (Cepheid), Check-Direct CPE (Check-Points Health), and an antibiotic resistance TaqMan assay (ThermoFisher Scientific). Xpert Carba-R is a fully automated and integrated system for sample preparation, DNA extraction, amplification, and qualitative detection of target genes using multiplex real-time PCR assays with a <1-h turnaround time (214). The method can be used directly on swab specimens and has 96.6% sensitivity and 98.6% specificity (215). In the Check-Direct CPE kit, blaNDM and blaVIM are detected using the same fluorochrome, and it is not possible to differentiate these two genes on certain real-time PCR platforms, such as ABI 7500, but they can be differentiated using other platforms, such as the BD Max platform (216). The Check-Direct CPE kit has been evaluated for detection of carbapenemase genes, including blaNDM, in Enterobacteriaceae and P. aeruginosa (212, 216), with 100% sensitivity and specificity. The ePlex blood culture identification kit (GenMark) is a highly multiplexed, fully automated, one-step, single-use cartridge assay system that was announced recently. The kit incorporates blaNDM detection, but validation has not been reported in the literature (217).
The hyplex SuperBug ID test system (AmplexDiagnostics) and the AID carbapenemase line probe assay (Autoimmun Diagnostika) are two commercially available PCR assays for detecting multiple carbapenemase genes, including blaNDM. These assays use reverse hybridizations following multiplex PCR. Both assays have 100% sensitivity and specificity for detecting blaNDM (218–220). A locked nucleic acid (LNA)-based quantitative real-time PCR assay has been developed to simultaneously detect multiple antimicrobial resistance genes, including blaNDM, directly from positive blood cultures but has been tested only on several NDM-positive strains (221). A long-fragment real-time quantitative PCR–combined in vitro protein expression (PCR-P) method has been developed for detection of blaNDM-1. PCR-P is able to detect blaNDM-1 variants that have led to changes of function by measuring rates of degradation of imipenem (222).
Loop-mediated isothermal amplification.
Loop-mediated isothermal amplification (LAMP) has been used for the rapid and sensitive detection of blaNDM (223, 224). LAMP does not require expensive thermocyclers, is quicker to perform than conventional PCR, and has been shown to exhibit higher sensitivity for detecting blaNDM (223–225). In addition, LAMP does not require gel electrophoresis, making it convenient in clinical laboratories. A commercially available LAMP system called Eazyplex (AmplexDiagnostics) has been evaluated for detecting carbapenemase genes in Acinetobacter (226, 227), Enterobacteriaceae (216, 227, 228), and P. aeruginosa (216, 227, 228), with 100% sensitivity and specificity for blaNDM. LAMP has been evaluated only for detecting blaNDM-1, and since only 4 or 6 primers can be used in LAMP, it may not be able to detect all blaNDM variants due to the possibility of nucleotide mutations occurring in primer binding regions. Due to the extremely high amplification efficiency of LAMP, extra care should also be taken to avoid contamination (223).
DNA microarray.
DNA microarrays can simultaneously detect a vast number of genes. DNA microarrays for detecting antimicrobial resistance genes, including blaNDM, have been developed (229) but may not be appropriate for specifically detecting blaNDM alone, as PCR and LAMP are less expensive and simpler molecular methods. In addition, detection of DNA hybridization with electrochemical impedance spectroscopy (EIS) has also been developed to specially detect blaNDM (196). DNA microarrays including blaNDM in the target panel have become commercially available, including the Verigene Gram-negative blood culture nucleic acid test (BC-GN; Nanosphere), Check-MDR CT102 and Check-MDR CT103 assays (Check-Points Health), and the CarbDetect AS-1 kit and CarbDetect AS-2 kit (Alere Technologies). BC-GN and Check-MDR CT102 detect genes encoding ESBLs and major types of carbapenemases (IMP, KPC, VIM, NDM, and OXA-48), while Check-MDR CT103 also targets genes encoding plasmid-mediated AmpC cephalosporinases on the basis of CT102. CarbDetect kits also target genes mediating resistance to aminoglycosides, quinolones, macrolides, sulfonamides, and trimethoprim. Both CT102 and CT103 have been evaluated using collections including NDM-positive strains and exhibit 100% sensitivity and specificity (230, 231). BC-GN has 96.2% sensitivity for detecting blaNDM and gives false-negative results for several NDM-positive strains (232). DNA microarrays have limitations, including high costs, long turnaround times, and inflexibility with respect to adding new targets once an array is established (233).
Whole-genome sequencing.
WGS is being increasingly used in health care settings. It can be used for many purposes, including detecting genes encoding antimicrobial resistance. The cost of next-generation WGS, commonly the MiSeq and HiSeq platforms (Illumina), has significantly dropped to typically $74 per bacterial genome in 2018. Several databases of antimicrobial resistance are available for detecting known antimicrobial resistance genes. The most-used examples are ResFinder (https://cge.cbs.dtu.dk/services/ResFinder/) (234), ARDB (Antibiotic Resistance Genes Database) (https://ardb.cbcb.umd.edu/) (which is not maintained at present) (235), and CARD (Comprehensive Antibiotic Resistance Database) (https://card.mcmaster.ca/) (236). By querying the databases, next-generation WGS can be used to detect all known antimicrobial resistance genes and any new variants of a certain gene, such as blaNDM, which could be missed by many other commonly used molecular methods such as real-time PCR and DNA microarrays. Genome sequences generated by WGS also allow precise species identification and strain typing for surveillance and tracking of the transmission of certain strains, critical for epidemiology and infection control (108, 237–239). The complete sequence of plasmids carrying certain antimicrobial resistance genes, such as blaNDM, can be further obtained using long-read sequencing platforms such as PacBio (Pacific Biosciences) and MinION (Nanopore). These platforms can provide complementary information on the transmission of antimicrobial resistance in addition to strain typing. MinION is portable and is particularly useful in health care settings, but the cost is still relatively high at present. Metagenomic sequencing has also been developed for sequencing total DNA directly from clinical samples. This can also detect antimicrobial resistance genes such as blaNDM. However, metagenomic sequencing needs much higher sequencing depth (costlier), and the analysis of metagenomic data is much more complex than WGS for single isolates (237). WGS is promising, but several major aspects, such as the cost, bioinformatics pipelines, and turnaround time, need to be improved before it can be used routinely for diagnosis and detection in health care settings (237).
TREATMENT OPTIONS AGAINST NDM-POSITIVE STRAINS
Treatment for CRE infections has been reviewed extensively (240–244). However, CRE strains included in previous studies are commonly KPC producers, or their type of carbapenemase has not been specified. In this review, we focus on treatment for NDM-positive strains rather than CRE as a whole.
Aztreonam-Avibactam or Aztreonam Combined with Ceftazidime-Avibactam
Although aztreonam is stable against MBLs, NDM-producing strains usually have ESBLs and/or AmpC enzymes that are able to hydrolyze aztreonam. Aztreonam alone therefore has limited clinical utility against NDM-producing strains. Avibactam is a non-β-lactam β-lactamase inhibitor with the ability to inhibit most serine β-lactamases, such as class A (e.g., KPC, CTX-M, TEM, and SHV), class C (AmpC), and some class D (e.g., OXA-48) enzymes. However, avibactam has no ability to inhibit MBLs, including NDM, and cannot effectively protect β-lactams from the hydrolysis of MBLs (245). The combination of avibactam with aztreonam can protect the latter from the hydrolysis of ESBLs and AmpC and therefore can expand the spectrum of aztreonam. The combination of aztreonam and avibactam has demonstrated potent in vitro activity against NDM-positive ESBL-producing Enterobacteriaceae (246). Aztreonam-avibactam is currently in clinical development and is not available for clinical use. Ceftazidime-avibactam has been approved for clinical use and is highly effective against KPC-positive CRE but has no activity against NDM-positive strains. In vitro studies have demonstrated synergistic activity and a bactericidal effect of the combination of ceftazidime-avibactam and aztreonam against CRE (247, 248). A small case series of 10 patients revealed that 7 of the patients survived after receiving the combination of aztreonam and ceftazidime-avibactam. The other three patients died as a result of other underlying conditions and therefore should not be considered a failure of the combination treatment (249). Several case reports have also demonstrated that the combination of aztreonam and ceftazidime-avibactam can successfully treat infections with NDM-positive CRE strains (247, 250). This combination could be considered if there are no alternative therapeutic options, and its efficacy warrants further, large-scale studies.
Polymyxins (Colistin and Polymyxin B) Alone and in Combination
Polymyxins are the current mainstay choice of antimicrobial agents against CRE and carbapenem-resistant A. baumannii, including NDM-positive strains. The treatment of infections due to multidrug-resistant organisms (MDRO) with polymyxins has been reviewed previously (251–253), and there are many studies on the efficacy of colistin against CRE, most of which have KPC rather than NDM (254, 255). In this review, we focus on treatment against NDM-positive strains only.
Polymyxin E (colistin) and polymyxin B have been evaluated for the treatment of infections caused by NDM-positive strains. NDM-positive strains are usually susceptible to polymyxins. There is a case report that colistin alone has been used successfully for treatment of a patient with a polymicrobial infection, including an NDM-positive Vibrio cholerae strain, with an increased dose (from 1 million units [MU] to 2 MU three times a day) and monitoring serum concentrations (256). However, an in vitro time-kill assay revealed that although initial killing of bacterial cells could be achieved by colistin alone, considerable regrowth occurs at 24 h, and resistant subpopulations are frequently detected after exposure to colistin alone (257). A murine infection model demonstrated that unlike in combination with amikacin, colistin alone is unable to achieve 1.5- to 2.8-log10 killing after 24 h of therapy (258). Another study using murine models revealed that colistin alone was inappropriate for treating pneumonia due to NDM-positive K. pneumoniae, although the strain was susceptible to colistin in vitro (259). The use of colistin has also been hampered by the occurrence of renal toxicity (260, 261) and, to a lesser extent, neurological adverse effects (262). Many NDM-positive Enterobacteriaceae strains have become resistant to colistin by acquiring plasmid-borne mcr genes or have mutations/interruptions of chromosomal genes such as phoP-phoQ (encoding a two-component system) and mgrB (a regulator of phoP-phoQ) (263–265).
Evidence suggests that treatment with colistin-based combinations may offer a benefit compared to colistin alone. Colistin is usually recommended in combination with other agents for treatment (266). Although various colistin-based combinations have been examined in vitro and occasionally in vivo, it remains unclear what the best combination is, as studies are usually done with small sample sizes or single case reports, are heterogeneous in methodology, and have generated inconsistent findings.
A patient with complicated health care-associated cystitis due to an NDM-positive E. coli strain recovered after receiving colistin and rifampin (267). The combination of colistin, rifampin, and meropenem was successfully used to treat pyoderma caused by multiple microorganisms, including an NDM-positive E. coli strain, in a 49-year-old male patient (268). A patient with acute pyelonephritis due to NDM-positive P. aeruginosa recovered when treated with colistin combined with aztreonam (269). Polymyxin B in combination with aztreonam and meropenem rescued a 65-year-old patient with acute myeloid leukemia from bloodstream infection caused by an NDM-positive K. pneumoniae strain (270).
The colistin-tigecycline combination was not bactericidal against two NDM-positive colistin-susceptible K. pneumoniae strains in a 24-h time-kill assay (271). Another study revealed that the addition of tigecycline to colistin did not produce increased bacterial killing. Instead, it may cause antagonism at lower concentrations (263). In a study including 28 NDM-positive Enterobacteriaceae strains, in vitro synergistic activity was observed with colistin plus tigecycline in very rare cases (272).
The combination of colistin and fosfomycin achieved increased bacterial killing and decreased the chance of emergence of resistance compared to either agent alone in 6 NDM-positive Enterobacteriaceae strains (273). Synergistic or bactericidal activity is present for fosfomycin and colistin in a 24-h time-kill assay (271). However, in another study including 28 NDM-positive Enterobacteriaceae strains, in vitro synergistic activity was observed for colistin plus fosfomycin only in very rare cases (272).
A time-kill assay revealed that the addition of amikacin is able to restore the susceptibility of four NDM-positive and mcr-positive E. coli strains to colistin (258). However, another time-kill assay demonstrated that the combination of amikacin and polymyxin B failed to eradicate NDM-positive and mcr-positive E. coli strains but that the addition of aztreonam with amikacin and polymyxin achieved eradication (274).
Tigecycline
The susceptibility of NDM-positive Enterobacteriaceae strains to tigecycline varies significantly. Some studies report that these strains are mostly susceptible to tigecycline in vitro (275, 276), while a multicenter study in China found that most NDM-positive strains were nonsusceptible to tigecycline (38). Nonetheless, tigecycline does not have desirable pharmacokinetic properties and is a bacteriostatic agent. There are concerns that in vitro susceptibility to tigecycline may not be translated to in vivo efficacy (277). A previous study demonstrated that treatment with tigecycline was associated with high mortality rates (40.1%) when used for treatment of infections due to CRE (most of which are likely to have KPC rather than NDM), whereas the mortality rate for inactive therapy was 46.1% (278). Both in vivo and clinical studies on tigecycline against NDM-positive strains are scarce. In an in vivo study using a murine infection model, a high-dose tigecycline scheme was effective for treating pneumonia due to NDM-positive E. coli and K. pneumoniae and was more active than colistin (259). The combination of tigecycline and levofloxacin was used to successfully treat a patient with hospital-acquired pneumonia caused by NDM-positive Raoultella planticola (279). However, during treatment against NDM-positive strains, high-level tigecycline resistance can emerge rapidly (280). Nonetheless, these limited data fail to provide a convincing argument for or against the use of tigecycline for treating infections due to NDM-positive strains, and further studies are therefore required.
Eravacycline
Eravacycline is a novel synthetic fluorotetracycline with potency that is 2- to 4-fold higher than that of tigecycline in NDM-positive strains. In an in vitro analysis of 2,644 Gram-negative pathogens, eravacycline demonstrated excellent activity against 18 E. coli strains that had carbapenem resistance associated with OXA and NDM (281). Eravacycline was approved for treating adults with complicated intra-abdominal infections by the FDA in August 2018. Eravacycline is also currently being tested against complicated urinary tract infections in a clinical trial (ClinicalTrials.gov registration no. NCT01978938).
Dual Carbapenems
The rationale for using two carbapenems together is to provide a competitive substrate for the β-lactamase. These studies are mainly aimed at CRE as a whole, most of which have serine-based β-lactamases, such as KPC and OXA-48, rather than NDM.
Carbapenem MICs for carbapenemase-producing K. pneumoniae isolates may vary within a broad range of values, from 0.12 to >256 mg/liter. This variation depends on the clonal background of the bacterial isolates and the type of carbapenemase produced. Isolates producing NDM usually have high carbapenem MICs (≥32 mg/liter) (33, 282). This has made dual-carbapenem-based treatment more challenging for NDM-positive strains.
Twenty carbapenem-resistant K. pneumoniae (CRKP) clinical strains, 6 of which were NDM positive, were tested with dual-carbapenem (any two of doripenem, ertapenem, imipenem, and meropenem) combinations. The data strongly support the hypothesis that dual-carbapenem combinations might be effective against KPC and OXA-48, but no synergy was observed for any of the NDM-positive strains (283), which might be due to the different action mechanism of MBLs compared to those of serine-based carbapenemases (15). NDM-positive Enterobacteriaceae were tested in immunocompetent and neutropenic murine thigh infection models using humanized regimens of standard (500 mg given every 8 h) and high-dose, prolonged infusion (2 g given every 8 h; 4-h infusion) of doripenem and 1 g of ertapenem given intravenously every 24 h. Doripenem and ertapenem demonstrated efficacy against several NDM-positive strains, especially using high-dose and prolonged infusion (284). The findings in this in vivo study are inconsistent with those of the in vitro study (283). Dual carbapenems (meropenem and ertapenem) plus fosfomycin were used to successfully treat urinary tract infections caused by NDM-positive Enterobacteriaceae in two patients (285). In general, there are very few studies of dual carbapenems against NDM-positive strains, and the current evidence is contradictory. More studies are therefore required.
Aminoglycosides
Susceptibility to aminoglycosides may be unpredictable and can vary according to the strain type. Aminoglycosides may be considered a viable option for combination therapy against NDM-positive strains. Plazomicin is a synthetic derivative of sisomicin that evades many aminoglycoside-modifying enzymes but is not active against bacterial strains having 16S rRNA methyltransferases (286). However, NDM-positive strains are usually resistant to plazomicin (MIC ≥ 64 mg/liter) (287). Apramycin is of the 4-monosubstituted deoxystreptamine (DOS) subclass and is active against ribosomes modified by all 16S rRNA methyltransferases except NpmA (288), which is not common in the Enterobacteriaceae. An in vitro study demonstrated that almost 90% of NDM-positive strains are susceptible to apramycin (289). However, apramycin is a veterinary agent and has not been approved for clinical use, likely due to its narrow therapeutic index (287). Apramycin therefore warrants further investigations as a repurposed agent against CRE, including NDM-positive strains.
Fosfomycin
Fosfomycin is available as an oral agent in the United States and is also available for intravenous use in Europe and China. Few large-scale studies have addressed the susceptibility of NDM-positive Enterobacteriaceae to fosfomycin (267). Sufficient data are lacking to support the use of fosfomycin alone, but fosfomycin-containing combination therapy has demonstrated promising results, as mentioned above (273).
New Antimicrobial Agents in Development
Cefiderocol.
Cefiderocol (S-649266) (the chemical structure is shown in Fig. S1 in the supplemental material) is a novel catechol siderophore cephalosporin and is usually stable against the hydrolysis of carbapenemases, including MBLs (290). When tested on 49 NDM-producing strains, MICs for most strains (44/49; 89.8%) were 2 to 4 mg/liter, but those for 5 strains were ≥16 mg/liter. Although breakpoints to define susceptibility have not been established, the high MICs suggest that the 5 strains were likely resistant to cefiderocol. Cefiderocol demonstrated bactericidal activity against an NDM-1-producing K. pneumoniae strain in a rat lung infection model (the cefiderocol MIC was 8 mg/liter) (291). A phase 2 randomized study demonstrated that cefiderocol was noninferior to imipenem for treating patients with complicated urinary tract infections caused by carbapenem-susceptible Gram-negative bacteria (292). Cefiderocol is currently being tested for the treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria and nosocomial pneumonia caused by Gram-negative bacteria in two clinical trials (ClinicalTrials.gov registration no. NCT02714595 and NCT03032380).
LYS228.
LYS228 (Fig. S1) is a novel monobactam stable against the hydrolysis of MBLs and serine carbapenemases. In vitro studies revealed that it has potent activity against the majority of ESBL-producing Enterobacteriaceae and CRE strains tested, including NDM-positive ones (293). LYS228 is currently being tested for treating patients with complicated intra-abdominal infections in a clinical trial (ClinicalTrials.gov registration no. NCT03354754).
Odilorhabdins.
Odilorhabdins are a new antibiotic class against both Gram-positive and Gram-negative pathogens, which act on ribosomes to inhibit bacterial translation (294). A compound of this class, named NOSO-502 (Fig. S1), has activity against CRE strains producing NDM, KPC, AmpC, or OXA enzymes (295), demonstrated using a murine systemic infection model (296).
Piscidins.
Tilapia piscidin 3 (TP3) and tilapia piscidin 4 (TP4) are two antimicrobial peptides (Fig. S1) from fish that exhibit strong activity against NDM-positive K. pneumoniae in vitro. Administration of TP3 (150 μg/mouse) or TP4 (50 μg/mouse) is able to significantly increase survival in a murine sepsis model, and TP4 was more effective than tigecycline at reducing CFU counts in several organs. TP3 and TP4 were shown to be nontoxic and have the potential for use in combating NDM-positive strains (297).
Photoactivated 2,3-distyrylindoles.
A compound based on the 2,3-distyrylindole scaffold has been found to exhibit activity against various MDR Gram-negative bacteria, including NDM-positive Enterobacteriaceae (298). The compound exhibited bactericidal properties at a concentration of 5 μM and in the presence of colistin at nonbactericidal concentration of 1.25 μg/ml. This resulted in a 7- to 9-log reduction in bacterial counts of NDM-positive Enterobacteriaceae via disruption of the bacterial cell membrane (298). The photoactivated 2,3-distyrylindole-based compounds may have the potential to be applied topically.
Broad-Spectrum β-Lactamase Inhibitors
VNRX-5133.
VNRX-5133 (see Fig. S1 in the supplemental material) is a second-generation boronate in development with cefepime. It is a new broad-spectrum β-lactamase inhibitor with direct inhibitory activity against Ambler class A, B (including NDM and VIM but not IMP), C, and D β-lactamases. However, MICs of cefepime–VNRX-5133 (1:1 ratio) for some NDM-producing strains are around 8 mg/liter (299). VNRX-5133 is currently being tested for safety, pharmacokinetics, and drug-drug interactions in several clinical trials (ClinicalTrials.gov registration no. NCT03690362, NCT02955459, and NCT03332732).
Other Non-β-Lactam Serine β-Lactamase Inhibitors in Development
Relebactam (MK7655) (300) and nacubactam (RG6080 or OP0595) (301) are two non-β-lactam β-lactamase inhibitors (see Fig. S1 in the supplemental material), like avibactam, and can inhibit serine β-lactamases but cannot inhibit MBLs. Relebactam and nacubactam are being developed in combination with imipenem and meropenem, respectively, and imipenem-relebactam has successfully completed a phase 3 trial. Although relebactam and nacubactam have no activity against NDM-positive strains, their combination with aztreonam may have potential, like aztreonam-avibactam (see above), against NDM-positive strains.
MBL Inhibitors
An attractive strategy to combat NDM-positive bacteria is to develop MBL (including NDM) inhibitors. A number of NDM inhibitors of various classes have been identified and characterized, but none of the compounds have been approved for clinical use thus far. The inhibitors have been summarized in several excellent reviews (19, 302, 303). In this review, we summarize these inhibitors, including those that have been developed in the past 3 years, in Table 4.
TABLE 4.
NDM inhibitors reported
| Inhibitor(s) | Description | Reference(s) |
|---|---|---|
| Sulfur-containing inhibitors | ||
| Bisthiazolidines | Penicillin analogs containing thiol | 404 |
| Captopril and various analogs | Angiotensin-converting enzyme inhibitor | 405, 406 |
| Thiorphan | Active metabolite of racecadotril, a peripherally acting enkephalinase inhibitor | 407 |
| Thiophenecarboxylic acid derivatives | Sulfur atom of the thiophene ring | 408 |
| Thioazoles and thiophene-containing amino acid thioesters | 409, 410 | |
| Tryptophan-containing compound | Thiol-containing amides | 411 |
| Sulfonamides | 412 | |
| Thiol-containing compounds | 413 | |
| ANT431 | Pyridine-2-carboxylic acid derivative | 304 |
| Metal-complexing agents | ||
| 1,4,7-Triazacyclononane-1,4,7-triacetic acid | Metal chelators | 414 |
| 1,4,7,10-Tetraazacyclononane-1,4,7,10-tetraacetic acid | Metal chelators | 414 |
| Calcium disodium EDTA | Metal chelators | 306, 415, 416 |
| ME1071 (disodium 2,3-diethylmaleate) | Maleic acid derivative | 287 |
| Colloidal bismuth subcitrate and related Bi(III) compounds | Anti-Helicobacter pylori drug | 417 |
| Inhibitor 36 | 2,6-Dipicolinic acid derivative | 418 |
| Aspergillomarasmine A | Extract of a strain of the fungus Aspergillus versicolor | 305 |
| Chromenes | ||
| Chromenone compound | 419 | |
| Chromenone and chromeno[3,2-c]pyridine compound | 2 polyketides isolated from Penicillium sp. from the rhizosphere soil of the plant Picea asperata | 420 |
| 3-Formylchromone | 421 | |
| 3-Cyanochromone | 421 | |
| Thiol-modifying agents | ||
| p-Chloromer-curibenzoic acid and sodium nitroprusside [Na2Fe(CN)5NO·2H2O] | Thiol-modifying agents | 422 |
| Ebselen | Selenium-containing molecule | 423 |
| Covalent irreversible inhibition by β-lactams | ||
| Supratherapeutic doses of β-lactams such as cephalothin and moxalactam | Key residue for interaction with the substrate | 424 |
| β-Phospholactam | Tetrahedral transition state analog | 425 |
| 4-Chloroisoquinolinols | 426 | |
| Aminoimidazoles | 427 | |
| Synthetic nucleotide analogs | ||
| Peptide-conjugated phosphorodiamidate morpholino oligomer | 428 | |
Many of the NDM inhibitors have been tested only in vitro. However, several, including ANT431 (304), aspergillomarasmine A (305), calcium disodium EDTA (306), and colloidal bismuth subcitrate (CBS) (an anti-Helicobacter pylori drug) (307), have also been studied using in vivo models and have been shown to be efficacious. None of the NDM inhibitors have been approved for clinical use yet.
Antimicrobial Adjuvants in Development
With limited treatment options available, we are in urgent need of new therapeutic options. One approach to combat this growing problem is the use of combinations containing adjuvants. Antimicrobial adjuvants include not only antimicrobial agents but also compounds from plants.
Organic acid.
The antibacterial activity of organic acids and their combinations against NDM-positive E. coli was tested by a disc diffusion method. The MIC of colistin is reduced from 8 to 0.5 µg/ml in the presence of 320 µg/ml oxalic and succinic acids. The addition of oxalic and succinic acids may have the potential for reducing the dose of colistin (308).
Macromolecules.
Membrane-active macromolecules (MAMs) have been found to enhance the uptake of tetracycline by bacterial cells and therefore are able to resensitize NDM-positive strains to tetracycline (309). The MAM-tetracycline combination was bactericidal, in contrast to the bacteriostatic effect of tetracycline alone (309).
Copper ions and coordination complexes.
A study found that copper is able to directly inhibit the activity of NDM-1. Copper shows synergy with ertapenem and meropenem against NDM-positive E. coli in standard checkerboard assays. The synergy between copper and carbapenems has also been confirmed using a low concentration (10 μM) of copper with the FDA-approved copper-pyrithione coordination complex. Copper coordination complexes therefore have potential as novel carbapenemase adjuvants (307).
Zidebactam and WCK 5153.
Zidebactam and WCK 5153 are novel β-lactam enhancers that are bicyclo-acyl hydrazides (BCHs), derivatives of the diazabicyclooctane (DBO) scaffold. They are targeted for the treatment of serious infections caused by MDR Gram-negative pathogens, including NDM-positive strains. Zidebactam and WCK 5153 exhibit specific penicillin binding protein 2 (PBP2) inhibition but do not inhibit MBLs. Time-kill assays and live-dead staining revealed the bactericidal activity of zidebactam and WCK 5153. Zidebactam and WCK 5153 restored susceptibility to β-lactams in P. aeruginosa mutant strains and represent a promising β-lactam “enhancer-based” approach to treat MDR P. aeruginosa infections, bypassing the need for MBL inhibition (310). The combination of cefepime and zidebactam showed potent activity against Enterobacteriaceae and P. aeruginosa strains producing various clinically relevant β-lactamases, including ESBLs, KPCs, AmpC, and MBLs (including NDM) (311).
Plant derivatives.
In one particular study of note, ethanol extracts from the leaves of 240 medicinal plant species were screened for antibacterial activity (312). The extracts from Combretum albidum G. Don, Hibiscus acetosella Welw. ex Hiern, Hibiscus cannabinus L., Hibiscus furcatus Willd., Punica granatum L., and Tamarindus indica L. inhibited the NDM-1 enzyme in vitro and showed synergistic effects when combined with colistin (312). In another study, magnolol, from the bark of magnolia trees, significantly inhibited NDM-1 enzyme activity and was able to restore the activity of meropenem against NDM-positive E. coli (28). Molecular modeling and a mutational analysis demonstrated that magnolol binds directly to the catalytic pocket of NDM-1, thereby blocking the binding of the substrate to NDM-1 and leading to its inactivation (28).
Immunotherapeutic Agents
Nonantimicrobial immunotherapeutic agents represent a new, alternative way to treat infections due to NDM-positive strains. Two recombinant human cysteine proteinase inhibitors, cystatin 9 (rCST9) and cystatin C (rCSTC), have been tested using murine pneumonia models of infection with NDM-positive K. pneumoniae (313). That study demonstrated that the combination of rCST9 and rCSTC led to significantly improved survival compared to that of infected mice treated with one of the inhibitors or without treatment (313). This suggests that rCST9-rCSTC is a promising therapeutic candidate for treating bacterial pneumonia (313).
INFECTION PREVENTION AND CONTROL FOR NDM-POSITIVE STRAINS
Outbreaks
Many outbreaks caused by NDM-producing strains of Enterobacteriaceae and A. baumannii have been reported in the literature (Table 5). Many reports describe only the microbiological aspects of the outbreak and fail to provide full information regarding outbreak investigations. The source of outbreaks has often been identified as a contaminated near-patient environment or communal medical device (Table 5). Outbreaks have also been seen in patients sharing rooms with those infected or colonized by NDM-positive strains (314, 315).
TABLE 5.
Outbreaks of NDM-positive strains reported in the literaturea
| Yr(s) | Country | Unit(s) of isolation | Age group | Infection site and/or specimen type | Species | ST(s) | No. of cases | Source(s) | Control measure(s) | Reference(s) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2014–2015 | Belgium | / | / | / | K. pneumoniae | ST716 | 29 | / | / | 429 |
| 2012 | Bulgaria | ICU, hepatology | / | / | E. coli | ST101 | 12 | / | / | 430 |
| 2015–2016 | Bulgaria | ICU, other wards | / | Abscess, BSI, pneumonia, UTI | K. pneumoniae | ST11 | 14 | / | Frequent hand hygiene audits; educational session at the ward level; microbiological investigation of HCWs, equipment, and hospital environment; additional disinfection in affected units; and patient cohorting or isolation when available | 316 |
| 2011 | Canada | Respiratory | Adult | Blood, wound | K. pneumoniae | ST231 | 5 | / | Standard control measures, including active screening | 323 |
| 2011 | Canada | / | / | BSI, UTI, colonization | K. pneumoniae | / | 7 | Environment contamination | Contact precautions, screening of contacts, and strengthening of environmental cleaning | 314 |
| 2011–2012 | China | / | Adult | VAP | K. pneumoniae | ST147 | 3 | / | / | 431 |
| 2012 | China | Neonatal | Neonate | Blood, sputum | K. pneumoniae | ST17, ST20 | 18 | Bed railing | / | 432 |
| 2012–2013 | China | Neonatal | Neonate | Pneumonia, sepsis | K. pneumoniae | ST17 | 7 | / | / | 433 |
| 2014 | China | Hospital wide, mainly NICU and neonatal | Neonate | Sputum, urine, colonization | K. pneumoniae | ST37, ST76 | 22 | / | Strengthening standard control measures | 317 |
| 2014 | China | Neonatal | Neonate | Pneumonia, sepsis | K. pneumoniae | ST1419 | 5 | / | / | 434 |
| 2014 | China | NICU | Neonate | Blood, sputum, stool | K. pneumoniae | ST105 | 17 | Incubator water | / | 435 |
| 2015 | China | Neonatal | Neonate | Aspiration catheter, BSI, UTI | K. pneumoniae | ST20 | 4 | Radiant warmer and nurses’ hands | Strengthening control measures, including monitoring hand hygiene compliance by video; placing patients in single rooms; extensive cleaning of shared equipment; and maximum use of disposable materials | 318 |
| 2015 | China | / | Adult | / | E. cloacae complex | ST88 | 4 | / | / | 436 |
| 2011–2012 | Colombia | Neonatal | Neonate | BSI, necrotizing enterocolitis | K. pneumoniae | ST1043 | 6 | / | Hand hygiene, patient isolation, contact precautions, supervised disinfection | 324 |
| 2013 | Denmark | Hematology | / | UTI, wound | C. freundii | ST18 | 7 | / | Strengthening control measures, including isolation, contact precautions, screening for colonizers and contaminated environment, and cleaning | 325 |
| 2013 | France | SICU | Adult | BSI, pneumonia, colonization | A. baumannii | ST85 | 7 | / | / | 437 |
| 2010 | Greece | Hematology | / | / | K. pneumoniae | ST11 | 4 | / | / | 48 |
| 2010 | Greece | Hospital wide | / | Mainly blood or urine | K. pneumoniae | ST11 | 67 | / | / | 48 |
| 2009 | India | Neonatal | Neonate | BSI | E. coli | / | 4 | / | / | 438 |
| 2012 | India | NICU | Neonate | Sepsis | K. pneumoniae | / | 6 | / | / | 439 |
| 2015 | Iran | ICU | / | / | K. pneumoniae | ST893 | 29 | / | / | 440 |
| 2014–2015 | Ireland | Hemodialysis | Adult | UTI, colonization | K. pneumoniae | / | 9 | / | Measures including screening contacts, distributing information leaflets, using long-sleeved disposable gowns and gloves, single-room isolation, chlorhexidine bathing, flagging on the ICNet system, education for HCWs, hand hygiene audit, and enhanced cleaning (twice daily) | 319) |
| 2011 | Italy | Neonatal | Neonate | Bile, sputum, urine | K. pneumoniae | / | 6 | / | / | 47 |
| 2013 | South Korea | Geratology | Elderly | Colonization | E. coli | ST101 | 4 | Sharing room | Strengthening control measures, including contact precautions and rigorous cleaning | 315 |
| 2016–2017 | South Korea | SICU | / | CAUTI | Providencia rettgeri | / | 8 | / | Strengthening control measures, including isolation, contact precautions, and environmental cleaning | 322 |
| 2014 | Kuwait | / | Adult | Blood, CVC, tip tissue | K. pneumoniae | / | 3 | / | / | 441 |
| 2012 | Nepal | Nurseries, NICU, PICU | Child, neonate | Mainly blood | K. pneumoniae | ST15 | 31 | / | Closing NICU and PICU | 333 |
| 2012 | Nepal | HDU | Child | Blood | K. pneumoniae | ST1559 | 10 | / | / | 333 |
| 2013 | Mexico | ICU | Adult | / | K. pneumoniae | ST22 | 3 | / | Using hydrogen peroxide-based disinfection | 326 |
| 2014–2015 | Mexico | / | / | / | K. pneumoniae | ST392 | 28 | / | / | 442 |
| 2012–2013 | Nepal | Neonatal | Neonate | Blood, urine | E. cloacae complex | / | 19 | Contaminated soap dispensers | / | 443 |
| 2015 | Netherlands | / | / | / | K. pneumoniae | / | 29 | / | / | 330 |
| 2015 | Netherlands | / | / | / | K. pneumoniae | ST873 | 26 | / | / | 90 |
| 2014 | Singapore | General | Adult | UTI, colonization | E. cloacae complex | / | 4 | Via health care staff | Strengthening control measures, especially pertaining to hand hygiene and ward staffing | 320 |
| 2014–2015 | Tunisia | / | Adult, child | Blood, catheter, urine, wound | K. pneumoniae | ST147, ST307 | 19 | / | / | 444 |
| 2016 | Turkey | ICU, NICU | Adult, neonate | BSI, empyema, tracheal aspirate, UTI | K. pneumoniae | ST11 | 6 | / | / | 6 |
| 2010 | UK | / | Adult | UTI | Klebsiella spp. | 12 | Urology endoscopic camera head | Sterilization or use of single-use sterile disposable plastic camera sheaths | 445 | |
| 2014–2015 | UK | / | / | / | K. pneumoniae | / | 40 | / | Enhanced screening (including renal outpatients), contact precautions, enhanced chlorine disinfection of the environment, labeling of electronic case notes for identification of readmission, regular teleconference calls internally and externally, and enhanced antimicrobial stewardship; later, hydrogen peroxide vapor and ward-based monitoring of hand and environmental hygiene were implemented | 321 |
| / | UK | / | Adult | / | E. cloacae complex | / | 3 | Ceramic sluice sink to dispose of waste material | Replacement of the whole sink unit in the sluice, control bundle | 446 |
| 2012 | USA | Burn/trauma ICU, SICU, cardiology, and rehabilitation | Adult | Pneumonia, colonization | K. pneumoniae | / | 8 | Timely surveillance cultures combined with targeted control measures | 447 | |
| 2013 | USA | / | Adult | / | E. coli | / | 48 | Duodenoscopy | Change of the reprocessing procedure from automated high-level disinfection to gas sterilization with ethylene oxide | 328, 329 |
Abbreviations: BSI, bloodstream infection; CAUTI, catheter-associated urinary tract infection; CVC, central venous catheter; HCWs, health care workers; HDU, high-dependence unit; SICU, surgical intensive care unit; NICU, neonatal intensive care unit; PICU, pediatric intensive care unit; VAP, ventilator-associated pneumonia; UTI, urinary tract infection; /, no information provided.
Outbreaks are generally controlled by strengthening commonly used infection control measures, such as contact precautions, hand hygiene, environmental cleaning, isolation, and active surveillance, with or without implementing additional measures (314–325). It has been reported that outbreaks due to CRE have been effectively controlled by enhanced environmental disinfection using hydrogen peroxide (326). Particular efforts should be taken to investigate possible environmental reservoirs during outbreaks (327), and if the source is identified, targeted measures are required to control the outbreak. For instance, duodenoscopes have been identified as the contamination source for several outbreaks, which were subsequently controlled by enhanced cleaning and a change from high-level disinfection to gas sterilization (328, 329). A “search-and-destroy” approach has been successfully used to control outbreaks but can be costly (330). The sources of outbreaks may not be identified in many cases. In such cases, the entire environment of affected wards may become heavily contaminated, and temporary ward closures have been recommended for enhanced cleaning and limiting the further spread of the pathogen (327, 331–333). External audits of infection control measures and extensive health service-wide education have also been recommended (334). If outbreaks persist despite the implementation of measures, screening of health care workers for carriage of MDRO is advised (332, 334), but the true relative value of this exercise is yet to be proven.
Infection Prevention and Control
The reservoirs and the mode of transmission of MDRO, including NDM-positive strains, are largely underinvestigated (335). Based on outbreak investigations, the source of NDM-positive strains in health care settings has been suggested to be patients infected or colonized with the strains or contaminated environment or devices (Table 5) (336). Unlike blaKPC-2, the spread of blaNDM is largely due to horizontal transfer, which is mainly mediated by plasmids but can also be mediated by OMVs, at least in Acinetobacter (60). The transmission of NDM-positive strains in health care settings is believed to be due to close contact (331). Hospitalized patients are the most vulnerable populations for infection or colonization by NDM-positive strains. Healthy persons can also acquire NDM-positive strains, although colonization is usually for a short period of time (less than 1 month, shorter than the period seen for hospitalized patients [see below]) (57).
Several guidance documents are available to address the prevention and control of carbapenem-resistant Gram-negative bacteria in health care settings. The CDC’s guidance for the control of CRE in health care facilities was published in 2012 and updated in 2015 (336). The European Centre for Disease Prevention and Control (ECDC) issued its guidance on prevention of the entry of CRE into health care settings in 2017 (337). The World Health Organization (WHO) also released global guidance on the prevention and control of carbapenem-resistant Enterobacteriaceae, A. baumannii, and P. aeruginosa in 2017 (331). The WHO guidance also identified research gaps and the need for further research. Measures (summarized in Appendix S2 in the supplemental material) recommended by these guidance documents are evidence based and are overall highly similar (Table 6), although the emphasized points and the terminology are slightly different. In addition to the guidance documents, the Society for Healthcare Epidemiology of America (SHEA) established a step-by-step strategic roadmap for CRE infection control based on evidence and expert opinion (327). This roadmap has six successional steps, including determining whether CRE have been isolated, determining affected wards and the occurrence of intrafacility transmission, implementing early CRE detection and CRE containment measures, enhancing existing infection control requirements, regional strategy, and investigating for community spread of CRE, which should be applied in different situations, such as sporadic cases, single-hospital outbreaks, and settings of endemicity (327). However, as the WHO guidance has identified, the quality of the evidence for the recommended measures in guidance is usually very low or low (331). The efficacy of the recommended measures within the guidelines remains to be verified in health care settings. In resource-limited settings, such as health care institutions in the developing world, overcrowding, shortage of staffing, and poor hygiene may be the critical factors contributing to the spread of NDM-positive strains. Such factors may not be addressed by studies that have been performed in developed countries and have been included as evidence during guideline development.
TABLE 6.
Infection control measures for carbapenem-resistant Enterobacteriaceae and other Gram-negative bacteriaa
| Measure(s) | Recommendation in guidance by: |
||
|---|---|---|---|
| CDC 2015b | ECDC 2017c | WHO 2017d | |
| Hand hygiene | + | + | + |
| Antimicrobial stewardship | + | + | − |
| Health care personnel education | + | − | − |
| Monitoring, auditing, and feedback | − | − | + |
| Minimization of use of invasive devices | + | − | − |
| Microbiological capacity | − | + | − |
| Notification from laboratory | + | − | − |
| Contact precautions | + | + | + |
| Patient isolation or cohorting | + | + | + |
| Nurse cohorting | + | + | − |
| Active surveillance for patients | + | + | + |
| Screening contacts | + | + | − |
| Surveillance cultures of the environment | − | − | + |
| Environmental cleaning | + | − | + |
| Communication at discharge and transfer | + | + | − |
| Chlorhexidine bathing | + | − | − |
CDC and ECDC guidance documents are only for CRE, while WHO guidance also covers carbapenem-resistant A. baumannii and P. aeruginosa. “−” does not mean its absence in the guidance but indicates that it has not been highlighted as an independent measure.
See reference 336.
See reference 337. ECDC guidance is only for the prevention of the entry of CRE and does not address the control measures for patients with CRE infection or colonization.
See reference 331.
There are several other major challenges to the prevention and control of colonization and infection due to NDM-positive strains in health care settings. One such challenge is long-term carriage (colonization) in human hosts. It is well known that CRE can colonize the human gut for extended periods of time (338, 339), with reports of colonization for more than 3 years (340). Although studies on the duration of colonization usually do not specifically target NDM-positive strains, prolonged colonization by an NDM-positive strain for months was previously reported (341). A study in South Korea reveals that NDM-positive strains are difficult to clear, as only 11.3% (12/106) of colonized patients had no such strains upon discharge from the hospital (342). These colonized patients could serve as cryptic sources of further transmission of NDM-positive strains. Another particular challenge for infection control is the implementation of recommended measures. Even within a single geographic area in developed countries, the infection control strategies and practices against CRE vary remarkably (343). As stated in the WHO guidance (331), compliance should be monitored and analyzed to identify barriers to the implementation of countermeasures. A strategic roadmap for the control of CRE based on the best available evidence and expert opinion has been developed to facilitate implementation (327), and the WHO guidance has also detailed ways to help implementation (331).
The WHO guidance states that the same infection control measures should apply to carbapenem-resistant organisms regardless of the resistance mechanisms (331). NDM- and KPC-positive strains have some important differences, however. Unlike the well-known international spread of KPC-positive strains of K. pneumoniae clonal complex 258 (including ST258, ST11, and a few closely related STs), the spread of NDM-positive strains is less clonal, as there is no sustained global spread of certain high-risk clones. The spread of blaNDM is primarily mediated by plasmids. blaNDM is mainly seen in the Enterobacteriaceae, which usually colonize the gut of warm-blooded animals, including humans. blaNDM has been found in various species of the Enterobacteriaceae. This suggests that the human gut may serve as a hot spot for the spread of blaNDM in health care settings. However, the predisposing factors of colonization by NDM-positive strains remain largely underinvestigated. The avoidance of disturbance of the gut microflora and selective decontamination may be effective measures against NDM-positive strains and warrant further investigation.
CONCLUDING REMARKS
NDM-positive strains are continuing to spread worldwide despite continuous efforts and remain a critical challenge for clinical treatment and a significant threat to public health. The wide dissemination of blaNDM genes is largely mediated by certain plasmids, particularly those of the IncX3 type. NDM enzymes are continually evolving to generate new variants, some of which have obtained enhanced carbapenemase activity. No treatment targeting NDM has been approved for clinical use at present, although many new agents with various mechanisms of action are under investigation or in development. Polymyxins remain the mainstream choice to treat infections caused by NDM-positive strains at present, while aztreonam-avibactam is a promising alternative option. The ultimate success of the fight against NDM also relies on effective infection control practice in addition to the development of antimicrobial agents. Awareness toward infection control and compliance with control measures still need to be significantly enhanced in health care settings.
There are a few notable research gaps regarding NDM enzymes and NDM-positive strains. First, very few studies have addressed the epidemiology of NDM-positive strains worldwide. Ten years after their initial discovery, we are still not sure whether NDM-positive strains are continuing to increase in prevalence, have reached a plateau, or are decreasing. Second, beyond health care settings, the spread of NDM-positive strains in the community remains unclear, although there are several reports that NDM-positive strains have been detected in healthy individuals (344). Third, the exact origin of blaNDM remains unknown. Is it from the chromosome of a bacterial species, like many other β-lactamase genes such as blaCTX-M? If so, what is the species? Fourth, although it is well known that infections caused by CRE are associated with high mortality rates (345, 346), CRE mortality studies usually refer to KPC-positive strains, or the carbapenemase type has not been specified. It is surprising that studies on the impact of the presence of NDM on mortality are scarce, and such studies are much needed. Fifth, the optimal treatment of infections caused by NDM-positive strains remains to be determined. Although polymyxins remain the mainstream choice, there are intrinsic limitations, such as toxicities, the absence of optimal dosage schemes, and the presence of heterogeneous resistance. There are still no treatments specifically targeted toward NDM. Sixth, almost all recommended infection control measures against carbapenem-resistant Gram-negative bacteria are based on low- or very-low-quality studies. Therefore, more well-designed prospective studies are needed to establish more-targeted and effective measures. Finally, although several guidance documents have stated that CRE should be regarded as a whole in terms of infection control, NDM- and KPC-positive strains have significant differences. Whether targeted measures should be established to control NDM-positive strains and what these measures might be remain to be determined.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a grant from the National Natural Science Foundation of China (project no. 81772233) and a joint grant from the National Natural Science Foundation of China (project no. 81661130159) and the Newton Advanced Fellowship, Royal Society, United Kingdom (NA150363). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
We declare no conflict of interest.
Biographies

Wenjing Wu, M.Med., M.B.B.S., obtained her master’s degree from Sichuan University and her M.B.B.S. from Luzhou Medical University (Sichuan, China). She is developing her Ph.D. thesis about the epidemiology and clonal background of carbapenemase-producing Enterobacter spp. at the Center of Infectious Diseases, West China Hospital, Sichuan University.

Yu Feng, H.B.Sc., a young scientist who has been working at the Center of Infectious Diseases, West China Hospital of Sichuan University, as a research assistant since 2014, received 4 years of training in the fields of microbiology, genetics, and biochemistry from the University of Adelaide, Australia, and was admitted to the Honours Degree of Bachelor of Science with First Class Honours at the end of 2013. As a researcher in the hospital, his interests are in dealing with clinical isolates, especially MDR strains from the family Enterobacteriaceae, focusing on the epidemiology status and resistance mechanism. Moreover, he has gained valuable and practical knowledge of bioinformatics from a variety of training programs as well as long-term working experience and is currently responsible for in silico works of the laboratory.

Guangmin Tang, M.D., is a specialist in infectious diseases. She completed her resident training at West China Hospital and obtained her medical degree from Sichuan University. As an attending physician, she has been a faculty member of the Center of Infectious Diseases at West China Hospital, Sichuan University, since 2010. Her research interests have focused on the treatment of patients with multidrug-resistant Gram-negative infections. Her M.D. thesis was about the molecular basis of antimicrobial resistance of carbapenem-resistant Klebsiella pneumoniae (CRKP) and treatment of infections caused by CRKP. She is also involved in various clinical research projects on new antimicrobial agents.

Fu Qiao, M.Med., obtained his master’s degree from Sichuan University. He has been a practitioner in infection prevention and control since 2005 and is currently the vice director of the IPC department at West China Hospital, Sichuan University, China. He is a member of the Youth Committee, Nosocomial Infection Control Commission, Chinese Preventive Medicine Association. He was also an International Ambassador of SHEA 2016 and has published 18 peer-reviewed papers as the first author. His main interests are the epidemiology and prevention measures of infections caused by multidrug-resistant organisms.

Alan McNally, Ph.D., is the Director of the Institute of Microbiology and Infection within the College of Medical and Dental Sciences at the University of Birmingham. He studied for a B.Sc. in molecular microbiology at the University of Glasgow, before moving to the University of Edinburgh to undertake a Ph.D. in virulence gene regulation. Postdoctoral positions at the University of Bristol and then the Veterinary Laboratories Agency in Surrey, United Kingdom, followed, working on subjects from bacterial pathogenesis to avian influenza molecular epidemiology. This led to an academic position at Nottingham Trent University, first as a senior lecturer and then as Reader, where he developed his research expertise in microbial genomics and evolution. He moved to the University of Birmingham in 2016 and was promoted to Director of the Institute in 2018. He now combines his background in classical microbiology with his expertise in genomics gained over the last 12 years to study the evolution of antimicrobial resistance.

Zhiyong Zong, Ph.D., M.B.B.S., is a specialist in infectious diseases. He is Head of the Infection Control Department, Director of the Center for Pathogen Research, and a Professor at West China Hospital, Sichuan University (Chengdu, China). He obtained his M.B.B.S. from the West China University for Medical Sciences (Chengdu, China) and completed his medical training at West China Hospital. He obtained his Ph.D. from the University of Sydney (Sydney, Australia). He receives the Newtown Advanced Fellowship from the Royal Society (United Kingdom) and was the International Ambassador of the Society for Hospital Epidemiology of America (SHEA) in 2011. His main research interests are the epidemiology and mechanisms of antimicrobial resistance in the Enterobacteriaceae and Acinetobacter spp. and the prevention and control of multidrug-resistant organisms. He has authored more than 100 peer-reviewed papers in scientific journals.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/CMR.00115-18.
REFERENCES
- 1.Nordmann P, Poirel L, Walsh TR, Livermore DM. 2011. The emerging NDM carbapenemases. Trends Microbiol 19:588–595. doi: 10.1016/j.tim.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Yong D, Toleman MA, Giske CG, Cho HS, Sundman K, Lee K, Walsh TR. 2009. Characterization of a new metallo-β-lactamase gene, blaNDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother 53:5046–5054. doi: 10.1128/AAC.00774-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marrs ECL, Day KM, Perry JD. 2014. In vitro activity of mecillinam against Enterobacteriaceae with NDM-1 carbapenemase. J Antimicrob Chemother 69:2873–2875. doi: 10.1093/jac/dku204. [DOI] [PubMed] [Google Scholar]
- 4.Nicolau DP. 2008. Carbapenems: a potent class of antibiotics. Expert Opin Pharmacother 9:23–37. doi: 10.1517/14656566.9.1.23. [DOI] [PubMed] [Google Scholar]
- 5.Breilh D, Texier-Maugein J, Allaouchiche B, Saux MC, Boselli E. 2013. Carbapenems. J Chemother 25:1–17. doi: 10.1179/1973947812Y.0000000032. [DOI] [PubMed] [Google Scholar]
- 6.Guducuoglu H, Gursoy NC, Yakupogullari Y, Parlak M, Karasin G, Sunnetcioglu M, Otlu B. 2018. Hospital outbreak of a colistin-resistant, NDM-1- and OXA-48-producing Klebsiella pneumoniae: high mortality from pandrug resistance. Microb Drug Resist 24:966–972. doi: 10.1089/mdr.2017.0173. [DOI] [PubMed] [Google Scholar]
- 7.Moellering RC., Jr. 2010. NDM-1—a cause for worldwide concern. N Engl J Med 363:2377–2379. doi: 10.1056/NEJMp1011715. [DOI] [PubMed] [Google Scholar]
- 8.Dortet L, Poirel L, Nordmann P. 2014. Worldwide dissemination of the NDM-type carbapenemases in Gram-negative bacteria. Biomed Res Int 2014:249856. doi: 10.1155/2014/249856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bush K. 2013. The ABCD’s of β-lactamase nomenclature. J Infect Chemother 19:549–559. doi: 10.1007/s10156-013-0640-7. [DOI] [PubMed] [Google Scholar]
- 10.Ambler RP, Coulson AF, Frere JM, Ghuysen JM, Joris B, Forsman M, Levesque RC, Tiraby G, Waley SG. 1991. A standard numbering scheme for the class A β-lactamases. Biochem J 276:269–270. doi: 10.1042/bj2760269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matagne A, Dubus A, Galleni M, Frere JM. 1999. The β-lactamase cycle: a tale of selective pressure and bacterial ingenuity. Nat Prod Rep 16:1–19. [DOI] [PubMed] [Google Scholar]
- 12.Garau G, Garcia-Saez I, Bebrone C, Anne C, Mercuri P, Galleni M, Frere JM, Dideberg O. 2004. Update of the standard numbering scheme for class B β-lactamases. Antimicrob Agents Chemother 48:2347–2349. doi: 10.1128/AAC.48.7.2347-2349.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galleni M, Lamotte-Brasseur J, Rossolini GM, Spencer J, Dideberg O, Frere JM, Metallo-β-Lactamases Working Group. 2001. Standard numbering scheme for class B β-lactamases. Antimicrob Agents Chemother 45:660–663. doi: 10.1128/AAC.45.3.660-663.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frere JM, Galleni M, Bush K, Dideberg O. 2005. Is it necessary to change the classification of β-lactamases? J Antimicrob Chemother 55:1051–1053. [DOI] [PubMed] [Google Scholar]
- 15.Palzkill T. 2013. Metallo-β-lactamase structure and function. Ann N Y Acad Sci 1277:91–104. doi: 10.1111/j.1749-6632.2012.06796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bush K, Jacoby GA. 2010. Updated functional classification of β-lactamases. Antimicrob Agents Chemother 54:969–976. doi: 10.1128/AAC.01009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mojica MF, Bonomo RA, Fast W. 2016. B1-metallo-β-lactamases: where do we stand? Curr Drug Targets 17:1029–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meini MR, Llarrull LI, Vila AJ. 2015. Overcoming differences: the catalytic mechanism of metallo-β-lactamases. FEBS Lett 589:3419–3432. doi: 10.1016/j.febslet.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groundwater PW, Xu S, Lai F, Varadi L, Tan J, Perry JD, Hibbs DE. 2016. New Delhi metallo-β-lactamase-1: structure, inhibitors and detection of producers. Future Med Chem 8:993–1012. doi: 10.4155/fmc-2016-0015. [DOI] [PubMed] [Google Scholar]
- 20.Kim Y, Cunningham MA, Mire J, Tesar C, Sacchettini J, Joachimiak A. 2013. NDM-1, the ultimate promiscuous enzyme: substrate recognition and catalytic mechanism. FASEB J 27:1917–1927. doi: 10.1096/fj.12-224014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H, Hao Q. 2011. Crystal structure of NDM-1 reveals a common β-lactam hydrolysis mechanism. FASEB J 25:2574–2582. doi: 10.1096/fj.11-184036. [DOI] [PubMed] [Google Scholar]
- 22.King D, Strynadka N. 2011. Crystal structure of New Delhi metallo-β-lactamase reveals molecular basis for antibiotic resistance. Protein Sci 20:1484–1491. doi: 10.1002/pro.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez LJ, Bahr G, Nakashige TG, Nolan EM, Bonomo RA, Vila AJ. 2016. Membrane anchoring stabilizes and favors secretion of New Delhi metallo-β-lactamase. Nat Chem Biol 12:516–522. doi: 10.1038/nchembio.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crowder MW, Spencer J, Vila AJ. 2006. Metallo-β-lactamases: novel weaponry for antibiotic resistance in bacteria. Acc Chem Res 39:721–728. doi: 10.1021/ar0400241. [DOI] [PubMed] [Google Scholar]
- 25.Bahr G, Vitor-Horen L, Bethel CR, Bonomo RA, Gonzalez LJ, Vila AJ. 2018. Clinical evolution of New Delhi metallo-β-lactamase (NDM) optimizes resistance under Zn(II) deprivation. Antimicrob Agents Chemother 62:e01849-17. doi: 10.1128/AAC.01849-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hornsey M, Phee L, Wareham DW. 2011. A novel variant, NDM-5, of the New Delhi metallo-β-lactamase in a multidrug-resistant Escherichia coli ST648 isolate recovered from a patient in the United Kingdom. Antimicrob Agents Chemother 55:5952–5954. doi: 10.1128/AAC.05108-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Z, Wang Y, Walsh TR, Liu D, Shen Z, Zhang R, Yin W, Yao H, Li J, Shen J. 2017. Plasmid-mediated novel blaNDM-17 gene encoding a carbapenemase with enhanced activity in a sequence type 48 Escherichia coli strain. Antimicrob Agents Chemother 61:e02233-16. doi: 10.1128/AAC.02233-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Z, Li J, Wang X, Liu D, Ke Y, Wang Y, Shen J. 2018. Novel variant of New Delhi metallo-β-lactamase, NDM-20, in Escherichia coli. Front Microbiol 9:248. doi: 10.3389/fmicb.2018.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu L, Feng Y, McNally A, Zong Z. 2018. blaNDM-21, a new variant of blaNDM in an Escherichia coli clinical isolate carrying blaCTX-M-55 and rmtB. J Antimicrob Chemother 73:2336–2339. doi: 10.1093/jac/dky226. [DOI] [PubMed] [Google Scholar]
- 30.Cheng Z, Thomas PW, Ju L, Bergstrom A, Mason K, Clayton D, Miller C, Bethel CR, VanPelt J, Tierney DL, Page RC, Bonomo RA, Fast W, Crowder MW. 2018. Evolution of New Delhi metallo-β-lactamase (NDM) in the clinic: effects of NDM mutations on stability, zinc affinity, and mono-zinc activity. J Biol Chem 293:12606–12618. doi: 10.1074/jbc.RA118.003835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nordmann P, Boulanger AE, Poirel L. 2012. NDM-4 metallo-β-lactamase with increased carbapenemase activity from Escherichia coli. Antimicrob Agents Chemother 56:2184–2186. doi: 10.1128/AAC.05961-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zou D, Huang Y, Zhao X, Liu W, Dong D, Li H, Wang X, Huang S, Wei X, Yan X, Yang Z, Tong Y, Huang L, Yuan J. 2015. A novel New Delhi metallo-β-lactamase variant, NDM-14, isolated in a Chinese hospital possesses increased enzymatic activity against carbapenems. Antimicrob Agents Chemother 59:2450–2453. doi: 10.1128/AAC.05168-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, Chaudhary U, Doumith M, Giske CG, Irfan S, Krishnan P, Kumar AV, Maharjan S, Mushtaq S, Noorie T, Paterson DL, Pearson A, Perry C, Pike R, Rao B, Ray U, Sarma JB, Sharma M, Sheridan E, Thirunarayan MA, Turton J, Upadhyay S, Warner M, Welfare W, Livermore DM, Woodford N. 2010. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis 10:597–602. doi: 10.1016/S1473-3099(10)70143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karlowsky JA, Lob SH, Kazmierczak KM, Badal RE, Young K, Motyl MR, Sahm DF. 2017. In vitro activity of imipenem against carbapenemase-positive Enterobacteriaceae isolates collected by the SMART global surveillance program from 2008 to 2014. J Clin Microbiol 55:1638–1649. doi: 10.1128/JCM.02316-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kazmierczak KM, Rabine S, Hackel M, McLaughlin RE, Biedenbach DJ, Bouchillon SK, Sahm DF, Bradford PA. 2016. Multiyear, multinational survey of the incidence and global distribution of metallo-β-lactamase-producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother 60:1067–1078. doi: 10.1128/AAC.02379-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perry JD, Naqvi SH, Mirza IA, Alizai SA, Hussain A, Ghirardi S, Orenga S, Wilkinson K, Woodford N, Zhang J, Livermore DM, Abbasi SA, Raza MW. 2011. Prevalence of faecal carriage of Enterobacteriaceae with NDM-1 carbapenemase at military hospitals in Pakistan, and evaluation of two chromogenic media. J Antimicrob Chemother 66:2288–2294. doi: 10.1093/jac/dkr299. [DOI] [PubMed] [Google Scholar]
- 37.Hu X, Xu X, Wang X, Xue W, Zhou H, Zhang L, Ma Q, Zhao R, Li G, Li P, Zhang C, Shi Y, Wang J, Jia L, Hao R, Wang L, Zou D, Liu X, Qiu S, Song H, Sun Y. 2017. Diversity of New Delhi metallo-β-lactamase-producing bacteria in China. Int J Infect Dis 55:92–95. doi: 10.1016/j.ijid.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 38.Zhang R, Liu L, Zhou H, Chan EW, Li J, Fang Y, Li Y, Liao K, Chen S. 2017. Nationwide surveillance of clinical carbapenem-resistant Enterobacteriaceae (CRE) strains in China. EBioMedicine 19:98–106. doi: 10.1016/j.ebiom.2017.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grundmann H, Glasner C, Albiger B, Aanensen DM, Tomlinson CT, Andrasević AT, Cantón R, Carmeli Y, Friedrich AW, Giske CG, Glupczynski Y, Gniadkowski M, Livermore DM, Nordmann P, Poirel L, Rossolini GM, Seifert H, Vatopoulos A, Walsh T, Woodford N, Monnet DL, European Survey of Carbapenemase-Producing Enterobacteriaceae Working Group. 2017. Occurrence of carbapenemase-producing Klebsiella pneumoniae and Escherichia coli in the European Survey of Carbapenemase-Producing Enterobacteriaceae (EuSCAPE): a prospective, multinational study. Lancet Infect Dis 17:153–163. doi: 10.1016/S1473-3099(16)30257-2. [DOI] [PubMed] [Google Scholar]
- 40.Zong Z, Zhang X. 2013. blaNDM-1-carrying Acinetobacter johnsonii detected in hospital sewage. J Antimicrob Chemother 68:1007–1010. doi: 10.1093/jac/dks505. [DOI] [PubMed] [Google Scholar]
- 41.Zhang C, Qiu S, Wang Y, Qi L, Hao R, Liu X, Shi Y, Hu X, An D, Li Z, Li P, Wang L, Cui J, Wang P, Huang L, Klena JD, Song H. 2014. Higher isolation of NDM-1 producing Acinetobacter baumannii from the sewage of the hospitals in Beijing. PLoS One 8:e64857. doi: 10.1371/journal.pone.0064857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khan AU, Parvez S. 2014. Detection of blaNDM-4 in Escherichia coli from hospital sewage. J Med Microbiol 63:1404–1406. doi: 10.1099/jmm.0.076026-0. [DOI] [PubMed] [Google Scholar]
- 43.Daoud Z, Farah J, Sokhn ES, El Kfoury K, Dahdouh E, Masri K, Afif C, Abdel-Massih RM, Matar GM. 2018. Multidrug-resistant Enterobacteriaceae in Lebanese hospital wastewater: implication in the One Health Concept. Microb Drug Resist 24:166–174. doi: 10.1089/mdr.2017.0090. [DOI] [PubMed] [Google Scholar]
- 44.Carraro E, Bonetta S, Bonetta S. 2017. Hospital wastewater: existing regulations and current trends in management, p 1–16. In Verlicchi P. (ed), Hospital wastewaters: characteristics, management, treatment and environmental risks. Springer Nature, London, United Kingdom. [Google Scholar]
- 45.Canton R, Akova M, Carmeli Y, Giske CG, Glupczynski Y, Gniadkowski M, Livermore DM, Miriagou V, Naas T, Rossolini GM, Samuelsen O, Seifert H, Woodford N, Nordmann P, European Network on Carbapenemases. 2012. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin Microbiol Infect 18:413–431. doi: 10.1111/j.1469-0691.2012.03821.x. [DOI] [PubMed] [Google Scholar]
- 46.Struelens MJ, Monnet DL, Magiorakos AP, Santos O’Connor F, Giesecke J, European NDM-1 Survey Participants. 2010. New Delhi metallo-β-lactamase 1-producing Enterobacteriaceae: emergence and response in Europe. Euro Surveill 15(46):pii=19716 https://www.eurosurveillance.org/content/10.2807/ese.15.46.19716-en. [DOI] [PubMed] [Google Scholar]
- 47.Gaibani P, Ambretti S, Berlingeri A, Cordovana M, Farruggia P, Panico M, Landini MP, Sambri V. 2011. Outbreak of NDM-1-producing Enterobacteriaceae in northern Italy, July to August 2011. Euro Surveill 16(47):pii=20027 https://www.eurosurveillance.org/content/10.2807/ese.16.47.20027-en. [PubMed] [Google Scholar]
- 48.Voulgari E, Gartzonika C, Vrioni G, Politi L, Priavali E, Levidiotou-Stefanou S, Tsakris A. 2014. The Balkan region: NDM-1-producing Klebsiella pneumoniae ST11 clonal strain causing outbreaks in Greece. J Antimicrob Chemother 69:2091–2097. doi: 10.1093/jac/dku105. [DOI] [PubMed] [Google Scholar]
- 49.Mataseje LF, Peirano G, Church DL, Conly J, Mulvey M, Pitout JD. 2016. Colistin-nonsusceptible Pseudomonas aeruginosa sequence type 654 with blaNDM-1 arrives in North America. Antimicrob Agents Chemother 60:1794–1800. doi: 10.1128/AAC.02591-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peirano G, Ahmed-Bentley J, Fuller J, Rubin JE, Pitout JD. 2014. Travel-related carbapenemase-producing Gram-negative bacteria in Alberta, Canada: the first 3 years. J Clin Microbiol 52:1575–1581. doi: 10.1128/JCM.00162-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Doi Y, O’Hara JA, Lando JF, Querry AM, Townsend BM, Pasculle AW, Muto CA. 2014. Co-production of NDM-1 and OXA-232 by Klebsiella pneumoniae. Emerg Infect Dis 20:163–165. doi: 10.3201/eid2001.130904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee C-S, Vasoo S, Hu F, Patel R, Doi Y. 2014. Klebsiella pneumoniae ST147 coproducing NDM-7 carbapenemase and RmtF 16S rRNA methyltransferase in Minnesota. J Clin Microbiol 52:4109–4110. doi: 10.1128/JCM.01404-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li J-J, Munoz-Price LS, Spychala CN, DePascale D, Doi Y. 2016. New Delhi metallo-β-lactamase-1-producing Klebsiella pneumoniae, Florida, USA. Emerg Infect Dis 22:744–746. doi: 10.3201/eid2204.151176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McNulty CAM, Lecky DM, Xu-McCrae L, Nakiboneka-Ssenabulya D, Chung KT, Nichols T, Thomas HL, Thomas M, Alvarez-Buylla A, Turner K, Shabir S, Manzoor S, Smith S, Crocker L, Hawkey PM. 2018. CTX-M ESBL-producing Enterobacteriaceae: estimated prevalence in adults in England in 2014. J Antimicrob Chemother 73:1368–1388. doi: 10.1093/jac/dky007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arcilla MS, van Hattem JM, Haverkate MR, Bootsma MCJ, van Genderen PJJ, Goorhuis A, Grobusch MP, Lashof AMO, Molhoek N, Schultsz C, Stobberingh EE, Verbrugh HA, de Jong MD, Melles DC, Penders J. 2017. Import and spread of extended-spectrum β-lactamase-producing Enterobacteriaceae by international travellers (COMBAT study): a prospective, multicentre cohort study. Lancet Infect Dis 17:78–85. doi: 10.1016/S1473-3099(16)30319-X. [DOI] [PubMed] [Google Scholar]
- 56.Kantele A, Laaveri T, Mero S, Vilkman K, Pakkanen SH, Ollgren J, Antikainen J, Kirveskari J. 2015. Antimicrobials increase travelers’ risk of colonization by extended-spectrum β-lactamase-producing Enterobacteriaceae. Clin Infect Dis 60:837–846. doi: 10.1093/cid/ciu957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ruppe E, Armand-Lefevre L, Estellat C, El-Mniai A, Boussadia Y, Consigny PH, Girard PM, Vittecoq D, Bouchaud O, Pialoux G, Esposito-Farese M, Coignard B, Lucet JC, Andremont A, Matheron S. 2014. Acquisition of carbapenemase-producing Enterobacteriaceae by healthy travellers to India, France, February 2012 to March 2013. Euro Surveill 19(14):pii=20768. doi: 10.2807/1560-7917.ES2014.19.14.20768. [DOI] [PubMed] [Google Scholar]
- 58.Iredell J, Brown J, Tagg K. 2016. Antibiotic resistance in Enterobacteriaceae: mechanisms and clinical implications. BMJ 352:h6420. doi: 10.1136/bmj.h6420. [DOI] [PubMed] [Google Scholar]
- 59.Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chatterjee S, Mondal A, Mitra S, Basu S. 2017. Acinetobacter baumannii transfers the blaNDM-1 gene via outer membrane vesicles. J Antimicrob Chemother 72:2201–2207. doi: 10.1093/jac/dkx131. [DOI] [PubMed] [Google Scholar]
- 61.Krahn T, Wibberg D, Maus I, Winkler A, Bontron S, Sczyrba A, Nordmann P, Puhler A, Poirel L, Schluter A. 2016. Intraspecies transfer of the chromosomal Acinetobacter baumannii blaNDM-1 carbapenemase gene. Antimicrob Agents Chemother 60:3032–3040. doi: 10.1128/AAC.00124-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peirano G, Matsumura Y, Adams MD, Bradford P, Motyl M, Chen L, Kreiswirth BN, Pitout JDD. 2018. Genomic epidemiology of global carbapenemase-producing Enterobacter spp., 2008-2014. Emerg Infect Dis 24:1010–1019. doi: 10.3201/eid2406.171648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Giske CG, Froding I, Hasan CM, Turlej-Rogacka A, Toleman M, Livermore D, Woodford N, Walsh TR. 2012. Diverse sequence types of Klebsiella pneumoniae contribute to the dissemination of blaNDM-1 in India, Sweden, and the United Kingdom. Antimicrob Agents Chemother 56:2735–2738. doi: 10.1128/AAC.06142-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jain A, Hopkins KL, Turton J, Doumith M, Hill R, Loy R, Meunier D, Pike R, Livermore DM, Woodford N. 2014. NDM carbapenemases in the United Kingdom: an analysis of the first 250 cases. J Antimicrob Chemother 69:1777–1784. doi: 10.1093/jac/dku084. [DOI] [PubMed] [Google Scholar]
- 65.Yoon EJ, Yang JW, Kim JO, Lee H, Lee KJ, Jeong SH. 2018. Carbapenemase-producing Enterobacteriaceae in South Korea: a report from the national laboratory surveillance system. Future Microbiol 13:771–783. doi: 10.2217/fmb-2018-0022. [DOI] [PubMed] [Google Scholar]
- 66.Pedersen T, Sekyere JO, Govinden U, Moodley K, Sivertsen A, Samuelsen O, Essack SY, Sundsfjord A. 2018. Spread of plasmid-encoded NDM-1 and GES-5 carbapenemases among extensively drug-resistant and pandrug-resistant clinical Enterobacteriaceae in Durban, South Africa. Antimicrob Agents Chemother 62:e02178-17. doi: 10.1128/AAC.02178-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tran DN, Tran HH, Matsui M, Suzuki M, Suzuki S, Shibayama K, Pham TD, Van Phuong TT, Dang DA, Trinh HS, Loan CT, Nga LT, van Doorn HR, Wertheim HF. 2017. Emergence of New Delhi metallo-β-lactamase 1 and other carbapenemase-producing Acinetobacter calcoaceticus-baumannii complex among patients in hospitals in Ha Noi, Viet Nam. Eur J Clin Microbiol Infect Dis 36:219–225. doi: 10.1007/s10096-016-2784-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bonnin RA, Poirel L, Carattoli A, Nordmann P. 2012. Characterization of an IncFII plasmid encoding NDM-1 from Escherichia coli ST131. PLoS One 7:e34752. doi: 10.1371/journal.pone.0034752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peirano G, Schreckenberger PC, Pitout JD. 2011. Characteristics of NDM-1-producing Escherichia coli isolates that belong to the successful and virulent clone ST131. Antimicrob Agents Chemother 55:2986–2988. doi: 10.1128/AAC.01763-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang LH, Liu PP, Wei DD, Liu Y, Wan LG, Xiang TX, Zhang YJ. 2016. Clinical isolates of uropathogenic Escherichia coli ST131 producing NDM-7 metallo-β-lactamase in China. Int J Antimicrob Agents 48:41–45. doi: 10.1016/j.ijantimicag.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 71.Nicolas-Chanoine MH, Blanco J, Leflon-Guibout V, Demarty R, Alonso MP, Canica MM, Park YJ, Lavigne JP, Pitout J, Johnson JR. 2008. Intercontinental emergence of Escherichia coli clone O25:H4-ST131 producing CTX-M-15. J Antimicrob Chemother 61:273–281. doi: 10.1093/jac/dkm464. [DOI] [PubMed] [Google Scholar]
- 72.Rogers BA, Sidjabat HE, Paterson DL. 2011. Escherichia coli O25b-ST131: a pandemic, multiresistant, community-associated strain. J Antimicrob Chemother 66:1–14. doi: 10.1093/jac/dkq415. [DOI] [PubMed] [Google Scholar]
- 73.Zong Z, Fenn S, Connor C, Feng Y, McNally A. 2018. Complete genomic characterization of two Escherichia coli lineages responsible for a cluster of carbapenem-resistant infections in a Chinese hospital. J Antimicrob Chemother 73:2340–2346. doi: 10.1093/jac/dky210. [DOI] [PubMed] [Google Scholar]
- 74.Pitout JD, Nordmann P, Poirel L. 2015. Carbapenemase-producing Klebsiella pneumoniae, a key pathogen set for global nosocomial dominance. Antimicrob Agents Chemother 59:5873–5884. doi: 10.1128/AAC.01019-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen L, Mathema B, Chavda KD, DeLeo FR, Bonomo RA, Kreiswirth BN. 2014. Carbapenemase-producing Klebsiella pneumoniae: molecular and genetic decoding. Trends Microbiol 22:686–696. doi: 10.1016/j.tim.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Qi Y, Wei Z, Ji S, Du X, Shen P, Yu Y. 2011. ST11, the dominant clone of KPC-producing Klebsiella pneumoniae in China. J Antimicrob Chemother 66:307–312. doi: 10.1093/jac/dkq431. [DOI] [PubMed] [Google Scholar]
- 77.Adler A, Hussein O, Ben-David D, Masarwa S, Navon-Venezia S, Schwaber MJ, Carmeli Y, Setton E, Golan S, Brill S, Lipkin V, Frodin E, Mendelson G, Rave R, Yehuda N, Aizen I, Kaganski M, Gershkovich P, Sasson A, Yosef H, Stessman J, Zlatkin S, Or I, Lazary A, Weinberg I, Madjar J, Taichman S, Ben-Israel J, Vigder C, Bar’el C, Davidovitch Y, Charish L. 2015. Persistence of Klebsiella pneumoniae ST258 as the predominant clone of carbapenemase-producing Enterobacteriaceae in post-acute-care hospitals in Israel, 2008–13. J Antimicrob Chemother 70:89–92. doi: 10.1093/jac/dku333. [DOI] [PubMed] [Google Scholar]
- 78.Gomez-Simmonds A, Annavajhala MK, Wang Z, Macesic N, Hu Y, Giddins MJ, O’Malley A, Toussaint NC, Whittier S, Torres VJ, Uhlemann AC. 2018. Genomic and geographic context for the evolution of high-risk carbapenem-resistant Enterobacter cloacae complex clones ST171 and ST78. mBio 9:e00542-18. doi: 10.1128/mBio.00542-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jovcic B, Lepsanovic Z, Suljagic V, Rackov G, Begovic J, Topisirovic L, Kojic M. 2011. Emergence of NDM-1 metallo-β-lactamase in Pseudomonas aeruginosa clinical isolates from Serbia. Antimicrob Agents Chemother 55:3929–3931. doi: 10.1128/AAC.00226-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Janvier F, Jeannot K, Tessé S, Robert-Nicoud M, Delacour H, Rapp C, Mérens A. 2013. Molecular characterization of blaNDM-1 in a sequence type 235 Pseudomonas aeruginosa isolate from France. Antimicrob Agents Chemother 57:3408–3411. doi: 10.1128/AAC.02334-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liew SM, Rajasekaram G, Puthucheary SD, Chua KH. 2018. Detection of VIM-2-, IMP-1- and NDM-1-producing multidrug-resistant Pseudomonas aeruginosa in Malaysia. J Glob Antimicrob Resist 13:271–273. doi: 10.1016/j.jgar.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 82.Baraniak A, Izdebski R, Fiett J, Gawryszewska I, Bojarska K, Herda M, Literacka E, Żabicka D, Tomczak H, Pewińska N, Szarata M, Ozorowski T, Milner A, Hryniewicz W, Gniadkowski M. 2016. NDM-producing Enterobacteriaceae in Poland, 2012-14: inter-regional outbreak of Klebsiella pneumoniae ST11 and sporadic cases. J Antimicrob Chemother 71:85–91. doi: 10.1093/jac/dkv282. [DOI] [PubMed] [Google Scholar]
- 83.Espinal P, Fugazza G, Lopez Y, Kasma M, Lerman Y, Malhotra-Kumar S, Goossens H, Carmeli Y, Vila J. 2011. Dissemination of an NDM-2-producing Acinetobacter baumannii clone in an Israeli rehabilitation center. Antimicrob Agents Chemother 55:5396–5398. doi: 10.1128/AAC.00679-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rahman M, Prasad KN, Gupta S, Singh S, Singh A, Pathak A, Gupta KK, Ahmad S, Gonzalez-Zorn B. 2018. Prevalence and molecular characterization of New Delhi metallo-β-lactamases in multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii from India. Microb Drug Resist 24:792–798. doi: 10.1089/mdr.2017.0078. [DOI] [PubMed] [Google Scholar]
- 85.Kumarasamy K, Kalyanasundaram A. 2012. Emergence of Klebsiella pneumoniae isolate co-producing NDM-1 with KPC-2 from India. J Antimicrob Chemother 67:243–244. doi: 10.1093/jac/dkr431. [DOI] [PubMed] [Google Scholar]
- 86.Walsh TR, Weeks J, Livermore DM, Toleman MA. 2011. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. Lancet Infect Dis 11:355–362. doi: 10.1016/S1473-3099(11)70059-7. [DOI] [PubMed] [Google Scholar]
- 87.Zujic Atalic V, Bedenic B, Kocsis E, Mazzariol A, Sardelic S, Barisic M, Plecko V, Bosnjak Z, Mijac M, Jajic I, Vranic-Ladavac M, Cornaglia G. 2014. Diversity of carbapenemases in clinical isolates of Enterobacteriaceae in Croatia—the results of a multicentre study. Clin Microbiol Infect 20:O894–O903. doi: 10.1111/1469-0691.12635. [DOI] [PubMed] [Google Scholar]
- 88.Dziri O, Alonso CA, Dziri R, Gharsa H, Maraoub A, Torres C, Chouchani C. 15 June 2018. Metallo-β-lactamases and class D carbapenemases in south-east Tunisia: implication of mobile genetic elements in their dissemination. Int J Antimicrob Agents doi: 10.1016/j.ijantimicag.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 89.Yoon EJ, Kang DY, Yang JW, Kim D, Lee H, Lee KJ, Jeong SH. 2018. New Delhi metallo-β-lactamase-producing Enterobacteriaceae in South Korea between 2010 and 2015. Front Microbiol 9:571. doi: 10.3389/fmicb.2018.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bosch T, Lutgens SPM, Hermans MHA, Wever PC, Schneeberger PM, Renders NHM, Leenders A, Kluytmans J, Schoffelen A, Notermans D, Witteveen S, Bathoorn E, Schouls LM. 2017. Outbreak of NDM-1-producing Klebsiella pneumoniae in a Dutch hospital, with interspecies transfer of the resistance plasmid and unexpected occurrence in unrelated health care centers. J Clin Microbiol 55:2380–2390. doi: 10.1128/JCM.00535-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rasheed JK, Kitchel B, Zhu W, Anderson KF, Clark NC, Ferraro MJ, Savard P, Humphries RM, Kallen AJ, Limbago BM. 2013. New Delhi metallo-β-lactamase-producing Enterobacteriaceae, United States. Emerg Infect Dis 19:870–878. doi: 10.3201/eid1906.121515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kieffer N, Nordmann P, Aires-de-Sousa M, Poirel L. 2016. High prevalence of carbapenemase-producing Enterobacteriaceae among hospitalized children in Luanda, Angola. Antimicrob Agents Chemother 60:6189–6192. doi: 10.1128/AAC.01201-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Becker L, Kaase M, Pfeifer Y, Fuchs S, Reuss A, von Laer A, Sin MA, Korte-Berwanger M, Gatermann S, Werner G. 2018. Genome-based analysis of carbapenemase-producing Klebsiella pneumoniae isolates from German hospital patients, 2008-2014. Antimicrob Resist Infect Control 7:62. doi: 10.1186/s13756-018-0352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu W, Feng Y, Carattoli A, Zong Z. 2015. Characterization of an Enterobacter cloacae strain producing both KPC and NDM carbapenemases by whole-genome sequencing. Antimicrob Agents Chemother 59:6625–6628. doi: 10.1128/AAC.01275-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wailan AM, Paterson DL, Kennedy K, Ingram PR, Bursle E, Sidjabat HE. 2016. Genomic characteristics of NDM-producing Enterobacteriaceae isolates in Australia and their blaNDM genetic contexts. Antimicrob Agents Chemother 60:136–141. doi: 10.1128/AAC.01243-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dolejska M, Villa L, Poirel L, Nordmann P, Carattoli A. 2013. Complete sequencing of an IncHI1 plasmid encoding the carbapenemase NDM-1, the ArmA 16S RNA methylase and a resistance-nodulation-cell division/multidrug efflux pump. J Antimicrob Chemother 68:34–39. doi: 10.1093/jac/dks357. [DOI] [PubMed] [Google Scholar]
- 97.Al-Baloushi AE, Pal T, Ghazawi A, Sonnevend A. 2018. Genetic support of carbapenemases in double carbapenemase producer Klebsiella pneumoniae isolated in the Arabian Peninsula. Acta Microbiol Immunol Hung 65:135–150. doi: 10.1556/030.65.2018.005. [DOI] [PubMed] [Google Scholar]
- 98.Sartor AL, Raza MW, Abbasi SA, Day KM, Perry JD, Paterson DL, Sidjabat HE. 2014. Molecular epidemiology of NDM-1-producing Enterobacteriaceae and Acinetobacter baumannii isolates from Pakistan. Antimicrob Agents Chemother 58:5589–5593. doi: 10.1128/AAC.02425-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Poirel L, Al Maskari Z, Al Rashdi F, Bernabeu S, Nordmann P. 2011. NDM-1-producing Klebsiella pneumoniae isolated in the Sultanate of Oman. J Antimicrob Chemother 66:304–306. doi: 10.1093/jac/dkq428. [DOI] [PubMed] [Google Scholar]
- 100.Netikul T, Sidjabat HE, Paterson DL, Kamolvit W, Tantisiriwat W, Steen JA, Kiratisin P. 2014. Characterization of an IncN2-type blaNDM-1-carrying plasmid in Escherichia coli ST131 and Klebsiella pneumoniae ST11 and ST15 isolates in Thailand. J Antimicrob Chemother 69:3161–3163. doi: 10.1093/jac/dku275. [DOI] [PubMed] [Google Scholar]
- 101.Quiles MG, Rocchetti TT, Fehlberg LC, Kusano EJ, Chebabo A, Pereira RM, Gales AC, Pignatari AC. 2015. Unusual association of NDM-1 with KPC-2 and armA among Brazilian Enterobacteriaceae isolates. Braz J Med Biol Res 48:174–177. doi: 10.1590/1414-431X20144154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kocsis E, Guzvinec M, Butic I, Kresic S, Crnek SS, Tambic A, Cornaglia G, Mazzariol A. 2016. blaNDM-1 carriage on IncR plasmid in Enterobacteriaceae strains. Microb Drug Resist 22:123–128. doi: 10.1089/mdr.2015.0083. [DOI] [PubMed] [Google Scholar]
- 103.Ho PL, Li Z, Lo WU, Cheung YY, Lin CH, Sham PC, Cheng VC, Ng TK, Que TL, Chow KH. 2012. Identification and characterization of a novel incompatibility group X3 plasmid carrying blaNDM-1 in Enterobacteriaceae isolates with epidemiological links to multiple geographical areas in China. Emerg Microbes Infect 1:e39. doi: 10.1038/emi.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Feng Y, Yang P, Wang X, Zong Z. 2016. Characterization of Acinetobacter johnsonii isolate XBB1 carrying nine plasmids and encoding NDM-1, OXA-58 and PER-1 by genome sequencing. J Antimicrob Chemother 71:71–75. doi: 10.1093/jac/dkv324. [DOI] [PubMed] [Google Scholar]
- 105.Villa L, Garcia-Fernandez A, Fortini D, Carattoli A. 2010. Replicon sequence typing of IncF plasmids carrying virulence and resistance determinants. J Antimicrob Chemother 65:2518–2529. doi: 10.1093/jac/dkq347. [DOI] [PubMed] [Google Scholar]
- 106.Ambrose SJ, Harmer CJ, Hall RM. 2018. Compatibility and entry exclusion of IncA and IncC plasmids revisited: IncA and IncC plasmids are compatible. Plasmid 96–97:7–12. doi: 10.1016/j.plasmid.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 107.Zou D, Huang Y, Liu W, Yang Z, Dong D, Huang S, He X, Ao D, Liu N, Wang S, Wang Y, Tong Y, Yuan J, Huang L. 2017. Complete sequences of two novel blaNDM-1-harbouring plasmids from two Acinetobacter towneri isolates in China associated with the acquisition of Tn125. Sci Rep 7:9405. doi: 10.1038/s41598-017-09624-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marquez-Ortiz RA, Haggerty L, Olarte N, Duarte C, Garza-Ramos U, Silva-Sanchez J, Castro BE, Sim EM, Beltran M, Moncada MV, Valderrama A, Castellanos JE, Charles IG, Vanegas N, Escobar-Perez J, Petty NK. 2017. Genomic epidemiology of NDM-1-encoding plasmids in Latin American clinical isolates reveals insights into the evolution of multidrug resistance. Genome Biol Evol 9:1725–1741. doi: 10.1093/gbe/evx115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jones LS, Toleman MA, Weeks JL, Howe RA, Walsh TR, Kumarasamy KK. 2014. Plasmid carriage of blaNDM-1 in clinical Acinetobacter baumannii isolates from India. Antimicrob Agents Chemother 58:4211–4213. doi: 10.1128/AAC.02500-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hu H, Hu Y, Pan Y, Liang H, Wang H, Wang X, Hao Q, Yang X, Yang X, Xiao X, Luan C, Yang Y, Cui Y, Yang R, Gao GF, Song Y, Zhu B. 2012. Novel plasmid and its variant harboring both a blaNDM-1 gene and type IV secretion system in clinical isolates of Acinetobacter lwoffii. Antimicrob Agents Chemother 56:1698–1702. doi: 10.1128/AAC.06199-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bertini A, Poirel L, Mugnier PD, Villa L, Nordmann P, Carattoli A. 2010. Characterization and PCR-based replicon typing of resistance plasmids in Acinetobacter baumannii. Antimicrob Agents Chemother 54:4168–4177. doi: 10.1128/AAC.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Poirel L, Bonnin RA, Nordmann P. 2011. Analysis of the resistome of a multidrug-resistant NDM-1-producing Escherichia coli strain by high-throughput genome sequencing. Antimicrob Agents Chemother 55:4224–4229. doi: 10.1128/AAC.00165-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Poirel L, Lagrutta E, Taylor P, Pham J, Nordmann P. 2010. Emergence of metallo-β-lactamase NDM-1-producing multidrug-resistant Escherichia coli in Australia. Antimicrob Agents Chemother 54:4914–4916. doi: 10.1128/AAC.00878-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Toleman MA, Walsh TR. 2008. Evolution of the ISCR3 group of ISCR elements. Antimicrob Agents Chemother 52:3789–3791. doi: 10.1128/AAC.00479-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Partridge SR, Iredell JR. 2012. Genetic contexts of blaNDM-1. Antimicrob Agents Chemother 56:6065–6067. doi: 10.1128/AAC.00117-12 (Reply, 56:6071. doi:.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Toleman MA, Spencer J, Jones L, Walsh TR. 2012. blaNDM-1 is a chimera likely constructed in Acinetobacter baumannii. Antimicrob Agents Chemother 56:2773–2776. doi: 10.1128/AAC.06297-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lambert T, Gerbaud G, Bouvet P, Vieu JF, Courvalin P. 1990. Dissemination of amikacin resistance gene aphA6 in Acinetobacter spp. Antimicrob Agents Chemother 34:1244–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nigro SJ, Post V, Hall RM. 2011. Aminoglycoside resistance in multiply antibiotic-resistant Acinetobacter baumannii belonging to global clone 2 from Australian hospitals. J Antimicrob Chemother 66:1504–1509. doi: 10.1093/jac/dkr163. [DOI] [PubMed] [Google Scholar]
- 119.Toleman MA, Bennett PM, Walsh TR. 2006. ISCR elements: novel gene-capturing systems of the 21st century? Microbiol Mol Biol Rev 70:296–316. doi: 10.1128/MMBR.00048-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Toleman MA, Walsh TR. 2011. Combinatorial events of insertion sequences and ICE in Gram-negative bacteria. FEMS Microbiol Rev 35:912–935. doi: 10.1111/j.1574-6976.2011.00294.x. [DOI] [PubMed] [Google Scholar]
- 121.Poirel L, Bonnin RA, Boulanger A, Schrenzel J, Kaase M, Nordmann P. 2012. Tn125-related acquisition of blaNDM-like genes in Acinetobacter baumannii. Antimicrob Agents Chemother 56:1087–1089. doi: 10.1128/AAC.05620-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bonnin RA, Poirel L, Nordmann P. 2014. New Delhi metallo-β-lactamase-producing Acinetobacter baumannii: a novel paradigm for spreading antibiotic resistance genes. Future Microbiol 9:33–41. doi: 10.2217/fmb.13.69. [DOI] [PubMed] [Google Scholar]
- 123.Feng Y, Liu L, McNally A, Zong Z. 2018. Coexistence of two blaNDM-5 genes on an IncF plasmid as revealed by Nanopore sequencing. Antimicrob Agents Chemother 62:e00110-18. doi: 10.1128/AAC.00110-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sekizuka T, Matsui M, Yamane K, Takeuchi F, Ohnishi M, Hishinuma A, Arakawa Y, Kuroda M. 2011. Complete sequencing of the blaNDM-1-positive IncA/C plasmid from Escherichia coli ST38 isolate suggests a possible origin from plant pathogens. PLoS One 6:e25334. doi: 10.1371/journal.pone.0025334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Campos JC, da Silva MJF, dos Santos PRN, Barros EM, Pereira MDO, Seco BMS, Magagnin CM, Leiroz LK, de Oliveira TGM, de Faria-Júnior C, Cerdeira LT, Barth AL, Sampaio SCF, Zavascki AP, Poirel L, Sampaio JLM. 2015. Characterization of Tn3000, a transposon responsible for blaNDM-1 dissemination among Enterobacteriaceae in Brazil, Nepal, Morocco, and India. Antimicrob Agents Chemother 59:7387–7395. doi: 10.1128/AAC.01458-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Girlich D, Dortet L, Poirel L, Nordmann P. 2015. Integration of the blaNDM-1 carbapenemase gene into Proteus genomic island 1 (PGI1-PmPEL) in a Proteus mirabilis clinical isolate. J Antimicrob Chemother 70:98–102. doi: 10.1093/jac/dku371. [DOI] [PubMed] [Google Scholar]
- 127.Shen P, Yi M, Fu Y, Ruan Z, Du X, Yu Y, Xie X. 2017. Detection of an Escherichia coli sequence type 167 strain with two tandem copies of blaNDM-1 in the chromosome. J Clin Microbiol 55:199–205. doi: 10.1128/JCM.01581-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jovcic B, Lepsanovic Z, Begovic J, Rakonjac B, Perovanovic J, Topisirovic L, Kojic M. 2013. The clinical isolate Pseudomonas aeruginosa MMA83 carries two copies of the blaNDM-1 gene in a novel genetic context. Antimicrob Agents Chemother 57:3405–3407. doi: 10.1128/AAC.02312-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huang TW, Wang JT, Lauderdale TL, Liao TL, Lai JF, Tan MC, Lin AC, Chen YT, Tsai SF, Chang SC. 2013. Complete sequences of two plasmids in a blaNDM-1-positive Klebsiella oxytoca isolate from Taiwan. Antimicrob Agents Chemother 57:4072–4076. doi: 10.1128/AAC.02266-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Szuplewska M, Ludwiczak M, Lyzwa K, Czarnecki J, Bartosik D. 2014. Mobility and generation of mosaic non-autonomous transposons by Tn3-derived inverted-repeat miniature elements (TIMEs). PLoS One 9:e105010. doi: 10.1371/journal.pone.0105010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang X, Feng Y, Zhou W, McNally A, Zong Z. 2018. Cryptic transmission of ST405 Escherichia coli carrying blaNDM-4 in hospital. Sci Rep 8:390. doi: 10.1038/s41598-017-18910-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wailan AM, Sidjabat HE, Yam WK, Alikhan NF, Petty NK, Sartor AL, Williamson DA, Forde BM, Schembri MA, Beatson SA, Paterson DL, Walsh TR, Partridge SR. 2016. Mechanisms involved in acquisition of blaNDM genes by IncA/C2 and IncFIIY plasmids. Antimicrob Agents Chemother 60:4082–4088. doi: 10.1128/AAC.00368-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen L, Peirano G, Lynch T, Chavda KD, Gregson DB, Church DL, Conly J, Kreiswirth BN, Pitout JD. 2015. Molecular characterization by using next-generation sequencing of plasmids containing blaNDM-7 in Enterobacteriaceae from Calgary, Canada. Antimicrob Agents Chemother 60:1258–1263. doi: 10.1128/AAC.02661-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Di DY, Jang J, Unno T, Hur HG. 2017. Emergence of Klebsiella variicola positive for NDM-9, a variant of New Delhi metallo-β-lactamase, in an urban river in South Korea. J Antimicrob Chemother 72:1063–1067. doi: 10.1093/jac/dkw547. [DOI] [PubMed] [Google Scholar]
- 135.Lv J, Qi X, Zhang D, Zheng Z, Chen Y, Guo Y, Wang S, Chen L, Kreiswirth BN, Tang YW, Chen Z, Hu L, Wang L, Yu F. 2016. First report of complete sequence of a blaNDM-13-harboring plasmid from an Escherichia coli ST5138 clinical isolate. Front Cell Infect Microbiol 6:130. doi: 10.3389/fcimb.2016.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lynch T, Chen L, Peirano G, Gregson DB, Church DL, Conly J, Kreiswirth BN, Pitout JD. 2016. Molecular evolution of a Klebsiella pneumoniae ST278 isolate harboring blaNDM-7 and involved in nosocomial transmission. J Infect Dis 214:798–806. doi: 10.1093/infdis/jiw240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Huang TW, Chen TL, Chen YT, Lauderdale TL, Liao TL, Lee YT, Chen CP, Liu YM, Lin AC, Chang YH, Wu KM, Kirby R, Lai JF, Tan MC, Siu LK, Chang CM, Fung CP, Tsai SF. 2013. Copy number change of the NDM-1 sequence in a multidrug-resistant Klebsiella pneumoniae clinical isolate. PLoS One 8:e62774. doi: 10.1371/journal.pone.0062774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gottig S, Riedel-Christ S, Saleh A, Kempf VA, Hamprecht A. 2016. Impact of blaNDM-1 on fitness and pathogenicity of Escherichia coli and Klebsiella pneumoniae. Int J Antimicrob Agents 47:430–435. doi: 10.1016/j.ijantimicag.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 139.Lee K, Chong Y, Shin HB, Kim YA, Yong D, Yum JH. 2001. Modified Hodge and EDTA-disk synergy tests to screen metallo-β-lactamase-producing strains of Pseudomonas and Acinetobacter species. Clin Microbiol Infect 7:88–91. [DOI] [PubMed] [Google Scholar]
- 140.Nordmann P, Poirel L, Dortet L. 2012. Rapid detection of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis 18:1503–1507. doi: 10.3201/eid1809.120355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mancini S, Kieffer N, Poirel L, Nordmann P. 2017. Evaluation of the RAPIDEC CARBA NP and β-CARBA tests for rapid detection of carbapenemase-producing Enterobacteriaceae. Diagn Microbiol Infect Dis 88:293–297. doi: 10.1016/j.diagmicrobio.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 142.Hinic V, Reist J, Egli A. 2018. Evaluation of the rapid biochemical β-CARBA test for detection of carbapenemase-producing Gram-negative bacteria. J Microbiol Methods 144:44–46. doi: 10.1016/j.mimet.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 143.van der Zwaluw K, de Haan A, Pluister GN, Bootsma HJ, de Neeling AJ, Schouls LM. 2015. The carbapenem inactivation method (CIM), a simple and low-cost alternative for the Carba NP test to assess phenotypic carbapenemase activity in Gram-negative rods. PLoS One 10:e0123690. doi: 10.1371/journal.pone.0123690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pierce VM, Simner PJ, Lonsway DR, Roe-Carpenter DE, Johnson JK, Brasso WB, Bobenchik AM, Lockett ZC, Charnot-Katsikas A, Ferraro MJ, Thomson RB Jr, Jenkins SG, Limbago BM, Das S. 2017. Modified carbapenem inactivation method for phenotypic detection of carbapenemase production among Enterobacteriaceae. J Clin Microbiol 55:2321–2333. doi: 10.1128/JCM.00193-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Oviano M, Bou G. 2017. Imipenem-avibactam: a novel combination for the rapid detection of carbapenemase activity in Enterobacteriaceae and Acinetobacter baumannii by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Diagn Microbiol Infect Dis 87:129–132. doi: 10.1016/j.diagmicrobio.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 146.Wang WJ, Wang Q, Zhang Y, Lu R, Zhang YL, Yang KW, Lei JE, He Y. 2017. Characterization of β-lactamase activity using isothermal titration calorimetry. Biochim Biophys Acta 1861:2031–2038. doi: 10.1016/j.bbagen.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 147.Bonnin RA, Naas T, Poirel L, Nordmann P. 2012. Phenotypic, biochemical, and molecular techniques for detection of metallo-β-lactamase NDM in Acinetobacter baumannii. J Clin Microbiol 50:1419–1421. doi: 10.1128/JCM.06276-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.CLSI. 2018. Performance standards for antimicrobial susceptibility testing; twenty-eighth informational supplement. M100-S28. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 149.CLSI. 2009. Performance standards for antimicrobial susceptibility testing; twenty-fifth informational supplement. M100-S19. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 150.Tamma PD, Simner PJ. 25 October 2018. Phenotypic detection of carbapenemase-producing organisms from clinical isolates. J Clin Microbiol doi: 10.1128/JCM.01140-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dortet L, Poirel L, Nordmann P. 2012. Rapid detection of carbapenemase-producing Pseudomonas spp. J Clin Microbiol 50:3773–3776. doi: 10.1128/JCM.01597-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rudresh SM, Ravi GS, Sunitha L, Hajira SN, Kalaiarasan E, Harish BN. 2017. Simple, rapid, and cost-effective modified Carba NP test for carbapenemase detection among Gram-negative bacteria. J Lab Physicians 9:303–307. doi: 10.4103/JLP.JLP_138_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.AbdelGhani S, Thomson GK, Snyder JW, Thomson KS, Onderdonk AB. 2015. Comparison of the Carba NP, modified Carba NP, and updated Rosco Neo-Rapid Carb kit tests for carbapenemase detection. J Clin Microbiol 53:3539–3542. doi: 10.1128/JCM.01631-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tamma PD, Opene BN, Gluck A, Chambers KK, Carroll KC, Simner PJ. 2017. Comparison of 11 phenotypic assays for accurate detection of carbapenemase-producing Enterobacteriaceae. J Clin Microbiol 55:1046–1055. doi: 10.1128/JCM.02338-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Simner PJ, Johnson JK, Brasso WB, Anderson K, Lonsway DR, Pierce VM, Bobenchik AM, Lockett ZC, Charnot-Katsikas A, Westblade LF, Yoo BB, Jenkins SG, Limbago BM, Das S, Roe-Carpenter DE. 2018. Multicenter evaluation of the modified carbapenem inactivation method and the Carba NP for detection of carbapenemase-producing Pseudomonas aeruginosa and Acinetobacter baumannii. J Clin Microbiol 56:e01369-17. doi: 10.1128/JCM.01369-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gallagher LC, Roundtree SS, Lancaster DP, Rudin SD, Bard JD, Roberts AL, Marshall SH, Bonomo RA, Sullivan KV. 2015. Performance of the CLSI Carba NP and the Rosco Carb screen assays using North American carbapenemase-producing Enterobacteriaceae and Pseudomonas aeruginosa isolates. J Clin Microbiol 53:3370–3373. doi: 10.1128/JCM.01547-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yamada K, Kashiwa M, Arai K, Nagano N, Saito R. 2016. Comparison of the modified-Hodge test, Carba NP test, and carbapenem inactivation method as screening methods for carbapenemase-producing Enterobacteriaceae. J Microbiol Methods 128:48–51. doi: 10.1016/j.mimet.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 158.Srisrattakarn A, Lulitanond A, Wilailuckana C, Charoensri N, Wonglakorn L, Piyapatthanakul S, Supajeen A, Chanawong A. 2016. Modification and evaluation of the Carba NP test by use of paper strip for simple and rapid detection of carbapenemase-producing Enterobacteriaceae. World J Microbiol Biotechnol 32:117. doi: 10.1007/s11274-016-2064-x. [DOI] [PubMed] [Google Scholar]
- 159.Bogaerts P, Yunus S, Massart M, Huang TD, Glupczynski Y. 2016. Evaluation of the BYG Carba test, a new electrochemical assay for rapid laboratory detection of carbapenemase-producing Enterobacteriaceae. J Clin Microbiol 54:349–358. doi: 10.1128/JCM.02404-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Srisrattakarn A, Lulitanond A, Wilailuckana C, Charoensri N, Daduang J, Chanawong A. 2017. A novel GoldNano Carb test for rapid phenotypic detection of carbapenemases, particularly OXA type, in Enterobacteriaceae, Pseudomonas aeruginosa and Acinetobacter spp. J Antimicrob Chemother 72:2519–2527. doi: 10.1093/jac/dkx156. [DOI] [PubMed] [Google Scholar]
- 161.Bonnin RA, Nordmann P, Poirel L. 2013. Screening and deciphering antibiotic resistance in Acinetobacter baumannii: a state of the art. Expert Rev Anti Infect Ther 11:571–583. doi: 10.1586/eri.13.38. [DOI] [PubMed] [Google Scholar]
- 162.Dortet L, Poirel L, Errera C, Nordmann P. 2014. CarbAcineto NP test for rapid detection of carbapenemase-producing Acinetobacter spp. J Clin Microbiol 52:2359–2364. doi: 10.1128/JCM.00594-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Literacka E, Herda M, Baraniak A, Żabicka D, Hryniewicz W, Skoczyńska A, Gniadkowski M. 2017. Evaluation of the Carba NP test for carbapenemase detection in Enterobacteriaceae, Pseudomonas spp. and Acinetobacter spp., and its practical use in the routine work of a national reference laboratory for susceptibility testing. Eur J Clin Microbiol Infect Dis 36:2281–2287. doi: 10.1007/s10096-017-3062-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dortet L, Agathine A, Naas T, Cuzon G, Poirel L, Nordmann P. 2015. Evaluation of the RAPIDEC CARBA NP, the Rapid CARB Screen and the Carba NP test for biochemical detection of carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother 70:3014–3022. doi: 10.1093/jac/dkv213. [DOI] [PubMed] [Google Scholar]
- 165.Kabir MH, Meunier D, Hopkins KL, Giske CG, Woodford N. 2016. A two-centre evaluation of RAPIDEC CARBA NP for carbapenemase detection in Enterobacteriaceae, Pseudomonas aeruginosa and Acinetobacter spp. J Antimicrob Chemother 71:1213–1216. doi: 10.1093/jac/dkv468. [DOI] [PubMed] [Google Scholar]
- 166.Novais A, Brilhante M, Pires J, Peixe L. 2015. Evaluation of the recently launched Rapid Carb Blue kit for detection of carbapenemase-producing Gram-negative bacteria. J Clin Microbiol 53:3105–3107. doi: 10.1128/JCM.01170-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Camelena F, Cointe A, Mathy V, Hobson C, Doit C, Bercot B, Decre D, Podglajen I, Dortet L, Monjault A, Bidet P, Bonacorsi S, Birgy A. 2018. Within-a-day detection and rapid characterization of carbapenemase by use of a new carbapenem inactivation method-based test, CIMplus. J Clin Microbiol 56:e00137-18. doi: 10.1128/JCM.00137-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Aguirre-Quiñonero A, Cano ME, Gamal D, Calvo J, Martínez-Martínez L. 2017. Evaluation of the carbapenem inactivation method (CIM) for detecting carbapenemase activity in enterobacteria. Diagn Microbiol Infect Dis 88:214–218. doi: 10.1016/j.diagmicrobio.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 169.Yamada K, Kashiwa M, Arai K, Nagano N, Saito R. 2017. Evaluation of the modified carbapenem inactivation method and sodium mercaptoacetate-combination method for the detection of metallo-β-lactamase production by carbapenemase-producing Enterobacteriaceae. J Microbiol Methods 132:112–115. doi: 10.1016/j.mimet.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 170.Kuchibiro T, Komatsu M, Yamasaki K, Nakamura T, Nishio H, Nishi I, Kimura K, Niki M, Ono T, Sueyoshi N, Kita M, Kida K, Ohama M, Satoh K, Toda H, Mizutani T, Fukuda N, Sawa K, Nakai I, Kofuku T, Orita T, Watari H, Shimura S, Fukuda S, Nakamura A, Wada Y. 2018. Evaluation of the modified carbapenem inactivation method for the detection of carbapenemase-producing Enterobacteriaceae. J Infect Chemother 24:262–266. doi: 10.1016/j.jiac.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 171.Laolerd W, Akeda Y, Preeyanon L, Ratthawongjirakul P, Santanirand P. 2018. Carbapenemase-producing carbapenem-resistant Enterobacteriaceae from Bangkok, Thailand, and their detection by the carba np and modified carbapenem inactivation method tests. Microb Drug Resist 24:1006–1011. doi: 10.1089/mdr.2018.0080. [DOI] [PubMed] [Google Scholar]
- 172.CLSI. 2017. Performance standards for antimicrobial susceptibility testing; twenty-seventh informational supplement. M100-S27. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 173.Muntean MM, Muntean AA, Gauthier L, Creton E, Cotellon G, Popa MI, Bonnin RA, Naas T. 2018. Evaluation of the rapid carbapenem inactivation method (rCIM): a phenotypic screening test for carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother 73:900–908. doi: 10.1093/jac/dkx519. [DOI] [PubMed] [Google Scholar]
- 174.Uechi K, Tada T, Shimada K, Kuwahara-Arai K, Arakaki M, Tome T, Nakasone I, Maeda S, Kirikae T, Fujita J, Munson E. 2017. A modified carbapenem inactivation method, CIMTris, for carbapenemase production in Acinetobacter and Pseudomonas species. J Clin Microbiol 55:3405–3410. doi: 10.1128/JCM.00893-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lasserre C, De Saint Martin L, Cuzon G, Bogaerts P, Lamar E, Glupczynski Y, Naas T, Tandé D. 2015. Efficient detection of carbapenemase activity in Enterobacteriaceae by matrix-assisted laser desorption ionization–time of flight mass spectrometry in less than 30 minutes. J Clin Microbiol 53:2163–2171. doi: 10.1128/JCM.03467-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hrabak J, Studentova V, Walkova R, Zemlickova H, Jakubu V, Chudackova E, Gniadkowski M, Pfeifer Y, Perry JD, Wilkinson K, Bergerova T. 2012. Detection of NDM-1, VIM-1, KPC, OXA-48, and OXA-162 carbapenemases by matrix-assisted laser desorption ionization–time of flight mass spectrometry. J Clin Microbiol 50:2441–2443. doi: 10.1128/JCM.01002-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ghebremedhin B, Halstenbach A, Smiljanic M, Kaase M, Ahmad-Nejad P. 2016. MALDI-TOF MS based carbapenemase detection from culture isolates and from positive blood culture vials. Ann Clin Microbiol Antimicrob 15:5. doi: 10.1186/s12941-016-0120-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kempf M, Bakour S, Flaudrops C, Berrazeg M, Brunel JM, Drissi M, Mesli E, Touati A, Rolain JM. 2012. Rapid detection of carbapenem resistance in Acinetobacter baumannii using matrix-assisted laser desorption ionization-time of flight mass spectrometry. PLoS One 7:e31676. doi: 10.1371/journal.pone.0031676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Knox J, Jadhav S, Sevior D, Agyekum A, Whipp M, Waring L, Iredell J, Palombo E. 2014. Phenotypic detection of carbapenemase-producing Enterobacteriaceae by use of matrix-assisted laser desorption ionization–time of flight mass spectrometry and the Carba NP test. J Clin Microbiol 52:4075–4077. doi: 10.1128/JCM.02121-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hrabak J, Chudackova E, Walkova R. 2013. Matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry for detection of antibiotic resistance mechanisms: from research to routine diagnosis. Clin Microbiol Rev 26:103–114. doi: 10.1128/CMR.00058-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dortet L, Tande D, de Briel D, Bernabeu S, Lasserre C, Gregorowicz G, Jousset AB, Naas T. 2018. MALDI-TOF for the rapid detection of carbapenemase-producing Enterobacteriaceae: comparison of the commercialized MBT STAR-Carba IVD kit with two in-house MALDI-TOF techniques and the RAPIDEC CARBA NP. J Antimicrob Chemother 73:2352–2359. doi: 10.1093/jac/dky209. [DOI] [PubMed] [Google Scholar]
- 182.Motara H, Mistry D, Brown DR, Cryan RA, Nigen M, Page MI. 2014. pH and basicity of ligands control the binding of metal-ions to B. cereus B1 β-lactamase. Chem Sci 5:3120–3129. doi: 10.1039/C4SC00601A. [DOI] [Google Scholar]
- 183.Aguirre-Quinonero A, Martinez-Martinez L. 2017. Non-molecular detection of carbapenemases in Enterobacteriaceae clinical isolates. J Infect Chemother 23:1–11. doi: 10.1016/j.jiac.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 184.Hammoudi D, Moubareck CA, Sarkis DK. 2014. How to detect carbapenemase producers? A literature review of phenotypic and molecular methods. J Microbiol Methods 107:106–118. doi: 10.1016/j.mimet.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 185.Franklin C, Liolios L, Peleg AY. 2006. Phenotypic detection of carbapenem-susceptible metallo-β-lactamase-producing Gram-negative bacilli in the clinical laboratory. J Clin Microbiol 44:3139–3144. doi: 10.1128/JCM.00879-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Segal H, Elisha BG. 2005. Use of Etest MBL strips for the detection of carbapenemases in Acinetobacter baumannii. J Antimicrob Chemother 56:598. doi: 10.1093/jac/dki265. [DOI] [PubMed] [Google Scholar]
- 187.Nordmann P, Poirel L, Carrer A, Toleman MA, Walsh TR. 2011. How to detect NDM-1 producers. J Clin Microbiol 49:718–721. doi: 10.1128/JCM.01773-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fujisaki M, Sadamoto S, Hishinuma A. 2013. Evaluation of the double-disk synergy test for New Delhi metallo-β-lactamase-1 and other metallo-β-lactamase producing Gram-negative bacteria by using metal-ethylenediaminetetraacetic acid complexes. Microbiol Immunol 57:346–352. doi: 10.1111/1348-0421.12042. [DOI] [PubMed] [Google Scholar]
- 189.Ohsaki Y, Kubo R, Hobson J, Davies M, Osaka S, Hayashi W, Taniguchi Y, Koide S, Nagano Y, Nagano N. 2018. MASTDISCS combi Carba plus, a simple method for discriminating carbapenemase-producing Enterobacteriaceae, including OXA-48-type producers. Microbiol Immunol 62:60–65. doi: 10.1111/1348-0421.12553. [DOI] [PubMed] [Google Scholar]
- 190.Sakanashi D, Kawachi M, Uozumi Y, Nishio M, Hara Y, Suematsu H, Hagihara M, Nishiyama N, Asai N, Koizumi Y, Yamagishi Y, Mikamo H. 2017. Evaluation of commercial phenotypic assays for the detection of IMP- or New Delhi metallo-β-lactamase-producing Enterobacteriaceae isolates in Japan. J Infect Chemother 23:474–480. doi: 10.1016/j.jiac.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 191.Hansen F, Hammerum AM, Skov R, Haldorsen B, Sundsfjord A, Samuelsen O. 2014. Evaluation of the total MBL confirm kit (ROSCO) for detection of metallo-β-lactamases in Pseudomonas aeruginosa and Acinetobacter baumannii. Diagn Microbiol Infect Dis 79:486–488. doi: 10.1016/j.diagmicrobio.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 192.Moubareck CA, Mouftah SF, Pal T, Ghazawi A, Halat DH, Nabi A, AlSharhan MA, AlDeesi ZO, Peters CC, Celiloglu H, Sannegowda M, Sarkis DK, Sonnevend A. 2018. Clonal emergence of Klebsiella pneumoniae ST14 co-producing OXA-48-type and NDM carbapenemases with high rate of colistin resistance in Dubai, United Arab Emirates. Int J Antimicrob Agents 52:90–95. doi: 10.1016/j.ijantimicag.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 193.Dortet L, Poirel L, Nordmann P. 2012. Rapid identification of carbapenemase types in Enterobacteriaceae and Pseudomonas spp. by using a biochemical test. Antimicrob Agents Chemother 56:6437–6440. doi: 10.1128/AAC.01395-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Maurer FP, Castelberg C, Quiblier C, Bloemberg GV, Hombach M. 2015. Evaluation of carbapenemase screening and confirmation tests with Enterobacteriaceae and development of a practical diagnostic algorithm. J Clin Microbiol 53:95–104. doi: 10.1128/JCM.01692-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Oviano M, Barba MJ, Fernandez B, Ortega A, Aracil B, Oteo J, Campos J, Bou G. 2016. Rapid detection of OXA-48-producing Enterobacteriaceae by matrix-assisted laser desorption ionization–time of flight mass spectrometry. J Clin Microbiol 54:754–759. doi: 10.1128/JCM.02496-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhang YJ, Wang WM, Oelschlaeger P, Chen C, Lei JE, Lv M, Yang KW. 5 November 2018. Real-time monitoring of NDM-1 activity in live bacterial cells by isothermal titration calorimetry: a new approach to measure inhibition of antibiotic-resistant bacteria. ACS Infect Dis doi: 10.1021/acsinfecdis.8b00147. [DOI] [PubMed] [Google Scholar]
- 197.Boutal H, Naas T, Devilliers K, Oueslati S, Dortet L, Bernabeu S, Simon S, Volland H. 2017. Development and validation of a lateral flow immunoassay for rapid detection of NDM-producing Enterobacteriaceae. J Clin Microbiol 55:2018–2029. doi: 10.1128/JCM.00248-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Saleh A, Gottig S, Hamprecht AG. 2018. Multiplex immunochromatographic detection of OXA-48, KPC, and NDM carbapenemases: impact of inoculum, antibiotics, and agar. J Clin Microbiol 56:e00050-18. doi: 10.1128/JCM.00050-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Boutal H, Vogel A, Bernabeu S, Devilliers K, Creton E, Cotellon G, Plaisance M, Oueslati S, Dortet L, Jousset A, Simon S, Naas T, Volland H. 2018. A multiplex lateral flow immunoassay for the rapid identification of NDM-, KPC-, IMP- and VIM-type and OXA-48-like carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother 73:909–915. doi: 10.1093/jac/dkx521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Glupczynski Y, Jousset A, Evrard S, Bonnin RA, Huang TD, Dortet L, Bogaerts P, Naas T. 2017. Prospective evaluation of the OKN K-SeT assay, a new multiplex immunochromatographic test for the rapid detection of OXA-48-like, KPC and NDM carbapenemases. J Antimicrob Chemother 72:1955–1960. doi: 10.1093/jac/dkx089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hamprecht A, Vehreschild JJ, Seifert H, Saleh A. 2018. Rapid detection of NDM, KPC and OXA-48 carbapenemases directly from positive blood cultures using a new multiplex immunochromatographic assay. PLoS One 13:e0204157. doi: 10.1371/journal.pone.0204157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hopkins KL, Meunier D, Naas T, Volland H, Woodford N. 2018. Evaluation of the NG-Test CARBA 5 multiplex immunochromatographic assay for the detection of KPC, OXA-48-like, NDM, VIM and IMP carbapenemases. J Antimicrob Chemother 73:3523–3526. doi: 10.1093/jac/dky342. [DOI] [PubMed] [Google Scholar]
- 203.Wareham DW, Abdul Momin MH. 2017. Rapid detection of carbapenemases in Enterobacteriaceae: evaluation of the Resist-3 O.K.N. (OXA-48, KPC, NDM) lateral flow multiplexed assay. J Clin Microbiol 55:1223–1225. doi: 10.1128/JCM.02471-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kolenda C, Benoit R, Carricajo A, Bonnet R, Dauwalder O, Laurent F. 25 October 2018. Evaluation of the new multiplex immunochromatographic O.K.N.V. K-Set assay for the rapid detection of OXA-48-like, KPC, NDM, and VIM carbapenemases. J Clin Microbiol doi: 10.1128/JCM.01247-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Nordmann P, Gniadkowski M, Giske CG, Poirel L, Woodford N, Miriagou V, European Network on Carbapenemases. 2012. Identification and screening of carbapenemase-producing Enterobacteriaceae. Clin Microbiol Infect 18:432–438. doi: 10.1111/j.1469-0691.2012.03815.x. [DOI] [PubMed] [Google Scholar]
- 206.Bialvaei AZ, Kafil HS, Asgharzadeh M, Yousef Memar M, Yousefi M. 2016. Current methods for the identification of carbapenemases. J Chemother 28:1–19. doi: 10.1179/1973947815Y.0000000063. [DOI] [PubMed] [Google Scholar]
- 207.Naas T, Ergani A, Carrer A, Nordmann P. 2011. Real-time PCR for detection of NDM-1 carbapenemase genes from spiked stool samples. Antimicrob Agents Chemother 55:4038–4043. doi: 10.1128/AAC.01734-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zheng F, Sun J, Cheng C, Rui Y. 2013. The establishment of a duplex real-time PCR assay for rapid and simultaneous detection of blaNDM and blaKPC genes in bacteria. Ann Clin Microbiol Antimicrob 12:30. doi: 10.1186/1476-0711-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Poirel L, Walsh TR, Cuvillier V, Nordmann P. 2011. Multiplex PCR for detection of acquired carbapenemase genes. Diagn Microbiol Infect Dis 70:119–123. doi: 10.1016/j.diagmicrobio.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 210.CDC. 2011. Multiplex real-time PCR detection of Klebsiella pneumoniae carbapenemase (KPC) and New Delhi metallo-β-lactamase (NDM-1). CDC, Atlanta, GA: https://www.cdc.gov/hai/settings/lab/kpc-ndm1-lab-protocol.html. [Google Scholar]
- 211.Lee TD, Adie K, McNabb A, Purych D, Mannan K, Azana R, Ng C, Tang P, Hoang LM. 2015. Rapid detection of KPC, NDM, and OXA-48-like carbapenemases by real-time PCR from rectal swab surveillance samples. J Clin Microbiol 53:2731–2733. doi: 10.1128/JCM.01237-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Nijhuis R, Samuelsen O, Savelkoul P, van Zwet A. 2013. Evaluation of a new real-time PCR assay (Check-Direct CPE) for rapid detection of KPC, OXA-48, VIM, and NDM carbapenemases using spiked rectal swabs. Diagn Microbiol Infect Dis 77:316–320. doi: 10.1016/j.diagmicrobio.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 213.Lund M, Petersen MB, Jorgensen AL, Paulmann D, Wang M. 2018. Rapid real-time PCR for the detection of IMP, NDM, VIM, KPC and OXA-48 carbapenemase genes in isolates and spiked stool samples. Diagn Microbiol Infect Dis 92:8–12. doi: 10.1016/j.diagmicrobio.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 214.AlTamimi M, AlSalamah A, AlKhulaifi M, AlAjlan H. 2017. Comparison of phenotypic and PCR methods for detection of carbapenemases production by Enterobacteriaceae. Saudi J Biol Sci 24:155–161. doi: 10.1016/j.sjbs.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Tato M, Ruiz-Garbajosa P, Traczewski M, Dodgson A, McEwan A, Humphries R, Hindler J, Veltman J, Wang H, Cantón R. 2016. Multisite evaluation of Cepheid Xpert Carba-R assay for detection of carbapenemase-producing organisms in rectal swabs. J Clin Microbiol 54:1814–1819. doi: 10.1128/JCM.00341-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Findlay J, Hopkins KL, Meunier D, Woodford N. 2015. Evaluation of three commercial assays for rapid detection of genes encoding clinically relevant carbapenemases in cultured bacteria. J Antimicrob Chemother 70:1338–1342. doi: 10.1093/jac/dku571. [DOI] [PubMed] [Google Scholar]
- 217.Poole S, Kidd SP, Saeed K. 2018. A review of novel technologies and techniques associated with identification of bloodstream infection etiologies and rapid antimicrobial genotypic and quantitative phenotypic determination. Expert Rev Mol Diagn 18:543–555. doi: 10.1080/14737159.2018.1480369. [DOI] [PubMed] [Google Scholar]
- 218.Rosner S, Gehlweiler K, Kusters U, Kolbert M, Hubner K, Pfennigwerth N, Mack D. 2018. Comparison of two commercial carbapenemase gene confirmatory assays in multiresistant Enterobacteriaceae and Acinetobacter baumannii-complex. PLoS One 13:e0197839. doi: 10.1371/journal.pone.0197839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Bloemberg GV, Braun-Kiewnick A, Tedrup J, Meijerink C, Durer E, Ritter C, Keller PM, Hombach M. 2017. Evaluation of the AID carbapenemase line probe assay for rapid detection and identification of carbapenemase genes in Gram-negative bacilli. J Antimicrob Chemother 72:1948–1954. doi: 10.1093/jac/dkx100. [DOI] [PubMed] [Google Scholar]
- 220.Kaase M, Szabados F, Wassill L, Gatermann SG. 2012. Detection of carbapenemases in Enterobacteriaceae by a commercial multiplex PCR. J Clin Microbiol 50:3115–3118. doi: 10.1128/JCM.00991-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zhu L, Shen D, Zhou Q, Li Z, Fang X, Li QZ. 2015. A locked nucleic acid (LNA)-based real-time PCR assay for the rapid detection of multiple bacterial antibiotic resistance genes directly from positive blood culture. PLoS One 10:e0120464. doi: 10.1371/journal.pone.0120464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Huang L, Hu X, Zhou M, Yang Y, Qiao J, Wang D, Yu J, Cui Z, Zhang Z, Zhang XE, Wei H. 2014. Rapid detection of New Delhi metallo-β-lactamase gene and variants coding for carbapenemases with different activities by use of a PCR-based in vitro protein expression method. J Clin Microbiol 52:1947–1953. doi: 10.1128/JCM.03363-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Liu W, Zou D, Li Y, Wang X, He X, Wei X, Shao C, Li X, Shang W, Yu K, Liu D, Li Y, Guo J, Yin Z, Yuan J. 2012. Sensitive and rapid detection of the New Delhi metallo-β-lactamase gene by loop-mediated isothermal amplification. J Clin Microbiol 50:1580–1585. doi: 10.1128/JCM.06647-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Qi J, Du Y, Zhu X, Bai H, Luo Y, Liu Y. 2012. A loop-mediated isothermal amplification method for rapid detection of NDM-1 gene. Microb Drug Resist 18:359–363. doi: 10.1089/mdr.2011.0220. [DOI] [PubMed] [Google Scholar]
- 225.Solanki R, Vanjari L, Ede N, Gungi A, Soory A, Vemu L. 2013. Evaluation of LAMP assay using phenotypic tests and conventional PCR for detection of blaNDM-1 and blaKPC genes among carbapenem-resistant clinical Gram-negative isolates. J Med Microbiol 62:1540–1544. doi: 10.1099/jmm.0.059907-0. [DOI] [PubMed] [Google Scholar]
- 226.Vergara A, Zboromyrska Y, Mosqueda N, Morosini MI, Garcia-Fernandez S, Roca I, Canton R, Marco F, Vila J. 2014. Evaluation of a loop-mediated isothermal amplification-based methodology to detect carbapenemase carriage in Acinetobacter clinical isolates. Antimicrob Agents Chemother 58:7538–7540. doi: 10.1128/AAC.03870-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Cheng C, Zheng F, Rui Y. 2014. Rapid detection of blaNDM, blaKPC, blaIMP, and blaVIM carbapenemase genes in bacteria by loop-mediated isothermal amplification. Microb Drug Resist 20:533–538. doi: 10.1089/mdr.2014.0040. [DOI] [PubMed] [Google Scholar]
- 228.Srisrattakarn A, Lulitanond A, Wilailuckana C, Charoensri N, Wonglakorn L, Saenjamla P, Chaimanee P, Daduang J, Chanawong A. 2017. Rapid and simple identification of carbapenemase genes, blaNDM, blaOXA-48, blaVIM, blaIMP-14 and blaKPC groups, in Gram-negative bacilli by in-house loop-mediated isothermal amplification with hydroxynaphthol blue dye. World J Microbiol Biotechnol 33:130. doi: 10.1007/s11274-017-2295-5. [DOI] [PubMed] [Google Scholar]
- 229.Dally S, Lemuth K, Kaase M, Rupp S, Knabbe C, Weile J. 2013. DNA microarray for genotyping antibiotic resistance determinants in Acinetobacter baumannii clinical isolates. Antimicrob Agents Chemother 57:4761–4768. doi: 10.1128/AAC.00863-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Naas T, Cuzon G, Bogaerts P, Glupczynski Y, Nordmann P. 2011. Evaluation of a DNA microarray (Check-MDR CT102) for rapid detection of TEM, SHV, and CTX-M extended-spectrum β-lactamases and of KPC, OXA-48, VIM, IMP, and NDM-1 carbapenemases. J Clin Microbiol 49:1608–1613. doi: 10.1128/JCM.02607-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Cuzon G, Naas T, Bogaerts P, Glupczynski Y, Nordmann P. 2012. Evaluation of a DNA microarray for the rapid detection of extended-spectrum β-lactamases (TEM, SHV and CTX-M), plasmid-mediated cephalosporinases (CMY-2-like, DHA, FOX, ACC-1, ACT/MIR and CMY-1-like/MOX) and carbapenemases (KPC, OXA-48, VIM, IMP and NDM). J Antimicrob Chemother 67:1865–1869. doi: 10.1093/jac/dks156. [DOI] [PubMed] [Google Scholar]
- 232.Ledeboer NA, Lopansri BK, Dhiman N, Cavagnolo R, Carroll KC, Granato P, Thomson R Jr, Butler-Wu SM, Berger H, Samuel L, Pancholi P, Swyers L, Hansen GT, Tran NK, Polage CR, Thomson KS, Hanson ND, Winegar R, Buchan BW. 2015. Identification of Gram-negative bacteria and genetic resistance determinants from positive blood culture broths by use of the Verigene Gram-negative blood culture multiplex microarray-based molecular assay. J Clin Microbiol 53:2460–2472. doi: 10.1128/JCM.00581-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Fleece ME, Pholwat S, Mathers AJ, Houpt ER. 2018. Molecular diagnosis of antimicrobial resistance in Escherichia coli. Expert Rev Mol Diagn 18:207–217. doi: 10.1080/14737159.2018.1439381. [DOI] [PubMed] [Google Scholar]
- 234.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, Aarestrup FM, Larsen MV. 2012. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Liu B, Pop M. 2009. ARDB—Antibiotic Resistance Genes Database. Nucleic Acids Res 37:D443–D447. doi: 10.1093/nar/gkn656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Jia B, Raphenya AR, Alcock B, Waglechner N, Guo P, Tsang KK, Lago BA, Dave BM, Pereira S, Sharma AN, Doshi S, Courtot M, Lo R, Williams LE, Frye JG, Elsayegh T, Sardar D, Westman EL, Pawlowski AC, Johnson TA, Brinkman FS, Wright GD, McArthur AG. 2017. CARD 2017: expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res 45:D566–D573. doi: 10.1093/nar/gkw1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Balloux F, Bronstad Brynildsrud O, van Dorp L, Shaw LP, Chen H, Harris KA, Wang H, Eldholm V. 4 September 2018. From theory to practice: translating whole-genome sequencing (WGS) into the clinic. Trends Microbiol doi: 10.1016/j.tim.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Rossen JWA, Friedrich AW, Moran-Gilad J, ESCMID Study Group for Genomic and Molecular Diagnostics. 2018. Practical issues in implementing whole-genome-sequencing in routine diagnostic microbiology. Clin Microbiol Infect 24:355–360. doi: 10.1016/j.cmi.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 239.Khong WX, Xia E, Marimuthu K, Xu W, Teo YY, Tan EL, Neo S, Krishnan PU, Ang BS, Lye DC, Chow AL, Ong RT, Ng OT. 2016. Local transmission and global dissemination of New Delhi metallo-β-lactamase (NDM): a whole genome analysis. BMC Genomics 17:452. doi: 10.1186/s12864-016-2740-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Iovleva A, Doi Y. 2017. Carbapenem-resistant Enterobacteriaceae. Clin Lab Med 37:303–315. doi: 10.1016/j.cll.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Morrill HJ, Pogue JM, Kaye KS, LaPlante KL. 2015. Treatment options for carbapenem-resistant Enterobacteriaceae infections. Open Forum Infect Dis 2:ofv050. doi: 10.1093/ofid/ofv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Temkin E, Adler A, Lerner A, Carmeli Y. 2014. Carbapenem-resistant Enterobacteriaceae: biology, epidemiology, and management. Ann N Y Acad Sci 1323:22–42. doi: 10.1111/nyas.12537. [DOI] [PubMed] [Google Scholar]
- 243.Rafailidis PI, Falagas ME. 2014. Options for treating carbapenem-resistant Enterobacteriaceae. Curr Opin Infect Dis 27:479–483. doi: 10.1097/QCO.0000000000000109. [DOI] [PubMed] [Google Scholar]
- 244.Trecarichi EM, Tumbarello M. 2017. Therapeutic options for carbapenem-resistant Enterobacteriaceae infections. Virulence 8:470–484. doi: 10.1080/21505594.2017.1292196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Abboud MI, Damblon C, Brem J, Smargiasso N, Mercuri P, Gilbert B, Rydzik AM, Claridge TD, Schofield CJ, Frere JM. 2016. Interaction of avibactam with class B metallo-β-lactamases. Antimicrob Agents Chemother 60:5655–5662. doi: 10.1128/AAC.00897-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Karlowsky JA, Kazmierczak KM, de Jonge BLM, Hackel MA, Sahm DF, Bradford PA. 2017. In vitro activity of aztreonam-avibactam against Enterobacteriaceae and Pseudomonas aeruginosa isolated by clinical laboratories in 40 countries from 2012 to 2015. Antimicrob Agents Chemother 61:e00472-17. doi: 10.1128/AAC.00472-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Marshall S, Hujer AM, Rojas LJ, Papp-Wallace KM, Humphries RM, Spellberg B, Hujer KM, Marshall EK, Rudin SD, Perez F, Wilson BM, Wasserman RB, Chikowski L, Paterson DL, Vila AJ, van Duin D, Kreiswirth BN, Chambers HF, Fowler VG Jr, Jacobs MR, Pulse ME, Weiss WJ, Bonomo RA. 2017. Can ceftazidime-avibactam and aztreonam overcome β-lactam resistance conferred by metallo-β-lactamases in Enterobacteriaceae? Antimicrob Agents Chemother 61:e02243-16. doi: 10.1128/AAC.02243-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Wenzler E, Deraedt MF, Harrington AT, Danizger LH. 2017. Synergistic activity of ceftazidime-avibactam and aztreonam against serine and metallo-β-lactamase-producing gram-negative pathogens. Diagn Microbiol Infect Dis 88:352–354. doi: 10.1016/j.diagmicrobio.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 249.Shaw E, Rombauts A, Tubau F, Padullés A, Càmara J, Lozano T, Cobo-Sacristán S, Sabe N, Grau I, Rigo-Bonnin R, Dominguez MA, Carratalà J. 2018. Clinical outcomes after combination treatment with ceftazidime/avibactam and aztreonam for NDM-1/OXA-48/CTX-M-15-producing Klebsiella pneumoniae infection. J Antimicrob Chemother 73:1104–1106. doi: 10.1093/jac/dkx496. [DOI] [PubMed] [Google Scholar]
- 250.Davido B, Fellous L, Lawrence C, Maxime V, Rottman M, Dinh A. 2017. Ceftazidime-avibactam and aztreonam, an interesting strategy to overcome β-lactam resistance conferred by metallo-β-lactamases in Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother 61:e01008-17. doi: 10.1128/AAC.01008-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Vardakas KZ, Mavroudis AD, Georgiou M, Falagas ME. 2018. Intravenous colistin combination antimicrobial treatment vs. monotherapy: a systematic review and meta-analysis. Int J Antimicrob Agents 51:535–547. doi: 10.1016/j.ijantimicag.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 252.Zayyad H, Eliakim-Raz N, Leibovici L, Paul M. 2017. Revival of old antibiotics: needs, the state of evidence and expectations. Int J Antimicrob Agents 49:536–541. doi: 10.1016/j.ijantimicag.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 253.Zhang X, Guo F, Shao H, Zheng X. 2017. Clinical translation of polymyxin-based combination therapy: facts, challenges and future opportunities. J Infect 74:118–130. doi: 10.1016/j.jinf.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 254.Jacobs DM, Safir MC, Huang D, Minhaj F, Parker A, Rao GG. 2017. Triple combination antibiotic therapy for carbapenemase-producing Klebsiella pneumoniae: a systematic review. Ann Clin Microbiol Antimicrob 16:76. doi: 10.1186/s12941-017-0249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Antachopoulos C, Iosifidis E. 2017. Colistin use in neonates and children with infections due to carbapenem-resistant bacteria. Pediatr Infect Dis J 36:905–907. doi: 10.1097/INF.0000000000001655. [DOI] [PubMed] [Google Scholar]
- 256.Darley E, Weeks J, Jones L, Daniels V, Wootton M, MacGowan A, Walsh T. 2012. NDM-1 polymicrobial infections including Vibrio cholerae. Lancet 380:1358. doi: 10.1016/S0140-6736(12)60911-8. [DOI] [PubMed] [Google Scholar]
- 257.Lagerback P, Khine WW, Giske CG, Tangden T. 2016. Evaluation of antibacterial activities of colistin, rifampicin and meropenem combinations against NDM-1-producing Klebsiella pneumoniae in 24 h in vitro time-kill experiments. J Antimicrob Chemother 71:2321–2325. doi: 10.1093/jac/dkw213. [DOI] [PubMed] [Google Scholar]
- 258.Zhou YF, Tao MT, Feng Y, Yang RS, Liao XP, Liu YH, Sun J. 2017. Increased activity of colistin in combination with amikacin against Escherichia coli co-producing NDM-5 and MCR-1. J Antimicrob Chemother 72:1723–1730. doi: 10.1093/jac/dkx038. [DOI] [PubMed] [Google Scholar]
- 259.Docobo-Pérez F, Nordmann P, Domínguez-Herrera J, López-Rojas R, Smani Y, Poirel L, Pachón J. 2012. Efficacies of colistin and tigecycline in mice with experimental pneumonia due to NDM-1-producing strains of Klebsiella pneumoniae and Escherichia coli. Int J Antimicrob Agents 39:251–254. doi: 10.1016/j.ijantimicag.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 260.Ortwine JK, Kaye KS, Li J, Pogue JM. 2015. Colistin: understanding and applying recent pharmacokinetic advances. Pharmacotherapy 35:11–16. doi: 10.1002/phar.1484. [DOI] [PubMed] [Google Scholar]
- 261.Wolinsky E, Hines JD. 1962. Neurotoxic and nephrotoxic effects of colistin in patients with renal disease. N Engl J Med 266:759–762. doi: 10.1056/NEJM196204122661505. [DOI] [PubMed] [Google Scholar]
- 262.Stone NRH, Woodford N, Livermore DM, Howard J, Pike R, Mushtaq S, Perry C, Hopkins S. 2011. Breakthrough bacteraemia due to tigecycline-resistant Escherichia coli with New Delhi metallo-β-lactamase (NDM)-1 successfully treated with colistin in a patient with calciphylaxis. J Antimicrob Chemother 66:2677–2678. doi: 10.1093/jac/dkr337. [DOI] [PubMed] [Google Scholar]
- 263.Albur M, Noel A, Bowker K, MacGowan A. 2012. Bactericidal activity of multiple combinations of tigecycline and colistin against NDM-1-producing Enterobacteriaceae. Antimicrob Agents Chemother 56:3441–3443. doi: 10.1128/AAC.05682-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Poirel L, Jayol A, Nordmann P. 2017. Polymyxins: antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes. Clin Microbiol Rev 30:557–596. doi: 10.1128/CMR.00064-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Tansarli GS, Papaparaskevas J, Balaska M, Samarkos M, Pantazatou A, Markogiannakis A, Mantzourani M, Polonyfi K, Daikos GL. 28 June 2018. Colistin resistance in carbapenemase-producing Klebsiella pneumoniae bloodstream isolates: evolution over 15 years and temporal association with colistin use by time series analysis. Int J Antimicrob Agents doi: 10.1016/j.ijantimicag.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 266.Biswas S, Brunel JM, Dubus JC, Reynaud-Gaubert M, Rolain JM. 2012. Colistin: an update on the antibiotic of the 21st century. Expert Rev Anti Infect Ther 10:917–934. doi: 10.1586/eri.12.78. [DOI] [PubMed] [Google Scholar]
- 267.Rogers BA, Sidjabat HE, Silvey A, Anderson TL, Perera S, Li J, Paterson DL. 2013. Treatment options for New Delhi metallo-β-lactamase-harboring Enterobacteriaceae. Microb Drug Resist 19:100–103. doi: 10.1089/mdr.2012.0063. [DOI] [PubMed] [Google Scholar]
- 268.Nakamura I, Sakamoto N, Ida Y, Imai R, Aoki K, Ando R, Yamaguchi T, Matsumura H, Matsumoto T. 2015. Combination therapy against polymicrobial infection, including by NDM-1-producing Enterobacteriaceae resistant to colistin. Antimicrob Agents Chemother 59:5092–5093. doi: 10.1128/AAC.00746-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Flateau C, Janvier F, Delacour H, Males S, Ficko C, Andriamanantena D, Jeannot K, Merens A, Rapp C. 2012. Recurrent pyelonephritis due to NDM-1 metallo-β-lactamase producing Pseudomonas aeruginosa in a patient returning from Serbia, France, 2012. Euro Surveill 17(45):pii=20311 https://www.eurosurveillance.org/content/10.2807/ese.17.45.20311-en. [PubMed] [Google Scholar]
- 270.Chien JM, Koh TH, Chan KS, Chuah TH, Tan TT. 2012. Successful treatment of NDM-1 Klebsiella pneumoniae bacteraemia in a neutropenic patient. Scand J Infect Dis 44:312–314. doi: 10.3109/00365548.2011.633549. [DOI] [PubMed] [Google Scholar]
- 271.Tangden T, Hickman RA, Forsberg P, Lagerback P, Giske CG, Cars O. 2014. Evaluation of double- and triple-antibiotic combinations for VIM- and NDM-producing Klebsiella pneumoniae by in vitro time-kill experiments. Antimicrob Agents Chemother 58:1757–1762. doi: 10.1128/AAC.00741-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Berçot B, Poirel L, Dortet L, Nordmann P. 2011. In vitro evaluation of antibiotic synergy for NDM-1-producing Enterobacteriaceae. J Antimicrob Chemother 66:2295–2297. doi: 10.1093/jac/dkr296. [DOI] [PubMed] [Google Scholar]
- 273.Albur MS, Noel A, Bowker K, MacGowan A. 2015. The combination of colistin and fosfomycin is synergistic against NDM-1-producing Enterobacteriaceae in in vitro pharmacokinetic/pharmacodynamic model experiments. Int J Antimicrob Agents 46:560–567. doi: 10.1016/j.ijantimicag.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 274.Bulman ZP, Liang C, Walsh TJ, Satlin MJ, Qian Y, Bulitta JB, Peloquin CA, Holden PN, Nation RL, Jian L. 2017. Polymyxin combinations combat Escherichia coli harboring mcr-1 and blaNDM-5: preparation for a postantibiotic era. mBio 8:e00540-17. doi: 10.1128/mBio.00540-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Holman AM, Allyn J, Miltgen G, Lugagne N, Traversier N, Picot S, Lignereux A, Oudin C, Belmonte O, Allou N. 2017. Surveillance of carbapenemase-producing Enterobacteriaceae in the Indian Ocean region between January 2010 and December 2015. Med Mal Infect 47:333–339. doi: 10.1016/j.medmal.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 276.Nordmann P, Naas T, Poirel L. 2011. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis 17:1791–1798. doi: 10.3201/eid1710.110655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Monogue ML, Abbo LM, Rosa R, Camargo JF, Martinez O, Bonomo RA, Nicolau DP. 2017. In vitro discordance with in vivo activity: humanized exposures of ceftazidime-avibactam, aztreonam, and tigecycline alone and in combination against New Delhi metallo-β-lactamase-producing Klebsiella pneumoniae in a murine lung infection model. Antimicrob Agents Chemother 61:e00486-17. doi: 10.1128/AAC.00486-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Tzouvelekis LS, Markogiannakis A, Piperaki E, Souli M, Daikos GL. 2014. Treating infections caused by carbapenemase-producing Enterobacteriaceae. Clin Microbiol Infect 20:862–872. doi: 10.1111/1469-0691.12697. [DOI] [PubMed] [Google Scholar]
- 279.Pan Z, Liu R, Zhang P, Zhou H, Fu Y, Zhou J. 2017. Combination of tigecycline and levofloxacin for successful treatment of nosocomial pneumonia caused by New Delhi metallo-β-lactamase-1-producing Raoultella planticola. Microb Drug Resist 23:127–131. doi: 10.1089/mdr.2015.0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Li X, Mu X, Yang Y, Hua X, Yang Q, Wang N, Du X, Ruan Z, Shen X, Yu Y. 2016. Rapid emergence of high-level tigecycline resistance in Escherichia coli strains harbouring blaNDM-5 in vivo. Int J Antimicrob Agents 47:324–327. doi: 10.1016/j.ijantimicag.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 281.Martirosov DM, Lodise TP. 2016. Emerging trends in epidemiology and management of infections caused by carbapenem-resistant Enterobacteriaceae. Diagn Microbiol Infect Dis 85:266–275. doi: 10.1016/j.diagmicrobio.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 282.Daikos GL, Markogiannakis A. 2011. Carbapenemase-producing Klebsiella pneumoniae: (when) might we still consider treating with carbapenems? Clin Microbiol Infect 17:1135–1141. doi: 10.1111/j.1469-0691.2011.03553.x. [DOI] [PubMed] [Google Scholar]
- 283.Poirel L, Kieffer N, Nordmann P. 2016. In vitro evaluation of dual carbapenem combinations against carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother 71:156–161. doi: 10.1093/jac/dkv294. [DOI] [PubMed] [Google Scholar]
- 284.Wiskirchen DE, Nordmann P, Crandon JL, Nicolau DP. 2014. In vivo efficacy of human simulated regimens of carbapenems and comparator agents against NDM-1-producing Enterobacteriaceae. Antimicrob Agents Chemother 58:1671–1677. doi: 10.1128/AAC.01946-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Rosa R, Rudin SD, Rojas LJ, Hujer AM, Perez-Cardona A, Perez F, Bonomo RA, Martinez O, Abbo LM, Camargo JF. 2018. “Double carbapenem” and oral fosfomycin for the treatment of complicated urinary tract infections caused by blaNDM-harboring Enterobacteriaceae in kidney transplantation. Transpl Infect Dis 20:e12795. doi: 10.1111/tid.12795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Zhang Y, Kashikar A, Bush K. 2017. In vitro activity of plazomicin against β-lactamase-producing carbapenem-resistant Enterobacteriaceae (CRE). J Antimicrob Chemother 72:2792–2795. doi: 10.1093/jac/dkx261. [DOI] [PubMed] [Google Scholar]
- 287.Livermore DM, Mushtaq S, Warner M, Zhang JC, Maharjan S, Doumith M, Woodford N. 2011. Activity of aminoglycosides, including ACHN-490, against carbapenem-resistant Enterobacteriaceae isolates. J Antimicrob Chemother 66:48–53. doi: 10.1093/jac/dkq408. [DOI] [PubMed] [Google Scholar]
- 288.Doi Y, Wachino J-I, Arakawa Y. 2016. Aminoglycoside resistance: the emergence of acquired 16S ribosomal RNA methyltransferases. Infect Dis Clin North Am 30:523–537. doi: 10.1016/j.idc.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Hu Y, Liu L, Zhang X, Feng Y, Zong Z. 2017. In vitro activity of neomycin, streptomycin, paromomycin and apramycin against carbapenem-resistant Enterobacteriaceae clinical strains. Front Microbiol 8:2275. doi: 10.3389/fmicb.2017.02275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Kohira N, West J, Ito A, Ito-Horiyama T, Nakamura R, Sato T, Rittenhouse S, Tsuji M, Yamano Y. 2016. In vitro antimicrobial activity of a siderophore cephalosporin, S-649266, against Enterobacteriaceae clinical isolates, including carbapenem-resistant strains. Antimicrob Agents Chemother 60:729–734. doi: 10.1128/AAC.01695-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Matsumoto S, Singley CM, Hoover J, Nakamura R, Echols R, Rittenhouse S, Tsuji M, Yamano Y. 2017. Efficacy of cefiderocol against carbapenem-resistant Gram-negative bacilli in immunocompetent-rat respiratory tract infection models recreating human plasma pharmacokinetics. Antimicrob Agents Chemother 61:e00700-17. doi: 10.1128/AAC.00700-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Portsmouth S, van Veenhuyzen D, Echols R, Machida M, Ferreira JCA, Ariyasu M, Tenke P, Nagata TD. 25 October 2018. Cefiderocol versus imipenem-cilastatin for the treatment of complicated urinary tract infections caused by Gram-negative uropathogens: a phase 2, randomised, double-blind, non-inferiority trial. Lancet Infect Dis doi: 10.1016/S1473-3099(18)30554-1. [DOI] [PubMed] [Google Scholar]
- 293.Blais J, Lopez S, Li C, Ruzin A, Ranjitkar S, Dean CR, Leeds JA, Casarez A, Simmons RL, Reck F. 24 September 2018. In vitro activity of LYS228, a novel monobactam antibiotic, against multidrug-resistant Enterobacteriaceae. Antimicrob Agents Chemother doi: 10.1128/AAC.00552-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Pantel L, Florin T, Dobosz-Bartoszek M, Racine E, Sarciaux M, Serri M, Houard J, Campagne JM, de Figueiredo RM, Midrier C, Gaudriault S, Givaudan A, Lanois A, Forst S, Aumelas A, Cotteaux-Lautard C, Bolla JM, Vingsbo Lundberg C, Huseby DL, Hughes D, Villain-Guillot P, Mankin AS, Polikanov YS, Gualtieri M. 2018. Odilorhabdins, antibacterial agents that cause miscoding by binding at a new ribosomal site. Mol Cell 70:83.e7–94.e7. doi: 10.1016/j.molcel.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 295.Racine E, Nordmann P, Pantel L, Sarciaux M, Serri M, Houard J, Villain-Guillot P, Demords A, Vingsbo Lundberg C, Gualtieri M. 27 August 2018. In vitro and in vivo characterization of NOSO-502, a novel inhibitor of bacterial translation. Antimicrob Agents Chemother doi: 10.1128/AAC.01016-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Zhao M, Lepak AJ, Marchillo K, VanHecker J, Andes DR. 27 August 2018. In vivo pharmacodynamic characterization of a novel odilorhabdin antibiotic, NOSO-502, against Escherichia coli and Klebsiella pneumoniae in a murine thigh infection model. Antimicrob Agents Chemother doi: 10.1128/AAC.01067-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Pan CY, Chen JC, Chen TL, Wu JL, Hui CF, Chen JY. 2015. Piscidin is highly active against carbapenem-resistant Acinetobacter baumannii and NDM-1-producing Klebsiella pneumonia in a systemic septicaemia infection mouse model. Mar Drugs 13:2287–2305. doi: 10.3390/md13042287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Edwards L, Turner D, Champion C, Khandelwal M, Zingler K, Stone C, Rajapaksha RD, Yang J, Ranasinghe MI, Kornienko A, Frolova LV, Rogelj S. 2018. Photoactivated 2,3-distyrylindoles kill multi-drug resistant bacteria. Bioorg Med Chem Lett 28:1879–1886. doi: 10.1016/j.bmcl.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Livermore D. 2018. The 2018 Garrod Lecture: Preparing for the Black Swans of resistance. J Antimicrob Chemother 73:2907–2915. doi: 10.1093/jac/dky265. [DOI] [PubMed] [Google Scholar]
- 300.Mangion IK, Ruck RT, Rivera N, Huffman MA, Shevlin M. 2011. A concise synthesis of a β-lactamase inhibitor. Org Lett 13:5480–5483. doi: 10.1021/ol202195n. [DOI] [PubMed] [Google Scholar]
- 301.Monogue ML, Giovagnoli S, Bissantz C, Zampaloni C, Nicolau DP. 27 August 2018. In vivo efficacy of meropenem, with a novel non-β-lactam β-lactamase inhibitor, nacubactam, against Gram-negative organisms exhibiting various resistance mechanisms in a murine complicated urinary tract infection model. Antimicrob Agents Chemother doi: 10.1128/AAC.02596-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.King DT, Strynadka NC. 2013. Targeting metallo-β-lactamase enzymes in antibiotic resistance. Future Med Chem 5:1243–1263. doi: 10.4155/fmc.13.55. [DOI] [PubMed] [Google Scholar]
- 303.Ju LC, Cheng Z, Fast W, Bonomo RA, Crowder MW. 2018. The continuing challenge of metallo-β-lactamase inhibition: mechanism matters. Trends Pharmacol Sci 39:635–647. doi: 10.1016/j.tips.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Everett M, Sprynski N, Coelho A, Castandet J, Bayet M, Bougnon J, Lozano C, Davies DT, Leiris S, Zalacain M, Morrissey I, Magnet S, Holden K, Warn P, De Luca F, Docquier JD, Lemonnier M. 2018. Discovery of a novel metallo-β-lactamase inhibitor that potentiates meropenem activity against carbapenem-resistant Enterobacteriaceae. Antimicrob Agents Chemother 62:e00074-18. doi: 10.1128/AAC.00074-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.King AM, Reid-Yu SA, Wang W, King DT, De Pascale G, Strynadka NC, Walsh TR, Coombes BK, Wright GD. 2014. Aspergillomarasmine A overcomes metallo-β-lactamase antibiotic resistance. Nature 510:503–506. doi: 10.1038/nature13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Yoshizumi A, Ishii Y, Kimura S, Saga T, Harada S, Yamaguchi K, Tateda K, Livermore DM, Woodford N, Livermore DM. 2013. Efficacies of calcium-EDTA in combination with imipenem in a murine model of sepsis caused by Escherichia coli with NDM-1 β-lactamase. J Infect Chemother 19:992–995. doi: 10.1007/s10156-012-0528-y. [DOI] [PubMed] [Google Scholar]
- 307.Djoko KY, Achard MES, Phan MD, Lo AW, Miraula M, Prombhul S, Hancock SJ, Peters KM, Sidjabat HE, Harris PN, Mitic N, Walsh TR, Anderson GJ, Shafer WM, Paterson DL, Schenk G, McEwan AG, Schembri MA. 2018. Copper ions and coordination complexes as novel carbapenem adjuvants. Antimicrob Agents Chemother 62:e02280-17. doi: 10.1128/AAC.02280-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Kumar R, Chandar B, Parani M. 2018. Use of succinic & oxalic acid in reducing the dosage of colistin against New Delhi metallo-β-lactamase-1 bacteria. Indian J Med Res 147:97–101. doi: 10.4103/ijmr.IJMR_1407_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Uppu DS, Manjunath GB, Yarlagadda V, Kaviyil JE, Ravikumar R, Paramanandham K, Shome BR, Haldar J. 2015. Membrane-active macromolecules resensitize NDM-1 Gram-negative clinical isolates to tetracycline antibiotics. PLoS One 10:e0119422. doi: 10.1371/journal.pone.0119422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Moya B, Barcelo IM, Bhagwat S, Patel M, Bou G, Papp-Wallace KM, Bonomo RA, Oliver A. 2017. WCK 5107 (zidebactam) and WCK 5153 are novel inhibitors of PBP2 showing potent “β-lactam enhancer” activity against Pseudomonas aeruginosa, including multidrug-resistant metallo-β-lactamase-producing high-risk clones. Antimicrob Agents Chemother 61:e02529-16. doi: 10.1128/AAC.02529-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Sader HS, Rhomberg PR, Flamm RK, Jones RN, Castanheira M. 2017. WCK 5222 (cefepime/zidebactam) antimicrobial activity tested against Gram-negative organisms producing clinically relevant β-lactamases. J Antimicrob Chemother 72:1696–1703. doi: 10.1093/jac/dkx050. [DOI] [PubMed] [Google Scholar]
- 312.Chandar B, Poovitha S, Ilango K, MohanKumar R, Parani M. 2017. Inhibition of New Delhi metallo-β-lactamase 1 (NDM-1) producing Escherichia coli IR-6 by selected plant extracts and their synergistic actions with antibiotics. Front Microbiol 8:1580. doi: 10.3389/fmicb.2017.01580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Holloway AJ, Yu J, Arulanandam BP, Hoskinson SM, Eaves-Pyles T. 2018. Cystatins 9 and C as a novel immunotherapy treatment that protects against multidrug-resistant New Delhi metallo-β-lactamase-1-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 62:e01900-17. doi: 10.1128/AAC.01900-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Lowe CF, Kus JV, Salt N, Callery S, Louie L, Khan MA, Vearncombe M, Simor AE. 2013. Nosocomial transmission of New Delhi metallo-β-lactamase-1-producing Klebsiella pneumoniae in Toronto, Canada. Infect Control Hosp Epidemiol 34:49–55. doi: 10.1086/668778. [DOI] [PubMed] [Google Scholar]
- 315.Yoo JS, Kim HM, Koo HS, Yang JW, Yoo JI, Kim HS, Park HK, Lee YS. 2013. Nosocomial transmission of NDM-1-producing Escherichia coli ST101 in a Korean hospital. J Antimicrob Chemother 68:2170–2172. doi: 10.1093/jac/dkt126. [DOI] [PubMed] [Google Scholar]
- 316.Savov E, Politi L, Spanakis N, Trifonova A, Kioseva E, Tsakris A. 2018. NDM-1 hazard in the Balkan states: evidence of the first outbreak of NDM-1-producing Klebsiella pneumoniae in Bulgaria. Microb Drug Resist 24:253–259. doi: 10.1089/mdr.2017.0230. [DOI] [PubMed] [Google Scholar]
- 317.Zhu J, Sun L, Ding B, Yang Y, Xu X, Liu W, Zhu D, Yang F, Zhang H, Hu F. 2016. Outbreak of NDM-1-producing Klebsiella pneumoniae ST76 and ST37 isolates in neonates. Eur J Clin Microbiol Infect Dis 35:611–618. doi: 10.1007/s10096-016-2578-z. [DOI] [PubMed] [Google Scholar]
- 318.Yu J, Tan K, Rong Z, Wang Y, Chen Z, Zhu X, Wu L, Tan L, Xiong W, Sun Z, Chen L. 2016. Nosocomial outbreak of KPC-2- and NDM-1-producing Klebsiella pneumoniae in a neonatal ward: a retrospective study. BMC Infect Dis 16:563. doi: 10.1186/s12879-016-1870-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.O’Connor C, Cormican M, Boo TW, McGrath E, Slevin B, O’Gorman A, Commane M, Mahony S, O’Donovan E, Powell J, Monahan R, Finnegan C, Kiernan MG, Coffey JC, Power L, O’Connell NH, Dunne CP. 2016. An Irish outbreak of New Delhi metallo-β-lactamase (NDM)-1 carbapenemase-producing Enterobacteriaceae: increasing but unrecognized prevalence. J Hosp Infect 94:351–357. doi: 10.1016/j.jhin.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 320.Ho HJ, Toh CY, Ang B, Krishnan P, Lin RT, La MV, Chow A. 2016. Outbreak of New Delhi metallo-β-lactamase-1-producing Enterobacter cloacae in an acute care hospital general ward in Singapore. Am J Infect Control 44:177–182. doi: 10.1016/j.ajic.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 321.Otter JA, Doumith M, Davies F, Mookerjee S, Dyakova E, Gilchrist M, Brannigan ET, Bamford K, Galletly T, Donaldson H, Aanensen DM, Ellington MJ, Hill R, Turton JF, Hopkins KL, Woodford N, Holmes A. 2017. Emergence and clonal spread of colistin resistance due to multiple mutational mechanisms in carbapenemase-producing Klebsiella pneumoniae in London. Sci Rep 7:12711. doi: 10.1038/s41598-017-12637-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Shin S, Jeong SH, Lee H, Hong JS, Park MJ, Song W. 2018. Emergence of multidrug-resistant Providencia rettgeri isolates co-producing NDM-1 carbapenemase and PER-1 extended-spectrum β-lactamase causing a first outbreak in Korea. Ann Clin Microbiol Antimicrob 17:20. doi: 10.1186/s12941-018-0272-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Borgia S, Lastovetska O, Richardson D, Eshaghi A, Xiong J, Chung C, Baqi M, McGeer A, Ricci G, Sawicki R, Pantelidis R, Low DE, Patel SN, Melano RG. 2012. Outbreak of carbapenem-resistant enterobacteriaceae containing blaNDM-1, Ontario, Canada. Clin Infect Dis 55:e109–e117. doi: 10.1093/cid/cis737. [DOI] [PubMed] [Google Scholar]
- 324.Escobar Perez JA, Olarte Escobar NM, Castro-Cardozo B, Valderrama Marquez IA, Garzon Aguilar MI, Martinez de la Barrera L, Barrero Barreto ER, Marquez-Ortiz RA, Moncada Guayazan MV, Vanegas Gomez N. 2013. Outbreak of NDM-1-producing Klebsiella pneumoniae in a neonatal unit in Colombia. Antimicrob Agents Chemother 57:1957–1960. doi: 10.1128/AAC.01447-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Hammerum AM, Hansen F, Nielsen HL, Jakobsen L, Stegger M, Andersen PS, Jensen P, Nielsen TK, Hansen LH, Hasman H, Fuglsang-Damgaard D. 2016. Use of WGS data for investigation of a long-term NDM-1-producing Citrobacter freundii outbreak and secondary in vitro spread of blaNDM-1 to Escherichia coli, Klebsiella pneumoniae and Klebsiella oxytoca. J Antimicrob Chemother 71:3117–3124. doi: 10.1093/jac/dkw289. [DOI] [PubMed] [Google Scholar]
- 326.Torres-Gonzalez P, Bobadilla-Del Valle M, Tovar-Calderon E, Leal-Vega F, Hernandez-Cruz A, Martinez-Gamboa A, Niembro-Ortega MD, Sifuentes-Osornio J, Ponce-de-Leon A. 2015. Outbreak caused by Enterobacteriaceae harboring NDM-1 metallo-β-lactamase carried in an IncFII plasmid in a tertiary care hospital in Mexico City. Antimicrob Agents Chemother 59:7080–7083. doi: 10.1128/AAC.00055-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Friedman ND, Carmeli Y, Walton AL, Schwaber MJ. 2017. Carbapenem-resistant Enterobacteriaceae: a strategic roadmap for infection control. Infect Control Hosp Epidemiol 38:580–594. doi: 10.1017/ice.2017.42. [DOI] [PubMed] [Google Scholar]
- 328.Epstein L, Hunter JC, Arwady MA, Tsai V, Stein L, Gribogiannis M, Frias M, Guh AY, Laufer AS, Black S, Pacilli M, Moulton-Meissner H, Rasheed JK, Avillan JJ, Kitchel B, Limbago BM, MacCannell D, Lonsway D, Noble-Wang J, Conway J, Conover C, Vernon M, Kallen AJ. 2014. New Delhi metallo-β-lactamase-producing carbapenem-resistant Escherichia coli associated with exposure to duodenoscopes. JAMA 312:1447–1455. doi: 10.1001/jama.2014.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.CDC. 2014. Notes from the field: New Delhi metallo-β-lactamase-producing Escherichia coli associated with endoscopic retrograde cholangiopancreatography—Illinois, 2013. MMWR Morb Mortal Wkly Rep 62:1051. [PMC free article] [PubMed] [Google Scholar]
- 330.Mollers M, Lutgens SP, Schoffelen AF, Schneeberger PM, Suijkerbuijk AWM. 2017. Cost of nosocomial outbreak caused by NDM-1-containing Klebsiella pneumoniae in the Netherlands, October 2015-January 2016. Emerg Infect Dis 23:1574–1576. doi: 10.3201/eid2309.161710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.World Health Organization. 2017. Guidelines for the prevention and control of carbapenem-resistant Enterobacteriaceae, Acinetobacter baumannii and Pseudomonas aeruginosa in health care facilities. World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- 332.Royal College of Physicians Ireland. 2012. Guidelines for the prevention and control of multi-drug resistant organisms (MDRO) excluding MRSA in the healthcare setting. Royal College of Physicians Ireland, Dublin, Ireland. [Google Scholar]
- 333.Chung The H, Karkey A, Pham Thanh D, Boinett CJ, Cain AK, Ellington M, Baker KS, Dongol S, Thompson C, Harris SR, Jombart T, Le Thi Phuong T, Tran Do Hoang N, Ha Thanh T, Shretha S, Joshi S, Basnyat B, Thwaites G, Thomson NR, Rabaa MA, Baker S. 2015. A high-resolution genomic analysis of multidrug-resistant hospital outbreaks of Klebsiella pneumoniae. EMBO Mol Med 7:227–239. doi: 10.15252/emmm.201404767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Department of Health and Human Services. 2015. Victorian guideline on carbapenemase producing Enterobacteriaceae for health services. Department of Health and Human Services, Melbourne, Victoria, Australia. [Google Scholar]
- 335.Tacconelli E, Cataldo MA, Dancer SJ, De Angelis G, Falcone M, Frank U, Kahlmeter G, Pan A, Petrosillo N, Rodriguez-Bano J, Singh N, Venditti M, Yokoe DS, Cookson B, European Society of Clinical Microbiology. 2014. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin Microbiol Infect 20:1–55. doi: 10.1111/1469-0691.12427. [DOI] [PubMed] [Google Scholar]
- 336.CDC. 2015. Facility guidance for control of carbapenem-resistant Enterobacteriaceae (CRE) November 2015 update. CDC, Atlanta, GA. [Google Scholar]
- 337.Magiorakos AP, Burns K, Rodriguez Bano J, Borg M, Daikos G, Dumpis U, Lucet JC, Moro ML, Tacconelli E, Simonsen GS, Szilagyi E, Voss A, Weber JT. 2017. Infection prevention and control measures and tools for the prevention of entry of carbapenem-resistant Enterobacteriaceae into healthcare settings: guidance from the European Centre for Disease Prevention and Control. Antimicrob Resist Infect Control 6:113. doi: 10.1186/s13756-017-0259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Haverkate MR, Weiner S, Lolans K, Moore NM, Weinstein RA, Bonten MJ, Hayden MK, Bootsma MC. 2016. Duration of colonization with Klebsiella pneumoniae carbapenemase-producing bacteria at long-term acute care hospitals in Chicago, Illinois. Open Forum Infect Dis 3:ofw178. doi: 10.1093/ofid/ofw178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Feldman N, Adler A, Molshatzki N, Navon-Venezia S, Khabra E, Cohen D, Carmeli Y. 2013. Gastrointestinal colonization by KPC-producing Klebsiella pneumoniae following hospital discharge: duration of carriage and risk factors for persistent carriage. Clin Microbiol Infect 19:E190–E196. doi: 10.1111/1469-0691.12099. [DOI] [PubMed] [Google Scholar]
- 340.Lubbert C, Lippmann N, Busch T, Kaisers UX, Ducomble T, Eckmanns T, Rodloff AC. 2014. Long-term carriage of Klebsiella pneumoniae carbapenemase-2-producing K pneumoniae after a large single-center outbreak in Germany. Am J Infect Control 42:376–380. doi: 10.1016/j.ajic.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 341.D’Andrea MM, Venturelli C, Giani T, Arena F, Conte V, Bresciani P, Rumpianesi F, Pantosti A, Narni F, Rossolini GM. 2011. Persistent carriage and infection by multidrug-resistant Escherichia coli ST405 producing NDM-1 carbapenemase: report on the first Italian cases. J Clin Microbiol 49:2755–2758. doi: 10.1128/JCM.00016-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Lim YJ, Park HY, Lee JY, Kwak SH, Kim MN, Sung H, Kim SH, Choi SH. 2 June 2018. Clearance of carbapenemase-producing Enterobacteriaceae (CPE) carriage: a comparative study of NDM-1 and KPC CPE. Clin Microbiol Infect doi: 10.1016/j.cmi.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 343.Lowe C, Katz K, McGeer A, Muller MP, Toronto ESBL Working Group. 2012. Disparity in infection control practices for multidrug-resistant Enterobacteriaceae. Am J Infect Control 40:836–839. doi: 10.1016/j.ajic.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 344.Zhong LL, Zhang YF, Doi Y, Huang X, Zhang XF, Zeng KJ, Shen C, Patil S, Xing Y, Zou Y, Tian GB. 2017. Coproduction of MCR-1 and NDM-1 by colistin-resistant Escherichia coli isolated from a healthy individual. Antimicrob Agents Chemother 61:e01962-16. doi: 10.1128/AAC.01962-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Xu L, Sun X, Ma X. 2017. Systematic review and meta-analysis of mortality of patients infected with carbapenem-resistant Klebsiella pneumoniae. Ann Clin Microbiol Antimicrob 16:18. doi: 10.1186/s12941-017-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Gomez-Simmonds A, Uhlemann AC. 2017. Clinical implications of genomic adaptation and evolution of carbapenem-resistant Klebsiella pneumoniae. J Infect Dis 215:S18–S27. doi: 10.1093/infdis/jiw378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Pereira PS, Albano RM, Asensi MD, Carvalho-Assef AP. 2015. Draft genome sequences of three NDM-1-producing Enterobacteriaceae species isolated from Brazil. Mem Inst Oswaldo Cruz 110:580–582. doi: 10.1590/0074-02760150081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Khajuria A, Praharaj AK, Grover N, Kumar M. 2013. First report of blaNDM-1 in Raoultella ornithinolytica. Antimicrob Agents Chemother 57:1092–1093. doi: 10.1128/AAC.02147-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Sun F, Yin Z, Feng J, Qiu Y, Zhang D, Luo W, Yang H, Yang W, Wang J, Chen W, Xia P, Zhou D. 2015. Production of plasmid-encoding NDM-1 in clinical Raoultella ornithinolytica and Leclercia adecarboxylata from China. Front Microbiol 6:458. doi: 10.3389/fmicb.2015.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Wang J, Yuan M, Chen H, Chen X, Jia Y, Zhu X, Bai L, Bai X, Fanning S, Lu J, Li J. 2017. First report of Klebsiella oxytoca strain simultaneously producing NDM-1, IMP-4, and KPC-2 carbapenemases. Antimicrob Agents Chemother 61:e00877-17. doi: 10.1128/AAC.00877-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Khajuria A, Praharaj AK, Kumar M, Grover N. 2014. Emergence of NDM-1 in a clinical isolate of Pantoea agglomerans from India. J Glob Antimicrob Resist 2:340–341. doi: 10.1016/j.jgar.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 352.Cavalie L, Mantion B, Fayet O, Prere MF. 2016. blaNDM-1-positive Citrobacter sedlakii: emergence after horizontal gene transfer from Klebsiella pneumoniae in the human intestinal tract. Int J Antimicrob Agents 47:411–413. doi: 10.1016/j.ijantimicag.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 353.Phan HTT, Stoesser N, Maciuca IE, Toma F, Szekely E, Flonta M, Hubbard ATM, Pankhurst L, Do T, Peto TEA, Walker AS, Crook DW, Timofte D. 2017. Illumina short-read and MinION long-read WGS to characterize the molecular epidemiology of an NDM-1 Serratia marcescens outbreak in Romania. J Antimicrob Chemother 73:672–679. doi: 10.1093/jac/dkx456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Day MR, Meunier D, Doumith M, de Pinna E, Woodford N, Hopkins KL. 2015. Carbapenemase-producing Salmonella enterica isolates in the UK. J Antimicrob Chemother 70:2165–2167. doi: 10.1093/jac/dkv075. [DOI] [PubMed] [Google Scholar]
- 355.Zheng B, Xu H, Yu X, Lv T, Jiang X, Cheng H, Zhang J, Chen Y, Huang C, Xiao Y. 2018. Identification and genomic characterization of a KPC-2-, NDM-1- and NDM-5-producing Klebsiella michiganensis isolate. J Antimicrob Chemother 73:536–538. doi: 10.1093/jac/dkx415. [DOI] [PubMed] [Google Scholar]
- 356.Ahmad N, Ali SM, Khan AU. 2017. First reported New Delhi metallo-β-lactamase-1-producing Cedecea lapagei. Int J Antimicrob Agents 49:118–119. doi: 10.1016/j.ijantimicag.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 357.Pereira PS, Borghi M, Albano RM, Lopes JC, Silveira MC, Marques EA, Oliveira JC, Asensi MD, Carvalho-Assef AP. 2015. Coproduction of NDM-1 and KPC-2 in Enterobacter hormaechei from Brazil. Microb Drug Resist 21:234–236. doi: 10.1089/mdr.2014.0171. [DOI] [PubMed] [Google Scholar]
- 358.Shen Y, Xiao WQ, Gong JM, Pan J, Xu QX. 2017. Detection of New Delhi metallo-β-lactamase (encoded by blaNDM-1) in Enterobacter aerogenes in China. J Clin Lab Anal 31:e22044. doi: 10.1002/jcla.22044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Pasteran F, Meo A, Gomez S, Derdoy L, Albronoz E, Faccone D, Guerriero L, Archuby D, Tarzia A, Lopez M, Corso A. 2014. Emergence of genetically related NDM-1-producing Providencia rettgeri strains in Argentina. J Glob Antimicrob Resist 2:344–345. doi: 10.1016/j.jgar.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 360.Manageiro V, Sampaio DA, Pereira P, Rodrigues P, Vieira L, Palos C, Canica M. 2015. Draft genome sequence of the first NDM-1-producing Providencia stuartii strain isolated in Portugal. Genome Announc 3:e01077-15. doi: 10.1128/genomeA.01077-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Seija V, Medina Presentado JC, Bado I, Papa Ezdra R, Batista N, Gutierrez C, Guirado M, Vidal M, Nin M, Vignoli R. 2015. Sepsis caused by New Delhi metallo-β-lactamase (blaNDM-1) and qnrD-producing Morganella morganii, treated successfully with fosfomycin and meropenem: case report and literature review. Int J Infect Dis 30:20–26. doi: 10.1016/j.ijid.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 362.Bosnjak Z, Plecko V, Budimir A, Marekovic I, Bedenic B. 2014. First report of NDM-1-producing Acinetobacter guillouiae. Chemotherapy 60:250–252. doi: 10.1159/000381256. [DOI] [PubMed] [Google Scholar]
- 363.Pasteran F, Mora MM, Albornoz E, Faccone D, Franco R, Ortellado J, Melgarejo N, Gomez S, Riquelme I, Matheu J, Ramon-Pardo P, Corso A. 2014. Emergence of genetically unrelated NDM-1-producing Acinetobacter pittii strains in Paraguay. J Antimicrob Chemother 69:2575–2578. doi: 10.1093/jac/dku139. [DOI] [PubMed] [Google Scholar]
- 364.Montaña S, Cittadini R, Del Castillo M, Uong S, Lazzaro T, Almuzara M, Barberis C, Vay C, Ramírez MS. 2016. Presence of New Delhi metallo-β-lactamase gene (NDM-1) in a clinical isolate of Acinetobacter junii in Argentina. New Microbes New Infect 11:43–44. doi: 10.1016/j.nmni.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Montana S, Palombarani S, Carulla M, Kunst A, Rodriguez CH, Nastro M, Vay C, Ramirez MS, Almuzara M. 2018. First case of bacteraemia due to Acinetobacter schindleri harbouring blaNDM-1 in an immunocompromised patient. New Microbes New Infect 21:28–30. doi: 10.1016/j.nmni.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Brovedan M, Marchiaro PM, Moran-Barrio J, Revale S, Cameranesi M, Brambilla L, Viale AM, Limansky AS. 2016. Draft genome sequence of Acinetobacter bereziniae HPC229, a carbapenem-resistant clinical strain from Argentina harboring blaNDM-1. Genome Announc 4:e00117-16. doi: 10.1128/genomeA.00117-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Islam MA, Islam M, Hasan R, Hossain MI, Nabi A, Rahman M, Goessens WHF, Endtz HP, Boehm AB, Faruque SM. 2017. Environmental spread of New Delhi metallo-β-lactamase-1-producing multidrug-resistant bacteria in Dhaka, Bangladesh. Appl Environ Microbiol 83:e00793-17. doi: 10.1128/AEM.00793-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Chen Y, Yan Z, Wang M, Zheng X, Lu Y, Lin S. 2014. Draft genome sequence of a multidrug-resistant blaNDM-1-producing Acinetobacter soli isolate in China. Indian J Microbiol 54:474–475. doi: 10.1007/s12088-014-0469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Sun Y, Ji X, Liu Y, Liu Q, Guo X, Liu J, Xu L, Zhu L, Zhou W, Feng S. 2015. New Delhi metallo-β-lactamase-1-producing Acinetobacter lwoffii of companion animal origin in China. Indian J Med Microbiol 33:615–617. doi: 10.4103/0255-0857.167333. [DOI] [PubMed] [Google Scholar]
- 370.Wang B, Sun D. 2015. Detection of NDM-1 carbapenemase-producing Acinetobacter calcoaceticus and Acinetobacter junii in environmental samples from livestock farms. J Antimicrob Chemother 70:611–613. doi: 10.1093/jac/dku405. [DOI] [PubMed] [Google Scholar]
- 371.Zhang R, Hu YY, Yang XF, Gu DX, Zhou HW, Hu QF, Zhao K, Yu SF, Chen GX. 2014. Emergence of NDM-producing non-baumannii Acinetobacter spp. isolated from China. Eur J Clin Microbiol Infect Dis 33:853–860. doi: 10.1007/s10096-013-2024-4. [DOI] [PubMed] [Google Scholar]
- 372.Mathlouthi N, El Salabi AA, Ben Jomaa-Jemili M, Bakour S, Al-Bayssari C, Zorgani AA, Kraiema A, Elahmer O, Okdah L, Rolain JM, Chouchani C. 2016. Early detection of metallo-β-lactamase NDM-1- and OXA-23 carbapenemase-producing Acinetobacter baumannii in Libyan hospitals. Int J Antimicrob Agents 48:46–50. doi: 10.1016/j.ijantimicag.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 373.Fu Y, Du X, Ji J, Chen Y, Jiang Y, Yu Y. 2012. Epidemiological characteristics and genetic structure of blaNDM-1 in non-baumannii Acinetobacter spp. in China. J Antimicrob Chemother 67:2114–2122. doi: 10.1093/jac/dks192. [DOI] [PubMed] [Google Scholar]
- 374.Fu Y, Liu L, Li X, Chen Y, Jiang Y, Wang Y, Yu Y, Xie X. 2015. Spread of a common blaNDM-1-carrying plasmid among diverse Acinetobacter species. Infect Genet Evol 32:30–33. doi: 10.1016/j.meegid.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 375.Tada T, Shimada K, Satou K, Hirano T, Pokhrel BM, Sherchand JB, Kirikae T. 2017. Pseudomonas aeruginosa clinical isolates in Nepal coproducing metallo-β-lactamases and 16S rRNA methyltransferases. Antimicrob Agents Chemother 61:e00694-17. doi: 10.1128/AAC.00694-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Bhattacharya D, Dey S, Kadam S, Kalal S, Jali S, Koley H, Sinha R, Nag D, Kholkute SD, Roy S. 2015. Isolation of NDM-1-producing multidrug-resistant Pseudomonas putida from a paediatric case of acute gastroenteritis, India. New Microbes New Infect 5:5–9. doi: 10.1016/j.nmni.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Furlan JPR, Pitondo-Silva A, Stehling EG. 2018. Detection of blaNDM-1 in Stenotrophomonas maltophilia isolated from Brazilian soil. Mem Inst Oswaldo Cruz 113:e170558. doi: 10.1590/0074-02760170558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Briet A, Helsens N, Delannoy S, Debuiche S, Brisabois A, Midelet G, Granier SA. 22 May 2018. NDM-1-producing Vibrio parahaemolyticus isolated from imported seafood. J Antimicrob Chemother doi: 10.1093/jac/dky200. [DOI] [PubMed] [Google Scholar]
- 379.Chowdhury G, Pazhani GP, Sarkar A, Rajendran K, Mukhopadhyay AK, Bhattacharya MK, Ghosh A, Ramamurthy T. 2016. Carbapenem resistance in clonally distinct clinical strains of Vibrio fluvialis isolated from diarrheal samples. Emerg Infect Dis 22:1754–1761. doi: 10.3201/eid2210.151612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Kaase M, Nordmann P, Wichelhaus TA, Gatermann SG, Bonnin RA, Poirel L. 2011. NDM-2 carbapenemase in Acinetobacter baumannii from Egypt. J Antimicrob Chemother 66:1260–1262. doi: 10.1093/jac/dkr135. [DOI] [PubMed] [Google Scholar]
- 381.Kumar M. 2016. Identification of a novel NDM variant, blaNDM-3, from a multidrug-resistant Acinetobacter baumannii. Infect Control Hosp Epidemiol 37:747–748. doi: 10.1017/ice.2016.66. [DOI] [PubMed] [Google Scholar]
- 382.Papagiannitsis CC, Studentova V, Chudackova E, Bergerova T, Hrabak J, Radej J, Novak I. 2013. Identification of a New Delhi metallo-β-lactamase-4 (NDM-4)-producing Enterobacter cloacae from a Czech patient previously hospitalized in Sri Lanka. Folia Microbiol (Praha) 58:547–549. doi: 10.1007/s12223-013-0247-5. [DOI] [PubMed] [Google Scholar]
- 383.Tada T, Tsuchiya M, Shimada K, Nga TTT, Thu LTA, Phu TT, Ohmagari N, Kirikae T. 2017. Dissemination of carbapenem-resistant Klebsiella pneumoniae clinical isolates with various combinations of carbapenemases (KPC-2, NDM-1, NDM-4, and OXA-48) and 16S rRNA methylases (RmtB and RmtC) in Vietnam. BMC Infect Dis 17:467. doi: 10.1186/s12879-017-2570-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Shrestha B, Tada T, Shimada K, Shrestha S, Ohara H, Pokhrel BM, Sherchand JB, Kirikae T. 2017. Emergence of various NDM-type-metallo-β-lactamase-producing Escherichia coli clinical isolates in Nepal. Antimicrob Agents Chemother 61:e01425-17. doi: 10.1128/AAC.01425-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Li X, Fu Y, Shen M, Huang D, Du X, Hu Q, Zhou Y, Wang D, Yu Y. 2018. Dissemination of blaNDM-5 gene via an IncX3-type plasmid among non-clonal Escherichia coli in China. Antimicrob Resist Infect Control 7:59. doi: 10.1186/s13756-018-0349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Valentin T, Feierl G, Masoud-Landgraf L, Kohek P, Luxner J, Zarfel G. 2018. Proteus mirabilis harboring carbapenemase NDM-5 and ESBL VEB-6 detected in Austria. Diagn Microbiol Infect Dis 91:284–286. doi: 10.1016/j.diagmicrobio.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 387.Williamson DA, Sidjabat HE, Freeman JT, Roberts SA, Silvey A, Woodhouse R, Mowat E, Dyet K, Paterson DL, Blackmore T, Burns A, Heffernan H. 2012. Identification and molecular characterisation of New Delhi metallo-β-lactamase-1 (NDM-1)- and NDM-6-producing Enterobacteriaceae from New Zealand hospitals. Int J Antimicrob Agents 39:529–533. doi: 10.1016/j.ijantimicag.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 388.Tada T, Miyoshi-Akiyama T, Dahal RK, Sah MK, Ohara H, Kirikae T, Pokhrel BM. 2013. NDM-8 metallo-β-lactamase in a multidrug-resistant Escherichia coli strain isolated in Nepal. Antimicrob Agents Chemother 57:2394–2396. doi: 10.1128/AAC.02553-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Liu BT, Song FJ, Zou M, Hao ZH, Shan H. 2017. Emergence of colistin resistance gene mcr-1 in Cronobacter sakazakii producing NDM-9 and in Escherichia coli from the same animal. Antimicrob Agents Chemother 61:e01444-16. doi: 10.1128/AAC.01444-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Wang X, Li H, Zhao C, Chen H, Liu J, Wang Z, Wang Q, Zhang Y, He W, Zhang F, Wang H. 2014. Novel NDM-9 metallo-β-lactamase identified from a ST107 Klebsiella pneumoniae strain isolated in China. Int J Antimicrob Agents 44:90–91. doi: 10.1016/j.ijantimicag.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 391.Lai CC, Chuang YC, Chen CC, Tang HJ. 2017. Coexistence of MCR-1 and NDM-9 in a clinical carbapenem-resistant Escherichia coli isolate. Int J Antimicrob Agents 49:517–518. doi: 10.1016/j.ijantimicag.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 392.Khajuria A, Praharaj AK, Kumar M, Grover N. 2016. Presence of a novel variant NDM-10, of the New Delhi metallo-β-lactamase in a Klebsiella pneumoniae isolate. Indian J Med Microbiol 34:121–123. doi: 10.4103/0255-0857.174101. [DOI] [PubMed] [Google Scholar]
- 393.Rahman M, Mukhopadhyay C, Rai RP, Singh S, Gupta S, Singh A, Pathak A, Prasad KN. 12 April 2018. Novel variant NDM-11 and other NDM-1 variants in multidrug resistant Escherichia coli from South India. J Glob Antimicrob Resist doi: 10.1016/j.jgar.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 394.Singh-Moodley A, Perovic O. 2016. Antimicrobial susceptibility testing in predicting the presence of carbapenemase genes in Enterobacteriaceae in South Africa. BMC Infect Dis 16:536. doi: 10.1186/s12879-016-1858-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Kazi M, Drego L, Nikam C, Ajbani K, Soman R, Shetty A, Rodrigues C. 2015. Molecular characterization of carbapenem-resistant Enterobacteriaceae at a tertiary care laboratory in Mumbai. Eur J Clin Microbiol Infect Dis 34:467–472. doi: 10.1007/s10096-014-2249-x. [DOI] [PubMed] [Google Scholar]
- 396.Rojas LJ, Wright MS, De La Cadena E, Motoa G, Hujer KM, Villegas MV, Adams MD, Bonomo RA. 2016. Initial assessment of the molecular epidemiology of blaNDM-1 in Colombia. Antimicrob Agents Chemother 60:4346–4350. doi: 10.1128/AAC.03072-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Liu BT, Song FJ, Zou M, Zhang QD, Shan H. 2017. High incidence of Escherichia coli strains coharboring mcr-1 and blaNDM from chickens. Antimicrob Agents Chemother 61:e02347-16. doi: 10.1128/AAC.02347-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Pitart C, Sole M, Roca I, Roman A, Moreno A, Vila J, Marco F. 2015. Molecular characterization of blaNDM-5 carried on an IncFII plasmid in an Escherichia coli isolate from a nontraveler patient in Spain. Antimicrob Agents Chemother 59:659–662. doi: 10.1128/AAC.04040-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Rahman M, Shukla SK, Prasad KN, Ovejero CM, Pati BK, Tripathi A, Singh A, Srivastava AK, Gonzalez-Zorn B. 2014. Prevalence and molecular characterisation of New Delhi metallo-β-lactamases NDM-1, NDM-5, NDM-6 and NDM-7 in multidrug-resistant Enterobacteriaceae from India. Int J Antimicrob Agents 44:30–37. doi: 10.1016/j.ijantimicag.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 400.Mediavilla JR, Patrawalla A, Chen L, Chavda KD, Mathema B, Vinnard C, Dever LL, Kreiswirth BN. 2016. Colistin- and carbapenem-resistant Escherichia coli harboring mcr-1 and blaNDM-5, causing a complicated urinary tract infection in a patient from the United States. mBio 7:e01191-16. doi: 10.1128/mBio.01191-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Chou A, Roa M, Evangelista MA, Sulit AK, Lagamayo E, Torres BC, Klinzing DC, Daroy ML, Navoa-Ng J, Sucgang R, Zechiedrich L. 2016. Emergence of Klebsiella pneumoniae ST273 carrying blaNDM-7 and ST656 carrying blaNDM-1 in Manila, Philippines. Microb Drug Resist 22:585–588. doi: 10.1089/mdr.2015.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Gottig S, Hamprecht AG, Christ S, Kempf VA, Wichelhaus TA. 2013. Detection of NDM-7 in Germany, a new variant of the New Delhi metallo-β-lactamase with increased carbapenemase activity. J Antimicrob Chemother 68:1737–1740. doi: 10.1093/jac/dkt088. [DOI] [PubMed] [Google Scholar]
- 403.Tada T, Shrestha B, Miyoshi-Akiyama T, Shimada K, Ohara H, Kirikae T, Pokhrel BM. 2014. NDM-12, a novel New Delhi metallo-β-lactamase variant from a carbapenem-resistant Escherichia coli clinical isolate in Nepal. Antimicrob Agents Chemother 58:6302–6305. doi: 10.1128/AAC.03355-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Gonzalez MM, Kosmopoulou M, Mojica MF, Castillo V, Hinchliffe P, Pettinati I, Brem J, Schofield CJ, Mahler G, Bonomo RA, Llarrull LI, Spencer J, Vila AJ. 2015. Bisthiazolidines: a substrate-mimicking scaffold as an inhibitor of the NDM-1 carbapenemase. ACS Infect Dis 1:544–554. doi: 10.1021/acsinfecdis.5b00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Li N, Xu Y, Xia Q, Bai C, Wang T, Wang L, He D, Xie N, Li L, Wang J, Zhou H-G, Xu F, Yang C, Zhang Q, Yin Z, Guo Y, Chen Y. 2014. Simplified captopril analogues as NDM-1 inhibitors. Bioorg Med Chem Lett 24:386–389. doi: 10.1016/j.bmcl.2013.10.068. [DOI] [PubMed] [Google Scholar]
- 406.Ehlers MR, Riordan JF. 1991. Angiotensin-converting enzyme: zinc- and inhibitor-binding stoichiometries of the somatic and testis isozymes. Biochemistry 30:7118–7126. doi: 10.1021/bi00243a012. [DOI] [PubMed] [Google Scholar]
- 407.Klingler F-M, Wichelhaus TA, Frank D, Cuesta-Bernal J, El-Delik J, Müller HF, Sjuts H, Göttig S, Koenigs A, Pos KM, Pogoryelov D, Proschak E. 2015. Approved drugs containing thiols as inhibitors of metallo-β-lactamases: strategy to combat multidrug-resistant bacteria. J Med Chem 58:3626–3630. doi: 10.1021/jm501844d. [DOI] [PubMed] [Google Scholar]
- 408.Shen B, Yu Y, Chen H, Cao X, Lao X, Fang Y, Shi Y, Chen J, Zheng H. 2013. Inhibitor discovery of full-length New Delhi metallo-β-lactamase-1 (NDM-1). PLoS One 8:e62955. doi: 10.1371/journal.pone.0062955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Zhang YL, Yang KW, Zhou YJ, Lacuran AE, Oelschlaeger P, Crowder MW. 2014. Diaryl-substituted azolylthioacetamides: inhibitor discovery of New Delhi metallo-β-lactamase-1 (NDM-1). ChemMedChem 9:2445–2448. doi: 10.1002/cmdc.201402249. [DOI] [PubMed] [Google Scholar]
- 410.Liu XL, Shi Y, Kang JS, Oelschlaeger P, Yang KW. 2015. Amino acid thioester derivatives: a highly promising scaffold for the development of metallo-β-lactamase 1 inhibitors. ACS Med Chem Lett 6:660–664. doi: 10.1021/acsmedchemlett.5b00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Ma J, Cao Q, Mcleod SM, Ferguson K, Gao N, Breeze AL, Hu J. 2015. Target-based whole-cell screening by 1H NMR spectroscopy. Angew Chem Int Ed Engl 54:4764–4767. doi: 10.1002/anie.201410701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Wang X, Lu M, Shi Y, Ou Y, Cheng X. 2015. Discovery of novel New Delhi metallo-β-lactamases-1 inhibitors by multistep virtual screening. PLoS One 10:e0118290. doi: 10.1371/journal.pone.0118290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Spyrakis F, Celenza G, Marcoccia F, Santucci M, Cross S, Bellio P, Cendron L, Perilli M, Tondi D. 2018. Structure-based virtual screening for the discovery of novel inhibitors of New Delhi metallo-β-lactamase-1. ACS Med Chem Lett 9:45–50. doi: 10.1021/acsmedchemlett.7b00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Somboro AM, Tiwari D, Bester LA, Parboosing R, Chonco L, Kruger HG, Arvidsson PI, Govender T, Naicker T, Essack SY. 2015. NOTA: a potent metallo-β-lactamase inhibitor. J Antimicrob Chemother 70:1594–1596. doi: 10.1093/jac/dku538. [DOI] [PubMed] [Google Scholar]
- 415.Drawz SM, Papp-Wallace KM, Bonomo RA. 2014. New β-lactamase inhibitors: a therapeutic renaissance in an MDR world. Antimicrob Agents Chemother 58:1835–1846. doi: 10.1128/AAC.00826-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Ma J, McLeod S, MacCormack K, Sriram S, Gao N, Breeze AL, Hu J. 2014. Real-time monitoring of New Delhi metallo-β-lactamase activity in living bacterial cells by 1H NMR spectroscopy. Angew Chem Int Ed Engl 53:2130–2133. doi: 10.1002/anie.201308636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Wang R, Lai TP, Gao P, Zhang H, Ho PL, Woo PC, Ma G, Kao RY, Li H, Sun H. 2018. Bismuth antimicrobial drugs serve as broad-spectrum metallo-β-lactamase inhibitors. Nat Commun 9:439. doi: 10.1038/s41467-018-02828-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Chen AY, Thomas PW, Stewart AC, Bergstrom A, Cheng Z, Miller C, Bethel CR, Marshall SH, Credille CV, Riley CL, Page RC, Bonomo RA, Crowder MW, Tierney DL, Fast W, Cohen SM. 2017. Dipicolinic acid derivatives as inhibitors of New Delhi metallo-β-lactamase-1. J Med Chem 60:7267–7283. doi: 10.1021/acs.jmedchem.7b00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Klingler FM, Moser D, Büttner D, Wichelhaus TA, Löhr F, Dötsch V, Proschak E. 2015. Probing metallo-β-lactamases with molecular fragments identified by consensus docking. Bioorg Med Chem Lett 25:5243–5246. doi: 10.1016/j.bmcl.2015.09.056. [DOI] [PubMed] [Google Scholar]
- 420.Gan M, Liu Y, Bai Y, Guan Y, Li L, Gao R, He W, You X, Li Y, Yu L, Xiao C. 2013. Polyketides with New Delhi metallo-β-lactamase 1 inhibitory activity from Penicillium sp. J Nat Prod 76:1535–1540. doi: 10.1021/np4000944. [DOI] [PubMed] [Google Scholar]
- 421.Christopeit T, Albert A, Leiros HS. 2016. Discovery of a novel covalent non-β-lactam inhibitor of the metallo-β-lactamase NDM-1. Bioorg Med Chem 24:2947–2953. doi: 10.1016/j.bmc.2016.04.064. [DOI] [PubMed] [Google Scholar]
- 422.Thomas PW, Spicer T, Cammarata M, Brodbelt JS, Hodder P, Fast W. 2013. An altered zinc-binding site confers resistance to a covalent inactivator of New Delhi metallo-β-lactamase-1 (NDM-1) discovered by high-throughput screening. Bioorg Med Chem 21:3138–3146. doi: 10.1016/j.bmc.2013.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Chiou J, Wan S, Chan KF, So PK, He D, Chan EW, Chan TH, Wong KY, Tao J, Chen S. 2015. Ebselen as a potent covalent inhibitor of New Delhi metallo-β-lactamase (NDM-1). Chem Commun (Camb) 51:9543–9546. doi: 10.1039/c5cc02594j. [DOI] [PubMed] [Google Scholar]
- 424.Thomas PW, Cammarata M, Brodbelt JS, Fast W. 2014. Covalent inhibition of New Delhi metallo-β-lactamase-1 (NDM-1) by cefaclor. Chembiochem 15:2541–2548. doi: 10.1002/cbic.201402268. [DOI] [PubMed] [Google Scholar]
- 425.Yang KW, Feng L, Yang SK, Aitha M, Lacuran AE, Oelschlaeger P, Crowder MW. 2013. New β-phospholactam as a carbapenem transition state analog: synthesis of a broad-spectrum inhibitor of metallo-β-lactamases. Bioorg Med Chem Lett 23:5855–5859. doi: 10.1016/j.bmcl.2013.08.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Berkel SSV, Brem J, Rydzik AM, Salimraj R, Cain R, Verma A, Owens RJ, Fishwick CWG, Spencer J, Schofield CJ. 2013. Assay platform for clinically relevant metallo-β-lactamases. J Med Chem 56:6945–6953. doi: 10.1021/jm400769b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Worthington RJ, Bunders CA, Reed CS, Melander C. 2012. Small molecule suppression of carbapenem resistance in NDM-1 producing Klebsiella pneumoniae. ACS Med Chem Lett 3:357–361. doi: 10.1021/ml200290p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Sully EK, Geller BL, Li L, Moody CM, Bailey SM, Moore AL, Wong M, Nordmann P, Daly SM, Sturge CR. 2016. Peptide-conjugated phosphorodiamidate morpholino oligomer (PPMO) restores carbapenem susceptibility to NDM-1-positive pathogens in vitro and in vivo. J Antimicrob Chemother 72:782–790. doi: 10.1093/jac/dkw476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Heinrichs A, Argudin MA, De Mendonca R, Deplano A, Roisin S, Dodemont M, Coussement J, Filippin L, Dombrecht J, De Bruyne K, Huang TD, Supply P, Byl B, Glupczynski Y, Denis O. 18 July 2018. An outpatient clinic as a potential site of transmission for an outbreak of NDM-producing Klebsiella pneumoniae ST716: a study using whole-genome sequencing. Clin Infect Dis doi: 10.1093/cid/ciy581. [DOI] [PubMed] [Google Scholar]
- 430.Poirel L, Savov E, Nazli A, Trifonova A, Todorova I, Gergova I, Nordmann P. 2014. Outbreak caused by NDM-1- and RmtB-producing Escherichia coli in Bulgaria. Antimicrob Agents Chemother 58:2472–2474. doi: 10.1128/AAC.02571-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Wang X, Xu X, Li Z, Chen H, Wang Q, Yang P, Zhao C, Ni M, Wang H. 2014. An outbreak of a nosocomial NDM-1-producing Klebsiella pneumoniae ST147 at a teaching hospital in mainland China. Microb Drug Resist 20:144–149. doi: 10.1089/mdr.2013.0100. [DOI] [PubMed] [Google Scholar]
- 432.Jin Y, Shao C, Li J, Fan H, Bai Y, Wang Y. 2015. Outbreak of multidrug resistant NDM-1-producing Klebsiella pneumoniae from a neonatal unit in Shandong Province, China. PLoS One 10:e0119571. doi: 10.1371/journal.pone.0119571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Zhang X, Li X, Wang M, Yue H, Li P, Liu Y, Cao W, Yao D, Liu L, Zhou X, Zheng R, Bo T. 2015. Outbreak of NDM-1-producing Klebsiella pneumoniae causing neonatal infection in a teaching hospital in mainland China. Antimicrob Agents Chemother 59:4349–4351. doi: 10.1128/AAC.03868-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Yu J, Wang Y, Chen Z, Zhu X, Tian L, Li L, Sun Z. 2017. Outbreak of nosocomial NDM-1-producing Klebsiella pneumoniae ST1419 in a neonatal unit. J Glob Antimicrob Resist 8:135–139. doi: 10.1016/j.jgar.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 435.Zheng R, Zhang Q, Guo Y, Feng Y, Liu L, Zhang A, Zhao Y, Yang X, Xia X. 2016. Outbreak of plasmid-mediated NDM-1-producing Klebsiella pneumoniae ST105 among neonatal patients in Yunnan, China. Ann Clin Microbiol Antimicrob 15:10. doi: 10.1186/s12941-016-0124-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Jia X, Dai W, Ma W, Yan J, He J, Li S, Li C, Yang S, Xu X, Sun S, Shi J, Zhang L. 2018. Carbapenem-resistant E. cloacae in southwest China: molecular analysis of resistance and risk factors for infections caused by NDM-1-producers. Front Microbiol 9:658. doi: 10.3389/fmicb.2018.00658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Decousser JW, Jansen C, Nordmann P, Emirian A, Bonnin RA, Anais L, Merle JC, Poirel L. 2013. Outbreak of NDM-1-producing Acinetobacter baumannii in France, January to May 2013. Euro Surveill 18(31):pii=20547. doi: 10.2807/1560-7917.ES2013.18.31.20547. [DOI] [PubMed] [Google Scholar]
- 438.Roy S, Singh AK, Viswanathan R, Nandy RK, Basu S. 2011. Transmission of imipenem resistance determinants during the course of an outbreak of NDM-1 Escherichia coli in a sick newborn care unit. J Antimicrob Chemother 66:2773–2780. doi: 10.1093/jac/dkr376. [DOI] [PubMed] [Google Scholar]
- 439.Khajuria A, Praharaj AK, Kumar M, Grover N, Aggarwal A. 2014. Multidrug resistant NDM-1 metallo-β-lactamase producing Klebsiella pneumoniae sepsis outbreak in a neonatal intensive care unit in a tertiary care center at central India. Indian J Pathol Microbiol 57:65–68. doi: 10.4103/0377-4929.130900. [DOI] [PubMed] [Google Scholar]
- 440.Solgi H, Badmasti F, Giske CG, Aghamohammad S, Shahcheraghi F. 2018. Molecular epidemiology of NDM-1- and OXA-48-producing Klebsiella pneumoniae in an Iranian hospital: clonal dissemination of ST11 and ST893. J Antimicrob Chemother 73:1517–1524. doi: 10.1093/jac/dky081. [DOI] [PubMed] [Google Scholar]
- 441.Jamal WY, Albert MJ, Rotimi VO. 2016. High prevalence of New Delhi metallo-β-lactamase-1 (NDM-1) producers among carbapenem-resistant Enterobacteriaceae in Kuwait. PLoS One 11:e0152638. doi: 10.1371/journal.pone.0152638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Bocanegra-Ibarias P, Garza-González E, Morfín-Otero R, Barrios H, Villarreal-Treviño L, Rodríguez-Noriega E, Garza-Ramos U, Petersen-Morfin S, Silva-Sanchez J. 2017. Molecular and microbiological report of a hospital outbreak of NDM-1-carrying Enterobacteriaceae in Mexico. PLoS One 12:e0179651. doi: 10.1371/journal.pone.0179651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Stoesser N, Sheppard AE, Shakya M, Sthapit B, Thorson S, Giess A, Kelly D, Pollard AJ, Peto TE, Walker AS, Crook DW. 2015. Dynamics of MDR Enterobacter cloacae outbreaks in a neonatal unit in Nepal: insights using wider sampling frames and next-generation sequencing. J Antimicrob Chemother 70:1008–1015. doi: 10.1093/jac/dku521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Hamzaoui Z, Ocampo-Sosa A, Maamar E, Fernandez MM, Ferjani S, Hammami S, Harbaoui S, Genel N, Arlet G, Saidani M, Slim A, Boutiba-Ben Boubaker I, Martinez-Martinez L. 9 October 2018. An outbreak of NDM-1-producing Klebsiella pneumoniae, associated with OmpK35 and OmpK36 porin loss in Tunisia. Microb Drug Resist doi: 10.1089/mdr.2017.0165. [DOI] [PubMed] [Google Scholar]
- 445.Koo VS, O’Neill P, Elves A. 2012. Multidrug-resistant NDM-1 Klebsiella outbreak and infection control in endoscopic urology. BJU Int 110:E922–E926. doi: 10.1111/j.1464-410X.2012.11556.x. [DOI] [PubMed] [Google Scholar]
- 446.Mahida N, Clarke M, White G, Vaughan N, Boswell T. 2017. Outbreak of Enterobacter cloacae with New Delhi metallo-β-lactamase (NDM)-1: challenges in epidemiological investigation and environmental decontamination. J Hosp Infect 97:64–65. doi: 10.1016/j.jhin.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 447.Epson EE, Pisney LM, Wendt JM, MacCannell DR, Janelle SJ, Kitchel B, Rasheed JK, Limbago BM, Gould CV, Kallen AJ, Barron MA, Bamberg WM. 2014. Carbapenem-resistant Klebsiella pneumoniae producing New Delhi metallo-β-lactamase at an acute care hospital, Colorado, 2012. Infect Control Hosp Epidemiol 35:390–397. doi: 10.1086/675607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.