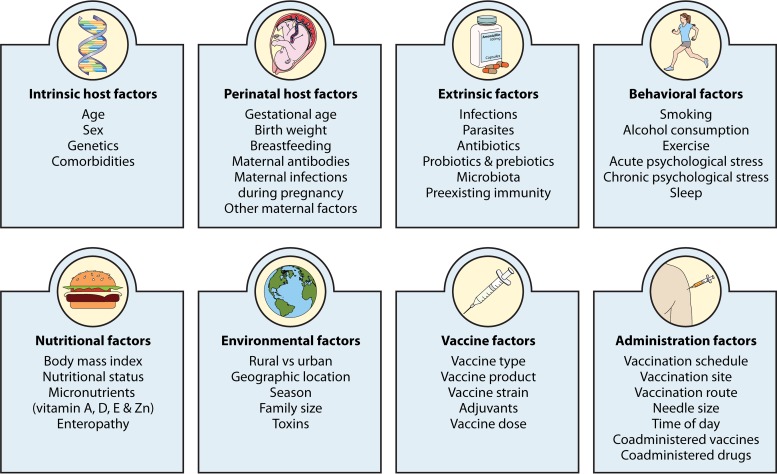
There is substantial variation between individuals in the immune response to vaccination. In this review, we provide an overview of the plethora of studies that have investigated factors that influence humoral and cellular vaccine responses in humans.
KEYWORDS: antibodies, cellular, cytokines, humoral, immunization, immunoglobulin
SUMMARY
There is substantial variation between individuals in the immune response to vaccination. In this review, we provide an overview of the plethora of studies that have investigated factors that influence humoral and cellular vaccine responses in humans. These include intrinsic host factors (such as age, sex, genetics, and comorbidities), perinatal factors (such as gestational age, birth weight, feeding method, and maternal factors), and extrinsic factors (such as preexisting immunity, microbiota, infections, and antibiotics). Further, environmental factors (such as geographic location, season, family size, and toxins), behavioral factors (such as smoking, alcohol consumption, exercise, and sleep), and nutritional factors (such as body mass index, micronutrients, and enteropathy) also influence how individuals respond to vaccines. Moreover, vaccine factors (such as vaccine type, product, adjuvant, and dose) and administration factors (schedule, site, route, time of vaccination, and coadministered vaccines and other drugs) are also important. An understanding of all these factors and their impacts in the design of vaccine studies and decisions on vaccination schedules offers ways to improve vaccine immunogenicity and efficacy.
INTRODUCTION
Vaccination is the most cost-effective life-saving medical intervention and is estimated to save at least 2.5 million lives each year (1, 2). Protection induced by vaccinations is mediated through a complex interplay between innate, humoral, and cell-mediated immunity (3, 4). Methods to quantify vaccine responses include measuring geometric mean antibody titers (GMTs), seroconversion rates (SCRs), seroprotection rates (SPRs), functional antibodies (by flow cytometric opsonophagocytosis assays), antibody avidity, B and T cell activation, lymphoproliferation, and cytokine responses. There is substantial variation between individuals in the immune response to vaccination, in both quantity and quality. For example, the antibody responses to yellow fever (YF) vaccination vary >10-fold between individuals (5), those to 7- and 13-valent conjugated pneumococcal (PCV7 and PCV13) and Haemophilus influenzae type b (Hib) vaccination up to 40-fold (6), and those to trivalent inactivated influenza vaccine (TIV) (7) and hepatitis B (HepB) vaccination >100-fold (6, 8). Similarly, cytokine responses to Mycobacterium bovis bacillus Calmette-Guérin (BCG) vaccination vary up to 10-fold (9). Other examples of differences in the quality of vaccine responses include a lower avidity of antibodies (10) or strength of cell-mediated immune responses (11) in neonates. These variations in vaccine responses have consequences for both protective efficacy and the duration of protection. Worryingly, a significant proportion of vaccine-preventable infections occur in vaccinated individuals (12). It is estimated that large numbers of vaccinated children are unprotected due to vaccine ineffectiveness, including 77 million from tuberculosis (TB) (following BCG vaccination), 19 million from measles, 18 million from poliomyelitis (following vaccination with inactivated polio vaccine [IPV]), and 10 million from pertussis and from pneumococcus (13).
In this review, we provide a general overview of factors that influence the immune response to vaccination (Fig. 1). A greater understanding of these factors offers opportunities to improve vaccine immunogenicity and efficacy.
FIG 1.
Factors that influence the immune response to vaccination.
FACTORS INFLUENCING VACCINE RESPONSES
Intrinsic Host Factors
Age.
Age is an important factor that influences vaccine responses, especially in the extreme ages of life. Infants should receive immunizations as early as possible to minimize the time that they are susceptible to infections. However, neonates have a lower level of antibody production and, moreover, passively acquired maternal antibodies interfere with vaccine responses (14; P. Zimmermann, K. Perrett, N. Messina, S. Donath, N. Ritz, F. R. M. van der Klis, and N. Curtis, submitted for publication). Additionally, cell-mediated immune responses are less strong, and the response to T-independent polysaccharide antigens is poor (11). Studies in the 1950s sought to establish the optimal age to start vaccination (15, 16). These studies showed, for example, that oral polio vaccine (OPV) given during the first week of life leads to adequate serum antibody responses in only 30% to 70% of infants but that, when it is given after 4 to 8 weeks of age, it leads to adequate responses in nearly all infants (15). Similarly, the diphtheria-tetanus-pertussis (DTP) vaccine is less effective when the first dose is given within the first week of life than when it is given at 6 months of age (16) (Table 1). Results of studies investigating the immunogenicity of BCG given at different ages are conflicting, with some studies showing better immunogenicity when the vaccine is given after the age of 2 months than when it is given at birth (17) and others reporting lower immunogenicity when the vaccine is given at 4 months than when it is given at birth (18). HepB vaccine given in the first year of life leads to lower long-term antibody responses than those obtained when it is given later in childhood: only 40% of adolescents who were HepB vaccinated at birth had antibody levels of >10 mIU/ml at the age of 15 years (19), and after a booster dose, antibodies rose above this protective level in only half of individuals (19, 20). However, importantly, in contrast to antibody levels, the number of memory B cells does not decrease over time (21), and hence booster doses of this vaccine are not necessary.
TABLE 1.
Results with regard to age from studies investigating intrinsic host factors that influence vaccine responsesa
| Type of response | Vaccine: measurement [reference(s)] |
||
|---|---|---|---|
| Effective when given at birth | Lower vaccine responses in neonates and young infants | Lower vaccine responses in elderly people | |
| Humoral responses | |||
| Cellular responses | BCG: Th1, Th2, Treg responses (18) | BCG: CD4 T cell response (17) | TIV: Th cell and cytotoxic T cell responses (86), lymphoproliferative responses (87) |
| Cytokine responses | BCG: IL-6 and IL-17 production (18) | TIV: IL-2 (85), IL-10 (87), and IFN-γ (87) production | |
AGGs, agglutinogens; aP, acellular pertussis; BCG, bacillus Calmette-Guérin; CD, cluster of differentiation; FHA, filamentous hemagglutinin; GMTs, geometric mean antibody titers; HepA, hepatitis A; HepB, hepatitis B; Hib, Haemophilus influenzae type b; HLA, human leukocyte antigen; HSV, herpes simplex virus; MHC, major histocompatibility complex; IFN, interferon; IL, interleukin; IPV, inactivated polio vaccine; MCV, meningococcal conjugate vaccine; MPV, meningococcal polysaccharide vaccine; OCV, oral cholera vaccine; OPV, oral polio vaccine; ORV, oral rotavirus vaccine; PCV7, 7-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine; PPV23, 23-valent pneumococcal polysaccharide vaccine; PRN, pertactin; PRR, pattern recognition receptor; PT, pertussis toxin; SCRs, seroconversion rates; SPRs, seroprotection rates; TBE, tick-borne encephalitis; TGF, transforming growth factor; Th, T helper; TIV, trivalent inactivated influenza vaccine; TLR, Toll-like receptor; TNF, tumor necrosis factor; Treg, T regulatory cell; wP, whole-cell pertussis.
The most-studied vaccine in relation to the effect of age on vaccine responses, by far, is the measles vaccine (10, 22–48). A meta-analysis of 20 studies shows that the proportion of infants seroconverting after one dose of measles vaccination increases from 50% at 4 months of age to 85% at 8 months and that GMTs are lower in children who receive their first dose before 9 months of age than in those who receive their first dose at an older age (49). Additionally, antibodies wane significantly more quickly in infants who receive the first dose of measles vaccine before the age of 9 months (49), and antibodies have significantly lower avidity when the first dose of measles vaccine is given before the age of 6 months than when it is given at 9 to 12 months of age (10). A recent large study summarizing randomized controlled trials (RCTs) shows that children who receive their first measles vaccine before the age of 12 months have lower SCRs and geometric mean concentrations (GMCs) than those of children who receive their first measles vaccine after the age of 15 months (50). Lower antibody concentrations persist after the second dose of measles vaccine (49, 50). In contrast, age at first measles vaccination does not influence cellular responses: no differences in in vitro T cell proliferation in response to measles virus were observed after starting vaccination at 4, 6, 9, or 12 months (30, 37, 38, 51).
Despite the fact that many vaccines have lower immunogenicity in neonates, it has been shown that for acellular pertussis (aP) (52, 53), BCG (54), HepB (55), and OPV (15) vaccines, vaccination can be effective in the neonatal age group when adjuvants and formulations are adapted to the function of the early immune system (56) (Table 1). The early neonatal immune system shows suboptimal interaction between antigen-presenting cells and T cells, leading to impairment of CD4 and CD8 T cell function and a polarization toward T helper type 2 (Th2) cells (57) and toward induction of memory B cells rather than antibody-secreting plasma cells (58, 59). T cells largely remain unaffected by passively acquired maternal antibodies.
In addition to those in early life, vaccine responses are also diminished in the elderly, who also have more rapid waning of antibodies. Elderly people have lower antibody responses to diphtheria (60), hepatitis A (HepA) (61–65), HepB (61, 62, 66–81), pneumococcal polysaccharide vaccine (PPV23) (82–84), TIV (85–87), tick-borne encephalitis (TBE) (88), and tetanus (60, 88, 89) vaccination (Table 1). After vaccination with TIV, elderly people also have lower cellular vaccine responses (85–87, 90). In contrast, one study reports higher antibody responses to TIV vaccination in elderly people, with a higher avidity of antibodies and slower antibody dissociation (91). Aging is accompanied by a shift toward anti-inflammatory interleukin-10 (IL-10), which is associated with a decline in CD8 T cells responsible for clearing influenza virus. Hence, adjuvants in TIV that stimulate inflammatory cytokines and suppress the IL-10 response would potentially enhance protection in this age group (92).
Notably, the influence of age on vaccine responses is not seen just at the extremes of age. For example, antibody responses to TIV are higher in children of 2 to 4 years of age than in younger children (93), and GMTs after meningococcal conjugate vaccine (MCV-C) vaccination are higher in children over the age of 10 years than in younger children (94). The findings of studies that investigated the influence of age on vaccine responses are summarized in Table 1.
Sex.
The findings across studies and meta-analyses investigating the effect of sex on vaccine responses are largely consistent (Table 2). Females have higher antibody responses to dengue (95), HepA (61–65, 96–104), HepB (61, 62, 66–78, 80, 100, 101, 105–117), Hib (118), IPV (119), rabies (120–122), smallpox (123), and TIV (124–134) vaccination, while males have higher antibody responses to diphtheria (135–138), MCV-A (139), PCV7 (140), PPV23 (83, 122, 140–144), and tetanus (88, 89, 119) vaccination. Additionally, females also have higher cellular responses to a herpes simplex virus (HSV) vaccine that is not currently used (145). Only a few studies report opposite findings, with higher antibody responses to diphtheria (60, 146), PCV13 (118), and tetanus (60, 147) vaccination in females, higher antibody responses to TIV (91) vaccination in males, or no difference between sexes in antibody responses to rabies (148) vaccination (Table 2).
TABLE 2.
Results with regard to sex from studies investigating intrinsic host factors that influence vaccine responses
| Type of response | Vaccine and age group: measurement [reference(s)] |
|||
|---|---|---|---|---|
| Higher vaccine responses in females | Higher vaccine responses in males | No difference in vaccine responses between females and males | Inconsistent findings in vaccine responses between females and males | |
| Humoral responses |
|
|
|
|
| Cellular responses |
|
|
||
| Cytokine responses | ||||
Notably, even though females have an overall tendency to have higher antibody responses, faster waning of antibodies has been shown following HepA (103) and PPV23 (83, 144) vaccination.
Studies investigating sex differences in response to the measles-mumps-rubella (MMR) vaccine report less consistent findings. Some studies report higher GMTs or SPRs after measles (149), mumps (150), and rubella (151) vaccination and higher cytokine responses after measles (152) vaccination in females, while other studies report transiently higher GMTs and lymphoproliferative responses after rubella (153) vaccination and higher SCRs after measles (154) vaccination in males or no sex differences in GMTs after mumps (155) and rubella (156) vaccination or cytokine responses after rubella (156) vaccination (Table 2).
Reported sex differences in antibody responses to YF vaccine vary depending on the vaccine used (157–159). Interestingly, 3 to 10 days after YF vaccination, expression of 660 genes changes in women, while only 67 genes are expressed differently in men (160). Many of these differentially expressed genes are involved in the early innate immune response (160).
Genetics.
Different ethnic groups living in the same location have varied responses to vaccination (64, 89, 161–166) and decline of antibodies (89), indicating a genetic influence on vaccine responses. Studies of twins estimate the degree of heritability to be 36 to 90% for humoral responses (167–173) and 39 to 90% for cellular responses, depending on the specific vaccine (167, 169) (Table 3).
TABLE 3.
Results with regard to genetics from studies investigating intrinsic host factors that influence vaccine responses
| Type of response | Vaccine (estimated degree of heritability in vaccine responses [%]) [reference(s)] | Variation in vaccine response and vaccine: MHC genes for which polymorphisms have been identified to be associated with variation in vaccine response [reference(s)] | Variation in vaccine response and vaccine: PRR genes for which polymorphisms have been identified to be associated with variation in vaccine response [reference(s)] | Variation in vaccine response and vaccine: proteins for which single-nucleotide polymorphisms in genes are associated with variations in vaccine responses [reference(s)] |
|---|---|---|---|---|
| Humoral responses |
|
|
||
| Cellular responses |
|
|
||
| Cytokine responses |
|
|
|
One mechanism identified with variations in humoral (150, 174–190) and cellular (145, 150, 186, 191) vaccine responses is polymorphism in major histocompatibility complex (MHC) genes (summarized in Table 3). Further genetic factors are polymorphisms in pattern recognition receptors (PRRs), such as Toll-like receptor (TLR) or RIG-like receptor (RLR) genes (192–195) (Table 3). In addition, many single-nucleotide polymorphisms (SNPs) in other genes, for example, those coding for cytokines or cytokine, viral, or vitamin receptors, are also associated with variations in vaccine responses (182, 190, 192, 193, 196–211) (Table 3). Further, different expression levels of genes also influence vaccine responses. For example, individuals with increased expression of genes involved in early interferon signaling, antigen processing, and antigen presentation have higher antibody responses to TIV (194, 212).
Blood group antigens are important receptors or coreceptors for microorganisms. Additionally, they can also modify the innate immune response to infection (213). It is therefore likely that blood group antigens influence responses to vaccination. It has been suggested that a cholera subunit-killed oral vaccine is less efficient in individuals with blood group O (214, 215). In contrast, the oral cholera vaccine (OCV) has been reported to induce higher antibody concentrations in individuals with blood group O (216). This has not, however, been a consistent finding (217).
Comorbidities.
Numerous studies have investigated vaccine responses in children with celiac disease (CD) (218–233) (Table 4). Most found that children with CD have lower antibody responses to HepB vaccination (218–228, 231–233), with more rapid waning of antibodies (227). A meta-analysis shows that, in retrospective studies, the SCR to HepB vaccination in children with CD is 54%, and that in prospective studies is 66% (compared to 95% in healthy individuals) (234). It has been postulated that the presence of HLA-DQ2 or -DQ8, which confers a genetic predisposition to CD, may be the driver of the lower responses to HepB vaccination in CD patients. However, the presence of HLA-DQ2 is associated with higher antibody responses to HepB vaccination (55). Nevertheless, HLA-DR3, -DR4, and -DR7, which can also be found in patients with CD (229) and patients with diabetes mellitus (DM), are associated with lower responses to HepB vaccination (183, 184, 235). Children with CD and DM have lower SPRs after HepB vaccination than those of children that have DM only (228), suggesting that gluten might be a factor in decreasing HepB vaccine efficacy, and some studies suggest that the response rate to HepB vaccination correlates with gluten intake (220, 223). Children with CD who are on a strict gluten-free diet have no difference in SPRs to HepB vaccination compared to those of healthy children (222, 226). Apart from lower antibody responses to HepB vaccination, one study also showed that CD patients are less responsive to HepA vaccination (225). However, another study did not find a difference in antibody responses to HepA vaccination in CD patients compared to healthy controls (229). There is also no difference in antibody responses to tetanus (219), measles (236), rubella (219), Hib (219), and TIV (230) vaccination between children with CD and healthy children.
TABLE 4.
Results with regard to comorbidities from studies investigating intrinsic host factors that influence vaccine responses
| Type of response | Variation in vaccine response, vaccine, age group, measurement [reference(s)] |
|||
|---|---|---|---|---|
| Celiac disease | Diabetes mellitus | Chronic renal failure requiring hemodialysis | Chronic liver failure | |
| Humoral responses |
|
|
|
|
| Cytokine responses | ||||
Children with DM have lower antibody responses to HepB (232, 237–239) vaccination, and possibly PPV23 (240), rubella (241), and measles (241) vaccination, but not to diphtheria (240, 241), Hib (240), PCV7 (240), pertussis (241), or tetanus (240, 241) vaccination. Adults with DM also have lower antibody responses to HepB (242–245) vaccination. Adding selenium to the HepB vaccine can increase antibody responses in patients with DM (246). It is unclear whether DM influences the antibody response to TIV vaccination. Some studies report lower responses after vaccination with a monovalent influenza vaccine in adults (90), while studies of elderly people with type II DM show no difference in antibody or cytokine responses to TIV vaccination (247, 248) (Table 4).
Adults with chronic renal failure who are on hemodialysis have lower antibody responses to diphtheria (249–251), HepB (252–255), and tetanus (249–251) vaccination, with faster waning of antibodies (252–255), especially when they suffer from additional diabetes mellitus (256–259). Children on hemodialysis have lower antibody responses to poliovirus type 2 (260). SPR after HepB vaccination is inversely associated with glomerular filtration rate (253). Factors responsible for lower vaccine responses in patients with chronic renal failure include malnutrition, uremia, and a generalized immunosuppressive state. Patients with chronic renal failure benefit from an increased vaccination dose (259).
Adults with chronic liver disease have lower GMTs after HepA vaccination (102, 261, 262) (with similar SPRs [102, 262] or lower SPRs [263, 264]) and lower GMTs and SPRs after HepB vaccination (242) than those of healthy adults. Children with chronic liver disease also have lower GMTs (265) and SPRs (263, 265) after HepB vaccination (265), with no difference in SCRs. However, in contrast, they have higher antibody responses to IPV and diphtheria (260) vaccination (Table 4).
Perinatal Host Factors
Gestational age.
Preterm infants are at increased risk of infections, including vaccine-preventable infections. Differences in the immune system which render preterm infants less responsive to vaccination include diminished pathogen recognition by dendritic cells and macrophages and diminished T cell activity (especially Th1 activity) and diminished B cell interaction with T cells (266). Variations in vaccine responses between term and preterm infants depend on the specific vaccine. In comparison to those of term infants, preterm infants have significantly lower antibody levels after the first dose of DTP (267). Significantly lower GMTs also persist after primary immunization with three doses for diphtheria (268), HepB (268–270), Hib (269, 271–275), PCV7 (276, 277), poliovirus type 3 (269), and pertussis (267, 268, 278–280) vaccines. However, preterm infants reach sufficient SPRs for most vaccines (Table 5), with the notable exception of responses to HepB (269), Hib (269, 271–275), pertussis (279), and poliovirus type 3 (281) vaccines. In addition to having lower GMTs and SPRs, preterm infants can also have quicker waning of antibodies, as shown for antibodies produced in response to MCV-C (282) vaccination.
TABLE 5.
Results with regard to infant factors from studies investigating perinatal factors that influence vaccine responses
| Type of response | Timing of response, vaccine response, vaccine, age, measurement [reference(s)] |
||
|---|---|---|---|
| Gestational age | Birth weight | Feeding | |
| Humoral responses |
|
|
|
Preterm infants have lower vaccine responses not only after primary immunization but also after booster doses. At 2 to 3 years of age, they have lower antibody responses to HepB (270, 283), Hib (283), and poliovirus type 3 (283) vaccination, and at the age of 5 years, they have lower responses to HepB (283) and pertussis (284) vaccination, as well as reduced lymphoproliferation and cytokine responses in the latter case.
Birth weight.
One month after the third dose of DTaP-Hib-IPV-HepB vaccination given at 2, 4, and 6 months, very-low-birth-weight (VLBW; less than 1.5 kg) infants have significantly lower GMTs to aP and poliovirus type 3 and lower SPRs to Hib than infants with a low birth weight (LBW; 1.5 to 2 kg), while both groups have similar GMTs and SPRs after diphtheria, tetanus, and HepB (285) vaccination. However, 1 month after a booster dose given at the age of 18 to 24 months, the SPR to HepB in VLBW infants is lower than that in LBW infants, and there is a trend toward lower GMTs for all other vaccine components (285) (Table 5). Similarly, 1 month after completing a 3-dose HepB schedule given at 0, 1, and 6 months, LBW infants have lower GMTs to HepB vaccine than infants with a normal birth weight (286). A decreasing birth weight is associated with a progressive reduction in antibody responses to Hib (272) vaccination. However, there is no difference in GMTs after a 3-dose schedule of PCV7 vaccine between LBW infants and infants with a normal weight (277) and no correlation between birth weight and SCRs after measles vaccination (287).
In one study, the antibody response to Salmonella enterica serovar Typhi vaccination (but not rabies vaccination) in adults was reported to be related to birth weight (288).
Breastfeeding.
After routine vaccination, compared to formula-fed infants, breastfed infants have higher serum IgG levels after diphtheria (289), Hib (290, 291), and OPV (289, 292) vaccination, higher salivary IgA levels after tetanus, diphtheria, and OPV vaccination (289), and higher stool IgM levels after tetanus and OPV vaccination (289) (Table 5).
While one meta-analysis summarizing studies on the effect of breastfeeding on the antibody response to oral rotavirus vaccine (ORV) reports reduced SCRs in breastfed infants in all included studies (293), another meta-analysis reports decreased responses in only 3 of 16 studies (294). However, in all the included studies, only one dose of ORV was given, and infants with mixed feeding were sometimes classified as breastfed and sometimes as nonbreastfed. More recent studies show that higher levels of rotavirus-specific IgA in breast milk lead to lower SCRs in infants after ORV (a 2-fold increase is associated with a 22% decrease in SCR) (295). IgA levels in breast milk depend on geographic location: Indian women have higher levels than those in Korean and Vietnamese women and American women (296). Additionally, the neutralizing activity of IgA in breast milk from Indian women is higher than that in breast milk from American women (296). Three studies investigating withholding breastfeeding for an hour before and after ORV showed no effect on rotavirus-specific IgA SCRs in infants (297–299).
Maternal antibodies.
Preexisting maternal antibodies inhibit infant antibody responses to vaccination (14) (Table 6). After primary immunization, a 2-fold higher maternal antibody level results in approximately one-fourth lower postvaccination GMTs in infants (28% to IPV, 24% to diphtheria, 22% to pertactin, and 13% to tetanus). Maternal antibodies also influence infant vaccine responses after booster doses in the second year of life (14).
TABLE 6.
Results with regard to maternal factors from studies investigating perinatal factors that influence vaccine responses
| Type of response | Vaccine response and vaccine: measurement [reference(s)] |
|||
|---|---|---|---|---|
| Maternal antibodies associated with lower infant responses to vaccination | Maternal high body mass index | Maternal malnutrition | Antenatal steroids | |
| Humoral responses |
|
|
||
| Cytokine responses |
|
|||
A meta-analysis summarizing 16 studies investigating the influence of maternal antibodies on responses to measles vaccination shows that the presence of maternal antibodies decreases SCRs, on average, by 33.2% (49). However, maternal antibodies to measles virus in infants decrease quickly, and by the age of 5 months, only 12% of infants have protective levels. Maternal age is the only factor associated with maternal measles antibody levels (300, 301), while maternal weight has no influence (300). Measles-vaccinated women have significantly lower GMTs than those of women with naturally acquired immunity (301, 302).
Maternal antibodies against HepA in infants remain high during the first 6 months but then drop quickly, with only approximately 37% of infants having protective titers by the age of 12 months (303, 304). When the HepA vaccine is given to infants within the first 6 months of life, GMTs in those without maternal antibodies are significantly higher at the age of 12 to 15 months than those in infants with maternal antibodies. However, there is no difference in SPRs (305, 306). For ORV, transplacental transfer of maternal antibodies (307–309) and transfer of antibodies through breast milk (295, 296, 310) lead to decreased infant vaccine responses, in a dose-dependent matter.
The amount of maternal antibodies transferred to infants depends on several factors, including maternal antibody levels, IgG subclass, gestational age, and placental characteristics (311). The transfer of maternal antibodies can be optimized by maternal vaccination during pregnancy. This strategy is currently recommended for vaccination against tetanus, pertussis, and influenza. However, appropriate timing of vaccination during pregnancy is important. For example, Tdap given during the second trimester of pregnancy leads to significantly higher neonatal antibodies than those obtained when it is given during the third trimester (312).
Maternal infections during pregnancy.
Most studies do not find an association between maternal infection with filariae, hookworm, malaria parasites, Mansonella perstans, or Schistosoma mansoni during pregnancy and infant antibody responses to diphtheria (313, 314), HepB (313–315), Hib (314), pertussis (314), and tetanus (313, 316) vaccination. However, one study shows that maternal infection with malaria parasites, filariae, or hookworm is independently associated with lower infant antibody responses to Hib vaccination (313), while another study shows that maternal infection with S. mansoni is associated with lower infant antibody responses to HepB vaccination (317). Maternal malaria during pregnancy is associated with lower infant SPRs after measles vaccination (318). The presence of multiple maternal infections is associated with lower infant antibody responses to Hib, diphtheria, and tetanus (313) vaccination. In contrast, maternal infection with Strongyloides during pregnancy is associated with higher antibody responses to Hib, HepB, and pertussis toxin (PT) (314) vaccination.
Maternal infection with M. perstans during pregnancy is associated with higher infant IL-10 responses after BCG and tetanus vaccination, but there are no differences in gamma interferon (IFN-γ), IL-5, and IL-13 (319) production. Maternal infection with filariae (320), Trypanosoma cruzi (321), or S. mansoni (320) during pregnancy is associated with lower IFN-γ production in children after BCG vaccination, while other helminth infections show no effect on infant responses to BCG vaccination (319, 322, 323). Empiric treatment of mothers with albendazole or praziquantel during pregnancy does not influence infant vaccine responses to BCG (324), diphtheria (314), HepB (314), Hib (314), measles (324), pertussis (314), or tetanus (324) vaccination (Table 7).
TABLE 7.
Results with regard to maternal infections from studies investigating perinatal factors that influence vaccine responses
| Type of response | Vaccine response and vaccine: measurement [reference(s)] |
|||||||
|---|---|---|---|---|---|---|---|---|
| Filariasis | Helminths | Hookworm | Malaria | Mansonella perstans | Schistosomiasis | Strongyloidiasis | Trypanosoma cruzi | |
| Humoral responses |
|
|
|
|
||||
| Cytokine responses |
|
|
|
|
||||
Other maternal factors.
In infants, cytokine responses to BCG vaccination correlate inversely with maternal body mass index (BMI) (325). On the other hand, maternal malnutrition is associated with lower infant antibody responses to OPV and ORV vaccination, while it does not influence responses to parenteral vaccines (326).
It has been suggested that low maternal education status is associated with higher infant GMTs to tetanus vaccination (327) and lower infant IFN-γ and IL-5 production after tetanus vaccination (319), while a lower maternal socioeconomic status is associated with lower infant IL-10 production after tetanus vaccination (319) (Table 8). However, this might be confounded by other factors, such as malnourishment and nonadherence to vaccination schedules.
TABLE 8.
Results with regard to other maternal factors from studies investigating perinatal factors that influence vaccine responses
| Type of response | Vaccine response and vaccine: measurement [reference(s)] |
|||
|---|---|---|---|---|
| Low maternal education status | Low maternal socioeconomic status | Maternal treatment with albendazole or praziquantel during pregnancy | Maternal vaccination | |
| Humoral responses |
|
|
||
| Cytokine responses |
|
|||
Maternal tetanus immunization during pregnancy is associated with higher infant antibody responses to tetanus vaccination, while the presence of a maternal BCG scar is associated with lower infant IL-5 (319), IL-10 (323), and IL-13 (319) responses after BCG vaccination (319).
Infants who are exposed to HIV during pregnancy but remain uninfected have lower IFN-γ, IL-5, and IL-13 responses after tetanus and BCG vaccination (319). Antenatal, but not postnatal, steroids decrease infant antibody production in response to tetanus vaccination (280) (Table 6).
Extrinsic Factors
Infections.
Most studies show no difference in antibody responses to MMR vaccination between children with diarrhea (287, 328), fever (287, 328), or afebrile upper respiratory tract infections (URTI) (329–332) and healthy children. However, studies have reported lower SCRs after measles (330) and varicella (331) vaccination for children with URTI (without differences in GMTs), lower GMTs after Hib vaccination in children with fever (333), and lower GMTs and SCRs for children with diarrhea after OPV vaccination (334, 335). Concurrent infection with nonpoliovirus enteroviruses at the time of vaccination with OPV reduces SCRs against poliovirus type 1 but not type 2 or 3 (334) (Table 9).
TABLE 9.
Results with regard to symptoms or infections from studies investigating extrinsic factors that influence vaccine responses
| Type of response | Vaccine response, vaccine, age group, measurement [reference(s)] |
||||||
|---|---|---|---|---|---|---|---|
| Diarrhea | Fever | URTI | CMV infection | EBV infection | Hepatitis B virus infection | Hepatitis C virus infection | |
| Humoral responses |
|
|
|
|
|||
| Cellular responses | |||||||
Children who are infected with Epstein-Barr virus (EBV) have reduced antibody responses to meningococcal polysaccharide (MPV) and measles vaccination. However, infants who are infected with both EBV and cytomegalovirus (CMV) have responses similar to those of uninfected infants, suggesting that the effects of EBV infection might be countered by CMV infection (336). Children who are infected with CMV also have higher GMTs after vaccination with measles vaccine (337), while results from studies investigating vaccine responses in CMV-seropositive adults are conflicting. Studies report higher antibody responses to TIV vaccination in young adults (GMTs) (338) and elderly people (SCRs) (248) who are seropositive for CMV than in seronegative individuals, while other studies show reduced antibody responses (SCRs) (339, 340), lower B and T cell activation (341), and switched memory B cells (339) in response to TIV vaccination in CMV-seropositive individuals (Table 9).
Adults who are infected with HIV have lower SPRs after vaccination with HepA vaccine. A lower viral load and higher CD4 cell count are predictors of a higher response to vaccination (104). Pregnant women who are infected with HIV have lower cytokine responses to BCG (319, 323) and tetanus (319) vaccination. Infants who are infected with HIV have lower cytokine responses after vaccination with BCG (319, 323), measles (337), and tetanus (319) vaccines and lower antibody responses after vaccination with measles vaccine (318). Children who are infected with HIV have quicker waning of antibodies after vaccination with HepA vaccine (342).
Individuals who are chronically infected with either hepatitis B or hepatitis C virus have lower antibody responses to HepA vaccination (102, 263, 343, 344).
Parasites.
Infants who are symptomatically infected with malaria parasites have lower antibody responses to Hib (333), measles (318), MCV-C (345), Salmonella Typhi (intradermal), and tetanus (345–347) vaccination and lower cytokine responses (IFN-γ, IL-5, and IL-13) after vaccination with tetanus (319) and BCG (319, 323) vaccines (Table 10). Children with asymptomatic malaria have lower GMTs after MCV-C (94, 348) and aP (349) vaccination but no difference in antibody responses to tetanus vaccination (350, 351). Malaria prophylaxis given to children prior to vaccination has no influence on GMTs or SCRs after diphtheria (352–355), IPV (355), measles (352, 353, 356), OCV (357), OPV (353), pertussis (352), Salmonella Typhi (353, 357), or tetanus (352–355, 358, 359) vaccination. Malaria prophylaxis also has no influence on the size of the tuberculin skin reaction after BCG vaccination (353). However, GMTs after vaccination with MCV-A and MCV-C are (transiently) higher in children who are on malaria prophylaxis (348, 353).
TABLE 10.
Results with regard to parasitic infections from studies investigating extrinsic factors that influence vaccine responses
| Type of response | Vaccine response, vaccine, age group, measurement [reference(s)] |
|||||
|---|---|---|---|---|---|---|
| HIV infection | Ascaris lumbricoides | Malaria | Onchocerca volvulus | Schistosoma mansoni | Trypanosoma cruzi | |
| Humoral responses |
|
|
||||
| Cellular responses |
|
|||||
| Cytokine responses |
|
|
||||
Individuals who are infected with Onchocerca volvulus produce fewer antibodies and have less proliferation of mononuclear cells and lower IFN-γ production (360) after tetanus vaccination, and individuals who are infected with S. mansoni produce less IFN-γ (361) after tetanus vaccination. In children and adults with Ascaris lumbricoides infection, antibody and cytokine responses (IFN-γ and IL-2) to OCV are diminished but can be partially restored by albendazole treatment prior to vaccination (362, 363). Childhood helminth infections are not associated with cytokine responses after BCG vaccination. Empiric quarterly albendazole treatment during the first 5 years of age leads to less IFN-γ and IL-13 production after vaccination with BCG (323). In contrast, albendazole treatment in individuals with proven helminth infection leads to more T cell proliferation (364) and IFN-γ (364, 365) and IL-12 (365) production after BCG vaccination but to less transforming growth factor beta (TGF-β) and concanavalin A production (365) (Table 11). Infants who are congenitally infected with Trypanosoma cruzi have stronger in vitro IFN-γ responses after vaccination with diphtheria, HepB, and tetanus vaccines (321) (Table 10).
TABLE 11.
Results with regard to antiparasitic treatment from studies investigating extrinsic factors that influence vaccine responses
| Type of response | Vaccine response, vaccine, age group, measurement [reference(s)] |
||||
|---|---|---|---|---|---|
| Responses after treatment with albendazole | Responses on prophylaxis with amodiaquine | Responses on prophylaxis with atovaquone-proguanil | Responses on prophylaxis with chloroquine | Responses on prophylaxis with sulfadoxine-pyrimethamine | |
| Humoral responses |
|
|
|
||
| Cellular responses |
|
|
|||
| Cytokine responses |
|
||||
Antibiotics, probiotics, and prebiotics.
There are no studies of humans that have investigated the effects of antibiotics on vaccine responses. However, a study in mice showed that administration of clarithromycin or doxycycline leads to lower antibody responses after vaccination with a HepB, tetanus, or PPV23 vaccine, while administration of ampicillin leads to higher antibody responses after vaccination with a live attenuated Salmonella Typhi vaccine (366). A study in calves showed that concurrent administration of oxytetracycline leads to lower antibody responses to subcutaneous vaccination with Brucella abortus vaccine (367).
The effects of probiotics on vaccine responses are detailed in a recent systematic review (368). A beneficial effect of probiotics has been reported for responses to diphtheria (369), HepA (370), Hib (371), IPV (372), OCV (373), ORV (374), OPV (375), and TIV (376–380) vaccination. However, the choice of probiotic strains and dose as well as the duration and timing of administration varied largely between studies.
Studies investigating the effects of nutritional supplements containing a mix of probiotics (Lactobacillus paracasei [381] or Bifidobacterium longum bv. infantis [341, 382]) and prebiotics (fructo-oligosaccharides [381] or gluco-oligosaccharide [341, 382]) show no effect of the supplements on antibody responses to TIV (381–383) or PPV23 (381, 383) vaccination or on B or T cell responses (341) to TIV vaccination.
Microbiota.
There is an association between the intestinal microbiota and vaccine responses (384). In infants, a higher relative abundance of actinobacteria (especially the species B. longum) has been associated with either higher antibody or cellular vaccine responses to BCG (385), HepB (385), IPV (372), OPV (385), and tetanus (385) vaccination. In contrast, a higher relative abundance of proteobacteria (especially Pseudomonadales) has been associated with lower responses to these vaccines (385). Additionally, a higher relative abundance of Firmicutes bacteria (families Lachnospiraceae and Ruminococcacae) has been associated with higher humoral responses to ORV (386) in infants and cellular responses to Salmonella Typhi (387) in adults. Additionally, in infants, one study found a higher relative abundance of Bacteroidetes organisms to be associated with lower humoral responses to ORV (386), while another study did not find any association between the composition of the intestinal microbiota and responses to ORV (388) (Table 12). Children with small bowel bacterial overgrowth have reduced antibody responses to OCV (389).
TABLE 12.
Results with regard to microbiota from studies investigating extrinsic factors that influence vaccine responses
| Type of response | Bacterium/bacterial group, age group, affected vaccine response (reference) |
||
|---|---|---|---|
| Higher relative abundance in intestinal microbiota associated with higher vaccine responses | Higher relative abundance in intestinal microbiota associated with lower vaccine responses | Higher relative abundance in nasopharyngeal microbiota associated with higher vaccine responses | |
| Humoral responses |
|
|
|
| Cellular responses |
|
||
The one study which investigated the influence of the respiratory microbiota on vaccine responses shows a positive association between the presence of Bacteroides ovatus, Lactobacillus helveticus, Prevotella melaninogenica, Streptococcus infantis, and Veillonella dispar in the nasopharynx and influenza virus-specific H1 IgA levels in nasal washings after vaccination with live attenuated influenza virus (LAIV) (390).
Preexisting immunity.
Individuals with higher prevaccination tetanus antibody titers have higher SPRs after booster vaccination (60), while the antibody response to diphtheria is not affected by prevaccination titers (391). Similarly, the antibody response to TIV is positively associated with prevaccination titers (93). Elderly people who have low antibody titers against influenza virus before vaccination might not seroconvert after one dose of TIV (392). Further, priming with an antigen different from that included in the TIV vaccine leads to lower SCRs and GMTs (393, 394) than those obtained after priming with similar antigens. Further evidence that preexisting immunity influences vaccine responses comes from studies showing that individuals who have antibodies against flaviviruses have higher antibody responses to dengue fever vaccination (95). Previous exposure to Hib-like environmental bacteria increases antibody responses to Hib vaccination (327). In contrast, there is evidence that lower cytokine production after BCG vaccination can be explained partly by previous exposure to other mycobacteria (395–397). However, this is not the case in all studies (398). The evidence for antibody responses to HepB vaccination is also conflicting, with one study showing higher antibody responses in those who are anti-HBc positive (242) and another showing lower responses (72). For OCV, SCRs have been observed to be lower for individuals with preexisting immunity (399).
Behavioral Factors
Smoking.
Smoking leads to lower antibody responses to HepB vaccination in some (66, 68, 69, 74, 75, 78, 80, 400) but not all studies (81, 100, 401, 402) and to increased antibody responses to YF vaccination (157). Smoking has no influence on GMTs after human papillomavirus (HPV) vaccination, but it increases the risk of having low-avidity antibodies after vaccination (403). Smokers have higher SCRs after vaccination with LAIV, but waning of antibodies is quicker in smokers than in nonsmokers after vaccination with LAIV and TIV (404) (Table 13).
TABLE 13.
Results from studies investigating behavioral factors that influence vaccine responses
| Type of response | Vaccine response, vaccine, age group, task or characteristic, measurement [reference(s)] |
|||||
|---|---|---|---|---|---|---|
| Smoking | Alcohol consumption | Exercise | Acute psychological stress | Chronic psychological stress | Sleep deprivation | |
| Humoral responses |
|
|
|
|
|
|
| Cellular responses |
|
|||||
| Cytokine responses |
|
|
||||
Alcohol consumption.
Alcohol intake has been found not to influence antibody responses to HepA (405) or HepB (68, 69, 81, 402, 406) vaccination but might influence responses to pneumococcus vaccination (142). After vaccination with PPV23, alcoholics have lower SCRs to serotypes 3, 4, 7F, 8, and 19F (however, this is significant only for serotypes 3 and 19F) (142) (Table 13).
Exercise.
A small study showed that antibody responses to tetanus vaccination were higher in runners vaccinated after they completed a marathon than in a nonrunner control group (407). However, a larger study found no differences in antibody responses to diphtheria, tetanus, and PPV23 vaccines given to triathletes after they completed a race compared to the responses in triathletes vaccinated when they had not been exercising or in vaccinated sedentary controls (408). A further study reports higher GMTs to TIV (only to A/Panama [H3N2]) in females who used an ergometer in the 45 min before vaccination (409), and one study showed that active participants over the age of 62 years (defined as more than 20 min of vigorous exercise three or more times per week) had higher antibody responses to TIV than those of sedentary participants (410), while other studies with interventions to increase physical activity in adults do not show an association between increasing exercise and antibody responses to TIV (411) or PPV23 (412) vaccination (Table 13).
One study showed that eccentric exercise of the arm before administration of TIV leads to increased interferon gamma production (with the increase in cytokine production correlating with the increase in arm circumference) in males and antibody production in females (124). The increase in antibody response to TIV vaccination through exercise varies among different vaccine strains, with low-immunogenicity strains being particularly responsive to augmentation through exercise (127). However, a follow-up study could not confirm an association between eccentric exercise of the arm and the humoral or cellular vaccine response to TIV (413) (Table 13).
Acute psychological stress.
In elderly people, positive mood on the day of vaccination is associated with higher antibody responses to TIV (414). In contrast, mental stress before vaccination leads to higher GMTs after TIV vaccination in females (only to A/Panama [H3N2]) (409) and to higher GMTs to MCV-A in males (139). Individuals who have greater blood pressure reactions (with evidence of delayed diastolic blood pressure recovery) toward the end of a mental arithmetic stress task that causes aversive stimuli have higher SCRs after vaccination with MPV-A (415) and TIV (to A/Panama [H3N2] and B/Shandong) (Table 13).
Chronic psychological stress.
Many studies report (mostly negative) associations between chronic psychological stress and vaccine responses. For example, elderly people who suffer from stress by being caregivers of relatives with dementia have lower antibody responses (416–419), less IL-1β and IL-2 production (410, 416, 417), and more IL-6 production (419) after vaccination with TIV, as well as lower antibody responses after vaccination with PPV23 (420). In contrast, greater optimism in elderly people is associated with higher antibody responses (411) and more IL-10 production (410) after vaccination with TIV. Adults with high perceived stress, stressful life events, or loneliness also have lower antibody (410, 421–424) and cellular (425) responses after vaccination with TIV. This is not the case for adults who care for patients with multiple sclerosis (426). Responses to influenza A/H1N1 and B viruses appear to be more sensitive to the influence of psychological stress than those to A/H3N2 viruses (427). Having better social support or being married is associated with higher antibody responses to HepB (417) and TIV (423, 428) vaccination, while bereavement is associated with lower antibody responses to TIV (428) (however, one study reports lower antibody responses to TIV vaccination in patients with better social support [429]). Mindfulness-based stress reduction exercises do not influence antibody responses to TIV vaccination (411).
Young adults with stressful life events, chronic stress, and a low level of psychological well-being can have lower antibody responses to HepB (430–433) and MCV-C (434) vaccination. However, in contrast, one study shows higher antibody responses to HepB vaccination in young adults with chronic stress (435), and some studies do not find any associations between psychological stress and antibody responses to HepB vaccination (436, 437). Antibody responses might also be influenced by coping mechanisms. For example, individuals who accept the reality of stressful situations have higher antibody responses to HepB vaccination, while individuals who cope by substance use (430) or have high trait negative affect (436) have lower responses. High internalizing behavior, neuroticism, and low self-esteem are associated with lower antibody responses to rubella vaccination (438), while children with internalizing mood symptoms (depressive or anxious symptoms) have higher antibody responses to MCV-4 (439). One study found no association between coping mechanisms and antibody responses to HepB vaccination (432).
Observed negative/conflict behaviors in an interaction task between parents and children predicts lower antibody responses to serotype C after MCV-4 vaccination in children (440). Children who have higher salivary cortisol levels after starting kindergarten have lower antibody responses to PPV23 (441) (Table 13).
Sleep.
Short sleep duration, but not subjective sleep quality, in the week around vaccination is associated with lower antibody responses to HepB vaccination (442). Deliberate sleep deprivation during the night after HepA vaccination is associated with lower antibody levels (443, 444) and specific Th cell responses (444), while high sleep slow-wave activity is associated with higher antibody responses (444). Deliberate sleep deprivation 1 week after vaccination with TIV is also associated with lower antibody responses (445), while no association is found between sleep duration (414) or obstructive sleep apnea (446) and antibody responses to TIV vaccination (Table 13).
Nutritional Factors
Body mass index.
Many studies in adults show that an increase in body mass index (BMI) is inversely correlated with antibody responses to HepA (61, 447) and HepB (61, 66, 68, 77, 78, 80, 100, 259, 401, 402, 448–454) vaccination. After vaccination with TIV, an increase in BMI is initially correlated with higher antibody responses. However, 12 months after vaccination, a higher BMI is associated with a greater decline in antibodies, and obese individuals also have fewer specific CD8 T cells and less IFN-γ production (449) (Table 14).
TABLE 14.
Results from studies investigating nutritional factors that influence vaccine responses
| Type of response | Vaccine response, vaccine, age group, measurement [reference(s)] |
|||
|---|---|---|---|---|
| Higher body mass index | Malnourishment | Low vitamin D levels | Enteropathy | |
| Humoral responses |
|
|
|
|
| Cellular responses |
|
|
||
| Cytokine responses |
|
|||
Nutritional status.
Many vaccine responses are not influenced by malnourishment. However, malnourished children are reported to have lower antibody responses to HepB (455), MPVS (456), measles (318, 457–459), OPV (335), pertussis (460), Salmonella Typhi (461), and tetanus (462) vaccination. Additionally, malnourished children have smaller tuberculin skin reactions after BCG vaccination (463–465) (Table 14).
Micronutrients (vitamins A, D, and E and zinc).
Vitamin D plays an important role in innate, humoral, and cellular immune responses (466). However, its role in influencing vaccine responses is not clear (466). Most studies reporting on the effects of vitamin D on vaccine responses have investigated responses to TIV. The results of these studies are conflicting (Table 14). In adults, there is no association between vitamin D levels or administration of vitamin D and antibody responses to TIV (467–469). In children, some studies find no association between vitamin D levels or administration of vitamin D and antibody responses to TIV (470, 471), while others show a trend toward decreased SPRs against TIV in children with vitamin D deficiency (472). In contrast, after vaccination with LAIV, higher antibody responses against two influenza B virus strains were observed in children with low vitamin D levels, while there was no association between vitamin D levels and GMTs against the A strain of the LAIV vaccine (471). In adult dialysis patients, parenteral calcitriol treatment leads to higher antibody responses to TIV (473). Additionally, there is also a clear association between vitamin D deficiency in hemodialysis patients and diminished antibody responses to HepB vaccine (474). In children with idiopathic nephrotic syndrome, there is no correlation between vitamin D levels and antibody responses to PPV23 (475). In asplenic patients vaccinated against Hib, MCV-C, and PCV7, there is no correlation between vitamin D levels and vaccine antibody levels (476). Adults receiving vitamin D supplementation before a booster dose of tetanus have higher antibody levels against tetanus (477). Infants receiving supplementation with vitamins A and D have stronger tuberculin skin reactions (478) and lower IFN-γ responses after BCG vaccination.
Studies investigating coadministration of vitamin A and measles vaccine also report conflicting results. Vitamin A given simultaneously with a measles vaccine at 6 months of age leads to lower SCRs (154), but only in infants with maternal antibodies. Vitamin A given simultaneously with measles vaccination at 9 months of age does not lead to a difference in GMTs and SCRs at the age of 12 months (479) but does lead to higher GMTs at the ages of 18 months (35) and 6 to 8 years (480), especially in boys. Vitamin A given simultaneously with measles vaccination at 9 months of age to malnourished infants leads to significantly higher GMTs (479). In children with and without vitamin A deficiency, there is no difference in antibody responses to diphtheria and tetanus vaccination (481). In elderly people who are not vitamin deficient, there is no association between levels of vitamin A, vitamin E, or zinc and antibody responses to TIV (482). Polymorphisms of vitamin A and D receptor are associated with lower antibody responses to HepB vaccination (483) and with variations in cytokine responses after rubella and measles vaccination (194, 484).
Enteropathy.
Enteropathy is defined as intestinal inflammation not necessarily associated with diarrhea. It occurs more frequently in individuals exposed to poor sanitary conditions. It is often proposed that enteropathy is one of the mechanisms behind the lower effectiveness of oral vaccines in developing countries. However, only one study has investigated the effect of enteropathy on vaccine responses. That study found that infants with enteropathy have lower SPRs against OPV and ORV but not against parenteral vaccines (326).
Environmental Factors
Rural versus urban environment.
Children living in rural areas have higher antibody responses to tetanus (351) and an experimental malaria vaccine (485, 486). They have a Th1-skewed response (IL-5 production) to tetanus and TIV vaccination, while children living in semiurban areas have a Th2-skewed response (IFN-γ production) (351, 487).
In contrast, humoral and cellular responses to TIV are lower in children living in rural areas than in those living in semiurban areas (487). Similarly, antibody responses to HepB vaccination (161, 486, 488) and cytokine responses after vaccination with BCG (323) are lower in children and adults living in rural areas.
Geographic location.
Children living in developing countries have higher antibody responses to Hib (489), diphtheria (489), PCV7 (490), and pertussis (489) vaccination but lower antibody responses to measles (491–493), OCV (494), OPV (495, 496), and Salmonella Typhi (497) vaccination. Adults in developing countries have lower antibody responses to HepB vaccination (498). Children living in developed countries have a Th2-skewed response to BCG vaccination, with more IFN-γ production (396, 398, 499, 500), while children in developing countries have a Th1-skewed response (499).
Season.
There is an association between month of administration and antibody responses to pertussis (460), PPV23 (146), rabies (146), and Salmonella Typhi (146) vaccination but no association between month of vaccine administration and antibody responses to diphtheria (146), HepB (146), and tetanus (146) vaccination (Table 15). African infants born in the wet season have stronger CD154 vaccine responses to BCG than those of infants born in the dry season (325). In adolescents, antibody responses (GMTs) to HepB vaccination are lower in individuals who receive their first and second doses in summer than in those who receive them in winter. However, after the third dose, no statistically significant difference in GMTs is found. It has been suggested that these variations in vaccine responses may be explained through differences in exposure to UV irradiation (501). Nevertheless, exposing healthy volunteers to UVB on five consecutive days before HepB vaccination does not lead to a difference in GMTs or cellular vaccine responses (in a lymphocyte stimulation test) between UVB- and non-UVB-exposed individuals (502).
TABLE 15.
Results from studies investigating environmental factors that influence vaccine responses
| Type of response | Vaccine response, vaccine, age group, measurement [reference(s)] |
||||||
|---|---|---|---|---|---|---|---|
| Living in rural areas | Living in developing countries | Season | Larger family size | Arsenic exposure | Lead exposure | Polychlorinated biphenyls and dioxins exposure | |
| Humoral responses |
|
|
|
|
|
|
|
| Cellular responses |
|
||||||
| Cytokine responses |
|
||||||
Family size.
A larger number of people living in the household is associated with superior antibody responses to Hib vaccine (327).
Toxins.
One study compared vaccine responses in lead-exposed children with metabolic impairment to those in healthy children and did not find a difference in antibody responses to tetanus (503). Another study showed that early-life arsenic exposure leads to decreased antibody levels to mumps virus after vaccination with MMR (504). It has also been suggested that pre- and postnatal (breast milk) exposure to polychlorinated biphenyls and dioxins leads to lower antibody responses to measles and mumps viruses (505).
Vaccine Factors
Vaccine type, product, and strain.
Immune responses vary widely with different vaccine types and products. For example, live vaccines usually induce high vaccine responses leading to life-long protection, often after just one dose, while inactivated, subunit, or toxoid vaccines usually require several doses, including booster doses, to achieve similar protection. Responses to different subunit vaccines also vary. Polysaccharide vaccines induce a T cell-independent vaccine response which does not induce immune memory and is therefore short-lived. Such vaccines are not protective in children under 2 years of age and do not decrease nasopharyngeal carriage. In contrast, polysaccharide-protein-conjugated vaccines have superior immunogenicity, protect infants under 2 years of age, and reduce nasopharyngeal carriage. The responses to similar vaccines against the same disease can also vary according to specific product or strain. This has been shown, for example, for BCG (323, 506, 507), HepB (107, 508), TIV (86), PCV7 (490), rabies (509), and measles (49) vaccines. However, detailed discussion of this topic is beyond the scope of this review.
Adjuvants.
Adjuvants are added to vaccines to elicit stronger immune responses. There is great variation in the magnitude and quality of vaccine responses depending on which adjuvants are added to a vaccine (510). For example, the new adjuvant MF59 increases antibody affinity and cross-protection against TIV (511, 512), and compared to those obtained with the standard HepB vaccine, a vaccine containing the new adjuvant AS04 induces higher SPRs and GMTs (513).
Vaccine dose.
A study investigating IPV showed that there is a linear relationship between the dose of administered antigen and antibody responses (514). With ORV, the inhibitory effect of transplacental or breast milk-transferred maternal antibodies can be overcome by increasing the vaccine dose (309).
In adolescents receiving HepB vaccination, 10 μg instead of the more commonly used 20-μg dose is sufficient to induce seroprotection (454). However, it leads to lower GMTs (71, 515) and SCRs (71). In patients with end-stage kidney disease, 80 μg of HepB vaccine increases the likelihood of persistent protective antibodies (259).
For the Hib vaccine, it has been shown that even one-eighth the usually given dose leads to adequate antibody titers associated with long-term immunity (516).
For TIV, it has been shown for elderly people that higher doses of antigens are associated with higher antibody responses (130, 517), while in previously immunized participants of 18 to 49 years of age, half the usual vaccine dose leads to comparable SCRs and GMTs, especially in females (126).
Administration Factors
Vaccination schedule.
A further factor influencing vaccine responses is the spacing of vaccine doses. Schedules that have longer intervals between vaccine doses usually lead to higher immune responses. For example, antibody responses to aP are significantly higher after a 2-month to 4- to 6-month schedule than after an accelerated 2-month to 3- to 4-month schedule (349). Similarly, antibody responses to DTP vaccination are higher after a 3-month to 5- to 9-month schedule than after an accelerated 2-month to 3- to 4-month schedule (518). For HepB vaccination, increased time between the first and second doses or the second and third doses also correlates with increasing GMTs and SPRs (117, 401). However, one study showed lower GMTs after HepB vaccination on a 0-month to 12- to 24-month schedule than after a 0-month to 1- to 6-month schedule (448), and similarly, another study also showed that individuals who have an interval of more than 1 month between the first and second doses have lower SPRs (79).
In contrast, GMTs after HepA vaccination are higher after a 0-month to 1- to 12-month schedule than after a 0-month to 6- to 12-month schedule (99) or a 0-month to 1- to 2-month schedule (64).
In infants receiving two doses of measles, mumps, rubella, and varicella virus vaccination (MMRV), GMTs are higher to all components of the vaccine when the doses are given 12 months apart instead of 4 weeks apart (519). When influenza vaccination is given to children below the age of 24 months, there is no difference in antibody responses if one priming dose is given in spring followed by the second dose in fall or if both doses are given in fall (520).
Vaccination site.
In adults, HepB vaccination given in the buttock area leads to lower responses than those obtained when it is given in the upper arm (521–524). However, this is not the case for infants (525). In children receiving Hib vaccination, there is also no difference in GMTs between children who receive the vaccination in the vastus lateralis and those who receive it in the deltoid muscle (526). Similarly, there is no difference in GMTs after HepB vaccination in infants who receive the vaccine in the anterolateral thigh compared to those who receive it in the ventrogluteal region (8).
Vaccination route.
Intramuscular administration of TIV leads to higher antibody responses than those obtained by subcutaneous administration (125). However, there is no difference in antibody responses to diphtheria (137), PPV23 (143), or tetanus (137) vaccination after intramuscular or subcutaneous administration. For HepB and rabies vaccines, a lower dose given intradermally induces an antibody response similar to that induced by a larger intramuscular dose (148, 527–529). Intramuscular vaccination relies on a T cell-mediated response, while intradermal vaccination activates a dendritic cell-mediated response, which requires lower antigen doses (530). Individuals who do not respond to intramuscular HepB vaccination might respond to intradermal administration (531, 532). While 5 μg given intradermally leads to higher SCRs than those obtained with 40 μg given intramuscularly (533), 2 μg given intradermally leads to lower SPRs (74) and GMTs (75) than those obtained with 20 μg given intramuscularly (75). There is no difference in time required to seroconvert or time to peak antibody titer (75).
Needle size.
The size of the needle used to administer vaccines has not been found to influence vaccine responses (534, 535).
Time of day.
Studies investigating diurnal variations in antibody responses to TIV in adults show conflicting results. One study showed that individuals receiving the vaccine in the morning have significantly higher antibody responses to the H1N1 A strain and a trend toward higher responses to the B strain, while the time of vaccine administration has no influence on responses to the H3N2 A strain (536). A second study indicated that diurnal variations in antibody responses after TIV are strain specific (537). Additionally, a third study showed higher GMTs to TIV after morning (compared to afternoon) vaccination, but only in men (538). The same has been shown for GMTs after HepA vaccination, but again only in men (538).
Coadministered vaccines.
A vast number of studies have investigated the influence of concurrent administration of live vaccines on vaccine responses. Two meta-analyses summarized RCTs comparing vaccine responses in infants receiving either one dose of MMRV or MMR and V as separate vaccines (539, 540). The first meta-analysis, which included 10 RCTs, did not find any difference in GMTs to any of the vaccine components (540). However, the second meta-analysis, which included 24 RCTs, showed that after a single vaccination in children of 9 to 24 months of age, GMTs against mumps and varicella viruses are comparable between infants receiving MMRV and those receiving MMR and V (MMR+V) or MMR. In contrast, individuals receiving MMRV achieve significantly higher GMTs to measles virus than those in individuals receiving MMR+V or MMR. GMTs to rubella virus are reduced in those receiving MMRV compared to those in individuals receiving MMR+V or MMR (539). MMRV also leads to lower SCRs than those with MMR+V or MMR, but this is likely confounded by lower virus antigen doses in early vaccines (539). In investigations of immune responses after two vaccine doses, similar GMTs to most vaccine viruses are observed (541–545). However, there is a tendency toward higher varicella (541, 542, 544, 545) and mumps (541, 543–545) GMTs and lower measles (542–544) and rubella (541, 544, 545) GMTs in groups receiving MMRV as a second dose than in those receiving MMR+V. Concurrent administration of LAIV with MMR+V does not influence GMTs or SPRs against any of the vaccine components (546). YF vaccine given simultaneously with the MMR vaccine leads to lower SCRs than those obtained when the vaccines are given 1 month apart (61% versus 71% for mumps, 90% versus 97% for rubella, and 70% versus 87% for YF), while SCRs for measles are not influenced by simultaneous vaccination (547). One study suggests that a live attenuated Japanese encephalitis (JE) vaccine given 30 days before a YF vaccine might interfere with SCRs to YF vaccination (548). However, a subsequent study did not find a difference in SCRs to YF vaccine whether JE was given 30 days before, simultaneously with, or after the vaccine. For JE, even though there were also no significant differences in SCRs between individuals given the two vaccinations at the same time and those receiving them 30 days apart, GMTs were significantly higher in participants who receive JE 30 days before YF rather than the other way around or at the same time (549).
Studies show that simultaneous administration of nonlive vaccines with MMR or V vaccine does not influence the response to any of the vaccine components. This includes 7-valent conjugated pneumococcal (Pn) (550–552), 10-valent Pn-Hib (553), meningococcal C (MenC) (554), DTaP-HBV-IPV-Hib (555), and HepA (551, 552) vaccines.
An additional dose of acellular pertussis (aP) vaccine at birth might lead to subsequently lower HepB (52, 53), Hib (52, 53), and diphtheria (556) GMTs.
Studies investigating the influence of coadministering BCG vaccine show that it leads to significantly higher levels of antibodies against HepB, OPV, PCV7, and TIV but not against DTaP-Hib or Salmonella Typhi (557).
There are several studies which show that concomitant administration of OPV reduces vaccine responses to ORV (558, 559). Infants who receive the two vaccines concomitantly are less likely to seroconvert than those who receive both vaccines in a staggered manner (558). The interference is greater after the first dose of OPV, presumably because the first dose is associated with the greatest intestinal replication of vaccine poliovirus strains (559). However, when the vaccines are given concomitantly after the age of 3 months, neither OPV nor ORV influences vaccine responses (560).
There is less interference reported with coadministration of nonlive vaccines. Coadministration of PCV7 vaccine with either acellular pertussis or whole-cell pertussis vaccine has no effect on the immunogenicity of the PCV7 vaccine, expect for that against serotype 14, for which the specific antibody response is lower when the vaccine is given with the acellular pertussis vaccine (490). One RCT showed that when DPT-IPV and Hib are given together as a single dose, the antibody response to tetanus is significantly lower than that obtained when DPT-IPV is given at an injection site different from that used for Hib or given 6 months apart (561). A Cochrane review summarizing studies that compared the immunogenicities of the combined DTP-HBV-Hib vaccine and the DTP-HBV and Hib vaccines given at separate sites did not find conclusive results, as only one study reported significantly lower immunological responses to Hib and tetanus when the vaccine components were given in a combined vaccine (562).
Coadministered drugs.
Several studies have investigated the effect of concurrent administration of paracetamol around the time of vaccination. A review summarizing 13 RCTs which investigated the influence of prophylactic antipyretic administration after vaccination shows that infants receiving paracetamol after each of 3 doses of routine vaccination have lower GMTs to all Pn serotypes, Hib, diphtheria, tetanus, and pertactin but not to pertussis toxin, filamentous hemagglutinin (FHA), HepB, or IPV. After a booster dose, lower GMTs persisted in the prophylactic paracetamol group for all Pn serotypes, diphtheria, and tetanus (563). The one study that investigated the effect of ibuprofen administration around the time of vaccination shows that GMTs to FHA and tetanus are diminished after primary administration but not after a booster dose, while responses to conjugated Pn vaccine are not affected (564). Further, a recent large study in elderly individuals showed that the intake of statins diminishes GMTs to TIV (565). Similarly, preterm infants who receive dexamethasone for chronic lung disease have significantly lower GMTs against Hib after basic immunization with 3 doses (566).
CONCLUDING REMARKS
This review summarizes many important (and often unrecognized or underappreciated) factors that might affect how individuals respond to vaccines (Fig. 1). There is solid evidence that intrinsic factors, such as genetics, sex, age at time of vaccination, and comorbidities, as well as vaccine-related factors, such as choice of vaccine products, adjuvants, and vaccination schedule, strongly influence vaccine responses. Good evidence also exists for the interaction between maternal antibodies and vaccine responses in infants. In contrast, the available data on the influence of other perinatal factors, such as birth weight or feeding method, or on the influence of infections, antibiotics, the microbiota, and nutrition are less robust. For smoking, alcohol consumption, psychological stress, and exercise, the data from different studies are inconsistent. Many studies report differences in vaccine responses depending on geographic region. However, many other factors, such as preexisting immunity, nutritional status, and other behavioral factors, as well as genetics and the microbiota, might confound this observation. The potential for confounding also exists for many of the other factors discussed in this review, as the response to vaccination is complex, likely involving the interplay of multiple different factors. It is therefore important not to overinterpret findings from single studies.
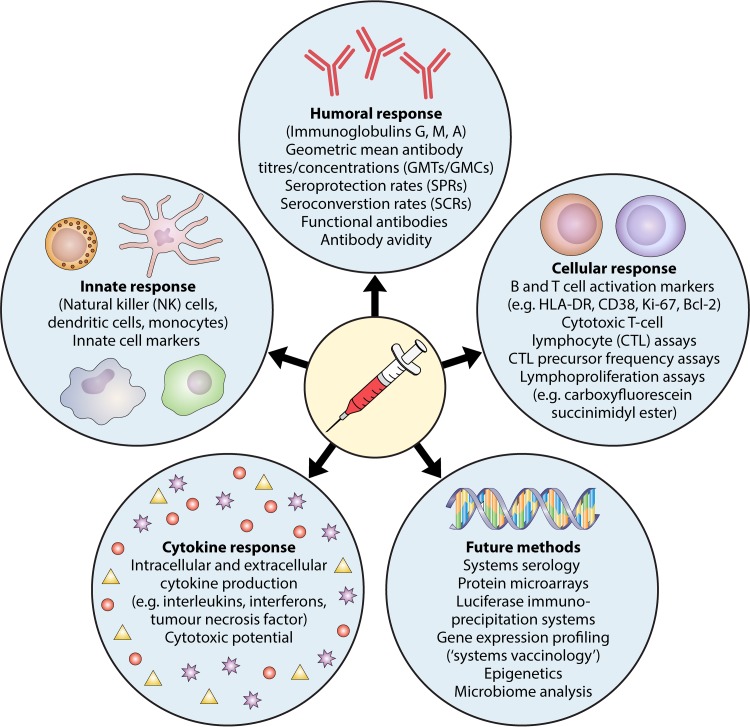
There are many different methods for quantifying vaccine responses (Fig. 2). However, most studies investigating vaccine responses quantify antibodies. The antibody concentration defined as a protective correlate varies depending on whether it relates to protection against colonization, mucosal disease, or invasive disease. However, vaccine responses are far more complex than just measurable antibody concentrations. Other parts of the immune system, such as innate, cellular, and cytokine responses, also play a crucial role in vaccine efficacy. The complex interplay between these different components of the immune system in vaccine responses is not fully understood, and surrogate markers of vaccine-induced protection are imperfect. As most vaccines induce very high antibody responses, small differences in antibody concentrations between groups of individuals may not be clinically significant in terms of protective efficacy, may be relevant only in individuals with poor responses, or may impact only the duration of protection. Moreover, the quality of antibody response is an important consideration, as only a subset of total detectable antibody can neutralize pathogens. SPRs or GMCs do not take this into account and are therefore imperfect correlates of protection. Further, the level of antibody response in vitro does not necessarily correlate with health outcomes. For example, seroconversion does not mean full protection against a disease, and nonseroconversion is not necessarily associated with susceptibility (567). Moreover, antibody levels wane with time, but seronegative individuals can still be protected through other immune mechanisms, as shown, for example, after HepB vaccination (568).
FIG 2.
Methods for quantifying vaccine responses.
This review provides an overview of the current evidence for factors that might influence vaccine responses and identifies factors that require further investigation. Important topics for future studies include the influences of the microbiota (intestinal and respiratory), concurrent infections, and antibiotics on vaccine responses. Further important lines of investigation include the association between preexisting immunity and vaccine responses, as well as the influence of behavioral factors. Understanding these interactions in more depth will open new avenues for improving vaccine immunogenicity and effectiveness as well as designing vaccine schedules that optimize the benefits of vaccination.
ACKNOWLEDGMENTS
P.Z. is supported by a Fellowship from the European Society of Pediatric Infectious Diseases and an International Research Scholarship from the University of Melbourne.
We declare that we have no competing interests.
P.Z. drafted the initial manuscript and approved the final manuscript as submitted. N.C. critically reviewed and revised the manuscript and approved the final manuscript as submitted.
Biographies

Petra Zimmermann, M.D., works as a Consultant in Paediatric Infectious Diseases at the Fribourg Hospital, Switzerland. She trained as a Paediatrician and Infectious Diseases Specialist at the University Children’s Hospital of Berne, Switzerland, and at the Royal Children’s Hospital in Melbourne, Australia. Dr. Zimmermann completed her thesis at the Clinic for Infectious Diseases and Travel Medicine at the University Hospital of Berne in 2013. Before her current position, she worked as a Consultant in Paediatric Infectious Diseases at the Children’s Hospital in Basel. Dr. Zimmermann is currently completing her Ph.D. at the University of Melbourne. Her research interests include vaccine responses, nonspecific effects of vaccines, and the association between the intestinal microbiome and immune responses.

Nigel Curtis is a clinician scientist (M.D., Ph.D.) who trained in the United Kingdom and Canada. He is now based in Australia, where he is Head of Infectious Diseases at the Royal Children’s Hospital Melbourne and Professor of Paediatric Infectious Diseases at the University of Melbourne. He is also Leader of the Infectious Diseases & Microbiology Research Group at the Murdoch Children’s Research Institute. His research interests focus on the innate and cellular immune responses to childhood infection to improve the diagnosis and prevention of infectious diseases. Prof. Curtis currently leads a large randomized controlled trial to investigate the immunomodulatory heterologous (nonspecific) effects of neonatal BCG vaccination, including its ability to prevent infections, allergic disease, and asthma. The laboratory aspects of this trial include analysis of the early infant microbiome to elucidate its role in the developing immune system and its influence on the development of allergic diseases.
REFERENCES
- 1.World Health Organization. 2009. State of the world’s vaccines and immunization. WHO; Geneva, Switzerland. [Google Scholar]
- 2.Anonymous. 1999. Impact of vaccines universally recommended for children—United States, 1990–1998. MMWR Morb Mortal Wkly Rep 48:243–248. [PubMed] [Google Scholar]
- 3.Pulendran B, Ahmed R. 2011. Immunological mechanisms of vaccination. Nat Immunol 12:509–517. doi: 10.1038/ni.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sallusto F, Lanzavecchia A, Araki K, Ahmed R. 2010. From vaccines to memory and back. Immunity 33:451–463. doi: 10.1016/j.immuni.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Querec TD, Akondy RS, Lee EK, Cao W, Nakaya HI, Teuwen D, Pirani A, Gernert K, Deng J, Marzolf B, Kennedy K, Wu H, Bennouna S, Oluoch H, Miller J, Vencio RZ, Mulligan M, Aderem A, Ahmed R, Pulendran B. 2009. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol 10:116–125. doi: 10.1038/ni.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ritz N, Mui M, Balloch A, Curtis N. 2013. Non-specific effect of Bacille Calmette-Guerin vaccine on the immune response to routine immunisations. Vaccine 31:3098–3103. doi: 10.1016/j.vaccine.2013.03.059. [DOI] [PubMed] [Google Scholar]
- 7.Nakaya HI, Hagan T, Duraisingham SS, Lee EK, Kwissa M, Rouphael N, Frasca D, Gersten M, Mehta AK, Gaujoux R, Li GM, Gupta S, Ahmed R, Mulligan MJ, Shen-Orr S, Blomberg BB, Subramaniam S, Pulendran B. 2015. Systems analysis of immunity to influenza vaccination across multiple years and in diverse populations reveals shared molecular signatures. Immunity 43:1186–1198. doi: 10.1016/j.immuni.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Junqueira AL, Tavares VR, Martins RM, Frauzino KV, da Costa e Silva AM, Minamisava R, Teles SA. 2010. Safety and immunogenicity of hepatitis B vaccine administered into ventrogluteal vs. anterolateral thigh sites in infants: a randomised controlled trial. Int J Nurs Stud 47:1074–1079. doi: 10.1016/j.ijnurstu.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Finan C, Ota MO, Marchant A, Newport MJ. 2008. Natural variation in immune responses to neonatal Mycobacterium bovis bacillus Calmette-Guerin (BCG) vaccination in a cohort of Gambian infants. PLoS One 3:e3485. doi: 10.1371/journal.pone.0003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nair N, Gans H, Lew-Yasukawa L, Long-Wagar AC, Arvin A, Griffin DE. 2007. Age-dependent differences in IgG isotype and avidity induced by measles vaccine received during the first year of life. J Infect Dis 196:1339–1345. doi: 10.1086/522519. [DOI] [PubMed] [Google Scholar]
- 11.Siegrist CA. 2007. The challenges of vaccine responses in early life: selected examples. J Comp Pathol 137(Suppl 1):S4–S9. doi: 10.1016/j.jcpa.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Poland GA, Jacobson RM. 1994. Failure to reach the goal of measles elimination. Apparent paradox of measles infections in immunized persons. Arch Intern Med 154:1815–1820. doi: 10.1001/archinte.1994.00420160048006. [DOI] [PubMed] [Google Scholar]
- 13.Grassly NC, Kang G, Kampmann B. 2015. Biological challenges to effective vaccines in the developing world. Philos Trans R Soc Lond B Biol Sci 370:20140138. doi: 10.1098/rstb.2014.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voysey M, Kelly DF, Fanshawe TR, Sadarangani M, O’Brien KL, Perera R, Pollard AJ. 2017. The influence of maternally derived antibody and infant age at vaccination on infant vaccine responses: an individual participant meta-analysis. JAMA Pediatr 171:637–646. doi: 10.1001/jamapediatrics.2017.0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halsey N, Galazka A. 1985. The efficacy of DPT and oral poliomyelitis immunization schedules initiated from birth to 12 weeks of age. Bull World Health Organ 63:1151–1169. [PMC free article] [PubMed] [Google Scholar]
- 16.di Sant’Agnese PA. 1950. Simultaneous immunization of newborn infants against diphtheria, tetanus, and pertussis; production of antibodies and duration of antibody levels in an eastern metropolitan area. Am J Public Health Nations Health 40:674–680. doi: 10.2105/AJPH.40.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kagina BM, Abel B, Bowmaker M, Scriba TJ, Gelderbloem S, Smit E, Erasmus M, Nene N, Walzl G, Black G, Hussey GD, Hesseling AC, Hanekom WA. 2009. Delaying BCG vaccination from birth to 10 weeks of age may result in an enhanced memory CD4 T cell response. Vaccine 27:5488–5495. doi: 10.1016/j.vaccine.2009.06.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burl S, Adetifa UJ, Cox M, Touray E, Ota MO, Marchant A, Whittle H, McShane H, Rowland-Jones SL, Flanagan KL. 2010. Delaying bacillus Calmette-Guerin vaccination from birth to 4 1/2 months of age reduces postvaccination Th1 and IL-17 responses but leads to comparable mycobacterial responses at 9 months of age. J Immunol 185:2620–2628. doi: 10.4049/jimmunol.1000552. [DOI] [PubMed] [Google Scholar]
- 19.Bialek SR, Bower WA, Novak R, Helgenberger L, Auerbach SB, Williams IT, Bell BP. 2008. Persistence of protection against hepatitis B virus infection among adolescents vaccinated with recombinant hepatitis B vaccine beginning at birth: a 15-year follow-up study. Pediatr Infect Dis J 27:881–885. doi: 10.1097/INF.0b013e31817702ba. [DOI] [PubMed] [Google Scholar]
- 20.Hammitt LL, Hennessy TW, Fiore AE, Zanis C, Hummel KB, Dunaway E, Bulkow L, McMahon BJ. 2007. Hepatitis B immunity in children vaccinated with recombinant hepatitis B vaccine beginning at birth: a follow-up study at 15 years. Vaccine 25:6958–6964. doi: 10.1016/j.vaccine.2007.06.059. [DOI] [PubMed] [Google Scholar]
- 21.West DJ, Calandra GB. 1996. Vaccine induced immunologic memory for hepatitis B surface antigen: implications for policy on booster vaccination. Vaccine 14:1019–1027. doi: 10.1016/0264-410X(96)00062-X. [DOI] [PubMed] [Google Scholar]
- 22.Abanamy A, Khalil M, Salman H, Abdel Azeem M. 1992. Vaccination of Saudi children against measles with Edmonston-Zagreb. Ann Saudi Med 12:110–111. doi: 10.5144/0256-4947.1992.110a. [DOI] [PubMed] [Google Scholar]
- 23.Anonymous. 1977. Measles immunity in the first year after birth and the optimum age for vaccination in Kenyan children. Collaborative study by the Ministry of Health of Kenya and the World Health Organization. Bull World Health Organ 55:21–31. [PMC free article] [PubMed] [Google Scholar]
- 24.Bolotovski VM, Grabowsky M, Clements CJ, Albrecht P, Brenner ER, Zargaryantzs AI, Litvinov SK, Mikheyeva IV. 1994. Immunization of 6 and 9 month old infants with AIK-C, Edmonston-Zagreb, Leningrad-16 and Schwarz strains of measles vaccine. Int J Epidemiol 23:1069–1077. doi: 10.1093/ije/23.5.1069. [DOI] [PubMed] [Google Scholar]
- 25.Carson MM, Spady DW, Albrecht P, Beeler JA, Thipphawong J, Barreto L, Grimsrud KM, Pabst HF. 1995. Measles vaccination of infants in a well-vaccinated population. Pediatr Infect Dis J 14:17–22. doi: 10.1097/00006454-199501000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Cutts FT, Nyandu B, Markowitz LE, Forsey T, Zell ER, Othepa O, Wilkins K. 1994. Immunogenicity of high-titre AIK-C or Edmonston-Zagreb vaccines in 3.5-month-old infants, and of medium- or high-titre Edmonston-Zagreb vaccine in 6-month-old infants, in Kinshasa, Zaire. Vaccine 12:1311–1316. doi: 10.1016/S0264-410X(94)80057-7. [DOI] [PubMed] [Google Scholar]
- 27.Díaz-Ortega JL, Zárate-Aquino ML, Valdespino-Gómez JL, Cardenas-Ayala VM, Ruíz-Matus C. 1986. Seroconversion with measles vaccine in children 8–18 months old. Bol Med Hosp Infant Mex 43:526–531. [PubMed] [Google Scholar]
- 28.Ekunwe EO. 1985. Separating the factors in measles vaccine failure. Ann Trop Paediatr 5:103–106. doi: 10.1080/02724936.1985.11748371. [DOI] [PubMed] [Google Scholar]
- 29.de Castro JF, Gomez JLV, Ortega JLD, Aquino MLZ. 1986. Diploid cell measles vaccine. JAMA 256:714. doi: 10.1001/jama.1986.03380060040014. [DOI] [PubMed] [Google Scholar]
- 30.Gans HA, Arvin AM, Galinus J, Logan L, DeHovitz R, Maldonado Y. 1998. Deficiency of the humoral immune response to measles vaccine in infants immunized at age 6 months. JAMA 280:527–532. doi: 10.1001/jama.280.6.527. [DOI] [PubMed] [Google Scholar]
- 31.He H, Chen E, Chen H, Wang Z, Li Q, Yan R, Guo J, Zhou Y, Pan J, Xie S. 2014. Similar immunogenicity of measles-mumps-rubella (MMR) vaccine administrated at 8 months versus 12 months age in children. Vaccine 32:4001–4005. doi: 10.1016/j.vaccine.2014.04.044. [DOI] [PubMed] [Google Scholar]
- 32.Job JS, John TJ, Joseph A. 1984. Antibody response to measles immunization in India. Bull World Health Organ 62:737–741. [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson CE, Nalin DR, Chui LW, Whitwell J, Marusyk RG, Kumar ML. 1994. Measles vaccine immunogenicity in 6- versus 15-month-old infants born to mothers in the measles vaccine era. Pediatrics 93:939–944. [PubMed] [Google Scholar]
- 34.Katiyar GP, Kaur G, Agarwal DK, Gulati AK, Kalra A, Agarwal KN. 1985. Sero-conversion response to Mevilin-L measles vaccine at age 6–15 months. Indian Pediatr 22:653–659. [PubMed] [Google Scholar]
- 35.Benn CS, Aaby P, Bale C, Olsen J, Michaelsen KF, George E, Whittle H. 1997. Randomised trial of effect of vitamin A supplementation on antibody response to measles vaccine in Guinea-Bissau, West Africa. Lancet 350:101–105. doi: 10.1016/S0140-6736(96)12019-5. [DOI] [PubMed] [Google Scholar]
- 36.Berry S, Hernandez H, Kanashiro R, Campos M, Azabache V, Gomez G, Gutierrez M, Weirs B, De Quadros C, Halsey N. 1992. Comparison of high titer Edmonston-Zagreb, Biken-CAM and Schwarz measles vaccines in Peruvian infants. Pediatr Infect Dis J 11:822–827. doi: 10.1097/00006454-199210000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Gans HA, Maldonado Y, Yasukawa LL, Beeler J, Audet S, Rinki MM, DeHovitz R, Arvin AM. 1999. IL-12, IFN-gamma, and T cell proliferation to measles in immunized infants. J Immunol 162:5569–5575. [PubMed] [Google Scholar]
- 38.Gans H, Yasukawa L, Rinki M, DeHovitz R, Forghani B, Beeler J, Audet S, Maldonado Y, Arvin AM. 2001. Immune responses to measles and mumps vaccination of infants at 6, 9, and 12 months. J Infect Dis 184:817–826. doi: 10.1086/323346. [DOI] [PubMed] [Google Scholar]
- 39.Gans HA, Yasukawa LL, Alderson A, Rinki M, DeHovitz R, Beeler J, Audet S, Maldonado Y, Arvin AM. 2004. Humoral and cell-mediated immune responses to an early 2-dose measles vaccination regimen in the United States. J Infect Dis 190:83–90. doi: 10.1086/421032. [DOI] [PubMed] [Google Scholar]
- 40.Garly ML, Bale C, Martins CL, Monteiro M, George E, Kidd M, Dias F, Aaby P, Whittle HC. 2001. Measles antibody responses after early two dose trials in Guinea-Bissau with Edmonston-Zagreb and Schwarz standard-titre measles vaccine: better antibody increase from booster dose of the Edmonston-Zagreb vaccine. Vaccine 19:1951–1959. doi: 10.1016/S0264-410X(00)00431-X. [DOI] [PubMed] [Google Scholar]
- 41.Hussey GD, Goddard EA, Hughes J, Ryon JJ, Kerran M, Carelse E, Strebel PM, Markowitz LE, Moodie J, Barron P, Latief Z, Sayed R, Beatty D, Griffin DE. 1996. The effect of Edmonston-Zagreb and Schwarz measles vaccines on immune response in infants. J Infect Dis 173:1320–1326. doi: 10.1093/infdis/173.6.1320. [DOI] [PubMed] [Google Scholar]
- 42.Jensen TG, Whittle H, Mordhorst CH, Pedersen IR, Thaarup J, Poulsen A, Sodemann M, Jakobsen M, Brink L, Gansted U, Permin A, Clotilde da Silva M, Aaby P. 1994. Trials of Edmonston-Zagreb measles vaccine in Guinea-Bissau: serological responses following vaccination with Edmonston-Zagreb strain at 4–8 months versus vaccination with Schwarz strain at 9–12 months of age. Vaccine 12:1026–1031. doi: 10.1016/0264-410X(94)90340-9. [DOI] [PubMed] [Google Scholar]
- 43.Kiepiela P, Coovadia HM, Loening WE, Coward P, Botha G, Hugo J, Becker PJ. 1991. Lack of efficacy of the standard potency Edmonston-Zagreb live, attenuated measles vaccine in African infants. Bull World Health Organ 69:221–227. [PMC free article] [PubMed] [Google Scholar]
- 44.Ko B, Roberts D, Begg N, Rao M, Teare L, Cohen B. 1999. Neutralising antibody responses to two doses of measles vaccine at 5 and 13 months of age in the United Kingdom. Commun Dis Public Health 2:203–206. [PubMed] [Google Scholar]
- 45.Lee YL, Black FL, Chen CL, Wu CL, Berman LL. 1983. The optimal age for vaccination against measles in an Asiatic city, Taipei, Taiwan: reduction of vaccine induced titre by residual transplacental antibody. Int J Epidemiol 12:340–343. doi: 10.1093/ije/12.3.340. [DOI] [PubMed] [Google Scholar]
- 46.Anonymous. 1982. Seroconversion rates and measles antibody titers induced by measles vaccine in Latin American children 6–12 months of age. Collaborative study by the Ministries of Health of Brazil, Chile, Costa Rica, Ecuador, and the Pan American Health Organization. Bull Pan Am Health Organ 16:272–285. [PubMed] [Google Scholar]
- 47.Rogers S, Sanders RC, Alpers MP. 1991. Immunogenicity of standard dose Edmonston-Zagreb measles vaccine in highland Papua New Guinean children from four months of age. J Trop Med Hyg 94:88–91. [PubMed] [Google Scholar]
- 48.Tidjani O, Grunitsky B, Guerin N, Levy-Bruhl D, Lecam N, Xuereff C, Tatagan K. 1989. Serological effects of Edmonston-Zagreb, Schwarz, and AIK-C measles vaccine strains given at ages 4–5 or 8–10 months. Lancet ii:1357–1360. [DOI] [PubMed] [Google Scholar]
- 49.Lochlainn LN, de Gier B, van der Maas N, Rots N, van Binnendijk R, de Melker H, Hahné S. 2015. Measles vaccination below 9 months of age: systematic literature review and meta‐analyses of effects and safety. Centre for Infectious Disease Control, National Institute for Public Health and the Environment (RIVM) Bilthoven, the Netherlands. [Google Scholar]
- 50.Carazo Perez S, De Serres G, Bureau A, Skowronski DM. 2017. Reduced antibody response to infant measles vaccination: effects based on type and timing of the first vaccine dose persist after the second dose. Clin Infect Dis 65:1094–1102. doi: 10.1093/cid/cix510. [DOI] [PubMed] [Google Scholar]
- 51.Njie-Jobe J, Nyamweya S, Miles DJ, van der Sande M, Zaman S, Touray E, Hossin S, Adetifa J, Palmero M, Burl S, Jeffries D, Rowland-Jones S, Flanagan K, Jaye A, Whittle H. 2012. Immunological impact of an additional early measles vaccine in Gambian children: responses to a boost at 3 years. Vaccine 30:2543–2550. doi: 10.1016/j.vaccine.2012.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knuf M, Schmitt HJ, Wolter J, Schuerman L, Jacquet JM, Kieninger D, Siegrist CA, Zepp F. 2008. Neonatal vaccination with an acellular pertussis vaccine accelerates the acquisition of pertussis antibodies in infants. J Pediatr 152:655–660. doi: 10.1016/j.jpeds.2007.09.034. [DOI] [PubMed] [Google Scholar]
- 53.Wood N, McIntyre P, Marshall H, Roberton D. 2010. Acellular pertussis vaccine at birth and one month induces antibody responses by two months of age. Pediatr Infect Dis J 29:209–215. doi: 10.1097/INF.0b013e3181bc98d5. [DOI] [PubMed] [Google Scholar]
- 54.Colditz GA, Berkey CS, Mosteller F, Brewer TF, Wilson ME, Burdick E, Fineberg HV. 1995. The efficacy of bacillus Calmette-Guerin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics 96:29–35. [PubMed] [Google Scholar]
- 55.Belloni C, Orsolini P, Martinetti M, Chirico G, Cerbo RM, Comolli G, Maccarini U, Barlassina C, Togni C, Polatti F. 1993. Control of hepatitis B: evaluation of two different vaccinal schedules in newborns from HBsAg negative mothers. New Microbiol 16:237–244. [PubMed] [Google Scholar]
- 56.Wood N, Siegrist CA. 2011. Neonatal immunization: where do we stand? Curr Opin Infect Dis 24:190–195. doi: 10.1097/QCO.0b013e328345d563. [DOI] [PubMed] [Google Scholar]
- 57.Levy O. 2007. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol 7:379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 58.Siegrist CA, Aspinall R. 2009. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol 9:185–194. doi: 10.1038/nri2508. [DOI] [PubMed] [Google Scholar]
- 59.Siegrist CA. 2001. Neonatal and early life vaccinology. Vaccine 19:3331–3346. doi: 10.1016/S0264-410X(01)00028-7. [DOI] [PubMed] [Google Scholar]
- 60.Bayas JM, Vilella A, Bertran MJ, Vidal J, Batalla J, Asenjo MA, Salleras LL. 2001. Immunogenicity and reactogenicity of the adult tetanus-diphtheria vaccine. How many doses are necessary? Epidemiol Infect 127:451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van der Wielen M, Van Damme P, Chlibek R, Smetana J, von Sonnenburg F. 2006. Hepatitis A/B vaccination of adults over 40 years old: comparison of three vaccine regimens and effect of influencing factors. Vaccine 24:5509–5515. doi: 10.1016/j.vaccine.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 62.Wolters B, Junge U, Dziuba S, Roggendorf M. 2003. Immunogenicity of combined hepatitis A and B vaccine in elderly persons. Vaccine 21:3623–3628. doi: 10.1016/S0264-410X(03)00399-2. [DOI] [PubMed] [Google Scholar]
- 63.Tanaka E, Sata M, Nakano H, Suzuki H, Tanikawa K. 1997. Antibody response to inactivated hepatitis A vaccine. Hepatol Res 9:103–112. doi: 10.1016/S1386-6346(97)00079-X. [DOI] [Google Scholar]
- 64.McMahon BJ, Williams J, Bulkow L, Snowball M, Wainwright R, Kennedy M, Krause D. 1995. Immunogenicity of an inactivated hepatitis A vaccine in Alaska Native children and Native and non-Native adults. J Infect Dis 171:676–679. doi: 10.1093/infdis/171.3.676. [DOI] [PubMed] [Google Scholar]
- 65.D’Acremont V, Herzog C, Genton B. 2006. Immunogenicity and safety of a virosomal hepatitis A vaccine (Epaxal) in the elderly. J Travel Med 13:78–83. doi: 10.1111/j.1708-8305.2006.00001.x. [DOI] [PubMed] [Google Scholar]
- 66.Yang S, Tian G, Cui Y, Ding C, Deng M, Yu C, Xu K, Ren J, Yao J, Li Y, Cao Q, Chen P, Xie T, Wang C, Wang B, Mao C, Ruan B, Jiang T, Li L. 2016. Factors influencing immunologic response to hepatitis B vaccine in adults. Sci Rep 6:27251. doi: 10.1038/srep27251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Westmoreland D, Player V, Heap DC, Hammond A. 1990. Immunization against hepatitis B—what can we expect? Results of a survey of antibody response to immunization in persons ‘at risk’ of occupational exposure to hepatitis B. Epidemiol Infect 104:499–509. doi: 10.1017/S0950268800047506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roome AJ, Walsh SJ, Cartter ML, Hadler JL. 1993. Hepatitis B vaccine responsiveness in Connecticut public safety personnel. JAMA 270:2931–2934. doi: 10.1001/jama.1993.03510240043029. [DOI] [PubMed] [Google Scholar]
- 69.Bock HL, Kruppenbacher J, Sanger R, Hobel W, Clemens R, Jilg W. 1996. Immunogenicity of a recombinant hepatitis B vaccine in adults. Arch Intern Med 156:2226–2231. doi: 10.1001/archinte.1996.00440180088011. [DOI] [PubMed] [Google Scholar]
- 70.Morris CA, Oliver PR, Reynolds F, Selkon JB. 1989. Intradermal hepatitis B immunization with yeast-derived vaccine: serological response by sex and age. Epidemiol Infect 103:387–394. doi: 10.1017/S0950268800030740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dentico P, Buongiorno R, Volpe A, Zavoianni A, Pastore G, Schiraldi O. 1992. Long-term immunogenicity safety and efficacy of a recombinant hepatitis B vaccine in healthy adults. Eur J Epidemiol 8:650–655. doi: 10.1007/BF00145379. [DOI] [PubMed] [Google Scholar]
- 72.Yen YH, Chen CH, Wang JH, Lee CM, Changchien CS, Lu SN. 2005. Study of hepatitis B (HB) vaccine non-responsiveness among health care workers from an endemic area (Taiwan). Liver Int 25:1162–1168. doi: 10.1111/j.1478-3231.2005.01157.x. [DOI] [PubMed] [Google Scholar]
- 73.Hess G, Hingst V, Cseke J, Bock HL, Clemens R. 1992. Influence of vaccination schedules and host factors on antibody response following hepatitis B vaccination. Eur J Clin Microbiol Infect Dis 11:334–340. doi: 10.1007/BF01962073. [DOI] [PubMed] [Google Scholar]
- 74.Struve J, Aronsson B, Frenning B, Granath F, von Sydow M, Weiland O. 1992. Intramuscular versus intradermal administration of a recombinant hepatitis B vaccine: a comparison of response rates and analysis of factors influencing the antibody response. Scand J Infect Dis 24:423–429. doi: 10.3109/00365549209052627. [DOI] [PubMed] [Google Scholar]
- 75.Coleman PJ, Shaw FE Jr, Serovich J, Hadler SC, Margolis HS. 1991. Intradermal hepatitis B vaccination in a large hospital employee population. Vaccine 9:723–727. doi: 10.1016/0264-410X(91)90287-G. [DOI] [PubMed] [Google Scholar]
- 76.Cleveland JL, Siew C, Lockwood SA, Gruninger SE, Chang SB, Neidle EA, Russell CM. 1994. Factors associated with hepatitis B vaccine response among dentists. J Dent Res 73:1029–1035. doi: 10.1177/00220345940730050301. [DOI] [PubMed] [Google Scholar]
- 77.Estévez ZC, Betancourt AA, Muzio González V, Baile NF, Silva CV, Bernal FH, Arias EP, Delhanty Fernández A, Olazábal NM, del Río Martín A, Batista LL, Véliz Ríos G, Hernández HH, Hernández AB, Lugo EP, de la Torre Cruz J, Batista Marchec BL, Falcón LA, Brito JT, León DO, Saura PL. 2007. Immunogenicity and safety assessment of the Cuban recombinant hepatitis B vaccine in healthy adults. Biologicals 35:115–122. doi: 10.1016/j.biologicals.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 78.Averhoff F, Mahoney F, Coleman P, Schatz G, Hurwitz E, Margolis H. 1998. Immunogenicity of hepatitis B vaccines. Implications for persons at occupational risk of hepatitis B virus infection. Am J Prev Med 15:1–8. doi: 10.1016/S0749-3797(98)00003-8. [DOI] [PubMed] [Google Scholar]
- 79.Sabido M, Gavalda L, Olona N, Ramon JM. 2007. Timing of hepatitis B vaccination: its effect on vaccine response in health care workers. Vaccine 25:7568–7572. doi: 10.1016/j.vaccine.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 80.Wood RC, MacDonald KL, White KE, Hedberg CW, Hanson M, Osterholm MT. 1993. Risk factors for lack of detectable antibody following hepatitis B vaccination of Minnesota health care workers. JAMA 270:2935–2939. doi: 10.1001/jama.1993.03510240047030. [DOI] [PubMed] [Google Scholar]
- 81.Tohme RA, Awosika-Olumo D, Nielsen C, Khuwaja S, Scott J, Xing J, Drobeniuc J, Hu DJ, Turner C, Wafeeg T, Sharapov U, Spradling PR. 2011. Evaluation of hepatitis B vaccine immunogenicity among older adults during an outbreak response in assisted living facilities. Vaccine 29:9316–9320. doi: 10.1016/j.vaccine.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rubins JB, Puri AK, Loch J, Charboneau D, MacDonald R, Opstad N, Janoff EN. 1998. Magnitude, duration, quality, and function of pneumococcal vaccine responses in elderly adults. J Infect Dis 178:431–440. doi: 10.1086/515644. [DOI] [PubMed] [Google Scholar]
- 83.Sankilampi U, Honkanen PO, Bloigu A, Herva E, Leinonen M. 1996. Antibody response to pneumococcal capsular polysaccharide vaccine in the elderly. J Infect Dis 173:387–393. doi: 10.1093/infdis/173.2.387. [DOI] [PubMed] [Google Scholar]
- 84.Konradsen H, Henrichsen J. 1991. Antibody response to pneumococcal vaccination in the elderly. Zentralbl Bakteriol 275:94–99. doi: 10.1016/S0934-8840(11)80772-3. [DOI] [PubMed] [Google Scholar]
- 85.McElhaney JE, Meneilly GS, Beattie BL, Helgason CD, Lee SF, Devine RD, Bleackley RC. 1992. The effect of influenza vaccination on IL2 production in healthy elderly: implications for current vaccination practices. J Gerontol 47:M3–M8. doi: 10.1093/geronj/47.1.M3. [DOI] [PubMed] [Google Scholar]
- 86.McElhaney JE, Upshaw CM, Hooton JW, Lechelt KE, Meneilly GS. 1998. Responses to influenza vaccination in different T-cell subsets: a comparison of healthy young and older adults. Vaccine 16:1742–1747. doi: 10.1016/S0264-410X(98)00133-9. [DOI] [PubMed] [Google Scholar]
- 87.Bernstein ED, Gardner EM, Abrutyn E, Gross P, Murasko DM. 1998. Cytokine production after influenza vaccination in a healthy elderly population. Vaccine 16:1722–1731. doi: 10.1016/S0264-410X(98)00140-6. [DOI] [PubMed] [Google Scholar]
- 88.Hainz U, Jenewein B, Asch E, Pfeiffer KP, Berger P, Grubeck-Loebenstein B. 2005. Insufficient protection for healthy elderly adults by tetanus and TBE vaccines. Vaccine 23:3232–3235. doi: 10.1016/j.vaccine.2005.01.085. [DOI] [PubMed] [Google Scholar]
- 89.Gergen PJ, McQuillan GM, Kiely M, Ezzati-Rice TM, Sutter RW, Virella G. 1995. A population-based serologic survey of immunity to tetanus in the United States. N Engl J Med 332:761–766. doi: 10.1056/NEJM199503233321201. [DOI] [PubMed] [Google Scholar]
- 90.Kao TM, Hsieh SM, Kung HC, Lee YC, Huang KC, Huang LM, Chang FY, Wang NC, Liu YC, Lee WS, Liu HE, Chen CI, Chen CH. 2010. Immune response of single dose vaccination against 2009 pandemic influenza A (H1N1) in the Taiwanese elderly. Vaccine 28:6159–6163. doi: 10.1016/j.vaccine.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 91.Khurana S, Verma N, Talaat KR, Karron RA, Golding H. 2012. Immune response following H1N1pdm09 vaccination: differences in antibody repertoire and avidity in young adults and elderly populations stratified by age and gender. J Infect Dis 205:610–620. doi: 10.1093/infdis/jir791. [DOI] [PubMed] [Google Scholar]
- 92.McElhaney JE, Zhou X, Talbot HK, Soethout E, Bleackley RC, Granville DJ, Pawelec G. 2012. The unmet need in the elderly: how immunosenescence, CMV infection, co-morbidities and frailty are a challenge for the development of more effective influenza vaccines. Vaccine 30:2060–2067. doi: 10.1016/j.vaccine.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mugitani A, Ito K, Irie S, Eto T, Ishibashi M, Ohfuji S, Fukushima W, Maeda A, Hirota Y. 2014. Immunogenicity of the trivalent inactivated influenza vaccine in young children less than 4 years of age, with a focus on age and baseline antibodies. Clin Vaccine Immunol 21:1253–1260. doi: 10.1128/CVI.00200-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Greenwood BM, Bradley AK, Blakebrough IS, Whittle HC, Marshall TF, Gilles HM. 1980. The immune response to a meningococcal polysaccharide vaccine in an African village. Trans R Soc Trop Med Hyg 74:340–346. doi: 10.1016/0035-9203(80)90095-4. [DOI] [PubMed] [Google Scholar]
- 95.Kanesa-Thasan N, Sun W, Ludwig GV, Rossi C, Putnak JR, Mangiafico JA, Innis BL, Edelman R. 2003. Atypical antibody responses in dengue vaccine recipients. Am J Trop Med Hyg 69:32–38. doi: 10.4269/ajtmh.2003.69.32. [DOI] [PubMed] [Google Scholar]
- 96.Wagner G, Lavanchy D, Darioli R, Pecoud A, Brulein V, Safary A, Frei PC. 1993. Simultaneous active and passive immunization against hepatitis A studied in a population of travellers. Vaccine 11:1027–1032. doi: 10.1016/0264-410X(93)90128-K. [DOI] [PubMed] [Google Scholar]
- 97.Chen XQ, Bulbul M, de Gast GC, van Loon AM, Nalin DR, van Hattum J. 1997. Immunogenicity of two versus three injections of inactivated hepatitis A vaccine in adults. J Hepatol 26:260–264. doi: 10.1016/S0168-8278(97)80039-6. [DOI] [PubMed] [Google Scholar]
- 98.Goubau P, Van Gerven V, Safary A, Delem A, Knops J, D’Hondt E, Andre FE, Desmyter J. 1992. Effect of virus strain and antigen dose on immunogenicity and reactogenicity of an inactivated hepatitis A vaccine. Vaccine 10:S114—S118. doi: 10.1016/0264-410X(92)90561-W. [DOI] [PubMed] [Google Scholar]
- 99.Sandman L, Davidson M, Krugman S. 1995. Inactivated hepatitis A vaccine: a safety and immunogenicity study in health professionals. J Infect Dis 171:S50–S52. doi: 10.1093/infdis/171.Supplement_1.S50. [DOI] [PubMed] [Google Scholar]
- 100.Hohler T, Groeger-Bicanic G, Hoet B, Stoffel M. 2007. Antibody persistence and immune memory elicited by combined hepatitis A and B vaccination in older adults. Vaccine 25:1503–1508. doi: 10.1016/j.vaccine.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 101.Reutter J, Bart PA, Francioli P, Safary A, Frei PC. 1998. Production of antibody to hepatitis A virus and hepatitis B surface antigen measured after combined hepatitis A/hepatitis B vaccination in 242 adult volunteers. J Viral Hepat 5:205–211. doi: 10.1046/j.1365-2893.1998.00101.x. [DOI] [PubMed] [Google Scholar]
- 102.Keeffe EB, Iwarson S, McMahon BJ, Lindsay KL, Koff RS, Manns M, Baumgarten R, Wiese M, Fourneau M, Safary A, Clemens R, Krause DS. 1998. Safety and immunogenicity of hepatitis A vaccine in patients with chronic liver disease. Hepatology 27:881–886. doi: 10.1002/hep.510270336. [DOI] [PubMed] [Google Scholar]
- 103.Bovier PA, Bock J, Loutan L, Farinelli T, Glueck R, Herzog C. 2002. Long-term immunogenicity of an inactivated virosome hepatitis A vaccine. J Med Virol 68:489–493. doi: 10.1002/jmv.10244. [DOI] [PubMed] [Google Scholar]
- 104.Weissman S, Feucht C, Moore BA. 2006. Response to hepatitis A vaccine in HIV-positive patients. J Viral Hepat 13:81–86. doi: 10.1111/j.1365-2893.2005.00658.x. [DOI] [PubMed] [Google Scholar]
- 105.Jilg W, Lorbeer B, Schmidt M, Wilske B, Zoulek G, Deinhardt F. 1984. Clinical evaluation of a recombinant hepatitis B vaccine. Lancet ii:1174–1175. [DOI] [PubMed] [Google Scholar]
- 106.Zeeshan M, Jabeen K, Ali AN, Ali AW, Farooqui SZ, Mehraj V, Zafar A. 2007. Evaluation of immune response to hepatitis B vaccine in health care workers at a tertiary care hospital in Pakistan: an observational prospective study. BMC Infect Dis 7:120. doi: 10.1186/1471-2334-7-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rendi-Wagner P, Kundi M, Stemberger H, Wiedermann G, Holzmann H, Hofer M, Wiesinger K, Kollaritsch H. 2001. Antibody-response to three recombinant hepatitis B vaccines: comparative evaluation of multicenter travel-clinic based experience. Vaccine 19:2055–2060. doi: 10.1016/S0264-410X(00)00410-2. [DOI] [PubMed] [Google Scholar]
- 108.Halota W, Muszyńska M, Pawłowska M. 2002. Hepatitis B virus serologic markers and anti-hepatitis B vaccination in patients with diabetes. Med Sci Monit 8:Cr516–Cr519. [PubMed] [Google Scholar]
- 109.Douvin C, Simon D, Charles MA, Deforges L, Bierling P, Lehner V, Budkowska A, Dhumeaux D. 1997. Hepatitis B vaccination in diabetic patients. Randomized trial comparing recombinant vaccines containing and not containing pre-S2 antigen. Diabetes Care 20:148–151. doi: 10.2337/diacare.20.2.148. [DOI] [PubMed] [Google Scholar]
- 110.Das K, Gupta RK, Kumar V, Kar P. 2003. Immunogenicity and reactogenicity of a recombinant hepatitis B vaccine in subjects over age of forty years and response of a booster dose among nonresponders. World J Gastroenterol 9:1132–1134. doi: 10.3748/wjg.v9.i5.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.West DJ, Snavely DB, Zajac BA, Brown GW, Babb CJ. 1990. Development and persistence of antibody in a high risk institutionalized population given plasma-derived hepatitis B vaccine. Vaccine 8:111–114. doi: 10.1016/0264-410X(90)90132-6. [DOI] [PubMed] [Google Scholar]
- 112.Stevens CE, Szmuness W, Goodman AI, Weseley SA, Fotino M. 1980. Hepatitis B vaccine: immune responses in haemodialysis patients. Lancet ii:1211–1213. [DOI] [PubMed] [Google Scholar]
- 113.Rogan PD, Duguid JK. 1991. Immunisation of staff of a regional blood transfusion centre with a recombinant hepatitis B vaccine. J Infect 22:5–9. doi: 10.1016/0163-4453(91)90786-R. [DOI] [PubMed] [Google Scholar]
- 114.Fang JW, Lai CL, Chung HT, Wu PC, Lau JY. 1994. Female children respond to recombinant hepatitis B vaccine with a higher titre than male. J Trop Pediatr 40:104–107. doi: 10.1093/tropej/40.2.104. [DOI] [PubMed] [Google Scholar]
- 115.Gold Y, Somech R, Mandel D, Peled Y, Reif S. 2003. Decreased immune response to hepatitis B eight years after routine vaccination in Israel. Acta Paediatr 92:1158–1162. [DOI] [PubMed] [Google Scholar]
- 116.Cook IF, Murtagh J. 2002. Comparative immunogenicity of hepatitis B vaccine administered into the ventrogluteal area and anterolateral thigh in infants. J Paediatr Child Health 38:393–396. doi: 10.1046/j.1440-1754.2002.00013.x. [DOI] [PubMed] [Google Scholar]
- 117.Keyserling HL, West DJ, Hesley TM, Bosley C, Wiens BL, Calandra GB. 1994. Antibody responses of healthy infants to a recombinant hepatitis B vaccine administered at two, four, and twelve or fifteen months of age. J Pediatr 125:67–69. doi: 10.1016/S0022-3476(94)70123-7. [DOI] [PubMed] [Google Scholar]
- 118.Nissen TN, Birk NM, Smits G, Jeppesen DL, Stensballe LG, Netea MG, van der Klis F, Benn CS, Pryds O. 2017. Bacille Calmette-Guerin (BCG) vaccination at birth and antibody responses to childhood vaccines. A randomised clinical trial. Vaccine 35:2084–2091. doi: 10.1016/j.vaccine.2017.02.048. [DOI] [PubMed] [Google Scholar]
- 119.Stark K, Schonfeld C, Barg J, Molz B, Vornwald A, Bienzle U. 1999. Seroprevalence and determinants of diphtheria, tetanus and poliomyelitis antibodies among adults in Berlin, Germany. Vaccine 17:844–850. doi: 10.1016/S0264-410X(98)00269-2. [DOI] [PubMed] [Google Scholar]
- 120.Shaw MM, Leggat PA, Williams ML. 2006. Intradermal pre-exposure rabies immunisation in New Zealand. Travel Med Infect Dis 4:29–33. doi: 10.1016/j.tmaid.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 121.Briggs DJ, Banzhoff A, Nicolay U, Sirikwin S, Dumavibhat B, Tongswas S, Wasi C. 2000. Antibody response of patients after postexposure rabies vaccination with small intradermal doses of purified chick embryo cell vaccine or purified Vero cell rabies vaccine. Bull World Health Organ 78:693–698. [PMC free article] [PubMed] [Google Scholar]
- 122.Moore SE, Goldblatt D, Bates CJ, Prentice AM. 2003. Impact of nutritional status on antibody responses to different vaccines in undernourished Gambian children. Acta Paediatr 92:170–176. [DOI] [PubMed] [Google Scholar]
- 123.Kennedy RB, Ovsyannikova IG, Pankratz VS, Vierkant RA, Jacobson RM, Ryan MA, Poland GA. 2009. Gender effects on humoral immune responses to smallpox vaccine. Vaccine 27:3319–3323. doi: 10.1016/j.vaccine.2009.01.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Edwards KM, Burns VE, Allen LM, McPhee JS, Bosch JA, Carroll D, Drayson M, Ring C. 2007. Eccentric exercise as an adjuvant to influenza vaccination in humans. Brain Behav Immun 21:209–217. doi: 10.1016/j.bbi.2006.04.158. [DOI] [PubMed] [Google Scholar]
- 125.Cook IF, Barr I, Hartel G, Pond D, Hampson AW. 2006. Reactogenicity and immunogenicity of an inactivated influenza vaccine administered by intramuscular or subcutaneous injection in elderly adults. Vaccine 24:2395–2402. doi: 10.1016/j.vaccine.2005.11.057. [DOI] [PubMed] [Google Scholar]
- 126.Engler RJ, Nelson MR, Klote MM, VanRaden MJ, Huang CY, Cox NJ, Klimov A, Keitel WA, Nichol KL, Carr WW, Treanor JJ. 2008. Half- vs full-dose trivalent inactivated influenza vaccine (2004–2005): age, dose, and sex effects on immune responses. Arch Intern Med 168:2405–2414. doi: 10.1001/archinternmed.2008.513. [DOI] [PubMed] [Google Scholar]
- 127.Edwards KM, Campbell JP, Ring C, Drayson MT, Bosch JA, Downes C, Long JE, Lumb JA, Merry A, Paine NJ, Burns VE. 2010. Exercise intensity does not influence the efficacy of eccentric exercise as a behavioural adjuvant to vaccination. Brain Behav Immun 24:623–630. doi: 10.1016/j.bbi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 128.Booy R, Khandaker G, Heron LG, Yin J, Doyle B, Tudo KK, Hueston L, Gilbert GL, Macintyre CR, Dwyer DE. 2011. Cross-reacting antibodies against the pandemic (H1N1) 2009 influenza virus in older Australians. Med J Aust 194:19–23. [DOI] [PubMed] [Google Scholar]
- 129.Talaat KR, Greenberg ME, Lai MH, Hartel GF, Wichems CH, Rockman S, Jeanfreau RJ, Ghosh MR, Kabongo ML, Gittleson C, Karron RA. 2010. A single dose of unadjuvanted novel 2009 H1N1 vaccine is immunogenic and well tolerated in young and elderly adults. J Infect Dis 202:1327–1337. doi: 10.1086/656601. [DOI] [PubMed] [Google Scholar]
- 130.Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. 2009. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis 200:172–180. doi: 10.1086/599790. [DOI] [PubMed] [Google Scholar]
- 131.Furman D, Hejblum BP, Simon N, Jojic V, Dekker CL, Thiebaut R, Tibshirani RJ, Davis MM. 2014. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci U S A 111:869–874. doi: 10.1073/pnas.1321060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O’Fallon WM, Weyand CM. 2001. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J Virol 75:12182–12187. doi: 10.1128/JVI.75.24.12182-12187.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Couch RB, Winokur P, Brady R, Belshe R, Chen WH, Cate TR, Sigurdardottir B, Hoeper A, Graham IL, Edelman R, He F, Nino D, Capellan J, Ruben FL. 2007. Safety and immunogenicity of a high dosage trivalent influenza vaccine among elderly subjects. Vaccine 25:7656–7663. doi: 10.1016/j.vaccine.2007.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hui SL, Chu LW, Peiris JS, Chan KH, Chu D, Tsui W. 2006. Immune response to influenza vaccination in community-dwelling Chinese elderly persons. Vaccine 24:5371–5380. doi: 10.1016/j.vaccine.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 135.Miller E, Rush M, Morgan-Capner P, Hutchinson D, Hindle L. 1994. Immunity to diphtheria in adults in England. BMJ 308:598. doi: 10.1136/bmj.308.6928.598b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cohen D, Katzenelson E, Green M, Slepon R, Bercovier H, Danon Y. 1991. Prevalence and correlates of diphtheria toxin antibodies among young adults in Israel. J Infect 23:117–121. doi: 10.1016/0163-4453(91)91929-R. [DOI] [PubMed] [Google Scholar]
- 137.Mark A, Carlsson RM, Granstrom M. 1999. Subcutaneous versus intramuscular injection for booster DT vaccination of adolescents. Vaccine 17:2067–2072. doi: 10.1016/S0264-410X(98)00410-1. [DOI] [PubMed] [Google Scholar]
- 138.Bjorkholm B, Hagberg L, Sundbeck G, Granstrom M. 2000. Booster effect of low doses of tetanus toxoid in elderly vaccinees. Eur J Clin Microbiol Infect Dis 19:195–199. [DOI] [PubMed] [Google Scholar]
- 139.Edwards KM, Burns VE, Adkins AE, Carroll D, Drayson M, Ring C. 2008. Meningococcal A vaccination response is enhanced by acute stress in men. Psychosom Med 70:147–151. doi: 10.1097/PSY.0b013e318164232e. [DOI] [PubMed] [Google Scholar]
- 140.Goldblatt D, Southern J, Andrews N, Ashton L, Burbidge P, Woodgate S, Pebody R, Miller E. 2009. The immunogenicity of 7-valent pneumococcal conjugate vaccine versus 23-valent polysaccharide vaccine in adults aged 50–80 years. Clin Infect Dis 49:1318–1325. doi: 10.1086/606046. [DOI] [PubMed] [Google Scholar]
- 141.Brandão AP, de Oliveira TC, de Cunto Brandileone MC, Gonçalves JE, Yara TI, Simonsen V. 2004. Persistence of antibody response to pneumococcal capsular polysaccharides in vaccinated long term-care residents in Brazil. Vaccine 23:762–768. doi: 10.1016/j.vaccine.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 142.McMahon BJ, Parkinson AJ, Bulkow L, Davidson M, Wainwright K, Wolfe P, Schiffman GS. 1993. Immunogenicity of the 23-valent pneumococcal polysaccharide vaccine in Alaska Native chronic alcoholics compared with nonalcoholic Native and non-Native controls. Am J Med 95:589–594. doi: 10.1016/0002-9343(93)90354-R. [DOI] [PubMed] [Google Scholar]
- 143.Cook IF, Pond D, Hartel G. 2007. Comparative reactogenicity and immunogenicity of 23 valent pneumococcal vaccine administered by intramuscular or subcutaneous injection in elderly adults. Vaccine 25:4767–4774. doi: 10.1016/j.vaccine.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 144.Roghmann KJ, Tabloski PA, Bentley DW, Schiffman G. 1987. Immune response of elderly adults to pneumococcus: variation by age, sex, and functional impairment. J Gerontol 42:265–270. doi: 10.1093/geronj/42.3.265. [DOI] [PubMed] [Google Scholar]
- 145.Zhang X, Castelli FA, Zhu X, Wu M, Maillere B, BenMohamed L. 2008. Gender-dependent HLA-DR-restricted epitopes identified from herpes simplex virus type 1 glycoprotein D. Clin Vaccine Immunol 15:1436–1449. doi: 10.1128/CVI.00123-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Moore SE, Collinson AC, Fulford AJ, Jalil F, Siegrist CA, Goldblatt D, Hanson LA, Prentice AM. 2006. Effect of month of vaccine administration on antibody responses in The Gambia and Pakistan. Trop Med Int Health 11:1529–1541. doi: 10.1111/j.1365-3156.2006.01700.x. [DOI] [PubMed] [Google Scholar]
- 147.Marvell DM, Parish HJ. 1940. Tetanus prophylaxis and circulating antitoxin in men and women. Br Med J 2:891–895. doi: 10.1136/bmj.2.4173.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lau C, Sisson J. 2002. The effectiveness of intradermal pre-exposure rabies vaccination in an Australian travel medicine clinic. J Travel Med 9:285–288. [DOI] [PubMed] [Google Scholar]
- 149.Green MS, Shohat T, Lerman Y, Cohen D, Slepon R, Duvdevani P, Varsano N, Dagan R, Mendelson E. 1994. Sex differences in the humoral antibody response to live measles vaccine in young adults. Int J Epidemiol 23:1078–1081. doi: 10.1093/ije/23.5.1078. [DOI] [PubMed] [Google Scholar]
- 150.Ovsyannikova IG, Jacobson RM, Dhiman N, Vierkant RA, Pankratz VS, Poland GA. 2008. Human leukocyte antigen and cytokine receptor gene polymorphisms associated with heterogeneous immune responses to mumps viral vaccine. Pediatrics 121:e1091–e1099. doi: 10.1542/peds.2007-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dominguez A, Plans P, Costa J, Torner N, Cardenosa N, Batalla J, Plasencia A, Salleras L. 2006. Seroprevalence of measles, rubella, and mumps antibodies in Catalonia, Spain: results of a cross-sectional study. Eur J Clin Microbiol Infect Dis 25:310–317. doi: 10.1007/s10096-006-0133-z. [DOI] [PubMed] [Google Scholar]
- 152.Umlauf BJ, Haralambieva IH, Ovsyannikova IG, Kennedy RB, Pankratz VS, Jacobson RM, Poland GA. 2012. Associations between demographic variables and multiple measles-specific innate and cell-mediated immune responses after measles vaccination. Viral Immunol 25:29–36. doi: 10.1089/vim.2011.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mitchell LA. 1999. Sex differences in antibody- and cell-mediated immune response to rubella re-immunisation. J Med Microbiol 48:1075–1080. doi: 10.1099/00222615-48-12-1075. [DOI] [PubMed] [Google Scholar]
- 154.Semba RD, Munasir Z, Beeler J, Akib A, Muhilal AS, Sommer A. 1995. Reduced seroconversion to measles in infants given vitamin A with measles vaccination. Lancet 345:1330–1332. [DOI] [PubMed] [Google Scholar]
- 155.Davidkin I, Valle M, Julkunen I. 1995. Persistence of anti-mumps virus antibodies after a two-dose MMR vaccination. A nine-year follow-up. Vaccine 13:1617–1622. doi: 10.1016/0264-410X(95)00064-8. [DOI] [PubMed] [Google Scholar]
- 156.Ovsyannikova IG, Jacobson RM, Ryan JE, Dhiman N, Vierkant RA, Poland GA. 2007. Relationship between HLA polymorphisms and gamma interferon and interleukin-10 cytokine production in healthy individuals after rubella vaccination. Clin Vaccine Immunol 14:115–122. doi: 10.1128/CVI.00247-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Monath TP, Nichols R, Archambault WT, Moore L, Marchesani R, Tian J, Shope RE, Thomas N, Schrader R, Furby D, Bedford P. 2002. Comparative safety and immunogenicity of two yellow fever 17D vaccines (ARILVAX and YF-VAX) in a phase III multicenter, double-blind clinical trial. Am J Trop Med Hyg 66:533–541. doi: 10.4269/ajtmh.2002.66.533. [DOI] [PubMed] [Google Scholar]
- 158.Pfister M, Kursteiner O, Hilfiker H, Favre D, Durrer P, Ennaji A, L’Age-Stehr J, Kaufhold A, Herzog C. 2005. Immunogenicity and safety of BERNA-YF compared with two other 17D yellow fever vaccines in a phase 3 clinical trial. Am J Trop Med Hyg 72:339–346. doi: 10.4269/ajtmh.2005.72.339. [DOI] [PubMed] [Google Scholar]
- 159.Camacho LAB, Freire MDS, Leal MDL, Aguiar SG, Nascimento JP, Iguchi T, Lozana JDA, Farias RH. 2004. Immunogenicity of WHO-17D and Brazilian 17DD yellow fever vaccines: a randomized trial. Rev Saude Publica 38:671–678. doi: 10.1590/S0034-89102004000500009. [DOI] [PubMed] [Google Scholar]
- 160.Klein SL, Jedlicka A, Pekosz A. 2010. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis 10:338–349. doi: 10.1016/S1473-3099(10)70049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang LY, Hu CT, Ho TY, Lin HH. 2006. Geographic and ethnic variations of long-term efficacy and immunogenicity of hepatitis B vaccination in Hualien, a HBV hyperendemic area. Vaccine 24:4427–4432. doi: 10.1016/j.vaccine.2005.12.069. [DOI] [PubMed] [Google Scholar]
- 162.Asturias EJ, Mayorga C, Caffaro C, Ramirez P, Ram M, Verstraeten T, Clemens R, Halsey NA. 2009. Differences in the immune response to hepatitis B and Haemophilus influenzae type b vaccines in Guatemalan infants by ethnic group and nutritional status. Vaccine 27:3650–3654. doi: 10.1016/j.vaccine.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 163.Haralambieva IH, Ovsyannikova IG, Umlauf BJ, Vierkant RA, Shane Pankratz V, Jacobson RM, Poland GA. 2011. Genetic polymorphisms in host antiviral genes: associations with humoral and cellular immunity to measles vaccine. Vaccine 29:8988–8997. doi: 10.1016/j.vaccine.2011.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Haralambieva IH, Ovsyannikova IG, Kennedy RB, Larrabee BR, Shane Pankratz V, Poland GA. 2013. Race and sex-based differences in cytokine immune responses to smallpox vaccine in healthy individuals. Hum Immunol 74:1263–1266. doi: 10.1016/j.humimm.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Haralambieva IH, Salk HM, Lambert ND, Ovsyannikova IG, Kennedy RB, Warner ND, Pankratz VS, Poland GA. 2014. Associations between race, sex and immune response variations to rubella vaccination in two independent cohorts. Vaccine 32:1946–1953. doi: 10.1016/j.vaccine.2014.01.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hsu LC, Lin SR, Hsu HM, Chao WH, Hsieh JT, Wang MC, Lu CF, Chang YH, Ho MS. 1996. Ethnic differences in immune responses to hepatitis B vaccine. Am J Epidemiol 143:718–724. doi: 10.1093/oxfordjournals.aje.a008805. [DOI] [PubMed] [Google Scholar]
- 167.Kennedy RB, Ovsyannikova IG, Haralambieva IH, O’Byrne MM, Jacobson RM, Pankratz VS, Poland GA. 2012. Multigenic control of measles vaccine immunity mediated by polymorphisms in measles receptor, innate pathway, and cytokine genes. Vaccine 30:2159–2167. doi: 10.1016/j.vaccine.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tan PL, Jacobson RM, Poland GA, Jacobsen SJ, Pankratz VS. 2001. Twin studies of immunogenicity—determining the genetic contribution to vaccine failure. Vaccine 19:2434–2439. doi: 10.1016/S0264-410X(00)00468-0. [DOI] [PubMed] [Google Scholar]
- 169.Newport MJ, Goetghebuer T, Weiss HA, Whittle H, Siegrist CA, Marchant A. 2004. Genetic regulation of immune responses to vaccines in early life. Genes Immun 5:122–129. doi: 10.1038/sj.gene.6364051. [DOI] [PubMed] [Google Scholar]
- 170.Hohler T, Reuss E, Evers N, Dietrich E, Rittner C, Freitag CM, Vollmar J, Schneider PM, Fimmers R. 2002. Differential genetic determination of immune responsiveness to hepatitis B surface antigen and to hepatitis A virus: a vaccination study in twins. Lancet 360:991–995. doi: 10.1016/S0140-6736(02)11083-X. [DOI] [PubMed] [Google Scholar]
- 171.Yan K, Cai W, Cao F, Sun H, Chen S, Xu R, Wei X, Shi X, Yan W. 2013. Genetic effects have a dominant role on poor responses to infant vaccination to hepatitis B virus. J Hum Genet 58:293–297. doi: 10.1038/jhg.2013.18. [DOI] [PubMed] [Google Scholar]
- 172.Lee YC, Newport MJ, Goetghebuer T, Siegrist CA, Weiss HA, Pollard AJ, Marchant A. 2006. Influence of genetic and environmental factors on the immunogenicity of Hib vaccine in Gambian twins. Vaccine 24:5335–5340. doi: 10.1016/j.vaccine.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 173.Klein NP, Fireman B, Enright A, Ray P, Black S, Dekker CL. 2007. A role for genetics in the immune response to the varicella vaccine. Pediatr Infect Dis J 26:300–305. doi: 10.1097/01.inf.0000257454.74513.07. [DOI] [PubMed] [Google Scholar]
- 174.McDermott AB, Zuckerman JN, Sabin CA, Marsh SG, Madrigal JA. 1997. Contribution of human leukocyte antigens to the antibody response to hepatitis B vaccination. Tissue Antigens 50:8–14. doi: 10.1111/j.1399-0039.1997.tb02827.x. [DOI] [PubMed] [Google Scholar]
- 175.Amirzargar AA, Mohseni N, Shokrgozar MA, Arjang Z, Ahmadi N, Yousefi Behzadi M, Amanzadeh A, Shokri F. 2008. HLA-DRB1, DQA1 and DQB1 alleles and haplotypes frequencies in Iranian healthy adult responders and non-responders to recombinant hepatitis B vaccine. Iran J Immunol 5:92–99. IJIv5i2A3. [PubMed] [Google Scholar]
- 176.Qian Y, Zhang L, Liang XM, Hou JL, Luo KX. 2002. Association of immune response to hepatitis B vaccine with HLA-DRB1*02, 07, 09 genes in the population of Han nationality in Guangdong Province. Di Yi Jun Yi Da Xue Xue Bao 22:67–69. [PubMed] [Google Scholar]
- 177.Höhler T, Meyer CU, Notghi A, Stradmann-Bellinghausen B, Schneider PM, Starke R, Zepp F, Sänger R, Clemens R, Meyer Zum Büschenfelde KH, Rittner C. 1998. The influence of major histocompatibility complex class II genes and T-cell Vbeta repertoire on response to immunization with HBsAg. Hum Immunol 59:212–218. doi: 10.1016/S0198-8859(98)00014-7. [DOI] [PubMed] [Google Scholar]
- 178.Lango-Warensjo A, Cardell K, Lindblom B. 1998. Haplotypes comprising subtypes of the DQB1*06 allele direct the antibody response after immunisation with hepatitis B surface antigen. Tissue Antigens 52:374–380. doi: 10.1111/j.1399-0039.1998.tb03058.x. [DOI] [PubMed] [Google Scholar]
- 179.Martinetti M, De Silvestri A, Belloni C, Pasi A, Tinelli C, Pistorio A, Salvaneschi L, Rondini G, Avanzini MA, Cuccia M. 2000. Humoral response to recombinant hepatitis B virus vaccine at birth: role of HLA and beyond. Clin Immunol 97:234–240. doi: 10.1006/clim.2000.4933. [DOI] [PubMed] [Google Scholar]
- 180.Martinetti M, Cuccia M, Daielli C, Ambroselli F, Gatti C, Pizzochero C, Belloni C, Orsolini P, Salvaneschi L. 1995. Anti-HBV neonatal immunization with recombinant vaccine. Part II. Molecular basis of the impaired alloreactivity. Vaccine 13:555–560. doi: 10.1016/0264-410X(94)00044-N. [DOI] [PubMed] [Google Scholar]
- 181.Hohler T, Stradmann-Bellinghausen B, Starke R, Sanger R, Victor A, Rittner C, Schneider PM. 2002. C4A deficiency and nonresponse to hepatitis B vaccination. J Hepatol 37:387–392. doi: 10.1016/S0168-8278(02)00205-2. [DOI] [PubMed] [Google Scholar]
- 182.Davila S, Froeling FE, Tan A, Bonnard C, Boland GJ, Snippe H, Hibberd ML, Seielstad M. 2010. New genetic associations detected in a host response study to hepatitis B vaccine. Genes Immun 11:232–238. doi: 10.1038/gene.2010.1. [DOI] [PubMed] [Google Scholar]
- 183.Varla-Leftherioti M, Papanicolaou M, Spyropoulou M, Vallindra H, Tsiroyianni P, Tassopoulos N, Kapasouri H, Stavropoulos-Giokas C. 1990. HLA-associated non-responsiveness to hepatitis B vaccine. Tissue Antigens 35:60–63. doi: 10.1111/j.1399-0039.1990.tb01757.x. [DOI] [PubMed] [Google Scholar]
- 184.Craven DE, Awdeh ZL, Kunches LM, Yunis EJ, Dienstag JL, Werner BG, Polk BF, Syndman DR, Platt R, Crumpacker CS. 1986. Nonresponsiveness to hepatitis B vaccine in health care workers. Results of revaccination and genetic typings. Ann Intern Med 105:356–360. doi: 10.7326/0003-4819-105-3-356. [DOI] [PubMed] [Google Scholar]
- 185.Poland GA, Ovsyannikova IG, Jacobson RM, Vierkant RA, Jacobsen SJ, Pankratz VS, Schaid DJ. 2001. Identification of an association between HLA class II alleles and low antibody levels after measles immunization. Vaccine 20:430–438. doi: 10.1016/S0264-410X(01)00346-2. [DOI] [PubMed] [Google Scholar]
- 186.Gelder CM, Lambkin R, Hart KW, Fleming D, Williams OM, Bunce M, Welsh KI, Marshall SE, Oxford J. 2002. Associations between human leukocyte antigens and nonresponsiveness to influenza vaccine. J Infect Dis 185:114–117. doi: 10.1086/338014. [DOI] [PubMed] [Google Scholar]
- 187.Moss AJ, Gaughran FP, Karasu A, Gilbert AS, Mann AJ, Gelder CM, Oxford JS, Stephens HA, Lambkin-Williams R. 2013. Correlation between human leukocyte antigen class II alleles and HAI titers detected post-influenza vaccination. PLoS One 8:e71376. doi: 10.1371/journal.pone.0071376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lambert ND, Haralambieva IH, Kennedy RB, Ovsyannikova IG, Pankratz VS, Poland GA. 2015. Polymorphisms in HLA-DPB1 are associated with differences in rubella virus-specific humoral immunity after vaccination. J Infect Dis 211:898–905. doi: 10.1093/infdis/jiu553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Haralambieva IH, Oberg AL, Ovsyannikova IG, Kennedy RB, Grill DE, Middha S, Bot BM, Wang VW, Smith DI, Jacobson RM, Poland GA. 2013. Genome-wide characterization of transcriptional patterns in high and low antibody responders to rubella vaccination. PLoS One 8:e62149. doi: 10.1371/journal.pone.0062149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ovsyannikova IG, Vierkant RA, Pankratz VS, Jacobson RM, Poland GA. 2010. Extended LTA, TNF, LST1 and HLA gene haplotypes and their association with rubella vaccine-induced immunity. PLoS One 5:e11806. doi: 10.1371/journal.pone.0011806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ovsyannikova IG, Vierkant RA, Pankratz VS, O’Byrne MM, Jacobson RM, Poland GA. 2009. HLA haplotype and supertype associations with cellular immune responses and cytokine production in healthy children after rubella vaccine. Vaccine 27:3349–3358. doi: 10.1016/j.vaccine.2009.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Dhiman N, Ovsyannikova IG, Vierkant RA, Ryan JE, Pankratz VS, Jacobson RM, Poland GA. 2008. Associations between SNPs in Toll-like receptors and related intracellular signaling molecules and immune responses to measles vaccine: preliminary results. Vaccine 26:1731–1736. doi: 10.1016/j.vaccine.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Moore CE, Hennig BJ, Perrett KP, Hoe JC, Lee SJ, Fletcher H, Brocklebank D, O’Connor D, Snape MD, Hall AJ, Segal S, Hill AV, Pollard AJ. 2012. Single nucleotide polymorphisms in the Toll-like receptor 3 and CD44 genes are associated with persistence of vaccine-induced immunity to the serogroup C meningococcal conjugate vaccine. Clin Vaccine Immunol 19:295–303. doi: 10.1128/CVI.05379-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ovsyannikova IG, Dhiman N, Haralambieva IH, Vierkant RA, O’Byrne MM, Jacobson RM, Poland GA. 2010. Rubella vaccine-induced cellular immunity: evidence of associations with polymorphisms in the Toll-like, vitamin A and D receptors, and innate immune response genes. Hum Genet 127:207–221. doi: 10.1007/s00439-009-0763-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Randhawa AK, Shey MS, Keyser A, Peixoto B, Wells RD, de Kock M, Lerumo L, Hughes J, Hussey G, Hawkridge A, Kaplan G, Hanekom WA, Hawn TR. 2011. Association of human TLR1 and TLR6 deficiency with altered immune responses to BCG vaccination in South African infants. PLoS Pathog 7:e1002174. doi: 10.1371/journal.ppat.1002174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yucesoy B, Johnson VJ, Fluharty K, Kashon ML, Slaven JE, Wilson NW, Weissman DN, Biagini RE, Germolec DR, Luster MI. 2009. Influence of cytokine gene variations on immunization to childhood vaccines. Vaccine 27:6991–6997. doi: 10.1016/j.vaccine.2009.09.076. [DOI] [PubMed] [Google Scholar]
- 197.Lin YJ, Lan YC, Huang YC, Lin TH, Huang SM, Lai CC, Liu CS, Lin CW, Chen SY, Tsai FJ. 2012. Effects of cytokine and cytokine receptor gene variation on high anti-HB titers: following up on Taiwan’s neonatal hepatitis B immunization program. Clin Chim Acta 413:1194–1198. doi: 10.1016/j.cca.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 198.Hohler T, Reuss E, Freitag CM, Schneider PM. 2005. A functional polymorphism in the IL-10 promoter influences the response after vaccination with HBsAg and hepatitis A. Hepatology 42:72–76. doi: 10.1002/hep.20740. [DOI] [PubMed] [Google Scholar]
- 199.Egli A, Santer DM, O’Shea D, Barakat K, Syedbasha M, Vollmer M, Baluch A, Bhat R, Groenendyk J, Joyce MA, Lisboa LF, Thomas BS, Battegay M, Khanna N, Mueller T, Tyrrell DL, Houghton M, Humar A, Kumar D. 2014. IL-28B is a key regulator of B- and T-cell vaccine responses against influenza. PLoS Pathog 10:e1004556. doi: 10.1371/journal.ppat.1004556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ovsyannikova IG, Haralambieva IH, Vierkant RA, O’Byrne MM, Jacobson RM, Poland GA. 2011. The association of CD46, SLAM and CD209 cellular receptor gene SNPs with variations in measles vaccine-induced immune responses: a replication study and examination of novel polymorphisms. Hum Hered 72:206–223. doi: 10.1159/000331585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Dhiman N, Poland GA, Cunningham JM, Jacobson RM, Ovsyannikova IG, Vierkant RA, Wu Y, Pankratz VS. 2007. Variations in measles vaccine-specific humoral immunity by polymorphisms in SLAM and CD46 measles virus receptors. J Allergy Clin Immunol 120:666–672. doi: 10.1016/j.jaci.2007.04.036. [DOI] [PubMed] [Google Scholar]
- 202.Dhiman N, Ovsyannikova IG, Cunningham JM, Vierkant RA, Kennedy RB, Pankratz VS, Poland GA, Jacobson RM. 2007. Associations between measles vaccine immunity and single-nucleotide polymorphisms in cytokine and cytokine receptor genes. J Infect Dis 195:21–29. doi: 10.1086/510596. [DOI] [PubMed] [Google Scholar]
- 203.Haralambieva IH, Ovsyannikova IG, Kennedy RB, Vierkant RA, Pankratz VS, Jacobson RM, Poland GA. 2011. Associations between single nucleotide polymorphisms and haplotypes in cytokine and cytokine receptor genes and immunity to measles vaccination. Vaccine 29:7883–7895. doi: 10.1016/j.vaccine.2011.08.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.White SJ, Haralambieva IH, Ovsyannikova IG, Vierkant RA, O’Byrne MM, Poland GA. 2012. Replication of associations between cytokine and cytokine receptor single nucleotide polymorphisms and measles-specific adaptive immunophenotypic extremes. Hum Immunol 73:636–640. doi: 10.1016/j.humimm.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wiertsema SP, Baynam G, Khoo SK, Veenhoven RH, van Heerbeek N, Zhang G, Laing IA, Rijkers GT, Goldblatt J, Sanders EA, Le Souëf PN. 2007. Impact of genetic variants in IL-4, IL-4 RA and IL-13 on the anti-pneumococcal antibody response. Vaccine 25:306–313. doi: 10.1016/j.vaccine.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 206.Haralambieva IH, Lambert ND, Ovsyannikova IG, Kennedy RB, Larrabee BR, Pankratz VS, Poland GA. 2014. Associations between single nucleotide polymorphisms in cellular viral receptors and attachment factor-related genes and humoral immunity to rubella vaccination. PLoS One 9:e99997. doi: 10.1371/journal.pone.0099997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Pankratz VS, Vierkant RA, O’Byrne MM, Ovsyannikova IG, Poland GA. 2010. Associations between SNPs in candidate immune-relevant genes and rubella antibody levels: a multigenic assessment. BMC Immunol 11:48. doi: 10.1186/1471-2172-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Haralambieva IH, Ovsyannikova IG, Dhiman N, Kennedy RB, O’Byrne M, Pankratz VS, Jacobson RM, Poland GA. 2011. Common SNPs/haplotypes in IL18R1 and IL18 genes are associated with variations in humoral immunity to smallpox vaccination in Caucasians and African Americans. J Infect Dis 204:433–441. doi: 10.1093/infdis/jir268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Clifford HD, Richmond P, Khoo SK, Zhang G, Yerkovich ST, Le Souef PN, Hayden CM. 2011. SLAM and DC-SIGN measles receptor polymorphisms and their impact on antibody and cytokine responses to measles vaccine. Vaccine 29:5407–5413. doi: 10.1016/j.vaccine.2011.05.068. [DOI] [PubMed] [Google Scholar]
- 210.Kennedy RB, Ovsyannikova IG, Haralambieva IH, Lambert ND, Pankratz VS, Poland GA. 2014. Genetic polymorphisms associated with rubella virus-specific cellular immunity following MMR vaccination. Hum Genet 133:1407–1417. doi: 10.1007/s00439-014-1471-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kennedy RB, Ovsyannikova IG, Haralambieva IH, Lambert ND, Pankratz VS, Poland GA. 2014. Genome-wide SNP associations with rubella-specific cytokine responses in measles-mumps-rubella vaccine recipients. Immunogenetics 66:493–499. doi: 10.1007/s00251-014-0776-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Bucasas KL, Franco LM, Shaw CA, Bray MS, Wells JM, Nino D, Arden N, Quarles JM, Couch RB, Belmont JW. 2011. Early patterns of gene expression correlate with the humoral immune response to influenza vaccination in humans. J Infect Dis 203:921–929. doi: 10.1093/infdis/jiq156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Cooling L. 2015. Blood groups in infection and host susceptibility. Clin Microbiol Rev 28:801–870. doi: 10.1128/CMR.00109-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Clemens JD, Sack DA, Harris JR, Chakraborty J, Khan MR, Huda S, Ahmed F, Gomes J, Rao MR, Svennerholm AM. 1989. ABO blood groups and cholera: new observations on specificity of risk and modification of vaccine efficacy. J Infect Dis 159:770–773. doi: 10.1093/infdis/159.4.770. [DOI] [PubMed] [Google Scholar]
- 215.Richie EE, Punjabi NH, Sidharta YY, Peetosutan KK, Sukandar MM, Wasserman SS, Lesmana MM, Wangsasaputra FF, Pandam SS, Levine MM, O’Hanley PP, Cryz SJ, Simanjuntak CH. 2000. Efficacy trial of single-dose live oral cholera vaccine CVD 103-HgR in North Jakarta, Indonesia, a cholera-endemic area. Vaccine 18:2399–2410. doi: 10.1016/S0264-410X(00)00006-2. [DOI] [PubMed] [Google Scholar]
- 216.Lagos R, Avendano A, Prado V, Horwitz I, Wasserman S, Losonsky G, Cryz S Jr, Kaper JB, Levine MM. 1995. Attenuated live cholera vaccine strain CVD 103-HgR elicits significantly higher serum vibriocidal antibody titers in persons of blood group O. Infect Immun 63:707–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ramamurthy T, Wagener D, Chowdhury G, Majumder PP. 2010. A large study on immunological response to a whole-cell killed oral cholera vaccine reveals that there are significant geographical differences in response and that O blood group individuals do not elicit a higher response. Clin Vaccine Immunol 17:1232–1237. doi: 10.1128/CVI.00123-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ahishali E, Boztas G, Akyuz F, Ibrisim D, Poturoglu S, Pinarbasi B, Ozdil S, Mungan Z. 2008. Response to hepatitis B vaccination in patients with celiac disease. Dig Dis Sci 53:2156–2159. doi: 10.1007/s10620-007-0128-3. [DOI] [PubMed] [Google Scholar]
- 219.Park SD, Markowitz J, Pettei M, Weinstein T, Sison CP, Swiss SR, Levine J. 2007. Failure to respond to hepatitis B vaccine in children with celiac disease. J Pediatr Gastroenterol Nutr 44:431–435. doi: 10.1097/MPG.0b013e3180320654. [DOI] [PubMed] [Google Scholar]
- 220.Nemes E, Lefler E, Szegedi L, Kapitany A, Kovacs JB, Balogh M, Szabados K, Tumpek J, Sipka S, Korponay SIR. 2008. Gluten intake interferes with the humoral immune response to recombinant hepatitis B vaccine in patients with celiac disease. Pediatrics 121:e1570–e1576. doi: 10.1542/peds.2007-2446. [DOI] [PubMed] [Google Scholar]
- 221.Balamtekın N, Uslu N, Baysoy G, Saltik-Temızel I, Demır H, Yüce A. 2011. Responsiveness of children with celiac disease to different hepatitis B vaccination protocols. Turk J Gastroenterol 22:27–31. doi: 10.4318/tjg.2011.0152. [DOI] [PubMed] [Google Scholar]
- 222.Ertem D, Gonen I, Tanidir C, Ugras M, Yildiz A, Pehlivanoğlu E, Eksioglu-Demiralp E. 2010. The response to hepatitis B vaccine: does it differ in celiac disease? Eur J Gastroenterol Hepatol 22:787–793. doi: 10.1097/MEG.0b013e32832e9d41. [DOI] [PubMed] [Google Scholar]
- 223.Ertekin V, Tosun MS, Selimoglu MA. 2011. Is there need for a new hepatitis B vaccine schedule for children with celiac disease? Hepat Mon 11:634–637. doi: 10.5812/kowsar.1735143X.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Leonardi S, Spina M, Spicuzza L, Rotolo N, La Rosa M. 2009. Hepatitis B vaccination failure in celiac disease: is there a need to reassess current immunization strategies? Vaccine 27:6030–6033. doi: 10.1016/j.vaccine.2009.07.099. [DOI] [PubMed] [Google Scholar]
- 225.Urganci N, Kalyoncu D. 2013. Response to hepatitis A and B vaccination in pediatric patients with celiac disease. J Pediatr Gastroenterol Nutr 56:408–411. doi: 10.1097/MPG.0b013e31827af200. [DOI] [PubMed] [Google Scholar]
- 226.Zingone F, Capone P, Tortora R, Rispo A, Morisco F, Caporaso N, Imperatore N, De Stefano G, Iovino P, Ciacci C. 2013. Role of gluten intake at the time of hepatitis B virus vaccination in the immune response of celiac patients. Clin Vaccine Immunol 20:660–662. doi: 10.1128/CVI.00729-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zingone F, Morisco F, Zanetti A, Romano L, Portella G, Capone P, Andreozzi P, Tortora R, Ciacci C. 2011. Long-term antibody persistence and immune memory to hepatitis B virus in adult celiac patients vaccinated as adolescents. Vaccine 29:1005–1008. doi: 10.1016/j.vaccine.2010.11.060. [DOI] [PubMed] [Google Scholar]
- 228.Leonardi S, Filippelli M, Manti S, Cuppari C, Salpietro C. 2015. Extending the debate on poor response to hepatitis B virus vaccination in children with celiac disease: which question remains? Hepat Mon 15:e30888. doi: 10.5812/hepatmon.30888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sari S, Dalgic B, Basturk B, Gonen S, Soylemezoglu O. 2011. Immunogenicity of hepatitis A vaccine in children with celiac disease. J Pediatr Gastroenterol Nutr 53:532–535. doi: 10.1097/MPG.0b013e318223b3ed. [DOI] [PubMed] [Google Scholar]
- 230.Schappi MG, Meier S, Bel M, Siegrist CA, Posfay-Barbe KM. 2012. Protective antibody responses to influenza A/H1N1/09 vaccination in children with celiac disease. J Pediatr Gastroenterol Nutr 54:817–819. doi: 10.1097/MPG.0b013e318248e7be. [DOI] [PubMed] [Google Scholar]
- 231.Filippelli M, Garozzo MT, Capizzi A, Spina M, Manti S, Tardino L, Salpietro C, Leonardi S. 2016. Immune response to hepatitis B virus vaccine in celiac subjects at diagnosis. World J Hepatol 8:1105–1109. doi: 10.4254/wjh.v8.i26.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zanoni G, Contreas G, Valletta E, Gabrielli O, Mengoli C, Veneri D. 2015. Normal or defective immune response to hepatitis B vaccine in patients with diabetes and celiac disease. Hum Vaccin Immunother 11:58–62. doi: 10.4161/hv.34309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Walkiewicz-Jedrzejczak D, Egberg M, Nelson C, Eickoff J. 2014. Evaluation of the response to vaccination with hepatitis B vaccine in pediatric patients diagnosed with celiac disease. SAGE Open Med 2:2050312114563346. doi: 10.1177/2050312114563346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Opri R, Veneri D, Mengoli C, Zanoni G. 2015. Immune response to hepatitis B vaccine in patients with celiac disease: a systematic review and meta-analysis. Hum Vaccin Immunother 11:2800–2805. doi: 10.1080/21645515.2015.1069448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Alper CA, Kruskall MS, Marcus-Bagley D, Craven DE, Katz AJ, Brink SJ, Dienstag JL, Awdeh Z, Yunis EJ. 1989. Genetic prediction of nonresponse to hepatitis B vaccine. N Engl J Med 321:708–712. doi: 10.1056/NEJM198909143211103. [DOI] [PubMed] [Google Scholar]
- 236.Leonardi S, Longo R, Cotugno M, Tardino L, Spina M, Lionetti E, La Rosa M. 2011. Vaccination and celiac disease: results of a retrospective study. Minerva Pediatr 63:363–367. [PubMed] [Google Scholar]
- 237.Elrashidy H, Elbahrawy A, El-Didamony G, Mostafa M, George NM, Elwassief A, Saeid Mohamed AG, Elmestikawy A, Morsy MH, Hashim A, Abdelbasseer MA. 2013. Antibody levels against hepatitis B virus after hepatitis B vaccination in Egyptian diabetic children and adolescents. Hum Vaccin Immunother 9:2002–2006. doi: 10.4161/hv.25426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Leonardi S, Vitaliti G, Garozzo MT, Miraglia del Giudice M, Marseglia G, La Rosa M. 2012. Hepatitis B vaccination failure in children with diabetes mellitus? The debate continues. Hum Vaccin Immunother 8:448–452. doi: 10.4161/hv.19107. [DOI] [PubMed] [Google Scholar]
- 239.Arslanoğlu I, Cetin B, Işgüven P, Karavuş M. 2002. Anti-HBs response to standard hepatitis B vaccination in children and adolescents with diabetes mellitus. J Pediatr Endocrinol Metab 15:389–395. [DOI] [PubMed] [Google Scholar]
- 240.Eisenhut M, Chesover A, Misquith R, Nathwani N, Walters A. 2016. Antibody responses to immunizations in children with type I diabetes mellitus: a case-control study. Clin Vaccine Immunol 23:873–877. doi: 10.1128/CVI.00400-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Dashti AS, Alaei MR, Musavi Z, Faramarzi R, Mansouri F, Nasimfar A. 2015. Serological response to vaccines in children with diabetes. Roum Arch Microbiol Immunol 74:112–117. [PubMed] [Google Scholar]
- 242.Gutierrez Domingo I, Pascasio Acevedo JM, Alcalde Vargas A, Ramos Cuadra A, Ferrer Ríos MT, Sousa Martin JM, Sayago Mota M, Giráldez Gallego A, Suárez Artacho G. 2012. Response to vaccination against hepatitis B virus with a schedule of four 40-μg doses in cirrhotic patients evaluated for liver transplantation: factors associated with a response. Transplant Proc 44:1499–1501. doi: 10.1016/j.transproceed.2012.05.071. [DOI] [PubMed] [Google Scholar]
- 243.Pozzilli P, Arduini P, Visalli N, Sutherland J, Pezzella M, Galli C, Corradini SG, Biasio L, Gale EA, Andreani D. 1987. Reduced protection against hepatitis B virus following vaccination in patients with type 1 (insulin-dependent) diabetes. Diabetologia 30:817–819. [DOI] [PubMed] [Google Scholar]
- 244.Wismans PJ, van Hattum J, de Gast GC, Bouter KP, Diepersloot RJ, Maikoe T, Mudde GC. 1991. A prospective study of in vitro anti-HBs producing B cells (spot-ELISA) following primary and supplementary vaccination with a recombinant hepatitis B vaccine in insulin dependent diabetic patients and matched controls. J Med Virol 35:216–222. doi: 10.1002/jmv.1890350313. [DOI] [PubMed] [Google Scholar]
- 245.Bouter KP, Diepersloot RJ, Wismans PJ, Gmelig Meyling FH, Hoekstra JB, Heijtink RA, van Hattum J. 1992. Humoral immune response to a yeast-derived hepatitis B vaccine in patients with type 1 diabetes mellitus. Diabet Med 9:66–69. doi: 10.1111/j.1464-5491.1992.tb01717.x. [DOI] [PubMed] [Google Scholar]
- 246.Janbakhsh A, Mansouri F, Vaziri S, Sayad B, Afsharian M, Rahimi M, Shahebrahimi K, Salari F. 2013. Effect of selenium on immune response against hepatitis B vaccine with accelerated method in insulin-dependent diabetes mellitus patients. Caspian J Intern Med 4:603–606. [PMC free article] [PubMed] [Google Scholar]
- 247.McElhaney JE, Pinkoski MJ, Au D, Lechelt KE, Bleackley RC, Meneilly GS. 1996. Helper and cytotoxic T lymphocyte responses to influenza vaccination in healthy compared to diabetic elderly. Vaccine 14:539–544. doi: 10.1016/0264-410X(95)00219-Q. [DOI] [PubMed] [Google Scholar]
- 248.McElhaney JE, Garneau H, Camous X, Dupuis G, Pawelec G, Baehl S, Tessier D, Frost EH, Frasca D, Larbi A, Fulop T. 2015. Predictors of the antibody response to influenza vaccination in older adults with type 2 diabetes. BMJ Open Diabetes Res Care 3:e000140. doi: 10.1136/bmjdrc-2015-000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Girndt M, Pietsch M, Kohler H. 1995. Tetanus immunization and its association to hepatitis B vaccination in patients with chronic renal failure. Am J Kidney Dis 26:454–460. doi: 10.1016/0272-6386(95)90491-3. [DOI] [PubMed] [Google Scholar]
- 250.Kruger S, Seyfarth M, Sack K, Kreft B. 1999. Defective immune response to tetanus toxoid in hemodialysis patients and its association with diphtheria vaccination. Vaccine 17:1145–1150. doi: 10.1016/S0264-410X(98)00334-X. [DOI] [PubMed] [Google Scholar]
- 251.Guerin A, Buisson Y, Nutini MT, Saliou P, London G, Marchais S. 1992. Response to vaccination against tetanus in chronic haemodialysed patients. Nephrol Dial Transplant 7:323–326. doi: 10.1093/oxfordjournals.ndt.a092136. [DOI] [PubMed] [Google Scholar]
- 252.Buti M, Viladomiu L, Jardi R, Olmos A, Rodriguez JA, Bartolome J, Esteban R, Guardia J. 1992. Long-term immunogenicity and efficacy of hepatitis B vaccine in hemodialysis patients. Am J Nephrol 12:144–147. doi: 10.1159/000168436. [DOI] [PubMed] [Google Scholar]
- 253.DaRoza G, Loewen A, Djurdjev O, Love J, Kempston C, Burnett S, Kiaii M, Taylor PA, Levin A. 2003. Stage of chronic kidney disease predicts seroconversion after hepatitis B immunization: earlier is better. Am J Kidney Dis 42:1184–1192. doi: 10.1053/j.ajkd.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 254.Kruger S, Muller-Steinhardt M, Kirchner H, Kreft B. 2001. A 5-year follow-up on antibody response after diphtheria and tetanus vaccination in hemodialysis patients. Am J Kidney Dis 38:1264–1270. doi: 10.1053/ajkd.2001.29223. [DOI] [PubMed] [Google Scholar]
- 255.Ramezani A, Eslamifar A, Banifazl M, Ahmadi F, Maziar S, Razeghi E, Kalantar E, Amirkhani A, Aghakhani A. 2009. Efficacy and long-term immunogenicity of hepatitis B vaccine in haemodialysis patients. Int J Clin Pract 63:394–397. doi: 10.1111/j.1742-1241.2007.01470.x. [DOI] [PubMed] [Google Scholar]
- 256.Alavian SM, Tabatabaei SV. 2010. The effect of diabetes mellitus on immunological response to hepatitis B virus vaccine in individuals with chronic kidney disease: a meta-analysis of current literature. Vaccine 28:3773–3777. doi: 10.1016/j.vaccine.2010.03.038. [DOI] [PubMed] [Google Scholar]
- 257.Fabrizi F, Dixit V, Martin P, Messa P. 2011. Meta-analysis: the impact of diabetes mellitus on the immunological response to hepatitis B virus vaccine in dialysis patients. Aliment Pharmacol Ther 33:815–821. doi: 10.1111/j.1365-2036.2011.04589.x. [DOI] [PubMed] [Google Scholar]
- 258.Ocak S, Eskiocak AF. 2008. The evaluation of immune responses to hepatitis B vaccination in diabetic and non-diabetic haemodialysis patients and the use of tetanus toxoid. Nephrology (Carlton) 13:487–491. doi: 10.1111/j.1440-1797.2008.00936.x. [DOI] [PubMed] [Google Scholar]
- 259.Chow KM, Law MC, Leung CB, Szeto CC, Li PK. 2006. Antibody response to hepatitis B vaccine in end-stage renal disease patients. Nephron Clin Pract 103:c89–c93. doi: 10.1159/000092016. [DOI] [PubMed] [Google Scholar]
- 260.Balloni A, Assael BM, Ghio L, Pedrazzi C, Nebbia G, Gridelli B, Melada E, Panuccio A, Foti M, Barbi M, Luraschi C. 1999. Immunity to poliomyelitis, diphtheria and tetanus in pediatric patients before and after renal or liver transplantation. Vaccine 17:2507–2511. doi: 10.1016/S0264-410X(99)00064-X. [DOI] [PubMed] [Google Scholar]
- 261.Lee S. 2000. Hepatitis A vaccination in patients with chronic liver disease in Taiwan. J Viral Hepat 7:19–21. doi: 10.1046/j.1365-2893.2000.00015.x. [DOI] [PubMed] [Google Scholar]
- 262.Tsang SW, Sung JJ. 1999. Inactivated hepatitis A vaccine in Chinese patients with chronic hepatitis B infection. Aliment Pharmacol Ther 13:1445–1449. doi: 10.1046/j.1365-2036.1999.00628.x. [DOI] [PubMed] [Google Scholar]
- 263.Kallinowski B, Jilg W, Buchholz L, Stremmel W, Engler S. 2003. Immunogenicity of an accelerated vaccination regime with a combined hepatitis A/B vaccine in patients with chronic hepatitis C. Z Gastroenterol 41:983–990. doi: 10.1055/s-2003-42929. [DOI] [PubMed] [Google Scholar]
- 264.Dumot JA, Barnes DS, Younossi Z, Gordon SM, Avery RK, Domen RE, Henderson JM, Carey WD. 1999. Immunogenicity of hepatitis A vaccine in decompensated liver disease. Am J Gastroenterol 94:1601–1604. doi: 10.1111/j.1572-0241.1999.01150.x. [DOI] [PubMed] [Google Scholar]
- 265.Ferreira CT, da Silveira TR, Vieira SM, Taniguchi A, Pereira-Lima J. 2003. Immunogenicity and safety of hepatitis A vaccine in children with chronic liver disease. J Pediatr Gastroenterol Nutr 37:258–261. doi: 10.1097/00005176-200309000-00011. [DOI] [PubMed] [Google Scholar]
- 266.Baxter D. 2010. Impaired functioning of immune defenses to infection in premature and term infants and their implications for vaccination. Hum Vaccin 6:494–505. doi: 10.4161/hv.6.6.12008. [DOI] [PubMed] [Google Scholar]
- 267.Bernbaum JC, Daft A, Anolik R, Samuelson J, Barkin R, Douglas S, Polin R. 1985. Response of preterm infants to diphtheria-tetanus-pertussis immunizations. J Pediatr 107:184–188. doi: 10.1016/S0022-3476(85)80122-0. [DOI] [PubMed] [Google Scholar]
- 268.Faldella G, Alessandroni R, Magini GM, Perrone A, Sabatini MR, Vancini A, Salvioli GP. 1998. The preterm infant’s antibody response to a combined diphtheria, tetanus, acellular pertussis and hepatitis B vaccine. Vaccine 16:1646–1649. doi: 10.1016/S0264-410X(98)00060-7. [DOI] [PubMed] [Google Scholar]
- 269.Omenaca F, Garcia-Sicilia J, Garcia-Corbeira P, Boceta R, Romero A, Lopez G, Dal-Re R. 2005. Response of preterm newborns to immunization with a hexavalent diphtheria-tetanus-acellular pertussis-hepatitis B virus-inactivated polio and Haemophilus influenzae type b vaccine: first experiences and solutions to a serious and sensitive issue. Pediatrics 116:1292–1298. doi: 10.1542/peds.2004-2336. [DOI] [PubMed] [Google Scholar]
- 270.Linder N, Vishne TH, Levin E, Handsher R, Fink-Kremer I, Waldman D, Levine A, Ashkenazi S, Sirota L. 2002. Hepatitis B vaccination: long-term follow-up of the immune response of preterm infants and comparison of two vaccination protocols. Infection 30:136–139. doi: 10.1007/s15010-002-2068-3. [DOI] [PubMed] [Google Scholar]
- 271.Berrington JE, Cant AJ, Matthews JN, O’Keeffe M, Spickett GP, Fenton AC. 2006. Haemophilus influenzae type b immunization in infants in the United Kingdom: effects of diphtheria/tetanus/acellular pertussis/Hib combination vaccine, significant prematurity, and a fourth dose. Pediatrics 117:e717–e724. doi: 10.1542/peds.2005-0348. [DOI] [PubMed] [Google Scholar]
- 272.Omenaca F, Garcia-Sicilia J, Garcia-Corbeira P, Boceta R, Torres V. 2007. Antipolyribosyl ribitol phosphate response of premature infants to primary and booster vaccination with a combined diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated polio virus/Haemophilus influenzae type b vaccine. Pediatrics 119:e179–e185. doi: 10.1542/peds.2005-2907. [DOI] [PubMed] [Google Scholar]
- 273.Slack MH, Schapira D, Thwaites RJ, Burrage M, Southern J, Andrews N, Borrow R, Goldblatt D, Miller E. 2001. Immune response of premature infants to meningococcal serogroup C and combined diphtheria-tetanus toxoids-acellular pertussis-Haemophilus influenzae type b conjugate vaccines. J Infect Dis 184:1617–1620. doi: 10.1086/324666. [DOI] [PubMed] [Google Scholar]
- 274.Munoz A, Salvador A, Brodsky NL, Arbeter AM, Porat R. 1995. Antibody response of low birth weight infants to Haemophilus influenzae type b polyribosylribitol phosphate-outer membrane protein conjugate vaccine. Pediatrics 96:216–219. [PubMed] [Google Scholar]
- 275.Kristensen K, Gyhrs A, Lausen B, Barington T, Heilmann C. 1996. Antibody response to Haemophilus influenzae type b capsular polysaccharide conjugated to tetanus toxoid in preterm infants. Pediatr Infect Dis J 15:525–529. doi: 10.1097/00006454-199606000-00010. [DOI] [PubMed] [Google Scholar]
- 276.Ruggeberg JU, Collins C, Clarke P, Johnson N, Sinha R, Everest N, Chang J, Stanford E, Balmer P, Borrow R, Martin S, Robinson MJ, Moxon ER, Pollard AJ, Heath PT. 2007. Immunogenicity and induction of immunological memory of the heptavalent pneumococcal conjugate vaccine in preterm UK infants. Vaccine 25:264–271. doi: 10.1016/j.vaccine.2006.07.036. [DOI] [PubMed] [Google Scholar]
- 277.Shinefield H, Black S, Ray P, Fireman B, Schwalbe J, Lewis E. 2002. Efficacy, immunogenicity and safety of heptavalent pneumococcal conjugate vaccine in low birth weight and preterm infants. Pediatr Infect Dis J 21:182–186. doi: 10.1097/00006454-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 278.Ramsay ME, Miller E, Ashworth LA, Coleman TJ, Rush M, Waight PA. 1995. Adverse events and antibody response to accelerated immunisation in term and preterm infants. Arch Dis Child 72:230–232. doi: 10.1136/adc.72.3.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Schloesser RL, Fischer D, Otto W, Rettwitz-Volk W, Herden P, Zielen S. 1999. Safety and immunogenicity of an acellular pertussis vaccine in premature infants. Pediatrics 103:e60. doi: 10.1542/peds.103.5.e60. [DOI] [PubMed] [Google Scholar]
- 280.Slack MH, Schapira D, Thwaites RJ, Schapira C, Bamber J, Burrage M, Southern J, Andrews N, Miller E. 2004. Acellular pertussis vaccine given by accelerated schedule: response of preterm infants. Arch Dis Child Fetal Neonatal Ed 89:F57–F60. doi: 10.1136/fn.89.1.F57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.D’Angio CT, Maniscalco WM, Pichichero ME. 1995. Immunologic response of extremely premature infants to tetanus, Haemophilus influenzae, and polio immunizations. Pediatrics 96:18–22. [PubMed] [Google Scholar]
- 282.Esposito S, Corbellini B, Bosis S, Pugni L, Tremolati E, Tagliabue C, Toneatto D, Mosca F, Principi N. 2007. Immunogenicity, safety and tolerability of meningococcal C CRM197 conjugate vaccine administered 3, 5 and 11 months post-natally to pre- and full-term infants. Vaccine 25:4889–4894. doi: 10.1016/j.vaccine.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 283.Omeñaca F, Garcia-Sicilia J, Boceta R, Sistiaga-Hernando A, García-Corbeira P. 2007. Antibody persistence and booster vaccination during the second and fifth years of life in a cohort of children who were born prematurely. Pediatr Infect Dis J 26:824–829. doi: 10.1097/INF.0b013e318124a9c8. [DOI] [PubMed] [Google Scholar]
- 284.Esposito S, Faldella G, Giammanco A, Bosis S, Friscia O, Clerici M, Principi N. 2002. Long-term pertussis-specific immune responses to a combined diphtheria, tetanus, tricomponent acellular pertussis and hepatitis B vaccine in pre-term infants. Vaccine 20:2928–2932. doi: 10.1016/S0264-410X(02)00230-X. [DOI] [PubMed] [Google Scholar]
- 285.Vazquez L, Garcia F, Ruttimann R, Coconier G, Jacquet JM, Schuerman L. 2008. Immunogenicity and reactogenicity of DTPa-HBV-IPV/Hib vaccine as primary and booster vaccination in low-birth-weight premature infants. Acta Paediatr 97:1243–1249. doi: 10.1111/j.1651-2227.2008.00884.x. [DOI] [PubMed] [Google Scholar]
- 286.Han K, Shao X, Zheng H, Wu C, Zhu J, Zheng X, Zhang Y. 2012. Revaccination of non- and low- responders after a standard three dose hepatitis B vaccine schedule. Hum Vaccin Immunother 8:1845–1849. doi: 10.4161/hv.21818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Halsey NA, Boulos R, Mode F, Andre J, Bowman L, Yaeger RG, Toureau S, Rohde J, Boulos C. 1985. Response to measles vaccine in Haitian infants 6 to 12 months old. Influence of maternal antibodies, malnutrition, and concurrent illnesses. N Engl J Med 313:544–549. doi: 10.1056/NEJM198508293130904. [DOI] [PubMed] [Google Scholar]
- 288.Moore SE, Jalil F, Ashraf R, Szu SC, Prentice AM, Hanson LA. 2004. Birth weight predicts response to vaccination in adults born in an urban slum in Lahore, Pakistan. Am J Clin Nutr 80:453–459. doi: 10.1093/ajcn/80.2.453. [DOI] [PubMed] [Google Scholar]
- 289.Pickering LK, Granoff DM, Erickson JR, Masor ML, Cordle CT, Schaller JP, Winship TR, Paule CL, Hilty MD. 1998. Modulation of the immune system by human milk and infant formula containing nucleotides. Pediatrics 101:242–249. doi: 10.1542/peds.101.2.242. [DOI] [PubMed] [Google Scholar]
- 290.Pabst HF, Spady DW. 1990. Effect of breast-feeding on antibody response to conjugate vaccine. Lancet 336:269–270. doi: 10.1016/0140-6736(90)91802-H. [DOI] [PubMed] [Google Scholar]
- 291.Silfverdal SA, Bodin L, Ulanova M, Hahn-Zoric M, Hanson LA, Olcen P. 2002. Long term enhancement of the IgG2 antibody response to Haemophilus influenzae type b by breast-feeding. Pediatr Infect Dis J 21:816–821. doi: 10.1097/01.inf.0000027668.74570.96. [DOI] [PubMed] [Google Scholar]
- 292.Hahn-Zoric M, Fulconis F, Minoli I, Moro G, Carlsson B, Böttiger M, Räihä N, Hanson LA. 1990. Antibody responses to parenteral and oral vaccines are impaired by conventional and low protein formulas as compared to breast-feeding. Acta Paediatr Scand 79:1137–1142. [DOI] [PubMed] [Google Scholar]
- 293.Pichichero ME. 1990. Effect of breast-feeding on oral rhesus rotavirus vaccine seroconversion: a metaanalysis. J Infect Dis 162:753–755. doi: 10.1093/infdis/162.3.753. [DOI] [PubMed] [Google Scholar]
- 294.Glass RI, Ing DJ, Stoll BJ, Ing RT. 1991. Immune response to rotavirus vaccines among breast-fed and nonbreast-fed children. Adv Exp Med Biol 310:249–254. doi: 10.1007/978-1-4615-3838-7_33. [DOI] [PubMed] [Google Scholar]
- 295.Chilengi R, Simuyandi M, Beach L, Mwila K, Becker-Dreps S, Emperador DM, Velasquez DE, Bosomprah S, Jiang B. 2016. Association of maternal immunity with rotavirus vaccine immunogenicity in Zambian infants. PLoS One 11:e0150100. doi: 10.1371/journal.pone.0150100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Moon SS, Wang Y, Shane AL, Nguyen T, Ray P, Dennehy P, Baek LJ, Parashar U, Glass RI, Jiang B. 2010. Inhibitory effect of breast milk on infectivity of live oral rotavirus vaccines. Pediatr Infect Dis J 29:919–923. doi: 10.1097/INF.0b013e3181e232ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Ali A, Kazi AM, Cortese MM, Fleming JA, Moon S, Parashar UD, Jiang B, McNeal MM, Steele D, Bhutta Z, Zaidi AK. 2015. Impact of withholding breastfeeding at the time of vaccination on the immunogenicity of oral rotavirus vaccine—a randomized trial. PLoS One 10:e0127622. doi: 10.1371/journal.pone.0127622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Rongsen-Chandola T, Strand TA, Goyal N, Flem E, Rathore SS, Arya A, Winje BA, Lazarus R, Shanmugasundaram E, Babji S, Sommerfelt H, Vainio K, Kang G, Bhandari N. 2014. Effect of withholding breastfeeding on the immune response to a live oral rotavirus vaccine in North Indian infants. Vaccine 32:A134–A139. doi: 10.1016/j.vaccine.2014.04.078. [DOI] [PubMed] [Google Scholar]
- 299.Groome MJ, Moon SS, Velasquez D, Jones S, Koen A, van Niekerk N, Jiang B, Parashar UD, Madhi SA. 2014. Effect of breastfeeding on immunogenicity of oral live-attenuated human rotavirus vaccine: a randomized trial in HIV-uninfected infants in Soweto, South Africa. Bull World Health Organ 92:238–245. doi: 10.2471/BLT.13.128066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.de Francisco A, Hall AJ, Unicomb L, Chakraborty J, Yunus M, Sack RB. 1998. Maternal measles antibody decay in rural Bangladeshi infants—implications for vaccination schedules. Vaccine 16:564–568. doi: 10.1016/S0264-410X(97)00245-4. [DOI] [PubMed] [Google Scholar]
- 301.Markowitz LE, Albrecht P, Rhodes P, Demonteverde R, Swint E, Maes EF, Powell C, Patriarca PA. 1996. Changing levels of measles antibody titers in women and children in the United States: impact on response to vaccination. Kaiser Permanente Measles Vaccine Trial Team. Pediatrics 97:53–58. [PubMed] [Google Scholar]
- 302.Leuridan E, Hens N, Hutse V, Ieven M, Aerts M, Van Damme P. 2010. Early waning of maternal measles antibodies in era of measles elimination: longitudinal study. BMJ 340:c1626. doi: 10.1136/bmj.c1626. [DOI] [PubMed] [Google Scholar]
- 303.Derya A, Necmi A, Emre A, Akgun Y. 2005. Decline of maternal hepatitis A antibodies during the first 2 years of life in infants born in Turkey. Am J Trop Med Hyg 73:457–459. doi: 10.4269/ajtmh.2005.73.457. [DOI] [PubMed] [Google Scholar]
- 304.Lieberman JM, Chang SJ, Partridge S, Hollister JC, Kaplan KM, Jensen EH, Kuter B, Ward JI. 2002. Kinetics of maternal hepatitis A antibody decay in infants: implications for vaccine use. Pediatr Infect Dis J 21:347–348. doi: 10.1097/00006454-200204000-00017. [DOI] [PubMed] [Google Scholar]
- 305.Letson GW, Shapiro CN, Kuehn D, Gardea C, Welty TK, Krause DS, Lambert SB, Margolis HS. 2004. Effect of maternal antibody on immunogenicity of hepatitis A vaccine in infants. J Pediatr 144:327–332. doi: 10.1016/j.jpeds.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 306.Lieberman JM, Marcy M, Partridge S, Ward JI. 1998. Evaluation of a HA vaccine in infants: effect of maternal antibodies on antibody response, [abstract]. In Program and abstracts of the 36th annual meeting of the Infectious Diseases Society of America, Denver, CO Infectious Diseases Society of America, Alexandria, VA. [Google Scholar]
- 307.Moon SS, Groome MJ, Velasquez DE, Parashar UD, Jones S, Koen A, van Niekerk N, Jiang B, Madhi SA. 2016. Prevaccination rotavirus serum IgG and IgA are associated with lower immunogenicity of live, oral human rotavirus vaccine in South African infants. Clin Infect Dis 62:157–165. doi: 10.1093/cid/civ828. [DOI] [PubMed] [Google Scholar]
- 308.Becker-Dreps S, Vilchez S, Velasquez D, Moon SS, Hudgens MG, Zambrana LE, Jiang B. 2015. Rotavirus-specific IgG antibodies from mothers’ serum may inhibit infant immune responses to the pentavalent rotavirus vaccine. Pediatr Infect Dis J 34:115–116. doi: 10.1097/INF.0000000000000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Appaiahgari MB, Glass R, Singh S, Taneja S, Rongsen-Chandola T, Bhandari N, Mishra S, Vrati S. 2014. Transplacental rotavirus IgG interferes with immune response to live oral rotavirus vaccine ORV-116E in Indian infants. Vaccine 32:651–656. doi: 10.1016/j.vaccine.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 310.Moon SS, Tate JE, Ray P, Dennehy PH, Archary D, Coutsoudis A, Bland R, Newell ML, Glass RI, Parashar U, Jiang B. 2013. Differential profiles and inhibitory effect on rotavirus vaccines of nonantibody components in breast milk from mothers in developing and developed countries. Pediatr Infect Dis J 32:863–870. doi: 10.1097/INF.0b013e318290646d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.de Voer RM, van der Klis FR, Nooitgedagt JE, Versteegh FG, van Huisseling JC, van Rooijen DM, Sanders EA, Berbers GA. 2009. Seroprevalence and placental transportation of maternal antibodies specific for Neisseria meningitidis serogroup C, Haemophilus influenzae type B, diphtheria, tetanus, and pertussis. Clin Infect Dis 49:58–64. doi: 10.1086/599347. [DOI] [PubMed] [Google Scholar]
- 312.Eberhardt CS, Blanchard-Rohner G, Lemaitre B, Boukrid M, Combescure C, Othenin-Girard V, Chilin A, Petre J, de Tejada BM, Siegrist CA. 2016. Maternal immunization earlier in pregnancy maximizes antibody transfer and expected infant seropositivity against pertussis. Clin Infect Dis 62:829–836. doi: 10.1093/cid/ciw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Malhotra I, McKibben M, Mungai P, McKibben E, Wang X, Sutherland LJ, Muchiri EM, King CH, King CL, LaBeaud AD. 2015. Effect of antenatal parasitic infections on anti-vaccine IgG levels in children: a prospective birth cohort study in Kenya. PLoS Negl Trop Dis 9:e0003466. doi: 10.1371/journal.pntd.0003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Nash S, Mentzer AJ, Lule SA, Kizito D, Smits G, van der Klis FR, Elliott AM. 2017. The impact of prenatal exposure to parasitic infections and to anthelminthic treatment on antibody responses to routine immunisations given in infancy: secondary analysis of a randomised controlled trial. PLoS Negl Trop Dis 11:e0005213. doi: 10.1371/journal.pntd.0005213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Bassily S, Kotkat A, Hyams KC, Youssef FG, El-Masry NA, Arthur R, Imam IZ, Brown FM. 1997. Immunogenicity of recombinant hepatitis B vaccine among infants of mothers with active schistosomiasis. Am J Trop Med Hyg 57:197–199. doi: 10.4269/ajtmh.1997.57.197. [DOI] [PubMed] [Google Scholar]
- 316.Brabin BJ, Nagel J, Hagenaars AM, Ruitenberg E, van Tilborgh AM. 1984. The influence of malaria and gestation on the immune response to one and two doses of adsorbed tetanus toxoid in pregnancy. Bull World Health Organ 62:919–930. [PMC free article] [PubMed] [Google Scholar]
- 317.Ghaffar YA, Kamel M, el-Sobky M, Bahnasy R, Strickland GT. 1989. Response to hepatitis B vaccine in infants born to mothers with schistosomiasis. Lancet ii:272. [DOI] [PubMed] [Google Scholar]
- 318.Kizito D, Tweyongyere R, Namatovu A, Webb EL, Muhangi L, Lule SA, Bukenya H, Cose S, Elliott AM. 2013. Factors affecting the infant antibody response to measles immunisation in Entebbe-Uganda. BMC Public Health 13:619. doi: 10.1186/1471-2458-13-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Elliott AM, Mawa PA, Webb EL, Nampijja M, Lyadda N, Bukusuba J, Kizza M, Namujju PB, Nabulime J, Ndibazza J, Muwanga M, Whitworth JA. 2010. Effects of maternal and infant co-infections, and of maternal immunisation, on the infant response to BCG and tetanus immunisation. Vaccine 29:247–255. doi: 10.1016/j.vaccine.2010.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Malhotra I, Mungai P, Wamachi A, Kioko J, Ouma JH, Kazura JW, King CL. 1999. Helminth- and bacillus Calmette-Guerin-induced immunity in children sensitized in utero to filariasis and schistosomiasis. J Immunol 162:6843–6848. [PubMed] [Google Scholar]
- 321.Dauby N, Alonso-Vega C, Suarez E, Flores A, Hermann E, Cordova M, Tellez T, Torrico F, Truyens C, Carlier Y. 2009. Maternal infection with Trypanosoma cruzi and congenital Chagas disease induce a trend to a type 1 polarization of infant immune responses to vaccines. PLoS Negl Trop Dis 3:e571. doi: 10.1371/journal.pntd.0000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Djuardi Y, Sartono E, Wibowo H, Supali T, Yazdanbakhsh M. 2010. A longitudinal study of BCG vaccination in early childhood: the development of innate and adaptive immune responses. PLoS One 5:e14066. doi: 10.1371/journal.pone.0014066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Lule SA, Mawa PA, Nkurunungi G, Nampijja M, Kizito D, Akello F, Muhangi L, Elliott AM, Webb EL. 2015. Factors associated with tuberculosis infection, and with anti-mycobacterial immune responses, among five year olds BCG-immunised at birth in Entebbe, Uganda. Vaccine 33:796–804. doi: 10.1016/j.vaccine.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Webb EL, Mawa PA, Ndibazza J, Kizito D, Namatovu A, Kyosiimire-Lugemwa J, Nanteza B, Nampijja M, Muhangi L, Woodburn PW, Akurut H, Mpairwe H, Akello M, Lyadda N, Bukusuba J, Kihembo M, Kizza M, Kizindo R, Nabulime J, Ameke C, Namujju PB, Tweyongyere R, Muwanga M, Whitworth JA, Elliott AM. 2011. Effect of single-dose anthelmintic treatment during pregnancy on an infant’s response to immunisation and on susceptibility to infectious diseases in infancy: a randomised, double-blind, placebo-controlled trial. Lancet 377:52–62. doi: 10.1016/S0140-6736(10)61457-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Miles DJ, van der Sande M, Crozier S, Ojuola O, Palmero MS, Sanneh M, Touray ES, Rowland-Jones S, Whittle H, Ota M, Marchant A. 2008. Effects of antenatal and postnatal environments on CD4 T-cell responses to Mycobacterium bovis BCG in healthy infants in the Gambia. Clin Vaccine Immunol 15:995–1002. doi: 10.1128/CVI.00037-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Naylor C, Lu M, Haque R, Mondal D, Buonomo E, Nayak U, Mychaleckyj JC, Kirkpatrick B, Colgate R, Carmolli M, Dickson D, van der Klis F, Weldon W, Steven Oberste M, Ma JZ, Petri WA Jr. 2015. Environmental enteropathy, oral vaccine failure and growth faltering in infants in Bangladesh. EBioMedicine 2:1759–1766. doi: 10.1016/j.ebiom.2015.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Levine OS, Granoff DM, Lagos R, Fritzell B, Levine MM. 1997. Factors associated with superior antibody responses to a single dose of Haemophilus influenzae type b-tetanus toxoid conjugate vaccine administered to Chilean infants at 2 months of age. Vaccine 15:325–328. doi: 10.1016/S0264-410X(96)00161-2. [DOI] [PubMed] [Google Scholar]
- 328.Ndikuyeze A, Munoz A, Stewart J, Modlin J, Heymann D, Herrmann KL, Polk BF. 1988. Immunogenicity and safety of measles vaccine in ill African children. Int J Epidemiol 17:448–455. doi: 10.1093/ije/17.2.448. [DOI] [PubMed] [Google Scholar]
- 329.Cilla G, Pena B, Marimon JM, Perez-Trallero E. 1996. Serologic response to measles-mumps-rubella vaccine among children with upper respiratory tract infection. Vaccine 14:492–494. doi: 10.1016/0264-410X(95)00234-R. [DOI] [PubMed] [Google Scholar]
- 330.Krober MS, Stracener CE, Bass JW. 1991. Decreased measles antibody response after measles-mumps-rubella vaccine in infants with colds. JAMA 265:2095–2096. doi: 10.1001/jama.1991.03460160073032. [DOI] [PubMed] [Google Scholar]
- 331.Dennehy PH, Saracen CL, Peter G. 1994. Seroconversion rates to combined measles-mumps-rubella-varicella vaccine of children with upper respiratory tract infection. Pediatrics 94:514–516. [PubMed] [Google Scholar]
- 332.Ratnam S, West R, Gadag V. 1995. Measles and rubella antibody response after measles-mumps-rubella vaccination in children with afebrile upper respiratory tract infection. J Pediatr 127:432–434. doi: 10.1016/S0022-3476(95)70077-3. [DOI] [PubMed] [Google Scholar]
- 333.Usen S, Milligan P, Ethevenaux C, Greenwood B, Mulholland K. 2000. Effect of fever on the serum antibody response of Gambian children to Haemophilus influenzae type b conjugate vaccine. Pediatr Infect Dis J 19:444–449. doi: 10.1097/00006454-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 334.Parker EP, Kampmann B, Kang G, Grassly NC. 2014. Influence of enteric infections on response to oral poliovirus vaccine: a systematic review and meta-analysis. J Infect Dis 210:853–864. doi: 10.1093/infdis/jiu182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Haque R, Snider C, Liu Y, Ma JZ, Liu L, Nayak U, Mychaleckyj JC, Korpe P, Mondal D, Kabir M, Alam M, Pallansch M, Oberste MS, Weldon W, Kirkpatrick BD, Petri WA Jr. 2014. Oral polio vaccine response in breast fed infants with malnutrition and diarrhea. Vaccine 32:478–482. doi: 10.1016/j.vaccine.2013.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Holder B, Miles DJ, Kaye S, Crozier S, Mohammed NI, Duah NO, Roberts E, Ojuola O, Palmero MS, Touray ES, Waight P, Cotten M, Rowland-Jones S, van der Sande M, Whittle H. 2010. Epstein-Barr virus but not cytomegalovirus is associated with reduced vaccine antibody responses in Gambian infants. PLoS One 5:e14013. doi: 10.1371/journal.pone.0014013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Miles DJ, Sanneh M, Holder B, Crozier S, Nyamweya S, Touray ES, Palmero MS, Zaman SM, Rowland-Jones S, van der Sande M, Whittle H. 2008. Cytomegalovirus infection induces T-cell differentiation without impairing antigen-specific responses in Gambian infants. Immunology 124:388–400. doi: 10.1111/j.1365-2567.2007.02787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Furman D, Jojic V, Sharma S, Shen-Orr SS, Angel CJ, Onengut-Gumuscu S, Kidd BA, Maecker HT, Concannon P, Dekker CL, Thomas PG, Davis MM. 2015. Cytomegalovirus infection enhances the immune response to influenza. Sci Transl Med 7:281ra243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Frasca D, Diaz A, Romero M, Landin AM, Blomberg BB. 2015. Cytomegalovirus (CMV) seropositivity decreases B cell responses to the influenza vaccine. Vaccine 33:1433–1439. doi: 10.1016/j.vaccine.2015.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Trzonkowski P, Myśliwska J, Szmit E, Wieckiewicz J, Lukaszuk K, Brydak LB, Machała M, Myśliwski A. 2003. Association between cytomegalovirus infection, enhanced proinflammatory response and low level of anti-hemagglutinins during the anti-influenza vaccination—an impact of immunosenescence. Vaccine 21:3826–3836. doi: 10.1016/S0264-410X(03)00309-8. [DOI] [PubMed] [Google Scholar]
- 341.Enani S, Przemska-Kosicka A, Childs CE, Maidens C, Dong H, Conterno L, Tuohy K, Todd S, Gosney M, Yaqoob P. 2018. Impact of ageing and a synbiotic on the immune response to seasonal influenza vaccination; a randomised controlled trial. Clin Nutr 37:443–451. doi: 10.1016/j.clnu.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Gouvêa ADFTB, Pinto MIDM, Miyamoto M, Machado DM, Pessoa SD, Carmo FBD, Beltrão SCDV, Succi RCDM. 2015. Persistence of hepatitis A virus antibodies after primary immunization and response to revaccination in children and adolescents with perinatal HIV exposure. Rev Paul Pediatr 33:142–149. doi: 10.1016/j.rpped.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Lee SD, Chan CY, Yu MI, Wang YJ, Chang FY, Lo KJ, Safary A. 1997. Safety and immunogenicity of inactivated hepatitis A vaccine in patients with chronic liver disease. J Med Virol 52:215–218. doi:. [DOI] [PubMed] [Google Scholar]
- 344.Jabłonowska E, Kuydowicz J. 2014. Durability of response to vaccination against viral hepatitis A in HIV-infected patients: a 5-year observation. Int J STD AIDS 25:745–750. doi: 10.1177/0956462413518902. [DOI] [PubMed] [Google Scholar]
- 345.Williamson WA, Greenwood BM. 1978. Impairment of the immune response to vaccination after acute malaria. Lancet i:1328–1329. [DOI] [PubMed] [Google Scholar]
- 346.Greenwood BM, Bradley-Moore AM, Bryceson AD, Palit A. 1972. Immunosuppression in children with malaria. Lancet i:169–172. [DOI] [PubMed] [Google Scholar]
- 347.McGregor IA, Barr M. 1962. Antibody response to tetanus toxoid inoculation in malarious and non-malarious Gambian children. Trans R Soc Trop Med Hyg 56:364–367. doi: 10.1016/0035-9203(62)90005-6. [DOI] [Google Scholar]
- 348.Greenwood AM, Greenwood BM, Bradley AK, Ball PAJ, Gilles HM. 1981. Enhancement of the immune response to meningococcal polysaccharide vaccine in a malaria endemic area by administration of chloroquine. Ann Trop Med Parasitol 75:261–263. doi: 10.1080/00034983.1981.11687439. [DOI] [Google Scholar]
- 349.Simondon F, Preziosi MP, Pinchinat S, Yam A, Chabirand L, Wassilak S, Pines E, Trape JF, Salomon H, Hoffenbach A. 1999. Randomised study of the possible adjuvant effect of BCG vaccine on the immunogenicity of diphtheria-tetanus-acellular pertussis vaccine in Senegalese infants. Eur J Clin Microbiol Infect Dis 18:23–29. doi: 10.1007/s100960050221. [DOI] [PubMed] [Google Scholar]
- 350.Corrigall RJ. 1988. Asymptomatic malaria parasitaemia and the antibody response to tetanus toxoid vaccination. Trans R Soc Trop Med Hyg 82:540–541. doi: 10.1016/0035-9203(88)90497-X. [DOI] [PubMed] [Google Scholar]
- 351.van Riet E, Retra K, Adegnika AA, Jol-van der Zijde CM, Uh HW, Lell B, Issifou S, Kremsner PG, Yazdanbakhsh M, van Tol MJ, Hartgers FC. 2008. Cellular and humoral responses to tetanus vaccination in Gabonese children. Vaccine 26:3690–3695. doi: 10.1016/j.vaccine.2008.04.067. [DOI] [PubMed] [Google Scholar]
- 352.Rosen JB, Breman JG, Manclark CR, Meade BD, Collins WE, Lobel HO, Saliou P, Roberts JM, Campaore P, Miller MA. 2005. Malaria chemoprophylaxis and the serologic response to measles and diphtheria-tetanus-whole-cell pertussis vaccines. Malar J 4:53. doi: 10.1186/1475-2875-4-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Bradley-Moore AM, Greenwood BM, Bradley AK, Bartlett A, Bidwell DE, Voller A, Craske J, Kirkwood BR, Gilles HM. 1985. Malaria chemoprophylaxis with chloroquine in young Nigerian children. II. Effect on the immune response to vaccination. Ann Trop Med Parasitol 79:563–573. doi: 10.1080/00034983.1985.11811963. [DOI] [PubMed] [Google Scholar]
- 354.Schellenberg D, Menendez C, Kahigwa E, Aponte J, Vidal J, Tanner M, Mshinda H, Alonso P. 2001. Intermittent treatment for malaria and anaemia control at time of routine vaccinations in Tanzanian infants: a randomised, placebo-controlled trial. Lancet 357:1471–1477. doi: 10.1016/S0140-6736(00)04643-2. [DOI] [PubMed] [Google Scholar]
- 355.Massaga JJ, Kitua AY, Lemnge MM, Akida JA, Malle LN, Ronn AM, Theander TG, Bygbjerg IC. 2003. Effect of intermittent treatment with amodiaquine on anaemia and malarial fevers in infants in Tanzania: a randomised placebo-controlled trial. Lancet 361:1853–1860. doi: 10.1016/S0140-6736(03)13504-0. [DOI] [PubMed] [Google Scholar]
- 356.Monjour L, Bourdillon F, Froment A, Daniel-Ribeiro C, Tirard S, Datry A, Chastang C, Tselentis Y, Gentilini M. 1985. Measles vaccination in the Sudan-Sahel region of Africa. Absence of the immunodepressive effect of malaria. Pathol Biol (Paris) 33:232–235. [PubMed] [Google Scholar]
- 357.Faucher JF, Binder R, Missinou MA, Matsiegui PB, Gruss H, Neubauer R, Lell B, Que JU, Miller GB, Kremsner PG. 2002. Efficacy of atovaquone/proguanil for malaria prophylaxis in children and its effect on the immunogenicity of live oral typhoid and cholera vaccines. Clin Infect Dis 35:1147–1154. doi: 10.1086/342908. [DOI] [PubMed] [Google Scholar]
- 358.Monjour L, Bourdillon F, Korinek AM, Aubonnet-Laignel A, Brousse G, Gentilini M, Bayard P, Ballet JJ. 1988. Humoral immunity, 5 years after anti-tetanus vaccination, in a group of malaria-infected and malnourished African children. Pathol Biol (Paris) 36:235–239. [PubMed] [Google Scholar]
- 359.Monjour L, Bourdillon F, Schlumberger M, Fayet MT, Michon C, Ballet JJ, Gouba E, Gentilini M. 1982. Humoral and cellular immunity following antitetanus vaccination in malnourished and malaria-induced African children. 1. Study of the antitetanus antibody response. Bull World Health Organ 60:589–596. [PMC free article] [PubMed] [Google Scholar]
- 360.Cooper PJ, Espinel I, Paredes W, Guderian RH, Nutman TB. 1998. Impaired tetanus-specific cellular and humoral responses following tetanus vaccination in human onchocerciasis: a possible role for interleukin-10. J Infect Dis 178:1133–1138. doi: 10.1086/515661. [DOI] [PubMed] [Google Scholar]
- 361.Sabin EA, Araujo MI, Carvalho EM, Pearce EJ. 1996. Impairment of tetanus toxoid-specific Th1-like immune responses in humans infected with Schistosoma mansoni. J Infect Dis 173:269–272. doi: 10.1093/infdis/173.1.269. [DOI] [PubMed] [Google Scholar]
- 362.Cooper PJ, Chico M, Sandoval C, Espinel I, Guevara A, Levine MM, Griffin GE, Nutman TB. 2001. Human infection with Ascaris lumbricoides is associated with suppression of the interleukin-2 response to recombinant cholera toxin B subunit following vaccination with the live oral cholera vaccine CVD 103-HgR. Infect Immun 69:1574–1580. doi: 10.1128/IAI.69.3.1574-1580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Cooper PJ, Chico ME, Losonsky G, Sandoval C, Espinel I, Sridhara R, Aguilar M, Guevara A, Guderian RH, Levine MM, Griffin GE, Nutman TB. 2000. Albendazole treatment of children with ascariasis enhances the vibriocidal antibody response to the live attenuated oral cholera vaccine CVD 103-HgR. J Infect Dis 182:1199–1206. doi: 10.1086/315837. [DOI] [PubMed] [Google Scholar]
- 364.Elias D, Wolday D, Akuffo H, Petros B, Bronner U, Britton S. 2001. Effect of deworming on human T cell responses to mycobacterial antigens in helminth-exposed individuals before and after bacille Calmette-Guerin (BCG) vaccination. Clin Exp Immunol 123:219–225. doi: 10.1046/j.1365-2249.2001.01446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Elias D, Britton S, Aseffa A, Engers H, Akuffo H. 2008. Poor immunogenicity of BCG in helminth infected population is associated with increased in vitro TGF-beta production. Vaccine 26:3897–3902. doi: 10.1016/j.vaccine.2008.04.083. [DOI] [PubMed] [Google Scholar]
- 366.Woo PC, Tsoi HW, Wong LP, Leung HC, Yuen KY. 1999. Antibiotics modulate vaccine-induced humoral immune response. Clin Diagn Lab Immunol 6:832–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Smith RA, Thedford TR, Espe BH, Woodson PD, Burrows GE. 1983. Effect of oxytetracycline administration on antibody response to Brucella abortus vaccination in calves. J Am Vet Med Assoc 183:70–71. [PubMed] [Google Scholar]
- 368.Zimmermann P, Curtis N. 2018. The influence of probiotics on vaccine responses—a systematic review. Vaccine 36:207–213. doi: 10.1016/j.vaccine.2017.08.069. [DOI] [PubMed] [Google Scholar]
- 369.West CE, Gothefors L, Granstrom M, Kayhty H, Hammarstrom ML, Hernell O. 2007. Effects of feeding probiotics during weaning on infections and antibody responses to diphtheria, tetanus and Hib vaccines. Pediatr Allergy Immunol 19:53–60. doi: 10.1111/j.1399-3038.2007.00583.x. [DOI] [PubMed] [Google Scholar]
- 370.Redondo N, Nova E, Gheorghe A, Diaz LE, Hernandez A, Marcos A. 2017. Evaluation of Lactobacillus coryniformis CECT5711 strain as a coadjuvant in a vaccination process: a randomised clinical trial in healthy adults. Nutr Metab (Lond) 14:2. doi: 10.1186/s12986-016-0154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Kukkonen K, Nieminen T, Poussa T, Savilahti E, Kuitunen M. 2006. Effect of probiotics on vaccine antibody responses in infancy—a randomized placebo-controlled double-blind trial. Pediatr Allergy Immunol 17:416–421. doi: 10.1111/j.1399-3038.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 372.Mullie C, Yazourh A, Thibault H, Odou MF, Singer E, Kalach N, Kremp O, Romond MB. 2004. Increased poliovirus-specific intestinal antibody response coincides with promotion of Bifidobacterium longum-infantis and Bifidobacterium breve in infants: a randomized, double-blind, placebo-controlled trial. Pediatr Res 56:791–795. doi: 10.1203/01.PDR.0000141955.47550.A0. [DOI] [PubMed] [Google Scholar]
- 373.Paineau D, Carcano D, Leyer G, Darquy S, Alyanakian MA, Simoneau G, Bergmann JF, Brassart D, Bornet F, Ouwehand AC. 2008. Effects of seven potential probiotic strains on specific immune responses in healthy adults: a double-blind, randomized, controlled trial. FEMS Immunol Med Microbiol 53:107–113. doi: 10.1111/j.1574-695X.2008.00413.x. [DOI] [PubMed] [Google Scholar]
- 374.Isolauri E, Joensuu J, Suomalainen H, Luomala M, Vesikari T. 1995. Improved immunogenicity of oral D × RRV reassortant rotavirus vaccine by Lactobacillus casei GG. Vaccine 13:310–312. doi: 10.1016/0264-410X(95)93319-5. [DOI] [PubMed] [Google Scholar]
- 375.de Vrese M, Rautenberg P, Laue C, Koopmans M, Herremans T, Schrezenmeir J. 2005. Probiotic bacteria stimulate virus-specific neutralizing antibodies following a booster polio vaccination. Eur J Nutr 44:406–413. doi: 10.1007/s00394-004-0541-8. [DOI] [PubMed] [Google Scholar]
- 376.Bosch M, Mendez M, Perez M, Farran A, Fuentes MC, Cune J. 2012. Lactobacillus plantarum CECT7315 and CECT7316 stimulate immunoglobulin production after influenza vaccination in elderly. Nutr Hosp 27:504–509. doi: 10.1590/S0212-16112012000200023. [DOI] [PubMed] [Google Scholar]
- 377.Rizzardini G, Eskesen D, Calder PC, Capetti A, Jespersen L, Clerici M. 2012. Evaluation of the immune benefits of two probiotic strains Bifidobacterium animalis ssp. lactis, BB-12(R) and Lactobacillus paracasei ssp. paracasei, L. casei 431(R) in an influenza vaccination model: a randomised, double-blind, placebo-controlled study. Br J Nutr 107:876–884. doi: 10.1017/S000711451100420X. [DOI] [PubMed] [Google Scholar]
- 378.Davidson LE, Fiorino AM, Snydman DR, Hibberd PL. 2011. Lactobacillus GG as an immune adjuvant for live-attenuated influenza vaccine in healthy adults: a randomized double-blind placebo-controlled trial. Eur J Clin Nutr 65:501–507. doi: 10.1038/ejcn.2010.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Boge T, Remigy M, Vaudaine S, Tanguy J, Bourdet-Sicard R, van der Werf S. 2009. A probiotic fermented dairy drink improves antibody response to influenza vaccination in the elderly in two randomised controlled trials. Vaccine 27:5677–5684. doi: 10.1016/j.vaccine.2009.06.094. [DOI] [PubMed] [Google Scholar]
- 380.Olivares M, Díaz-Ropero MP, Sierra S, Lara-Villoslada F, Fonollá J, Navas M, Rodríguez JM, Xaus J. 2007. Oral intake of Lactobacillus fermentum CECT5716 enhances the effects of influenza vaccination. Nutrition 23:254–260. doi: 10.1016/j.nut.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 381.Bunout D, Barrera G, Hirsch S, Gattas V, de la Maza MP, Haschke F, Steenhout P, Klassen P, Hager C, Avendaño M, Petermann M, Muñoz C. 2004. Effects of a nutritional supplement on the immune response and cytokine production in free-living Chilean elderly. JPEN J Parenter Enteral Nutr 28:348–354. doi: 10.1177/0148607104028005348. [DOI] [PubMed] [Google Scholar]
- 382.Przemska-Kosicka A, Childs CE, Enani S, Maidens C, Dong H, Dayel IB, Tuohy K, Todd S, Gosney MA, Yaqoob P. 2016. Effect of a synbiotic on the response to seasonal influenza vaccination is strongly influenced by degree of immunosenescence. Immun Ageing 13:6. doi: 10.1186/s12979-016-0061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Bunout D, Hirsch S, Pia de la Maza M, Munoz C, Haschke F, Steenhout P, Klassen P, Barrera G, Gattas V, Petermann M. 2002. Effects of prebiotics on the immune response to vaccination in the elderly. JPEN J Parenter Enteral Nutr 26:372–376. doi: 10.1177/0148607102026006372. [DOI] [PubMed] [Google Scholar]
- 384.Zimmermann P, Curtis N. 2018. The influence of the intestinal microbiome on vaccine responses. Vaccine 36:4433–4439. doi: 10.1016/j.vaccine.2018.04.066. [DOI] [PubMed] [Google Scholar]
- 385.Huda MN, Lewis Z, Kalanetra KM, Rashid M, Ahmad SM, Raqib R, Qadri F, Underwood MA, Mills DA, Stephensen CB. 2014. Stool microbiota and vaccine responses of infants. Pediatrics 134:e362–e372. doi: 10.1542/peds.2013-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Harris VC, Armah G, Fuentes S, Korpela KE, Parashar U, Victor JC, Tate J, de Weerth C, Giaquinto C, Wiersinga WJ, Lewis KD, de Vos WM. 2017. Significant correlation between the infant gut microbiome and rotavirus vaccine response in rural Ghana. J Infect Dis 215:34–41. doi: 10.1093/infdis/jiw518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Eloe-Fadrosh EA, McArthur MA, Seekatz AM, Drabek EF, Rasko DA, Sztein MB, Fraser CM. 2013. Impact of oral typhoid vaccination on the human gut microbiota and correlations with S. Typhi-specific immunological responses. PLoS One 8:e62026. doi: 10.1371/journal.pone.0062026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Parker EPK, Praharaj I, Zekavati A, Lazarus RP, Giri S, Operario DJ, Liu J, Houpt E, Iturriza-Gomara M, Kampmann B, John J, Kang G, Grassly NC. 2018. Influence of the intestinal microbiota on the immunogenicity of oral rotavirus vaccine given to infants in south India. Vaccine 36:264–272. doi: 10.1016/j.vaccine.2017.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Donowitz JR, Petri WA Jr. 2015. Pediatric small intestine bacterial overgrowth in low-income countries. Trends Mol Med 21:6–15. doi: 10.1016/j.molmed.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Salk HM, Simon WL, Lambert ND, Kennedy RB, Grill DE, Kabat BF, Poland GA. 2016. Taxa of the nasal microbiome are associated with influenza-specific IgA response to live attenuated influenza vaccine. PLoS One 11:e0162803. doi: 10.1371/journal.pone.0162803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Pedrazzi C, Ghio L, Balloni A, Panuccio A, Foti M, Edefonti A, Assael BM. 1999. Duration of immunity to diphtheria and tetanus in young kidney transplant patients. Pediatr Transplant 3:109–114. doi: 10.1034/j.1399-3046.1999.00013.x. [DOI] [PubMed] [Google Scholar]
- 392.Matsushita M, Takeuchi S, Kumagai N, Uehara Y, Matsushita C, Arise K, Seo H, Awatani T. 2012. Prevaccination antibody titers can estimate the immune response to influenza vaccine in a rural community-dwelling elderly population. Vaccine 30:1101–1107. doi: 10.1016/j.vaccine.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 393.Walter EB, Neuzil KM, Zhu Y, Fairchok MP, Gagliano ME, Monto AS, Englund JA. 2006. Influenza vaccine immunogenicity in 6- to 23-month-old children: are identical antigens necessary for priming? Pediatrics 118:e570–e578. doi: 10.1542/peds.2006-0198. [DOI] [PubMed] [Google Scholar]
- 394.Englund JA, Walter EB, Gbadebo A, Monto AS, Zhu Y, Neuzil KM. 2006. Immunization with trivalent inactivated influenza vaccine in partially immunized toddlers. Pediatrics 118:e579–e585. doi: 10.1542/peds.2006-0201. [DOI] [PubMed] [Google Scholar]
- 395.Fine PE. 1995. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346:1339–1345. doi: 10.1016/S0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- 396.Black GF, Weir RE, Floyd S, Bliss L, Warndorff DK, Crampin AC, Ngwira B, Sichali L, Nazareth B, Blackwell JM, Branson K, Chaguluka SD, Donovan L, Jarman E, King E, Fine PE, Dockrell HM. 2002. BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: two randomised controlled studies. Lancet 359:1393–1401. doi: 10.1016/S0140-6736(02)08353-8. [DOI] [PubMed] [Google Scholar]
- 397.Abubakar I, Pimpin L, Ariti C, Beynon R, Mangtani P, Sterne JA, Fine PE, Smith PG, Lipman M, Elliman D, Watson JM, Drumright LN, Whiting PF, Vynnycky E, Rodrigues LC. 2013. Systematic review and meta-analysis of the current evidence on the duration of protection by bacillus Calmette-Guerin vaccination against tuberculosis. Health Technol Assess 17:1–372. 10.3310/hta17370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Weir RE, Black GF, Nazareth B, Floyd S, Stenson S, Stanley C, Branson K, Sichali L, Chaguluka SD, Donovan L, Crampin AC, Fine PE, Dockrell HM. 2006. The influence of previous exposure to environmental mycobacteria on the interferon-gamma response to bacille Calmette-Guerin vaccination in southern England and northern Malawi. Clin Exp Immunol 146:390–399. doi: 10.1111/j.1365-2249.2006.03222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Anh DD, Canh DG, Lopez AL, Thiem VD, Long PT, Son NH, Deen J, von Seidlein L, Carbis R, Han SH, Shin SH, Attridge S, Holmgren J, Clemens J. 2007. Safety and immunogenicity of a reformulated Vietnamese bivalent killed, whole-cell, oral cholera vaccine in adults. Vaccine 25:1149–1155. doi: 10.1016/j.vaccine.2006.09.049. [DOI] [PubMed] [Google Scholar]
- 400.Winter AP, Follett EA, McIntyre J, Stewart J, Symington IS. 1994. Influence of smoking on immunological responses to hepatitis B vaccine. Vaccine 12:771–772. doi: 10.1016/0264-410X(94)90283-6. [DOI] [PubMed] [Google Scholar]
- 401.Middleman AB, Kozinetz CA, Robertson LM, DuRant RH, Emans SJ. 2001. The effect of late doses on the achievement of seroprotection and antibody titer levels with hepatitis B immunization among adolescents. Pediatrics 107:1065–1069. doi: 10.1542/peds.107.5.1065. [DOI] [PubMed] [Google Scholar]
- 402.Sheffield JS, Hickman A, Tang J, Moss K, Kourosh A, Crawford NM, Wendel GD Jr. 2011. Efficacy of an accelerated hepatitis B vaccination program during pregnancy. Obstet Gynecol 117:1130–1135. doi: 10.1097/AOG.0b013e3182148efe. [DOI] [PubMed] [Google Scholar]
- 403.Namujju PB, Pajunen E, Simen-Kapeu A, Hedman L, Merikukka M, Surcel HM, Kirnbauer R, Apter D, Paavonen J, Hedman K, Lehtinen M. 2014. Impact of smoking on the quantity and quality of antibodies induced by human papillomavirus type 16 and 18 AS04-adjuvanted virus-like-particle vaccine—a pilot study. BMC Res Notes 7:445. doi: 10.1186/1756-0500-7-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.MacKenzie JS, MacKenzie IH, Holt PG. 1976. The effect of cigarette smoking on susceptibility to epidemic influenza and on serological responses to live attenuated and killed subunit influenza vaccines. J Hyg (Lond) 77:409–417. doi: 10.1017/S0022172400055790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Lim J, Song YJ, Park WS, Sohn H, Lee MS, Shin DH, Kim CB, Kim H, Oh GJ, Ki M. 2014. The immunogenicity of a single dose of hepatitis A virus vaccines (Havrix(R) and Epaxal(R)) in Korean young adults. Yonsei Med J 55:126–131. doi: 10.3349/ymj.2014.55.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Oliveira LC, Silva TE, Alves MH. 2007. Response to hepatitis B vaccine in alcoholics without clinically evident liver cirrhosis. Arq Gastroenterol 44:195–200. doi: 10.1590/S0004-28032007000300003. [DOI] [PubMed] [Google Scholar]
- 407.Eskola J, Ruuskanen O, Soppi E, Viljanen MK, Jarvinen M, Toivonen H, Kouvalainen K. 1978. Effect of sport stress on lymphocyte transformation and antibody formation. Clin Exp Immunol 32:339–345. [PMC free article] [PubMed] [Google Scholar]
- 408.Bruunsgaard H, Hartkopp A, Mohr T, Konradsen H, Heron I, Mordhorst CH, Pedersen BK. 1997. In vivo cell-mediated immunity and vaccination response following prolonged, intense exercise. Med Sci Sports Exerc 29:1176–1181. doi: 10.1097/00005768-199709000-00009. [DOI] [PubMed] [Google Scholar]
- 409.Edwards KM, Burns VE, Reynolds T, Carroll D, Drayson M, Ring C. 2006. Acute stress exposure prior to influenza vaccination enhances antibody response in women. Brain Behav Immun 20:159–168. doi: 10.1016/j.bbi.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 410.Kohut ML, Cooper MM, Nickolaus MS, Russell DR, Cunnick JE. 2002. Exercise and psychosocial factors modulate immunity to influenza vaccine in elderly individuals. J Gerontol A Biol Sci Med Sci 57:M557–M562. doi: 10.1093/gerona/57.9.M557. [DOI] [PubMed] [Google Scholar]
- 411.Hayney MS, Coe CL, Muller D, Obasi CN, Backonja U, Ewers T, Barrett B. 2014. Age and psychological influences on immune responses to trivalent inactivated influenza vaccine in the meditation or exercise for preventing acute respiratory infection (MEPARI) trial. Hum Vaccin Immunother 10:83–91. doi: 10.4161/hv.26661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Long JE, Ring C, Bosch JA, Eves F, Drayson MT, Calver R, Say V, Allen D, Burns VE. 2013. A life-style physical activity intervention and the antibody response to pneumococcal vaccination in women. Psychosom Med 75:774–782. doi: 10.1097/PSY.0b013e3182a0b664. [DOI] [PubMed] [Google Scholar]
- 413.Campbell JP, Edwards KM, Ring C, Drayson MT, Bosch JA, Inskip A, Long JE, Pulsford D, Burns VE. 2010. The effects of vaccine timing on the efficacy of an acute eccentric exercise intervention on the immune response to an influenza vaccine in young adults. Brain Behav Immun 24:236–242. doi: 10.1016/j.bbi.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 414.Ayling K, Fairclough L, Tighe P, Todd I, Halliday V, Garibaldi J, Royal S, Hamed A, Buchanan H, Vedhara K. 2018. Positive mood on the day of influenza vaccination predicts vaccine effectiveness: a prospective observational cohort study. Brain Behav Immun 67:314–323. doi: 10.1016/j.bbi.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 415.Phillips AC, Carroll D, Burns VE, Drayson M. 2009. Cardiovascular activity and the antibody response to vaccination. J Psychosom Res 67:37–43. doi: 10.1016/j.jpsychores.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 416.Kiecolt-Glaser JK, Glaser R, Gravenstein S, Malarkey WB, Sheridan J. 1996. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci U S A 93:3043–3047. doi: 10.1073/pnas.93.7.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Glaser R, Kiecolt-Glaser JK, Malarkey WB, Sheridan JF. 1998. The influence of psychological stress on the immune response to vaccines. Ann N Y Acad Sci 840:649–655. doi: 10.1111/j.1749-6632.1998.tb09603.x. [DOI] [PubMed] [Google Scholar]
- 418.Vedhara K, Cox NK, Wilcock GK, Perks P, Hunt M, Anderson S, Lightman SL, Shanks NM. 1999. Chronic stress in elderly carers of dementia patients and antibody response to influenza vaccination. Lancet 353:627–631. doi: 10.1016/S0140-6736(98)06098-X. [DOI] [PubMed] [Google Scholar]
- 419.Segerstrom SC, Schipper LJ, Greenberg RN. 2008. Caregiving, repetitive thought, and immune response to vaccination in older adults. Brain Behav Immun 22:744–752. doi: 10.1016/j.bbi.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Glaser R, Sheridan J, Malarkey WB, MacCallum RC, Kiecolt-Glaser JK. 2000. Chronic stress modulates the immune response to a pneumococcal pneumonia vaccine. Psychosom Med 62:804–807. doi: 10.1097/00006842-200011000-00010. [DOI] [PubMed] [Google Scholar]
- 421.Burns VE, Carroll D, Drayson M, Whitham M, Ring C. 2003. Life events, perceived stress and antibody response to influenza vaccination in young, healthy adults. J Psychosom Res 55:569–572. doi: 10.1016/S0022-3999(03)00073-4. [DOI] [PubMed] [Google Scholar]
- 422.Miller GE, Cohen S, Pressman S, Barkin A, Rabin BS, Treanor JJ. 2004. Psychological stress and antibody response to influenza vaccination: when is the critical period for stress, and how does it get inside the body? Psychosom Med 66:215–223. doi: 10.1097/01.psy.0000116718.54414.9e. [DOI] [PubMed] [Google Scholar]
- 423.Phillips AC, Burns VE, Carroll D, Ring C, Drayson M. 2005. The association between life events, social support, and antibody status following thymus-dependent and thymus-independent vaccinations in healthy young adults. Brain Behav Immun 19:325–333. doi: 10.1016/j.bbi.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 424.Pressman SD, Cohen S, Miller GE, Barkin A, Rabin BS, Treanor JJ. 2005. Loneliness, social network size, and immune response to influenza vaccination in college freshmen. Health Psychol 24:297–306. doi: 10.1037/0278-6133.24.3.297. [DOI] [PubMed] [Google Scholar]
- 425.Kosor Krnic E, Gagro A, Kozaric-Kovacic D, Vilibic M, Grubisic-Ilic M, Folnegovic-Smalc V, Drazenovic V, Cecuk-Jelicic E, Gjenero-Margan I, Kuzman I, Jeren T, Sabioncello A, Kerhin-Brkljacic V, Kaic B, Markotic A, Gotovac K, Rabatic S, Mlinaric-Galinovic G, Dekaris D. 2007. Outcome of influenza vaccination in combat-related post-traumatic stress disorder (PTSD) patients. Clin Exp Immunol 149:303–310. doi: 10.1111/j.1365-2249.2007.03410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Vedhara K, McDermott MP, Evans TG, Treanor JJ, Plummer S, Tallon D, Cruttenden KA, Schifitto G. 2002. Chronic stress in nonelderly caregivers: psychological, endocrine and immune implications. J Psychosom Res 53:1153–1161. doi: 10.1016/S0022-3999(02)00343-4. [DOI] [PubMed] [Google Scholar]
- 427.Pedersen AF, Zachariae R, Bovbjerg DH. 2009. Psychological stress and antibody response to influenza vaccination: a meta-analysis. Brain Behav Immun 23:427–433. doi: 10.1016/j.bbi.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 428.Phillips AC, Carroll D, Burns VE, Ring C, Macleod J, Drayson M. 2006. Bereavement and marriage are associated with antibody response to influenza vaccination in the elderly. Brain Behav Immun 20:279–289. doi: 10.1016/j.bbi.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 429.Moynihan JA, Larson MR, Treanor J, Duberstein PR, Power A, Shore B, Ader R. 2004. Psychosocial factors and the response to influenza vaccination in older adults. Psychosom Med 66:950–953. doi: 10.1097/01.psy.0000140001.49208.2d. [DOI] [PubMed] [Google Scholar]
- 430.Burns VE, Carroll D, Ring C, Harrison LK, Drayson M. 2002. Stress, coping, and hepatitis B antibody status. Psychosom Med 64:287–293. doi: 10.1097/00006842-200203000-00012. [DOI] [PubMed] [Google Scholar]
- 431.Glaser R, Kiecolt-Glaser JK, Bonneau RH, Malarkey W, Kennedy S, Hughes J. 1992. Stress-induced modulation of the immune response to recombinant hepatitis B vaccine. Psychosom Med 54:22–29. doi: 10.1097/00006842-199201000-00005. [DOI] [PubMed] [Google Scholar]
- 432.Jabaaij L, Grosheide PM, Heijtink RA, Duivenvoorden HJ, Ballieux RE, Vingerhoets AJ. 1993. Influence of perceived psychological stress and distress on antibody response to low dose rDNA hepatitis B vaccine. J Psychosom Res 37:361–369. doi: 10.1016/0022-3999(93)90138-6. [DOI] [PubMed] [Google Scholar]
- 433.Petrie KJ, Booth RJ, Pennebaker JW, Davison KP, Thomas MG. 1995. Disclosure of trauma and immune response to a hepatitis B vaccination program. J Consult Clin Psychol 63:787–792. doi: 10.1037/0022-006X.63.5.787. [DOI] [PubMed] [Google Scholar]
- 434.Burns VE, Drayson M, Ring C, Carroll D. 2002. Perceived stress and psychological well-being are associated with antibody status after meningitis C conjugate vaccination. Psychosom Med 64:963–970. [DOI] [PubMed] [Google Scholar]
- 435.Petry LJ, Weems LB II, Livingstone JN II.. 1991. Relationship of stress, distress, and the immunologic response to a recombinant hepatitis B vaccine. J Fam Pract 32:481–486. [PubMed] [Google Scholar]
- 436.Marsland AL, Cohen S, Rabin BS, Manuck SB. 2001. Associations between stress, trait negative affect, acute immune reactivity, and antibody response to hepatitis B injection in healthy young adults. Health Psychol 20:4–11. doi: 10.1037/0278-6133.20.1.4. [DOI] [PubMed] [Google Scholar]
- 437.Jabaaij L, van Hattum J, Vingerhoets JJ, Oostveen FG, Duivenvoorden HJ, Ballieux RE. 1996. Modulation of immune response to rDNA hepatitis B vaccination by psychological stress. J Psychosom Res 41:129–137. doi: 10.1016/0022-3999(96)00123-7. [DOI] [PubMed] [Google Scholar]
- 438.Morag M, Morag A, Reichenberg A, Lerer B, Yirmiya R. 1999. Psychological variables as predictors of rubella antibody titers and fatigue—a prospective, double blind study. J Psychiatr Res 33:389–395. doi: 10.1016/S0022-3956(99)00010-2. [DOI] [PubMed] [Google Scholar]
- 439.O’Connor TG, Moynihan JA, Wyman PA, Carnahan J, Lofthus G, Quataert SA, Bowman M, Caserta MT. 2014. Depressive symptoms and immune response to meningococcal conjugate vaccine in early adolescence. Dev Psychopathol 26:1567–1576. doi: 10.1017/S0954579414001242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.O’Connor TG, Wang H, Moynihan JA, Wyman PA, Carnahan J, Lofthus G, Quataert SA, Bowman M, Burke AS, Caserta MT. 2015. Observed parent-child relationship quality predicts antibody response to vaccination in children. Brain Behav Immun 48:265–273. doi: 10.1016/j.bbi.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Boyce WT, Adams S, Tschann JM, Cohen F, Wara D, Gunnar MR. 1995. Adrenocortical and behavioral predictors of immune responses to starting school. Pediatr Res 38:1009–1017. doi: 10.1203/00006450-199512000-00030. [DOI] [PubMed] [Google Scholar]
- 442.Prather AA, Hall M, Fury JM, Ross DC, Muldoon MF, Cohen S, Marsland AL. 2012. Sleep and antibody response to hepatitis B vaccination. Sleep 35:1063–1069. doi: 10.5665/sleep.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Lange T, Perras B, Fehm HL, Born J. 2003. Sleep enhances the human antibody response to hepatitis A vaccination. Psychosom Med 65:831–835. doi: 10.1097/01.PSY.0000091382.61178.F1. [DOI] [PubMed] [Google Scholar]
- 444.Lange T, Dimitrov S, Bollinger T, Diekelmann S, Born J. 2011. Sleep after vaccination boosts immunological memory. J Immunol 187:283–290. doi: 10.4049/jimmunol.1100015. [DOI] [PubMed] [Google Scholar]
- 445.Spiegel K, Sheridan JF, Van Cauter E. 2002. Effect of sleep deprivation on response to immunization. JAMA 288:1471–1472. doi: 10.1001/jama.288.12.1469. [DOI] [PubMed] [Google Scholar]
- 446.Dopp JM, Wiegert NA, Moran JJ, Muller D, Weber S, Hayney MS. 2007. Humoral immune responses to influenza vaccination in patients with obstructive sleep apnea. Pharmacotherapy 27:1483–1489. doi: 10.1592/phco.27.11.1483. [DOI] [PubMed] [Google Scholar]
- 447.Reuman PD, Kubilis P, Hurni W, Brown L, Nalin D. 1997. The effect of age and weight on the response to formalin inactivated, alum-adjuvanted hepatitis A vaccine in healthy adults. Vaccine 15:1157–1161. doi: 10.1016/S0264-410X(96)00310-6. [DOI] [PubMed] [Google Scholar]
- 448.Halsey NA, Moulton LH, O’Donovan JC, Walcher JR, Thoms ML, Margolis HS, Krause DS. 1999. Hepatitis B vaccine administered to children and adolescents at yearly intervals. Pediatrics 103:1243–1247. doi: 10.1542/peds.103.6.1243. [DOI] [PubMed] [Google Scholar]
- 449.Sheridan PA, Paich HA, Handy J, Karlsson EA, Hudgens MG, Sammon AB, Holland LA, Weir S, Noah TL, Beck MA. 2012. Obesity is associated with impaired immune response to influenza vaccination in humans. Int J Obes 36:1072–1077. doi: 10.1038/ijo.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Talbot HK, Coleman LA, Crimin K, Zhu Y, Rock MT, Meece J, Shay DK, Belongia EA, Griffin MR. 2012. Association between obesity and vulnerability and serologic response to influenza vaccination in older adults. Vaccine 30:3937–3943. doi: 10.1016/j.vaccine.2012.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Young KM, Gray CM, Bekker LG. 2013. Is obesity a risk factor for vaccine non-responsiveness? PLoS One 8:e82779. doi: 10.1371/journal.pone.0082779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Weber DJ, Rutala WA, Samsa GP, Bradshaw SE, Lemon SM. 1986. Impaired immunogenicity of hepatitis B vaccine in obese persons. N Engl J Med 314:1393. doi: 10.1056/NEJM198605223142120. [DOI] [PubMed] [Google Scholar]
- 453.Simó Miñana J, Gaztambide Ganuza M, Fernández Millán P, Peña Fernández M. 1996. Hepatitis B vaccine immunoresponsiveness in adolescents: a revaccination proposal after primary vaccination. Vaccine 14:103–106. doi: 10.1016/0264-410X(95)00176-2. [DOI] [PubMed] [Google Scholar]
- 454.Ul-Haq N, Hasnain SS, Umar M, Abbas Z, Valenzuela-Silva C, Lopez-Saura P. 2003. Immunogenicity of 10 and 20 microgram hepatitis B vaccine in a two-dose schedule. Vaccine 21:3179–3185. doi: 10.1016/S0264-410X(03)00232-9. [DOI] [PubMed] [Google Scholar]
- 455.el-Gamal Y, Aly RH, Hossny E, Afify E, el-Taliawy D. 1996. Response of Egyptian infants with protein calorie malnutrition to hepatitis B vaccination. J Trop Pediatr 42:144–145. doi: 10.1093/tropej/42.3.144. [DOI] [PubMed] [Google Scholar]
- 456.Mohammed I, Damisah MM. 1982. The immunological response to polyvalent meningococcal vaccine in Bauchi State, Nigeria. Trans R Soc Trop Med Hyg 76:351–353. doi: 10.1016/0035-9203(82)90188-2. [DOI] [PubMed] [Google Scholar]
- 457.Salimonu LS, Johnson AO, Williams AI, Adeleye GI, Osunkoya BO. 1982. Lymphocyte subpopulations and antibody levels in immunized malnourished children. Br J Nutr 48:7–14. doi: 10.1079/BJN19820082. [DOI] [PubMed] [Google Scholar]
- 458.Idris S, El Seed AM. 1983. Measles vaccination in severely malnourished Sudanese children. Ann Trop Paediatr 3:63–67. doi: 10.1080/02724936.1983.11748270. [DOI] [PubMed] [Google Scholar]
- 459.Powell GM. 1982. Response to live attenuated measles vaccine in children with severe kwashiorkor. Ann Trop Paediatr 2:143–145. doi: 10.1080/02724936.1982.11748247. [DOI] [PubMed] [Google Scholar]
- 460.Gaayeb L, Pincon C, Cames C, Sarr JB, Seck M, Schacht AM, Remoue F, Hermann E, Riveau G. 2014. Immune response to Bordetella pertussis is associated with season and undernutrition in Senegalese children. Vaccine 32:3431–3437. doi: 10.1016/j.vaccine.2014.03.086. [DOI] [PubMed] [Google Scholar]
- 461.Suskind R, Sirishinha S, Vithayasai V, Edelman R, Damrongsak D, Charupatana C, Olson RE. 1976. Immunoglobulins and antibody response in children with protein-calorie malnutrition. Am J Clin Nutr 29:836–841. doi: 10.1093/ajcn/29.8.836. [DOI] [PubMed] [Google Scholar]
- 462.Brussow H, Sidoti J, Dirren H, Freire WB. 1995. Effect of malnutrition in Ecuadorian children on titers of serum antibodies to various microbial antigens. Clin Diagn Lab Immunol 2:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Harland PS. 1965. Tuberculin reactions in malnourished children. Lancet ii:719–721. [DOI] [PubMed] [Google Scholar]
- 464.McMurray DN, Loomis SA, Casazza LJ, Rey H, Miranda R. 1981. Development of impaired cell-mediated immunity in mild and moderate malnutrition. Am J Clin Nutr 34:68–77. doi: 10.1093/ajcn/34.1.68. [DOI] [PubMed] [Google Scholar]
- 465.Ziegler HD, Ziegler PB. 1975. Depression of tuberculin reaction in mild and moderate protein-calorie malnourished children following BCG vaccination. Johns Hopkins Med J 137:59–64. [PubMed] [Google Scholar]
- 466.Sadarangani SP, Whitaker JA, Poland GA. 2015. “Let there be light”: the role of vitamin D in the immune response to vaccines. Expert Rev Vaccines 14:1427–1440. doi: 10.1586/14760584.2015.1082426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Sundaram ME, Talbot HK, Zhu Y, Griffin MR, Spencer S, Shay DK, Coleman LA. 2013. Vitamin D is not associated with serologic response to influenza vaccine in adults over 50 years old. Vaccine 31:2057–2061. doi: 10.1016/j.vaccine.2013.02.028. [DOI] [PubMed] [Google Scholar]
- 468.Kriesel JD, Spruance J. 1999. Calcitriol (1,25-dihydroxy-vitamin D3) coadministered with influenza vaccine does not enhance humoral immunity in human volunteers. Vaccine 17:1883–1888. doi: 10.1016/S0264-410X(98)00476-9. [DOI] [PubMed] [Google Scholar]
- 469.Cooper C, Thorne A. 2011. Vitamin D supplementation does not increase immunogenicity of seasonal influenza vaccine in HIV-infected adults. HIV Clin Trials 12:275–276. doi: 10.1310/hct1205-275. [DOI] [PubMed] [Google Scholar]
- 470.Principi N, Marchisio P, Terranova L, Zampiero A, Baggi E, Daleno C, Tirelli S, Pelucchi C, Esposito S. 2013. Impact of vitamin D administration on immunogenicity of trivalent inactivated influenza vaccine in previously unvaccinated children. Hum Vaccin Immunother 9:969–974. doi: 10.4161/hv.23540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Lin CJ, Martin JM, Cole KS, Zimmerman RK, Susick M, Moehling KK, Levine MZ, Spencer S, Flannery B, Nowalk MP. 2017. Are children’s vitamin D levels and BMI associated with antibody titers produced in response to 2014-2015 influenza vaccine? Hum Vaccin Immunother 13:1661–1665. doi: 10.1080/21645515.2017.1299837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Science M, Maguire JL, Russell ML, Smieja M, Walter SD, Loeb M. 2014. Serum 25-hydroxyvitamin D level and influenza vaccine immunogenicity in children and adolescents. PLoS One 9:e83553. doi: 10.1371/journal.pone.0083553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Antonen JA, Hannula PM, Pyhala R, Saha HH, Ala-Houhala IO, Pasternack AI. 2000. Adequate seroresponse to influenza vaccination in dialysis patients. Nephron 86:56–61. doi: 10.1159/000045713. [DOI] [PubMed] [Google Scholar]
- 474.Zitt E, Sprenger-Mahr H, Knoll F, Neyer U, Lhotta K. 2012. Vitamin D deficiency is associated with poor response to active hepatitis B immunisation in patients with chronic kidney disease. Vaccine 30:931–935. doi: 10.1016/j.vaccine.2011.11.086. [DOI] [PubMed] [Google Scholar]
- 475.Aoun B, Dourthe ME, Davourie Salandre A, Souberbielle JC, Ulinski T. 2012. Do vitamin D plasma levels impact vaccine response in children with idiopathic nephrotic syndrome? Pediatr Nephrol 27:2161–2162. doi: 10.1007/s00467-012-2273-y. [DOI] [PubMed] [Google Scholar]
- 476.Peelen E, Rijkers G, Meerveld-Eggink A, Meijvis S, Vogt M, Cohen Tervaert JW, Hupperts R, Damoiseaux J. 2013. Relatively high serum vitamin D levels do not impair the antibody response to encapsulated bacteria. Eur J Clin Microbiol Infect Dis 32:61–69. doi: 10.1007/s10096-012-1714-7. [DOI] [PubMed] [Google Scholar]
- 477.Heine G, Drozdenko G, Lahl A, Unterwalder N, Mei H, Volk HD, Dorner T, Radbruch A, Worm M. 2011. Efficient tetanus toxoid immunization on vitamin D supplementation. Eur J Clin Nutr 65:329–334. doi: 10.1038/ejcn.2010.276. [DOI] [PubMed] [Google Scholar]
- 478.Zheng Y, Li XG, Wang QZ, Ma AG, Bygbjerg IC, Sun YY, Li Y, Zheng MC, Wang X. 2014. Enhancement of vitamin A combined vitamin D supplementation on immune response to bacille Calmette-Guerin vaccine revaccinated in Chinese infants. Asian Pac J Trop Med 7:130–135. doi: 10.1016/S1995-7645(14)60008-0. [DOI] [PubMed] [Google Scholar]
- 479.Bahl R, Kumar R, Bhandari N, Kant S, Srivastava R, Bhan MK. 1999. Vitamin A administered with measles vaccine to nine-month-old infants does not reduce vaccine immunogenicity. J Nutr 129:1569–1573. doi: 10.1093/jn/129.8.1569. [DOI] [PubMed] [Google Scholar]
- 480.Benn CS, Balde A, George E, Kidd M, Whittle H, Lisse IM, Aaby P. 2002. Effect of vitamin A supplementation on measles-specific antibody levels in Guinea-Bissau. Lancet 359:1313–1314. doi: 10.1016/S0140-6736(02)08274-0. [DOI] [PubMed] [Google Scholar]
- 481.Kutty PM, Mohanram M, Vinodini R. 1981. Humoral immune response in vitamin A deficient children. Acta Vitaminol Enzymol 3:231–235. [PubMed] [Google Scholar]
- 482.Sundaram ME, Meydani SN, Vandermause M, Shay DK, Coleman LA. 2014. Vitamin E, vitamin A, and zinc status are not related to serologic response to influenza vaccine in older adults: an observational prospective cohort study. Nutr Res 34:149–154. doi: 10.1016/j.nutres.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 483.Grzegorzewska AE, Jodłowska E, Mostowska A, Sowińska A, Jagodziński PP. 2014. Single nucleotide polymorphisms of vitamin D binding protein, vitamin D receptor and retinoid X receptor alpha genes and response to hepatitis B vaccination in renal replacement therapy patients. Expert Rev Vaccines 13:1395–1403. doi: 10.1586/14760584.2014.962521. [DOI] [PubMed] [Google Scholar]
- 484.Ovsyannikova IG, Haralambieva IH, Vierkant RA, O’Byrne MM, Jacobson RM, Poland GA. 2012. Effects of vitamin A and D receptor gene polymorphisms/haplotypes on immune responses to measles vaccine. Pharmacogenet Genomics 22:20–31. doi: 10.1097/FPC.0b013e32834df186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Imboumy-Limoukou RK, Oyegue-Liabagui SL, Ndidi S, Pegha-Moukandja I, Kouna CL, Galaway F, Florent I, Lekana-Douki JB. 2016. Comparative antibody responses against three antimalarial vaccine candidate antigens from urban and rural exposed individuals in Gabon. Eur J Microbiol Immunol 6:287–297. doi: 10.1556/1886.2016.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Alssamei FA, Al-Sonboli NA, Alkumaim FA, Alsayaad NS, Al-Ahdal MS, Higazi TB, Elagib AA. 2017. Assessment of immunization to hepatitis B vaccine among children under five years in rural areas of Taiz, Yemen. Hepat Res Treat 2017:2131627. doi: 10.1155/2017/2131627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.van Riet E, Adegnika AA, Retra K, Vieira R, Tielens AG, Lell B, Issifou S, Hartgers FC, Rimmelzwaan GF, Kremsner PG, Yazdanbakhsh M. 2007. Cellular and humoral responses to influenza in Gabonese children living in rural and semi-urban areas. J Infect Dis 196:1671–1678. doi: 10.1086/522010. [DOI] [PubMed] [Google Scholar]
- 488.Edstam JS, Dulmaa N, Nymadawa P, Rinchin A, Khulan J, Kimball AM. 2002. Comparison of hepatitis B vaccine coverage and effectiveness among urban and rural Mongolian 2-year-olds. Prev Med 34:207–214. doi: 10.1006/pmed.2001.0972. [DOI] [PubMed] [Google Scholar]
- 489.Hoppenbrouwers K, Kanra G, Roelants M, Ceyhan M, Vandermeulen C, Yurdakok K, Silier T, Dupuy M, Pehlivan T, Ozmert E, Desmyter J. 1999. Priming effect, immunogenicity and safety of an Haemophilus influenzae type b-tetanus toxoid conjugate (PRP-T) and diphtheria-tetanus-acellular pertussis (DTaP) combination vaccine administered to infants in Belgium and Turkey. Vaccine 17:875–886. doi: 10.1016/S0264-410X(98)00273-4. [DOI] [PubMed] [Google Scholar]
- 490.Park DE, Johnson TS, Nonyane BAS, Chandir S, Conklin L, Fleming-Dutra KE, Loo JD, Goldblatt D, Whitney CG, O’Brien KL, Deloria Knoll M. 2014. The differential impact of coadministered vaccines, geographic region, vaccine product and other covariates on pneumococcal conjugate vaccine immunogenicity. Pediatr Infect Dis J 33:S130–S139. doi: 10.1097/INF.0000000000000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Onoja AL, Adu FD, Tomori O. 1992. Evaluation of measles vaccination programme conducted in two separate health centres. Vaccine 10:49–52. doi: 10.1016/0264-410X(92)90419-K. [DOI] [PubMed] [Google Scholar]
- 492.Adu FD, Akinwolere OA, Tomori O, Uche LN. 1992. Low seroconversion rates to measles vaccine among children in Nigeria. Bull World Health Organ 70:457–460. [PMC free article] [PubMed] [Google Scholar]
- 493.Fowotade A, Okonko IO, Nwabuisi C, Bakare RA, Fadeyi A, Adu FD. 2015. Measles vaccine potency and sero-conversion rates among infants receiving measles immunization in Ilorin, Kwara State, Nigeria. J Immunoassay Immunochem 36:195–209. doi: 10.1080/15321819.2014.920713. [DOI] [PubMed] [Google Scholar]
- 494.Levine MM. 2010. Immunogenicity and efficacy of oral vaccines in developing countries: lessons from a live cholera vaccine. BMC Biol 8:129. doi: 10.1186/1741-7007-8-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Patriarca PA, Wright PF, John TJ. 1991. Factors affecting the immunogenicity of oral poliovirus vaccine in developing countries: review. Rev Infect Dis 13:926–939. doi: 10.1093/clinids/13.5.926. [DOI] [PubMed] [Google Scholar]
- 496.Triki H, Abdallah MV, Ben Aissa R, Bouratbine A, Ben Ali Kacem M, Bouraoui S, Koubaa C, Zouari S, Mohsni E, Crainic R, Dellagi K. 1997. Influence of host related factors on the antibody response to trivalent oral polio vaccine in Tunisian infants. Vaccine 15:1123–1129. doi: 10.1016/S0264-410X(97)00001-7. [DOI] [PubMed] [Google Scholar]
- 497.Mirza NB, Wamola IA, Estambale BA, Mbithi E, Poillet M. 1995. Typhim Vi vaccine against typhoid fever: a clinical trial in Kenya. East Afr Med J 72:162–164. [PubMed] [Google Scholar]
- 498.Tabor E, Gerety RJ, Cairns J, Bayley AC. 1990. Antibody responses of adults, adolescents, and children to a plasma-derived hepatitis B vaccine in a rural African setting. J Med Virol 32:134–138. doi: 10.1002/jmv.1890320212. [DOI] [PubMed] [Google Scholar]
- 499.Lalor MK, Floyd S, Gorak-Stolinska P, Ben-Smith A, Weir RE, Smith SG, Newport MJ, Blitz R, Mvula H, Branson K, McGrath N, Crampin AC, Fine PE, Dockrell HM. 2011. BCG vaccination induces different cytokine profiles following infant BCG vaccination in the UK and Malawi. J Infect Dis 204:1075–1085. doi: 10.1093/infdis/jir515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Lalor MK, Ben-Smith A, Gorak-Stolinska P, Weir RE, Floyd S, Blitz R, Mvula H, Newport MJ, Branson K, McGrath N, Crampin AC, Fine PE, Dockrell HM. 2009. Population differences in immune responses to bacille Calmette-Guerin vaccination in infancy. J Infect Dis 199:795–800. doi: 10.1086/597069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Termorshuizen F, Garssen J, Norval M, Koulu L, Laihia J, Leino L, Jansen CT, De Gruijl F, Gibbs NK, De Simone C, Van Loveren H. 2002. A review of studies on the effects of ultraviolet irradiation on the resistance to infections: evidence from rodent infection models and verification by experimental and observational human studies. Int Immunopharmacol 2:263–275. doi: 10.1016/S1567-5769(01)00178-3. [DOI] [PubMed] [Google Scholar]
- 502.Sleijffers A, Garssen J, de Gruijl FR, Boland GJ, van Hattum J, van Vloten WA, van Loveren H. 2001. Influence of ultraviolet B exposure on immune responses following hepatitis B vaccination in human volunteers. J Invest Dermatol 117:1144–1150. doi: 10.1046/j.0022-202x.2001.01542.x. [DOI] [PubMed] [Google Scholar]
- 503.Reigart JR, Graber CD. 1976. Evaluation of the humoral immune response of children with low lead exposure. Bull Environ Contam Toxicol 16:112–117. doi: 10.1007/BF01753115. [DOI] [PubMed] [Google Scholar]
- 504.Raqib R, Ahmed S, Ahsan KB, Kippler M, Akhtar E, Roy AK, Lu Y, Arifeen SE, Wagatsuma Y, Vahter M. 2017. Humoral immunity in arsenic-exposed children in rural Bangladesh: total immunoglobulins and vaccine-specific antibodies. Environ Health Perspect 125:067006. doi: 10.1289/EHP318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Weisglas-Kuperus N, Patandin S, Berbers GA, Sas TC, Mulder PG, Sauer PJ, Hooijkaas H. 2000. Immunologic effects of background exposure to polychlorinated biphenyls and dioxins in Dutch preschool children. Environ Health Perspect 108:1203–1207. doi: 10.1289/ehp.001081203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Ritz N, Dutta B, Donath S, Casalaz D, Connell TG, Tebruegge M, Robins-Browne R, Hanekom WA, Britton WJ, Curtis N. 2012. The influence of bacille Calmette-Guerin vaccine strain on the immune response against tuberculosis: a randomized trial. Am J Respir Crit Care Med 185:213–222. doi: 10.1164/rccm.201104-0714OC. [DOI] [PubMed] [Google Scholar]
- 507.Ritz N, Hanekom WA, Robins-Browne R, Britton WJ, Curtis N. 2008. Influence of BCG vaccine strain on the immune response and protection against tuberculosis. FEMS Microbiol Rev 32:821–841. doi: 10.1111/j.1574-6976.2008.00118.x. [DOI] [PubMed] [Google Scholar]
- 508.Kao JT, Wang JH, Hung CH, Yen YH, Hung SF, Hu TH, Lee CM, Lu SN. 2009. Long-term efficacy of plasma-derived and recombinant hepatitis B vaccines in a rural township of Central Taiwan. Vaccine 27:1858–1862. doi: 10.1016/j.vaccine.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 509.Fang Y, Chen L, Liu MQ, Zhu ZG, Zhu ZR, Hu Q. 2014. Comparison of safety and immunogenicity of PVRV and PCECV immunized in patients with WHO category II animal exposure: a study based on different age groups. PLoS Negl Trop Dis 8:e3412. doi: 10.1371/journal.pntd.0003412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Levy O, Goriely S, Kollmann TR. 2013. Immune response to vaccine adjuvants during the first year of life. Vaccine 31:2500–2505. doi: 10.1016/j.vaccine.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Khurana S, Verma N, Yewdell JW, Hilbert AK, Castellino F, Lattanzi M, Del Giudice G, Rappuoli R, Golding H. 2011. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci Transl Med 3:85ra48. doi: 10.1126/scitranslmed.3002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Mosca F, Tritto E, Muzzi A, Monaci E, Bagnoli F, Iavarone C, O’Hagan D, Rappuoli R, De Gregorio E. 2008. Molecular and cellular signatures of human vaccine adjuvants. Proc Natl Acad Sci U S A 105:10501–10506. doi: 10.1073/pnas.0804699105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Jacques P, Moens G, Desombere I, Dewijngaert J, Leroux-Roels G, Wettendorff M, Thoelen S. 2002. The immunogenicity and reactogenicity profile of a candidate hepatitis B vaccine in an adult vaccine non-responder population. Vaccine 20:3644–3649. doi: 10.1016/S0264-410X(02)00397-3. [DOI] [PubMed] [Google Scholar]
- 514.Salk J, Cohen H, Fillastre C, Stoeckel P, Rey JL, Schlumberger M, Nicolas A, van Steenis G, van Wezel AL, Triau R, Saliou P, Barry LF, Moreau JP, Mérieux C. 1978. Killed poliovirus antigen titration in humans. Dev Biol Stand 41:119–132. [PubMed] [Google Scholar]
- 515.Chiaramonte M, Majori S, Ngatchu T, Moschen ME, Baldo V, Renzulli G, Simoncello I, Rocco S, Bertin T, Naccarato R, Trivello R. 1996. Two different dosages of yeast derived recombinant hepatitis B vaccines: a comparison of immunogenicity. Vaccine 14:135–137. doi: 10.1016/0264-410X(95)00148-T. [DOI] [PubMed] [Google Scholar]
- 516.Punjabi NH, Richie EL, Simanjuntak CH, Harjanto SJ, Wangsasaputra F, Arjoso S, Rofiq A, Prijanto M, Julitasari Yela U, Herzog C, Cryz SJ. 2006. Immunogenicity and safety of four different doses of Haemophilus influenzae type b-tetanus toxoid conjugated vaccine, combined with diphtheria-tetanus-pertussis vaccine (DTP-Hib), in Indonesian infants. Vaccine 24:1776–1785. doi: 10.1016/j.vaccine.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 517.DiazGranados CA, Dunning AJ, Jordanov E, Landolfi V, Denis M, Talbot HK. 2013. High-dose trivalent influenza vaccine compared to standard dose vaccine in elderly adults: safety, immunogenicity and relative efficacy during the 2009-2010 season. Vaccine 31:861–866. doi: 10.1016/j.vaccine.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 518.Booy R, Aitken SJ, Taylor S, Tudor-Williams G, Macfarlane JA, Moxon ER, Ashworth LA, Mayon-White RT, Griffiths H, Chapel HM. 1992. Immunogenicity of combined diphtheria, tetanus, and pertussis vaccine given at 2, 3, and 4 months versus 3, 5, and 9 months of age. Lancet 339:507–510. doi: 10.1016/0140-6736(92)90336-2. [DOI] [PubMed] [Google Scholar]
- 519.Rumke HC, Loch HP, Hoppenbrouwers K, Vandermeulen C, Malfroot A, Helm K, Douha M, Willems P. 2011. Immunogenicity and safety of a measles-mumps-rubella-varicella vaccine following a 4-week or a 12-month interval between two doses. Vaccine 29:3842–3849. doi: 10.1016/j.vaccine.2011.02.067. [DOI] [PubMed] [Google Scholar]
- 520.Englund JA, Walter EB, Fairchok MP, Monto AS, Neuzil KM. 2005. A comparison of 2 influenza vaccine schedules in 6- to 23-month-old children. Pediatrics 115:1039–1047. doi: 10.1542/peds.2004-2373. [DOI] [PubMed] [Google Scholar]
- 521.Ukena T, Esber H, Bessette R, Parks T, Crocker B, Shaw FE Jr. 1985. Site of injection and response to hepatitis B vaccine. N Engl J Med 313:579–580. doi: 10.1056/NEJM198508293130911. [DOI] [PubMed] [Google Scholar]
- 522.Anonymous. 1985. Suboptimal response to hepatitis B vaccine given by injection into the buttock. MMWR Morb Mortal Wkly Rep 34:105–108, 113. [PubMed] [Google Scholar]
- 523.Shaw FE Jr, Guess HA, Roets JM, Mohr FE, Coleman PJ, Mandel EJ, Roehm RR Jr, Talley WS, Hadler SC. 1989. Effect of anatomic injection site, age and smoking on the immune response to hepatitis B vaccination. Vaccine 7:425–430. doi: 10.1016/0264-410X(89)90157-6. [DOI] [PubMed] [Google Scholar]
- 524.Weber DJ, Rutala WA, Kenyear SA, Lemon SM. 1986. Response to deltoid muscle injection of hepatitis B vaccine after failure to respond to gluteal injections. JAMA 255:2157. doi: 10.1001/jama.1986.03370160055008. [DOI] [PubMed] [Google Scholar]
- 525.Alves AS, Nascimento CM, Granato CH, Sato HK, Morgato MF, Pannuti CS. 2001. Hepatitis B vaccine in infants: a randomized controlled trial comparing gluteal versus anterolateral thigh muscle administration. Rev Inst Med Trop Sao Paulo 43:139–143. doi: 10.1590/S0036-46652001000300004. [DOI] [PubMed] [Google Scholar]
- 526.Shi N, Luo F, Li L, Zheng D, Zhang Z, Wang Z, Yang L, Liu Z, Ai X, Bai Y, Lu Q. 2013. A randomized, controlled, blinded study of the safety and immunogenicity of Haemophilus influenzae type b conjugate vaccine injected at different intramuscular sites in Chinese infants. Hum Vaccin Immunother 9:2311–2315. doi: 10.4161/hv.25526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Cardell K, Fryden A, Normann B. 1999. Intradermal hepatitis B vaccination in health care workers. Response rate and experiences from vaccination in clinical practise. Scand J Infect Dis 31:197–200. [DOI] [PubMed] [Google Scholar]
- 528.Mills DJ, Lau CL, Fearnley EJ, Weinstein P. 2011. The immunogenicity of a modified intradermal pre-exposure rabies vaccination schedule—a case series of 420 travelers. J Travel Med 18:327–332. doi: 10.1111/j.1708-8305.2011.00540.x. [DOI] [PubMed] [Google Scholar]
- 529.Warrell MJ, Suntharasamai P, Nicholson KG, Warrell DA, Chanthavanich P, Viravan C, Sinhaseni A, Phanfung R, Xueref C, Vincent-Falquet JC. 1984. Multi-site intradermal and multi-site subcutaneous rabies vaccination: improved economical regimens. Lancet 323:874–876. doi: 10.1016/S0140-6736(84)91340-0. [DOI] [PubMed] [Google Scholar]
- 530.Dubois B, Bridon JM, Fayette J, Barthelemy C, Banchereau J, Caux C, Briere F. 1999. Dendritic cells directly modulate B cell growth and differentiation. J Leukoc Biol 66:224–230. doi: 10.1002/jlb.66.2.224. [DOI] [PubMed] [Google Scholar]
- 531.Playford EG, Hogan PG, Bansal AS, Harrison K, Drummond D, Looke DF, Whitby M. 2002. Intradermal recombinant hepatitis B vaccine for healthcare workers who fail to respond to intramuscular vaccine. Infect Control Hosp Epidemiol 23:87–90. doi: 10.1086/502012. [DOI] [PubMed] [Google Scholar]
- 532.Leonardi S, Del Giudice MM, Spicuzza L, Spina M, La Rosa M. 2010. Hepatitis B vaccine administered by intradermal route in patients with celiac disease unresponsive to the intramuscular vaccination schedule: a pilot study. Am J Gastroenterol 105:2117–2119. doi: 10.1038/ajg.2010.195. [DOI] [PubMed] [Google Scholar]
- 533.Charest AF, McDougall J, Goldstein MB. 2000. A randomized comparison of intradermal and intramuscular vaccination against hepatitis B virus in incident chronic hemodialysis patients. Am J Kidney Dis 36:976–982. doi: 10.1053/ajkd.2000.19099. [DOI] [PubMed] [Google Scholar]
- 534.Diggle L, Deeks JJ, Pollard AJ. 2006. Effect of needle size on immunogenicity and reactogenicity of vaccines in infants: randomised controlled trial. BMJ 333:571. doi: 10.1136/bmj.38906.704549.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Beirne PV, Hennessy S, Cadogan SL, Shiely F, Fitzgerald T, MacLeod F. 2015. Needle size for vaccination procedures in children and adolescents. Cochrane Database Syst Rev 2015:CD010720. doi: 10.1002/14651858.CD010720.pub2. [DOI] [PubMed] [Google Scholar]
- 536.Long JE, Drayson MT, Taylor AE, Toellner KM, Lord JM, Phillips AC. 2016. Morning vaccination enhances antibody response over afternoon vaccination: a cluster-randomised trial. Vaccine 34:2679–2685. doi: 10.1016/j.vaccine.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Langlois PH, Smolensky MH, Glezen WP, Keitel WA. 1995. Diurnal variation in responses to influenza vaccine. Chronobiol Int 12:28–36. doi: 10.3109/07420529509064497. [DOI] [PubMed] [Google Scholar]
- 538.Phillips AC, Gallagher S, Carroll D, Drayson M. 2008. Preliminary evidence that morning vaccination is associated with an enhanced antibody response in men. Psychophysiology 45:663–666. doi: 10.1111/j.1469-8986.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 539.Ma SJ, Li X, Xiong YQ, Yao AL, Chen Q. 2015. Combination measles-mumps-rubella-varicella vaccine in healthy children: a systematic review and meta-analysis of immunogenicity and safety. Medicine (Baltimore, MD) 94:e1721. doi: 10.1097/MD.0000000000001721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Leung JH, Hirai HW, Tsoi KK. 2015. Immunogenicity and reactogenicity of tetravalent vaccine for measles, mumps, rubella and varicella (MMRV) in healthy children: a meta-analysis of randomized controlled trials. Expert Rev Vaccines 14:1149–1157. doi: 10.1586/14760584.2015.1057572. [DOI] [PubMed] [Google Scholar]
- 541.Goh P, Lim FS, Han HH, Willems P. 2007. Safety and immunogenicity of early vaccination with two doses of tetravalent measles-mumps-rubella-varicella (MMRV) vaccine in healthy children from 9 months of age. Infection 35:326–333. doi: 10.1007/s15010-007-6337-z. [DOI] [PubMed] [Google Scholar]
- 542.Reisinger KS, Brown ML, Xu J, Sullivan BJ, Marshall GS, Nauert B, Matson DO, Silas PE, Schodel F, Gress JO, Kuter BJ. 2006. A combination measles, mumps, rubella, and varicella vaccine (ProQuad) given to 4- to 6-year-old healthy children vaccinated previously with M-M-RII and Varivax. Pediatrics 117:265–272. doi: 10.1542/peds.2005-0092. [DOI] [PubMed] [Google Scholar]
- 543.Gillet Y, Steri GC, Behre U, Arsene JP, Lanse X, Helm K, Esposito S, Meister N, Desole MG, Douha M, Willems P. 2009. Immunogenicity and safety of measles-mumps-rubella-varicella (MMRV) vaccine followed by one dose of varicella vaccine in children aged 15 months–2 years or 2–6 years primed with measles-mumps-rubella (MMR) vaccine. Vaccine 27:446–453. doi: 10.1016/j.vaccine.2008.10.064. [DOI] [PubMed] [Google Scholar]
- 544.Halperin SA, Ferrera G, Scheifele D, Predy G, Stella G, Cuccia M, Douha M, Willems P. 2009. Safety and immunogenicity of a measles-mumps-rubella-varicella vaccine given as a second dose in children up to six years of age. Vaccine 27:2701–2706. doi: 10.1016/j.vaccine.2009.02.044. [DOI] [PubMed] [Google Scholar]
- 545.Lalwani S, Chatterjee S, Balasubramanian S, Bavdekar A, Mehta S, Datta S, Povey M, Henry O. 2015. Immunogenicity and safety of early vaccination with two doses of a combined measles-mumps-rubella-varicella vaccine in healthy Indian children from 9 months of age: a phase III, randomised, non-inferiority trial. BMJ Open 5:e007202. doi: 10.1136/bmjopen-2014-007202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Nolan T, Bernstein DI, Block SL, Hilty M, Keyserling HL, Marchant C, Marshall H, Richmond P, Yogev R, Cordova J, Cho I, Mendelman PM. 2008. Safety and immunogenicity of concurrent administration of live attenuated influenza vaccine with measles-mumps-rubella and varicella vaccines to infants 12 to 15 months of age. Pediatrics 121:508–516. doi: 10.1542/peds.2007-1064. [DOI] [PubMed] [Google Scholar]
- 547.Nascimento Silva JR, Camacho LAB, Siqueira MM, Freire MDS, Castro YP, Maia MDLS, Yamamura AMY, Martins RM, Leal MDLF. 2011. Mutual interference on the immune response to yellow fever vaccine and a combined vaccine against measles, mumps and rubella. Vaccine 29:6327–6334. doi: 10.1016/j.vaccine.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 548.Monath TP, Guirakhoo F, Nichols R, Yoksan S, Schrader R, Murphy C, Blum P, Woodward S, McCarthy K, Mathis D, Johnson C, Bedford P. 2003. Chimeric live, attenuated vaccine against Japanese encephalitis (ChimeriVax-JE): phase 2 clinical trials for safety and immunogenicity, effect of vaccine dose and schedule, and memory response to challenge with inactivated Japanese encephalitis antigen. J Infect Dis 188:1213–1230. doi: 10.1086/378356. [DOI] [PubMed] [Google Scholar]
- 549.Nasveld PE, Marjason J, Bennett S, Aaskov J, Elliott S, McCarthy K, Kanesa-Thasan N, Feroldi E, Reid M. 2010. Concomitant or sequential administration of live attenuated Japanese encephalitis chimeric virus vaccine and yellow fever 17D vaccine: randomized double-blind phase II evaluation of safety and immunogenicity. Hum Vaccin 6:906–914. doi: 10.4161/hv.6.11.12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Black SB, Cimino CO, Hansen J, Lewis E, Ray P, Corsaro B, Graepel J, Laufer D. 2006. Immunogenicity and safety of measles-mumps-rubella, varicella and Haemophilus influenzae type b vaccines administered concurrently with a fourth dose of heptavalent pneumococcal conjugate vaccine compared with the vaccines administered without heptavalent pneumococcal conjugate vaccine. Pediatr Infect Dis J 25:306–311. doi: 10.1097/01.inf.0000207409.92198.6f. [DOI] [PubMed] [Google Scholar]
- 551.Blatter MM, Klein NP, Shepard JS, Leonardi M, Shapiro S, Schear M, Mufson MA, Martin JM, Varman M, Grogg S, London A, Cambron P, Douha M, Nicholson O, da Costa C, Innis BL. 2012. Immunogenicity and safety of two tetravalent (measles, mumps, rubella, varicella) vaccines coadministered with hepatitis A and pneumococcal conjugate vaccines to children twelve to fourteen months of age. Pediatr Infect Dis J 31:e133–e140. doi: 10.1097/INF.0b013e318259fc8a. [DOI] [PubMed] [Google Scholar]
- 552.Yetman RJ, Shepard JS, Duke A, Stek JE, Petrecz M, Klopfer SO, Kuter BJ, Schodel FP, Lee AW. 2013. Concomitant administration of hepatitis A vaccine with measles/mumps/rubella/varicella and pneumococcal vaccines in healthy 12- to 23-month-old children. Hum Vaccin Immunother 9:1691–1697. doi: 10.4161/hv.24873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Vesikari T, Karvonen A, Lindblad N, Korhonen T, Lommel P, Willems P, Dieussaert I, Schuerman L. 2010. Safety and immunogenicity of a booster dose of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine coadministered with measles-mumps-rubella-varicella vaccine in children aged 12 to 16 months. Pediatr Infect Dis J 29:e47–e56. doi: 10.1097/INF.0b013e3181dffabf. [DOI] [PubMed] [Google Scholar]
- 554.Durando P, Esposito S, Bona G, Cuccia M, Desole MG, Ferrera G, Gabutti G, Pellegrino A, Salvini F, Henry O, Povey M, Marchetti F. 2016. The immunogenicity and safety of a tetravalent measles-mumps-rubella-varicella vaccine when co-administered with conjugated meningococcal C vaccine to healthy children: a phase IIIb, randomized, multi-center study in Italy. Vaccine 34:4278–4284. doi: 10.1016/j.vaccine.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 555.Dhillon S, Curran MP. 2008. Live attenuated measles, mumps, rubella, and varicella zoster virus vaccine (Priorix-Tetra). Paediatr Drugs 10:337–347. doi: 10.2165/00148581-200810050-00007. [DOI] [PubMed] [Google Scholar]
- 556.Halasa NB, O’Shea A, Shi JR, LaFleur BJ, Edwards KM. 2008. Poor immune responses to a birth dose of diphtheria, tetanus, and acellular pertussis vaccine. J Pediatr 153:327–332. doi: 10.1016/j.jpeds.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Zimmermann P, Curtis N. 2018. The influence of BCG on vaccine responses—a systematic review. Expert Rev Vaccines 17:547–554. doi: 10.1080/14760584.2018.1483727. [DOI] [PubMed] [Google Scholar]
- 558.Emperador DM, Velasquez DE, Estivariz CF, Lopman B, Jiang B, Parashar U, Anand A, Zaman K. 2016. Interference of monovalent, bivalent, and trivalent oral poliovirus vaccines on monovalent rotavirus vaccine immunogenicity in rural Bangladesh. Clin Infect Dis 62:150–156. doi: 10.1093/cid/civ807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Patel M, Steele AD, Parashar UD. 2012. Influence of oral polio vaccines on performance of the monovalent and pentavalent rotavirus vaccines. Vaccine 30(Suppl 1):A30–A35. doi: 10.1016/j.vaccine.2011.11.093. [DOI] [PubMed] [Google Scholar]
- 560.Zaman K, Sack DA, Yunus M, Arifeen SE, Podder G, Azim T, Luby S, Breiman RF, Neuzil K, Datta SK, Delem A, Suryakiran PV, Bock HL. 2009. Successful co-administration of a human rotavirus and oral poliovirus vaccines in Bangladeshi infants in a 2-dose schedule at 12 and 16 weeks of age. Vaccine 27:1333–1339. doi: 10.1016/j.vaccine.2008.12.059. [DOI] [PubMed] [Google Scholar]
- 561.Labadie J, Sundermann LC, Ru¨mke HC, DPT-IPV Hib Vaccine Study Group. 1996. Multi-center study on the simultaneous administration of DPT-IPV and Hib PRP-T vaccines. Part 1. Immunogenicity. RIVM report 124001003. National Institute of Public Health and the Environment; Bilthoven, The Netherlands. [Google Scholar]
- 562.Bar-On ES, Goldberg E, Hellmann S, Leibovici L. 2012. Combined DTP-HBV-HIB vaccine versus separately administered DTP-HBV and HIB vaccines for primary prevention of diphtheria, tetanus, pertussis, hepatitis B and Haemophilus influenzae B (HIB). Cochrane Database Syst Rev 2012:CD005530. doi: 10.1002/14651858.CD005530.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Das RR, Panigrahi I, Naik SS. 2014. The effect of prophylactic antipyretic administration on post-vaccination adverse reactions and antibody response in children: a systematic review. PLoS One 9:e106629. doi: 10.1371/journal.pone.0106629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Wysocki J, Center KJ, Brzostek J, Majda-Stanislawska E, Szymanski H, Szenborn L, Czajka H, Hasiec B, Dziduch J, Jackowska T, Witor A, Kopińska E, Konior R, Giardina PC, Sundaraiyer V, Patterson S, Gruber WC, Scott DA, Gurtman A. 2017. A randomized study of fever prophylaxis and the immunogenicity of routine pediatric vaccinations. Vaccine 35:1926–1935. doi: 10.1016/j.vaccine.2017.02.035. [DOI] [PubMed] [Google Scholar]
- 565.Black S, Nicolay U, Del Giudice G, Rappuoli R. 2016. Influence of statins on influenza vaccine response in elderly individuals. J Infect Dis 213:1224–1228. doi: 10.1093/infdis/jiv456. [DOI] [PubMed] [Google Scholar]
- 566.Robinson MJ, Campbell F, Powell P, Sims D, Thornton C. 1999. Antibody response to accelerated Hib immunisation in preterm infants receiving dexamethasone for chronic lung disease. Arch Dis Child Fetal Neonatal Ed 80:F69–F71. doi: 10.1136/fn.80.1.F69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Aaby P, Martins CL, Garly ML, Rodrigues A, Benn CS, Whittle H. 2012. The optimal age of measles immunisation in low-income countries: a secondary analysis of the assumptions underlying the current policy. BMJ Open 2:e000761 10.1136/bmjopen-2011-000761. doi: 10.1136/bmjopen-2011-000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.van der Sande MA, Waight P, Mendy M, Rayco-Solon P, Hutt P, Fulford T, Doherty C, McConkey SJ, Jeffries D, Hall AJ, Whittle HC. 2006. Long-term protection against carriage of hepatitis B virus after infant vaccination. J Infect Dis 193:1528–1535. doi: 10.1086/503433. [DOI] [PubMed] [Google Scholar]
- 569.Ovsyannikova IG, Pankratz VS, Vierkant RA, Jacobson RM, Poland GA. 2006. Human leukocyte antigen haplotypes in the genetic control of immune response to measles-mumps-rubella vaccine. J Infect Dis 193:655–663. doi: 10.1086/500144. [DOI] [PubMed] [Google Scholar]
- 570.Watanabe H, Okumura M, Hirayama K, Sasazuki T. 1990. HLA-Bw54-DR4-DRw53-DQw4 haplotype controls nonresponsiveness to hepatitis-B surface antigen via CD8-positive suppressor T cells. Tissue Antigens 36:69–74. doi: 10.1111/j.1399-0039.1990.tb01802.x. [DOI] [PubMed] [Google Scholar]
- 571.Egea E, Iglesias A, Salazar M, Morimoto C, Kruskall MS, Awdeh Z, Schlossman SF, Alper CA, Yunis EJ. 1991. The cellular basis for lack of antibody response to hepatitis B vaccine in humans. J Exp Med 173:531–538. doi: 10.1084/jem.173.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Marseglia G, Alibrandi A, d’Annunzio G, Gulminetti R, Avanzini MA, Marconi M, Tinelli C, Lorini R. 2000. Long term persistence of anti-HBs protective levels in young patients with type 1 diabetes after recombinant hepatitis B vaccine. Vaccine 19:680–683. doi: 10.1016/S0264-410X(00)00268-1. [DOI] [PubMed] [Google Scholar]
- 573.Schmid S, Molteni A, Fuchtenbusch M, Naserke HE, Ziegler AG, Bonifacio E. 2002. Reduced IL-4 associated antibody responses to vaccine in early pre-diabetes. Diabetologia 45:677–685. doi: 10.1007/s00125-002-0816-7. [DOI] [PubMed] [Google Scholar]
- 574.Smolen P, Bland R, Heiligenstein E, Lawless MR, Dillard R, Abramson J. 1983. Antibody response to oral polio vaccine in premature infants. J Pediatr 103:917–919. doi: 10.1016/S0022-3476(83)80714-8. [DOI] [PubMed] [Google Scholar]
- 575.Krugman S, Warren J, Eiger MS, Berman PH, Michaels RM, Sabin AB. 1961. Immunization with live attenuated poliovirus vaccine. Am J Dis Child 101:23–29. [DOI] [PubMed] [Google Scholar]
- 576.Greenwood BM, Bradley-Moore AM, Bradley AK, Kirkwood BR, Gilles HM. 1986. The immune response to vaccination in undernourished and well-nourished Nigerian children. Ann Trop Med Parasitol 80:537–544. doi: 10.1080/00034983.1986.11812062. [DOI] [PubMed] [Google Scholar]
- 577.Karimi M, Raee A, Baghianimoghadam B, Fallahzadeh MH. 2013. Vaccine-induced anti-HBs level in 5-6 year-old malnourished children. Hepat Mon 13:e7048. doi: 10.5812/hepatmon.7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Ifekwunigwe AE, Grasset N, Glass R, Foster S. 1980. Immune responses to measles and smallpox vaccinations in malnourished children. Am J Clin Nutr 33:621–624. doi: 10.1093/ajcn/33.3.621. [DOI] [PubMed] [Google Scholar]
- 579.Ekunwe EO. 1985. Malnutrition and seroconversion following measles immunization. J Trop Pediatr 31:290–291. doi: 10.1093/tropej/31.6.290. [DOI] [PubMed] [Google Scholar]
- 580.Marshall R, Habicht JP, Landrigan PJ, Foege WH, Delgado H. 1974. Effectiveness of measles vaccine given simultaneously with DTP. J Trop Pediatr Environ Child Health 20:126–129. [DOI] [PubMed] [Google Scholar]
- 581.Kielmann AA, Uberoi IS, Chandra RK, Mehra VL. 1976. The effect of nutritional status on immune capacity and immune responses in preschool children in a rural community in India. Bull World Health Organ 54:477–483. [PMC free article] [PubMed] [Google Scholar]
- 582.Ballet JJ, Agrapart M, Monjour L, Bourdillon F, Karam M, Kyelem JM, Stoeckel P. 1982. Humoral and cellular immunity following antitetanus vaccination in malnourished and malaria-induced African children. 2. In vitro study of non-specific and specific cellular responses to tetanus anatoxin. Bull World Health Organ 60:597–604. [PMC free article] [PubMed] [Google Scholar]