Gut bacteria play a key role in initiating and maintaining the inflammatory process in the gut tissues of inflammatory bowel disease (IBD) patients, by supplying antigens or other stimulatory factors that trigger immune cell activation. Changes in the composition of the intestinal microbiota in IBD patients compared to that in healthy controls and a reduced diversity of intestinal microbial species are linked to the pathogenesis of IBD.
KEYWORDS: carcinoembryonic antigen-related cell adhesion molecules 6, Crohn’s disease, diet, Escherichia coli, tight junction, ulcerative colitis, inflammatory bowel disease, interleukins, probiotics, tumor necrosis factor receptors
SUMMARY
Gut bacteria play a key role in initiating and maintaining the inflammatory process in the gut tissues of inflammatory bowel disease (IBD) patients, by supplying antigens or other stimulatory factors that trigger immune cell activation. Changes in the composition of the intestinal microbiota in IBD patients compared to that in healthy controls and a reduced diversity of intestinal microbial species are linked to the pathogenesis of IBD. Adherent invasive Escherichia coli (AIEC) has been linked to Crohn’s disease (CD) patients, while diffusely adherent E. coli (DAEC) has been associated with ulcerative colitis (UC). Bacteriological analysis of intestinal biopsy specimens and fecal samples from IBD patients shows an increased number of E. coli strains belonging to the B2 phylogenetic group, which are typically known as extraintestinal pathogenic E. coli (ExPEC). Results from studies of both cell cultures and animal models reveal pathogenic features of these E. coli pathobionts, which may link them to IBD pathogenesis. This suggests that IBD-associated E. coli strains play a facilitative role during IBD flares. In this review, we explain IBD-associated E. coli and its role in IBD pathogenesis.
INTRODUCTION
Inflammatory bowel disease (IBD) is a family of chronic inflammatory diseases of the gastrointestinal tract. IBD has traditionally been divided into Crohn’s disease (CD) and ulcerative colitis (UC) (1). Crohn’s disease and ulcerative colitis are differentiated by their clinical manifestations and hypothesized pathogenic mechanisms. UC is a relapsing, nontransmural, chronic inflammatory disease that is restricted to the colon and during flares is characterized by bloody diarrhea. CD is a chronic, segmental, localized granulomatous disease that can affect all parts along the entire gastrointestinal tract, from the mouth to the anus. The clinical presentation depends on disease location and may include diarrhea, abdominal pain, fever, clinical signs of bowel obstruction, and anal passage of blood, mucus, or both (1).
Ulcerative colitis and Crohn’s disease may appear at any age, but most patients are diagnosed in their third decade of life (2). The prevalence of ulcerative colitis and Crohn’s disease is highest in the industrialized Western countries and has increased 2- to 3-fold in Europe and the United States since the early 1970s (2, 3). The prevalence of IBD in Northern Europe varies from 35 to 50 cases per 100,000 inhabitants for ulcerative colitis and from 30 to 100 cases per 100,000 inhabitants for Crohn’s disease (3). The prevalence of IBD in the United States was recently estimated to be as high as 241.3 and 263 cases of CD and UC per 100,000 people, respectively (4).
The exact etiology of IBD is still unclear, but studies indicate several possible links to genetics (5), immunology (6–8), nutrition (9, 10), bacteria (11, 12), viruses (13, 14), and other environmental factors (15, 16). Animal model studies suggest that inflammation in IBD patients most likely arises as a result of either exaggerated effector T-cell function or poor regulatory T-cell function, leading to the overproduction of proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) and interleukin-12 (IL-12), and/or the impaired production or function of known regulatory/immunosuppressive cytokines, such as IL-10 (6, 17). The gut microbiota is necessary to initiate or maintain the intestinal inflammatory process by providing antigens or other stimulatory factors, which can trigger inflammatory defenses that prove maladaptive in IBD (6). However, so far, there is no specific pathogenic microorganism directly linked to IBD (6). Experimental models have repeatedly revealed that the intestinal microbiota plays a role in IBD, while placebo-controlled studies of antibiotic treatment show some benefit in promoting remission of IBD. However, most of the clinical trials of antibiotic treatment of IBD published so far have involved limited numbers of patients treated for only a short time. To define the effect of antibiotics in the management of IBD, more randomized controlled trials (RCTs) of antibiotics need to be carried out (18–20). Studies have also noted an aberrant fecal microbiota in IBD patients compared to that found in healthy controls, while a reduced diversity of the conventional intestinal microbiota has been linked to IBD (21–23). Interestingly, bacteriological analyses of biopsy specimens and fecal samples from IBD patients show increased numbers of Escherichia coli isolates belonging to the B2 phylogenetic group that harbors extraintestinal pathogenic E. coli (ExPEC) genes (24, 25). In this review, we define IBD-associated E. coli and evaluate its potential role in IBD pathogenesis, disease relapses, and remission. For this purpose, the focus is on both pathogenic E. coli strains known to cause clinical disease and E. coli strains that are better described as pathobionts, which are bacteria linked to immune-mediated diseases and depending on genetic defects or other environmental factors causing disease.
INFLAMMATORY BOWEL DISEASE AND THE GUT MICROBIOTA
Because IBD is an inflammatory disease of the gastrointestinal tract, it has been speculated that luminal factors are involved. Therefore, gastrointestinal bacteria are frequently suspected as the cause of IBD relapses. Some IBD patients experience clinical improvement when they receive antibiotics, such as ciprofloxacin or rifaximin (26). Microbiological findings for IBD patients with active disease show a reduction of the resident aerobic and anaerobic microbiota, such as Faecalibacterium prausnitzii, belonging to clostridial cluster IV (27) (compared to that in healthy controls), and an increase in potentially pathogenic microorganisms, such as Klebsiella, Enterobacter, Proteus, and fungi (19). Studies also show a significant reduction in lactobacilli and bifidobacteria as well as an increase in Bacteroides in the intestines of IBD patients (22). The decreased prevalence of lactobacilli and bifidobacteria might play an important role in the etiology of IBD, since these bacteria have immunoregulatory effects and therefore contribute to intestinal host defenses through their interactions with the immune system (28, 29). The reduced prevalence of butyrate-producing bacteria (such as those belonging to the clostridial group) in the guts of IBD patients with active disease leads to reduced levels of butyrate. This may worsen IBD, since butyrate normally serves as an inhibitor of proinflammatory cytokine expression in the intestinal mucosa as well as a stimulator of mucin and antimicrobial peptide production and a strengthener of epithelial barrier integrity by increasing the expression of tight junction (TJ) proteins (30).
Several microorganisms have been suggested to play a role in the pathogenesis of IBD. Mycoplasma spp. (31), Mycobacterium spp. (32), Clostridioides difficile (33), Salmonella spp. (34), Listeria monocytogenes (35), Aeromonas hydrophila (36), Proteus spp. (37), E. coli (38, 39), and some viruses (40) have all been linked to IBD and are suspected to play a causal role in disease relapses. On looking at these possible IBD pathogenesis links, it is currently unresolved whether the major part is played by specific microorganisms or by the reduced diversity of the microbiota as such. Interestingly, a recent paper described both reduced microbiota diversity and an increased frequency of virulence markers primarily linked to E. coli as being associated with both ulcerative colitis and Crohn’s disease (41).
E. COLI IN INTESTINAL DISORDERS
E. coli is a predominantly facultative anaerobic Gram-negative bacterium which colonizes the intestinal tract of human infants immediately after birth and helps to maintain normal intestinal homeostasis (42). E. coli strains are classified—on the basis of genetic and clinical criteria—into the following three major groups: (i) commensal strains found in the human and animal gut (lacking specialized virulence factors), (ii) intestinal pathogenic strains (diarrheagenic), and (iii) extraintestinal pathogenic E. coli (ExPEC) (43). While the diarrheagenic E. coli strains have not been linked to IBD, they have been clearly shown to promote intestinal inflammation and pathophysiology. Six well-known intestinal pathogenic E. coli types are enteropathogenic E. coli (EPEC), Shiga toxin-producing E. coli (STEC), enterotoxigenic E. coli (ETEC), enteroaggregative E. coli (EAEC), enteroinvasive E. coli (EIEC), and diffusely adherent E. coli (DAEC) (44). These E. coli strains cause gastrointestinal diseases ranging from self-limiting diarrhea to hemorrhagic colitis (44).
EPEC
EPEC was the first pathotype of E. coli described (in 1945) and was isolated from the intestines of infants with diarrhea in the United Kingdom (44, 45). EPEC adheres to epithelial cells via the BFP type IV pilus (46, 47) followed by activation of its type III secretion system. As a result of this activation, protein kinase C, protein tyrosine kinase(s), phospholipase Cγ, myosin light chain kinase, and mitogen-activated protein (MAP) accumulate under the attached bacteria (46). Thereafter, various effector proteins—including EspF, Map, EspG, EspH, and Tir—are translocated into the infected host cell, which increases intracellular calcium (Ca2+) and triggers depolymerization of the microvillus actin, leading to the formation of the characteristic pedestal complex (48, 49). This leads to increased permeability within the intestinal epithelium due to loosened tight junctions and the activation of NF-κB, followed by production of IL-8 and the transmigration of polymorphonuclear leukocytes (PMNs) across the epithelium and into the intestinal lumen (44). Diarrhea usually results from increased ion secretion, increased intestinal permeability, intestinal inflammation, and a loss of absorptive surface area resulting from microvillus effacement (44).
ETEC
ETEC is primarily associated with high mortality in children under 5 years of age, as well as being a frequent cause of diarrhea in tourists visiting developing countries (50). The reasons for ETEC infections occurring predominantly in countries with warm climates are still unknown, but it is likely that water contaminated by human or animal sewage is an important means of spreading the infection (50). ETEC strains produce a heat-stable enterotoxin (ST) and a heat-labile cholera toxin-like enterotoxin (LT) (44). The heat-labile toxin consists of an A subunit and five identical B subunits. The B subunits promote binding of the holotoxin to the cell surface gangliosides GM1 and GD1b, while the A subunit promotes the enzymatic activity of the toxin (44, 51). The heat-stable enterotoxin STb has also been associated with human disease (44). It stimulates the intestinal brush border guanylate cyclase C (GC-C) receptor, which increases levels of the intracellular messenger cyclic GMP (50). Cyclic GMP mediates reduced absorption of sodium and chloride ions and increased secretion of bicarbonate and chloride ions, ultimately resulting in watery diarrhea (50). Colonization factor CFA/I also plays an important role in the pathogenesis of STh-ETEC diarrhea by facilitating microbial adherence to the intestinal mucosa.
EIEC
Molecular studies and phenotyping results have shown that E. coli strains are closely related (sister species) to Shigella spp. (52). In fact, enteroinvasive E. coli (EIEC) shares many properties with Shigella, including virulence mechanisms (53). They both possess a large invasion plasmid encoding the Mxi-Spa type III secretion system and invasion plasmid antigen (Ipa) effectors, which enable bacterial invasion of eukaryotic cells (53). The plasmid also encodes IcsA, which enables bacteria to spread to other cells in vivo while avoiding the immune system (53). EIEC/Shigella pathogenesis mechanisms initially involve epithelial cell penetration followed by lysis of the endocytic vacuole, intracellular replication, directional movement through the cytoplasm, extension into/invasion of adjacent epithelial cells (54), and, finally, the induction of apoptosis in infected macrophages and the release of IL-1β (55). EIEC may on occasion cause inflammatory colitis, dysentery, and watery diarrhea (55).
EAEC
EAEC is associated with chronic diarrheal disease in developing countries and in immunocompromised patients (45, 55). EAEC was first defined in 1987 (56) as an E. coli strain with the ability to adhere to Hep-2 cells in a stacked-brick-like configuration (57). EAEC pathogenic mechanisms initially involve bacterial adherence to the intestinal mucosa via aggregative adherence fimbriae (AAFs). EAEC surface structures along with its release of flagellin cause inflammation by inducing the release of the chemokine IL-8, stimulating neutrophil transmigration across the epithelium and, as a result, tissue damage (58). The aggR gene in EAEC regulates expression of multiple virulence factors, such as AAFs, as well as the ability to form biofilms (e.g., see reference 59). The toxins associated with EAEC are Pic, an autotransporter protease with mucinase activity (60); EAST1, a homologue of STa toxin which can cause watery diarrhea (61); and Pet, an autotransporter with enterotoxic activity which causes cytoskeletal changes (62).
STEC/Verocytotoxigenic E. coli (VTEC)
STEC is best known as the cause of hemorrhagic colitis and hemolytic-uremic syndrome (HUS), for which contaminated food is an important source of infection (63). Escherichia coli producing a toxin similar to Shiga toxin (Stx; produced by Shigella dysenteriae) is a common cause of HUS in children and often manifests with bloody diarrhea and acute renal failure (64, 65). STEC was first discovered in 1982, when Karmali et al. (66) found increased Stx activity in the fecal filtrates of children with HUS who were infected with E. coli serotype O157:H7. Later, additional STEC serotypes were discovered, such as serotypes O145 and O121 (64, 67). The pathogenic mechanisms of STEC start when Stx-E. coli is ingested and then adheres to gastrointestinal epithelial cells via the bacterial outer membrane protein intimin (68), similar to the adherence mechanisms of EPEC. The STEC toxin is then transported into the kidney via blood or by transmigration of neutrophils (PMNs) (69), or it binds to blood platelets (68) and erythrocytes (70).
ExPEC
ExPEC causes diseases outside the gut, such as in the urinary tract, and also causes infections of the central nervous system, the circulatory system, and the respiratory tract (43, 71, 72). Clermont et al. (73) divided E. coli into four main phylogenetic groups, A, B1, B2, and D, based on the following three genes: chuA (heme transport gene), yjaA (unknown function), and TspE4.C2 (anonymous DNA fragments). The most virulent extraintestinal E. coli strains belong to phylogenetic group B2 (harboring chuA and yjaA). The E. coli strains displaying less virulence during extraintestinal infections belong to phylogenetic group D (harboring yjaA), and finally, the various commensal strains of E. coli belong to groups A (harboring none of the above-mentioned genes) and B1 (harboring TspE4.C2) (73). Recently, E. coli phylogenetic groups E (harboring the arpA [unknown function], chuA, and TspE4.C2 genes) and F (harboring chuA), known as sister groups to phylogenetic group B2, and phylogenetic group C (harboring the arpA and yjaA genes), closely related to phylogenetic group B1, were described (74).
Johnson et al. (75) defined E. coli isolates harboring at least two of the following virulence genes as ExPEC strains (76): sfa/foc (S and FIC fimbria subunit), papA or papC (P fimbriae), afa/dra (Dr-antigen-binding adhesins), iutA (aerobactin; iron acquisition system), and kpsMTII (capsule; host defense avoidance mechanisms). Virulence markers such as hly (toxin; hemolysin) and/or ompT (outer membrane protease T subunit) have also been linked to ExPEC strains (77).
E. COLI STRAINS ASSOCIATED WITH CROHN’S DISEASE
Since the 1970s, E. coli has been suspected as a possible reason for the onset of disease in IBD patients (78). Several studies have found increased numbers of E. coli strains with virulence properties isolated from IBD patients compared to those from healthy controls, especially when focusing on IBD patients during disease relapses (78, 79). In 1978, Keighley et al. (80) observed a modification of luminal bacterial concentrations in CD patients, with evidence of a dramatic increase in E. coli strains. Burke and Axon (78) showed a significantly larger proportion of adhesive E. coli strains present in active CD (CDA) patients than in a control group (79). Ilnyckyj et al. (81) published a case report in which infection with E. coli O157:H7 (82) mimicked right-sided colonic CD. In 1998, Darfeuille-Michaud et al. (83) showed a high prevalence of E. coli isolated from ileal biopsy specimens from CD patients, i.e., 100% prevalence in early lesions and 65% prevalence in chronic lesions. These findings suggested that E. coli might participate in initiation as well as acting as a chronic promoter of the inflammatory processes in CD.
Martin et al. (84) showed increased mucosa-associated Gram-negative bacteria in colonic biopsy specimens obtained from patients with CD, of which 73% were identified as E. coli. A number of studies indicate that there is a link between the prevalence of E. coli and IBD relapses (85, 86). One of the histological characteristics of CD is the presence of epithelioid granulomatous inflammation of the intestine (87, 88). Adherent invasive E. coli (AIEC), which is linked to CD, causes the same histological characteristic in vitro, such as forming multinucleated giant cells, along with the subsequent recruitment of lymphocytes (89).
Epithelium-associated invasive E. coli has frequently been isolated from the ileal and colonic mucosa of CD patients and has been shown to often possess the ability to bind to intestinal epithelial cell monolayers as well as to synthesize alpha-hemolysin (90, 91). Colonic biopsy specimens from patients with CD show specific pathogenic strains of E. coli with the ability to infect and invade host cells, where they multiply and damage host tissues (85, 86).
In 1976, Schussler et al. (91) showed a significant elevation of antibody titers against the lipid A and O antigens of E. coli in CD patient groups compared to those in healthy controls as well as those in UC and acute enteritis groups and suggested that these titers may function as a potential marker to differentiate CD and UC. E. coli antigens have been detected in 57% of CD patient biopsy or resection specimens (35), while polyclonal antibodies against E. coli were detected in macrophages within the lamina propria, in the germinal centers of mesenteric lymph nodes, and in giant cells along fissures, below ulcers, and in granulomas. Additionally, increased numbers of antibodies against the E. coli outer membrane protein C were detected in 37 to 55% of patients with CD (92, 93). Most of these CD studies are association studies that do not clarify if the infection with AIEC or the intestinal inflammation came first.
E. COLI ASSOCIATED WITH ULCERATIVE COLITIS
Tabaqchal et al. (94) showed that the majority of IBD patients display increased positive antibody reactions to a variety of Escherichia coli O antigens, such as those of the O1, O2, O6, O18 (O18ac and O18ab), and O75 serotypes, compared to those of a control group. These serotypes are mostly associated with urinary tract infections and originate from the fecal microbiota (82, 94). Serotyping of E. coli strains isolated from IBD patients showed that 83% of E. coli isolates from active UC (UCA) cases, 33% from inactive UC (UCI) cases, 50% from CDA cases, and 33% from inactive CD (CDI) cases harbor O1, O2, O6, O18 (O18ab and O18ac), or O75 genes, which are linked to urinary tract infections and thereby belong to the ExPEC group (25). However, only 22% of E. coli isolates from healthy controls harbored one of the above-mentioned O antigens.
In 1987, Burke and Axon (95) showed that isolated E. coli strains from the stool of UC patients were predominantly diffusely adherent E. coli (DAEC) with both enterotoxigenic (96) and enteropathogenic (97) properties, in contrast to isolates from healthy persons. Bacteriological analysis of rectal biopsy specimens or fecal samples from UCA patients showed distinct variability among the mucosal bacteria and increased numbers of E. coli strains of the B2 and D phylogenetic groups (24, 25). A previous study showed that UCA patients colonized with B2 E. coli display increased burdens of inflammation as measured by the colitis activity index (CAI) and fecal calprotectin levels (98). Mirsepasi-Lauridsen et al. (38) showed that UC-associated E. coli p19A, an ExPEC strain harboring alpha-hemolysin, dissolved the TJ protein occludin in cell lines and disrupted the TJ in Caco-2 cells in vitro, followed by increasing barrier permeability (38). Additionally, the UC-associated E. coli strain p19A induces cell death in dendritic cells and stimulates the release of the cytokines TNF-α, IL-6, and IL-23 (99). As is the case with AIEC and CD, the link between DAEC and/or B2 E. coli is associative, and no data so far reveal a causative link.
ANTIBIOTIC AND PROBIOTIC TREATMENT OF IBD
Since gut bacteria are suspected to play a central role in the pathogenesis of IBD, antibiotics have often been used as a therapeutic option (18, 100, 101). One of the antibiotic combinations used as a therapeutic option for CD is clofazimine together with clarithromycin and rifabutin, since they are effective against Mycobacterium paratuberculosis, which is speculated to be a possible cause of CD (102). Clofazimine together with clarithromycin and rifabutin is effective at inducing remission when used concurrently with a course of corticosteroids. Nevertheless, the combination of antibiotics used concurrently with a course of corticosteroids has several disadvantages (103), such as the masking of any infection by suppressing the symptoms and signs of inflammation as well as an increased risk of bleeding. However, a meta-analysis of the usage of broad-spectrum antibiotics in CD patients showed that metronidazole and ciprofloxacin are the most effective at promoting clinical improvement (20, 104). A 3-month follow-up of E. coli present in the intestine showed that 1 week of ciprofloxacin treatment in patients with UCA was not effective against E. coli strains of the B2 phylogenetic group (98).
Placebo-controlled studies of IBD or irritable bowel syndrome patients indicated that probiotic treatment significantly reduced small bowel permeability (lactulose/mannitol ratio) and induced remission in IBD patients (103, 105). The probiotic E. coli strain Nissle 1917 (19, 106) was isolated during World War I from the feces of a German soldier who seemed to be protected from infectious diarrheal disease (107). Genomic studies of E. coli strain Nissle 1917 showed that in contrast to other commensal E. coli strains, it expresses microcins, adhesins, and at least six different iron uptake systems (including enterobactin, salmochelin, aerobactin, yersiniabactin, and EfeU) for the generation of energy through ATP. Nevertheless, it lacks prominent virulence factors, such as hlyA (108–112).
Studies showed that E. coli Nissle 1917, which interestingly belongs to the B2 phylogenetic group (ExPEC), has immunoregulatory properties, such as decreasing the number of T cells within the intestinal mucosa as well as reducing the secretion of proinflammatory cytokines, such as IL-2, gamma interferon (IFN-γ), and TNF-α, while stimulating the secretion of regulatory proteins, such as IL-10 and IL-1β (112–114). Schultz et al. demonstrated that E. coli Nissle 1917 is effective at preventing colitis in different murine models of colitis (115). Schlee et al. (116) showed that E. coli Nissle 1917 induces the expression of human β-defensin 2 (hBD-2), a human antimicrobial peptide, which helps to reinforce the intestinal mucosal barrier by limiting bacterial adherence as well as bacterial invasion of the gut mucosa. In vivo models have shown that E. coli Nissle 1917 protects against infections with Salmonella enterica (117) and Candida albicans (118). In vitro models using various cells have shown that E. coli Nissle 1917 inhibits invasion by Salmonella enterica, Yersinia enterocolitica, Shigella flexneri, Listeria pneumophila, and L. monocytogenes (119) and invasion of host cells by AIEC (120).
A number of clinical trials suggest that E. coli Nissle 1917 is as effective as mesalazine at maintaining remission in UC patients (121–125), but there are some disputed points to discuss. In a study by Kruis et al. (121), only UC patients with inactive disease or those in remission were included. In a study by Rembacken et al. (125), UC patients with active disease were treated with corticosteroids, which diminish the signs/symptoms of any infection/inflammation that might be caused by the use of E. coli Nissle 1917 as an add-on treatment. Yet a randomized double-blind study of E. coli Nissle 1917 given as an add-on treatment to patients with active UC showed that fewer patients treated with E. coli Nissle 1917 had symptomatic remission and that patients treated with E. coli Nissle 1917 often withdrew from the study (98, 126). Considering these troubling findings, larger studies are needed to confirm any potential beneficial effects of E. coli Nissle 1917 in IBD. Studies show that exposure to antibiotics during pregnancy significantly increases the risk of CD development in children (127). The first year of life is critical for newborn children to develop their gut commensal microbiota. Studies show that the use of antibiotics in the first year of life significantly increases the risk of developing pediatric IBD (128). In addition, antibiotic usage for CD has been shown to decrease the number of beneficial bacteria belonging to genera such as Lactobacillus, Bacteroides, and Bifidobacterium, creating an environment for an increased prevalence of pathogenic bacteria, such as invasive E. coli, to adhere to—and invade—the intestinal epithelium (129). Thus, neither antibiotic nor probiotic studies exist that convincingly clarify if modulation or eradication of E. coli in IBD patients will lead to control of inflammation.
ROLE OF DIET IN CONTROLLING BACTERIAL CONTRIBUTIONS TO IBD
In the last several decades, there has been significant speculation regarding the role of diet and environmental factors in IBD. Food and clean water consumption plays an important role in shaping intestinal bacterial colonization. Diet has a significant influence on the composition of the intestinal microbiota early in life. Studies show that a high daily intake of fast food, which is rich in fats (pork, beef, corn, sunflower oils, and margarines) and digestible sugar, increases the risk of IBD (130). However, diets rich in olive oil, fish, fruits (131), and nondigestible fibers, such as vegetables and whole-wheat bread, seem to be protective against IBD (132). Dietary carbohydrates, starches, and fibers are substrates for fermentation that produce short-chain fatty acids (SCFA), such as acetate, propionate, and butyrate. The rate of SCFA production depends on the species and amount of microbiota in the colon. SCFA have anti-inflammatory effects (133) and contribute to the inhibition of E. coli growth in the gut (134). In vivo studies show that high-fat/high-sugar diets cause microbial dysbiosis, decreased mucus layer thickness, increased permeability, and increased susceptibility to colonization with pathogenic E. coli (135). Modulation of the gut microbiota in IBD influences the levels of critical vitamins and minerals in IBD patients, such as the bioavailability of vitamin K (136). A decreased prevalence of butyrate-producing bacteria, such as Clostridiales species, in IBD patients with active disease explains the decreased amounts of SCFA (such as butyrate) in their fecal samples, which would normally contribute to inhibition of E. coli growth in IBD (134, 137). Butyrate serves as a major source of energy for colonic epithelial cells (138) and as an inhibitor of proinflammatory cytokine expression in the intestinal mucosa (139). One of the most common complications of IBD is anemia, caused by iron, zinc, folate, and vitamin B12 deficiency (140). Zinc is critically involved in DNA replication and transcription and has immunoregulatory effects (141). Iron, folate, and vitamin B12 are critical for hemoglobin/blood cell formation. Decreased levels of vitamin D are associated with IBD (140), since vitamin D regulates the gut barrier function by inducing E-cadherin transcripts in gut epithelial cells (142), suppresses the proliferation of T cells in vitro (143), and induces production of several antimicrobial peptides, such as β-defensins and cathelicidin (144). Nutritional therapy has been shown to be more effective than corticosteroids for healing the mucosa (145). The evidence indicates that nutritional therapy has some prebiotic properties, which enables the modulation of the gut microbiota and regulation of the immune defense in IBD.
IBD AND E. COLI IN IN VIVO MODELS
Overall, IBD animal models can be divided into the following five categories: antigen-induced colitis and colitis induced by microbiota (146), chemically induced forms of colitis (147), genetically modified colitis models (148), adaptive infection models (149, 150), and spontaneous colitis models (151, 152).
The most important discoveries made by use of IBD animal models are that germfree animals generally do not develop intestinal inflammation and that spontaneous gut inflammation requires a certain genetic background. T cells are involved in most IBD animal models, and interactions between T cells and dendritic cells seem to be crucial for the initiation and perpetuation of inflammation (153). Ulceration of lymphoid follicles and the involvement of Peyer’s patches have been reported for CD patients (154, 155). In CD patients, inflammation in Peyer’s patches might be caused by the passage of particulate matter/bacteria from the bowel lumen into the lymphoid tissue of the mucosa and thereby into the lymphatic system of the gut (156). Chassaing et al. (157) showed that AIEC cells harboring long polar fimbriae (LPF) colonize the Peyer’s patches of Nod2−/− mice. Experimental animal models have repeatedly revealed that the intestinal microbiota plays a major role in IBD. In addition, genetically susceptible IBD mouse models indicate a key role for dysfunctional, unregulated, T-cell-mediated immune responses in IBD pathogenesis (158). For example, IL-10 stimulates the development of humoral Th2 cytokine-driven immune responses and prevents development of Th1 immune responses by reducing the ability of macrophages to produce IL-12, which is a key inducer of Th1 immune responses (159). In vivo models have shown that IL-10-deficient mice colonized with nonpathogenic E. coli strains develop distal colitis and produce high levels of IFN-γ and IL-4 as a result (160).
A previous study showed that a UC-associated E. coli strain (p19A) colonized both the intestines and extraintestinal tissues of dextran sulfate sodium-treated C57BL/6 mice, causing systemic infection (161). IBD animal models have so far given us a better understanding of IBD pathogenesis, such as the involvement of the intestinal microbiota and the importance of T-helper cells in driving gut inflammation in vulnerable hosts. In IBD mouse models, commensal E. coli strains do not seem to have the ability to induce and maintain chronic inflammation as IBD-associated E. coli pathobionts; however, more studies are needed to confirm this outcome (161).
SUGGESTED MECHANISMS OF IBD-ASSOCIATED E. COLI PATHOGENESIS
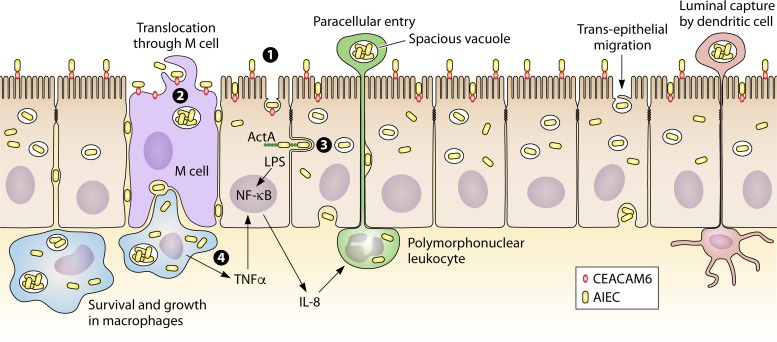
One of the E. coli types linked to CD is AIEC (162). In terms of pathogenesis, it is described that AIEC affects host cell processes such as protein synthesis, signal transduction, cell division, ion secretion, transcription, cytoskeletal function, and mitochondrial function (44, 163). Studies on AIEC show that it is able to adhere to the intestinal mucosa by binding to carcinoembryonic antigen-related cell adhesion molecules 6 (CEACAM6), invade intestinal epithelial cells by using host cell actin microfilaments and microtubules, replicate intracellularly, translocate across the human intestinal barrier, and move into deeper tissues (162, 164–168) (Fig. 1). Studies also show that AIEC is able to survive within macrophages, stimulate TNF-α production, and promote a granulomatous inflammatory response (89, 168). However, at present, there is still little known about the in vivo pathogenesis of AIEC or any genes that are specific for AIEC, as has been described for other E. coli pathogens, such as ETEC and EAEC. So far, the properties/fitness characteristics linked to AIEC are also found in other E. coli strains and therefore do not specifically belong to AIEC. More studies are needed to characterize AIEC as well as its potential role in CD and markers that can be used to specifically identify AIEC.
FIG 1.
Invasion of host cells by AIEC in CD. Abnormal colonization of the ileal mucosa is initiated by the interaction of AIEC with intestinal epithelial cells. (1) AIEC binds to CEACAM6, which is upregulated in the CD patient’s ileum. (2) By using a macropinocytosis-like process, AIEC enters, survives, and replicates inside the host cell cytoplasm after lysis of the endocytic vacuole. (3) By using its invasive ability and host cell actin microfilaments and microtubules, it crosses the intestinal barrier through intestinal epithelial cells or through M cells, invades host cells, and translocates to other cells. (4) AIEC is able to survive extensively within resident macrophages and dendritic cells and induces the secretion of large amounts of TNF-α and granulomatous inflammation.
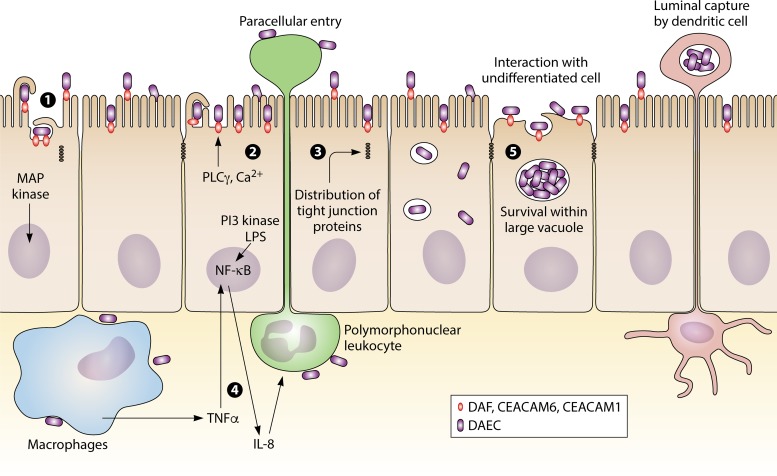
Another E. coli type, associated with UC rather than CD, is DAEC, which is an ExPEC strain expressing afimbrial adhesins (afa) (25, 96, 169). The involvement of DAEC harboring Afa/Dr in diarrhea was controversially demonstrated in polarized monolayers of intestinal T84 cells (170, 171). DAEC has also been shown to adhere to the colonic mucosae of UC patients and to promote proinflammatory responses via the interaction of its bacterial adhesins with membrane-bound host receptors (171). Studies on the pathogenesis of DAEC show that DAEC initiates its interactions with fully differentiated epithelial cells through bacterial recognition of decay/accelerating factor (DAF), CEACAM1, or CEACAM6 (by Afa/DrCEA adhesins) (Fig. 2). Le Bouguenec and Servin showed that DAEC interferes with host cell signaling pathways (169), inducing the rearrangement of brush border-associated F-actin and villin cytoskeletal proteins and the loss of the epithelial cell microvilli (169). It was also shown that DAEC induces the secretion of cytokines, including IL-8, TNF-α, and IL-1β, and induces changes in the distribution of tight junction-associated proteins, which leads to increased paracellular permeability (169). However, there are only limited studies associating DAEC with UC, and there are no epidemiology reports directly linking UC with DAEC. Moreover, there are no specific genes found in DAEC strains that enable one to specifically identify DAEC in UC patients. Clearly, more studies are needed to clarify the potential role of DAEC in pathogenesis of UC.
FIG 2.
Infection with DAEC in UC. (1) Infection with Afa/Dr DAEC starts by bacterial interaction with fully differentiated epithelial cells via bacterial recognition of DAF, CEACAM1, or CEACAM6. (2) DAEC interferes with host cell signaling pathways involving protein tyrosine kinases(s), phospholipase Cγ, phosphatidylinositol 3-kinase (PI3 kinase), and protein kinase C, followed by an increase of Ca2+ in the host cell. Increased Ca2+ in the host cell induces rearrangements of brush border-associated F-actin and villin cytoskeletal proteins, which results in the loss of the epithelial cell microvilli. (3) Changes in the distribution of tight junction-associated proteins leads to paracellular permeability. (4) Activated MAP kinase-dependent signaling pathways induce secretion of cytokines, such as IL-8, TNF-α, and IL-1β, causing an upregulation of DAF and major histocompatibility complex (MHC) class I chain-like gene A. (5) DAEC interacts with undifferentiated cells via recognition of DAF by Afa/Dr adhesins followed by invasion of the host cell. DAEC survives within a large vacuole and spreads to other epithelial cells after host cell apoptosis.
DISCUSSION
Recently developed concepts in IBD pathogenesis include defective innate immune function resulting in diminished bacterial killing as well as functional alterations in the composition of the commensal bacteria, such as E. coli, which in turn leads to their enhanced adherence and invasion of epithelial cells, bacterial persistence within intestinal epithelial and phagocytic cells, and metabolic derangements that negatively influence epithelial cell function.
E. coli possesses three nitrate reductases and three nitric oxide reductases. E. coli strains are thus able to convert nonfermentable nutrients/nitrates to fermentable nitrates, which is a nutritional benefit possessed by only a few bacteria. During IBD or infectious gastroenteritis, the host inflammatory response in the gut generates high levels of nonfermentable nitrate, which can serve as a substrate for nitrate respiration, enabling the overgrowth of commensal E. coli/Enterobacteriaceae in the lumen of the inflamed gut (172). This might explain the reduced intestinal bacterial diversity in IBD patients compared to that in healthy controls (27, 172–174), as it may be caused by intestinal overgrowth of aggressive bacteria in IBD patients which are normally in symbiotic balance with other intestinal bacteria in healthy individuals. Whether nitrate respiration specifically benefits IBD-associated E. coli has yet to be studied. However, reduction of the Enterobacteriaceae level by treatment with tungstate (an inhibitor of molybdenum cofactor-dependent microbial respiratory pathways) reduced the severity of intestinal inflammation in murine models of colitis, suggesting that the inflammation is dysbiosis driven (175).
The potential causal role of IBD-associated E. coli in the pathophysiology of IBD has been linked to the ability to adhere to and invade epithelial cells and multiply within macrophages, such as that seen with the prototypical AIEC strain, LF82. Our studies have shown that UC-associated E. coli (p19A) from the B2 phylogenetic group (ExPEC), harboring two alpha-hemolysin genes, induces cell death in dendritic cells, stimulates the release of the cytokines TNF-α, IL-6, and IL-23 (99), and causes rapid loss of the TJ integrity in differentiated Caco2-cell monolayers (38). The difference between p19A and strain LF82 is that LF82 does not disturb the epithelial TJ, obviously indicating that these two IBD-associated strains, both from the B2 phylogenetic group, differ in their pathogenic mechanisms. However, neither AIEC nor intestinal ExPEC has been well characterized, making it difficult to identify these strains in the course of IBD. So it is still unknown if ExPEC causes intestinal infections, and AIEC is described only by its phenotype, as not a single specific gene is linked to AIEC to enable us to identify AIEC in IBD. Additionally, there is still a lack of epidemiological studies, specifically on the prevalence of AIEC and and/or ExPEC in IBD in relation to disease onset and disease burden. What we have learned from animal models is that a combination of a genetic/immune defect and E. coli pathobionts plays an essential role in IBD.
Taken together, these findings suggest that IBD-associated E. coli might play a role in the pathogenesis of IBD and might also play a role in disease relapses in IBD patients. Additional studies of the gut microbiota and IBD-associated E. coli strains are needed to confirm whether IBD reflects an abnormal host response to commensal bacteria or if the acquisition of pathogenic features by specific E. coli strains drives the onset of IBD. However, clinical experience with treatment effects (though variable) of both immunosuppressants and antibiotics (and probiotics) suggests that the nexus of IBD pathogenesis lies in the interactions between the predisposing host genetic factors and the host immune response to intestinal bacteria. More epidemiology, animal, and treatment studies are still needed to convincingly verify the possibly important role of E. coli pathobionts in IBD pathogenesis.
ACKNOWLEDGMENTS
We are very grateful for funding by the Torben and Alice Frimodts Foundation, Lundbeckfonden, and the Met-Vet-Net Association to H.C.M.-L. and from Copenhagen University to Anders Løbner-Olesen.
We are grateful to Marian Jørgensen for her linguistic review and to Betina Hebbelstrup Jensen for helpful discussions and support.
B.A.V. is the Children with Intestinal and Liver Disorders (CHILD) Foundation Research Chair in Pediatric Gastroenterology.
Biographies

Hengameh Chloé Mirsepasi-Lauridsen completed her M.Sc. (Eng.) in biotechnology at the Technical University of Denmark. As a researcher and clinical project leader at Statens Serum Institut since 2009, she has focused on the clinical implications associated with the intestinal microbiota in inflammatory bowel disease. She obtained her Ph.D. in microbiology with a thesis entitled “Inflammatory Bowel Disease Associated with Virulence Factors in Escherichia coli.”

Bruce Andrew Vallance completed his Ph.D. training in gastrointestinal inflammation at McMaster University’s Intestinal Disease Research Program under the supervision of Stephen Collins and then pursued studies on disease-causing bacteria at the University of British Columbia’s Michael Smith Laboratories with Brett Finlay. As a full professor at the University of British Columbia, Dr. Vallance is well recognized for his expertise in the study and modeling of IBD and enteric bacterial infections and was named the CHILD Foundation Research Scholar in 2003 and the Canada Research Chair in Pediatric Gastroenterology and a Michael Smith Research Scholar in 2004. Dr. Vallance was also named the Canadian Association of Gastroenterology’s Young Investigator in 2007. He has authored more than 120 peer-reviewed papers, over 150 abstracts, and 8 book chapters addressing the mechanisms underlying inflammatory bowel diseases as well as bacterial and parasitic infectious diseases of the gastrointestinal tract.

Karen Angeliki Krogfelt, M.Sc. (Eng.), Ph.D., is an adjunct professor at the Institute of Systems Biology, Technical University of Denmark, DTU, and head of a research unit in the Department of Microbiology, Statens Serum Institut. She is involved in teaching master’s courses and has supervised numerous master’s and Ph.D. students. Her main research activities focus on the pathogenic mechanisms related mainly to intestinal bacterial infections and on assessing the development of the gut microbiota and its relationship to pathogenesis. She also uses characterization of specific virulence factors involved in pathogenesis for the development of diagnostic methods for bacterial infections.

Andreas Munk Petersen completed his M.D. and Ph.D. in gastroenterology at the University of Copenhagen. He completed a residency in internal medicine and gastroenterology at Herlev University Hospital, Denmark, before continuing his employment as a consultant at Hvidovre University Hospital, Denmark. His research focuses on the interfaces between microbiology and gastroenterology.
REFERENCES
- 1.Baumgart DC, Sandborn WJ. 2007. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet 369:1641–1657. doi: 10.1016/S0140-6736(07)60751-X. [DOI] [PubMed] [Google Scholar]
- 2.Loftus CG, Loftus EV Jr, Harmsen WS, Zinsmeister AR, Tremaine WJ, Melton LJ III, Sandborn WJ. 2007. Update on the incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota, 1940–2000. Inflamm Bowel Dis 13:254–261. doi: 10.1002/ibd.20029. [DOI] [PubMed] [Google Scholar]
- 3.Annese V, Latiano A, Andriulli A. 2003. Genetics of inflammatory bowel disease: the beginning of the end or the end of the beginning? Dig Liver Dis 35:442–449. doi: 10.1016/S1590-8658(03)00213-5. [DOI] [PubMed] [Google Scholar]
- 4.Kappelman MD, Moore KR, Allen JK, Cook SF. 2013. Recent trends in the prevalence of Crohn’s disease and ulcerative colitis in a commercially insured US population. Dig Dis Sci 58:519–525. doi: 10.1007/s10620-012-2371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGovern DPB, Kugathasan S, Cho JH. 2015. Genetics of inflammatory bowel diseases. Gastroenterology 149:1163–1176. doi: 10.1053/j.gastro.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouma G, Strober W. 2003. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol 3:521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 7.Nuding S, Fellermann K, Wehkamp J, Stange EF. 2007. Reduced mucosal antimicrobial activity in Crohn’s disease of the colon. Gut 56:1240–1247. doi: 10.1136/gut.2006.118646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelsen JR, Dawany N, Moran CJ, Petersen B-S, Sarmady M, Sasson A, Pauly-Hubbard H, Martinez A, Maurer K, Soong J, Rappaport E, Franke A, Keller A, Winter HS, Mamula P, Piccoli D, Artis D, Sonnenberg GF, Daly M, Sullivan KE, Baldassano RN, Devoto M. 2015. Exome sequencing analysis reveals variants in primary immunodeficiency genes in patients with very early onset inflammatory bowel disease. Gastroenterology 149:1415–1424. doi: 10.1053/j.gastro.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hebuterne X, Filippi J, Al Jaouni R, Schneider S. 2009. Nutritional consequences and nutrition therapy in Crohn’s disease. Gastroenterol Clin Biol 33:S235–S244. doi: 10.1016/S0399-8320(09)73159-8. [DOI] [PubMed] [Google Scholar]
- 10.Wędrychowicz A, Zając A, Tomasik P. 2016. Advances in nutritional therapy in inflammatory bowel diseases: review. World J Gastroenterol 22:1045–1066. doi: 10.3748/wjg.v22.i3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Png CW, Linden SK, Gilshenan KS, Zoetendal EG, McSweeney CS, Sly LI, McGuckin MA, Florin TH. 2010. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am J Gastroenterol 105:2420–2428. doi: 10.1038/ajg.2010.281. [DOI] [PubMed] [Google Scholar]
- 12.Wlodarska M, Kostic AD, Xavier RJ. 2015. An integrative view of microbiome-host interactions in inflammatory bowel diseases. Cell Host Microbe 17:577–591. doi: 10.1016/j.chom.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan RR, Lawson AD, Minnich LL, Martin K, Nasir A, Emmett MK, Welch CA, Udall JN Jr.. 2009. Gastrointestinal norovirus infection associated with exacerbation of inflammatory bowel disease. J Pediatr Gastroenterol Nutr 48:328–333. doi: 10.1097/MPG.0b013e31818255cc. [DOI] [PubMed] [Google Scholar]
- 14.Ciccocioppo R, Racca F, Paolucci S, Campanini G, Pozzi L, Betti E, Riboni R, Vanoli A, Baldanti F, Corazza GR. 2015. Human cytomegalovirus and Epstein-Barr virus infection in inflammatory bowel disease: need for mucosal viral load measurement. World J Gastroenterol 21:1915–1926. doi: 10.3748/wjg.v21.i6.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hume G, Radford-Smith GL. 2002. The pathogenesis of Crohn’s disease in the 21st century. Pathology 34:561–567. [PubMed] [Google Scholar]
- 16.Frolkis A, Dieleman LA, Barkema HW, Panaccione R, Ghosh S, Fedorak RN, Madsen K, Kaplan GG. 2013. Environment and the inflammatory bowel diseases. Can J Gastroenterol 27:e18–e24. doi: 10.1155/2013/102859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Westall FC. 2006. Integrating theories of the etiology of Crohn’s disease. On the etiology of Crohn’s disease: questioning the hypotheses. Med Sci Monit 12:LE5–LE6. (Letter.) [PubMed] [Google Scholar]
- 18.Macfarlane S, Steed H, Macfarlane GT. 2009. Intestinal bacteria and inflammatory bowel disease. Crit Rev Clin Lab Sci 46:25–54. doi: 10.1080/10408360802485792. [DOI] [PubMed] [Google Scholar]
- 19.Schultz M, Munro K, Tannock GW, Melchner I, Gottl C, Schwietz H, Scholmerich J, Rath HC. 2004. Effects of feeding a probiotic preparation (SIM) containing inulin on the severity of colitis and on the composition of the intestinal microflora in HLA-B27 transgenic rats. Clin Diagn Lab Immunol 11:581–587. doi: 10.1128/CDLI.11.3.581-587.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan KJ, Ullman TA, Ford AC, Abreu MT, Abadir A, Marshall JK, Talley NJ, Moayyedi P. 2011. Antibiotic therapy in inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol 106:661–673. doi: 10.1038/ajg.2011.72. [DOI] [PubMed] [Google Scholar]
- 21.Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, Nalin R, Jarrin C, Chardon P, Marteau P, Roca J, Dore J. 2006. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut 55:205–211. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braun A, Treede I, Gotthardt D, Tietje A, Zahn A, Ruhwald R, Schoenfeld U, Welsch T, Kienle P, Erben G, Lehmann WD, Fuellekrug J, Stremmel W, Ehehalt R. 2009. Alterations of phospholipid concentration and species composition of the intestinal mucus barrier in ulcerative colitis: a clue to pathogenesis. Inflamm Bowel Dis 15:1705–1720. doi: 10.1002/ibd.20993. [DOI] [PubMed] [Google Scholar]
- 23.Mirsepasi H, Persson S, Struve C, Andersen LOB, Petersen AM, Krogfelt KA. 2014. Microbial diversity in fecal samples depends on DNA extraction method: easyMag DNA extraction compared to QIAamp DNA stool mini kit extraction. BMC Res Notes 7:50. doi: 10.1186/1756-0500-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotlowski R, Bernstein CN, Sepehri S, Krause DO. 2007. High prevalence of Escherichia coli belonging to the B2+D phylogenetic group in inflammatory bowel disease. Gut 56:669–675. doi: 10.1136/gut.2006.099796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petersen AM, Nielsen EM, Litrup E, Brynskov J, Mirsepasi H, Krogfelt KA. 2009. A phylogenetic group of Escherichia coli associated with active left-sided inflammatory bowel disease. BMC Microbiol 9:171–179. doi: 10.1186/1471-2180-9-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rahimi R, Nikfar S, Rezaie A, Abdollahi M. 2006. A meta-analysis of broad-spectrum antibiotic therapy in patients with active Crohn’s disease. Clin Ther 28:1983–1988. doi: 10.1016/j.clinthera.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 27.Fava F, Danese S. 2011. Intestinal microbiota in inflammatory bowel disease: friend of foe? World J Gastroenterol 17:557–566. doi: 10.3748/wjg.v17.i5.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Lafuente A, Antolin M, Guarner F, Crespo E, Salas A, Forcada P, Laguarda M, Gavalda J, Baena JA, Vilaseca J, Malagelada JR. 1997. Incrimination of anaerobic bacteria in the induction of experimental colitis. Am J Physiol 272:G10–G15. doi: 10.1152/ajpgi.1997.272.1.G10. [DOI] [PubMed] [Google Scholar]
- 29.Wang W, Chen L, Zhou R, Wang X, Song L, Huang S, Wang G, Xia B. 2014. Increased proportions of Bifidobacterium and the Lactobacillus group and loss of butyrate-producing bacteria in inflammatory bowel disease. J Clin Microbiol 52:398–406. doi: 10.1128/JCM.01500-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geirnaert A, Calatayud M, Grootaert C, Laukens D, Devriese S, Smagghe G, De Vos M, Boon N, Van De Wiele T. 2017. Butyrate-producing bacteria supplemented in vitro to Crohn’s disease patient microbiota increased butyrate production and enhanced intestinal epithelial barrier integrity. Sci Rep 7:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sokol H, Seksik P, Rigottier-Gois L, Lay C, Lepage P, Podglajen I, Marteau P, Doré J. 2006. Specificities of the fecal microbiota in inflammatory bowel disease. Inflamm Bowel Dis 12:106–111. doi: 10.1097/01.MIB.0000200323.38139.c6. [DOI] [PubMed] [Google Scholar]
- 32.Hermon-Taylor J, Barnes N, Clarke C, Finlayson C. 1998. Mycobacterium paratuberculosis cervical lymphadenitis, followed five years later by terminal ileitis similar to Crohn’s disease. BMJ 316:449–453. doi: 10.1136/bmj.316.7129.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dorman SA, Liggoria E, Winn WC Jr, Beeken WL. 1982. Isolation of Clostridium difficile from patients with inactive Crohn’s disease. Gastroenterology 82:1348–1351. [PubMed] [Google Scholar]
- 34.Taylor-Robinson S, Miles R, Whitehead A, Dickinson RJ. 1989. Salmonella infection and ulcerative colitis. Lancet 1:1145. doi: 10.1016/S0140-6736(89)92428-8. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, van Kruiningen HJ, West AB, Cartun RW, Cortot A, Colombel JF. 1995. Immunocytochemical evidence of Listeria, Escherichia coli, and Streptococcus antigens in Crohn’s disease. Gastroenterology 108:1396–1404. doi: 10.1016/0016-5085(95)90687-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doman DB, Golding MI, Goldberg HJ, Doyle RB. 1989. Aeromonas hydrophila colitis presenting as medically refractory inflammatory bowel disease. Am J Gastroenterol 84:83–85. [PubMed] [Google Scholar]
- 37.Kanareykina SK, Misautova AA, Zlatkina AR, Levina EN. 1987. Proteus dysbioses in patients with ulcerative colitis. Nahrung 31:557–561. doi: 10.1002/food.19870310570. [DOI] [PubMed] [Google Scholar]
- 38.Mirsepasi-Lauridsen HC, Du Z, Struve C, Charbon G, Karczewski J, Krogfelt KA, Petersen AM, Wells JM. 2016. Secretion of alpha-hemolysin by Escherichia coli disrupts tight junctions in ulcerative colitis patients. Clin Transl Gastroenterol 7:e149. doi: 10.1038/ctg.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mirsepasi-Lauridsen HC, Yang H, Bosman E, Struve C, Yu H, Wu X, Ma C, Fotovati A, Petersen AM, Jacobson K, Krogfelt KA, Vallance B. 2017. Ulcerative colitis-associated Escherichia coli colonize the intestinal mucosa of susceptible host and promote colitis via hemolysin production. Gastroenterology 151:S-821–S-822. doi: 10.1016/S0016-5085(17)32838-X. [DOI] [Google Scholar]
- 40.Kangro HO, Chong SK, Hardiman A, Heath RB, Walker-Smith JA. 1990. A prospective study of viral and mycoplasma infections in chronic inflammatory bowel disease. Gastroenterology 98:549–553. doi: 10.1016/0016-5085(90)90272-3. [DOI] [PubMed] [Google Scholar]
- 41.Moustafa A, Li W, Anderson EL, Wong EHM, Dulai PS, Sandborn WJ, Biggs W, Yooseph S, Jones MB, Venter JC, Nelson KE, Chang JT, Telenti A, Boland BS. 2018. Genetic risk, dysbiosis, and treatment stratification using host genome and gut microbiome in inflammatory bowel disease. Clin Trans Gastroenterol 9:e132–e138. doi: 10.1038/ctg.2017.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelly D, King T, Aminov R. 2007. Importance of microbial colonization of the gut in early life to the development of immunity. Mutat Res 622:58–69. doi: 10.1016/j.mrfmmm.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 43.Russo TA, Johnson JR. 2000. Proposal for a new inclusive designation for extraintestinal pathogenic isolates of Escherichia coli: ExPEC. J Infect Dis 181:1753–1754. doi: 10.1086/315418. [DOI] [PubMed] [Google Scholar]
- 44.Kaper JB, Nataro JP, Mobley HL. 2004. Pathogenic Escherichia coli. Nat Rev Microbiol 2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 45.Jensen BH, Olsen KEP, Struve C, Krogfelt KA, Petersen AM. 2014. Epidemiology and clinical manifestations of enteroaggregative Escherichia coli. Clin Microbiol Rev 27:614–630. doi: 10.1128/CMR.00112-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mora A, Blanco M, Yamamoto D, Dahbi G, Blanco JE, Lopez C, Alonso MP, Vieira MA, Hernandes RT, Abe CM, Piazza RM, Lacher DW, Elias WP, Gomes TA, Blanco J. 2009. HeLa-cell adherence patterns and actin aggregation of enteropathogenic Escherichia coli (EPEC) and Shiga-toxin-producing E. coli (STEC) strains carrying different eae and tir alleles. Int Microbiol 12:243–251. [PubMed] [Google Scholar]
- 47.Hernandes RT, Elias WP, Vieira MA, Gomes TA. 2009. An overview of atypical enteropathogenic Escherichia coli. FEMS Microbiol Lett 297:137–149. doi: 10.1111/j.1574-6968.2009.01664.x. [DOI] [PubMed] [Google Scholar]
- 48.Hardwidge PR, Deng W, Vallance BA, Rodriguez-Escudero I, Cid VJ, Molina M, Finlay BB. 2005. Modulation of host cytoskeleton function by the enteropathogenic Escherichia coli and Citrobacter rodentium effector protein EspG. Infect Immun 73:2586–2594. doi: 10.1128/IAI.73.5.2586-2594.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reis RS, Horn F. 2010. Enteropathogenic Escherichia coli, Samonella, Shigella and Yersinia: cellular aspects of host-bacteria interactions in enteric diseases. Gut Pathog 2:8. doi: 10.1186/1757-4749-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taxt A, Aasland R, Sommerfelt H, Nataro J, Puntervoll P. 2010. Heat-stable enterotoxin of enterotoxigenic Escherichia coli as a vaccine target. Infect Immun 78:1824–1831. doi: 10.1128/IAI.01397-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sears CL, Kaper JB. 1996. Enteric bacterial toxins: mechanisms of action and linkage to intestinal secretion. Microbiol Rev 60:167–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zuo G, Xu Z, Hao B. 2013. Shigella strains are not clones of Escherichia coli but sister species in the genus Escherichia. Genomics Proteomics Bioinformatics 11:61–65. doi: 10.1016/j.gpb.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okeke IN. 2009. Diarrheagenic Escherichia coli in sub-Saharan Africa: status, uncertainties and necessities. J Infect Dev Ctries 3:817–842. [DOI] [PubMed] [Google Scholar]
- 54.Sansonetti P. 2002. Host-pathogen interactions: the seduction of molecular cross talk. Gut 50:III2–III8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaper JB. 2005. Pathogenic Escherichia coli. Int J Med Microbiol 295:355–356. doi: 10.1016/j.ijmm.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 56.Nataro JP, Kaper JB, Robins-Browne R, Prado V, Vial P, Levine MM. 1987. Patterns of adherence of diarrheagenic Escherichia coli to HEp-2 cells. Pediatr Infect Dis J 6:829–831. doi: 10.1097/00006454-198709000-00008. [DOI] [PubMed] [Google Scholar]
- 57.Nataro JP, Kaper JB. 1998. Diarrheagenic Escherichia coli. Clin Microbiol Rev 11:142–201. doi: 10.1128/CMR.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steiner TS, Nataro JP, Poteet-Smith CE, Smith JA, Guerrant RL. 2000. Enteroaggregative Escherichia coli expresses a novel flagellin that causes IL-8 release from intestinal epithelial cells. J Clin Invest 105:1769–1777. doi: 10.1172/JCI8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morin N, Santiago AE, Ernst RK, Guillot SJ, Nataro P. 2013. Characterization of the AggR regulon in enteroaggregative Escherichia coli. Infect Immun 81:122–132. doi: 10.1128/IAI.00676-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boisen N, Ruiz-Perez F, Scheutz F, Krogfelt KA, Nataro JP. 2009. Short report: high prevalence of serine protease autotransporter cytotoxins among strains of enteroaggregative Escherichia coli. Am J Trop Med Hyg 80:294–301. doi: 10.4269/ajtmh.2009.80.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Savarino SJ, Fasano A, Watson J, Martin BM, Levine MM, Guandalini S, Guerry P. 1993. Enteroaggregative Escherichia coli heat-stable enterotoxin 1 represents another subfamily of E. coli heat-stable toxin. Proc Natl Acad Sci U S A 90:3093–3097. doi: 10.1073/pnas.90.7.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Navarro-Garcia F, Eslava C, Villaseca JM, Lopez-Revilla R, Czeczulin JR, Srinivas S, Nataro JP, Cravioto A. 1998. In vitro effects of a high-molecular-weight heat-labile enterotoxin from enteroaggregative Escherichia coli. Infect Immun 66:3149–3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nash JH, Villegas A, Kropinski AM, Aguilar-Valenzuela R, Konczy P, Mascarenhas M, Ziebell K, Torres AG, Karmali MA, Coombes BK. 2010. Genome sequence of adherent-invasive Escherichia coli and comparative genomic analysis with other E. coli pathotypes. BMC Genomics 11:667. doi: 10.1186/1471-2164-11-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sonntag AK, Prager R, Bielaszewska M, Zhang W, Fruth A, Tschape H, Karch H. 2004. Phenotypic and genotypic analyses of enterohemorrhagic Escherichia coli O145 strains from patients in Germany. J Clin Microbiol 42:954–962. doi: 10.1128/JCM.42.3.954-962.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thorpe CM. 2004. Shiga toxin-producing Escherichia coli infection. Clin Infect Dis 38:1298–1303. doi: 10.1086/383473. [DOI] [PubMed] [Google Scholar]
- 66.Karmali RA, Muse P, Allen G, Louis T. 1982. Macrophage production of prostaglandins: effects of fetal calf serum and diazepam. Use of an improved method for extracting 6-keto-PGF1 alpha. Prostaglandins Leukot Med 8:565–577. [PubMed] [Google Scholar]
- 67.McCarthy TA, Barrett NL, Hadler JL, Salsbury B, Howard RT, Dingman DW, Brinkman CD, Bibb WF, Cartter ML. 2001. Hemolytic-uremic syndrome and Escherichia coli O121 at a lake in Connecticut, 1999. Pediatrics 108:E59. doi: 10.1542/peds.108.4.e59. [DOI] [PubMed] [Google Scholar]
- 68.Karmali MA, Gannon V, Sargeant JM. 2010. Verocytotoxin-producing Escherichia coli (VTEC). Vet Microbiol 140:360–370. doi: 10.1016/j.vetmic.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 69.Hurley BP, Thorpe CM, Acheson DW. 2001. Shiga toxin translocation across intestinal epithelial cells is enhanced by neutrophil transmigration. Infect Immun 69:6148–6155. doi: 10.1128/IAI.69.10.6148-6155.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bolton DJ. 2011. Verocytotoxigenic (Shiga toxin-producing) Escherichia coli: virulence factors and pathogenicity in the farm to fork paradigm. Foodborne Pathog Dis 8:357–365. doi: 10.1089/fpd.2010.0699. [DOI] [PubMed] [Google Scholar]
- 71.Johnson JR, Russo TA. 2002. Uropathogenic Escherichia coli as agents of diverse non-urinary tract extraintestinal infections. J Infect Dis 186:859–864. doi: 10.1086/342490. [DOI] [PubMed] [Google Scholar]
- 72.Russo TA, Johnson JR. 2003. Medical and economic impact of extraintestinal infections due to Escherichia coli: focus on an increasingly important endemic problem. Microbes Infect 5:449–456. doi: 10.1016/S1286-4579(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 73.Clermont O, Bonacorsi S, Bingen E. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl Environ Microbiol 66:4555–4558. doi: 10.1128/AEM.66.10.4555-4558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Clermont O, Christenson JK, Denamur E, Gordon DM. 2013. The Clermont Escherichia coli phylo-typing method revisited: improvement of specificity and detection of new phylo-groups. Environ Microbiol Rep 5:58–65. doi: 10.1111/1758-2229.12019. [DOI] [PubMed] [Google Scholar]
- 75.Johnson JR, Gajewski A, Lesse AJ, Russo TA. 2003. Extraintestinal pathogenic Escherichia coli as a cause of invasive nonurinary infections. J Clin Microbiol 41:5798–5802. doi: 10.1128/JCM.41.12.5798-5802.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sabate M, Moreno E, Perez T, Andreu A, Prats G. 2006. Pathogenicity island markers in commensal and uropathogenic Escherichia coli isolates. Clin Microbiol Infect 12:880–886. doi: 10.1111/j.1469-0691.2006.01461.x. [DOI] [PubMed] [Google Scholar]
- 77.Johnson JR, Russo TA. 2005. Molecular epidemiology of extraintestinal pathogenic (uropathogenic) Escherichia coli. Int J Med Microbiol 295:383–404. doi: 10.1016/j.ijmm.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 78.Burke DA, Axon AT. 1988. Adhesive Escherichia coli in inflammatory bowel disease and infective diarrhoea. BMJ 297:102–104. doi: 10.1136/bmj.297.6641.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Giaffer MH, Holdsworth CD, Duerden BI. 1992. Virulence properties of Escherichia coli strains isolated from patients with inflammatory bowel disease. Gut 33:646–650. doi: 10.1136/gut.33.5.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Keighley MR, Arabi Y, Dimock F, Burdon DW, Allan RN, Alexander-Williams J. 1978. Influence of inflammatory bowel disease on intestinal microflora. Gut 19:1099–1104. doi: 10.1136/gut.19.12.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ilnyckyj A, Greenberg H, Bernstein CN. 1997. Escherichia coli O157:H7 infection mimicking Crohn’s disease. Gastroenterology 112:995–999. doi: 10.1053/gast.1997.v112.pm9041263. [DOI] [PubMed] [Google Scholar]
- 82.Bielaszewska M, Schiller R, Lammers L, Bauwens A, Fruth A, Middendorf B, Schmidt MA, Tarr PI, Dobrindt U, Karch H, Mellmann A. 2014. Heteropathogenic virulence and phylogeny reveal phased pathogenic metamorphosis in Escherichia coli O2:H6. EMBO Mol Med 6:347–357. doi: 10.1002/emmm.201303133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Darfeuille-Michaud A, Neut C, Barnich N, Lederman E, Di Martino P, Desreumaux P, Gambiez L, Joly B, Cortot A, Colombel JF. 1998. Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn’s disease. Gastroenterology 115:1405–1413. doi: 10.1016/S0016-5085(98)70019-8. [DOI] [PubMed] [Google Scholar]
- 84.Martin HM, Campbell BJ, Hart CA, Mpofu C, Nayar M, Singh R, Englyst H, Williams HF, Rhodes JM. 2004. Enhanced Escherichia coli adherence and invasion in Crohn’s disease and colon cancer. Gastroenterology 127:80–93. doi: 10.1053/j.gastro.2004.03.054. [DOI] [PubMed] [Google Scholar]
- 85.La Ferla K, Seegert D, Schreiber S. 2004. Activation of NF-kappaB in intestinal epithelial cells by E. coli strains isolated from the colonic mucosa of IBD patients. Int J Colorectal Dis 19:334–342. doi: 10.1007/s00384-004-0583-7. [DOI] [PubMed] [Google Scholar]
- 86.Boudeau J, Glasser AL, Masseret E, Joly B, Darfeuille-Michaud A. 1999. Invasive ability of an Escherichia coli strain isolated from the ileal mucosa of a patient with Crohn’s disease. Infect Immun 67:4499–4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Greenstein RJ. 2003. Is Crohn’s disease caused by a mycobacterium? Comparisons with leprosy, tuberculosis, and Johne’s disease. Lancet Infect Dis 3:507–514. doi: 10.1016/S1473-3099(03)00724-2. [DOI] [PubMed] [Google Scholar]
- 88.Scanu AM, Bull TJ, Cannas S, Sanderson JD, Sechi LA, Dettori G, Zanetti S, Hermon-Taylor J. 2007. Mycobacterium avium subspecies paratuberculosis infection in cases of irritable bowel syndrome and comparison with Crohn’s disease and Johne’s disease: common neural and immune pathogenicities. J Clin Microbiol 45:3883–3890. doi: 10.1128/JCM.01371-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meconi S, Vercellone A, Levillain F, Payre B, Al Saati T, Capilla F, Desreumaux P, Darfeuille-Michaud A, Altare F. 2007. Adherent-invasive Escherichia coli isolated from Crohn’s disease patients induce granulomas in vitro. Cell Microbiol 9:1252–1261. doi: 10.1111/j.1462-5822.2006.00868.x. [DOI] [PubMed] [Google Scholar]
- 90.Sartor RB, Muehlbauer M. 2007. Microbial host interactions in IBD: implications for pathogenesis and therapy. Curr Gastroenterol Rep 9:497–507. doi: 10.1007/s11894-007-0066-4. [DOI] [PubMed] [Google Scholar]
- 91.Schussler P, Kruis W, Marget W. 1976. Lipid A antibody titers and O antibody titers in Crohn’s disease, ulcerative colitis and acute enteritis [author’s translation]. Med Klin 71:1898–1902. [PubMed] [Google Scholar]
- 92.Landers CJ, Cohavy O, Misra R, Yang H, Lin YC, Braun J, Targan SR. 2002. Selected loss of tolerance evidenced by Crohn’s disease-associated immune responses to auto- and microbial antigens. Gastroenterology 123:689–699. doi: 10.1053/gast.2002.35379. [DOI] [PubMed] [Google Scholar]
- 93.Mow WS, Vasiliauskas EA, Lin YC, Fleshner PR, Papadakis KA, Taylor KD, Landers CJ, Abreu-Martin MT, Rotter JI, Yang H, Targan SR. 2004. Association of antibody responses to microbial antigens and complications of small bowel Crohn’s disease. Gastroenterology 126:414–424. doi: 10.1053/j.gastro.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 94.Tabaqchali S, O’Donoghue DP, Bettelheim KA. 1978. Escherichia coli antibodies in patients with inflammatory bowel disease. Gut 19:108–113. doi: 10.1136/gut.19.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Burke DA, Axon AT. 1987. Ulcerative colitis and Escherichia coli with adhesive properties. J Clin Pathol 40:782–786. doi: 10.1136/jcp.40.7.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Satterwhite TK, Evans DG, DuPont HL, Evans DJ. 1978. Role of Escherichia coli colonisation factor antigen in acute diarrhoea. Lancet ii:181–184. [DOI] [PubMed] [Google Scholar]
- 97.Levine MM, Nataro JP, Karch H, Baldini MM, Kaper JB, Black RE, Clements ML, O’Brien AD. 1985. The diarrheal response of humans to some classic serotypes of enteropathogenic Escherichia coli is dependent on a plasmid encoding an enteroadhesiveness factor. J Infect Dis 152:550–559. doi: 10.1093/infdis/152.3.550. [DOI] [PubMed] [Google Scholar]
- 98.Mirsepasi-Lauridsen HC, Halkjaer SI, Mortensen EM, Lydolph MC, Nordgaard-Lassen I, Krogfelt KA, Petersen AM. 2016. Extraintestinal pathogenic Escherichia coli are associated with intestinal inflammation in patients with ulcerative colitis. Sci Rep 6:31152. doi: 10.1038/srep31152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jensen SR, Mirsepasi-Lauridsen HC, Thysen AH, Brynskov J, Krogfelt KA, Petersen AM, Pedersen AE, Brix S. 2015. Distinct inflammatory and cytopathic characteristics of Escherichia coli isolates from inflammatory bowel disease patients. Int J Med Microbiol 305:925–936. doi: 10.1016/j.ijmm.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 100.Prantera C, Berto E, Scribano ML, Falasco G. 1998. Use of antibiotics in the treatment of active Crohn’s disease: experience with metronidazole and ciprofloxacin. Ital J Gastroenterol Hepatol 30:602–606. [PubMed] [Google Scholar]
- 101.Hudson MJ, Hill MJ, Elliott PR, Berghouse LM, Burnham WR, Lennard-Jones JE. 1984. The microbial flora of the rectal mucosa and faeces of patients with Crohn’s disease before and during antimicrobial chemotherapy. J Med Microbiol 18:335–345. doi: 10.1099/00222615-18-3-335. [DOI] [PubMed] [Google Scholar]
- 102.Shafran I, Kugler L, El-Zaatari FAK, Naser SA, Sandoval J. 2002. Open clinical trial of rifabutin and clarithromycin therapy in Crohn’s. Dig Liver Dis 34:22–28. doi: 10.1016/S1590-8658(02)80055-X. [DOI] [PubMed] [Google Scholar]
- 103.Prantera C, Scribano ML. 2009. Antibiotics and probiotics in inflammatory bowel disease: why, when, and how. Curr Opin Gastroenterol 25:329–333. doi: 10.1097/MOG.0b013e32832b20bf. [DOI] [PubMed] [Google Scholar]
- 104.Greenbloom SL, Steinhart AH, Greenberg GR. 1998. Combination ciprofloxacin and metronidazole for active Crohn’s disease. Can J Gastroenterol 12:53–56. doi: 10.1155/1998/349460. [DOI] [PubMed] [Google Scholar]
- 105.Mennigen R, Nolte K, Rijcken E, Utech M, Loeffler B, Senninger N, Bruewer M. 2009. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol 296:G1140–G1149. doi: 10.1152/ajpgi.90534.2008. [DOI] [PubMed] [Google Scholar]
- 106.Schultz M. 2008. Clinical use of E. coli Nissle 1917 in inflammatory bowel disease. Inflamm Bowel Dis 14:1012–1018. doi: 10.1002/ibd.20377. [DOI] [PubMed] [Google Scholar]
- 107.Wehkamp J, Harder J, Wehkamp K, Wehkamp-von Meissner B, Schlee M, Enders C, Sonnenborn U, Nuding S, Bengmark S, Fellermann K, Schröder JM, Stange EF. 2004. NF-kappaB- and AP-1-mediated induction of human beta defensin-2 in intestinal epithelial cells by Escherichia coli Nissle 1917: a novel effect of a probiotic bacterium. Infect Immun 72:5750–5758. doi: 10.1128/IAI.72.10.5750-5758.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Grozdanov L, Zahringer U, Blum-Oehler G, Brade L, Henne A, Knirel YA, Schombel U, Schulze J, Sonnenborn U, Gottschalk G, Hacker J, Rietschel ET, Dobrindt U. 2002. A single nucleotide exchange in the wzy gene is responsible for the semirough O6 lipopolysaccharide phenotype and serum sensitivity of Escherichia coli strain Nissle 1917. J Bacteriol 184:5912–5925. doi: 10.1128/JB.184.21.5912-5925.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Grozdanov L, Raasch C, Schulze J, Sonnenborn U, Gottschalk G, Hacker J, Dobrindt U. 2004. Analysis of the genome structure of the nonpathogenic probiotic Escherichia coli strain Nissle 1917. J Bacteriol 186:5432–5441. doi: 10.1128/JB.186.16.5432-5441.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gupta N, Bostrom AG, Kirschner BS, Ferry GD, Winter HS, Baldassano RN, Gold BD, Abramson O, Smith T, Cohen SA, Heyman MB. 2007. Gender differences in presentation and course of disease in pediatric patients with Crohn disease. Pediatrics 120:e1418–e1425. doi: 10.1542/peds.2007-0905. [DOI] [PubMed] [Google Scholar]
- 111.Heimesaat MM, Fischer A, Jahn HK, Niebergall J, Freudenberg M, Blaut M, Liesenfeld O, Schumann RR, Gobel UB, Bereswill S. 2007. Exacerbation of murine ileitis by Toll-like receptor 4 mediated sensing of lipopolysaccharide from commensal Escherichia coli. Gut 56:941–948. doi: 10.1136/gut.2006.104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Helwig U, Lammers KM, Rizzello F, Brigidi P, Rohleder V, Caramelli E, Gionchetti P, Schrezenmeir J, Foelsch UR, Schreiber S, Campieri M. 2006. Lactobacilli, bifidobacteria and E. coli Nissle induce pro- and anti-inflammatory cytokines in peripheral blood mononuclear cells. World J Gastroenterol 12:5978–5986. doi: 10.3748/wjg.v12.i37.5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hibi T, Inoue N, Ogata H, Naganuma M. 2003. Introduction and overview: recent advances in the immunotherapy of inflammatory bowel disease. J Gastroenterol 38:36–42. [PubMed] [Google Scholar]
- 114.Hogaboam CM, Vallance BA, Kumar A, Addison CL, Graham FL, Gauldie J, Collins SM. 1997. Therapeutic effects of interleukin-4 gene transfer in experimental inflammatory bowel disease. J Clin Invest 100:2766–2776. doi: 10.1172/JCI119823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schultz M, Strauch UG, Linde H-J, Watzl S, Obermeier F, Göttl C, Dunger N, Grunwald N, Schölmerich J, Rath HC. 2004. Preventive effects of Escherichia coli strain Nissle 1917 on acute and chronic intestinal inflammation in two different murine models of colitis. Clin Diagn Lab Immunol 11:372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schlee M, Wehkamp J, Altenhoefer A, Oelschlaeger TA, Stange EF, Fellermann K. 2007. Induction of human beta-defensin 2 by the probiotic Escherichia coli Nissle 1917 is mediated through flagellin. Infect Immun 75:2399–2407. doi: 10.1128/IAI.01563-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mandel L, Trebichavsky I, Splichal I, Schulze J. 1995. Stimulation of intestinal immune cells by E. coli in gnotobiotic piglets. Adv Exp Med Biol 371A:463–464. [DOI] [PubMed] [Google Scholar]
- 118.Lorenz A. 1996. Establishment of E. coli Nissle 1917 and its interaction with Candida albicans in gnotobiotic rats. MicroecolTher 24:45–51. [Google Scholar]
- 119.Altenhoefer A, Oswald S, Sonnenborn U, Enders C, Schulze J, Hacker J, Oelschlaeger TA. 2004. The probiotic Escherichia coli strain Nissle 1917 interferes with invasion of human intestinal epithelial cells by different enteroinvasive bacterial pathogens. FEMS Immunol Med Microbiol 40:223–229. doi: 10.1016/S0928-8244(03)00368-7. [DOI] [PubMed] [Google Scholar]
- 120.Boudeau J, Glasser AL, Julien S, Colombel JF, Darfeuille-Michaud A. 2003. Inhibitory effect of probiotic Escherichia coli strain Nissle 1917 on adhesion to and invasion of intestinal epithelial cells by adherent-invasive E. coli strains isolated from patients with Crohn’s disease. Aliment Pharmacol Ther 18:45–56. doi: 10.1046/j.1365-2036.2003.01638.x. [DOI] [PubMed] [Google Scholar]
- 121.Kruis W, Schutz E, Fric P, Fixa B, Judmaier G, Stolte M. 1997. Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther 11:853–858. doi: 10.1046/j.1365-2036.1997.00225.x. [DOI] [PubMed] [Google Scholar]
- 122.Kruis W, Fric P, Pokrotnieks J, Lukas M, Fixa B, Kascak M, Kamm MA, Weismueller J, Beglinger C, Stolte M, Wolff C, Schulze J, Lukás M, Fixa B, Kascák C, Schulze J. 2004. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut 53:1617–1623. doi: 10.1136/gut.2003.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kuzela L, Kascak M, Vavrecka A. 2001. Induction and maintenance of remission with nonpathogenic Escherichia coli in patients with pouchitis. Am J Gastroenterol 96:3218–3219. doi: 10.1111/j.1572-0241.2001.05294.x. [DOI] [PubMed] [Google Scholar]
- 124.Malchow HA. 1997. Crohn’s disease and Escherichia coli. A new approach in therapy to maintain remission of colonic Crohn’s disease? J Clin Gastroenterol 25:653–658. doi: 10.1097/00004836-199712000-00021. [DOI] [PubMed] [Google Scholar]
- 125.Rembacken BJ, Snelling AM, Hawkey PM, Chalmers DM, Axon ATR. 1999. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet 354:635–639. doi: 10.1016/S0140-6736(98)06343-0. [DOI] [PubMed] [Google Scholar]
- 126.Petersen AM, Mirsepasi H, Halkjær SI, Mortensen EM, Nordgaard-Lassen I, Krogfelt KA. 2014. Ciprofloxacin and probiotic Escherichia coli Nissle add-on treatment in active ulcerative colitis: a double-blind randomized placebo controlled clinical trial. J Crohns Colitis 8:1498–1505. doi: 10.1016/j.crohns.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 127.Han DY, Fraser AG, Dryland P, Ferguson LR. 2010. Environmental factors in the development of chronic inflammation: a case-control study on risk factors for Crohn’s disease within New Zealand. Mutat Res 690:116–122. doi: 10.1016/j.mrfmmm.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 128.Shaw SY, Blanchard JF, Bernstein CN. 2010. Association between the use of antibiotics in the first year of life and pediatric inflammatory bowel disease. Am J Gastroenterol 105:2687–2692. doi: 10.1038/ajg.2010.398. [DOI] [PubMed] [Google Scholar]
- 129.De Vroey B, De Cassan C, Gower-Rousseau C, Colombel JF. 2010. Editorial: antibiotics earlier, IBD later. Am J Gastroenterol 105:2693–2696. doi: 10.1038/ajg.2010.396. [DOI] [PubMed] [Google Scholar]
- 130.Burisch J, Pedersen N, Cukovic-Cavka S, Turk N, Kaimakliotis I, Duricova D, Bortlik M, Shonová O, Vind I, Avnstrøm S, Thorsgaard N, Krabbe S, Andersen V, Dahlerup JF, Kjeldsen J, Salupere R, Olsen J, Nielsen KR, Manninen P, Collin P, Katsanos KH, Tsianos EV, Ladefoged K, Lakatos L, Ragnarsson G, Björnsson E, Bailey Y, O’Morain C, Schwartz D, Odes S, Giannotta M, Girardin G, Kiudelis G, Kupcinskas L, Turcan S, Barros L, Magro F, Lazar D, Goldis A, Nikulina I, Belousova E, Martinez-Ares D, Hernandez V, Almer S, Zhulina Y, Halfvarson J, Arebi N, Tsai HH, Sebastian S, Lakatos PL, Langholz E, Munkholm P. 2014. Environmental factors in a population-based inception cohort of inflammatory bowel disease patients in Europe—an ECCO-EpiCom study. J Crohns Colitis 8:607–616. doi: 10.1016/j.crohns.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 131.D’Souza S, Levy E, Mack D, Israel D, Lambrette P, Ghadirian P, Deslandres C, Morgan K, Seidman EG, Amre DK. 2008. Dietary patterns and risk for Crohn’s disease in children. Inflamm Bowel Dis 14:367–373. doi: 10.1002/ibd.20333. [DOI] [PubMed] [Google Scholar]
- 132.Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, De Silva P, Korzenik JR, Fuchs CS, Willett WC, Richter JM, Chan AT. 2013. A prospective study of long-term intake of dietary fiber and risk of Crohn’s disease and ulcerative colitis. Gastroenterology 145:970–977. doi: 10.1053/j.gastro.2013.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Viladomiu M, Hontecillas R, Yuan L, Lu P, Bassaganya-Riera J. 2013. Nutritional protective mechanisms against gut inflammation. J Nutr Biochem 24:929–939. doi: 10.1016/j.jnutbio.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wolin MJ. 1969. Volatile fatty acids and the inhibition of Escherichia coli growth by rumen fluid. Appl Microbiol 17:83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Martinez-Medina M, Denizot J, Dreux N, Robin F, Billard E, Bonnet R, Darfeuille-Michaud A, Barnich N. 2014. Western diet induces dysbiosis with increased E coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut 63:116–124. doi: 10.1136/gutjnl-2012-304119. [DOI] [PubMed] [Google Scholar]
- 136.Nowak JK, Grzybowska-Chlebowczyk U, Landowski P, Szaflarska-Poplawska A, Klincewicz B, Adamczak D, Banasiewicz T, Plawski A, Walkowiak J. 2014. Prevalence and correlates of vitamin K deficiency in children with inflammatory bowel disease. Sci Rep 4:4768. doi: 10.1038/srep04768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vernia P, Gnaedinger A, Hauck W, Breuer RI. 1988. Organic anions and the diarrhea of inflammatory bowel disease. Dig Dis Sci 33:1353–1358. doi: 10.1007/BF01536987. [DOI] [PubMed] [Google Scholar]
- 138.Fleming LL, Floch MH. 1986. Digestion and absorption of fiber carbohydrate in the colon. Am J Gastroenterol 81:507–511. [PubMed] [Google Scholar]
- 139.Segain J, Blétière DR, De Bourreille A, Leray V, Gervois N, Rosales C, Ferrier L, Bonnet C, Blottière HM, Galmiche J. 2000. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn’s disease. Gut 47:397–403. doi: 10.1136/gut.47.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vagianos K, Bector S, McConnell J, Bernstein CN. 2007. Nutrition assessment of patients with inflammatory bowel disease. JPEN J Parenter Enteral Nutr 31:311–319. doi: 10.1177/0148607107031004311. [DOI] [PubMed] [Google Scholar]
- 141.Ferguson LR, Peterman I, Hübner C, Philpott M, Shellin AN. 2007. Uncoupling gene-diet interactions in inflammatory bowel disease (IBD). Genes Nutr 2:71–73. doi: 10.1007/s12263-007-0012-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pálmer HG, González-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, Quintanilla M, Cano A, de Herreros AG, Lafarga M, Muñoz A. 2001. Vitamin D3 promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J Cell Biol 154:369–387. doi: 10.1083/jcb.200102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Muller K, Odum N, Bendtzen K. 1993. 1,25-Dihydroxyvitamin D3 selectively reduces interleukin-2 levels and proliferation of human T cell lines in vitro. Immunol Lett 35:177–182. doi: 10.1016/0165-2478(93)90088-J. [DOI] [PubMed] [Google Scholar]
- 144.Wang TT, Dabbas B, Laperriere D, Bitton AJ, Soualhine H, Tavera-Mendoza LE, Dionne S, Servant MJ, Bitton A, Seidman EG, Mader S, Behr MA, White JH. 2010. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin β2 innate immune pathway defective in Crohn disease. J Biol Chem 285:2227–2231. doi: 10.1074/jbc.C109.071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Borrelli O, Cordischi L, Cirulli M, Paganelli M, Labalestra V, Uccini S, Russo PM, Cucchiara S. 2006. Polymeric diet alone versus corticosteroids in the treatment of active pediatric Crohn’s disease: a randomized controlled open-label trial. Clin Gastroenterol Hepatol 4:744–753. doi: 10.1016/j.cgh.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 146.Iqbal N, Oliver JR, Wagner FH, Lazenby AS, Elson CO, Weaver CT. 2002. T helper 1 and T helper 2 cells are pathogenic in an antigen-specific model of colitis. J Exp Med 195:71–84. doi: 10.1084/jem.2001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Randhawa PK, Singh K, Singh N, Jaggi AS. 2014. A review on chemical-induced inflammatory bowel disease models in rodents. Korean J Physiol Pharmacol 18:279–288. doi: 10.4196/kjpp.2014.18.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mizoguchi A. 2004. Animal models of inflammatory bowel disease. Prog Mol Biol Transl Sci 50:81–92. [DOI] [PubMed] [Google Scholar]
- 149.Mansfield CS, James FE, Craven M, Davies DR, O’Hara AJ, Nicholls PK, Dogan B, MacDonough SP, Simpson KW. 2009. Remission of histiocytic ulcerative colitis in boxer dogs correlates with eradication of invasive intramucosal Escherichia coli. J Vet Intern Med 23:964–969. doi: 10.1111/j.1939-1676.2009.0363.x. [DOI] [PubMed] [Google Scholar]
- 150.Mansfield KG, Lin KC, Xia D, Newman JV, Schauer DB, MacKey J, Lackner AA, Carville A. 2001. Enteropathogenic Escherichia coli and ulcerative colitis in cotton-top tamarins (Saguinus oedipus). J Infect Dis 184:803–807. doi: 10.1086/322990. [DOI] [PubMed] [Google Scholar]
- 151.Kobayashi T, Steinbach EC, Russo SM, Matsuoka K, Nochi T, Maharshak N, Borst LB, Hostager B, Garcia-Martinez JV, Rothman PB, Kashiwada M, Sheikh SZ, Murray PJ, Plevy SE. 2014. NFIL3-deficient mice develop microbiota-dependent, IL-12/23-driven spontaneous colitis. J Immunol 192:1918–1927. doi: 10.4049/jimmunol.1301819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wald D, Qin J, Zhao Z, Qian Y, Naramura M, Tian L, Towne J, Sims JE, Stark GR, Li X. 2003. SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol 4:920–927. doi: 10.1038/ni968. [DOI] [PubMed] [Google Scholar]
- 153.Hoffmann JC, Pawlowski NN, Kühl AA, Höhne W, Zeitz M. 2002. –2003. Animal models of inflammatory bowel disease: an overview. Pathobiology 70:121–130. doi: 10.1159/000068143. [DOI] [PubMed] [Google Scholar]
- 154.Gullberg E, Söderholm JD. 2006. Peyer’s patches and M cells as potential sites of the inflammatory onset in Crohn’s disease. Ann N Y Acad Sci 1072:218–232. doi: 10.1196/annals.1326.028. [DOI] [PubMed] [Google Scholar]
- 155.Xavier RJ, Podolsky DK. 2007. Unravelling the pathogenesis of inflammatory bowel disease. Nature 448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 156.Morson BC. 1972. The early histological lesion of Crohn’s disease. Proc R Soc Med 65:71–72. [PMC free article] [PubMed] [Google Scholar]
- 157.Chassaing B, Rolhion N, De Vallée A, Salim SY, Prorok-Hamon M, Neut C, Campbell BJ, Söderholm JD, Hugot JP, Colombel JF, Darfeuille-Michaud A. 2011. Crohn disease-associated adherent-invasive E. coli bacteria target mouse and human Peyer’s patches via long polar fimbriae. J Clin Invest 121:966–975. doi: 10.1172/JCI44632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tlaskalová-Hogenová H, Štěpánková R, Hudcovic T, Tučková L, Cukrowska B, Lodinová-Žádníková R, Kozáková H, Rossmann P, Bártová J, Sokol D, Funda DP, Borovská D, Řeháková Z, Šinkora J, Hofman J, Drastich P, Kokešová A. 2004. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunol Lett 93:97–108. doi: 10.1016/j.imlet.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 159.Li MC, He SH. 2004. IL-10 and its related cytokines for treatment of inflammatory bowel disease. World J Gastroenterol 10:620–625. doi: 10.3748/wjg.v10.i5.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kim SC, Tonkonogy SL, Albright CA, Tsang J, Balish EJ, Braun J, Huycke MM, Sartor RB. 2005. Variable phenotypes of enterocolitis in interleukin 10-deficient mice monoassociated with two different commensal bacteria. Gastroenterology 128:891–906. doi: 10.1053/j.gastro.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 161.Lauridsen HC, Yang H, Bosman E, Yu H, Wu X, Ma C, Struve C, Petersen AM, Krogfelt KA, Jacobson K, Vallance B. 2015. Ulcerative colitis-associated Escherichia coli colonize the ileum and cecum of infected mice by adhering to the intestinal epithelium. Gastroenterology 148:S712. doi: 10.1016/S0016-5085(15)32421-5. [DOI] [Google Scholar]
- 162.Barnich N, Darfeuille-Michaud A. 2007. Adherent-invasive Escherichia coli and Crohn’s disease. Curr Opin Gastroenterol 23:16–20. doi: 10.1097/MOG.0b013e3280105a38. [DOI] [PubMed] [Google Scholar]
- 163.Torres AG, Zhou X, Kaper JB. 2005. Adherence of diarrheagenic Escherichia coli strains to epithelial cells. Infect Immun 73:18–29. doi: 10.1128/IAI.73.1.18-29.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Darfeuille-Michaud A. 2002. Adherent-invasive Escherichia coli: a putative new E. coli pathotype associated with Crohn’s disease. Int J Med Microbiol 292:185–193. doi: 10.1078/1438-4221-00201. [DOI] [PubMed] [Google Scholar]
- 165.Baumgart M, Dogan B, Rishniw M, Weitzman G, Bosworth B, Yantiss R, Orsi RH, Wiedmann M, McDonough P, Kim SG, Berg D, Schukken Y, Scherl E, Simpson KW. 2007. Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn’s disease involving the ileum. ISME J 1:403–418. doi: 10.1038/ismej.2007.52. [DOI] [PubMed] [Google Scholar]
- 166.Barnich N, Carvalho FA, Glasser AL, Darcha C, Jantscheff P, Allez M, Peeters H, Bommelaer G, Desreumaux P, Colombel JF, Darfeuille-Michaud A. 2007. CEACAM6 acts as a receptor for adherent-invasive E. coli, supporting ileal mucosa colonization in Crohn disease. J Clin Invest 117:1566–1574. doi: 10.1172/JCI30504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Barnich N, Bringer MA, Claret L, Darfeuille-Michaud A. 2004. Involvement of lipoprotein NlpI in the virulence of adherent invasive Escherichia coli strain LF82 isolated from a patient with Crohn’s disease. Infect Immun 72:2484–2493. doi: 10.1128/IAI.72.5.2484-2493.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Barnich N, Darfeuille-Michaud A. 2007. Role of bacteria in the etiopathogenesis of inflammatory bowel disease. World J Gastroenterol 13:5571–5576. doi: 10.3748/wjg.v13.i42.5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Le Bouguenec C, Servin AL. 2006. Diffusely adherent Escherichia coli strains expressing Afa/Dr adhesins (Afa/Dr DAEC): hitherto unrecognized pathogens. FEMS Microbiol Lett 256:185–194. doi: 10.1111/j.1574-6968.2006.00144.x. [DOI] [PubMed] [Google Scholar]
- 170.Betis F, Brest P, Hofman V, Guignot J, Kansau I, Rossi B, Servin A, Hofman P. 2003. Afa/Dr diffusely adhering Escherichia coli infection in T84 cell monolayers induces increased neutrophil transepithelial migration, which in turn promotes cytokine-dependent upregulation of decay-accelerating factor (CD55), the receptor for Afa/Dr adhesin. Infect Immun 71:1774–1783. doi: 10.1128/IAI.71.4.1774-1783.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Servin AL. 2005. Pathogenesis of Afa/Dr diffusely adhering Escherichia coli. Clin Microbiol Rev 18:264–292. doi: 10.1128/CMR.18.2.264-292.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Winter SE, Winter MG, Xavier MN, Thiennimitr P, Poon V, Keestra AM, Laughlin RC, Gomez G, Wu J, Lawhon SD, Popova IE, Parikh SJ, Adams LG, Tsolis RM, Stewart VJ, Bäumler AJ. 2013. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science 339:708–711. doi: 10.1126/science.1232467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI. 2009. A core gut microbiome in obese and lean twins. Nature 457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. 2006. Microbial ecology: human gut microbes associated with obesity. Nature 444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 175.Zhu W, Winter MG, Byndloss MX, Spiga L, Duerkop BA, Hughes ER, Büttner L, de Lima Romão E, Behrendt CL, Lopez CA, Sifuentes-Dominguez L, Huff-Hardy K, Wilson RP, Gillis CC, Tükel Ç, Koh AY, Burstein E, Hooper LV, Bäumler AJ, Winter SE. 2018. Precision editing of the gut microbiota ameliorates colitis. Nature 553:208–211. doi: 10.1038/nature25172. [DOI] [PMC free article] [PubMed] [Google Scholar]