Significance
In the present study, we found a significantly up-regulated CD3+CD4−CD8− T cell (double-negative T cell; DNT) population in the peripheral blood of patients with acute stroke compared with age-matched healthy subjects. Interestingly, infiltrating DNTs were mainly located close to microglia in the ischemic brain tissues of both stroke patients and middle cerebral artery occlusion mice. DNTs amplified the proinflammatory microglia and subsequently enhanced the neuroinflammation and exacerbated the brain injury after ischemic stroke. Mechanistically, DNT-intrinsic FasL and PTPN2 collaboratively regulate the level of CD86+ microglia via production of TNF-α. Taken together, these data reveal that infiltrating DNTs are the critical driving force in promoting microglia-mediated neuroinflammation and ischemic brain injury.
Keywords: ischemic stroke, neuroinflammation, DNTs, FasL, T cell-mediated immunity
Abstract
CD3+CD4−CD8− T cells (double-negative T cells; DNTs) have diverse functions in peripheral immune-related diseases by regulating immunological and inflammatory homeostasis. However, the functions of DNTs in the central nervous system remain unknown. Here, we found that the levels of DNTs were dramatically increased in both the brain and peripheral blood of stroke patients and in a mouse model in a time-dependent manner. The infiltrating DNTs enhanced cerebral immune and inflammatory responses and exacerbated ischemic brain injury by modulating the FasL/PTPN2/TNF-α signaling pathway. Blockade of this pathway limited DNT-mediated neuroinflammation and improved the outcomes of stroke. Our results identified a critical function of DNTs in the ischemic brain, suggesting that this unique population serves as an attractive target for the treatment of ischemic stroke.
Immune inflammation mediated by T cells that interact with microglia leads to brain damage after ischemic stroke (1). Previous studies assessing the mechanism by which T cells regulate immune and inflammatory responses in the brain during stroke have mainly focused on CD4+ helper and CD8+ cytotoxic T cells, which comprise ∼95% of T cells (2). Here, we found that CD3+CD4−CD8− T cells (double-negative T cells; DNTs), which account for 1 to 5% of the total T cell population in both naive mice and healthy humans (3–5), contribute to neuroinflammation and brain damage after ischemic stroke.
In this study, the level of DNTs was increased in both the peripheral blood and brain of stroke patients and middle cerebral artery occlusion (MCAO) mice. DNTs induced brain injury by promoting inflammatory microglial activation and neuroinflammation through the FasL/PTPN2/TNF-α pathway. This function of DNTs will shed light on the development of a therapeutic strategy for stroke.
Results
The Levels of DNTs Increase in the Brain and Peripheral Blood of Stroke Patients and MCAO Mice.
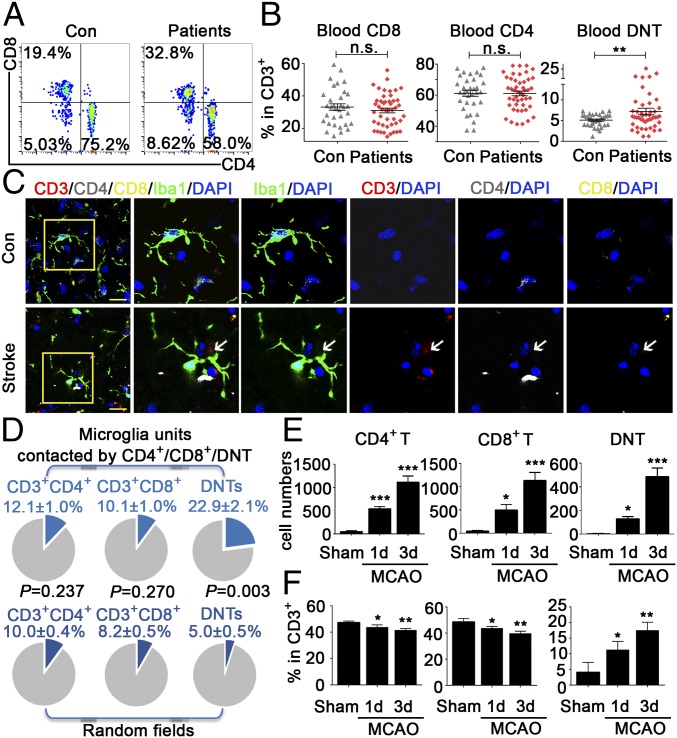
Previous study exploring the contribution of T cells to ischemic stroke has mainly focused on infiltrating CD3+CD8+ and CD3+CD4+ T cells (6–8). However, little has been studied about the impacts of DNTs on brain inflammation during ischemic stroke. Recent advances have uncovered that DNTs rapidly infiltrate the ischemic brain, but knowledge of their function in the CNS is still lacking (9, 10). We analyzed different subtypes of T cells in the peripheral blood of 47 patients with acute ischemic stroke and 33 healthy controls (SI Appendix, Tables S1 and S2). In the blood of healthy people, DNTs accounted for ∼4.98 ± 0.26% of CD3+ T cells. Surprisingly, compared with healthy controls, 68.09% (32/47) of stroke patients showed elevated levels of DNTs, and the DNT percentage was up to 7.05 ± 0.78% (Fig. 1 A and B). To explore the infiltration pattern of DNTs in the brain after stroke, we stained groups of postmortem brain sections from stroke (SI Appendix, Materials and Methods and Table S3). Immunofluorescence staining of patients’ brain slices showed DNTs located more around Iba-1+ microglia in ischemic penumbra than that in randomly captured microscopic fields (P = 0.003, 22.9 ± 2.1% surrounding microglia versus 5.0 ± 0.5% in random fields), suggesting a possible spatial and temporal cross-talk between DNTs and microglia after stroke. In contrast, this interesting phenomenon was not observed in CD4+ and CD8+ T cells between ischemic penumbra and randomly captured fields (Fig. 1 C and D and SI Appendix, Fig. S1 E and F).
Fig. 1.
Levels of DNTs increased in the brain and blood of stroke patients and MCAO mice. (A) Representative FACS plots of CD3+CD4−CD8− T cells (DNTs) in the blood of 47 stroke patients and 33 healthy controls. Con, control. (B) The percentages of DNTs, CD4+ T cells, and CD8+ T cells in human blood. (C) Confocal images of brain sections from stroke patients. (Scale bars, 20 µm.) The arrows indicate CD3+CD4−CD8− T cells in brain slices. n = 10 sections from six stroke patients. (D) Quantitation of CD4+ T cells, CD8+ T cells, and DNTs surrounding microglia in brains of stroke patients. We quantified 36 fields in 6 samples and counted 105 microglia in ischemic penumbra (Upper) and random fields (Lower). Blue sectors indicate percentages of the T cell subset around microglia. (E and F) The numbers and percentages of CD4+ T cells, CD8+ T cells, and CD4−CD8− T cells over the CD3+ T cell population in the mouse ischemic hemisphere 1 and 3 d after MCAO evaluated by FACS (n = 10 per group). *P < 0.05, **P < 0.01, and ***P < 0.001 compared with the sham group. n.s., not significant. The data are presented as the mean ± SD.
Moreover, we investigated the levels of DNTs in the ischemic brain and peripheral blood in mice. Consistently, we detected a prominent infiltration of different subtypes of T cells, including CD4+ T cells, CD8+ T cells, and DNTs, into the ischemic penumbra of wild-type mice 1 to 3 d after MCAO (Fig. 1 E and F and SI Appendix, Fig. S2A), a time when a pronounced inflammatory response occurs (11). Moreover, the percentage of DNTs in the blood was higher in ischemic mice than that in the sham group, and increased over time in the periphery (SI Appendix, Fig. S1 A and B). Although DNTs accounted for only ∼1 to 5% of the T cells in the peripheral blood (SI Appendix, Fig. S1 A and B) and were not the dominant population compared with CD4+ and CD8+ T cells in the brain after ischemia (Fig. 1E), they actively and profoundly accumulated over time (Fig. 1F). Furthermore, we discovered that DNTs had infiltrated the brain at day 1 and further increased at day 3 after stroke, suggesting that the infiltrating DNTs might actively migrate into the brain (Fig. 1 E and F). In addition, both the percentage and absolute number of DNTs increased dramatically over time, suggesting a critical role of DNTs during the progression of ischemic stroke (Fig. 1 E and F). Consistent with the data from stroke patients, DNTs increased in the ischemic penumbra of wild-type mice and located near the activated microglia, and this accumulation persisted for 3 d after reperfusion (SI Appendix, Fig. S2 B and C).
DNTs Promote Proinflammatory Microglial Activation After Ischemic Stroke.
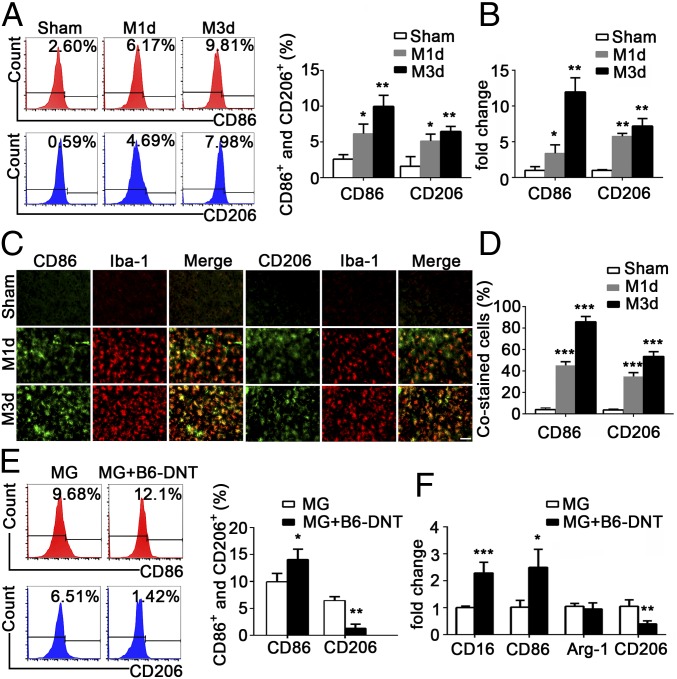
Flow cytometry was used to analyze the levels of proinflammatory and antiinflammatory microglia after stroke (SI Appendix, Fig. S3). The percentages of CD11b+CD45midCD86+ proinflammatory microglia and CD11b+CD45midCD206+ antiinflammatory microglia increased in the ischemic penumbra from day 1 to 3 after MCAO (Fig. 2A), but the former was present at higher levels. Consistently, the results from real-time PCR (Fig. 2B) and the immunofluorescence costaining for CD86+/Iba-1+ or CD206+/Iba-1+ (Fig. 2 C and D) further confirmed the up-regulation of CD86 and CD206 in the ischemic penumbra after MCAO.
Fig. 2.
DNTs promoted proinflammatory microglial activation during ischemic stroke. (A) Flow cytometry analysis of the percentages of CD11b+CD45midCD86+ proinflammatory and CD11b+CD45midCD206+ antiinflammatory microglia. (B) RT-PCR results showing the mRNA levels of the proinflammatory surface marker CD86 and the antiinflammatory surface marker CD206 in the ipsilateral cortex. (C) Images of the cortex costained for CD86 (green) and Iba-1 (red) or for CD206 (green) and Iba-1 (red). (Scale bar, 50 µm.) (D) The percentages of CD86+/Iba-1+ cells and CD206+/Iba-1+ cells are quantified. n = 10 animals per group. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with the sham group. (E) DNTs (5 × 105 cells) sorted from the spleens of C57BL/6J mice 3 d after MCAO were cocultured with microglia (5 × 105 cells) for 24 h. The percentages of CD86+ and CD206+ microglia were analyzed by flow cytometry. (F) RT-PCR results showing the mRNA levels of proinflammatory markers (CD16 and CD86) and antiinflammatory markers (Arg-1 and CD206). *P < 0.05, **P < 0.01, and ***P < 0.001 compared with the microglia (MG) group. The data are presented as the mean ± SD. M1d, day 1 of MCAO; M3d, day 3 of MCAO.
Next, we determined whether DNTs directly contributed to the activation of proinflammatory microglia. Interestingly, while the percentage of proinflammatory (CD86+) microglia increased, the percentage of antiinflammatory (CD206+) microglia decreased after coculture with DNTs isolated from wild-type spleens 3 d after MCAO (Fig. 2E). Similarly, the mRNA levels of proinflammatory microglial markers (CD16 and CD86) increased accompanied by a decreased antiinflammatory marker (CD206) after coculture with DNTs (Fig. 2F), suggesting the infiltrating DNTs contribute to the activation of proinflammatory microglia during acute ischemic stroke.
DNTs Aggravate Brain Injury After MCAO in Rag1−/− Mice.
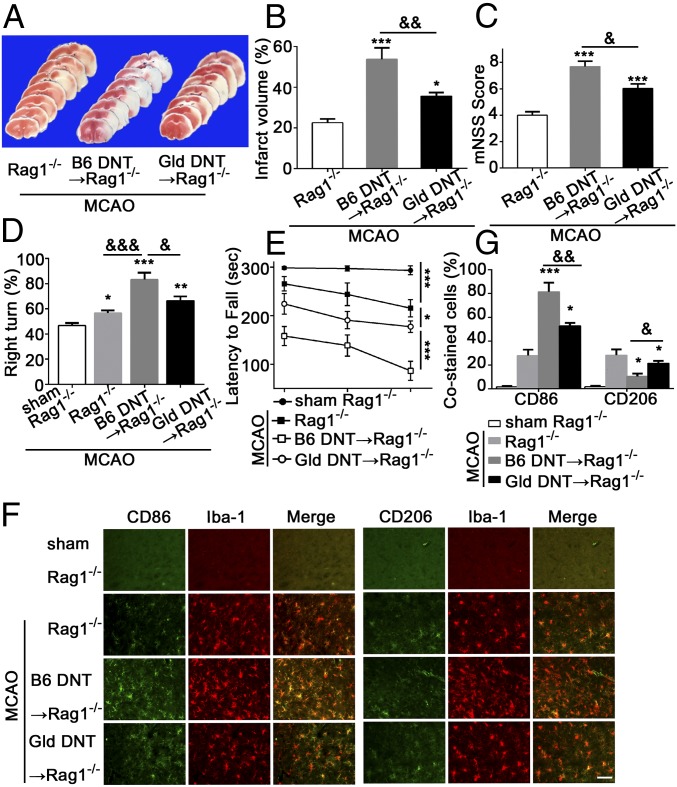
Rag1−/− mice (without mature T cells and B cells) were subjected to MCAO to further confirm the critical role of DNTs in microglial neuroinflammation after acute ischemic stroke. Rag1−/− mice were reconstituted with DNTs isolated from the spleen of B6 mice followed by induction of MCAO. As expected, the infarct volumes (Fig. 3 A and B), modified neurological severity scores (mNSS) (Fig. 3C), right-turn test (Fig. 3D), and latency to fall (Fig. 3E) in Rag1−/− mice receiving wild-type DNTs were significantly worse, suggesting that DNTs are an important factor contributing to ischemic brain injury. We then investigated the possible contributions of reconstituted DNTs to microglial activation in vivo. Consistently, the number of proinflammatory CD86+ microglia increased and the number of antiinflammatory CD206+ microglia decreased in Rag1−/− mice reconstituted with wild-type DNTs (Fig. 3 F and G), suggesting DNTs promote ischemic injury by activating brain proinflammatory microglia after acute stroke.
Fig. 3.
Rag1−/− mice reconstituted with DNTs with a FasL mutation showed reduced brain injury and microglial inflammation. (A and B) TTC staining showing the infarct volume in Rag1−/− mice receiving DNTs from C57BL/6J or gld mice 3 d after MCAO. (C–E) Neurological severity scores, corner test, and rotarod test in the indicated mice after MCAO. (F and G) Representative images and quantification of the cortex CD86+/Iba-1+ and CD206+/Iba-1+ microglia in the ischemic hemisphere. (Scale bar, 50 µm.) n = 10 animals per group. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with the Rag1−/− mice with MCAO; &P < 0.05, &&P < 0.01, and &&&P < 0.001 compared with Rag1−/− mice reconstituted with B6 DNTs. The data are presented as the mean ± SD.
DNT-Derived TNF-α Is a Major Contributor to Proinflammatory Microglial Activation.
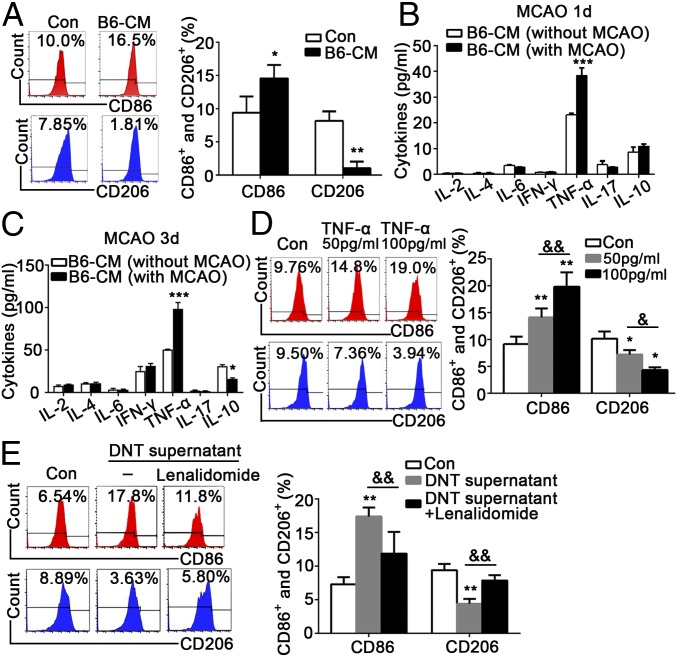
A critical question is the mechanism by which DNTs promote inflammatory microglial activation during ischemic stroke. Microglia were treated with conditioned medium (CM) collected from the DNTs of wild-type mice after stroke. Strikingly, this CM increased CD86+ microglial activation and decreased CD206+ microglial activation (Fig. 4A). Next, we screened the characteristic cytokines of DNTs in medium collected from cultured DNTs obtained from mice with or without MCAO. Our results revealed a significant increase in TNF-α levels in the DNT medium 1 and 3 d after MCAO (Fig. 4 B and C). In addition to TNF-α, we also noticed a mild reduced IL-10 in the DNT conditioned medium. To exclude the effects of reduced IL-10 on the microglial activation, we added exogenous IL-10 with different concentrations (ranging from 30 pg/mL to 30 ng/mL) to DNT conditioned medium and cocultured with microglia. Our data demonstrated that CD206+ microglia were increased only when exposed to higher-level IL-10 (30 ng/mL) but not to low levels (as we detected in DNT medium) (SI Appendix, Fig. S4), suggesting that decreased IL-10 in the medium failed to promote proinflammatory microglial polarization. Microglia were treated with exogenous TNF-α at two different doses (50 and 100 pg/mL) to further confirm the direct modulatory effect of TNF-α on microglia. As expected, the percentage of CD86+ microglia increased and CD206+ microglia decreased after TNF-α stimulation in a dose-dependent manner (Fig. 4D). In contrast, when the DNTs were treated with 5 µM lenalidomide, a specific TNF-α inhibitor, to repress TNF-α release, we found the percentage of CD86+ microglia was down-regulated and the percentage of CD206+ microglia was up-regulated (Fig. 4E). These results suggested that TNF-α plays an indispensable role in DNT-triggered activation of proinflammatory microglia.
Fig. 4.
DNT-derived TNF-α promoted proinflammatory microglial activation. The CM was collected from the cultured DNTs sorted from the spleens of C57BL/6J mice with or without MCAO and used to stimulate microglia for 24 h. (A) The percentages of CD86+ proinflammatory and CD206+ antiinflammatory microglia were determined using FACS. n = 12 animals per group. *P < 0.05 and **P < 0.01 compared with the control group (microglia only). (B and C) The cytokine profiles in the DNT CM before and after MCAO were evaluated by CBA. n = 12 animals per group. *P < 0.05 and ***P < 0.001 compared with the B6 CM (without MCAO) group. (D) The microglia were treated with or without TNF-α at two concentrations. The percentages of CD86+ proinflammatory and CD206+ antiinflammatory microglia were determined using FACS. n = 12 samples per group. *P < 0.05 and **P < 0.01 compared with the control group (without TNF-α); &P < 0.05 and &&P < 0.01 compared with the 50 pg/mL TNF-α–stimulated group. (E) CM from DNTs treated with or without the TNF-α inhibitor lenalidomide (5 µM) was applied to microglia. The percentages of CD86+ and CD206+ microglia were determined using FACS. n = 12 samples per group. **P < 0.01 compared with the control group (without DNT supernatant or lenalidomide); &&P < 0.01 compared with the DNT supernatant-stimulated group. The data are presented as the mean ± SD.
TNF-α Mediates Proinflammatory Microglial Activation Through FasL in DNTs After Ischemic Stroke.
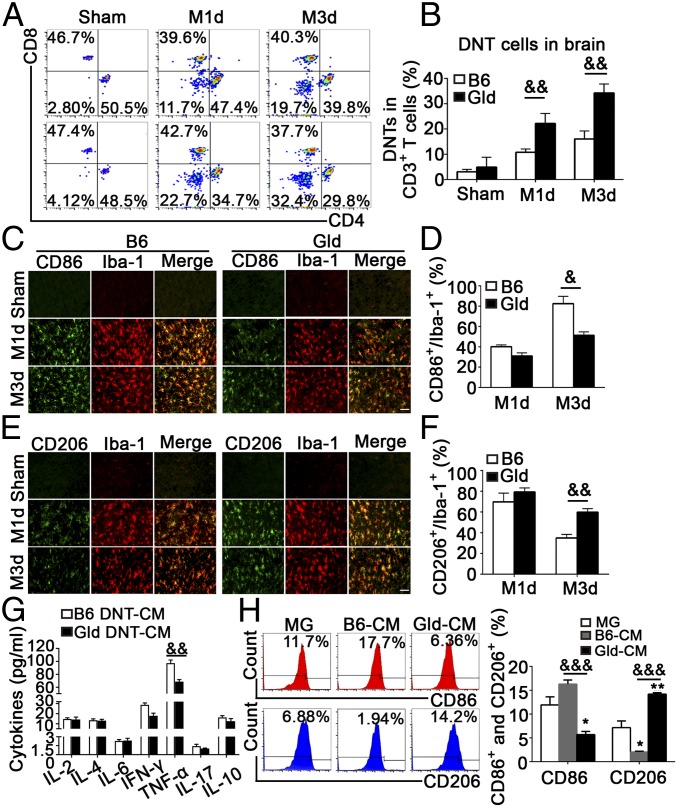
FasL has been reported to regulate T cell function via various signaling pathways (12). Thus, we hypothesized that FasL regulates the function of DNTs in modulating microglial activation. We analyzed the infiltration of DNTs into the ischemic penumbra after MCAO in FasL mutant mice (gld) and controls by flow cytometry. Of note, greater numbers of DNTs accumulated in the ischemic brains of gld mice than that in B6 mice after MCAO (Fig. 5 A and B). Moreover, while CD86+/Iba-1+ microglia were prominent in the ischemic brains of B6 mice after MCAO, this population was substantially reduced in gld mice (Fig. 5 C and D). However, a higher percentage of CD206+/Iba-1+ cells was detected in gld mice compared with that in B6 mice 3 d after MCAO (Fig. 5 E and F).
Fig. 5.
FasL deficiency inhibited TNF-α secretion from DNTs and attenuated proinflammatory microglial activation after stroke. (A and B) Representative FACS plots and quantification of CD4−CD8− T cells in the brain 1 and 3 d after MCAO. (C and D) Images and quantification of cortex CD86+/Iba-1+ microglia. (Scale bar, 50 µm.) n = 10 animals per group. (E and F) Images and quantification of cortex CD206+/Iba-1+ microglia. (Scale bar, 50 µm.) n = 10 animals per group. &P < 0.05 and &&P < 0.01 compared with the B6 MCAO group. DNTs obtained from C57BL/6J and gld mice 3 d after MCAO were cultured in vitro for 24 h. The DNT supernatant was collected and used to stimulate microglia for 24 h. (G) The cytokine profiles of the DNT supernatant were evaluated using CBA. (H) The percentages of CD86+ and CD206+ microglia were analyzed using FACS. n = 12 samples per group. *P < 0.05 and **P < 0.01 compared with the control group (microglia only); &&P < 0.01 and &&&P < 0.001 compared with the B6 group. The data are presented as the mean ± SD. M1d, day 1 of MCAO; M3d, day 3 of MCAO.
DNTs isolated from gld or wild-type B6 mice 3 d after MCAO were cocultured with wild-type primary microglia for 24 h. DNTs from the FasL mutant mice resulted in reduced CD86+microglial activation but enhanced CD206+ microglial activation (SI Appendix, Fig. S5A). Consistently, cytometric bead array (CBA) results (SI Appendix, Fig. S5B) revealed a 51.5% decrease of TNF-α in the supernatant of FasL-mutated DNTs compared with that from wild-type mice (85.32 ± 1.03 vs. 39.92 ± 1.23 pg/mL, P < 0.05). Moreover, significantly reduced TNF-α was detected in DNT medium from gld mice than that from B6 mice 3 d after MCAO (Fig. 5G). Additionally, gld DNT CM significantly down-regulated CD86+ microglia and up-regulated CD206+ microglia (Fig. 5H), suggesting FasL promoted TNF-α expression in DNTs.
Next, we reconstituted Rag1−/− mice with DNTs from gld mice followed by MCAO and found these mice demonstrated smaller infarcts and reduced neurological deficits than control mice reconstituted with B6 DNTs (Fig. 3 A–E). Additionally, the number of CD86+ microglia decreased and the number of CD206+ microglia increased in Rag1−/− mice reconstituted with gld DNTs (Fig. 3 F and G), suggesting DNT-promoted ischemic brain injury is at least partially reversed by blocking FasL.
To determine whether the results obtained from FasL mutant mice can be translated into therapy, anti-FasL antibodies were injected intraperitoneally into wild-type mice at 30 min, 24 h, and 48 h after MCAO. We found anti-FasL antibody massively penetrated the MCAO brain, effectively bound to DNTs, and significantly blocked FasL signaling (SI Appendix, Fig. S6). TTC (2,3,5-triphenyltetrazolium chloride) staining and behavior tests confirmed that FasL inhibition not only effectively promoted antiinflammatory microglial activation but also reduced ischemic brain injury (SI Appendix, Fig. S7 A–E). Furthermore, anti-FasL administration significantly inhibited proinflammatory microglial activation, suggesting FasL on DNTs promoted microglial neuroinflammation and stroke severity by enhancing TNF-α production (SI Appendix, Fig. S7 F and G).
The Key Role of PTPN2 in the FasL-Induced Inflammatory Function of DNTs After Ischemic Stroke.
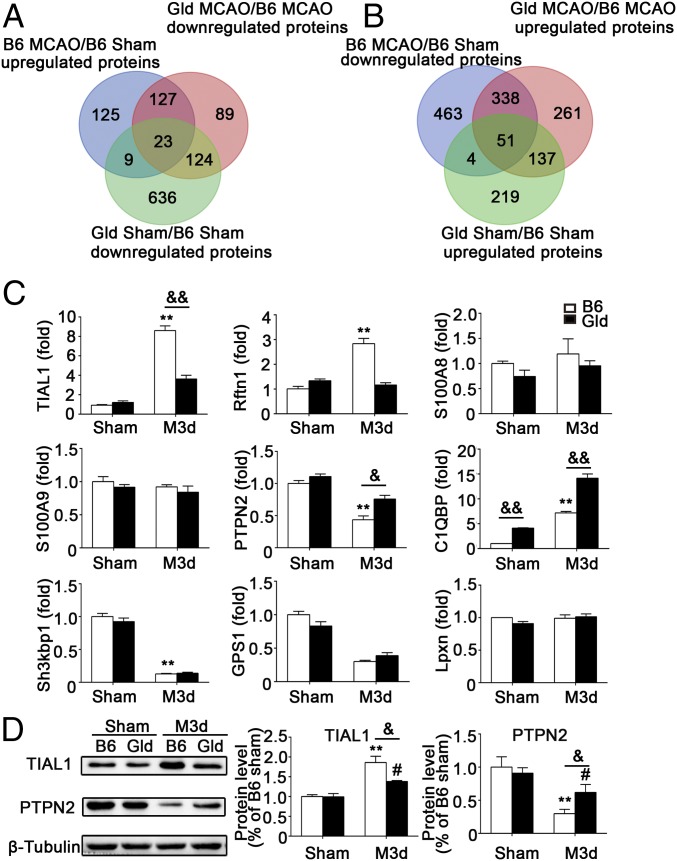
To identify the potential signaling pathways by which FasL specifically regulates TNF-α production in DNTs, protein mass spectrometry was performed to profile global molecular alterations in DNTs with or without FasL expression from B6 or gld mice 3 d after MCAO (SI Appendix, Figs. S8–S10). As shown by Venn diagram (Fig. 6 A and B), 127 of 726 (13.5%) MCAO-induced proteins were significantly decreased and 338 of 724 (46.7%) proteins were increased in DNTs isolated from gld mice compared with those from wild-type mice 3 d after MCAO. Pathway map analysis showed that nine significantly altered molecules were involved in inflammatory responses, including four proinflammatory proteins (TIAL1, RFTN1, S100A9, and S100A8) and five antiinflammatory proteins (PTPN2, C1QBP, SH3KBP1, GPS1, and LPXN), suggesting these proteins might be involved in the FasL signaling pathway in DNT-mediated TNF-α after MCAO.
Fig. 6.
Verification of differentially expressed inflammatory proteins identified in the proteomic analysis. Mass spectrometry was used to profile the global alterations in proteins expressed in DNTs isolated from B6 and gld mouse spleens 3 d after MCAO. (A) The Venn diagrams show that 127 proteins were up-regulated after MCAO, and the changes were reversed by the FasL mutation in gld mice. (B) Three hundred thirty-eight proteins were down-regulated after MCAO and rescued by the FasL mutation in gld mice. (C) RT-PCR analysis of four proinflammatory proteins (S100A9, S100A8, TIAL1, and RFTN1) and five antiinflammatory proteins (GPS1, SH3KBP1, PTPN2, C1QBP, and LPXN) in DNTs of the indicated mice. n = 9 mice per group. **P < 0.01 compared with the B6 sham group; &P < 0.05 and &&P < 0.01 compared with the B6 MCAO 3-d group. (D) Representative Western blots and quantification for PTPN2 and TIAL1. n = 9 samples per group. **P < 0.01 compared with the B6 sham group; #P < 0.05 compared with the gld sham group; &P < 0.05 compared with the B6 MCAO 3-d group. The data are presented as the mean ± SD. M1d, day 1 of MCAO; M3d, day 3 of MCAO.
Real-time PCR was performed to verify the mRNA levels of the nine proteins mentioned above in DNTs from B6 and gld mice 3 d after MCAO (Fig. 6C), and only three mRNAs (TIAL1, PTPN2, and C1QBP) were changed significantly. Because the C1QBP mRNA was differentially expressed between B6 and gld mice without MCAO, we selected TIAL1 and PTPN2 for further verification using Western blotting. Notably, significantly lower TIAL1 levels (P < 0.05) and higher levels of PTPN2 (P < 0.05) were detected in gld mice than in B6 mice 3 d after MCAO (Fig. 6D), suggesting FasL regulates the expression of PTPN2 and TIAL1 in DNTs.
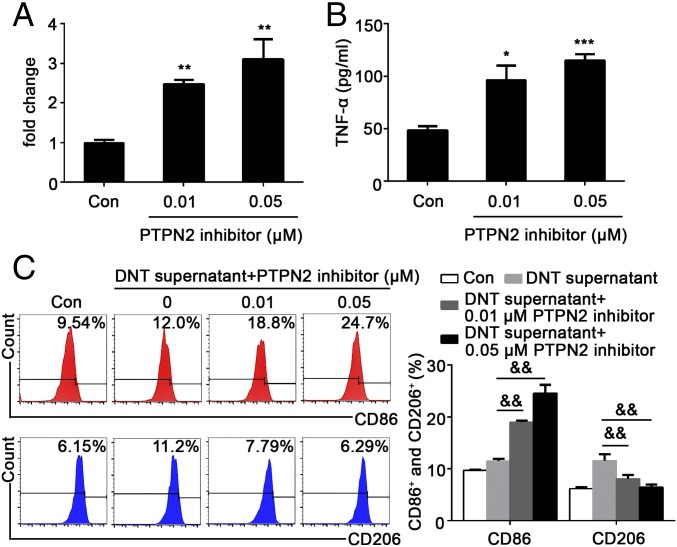
TNF-α Expression Is Regulated by PTPN2 in DNTs.
PTPN2 is strongly associated with the transcriptional regulation of inflammatory cytokines in pathological responses (13). Since increased PTPN2 expression was found in DNTs with a FasL mutation, we set out to investigate whether PTPN2 was involved in TNF-α production. DNTs from gld mice were pretreated with ethyl-3,4-dephosphostatin, a specific inhibitor of PTPN2, for 24 h. Real-time PCR and ELISA analysis demonstrated that the levels of the TNF-α mRNA and protein were significantly increased in DNTs treated with the PTPN2 inhibitor in a dose-dependent manner (Fig. 7 A and B), suggesting that PTPN2 is a negative regulator in DNTs of TNF-α production. Concomitantly, PTPN2 inhibitor pretreatment also reversed gld DNT CM-induced polarization of primary microglia (Fig. 7C). The percentage of CD86+ proinflammatory microglia was significantly increased and CD206+ antiinflammatory microglia significantly diminished when exposed to the supernatant of gld DNTs that were pretreated with PTPN2 inhibitor in vitro (Fig. 7C), suggesting the expression of DNT-derived TNF-α was negatively regulated by PTPN2. These results suggested that while FasL activation in DNTs promotes TNF-α production, PTPN2 serves as an intracellular negative regulator of FasL signaling to repress the secretion of TNF-α.
Fig. 7.
TNF-α expression is negatively regulated by PTPN2 in DNTs. (A and B) TNF-α mRNA and protein levels were analyzed by RT-PCR and ELISA, respectively. (C) The microglia were treated with CM from DNTs (with or without the PTPN2 inhibitor) for 24 h, followed by flow cytometry analysis of the percentage of CD86+ proinflammatory and CD206+ antiinflammatory microglia. n = 9 samples per group. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with the control group (microglia only); &&P < 0.01 compared with the DNT supernatant (without PTPN2 inhibition) group. The data are presented as the mean ± SD.
Discussion
Despite DNTs accounting for a small percentage (1 to 5%) of T cells in the peripheral blood and lymphoid organs, they play important roles in many diseases (3, 4). Few studies have evaluated the roles of DNTs in the brain. Here, we observed completely different distribution patterns among CD4+ T cells, CD8+ T cells, and DNTs in the ischemic brain. In both human and mouse peripheral blood samples, the percentage of DNTs, rather than CD4+ T cells or CD8+ T cells, increased after stroke, suggesting that DNTs play a crucial role in ischemic brain injury. Analysis of brain sections from stroke patients and MCAO mice demonstrated that DNTs were the major T cell subset located around microglia, suggesting DNTs might communicate with microglia to mount a unique immune response and amplify neuroinflammation.
It was reported that peripheral DNTs are mainly derived from the thymus and spleen (5), less from thymus-independent ways such as activated peripheral CD4+ (14) and/or CD8+ T cells (15, 16). However, the sources of DNTs in the diseased brain of ischemic stroke patients are still unknown. Ischemic stroke causes dramatic and acute brain inflammation (17), and DNTs might be attracted by the chemokines released in the brain and migrate to the inflamed lesion. In addition, previous evidence has shown that some unique T cell subpopulations like Th17 can convert to another T cell subset (such as Treg) in the brain during the resolution of neuroinflammation (18). However, whether DNTs can be converted from other T cell subsets during ischemic stroke is still to be elucidated. Moreover, stimuli like self-antigens exposed in the inflammatory CNS can also lead to T cell proliferation and expansion in situ (19, 20), which also serves as a possible manner for the accumulation of DNTs in the ischemic brain, which is beyond the scope of this study.
Several studies have reported that DNTs have distinct cytokine expression patterns depending on the inflammatory environment and associated exposed stimuli (3, 4, 21–23). Based on previous publications (15, 24, 25), we selected the key cytokines and assessed their production levels in both the MCAO model and cultured DNTs. Interestingly, the most increased inflammatory protein secreted by DNTs detected is TNF-α. Accumulating evidence showed ischemic stroke led to remarkable activation of the immune cells in the spleen and peripheral blood (26–29). In several pathological diseases, DNTs have been reported to produce TNF-α in the periphery, augment inflammation, and promote disease progression (30–35). Our results extended previous findings and demonstrated that DNTs in the ischemic brain promoted inflammatory microglial activation via production of TNF-α in vivo and in vitro.
PTPN2, also known as T cell protein tyrosine phosphatase, is highly expressed in hematopoietic cells, including T cells, and is generally considered a negative regulator during inflammatory responses (36). The antiinflammatory effects of PTPN2 correlated with the dephosphorylation of Src family kinases, including Lck (36), and various JAK/STAT family members, such as JAK1, JAK3, STAT1, STAT5, STAT6 (37–39), and c-Src, to attenuate TNF-α production in inflammatory processes (40). A T cell-specific PTPN2 deficiency in mice enhances the responses of CD4+ and CD8+ T cells to T cell receptor (TCR) stimulation, resulting in the development of widespread inflammation and autoimmunity with aging (41, 42). However, little is known about the association between PTPN2 expression and the TNF-α–mediated inflammatory responses in DNTs. In the present study, we observed a profound decrease in PTPN2 expression and increased levels of TNF-α in DNTs in an acute ischemic stroke model (3 d after MCAO). Strikingly, DNTs isolated from gld mice showed up-regulated PTPN2 and reduced TNF-α. Moreover, PTPN2 inhibition increased TNF-α secretion from DNTs, promoted proinflammatory microglial activation, and exacerbated brain injury. Taken together, DNT-intrinsic FasL and PTPN2 collaboratively regulate the levels of CD86+ microglia and CD206+ microglia via production of TNF-α.
Our study highlights that infiltrating DNTs are the critical driving force in promoting microglia-mediated neuroinflammation and ischemic brain injury, suggesting the pharmacological targeting of the FasL/PTPN2/TNF-α signaling cascade in DNTs represents an attractive therapeutic approach in ischemic stroke (SI Appendix, Fig. S11).
Materials and Methods
Patients.
The clinical study was approved by the Ethics Committee of Nanjing Drum Tower Hospital, Medical School of Nanjing University, with informed consent obtained from each patient. Detailed information of patients (SI Appendix, Tables S1 and S2) and study design is provided.
Human Postmortem Brain Tissue and Immunohistochemistry.
Brain sections from patients with ischemic stroke were obtained from the Sun Health Research Institute. Written informed consent was received from the participants before inclusion in this study or donation of tissues. Detailed protocols for brain tissue immunohistochemistry are provided (SI Appendix, Materials and Methods).
Ischemic Models in Mice and Neurological Function and Molecular Biology Assessments.
All animal experiments were approved by the Animal Care and Use Committee of the Model Animal Research Center, Nanjing University, and performed according to institutional guidelines. The MCAO model induction and behavior tests were performed as previously described (43). The procedures for RT-PCR, Western blotting, and flow cytometry are provided (SI Appendix, Materials and Methods).
Cell Cocultures in Vitro, CM Experiments, ELISA, and Cytometric Bead Array.
DNTs obtained after MCAO in mice were cocultured with primary microglia. Detailed protocols for the isolation of DNTs and microglia, CM preparation, TNF-α and IL-10 stimulation, ELISA, and CBA are provided (SI Appendix, Materials and Methods).
Proteomic Analysis.
We used mass spectrometry to analyze alterations in protein profile in DNTs from B6 and gld mice with or without MCAO treatment. A detailed description of the proteomic analysis is provided (SI Appendix, Materials and Methods).
Statistics.
Detailed descriptions of the statistical analyses are provided (SI Appendix, Materials and Methods). The data are presented as the mean ± SD. P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank the Translational Medicine Core facilities of the Medical School of Nanjing University for their support in the proteomic analysis. We thank Cun-Jin Zhang and Mei-Juan Zhang for editing the manuscript. This research was supported by the National Natural Science Foundation of China (81630028, 81230026, 81701168, and 81701170), Science and Technology Department of Jiangsu Province (BE2016610), and Jiangsu Province Key Medical Discipline (ZDXKA2016020).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1814394116/-/DCSupplemental.
References
- 1.Wang S, Zhang H, Xu Y. Crosstalk between microglia and T cells contributes to brain damage and recovery after ischemic stroke. Neurol Res. 2016;38:495–503. doi: 10.1080/01616412.2016.1188473. [DOI] [PubMed] [Google Scholar]
- 2.Liesz A, et al. Inhibition of lymphocyte trafficking shields the brain against deleterious neuroinflammation after stroke. Brain. 2011;134:704–720. doi: 10.1093/brain/awr008. [DOI] [PubMed] [Google Scholar]
- 3.Ford MS, et al. Peptide-activated double-negative T cells can prevent autoimmune type-1 diabetes development. Eur J Immunol. 2007;37:2234–2241. doi: 10.1002/eji.200636991. [DOI] [PubMed] [Google Scholar]
- 4.Young KJ, DuTemple B, Phillips MJ, Zhang L. Inhibition of graft-versus-host disease by double-negative regulatory T cells. J Immunol. 2003;171:134–141. doi: 10.4049/jimmunol.171.1.134. [DOI] [PubMed] [Google Scholar]
- 5.Brandt D, Hedrich CM. TCRαβ+CD3+CD4-CD8- (double negative) T cells in autoimmunity. Autoimmun Rev. 2018;17:422–430. doi: 10.1016/j.autrev.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Kleinschnitz C, et al. Early detrimental T-cell effects in experimental cerebral ischemia are neither related to adaptive immunity nor thrombus formation. Blood. 2010;115:3835–3842. doi: 10.1182/blood-2009-10-249078. [DOI] [PubMed] [Google Scholar]
- 7.Shichita T, et al. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat Med. 2009;15:946–950. doi: 10.1038/nm.1999. [DOI] [PubMed] [Google Scholar]
- 8.Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006;113:2105–2112. doi: 10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]
- 9.Gelderblom M, et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40:1849–1857. doi: 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]
- 10.Chu HX, et al. Immune cell infiltration in malignant middle cerebral artery infarction: Comparison with transient cerebral ischemia. J Cereb Blood Flow Metab. 2014;34:450–459. doi: 10.1038/jcbfm.2013.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, et al. DRα1-MOG-35-55 reduces permanent ischemic brain injury. Transl Stroke Res. 2017;8:284–293. doi: 10.1007/s12975-016-0514-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calmon-Hamaty F, Audo R, Combe B, Morel J, Hahne M. Targeting the Fas/FasL system in rheumatoid arthritis therapy: Promising or risky? Cytokine. 2015;75:228–233. doi: 10.1016/j.cyto.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Tiganis T, Bennett AM. Protein tyrosine phosphatase function: The substrate perspective. Biochem J. 2007;402:1–15. doi: 10.1042/BJ20061548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grishkan IV, Ntranos A, Calabresi PA, Gocke AR. Helper T cells down-regulate CD4 expression upon chronic stimulation giving rise to double-negative T cells. Cell Immunol. 2013;284:68–74. doi: 10.1016/j.cellimm.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crispín JC, Tsokos GC. Human TCR-alpha beta+ CD4- CD8- T cells can derive from CD8+ T cells and display an inflammatory effector phenotype. J Immunol. 2009;183:4675–4681. doi: 10.4049/jimmunol.0901533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedrich CM, et al. cAMP-responsive element modulator α (CREMα) trans-represses the transmembrane glycoprotein CD8 and contributes to the generation of CD3+CD4-CD8- T cells in health and disease. J Biol Chem. 2013;288:31880–31887. doi: 10.1074/jbc.M113.508655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang CJ, et al. TLR-stimulated IRAKM activates caspase-8 inflammasome in microglia and promotes neuroinflammation. J Clin Invest. 2018;128:5399–5412. doi: 10.1172/JCI121901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gagliani N, et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 2015;523:221–225. doi: 10.1038/nature14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramadan A, et al. In situ expansion of T cells that recognize distinct self-antigens sustains autoimmunity in the CNS. Brain. 2016;139:1433–1446. doi: 10.1093/brain/aww032. [DOI] [PubMed] [Google Scholar]
- 20.Jin WN, et al. Brain ischemia induces diversified neuroantigen-specific T-cell responses that exacerbate brain injury. Stroke. 2018;49:1471–1478. doi: 10.1161/STROKEAHA.118.020203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crispín JC, et al. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol. 2008;181:8761–8766. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jang HR, Gandolfo MT, Ko GJ, Racusen L, Rabb H. The effect of murine anti-thymocyte globulin on experimental kidney warm ischemia-reperfusion injury in mice. Transpl Immunol. 2009;22:44–54. doi: 10.1016/j.trim.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Alunno A, et al. IL-17-producing CD4-CD8- T cells are expanded in the peripheral blood, infiltrate salivary glands and are resistant to corticosteroids in patients with primary Sjogren’s syndrome. Ann Rheum Dis. 2013;72:286–292. doi: 10.1136/annrheumdis-2012-201511. [DOI] [PubMed] [Google Scholar]
- 24.Fischer K, et al. Isolation and characterization of human antigen-specific TCR alpha beta+ CD4(-)CD8- double-negative regulatory T cells. Blood. 2005;105:2828–2835. doi: 10.1182/blood-2004-07-2583. [DOI] [PubMed] [Google Scholar]
- 25.Brandt D, Sergon M, Abraham S, Mäbert K, Hedrich CM. TCR+CD3+CD4-CD8- effector T cells in psoriasis. Clin Immunol. 2017;181:51–59. doi: 10.1016/j.clim.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Niu FN, et al. Targeted mutation of Fas ligand gene attenuates brain inflammation in experimental stroke. Brain Behav Immun. 2012;26:61–71. doi: 10.1016/j.bbi.2011.07.235. [DOI] [PubMed] [Google Scholar]
- 27.Clausen BH, et al. Systemically administered anti-TNF therapy ameliorates functional outcomes after focal cerebral ischemia. J Neuroinflammation. 2014;11:203. doi: 10.1186/s12974-014-0203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li P, et al. Adoptive regulatory T-cell therapy preserves systemic immune homeostasis after cerebral ischemia. Stroke. 2013;44:3509–3515. doi: 10.1161/STROKEAHA.113.002637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Offner H, et al. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006;26:654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- 30.Zlotnik A, Godfrey DI, Fischer M, Suda T. Cytokine production by mature and immature CD4-CD8- T cells. Alpha beta-T cell receptor+ CD4-CD8- T cells produce IL-4. J Immunol. 1992;149:1211–1215. [PubMed] [Google Scholar]
- 31.Antonelli LR, et al. Disparate immunoregulatory potentials for double-negative (CD4- CD8-) alpha beta and gamma delta T cells from human patients with cutaneous leishmaniasis. Infect Immun. 2006;74:6317–6323. doi: 10.1128/IAI.00890-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gollob KJ, Antonelli LR, Faria DR, Keesen TS, Dutra WO. Immunoregulatory mechanisms and CD4-CD8- (double negative) T cell subpopulations in human cutaneous leishmaniasis: A balancing act between protection and pathology. Int Immunopharmacol. 2008;8:1338–1343. doi: 10.1016/j.intimp.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.D’Acquisto F, Crompton T. CD3+CD4-CD8- (double negative) T cells: Saviours or villains of the immune response? Biochem Pharmacol. 2011;82:333–340. doi: 10.1016/j.bcp.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 34.Lu Y, et al. Liver TCRγδ(+) CD3(+) CD4(-) CD8(-) T cells contribute to murine hepatitis virus strain 3-induced hepatic injury through a TNF-α-dependent pathway. Mol Immunol. 2012;52:229–236. doi: 10.1016/j.molimm.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 35.Mou Z, et al. MHC class II restricted innate-like double negative T cells contribute to optimal primary and secondary immunity to Leishmania major. PLoS Pathog. 2014;10:e1004396. doi: 10.1371/journal.ppat.1004396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiede F, et al. PTPN2 regulates T cell lineage commitment and αβ versus γδ specification. J Exp Med. 2017;214:2733–2758. doi: 10.1084/jem.20161903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fukushima A, et al. T-cell protein tyrosine phosphatase attenuates STAT3 and insulin signaling in the liver to regulate gluconeogenesis. Diabetes. 2010;59:1906–1914. doi: 10.2337/db09-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu X, et al. T-cell protein tyrosine phosphatase, distinctively expressed in activated-B-cell-like diffuse large B-cell lymphomas, is the nuclear phosphatase of STAT6. Mol Cell Biol. 2007;27:2166–2179. doi: 10.1128/MCB.01234-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.ten Hoeve J, et al. Identification of a nuclear Stat1 protein tyrosine phosphatase. Mol Cell Biol. 2002;22:5662–5668. doi: 10.1128/MCB.22.16.5662-5668.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Vliet C, et al. Selective regulation of tumor necrosis factor-induced Erk signaling by Src family kinases and the T cell protein tyrosine phosphatase. Nat Immunol. 2005;6:253–260. doi: 10.1038/ni1169. [DOI] [PubMed] [Google Scholar]
- 41.Wiede F, et al. T cell protein tyrosine phosphatase attenuates T cell signaling to maintain tolerance in mice. J Clin Invest. 2011;121:4758–4774. doi: 10.1172/JCI59492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zikherman J, Weiss A. Unraveling the functional implications of GWAS: How T cell protein tyrosine phosphatase drives autoimmune disease. J Clin Invest. 2011;121:4618–4621. doi: 10.1172/JCI60001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen ZB, et al. Human urinary kallidinogenase suppresses cerebral inflammation in experimental stroke and downregulates nuclear factor-kappaB. J Cereb Blood Flow Metab. 2010;30:1356–1365. doi: 10.1038/jcbfm.2010.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.